Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets
Abstract
1. Introduction
2. Discussion
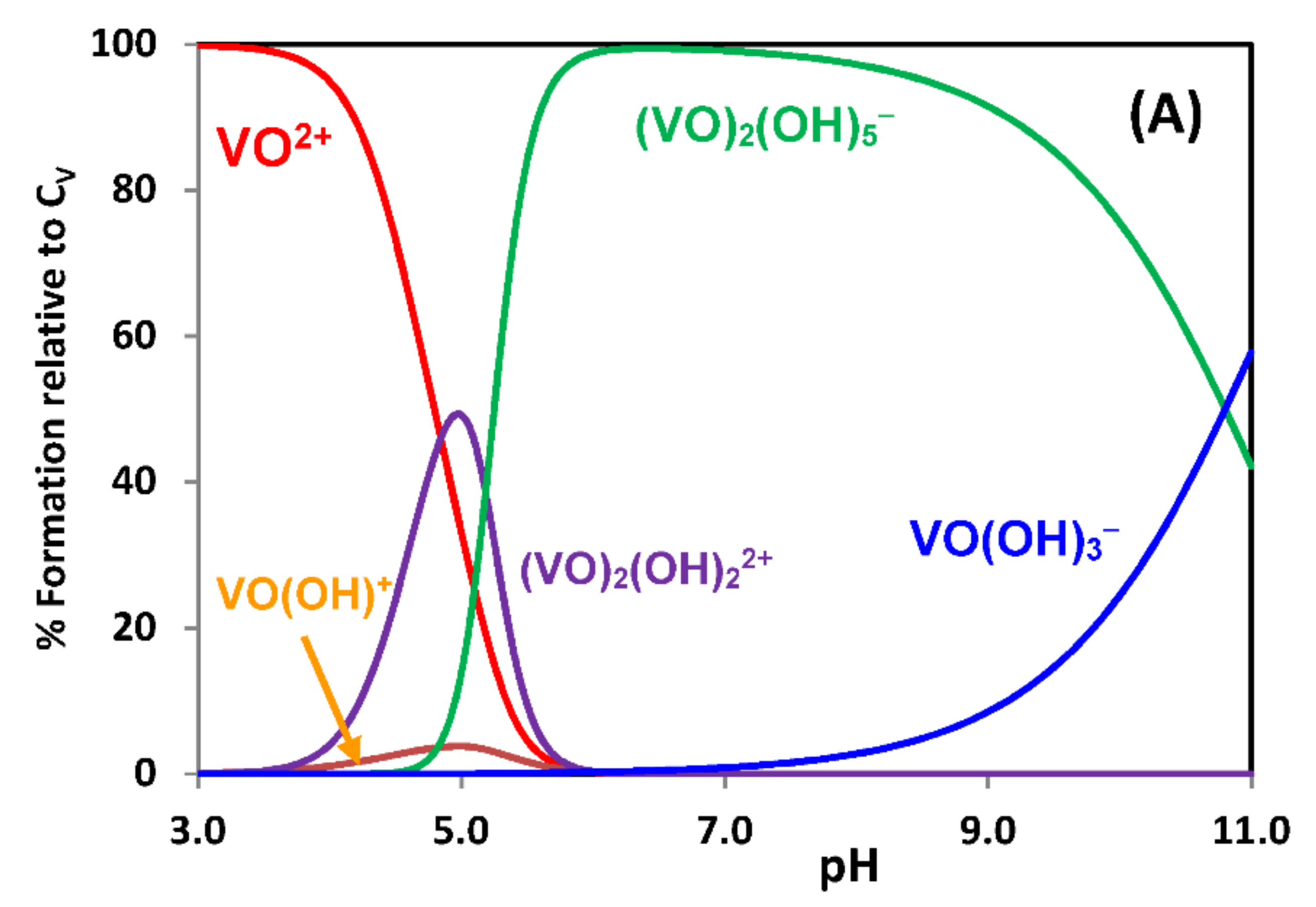
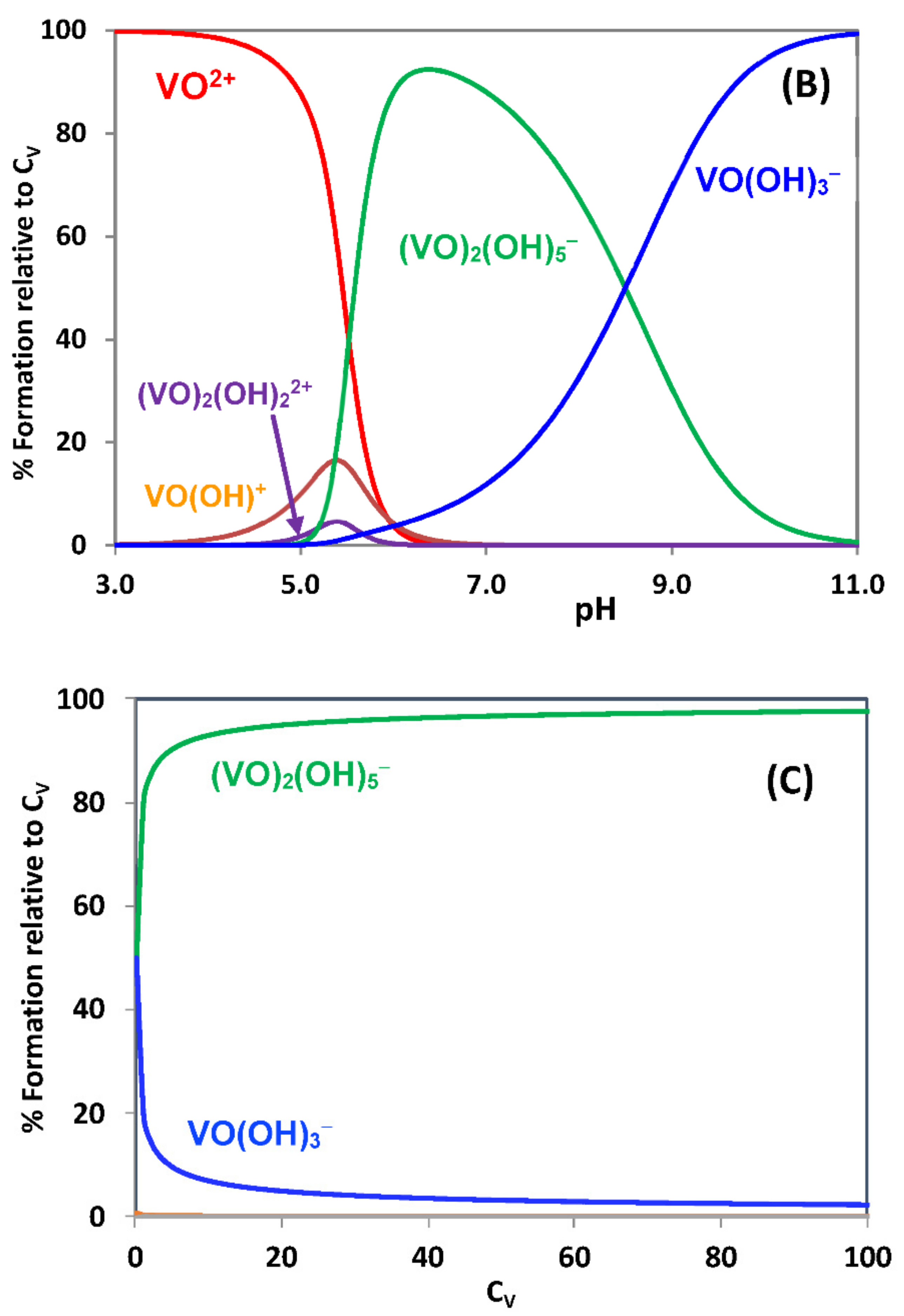
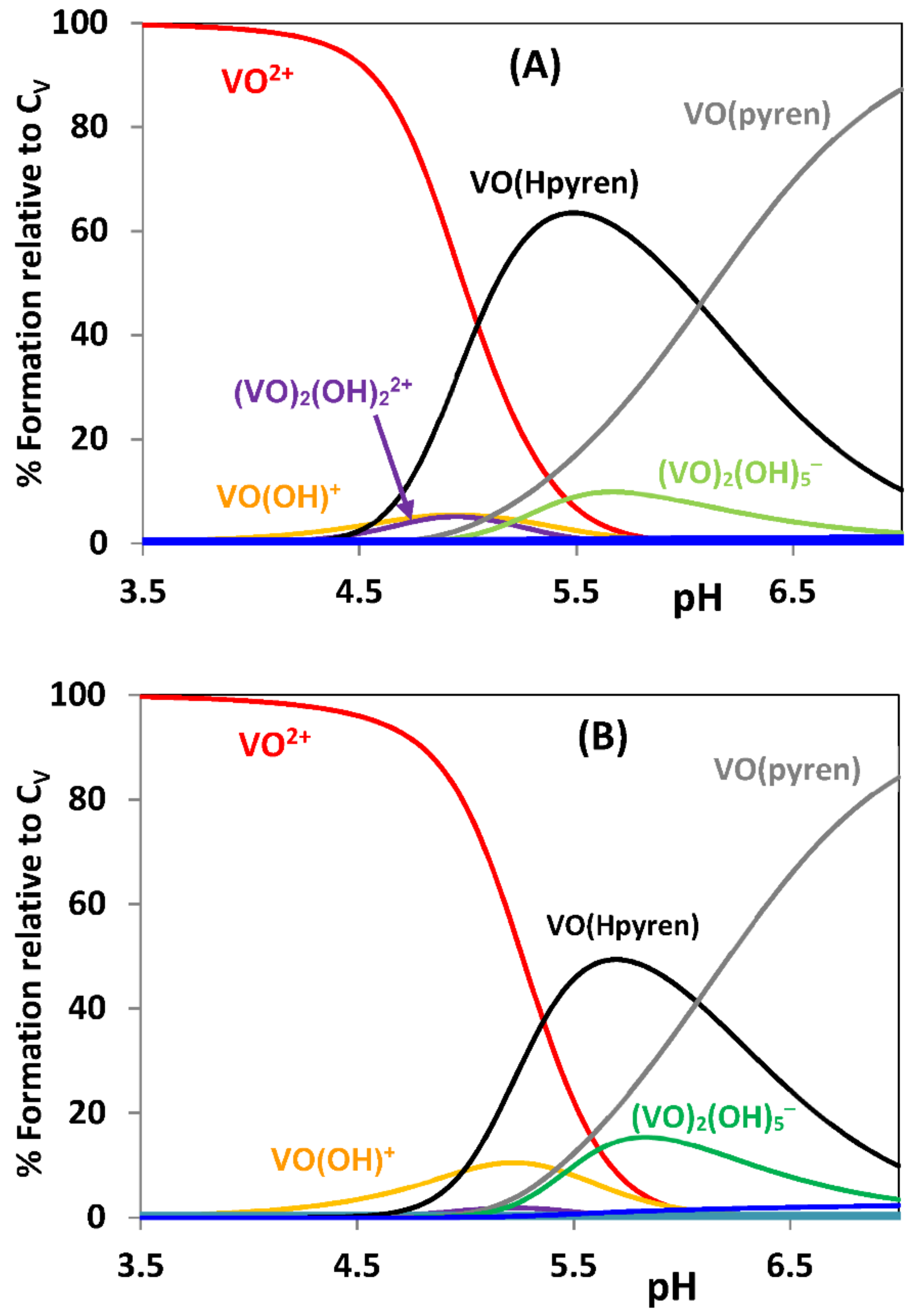
2.1. Hydrolytic Behavior of Oxidovanadium(IV) Ions
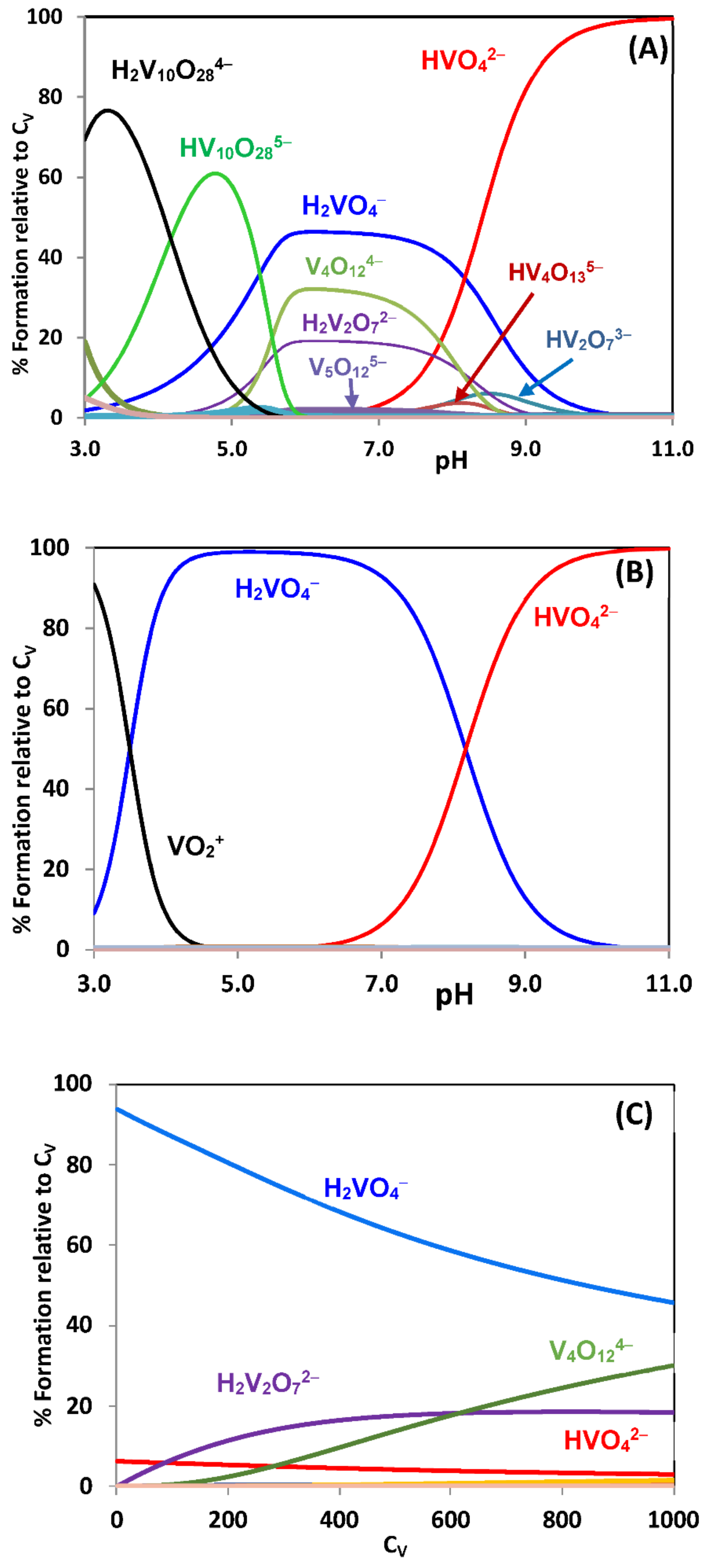
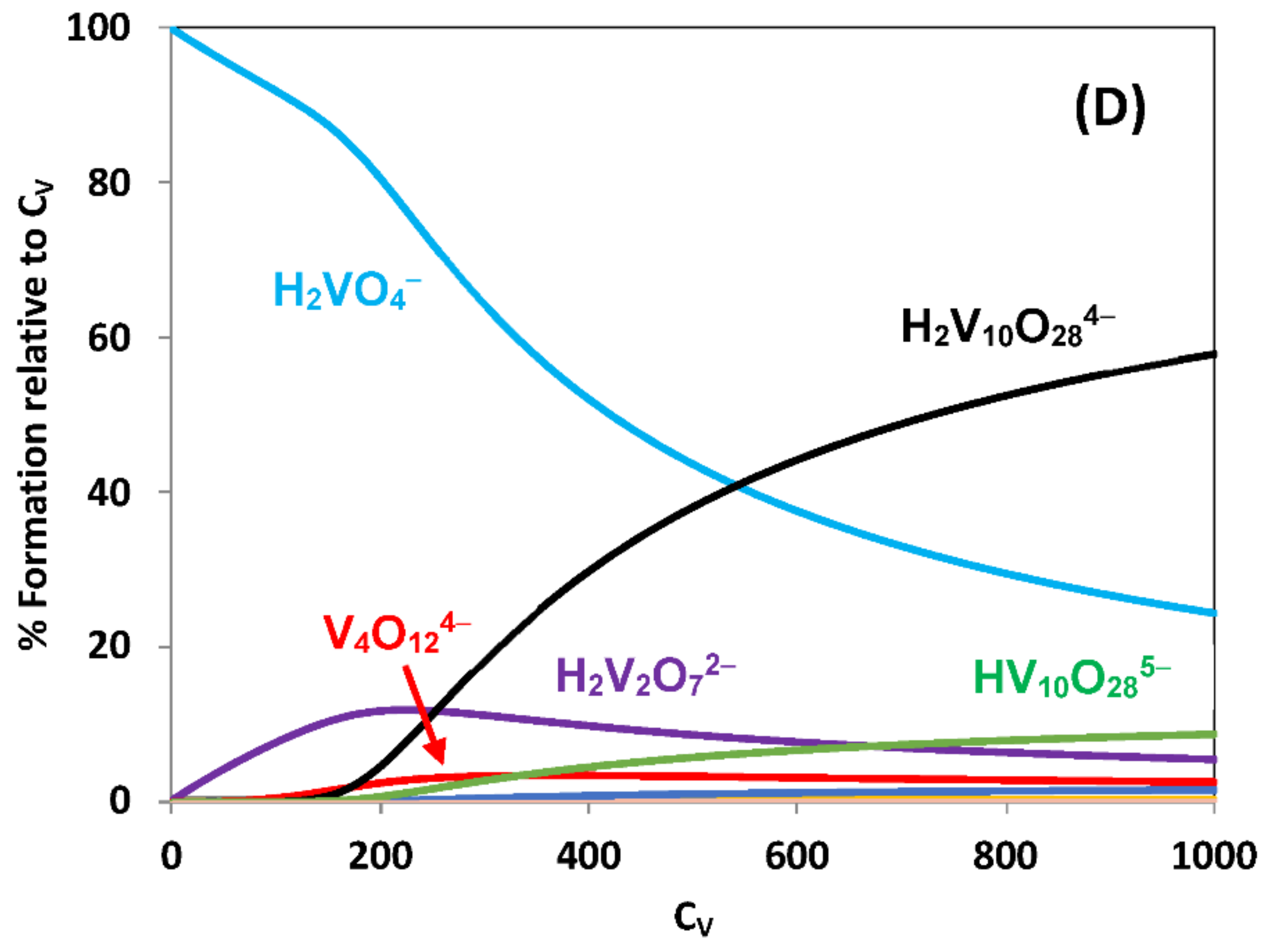
2.2. Hydrolytic Behavior of Oxidovanadium(V) Ions
2.3. Evaluation of Binding Constants of Metal Complexes with Bio-Macromolecules
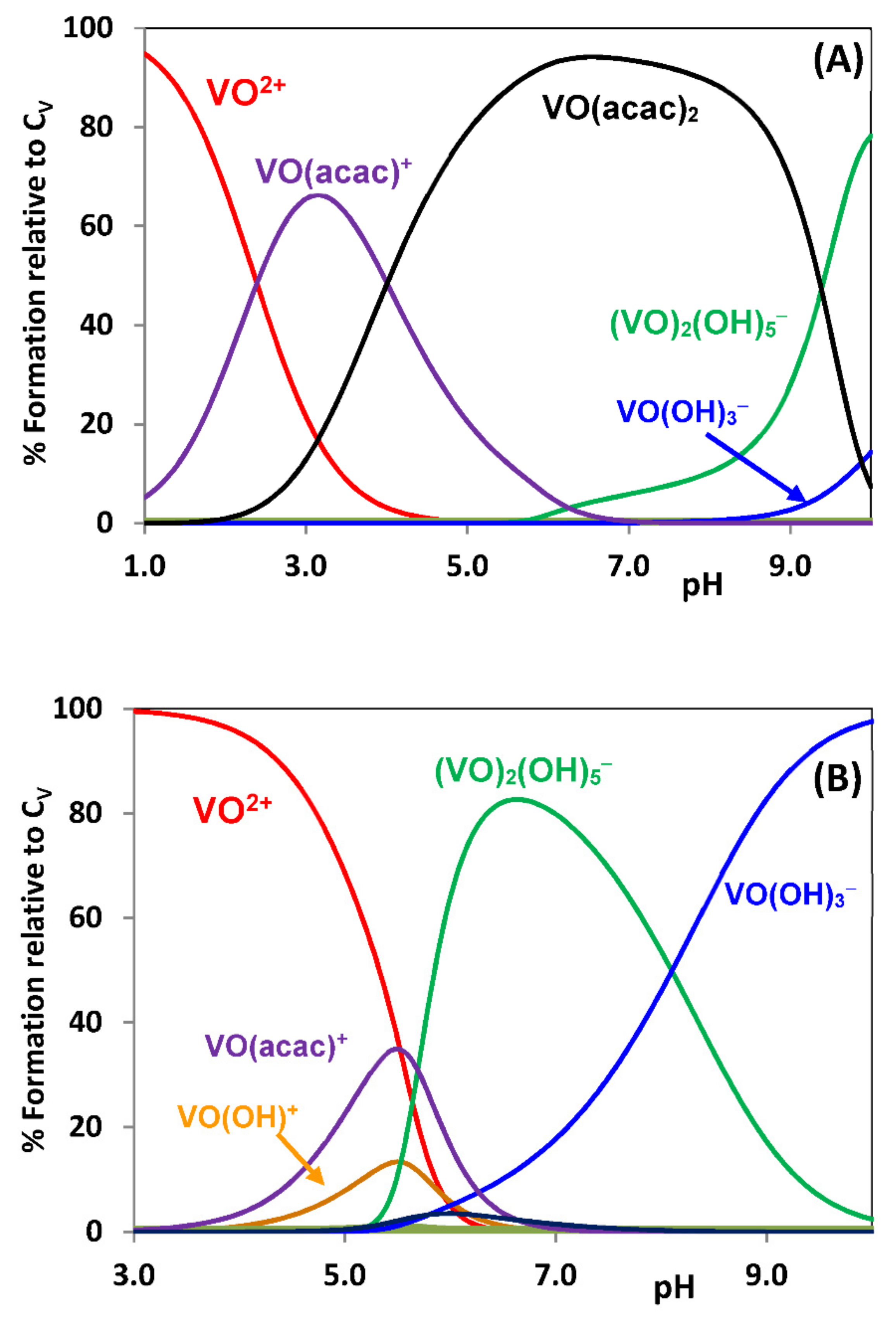
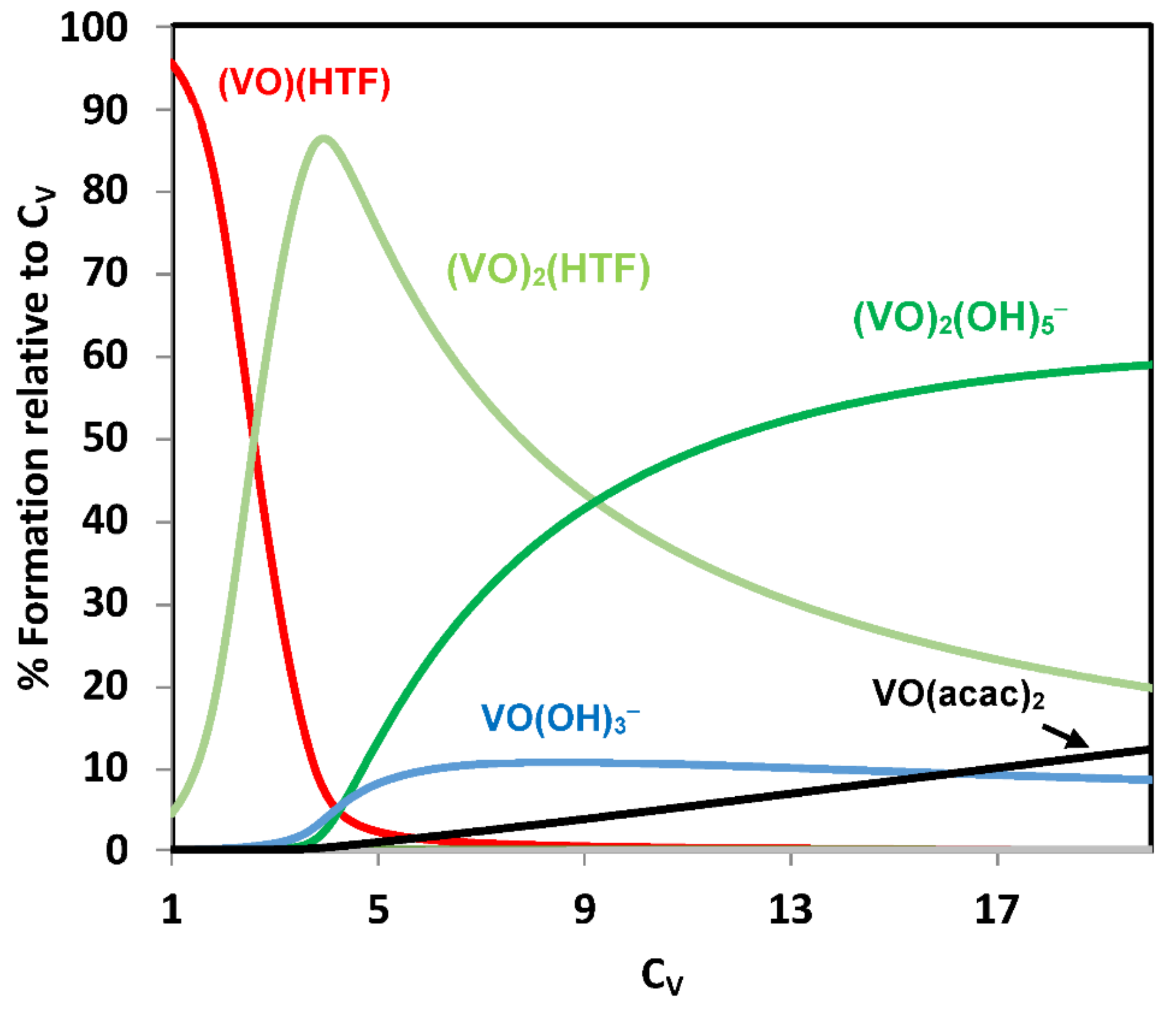
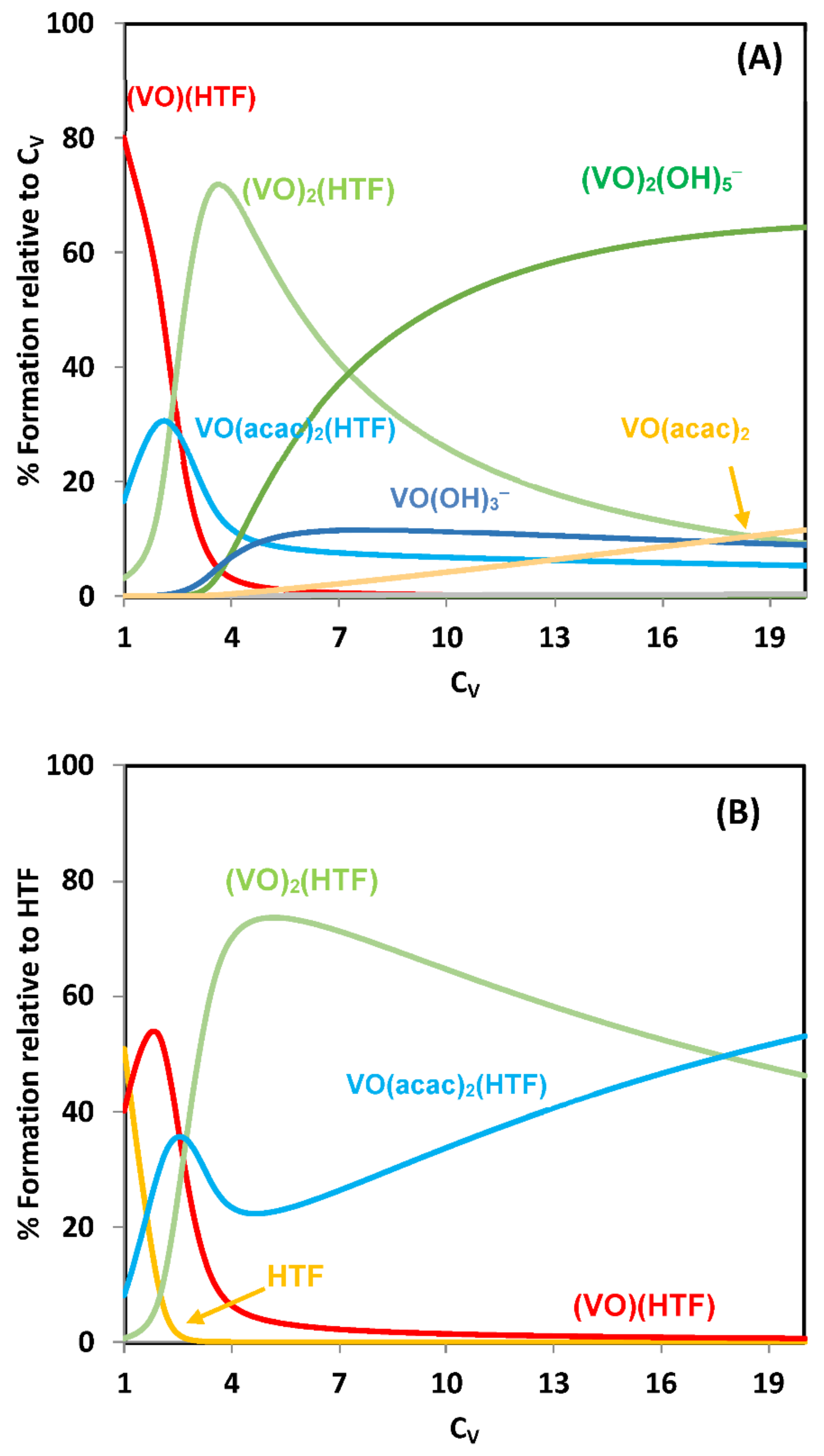
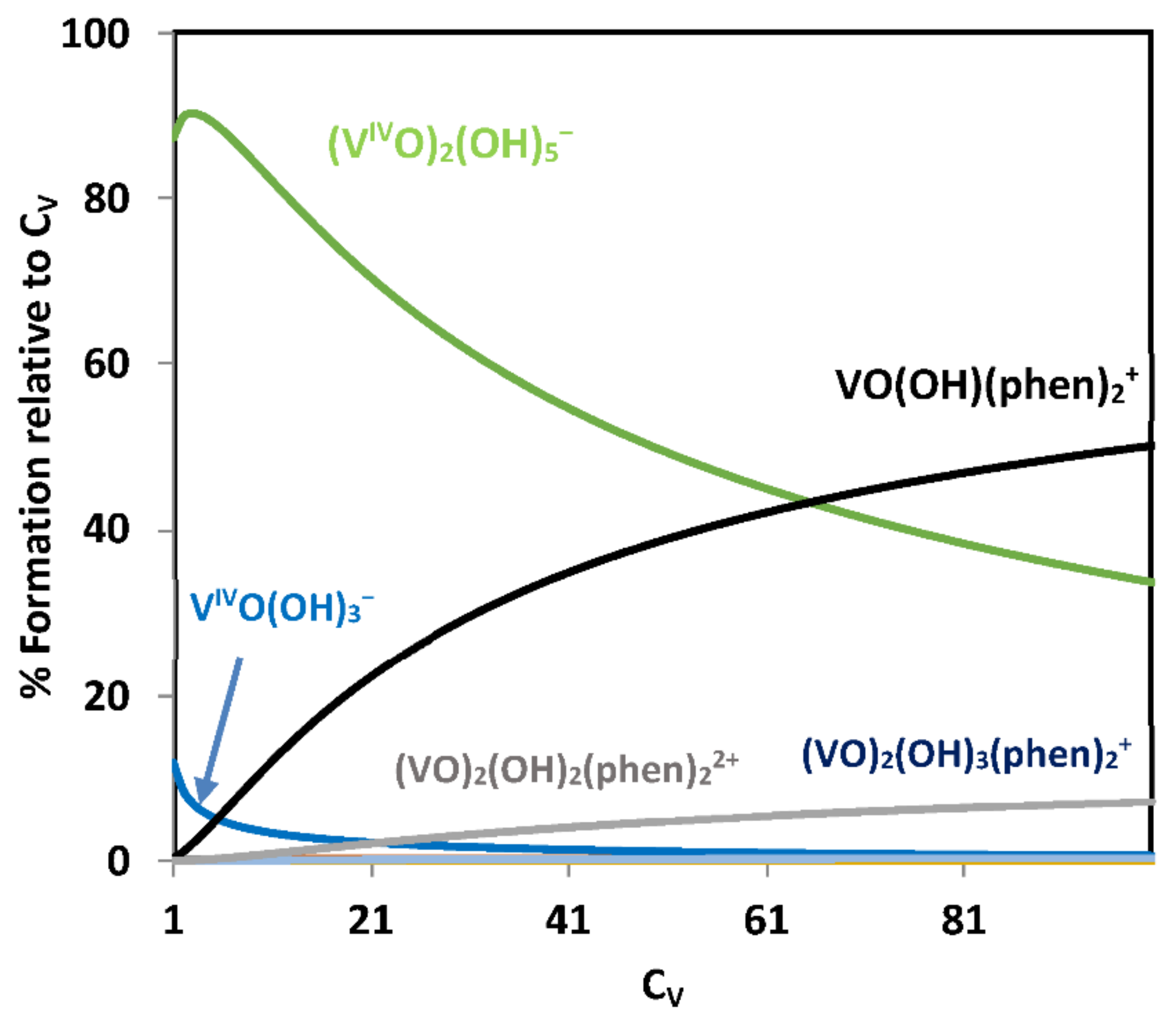
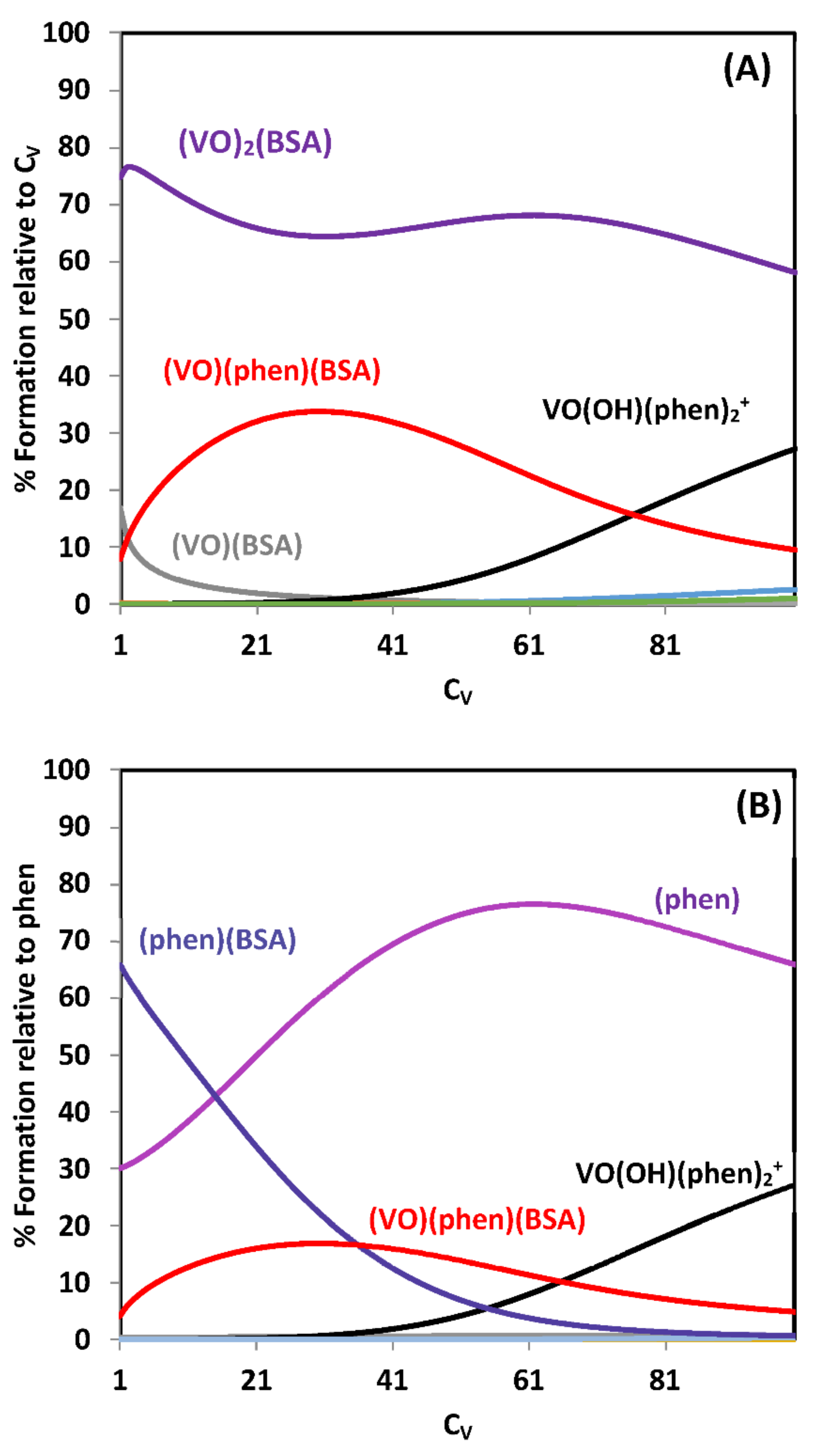
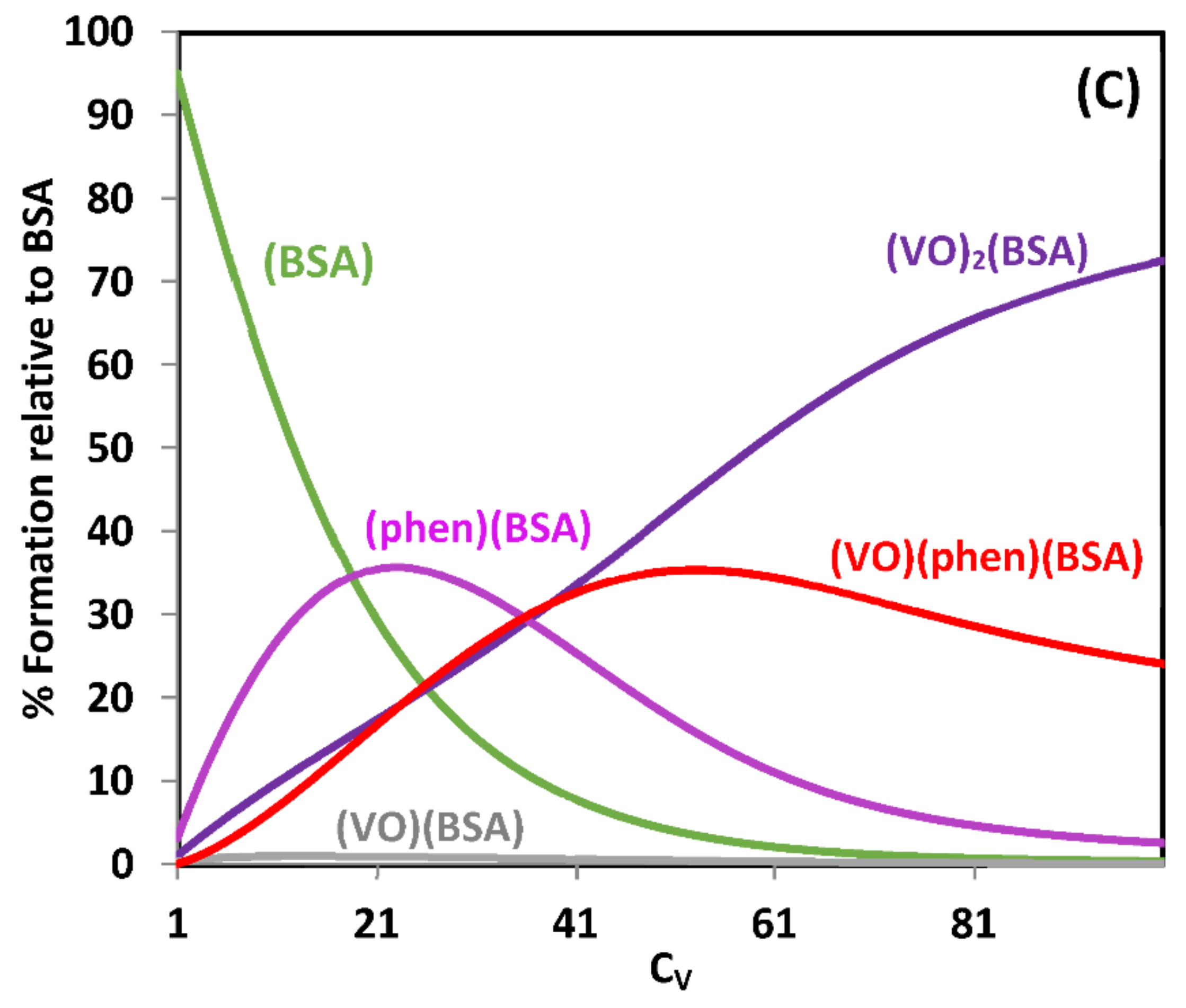
2.3.1. Binding to Proteins; Human Apo-Transferrin and [VIVO(acac)2] as an Example
2.3.2. Binding to DNA
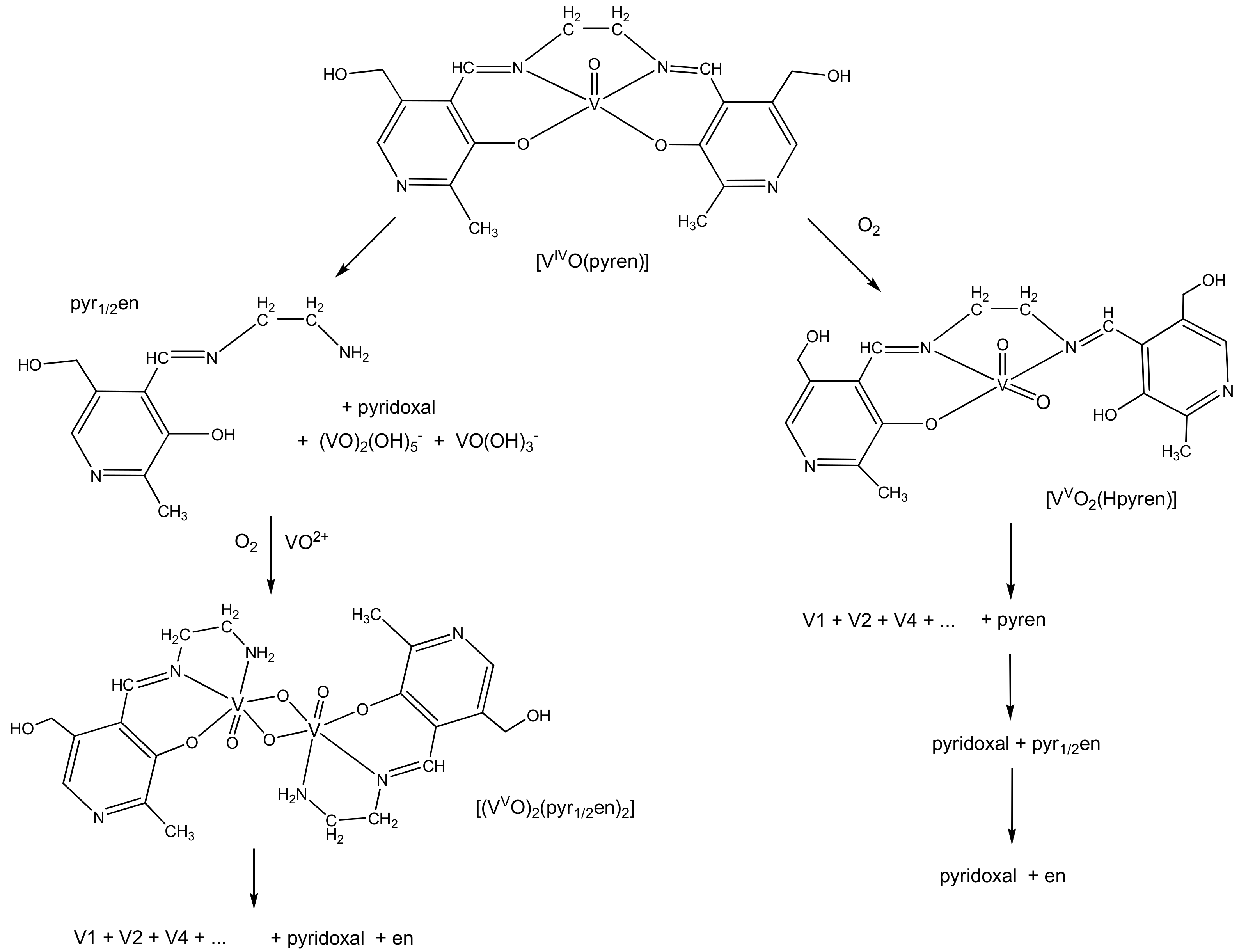
2.4. Behavior of Metal Complexes when Added to Incubation Media of Cells
2.5. Interactions of Metal Complexes with Biological Targets
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acac | acetylacetonate |
| alx | 3-hydroxy-5-methoxy-6-methyl-2-pentyl-4-pyrone |
| Amphen | 5-amino-1,10-phenanthroline |
| ATP | Adenosine triphosphate |
| Bipy | 2,2′-bipyridine |
| BSA | Bovine serum albumin |
| CD | Circular dichroism |
| CV | total vanadium concentration |
| dhp | 1,2-dimethyl-3-hydroxy-4(1H)-pyridinone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| EGTA | ethylene glycol bis(2-aminoethyl ether)tetraacetate |
| EPR | Electronic paramagnetic spectroscopy |
| FBS | Fetal bovine serum |
| HSA | Human serum albumin |
| IC50 | Minimum inhibitory concentration |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| Mal | maltolato |
| MEM | Minimum Essential Medium Eagle |
| Me2phen | 4,7-dimethyl-1,10-phenanthroline |
| MeOH | Methanol |
| NMR | Nuclear magnetic resonance |
| Phen | 1,10-phenanthroline |
| ROS | Reactive oxygen species |
| RPMI medium | Roswell Park Memorial Institute medium |
| SDS | Sodium dodecyl sulfate |
| Tris | Tris(hydroxymethyl)aminomethane |
References
- Williams, R.J.P.; Fraústo da Silva, J.J.R. The Natural Selection of the Chemical Elements—The Environment and Life’s Chemistry; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Williams, R.J.P.; Fraústo da Silva, J.J.R. The Chemistry of Evolution—The Development of our Ecosystem; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Pessoa, J.C.; Garribba, E.; Santos, M.F.A.; Santos-Silva, T. Vanadium and proteins: Uptake, transport, structure, activity and function. Coord. Chem. Rev. 2015, 301, 49–86. [Google Scholar] [CrossRef]
- Willsky, G.R.; Chi, L.H.; Godzala, M., III; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.; Hu, Z.; Crans, D.C. Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 2011, 255, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Trujillo, A.M.; Pharazyn, P.S.; Cohen, M.D. How environment affects drug activity: Localization, compartmentalization and reactions of a vanadium insulin-enhancing compound, dipicolinatooxovanadium (V). Coord. Chem. Rev. 2011, 255, 2178–2192. [Google Scholar] [CrossRef]
- Metelo, A.M.; Pérez-Carro, R.; Castro, M.M.C.A.; López-Larrubia, P. VO(dmpp)2 normalizes pre-diabetic parameters as assessed by in vivo magnetic resonance imaging and spectroscopy. J. Inorg. Biochem. 2012, 115, 44–49. [Google Scholar] [CrossRef]
- Trevino, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.C. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Goncalves, G.; Roy, S.; Correia, I.; Mehtab, S.; Santos, M.F.A.; Santos-Silva, T. New insights on vanadium binding to human serum transferrin. Inorg. Chim. Acta 2014, 420, 60–68. [Google Scholar] [CrossRef]
- Mehtab, S.; Goncalves, G.; Roy, S.; Tomaz, A.I.; Santos-Silva, T.; Santos, M.F.A.; Romão, M.J.; Jakusch, T.; Kiss, T.; Pessoa, J.C. Interaction of vanadium(IV) with human serum apo-transferrin. J. Inorg. Biochem. 2013, 121, 187–195. [Google Scholar] [CrossRef]
- Sanna, D.; Biro, L.; Buglyo, P.; Micera, G.; Garribba, E. Biotransformation of BMOV in the presence of blood serum proteins. Metallomics 2012, 4, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Micera, G.; Garribba, E. New Developments in the Comprehension of the Biotransformation and Transport of Insulin-Enhancing Vanadium Compounds in the Blood Serum. Inorg. Chem. 2010, 49, 174–187. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Tomaz, I. Transport of Therapeutic Vanadium and Ruthenium Complexes by Blood Plasma Components. Curr. Med. Chem. 2010, 17, 3701–3738. [Google Scholar] [CrossRef]
- Jakusch, T.; Hollender, D.; Enyedy, E.A.; Gonzalez, C.S.; Montes-Bayon, M.; Sanz-Medel, A.; Pessoa, J.C.; Tomaz, I.; Kiss, T. Biospeciation of various antidiabetic (VO)-O-IV compounds in serum. Dalton Trans. 2009, 2428–2437. [Google Scholar] [CrossRef]
- Chasteen, N.D.; Grady, J.K.; Holloway, C.E. Characterization of the binding, kinetics, and redox stability of vanadium(IV) and vanadium(V) protein complexes in serum. Inorg. Chem. 1986, 25, 2754–2760. [Google Scholar] [CrossRef]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.E.; McLeod, A.I.; Aitken, J.B.; Levina, A.; Lay, P.A. Vanadium(V) and -(IV) complexes of anionic polysaccharides: Controlled release pharmaceutical formulations and models of vanadium biotransformation products. J. Inorg. Biochem. 2015, 147, 227–234. [Google Scholar] [CrossRef]
- Pettersson, L.; Andersson, I.; Gorzsas, A. Speciation in peroxovanadate systems. Coord. Chem. Rev. 2003, 237, 77–87. [Google Scholar] [CrossRef]
- Elvingson, K.; Gonzalez Baro, A.; Pettersson, L. Speciation in Vanadium Bioinorganic Systems. 2. An NMR, ESR, and Potentiometric Study of the Aqueous H+-Vanadate-Maltol System. Inorg. Chem. 1996, 35, 3388–3393. [Google Scholar] [CrossRef]
- Vilas Boas, L.F.; Pessoa, J.C. Vanadium. In Comprehensive Coordination Chemistry; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; Volume 3, pp. 453–583. [Google Scholar]
- Ducret, L.P. Vanadium Tétravalent. Ann. Chim. S12 Chp. III 1951, 6, 723–737. [Google Scholar]
- Rossotti, F.J.C.; Rossotti, H.S. Studies on the Hydrolysis of Metal Ions. XII. The Hydrolysis of the Vanadium(IV) ion. Acta Chem. Scand. 1955, 9, 1177–1192. [Google Scholar] [CrossRef][Green Version]
- Iannuzzi, M.M.; Kubiak, C.P.; Rieger, P.H. Electron spin resonance line width studies of vanadium(IV) in acidic and basic aqueous solutions. J. Phys. Chem. 1976, 80, 541–545. [Google Scholar] [CrossRef]
- Iannuzzi, M.M.; Rieger, P.H. Nature of vanadium(IV) in basic aqueous solution. Inorg. Chem. 1975, 14, 2895–2899. [Google Scholar] [CrossRef]
- Copenhafer, W.C.; Rieger, P.H. Proton, deuteron, oxygen-17, and electron spin magnetic resonance of the trihydroxovanadyl(IV) ion. Kinetics and relaxation. Inorg. Chem. 1977, 16, 2431–2437. [Google Scholar] [CrossRef]
- Komura, K.; Hayashi, M.; Imanaga, H. Hydrolytic Behavior of Oxovanadium(IV) Ions. Bull. Chem. Soc. Jpn. 1977, 50, 2927–2931. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Boas, L.F.V.; Gillard, R.D.; Lancashire, R.J. Oxovanadium(Iv) and Amino Acids—I. The System L-Alanine+VO2+—A Potentiometric and Spectroscopic Study. Polyhedron 1988, 7, 1245–1262. [Google Scholar] [CrossRef]
- Costa Pessoa, J.; Vilas Boas, L.F.; Gillard, R.D. Oxovanadium (IV) and Aminoacids—II. The systems L-Serine and L-Threonine +VO2+; A Potentiometric and Spectroscopic Study. Polyhedron 1989, 8, 1173–1199. [Google Scholar] [CrossRef]
- Costa Pessoa, J.; Vilas Boas, L.F.; Gillard, R.D. Oxovanadium(IV) and Aminoacids—IV. The system L-Cysteine and D-Penicillamine +VO2+; A Potentiometric and Spectroscopic Study. Polyhedron 1990, 9, 2101–2125. [Google Scholar] [CrossRef]
- Costa Pessoa, J.; Marques, R.L.; Vilas Boas, L.F.; Gillard, R.D. Oxovanadium(IV) and Aminoacids—III. The system L-Aspartic Acid +VO2+; A Potentiometric and Spectroscopic Study. Polyhedron 1990, 9, 81–99. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef]
- Turner, T.L.; Nguyen, V.H.; McLauchlan, C.C.; Dymon, Z.; Dorsey, B.M.; Hooker, J.D.; Jones, M.A. Inhibitory effects of decavanadate on several enzymes and Leishmania tarentolae In Vitro. J. Inorg. Biochem. 2012, 108, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Ohlin, C.A. Decavanadate in vitro and in vivo effects: Facts and opinions. J. Inorg. Biochem. 2014, 137, 123–130. [Google Scholar] [CrossRef]
- Crans, D.C.; Peters, B.J.; Wu, X.; McLauchlan, C.C. Does anion-cation organization in Na+-containing X-ray crystal structures relate to solution interactions in inhomogeneous nanoscale environments: Sodium-decavanadate in solid state materials, minerals, and microemulsions. Coord. Chem. Rev. 2017, 344, 115–130. [Google Scholar] [CrossRef]
- Samart, N.; Althumairy, D.; Zhang, D.; Roess, D.A.; Crans, D.C. Initiation of a novel mode of membrane signaling: Vanadium facilitated signal transduction. Coord. Chem. Rev. 2020, 416, 213286. [Google Scholar] [CrossRef]
- Coutinho, A.A.; Prieto, M. Ribonuclease TI and Alcohol Dehydrogenase Fluorescence Quenching by Acrylamide. J. Chem. Ed. 1993, 70, 425–428. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Van de Weert, M.; Stella, L. Fluorescence quenching and ligand binding: A critical discussion of a popular methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- Galkina, P.A.; Proskurnin, M.A. Supramolecular interaction of transition metal complexes with albumins and DNA: Spectroscopic methods of estimation of binding parameters. Appl. Organomet. Chem. 2018, 32, e4150. [Google Scholar] [CrossRef]
- Correia, I.; Chorna, I.; Cavaco, I.; Roy, S.; Kuznetsov, M.L.; Ribeiro, N.; Justino, G.; Marques, F.; Santos-Silva, T.; Santos, M.F.A.; et al. Interaction of [(VO)-O-IV(acac)(2)] with Human Serum Transferrin and Albumin. Chem. Asian J. 2017, 12, 2062–2084. [Google Scholar] [CrossRef] [PubMed]
- Macii, F.; Biver, T. Spectrofluorimetric analysis of the binding of a target molecule to serum albumin: Tricky aspects and tips. J. Inorg. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Van de Weert, M. Fluorescence Quenching to Study Protein-ligand Binding: Common Errors. J. Fluoresc. 2010, 20, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Khan, A.R.; Mahroof-Tahir, M.; Mondal, S.; Miller, S.M.; la Cour, A.; Anderson, O.P.; Jakusch, T.; Kiss, T. Bis(acetylamido)oxovanadium(IV) complexes: Solid state and solution studies. J. Chem. Soc. Dalton Trans. 2001, 3337–3345. [Google Scholar] [CrossRef]
- Sanna, D.; Buglyo, P.; Micera, G.; Garribba, E. A quantitative study of the biotransformation of insulin-enhancing VO2+ compounds. J. Biol. Inorg. Chem. 2010, 15, 825–839. [Google Scholar] [CrossRef]
- Ivancsits, S.; Pilger, A.; Diem, E.; Schaffer, A.; Rüdiger, H.W. Vanadate induces DNA strand breaks in cultured human fibroblasts at doses relevant to occupational exposure. Mutation Res. 2002, 519, 25–35. [Google Scholar] [CrossRef]
- Desaulniers, D.; Cummings-Lorbetskie, C.; Leingartner, K.; Xiao, G.-H.; Zhou, G.; Parfett, C. Effects of vanadium (sodium metavanadate) and aflatoxin-B1 on cytochrome p450 activities, DNA damage and DNA methylation in human liver cell lines. Toxicol. In Vivo 2021, 70, 105036. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.; Blasiak, J. Vanadyl sulfate can differentially damage DNA in human lymphocytes and HeLa cells. Arch. Toxicol. 2004, 78, 7–15. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, H.; Mao, Y.; Ye, J.; Saffiotti, U. Vanadium(IV)-mediated free radical generation and related 2′-deoxyguanosine hydroxylation and DNA damage. Toxicology 1996, 106, 27–38. [Google Scholar] [CrossRef]
- Sakurai, H.; Nakai, M.; Miki, T.; Tsuchiya, K.; Takada, J.; Matsushit, R. DNA cleavage by hydroxyl radicals generated in a vanadyl ion-hydrogen peroxide system. Biochem. Biophys. Res. Commun. 1992, 189, 1090–1095. [Google Scholar] [CrossRef]
- Patra, D.; Biswas, N.; Kumari, B.; Das, P.; Sepay, N.; Chatterjee, S.; Drew, M.G.B.; Ghosh, T. A family of mixed-ligand oxidovanadium(V) complexes with aroylhydrazone ligands: A combined experimental and computational study on the electronic effects of para substituents of hydrazone ligands on the electronic properties, DNA binding and nuclease activities. RSC Adv. 2015, 5, 92456–92472. [Google Scholar]
- Butenko, N.; Pinheiro, J.P.; Da Silva, J.P.; Tomaz, A.I.; Correia, I.; Ribeiro, V.; Pessoa, J.C.; Cavaco, I. The effect of phosphate on the nuclease activity of vanadium compounds. J. Inorg. Biochem. 2015, 147, 165–176. [Google Scholar] [CrossRef]
- Butenko, N.; Tomaz, A.I.; Nouri, O.; Escribano, E.; Moreno, V.; Gama, S.; Ribeiro, V.; Telo, J.P.; Pessoa, J.C.; Cavaco, I. DNA cleavage activity of (VO)-O-IV(acac)(2) and derivatives. J. Inorg. Biochem. 2009, 103, 622–632. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Patra, A.K.; Nethaji, M.; Chakravarty, A.R. DNA Cleavage by New Oxovanadium(IV) Complexes of N-Salicylidene alfa-Amino Acids and Phenanthroline Bases in the Photodynamic Therapy Window. Inorg. Chem. 2007, 46, 11112–11121. [Google Scholar] [CrossRef]
- Palmajumder, E.; Sepay, N.; Mukherjea, K.K. Development of oxidovanadium and oxido-peroxido vanadium-based artificial DNA nucleases via multi spectroscopic investigations and theoretical simulation of DNA binding. J. Biomol. Struct. Dyn. 2018, 36, 919–927. [Google Scholar] [CrossRef]
- Verquin, G.; Fontaine, G.; Bria, M.; Zhilinskaya, E.Z.; Abi-Aad, E.; Aboukaïs, A.; Baldeyrou, B.; Bailly, C.; Bernier, J.-L. DNA modification by oxovanadium(IV) complexes of salen derivatives. J. Biol. Inorg. Chem. 2004, 9, 345–353. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Patra, A.K.; Chakravarty, A.R. Synthesis, structure, DNA binding and DNA cleavage activity of oxovanadium(IV) N-salicylidene-S-methyldithiocarbazate complexes of phenanthroline bases. J. Inorg. Biochem. 2008, 102, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Pandya, N.; Maity, N.C.; Kumar, M.; Patel, R.M.; Kureshy, R.I.; Abdi, S.H.R.; Mishra, S.; Das, S.; Bajaj, H.C. Influence of chirality of V(V) Schiff base complexes on DNA, BSA binding and cleavage activity. Eur. J. Med. Chem. 2011, 46, 5074–5085. [Google Scholar] [CrossRef]
- Saha, U.; Mukherjea, K.K. DNA binding and nuclease activity of an oxovanadium valinato-Schiff base complex. Intern. J. Biol. Macromol. 2014, 66, 166–171. [Google Scholar] [CrossRef]
- Correia, I.; Roy, S.; Matos, C.P.; Borovic, S.; Butenko, N.; Cavaco, I.; Marques, F.; Lorenzo, J.; Rodriguez, A.; Moreno, V.; et al. Vanadium(IV) and copper(II) complexes of salicylaldimines and aromatic heterocycles: Cytotoxicity: DNA binding and DNA cleavage properties. J. Inorg. Biochem. 2015, 147, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Kwong, D.W.J.; Chan, O.Y.; Wong, R.N.S.; Musser, S.M.; Vaca, L.; Chan, S.I. DNA-Photocleavage Activities of Vanadium(V)−Peroxo Complexes. Inorg. Chem. 1997, 36, 1276–1277. [Google Scholar] [CrossRef]
- Stemmler, A.; Burrows, C. Guanine versus deoxyribose damage in DNA oxidation mediated by vanadium(IV) and vanadium(V) complexes. J. Biol. Inorg. Chem. 2001, 6, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Scalese, G.; Machado, I.; Correia, I.; Pessoa, J.C.; Bilbao, L.; Pérez-Diaz, L.; Gambino, D. Exploring oxidovanadium(IV) homoleptic complexes with 8-hydroxyquinoline derivatives as prospective antitrypanosomal agents. New J. Chem. 2019, 43, 17756–17773. [Google Scholar] [CrossRef]
- Fernández, M.; Varela, J.; Correia, I.; Birriel, E.; Castiglioni, J.; Moreno, V.; Pessoa, J.C.; Cerecetto, H.; González, M.; Gambino, D. A new series of heteroleptic oxidovanadium(IV) compounds with phenanthroline-derived co-ligands: Selective Trypanosoma cruzi growth inhibitors. Dalton Trans. 2013, 42, 11900–11911. [Google Scholar] [CrossRef]
- Fernández, M.; Becco, L.; Correia, I.; Benítez, J.; Piro, O.E.; Echeverria, G.A.; Medeiros, A.; Comini, M.; Lavaggi, M.L.; González, M.; et al. Oxidovanadium(IV) and dioxidovanadium(V) complexes of tridentate salicylaldehyde semicarbazones: Searching for prospective antitrypanosomal agents. J. Inorg. Biochem. 2013, 127, 150–160. [Google Scholar] [CrossRef]
- Machado, I.; Fernández, M.; Becco, L.; Garat, B.; Brissos, R.F.; Zabarska, N.; Gamez, P.; Marques, F.; Correia, I.; Pessoa, J.C.; et al. New metal complexes of NNO tridentate ligands: Effect of metal center and co-ligand on biological activity. Inorg. Chim. Acta 2014, 420, 39–46. [Google Scholar] [CrossRef]
- Benítez, J.; Cavalcanti de Queiroz, A.; Correia, I.; Amaral Alves, M.; Alexandre-Moreira, M.S.; Barreiro, E.J.; Moreira Lima, L.J.; Varela, J.; González, M.H.; Cerecetto, H.H.; et al. New oxovanadium(IV) N-acylhydrazone complexes: Promising antileishmanial and antitrypanosomal agents. Eur. J. Med. Chem. 2013, 62, 20–27. [Google Scholar] [CrossRef]
- Ouameur, A.A.; Arakawa, H.; Tajmir-Riahi, H.A. Binding of oxovanadium ions to the major and minor grooves of DNA duplex: Stability and structural models. Biochem. Cell Biol. 2006, 84, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, A.; Sahoo, S.; Banerjee, S.; Bhattacharyya, A.; Garaia, A.; Karandeb, A.A.; Chakravarty, A.R. Crystal structure, DNA crosslinking and photo-induced cytotoxicity of oxovanadium(IV) conjugates of boron-dipyrromethene. J. Inorg. Biochem. 2020, 202, 110817. [Google Scholar] [CrossRef]
- Kiss, T.; Jakusch, T.; Hollender, D.; Dornyei, A.; Enyedy, E.A.; Pessoa, J.C.; Sakurai, H.; Sanz-Medel, A. Biospeciation of antidiabetic VO(IV) complexes. Coord. Chem. Rev. 2008, 252, 1153–1162. [Google Scholar] [CrossRef]
- Sanna, D.; Garribba, E.; Micera, G. Interaction of VO2+ ion with human serum transferrin and albumin. J. Inorg. Biochem. 2009, 103, 648–655. [Google Scholar] [CrossRef]
- Jakusch, T.; Pessoa, J.C.; Kiss, T. The speciation of vanadium in human serum. Coord. Chem. Rev. 2011, 255, 2218–2226. [Google Scholar] [CrossRef]
- Sanna, D.; Bíró, L.; Buglyó, P.; Micera, G.; Garribba, E. Transport of the anti-diabetic VO2+ complexes formed by pyrone derivatives in the blood serum. J. Inorg. Biochem. 2012, 115, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D. Potentiality of vanadium compounds as antiparasitic agents. Coord. Chem. Rev. 2011, 255, 2193–2203. [Google Scholar] [CrossRef]
- Benítez, J.; Correia, I.; Becco, L.; Fernández, M.; Garat, B.; Gallardo, H.; Conte, G.; Kuznetsov, M.L.; Neves, A.; Moreno, V.; et al. Searching for vanadium-based prospective agents against Trypanosoma cruzi: Oxidovanadium(IV) compounds with phenanthroline derivatives as ligands. Z. Anorg. Allg. Chem. 2013, 639, 1417–1425. [Google Scholar] [CrossRef]
- Rehder, D. Perspectives for vanadium in health issues. Future Med. Chem. 2016, 8, 325–338. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301, 87–105. [Google Scholar] [CrossRef]
- Benitez, J.; Guggeri, L.; Tomaz, I.; Pessoa, J.C.; Moreno, V.; Lorenzo, J.; Aviles, F.X.; Garat, B.; Gambino, D. A novel vanadyl complex with a polypyridyl DNA intercalator as ligand: A potential anti-protozoa and anti-tumor agent. J. Inorg. Biochem. 2009, 103, 1386–1394. [Google Scholar] [CrossRef]
- Benitez, J.; Becco, L.; Correia, I.; Leal, S.M.; Guiset, H.; Pessoa, J.C.; Lorenzo, J.; Tanco, S.; Escobar, P.; Moreno, V.; et al. Vanadium polypyridyl compounds as potential antiparasitic and antitumoral agents: New achievements. J. Inorg. Biochem. 2011, 105, 303–312. [Google Scholar] [CrossRef]
- Sakurai, H.; Tamura, H.; Okatami, K. Mechanism for a new antitumor vanadium complex hydroxyl radical-dependent DNA-cleavage by 1,10-phenanthroline-vanadyl complex in the presence of hydrogen peroxide. Biochem. Biophys. Res. Commun. 1995, 206, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Narla, R.K.; Dong, Y.; D’Cruz, O.J.; Navara, C.; Uckun, F.M. Bis(4,7-dimethyl-1,10-phenanthroline) Sulfatooxovanadium(IV) as a Novel Apoptosis-inducing Anticancer Agent. Clin. Cancer Res. 2000, 6, 1546–1556. [Google Scholar]
- Rehder, D. Implications of vanadium in technical applications and pharmaceutical issues. Inorg. Chim. Acta 2017, 455, 378–389. [Google Scholar] [CrossRef]
- Benitez, J.; Guggeri, L.; Tomaz, I.; Arrambide, G.; Navarro, M.; Pessoa, J.C.; Garat, B.; Garnbino, D. Design of vanadium mixed-ligand complexes as potential anti-protozoa agents. J. Inorg. Biochem. 2009, 103, 609–616. [Google Scholar] [CrossRef]
- Maurya, M.R.; Khan, A.A.; Azam, A.; Kumar, A.; Ranjan, S.; Mondal, N.; Pessoa, J.C. Dinuclear Oxidovanadium(IV) and Dioxidovanadium(V) Complexes of 5,5 ’-Methylenebis(dibasic tridentate) Ligands: Synthesis, Spectral Characterisation, Reactivity, and Catalytic and Antiamoebic Activities. Eur. J. Inorg. Chem. 2009, 2009, 5377–5390. [Google Scholar] [CrossRef]
- Maurya, M.R.; Khan, A.A.; Azam, A.; Ranjan, S.; Mondal, N.; Kumar, A.; Avecilla, F.; Pessoa, J.C. Vanadium complexes having [(VO)-O-IV](2+) and [(VO2)-O-V](+) cores with binucleating dibasic tetradentate ligands: Synthesis, characterization, catalytic and antiamoebic activities. Dalton Trans. 2010, 39, 1345–1360. [Google Scholar] [CrossRef]
- Leon, I.E.; Porro, V.; Di Virgilio, A.L.; Naso, L.G.; Williams, P.A.M.; Bollati-Fogolin, M.; Etcheverry, S.B. Antiproliferative and apoptosis-inducing activity of an oxidovanadium(IV) complex with the flavonoid silibinin against osteosarcoma cells. J. Biol. Inorg. Chem. 2014, 19, 59–74. [Google Scholar] [CrossRef]
- Leon, I.E.; Di Virgilio, A.L.; Porro, V.; Muglia, C.I.; Naso, L.G.; Williams, P.A.M.; Bollati-Fogolin, M.; Etcheverry, S.B. Antitumor properties of a vanadyl(IV) complex with the flavonoid chrysin [VO(chrysin)(2)EtOH](2) in a human osteosarcoma model: The role of oxidative stress and apoptosis. Dalton Trans. 2013, 42, 11868–11880. [Google Scholar] [CrossRef]
- Tasiopoulos, A.J.; Tolis, E.J.; Tsangaris, J.M.; Evangelou, A.; Woollins, J.D.; Slawin, A.M.; Pessoa, J.C.; Correia, I.; Kabanos, T.A. Model investigations for vanadium-protein interactions: Vanadium(III) compounds with dipeptides and their oxovanadium(IV) analogues. J. Biol. Inorg. Chem. 2002, 7, 363–374. [Google Scholar] [CrossRef]
- Dong, Y.H.; Narla, R.K.; Sudbeck, E.; Uckun, F.M. Synthesis, X-ray structure, and anti-leukemic activity of oxovanadium(IV) complexes. J. Inorg. Biochem. 2000, 78, 321–330. [Google Scholar] [CrossRef]
- Scalese, G.; Correia, I.; Benítez, J.; Rostán, S.; Marques, F.; Mendes, F.; Matos, A.P.; Pessoa, J.C.; Gambino, D. Evaluation of cellular uptake, cytotoxicity and cellular ultrastructural effects of heteroleptic oxidovanadium(IV) complexes of salicylaldimines and polypyridyl ligands. J. Inorg. Biochem. 2017, 166, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Narla, R.K.; Chen, C.L.; Dong, Y.H.; Uckun, F.M. In vivo antitumor activity of bis(4,7-dimethyl-1,10-phenanthroline) sulfatooxovanadium(IV) {METVAN [VO(SO4)(Me-2-Phen)(2)]}. Clin. Cancer Res. 2001, 7, 2124–2133. [Google Scholar] [PubMed]
- Narla, R.K.; Dong, Y.H.; Uckun, F.M. Apoptosis inducing novel anti-leukemic agent, bis(4,7-dimethyl-1,10 phenanthroline) sulfatooxovanadium(IV) [VO(SO4)(Me2-Phen)(2)] depolarizes mitochondrial membranes. Leuk. Lymphoma 2001, 41, 625. [Google Scholar] [CrossRef]
- Sanna, D.; Buglyo, P.; Tomaz, A.I.; Pessoa, J.C.; Borovic, S.; Micera, G.; Garribba, E. (VO)-O-IV and Cu-II complexation by ligands based on pyridine nitrogen donors. Dalton Trans. 2012, 41, 12824–12838. [Google Scholar] [CrossRef]
- Le, M.; Rathje, O.; Levina, A.; Lay, P.A. High cytotoxicity of vanadium(IV) complexes with 1,10-phenanthroline and related ligands is due to decomposition in cell culture medium. J. Biol. Inorg. Chem. 2017, 22, 663–672. [Google Scholar] [CrossRef]
- Nunes, P.; Correia, I.; Cavaco, I.; Marques, F.; Pinheiro, T.; Avecilla, F.; Costa Pessoa, J. Therapeutic potential of vanadium complexes with 1,10-phenanthroline ligands, quo vadis? Fate of complexes in cell media and cancer cells. J. Inorg. Biochem. 2021, 217, 111350. [Google Scholar] [CrossRef]
- Coyle, B.; Kavanagh, K.; McCann, M.; Devereux, M.; Geraghty, M. Mode of anti-fungal activity of 1,10-phenanthroline and its Cu(II), Mn(II) and Ag(I) complexes. Biometals 2003, 16, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Deegan, C.; Coyle, B.; McCann, M.; Devereux, M.; Egan, D.A. In Vitro anti-tumour effect of 1,10-phenanthroline-5,6-dione (phendione), [Cu(phendione)3](ClO4)2·4H2O and [Ag(phendione)2]ClO4 using human epithelial cell lines. Chem. Biol. Interact. 2006, 164, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Kellett, A.; O’Connor, M.; McCann, M.; Howe, O.; Casey, A.; McCarron, P.; Kavanagh, K.; McNamara, M.; Kennedy, S.; May, D.D.; et al. Water-soluble bis(1,10-phenanthroline) octanedioate Cu2+ and Mn2+ complexes with unprecedented nano and picomolar in vitro cytotoxicity: Promising leads for chemotherapeutic drug development. MedChemComm 2011, 2, 579–584. [Google Scholar] [CrossRef]
- McCann, M.; Santos, A.L.S.; da Silva, B.A.; Romanos, M.T.V.; Pyrrho, A.S.; Devereux, M.; Kavanagh, K.; Fichtner, I.; Kellett, A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(II) and silver(I) complexes. Toxicol. Res. UK 2012, 1, 47–54. [Google Scholar] [CrossRef]
- Stavrianopoulos, J.G.; Karkas, J.D.; Chargaff, E. DNA Polymerase of Chicken Embryo: Purification and Properties. Proc. Nat. Acad. Sci. USA 1972, 69, 1781–1785. [Google Scholar] [CrossRef]
- Falchuk, K.H.; Krishan, A. 1,10-Phenanthroline Inhibition of Lymphoblast Cell Cycle. Cancer Res. 1977, 37, 2050–2056. [Google Scholar] [PubMed]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Advanced DMEM (Dulbecco’s Modified Eagle’s Medium); Catalog Number 12491023; Thermo Fisher Scientific: Waltham, MA, USA, 2017.
- Sanna, D.; Ugone, V.; Serra, M.; Garribba, E. Speciation of potential anti-diabetic vanadium complexes in real serum samples. J. Inorg. Biochem. 2017, 173, 52–65. [Google Scholar] [CrossRef]
- Sanna, D.; Ugone, V.; Micera, G.; Buglyo, P.; Biro, L.; Garribba, E. Speciation in human blood of Metvan, a vanadium based potential anti-tumor drug. Dalton Trans. 2017, 46, 8950–8967. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Serra, M.; Micera, G.; Garribba, E. Interaction of Antidiabetic Vanadium Compounds with Hemoglobin and Red Blood Cells and Their Distribution between Plasma and Erythrocytes. Inorg. Chem. 2014, 53, 1449–1464. [Google Scholar] [CrossRef]
- Sanna, D.; Micera, G.; Garribba, E. Interaction of Insulin-Enhancing Vanadium Compounds with Human Serum holo-Transferrin. Inorg. Chem. 2013, 52, 11975–11985. [Google Scholar] [CrossRef]
- Levina, A.; McLeod, A.I.; Gasparini, S.J.; Nguyen, A.; De Silva, W.G.M.; Aitken, J.B.; Harris, H.H.; Glover, C.; Johannessen, B.; Lay, P.A. Reactivity and Speciation of Anti-Diabetic Vanadium Complexes in Whole Blood and Its Components: The Important Role of Red Blood Cells. Inorg. Chem. 2015, 54, 7753–7766. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, G.; Sanna, D.; Lubinu, G.; Maréchal, J.-D.; Garribba, E. Unveiling VIVO2+ binding modes to human serum albumins by an integrated spectroscopic-computational approach. Chem. Eur. J. 2020. [Google Scholar] [CrossRef]
- Cobbina, E.; Mehtab, S.; Correia, I.; Gonçalves, G.; Tomaz, I.; Cavaco, I.; Jakusch, T.; Enyedi, E.; Kiss, T.; Pessoa, J.C. Binding of Oxovanadium(IV) Complexes to Blood Serum Albumins. J. Mex. Chem. Soc. 2013, 57, 180–191. [Google Scholar] [CrossRef]
- Reytman, L.; Hochman, J.; Tshuva, E.Y. Anticancer diaminotris(phenolato) vanadium(V) complexes: Ligand-metal interplay. J. Coord. Chem. 2018, 71, 2003–2011. [Google Scholar] [CrossRef]
- Reytman, L.; Braitbard, O.; Hochman, J.; Tshuva, E.Y. Highly Effective and Hydrolytically Stable Vanadium(V) Amino Phenolato Antitumor Agents. Inorg. Chem. 2016, 55, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Mahroof-Tahir, M.; Brezina, D.; Fatima, N.; Choudhary, M.I. Atta-ur-Rahman Synthesis and characterization of mononuclear oxovanadium(IV) complexes and their enzyme inhibition studies with a carbohydrate metabolic enzyme, phosphodiesterase I. J. Inorg. Biochem. 2005, 99, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Hiromura, M.; Nakayama, A.; Adachi, Y.; Doi, M.; Sakurai, H. Action mechanism of bis(allixinato)oxovanadium(IV) as a novel potent insulin-mimetic complex: Regulation of GLUT4 translocation and FoxO1 transcription factor. J. Biol. Inorg. Chem. 2007, 12, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Pessoa, J.C.; Duarte, M.T.; Henriques, R.T.; Piedade, M.F.M.; Veiros, L.F.; Jakusch, T.; Kiss, T.; Dörnyei, A.; Castro, M.M.C.A.; et al. N,N’-ethylenebis(pyridoxylideneiminato) and N,N’-ethylenebis(pyridoxylaminato): Synthesis, characterisation, potentiometric, spectroscopic and DFT study of their vanadium(IV) and vanadium(V) complexes. Chem. Eur. J. 2004, 10, 2301–2317. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Basile, A.; Mazzone, A.; Morello, S.; Turco, M.C.; Pellecchia, C. Therapeutic Potential of a Pyridoxal-Based Vanadium(IV) Complex Showing Selective Cytotoxicity for Cancer Versus Healthy Cells. J. Cell Physiol. 2013, 228, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessoa, J.C.; Correia, I. Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets. Inorganics 2021, 9, 17. https://doi.org/10.3390/inorganics9020017
Pessoa JC, Correia I. Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets. Inorganics. 2021; 9(2):17. https://doi.org/10.3390/inorganics9020017
Chicago/Turabian StylePessoa, João Costa, and Isabel Correia. 2021. "Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets" Inorganics 9, no. 2: 17. https://doi.org/10.3390/inorganics9020017
APA StylePessoa, J. C., & Correia, I. (2021). Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets. Inorganics, 9(2), 17. https://doi.org/10.3390/inorganics9020017







