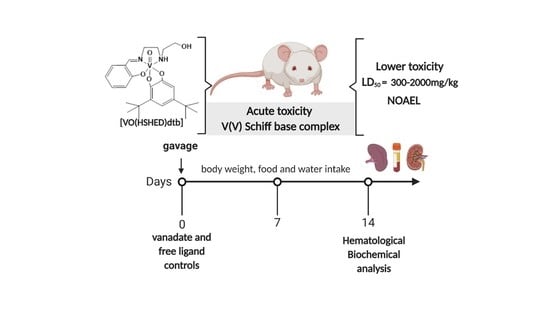
Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex
Abstract
:1. Introduction
2. Results
2.1. Synthesis of [VO(HSHED)dtb] Complex
2.2. Stability of [VO(HSHED)dtb] Complex
2.3. Acute Toxicity in Mice
2.4. Clinical Observations
2.5. Hematology and Biochemical Analysis
3. Discussion
4. Material and Methods
4.1. Reagents and Chemical Analysis
4.2. Synthesis of [VO2(HSHED)] Complex
4.3. Synthesis of [VO(HSHED)dtb] Complex
4.4. Stability of [VO(HSHED)dtb] Complex
4.5. Animals
4.6. Acute Toxicity in Mice
4.7. Biochemical Analysis: Determination of Serum Biomarkers for Liver and Kidney Functions
4.8. Hematology Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADME | Absorption, Distribution, Metabolism and Excretion |
| ALB | albumin |
| ALT | alanine aminotransferase |
| Alx | allixinate |
| ANOVA | one-way analysis of variance |
| AST | aspartate aminotransferase |
| BMOV | bis(maltolato)oxidovanadium(IV) |
| BUN | blood urea nitrogen |
| CRE | creatinine |
| Dipic | dipicolinate |
| Dtb | 3,5-di(t-butyl)catechol |
| EDTA | ethylenediamine tetraacetic acid |
| GHS | Globally Harmonized System |
| GLB | globulin |
| Hb | hemoglobin |
| HCT | hematocrit |
| HPLC | high performance liquid chromatography |
| i.p | intraperitoneal administration |
| LD50 | median lethal dose |
| LIKA | Laboratory of the immunopathology of Keizo Asami |
| L-Pheol-im | L-phenylalaninol |
| LYM | lymphocytes |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| NMR | nuclear magnetic ressonance |
| NOAEL | no-observed adverse-effect level |
| O.G | oral gavage |
| OECD | Organization for Economic Cooperation and Development |
| RBC | red blood cells |
| Rpm | rotation per minute |
| SD | standard deviation |
| STZ | streptozotocin |
| TP | total protein |
| UV-Vis | Ultraviolet-visible spectroscopy |
| WBC | white blood cells |
| 8HQ | hydroxyquinoline |
References
- Correia, I.; Adao, P.; Roy, S.; Wahba, M.; Matos, C.; Maurya, M.R.; Marques, F.; Pavan, F.R.; Leite, C.Q.F.; Avecilla, F.; et al. Hydroxyquinoline Derived Vanadium (IV and V) and Copper (II) Complexes as Potential Anti-Tuberculosis and Anti-Tumor Agents. J. Inorg. Biochem. 2014, 141, 83–93. [Google Scholar]
- Crans, D.C. Antidiabetic, Chemical, and Physical Properties of Organic Vanadates as Presumed Transition-State Inhibitors for Phosphatases. J. Org. Chem. 2015, 80, 11899–11915. [Google Scholar]
- McLauchlan, C.C.; Peters, B.J.; Willsky, G.R.; Crans, D.C. Vanadium–Phosphatase Complexes: Phosphatase Inhibitors Favor the Trigonal Bipyramidal Transition State Geometries. Coord. Chem. Rev. 2015, 301, 163–199. [Google Scholar]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium Compounds in Medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar]
- Thompson, K.H.; Liboiron, B.D.; Sun, Y.; Bellman, K.D.; Setyawati, I.A.; Patrick, B.O.; Karunaratne, V.; Rawji, G.; Wheeler, J.; Sutton, K.; et al. Preparation and Characterization of Vanadyl Complexes with Bidentate Maltol-Type Ligands; in vivo Comparisons of Anti-Diabetic Therapeutic Potential. J. Biol. Inorg. Chem. 2003, 8, 66–74. [Google Scholar]
- Wei, Y.B.; Yang, X.D. Synthesis, Characterization and Anti-Diabetic Therapeutic Potential of a New Benzyl Acid-Derivatized Kojic Acid Vanadyl Complex. BioMetals 2012, 25, 1261–1268. [Google Scholar]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The Chemistry and Biology of Vanadium Compounds in Cancer Therapeutics. Coord. Chem. Rev. 2015, 301, 87–105. [Google Scholar]
- Heyliger, C.E.; Tahiliani, A.G.; McNeill, J.H. Effect of Vanadate on Elevated Glucose and Depressed Cardiac Performance of Diabetic Rats. Science 1985, 227, 1474–1477. [Google Scholar]
- Thompson, K.H.; Orvig, C. Vanadium in Diabetes: 100 Years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar]
- Crans, D.C.; Henry, L.R.; Cardiff, G.; Posner, B.I. Developing Vanadium as an Antidiabetic or Anticancer Drug: A Clinical and Historical Perspective. Met. Ions Life Sci. 2019, 19, 203–230. [Google Scholar]
- Willsky, G.R.; Goldfine, A.B.; Kostyniak, P.J.; McNeill, J.H.; Yang, L.Q.; Khan, H.R.; Crans, D.C. Effect of Vanadium (IV) Compounds in the Treatment of Diabetes: In vivo and in vitro Studies with Vanadyl Sulfate and Bis(Maltolato)Oxovandium(IV). J. Inorg. Biochem. 2001, 85, 33–42. [Google Scholar]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar]
- Tolman, E.L.; Barris, E.; Burns, M.; Pansini, A.; Partridge, R. Effects of Vanadium on Glucose Metabolism in vitro. Life Sci. 1979, 25, 1159–1164. [Google Scholar]
- Crans, D.C.; Gambino, D.; Etcheverry, S.B. Vanadium Science: Chemistry, Catalysis, Materials, Biological and Medicinal Studies. New J. Chem. 2019, 43, 17535–17537. [Google Scholar]
- Ramanadham, S.; Mongold, J.J.; Brownsey, R.W.; Cros, G.H.; McNeill, J.H. Oral Vanadyl Sulfate in Treatment of Diabetes Mellitus in Rats. Am. J. Physiol. Circ. Physiol. 1989, 257, 904–911. [Google Scholar]
- Cam, M.C.; Pederson, R.A.; Brownsey, R.W.; McNeill, J.H. Long-Term Effectiveness of Oral Vanadyl Sulphate in Streptozotocin-Diabetic Rats. Diabetologia 1993, 36, 218–224. [Google Scholar]
- León, I.E.; Cadavid-Vargas, J.F.; Tiscornia, I.; Porro, V.; Castelli, S.; Katkar, P.; Desideri, A.; Bollati-Fogolin, M.; Etcheverry, S.B. Oxidovanadium(IV) Complexes with Chrysin and Silibinin: Anticancer Activity and Mechanisms of Action in a Human Colon Adenocarcinoma Model. J. Biol. Inorg. Chem. 2015, 20, 1175–1191. [Google Scholar]
- Selman, M.; Rousso, C.; Bergeron, A.; Son, H.H.; Krishnan, R.; El-sayes, N.A.; Varette, O.; Chen, A.; Le Boeuf, F.; Tzelepis, F.; et al. Multi-Modal Potentiation of Oncolytic Virotherapy by Vanadium Compounds. Mol. Ther. 2018, 26, 56–69. [Google Scholar]
- Levina, A.; Pires Vieira, A.; Wijetunga, A.; Kaur, R.; Koehn, J.T.; Crans, D.C.; Lay, P.A. A Short-Lived but Highly Cytotoxic Vanadium(V) Complex as a Potential Drug Lead for Brain Cancer Treatment by Intratumoral Injections. Angew. Chemie Int. Ed. 2020, 59, 15834–15838. [Google Scholar]
- Hiromura, M.; Adachi, Y.; Machida, M.; Hattori, M.; Sakurai, H. Glucose Lowering Activity by Oral Administration of Bis (Allixinato)Oxidovanadium (Iv) Complex in Streptozotocin-Induced Diabetic Mice and Gene Expression Profiling in Their Skeletal Muscles. Metallomics 2009, 1, 92–100. [Google Scholar]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium Treatment of Type 2 Diabetes: A View to the Future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar]
- Crans, D.C. Chemistry and Insulin-like Properties of Vanadium (IV) and Vanadium (V) Compounds. J. Inorg. Biochem. 2000, 80, 123–131. [Google Scholar]
- Boden, G.; Chen, X.; Ruiz, J.; Van Rossum, G.D.V.; Turco, S. Effects of Vanadyl Sulfate on Carbohydrate and Lipid Metabolism in Patients with Non-Insulin-Dependent Diabetes Mellitus. Metab. Clin. Exp. 1996, 45, 1130–1135. [Google Scholar]
- Willsky, G.R.; Chi, L.H.; Godzala, M.; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.; Hu, Z.; Crans, D.C. Anti-Diabetic Effects of a Series of Vanadium Dipicolinate Complexes in Rats with Streptozotocin-Induced Diabetes. Coord. Chem. Rev. 2011, 255, 2258–2269. [Google Scholar]
- Koyuturk, M.; Tunali, S.; Bolkent, S.; Yanardag, R. Effects of Vanadyl Sulfate on Liver of Streptozotocin-Induced Diabetic Rats. Biol. Trace Elem. Res. 2005, 104, 233–247. [Google Scholar]
- Sakurai, H.; Tsuchiya, K.; Nukatsuka, M.; Kawada, J.; Ishikawa, S.; Yoshida, H.; Komatsu, M. Insulin-Mimetic Action of Vanadyl Complexes. J. Clin. Biochem. Nutr. 1990, 8, 193–200. [Google Scholar]
- Crans, D.; Trujillo, A.; Pharazyn, P.; Cohen, M. How Environment Affects Drug Activity: Localization, Compartmentalization and Reactions of a Vanadium Insulin-Enhancing Compound, Dipicolinatooxovanadium(V). Coord. Chem. Rev. 2011, 255, 2178–2192. [Google Scholar]
- Bergeron, A.; Kostenkova, K.; Selman, M.; Murakami, H.; Owens, E.; Haribabu, N.; Arulanandam, R.; Diallo, J.S.; Crans, D. Enhancement of Oncolytic Virotherapy by Vanadium(V) Dipicolinates. BioMetals 2019, 32, 545–561. [Google Scholar]
- Chinedu, E.; David, A.; Fidelis, S. A New Method for Determining Acute Toxicity in Animal Models. Toxicol. Int. 2013, 20, 224–226. [Google Scholar]
- Erhirhie, E.O.; Ihekwereme, C.P.; Ilodigwe, E.E. Advances in Acute Toxicity Testing: Strengths, Weaknesses and Regulatory Acceptance. Interdiscip. Toxicol. 2018, 11, 5–12. [Google Scholar]
- Alhaji Saganuwan, S. Toxicity Studies of Drugs and Chemicals in Animals: An Overview. Bulg. J. Vet. Med. 2017, 20, 291–318. [Google Scholar]
- Llobet, J.M.; Domingo, J.L. Acute Toxicity of Vanadium Compounds in Rats and Mice. Toxicol. Lett. 1984, 23, 227–231. [Google Scholar]
- Mongold, J.J.; Cros, G.H.; Vian, L.; Tep, A.; Ramanadham, S.; Siou, G.; Diaz, J.; McNeill, J.H.; Serrano, J.J. Toxicological Aspects of Vanadyl Sulphate on Diabetic Rats: Effects on Vanadium Levels and Pancreatic B-Cell Morphology. Pharmacol. Toxicol. 1990, 67, 192–198. [Google Scholar]
- Domingo, J.L.; Gomez, M.; Sanchez, D.J.; Llobet, J.M.; Keen, C.L. Toxicology of Vanadium Compounds in Diabetic Rats: The Action of Chelating Agents on Vanadium Accumulation. Mol. Cell. Biochem. 1995, 153, 233–240. [Google Scholar]
- Doucette, K.A.; Hassell, K.N.; Crans, D.C. Selective Speciation Improves Efficacy and Lowers Toxicity of Platinum Anticancer and Vanadium Antidiabetic Drugs. J. Inorg. Biochem. 2016, 165, 56–70. [Google Scholar]
- Naso, L.; Ferrer, E.G.; Lezama, L.; Rojo, T.; Etcheverry, S.B.; Williams, P. Role of Oxidative Stress in the Antitumoral Action of a New Vanadyl(IV) Complex with the Flavonoid Chrysin in Two Osteoblast Cell Lines: Relationship with the Radical Scavenger Activity. J. Biol. Inorg. Chem. 2010, 15, 889–902. [Google Scholar]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar]
- Yilmaz-Ozden, T.; Kurt-Sirin, O.; Tunali, S.; Akev, N.; Can, A.; Yanardag, R. Ameliorative Effect of Vanadium on Oxidative Stress in Stomach Tissue of Diabetic Rats. Bosn. J. Basic Med. Sci. 2014, 14, 105–109. [Google Scholar]
- Cortizo, A.M.; Bruzzone, L.; Molinuevo, S.; Etcheverry, S.B. A Possible Role of Oxidative Stress in the Vanadium-Induced Cytotoxicity in the MC3T3E1 Osteoblast and UMR106 Osteosarcoma Cell Lines. Toxicology 2000, 147, 89–99. [Google Scholar]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stępniak, I. Molecular and Cellular Mechanisms of Cytotoxic Activity of Vanadium Compounds against Cancer Cells. Molecules 2020, 25, 1757. [Google Scholar]
- Rojas-Lemus, M.; Patricia, B.N.; Nelly, L.V.; Gonzalez-Villalva, A.; Gabriela, G.P.; Eugenia, C.V.; Otto, T.C.; Norma, R.F.; Brenda, C.T.; Martha, U.C.; et al. Oxidative Stress and Vanadium. In Antimutagens–Mechanisms of DNA Protection; Intech Open: London, UK, 2020. [Google Scholar]
- Irving, E.; Stoker, A.W. Vanadium Compounds as PTP Inhibitors. Molecules 2017, 22, 2269. [Google Scholar]
- Saibu, M.; Sagar, S.; Green, I.; Ameer, F.; Meyer, M. Evaluating the Cytotoxic Effects of Novel Quinone Compounds. Anticancer Res. 2014, 34, 4077–4086. [Google Scholar]
- Sanna, D.; Ugone, V.; Fadda, A.; Micera, G.; Garribba, E. Behavior of the Potential Antitumor VIVO Complexes Formed by Flavonoid Ligands. 3. Antioxidant Properties and Radical Production Capability. J. Inorg. Biochem. 2016, 161, 18–26. [Google Scholar]
- Dankhoff, K.; Ahmad, A.; Weber, B.; Biersack, B.; Schobert, R. Anticancer Properties of a New Non-Oxido Vanadium (IV) Complex with a Catechol-Modified 3,3′-Diindolylmethane Ligand. J. Inorg. Biochem. 2019, 194, 1–6. [Google Scholar]
- Li, M.; Wei, D.; Ding, W.; Baruah, B.; Crans, D.C. Anti-Diabetic Effects of Cesium Aqua (N,N′-Ethylene(Salicylideneiminato)-5-Sulfonato) Oxovanadium (IV) Dihydrate in Streptozotocin-Induced Diabetic Rats. Biol. Trace Elem. Res. 2008, 121, 226–232. [Google Scholar]
- Crans, D.C.; Mahroof-Tahir, M.; Johnson, M.D.; Wilkins, P.C.; Yang, L.; Robbins, K.; Johnson, A.; Alfano, J.A.; Godzala, M.E.; Austin, L.T.; et al. Vanadium (IV) and Vanadium (V) Complexes of Dipicolinic Acid and Derivatives. Synthesis, X-Ray Structure, Solution State Properties and Effects in Rats with STZ-Induced Diabetes. Inorg. Chim. Acta 2003, 356, 365–378. [Google Scholar]
- Tadele, K.T.; Tsega, T.W. Schiff Bases and Their Metal Complexes as Potential Anticancer Candidates: A Review of Recent Works. Anti Cancer Agents Med. Chem. 2019, 19, 1786–1795. [Google Scholar]
- Kowalski, S.; Wyrzykowski, D.; Hac, S.; Rychlowski, M.; Radomski, M.W.; Inkielewicz-Stepniak, I. New Oxidovanadium(IV) Coordination Complex Containing 2-Methylnitrilotriacetate Ligands Induces Cell Cycle Arrest and Autophagy in Human Pancreatic Ductal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2019, 20, 261. [Google Scholar]
- Lewis, N.A.; Liu, F.; Seymour, L.; Magnusen, A.; Erves, T.R.; Arca, J.F.; Beckford, F.A.; Venkatraman, R.; González-Sarrías, A.; Fronczek, F.R.; et al. Synthesis, Characterization, and Preliminary in vitro Studies of Vanadium(IV) Complexes with a Schiff Base and Thiosemicarbazones as Mixed-Ligands. Eur. J. Inorg. Chem. 2012, 2012, 664–677. [Google Scholar]
- Leon, I.E.; Cadavid-Vargas, J.F.; Di Virgilio, A.L.; Etcheverry, S.B. Vanadium, Ruthenium and Copper Compounds: A New Class of Nonplatinum Metallodrugs with Anticancer Activity. Curr. Med. Chem. 2017, 24, 112–148. [Google Scholar]
- Leon, I.E.; Díez, P.; Etcheverry, S.; Fuentes, M. Deciphering the Effect of an Oxovanadium(IV) Complex with the Flavonoid Chrysin (VOChrys) in Intracellular Cell Signalling Pathways in an Osteosarcoma Cell Line. Metallomics 2016, 8, 739–749. [Google Scholar]
- León, I.E.; Butenko, N.; Di Virgilio, A.L.; Muglia, C.I.; Baran, E.J.; Cavaco, I.; Etcheverry, S.B. Vanadium and Cancer Treatment: Antitumoral Mechanisms of Three Oxidovanadium(IV) Complexes on a Human Osteosarcoma Cell Line. J. Inorg. Biochem. 2014, 134, 106–117. [Google Scholar]
- Nica, S.; Rudolph, M.; Lippold, I.; Buchholz, A.; Görls, H.; Plass, W. Vanadium(V) Complex with Schiff-Base Ligand Containing a Flexible Amino Side Chain: Synthesis, Structure and Reactivity. J. Inorg. Biochem. 2015, 147, 193–203. [Google Scholar]
- Cornman, C.R.; Colpas, G.J.; Hoeschele, J.D.; Kampf, J.; Pecoraro, V.L. Implications for the Spectroscopic Assignment of Vanadium Biomolecules: Structural and Spectroscopic Characterization of Monooxovanadium(V) Complexes Containing Catecholate and Hydroximate Based Noninnocent Ligands. J. Am. Chem. Soc. 1992, 114, 9925–9933. [Google Scholar]
- Crans, D.C.; Koehn, J.T.; Petry, S.M.; Glover, C.M.; Wijetunga, A.; Kaur, R.; Levina, A.; Lay, P.A. Hydrophobicity May Enhance Membrane Affinity and Anti-Cancer Effects of Schiff Base Vanadium(v) Catecholate Complexes. Dalt. Trans. 2019, 48, 6383–6395. [Google Scholar]
- Boukhobza, I.; Crans, D.C. Application of HPLC to Measure Vanadium in Environmental, Biological and Clinical Matrices. Arabian J. Chem. 2020, 13, 1198–1228. [Google Scholar]
- Ugone, V.; Sanna, D.; Sciortino, G.; Crans, D.C.; Garibba, E. ESI-MS Study of the Interaction of Potential VIV Drugs. Inorg. Chem. 2020, 59, 9739–9755. [Google Scholar]
- Srivastava, A. Anti-Diabetic and Toxic Effects of Vanadium Compounds. Mol. Cell. Biochem. 2000, 206, 177–182. [Google Scholar]
- OECD. Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Lipnick, R.L.; Cotruvo, J.A.; Hill, R.N.; Bruce, R.D.; Stitzel, K.A.; Walker, A.P.; Chu, I.; Goddard, M.; Segal, L.; Springer, J.A.; et al. Comparison of the Up-and-down, Conventional LD50, and Fixed-Dose Acute Toxicity Procedures. Food Chem. Toxicol. 1995, 33, 223–231. [Google Scholar]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The Current State of Serum Biomarkers of Hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar]
- McGill, M.R. The Past and Present of Serum Aminotransferases and the Future of Liver Injury Biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar]
- Pan, X.; Chang, F.; Liu, Y.; Li, D.; Xu, A.; Shen, Y.; Huang, Z. Mouse Toxicity of Anabaena Flos-Aquae from Lake Dianchi, China. Environ. Toxicol. 2009, 24, 10–18. [Google Scholar]
- Edelstein, C.L. Biomarkers of Acute Kidney Injury. Adv. Chronic Kidney Dis. 2008, 15, 222–234. [Google Scholar]
- Cooper, R.G. Vanadium Pentoxide Inhalation. Indian J. Occup. Environ. Med. 2007, 11, 97–102. [Google Scholar]
- Crans, D.C.; Postal, K.; MacGregor, J.A. Vanadium–speciation Chemistry is Important when Assessing Health Effects on Living Systems. In Metal Toxicology Handbook; CRC Press: Boca Raton, FL, USA, 2020; Chapter 6. [Google Scholar]
- Gajens, J.; Meier, B.; Adachi, Y.; Sakurai, H.; Rehder, D. Characterization and Insulin-Mimetic Potential of Oxidovanadium (IV) Complexes Derived from Monoesters and -carboxylates of 2,5-Dipicolinic Acid. Eur. J. Inorg. Chem. 2006, 18, 3575–3585. [Google Scholar]
- Brichard, S.M.; Henquin, J.C. The Role of Vanadium in the Management of Diabetes. Trends Pharmacol. Sci. 1995, 16, 265–270. [Google Scholar]
- Hanson, G.R.; Sun, Y.; Orvig, C. Characterization of the Potent Insulin Mimetic Agent Bis (Maltolato) Oxovanadium (IV) (BMOV) in Solution by EPR Spectroscopy. Inorg. Chem. 1996, 35, 6507–6512. [Google Scholar]
- Pessoa, J.C.; Correa, I. Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets. Inorganics 2021, 9, 17. [Google Scholar]
- Wang, B.; Tanaka, K.; Morita, A.; Ninomiya, Y.; Maruyama, K.; Fujita, K.; Hosoi, Y.; Nenoi, M. Sodium Orthovanadate (Vanadate), a Potent Mitigator of Radiation-Induced Damage to the Hematopoietic System in Mice. J. Radiat. Res. 2013, 54, 620–629. [Google Scholar]
- Roy, S.; Majumdar, S.; Singh, A.K.; Ghosh, B.; Ghosh, N.; Manna, S.; Chakraborty, T.; Mallick, S. Synthesis, Characterization, Antioxidant Status, and Toxicity Study of Vanadium-Rutin Complex in Balb/c Mice. Biol. Trace Elem. Res. 2015, 166, 183–200. [Google Scholar]
- Sanchez, D.; Ortega, A.; Domingo, J.L.; Corbella, J. Developmental Toxicity Evaluation of Orthovanadate in the Mouse. Biol. Trace Elem. Res. 1991, 30, 219–226. [Google Scholar]
- Montaser, A.S.; Wassel, A.R.; Al-Shaye’a, O.N. Synthesis, characterization and antimicrobial activity of Schiff bases from chitosan and salicylaldehyde/TiO2 nanocomposite membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar]
- Kenkyūjo, K.G.S. Toxic and Hazardous Industrial Chemicals Safety Manual or Handling and Disposal with Toxicity and Hazard Data; The International Technical Information Institute: Tokyo, Japan, 1988; p. 591. [Google Scholar]
- Miadzvedski, I.; Nikolayuk, O.; Dubovik, B. Acute Toxicity of Spatially Hindered Derivatives of Aminophenol and Catechol. In Proceedings of the Actual Problems of Medicine, Grodno, Belarus, January 2013. [Google Scholar]
- Boehm, O.; Zur, B.; Koch, A.; Tran, N.; Freyenhagen, R.; Hartmann, M.; Zacharowski, K. Clinical Chemistry Reference Database for Wistar Rats and C57/BL6 Mice. Biol Chem. 2007, 388, 547–554. [Google Scholar]
- Reul, B.A.; Amin, S.S.; Buchet, J.; Ongemba, L.N.; Crans, D.C.; Brichard, S.M. Effects of Vanadium Complexes with Organic Ligands on Glucose Metabolism: A Comparison Study in Diabetic Rats. Br. J. Pharmacol. 1999, 126, 467–477. [Google Scholar]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of Metal Drugs, Supplements and Toxins in Media and Bodily Fluids Controls in vitro Activities. Coor. Chem. Rev. 2017, 352, 473–498. [Google Scholar]
- Elvingson, K.; Gonzalez Baro, A.; Pettersson, L. Speciation in Vanadium Bioinorganic Systems. 2. An NMR, ESR, and Potentiometric Study of the Aqueous H+-Vanadate-Maltol System. Inorg. Chem. 1996, 35, 3388–3393. [Google Scholar]
- Samart, N.; Arhouma, Z.; Kumar, S.; Murakami, H.A. Decavanadate Inhibits Mycobacterial Growth More Potently Than Other Oxovanadates. Front. Chem. 2018, 6, 1–16. [Google Scholar]
- Althumairy, D.; Postal, K.; Barisas, G.B.; Nunes, G.G.; Roess, D.A.; Crans, D.C. Polyoxometalates Function as Indirect Activators of a G Protein-Coupled Receptor. Metallomics 2020, 12, 1044–1061. [Google Scholar]
- Yoshikawa, Y.; Sakurai, H.; Crans, D.C.; Micera, G.; Garribba, E. Structural and Redox Requirements for the Action of Anti-Diabetic Vanadium Compounds. Dalt. Trans. 2014, 43, 6965–6972. [Google Scholar]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and Possible Benefits in the Light of a Comprehensive Overview of Its Pharmacotoxicological Mechanisms and Multi-Applications with a Summary of Further Research Trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar]
- Lima, L.M.A.; Belian, M.F.; Silva, W.E.; Postal, K.; Kostenkova, K.; Crans, D.C.; Rossiter, A.K.F.F.; da Silva Júnior, V.A. Vanadium (IV)-Diamine Complex with Hypoglycemic Activity and a Reduction in Testicular Atrophy. J. Inorg. Biochem. 2020, 216, 111312. [Google Scholar]
- Crans, D.C.; Shin, P.K.; Armstrong, K.B. Characterization of vanadium(V) complexes in aqueous solutions: Ethanolamine and glycine derived complexes. J. Am. Chem. Soc. 1994, 116, 1305–1315. [Google Scholar]
- Rehder, D.; Polenova, T.; Bühl, M. Vanadium-51 NMR. Annu. Rep. NMR Spectrosc. 2007, 62, 49–114. [Google Scholar]
- Li, X.; Lah, M.S.; Pecoraro, V.L. Vanadium Complexes of the Tridentate Schiff Base Ligand N-Salicylidene-N’-(2-Hydroxyethyl) Ethylenediamine: Acid-Base and Redox Conversion between Vanadium (IV) and Vanadium (V) Imino Phenolates. Inorg. Chem. 1988, 27, 4657–4664. [Google Scholar]
- Rajendiran, V.; Karthik, R.; Palaniandavar, M.; Stoeckli-Evans, H.; Periasamy, V.S.; Akbarsha, M.A.; Srinag, B.S.; Krishnamurthy, H. Mixed-ligand Copper (II)-phenolate Complexes: Effect of Coligand on Enhanced DNA and Protein Binding, DNA Cleavage, and Anticancer Activity K. Inorg. Chem. 2007, 46, 8208–8221. [Google Scholar]
- Chatterjee, P.B.; Goncharov-Zapata, O.; Quinn, L.L.; Hou, G.; Hamaed, H.; Schurko, R.W.; Polenova, T.; Crans, D.C. Characterization of Non-innocent Metal Complexes Using Solid–state NMR Spectroscopy: O-dioxolene Vanadium Complexes. Inorg. Chem. 2011, 50, 9794–9803. [Google Scholar]
- Hasegawa, R.; Nakaji, Y.; Kurokawa, Y.; Tobe, M. Acute Toxicity Tests on 113 Environmental Chemicals. Sci. Rep. Res. Inst. Tohoku Univ. Med. 1989, 36, 10–16. [Google Scholar]
- Barbosa, H.M.; Do Nascimento, J.N.; Araújo, T.A.S.; Duarte, F.S.; Albuquerque, U.P.; Vieira, J.R.C.; De Santana, E.R.B.; Yara, R.; Lima, C.S.A.; Gomes, D.A.; et al. Acute Toxicity and Cytotoxicity Effect of Ethanolic Extract of Spondias Tuberose Arruda Bark: Hematological, Biochemical and Histopathological Evaluation. An. Acad. Bras. Cienc. 2016, 88, 1993–2004. [Google Scholar]
- Howard, W.; Robinson, J.W.P.; Hogden, C.G. The Estimation of Albumin and Globulin in Blood. J. Bio. Chem. 1937, 120, 481–498. [Google Scholar]

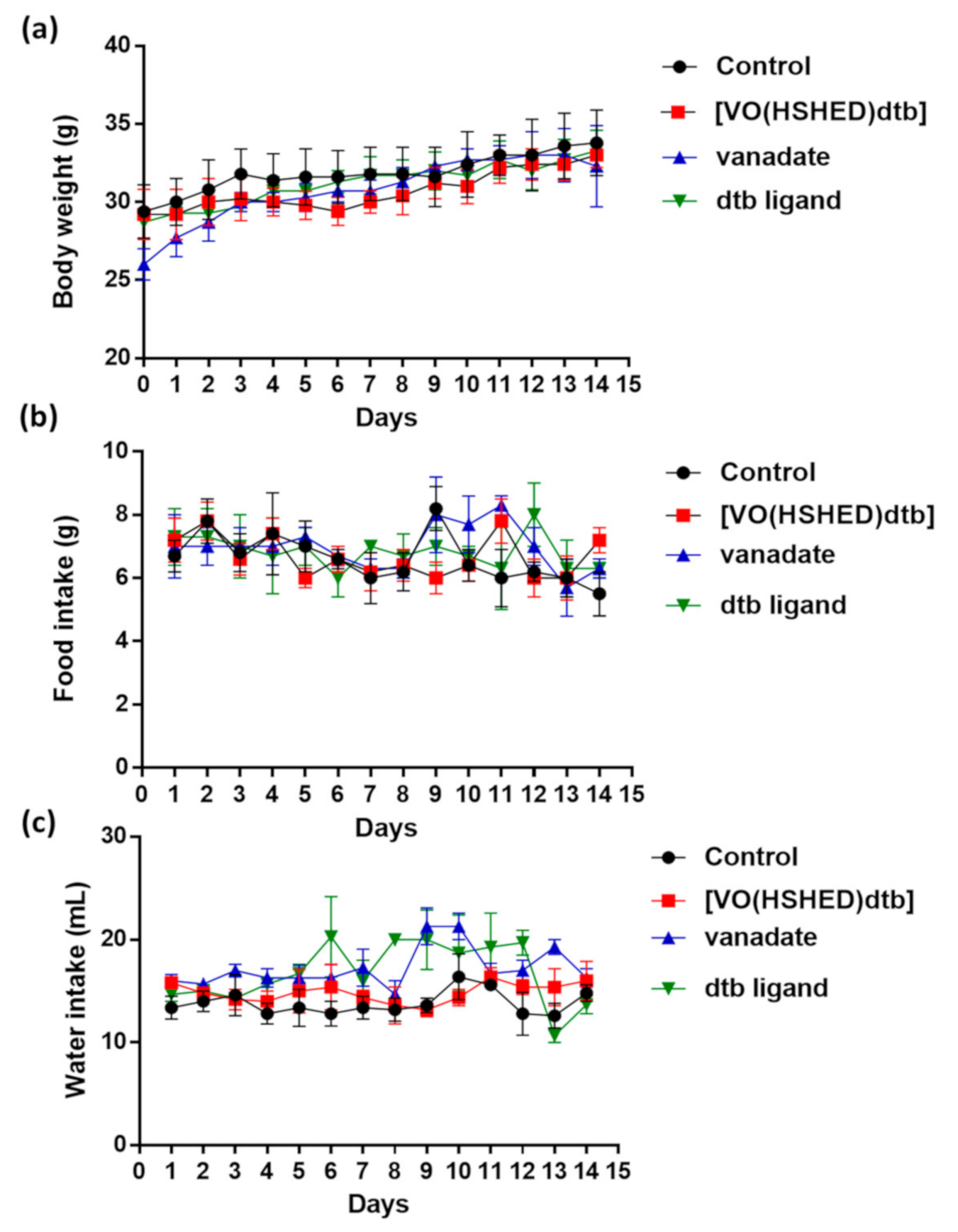


| Study Group | ||||
|---|---|---|---|---|
| Parameter | Control | [VO(HSHED)dtb] | Orthovanadate | 3,5-di(t-butyl)catechol |
| Body weight (g) | 31.8 ± 1.8 | 30.7 ± 1.0 | 30.8 ± 0.9 | 31.2 ± 0.7 |
| Weight gain (g) | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.2 0 ±.1 | 0.2 ± 0.1 |
| Food intake (g/24 h) | 6.8 ± 0.3 | 6.7 ± 0.3 | 7.0 ± 0.1 | 6.8 ± 0.3 |
| Water intake (mL/24 h) | 13.8 ± 0.8 | 15.0 ± 0.6 | 17.3 ± 0.5 * | 16.7 ± 1.5 |
| Study Group | ||||
|---|---|---|---|---|
| Biochemical Parameters | Control | [VO(HSHED)dtb] | Orthovanadate | 3,5-di(t-butyl)catechol |
| AST/ALT ratio | 1.1 ± 0.01 | 0.9 ± 0.02 | 1.0 ± 0.01 | 1.1 ± 0.01 |
| Albumin (g/dL) | 2.2 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| Total proteins (g/dL) | 5.7 ± 0.4 | 5.5 ± 0.2 | 6.5 ± 0.6 | 5.8 ± 0.3 |
| Globulin (g/dL) | 3.5 ± 0.3 | 3.4 ± 0.2 | 4.3 ± 0.5 * | 3.8 ± 0.3 |
| A/G ratio | 0.6 ± 0.01 | 0.6 ± 0.01 | 0.5 ± 0.02 | 0.6 ± 0.02 |
| Study Group | ||||
|---|---|---|---|---|
| Hematological Parameters | Control | [VO(HSHED)dtb] | Orthovanadate | 3,5-di(t-butyl)catechol |
| Red blood cell (1012/L) | 9.5 ± 0.2 | 9.3 ± 0.2 | 8.9 ± 0.2 | 9.7 ± 0.3 |
| Mean corpuscular volume (Fl) | 57.6 ± 0.2 | 56.2 ± 0.8 | 57.0 ± 0.6 | 56.3 ± 0.3 |
| Hemoglobin (g/dL) | 12.4 ± 0.5 | 12.5 ± 0.1 | 12.3 ± 0.1 | 13.4 ± 0.2 |
| Mean corpuscular hemoglobin (ρg) | 13.5 ± 0.5 | 15.4 ± 2.0 | 13.7 ± 0.3 | 13.8 ± 0.1 |
| mean corpuscular hemoglobin concentration (g/dL) | 23.5 ± 0.6 | 23.9 ± 0.2 | 23.9 ± 0.2 | 24.4 ± 0.1 |
| Hematocrit (%) | 52.6 ± 0.9 | 52.5 ± 0.5 | 51.6 ± 0.8 | 55.0 ± 1.6 |
| White blood cell (109/L) | 12.6 ± 1.3 | 8.6 ± 1.3 * | 12.7 ± 1.9 | 10.9 ± 1.1 |
| Lymphocytes (%) | 82.6 ± 0.8 | 81.8 ± 0.9 | 84.3 ± 1.5 | 83.2 ± 1.3 |
| Study Group | ||||
|---|---|---|---|---|
| Organs (g/100 g b.w) | Control | [VO(HSHED)dtb] | Orthovanadate | 3,5-di(t-butyl)catechol |
| Heart | 0.54 ± 0.03 | 0.53 ± 0.02 | 0.59 ± 0.04 | 0.60 ± 0.07 |
| Kidney | 5.75 ± 0.29 | 6.23 ± 0.38 | 6.11 ± 0.11 | 5.50 ± 0.54 |
| Liver | 1.36 ± 0.10 | 1.52 ± 0.08 | 1.70 ± 0.20 * | 1.61 ± 0.18 |
| Spleen | 0.48 ± 0.05 | 0.47 ± 0.03 | 0.66 ± 0.06 * | 0.59 ± 0.13 |
| Lung | 0.88 ± 0.11 | 0.79 ± 0.03 | 0.86 ± 0.14 | 0.75 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.M.A.; Murakami, H.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C.; Crans, D.C. Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex. Inorganics 2021, 9, 42. https://doi.org/10.3390/inorganics9060042
Lima LMA, Murakami H, Gaebler DJ, Silva WE, Belian MF, Lira EC, Crans DC. Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex. Inorganics. 2021; 9(6):42. https://doi.org/10.3390/inorganics9060042
Chicago/Turabian StyleLima, Lidiane M. A., Heide Murakami, D. Jackson Gaebler, Wagner E. Silva, Mônica F. Belian, Eduardo C. Lira, and Debbie C. Crans. 2021. "Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex" Inorganics 9, no. 6: 42. https://doi.org/10.3390/inorganics9060042
APA StyleLima, L. M. A., Murakami, H., Gaebler, D. J., Silva, W. E., Belian, M. F., Lira, E. C., & Crans, D. C. (2021). Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex. Inorganics, 9(6), 42. https://doi.org/10.3390/inorganics9060042