Effect of a Recombinant Human Basic Fibroblast Growth Factor 2 (rhFGF-2)-Impregnated Atelocollagen Sponge on Vertical Guided Bone Regeneration in a Rat Calvarial Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of FGF-2 Incorporated in the Atelocollagen Sponge
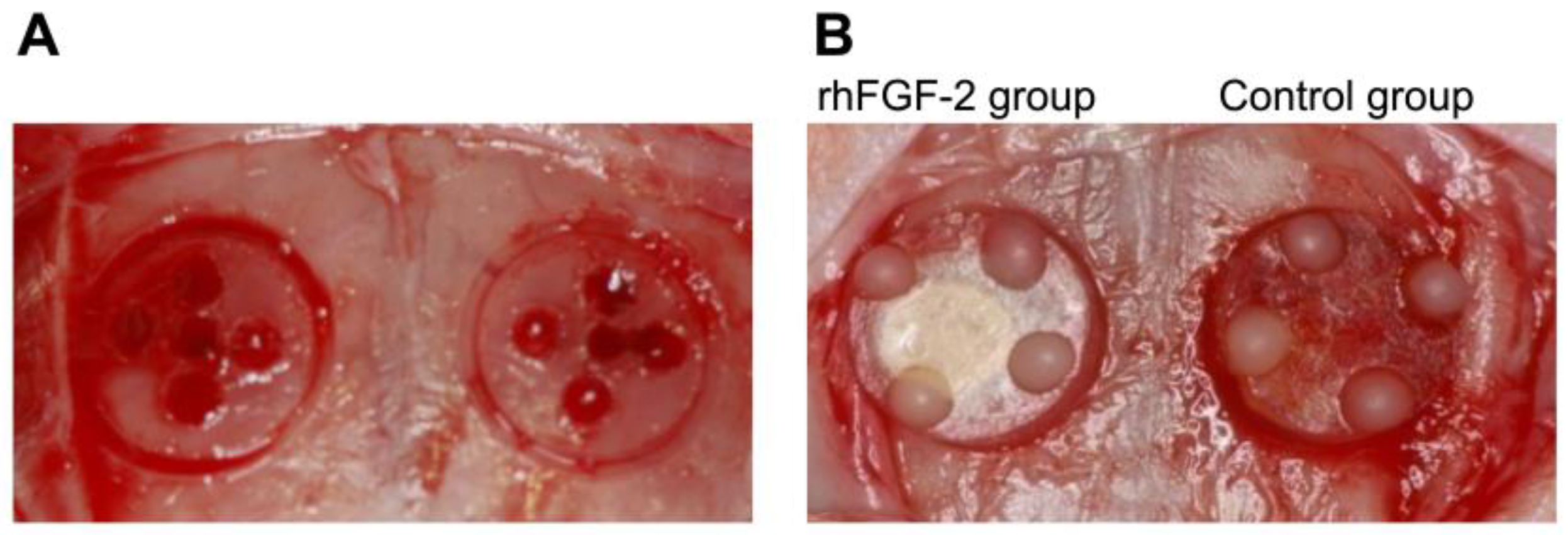
2.3. Surgical Procedures
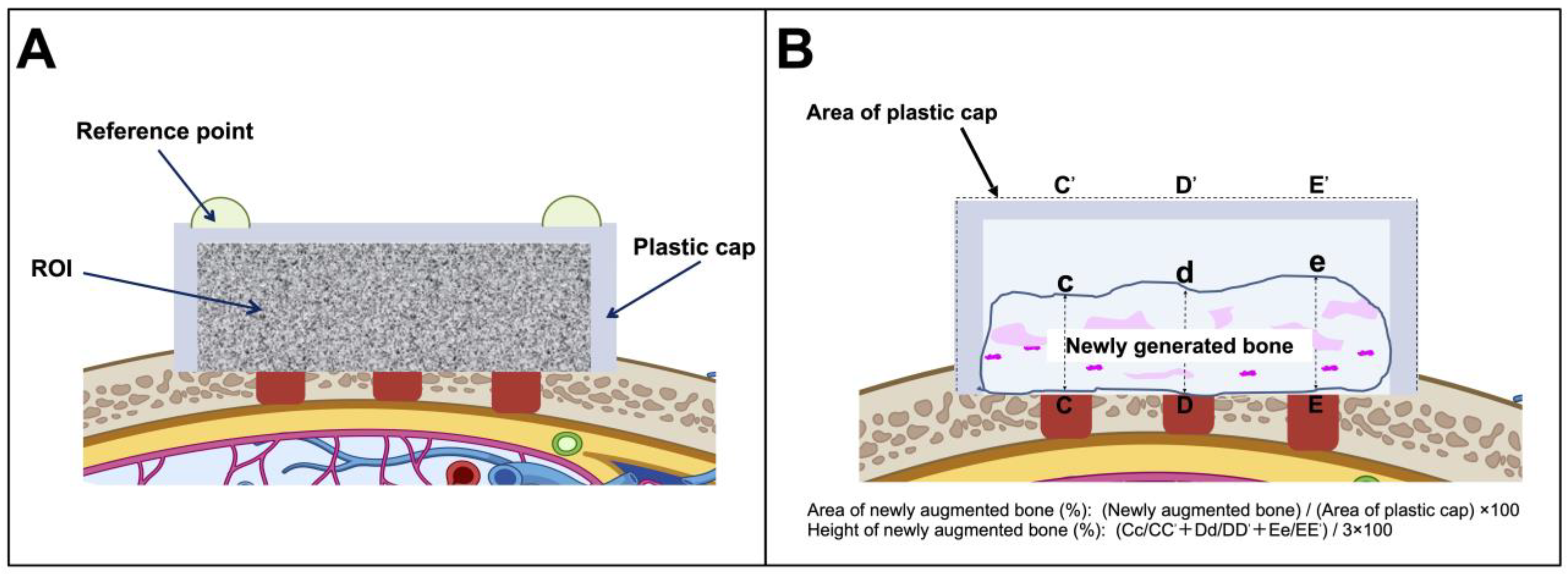
2.4. Micro-Computed Tomography Imaging
2.5. Histological and Histomorphometric Analyses
2.6. Statistical Analyses
3. Results
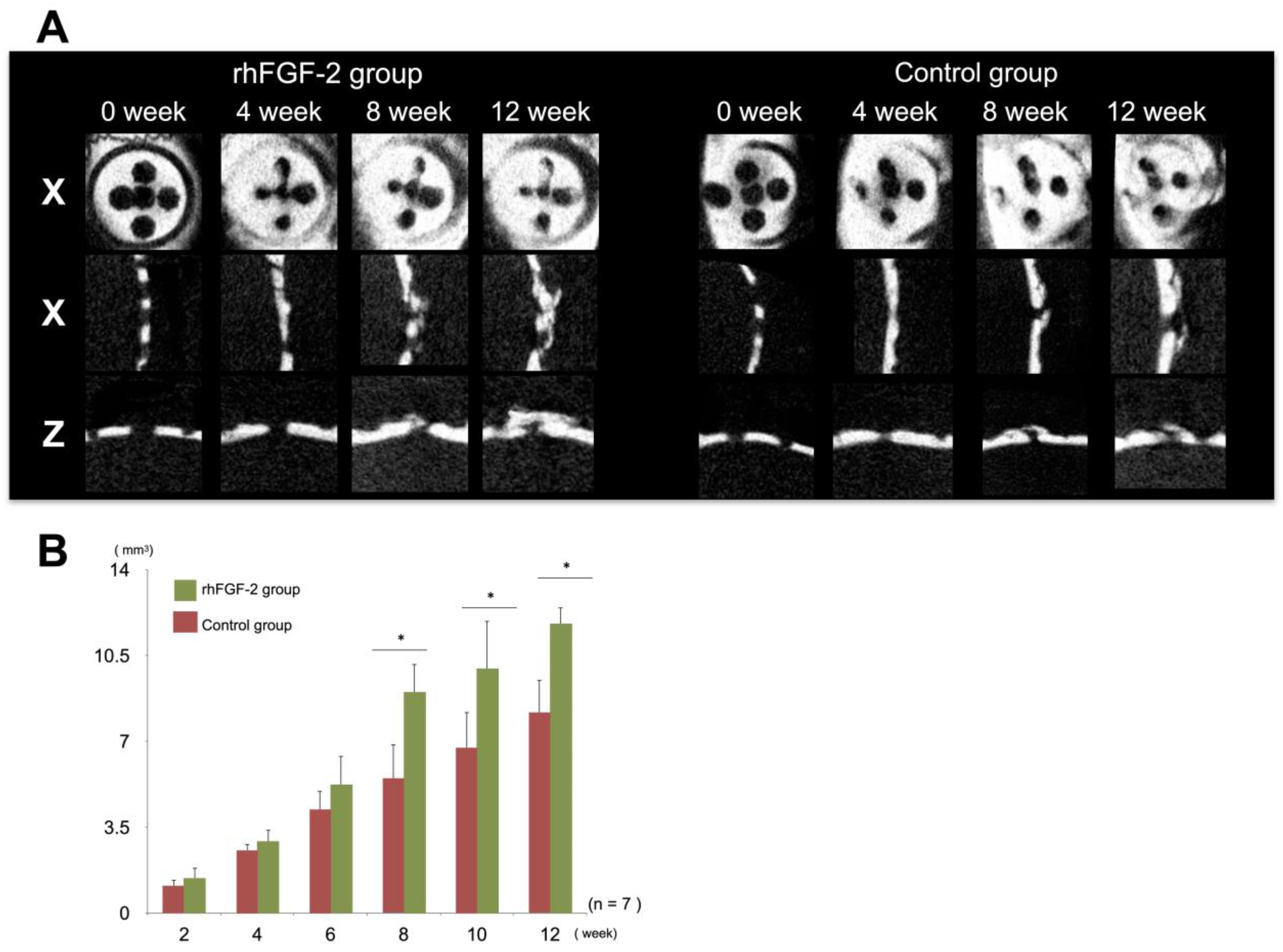
3.1. Micro-CT Images
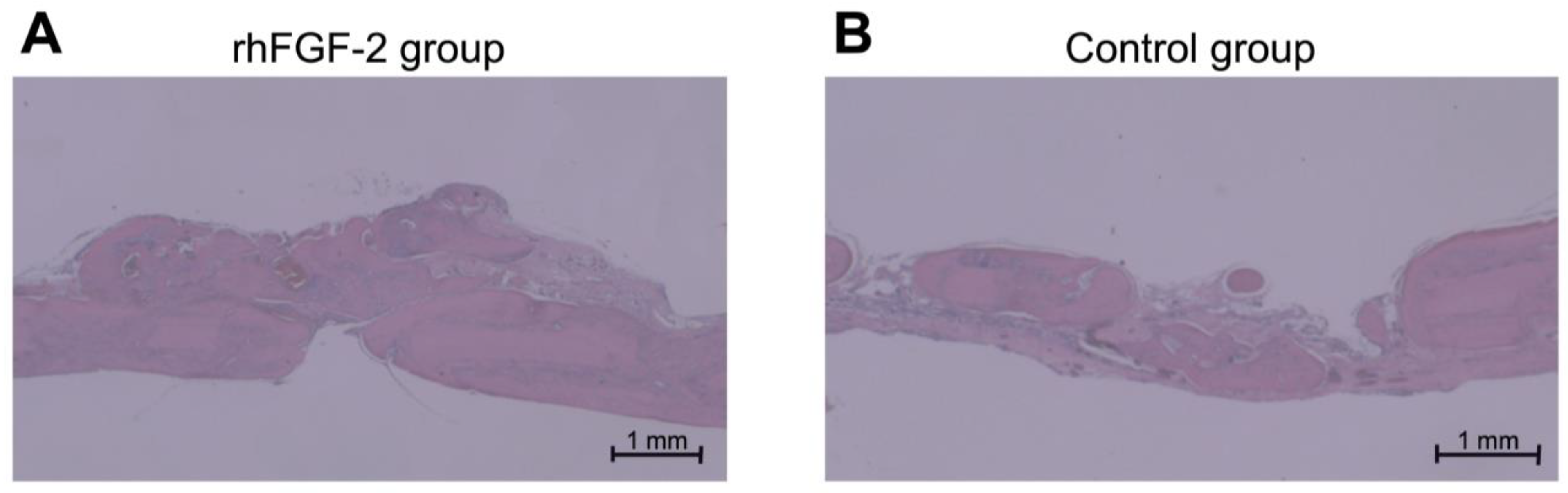
3.2. Histological Observation and Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FGF-2 | fibroblast growth factor 2 |
| Micro-CT | micro-computed tomography |
| BV | bone volume |
| GBR | guided bone regeneration |
| rhFGF-2 | recombinant human fibroblast growth factor 2 |
| β-TCP | β-tricalcium phosphate |
References
- Di Stefano, D.A.; Arosio, P.; Capparè, P.; Barbon, S.; Gherlone, E.F. Stability of Dental Implants and Thickness of Cortical Bone: Clinical Research and Future Perspectives. A Systematic Review. Materials 2021, 14, 7183. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 2012, 23 (Suppl. 5), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Funato, A.; Ishikura, C.; Naito, K.; Hasuike, A. Resorbable Membrane Pouch Technique for Single-Implant Placement in the Esthetic Zone: A Preliminary Technical Case Report. Bioengineering 2022, 9, 649. [Google Scholar] [CrossRef]
- Lekholm, U.; Adell, R.; Lindhe, J.; Brånemark, P.I.; Eriksson, B.; Rockler, B.; Lindvall, A.M.; Yoneyama, T. Marginal tissue reactions at osseointegrated titanium fixtures. (II) A cross-sectional retrospective study. Int. J. Oral Maxillofac. Surg. 1986, 15, 53–61. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E. Guided tissue regeneration for implants placed into extraction sockets and for implant dehiscences: Surgical techniques and case report. Int. J. Periodontics Restor. Dent. 1990, 10, 376–391. [Google Scholar]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided bone regeneration in implant dentistry: Basic principle, progress over 35 years, and recent research activities. Periodontology 2000 2023, 93, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Zhang, Y.; Luo, G.; Li, D.; Ma, Y.; Xiao, Y.; Xiao, L.; Wang, X. The decisive early phase of biomaterial-induced bone regeneration. Appl. Mater. Today 2024, 38, 102236. [Google Scholar] [CrossRef]
- Khojasteh, A.; Behnia, H.; Naghdi, N.; Esmaeelinejad, M.; Alikhassy, Z.; Stevens, M. Effects of different growth factors and carriers on bone regeneration: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e405–e423. [Google Scholar] [CrossRef]
- Che, Z.; Sun, Q.; Zhao, Z.; Wu, Y.; Xing, H.; Song, K.; Chen, A.; Wang, B.; Cai, M. Growth factor-functionalized titanium implants for enhanced bone regeneration: A review. Int. J. Biol. Macromol. 2024, 274, 133153. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Hayami, J.; Kita, Y. A therapy-resistant chronic leg ulcer treated successfully with topical basic fibroblast growth factor. J. Int. Med. Res. 2003, 31, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Bikfalvi, A.; Klein, S.; Pintucci, G.; Rifkin, D.B. Biological roles of fibroblast growth factor-2. Endocr. Rev. 1997, 18, 26–45. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Terashima, H.; Takedachi, M.; Maeda, K.; Nakamura, T.; Sawada, K.; Kobashi, M.; Awata, T.; Oohara, H.; Kawahara, T.; et al. Fibroblast growth factor-2 stimulates directed migration of periodontal ligament cells via PI3K/AKT signaling and CD44/hyaluronan interaction. J. Cell. Physiol. 2011, 226, 809–821. [Google Scholar] [CrossRef]
- Takayama, S.; Murakami, S.; Miki, Y.; Ikezawa, K.; Tasaka, S.; Terashima, A.; Asano, T.; Okada, H. Effects of basic fibroblast growth factor on human periodontal ligament cells. J. Periodontal Res. 1997, 32, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Akamatsu, M.; Kawanami, M.; Furuichi, Y.; Fujii, T.; Mori, M.; Kunimatsu, K.; Shimauchi, H.; Ogata, Y.; Yamamoto, M.; et al. Randomized Placebo-Controlled and Controlled Non-Inferiority Phase III Trials Comparing Trafermin, a Recombinant Human Fibroblast Growth Factor 2, and Enamel Matrix Derivative in Periodontal Regeneration in Intrabony Defects. J. Bone Miner. Res. 2016, 31, 806–814. [Google Scholar] [CrossRef]
- Kitamura, M.; Nakashima, K.; Kowashi, Y.; Fujii, T.; Shimauchi, H.; Sasano, T.; Furuuchi, T.; Fukuda, M.; Noguchi, T.; Shibutani, T.; et al. Periodontal tissue regeneration using fibroblast growth factor-2: Randomized controlled phase II clinical trial. PLoS ONE 2008, 3, e2611. [Google Scholar] [CrossRef]
- Kitamura, M.; Akamatsu, M.; Machigashira, M.; Hara, Y.; Sakagami, R.; Hirofuji, T.; Hamachi, T.; Maeda, K.; Yokota, M.; Kido, J.; et al. FGF-2 stimulates periodontal regeneration: Results of a multi-center randomized clinical trial. J. Dent. Res. 2011, 90, 35–40. [Google Scholar] [CrossRef]
- Saito, A.; Bizenjima, T.; Takeuchi, T.; Suzuki, E.; Sato, M.; Yoshikawa, K.; Kitamura, Y.; Matsugami, D.; Aoki, H.; Kita, D.; et al. Treatment of intrabony periodontal defects using rhFGF-2 in combination with deproteinized bovine bone mineral or rhFGF-2 alone: A 6-month randomized controlled trial. J. Clin. Periodontol. 2019, 46, 332–341. [Google Scholar] [CrossRef]
- Fukuba, S.; Akizuki, T.; Hoshi, S.; Matsuura, T.; Shujaa Addin, A.; Okada, M.; Tabata, Y.; Matsui, M.; Tabata, M.J.; Sugiura-Nakazato, M.; et al. Comparison between different isoelectric points of biodegradable gelatin sponges incorporating β-tricalcium phosphate and recombinant human fibroblast growth factor-2 for ridge augmentation: A preclinical study of saddle-type defects in dogs. J. Periodontal Res. 2019, 54, 278–285. [Google Scholar] [CrossRef]
- Hoshi, S.; Akizuki, T.; Matsuura, T.; Ikawa, T.; Kinoshita, A.; Oda, S.; Tabata, Y.; Matsui, M.; Izumi, Y. Ridge augmentation using recombinant human fibroblast growth factor-2 with biodegradable gelatin sponges incorporating β-tricalcium phosphate: A preclinical study in dogs. J. Periodontal Res. 2016, 51, 77–85. [Google Scholar] [CrossRef]
- Fukuba, S.; Akizuki, T.; Matsuura, T.; Okada, M.; Nohara, K.; Hoshi, S.; Shujaa Addin, A.; Iwata, T.; Izumi, Y. Effects of combined use of recombinant human fibroblast growth factor-2 and β-tricalcium phosphate on ridge preservation in dehiscence bone defects after tooth extraction: A split-mouth study in dogs. J. Periodontal Res. 2021, 56, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kochi, G.; Sato, S.; Fukuyama, T.; Morita, C.; Honda, K.; Arai, Y.; Ito, K. Analysis on the guided bone augmentation in the rat calvarium using a microfocus computerized tomography analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, e42–e48. [Google Scholar] [CrossRef]
- Oginuma, T.; Sato, S.; Udagawa, A.; Saito, Y.; Arai, Y.; Ito, K. Autogenous bone with or without hydroxyapatite bone substitute augmentation in rat calvarium within a plastic cap. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S107–S113. [Google Scholar] [CrossRef]
- Kubota, T.; Hasuike, A.; Naito, M.; Tsunori, K.; Min, S.; Sato, S. Enhancement of Bone Augmentation in Osteoporotic Conditions by the Intermittent Parathyroid Hormone: An Animal Study in the Calvarium of Ovariectomized Rat. Int. J. Oral Maxillofac. Implants 2018, 33, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Sato, S.; Oginuma, T.; Saito, Y.; Arai, Y.; Ito, K. Effects of nicotine on guided bone augmentation in rat calvarium. Clin. Oral Implants Res. 2013, 24, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, R.; Kikuzaki, K.; Kimoto, T.; Matsuura, T.; Chiba, D.; Wadamoto, M.; Sato, Y.; Maeda, M.; Sano, A.; Akagawa, Y. Controlled local application of basic fibroblast growth factor (FGF-2) accelerates the healing of GBR. An experimental study in beagle dogs. Clin. Oral Implants Res. 2000, 11, 345–353. [Google Scholar] [CrossRef]
- You, S.; Yu, F.; Fan, Q.; Xia, T.; Liang, L.; Yan, Q.; Zeng, H.; Shi, B. Radiographic comparison of atelocollagen versus deproteinized bovine bone minerals covered with a collagen membrane in alveolar ridge preservation: A retrospective study. BMC Oral Health 2023, 23, 901. [Google Scholar] [CrossRef]
- Yu, S.-J.; Moon, S.-S.; Jang, H.-S.; Han, K.-Y.; Hwang, K.-S.; Choi, S.-H.; Kwon, Y.-H.; Kim, B.-O. A clinical and histological evaluation for healing of dehiscence defects filled with an absorbable atelocollagen sponge in dogs. Tissue Eng. Regen. Med. 2012, 9, 320–327. [Google Scholar] [CrossRef]
- Kobayashi, N.; Miyaji, H.; Sugaya, T.; Kawanami, M. Bone Augmentation by Implantation of an FGF2-loaded Collagen Gel-sponge Composite Scaffold. J. Oral Tissue Eng. 2010, 8, 91–101. [Google Scholar] [CrossRef]
- Kigami, R.; Sato, S.; Tsuchiya, N.; Yoshimakai, T.; Arai, Y.; Ito, K. FGF-2 angiogenesis in bone regeneration within critical-sized bone defects in rat calvaria. Implant. Dent. 2013, 22, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Kigami, R.; Sato, S.; Tsuchiya, N.; Sato, N.; Suzuki, D.; Arai, Y.; Ito, K.; Ogiso, B. Effect of basic fibroblast growth factor on angiogenesis and bone regeneration in non-critical-size bone defects in rat calvaria. J. Oral. Sci. 2014, 56, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ito, T.; Okamoto, K.; Mima, T.; Uchida, K.; Siddiqui, Y.D.; Ito, M.; Tai, M.; Okubo, K.; Yamashiro, K.; et al. Acceleration of bone regeneration of horizontal bone defect in rats using collagen-binding basic fibroblast growth factor combined with collagen scaffolds. J. Periodontol. 2019, 90, 1043–1052. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Simeone, D.; Torge, D.; Macchiarelli, G.; Marchetti, E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials 2022, 15, 8284. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.; Chatzopoulou, E.; Chaussain, C.; Gorin, C. The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: A Review. Cells 2021, 10, 932. [Google Scholar] [CrossRef]
| Area of Newly Augmented Bone (%) | Height of Newly Augmented Bone (%) | |||
|---|---|---|---|---|
| 0.3% rhFGF-2 | 35.6 | (9.7) | 41.9 | (6.7) |
| Control | 9.1 | (3.2) | 13.4 | (7.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogure, K.; Hasuike, A.; Kurachi, R.; Igarashi, Y.; Idesawa, M.; Sato, S. Effect of a Recombinant Human Basic Fibroblast Growth Factor 2 (rhFGF-2)-Impregnated Atelocollagen Sponge on Vertical Guided Bone Regeneration in a Rat Calvarial Model. Dent. J. 2025, 13, 177. https://doi.org/10.3390/dj13040177
Kogure K, Hasuike A, Kurachi R, Igarashi Y, Idesawa M, Sato S. Effect of a Recombinant Human Basic Fibroblast Growth Factor 2 (rhFGF-2)-Impregnated Atelocollagen Sponge on Vertical Guided Bone Regeneration in a Rat Calvarial Model. Dentistry Journal. 2025; 13(4):177. https://doi.org/10.3390/dj13040177
Chicago/Turabian StyleKogure, Keisuke, Akira Hasuike, Risa Kurachi, Yasuyuki Igarashi, Masataka Idesawa, and Shuichi Sato. 2025. "Effect of a Recombinant Human Basic Fibroblast Growth Factor 2 (rhFGF-2)-Impregnated Atelocollagen Sponge on Vertical Guided Bone Regeneration in a Rat Calvarial Model" Dentistry Journal 13, no. 4: 177. https://doi.org/10.3390/dj13040177
APA StyleKogure, K., Hasuike, A., Kurachi, R., Igarashi, Y., Idesawa, M., & Sato, S. (2025). Effect of a Recombinant Human Basic Fibroblast Growth Factor 2 (rhFGF-2)-Impregnated Atelocollagen Sponge on Vertical Guided Bone Regeneration in a Rat Calvarial Model. Dentistry Journal, 13(4), 177. https://doi.org/10.3390/dj13040177








