Compatibility of Dual-Cure Core Materials with Self-Etching Adhesives
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHT | Butylated Hydroxytoluene |
| Bis-DMA | Bisphenol A Dimethacrylate |
| Bis-GMA | Bisphenol A Glycidyl Methacrylate |
| CHP | Cumene Hydroperoxide |
| DDDMA, | Dodecyl Dimethacrylate |
| DHEPT | N,N-Dihydroxyethyl-p-toluidine |
| EDMAB | Ethyl 4-Dimethylaminobenzoate |
| HDDMA | 1,6-Hexanediol Dimethacrylate |
| HEDMA | 2-Hydroxyethyl Methacrylate |
| MMHE | Methyl Methacrylate |
| NTG-GMA | Neopentyl Glycol Diglycidyl Methacrylate |
| PTU | Phenyl Thiourea |
| TEGDMA | Triethylene Glycol Dimethacrylate |
| TMPTMA | Trimethylolpropane Trimethacrylate |
| TPO | Diphenyl(2,4,6-trimethylbenzoyl)phosphine Oxide |
| UDMA | Urethane Dimethacrylate |
References
- Manning, K.E.; Yu, D.C.; Yu, H.C.; Kwan, E.W. Factors to consider for predictable post and core buildups of endodontically treated teeth. Part I: Basic theoretical concepts. J. Can. Dent. Assoc. 1995, 61, 693–695. [Google Scholar]
- Manning, K.E.; Yu, D.C.; Yu, H.C.; Kwan, E.W. Factors to consider for predictable post and core buildups of endodontically treated teeth. Part II: Clinical application of basic concepts. J. Can. Dent. Assoc. 1995, 61, 696–701. [Google Scholar]
- Zarow, M.; Dominiak, M.; Szczeklik, K.; Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Zamarripa-Calderón, J.E.; Kharouf, N.; Filtchev, D. Effect of composite core materials on fracture resistance of endodontically treated teeth: A systematic review and meta-analysis of in vitro studies. Polymers 2021, 13, 2251. [Google Scholar] [CrossRef]
- Kwon, T.Y.; Bagheri, R.; Kim, Y.K.; Kim, K.H.; Burrow, M.F. Cure mechanisms in materials for use in esthetic dentistry. J. Investig. Clin. Dent. 2012, 3, 3–16. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s pioneering acid-etch technique to self-adhering restoratives: A status perspective of rapidly advancing dental adhesive technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Watanabe, H.; Johnson, W.W.; Latta, M.A.; Miyazaki, M. Comparison between universal adhesives and two-step self-etch adhesives in terms of dentin bond fatigue durability in self-etch mode. Eur. J. Oral. Sci. 2017, 125, 215–222. [Google Scholar] [CrossRef]
- Sanares, A.M.; Itthagarun, A.; King, N.M.; Tay, F.R.; Pashley, D.H. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent. Mater. 2001, 17, 542–556. [Google Scholar] [CrossRef]
- Swift, E.J., Jr.; Perdigão, J.; Combe, E.C.; Simpson, C.H., 3rd; Nunes, M.F. Effects of restorative and adhesive curing methods on dentin bond strengths. Am. J. Dent. 2001, 14, 137–140. [Google Scholar]
- Walter, R.; Swift, E.J., Jr.; Ritter, A.V.; Bartholomew, W.W.; Gibson, C.G. Dentin bonding of an etch-and-rinse adhesive using self- and light-cured composites. Am. J. Dent. 2009, 22, 215–218. [Google Scholar]
- Tay, F.R.; King, N.M.; Suh, B.I.; Pashley, D.H. Effect of delayed activation of light-cured resin composites on bonding of all-in-one adhesives. J. Adhes. Dent. 2001, 3, 207–225. [Google Scholar]
- Bolhuis, P.B.; de Gee, A.J.; Kleverlaan, C.J.; El Zohairy, A.A.; Feilzer, A.J. Contraction stress and bond strength to dentin for compatible and incompatible combinations of bonding systems and chemical and light-cured core buildup resin composites. Dent. Mater. 2006, 22, 223–233. [Google Scholar] [CrossRef]
- O’Keefe, K.L.; Powers, J.M. Adhesion of resin composite core materials to dentin. Int. J. Prosthodont. 2001, 14, 451–456. [Google Scholar]
- Schittly, E.; Bouter, D.; Le Goff, S.; Degrange, M.; Attal, J.P. Compatibility of five self-etching adhesive systems with two resin luting cements. J. Adhes. Dent. 2010, 12, 137–142. [Google Scholar]
- Rathke, A.; Balz, U.; Muche, R.; Haller, B. Effects of self-curing activator and curing protocol on the bond strength of composite core buildups. J. Adhes. Dent. 2012, 14, 39–46. [Google Scholar]
- Mazzitelli, C.; Maravic, T.; Josic, U.; Mancuso, E.; Generali, L.; Checchi, V.; Breschi, L.; Mazzoni, A. Effect of adhesive strategy on resin cement bonding to dentin. J. Esthet. Restor. Dent. 2023, 35, 501–507. [Google Scholar] [CrossRef]
- Tagami, A.; Takahashi, R.; Nikaido, T.; Tagami, J. The effect of curing conditions on the dentin bond strength of two dual-cure resin cements. J. Prosthodont. Res. 2017, 61, 412–418. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Yiu, C.K.; Sanares, A.M.; Wei, S.H. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part I. Single-step self-etching adhesive. J. Adhes. Dent. 2003, 5, 27–40. [Google Scholar]
- Tay, F.R.; Suh, B.I.; Pashley, D.H.; Prati, C.; Chuang, S.F.; Li, F. Factors contributing to the incompatibility between simplified-step adhesives and self-cured or dual-cured composites. Part II. Single-bottle, total-etch adhesive. J. Adhes. Dent. 2003, 5, 91–105. [Google Scholar]
- Suh, B.I.; Feng, L.; Pashley, D.H.; Tay, F.R. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part III. Effect of acidic resin monomers. J. Adhes. Dent. 2003, 5, 267–282. [Google Scholar]
- Carvalho, R.M.; Garcia, F.C.; E Silva, S.M.; Castro, F.L. Critical appraisal: Adhesive-composite incompatibility, part I. J. Esthet. Restor. Dent. 2005, 17, 129–134. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Garcia, F.C.; e Silva, S.M.; Castro, F.L. Adhesive-composite incompatibility, part II. J. Esthet. Restor. Dent. 2005, 17, 191–195. [Google Scholar] [CrossRef]
- Kim, Y.K.; Chun, J.N.; Kwon, P.C.; Kim, K.H.; Kwon, T.Y. Polymerization kinetics of dual-curing adhesive systems when used solely or in conjunction with chemically-cured resin cement. J. Adhes. Dent. 2013, 15, 453–459. [Google Scholar]
- Bayindir, Y.Z.; Ölçer, E. Effect of dual-cure activators on dentin bond strengths of universal adhesives. Int. J. Appl. Dent. Sci. 2023, 9, 88–95. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Sutil, E.; Malaquias, P.; de Paris Matos, T.; de Souza, L.M.; Reis, A.; Perdigão, J.; Loguercio, A.D. Effect of self-curing activators and curing protocols on adhesive properties of universal adhesives bonded to dual-cured composites. Dent. Mater. 2017, 33, 775–787. [Google Scholar] [CrossRef]
- Michaud, P.L.; MacKenzie, A. Compatibility between dental adhesive systems and dual-polymerizing composite resins. J. Prosthet. Dent. 2016, 116, 597–602. [Google Scholar] [CrossRef]
- Al-Ansari, A.; Al-Harbi, F.; Baba, N.Z. In vitro evaluation of the bond strength of composite resin foundation materials to dentin. J. Prosthet. Dent. 2015, 114, 529–535. [Google Scholar] [CrossRef]
- Malaquias, P.; Gutiérrez, M.F.; Sutil, E.; Matos, T.P.; Hanzen, T.A.; Reis, A.; Perdigão, J.; Loguercio, A.D. Universal adhesives and dual-cured core buildup composite material: Adhesive properties. J. Appl. Oral. Sci. 2020, 30, e20200121. [Google Scholar] [CrossRef]
- Lamparth, I.; Fässler, P.; Schnur, T.; Thetiot, E.; Lalevée, J.; Catel, Y. Polymerizable thioureas as innovative reducing agents for self-cured and dual-cured dental materials. Dent. Mater. 2022, 38, 1108–1116. [Google Scholar] [CrossRef]
- Lamparth, I.; Angermann, J.; Fässler, P.; Schnur, T.; Graff, B.; Ohl, C.; Lalevée, J.; Catel, Y. Influence of the hydroperoxide structure on the reactivity and mechanical properties of self-cure dental composites. Dent. Mater. 2024, 40, 1191–1198. [Google Scholar] [CrossRef]
- Chen, X.; Durban, M.M. Dental Compositions and Methods of Use. U.S. Patent 11,259,995 B2, 1 March 2022. [Google Scholar]
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Enhancing the mechanical properties of glass-ionomer dental cements: A review. Materials 2020, 13, 2510. [Google Scholar] [CrossRef]
- ISO 29022; Dentistry–Adhesion–Notched-Edge Shear Bond Strength Test. The International Organization for Standardization: Geneva, Switzerland, 2013.
- 3M Scotchbond Universal Plus Technical Profile. Available online: https://multimedia.3m.com/mws/media/1910608O/technical-product-profile.pdf (accessed on 18 April 2025).
- Rueggeberg, F.A.; Caughman, W.F. The influence of light exposure on polymerization of dual-cure resin cements. Oper. Dent. 1993, 18, 48–55. [Google Scholar]

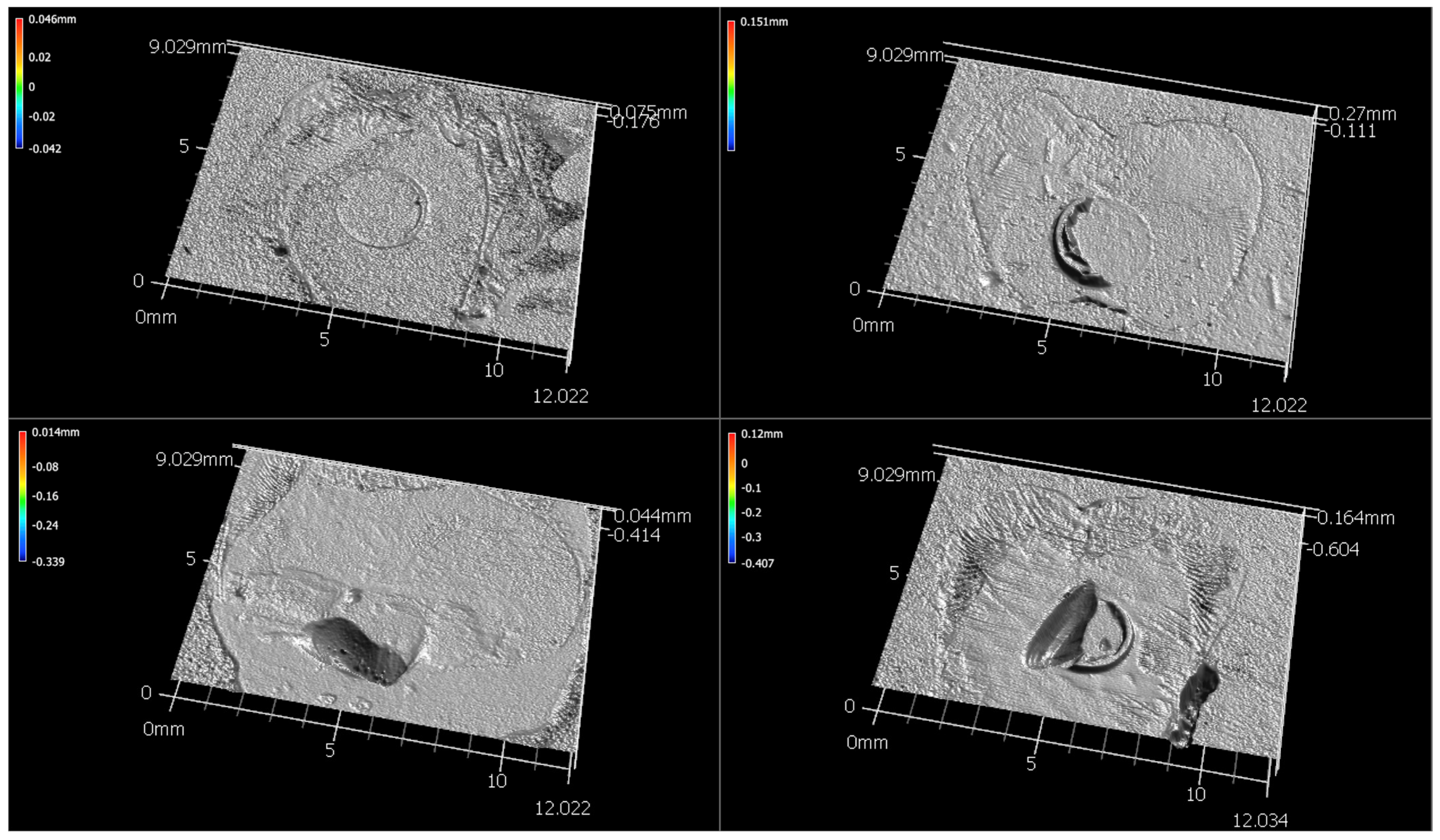
| Material Manufacturer LOT | Material Type | Relevant Material Composition |
|---|---|---|
| Activa BioActive-Restorative Pulpdent Corp Watertown, MA, USA LOT: 240129 | acid–base-containing | UDMA and other methacrylates; Modified polyacrylic acid; Silica; Sodium fluoride |
| Build-It FR Pentron (Kerr) Orange, CA, USA LOT: 9604454 | amine-containing | Bis-GMA, UDMA, HDDMA, Silane-treated barium boro-alumino silicate glass, Silane-treated chopped glass fibers, Pigments, UV absorbers, Initiators |
| Bulk EZ Plus Zest Dental Solutions Carlsbad, CA, USA LOT: L38KK | amine-free | Zirconia silica filler, Radiopaque filler, Ethoxylated Bis-DMA, TEGDMA, Bis-GMA, UDMA, Initiators (CHP, PTU, copper (II) catalyst) |
| CompCore AF Premier Dental Plymouth Meeting, PA, USA LOT: 5267-20QCW | amine-containing | Catalyst: TEGDMA; Benzoyl peroxide; Fumed silica Base: TEGDMA; Co-initiator; Photoinitiator; Fumed silica |
| Core Paste XP Syringe Den-Mat Lompoc, CA, USA LOT: 2419200010 | amine-containing | Ethoxylated Bis-DMA; TEGDMA; Silica; DHEPT; 2-(2′ hydroxy-5′-octylphenyl) benzotriazole; Benzoyl peroxide; TPO; BHT; Ethyl 4-dimethylaminobenzoate; Camphorquinone |
| Evanesce Bulk Fill Clinician’s Choice Lompoc, CA, USA LOT: 150CT24 | amine-free | Glass filler, TEGDMA, Titanium dioxide, CHP, BHT, Copper (II) catalyst, Iron oxide. |
| FluoroCore 2+ Dentsply Sirona Charlotte, NC, USA LOT: 00150296 | amine-containing | Base Paste: Silane-treated barium boro-alumino silicate glass; Fumed silica; Aluminum oxide; UDMA; Urethane modified Bis-GMA; Polymerizable methacrylate resins Catalyst Paste: Silane-treated barium boro-alumino silicate glass; Fumed silica; Aluminum oxide; Bis-GMA; Polymerizable dimethacrylate resin; Benzoyl peroxide |
| Geristore Den-Mat Lompoc, CA, USA LOT: 2408700069 | acid–base-containing | Benzoyl peroxide; Ethyl 4-dimethylaminobenzoate; Ethoxylated Bis-DMA; Polyacrylic acid; HEMA; NTG-GMA; Bis[2-[(2-methyl-1-oxoallyl)oxy]ethyl] dihydrogen; Tartaric acid; Camphorquinone; Silica; 2-(2′ hydroxy-5′-octylphenyl) benzotriazole; BHT |
| LuxaCore Z Dual DMG America Ridgefield Park, NJ, USA LOT: 296096 | amine-containing | Silica; Bis-GMA; UDMA; HDDMA; DDDMA; MMHE; EHDAB; Benzoyl peroxide |
| Multicore Flow Ivoclar Vivadent Schaan, Liechtenstein LOT: Z06NHR | amine-containing | Base: Ytterbium trifluoride; Bis-GMA; UDMA; TEGDMA Catalyst: Ytterbium trifluoride; Bis-GMA; UDMA; TEGDMA; Dibenzoyl peroxide |
| ParaCore Coltene Altstätten, Switzerland LOT: M66296 | amine-containing | UDMA; TMPTMA; Bis-GMA; TEGDMA; Dibenzoyl peroxide; Benzoyl peroxide; Sodium fluoride; Barium glass; Amorphous silica |
| Rebilda DC Voco GmbH Cuxhaven, Germany LOT: 2424665 | amine-containing | BHT; Amines; Benzoyl peroxide; Barium aluminum borosilicate glass; UDMA; HEDMA; DDDMA; Pyrogenic silica; Bis-GMA; Initiators; Stabilizers; Pigments. |
| Filtek Bulk Fill Flowable Universal Solventum St. Paul, MN, USA LOT: 10107433 | Light-cured bulk-fill composite | Silane-treated ceramic; UDMA; Substituted dimethacrylate; Ytterbium fluoride; Bis-GMA; Bis-EMA-6; TEGDMA; EDMAB |
| Material Manufacturer LOT | pH (Manufacturer Reported) | pH (Measured with Strips) | Bonding Protocol |
|---|---|---|---|
| OptiBond Universal Kerr Orange, CA, USA LOT: A172205 | Not reported | 1.0–1.5 | 20 s application, 5 s air thin, 5 s cure |
| Scotchbond Universal Plus Solventum St. Paul, MN, USA LOT: 10513692 | 2.730 | 3.0–3.5 | 20 s application, 5 s air thin, 10 s cure |
| Clearfil SE Bond 2 Kuraray Noritake Tokyo, Japan LOT: 3E0323 (Control) | Not reported | 4.0–4.5 (2nd bottle) | (1st bottle) 20 s application, air thin (2nd bottle) 20 s application, air thin, 10 s cure |
| OptiBond Universal | Scotchbond Universal Plus | Clearfil SE Bond 2 | Scotchbond Universal Plus (Core Light-Cured) | |
|---|---|---|---|---|
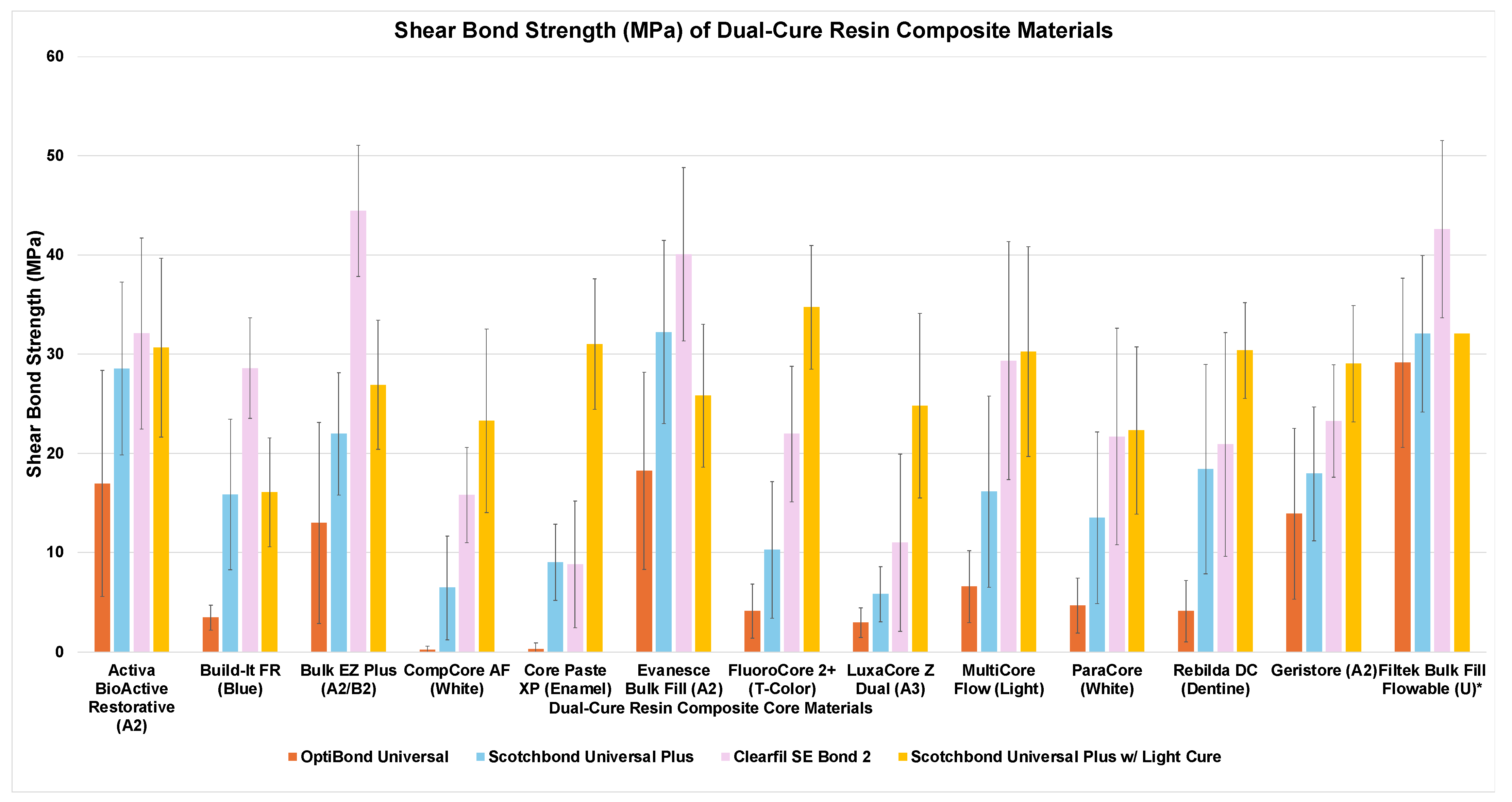
| Activa BioActive Restorative | 17.0 ± 11.4 CD,a | 28.5 ± 8.7 DE,b | 32.1 ± 9.7 CDE,b | 30.6 ± 9.0 BC,b |
| Build-It FR | 3.4 ± 1.3 AB,a | 15.8 ± 7.6 ABC,b | 28.6 ± 5.1 CD,c | 16.1 ± 5.5 A,b |
| Bulk EZ Plus | 13.0 ± 10.1 BCD,a | 22.0 ± 6.2 CD,b | 44.5 ± 6.6 E,c | 26.9 ± 6.5 ABC,b |
| CompCore AF | 0.2 ± 0.4 A,a | 6.4 ± 5.2 AB,b | 15.8 ± 4.8 AB,bc | 23.3 ± 9.3 ABC,c |
| Core Paste XP Syringe | 0.3 ± 0.6 A,a | 9.0 ± 3.8 ABC,a | 8.8 ± 6.4 A,a | 31.0 ± 6.6 BC,b |
| Evanesce Bulk Fill | 18.2 ± 9.9 DE,a | 32.2 ± 9.2 E,b | 40.1 ± 8.7 DE,b | 27.8 ± 4.3 ABC,b |
| FluoroCore 2+ | 4.1 ± 2.7 ABC,a | 10.2 ± 6.9 ABC,a | 21.9 ± 6.8 ABC,b | 34.7 ± 6.2 C,c |
| Geristore | 13.9 ± 8.6 BCD,a | 17.9 ± 6.8 BCD,ab | 23.3 ± 5.7 BC,bc | 29.0 ± 5.9 BC,c |
| LuxaCore Z Dual | 2.9 ± 1.5 AB,a | 5.8 ± 2.8 A,a | 11.0 ± 8.9 A,a | 24.8 ± 9.3 ABC,b |
| Multicore Flow | 6.6 ± 3.6 ABCD,a | 16.1 ± 9.6 ABCD,a | 29.3 ± 12 CD,b | 30.2 ± 10.6 BC,b |
| ParaCore | 4.7 ± 2.8 ABC,a | 13.5 ± 8.7 ABC,ab | 21.7 ± 10.9 ABC,b | 22.3 ± 8.4 BC,b |
| Rebilda DC | 4.1 ± 3.1 ABC,a | 18.4 ± 10.6 BCD,b | 20.9 ± 11.3 ABC,b | 30.4 ± 4.8 BC,c |
| Filtek Bulk Fill Flowable Universal * | 29.1 ± 8.5 E,a | 32.0 ± 7.9 E,a | 42.6 ± 8.9 E,b | NA |
| Mean Square | df | F | p | |
|---|---|---|---|---|
| Core Material | 1958.148 | 12 | 33.422 | <0.001 |
| Adhesive System | 8295.646 | 3 | 141.590 | <0.001 |
| Core Material × Adhesive System | 321.283 | 36 | 5.484 | <0.001 |
| OptiBond Universal | Scotchbond Universal Plus | Clearfil SE Bond 2 | Scotchbond Universal Plus (Core Light-Cured) | |
|---|---|---|---|---|
| Activa BioActive Restorative | 3:7 | 4:6 | 8:2 | 7:3 |
| Build-It FR | 0:10 | 0:10 | 4:6 | 0:10 |
| Bulk EZ Plus | 1:9 | 2:8 | 10:0 | 5:5 |
| CompCore AF | 0:10 | 0:10 | 0:10 | 1:9 |
| Core Paste XP Syringe | 0:10 | 0:10 | 0:10 | 6:4 |
| Evanesce Bulk Fill | 2:8 | 6:4 | 9:1 | 5:5 |
| FluoroCore 2+ | 0:10 | 0:10 | 1:9 | 8:2 |
| Geristore | 0:10 | 0:10 | 1:9 | 7:3 |
| LuxaCore Z Dual | 0:10 | 0:10 | 0:10 | 1:9 |
| Multicore Flow | 0:10 | 0:10 | 5:5 | 5:5 |
| ParaCore | 0:10 | 0:10 | 2:8 | 2:8 |
| Rebilda DC | 0:10 | 0:10 | 1:9 | 6:4 |
| Filtek Bulk Fill Universal | 5:5 | 7:3 | 9:1 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greene, Z.K.; Robles, A.A.; Lawson, N.C. Compatibility of Dual-Cure Core Materials with Self-Etching Adhesives. Dent. J. 2025, 13, 276. https://doi.org/10.3390/dj13070276
Greene ZK, Robles AA, Lawson NC. Compatibility of Dual-Cure Core Materials with Self-Etching Adhesives. Dentistry Journal. 2025; 13(7):276. https://doi.org/10.3390/dj13070276
Chicago/Turabian StyleGreene, Zachary K., Augusto A. Robles, and Nathaniel C. Lawson. 2025. "Compatibility of Dual-Cure Core Materials with Self-Etching Adhesives" Dentistry Journal 13, no. 7: 276. https://doi.org/10.3390/dj13070276
APA StyleGreene, Z. K., Robles, A. A., & Lawson, N. C. (2025). Compatibility of Dual-Cure Core Materials with Self-Etching Adhesives. Dentistry Journal, 13(7), 276. https://doi.org/10.3390/dj13070276






