Tunnel Technique in Bone Augmentation Procedures for Dental Implant Rehabilitation: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Criteria for Considering Studies for This Review
2.4. Outcome Measures
2.5. Data Collection
2.6. Assessment of Risk of Bias
2.7. Data Synthesis
3. Results
3.1. Search Results
3.2. Study and Patient Characteristics
3.3. Risk of Bias in Included Studies
3.4. Narrative Synthesis on the Success of the Bone Augmentation Procedure
3.5. Narrative Synthesis on the Complications
3.6. Narrative Synthesis on Graft Reabsorption
3.7. Narrative Synthesis on Histological Evaluation
3.8. Narrative Synthesis on the Primary Implant Stability
3.9. Narrative Synthesis on the Implant Survival
3.10. Narrative Synthesis on the Peri-Implant Bone Level Change
3.11. Narrative Synthesis on Operative Time
3.12. Narrative Synthesis on Vertical and Horizontal Bone Gain
3.13. Narrative Synthesis on Patient Comfort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. 2019, 31, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Molina, A.; Sanz, M. Complications in bone-grafting procedures: Classification and management. Periodontol. 2000 2022, 88, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.L.; Kapila, Y.; Lin, G.H. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Karmon, B.; Tavelli, L.; Rasperini, G. Tunnel Technique with a Subperiosteal Bag for Horizontal Ridge Augmentation. Int. J. Periodontics Restor. Dent. 2020, 40, 223–230. [Google Scholar] [CrossRef]
- Rothstein, S.S.; Paris, D.A.; Zacek, M.P. Use of hydroxylapatite for the augmentation of deficient alveolar ridges. J. Oral Maxillofac. Surg. 1984, 42, 224–230. [Google Scholar] [CrossRef]
- Vanassche, B.J.; Stoelinga, P.J.; de Koomen, H.A.; Blijdorp, P.A.; Schoenaers, J.H. Reconstruction of the severely resorbed mandible with interposed bone grafts and hydroxylapatite. A 2–3 year follow-up. Int. J. Oral Maxillofac. Surg. 1988, 17, 157–160. [Google Scholar] [CrossRef]
- Kent, J.N.; Finger, I.M.; Quinn, J.H.; Guerra, L.R. Hydroxylapatite alveolar ridge reconstruction: Clinical experiences, complications, and technical modifications. J. Oral Maxillofac. Surg. 1986, 44, 37–49. [Google Scholar] [CrossRef]
- Ylinen, P.; Suuronen, R.; Taurio, R.; Törmälä, P.; Rokkanen, P. Use of hydroxylapatite/ polymer-composite in facial bone augmentation. An experimental study. Int. J. Oral Maxillofac. Surg. 2002, 31, 405–409. [Google Scholar] [CrossRef]
- De Stavola, L.; Tunkel, J. Results of vertical bone augmentation with autogenous bone block grafts and the tunnel technique: A clinical prospective study of 10 consecutively treated patients. Int. J. Periodontics Restor. Dent. 2013, 33, 651–659. [Google Scholar] [CrossRef]
- Restoy-Lozano, A.; Dominguez-Mompell, J.L.; Infante-Cossio, P.; Lara-Chao, J.; Espin-Galvez, F.; Lopez-Pizarro, V. Reconstruction of mandibular vertical defects for dental implants with autogenous bone block grafts using a tunnel approach: Clinical study of 50 cases. Int. J. Oral Maxillofac. Surg. 2015, 44, 1416–1422. [Google Scholar] [CrossRef]
- Khoury, F.; Hanser, T. Three-Dimensional Vertical Alveolar Ridge Augmentation in the Posterior Maxilla: A 10-year Clinical Study. Int. J. Oral Maxillofac. Implants 2019, 34, 471–480. [Google Scholar] [CrossRef]
- Byun, S.H.; Kim, S.Y.; Lee, H.; Lim, H.K.; Kim, J.W.; Lee, U.L.; Lee, J.B.; Park, S.H.; Kim, S.J.; Song, J.D.; et al. Soft tissue expander for vertically atrophied alveolar ridges: Prospective, multicenter, randomized controlled trial. Clin. Oral Implants Res. 2020, 31, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and applications in craniofacial bone regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, Y.K.; Yun, P.Y. Minimal invasive horizontal ridge augmentation using subperiosteal tunneling technique. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 41. [Google Scholar] [CrossRef]
- Altiparmak, N.; Uckan, S.; Bayram, B.; Soydan, S. Comparison of Tunnel and Crestal Incision Techniques in Reconstruction of Localized Alveolar Defects. Int. J. Oral Maxillofac. Implants 2017, 32, 1103–1110. [Google Scholar] [CrossRef]
- Llamas-Monteagudo, O.; Girbés-Ballester, P.; Viña-Almunia, J.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Clinical parameters of implants placed in healed sites using flapped and flapless techniques: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e572–e581. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Choi, B.H.; Li, J.; Xuan, F.; Jeong, S.M. Blood vessels of the peri-implant mucosa: A comparison between flap and flapless procedures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 508–512. [Google Scholar] [CrossRef]
- Siu, T.L.; Dukka, H.; Saleh, M.H.A.; Tattan, M.; Dib, Z.; Ravidà, A.; Greenwell, H.; Wang, H.L.; Araujo, M.G. Flap versus flapless alveolar ridge preservation: A clinical and histological single-blinded, randomized controlled trial. J. Periodontol. 2023, 94, 184–192. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alfardan, L.; Alsabeeha, N.H.M. Flapped versus flapless alveolar ridge preservation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2022, 51, 133–142. [Google Scholar] [CrossRef]
- Majid, O.W. Does flapless immediate implant placement lead to significant preservation of buccal bone compared to flap surgical protocol? Evid. Based Dent. 2024, 25, 9–10. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Bmj 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Prasad, H.; Lynch, S. Sequential Human Histology Results of the Subperiosteal Minimally Invasive Aesthetic Ridge Augmentation Technique (SMART): A Chronologic Wound Healing Proof-of-Principle Study. Int. J. Periodontics Restor. Dent. 2024, 44, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Charavet, C.; Lecloux, G.; Vandenberghe, B.; Lambert, F. Buccal bone regeneration combined with piezocision in adult orthodontic patients: Clinical, 3D radiographic, and patient-reported outcomes. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.L.; Camelo, M.; Nevins, M.; Schupbach, P.; Friedland, B.; Camelo, J.M.; Kim, D.M. Minimally invasive alveolar ridge augmentation procedure (tunneling technique) using rhPDGF-BB in combination with three matrices: A case series. Int. J. Periodontics Restor. Dent. 2009, 29, 371–383. [Google Scholar]
- Kfir, E.; Kfir, V.; Eliav, E.; Kaluski, E. Minimally invasive guided bone regeneration. J. Oral Implantol. 2007, 33, 205–210. [Google Scholar] [CrossRef]
- Angelo, T.; Marcel, W.; Andreas, K.; Izabela, S. Biomechanical Stability of Dental Implants in Augmented Maxillary Sites: Results of a Randomized Clinical Study with Four Different Biomaterials and PRF and a Biological View on Guided Bone Regeneration. Biomed. Res. Int. 2015, 2015, 850340. [Google Scholar] [CrossRef]
- Soltan, M.; Smiler, D.; Soltan, C.; Prasad, H.S.; Rohrer, M.D. Bone grafting by means of a tunnel dissection: Predictable results using stem cells and matrix. Implant Dent. 2010, 19, 280–287. [Google Scholar] [CrossRef]
- Akhil, K.P.; Pramashivaiah, R.; Prabhuji, M.L.V.; Tasleem, R.; Almubarak, H.; Bahamdan, G.K.; Luke, A.M.; Shetty, K.P.; Snigdha, N.T.; Bhavikatti, S.K. Alveolar Ridge Augmentation Assessment Using a Minimalistic Approach, with and without Low-Level Laser Therapy (LLLT)—A Comparative Clinical Trial. Medicina 2023, 59, 1178. [Google Scholar] [CrossRef]
- Johnson, T.M.; Baron, D. Tunnel Access for Guided Bone Regeneration in the Maxillary Anterior. Clin. Adv. Periodontics 2018, 8, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Smiler, D.; Soltan, M.; Lee, J.W. A histomorphogenic analysis of bone grafts augmented with adult stem cells. Implant Dent. 2007, 16, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.L.; Heller, R.L. Tissue management protocol: “tunnel bone graft” technique. Dent. Today 2010, 29, 120–124. [Google Scholar] [PubMed]
- Khoury, F.; Hanser, T. 3D vertical alveolar crest augmentation in the posterior mandible using the tunnel technique: A 10-year clinical study. Int. J. Oral Implantol. 2022, 15, 111–126. [Google Scholar]
- Migliorati, M.; Amorfini, L.; Signori, A.; Biavati, A.S.; Benedicenti, S. Clinical and Aesthetic Outcome with Post-Extractive Implants with or without Soft Tissue Augmentation: A 2-Year Randomized Clinical Trial. Clin. Implant Dent. Relat. Res. 2015, 17, 983–995. [Google Scholar] [CrossRef]
- Papace, C.; Büsch, C.; Ristow, O.; Keweloh, M.; Hoffmann, J.; Mertens, C. The effect of different soft-tissue management techniques for alveolar ridge preservation: A randomized controlled clinical trial. Int. J. Implant Dent. 2021, 7, 113. [Google Scholar] [CrossRef]
- AlGhamdi, A.S. Post-surgical complications of symphyseal block graft with and without soft tissue grafting. Saudi Med. J. 2013, 34, 609–615. [Google Scholar]
- Mahn, D.H.; Woodside, J. Multidisciplinary rehabilitation: Tunnel grafting techniques. Dent. Today 2012, 31, 152, 154–155. [Google Scholar]
- Elaskary, A.T.; Gaweesh, Y.Y.; Maebed, M.A.; Cho, S.-C.; El Tantawi, M. A Novel Method for Immediate Implant Placement in Defective Fresh Extraction Sites. Int. J. Oral Maxillofac. Implants 2020, 35, 799–807. [Google Scholar] [CrossRef]
- Deeb, G.R.; Tran, D.; Carrico, C.K.; Block, E.; Laskin, D.M.; Deeb, J.G. How Effective Is the Tent Screw Pole Technique Compared to Other Forms of Horizontal Ridge Augmentation? J. Oral Maxillofac. Surg. 2017, 75, 2093–2098. [Google Scholar] [CrossRef]
- Byun, S.H.; Kim, S.H.; Cho, S.; Lee, H.; Lim, H.K.; Kim, J.W.; Lee, U.L.; Song, W.; Kim, S.J.; Kim, M.K.; et al. Tissue Expansion Improves the Outcome and Predictability for Alveolar Bone Augmentation: Prospective, Multicenter, Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1143. [Google Scholar] [CrossRef]
- Deeb, G.R.; Wilson, G.H.; Carrico, C.K.; Zafar, U.; Laskin, D.M.; Deeb, J.G. Is the Tunnel Technique More Effective Than Open Augmentation with a Titanium-Reinforced Polytetrafluoroethylene Membrane for Horizontal Ridge Augmentation? J. Oral Maxillofac. Surg. 2016, 74, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Wychowanski, P.; Woliński, J.; Morawiec, T.; Kownacki, P.; Starzynska, A.; Kosieradzki, M.; Fiedor, P. Preliminary Clinical Data and the Comparison of the Safety and Efficacy of Autogenous Bone Grafts Versus Xenograft Implantations in Vertical Bone Deficiencies Before Dental Implant Installation. Transplant. Proc. 2020, 52, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Bienz, S.P.; Figuero, E.; Jung, R.E.; Sanz-Martín, I. Efficacy of lateral bone augmentation performed simultaneously with dental implant placement: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 257–276. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.; Carvalho, P.H.A.; Quirino, L.C.; Torres, L.H.S.; Filho, V.A.P.; Gabrielli, M.F.R.; Gabrielli, M.A.C. Titanium Mesh Exposure After Bone Grafting: Treatment Approaches—A Systematic Review. Craniomaxillofacial Trauma Reconstr. 2022, 15, 397–405. [Google Scholar] [CrossRef]
- Asa’Ad, F.; Rasperini, G.; Pagni, G.; Rios, H.F.; Giannì, A.B. Pre-augmentation soft tissue expansion: An overview. Clin. Oral Implants Res. 2016, 27, 505–522. [Google Scholar] [CrossRef]
- Kofina, V.; Monfaredzadeh, M.; Rawal, S.Y.; Dentino, A.R.; Singh, M.; Tatakis, D.N. Patient-reported outcomes following guided bone regeneration: Correlation with clinical parameters. J. Dent. 2023, 136, 104605. [Google Scholar] [CrossRef]
- Gotfredsen, K. Patient-reported outcomes for bone regenerative procedures. Periodontol. 2000 2023, 93, 270–276. [Google Scholar] [CrossRef]
- Shi, J.Y.; Montero, E.; Wu, X.Y.; Palombo, D.; Wei, S.M.; Sanz-Sánchez, I. Bone preservation or augmentation simultaneous with or prior to dental implant placement: A systematic review of outcomes and outcome measures used in clinical trials in the last 10 years. Clin. Oral Implant. Res. 2023, 34, 68–83. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Monje, A. Efficacy of biologics for alveolar ridge preservation/reconstruction and implant site development: An American Academy of Periodontology best evidence systematic review. J. Periodontol. 2022, 93, 1827–1847. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Kwan, S.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Bone augmentation techniques for dental implant treatment. In Cochrane Database of Systematic Reviews; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y. Risk factors of perioperative hypertension in dental implant surgeries with bone augmentation. Beijing Da Xue Xue Bao Yi Xue Ban 2024, 56, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Hidajat, H. Extensive Autogenous Bone Augmentation and Implantation in Patients Under Bisphosphonate Treatment: A 15-Case Series. Int. J. Periodontics Restor. Dent. 2016, 36, 9–18. [Google Scholar] [CrossRef] [PubMed]
- De Riu, G.; Meloni, M.S.; Pisano, M.; Baj, A.; Tullio, A. Mandibular coronoid process grafting for alveolar ridge defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 430–436. [Google Scholar] [CrossRef]
- Hasson, O. Augmentation of deficient lateral alveolar ridge using the subperiosteal tunneling dissection approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 103, e14–e19. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Sanz, M.; Avila-Ortiz, G.; Berglundh, T.; Cairo, F.; Derks, J.; Figuero, E.; Graziani, F.; Guerra, F.; Heitz-Mayfield, L.; et al. Relevant domains, core outcome sets and measurements for implant dentistry clinical trials: The Implant Dentistry Core Outcome Set and Measurement (ID-COSM) international consensus report. J. Clin. Periodontol. 2023, 50 (Suppl. S25), 5–21. [Google Scholar] [CrossRef]
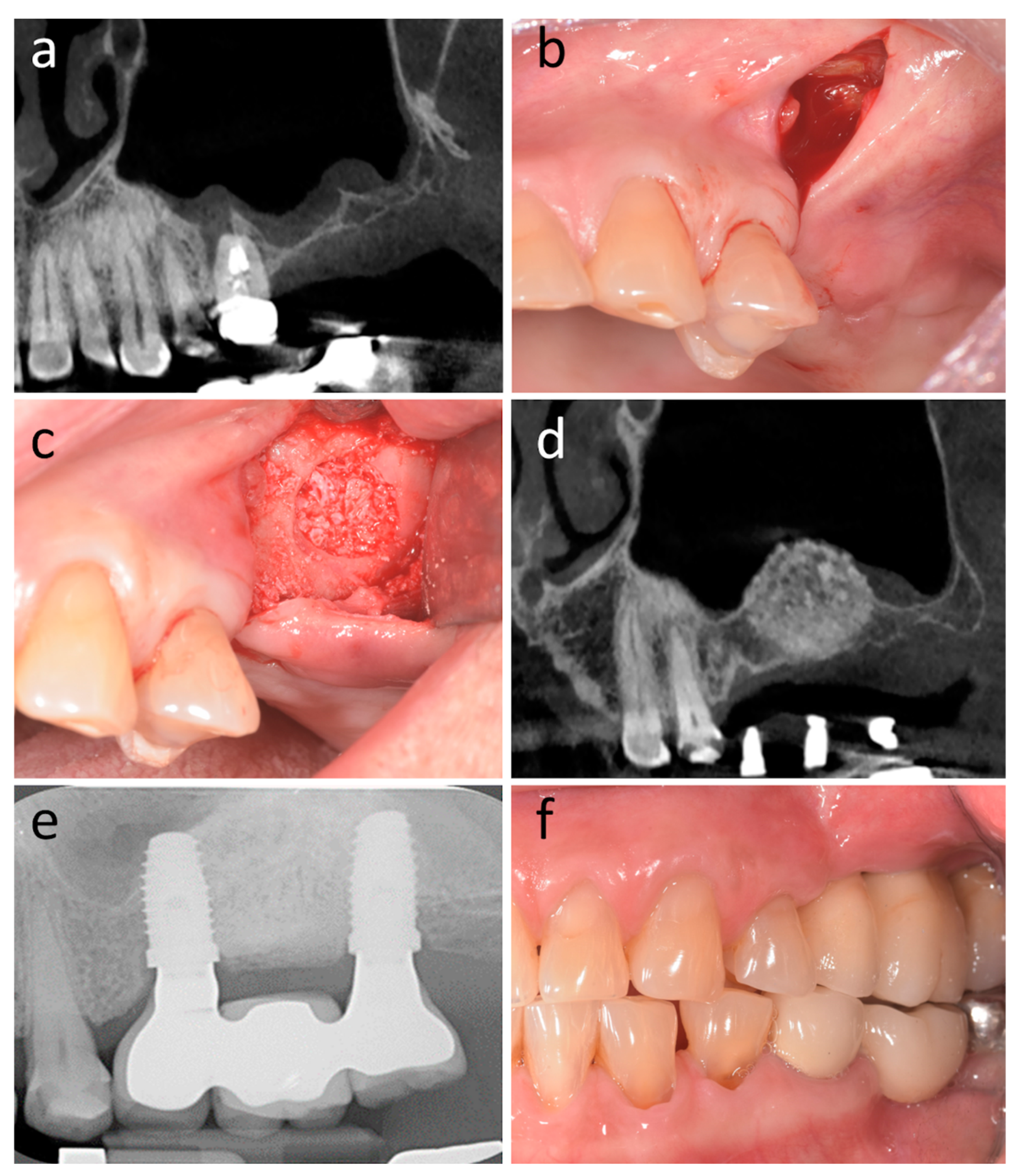

| Author(s), Year | Study Design | Males: Females | Age, Mean | Health Status | Smokers, n | Patients (Cases: Controls), n | Sites (Cases: Controls), n | Surgeons, n | Surgeon Experience | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Deeb et al., 2016 [43] | Retrospective cohort study | NR | >18 years | NR | NR | 52 (21:31) | 52 (21:31) | Multiple (unspecified number) | Senior level oral and maxillofacial surgery residents | 6 months |
| Altiparmak et al., 2017 [15] | Controlled prospective study | 29:39 | 41.5 years | ASA I (healthy subjects) | NR | 68 (NR) | 75 (38:37) | One | NR | 6 months |
| Wychowanski et al., 2020 [44] | Controlled prospective study | NR | NR | Immunocompromised subjects | NR | 30 (15:15) | 30 (15:15) | NR | NR | 24 months |
| Byun et al., 2020 [12] | Randomized controlled trial | 24:22 | 57.6 years | Among exclusion criteria: metabolic diseases, hemorrhagic diseases, specific therapies (e.g., drugs affecting bone metabolism) | None | 46 (23:23) | 46 (23:23) | Multiple (unspecified number) | NR | 7–19 months |
| Author(s), Year | Study Design | Tool | Domain | Risk of Bias | Overall Risk of Bias |
|---|---|---|---|---|---|
| Deeb et al., 2016 [43] | Retrospective cohort study | ROBINS-I | Bias due to confounding | Serious | Serious |
| Bias in selection of participants | Low | ||||
| Bias in classification of interventions | Low | ||||
| Bias due to deviations from intended interventions | Low | ||||
| Bias due to missing data | Low | ||||
| Bias in measurement of outcomes | Low | ||||
| Bias in selection of the reported result | Low | ||||
| Altiparmak et al., 2017 [15] | Controlled prospective study | ROBINS-I | Bias due to confounding | Serious | Serious |
| Bias in selection of participants | Low | ||||
| Bias in classification of interventions | Low | ||||
| Bias due to deviations from intended interventions | Low | ||||
| Bias due to missing data | Low | ||||
| Bias in measurement of outcomes | Low | ||||
| Bias in selection of the reported result | Serious | ||||
| Wychowanski et al., 2020 [44] | Controlled prospective study | ROBINS-I | Bias due to confounding | Serious | Serious |
| Bias in selection of participants | Low | ||||
| Bias in classification of interventions | Low | ||||
| Bias due to deviations from intended interventions | Serious | ||||
| Bias due to missing data | Unclear | ||||
| Bias in measurement of outcomes | Low | ||||
| Bias in selection of the reported result | Low | ||||
| Byun et al., 2020 [12] | Randomized controlled trial | RoB2 | Risk of bias arising from the randomization process | High | High |
| Risk of bias due to deviations from the intended interventions | High | ||||
| Risk of bias due to missing outcome data | Some concerns | ||||
| Risk of bias in measurement of the outcome | Some concerns | ||||
| Risk of bias in selection of the reported result | Low |
| Author(s), Year | Success of the Bone Augmentation Procedure, n/N (%) | Exposure of the Graft, n/N (%) | Temporary Paresthesia, n/N (%) | Permanent Paresthesia, n/N (%) | Graft Resorption, Mean (SD) | Primary Implant Stability, Mean (SD) | Implant Success/Survival rate, n/N (%) | Peri-Implant Bone Level Change, Mean (SD) | Operative Time, Median | Vertical Bone Gain, Mean (SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | T | C | T | C | T | C | T | C | T | C | T | C | |
| Deeb et al., 2016 [43] | 18/21 (86%) | 22/31 (71%) | 4/21 (19%) | 16/31 (52%) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Altiparmak et al., 2017 [15] | 37/38 (97%) | 34/37 (92%) | 5/38 (13%) | 15/37 (41%) | 4/38 (11%) | 6/37 (16%) | Nil | Nil | NR | NR | NR | NR | NR | NR | NR | NR | 95 min | 96 min | NR | NR |
| Wychowanski et al., 2020 [44] | NR | NR | 0/15 (0%) | 3/15 (20%) | NR | NR | NR | NR | NR | NR | −1.2 (1.6) | −3.2 (1.3) | 29/30 (97%) | 26/30 (87%) | <1.2 mm | <1.2 mm | NR | NR | 4.4 mm (1.5) | 4.3 mm (1.3) |
| Byun et al., 2020 [12] | NR | NR | 0/23 (0%) | 2/23 (9%) | NR | NR | NR | NR | 1.57 mm (1.03) | 2.32 mm (1.09) | NR | NR | NR | NR | 0.52 mm (0.21) | 0.41 mm (0.22) | NR | NR | 3.55 mm (NR) | 1.9 mm (NR) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivolella, S.; Brunello, G.; Castagna, D.A.; Cavallin, F.; Consolo, U. Tunnel Technique in Bone Augmentation Procedures for Dental Implant Rehabilitation: A Systematic Review. Dent. J. 2024, 12, 405. https://doi.org/10.3390/dj12120405
Sivolella S, Brunello G, Castagna DA, Cavallin F, Consolo U. Tunnel Technique in Bone Augmentation Procedures for Dental Implant Rehabilitation: A Systematic Review. Dentistry Journal. 2024; 12(12):405. https://doi.org/10.3390/dj12120405
Chicago/Turabian StyleSivolella, Stefano, Giulia Brunello, Dario Azeglio Castagna, Francesco Cavallin, and Ugo Consolo. 2024. "Tunnel Technique in Bone Augmentation Procedures for Dental Implant Rehabilitation: A Systematic Review" Dentistry Journal 12, no. 12: 405. https://doi.org/10.3390/dj12120405
APA StyleSivolella, S., Brunello, G., Castagna, D. A., Cavallin, F., & Consolo, U. (2024). Tunnel Technique in Bone Augmentation Procedures for Dental Implant Rehabilitation: A Systematic Review. Dentistry Journal, 12(12), 405. https://doi.org/10.3390/dj12120405








