Influence of Irrigant Activation Techniques on External Root Temperature Rise and Irrigation Penetration Depth in 3D-Printed Tooth Model: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
- (i)
- Sample selection:
- (ii)
- Sample preparation:
- (iii)
- Grouping:
- (iv)
- Irrigant Activation Protocol:
- (v)
- Temperature Measurement:
- (i)
- Sample size calculation:
- (ii)
- Sample preparation:
- (iii)
- Grouping:
- (iv)
- Depth of irrigation measurement:
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMH | Biofilm-Mimicking Hydrogel |
| PDL | Periodontal ligament |
References
- Schilder, H. Cleaning and shaping the root canal. Dent. Clin. N. Am. 1974, 18, 269–296. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Carvalho, C.R.; Belladonna, F.G.; Prado, M.C.; Lopes, R.T.; De-Deus, G.; Moreira, E.J.L. Micro-CT evaluation of different final irrigation protocols on the removal of hard-tissue debris from isthmus-containing mesial root of mandibular molars. Clin. Oral. Investig. 2019, 23, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, F.S., Jr.; Rôças, I.D.N.; Marceliano-Alves, M.F.; Pérez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral. Res. 2018, 32 (Suppl. 1), e65. [Google Scholar] [CrossRef] [PubMed]
- De Deus, Q.D. Frequency, location, and direction of the lateral, secondary, and accessory canals. J. Endod. 1975, 1, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Loghin, S.; Siqueira, J.F., Jr. Exuberant biofilm infection in a lateral canal as the cause of short-term endodontic treatment failure: Report of a case. J. Endod. 2013, 39, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Venturi, M.; Di Lenarda, R.; Prati, C.; Breschi, L. An in vitro model to investigate filling of lateral canals. J. Endod. 2005, 31, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.C.; Yoshioka, T.; Kobayashi, C.; Suda, H. Obturation of accessory canals after four different final irrigation regimes. J. Endod. 2002, 28, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Wang, Z.; Shen, Y.; Ma, J.; Cao, Y.; Haapasalo, M. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. J. Endod. 2014, 40, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Raducka, M.; Piszko, A.; Piszko, P.J.; Jawor, N.; Dobrzyński, M.; Grzebieluch, W.; Mikulewicz, M.; Skośkiewicz-Malinowska, K. Narrative review on methods of activating irrigation liquids for root canal treatment. Appl. Sci. 2023, 13, 7733. [Google Scholar] [CrossRef]
- Tay, F.R.; Gu, L.-S.; Schoeffel, G.J.; Wimmer, C.; Susin, L.; Zhang, K.; Arun, S.N.; Kim, J.; Looney, S.W.; Pashley, D.H. Effect of vapor lock on root canal debridement by using a side-vented needle for positive-pressure irrigant delivery. J. Endod. 2010, 36, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Brito, P.R.; Souza, L.C.; de Oliveira, J.C.M.; Alves, F.R.; De-Deus, G.; Lopes, H.P.; Siqueira, J.F. Comparison of the effectiveness of three irrigation techniques in reducing intracanal Enterococcus faecalis populations: An in vitro study. J. Endod. 2009, 35, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.; Almeida, B.M.; Neves, M.A.; Moreno, J.O.; Rôças, I.N.; Siqueira, J.F., Jr. Disinfecting oval-shaped root canals: Effectiveness of different supplementary approaches. J. Endod. 2011, 37, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.A.; Duarte, M.A.H.; Canali, L.C.F.; Zancan, R.F.; Vivan, R.R.; Bernardes, R.A.; Bramante, C.M. Comparative effectiveness of new mechanical irrigant agitating devices for debris removal from the canal and isthmus of mesial roots of mandibular molars. J. Endod. 2017, 43, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Macedo, R.G.; Verhaagen, B.; Wesselink, P.R.; Versluis, M.; van der Sluis, L.W. Influence of refreshment/activation cycles and temperature rise on the reaction rate of sodium hypochlorite with bovine dentine during ultrasonic activated irrigation. Int. Endod. J. 2014, 47, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bahcall, J.; Howard, P.; Miserendino, L.; Walia, H. Preliminary investigation of the histological effects of laser endodontic treatment on the periradicular tissues in dogs. J. Endod. 1992, 18, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, M.; Peters, O.A.; Paqué, F. Temperature changes during ultrasonic irrigation with different inserts and modes of activation. J. Endod. 2009, 35, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.D.; Rawal, S.Y. Severe periodontal damage by an ultrasonic endodontic device: A case report. Dent. Traumatol. 2007, 23, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Y.; Kimura, Y.; Kinoshita, J.; Ishizaki, N.T.; Matsumoto, K. Effects of diode laser irradiation on smear layer removal from root canal walls and apical leakage after obturation. Photomed. Laser Surg. 2005, 23, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Azma, E.; Safavi, N. Diode laser application in soft tissue oral surgery. J. Lasers Med. Sci. 2013, 4, 206–211. [Google Scholar] [PubMed] [PubMed Central]
- Hosseini Mehvar, B.; Azizi, A.; Lavaf, S.; Etemadi, A.; Hassani, A. Temperature rise and pain following the use of 810 and 980 nm diode lasers for second-stage dental implant surgery: A clinical trial. J Lasers Med. Sci. 2024, 15, e29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poly, A.; Marques, F.; Lee, J.; Setzer, F.C.; Karabucak, B. XP-Endo Finisher effectively reduces hard-tissue debris accumulated in root canals with isthmus after preparation with a reciprocating file system. Aust. Endod. J. 2023, 49, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.C.; Zuolo, M.L.; Arruda-Vasconcelos, R.; Marinho, A.C.S.; Louzada, L.M.; Francisco, P.A.; Pecorari, V.G.A.; Gomes, B.P.F.d.A. Effectiveness of XP-Endo Finisher in the reduction of bacterial load in oval-shaped root canals. Braz. Oral. Res. 2019, 33, e021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, T.; Guo, L. Effectiveness of XP-Endo Finisher and passive ultrasonic irrigation on intracanal medicament removal from root canals: A systematic review and meta-analysis. BMC Oral. Heal. 2021, 21, 294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reis, T.; Barbosa, C.; Franco, M.; Baptista, C.; Alves, N.; Castelo-Baz, P.; Martin-Cruces, J.; Martin-Biedma, B. 3D-Printed Teeth in Endodontics: Why, How, Problems and Future-A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 7966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balestra, D.; Lowther, M.; Goracci, C.; Mandurino, M.; Cortili, S.; Paolone, G.; Louca, C.; Vichi, A. 3D Printed Materials for Permanent Restorations in Indirect Restorative and Prosthetic Dentistry: A Critical Review of the Literature. Materials 2024, 17, 1380. [Google Scholar] [CrossRef]
- Macedo, R.G.; Robinson, J.P.; Verhaagen, B.; Walmsley, A.D.; Versluis, M.; Cooper, P.R.; van der Sluis, L.W.M. A novel methodology providing insights into removal of biofilm-mimicking hydrogel from lateral morphological features of the root canal during irrigation procedures. Int. Endod. J. 2014, 47, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Yanes, J.; Provenzano, J.C.; Marceliano-Alves, M.F.; Gazzaneo, I.; Pérez, A.R.; Gonçalves, L.S.; Siqueira, J.F. Distribution of sodium hypochlorite throughout the mesial root canal system of mandibular molars after adjunctive irrigant activation procedures: A micro-computed tomographic study. Clin. Oral. Investig. 2020, 24, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Q.; Yue, L.; Zou, X. Evaluation of sonic, ultrasonic, and laser irrigation activation systems to eliminate bacteria from the dentinal tubules of the root canal system. J. Appl. Oral. Sci. 2022, 30, e20220199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandhu, S.V.; Tiwari, R.; Bhullar, R.K.; Bansal, H.; Bhandari, R.; Kakkar, T.; Bhusri, R. Sterilization of extracted human teeth: A comparative analysis. J. Oral. Biol. Craniofac Res. 2012, 2, 170–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Intarasuksanti, C.; Prapayasatok, S.; Kampan, N.; Sirabanchongkran, S.; Mahakkanukrauh, P.; Sastraruji, T.; Khongkhunthian, P.; Kuharattanachai, K.; Tripuwabhrut, K. Effects of the cone-beam computed tomography protocol on the accuracy and image quality of root surface area measurements: An in vitro study. Imaging Sci. Dent. 2023, 53, 325–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adams, N.; Tomson, P. Access cavity preparation. Br. Dent. J. 2014, 216, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Spoorthy, E.; Velmurugan, N.; Ballal, S.; Nandini, S. Comparison of irrigant penetration up to working length and into simulated lateral canals using various irrigating techniques. Int. Endod. J. 2013, 46, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.M.; Markey, J.C.; Ebert-May, D. The other half of the story: Effect size analysis in quantitative research. CBE Life Sci. Educ. 2013, 12, 345–351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicholas, J.V.; White, D.R. An introduction to temperature measurement and calibration. In Traceable Temperatures; Wiley and Sons: Chichester, UK, 1994; pp. 239–270. [Google Scholar]
- Biagioni, P.A.; Longmore, R.B.; McGimpsey, J.G.; Lamey, P.J. Infrared thermography. Its role in dental research with particular reference to craniomandibular disorders. Dentomaxillofac Radiol. 1996, 25, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Mc Cullagh, J.J.P.; Setchell, D.J.; Gulabivala, K.; Hussey, D.L.; Biagioni, P.; Lamey, P.; Bailey, G. A comparison of thermocouple and infrared thermographic analysis of temperature rise on the root surface during the continuous wave of condensation technique. Int. Endod. J. 2000, 33, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Perlich, M.A.; Reader, A.; Foreman, D.W. A scanning electron microscopic investigation of accessory foramens on the pulpal floor of human molars. J. Endod. 1981, 7, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Vertucci, F.J.; Anthony, R.L. A scanning electron microscopic investigation of accessory foramens in the furcation and pulp chamber floor of molar teeth. Oral. Surg. Oral. Med. Oral. Pathol. 1986, 62, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Alfredo, E.; Marchesan, M.A.; Sousa-Neto, M.D.; Brugnera-Júnior, A.; Silva-Sousa, Y.T. Temperature variation at the external root surface during 980-nm diode laser irradiation in the root canal. J. Dent. 2008, 36, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, N.; Franzen, R.; Meister, J.; Vanweersch, L.; Mir, M. Temperature evolution on human teeth root surface after diode laser assisted endodontic treatment. Lasers Med. Sci. 2005, 20, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hmud, R.; Kahler, W.A.; Walsh, L.J. Temperature changes accompanying near infrared diode laser endodontic treatment of wet canals. J. Endod. 2010, 36, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Mejía, C.; Herrera, A.; Sánchez, A.I.; Moreno, S.; Moreno, F. Behavior in vitro of the dentin-enamel junction in human premolars submitted to high temperatures: Prediction of the maximum temperature based on logistic regression analysis. J. Forensic Odontostomatol. 2016, 34, 10–18. [Google Scholar] [PubMed] [PubMed Central]
- Kıvanc, B.H.; Arısu, H.D.; Sağlam, B.C.; Akça, G.; Gürel, M.A.; Görgül, G. Evaluation of Antimicrobial and Thermal Effects of Diode Laser on Root Canal Dentin. Niger. J. Clin. Pract. 2017, 20, 1527–1530. [Google Scholar] [CrossRef]
- Beer, F.; Farmakis, E.T.; Kopic, J.; Kurzmann, C.; Moritz, A. Temperature Development on the External Root Surface During Laser-Assisted Endodontic Treatment Applying a Microchopped Mode of a 980 nm Diode Laser. Photomed. Laser Surg. 2017, 35, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Antony Otero, C.; Marger, L.; Di Bella, E.; Felzer, A.; Krejci, I. Laser-activated irrigation. Front. Dent. Med. 2023, 4, 1010916. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Haupt, F.; Meinel, M.; Gunawardana, A.; Hülsmann, M. Effectiveness of different activated irrigation techniques on debris and smear layer removal from curved root canals: A SEM evaluation. Aust. Endod. J. 2020, 46, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Paixão, S.; Rodrigues, C.; Grenho, L.; Fernandes, M.H. Efficacy of sonic and ultrasonic activation during endodontic treatment: A meta-analysis of in vitro studies. Acta Odontol. Scand. 2022, 80, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.N.L.; Belladonna, F.G.; Zuolo, A.S.; Rodrigues, E.; Ehrhardt, I.C.; Souza, E.M.; De-Deus, G. Effectiveness of XP-endo Finisher and XP-endo Finisher R in removing root filling materials during retreatment of mandibular molars: A micro-CT study. Int. J. Dent. 2020, 2020, 8852527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bukiet, F.; Soler, T.; Guivarch, M.; Camps, J.; Tassery, H.; Cuisinier, F.; Candoni, N. Factors affecting the viscosity of sodium hypochlorite and their effect on irrigant flow. Int. Endod. J. 2013, 46, 954–961, Erratum in Int. Endod. J. 2016, 49, 912. [Google Scholar] [CrossRef] [PubMed]
- Virdee, S.S.; Farnell, D.J.J.; Silva, M.A.; Camilleri, J.; Cooper, P.R.; Tomson, P.L. The influence of irrigant activation, concentration and contact time on sodium hypochlorite penetration into root dentine: An ex vivo experiment. Int. Endod. J. 2020, 53, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, E.-G.C.; Skoulas, E.; Pepelassi, E.; Koidis, P.; Tzoutzas, I.G. The Use of Lasers in Dental Materials: A Review. Materials 2021, 14, 3370. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Ginjupalli, K.; George, S.D.; Chidangil, S.; Unnikrishnan, V.K. LASER as a tool for surface modification of dental biomaterials: A review. Heliyon 2023, 9, e17457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagunov, V.L.; Rybachuk, M.; Itthagarun, A.; Walsh, L.J.; George, R. Modification of dental enamel, dentin by an ultra-fast femtosecond laser irradiation: A systematic review. Opt. Laser Technol. 2022, 155, 108439. [Google Scholar] [CrossRef]
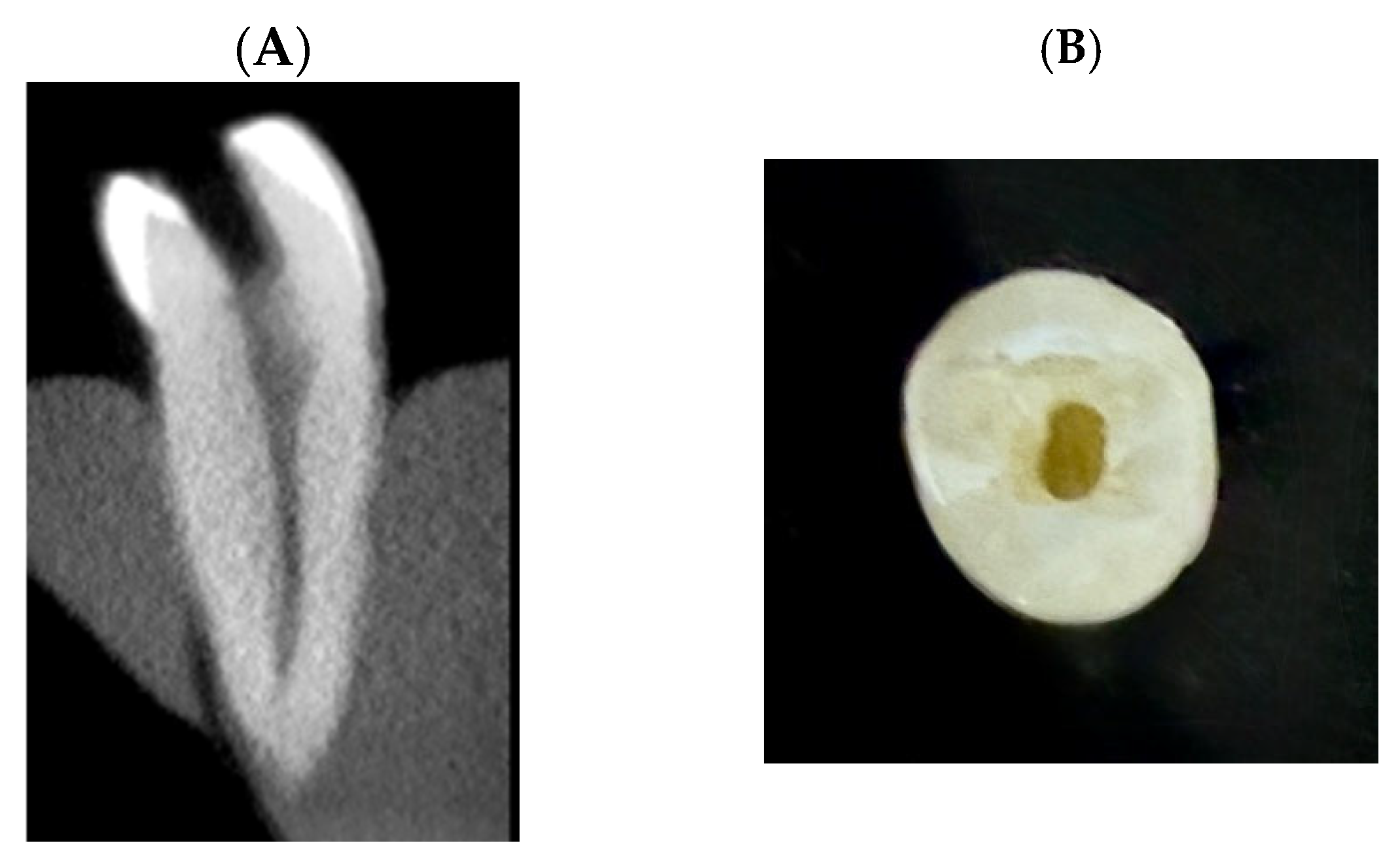

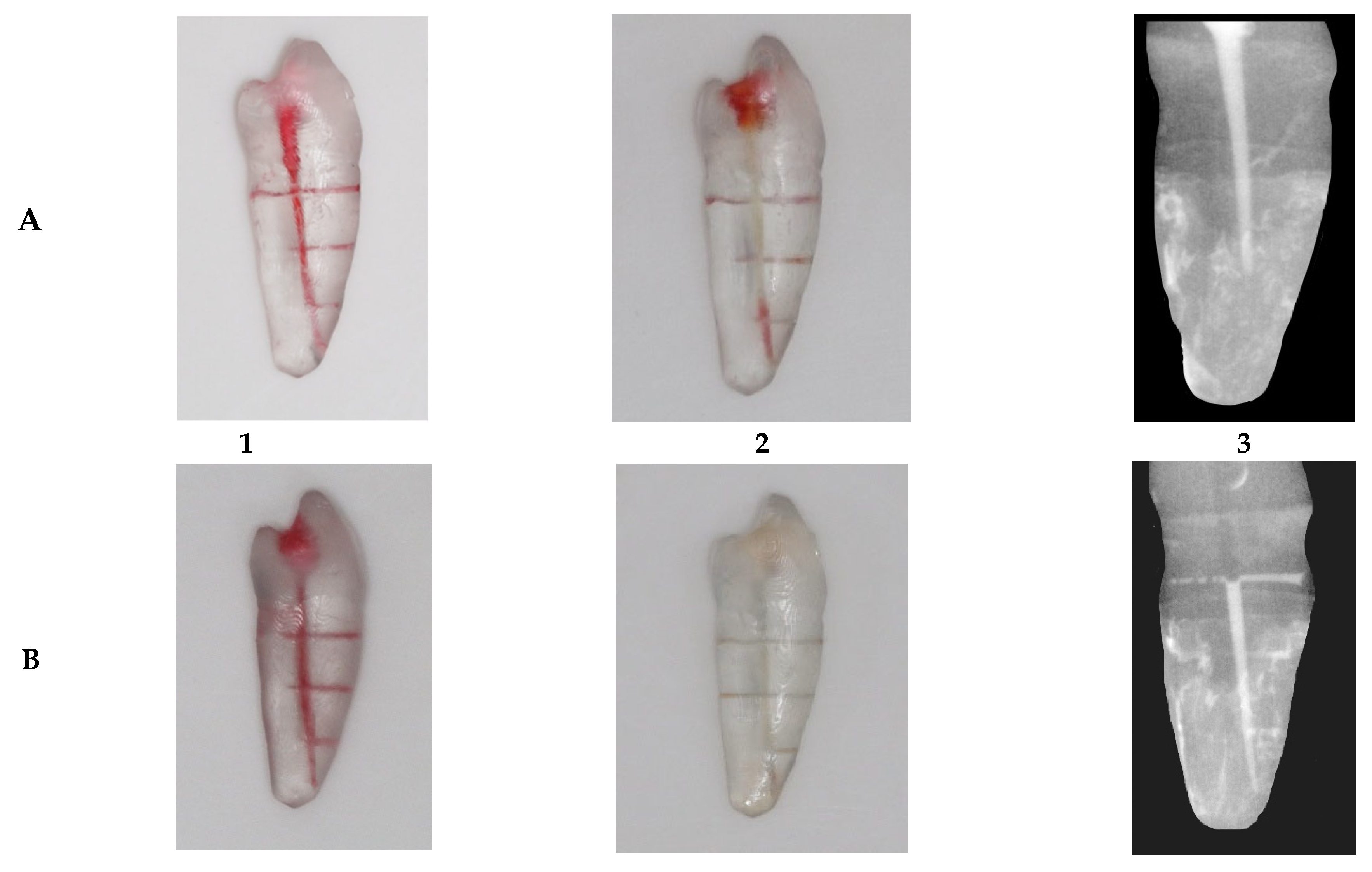
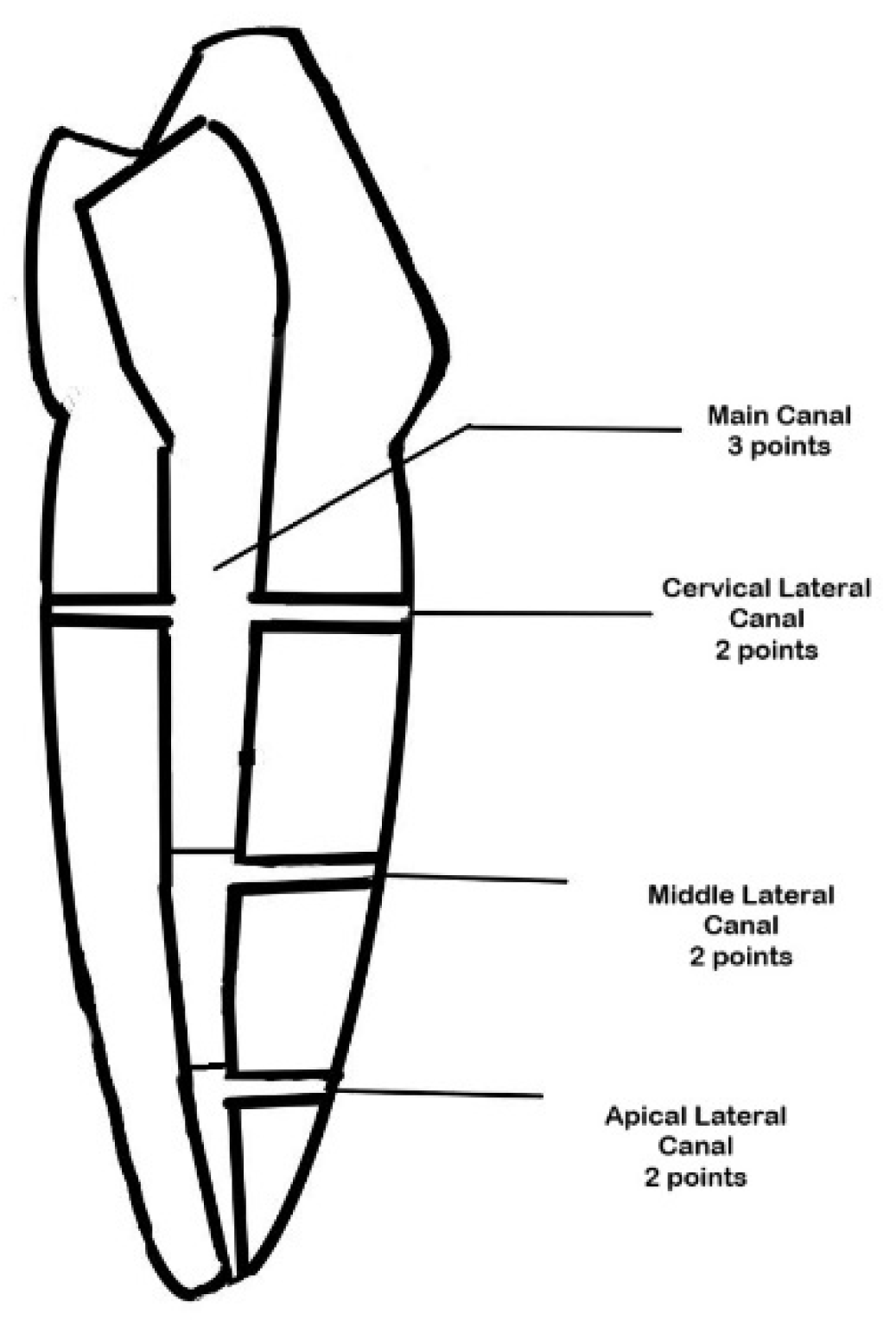
| Root Level | Root (B-L) | Root (M-D) | Lumen (B-L) | Lumen (M-D) |
|---|---|---|---|---|
| Cervical | 6.65 ± 0.263 | 4.32 ± 0.285 | 2.98 ± 0.226 | 1.50 ± 0.210 |
| Middle | 5.83 ± 0.221 | 3.24 ± 0.227 | 2.10 ± 0.258 | 1.26 ± 0.134 |
| Apical | 5.03 ± 0.383 | 2.80 ± 0.124 | 1.40 ± 0.182 | 0.90 ± 0.133 |
| Method | 30 Seconds (°C) Median (IQR) | 60 Seconds (°C) Median (IQR) | p-Value (30 s vs. 60 s) |
|---|---|---|---|
| Control | 0.28 (0.25–0.32) a | 0.33 (0.31–0.36) ae | 0.096 |
| Diode Laser | 4.50 (3.95–5.22) abcd | 8.00 (7.27–8.86) abcdf | 0.001 * |
| Ultrasonic | 0.92 (0.71–1.14) b | 2.10 (1.70–2.58) bef | 0.001 * |
| Sonic | 0.47 (0.38–0.56) d | 0.52 (0.43–0.62) c | 0.547 |
| XP-Finisher | 0.68 (0.54–0.83) c | 1.22 (0.96–1.48) d | 0.007 * |
| p-value (between groups) | <0.001 * | <0.001 * |
| Method | Without BMH (%) Median (IQR) | With BMH (%) Median (IQR) | p-Value (Without vs. With BMH) |
|---|---|---|---|
| Control | 65 (55–78) ac | 33 (33.33–44.44) abc | 0.0045 * |
| Diode Laser | 79 (72–87) bd | 36 (27–45) def | 0.0005 * |
| Ultrasonic | 87 (81–93) ab | 78 (66.66–88.88) ad | 0.121 |
| Sonic | 88 (81–95) cd | 83 (66.66–88.88) be | 0.122 |
| XP-Finisher | 68 (60–77)f | 63 (55.55–66.66) cf | 0.51 |
| p-value (between groups) | 0.022 * | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addokhi, A.; Rahoma, A.; Hanna, N.M.A.; Alonaizan, F.; Farooqi, F.; Rifaat, S. Influence of Irrigant Activation Techniques on External Root Temperature Rise and Irrigation Penetration Depth in 3D-Printed Tooth Model: An In Vitro Study. Dent. J. 2025, 13, 295. https://doi.org/10.3390/dj13070295
Addokhi A, Rahoma A, Hanna NMA, Alonaizan F, Farooqi F, Rifaat S. Influence of Irrigant Activation Techniques on External Root Temperature Rise and Irrigation Penetration Depth in 3D-Printed Tooth Model: An In Vitro Study. Dentistry Journal. 2025; 13(7):295. https://doi.org/10.3390/dj13070295
Chicago/Turabian StyleAddokhi, Ali, Ahmed Rahoma, Neveen M. A. Hanna, Faisal Alonaizan, Faraz Farooqi, and Shimaa Rifaat. 2025. "Influence of Irrigant Activation Techniques on External Root Temperature Rise and Irrigation Penetration Depth in 3D-Printed Tooth Model: An In Vitro Study" Dentistry Journal 13, no. 7: 295. https://doi.org/10.3390/dj13070295
APA StyleAddokhi, A., Rahoma, A., Hanna, N. M. A., Alonaizan, F., Farooqi, F., & Rifaat, S. (2025). Influence of Irrigant Activation Techniques on External Root Temperature Rise and Irrigation Penetration Depth in 3D-Printed Tooth Model: An In Vitro Study. Dentistry Journal, 13(7), 295. https://doi.org/10.3390/dj13070295






