Functionalizing NaGdF4:Yb,Er Upconverting Nanoparticles with Bone-Targeting Phosphonate Ligands: Imaging and In Vivo Biodistribution
Abstract
:1. Introduction
2. Results and Discussion
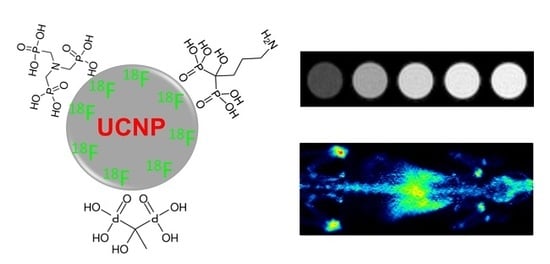
2.1. Synthesis and Surface Decoration of UCNPs NaGdF4:Yb,Er
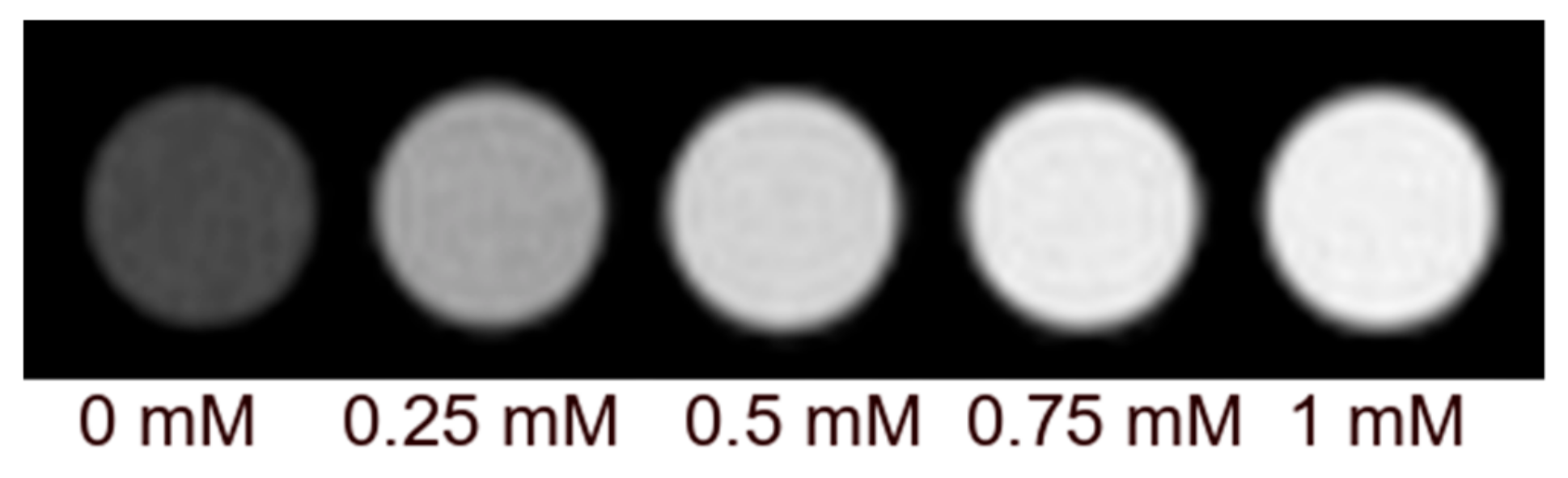
2.2. MRI Characterization and Interaction with Hydroxyapatite
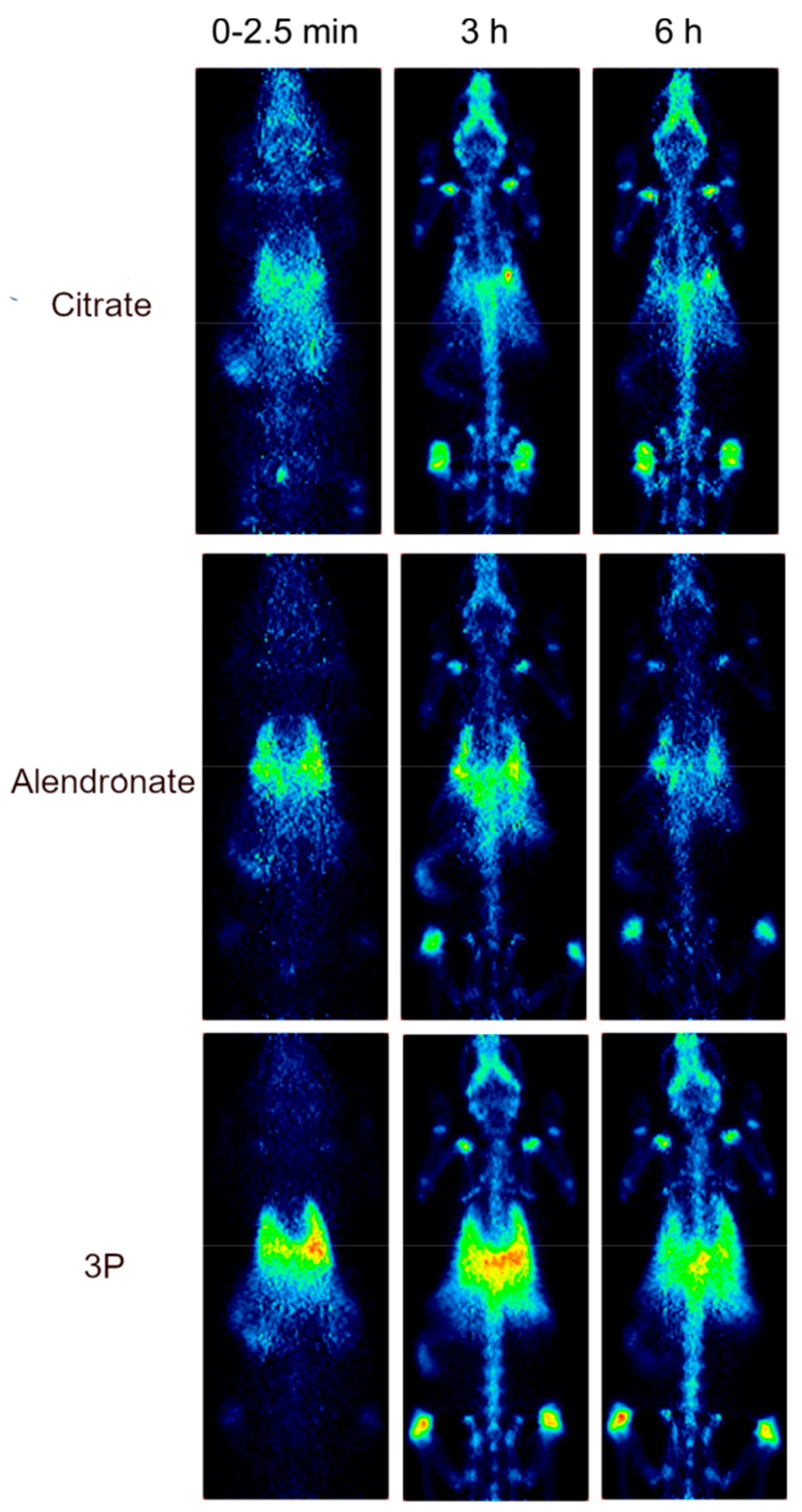
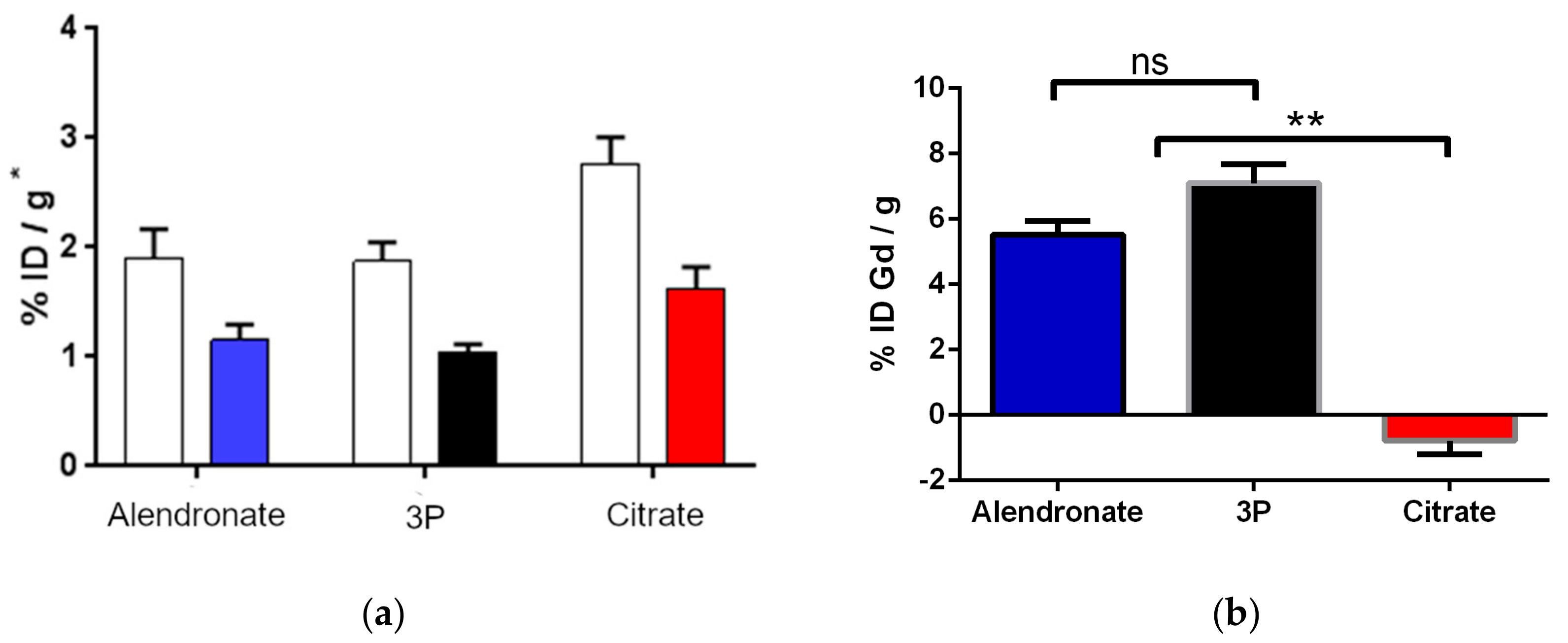
2.3. Biodistribution Study by Radiolabeling UCNPs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of UCNPs NaGdF4:Yb,Er
3.3. Functionalization of UCNPs/Ligand Exchange
3.4. Relaxivity Measurements and HA Binding
3.5. Labeling of UCNPs with 18F
3.6. Gd3+ Determination from Ex Vivo Femur Bones
3.7. Instrumentation
3.7.1. Transmission Electron Microscopy (TEM)
3.7.2. Fourier Transform Infrared (FTIR)
3.7.3. Dynamic Light Scattering
3.7.4. MRI
3.7.5. PET
3.7.6. ICP-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alonso-de Castro, S.; Ruggiero, E.; Ruiz-de-Angulo, A.; Rezabal, E.; Mareque-Rivas, J.C.; Lopez, X.; López-Gallego, F.; Salassa, L. Riboflavin as a bioorthogonal photocatalyst for the activation of a PtIV prodrug. Chem. Sci. 2017, 8, 4619–4625. [Google Scholar] [CrossRef]
- Alonso-de Castro, S.; Cortajarena, A.L.; López-Gallego, F.; Salassa, L. Bioorthogonal Catalytic Activation of Platinum and Ruthenium Anticancer Complexes by FAD and Flavoproteins. Angew. Chem. Int. Ed. 2018, 57, 3143–3147. [Google Scholar] [CrossRef] [PubMed]
- Alonso-de Castro, S.; Terenzi, A.; Hager, S.; Englinger, B.; Faraone, A.; Martínez, J.C.; Galanski, M.; Keppler, B.K.; Berger, W.; Salassa, L. Biological activity of PtIV prodrugs triggered by riboflavin-mediated bioorthogonal photocatalysis. Sci. Rep. 2018, 8, 17198. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, E.; Garino, C.; Mareque-Rivas, J.C.; Habtemariam, A.; Salassa, L. Upconverting Nanoparticles Prompt Remote Near-Infrared Photoactivation of Ru(II)–Arene Complexes. Chem. Eur. J. 2016, 22, 2801–2811. [Google Scholar] [CrossRef]
- Ruggiero, E.; Hernández-Gil, J.; Mareque-Rivas, J.C.; Salassa, L. Near infrared activation of an anticancer PtIV complex by Tm-doped upconversion nanoparticles. Chem. Commun. 2015, 51, 2091–2094. [Google Scholar] [CrossRef]
- Ruggiero, E.; Habtemariam, A.; Yate, L.; Mareque-Rivas, J.C.; Salassa, L. Near infrared photolysis of a Ru polypyridyl complex by upconverting nanoparticles. Chem. Commun. 2014, 50, 1715–1718. [Google Scholar] [CrossRef]
- Ruggiero, E.; Alonso-De Castro, S.; Habtemariam, A.; Salassa, L. Upconverting nanoparticles for the near infrared photoactivation of transition metal complexes: New opportunities and challenges in medicinal inorganic photochemistry. Dalton Trans. 2016, 45, 13012–13020. [Google Scholar] [CrossRef]
- Hemmer, E.; Acosta-Mora, P.; Méndez-Ramos, J.; Fischer, S. Optical nanoprobes for biomedical applications: Shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Li, F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Yang, Y.; Sun, Y.; Wu, Y.; Gao, Y.; Feng, W.; Li, F. Biodistribution of sub-10nm PEG-modified radioactive/upconversion nanoparticles. Biomaterials 2013, 34, 7127–7134. [Google Scholar] [CrossRef] [PubMed]
- Wise-Milestone, L.; Akens, M.K.; Lo, V.C.K.; Yee, A.J.; Wilson, B.C.; Whyne, C.M. Local treatment of mixed osteolytic/osteoblastic spinal metastases: Is photodynamic therapy effective? Breast Cancer Res. Treat. 2012, 133, 899–908. [Google Scholar] [CrossRef]
- Lo, V.C.K.; Akens, M.K.; Wise-Milestone, L.; Yee, A.J.M.; Wilson, B.C.; Whyne, C.M. The benefits of photodynamic therapy on vertebral bone are maintained and enhanced by combination treatment with bisphosphonates and radiation therapy. J. Orthop. Res. 2013, 31, 1398–1405. [Google Scholar] [CrossRef]
- Panahifar, A.; Mahmoudi, M.; Doschak, M.R. Synthesis and in Vitro Evaluation of Bone-Seeking Superparamagnetic Iron Oxide Nanoparticles as Contrast Agents for Imaging Bone Metabolic Activity. ACS Appl. Mater. Interfaces 2013, 5, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gangal, G.; Uludağ, H. ‘Magic bullets’ for bone diseases: Progress in rational design of bone-seeking medicinal agents. Chem. Soc. Rev. 2007, 36, 507–531. [Google Scholar] [CrossRef]
- Li, R.; Ji, Z.; Dong, J.; Chang, C.H.; Wang, X.; Sun, B.; Wang, M.; Liao, Y.-P.; Zink, J.I.; Nel, A.E.; et al. Enhancing the Imaging and Biosafety of Upconversion Nanoparticles through Phosphonate Coating. ACS Nano 2015, 9, 3293–3306. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.-C.; Carling, C.-J.; Chua, S.Y.; Wilson, D.; Johnsen, B.; Baillie, D.; Branda, N.R. Photomodulation of Fluorescent Upconverting Nanoparticle Markers in Live Organisms by Using Molecular Switches. Chem. Eur. J. 2012, 18, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.J.J.; Oakden, W.; Stanisz, G.J.; Scott Prosser, R.; van Veggel, F.C.J.M. Size-Tunable, Ultrasmall NaGdF4 Nanoparticles: Insights into Their T1 MRI Contrast Enhancement. Chem. Mater. 2011, 23, 3714–3722. [Google Scholar] [CrossRef]
- Wang, Z.; Carniato, F.; Xie, Y.; Huang, Y.; Li, Y.; He, S.; Zang, N.; Rinehart, J.D.; Botta, M.; Gianneschi, N.C. High Relaxivity Gadolinium-Polydopamine Nanoparticles. Small 2017, 13, 1701830. [Google Scholar] [CrossRef]
- Jacques, V.; Dumas, S.; Sun, W.-C.; Troughton, J.S.; Greenfield, M.T.; Caravan, P. High-relaxivity magnetic resonance imaging contrast agents. Part 2. Optimization of inner- and second-sphere relaxivity. Investig. Radiol. 2010, 45, 613–624. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Gorris, H.H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.; Bauer, H.; Mintorovitch, J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef]
- Pintaske, J.; Martirosian, P.; Graf, H.; Erb, G.; Lodemann, K.-P.; Claussen, C.D.; Schick, F. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in Human Blood Plasma at 0.2, 1.5, and 3 Tesla. Investig. Radiol. 2006, 41, 213–221. [Google Scholar] [CrossRef]
- Koziorowski, J.; Stanciu, A.; Gomez-Vallejo, V.; Llop, J. Radiolabeled Nanoparticles for Cancer Diagnosis and Therapy. Anticancer Agents Med. Chem. 2017, 17, 333–354. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, M.; Sun, Y.; Zhang, X.; Zhu, X.; Wu, Z.; Wu, D.; Li, F. Fluorine-18-labeled Gd3+/Yb3+/Er3+ co-doped NaYF4 nanophosphors for multimodality PET/MR/UCL imaging. Biomaterials 2011, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, M.; Liang, S.; Zhang, Y.; Li, C.; Mou, T.; Yang, W.; Zhang, X.; Li, B.; Huang, C.; et al. Fluorine-18 labeled rare-earth nanoparticles for positron emission tomography (PET) imaging of sentinel lymph node. Biomaterials 2011, 32, 2999–3007. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Li, C.; Zhou, J.; Li, C.; Yang, T.; Zhang, X.; Yi, T.; Wu, D.; Li, F. 18F-labeled magnetic-upconversion nanophosphors via rare-earth cation-assisted ligand assembly. ACS Nano 2011, 5, 3146–3157. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Han, G. Lanthanide-Doped Upconversion Nanoparticles for Imaging-Guided Drug Delivery and Therapy. In Advances in Nanotheranostics I: Design and Fabrication of Theranosic Nanoparticles; Dai, Z., Ed.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2016; pp. 139–164. ISBN 978-3-662-48544-6. [Google Scholar]
- Wang, J.; Zhang, L.; Peng, F.; Shi, X.; Leong, D.T. Targeting Endothelial Cell Junctions with Negatively Charged Gold Nanoparticles. Chem. Mater. 2018, 30, 3759–3767. [Google Scholar] [CrossRef]
- Tan, Y.L.; Leong, D.T.; Ho, H.K. Inorganic Nanomaterials as Highly Efficient Inhibitors of Cellular Hepatic Fibrosis. ACS Appl. Mater. Interfaces 2018, 10, 31938–31946. [Google Scholar] [CrossRef]
- Darrah, T.H.; Prutsman-Pfeiffer, J.J.; Poreda, R.J.; Campbell, M.E.; Hauschka, P.V.; Hannigan, R.E. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 2009, 1, 479–488. [Google Scholar] [CrossRef] [PubMed]


| r1 (mM−1 s−1) | |||
|---|---|---|---|
| H2O | MES Buffer | Human Serum | |
| Gd-UCNP-etidronate | 3.47 ± 0.02 | 8.47 ± 0.02 | 9.73 ± 0.02 |
| Gd-UCNP-alendronate | 0.59 ± 0.05 | 0.66 ± 0.03 | 0.27 ± 0.03 |
| Gd-UCNP-3P | 0.70 ± 0.01 | 0.69 ± 0.07 | 0.76 ± 0.05 |
| Parameter | Condition |
|---|---|
| Nebulizer gas flow | 1.075 L min−1 |
| Spray chamber | 2.70 °C |
| Extraction Lens | −111.3 V |
| CCT focus lens | 0.6 V |
| Plasma power | 1550 w |
| Cooling gas flow | 14 L min−1 |
| Auxiliary gas flow | 0.8 L min−1 |
| Colision gas flow | 4.733 L min−1 |
| Pole bias | −18 V |
| CCT bias | −21 V |
| Wash time | 30 s |
| Uptake time | 50 s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-de Castro, S.; Ruggiero, E.; Lekuona Fernández, A.; Cossío, U.; Baz, Z.; Otaegui, D.; Gómez-Vallejo, V.; Padro, D.; Llop, J.; Salassa, L. Functionalizing NaGdF4:Yb,Er Upconverting Nanoparticles with Bone-Targeting Phosphonate Ligands: Imaging and In Vivo Biodistribution. Inorganics 2019, 7, 60. https://doi.org/10.3390/inorganics7050060
Alonso-de Castro S, Ruggiero E, Lekuona Fernández A, Cossío U, Baz Z, Otaegui D, Gómez-Vallejo V, Padro D, Llop J, Salassa L. Functionalizing NaGdF4:Yb,Er Upconverting Nanoparticles with Bone-Targeting Phosphonate Ligands: Imaging and In Vivo Biodistribution. Inorganics. 2019; 7(5):60. https://doi.org/10.3390/inorganics7050060
Chicago/Turabian StyleAlonso-de Castro, Silvia, Emmanuel Ruggiero, Aitor Lekuona Fernández, Unai Cossío, Zuriñe Baz, Dorleta Otaegui, Vanessa Gómez-Vallejo, Daniel Padro, Jordi Llop, and Luca Salassa. 2019. "Functionalizing NaGdF4:Yb,Er Upconverting Nanoparticles with Bone-Targeting Phosphonate Ligands: Imaging and In Vivo Biodistribution" Inorganics 7, no. 5: 60. https://doi.org/10.3390/inorganics7050060
APA StyleAlonso-de Castro, S., Ruggiero, E., Lekuona Fernández, A., Cossío, U., Baz, Z., Otaegui, D., Gómez-Vallejo, V., Padro, D., Llop, J., & Salassa, L. (2019). Functionalizing NaGdF4:Yb,Er Upconverting Nanoparticles with Bone-Targeting Phosphonate Ligands: Imaging and In Vivo Biodistribution. Inorganics, 7(5), 60. https://doi.org/10.3390/inorganics7050060