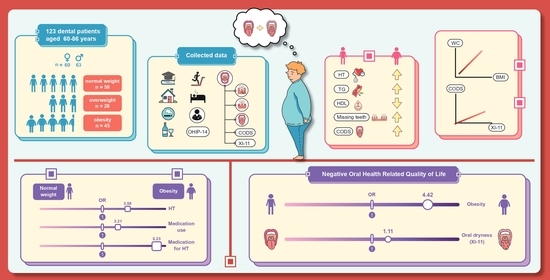
Increased Oral Dryness and Negative Oral Health-Related Quality of Life in Older People with Overweight or Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Assessment of General Health
2.3. Assessment of Patients’ Data
2.4. Assessment of Oral Health
2.5. Assessment of Oral Health-Related Quality of Life
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Characteristics of Oral Condition
Oral Mucosal Dryness
3.3. Oral Health-Related Quality of Life
3.4. Association of OHIP-14 with Relevant Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demographic, Population and Housing Report on the 2021 Survey of the Older Persons in Thailand. 2022. Available online: http://www.nso.go.th/sites/2014en/Pages/survey/Social/Demographic,%20Population%20and%20Housing/The-Survey-Of-Elderly-In-Thailand.aspx (accessed on 25 August 2022).
- Locker, D.; Clarke, M.; Payne, B. Self-perceived oral health status, psychological well-being, and life satisfaction in an older adult population. J. Dent. Res. 2000, 79, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Clementino, L.C.; de Souza, K.S.C.; Castelo-Branco, M.; Perazzo, M.F.; Ramos-Jorge, M.L.; Mattos, F.F.; Paiva, S.M.; Martins-Júnior, P.A. Top 100 most-cited oral health-related quality of life papers: Bibliometric analysis. Community Dent. Oral Epidemiol. 2022, 50, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Nammontri, O. Validation of the Thai version of the 14- item Oral Health Impact Profile (Thai OHIP-14) amongst the general Thai adult population in a community setting. J. Health Res. 2017, 31, 481–486. [Google Scholar]
- Rosli, T.I.; Chan, Y.M.; Kadir, R.A.; Hamid, T.A.A. Association between oral health-related quality of life and nutritional status among older adults in district of Kuala Pilah, Malaysia. BMC Public Health 2019, 19, 547. [Google Scholar] [CrossRef]
- Tenani, C.F.; De Checchi, M.H.R.; da Cunha, I.P.; Mendes, K.L.C.; Soares, G.H.; Michel-Crosato, E.; Jamieson, L.; Ju, X.; Mialhe, F.L. Factors associated with poor oral health-related quality of life among non-institutionalized Brazilian older adults: Oral health and quality of life in older adults. Spec Care Dentist. 2021, 41, 391–398. [Google Scholar] [CrossRef]
- Zhi, Q.H.; Si, Y.; Wang, X.; Tai, B.J.; Hu, D.Y.; Wang, B.; Zheng, S.G.; Liu, X.N.; Rong, W.S.; Wang, W.J.; et al. Determining the factors associated with oral health-related quality of life in Chinese elders: Findings from the fourth national survey. Community Dent. Oral Epidemiol. 2022, 50, 311–320. [Google Scholar] [CrossRef]
- Vu, H.; Vo, P.T.-D.; Kim, H.-D. Gender modified association of oral health indicators with oral health-related quality of life among Korean elders. BMC Oral Health. 2022, 22, 168. [Google Scholar]
- Bannwart, L.C.; de Moraes Melo Neto, C.L.; Goiato, M.C.; dos Santos, D.M.; da Silva Paiva, C.A.; de Araújo Moreno, N.V.; da Silva, E.V.F.; de Magalhães Bertoz, A.P. Oral health-related quality of life, dry mouth sensation, and level of anxiety in elderly patients rehabilitated with new removable dentures. Eur. J. Dent. 2022, 16, 351–359. [Google Scholar] [CrossRef]
- Dahl, K.E.; Wang, N.J.; Holst, D.; Ohrn, K. Oral health-related quality of life among adults 68-77 years old in Nord-Trøndelag, Norway. Int. J. Dent. Hyg. 2011, 9, 87–92. [Google Scholar] [CrossRef]
- Henni, S.H.; Skudutyte-Rysstad, R.; Ansteinsson, V.; Hellesø, R.; Hovden, E.A.S. Oral health and oral health-related quality of life among older adults receiving home health care services: A scoping review. Gerodontology 2022, 1–11. [Google Scholar] [CrossRef]
- Lindmark, U.; Ernsth Bravell, M.; Johansson, L.; Finkel, D. Oral health is essential for quality of life in older adults: A Swedish National Quality Register Study. Gerodontology 2021, 38, 191–198. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.F.S.; Wanderley, R.L.; de AraÚJo, E.C.F.; de Medeiros, M.M.D.; de Figueredo, O.M.C.; Pinheiro, M.A.; Rodrigues Garcia, R.C.M.; Cavalcanti, Y.W. Factors associated with oral health-related quality of life of institutionalized elders. Braz. Oral Res. 2021, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, K.; Armoon, B.; Higgs, P.; Bayat, A.H.; Mohammadi Gharehghani, M.A.; Hemmat, M.; Fakhri, Y.; Mohammadi, R.; Fattah Moghaddam, L.; Schroth, R.J. The association of oral health status and socio-economic determinants with oral health-related quality of life among the elderly: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2021, 19, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Thanakun, S.; Pornprasertsuk-Damrongsri, S.; Izumi, Y. Increased oral inflammation, leukocytes, and leptin, and lower adiponectin in overweight or obesity. Oral Dis. 2017, 23, 956–965. [Google Scholar] [CrossRef]
- Vu, G.T.; Little, B.B.; Esterhay, R.J.; Jennings, J.A.; Creel, L.; Gettleman, L. Oral health-related quality of life in US adults with type 2 diabetes. J. Public Health Dent. 2022, 82, 79–87. [Google Scholar] [CrossRef]
- Ruokonen, H.; Nylund, K.; Meurman, J.H.; Heikkinen, A.M.; Furuholm, J.; Sorsa, T.; Roine, R.; Ortiz, F. Oral symptoms and oral health-related quality of life in patients with chronic kidney disease from predialysis to posttransplantation. Clin. Oral Investig. 2019, 23, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Saboya, P.P.; Bodanese, L.C.; Zimmermann, P.R.; Gustavo, A.D.S.; Assumpção, C.M.; Londero, F. Metabolic syndrome and quality of life: A systematic review. Rev. Lat.-Am. De Enferm. 2016, 24, e2848. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, J.M.; Moura-Grec, P.G.D.; Freitas, A.R.D.; Sales-Peres, A.; Groppo, F.C.; Ceneviva, R.; Sales-Peres, S.H.D.C. Assessment of oral conditions and quality of life in morbid obese and normal weight individuals: A cross-sectional study. PLoS ONE. 2015, 10, e0129687. [Google Scholar]
- Tengku H, T.N.N.; Peh, W.Y.; Shoaib, L.A.; Baharuddin, N.A.; Vaithilingam, R.D.; Saub, R. Oral diseases and quality of life between obese and normal weight adolescents: A two-year observational study. Children 2021, 8, 435. [Google Scholar] [CrossRef]
- Pina, G.D.M.S.; Mota Carvalho, R.; Silva, B.S.D.F.; Almeida, F.T. Prevalence of hyposalivation in older people: A systematic review and meta-analysis. Gerodontology 2020, 37, 317–331. [Google Scholar] [CrossRef]
- Storbeck, T.; Qian, F.; Marek, C.; Caplan, D.; Marchini, L. Dose-dependent association between xerostomia and number of medications among older adults. Spec Care Dent. 2022, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Proença, L.; Oliveira, M.J.; Cavacas, M.A.; Amaro, L.; Águas, A.; Mendes, J.J. Perceived xerostomia, stress and periodontal status impact on elderly oral health-related quality of life: Findings from a cross-sectional survey. BMC Oral Health. 2020, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Temcharoen, P.; Kaewboonruang, P.; Pradipasen, M.; Srisorachart, S. The optimal cut-off points of body mass index which reflect the risk factors of cardiovascular disease in the urban Thai male population. J. Med. Assoc. Thai. 2009, 92 (Suppl. 7), S68–S74. [Google Scholar]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Landry, R.G.; Jean, M. Periodontal Screening and Recording (PSR) Index: Precursors, utility and limitations in a clinical setting. Int. J. Dent. 2002, 52, 35–40. [Google Scholar] [CrossRef]
- Thomson, W.M.; Chalmers, J.M.; Spencer, A.J.; Williams, S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent Health 1999, 16, 12–17. [Google Scholar]
- Osailan, S.M.; Pramanik, R.; Shirlaw, P.; Proctor, G.B.; Challacombe, S.J. Clinical assessment of oral dryness: Development of a scoring system related to salivary flow and mucosal wetness. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 597–603. [Google Scholar] [CrossRef]
- Slade, G.D. Derivation and validation of a short-form oral health impact profile. Community Dent. Oral Epidemiol. 1997, 25, 284–290. [Google Scholar] [CrossRef]
- Slade, G.D.; Nuttall, N.; Sanders, A.E.; Steele, J.G.; Allen, P.F.; Lahti, S. Impacts of oral disorders in the United Kingdom and Australia. Br. Dent. J. 2005, 198, 489–493. [Google Scholar] [CrossRef]
- Sakboonyarat, B.; Pornpongsawad, C.; Sangkool, T.; Phanmanas, C.; Kesonphaet, N.; Tangthongtawi, N.; Limsakul, A.; Assavapisitkul, R.; Thangthai, T.; Janenopparkarnjana, P.; et al. Trends, prevalence and associated factors of obesity among adults in a rural community in Thailand: Serial cross-sectional surveys, 2012 and 2018. BMC Public Health 2020, 20, 850. [Google Scholar] [CrossRef]
- Jitnarin, N.; Kosulwat, V.; Rojroongwasinkul, N.; Boonpraderm, A.; Haddock, C.K.; Poston, W.S.C. Prevalence of overweight and obesity in Thai population: Results of the National Thai Food Consumption Survey. Eat Weight Disord. 2011, 16, e242–e249. [Google Scholar] [CrossRef] [PubMed]
- Bajgai, G.P.; Okuma, N.; Khovidhunkit, S.-O.P.; Thanakun, S. Comparison of measured blood pressure levels, hypertension history, oral diseases, and associated factors among Thai dental patients. J. Oral Sci. 2022, 64, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Sanguansin, N.; Chinwanitcharoen, P.; Asavarachan, S.; Sasiwilasakorn, C.; Chaikornkij, V.; Thanakun, S.; Vuddhakanok, S. Emerged medically compromised conditions in Thai patients visiting a private dental school. World J. Dent. 2022, 13, 394–399. [Google Scholar] [CrossRef]
- Hall, J.E.; Do Carmo, J.M.; Da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Sato, M.; Kurokawa, A.; Sugimoto, H.; Yasuhara, Y.; Nakae, H.; Shinohara, Y.; Tanioka, T.; Iga, H.; Hinode, D.; Suzuki, Y.; et al. Relationship among health related quality of life, quality of sleep, and oral health condition. Health 2018, 10, 204–214. [Google Scholar] [CrossRef][Green Version]
- Grandner, M.A.; Chakravorty, S.; Perlis, M.L.; Oliver, L.; Gurubhagavatula, I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014, 15, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zhi, T.-F.; Sun, X.-M.; Li, S.-J.; Wang, Q.-S.; Cai, J.; Li, L.-Z.; Li, Y.-X.; Xu, M.-J.; Wang, Y.; Chu, X.F.; et al. Associations of sleep duration and sleep quality with life satisfaction in elderly Chinese: The mediating role of depression. Arch. Gerontol. Geriat. 2016, 65, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-P.; Yu, H.-C.; Lin, T.-H.; Wang, Y.-H.; Chang, Y.-C. Association between obesity and chronic periodontitis: A nationwide population-based cohort study in Taiwan. Medicine 2021, 100, e27506. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Peralta, F.S.; Nogueira, L.M.R.; Costa, F.O.; Aquino, D.R.; Rovai, E.S.; Cortelli, J.R. Periodontal therapy on the oral health-related quality of life of obese and non-obese individuals. Odontology 2021, 109, 956–964. [Google Scholar] [CrossRef]
- Wanichkittikul, N.; Laohapand, P.; Mansa-nguan, C.; Thanakun, S. Periodontal treatment improves serum levels of leptin, adiponectin, and C-reactive protein in Thai patients with overweight or obesity. Int. J. Dent. 2021, 2021, 6660097. [Google Scholar] [CrossRef] [PubMed]
- Somsak, K.; Kaewplung, O. The effects of the number of natural teeth and posterior occluding pairs on the oral health-related quality of life in elderly dental patients. Gerodontology 2016, 33, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Peres, K.G.; Peres, M.A. Retention of teeth and oral health–related quality of life. J. Dent. Res. 2016, 95, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Tôrres, L.H.d.N.; De Marchi, R.J.; Hilgert, J.B.; Hugo, F.N.; Ismail, A.I.; Antunes, J.L.F.; Sousa, M.d.L.R.d. Oral health and obesity in Brazilian elders: A longitudinal study. Community Dent. Oral Epidemiol. 2020, 48, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, M.J.; Kho, H.S. Oral health-related quality of life and associated factors in patients with xerostomia. Int. J. Dent. Hyg. 2021, 19, 313–322. [Google Scholar] [CrossRef]
- Jager, D.H.J.; Bots, C.P.; Forouzanfar, T.; Brand, H.S. Clinical oral dryness score: Evaluation of a new screening method for oral dryness. Odontology 2018, 106, 439–444. [Google Scholar] [CrossRef]
- Ohara, Y.; Kawai, H.; Shirobe, M.; Iwasaki, M.; Motokawa, K.; Edahiro, A.; Kim, H.; Fujiwara, Y.; Ihara, K.; Watanabe, Y.; et al. Association between dry mouth and physical frailty among community-dwelling older adults in Japan: The Otassha Study. Gerodontology 2022, 39, 41–48. [Google Scholar] [CrossRef]
- Saleh, J.; Figueiredo, M.A.Z.; Cherubini, K.; Salum, F.G. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2015, 60, 242–255. [Google Scholar] [PubMed]
| Participants (N = 123) | p-Value | |||
|---|---|---|---|---|
| Normal Weight (n = 50) | Overweight (n = 28) | Obesity (n = 45) | ||
| Age | 65 (63, 68) | 66 (62, 69) | 65 (61, 69) | 0.767 |
| Sex | ||||
| Male | 25 (50.0) | 14 (50.0) | 24 (53.3) | 0.939 |
| Female | 25 (50.0) | 14 (50.0) | 21 (46.7) | |
| BMI (kg/m2) | 21.1 (20.2, 22.0) | 24.2 (23.4, 24.8) | 27.6 (26.2, 29.6) | <0.001 * |
| WC (cm) | ||||
| Male | 80 (78, 82) | 89 (87, 91) | 97 (90, 102) | <0.001 * |
| Female | 75 (68, 78) | 83 (79, 87) | 87 (84, 96) | <0.001 * |
| FPG (mg/dL) | 100 (95, 112) | 100 (92, 117) | 104 (96, 115) | 0.335 |
| HDL (mg/dL) | ||||
| Male | 55 (47, 66) | 57 (52, 70) | 51 (45, 57) | 0.040 |
| Female | 75 (64, 81) | 62 (46, 74) | 52 (47, 61) | <0.001 * |
| TG (mg/dL) | 79 (63, 121) | 97(7, 134) | 116 (86, 178) | <0.001 * |
| Systolic BP (mmHg) | 121 (111, 131) | 132 (119, 138) | 128 (121, 141) | 0.017 * |
| Diastolic BP (mmHg) | 76 (64, 84) | 78 (70, 88) | 79 (74, 87) | 0.228 |
| Hypertension criteria | ||||
| Optimal HT <120 and/or <80 mmHg | 24 (48.0) | 6 (21.4) | 7 (15.6) | 0.002 * |
| Normal 120–129 and/or 80–84 mmHg | 7 (14.0) | 5 (17.9) | 16 (35.5) | |
| High normal 130–139 and/or 85–89 mmHg | 13 (26.0) | 8 (28.6) | 7 (15.6) | |
| Possible hypertension >140/90 mmHg | 6 (12.0) | 9 (32.1) | 15 (33.3) | |
| Number of medication use | ||||
| No | 28 (56.0) | 9 (32.1) | 13 (28.9) | 0.001 * |
| 1 group | 13 (26.0) | 12 (42.9) | 8 (17.8) | |
| ≥2 groups | 9 (18.0) | 7 (25.0) | 24 (53.3) | |
| Medication for diabetes mellitus | ||||
| No | 42 (84.0) | 26 (92.9) | 37 (82.2) | 0.430 |
| Yes | 8 (16.0) | 2 (7.1) | 8 (17.8) | |
| Medication for dyslipidemia | ||||
| No | 31 (62.0) | 16 (57.1) | 18 (40.0) | 0.088 |
| Yes | 19 (38.0) | 12 (42.9) | 27 (60.0) | |
| Medication for hypertension | ||||
| No | 41 (82.0) | 14 (50.0) | 20 (44.4) | <0.001 * |
| Yes | 9 (18.0) | 14 (50.0) | 25 (55.6) | |
| Participants (N = 123) | p-Value | |||
|---|---|---|---|---|
| Normal Weight (n = 50) | Overweight (n = 28) | Obesity (n = 45) | ||
| Number of tooth diseases | 9 (5, 13) | 10 (7, 15) | 11 (5, 16) | 0.366 |
| Number of missing teeth | 3 (1, 5) | 3 (0, 5) | 5 (2, 9) | 0.025 * |
| Periodontal status | ||||
| Gingivitis | 39 (78.0) | 17 (60.7) | 29 (64.4) | 0.198 |
| Periodontitis | 11 (22.0) | 11 (39.3) | 16 (35.6) | |
| Denture wear | ||||
| Upper | 11 (22.0) | 7 (25.0) | 11 (24.4) | 0.421 |
| Lower | 7 (14.0) | 5 (17.9) | 8 (17.8) | 0.171 |
| Total XI-11 score | 17 (12, 21) | 17 (14, 20) | 17 (15, 22) | 0.927 |
| Total CODS | 0 (0, 2) | 0 (0, 2) | 2 (0, 3) | 0.014 * |
| OHIP-14 | Participants (N = 123) | p-Value | ||
|---|---|---|---|---|
| Normal Weight (n = 50) | Overweight (n = 28) | Obesity (n = 45) | ||
| Score 0–3 | 42 (84.0) | 23 (82.1) | 25 (55.6) | 0.004 * |
| Score 4 | 8 (16.0) | 5 (17.9) | 20 (44.4) | |
| OHIP-14: Scoring 4 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Age (years) | 1.03 (0.95, 1.12) | 0.492 | 1.02 (0.94, 1.11) | 0.631 | 0.98 (0.88, 1.09) | 0.672 | 0.96 (0.86, 1.08) | 0.541 |
| Sex (female) | 1.38 (0.62, 3.07) | 0.434 | 1.49 (0.64, 3.48) | 0.352 | 1.64 (0.69, 3.94) | 0.266 | 1.29 (0.51, 3.22) | 0.593 |
| BMI | ||||||||
| Overweight | 1.13 (0.33, 3.88) | 0.846 | 1.24 (0.35, 4.34) | 0.737 | 1.41 (0.39, 5.08) | 0.603 | ||
| Obesity | 4.25 (1.62, 11.15) | 0.003 | 4.06 (1.51, 10.90) | 0.005 | 4.42 (1.57, 12.47) | 0.005 * | ||
| Number of missing teeth | 1.05 (0.95, 1.15) | 0.326 | 1.03 (0.93, 1.15) | 0.570 | ||||
| Number of teeth with pulpal diseases | 1.26 (0.89, 1.79) | 0.194 | 1.24 (0.79, 1.95) | 0.353 | ||||
| Total XI score | 1.11 (1.02, 1.20) | 0.013 * | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khongsirisombat, N.; Kiattavorncharoen, S.; Thanakun, S. Increased Oral Dryness and Negative Oral Health-Related Quality of Life in Older People with Overweight or Obesity. Dent. J. 2022, 10, 231. https://doi.org/10.3390/dj10120231
Khongsirisombat N, Kiattavorncharoen S, Thanakun S. Increased Oral Dryness and Negative Oral Health-Related Quality of Life in Older People with Overweight or Obesity. Dentistry Journal. 2022; 10(12):231. https://doi.org/10.3390/dj10120231
Chicago/Turabian StyleKhongsirisombat, Nattapat, Sirichai Kiattavorncharoen, and Supanee Thanakun. 2022. "Increased Oral Dryness and Negative Oral Health-Related Quality of Life in Older People with Overweight or Obesity" Dentistry Journal 10, no. 12: 231. https://doi.org/10.3390/dj10120231
APA StyleKhongsirisombat, N., Kiattavorncharoen, S., & Thanakun, S. (2022). Increased Oral Dryness and Negative Oral Health-Related Quality of Life in Older People with Overweight or Obesity. Dentistry Journal, 10(12), 231. https://doi.org/10.3390/dj10120231