Strike a Balance: Between Metals and Non-Metals, Metalloids as a Source of Anti-Infective Agents
Abstract
1. Introduction
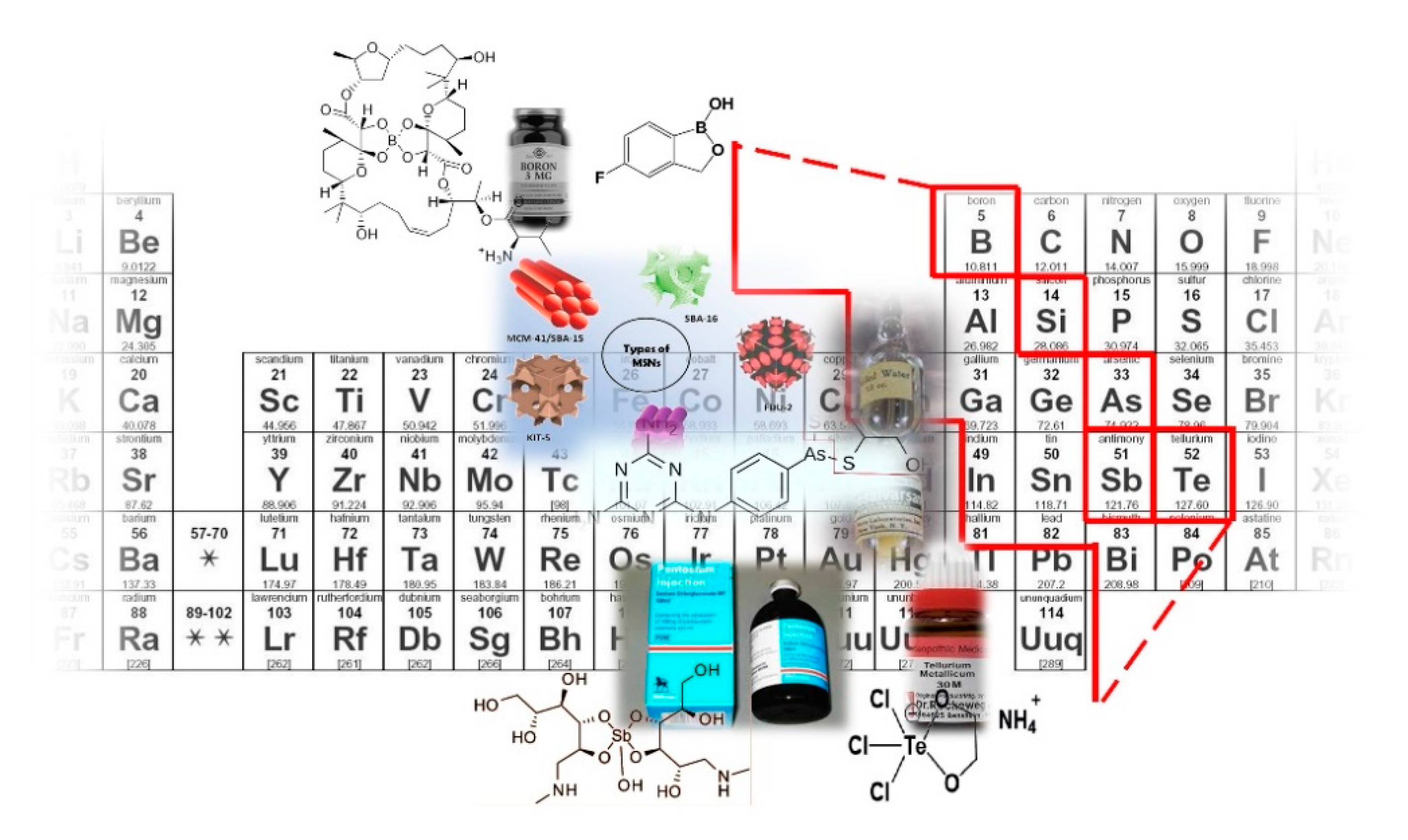
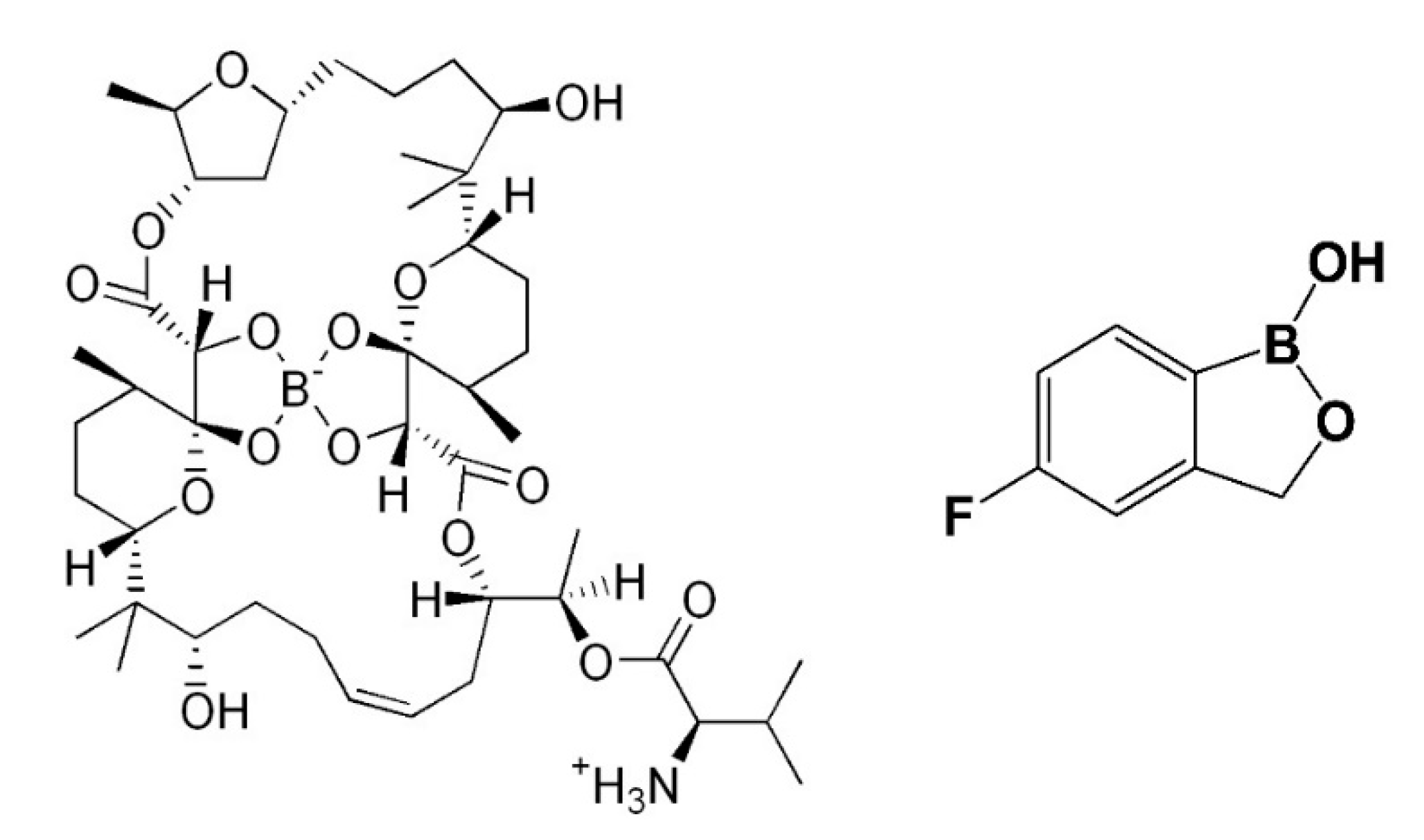
2. Boron
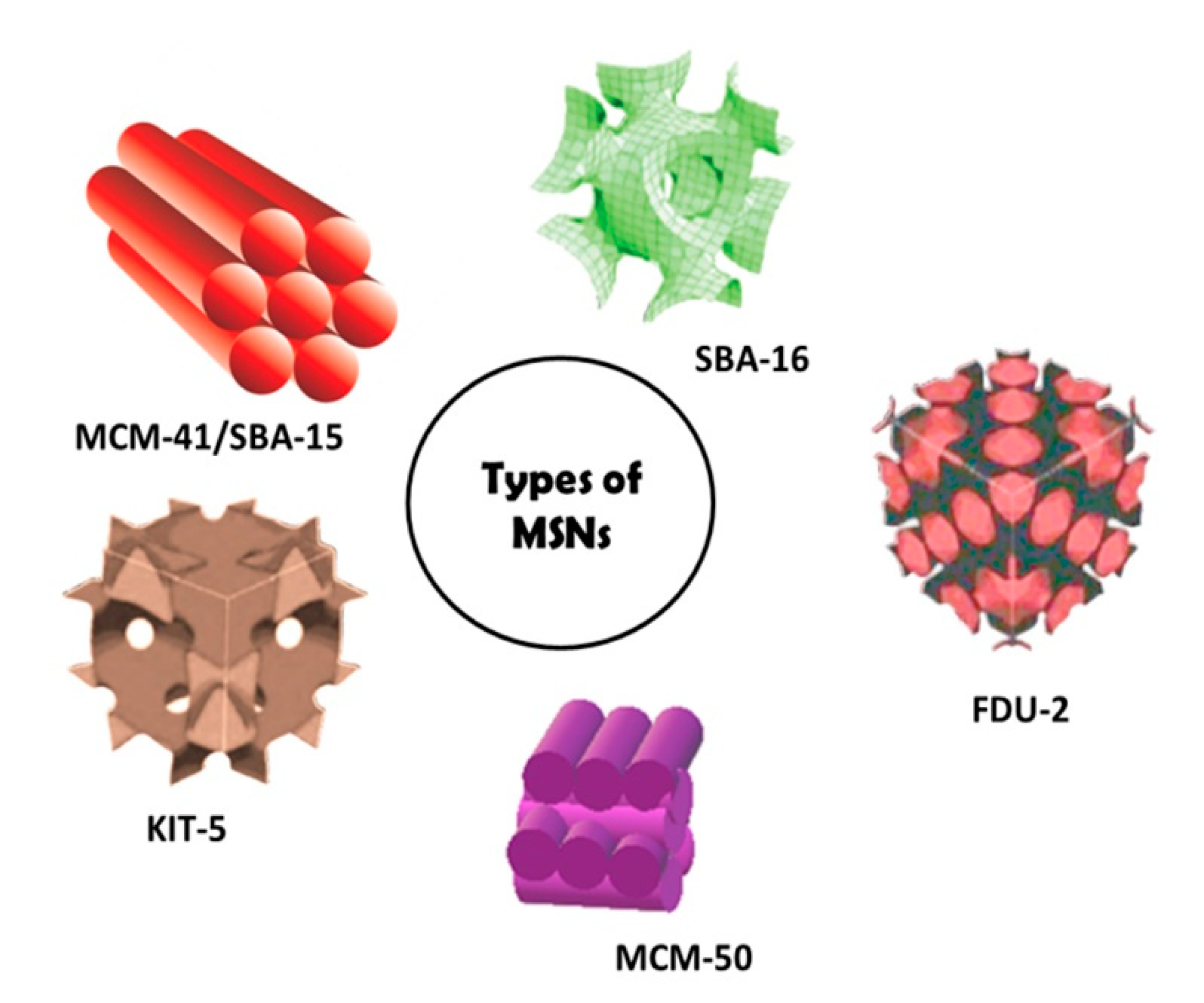
3. Silicon
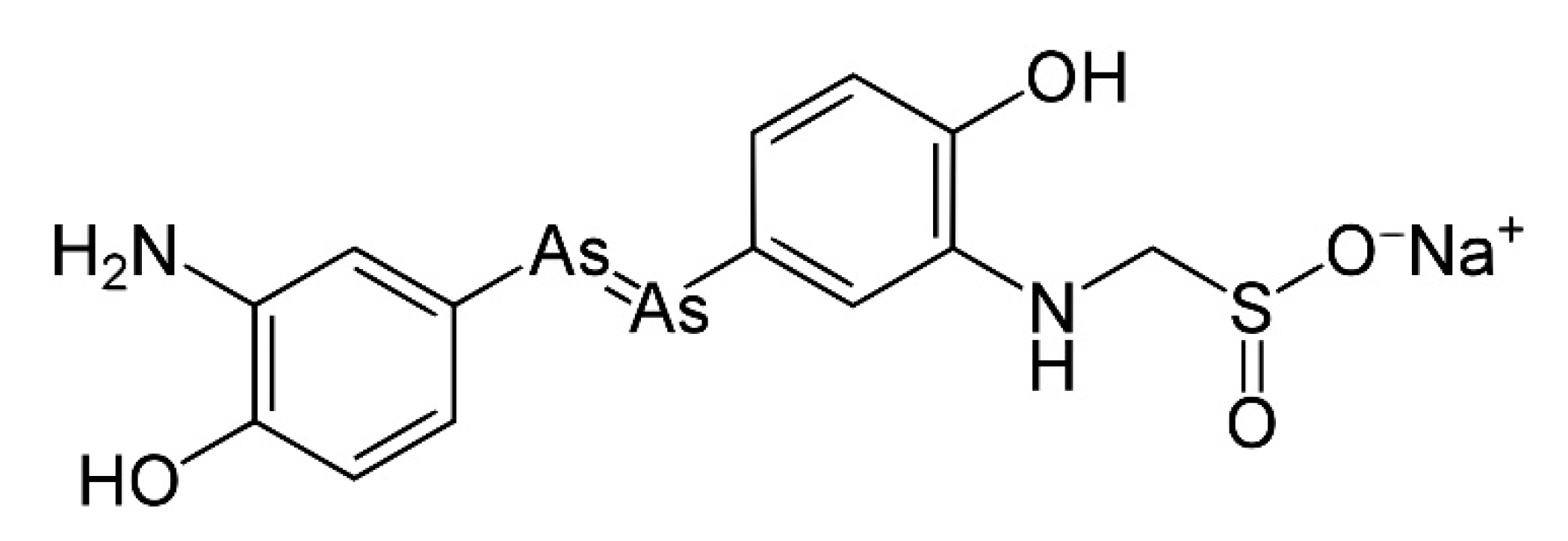
4. Arsenic
5. Antimony
6. Tellurium
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silvestry-Rodriguez, N.; Sicairos-Ruelas, E.E.; Gerba, C.P.; Bright, K.R. Silver as a Disinfectant. Rev. Environ. Contam. Toxicol. 2007, 191, 23–45. [Google Scholar] [CrossRef]
- Fricker, S.P. Medical Uses of Gold Compounds: Past, Present and Future. Gold Bull. 1996, 29, 53–60. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Marzo, T.; La Mendola, D. Biological Applications of Thiocarbohydrazones and Their Metal Complexes: A Perspective Review. Pharmaceuticals 2020, 13, 4. [Google Scholar] [CrossRef]
- Marzo, T.; La Mendola, D. The Effects on Angiogenesis of Relevant Inorganic Chemotherapeutics. Curr. Top. Med. Chem. 2020, 21, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Part (II) Agents, Nanoparticle Delivery, and Part (IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Pillozzi, S.; D’Amico, M.; Bartoli, G.; Gasparoli, L.; Petroni, G.; Crociani, O.; Marzo, T.; Guerriero, A.; Messori, L.; Severi, M.; et al. The Combined Activation of K Ca 3.1 and Inhibition of K V 11.1/Herg1 Currents Contribute to Overcome Cisplatin Resistance in Colorectal Cancer Cells. Br. J. Cancer 2018, 118, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Cirri, D.; Pillozzi, S.; Gabbiani, C.; Tricomi, J.; Bartoli, G.; Stefanini, M.; Michelucci, E.; Arcangeli, A.; Messori, L.; Marzo, T. PtI2 (DACH), the Iodido Analogue of Oxaliplatin as a Candidate for Colorectal Cancer Treatment: Chemical and Biological Features. Dalt. Trans. 2017, 46, 3311–3317. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Van Eldik, R.; Sadler, P. (Eds.) Medicinal Chemistry, 1st ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 75, Available online: www.elsevier.com/books/medicinal-chemistry/van-eldik/978-0-12-819196-5 (accessed on 5 May 2021).
- Barry, N.P.E.; Sadler, P.J. 100 Years of Metal Coordination Chemistry: From Alfred Werner to Anticancer Metallodrugs. Pure Appl. Chem. 2014, 86, 1897–1910. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Exploration of the Medical Periodic Table: Towards New Targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef]
- Cirri, D.; Pratesi, A.; Marzo, T.; Messori, L. Metallo Therapeutics for COVID-19. Exploiting Metal-Based Compounds for the Discovery of New Antiviral Drugs. Expert Opin. Drug. Discov. 2021, 16, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, R.; Chan, J.F.W.; Zhang, A.J.; Cheng, T.; Chik, K.K.H.; Ye, Z.W.; Wang, S.; Lee, A.C.Y.; Jin, L.; et al. Metallodrug Ranitidine Bismuth Citrate Suppresses SARS-Cov-2 Replication and Relieves Virus-Associated Pneumonia in Syrian Hamsters. Nat. Microbiol. 2020, 5, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Stone, S.; Natekar, J.; Kumari, P.; Arora, K.; Kumar, M. The FDA-Approved Gold Drug Auranofin Inhibits Novel Coronavirus (SARS-COV-2) Replication and Attenuates Inflammation in Human Cells. Virology 2020, 547, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Marzo, T.; Messori, L. A Role for Metal-Based Drugs in Fighting COVID-19 Infection? The Case of Auranofin. ACS Med. Chem. Lett. 2020, 11, 1067–1068. [Google Scholar] [CrossRef]
- Van Cleave, C.; Crans, D.C. The First-Row Transition Metals in the Periodic Table of Medicine. Inorganics 2019, 7, 111. [Google Scholar] [CrossRef]
- Vernon, R.E. Which Elements Are Metalloids? J. Chem. Educ. 2013, 90, 1703–1707. [Google Scholar] [CrossRef]
- Miller, J.S. Viewpoint: Metalloids—An Electronic Band Structure Perspective. Chem. A. Eur. J. 2019, 25, 11177–11179. [Google Scholar] [CrossRef]
- Lloyd, N.C.; Morgan, H.W.; Nicholson, B.K.; Ronimus, R.S. The Composition of Ehrlich’s Salvarsan: Resolution of a Century-Old Debate. Angew. Chem. Int. Ed. 2005, 44, 941–944. [Google Scholar] [CrossRef]
- Baker, S.J.; Ding, C.Z.; Akama, T.; Zhang, Y.K.; Hernandez, V.; Xia, Y. Therapeutic Potential of Boron-Containing Compounds. Future Med. Chem. 2009, 1, 1275–1288. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in Drug Design: Recent Advances in the Development of New Therapeutic Agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef]
- Ban, H.S.; Nakamura, H. Boron-Based Drug Design. Chem. Rec. 2015, 15, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Kohno, J.; Kawahata, T.; Otake, T.; Morimoto, M.; Mori, H.; Ueba, N.; Nishio, M.; Kinumaki, A.; Komatsubara, S.; Kawashima, K. Boromycin, an Anti-HIV Antibiotic. Biosci. Biotechnol. Biochem. 1996, 60, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.; Aziz, D.B.; Dick, T. Boromycin Kills Mycobacterial Persisters Without Detectable Resistance. Front. Microbiol. 2016, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, D.P.; Voyack, M.J. Onychomycosis: Current Trends in Diagnosis and Treatment. Am. Fam. Physician 2013, 88, 762–770. [Google Scholar]
- Markham, A. Tavaborole: First Global Approval. Drugs 2014, 74, 1555–1558. [Google Scholar] [CrossRef]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crépin, T.; Zhou, H.; Zhang, Y.K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An Antifungal Agent Inhibits an Aminoacyl-tRNA Synthetase by Trapping tRNA in the Editing Site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Jinna, S.; Finch, J. Spotlight on Tavaborole for the Treatment of Onychomycosis. Drug Des. Devel. Ther. 2015, 9, 6185–6190. [Google Scholar]
- Elewski, B.E.; Aly, R.; Baldwin, S.L.; González Soto, R.F.; Rich, P.; Weisfeld, M.; Wiltz, H.; Zane, L.T.; Pollak, R. Efficacy and Safety of Tavaborole Topical Solution, 5%, a Novel Boron-Based Antifungal Agent, for the Treatment of Toenail Onychomycosis: Results from 2 Randomized Phase-III Studies. J. Am. Acad. Dermatol. 2015, 73, 62–69. [Google Scholar] [CrossRef]
- Junold, K.; Baus, J.A.; Burschka, C.; Vent-Schmidt, T.; Riedel, S.; Tacke, R. Five-Coordinate Silicon(II) Compounds with Si-M Bonds (M = Cr, Mo, W, Fe): Bis[N,N′-Diisopropylbenzamidinato(-)]Silicon(II) as a Ligand in Transition-Metal Complexes. Inorg. Chem. 2013, 52, 11593–11599. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Bains, W.; Seager, S. On the Potential of Silicon as a Building Block for Life. Life 2020, 10, 84. [Google Scholar] [CrossRef]
- Reichstat, M.M.; Mioč, U.B.; Bogunovic, L.J.; Ribnikar, S.V. Theoretical Investigation of Intermolecular Hydrogen-Bonded Complexes in Systems: Substituted Carbinols (Silanols)-Ketones or Ethers. J. Mol. Struct. 1991, 244, 283–290. [Google Scholar] [CrossRef]
- Mills, J.S.; Showell, G.A. Exploitation of Silicon Medicinal Chemistry in Drug Discovery. Expert Opin. Investig. Drugs 2004, 13, 1149–1157. [Google Scholar] [CrossRef]
- Ramesh, R.; Reddy, D.S. Quest for Novel Chemical Entities through Incorporation of Silicon in Drug Scaffolds. J. Med. Chem. 2018, 61, 3779–3798. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities—Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Andersen, F.A. Final Report on the Safety Assessment of Aluminum Silicate, Calcium Silicate, Magnesium Aluminum Silicate, Magnesium Silicate, Magnesium Trisilicate, Sodium Magnesium Silicate, Zirconium Silicate, Attapulgite, Bentonite, Fuller’s Earth, Hectorite, Kaolin, Lithium Magnesium Silicate, Lithium Magnesium Sodium Silicate, Montmorillonite, Pyrophyllite, and Zeolite. Int. J. Toxicol. 2003, 22, 37–102. [Google Scholar] [CrossRef]
- Seljak, K.B.; Kocbek, P.; Gašperlin, M. Mesoporous Silica Nanoparticles as Delivery Carriers: An Overview of Drug Loading Techniques. J. Drug Deliv. Sci. Technol. 2020, 59, 101906. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, N.; Houlton, A.; Horrocks, B.R. Silicon Nanoparticles: Applications in Cell Biology and Medicine. Int. J. Nanomed. 2006, 1, 451–472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, X.; Tang, J.; Su, Y.; Peng, F.; Lu, Y.; Peng, R.; He, Y. A Silicon-Based Antibacterial Material Featuring Robust and High Antibacterial Activity. J. Mater. Chem. B 2014, 2, 691–697. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Smirnov, N.A.; Kudryashov, S.I.; Nastulyavichus, A.A.; Rudenko, A.A.; Saraeva, I.N.; Tolordava, E.R.; Gonchukov, S.A.; Romanova, Y.M.; Ionin, A.A.; Zayarny, D.A. Antibacterial Properties of Silicon Nanoparticles. Laser Phys. Lett. 2018, 15, 105602. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of Nanotoxicity: Generation of Reactive Oxygen Species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Grund, S.C.; Hanusch, K.; Wolf, H.U. Arsenic and Arsenic Compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2011, 100, 41–93. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small Organometallic Compounds as Antibacterial Agents. Dalt. Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Metalloid Compounds as Drugs. Res. Pharm. Sci. 2013, 8, 145–158. [Google Scholar] [PubMed]
- IARC Publications Website. Arsenic, Metals, Fibres, and Dusts. Available online: publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Arsenic-Metals-Fibres-And-Dusts-2012 (accessed on 5 May 2021).
- Gurnari, C.; De Bellis, E.; DIvona, M.; Ottone, T.; Lavorgna, S.; Voso, M.T. When Poisons Cure: The Case of Arsenic in Acute Promyelocytic Leukemia. Chemotherapy 2020, 64, 238–247. [Google Scholar] [CrossRef]
- Seixas, J.; Atouguia, J.; Josenando, T.; Vatunga, G.; Bilenge, C.M.M.; Lutumba, P.; Burri, C. Clinical Study on the Melarsoprol-Related Encephalopathic Syndrome: Risk Factors and HLA Association. Trop. Med. Infect. Dis. 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African Trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Blum, J.; Schmid, C.; Burri, C. Clinical Aspects of 2541 Patients with Second Stage Human African Trypanosomiasis. Acta Trop. 2006, 97, 55–64. [Google Scholar] [CrossRef]
- Fairlamb, A.H. Chemotherapy of Human African Trypanosomiasis: Current and Future Prospects. Trends Parasitol. 2003, 19, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Ericsson, Ö.; Burri, C. Investigations of the Metabolites of the Trypanocidal Drug Melarsoprol. Clin. Pharmacol. Ther. 2000, 67, 478–488. [Google Scholar] [CrossRef]
- Carter, N.S.; Fairlamb, A.H. Arsenical-Resistant Trypanosomes Lack an Unusual Adenosine Transporter. Nature 1993, 361, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Cerami, A. Metabolism and Functions of Trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992, 46, 695–729. [Google Scholar] [CrossRef]
- Marzo, T.; Ferraro, G.; Merlino, A.; Messori, L. Protein Metalation by Inorganic Anticancer Drugs. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; 2021; pp. 1–17. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119951438.eibc2747 (accessed on 16 May 2021). [CrossRef]
- Merlino, A.; Marzo, T.; Messori, L. Protein Metalation by Anticancer Metallodrugs: A Joint ESI MS and XRD Investigative Strategy. Chem. A. Eur. J. 2017, 23, 6942–6947. [Google Scholar] [CrossRef] [PubMed]
- Savadelis, M.D.; Day, K.M.; Bradner, J.L.; Wolstenholme, A.J.; Dzimianski, M.T.; Moorhead, A.R. Efficacy and Side Effects of Doxycycline Versus Minocycline in the Three-Dose Melarsomine Canine Adulticidal Heartworm Treatment Protocol. Parasites Vectors 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.L.; Yang, Q.; Yuan, P.; Wang, X.; Cheng, L. Associations of Essential and Toxic Metals/Metalloids in Whole Blood with Both Disease Severity and Mortality in Patients with COVID-19. FASEB J. 2021, 35, e21392. [Google Scholar] [CrossRef] [PubMed]
- Hansell, C. All Manner of Antimony. Nat. Chem. 2015, 7, 88. [Google Scholar] [CrossRef]
- Voyles, P.M.; Muller, D.A.; Grazul, J.L.; Citrin, P.H.; Gossmann, H.J.L. Atomic-Scale Imaging of Individual Dopant Atoms and Clusters in Highly N-Type Bulk Si. Nature 2002, 416, 826–829. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- World Health Organization. Available online: www.euro.who.int/__data/assets/pdf_file/0017/245330/Strategic-framework-for-leishmaniasis-control-in-the-WHO-European-Region-20142020.pdf (accessed on 16 May 2021).
- Ejov, M.; Dagne, D. Strategic Framework for Leishmaniasis Control in the WHO European Region. 2014–2020; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2014; ISBN 9789289050166. [Google Scholar]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Schistosomiasis. Available online: www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 16 May 2021).
- Jain, S.; Santana, W.; Dolabella, S.S.; Santos, A.L.S.; Souto, E.B.; Severino, P. Are Nanobiosensors an Improved Solution for Diagnosis of Leishmania? Pharmaceutics 2021, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent Antimonials: New Perspectives for Old Drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.K.; Sen, P.; Roy, S. Use of Antimony in the Treatment of Leishmaniasis: Current Status and Future Directions. Mol. Biol. Int. 2011, 2011, 1–23. [Google Scholar] [CrossRef]
- Ashutosh; Sundar, S.; Goyal, N. Molecular Mechanisms of Antimony Resistance in Leishmania. J. Med. Microbiol. 2007, 56, 143–153. [Google Scholar] [CrossRef]
- Yan, S.; Li, F.; Ding, K.; Sun, H. Reduction of Pentavalent Antimony by Trypanothione and Formation of a Binary and Ternary Complex of Antimony(III) and Trypanothione. J. Biol. Inorg. Chem. 2003, 8, 689–697. [Google Scholar] [CrossRef]
- Ferreira, C.D.S.; Pimenta, A.M.D.C.; Demicheli, C.; Frézard, F. Characterization of Reactions of Antimoniate and Meglumine Antimoniate with a Guanine Ribonucleoside at Different pH. BioMetals 2006, 19, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.L.; Berman, J.D.; Rainey, P.M. In vitro Antileishmanial Properties of Tri- and Pentavalent Antimonial Preparations. Antimicrob. Agents Chemother. 1995, 39, 1234–1239. [Google Scholar] [CrossRef]
- Cunha, R.L.O.R.; Gouvea, I.E.; Juliano, L. A Glimpse on Biological Activities of Tellurium Compounds. An. Acad. Bras. Cienc. 2009, 81, 393–407. [Google Scholar] [CrossRef]
- Halpert, G.; Sredni, B. The Effect of the Novel Tellurium Compound AS101 on Autoimmune Diseases. Autoimmun. Rev. 2014, 13, 1230–1235. [Google Scholar] [CrossRef]
- Nyska, A.; Waner, T.; Pirak, M.; Albeck, M.; Sredni, B. Toxicity Study in Rats of a Tellurium Based Immunomodulating Drug, AS-101: A Potential Drug for AIDS and Cancer Patients. Arch. Toxicol. 1989, 63, 386–393. [Google Scholar] [CrossRef]
- Zweibel, K. The Impact of Tellurium Supply on Cadmium Telluride Photovoltaics. Science 2010, 328, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Halpert, G.; Halperin Sheinfeld, M.; Monteran, L.; Sharif, K.; Volkov, A.; Nadler, R.; Schlesinger, A.; Barshak, I.; Kalechman, Y.; Blank, M.; et al. The Tellurium-Based Immunomodulator, AS101 Ameliorates Adjuvant-Induced Arthritis in Rats. Clin. Exp. Immunol. 2021, 203, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hachmo, Y.; Kalechman, Y.; Skornick, I.; Gafter, U.; Caspi, R.R.; Sredni, B. The Small Tellurium Compound AS101 Ameliorates Rat Crescentic Glomerulonephritis: Association with Inhibition of Macrophage Caspase-1 Activity Via Very Late Antigen-4 Inactivation. Front. Immunol. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Daniel-Hoffmann, M.; Sredni, B.; Nitzan, Y. Bactericidal Activity of the Organo-Tellurium Compound AS101 against Enterobacter Cloacae. J. Antimicrob. Chemother. 2012, 67, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, I.E.; Santos, J.A.N.; Burlandy, F.M.; Tersariol, I.L.S.; Da Silva, E.E.; Juliano, M.A.; Juliano, L.; Cunha, R.L.O.R. Poliovirus 3C Proteinase Inhibition by Organotelluranes. Biol. Chem. 2011, 392, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Vonsover, A.; Loya, S.; Sredni, B.; Albeck, M.; Gotlieb-Stematsky, T.; Araf, O.; Hizi, A. Inhibition of the Reverse Transcriptase Activity and Replication of Human Immunodeficiency Virus Type 1 by AS 101 In Vitro. AIDS Res. Hum. Retrovir. 1992, 8, 613–623. [Google Scholar] [CrossRef]
- Di Luca, M.; Marzo, T. Development of Effective Antibacterial Treatment: Lessons from the Past and Novel Approaches. Antibiotics 2021, 10, 230. [Google Scholar] [CrossRef]
- La Mendola, D.; Giacomelli, C.; Rizzarelli, E. Intracellular Bioinorganic Chemistry and Cross Talk Among Different-Omics. Curr. Top. Med. Chem. 2016, 16, 3103–3130. [Google Scholar] [CrossRef]



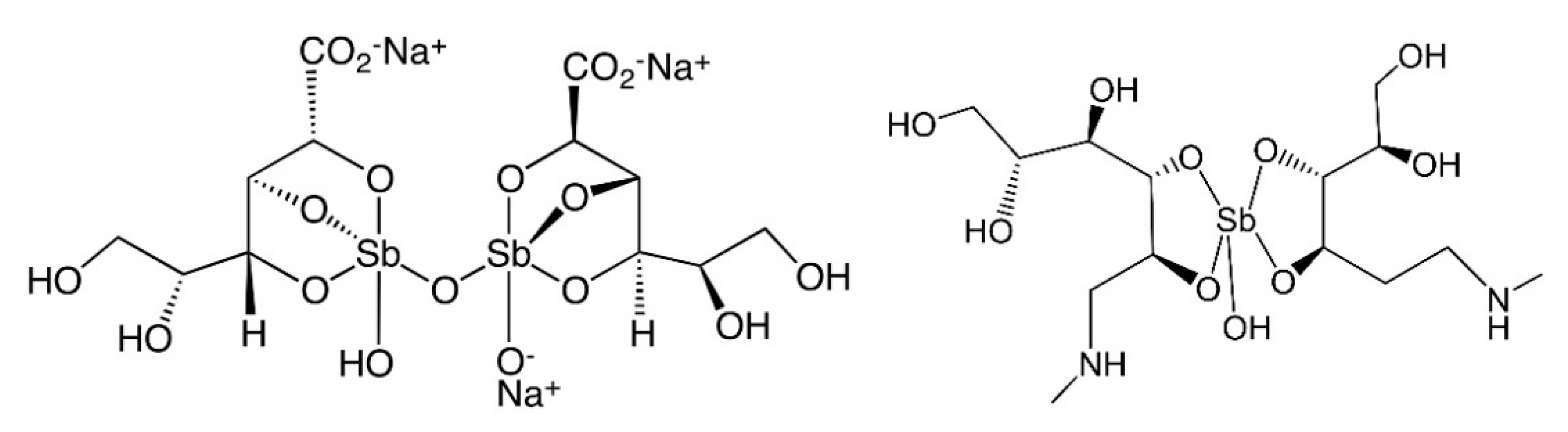
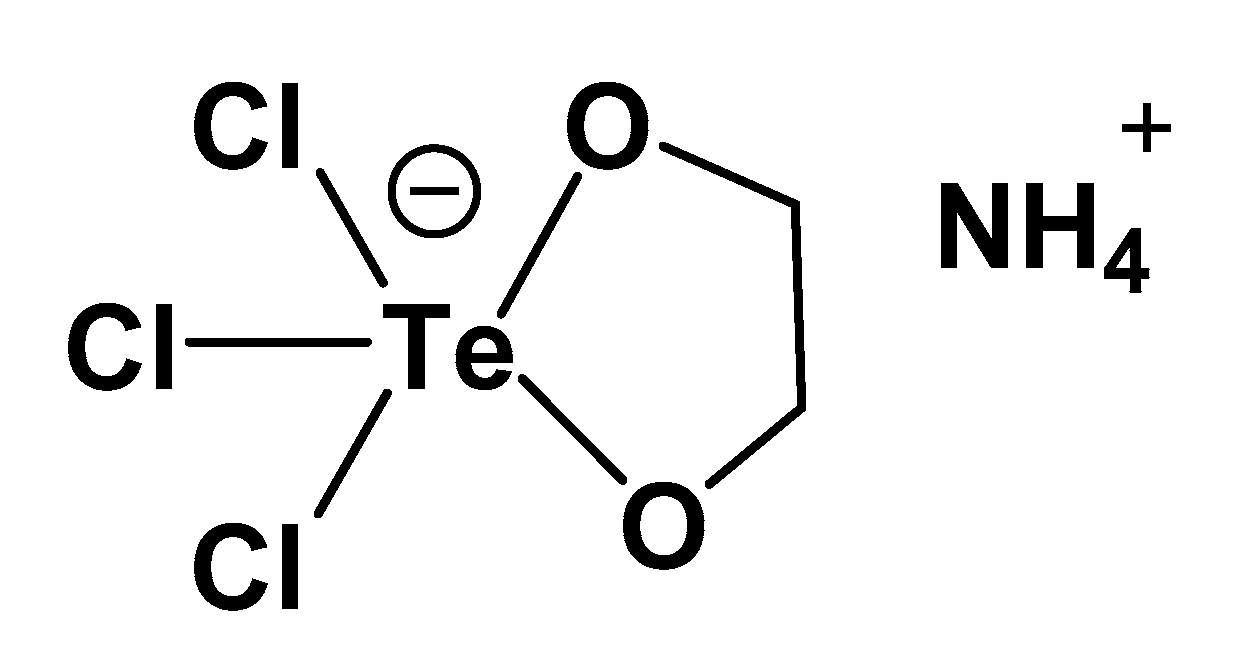
| Element | IP/(kcal/mol) | IP/(kJ/mol) | EN a | EN b |
|---|---|---|---|---|
| β-Boron | 193 | 800 | 2.0 | 2.04 |
| α-Silicon | 189 | 786 | 1.8 | 1.90 |
| α-Germanium | 184 | 762 | 1.8 | 2.01 |
| α-Arsenic | 228 | 944 | 2.0 | 2.18 |
| α-Antimony | 201 | 830 | 1.9 | 2.05 |
| α-Tellurium | 210 | 869 | 2.1 | 2.10 |
| Average | 201 | 832 | 1.9 | 2.05 |
| ClinicalTrials.gov Identifier | Status | Aim | Results |
|---|---|---|---|
| NCT03405818 | C (2018) | S/P | Y |
| NCT01302119 | C (2019) | S/E | Y |
| NCT01270971 | C (2019) | S/E | Y |
| NCT00680160 | C (2019) | A | NA |
| NCT00679601 | C (2018) | A | NA |
| NCT00680134 | C (2018) | S/E | NA |
| NCT01278394 | C (2018) | S/E | Y |
| NCT00679523 | C (2018) | S/E | NA |
| NCT00679965 | C (2018) | D | NA |
| NCT00679770 | C (2018) | D | NA |
| ClinicalTrials.gov Identifier | Drug(s) | Status | Aim | Results |
|---|---|---|---|---|
| NCT00480883 | Meglumine Antimoniate + Allopurinol | C (2010) | Co | NA |
| NCT01050777 | Nano-liposomal Meglumine Antimoniate | C (2012) | F | NA |
| NCT00317980 | Low-doses Meglumine Antimoniate | C (2009) | D | NA |
| NCT01301937 | Low-doses Meglumine Antimoniate | R | D | NA |
| NCT01301924 | Alternative doses of Meglumine Antimoniate | C (2018) | D | NA |
| NCT01381055 | Meglumine Antimoniate + Pentoxifylline | C (2017) | Co | NA |
| NCT00818818 | Low-doses Meglumine Antimoniate | C (2010) | D | NA |
| NCT00537953 | Meglumine Antimoniate + Miltefosine | U | D/Co | NA |
| NCT00657618 | Sodium Stibogluconate | C (2020) | S | Y |
| NCT02281669 | Sodium Stibogluconate | U | S | NA |
| NCT00508963 | Sodium Stibogluconate | A | E | NA |
| NCT00662012 | Sodium Stibogluconate | C (2021) | E | Y |
| NCT00255567 | Sodium Stibogluconate | C (2016) | E/S | NA |
| NCT01067443 | Sodium Stibogluconate + AmBisome® (Gilead Sciences, Inc., Foster City, CA, USA) | C (2017) | E/S/Co | NA |
| NCT03009422 | Sodium Stibogluconate + Fractional CO2 laser | U | E/Co | NA |
| NCT04699383 | Sodium Stibogluconate + Allopurinol | R | E | NA |
| ClinicalTrials.gov Identifier | Indication | Status | Aim | Results |
|---|---|---|---|---|
| NCT01555112 | HPV-condyloma acuminata | C (2013) | F/S/E | NA |
| NCT01943630 | External Genital Warts (HPV) | U | S/E | NA |
| NCT00001006 | HIV | C (2012) | E/S | NA |
| NCT00002266 | HIV | C (2005) | D/E | NA |
| NCT00002013 | HIV | C (2005) | Co/S/E/D/P | NA |
| NCT00002033 | HIV | C (2005) | Co/E/S | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzo, T.; La Mendola, D. Strike a Balance: Between Metals and Non-Metals, Metalloids as a Source of Anti-Infective Agents. Inorganics 2021, 9, 46. https://doi.org/10.3390/inorganics9060046
Marzo T, La Mendola D. Strike a Balance: Between Metals and Non-Metals, Metalloids as a Source of Anti-Infective Agents. Inorganics. 2021; 9(6):46. https://doi.org/10.3390/inorganics9060046
Chicago/Turabian StyleMarzo, Tiziano, and Diego La Mendola. 2021. "Strike a Balance: Between Metals and Non-Metals, Metalloids as a Source of Anti-Infective Agents" Inorganics 9, no. 6: 46. https://doi.org/10.3390/inorganics9060046
APA StyleMarzo, T., & La Mendola, D. (2021). Strike a Balance: Between Metals and Non-Metals, Metalloids as a Source of Anti-Infective Agents. Inorganics, 9(6), 46. https://doi.org/10.3390/inorganics9060046







