Abstract
This work summarizes the most commonly used in situ techniques for the study of Li-ion batteries from the micro to the atomic level. In situ analysis has attracted a great deal of interest owing to its ability to provide a wide range of information about the cycling behavior of batteries from the beginning until the end of cycling. The in situ techniques that are covered are: X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), and Scanning Transmission Electron Microscopy (STEM). An optimized setup is required to be able to use any of these in situ techniques in battery applications. Depending on the type of data required, the available setup, and the type of battery, more than one of these techniques might be needed. This study organizes these techniques from the micro to the atomic level, and shows the types of data that can be obtained using these techniques, their advantages and their challenges, and possible strategies for overcoming these challenges.
1. Introduction
Li-ion batteries have attracted a great deal of interest and are in high demand due to the rise of issues related to climate change. These batteries can be found in a variety of devices and machines that are used in our daily life, such as laptops, phones, and electric vehicles [1,2]. Therefore, it is of great importance to fully understand their cycling behavior and their failure mechanisms, in order to enhance their performance.
Using different characterization techniques to study Li-ion batteries has been shown to be an effective method of monitoring the cycling behavior and failure mechanisms of these batteries. However, the specific properties of Li present many challenges for the use of characterization techniques. Li sample preparation for characterization techniques is challenging due to Li being an ultra-soft material that is highly reactive with oxygen, hydrogen, carbon dioxide, and nitrogen [3,4]. The high reactivity of this material, along with its low melting temperature, also causes difficulties such as contamination or beam damage during characterization using electron or ion beams [3,4]. Therefore, to study materials containing Li, an appropriate characterization technique must be used that is in accordance with the desired information, and an optimal setup must be designed that is able to overcome the above-mentioned challenges during analysis. These characterization challenges are discussed in more detail later in this work.
Different materials containing Li are used in batteries as anodes and cathodes. The materials used for the anode include metallic Li, graphitic carbon, and Si. The most attractive materials for use as cathodes include Li cobalt oxide, NMC, LVO, and LFP. This work is organized on the basis of the different characterization techniques. Each technique is categorized on the basis of the types of analysis that can be conducted, and their advantages and disadvantages. To simplify the comparison between the different techniques, and to focus on the types of data that can be extracted from them, for each technique, the in situ and in operando analysis, as well as information on the mentioned anode and cathode materials, are given.
Below, a short summary of Li-ion batteries, their challenges, and different characterization techniques is given.
1.1. Li-Ion Batteries
Li-ion batteries consist of an anode electrode, a cathode electrode, and an electrolyte [1]. Each of these three parts need to be optimized to achieve a safe, cost-effective battery with high capacity [1,5]. Different batteries contain either a liquid or a solid electrolyte [1]. Solid electrolytes, including polymer and ceramic base electrolytes, have gained interest over liquid electrolytes due to the elimination of the flammability concerns imposed by liquid electrolytes, specifically in the presence of dendrites [1,6]. Solid electrolytes have also shown higher thermal and chemical stability [7].
During the charging of the first cycle, a passivation layer is formed on the surface of the anode called the solid electrolyte interphase (SEI), which is ionic conductive and an electron insulator [1,8]. The composition of this layer, its formation mechanism, and the deterioration of this layer during cycling play important roles in the life of the battery and its failure mechanism.
Dendrite formation is one of the main concerns in Li-ion battery applications. If the SEI layer does not cover the anode surface uniformly or undergoes deterioration during cycling, it will result in concentration of Li deposition in the damaged regions, causing dendrite formation and the subsequent short-circuit of the battery [9,10,11,12]. If these dendrites further detach from the anode surface, they produce “dead Li”, reducing battery efficiency [9,13].
It is crucial to understand the underlying mechanism for each of these phenomena occurring in the battery during cycling. There are many techniques specifically designed to address these chemical and structural evolutions in the battery. Some of these widely used techniques in the battery field and their applications are summarized below.
1.2. In Situ, In Operando, and Ex Situ Analysis
There are three different measurement techniques that can be used to study Li-ion batteries: in situ, in operando, and ex situ. In situ analysis refers to analyzing the battery after cycling is completed in the same cell and setup without moving the battery. In this technique, the battery is studied in its natural state [14]. In operando refers to conducting the battery analysis during cycling without pausing the cycling to conduct analysis [14]. Ex situ analysis refers to analyzing the battery after cycling is completed. In ex situ analysis, the cycling setup is separate from the characterization setup and a transfer holder is needed to move the battery. In many studies, in situ and in operando analysis are used interchangeably and are used to monitor the battery during cycling. In this work, these two terminologies are used interchangeably.
In situ analysis enables us to monitor the behavior of the battery from the beginning until the end of cycling as the chemical and microstructural changes are occurring, which provides more accurate results on the cycling behavior and the correlation between cycling parameters and battery evolution. Depending on the characterization method used, it is also possible to gather local information about a specific region of the battery while it is cycling. In the case of ex situ cycling, however, the cycling needs to be stopped to conduct further chemical or microstructural analysis, which might not provide accurate results on the sequence by which the battery is altered during cycling. To conduct ex situ analysis, an airtight transfer holder is also needed to transfer the battery, which could result in contamination of the battery due to the high reactivity of Li [15]. All in all, to obtain a complete understanding of the cycling behavior, these two techniques should be used together: in situ analysis to gather an overall understanding of the cycling and failure mechanism and conduct local investigation, and ex situ analysis to gather further in-depth information on the root cause of the specific changes observed on the battery using various characterization techniques. Some examples of these changes include the cause of dendrite initiation, grain boundary activities, phase separations, volume change, intercalation mechanism, solid electrolyte interphase (SEI) layer deterioration and reconstruction and chemical composition.
1.3. In Situ Characterization Techniques
The techniques that are covered in this study are: X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), and Scanning Transmission Electron Microscopy (STEM). The XRD technique provides information on the phase transformation and crystal structure of the material. In this technique, the diffracted X-rays that satisfy the Bragg’s law are collected by the detector, and the crystal structure is revealed with the peak positions and intensities from the spectrum [16]. Raman spectroscopy technique provides local information about the structure of the material. In this technique, only the inelastic Raman scattering signals are gathered by the detector [16]. The local structure is determined using the intensity vs. Raman shift spectrum [16]. The XPS technique has the ability to analyze the surface of the sample through the photoelectric effect. This technique can provide elemental analysis, which makes this technique suitable for studying the SEI layer of the battery [17]. SEM, TEM, and STEM techniques provide microstructural and chemical information about the surface and bulk of the sample through electron interaction with the specimen. SEM uses lower accelerating voltages (up to 30 kV) than TEM and provides information about the bulk sample [18]. TEM uses a high accelerating voltage (up to 300 kV) and provides information on a thin sample. STEM analysis also uses thin specimens, but with lower accelerating voltages.
2. In Situ Studies
2.1. In Situ XRD
The X-ray diffraction (XRD) technique is a useful tool for monitoring the phase transformations and the evolution of the crystal structures occurring in the electrodes when cycling a battery [16]. In this section, the types of results that can be achieved with in situ XRD analysis for battery applications are summarized for different cathode and anode materials.
2.2. Phase Transformation Analysis
Doron and Ein-Eli [19] studied Li intercalation in graphite electrodes using in situ XRD with four electrolytes: ethylene and diethyl carbonate mixtures (EC-DEC), propylene carbonate (PC), tetrahydrofuran (THF), and dimethyl carbonate (DMC). The graphite electrodes were stable in the systems containing EC-DEC and water-contaminated DMC, due to the formation of a passivation layer on the electrode before the start of intercalation [19]. The graphite electrode in the systems containing PC and THF electrolyte, however, deteriorated during cycling [19]. The use of the in situ XRD technique has been described as crucial for the study of Li intercalation in graphite because of the air sensitivity of these electrodes [19]. The study of the phase transformation during Li intercalation in graphite in DEC solution by Whitehead et al. [20] showed two plateaus in the chronopotentiometry analysis, which were correlated with the formation and movement of the Li–graphite intercalation compounds in the system. Chung et al. [21] used this technique to examine the impact of ZrO2 coating on the surface of LiCoO2 by monitoring the evolution of phase transformation in the cathode electrode. This study showed that the ZrO2 coating protects the cathode particles and lowers the decomposition of the electrolyte. Zhu et al. [22] in situ XRD investigation of LixMn1.5Ni0.5O4 doped with different metals for the purpose of observing the phase transformation in the cathode materials concluded that the improvement in the electrochemical behavior of the cathode when using Co metal was far more than in the case of using Al, Cu, or Mg metals. Zhu et al. [23] studied Li-S batteries using in situ XRD coupled with in situ Raman spectroscopy. The in situ XRD results showed the occurrence of phase transformation in the cathode during cycling [23]. Roberts [24] et al. studied the concentration gradient in the LiFePO4 (LFP) cathode material during discharge. This study showed that the cathode underwent a greater than 50% discharge during cycling, but when examining the concentration gradient, a limited Li insertion in the LFP electrode close to the current collector was observed [24].
2.3. Crystal Structure Analysis
The in situ XRD work on crystal structure analysis of the SEI layer formed on the epitaxial graphene on 6H-SiC (EG/SiC) Chattopadhyay et al. [25] revealed the presence of LiF crystallites in the SEI layer. Schweidler et al. [26] monitored the crystallographic and volume changes that could occur as a result of Li intercalation in the graphite anode during cycling. They observed expansion of the graphite electrode even before complete Li intercalation, which consequently affects the cycle life of the battery.
Mohanty et al. [27] monitored the change in the structure of Li1.2Co0.1Mn0.55Ni0.15O2 cathode during cycling. Their observations showed an increase in the c-lattice parameter during the first charge and a decrease during charging above 4.4 V. The discharge above and below 3.5 V resulted in an increase in the c-lattice parameter [27]. During the first cycle, the a-lattice parameter did not change during the plateau region. They also reported a decrease in the monoclinic phase as cycling was continued [27]. Liu et al. [28] examined the evolution of the structure of the LFP electrode with in situ XRD. This method provided information on the Li+ intercalation in the cathode electrode, and the results showed that the LFP cathode went through a two-phase reaction and a dual-phase solid-solution reaction mechanism [28]. The in situ XRD study by Dong et al. [29] of the LFP material at different pressures also showed an anisotropic decrease with increasing pressure.
2.4. XRD Advantages and Disadvantages
Using the XRD technique to study batteries requires a simple setup and battery design [16]. This advantage is of great importance, since the batteries require minimal sample manipulation prior to in situ cycling, making conducting the experiments easier than other techniques such as in situ SEM or TEM. XRD also makes it possible to study the bulk material [16]. Even though this technique provides fruitful information on phase transformation and the crystal structure of the bulk material, it cannot provide local information about specific phenomena that could occur nonuniformly during cycling, such as dendrite analysis, since the battery surface cannot be observed using this technique. Figure 1 shows an in situ XRD setup [27].
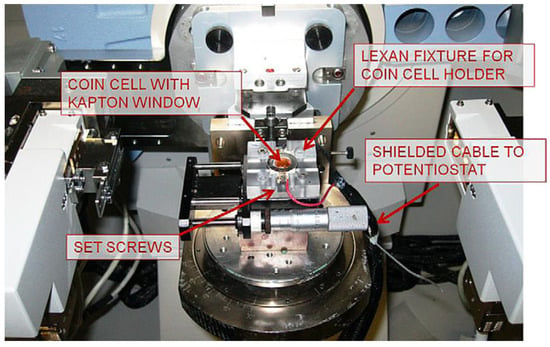
Figure 1.
In situ XRD setup Reprinted with permission from ref. [27]. Copyright 2012 Elsevier.
3. In Situ Raman Spectroscopy
Raman spectroscopy provides information about the structure of the material at the atomic level, as well as phase transformation [16]. This technique can be used to monitor the activities on the anode or the cathode. In this section, the types of analysis and the information that can be obtained using this technique are summarized.
3.1. Structural Analysis
The Raman spectroscopy study of Zhu et al. [30] on LiMn1.45Ni0.45M0.1O4 cathodes revealed changes in the local structure of the cathodes during cycling. This study showed the same reversible behavior for the Cr- and Co-doped cathodes during cycling. This technique also made it possible to monitor the Ni oxidation state and Li concentration during cycling [30]. The in situ Raman spectroscopy analysis of Li-S batteries by Zhu et al. [23] demonstrated the ability of this technique to be used for studying the species that are formed on the cathode and in the electrolyte.
Migge et al. used in situ Raman spectroscopy and confocal microscopy to study the intercalation of the ions in the graphite anode [31]. They studied the degree of intercalation by monitoring the Raman bands and used confocal microscopy to observe this phenomenon on the electrode. They reported inhomogeneous Li-ion intercalation during cycling [31]. Sole et al. [32] also monitored the insertion and extraction of Li in the graphite anode in a Li-ion battery during cycling to understand the ion intercalation mechanism. They showed the different steps of the formation of a single particle in the anode, and were able to monitor the double-resonance 2D band behavior during Li insertion and extraction in the graphite anode for the first time [32]. They coupled this technique with SEM to study the morphology of the electrode.
3.2. Raman Spectroscopy Advantages and Disadvantages
Raman spectroscopy is a non-destructive technique that can be used to analyze solid and liquid materials [16,32]. It provides information on changes in the local structure during battery cycling [16]. This technique can also provide images of the sample during cycling. It has, however, low spatial resolution. Figure 2 shows a schematic of an in situ Raman setup [32].
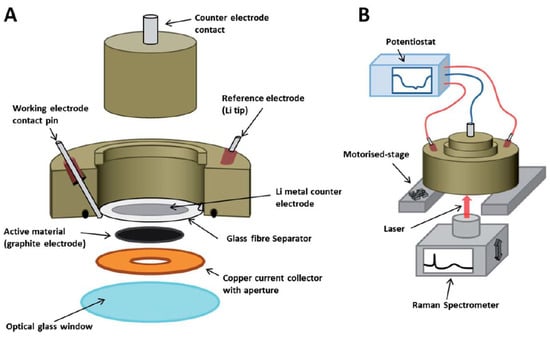
Figure 2.
Schematic of (A) the Raman setup, and (B) the setup during cycling. Reprinted with permission from ref. [32]. Copyright 2014 The Royal Society of Chemistry, Faraday Discussions.
4. In Situ XPS
In situ X-ray photoelectron spectroscopy (XPS) is a surface analysis tool widely used to investigate anodes such as lithium metal, lithium titanate (LTO) and graphite, and cathodes such as LiFePO4, LiCoO2, and LiNi1-x-yCoxMnyO2 (NMC) [33,34]. This technique can not only provide information on the surface composition of the lithium metal, such as the presence of Li2O, Li2CO3 and Li3N as layers, but can also provide chemical mapping to examine surface structures and irregularities such as those found in the LiAl alloy. It also provides information on the chemical reactivity and the associated stability of electrochemical interfaces over a wide spatial range, varying from the microscale (chemical state) to the mesoscale (elemental distribution). However, XPS is still underutilized as a characterization method for materials in Li-ion batteries and beyond, as well as in failure prediction and prevention.
Nandasiri et al. [17] studied the SEI layer in a Li-S battery with a Li anode using this technique. They divided the evolution of the SEI layer into three steps: (1) the primary phase, composed of Li compounds; (2) the secondary phase, which formed as the result of the reactions between the previous reaction products and the electrolyte; and (3) a dynamic monoanionic polysulfide fouling process [17].
XPS Advantages and Challenges
The advantage of this technique is the provision of a molecular-level understanding of the SEI layer. The disadvantage of XPS is the inability of this technique to provide micrographs of the entire surface of the battery, which would enable the user to choose specific regions on which to conduct local analysis. Figure 3 shows a schematic of an XPS sample holder [17].
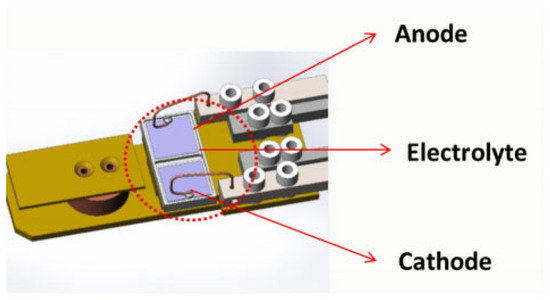
Figure 3.
Schematic of an XPS sample holder. Reprinted with permission from ref. [17]. Copyright 2017 American Chemical Society Publications.
5. In Situ SEM
Scanning electron microscopy (SEM) has been widely used to study different materials for battery applications. SEM provides high-resolution images that offer valuable information about the microstructure of different materials. SEM coupled with EDS and EBSD offers the ability to conduct chemical analysis and crystallographic information, respectively, both of which help understand the cause of observing each phenomenon during cycling. Studying batteries using in situ SEM provides information about the entire cycling behavior from the beginning until the failure of the battery. The batteries that can be investigated using in situ SEM are all-solid-state batteries.
5.1. Morphological Analysis
Studies have extracted extended morphological information during battery cycling from the battery surface and cross-section images: dendrite morphologies, anode, cathode, and electrolyte morphology change as the result of cycling and dendrite formation, and the solid electrolyte interphase (SEI).
The dendrite morphologies that have been reported on the basis of in situ SEM are: needle, mossy, and “multi-globular structure” [15,35,36,37]. Dolle et al. [35], Orisini et al. [37], and Golozar et al. [15] reported the needle and mossy morphologies. Dolle et al. [35] and Orisini et al. [37] observed the needle and mossy dendrites at high and low current densities, respectively. Their SEM analyses also showed the growth of a dendrite in the polymer electrolyte and the subsequent short circuit of the cell. To further analyze the observed morphologies, Golozar et al. [15] milled the needle dendrites using FIB and reported a hollow morphology for the dendrites. Harry et al. [36] reported dendrites with a “multi-globular structure” that were initiated from the impurities in the Li anode and polymer electrolyte interface in symmetrical Li cells. Figure 1 shows the different dendrite morphologies reported using SEM. Golozar et al. [38] also observed dendrites with mossy and needle morphologies in a symmetrical Li-metal cell with Li7La3Zr2O12 (LLZO) electrolyte. This study showed the formation and growth of the dendrites on the Li electrode from regions that thinning of the anode and consumption of Li had occurred [38]. Kaboli et al. [39] also reported mossy and needle dendrites in an all-solid-state battery with Li-metal anode, nickel-manganese-cobalt-oxide (NMC-622) cathode, and polymer electrolyte.
Morphological changes in the anode, cathode, and electrolyte have also been reported. Hovington et al. [40] monitored the thickness changes in the three electrodes (Li-metal anode, polymer electrolyte, LiFePO4 (LFP) or Li1.2V3O8 (LVO) cathode) during charging and discharging using a cross-section view of the battery. This study also showed the formation of isles on the Li-metal anode, resulting in the formation of solid polymer electrolyte (SPE) bridges in the battery [40]. Hovington et al. [41] also investigated the effect of the particle size of nano Si and SiO anode on the volume change during cycling. They reported that the formation of cracks on electrodes with small particle size was the result of electrochemical sintering [41]. Golozar et al. [42] were able to capture the evolution of these isles from the beginning of cycling until the end. The SEM images obtained in this study were used to construct videos that showed the complete cycling behavior. Videos showed consumption of Li in the vicinity of the isles and formation of dendrites on the fresh Li. Milling the isles using Fib also revealed their interior structure [42]. The morphological changes in the polymer electrolyte surrounding the dendrites was also captured and reported in the videos, and it was correlated with possible temperature increase, decomposition, and degassing of the polymer [42]. Milling the polymer electrolyte also showed the regions through which the dendrites perforated [15]. In situ SEM study of symmetrical Li cells with LLZO electrolyte conducted by Golozar et al. [38] revealed the Li consumption mechanism during cycling. The images captured the inhomogeneous thinning of Li anode with different sizes from different regions, some of which contained a few grains. The study by Kaboli et al. [39] on all-solid-state Li-metal batteries with NMC cathodes showed the thinning of the SPE during cycling as a consequence of chemical degradation. They also observed polymer degassing in the form of volcano-like features on the electrolyte.
5.2. Chemical and Crystallographic Analysis
Chemical analysis with SEM can be performed using EDS detectors. Li detection using EDS, however, faces many challenges, which are discussed in Section 5.3. Harry et al. [36] conducted chemical analysis on the interior of the “multi-globular structure” that was observed on the cycled cells. The EDS mapping results showed that these structures contain the electrolyte. The map also contained some white areas which were correlated with the presence of Li in these regions, which was not possible to detect due to detection limitation of the EDS detector [36]. Golozar et al. [15] were able to detect Li using a windowless EDS detector, which overcomes this detection limitation. They showed that the dendrites are comprised of Li, C, and O, and reported a carbide nature for the dendrites [15]. Golozar et al. [42] performed a chemical analysis of the cross-section of the Li-metal anode and the polymer electrolyte, showing the presence of salt in the vicinity of the isles formed on the anode and inhomogeneous salt distribution in the polymer, respectively. EDS mapping of symmetrical Li cells with LLZO electrolyte has also shown differences in the Li thickness on the anode, as well as the inhomogeneous distribution of Zr, La, and C in the cross-section of the electrolyte reported by Golozar et al. [38].
Kaboli et al. [43] used an in situ SEM technique to monitor the evolution of the passivation layer for Li-10 Mg alloy as a potential anode material at high temperature. The in situ SEM heat treatment of the alloy showed no change in the morphology of the surface passivation layer above the melting point while the bulk was melted. They employed electron backscatter diffraction (EBSD) crystal orientation mapping, which showed the formation of new grains after melting, confirming their observation [43].
5.3. SEM Advantages and Challenges
The advantages of using SEM to conduct in situ analysis include: high-resolution imaging, ability to conduct chemical analysis using EDS, crystallographic analysis using EBSD, gathering information on the depth of the sample when coupled with FIB, and minimal sample manipulation. This technique presents some challenges as well, especially in case of studying materials containing Li. A setup needs to be designed specifically for the purpose of conducting these experiments that enables the cycling of the battery in the microscope at the desired temperature and gathers images all through cycling at different time intervals from different regions of the battery. Li sample preparation is difficult because it is an ultra-soft metal and is highly reactive with oxygen, hydrogen, carbon dioxide, and nitrogen [3,4]. Therefore, it is crucial to perform sample preparation in a dry room or a glove box and to use an airtight transfer holder. It is also possible to encounter C and O in the SEM chamber [44,45]. To minimize this contamination, SEM analysis should be conducted in an ultra-high-vacuum microscope or at cryogenic temperature [44,46].
Li imaging is also challenging due to Li electron beam damage that could alter the surface [47]. This damage could be minimized using different techniques including using a low accelerating voltage and beam current, as well as lowering the sample holder temperature with liquid nitrogen [48].
Li chemical analysis using EDS is also challenging due to low fluorescence yield of Li that results in low signal generation and its low X-ray energy, which causes detection limitations of the X-ray [5,49]. To overcome the detection limitation a windowless EDS detector with extreme electronics (Oxford Instruments) can be used [5]. Analyzing Li in compounds is particularly difficult due to low and sometimes zero X-ray generation. In these situations, other techniques such as time-of-flight–secondary ion mass spectroscopy (TOF-SIMS) or electron energy loss spectroscopy (EELS) can be used.
Figure 4 shows the schematic of the surface and cross-section view assembly of the batteries as well as the setup used to study symmetrical Li cells with LLZO solid electrolyte [15,38].
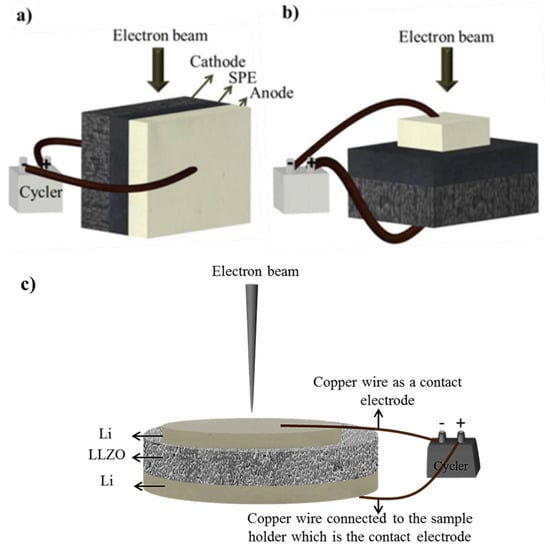
Figure 4.
Schematic of the (a) surface and (b) cross-section view assembly of the batteries. Reprinted with permission from ref. [15]. Copyright 2018 American Chemical Society Publications, and (c) the setup used to study symmetrical Li cells with LLZO solid electrolyte. Reprinted with permission from ref. [38]. Copyright 2020 Springer Nature.
6. In Situ TEM and STEM
Transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) techniques are useful to understand the cycling behavior of the battery at the atomic level. TEM and STEM can provide detailed information on the morphological changes in the battery, as well as conducting chemical and crystallographic analysis. In this section, the types of data that can be obtained with these techniques are discussed.
6.1. Morphological Analysis
The in situ TEM study by Kushima et al. [50] of the dendrites formed on a gold substrate during Li electrodeposition showed two different morphologies of whiskers at large overpotentials and “dense Eden-like clusters” at small overpotentials. This study also showed that the whiskers became hollow during dissolution, which was an indication of the formation of “dead Li”. Liu et al. [51] monitored the growth of Li fibers from nanowire anodes during charging They reported a directional growth of the fibers due to the electric field caused by the sharp tips, which confirms the safety concerns regarding dendrite growth in the batteries. Ghassemi et al. [52] were able to capture the growth of the fibers in a Li-ion battery using in situ TEM with the growth direction parallel to the applied electric field. They were also able to capture the formation of kinks in the fibers during growth.
6.2. Chemical and Crystallographic Analysis
Liu et al. [51] used EELS analysis to investigate the chemical composition of the fibers formed in the electrolyte during charging, which confirmed the presence of metallic Li. Wang et al. [53] studied the interface of the cathode and the electrolyte in a system containing LiCoO2 cathode, Si anode, and LiPON electrolyte with in situ STEM and electron energy loss spectroscopy (EELS). Chemical analysis of the layer formed on the cathode during cycling showed the presence of oxidized Co ions, Li oxide, and Li peroxide species [53].
As previously mentioned, Li is a beam sensitive material which results in characterization challenges when using high beam energies during TEM analysis. One method to overcome this challenge is cryogenic (cryo)-electron microscopy [54]. Using this technique, Wang et al. [54] showed that the electrodeposited Li is amorphous and is covered with an inhomogeneous SEI layer containing amorphous organic species and LiF with a short electrodeposition duration. Li et al. [55] also used cryo-TEM to study the atomic structure of dendrites. They reported dendrite growth along the <111>, <110>, and <211> directions as single-crystalline nanowires. They also reported change in the growth direction, where kinks were observed on the dendrites [55]. Both of these studies were conducted ex situ.
6.3. TEM and STEM Advantages and Challenges
The advantages of TEM are the ability to provide data on the morphology, chemistry, and crystalline structure with high spatial resolution at the atomic level [51,52].
Even though TEM is capable of providing images and information at the atomic level, as well as the possibility of conducting chemical analysis, it has some limitations that must be considered when using this technique to analyze different samples, in particular beam sensitive materials. To be able to use this technique, specific sample preparation procedures must be followed to make small-sized samples for imaging. This technique also uses high beam energies, which induce beam damage in beam-sensitive materials such as Li. Using STEM could reduce the beam damage of the samples, as it allows the user to work with lower beam energies. Figure 5 shows the schematic of in situ TEM biasing of nanobatteries [53].
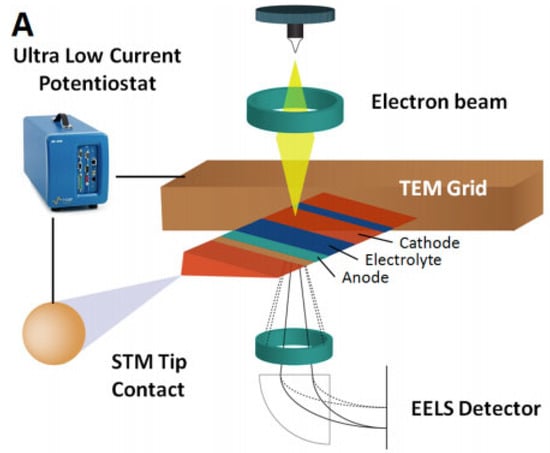
Figure 5.
Schematic of in situ TEM biasing of nanobattery. Reprinted with permission from ref. [53]. Copyright 2016 American Chemical Society Publications.
7. Conclusions and Perspective
In situ techniques can provide detailed information about the battery throughout cycling and show the correlation between the microstructural and chemical evolution and the cycling parameters. In situ studies provide the real behavior of the battery, as the cycling is observed in real time, and the provided data are not speculations. Each technique can provide specific information about different components of the battery, thus making them exclusively suitable for gathering a part of the information that might be needed. Therefore, the appropriate technique must be chosen based on the sample under study and the goal of the experiments. In some cases, more than one technique might be needed to extract the complete information. This work showed the most widely used in situ techniques for battery applications. The root cause of all the phenomenon observed in the battery can be explained at the atomic level, making STEM and TEM optimal methods for providing detailed information. If TEM is coupled with 3D atom probe, a comprehensive analysis can be conducted to show the cycling and failure mechanism. However, as mentioned in this work, there are many challenges concerning Li analysis using electron microscopy that need to be addressed, provided that these techniques are chosen.
Author Contributions
Conceptualization, M.G., K.Z. and R.G.; writing—original draft preparation, M.G. and R.G.; writing—review and editing, R.G. and K.Z.; supervision, K.Z.; project administration, K.Z. and R.G.; funding acquisition, R.G. and K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Council of Science and Engineering of Canada.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kalhammer, F.R. Polymer electrolytes and the electric vehicle. Solid State Ion. 2000, 135, 315–323. [Google Scholar] [CrossRef]
- Brodusch, N.; Zaghib, K.; Gauvin, R. Electron backscatter diffraction applied to lithium sheets prepared by broad ion beam milling. Microsc. Res. Tech. 2014, 78, 30–39. [Google Scholar] [CrossRef]
- Jeppson, D.; Ballif, J.; Yuan, W.; Chou, B. Lithium Literature Review: Lithium’s Properties and Interactions; Hanford Engineering Development Lab.: Richland, WA, USA, 1978. [Google Scholar]
- Hovington, P.; Timoshevskii, V.; Burgess, S.; Demers, H.; Statham, P.; Gauvin, R.; Zaghib, K. Can we detect Li KX-ray in lithium compounds using energy dispersive spectroscopy? Scanning 2016, 38, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, A.L.; Janek, J. Solid-state batteries enter EV fray. MRS Bull. 2014, 39, 1046–1047. [Google Scholar] [CrossRef] [Green Version]
- Commarieu, B.; Paolella, A.; Daigle, J.-C.; Zaghib, K. Toward high lithium conduction in solid polymer and polymer–ceramic batteries. Curr. Opin. Electrochem. 2018, 9, 56–63. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, Q. Dendrite-free lithium metal anodes: Stable solid electrolyte interphases for high-efficiency batteries. J. Mater. Chem. A 2015, 3, 7207–7209. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Rosso, M.; Brissot, C.; Teyssot, A.; Dollé, M.; Sannier, L.; Tarascon, J.-M.; Bouchet, R.; Lascaud, S. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 2006, 51, 5334–5340. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. Dendrite growth in lithium/polymer systems a propagation model for liquid electrolytes under galvanostatic conditions. J. Electrochem. Soc. 2003, 150, A1377–A1384. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Foroozan, T.; Sharifi-Asl, S.; Shahbazian-Yassar, R. Mechanistic understanding of Li dendrites growth by in- situ/operando imaging techniques. J. Power Sources 2020, 461, 228135. [Google Scholar] [CrossRef]
- Golozar, M.; Hovington, P.; Paolella, A.; Bessette, S.; Lagacé, M.; Bouchard, P.; Demers, H.; Gauvin, R.; Zaghib, K. In Situ Scanning Electron Microscopy Detection of Carbide Nature of Dendrites in Li–Polymer Batteries. Nano Lett. 2018, 18, 7583–7589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, D.; Paolella, A.; Gagnon, C.; Gariépy, V.; Vijh, A.; Zaghib, K. Application of Operando X-ray Diffraction and Raman Spectroscopies in Elucidating the Behavior of Cathode in Lithium-Ion Batteries. Front. Energy Res. 2018, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Nandasiri, M.I.; Forero, L.C.; Schwarz, A.M.; Shutthanandan, V.; Thevuthasan, S.; Balbuena, P.B.; Mueller, K.T.; Murugesan, V. In Situ Chemical Imaging of Solid-Electrolyte Interphase Layer Evolution in Li–S Batteries. Chem. Mater. 2017, 29, 4728–4737. [Google Scholar] [CrossRef]
- Goldstein, J.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Fiori, C.; Lifshin, E. Scanning Electron Microscopy and X-ray Microanalysis, 3rd ed.; Springer Science and Business Media: New York, NY, USA, 2003. [Google Scholar]
- Aurbach, D.; Ein-Eli, Y. The Study of Li-Graphite Intercalation Processes in Several Electrolyte Systems Using In Situ X-Ray Diffraction. J. Electrochem. Soc. 1995, 142, 1746–1752. [Google Scholar] [CrossRef]
- Whitehead, A.; Edström, K.; Rao, N.; Owen, J. In situ X-ray diffraction studies of a graphite-based Li-ion battery negative electrode. J. Power Sources 1996, 63, 41–45. [Google Scholar] [CrossRef]
- Chung, K.Y.; Yoon, W.-S.; McBreen, J.; Yang, X.-Q.; Oh, S.H.; Shin, H.C.; Cho, W.I.; Cho, B.W. In situ X-ray diffraction studies on the mechanism of capacity retention improvement by coating at the surface of LiCoO2. J. Power Sources 2007, 174, 619–623. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, D.; Trottier, J.; Gagnon, C.; Guerfi, A.; Julien, C.M.; Mauger, A.; Zaghib, K. Comparative studies of the phase evolution in M-doped LixMn1.5Ni0.5O4 (M = Co, Al, Cu and Mg) by in-situ X-ray diffraction. J. Power Sources 2014, 264, 290–298. [Google Scholar] [CrossRef]
- Zhu, W.; Paolella, A.; Kim, C.-S.; Liu, D.; Feng, Z.; Gagnon, C.; Trottier, J.; Vijh, A.; Guerfi, A.; Mauger, A.; et al. Investigation of the reaction mechanism of lithium sulfur batteries in different electrolyte systems by in situ Raman spectroscopy and in situ X-ray diffraction. Sustain. Energy Fuels 2017, 1, 737–747. [Google Scholar] [CrossRef]
- Roberts, M.R.; Madsen, A.; Nicklin, C.; Rawle, J.; Palmer, M.G.; Owen, J.R.; Hector, A.L. Direct Observation of Active Material Concentration Gradients and Crystallinity Breakdown in LiFePO4 Electrodes during Charge/Discharge Cycling of Lithium Batteries. J. Phys. Chem. C 2014, 118, 6548–6557. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Lipson, A.L.; Karmel, H.J.; Emery, J.D.; Fister, T.T.; Fenter, P.; Hersam, M.C.; Bedzyk, M.J. In Situ X-ray Study of the Solid Electrolyte Interphase (SEI) Formation on Graphene as a Model Li-ion Battery Anode. Chem. Mater. 2012, 24, 3038–3043. [Google Scholar] [CrossRef]
- Schweidler, S.; De Biasi, L.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Volume Changes of Graphite Anodes Revisited: A Combined Operando X-ray Diffraction and In Situ Pressure Analysis Study. J. Phys. Chem. C 2018, 122, 8829–8835. [Google Scholar] [CrossRef]
- Mohanty, D.; Kalnaus, S.; Meisner, R.A.; Rhodes, K.J.; Li, J.; Payzant, E.; Wood, D.; Daniel, C. Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction. J. Power Sources 2013, 229, 239–248. [Google Scholar] [CrossRef]
- Liu, Q.; He, H.; Li, Z.-F.; Liu, Y.; Ren, Y.; Lu, W.; Lu, J.; Stach, E.A.; Xie, J. Rate-Dependent, Li-Ion Insertion/Deinsertion Behavior of LiFePO4 Cathodes in Commercial 18650 LiFePO4 Cells. ACS Appl. Mater. Interfaces 2014, 6, 3282–3289. [Google Scholar] [CrossRef]
- Dong, H.; Guo, H.; He, Y.; Gao, J.; Han, W.; Lu, X.; Yan, S.; Yang, K.; Li, H.; Chen, D.; et al. Structural stability and Li-ion transport property of LiFePO4 under high-pressure. Solid State Ion. 2017, 301, 133–137. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, D.; Trottier, J.; Gagnon, C.; Howe, J.; Mauger, A.; Julien, C.M.; Zaghib, K. In-situ Raman spectroscopic investigation of LiMn1.45Ni0.45M0.1O4 (M = Cr, Co) 5 V cathode materials. J. Power Sources 2015, 298, 341–348. [Google Scholar] [CrossRef]
- Migge, S.; Sandmann, G.; Rahner, D.; Dietz, H.; Plieth, W. Studying lithium intercalation into graphite particles via in situ Raman spectroscopy and confocal microscopy. J. Solid State Electrochem. 2004, 9, 132–137. [Google Scholar] [CrossRef]
- Sole, C.; Drewett, N.E.; Hardwick, L.J. In situ Raman study of lithium-ion intercalation into microcrystalline graphite. Faraday Discuss. 2014, 172, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Shutthanandan, V.; Nandasiri, M.; Zheng, J.; Engelhard, M.H.; Xu, W.; Thevuthasan, S.; Murugesan, V. Applications of XPS in the characterization of Battery materials. J. Electron. Spectrosc. Relat. Phenom. 2019, 231, 2–10. [Google Scholar] [CrossRef]
- Baer, D.; Shutthanandan, V. Nano-Objects as Biomaterials: Immense Opportunities, Significant Challenges and the Important Use of Surface Analytical Methods; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2017.
- Dollé, M.; Sannier, L.; Beaudoin, B.; Trentin, M.; Tarascon, J.-M. Live Scanning Electron Microscope Observations of Dendritic Growth in Lithium/Polymer Cells. Electrochem. Solid-State Lett. 2002, 5, A286–A289. [Google Scholar] [CrossRef]
- Harry, K.J.; Liao, X.; Parkinson, D.Y.; Minor, A.M.; Balsara, N.P. Electrochemical Deposition and Stripping Behavior of Lithium Metal across a Rigid Block Copolymer Electrolyte Membrane. J. Electrochem. Soc. 2015, 162, A2699–A2706. [Google Scholar] [CrossRef]
- Orsini, F.; Du Pasquier, A.; Beaudouin, B.; Tarascon, J.; Trentin, M.; Langenhuizen, N.; de Beer, E.; Notten, P. In situ SEM study of the interfaces in plastic lithium cells. J. Power Sources 1999, 81-82, 918–921. [Google Scholar] [CrossRef]
- Golozar, M.; Paolella, A.; Demers, H.; Savoie, S.; Girard, G.; Delaporte, N.; Gauvin, R.; Guerfi, A.; Lorrmann, H.; Zaghib, K. Direct observation of lithium metal dendrites with ceramic solid electrolyte. Sci. Rep. 2020, 10, 18410. [Google Scholar] [CrossRef]
- Kaboli, S.; Demers, H.; Paolella, A.; Darwiche, A.; Dontigny, M.; Clément, D.; Guerfi, A.; Trudeau, M.; Goodenough, J.B.; Zaghib, K. Behavior of Solid Electrolyte in Li-Polymer Battery with NMC Cathode via In-Situ Scanning Electron Microscopy. Nano Lett. 2020, 20, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New lithium metal polymer solid state battery for an ultrahigh energy: Nano C-LiFePO4 versus nano Li1.2V3O8. Nano Lett. 2015, 15, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Hovington, P.; Dontigny, M.; Guerfi, A.; Trottier, J.; Lagacé, M.; Mauger, A.; Julien, C.; Zaghib, K. In situ Scanning electron microscope study and microstructural evolution of nano silicon anode for high energy Li-ion batteries. J. Power Sources 2014, 248, 457–464. [Google Scholar] [CrossRef]
- Golozar, M.; Paolella, A.; Demers, H.; Bessette, S.; Lagacé, M.; Bouchard, P.; Guerfi, A.; Gauvin, R.; Zaghib, K. In situ observation of solid electrolyte interphase evolution in a lithium metal battery. Commun. Chem. 2019, 2, 131. [Google Scholar] [CrossRef]
- Kaboli, S.; Noel, P.; Clément, D.; Demers, H.; Paolella, A.; Bouchard, P.; Trudeau, M.L.; Goodenough, J.B.; Zaghib, K. On high-temperature evolution of passivation layer in Li–10 wt% Mg alloy via in situ SEM-EBSD. Sci. Adv. 2020, 6, eabd5708. [Google Scholar] [CrossRef]
- OrsayPhysics. Scientific Overviews: What is UHV? Available online: http://www.orsayphysics.com/what-is-uhv (accessed on 7 September 2018).
- Li, Y.; Sun, Y.; Butz, B.; Yan, K.; Koh, A.L.; Zhao, J.; Pei, A.; Cui, Y. Revealing nanoscale passivation and corrosion mechanisms of reactive battery materials in gas environments. Nano Lett. 2017, 17, 5171–5178. [Google Scholar] [CrossRef] [PubMed]
- Hren, J.J. Barriers to AEM: Contamination and etching. In Introduction to Analytical Electron Microscopy; Hren, J.J., Goldstein, J.I., Joy, D.C., Eds.; Springer: Boston, MA, USA, 1979; pp. 481–505. [Google Scholar]
- Egerton, R. Radiation damage to organic and inorganic specimens in the TEM. Micron 2019, 119, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Egerton, R. Mechanisms of radiation damage in beam-sensitive specimens, for TEM accelerating voltages between 10 and 300 kV. Microsc. Res. Tech. 2012, 75, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.H.; Trehan, P.N.; Singh, N.; Chand, B.; Mehta, D.; Garg, M.L.; Garg, R.R.; Singh, S.; Puri, S. A review, bibliography, and tabulation of K, L, and higher atomic shell x-ray fluorescence yields. J. Phys. Chem. Ref. Data 1994, 23, 339–364. [Google Scholar] [CrossRef]
- Kushima, A.; So, K.P.; Su, C.; Bai, P.; Kuriyama, N.; Maebashi, T.; Fujiwara, Y.; Bazant, M.Z.; Li, J. Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: Root growth, dead lithium and lithium flotsams. Nano Energy 2017, 32, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.H.; Zhong, L.; Zhang, L.; Kushima, A.; Mao, S.X.; Li, J.; Ye, Z.Z.; Sullivan, J.P.; Huang, J.Y. Lithium fiber growth on the anode in a nanowire lithium ion battery during charging. Appl. Phys. Lett. 2011, 98, 183107. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi, H.M.; Au, M.; Chen, N.; Heiden, P.A.; Yassar, R.S. Real-time observation of lithium fibers growth inside a nanoscale lithium-ion battery. Appl. Phys. Lett. 2011, 99, 123113. [Google Scholar] [CrossRef]
- Wang, Z.; Santhanagopalan, D.; Zhang, W.; Wang, F.; Xin, H.L.; He, K.; Li, J.; Dudney, N.; Meng, Y.S. In Situ STEM-EELS Observation of Nanoscale Interfacial Phenomena in All-Solid-State Batteries. Nano Lett. 2016, 16, 3760–3767. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Alvarado, J.; Wang, S.; Sina, M.; Lu, B.; Bouwer, J.; Xu, W.; Xiao, J.; Zhang, J.-G.; et al. New Insights on the Structure of Electrochemically Deposited Lithium Metal and Its Solid Electrolyte Interphases via Cryogenic TEM. Nano Lett. 2017, 17, 7606–7612. [Google Scholar] [CrossRef]
- Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.L.; Joubert, L.M.; Chin, R.; Koh, A.L.; Yu, Y.; Perrino, J. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).