Association Between Oral Microbiota Dysbiosis and the Risk of Dementia: A Systematic Review
Abstract
1. Introduction
1.1. The Role of the Apolipoprotein
1.2. Research Question
1.3. Objective
2. Materials and Methods
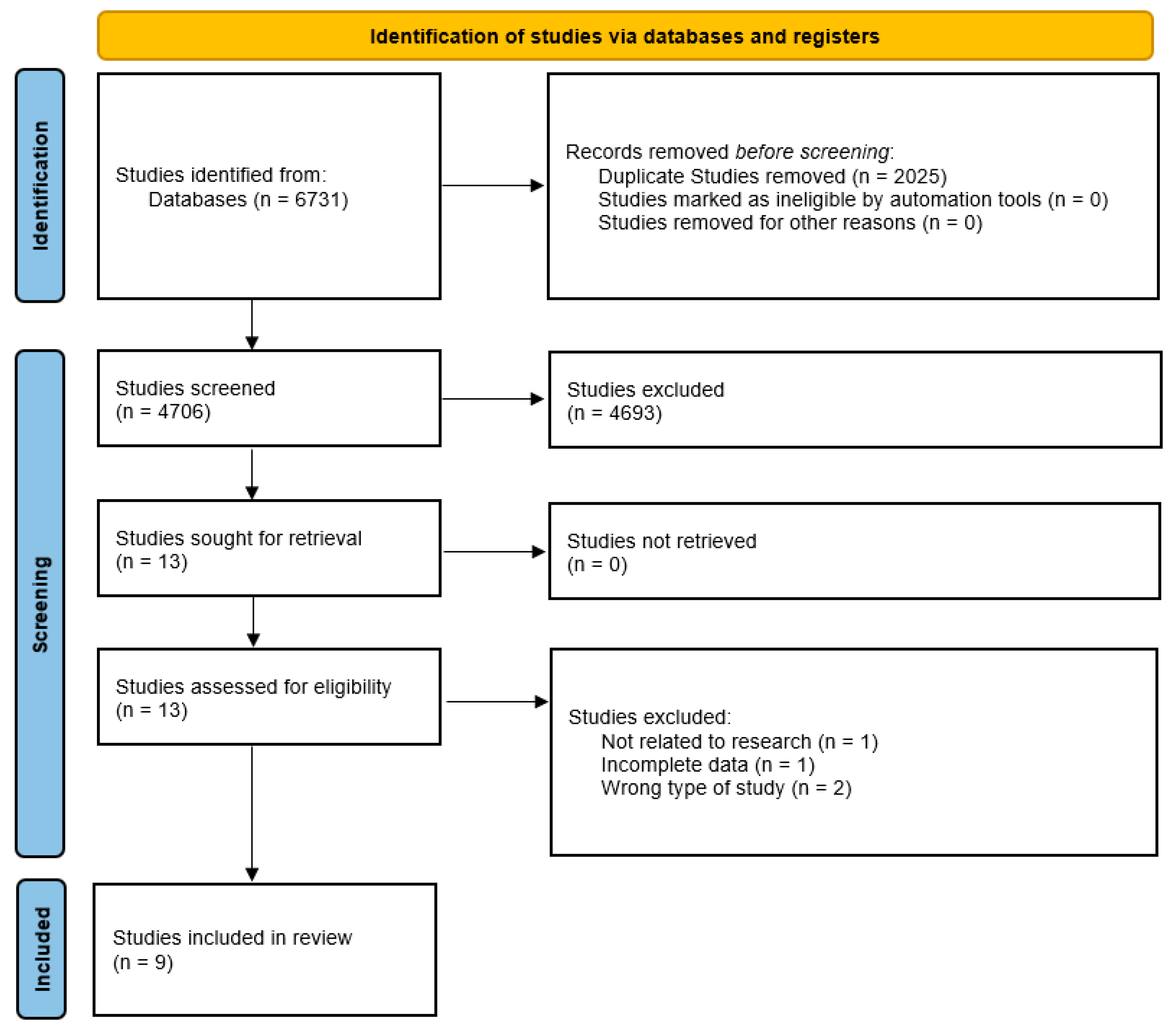
2.1. Study Design
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- -
- Population: Older adults (≥50 years) with a dementia diagnosis.
- -
- Intervention: Evaluation of the oral microbiota using genetic sequencing methods, bacterial culture, metagenomics, quantitative PCR, or other molecular analyses.
- -
- Comparator: Individuals without a dementia diagnosis.
- -
- Outcome: Measurement of the association between oral dysbiosis and the risk of dementia, cognitive decline, or neuroinflammatory biomarkers.
- -
- Study Design: Observational studies (cohort, case–control, cross-sectional) analyzing the relationship between oral microbiota and dementia.
2.2.2. Exclusion Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Processing
2.8. Ethical Considerations
3. Results
3.1. Summary of Study Designs and Their Impact on Research Robustness
3.1.1. Cross-Sectional Observational Studies (n = 6)
| Author/ Year | Country | N | n with CI * | n Without CI | Age Range | Type of CI Evaluated/ Stage, Grade | Neuropsychological Scales | Oral Microbiota Assessment Method | Sample Type | Bacteria Associated with Dysbiosis (Increase) | Bacteria Associated with Dysbiosis (Reduction) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Babenia [21] 2023 | Ukraine | 27 | 27 | No age range or average age is specified. | Alzheimer’s/ it does not specify differentiated clinical stages or RDA or MMSE values. | Not reported | Polymerase chain reaction (PCR) | Gingival fluid from periodontal pockets | Tannerella forsythia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans | Porphyromonas gingivalis | Tannerella forsythia and Fusobacterium nucleatum were detected in 100% of Alzheimer’s patients. The microbial composition suggests a possible link between oral dysbiosis and dementia pathogenesis. | |
| Bathini [22] 2020 | Switzerland | 80 | 38 | 42 | CNh: 67.0 ± 9.2 years CNr: 68.1 ± 10.0 years MCI: 73.2 ± 8.1 years AD: 71.1 ± 6.6 years | Alzheimer’s/ inclusion of patients in moderate-severe phase of AD. | Mini-Mental State Exam (MMSE), Clinical Dementia Rating (CDR), University of Pennsylvania Smell Identification Test (UPSIT) CNh (cognitively normal healthy): MMSE ≈ 28.4; CDR ≈ 0.0 CNr (cognitively normal at risk): MMSE ≈ 28.4; CDR ≈ 0.1 MCI: MMSE ≈ 22.5; CDR ≈ 0.8 AD: MMSE ≈ 14.2; CDR ≈ 1.4 | 16S rRNA Sequencing (Illumina MiSeq) | Saliva | Leptotrichia wadei (MCI), Cardiobacterium valvarum (AD) | Filifactor villosus, Filifactor alocis, Prevotella tannerae (MCI y AD), Porphyromonas gingivalis (MCI) | The salivary microbiota presents specific changes depending on the stage of dementia, with a reduction in periodontal bacteria and an increase in opportunistic species in the progression of the disease. |
| Chen [23] 2022 | China | 66 | 66 | 82.85 ± 6.00 years | All patients were diagnosed with mild Alzheimer’s disease | Mini-Mental State Examination (MMSE), Neuropsychiatric Inventory (NPI), Nursing Home Adjustment Scale (NHAS), Alzheimer’s Disease Cooperative Study-ADL (ADCS-ADL), Kayser-Jones Brief Oral Health Status Examination (BOHSE) | 16S rRNA Sequencing (Illumina MiSeq) | Subgingival biofilm | Alphaproteobacteria, Betaproteobacteria, Flavobacteria | Actinobacteria, Spirochaete, Synergistetes | The oral health intervention improved the oral microbiota and reduced cognitive impairment in patients with mild Alzheimer’s after 6 months of follow-up. | |
| Chen [24] 2024 | China | 165 | 125 | 40 | Normal controls: 67.45 ± 8.36 years SCD: 66.90 ± 7.98 years MCI: 66.33 ± 8.83 years Dementia: 68.44 ± 6.71 years | It does not report specific data on stage or severity, it only compares individuals with Alzheimer’s. | Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA) | 16S rRNA Sequencing (Illumina MiSeq) | Subgingival biofilm | Porphyromonas, Prevotella, Fusobacterium, Leptotrichia, Campylobacter, Selenomonas | Capnocytophaga, Saccharibacteria_genera_incertae_sedis, Lautropia, Granulicatella | Subgingival microbial composition is associated with different levels of cognitive function, suggesting a possible use as a biomarker of cognitive decline. |
| Da [25] 2023 | China | 94 | 47 | 47 | Not explicitly reported. | No classification is specified in degrees of severity; it is only reported as Alzheimer’s or MCI vs. controls. | Mini-Mental State Examination (MMSE), Auditory Verbal Learning Test, Trail-Making Test, RMB (Renminbi) Test | 16S rDNA Sequencing (Illumina MiSeq) | Unstimulated saliva | Veillonella unclassified_Veillonella, Fusobacterium sp._HMT_203 | Gemella haemolysans, Streptococcus gordonii | Older adults with mild cognitive impairment have an altered oral microbial composition compared to cognitively normal individuals. Gemella haemolysans and Streptococcus gordonii may be potential indicators of MCI. |
| Golipoor [27] 2024 | Iran | 152 | 76 | 76 | AD: 87.96 ± 7.91 years Non-AD: 85.18 ± 5.79 years | Alzheimer’s/ preclinical. Very mild cognitive decline/ mild to moderate cognitive decline and severe dementia. | Global Deterioration Scale (GDS) | Fungal culture on agar Sabouraud Chloramphenicol and PCR-Sequencing | Oral mucus swab | Candida albicans (AD: 80%, No AD: 40%), Candida glabrata (AD: 9%) | Increased fungal diversity in individuals without AD | Patients with AD have a higher prevalence of Candida spp. and a lower fungal diversity in the oral microbiota. Fungal microbiota analysis could be an early marker of the disease. |
| L’Heureux [14] 2025 | United Kingdom | 115 | 55 | 60 | Inclusion criteria: ≥50 years old. No mean age is reported. | Patients were classified as mild cognitive impairment (MCI). Patients with clinical Alzheimer’s were not included. | Mini-Mental State Examination (MMSE), Switching Stroop, Trail Making, Digit Span | 16S rRNA sequencing | Mouth rinse | Porphyromonas (MCI), Prevotella intermedia (APOE4+) | Neisseria, Haemophilus (associated with better cognition) | The oral microbiome of people with MCI exhibits a higher abundance of Porphyromonas and a lower level of Neisseria and Haemophilus, suggesting a link between oral dysbiosis and cognitive decline. |
| Liu [26] 2019 | China | 78 | 39 | 39 | The average age is not specified. | Alzheimer’s | Mini-Mental State Examination (MMSE), Neuropsychiatric Inventory (NPI), Clinical Dementia Rating (CDR), Activity of Daily Living Scale (ADL) | 16S rRNA sequencing | Unstimulated saliva | Moraxella, Leptotrichia, Sphaerochaeta | Rothia | Patients with AD have lower salivary microbial diversity and alterations in bacterial composition, with an increase in Moraxella and a reduction in Rothia. |
| Sritana [17] 2024 | Thailand | 100 | 56 | 44 | AD: 66.90 ± 7.06 years MCI: 68.50 ± 6.35 years Controls: 64.73 ± 4.78 years | Alzheimer’s/ average values are reported for AD patients. | Clinical Dementia Rating (CDR), Montreal Cognitive Assessment (MoCA), Mini-Mental State Examination (MMSE) | 16S rRNA Sequencing (PacBio SMRT) | Saliva | Fusobacteriota, Peptostreptococcaceae | Veillonella | Patients with AD show greater diversity of oral microbiota and elevated levels of Fusobacteriota and Peptostreptococcaceae, suggesting a possible role in the pathogenesis of the disease. |
3.1.2. Case–Control Studies (n = 2)
3.1.3. Randomized Controlled Trial (n = 1)
4. Discussion
4.1. Proposed Scheme on How Oral Microbial Dysbiosis May Influence the Development of Dementia
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chowdhry, A.; Kapoor, P.; Bhargava, D.; Bagga, D.K. Exploring the oral microbiome: An updated multidisciplinary oral healthcare perspective. Discoveries 2023, 11, e165. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Ding, T.; Li, H. Oral microbiome in human health and diseases. mLife 2024, 3, 367–383. [Google Scholar] [CrossRef]
- Na, H.S.; Jung, N.Y.; Song, Y.; Kim, S.Y.; Kim, H.J.; Lee, J.Y.; Chung, J. A distinctive subgingival microbiome in patients with periodontitis and Alzheimer’s disease compared with cognitively unimpaired periodontitis patients. J. Clin. Periodontol. 2023, 51, 43–53. [Google Scholar] [CrossRef]
- Spitzner, A.; Mieth, M.; Langan, E.A.; Büchler, M.W.; Michalski, C.; Billmann, F. Influence of dental status on postoperative complications in major visceral surgical and organ transplantation procedures—The bellydent retrospective observational study. Langenbeck’s Arch. Surg. 2024, 409, 284. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Morgadinho, J.; Halverson, T. Approach to the diagnosis and management of dysbiosis. Front. Nutr. 2024, 11, 1330903. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Orlandi, M.; Munoz Aguilera, E.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the treatment of periodontitis on systemic health and quality of life: A systematic review. J. Clin. Periodontol. 2022, 49, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Linden, G.J.; Lyons, A.; Scannapieco, F.A. Periodontal systemic associations: Review of the evidence. J. Periodontol. 2013, 84, S8–S19. [Google Scholar] [CrossRef]
- Zenobia, C.; Darveau, R.P. Does Oral Endotoxin Contribute to Systemic Inflammation? Front. Oral Health 2022, 3, 911420. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Kopra, E.; Pietiäinen, M.; Lehto, M.; Zaric, S.; Paju, S.; Salminen, A. Periodontitis and cardiometabolic disorders: The role of lipopolysaccharide and endotoxemia. Periodontology 2000 2022, 89, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Polizzi, A.; Viglianisi, G.; Leonforte, F.; Mascitti, M.; Isola, G. Impact of Periodontitis and Oral Dysbiosis Metabolites in the Modulation of Accelerating Ageing and Human Senescence. Metabolites 2025, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Peña-Durán, E.; García-Galindo, J.J.; López-Murillo, L.D.; Huerta-Huerta, A.; Balleza-Alejandri, L.R.; Beltrán-Ramírez, A.; Anaya-Ambriz, E.J.; Suárez-Rico, D.O. Microbiota and Inflammatory Markers: A Review of Their Interplay, Clinical Implications, and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 1773. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, J.E.; Corbett, A.; Ballard, C.; Vauzour, D.; Creese, B.; Winyard, P.G.; Jones, A.M.; Vanhatalo, A. Oral microbiome and nitric oxide biomarkers in older people with mild cognitive impairment and APOE4 genotype. PNAS Nexus 2025, 4, pgae543. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Popescu, C.; Munteanu, C.; Anghelescu, A.; Ciobanu, V.; Spînu, A.; Andone, I.; Mandu, M.; Bistriceanu, R.; Băilă, M.; Postoiu, R.-L.; et al. Novelties on Neuroinflammation in Alzheimer’s Disease–Focus on Gut and Oral Microbiota Involvement. Int. J. Mol. Sci. 2024, 25, 11272. [Google Scholar] [CrossRef]
- Sritana, N.; Phungpinij, A. Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2024, 21, 1242. [Google Scholar] [CrossRef]
- Lozupone, M.; Dibello, V.; Sardone, R.; Castellana, F.; Zupo, R.; Lampignano, L.; Bortone, I.; Daniele, A.; Bellomo, A.; Solfrizzi, V.; et al. The Impact of Apolipoprotein E (APOE) Epigenetics on Aging and Sporadic Alzheimer’s Disease. Biology 2023, 12, 1529. [Google Scholar] [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Babenia, H.; Harashchuk, I.; Shnaider, S.; Kotova, I.; Khrystova, M.; Savvova, A.; Korniichuk, O.; Babenia, H.O.; Harashchuk, I.V.; Shnaider, S.A.; et al. MOLECULAR GENETIC ASSESSMENT OF THE ORAL MICROBIOME IN PATIENTS WITH ALZHEIMER’S DISEASE. World Med. Biol. 2023, 85, 16–20. [Google Scholar] [CrossRef]
- Bathini, P.; Foucras, S.; Dupanloup, I.; Imeri, H.; Perna, A.; Berruex, J.; Doucey, M.; Annoni, J.; Alberi, L.; Bathini, P.; et al. Classifying dementia progression using microbial profiling of saliva. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12000. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Wu, X.; Xu, X.; Ji, X.; Wang, B.; Zhang, P.; Li, H. Effects of oral health intervention strategies on cognition and microbiota alterations in patients with mild Alzheimer’s disease: A randomized controlled trial. Geriatr. Nurs. 2022, 48, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Liu, J.; Hou, Z.; Wei, Y.; Chen, M.; Wang, B.; Cao, H.; Qiu, R.; Zhang, Y.; et al. Distinctive subgingival microbial signatures in older adults with different levels of cognitive function. J. Clin. Periodontol. 2024, 51, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Da, D.; Zhao, Q.; Zhang, H.; Wu, W.; Zeng, X.; Liang, X.; Jiang, Y.; Xiao, Z.; Yu, J.; Ding, S.; et al. Oral microbiome in older adults with mild cognitive impairment. J. Oral Microbiol. 2023, 15, 2173544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-X.; Jiao, B.; Liao, X.-X.; Guo, L.-N.; Yuan, Z.-H.; Wang, X.; Xiao, X.-W.; Zhang, X.-Y.; Tang, B.-S.; Shen, L. Analysis of Salivary Microbiome in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 633–640. [Google Scholar] [CrossRef]
- Golipoor, M.; Rafat, Z.; Saberi, A.; Roostaei, D.; Shabanpour, A.; Golipoor, M.; Rafat, Z.; Saberi, A.; Roostaei, D.; Shabanpour, A.-M. Comparing the frequency, antifungal susceptibility, and enzymatic profiles of the oral fungal composition in patients with and without Alzheimer’s disease admitted to a neurology clinic. Front. Cell. Infect. Microbiol. 2024, 14, 1477230. [Google Scholar] [CrossRef]
- Morales, H.; Herrero-Rivera, C.A.; Soler-Llompart, C.M.; Sala-Morales, A.C.; Olivieri-Henry, G.; Godoy-Vitorino, F.; Sepulveda, V. Oral Microbial Dysbiosis Associated with Alzheimer’s Dementia in Puerto Ricans: A Preliminary Report. Alzheimer’s Dement. 2024, 20, e095310. [Google Scholar] [CrossRef]
- Jungbauer, G.; Stähli, A.; Zhu, X.; Auber Alberi, L.; Sculean, A.; Eick, S. Periodontal microorganisms and Alzheimer disease—A causative relationship? Periodontology 2000 2022, 89, 59–82. [Google Scholar] [CrossRef]
- Wan, J.; Fan, H. Oral Microbiome and Alzheimer’s Disease. Microorganisms 2023, 11, 2550. [Google Scholar] [CrossRef]
- Pruntel, S.M.; Leusenkamp, L.A.; Zaura, E.; Vissink, A.; Visser, A. Oral Microbiota in Patients with Alzheimer’s Disease: A Systematic Review. Appl. Sci. 2024, 14, 8869. [Google Scholar] [CrossRef]
- McGuinness, B.; Passmore, A.P.; Holmes, C.; Teeling, J.; Linden, G.; Farsi, D.; McEvoy, C.T.; McKay, G.J.; Abadelkareem, R.; Patterson, C.; et al. Periodontitis and future cognitive decline. Alzheimer’s Dement. 2023, 19, e082957. [Google Scholar] [CrossRef]
- Jeong, S.-a.; Jung, B.-Y.; Lee, I.; Cha, J.H.; Choi, Y. PROFILING THE ORAL MICROBIOME IN OLDER ADULTS WITH DEMENTIA. Innov. Aging 2024, 8, 1268–1269. [Google Scholar] [CrossRef]
- Chaudhari, D.; Jain, S.; Labyak, C.; Golden, A.; GB, M.; Holland, P.; Masternak, M.; Yadav, H. UNIQUE MICROBIOME-PROTEOME SIGNATURES OF SALIVA IN PREDICTION OF COGNITIVE DECLINE IN OLDER ADULTS OF MIAGB COHORT. Innov. Aging 2023, 7, 932. [Google Scholar] [CrossRef]
- Loughman, A.; Adler, C.J.; Macpherson, H. Unlocking Modifiable Risk Factors for Alzheimer’s Disease: Does the Oral Microbiome Hold Some of the Keys? J. Alzheimer’s Dis. 2023, 92, 1111–1129. [Google Scholar] [CrossRef]
- Mao, S.; Huang, C.-P.; Lan, H.; Lau, H.-G.; Chiang, C.-P.; Chen, Y.-W. Association of periodontitis and oral microbiomes with Alzheimer’s disease: A narrative systematic review. J. Dent. Sci. 2022, 17, 1762–1779. [Google Scholar] [CrossRef]
- Hisamatsu, D.; Ogata, Y.; Takeshige-Amano, H.; Suda, W.; Hatano, T.; Asaoka, D.; Mabuchi, Y.; Naraoka, Y.; Sato, N.; Asada, T.; et al. Connection between drug-mediated neurotransmission and salivary microbiome. bioRxiv 2023. [Google Scholar] [CrossRef]
- Clasen, F.; Yildirim, S.; Arıkan, M.; Garcia-Guevara, F.; Hanoğlu, L.; Yılmaz, N.H.; Şen, A.; Demir, T.K.; Yıldız, Z.; Mardinoglu, A.; et al. Microbiome signatures of virulence in the oral-gut-brain axis influence Parkinson’s disease and cognitive decline pathophysiology. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wadop, Y.N.; Vasquez, E.L.; Mathews, J.J.; Muhammad, J.A.S.; Mavarez, R.P.; Satizabal, C.; Gonzales, M.M.; Tanner, J.; Maestre, G.; Fonteh, A.N.; et al. Differential Patterns of Gut and Oral Microbiomes in Hispanic Individuals with Cognitive Impairment. bioRxiv 2024. [Google Scholar] [CrossRef]
- Liu, S.; Dashper, S.G.; Zhao, R.; Yu, J.T. Association Between Oral Bacteria and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2023, 91, 129–150. [Google Scholar] [CrossRef]
- Tao, K.; Yuan, Y.; Xie, Q.; Dong, Z. Relationship between human oral microbiome dysbiosis and neuropsychiatric diseases: An updated overview. Behav. Brain Res. 2024, 471, 115111. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Jiao, Z.; Wang, L.; Zhao, Y.; Hu, W.; Chen, F.; Yu, J.; Zhang, X.; Zhao, P.; Jiang, H.; et al. Genetic evidence strengthens the bidirectional connection between oral health status and psychiatric disorders: A two-sample Mendelian randomization study. J. Affect. Disord. 2024, 351, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Cademartori, M.G.; Gastal, M.T.; Nascimento, G.G.; Demarco, F.F.; Corrêa, M.B. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
| Database | Formulation | Filters |
|---|---|---|
| Pubmed | ((“Oral microbiome” [Title/Abstract] OR “oral microbiota” [Title/Abstract] OR “oral bacteria” [Title/Abstract] OR “oral dysbiosis” [Title/Abstract]) AND (“Dementia” [Title/Abstract] OR “cognitive decline” [Title/Abstract] OR “Alzheimer’s disease” [Title/Abstract] OR “neurodegeneration” [Title/Abstract] OR “mild cognitive impairment” [Title/Abstract])) OR ((“Microbiota” [MeSH Terms] OR “Oral microbiome” [MeSH Terms] OR “Bacteria” [MeSH Terms]) AND (“Dementia” [MeSH Terms] OR “Alzheimer Disease” [MeSH Terms] OR “Neurodegenerative Diseases” [MeSH Terms])) | Clinical Study, Clinical Trial, Randomized Controlled Trial. |
| Scopus | (TITLE-ABS-KEY (“oral microbiome” OR “oral microbiota” OR “oral bacteria” OR “oral dysbiosis” OR “oral microbial community” OR “oral flora” OR “oral microbial diversity” OR “oral microbial ecosystem” OR “oral microbial composition” OR “oral microbiological profile” OR “oral pathogens” OR “oral microorganisms” OR “microbiota” OR “bacteria”) AND TITLE-ABS-KEY (“dementia” OR “cognitive decline” OR “Alzheimer’s disease” OR “neurodegeneration” OR “mild cognitive impairment” OR “neurocognitive disorders” OR “cognitive dysfunction” OR “neurodegenerative diseases” OR “cognitive disorders” OR “brain aging”)) | AND (LIMIT-TO (DOCTYPE, “ar”)) |
| WoS | (TS = (“oral microbiome” OR “oral microbiota” OR “oral bacteria” OR “oral dysbiosis” OR “oral microbial community” OR “oral flora” OR “oral microbial diversity” OR “oral microbial ecosystem” OR “oral microbial composition” OR “oral microbiological profile” OR “oral pathogens” OR “oral microorganisms” OR “microbiota” OR “bacteria”) AND TS = (“dementia” OR “cognitive decline” OR “Alzheimer’s disease” OR “neurodegeneration” OR “mild cognitive impairment” OR “neurocognitive disorders” OR “cognitive dysfunction” OR “neurodegenerative diseases” OR “cognitive disorders” OR “brain aging” OR “cognitive impairment”)) | Refined By:Document Types: Article |
| Embase | (‘oral microbiome’: ti,ab,kw OR ‘oral microbiota’: ti,ab,kw OR ‘oral bacteria’: ti,ab,kw OR ‘oral dysbiosis’: ti,ab,kw) AND (dementia: ti,ab,kw OR ‘cognitive decline’: ti,ab,kw OR ‘alzheimers disease’: ti,ab,kw OR neurodegeneration: ti,ab,kw OR ‘mild cognitive impairment’: ti,ab,kw) | |
| Cochrane Library | (“Oral microbiome” OR “oral microbiota” OR “oral bacteria” OR “oral dysbiosis”): ti,ab,kw AND (“Dementia” OR “cognitive decline” OR “Alzheimer’s disease” OR “neurodegeneration” OR “mild cognitive impairment”): ti,ab,kw (Word variations have been searched) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaple-Gil, A.M.; Santiesteban-Velázquez, M.; Urbizo Vélez, J.J. Association Between Oral Microbiota Dysbiosis and the Risk of Dementia: A Systematic Review. Dent. J. 2025, 13, 227. https://doi.org/10.3390/dj13060227
Chaple-Gil AM, Santiesteban-Velázquez M, Urbizo Vélez JJ. Association Between Oral Microbiota Dysbiosis and the Risk of Dementia: A Systematic Review. Dentistry Journal. 2025; 13(6):227. https://doi.org/10.3390/dj13060227
Chicago/Turabian StyleChaple-Gil, Alain Manuel, Meylin Santiesteban-Velázquez, and Joaquín Juan Urbizo Vélez. 2025. "Association Between Oral Microbiota Dysbiosis and the Risk of Dementia: A Systematic Review" Dentistry Journal 13, no. 6: 227. https://doi.org/10.3390/dj13060227
APA StyleChaple-Gil, A. M., Santiesteban-Velázquez, M., & Urbizo Vélez, J. J. (2025). Association Between Oral Microbiota Dysbiosis and the Risk of Dementia: A Systematic Review. Dentistry Journal, 13(6), 227. https://doi.org/10.3390/dj13060227











