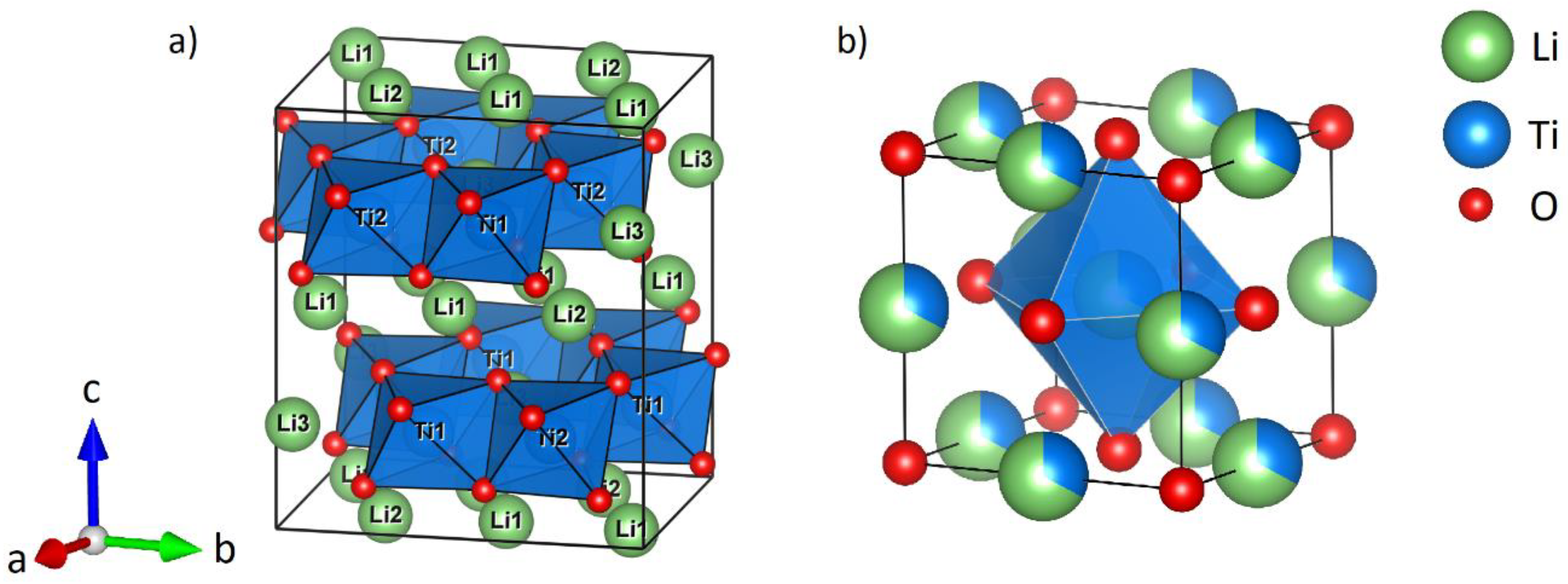
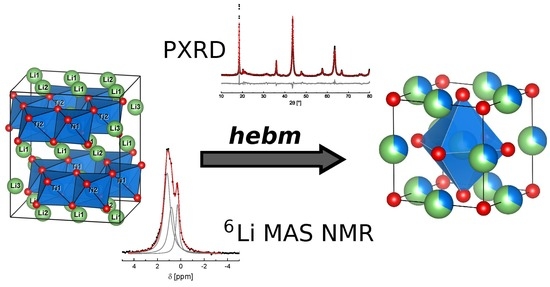
2.1. Mechanochemical Formation of α-Li2TiO3 from LiOH and TiO2
High energy ball milling of LiOH with rutile or anatase as titania precursors in yttrium stabilized zirconia (ZrO
2) and tungsten carbide/cobalt hard metal (WC) tools leads to the formation of new reflections in the X-ray diffractogram which may be indexed by a cubic unit cell, as already noted in a previous study [
19]. The new reflections could be assigned to either LiTiO
2 or Li
2TiO
3 with NaCl type structure (SG
Fm−3
m) and a refined lattice parameter
a varying between 4.15 and 4.16 Å, depending on the reaction conditions. While both compounds have the same structure type, a quantitative reduction of Ti
4+ to Ti
3+ is required to form LiTiO
2. This is unlikely under the given conditions, since a reducing agent or oxygen release would be necessary. However, the appearance of very small portions of Ti
3+ in TiO
2 has been described as a result of defect formation by mechanochemical methods [
20]. The mechanochemical formation of LiTiO
2 has been described before by milling of Li
2O, TiO
2 and Ti, with larger impurities of elemental iron from abrasion of the steel milling tools [
21]. Furthermore, LiTiO
2 is black in color [
22], while the products of our process are white if ZrO
2 tools are used or gray if WC tools are used. The grey color is based upon abrasion from the WC tools [
23]. Hence, the composition of the milling products corresponds to a composition close to Li
2TiO
3 with a mean Ti oxidation state of nearly IV. Due to the additional anatase to high pressure phase transformation [
2], milling products of LiOH and anatase show a complex phase composition. The Rietveld refinement of the milling product from ZrO
2 tools after 6 h of milling at 600 rpm is shown as an example in the
Supplementary Materials (Figure S1). The reflections of the high-pressure polymorph TiO
2-II overlap with the reflections of anatase and α-Li
2TiO
3. Additionally, milling with anatase as the titania precursor leads to a smaller portion of cubic phase after the same milling conditions.
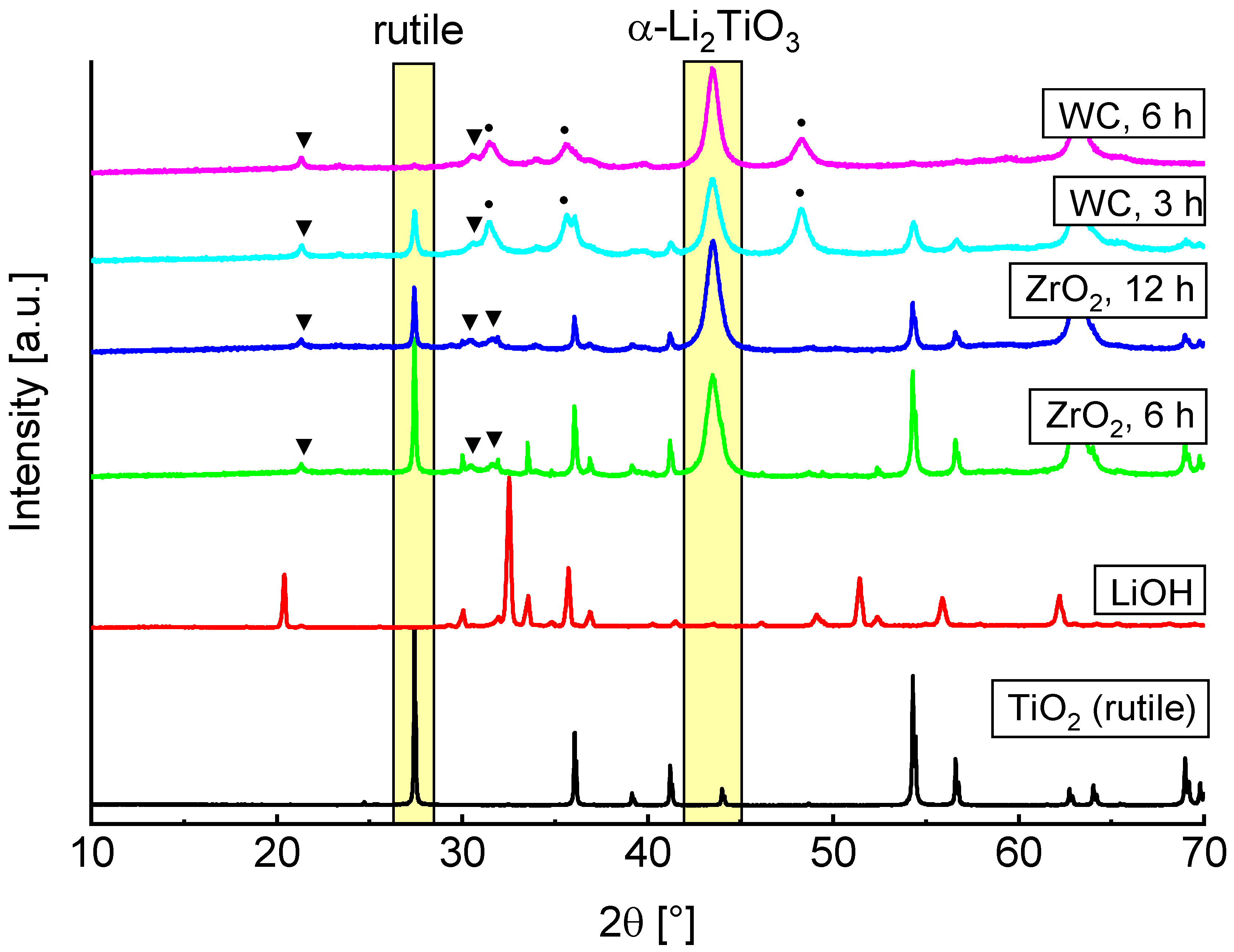
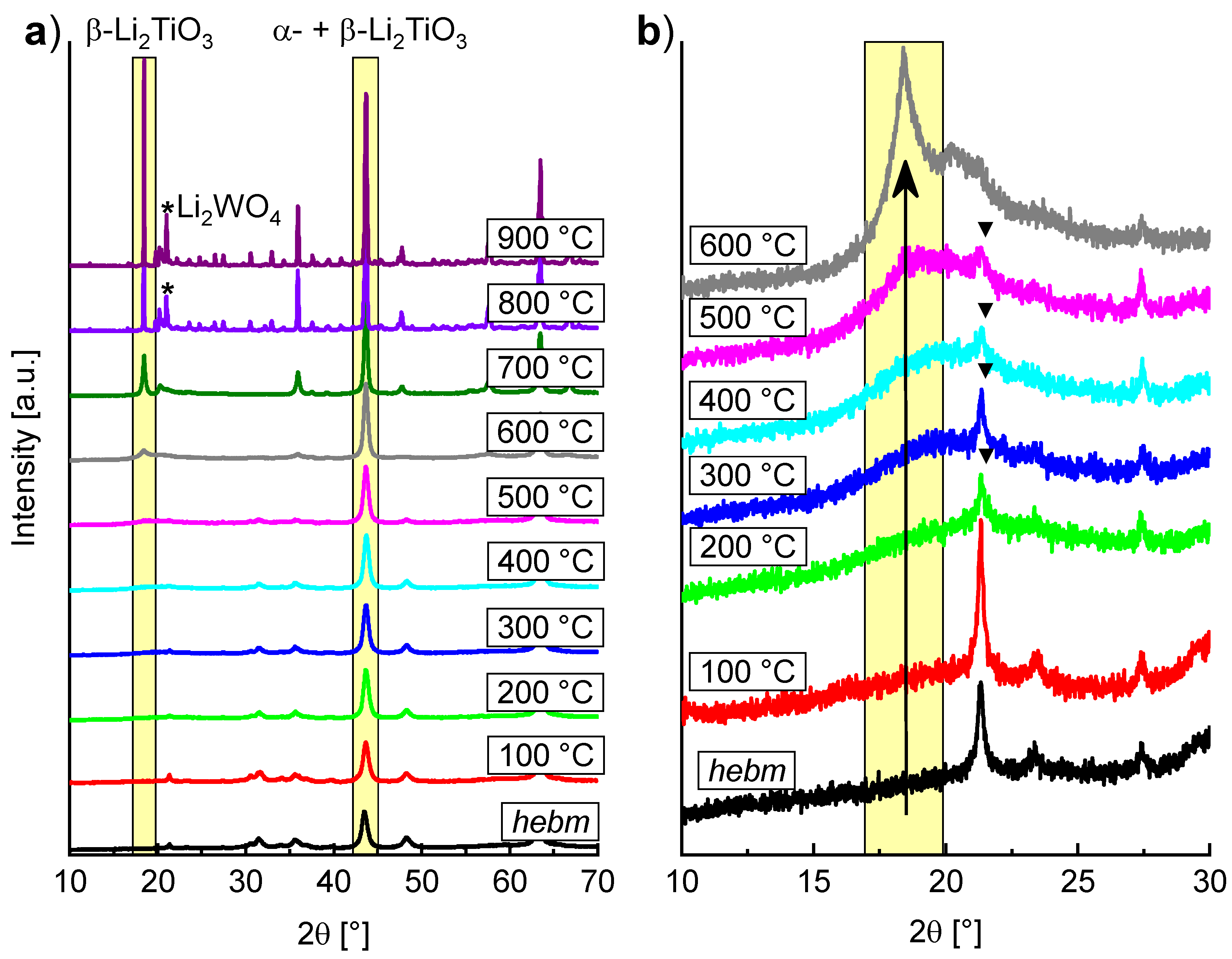
Based on the reported results we decided to only discuss the rutile ball milling more closely in the following sections. PXRD measurements of LiOH, rutile and the milling products with different parameters are shown in
Figure 2. Decrease of the TiO
2 main reflection and rise of new α-Li
2TiO
3 reflections are marked by yellow rectangles. Formation of Li
2CO
3 (marked by triangles) is caused by unreacted amorphous LiOH which forms crystalline Li
2CO
3 on contact with air, allowing for quantification by Rietveld refinement.
A faster formation of α-Li
2TiO
3 can be observed by use of WC milling tools. Rietveld refinements indicate an introduction of 2 to 4% crystalline WC from abrasion of the tools (marked by circles). After a milling time of 6 h no rutile reflections are visible, while these are still present after 12 h if ZrO
2 tools are used. This is most likely due to the different hardness of the milling tools and the resulting variations in impact energies. There are already reports in the literature which indicate that the milling tools can have a large influence on the formation of different phases [
3,
24].
Only results obtained by using WC tools are discussed more closely in the following, since they show a more efficient conversion to cubic Li
2TiO
3. Depending on the milling parameters, the refined structural parameters of the PXRD measurements are slightly different. The lattice parameter
a varies and the crystallite size is in the range of 10 to 15 nm. Since OH
− anions are readily available by using LiOH in the mechanochemical synthesis, substitution of O
2− by OH
− may be the case to some extent, resulting in a variation of the lattice parameter (4.15–4.16 Å) depending on the degree of substitution. Because only traces of H
2O were found during TG-IR investigation (
Supplementary Materials Figures S4 and S5) only a small amount of OH
− may be assumed but different OH
− contents caused by varying conditions of synthesis account for the different lattice parameters of the as prepared products.
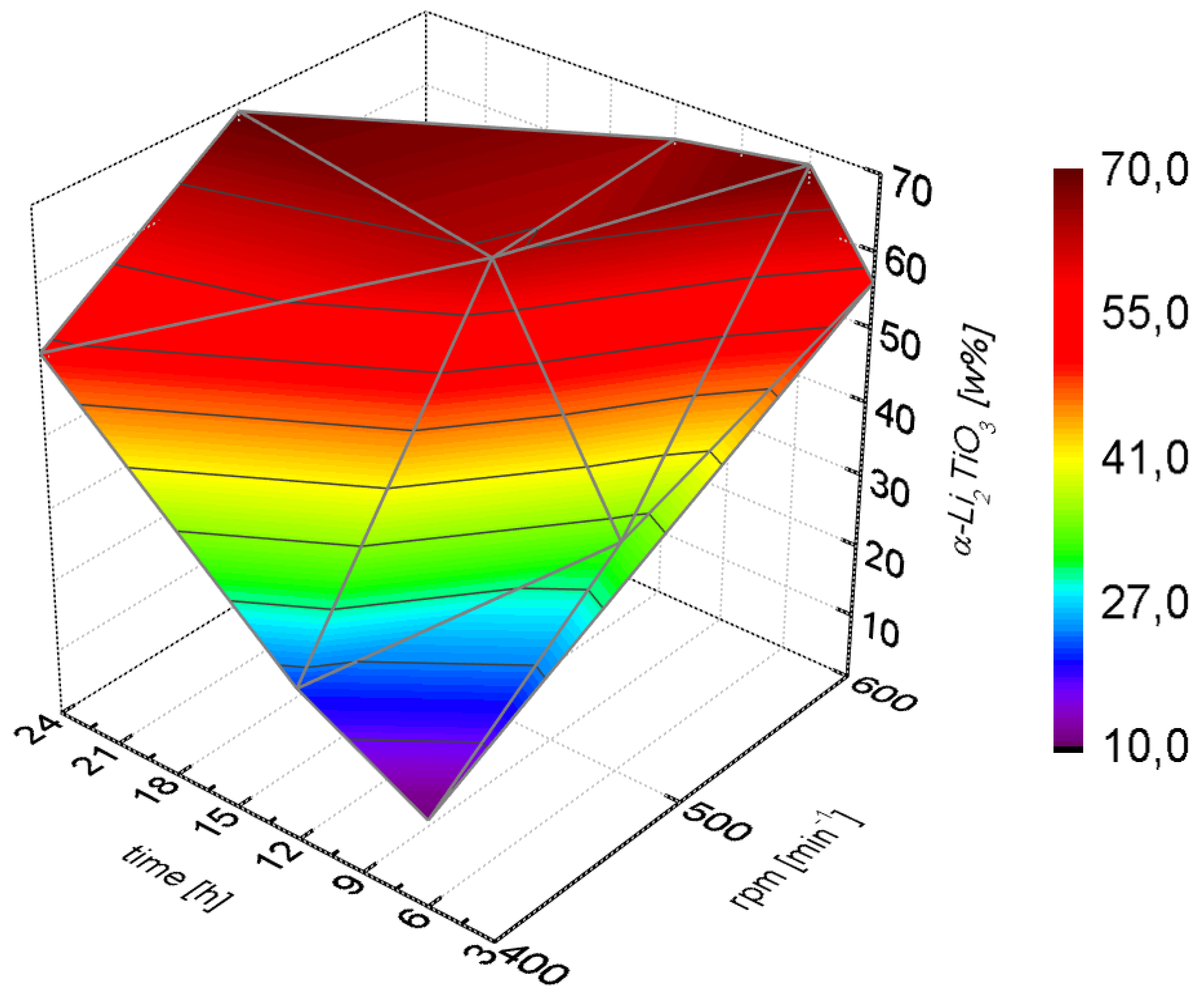
Higher amounts of cubic Li
2TiO
3 were only obtained after long milling times or high milling speeds (
Figure 3). The cubic phase formed slowly at lower rotational speeds (400 rpm) and even after 24 h a portion of 50% was not exceeded. At high milling speeds (600 rpm) a portion larger than 50% was already achieved after milling for only three hours.
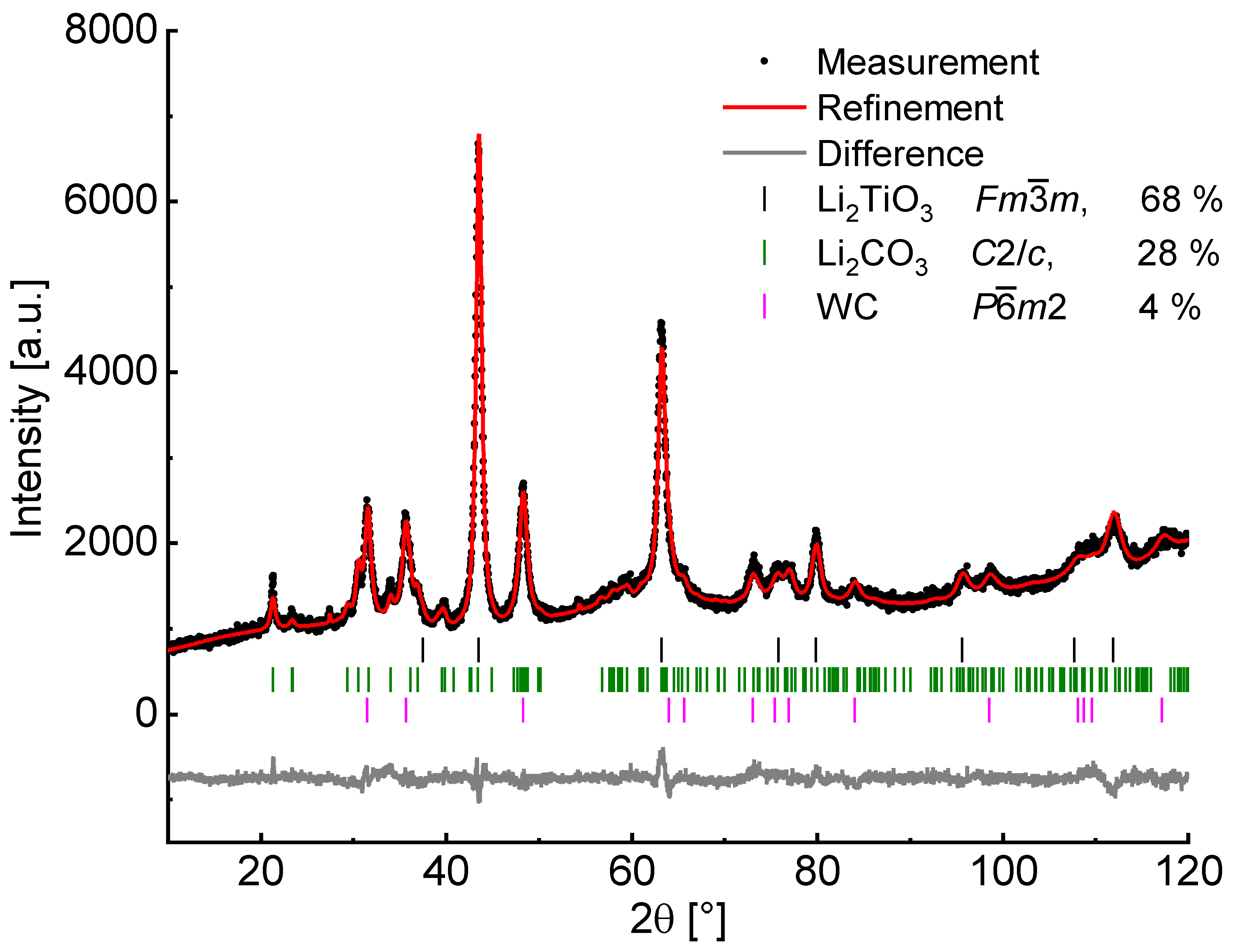
The percentage of α-Li
2TiO
3 after milling at 600 rpm for 6 h amounts to 68%, as analyzed by Rietveld refinement (
Figure 4) neglecting possibly existing amorphous matter. Since Li
2CO
3 is still present after complete conversion of TiO
2 and an increased baseline intensity is visible, a portion of amorphous material may be assumed. Handling of the milling product in a glovebox with subsequent measurement in an airtight dome sample holder (Rietveld refinement in
Supplementary Materials Figure S2) reveals no crystalline phase of LiOH nor Li
2CO
3, therefore Li
2CO
3 is formed after the milling process from the amorphous material by exposure to air. Measurements with an internal standard (50% of α-Al
2O
3) led to the identification of approximately 35% amorphous content, which may contain missing amounts of TiO
2.
If the amorphous content is assumed to contain the starting composition, the total formula may be written as Li
2−4xTi
1+xO
3 to consider a non-stoichiometry. A formula of Li
1.4Ti
1.15O
3 (
x = 0.15) is necessary to stay in the range of the starting composition with a Li to Ti ratio of 2:1. A constrained refinement of the Li and Ti occupancy on the
4a site of α-Li
2TiO
3 also suggests a lithium deficiency. However, to preserve electric neutrality one titanium substitutes four lithium atoms resulting in a small electronic contrast and, accordingly, these refinements rather represent a general tendency and no absolute values. Since Li
2CO
3 crystallized only after the reaction, the remaining amorphous content may be assumed to be only or mainly TiO
2 in which case a nearly stoichiometric compound with the formula Li
2TiO
3 would be obtained. Thermogravimetric analysis reveals a mass loss of 16.2% (
Supplementary Materials, Figure S4) upon heating to 1000 °C. IR coupling of the gas flow shows primarily characteristic bands of carbon dioxide besides traces of H
2O (
Supplementary Materials, Figure S5), indicating crystalline Li
2CO
3 as the main fraction responsible for the mass loss. Therefore, the amorphous background is assumed to be primarily TiO
2.
SEM images (
Supplementary Materials, Figure S3) indicate that large agglomerates with a seemingly granular surface were formed in the milling process. The primary grains of the agglomerates that form the surface are visible. The grain size of the primary grains is in the range of 20 nm and is of the same order as the refined crystallite sizes.
2.2. Thermal Transformation of α-Li2TiO3 to β-Li2TiO3
The thermal stability of mechanochemical formed α-Li
2TiO
3 (milling of LiOH and rutile in WC tools at 600 rpm for 6 h) was investigated by successive heating in 100 K steps for 1 h and subsequent PXRD measurement at ambient conditions after each step (
Figure 5). Between 300 and 500 °C, the formation of a broad reflection in the range around 20° 2θ indicates a short-range ordering phenomenon. Since the range in which the reflection is formed coincides with the position of the 002-reflection (18.5°) of β-Li
2TiO
3, the broad reflection is probably caused by the nucleation of the monoclinic phase. Sharp reflections of β-Li
2TiO
3 become evident between 500 and 600 °C. The transformation is completed at temperatures over 700 °C. Compared to the hydrothermal synthesis of α-Li
2TiO
3, sharp reflections of β-Li
2TiO
3 are visible at 420 °C and the transformation is nearly complete at 500 °C, with only minor amounts of the α-phase visible (estimated at 12%) [
14].
The lattice parameter
a of α-Li
2TiO
3 changes from 4.1588(2) to 4.1435(1) Å after heating to 200 °C for 1 h. This may be a consequence of the previously mentioned hypothetical OH
− contents in the lattice of an as-prepared α-Li
2TiO
3. Heating induces a transformation of OH
− to O
2− and H
2O, leaving the cubic phase with a smaller lattice parameter. Additionally, a high phase portion of α-Li
2TiO
3 has been obtained under these mild conditions, with only 7% Li
2CO
3 remaining and without affecting crystallite size of the cubic phase (Rietveld refinement shown in
Supplementary Materials, Figure S6).
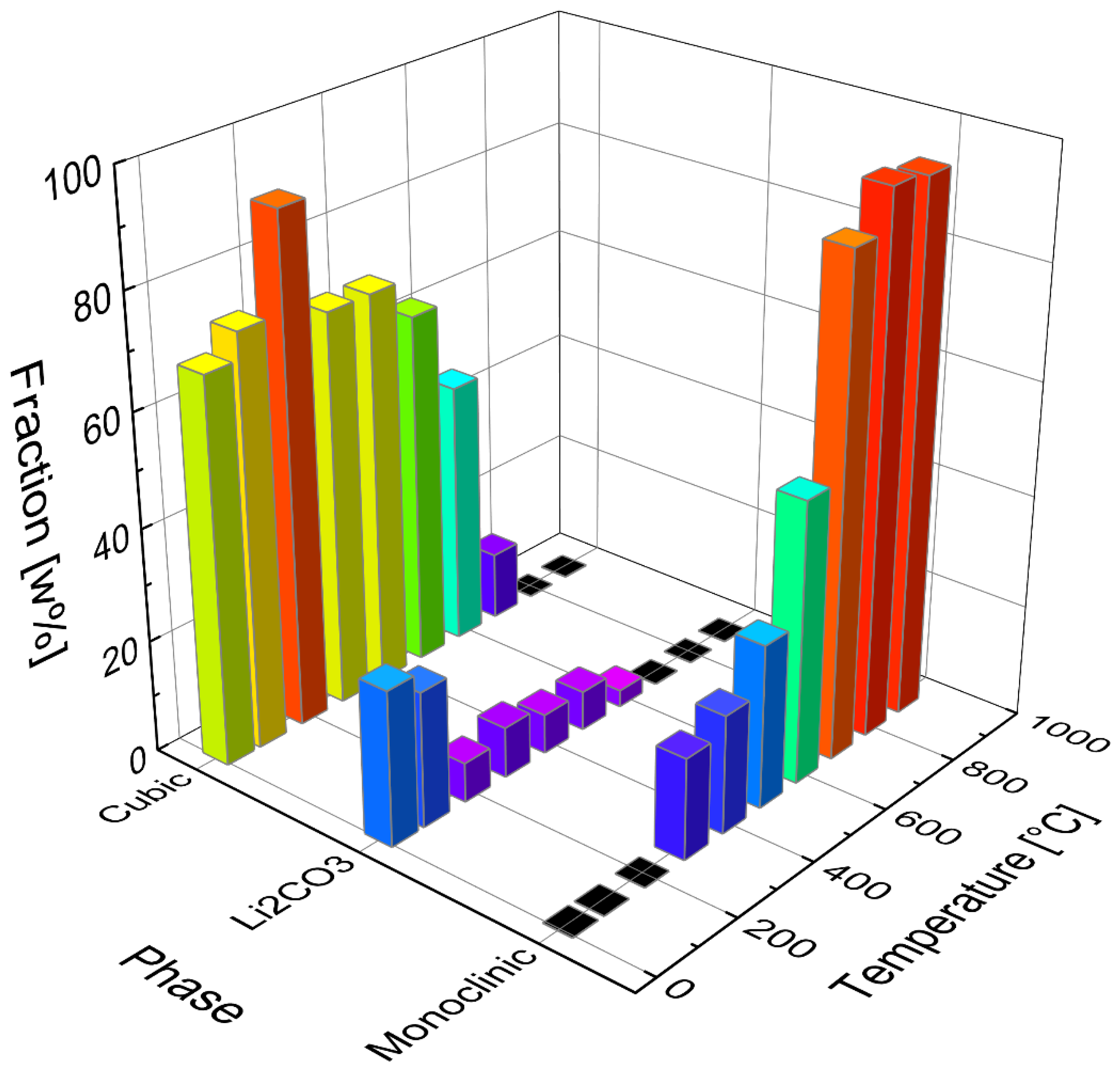
In the temperature range between 300 °C and 600 °C, a single fraction of β-Li
2TiO
3 was used in the refinements to estimate the proportion of cubic and monoclinic Li
2TiO
3. Starting with a temperature of 600 °C a three-fraction model for β-Li
2TiO
3 was used (described under
Section 3.4). Increasing amounts of 30 to 90% monoclinic fraction were refined in the temperature range between 300 and 700 °C, while the amount of α-Li
2TiO
3 was decreasing. However, up to 500 °C the intensities of the cubic reflections are hardly affected. This either indicates that the monoclinic ordering arises from byproducts without affecting the cubic structure, or that the ordering takes place continuously. The refined phase portions of α-Li
2TiO
3 (cubic) Li
2CO
3 and β-Li
2TiO
3 (monoclinic) are depicted in
Figure 6 with the corresponding calcination temperature.
Thermogravimetric analysis (
Supplementary Materials, Figure S4) between 25 and 1000 °C shows a mass loss up to the temperature range of 575 to 600 °C (attributed to Li
2CO
3). An exothermic signal arises in the calculated differential thermal analysis (DTA) curve, which correlates to the complete crystallization of β-Li
2TiO
3 as observed in the PXRD measurements. At 600 °C a composition with equal amounts of α- and β-Li
2TiO
3 was refined. Fitting with only the monoclinic phase led to an insufficient refinement (
GOF = 4.39) while inclusion of the cubic phase results in a greatly improved description (
GOF = 1.42). At 700 °C an abrupt increase to >90% β-Li
2TiO
3 occurs, with only minor amounts of the cubic phase remaining. Fitting with only the monoclinic structure results in a similar description (
GOF = 2.09) as by using both structures (
GOF = 1.93). Though the improvement of the description is small, this may still be attributed to remaining portions of the cubic phase. This leads to an overall interpretation that an ordering phenomenon takes place in the cubic phase at lower temperatures with a discrete structure change between 600 and 700 °C.
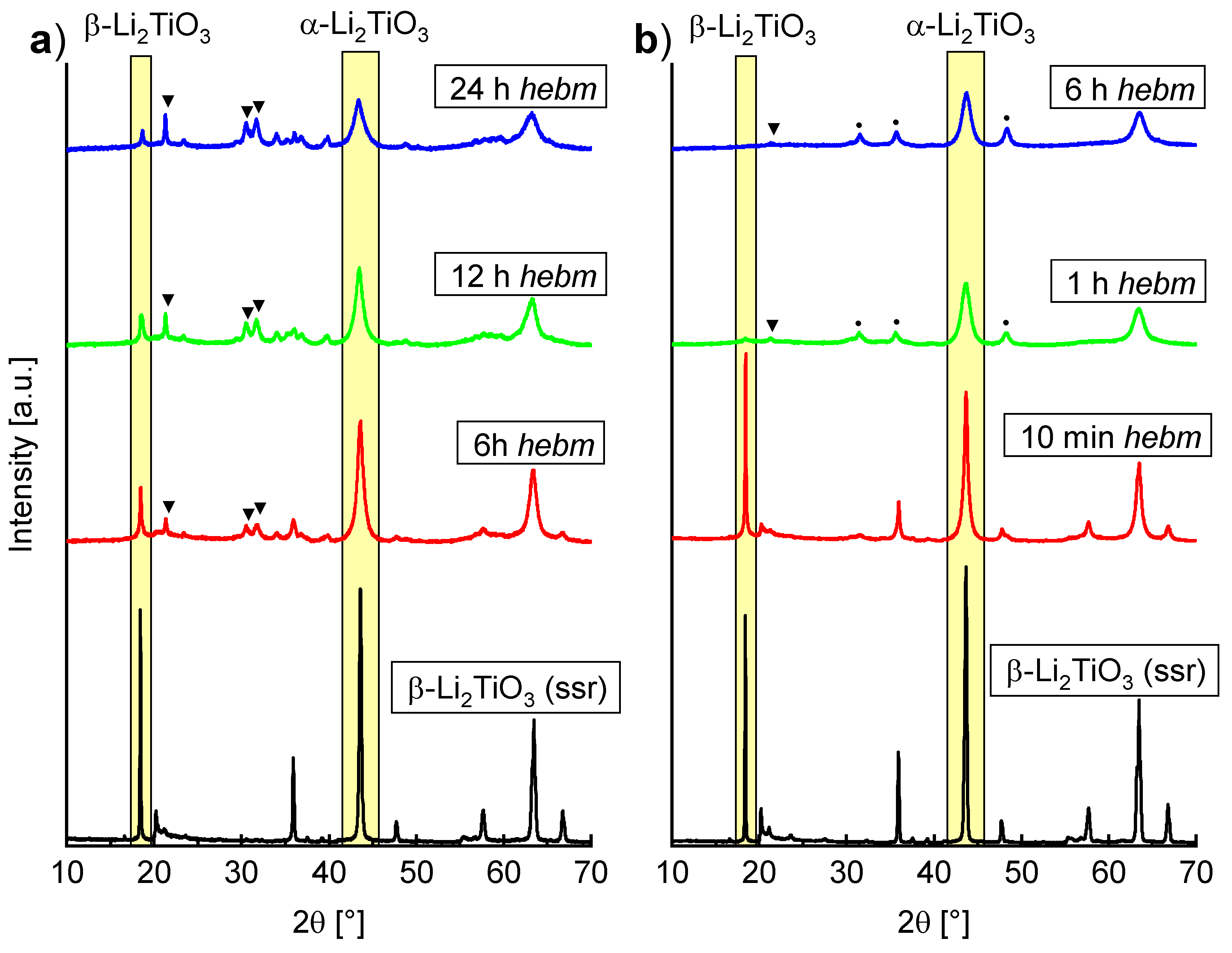
2.3. Mechanochemical Induced Phase Transformation of β-Li2TiO3 and Spinel Li4Ti5O12 to α-Li2TiO3
The ball milling-induced phase transformation from β- to α-Li
2TiO
3 has already been mentioned by Brandstätter et al. [
16], but no further investigation in terms of a quantitative conversion was conducted. In
Figure 7, PXRD measurements of β-Li
2TiO
3 before and after milling with (a) ZrO
2 tools and (b) WC tools at different parameters are shown. In both cases (a) and (b), a decrease of the β-Li
2TiO
3 main reflection can be seen. Milling with ZrO
2 tools at 600 rpm for 6 h leads to portions of 48% α-Li
2TiO
3, 25% β-Li
2TiO
3 and 28% Li
2CO
3. Prolonged milling leads to a further increase in intensity of Li
2CO
3 reflections. In contrast, the ball milling induced phase transformation is already detectable after milling for 10 min in WC tools. A portion of ca. 30% α-Li
2TiO
3 can be fitted, with small amounts of Li
2CO
3 (<10%). Even after milling for 1 h, only marginal amounts of ca. 5% β-Li
2TiO
3 remained. Milling with WC tools leads to complete disappearance of β-Li
2TiO
3 reflections after 6 h. Portions of 89% α-Li
2TiO
3, 8% Li
2CO
3 and 3% WC have been refined (Rietveld refinement in
Supplementary Materials, Figure S7). Similar to the mechanochemical synthesis from LiOH and rutile, an increase in baseline intensity suggests the formation of amorphous material. Refinement of β-Li
2TiO
3 (milling in WC tools for 6 h) with an internal standard (50% of α-Al
2O
3) suggests a portion of 27% amorphous background. In comparison to the mechanochemical synthesis from LiOH and rutile in WC tools, a smaller lattice parameter
a = 4.1463(2) Å is refined for α-Li
2TiO
3 if β-Li
2TiO
3 is used as an educt. This may also be a consequence of the previously mentioned hypothetical OH
− contents in the lattice. Since no OH
− is available in the mechanochemical-induced phase transformation of β- to α-Li
2TiO
3, a lattice parameter similar to the one of α-Li
2TiO
3 after heating to 200 °C is refined.
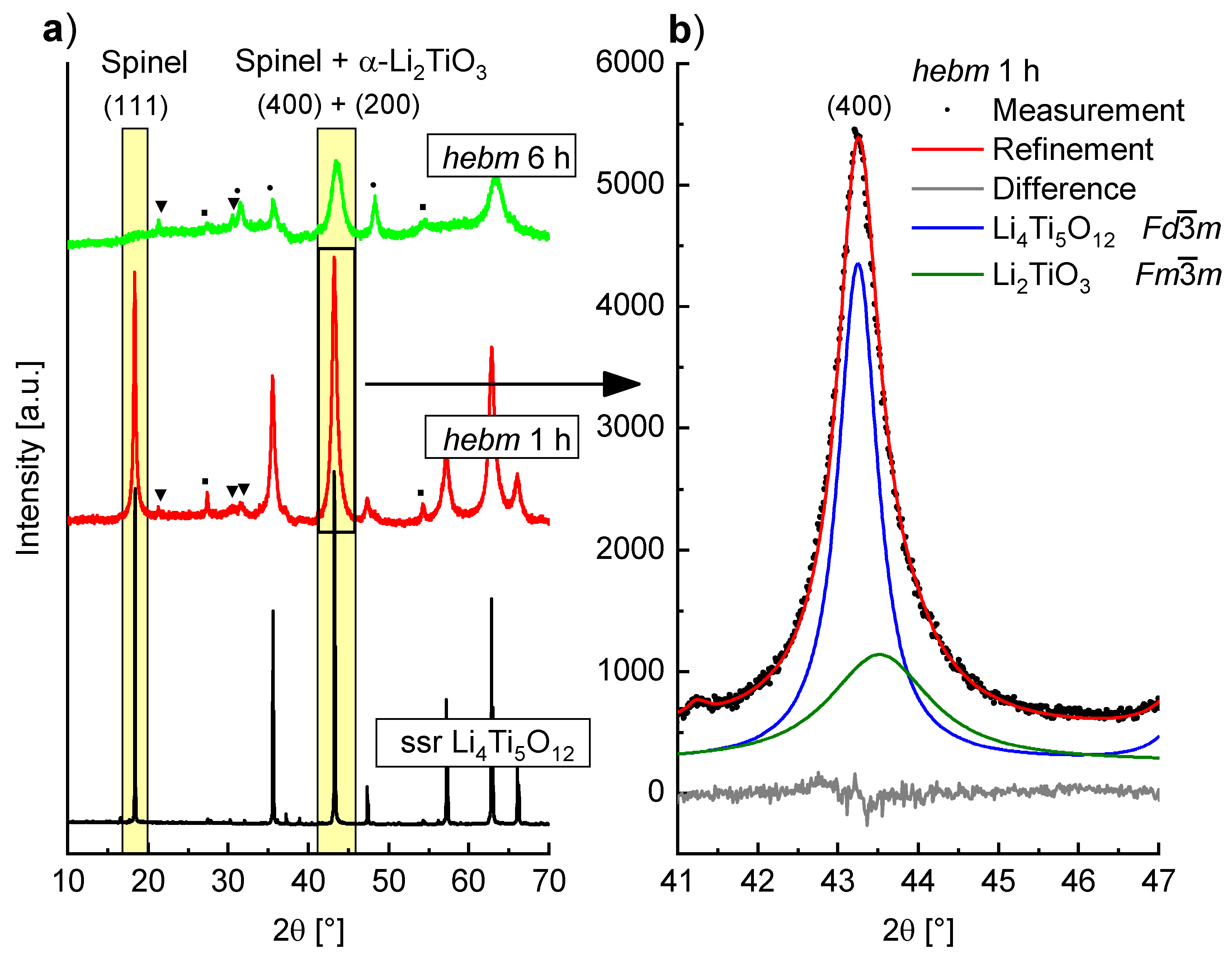
Formation of α-Li
2TiO
3 is also observed by milling of Li
4Ti
5O
12 with spinel type structure. PXRD measurements of Li
4Ti
5O
12 before and after milling in WC tools at 400 rpm are shown in
Figure 8a. After a milling time of 1 h a slight asymmetric broadening of the (400) reflection is visible, while the (111) reflection is not as strongly affected and shows little asymmetry. This is caused by the formation of α-Li
2TiO
3 whose reflections overlap with the spinel reflections. In
Figure 8b the Bragg intensities of the spinel (400) and the α-Li
2TiO
3 (200) reflection are shown with the resulting Rietveld fit. The (111) reflection of the spinel phase vanishes completely after a milling time of 6 h. Though a phase with cubic structure is formed, the intensities are very low and additional unidentified reflections are visible. Even after these mild milling conditions, the structure of the spinel phase is strongly altered, which may have large impact on the use of mechanochemically treated Li
4Ti
5O
12 as anode material in battery applications. It has already been established by impedance spectroscopy that milling of Li
4Ti
5O
12 leads to an increase in conductivity [
18]. This may be due to this partial structure transformation of spinel Li
4Ti
5O
12 to α-Li
2TiO
3.
2.4. 6Li SPE MAS NMR
6Li SPE MAS NMR experiments reveal additional insight into the milling-induced structure transformation. To illustrate the following interpretations, the unit cell of spinel Li
4Ti
5O
12 is shown in
Figure 9. (The unit cell of monoclinic β-Li
2TiO
3 is shown in
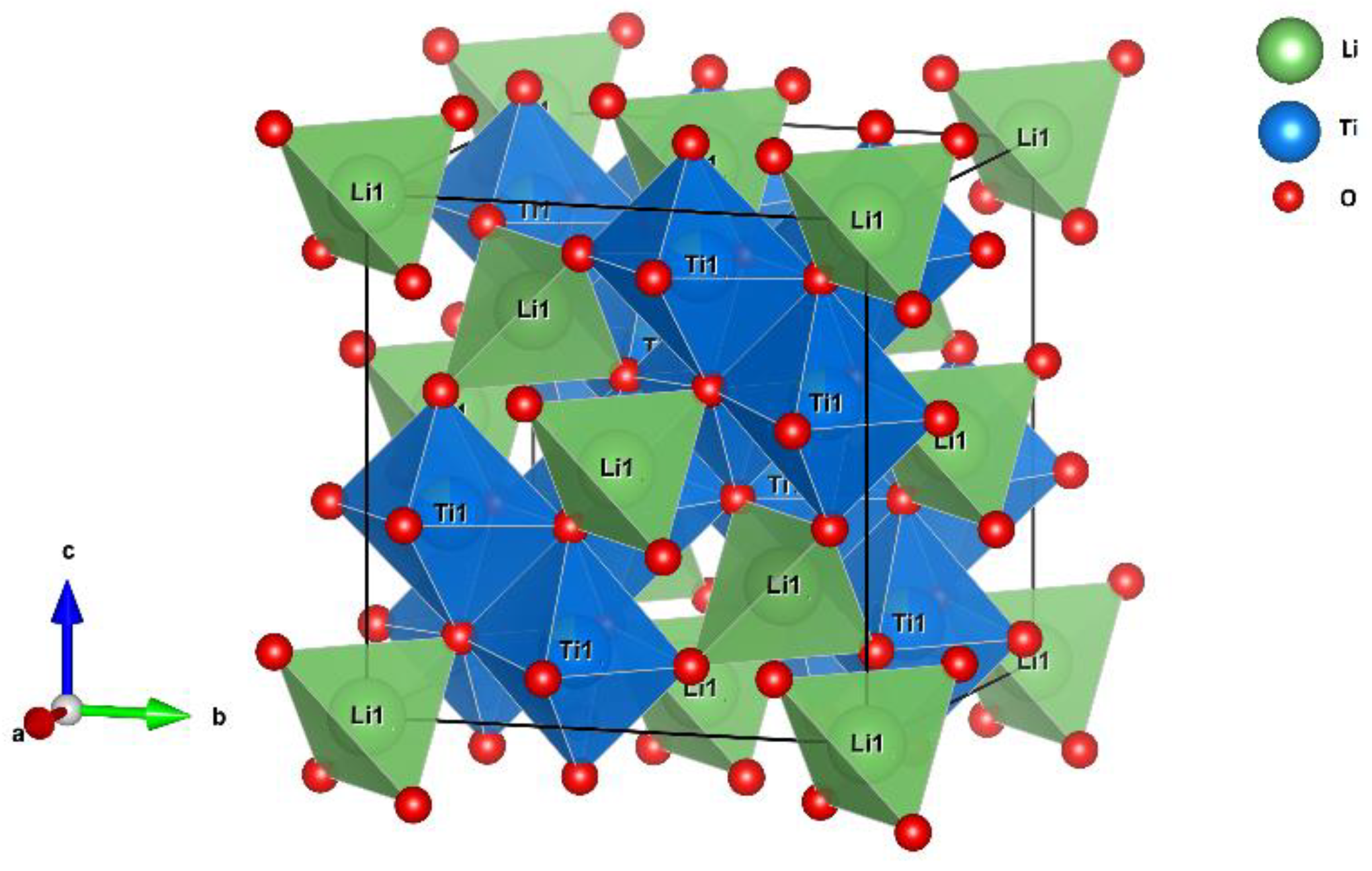
Figure 1.)
PXRD refinements of 900 °C sintered β-Li2TiO3 with constrained lithium site occupancy factors (total Li content is 2.0 Li per formula unit) show a full occupation of the 8f and 4d site (Li1 and Li2), while the 4e lithium site (Li3) and the two titanium 4e sites (Ti1 and Ti2) show a mixed occupation with 0.6 Li on the Li3 site, 0.3 Li on the Ti1 site and 0.1 Li on the Ti2 site. To obtain a sample with low Li3/Ti1/Ti2 anti-site mixing, several sintering steps at 1100 °C were necessary. A mixing of 0.1 Li on the Ti1 site and 0.1 Li on the Ti2 site remained.
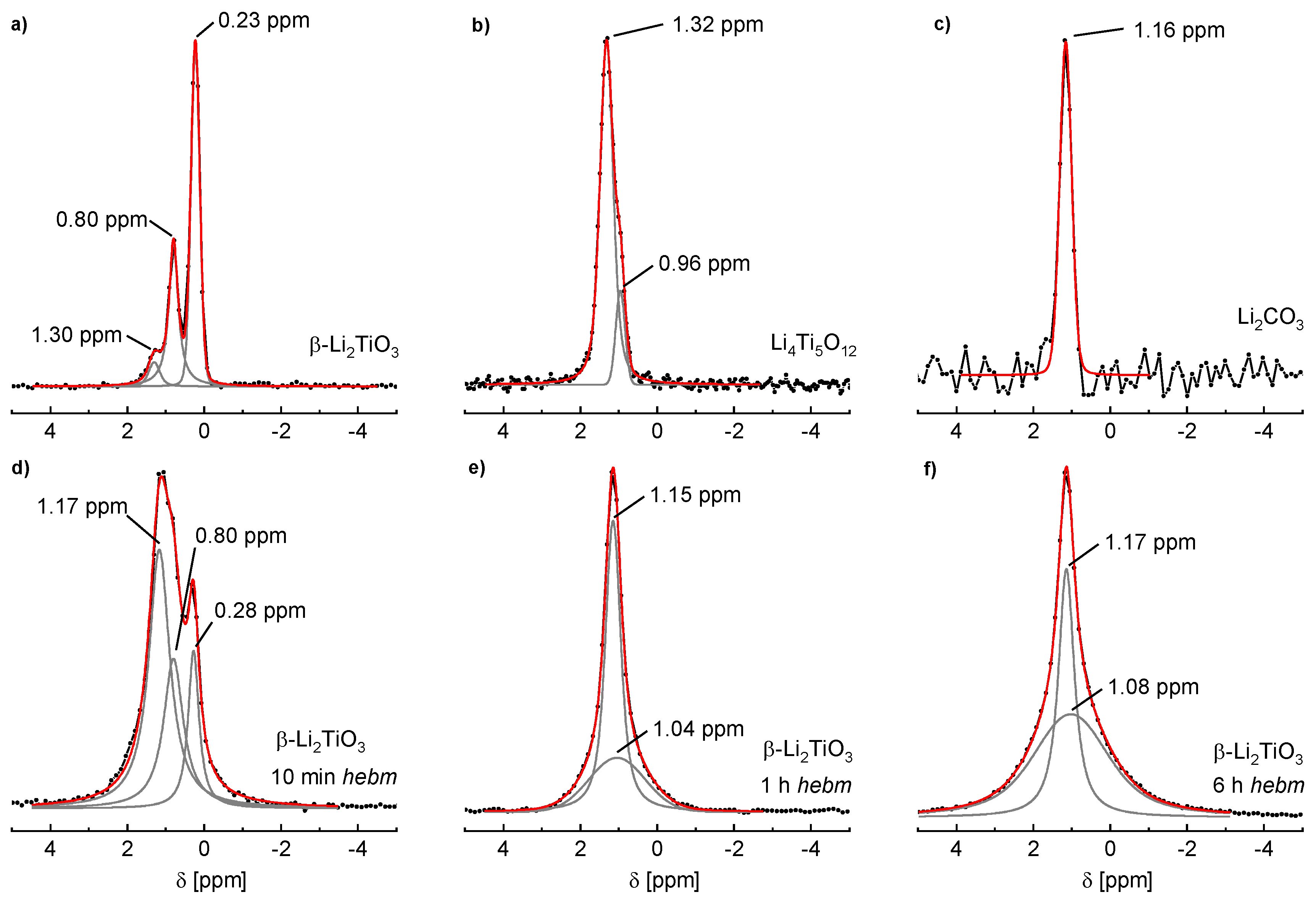
The
6Li NMR spectrum of 1100 °C sintered β-Li
2TiO
3 is shown in
Figure 10a where three signals are visible. Fitting of the line profile was performed with three independent Voigt functions, resulting in shifts of δ = 1.30, 0.80 and 0.23 ppm. The peak at 0.80 ppm is attributed to lithium on the 4
e site (Li3) in the LiTi
2 layer, the peak at 0.23 ppm originates from the two magnetically equivalent lithium sites 8
f (Li1) and 4
d (Li2) in the Li
2 layer [
25,
26]. The signals are consistent with values reported in the literature if an offset of ca. −1 ppm is considered due to the referencing against solid LiCl [
27]. The shift of the third signal matches a tetrahedral coordination of lithium [
25]. In
Figure 10b the
6Li spectrum of spinel Li
4Ti
5O
12 is shown. Li
4Ti
5O
12 may also be written as (Li)[Li
0.33Ti
1.66]O
4 to illustrate the occupation of 1 Li on the tetrahedral and ⅓ Li on the octahedral position. According to the literature, the peak at 1.32 ppm is attributed to tetrahedrally-coordinated lithium on the 8
a site, and the smaller peak at 0.96 ppm to octahedrally-coordinated lithium on the 16
d site [
28]. Additionally, the NMR spectrum of Li
2CO
3 reveals the solely tetrahedrally-coordinated lithium in this compound (
Figure 10c). A single shift of 1.16 ppm is observed, which is also in accordance with the literature [
28]. Since pristine compounds of β-Li
2TiO
3 and spinel Li
4Ti
5O
12 were yielded by solid state reactions, no Li
2CO
3 may be present (confirmed by PXRD measurements). The third signal of β-Li
2TiO
3 is therefore assigned to lithium on an interstitial position with tetrahedral coordination.
The spectrum of β-Li
2TiO
3 after 10 min of milling is shown in
Figure 10d. The signal formerly at δ = 1.30 ppm is shifted to 1.17 ppm, while the positions of the two other signals are hardly affected. Additionally, the signal intensities of β-Li
2TiO
3 are strongly altered. The signal at 1.17 ppm indicates that more than half the lithium atoms are tetrahedrally coordinated, while only few remain in octahedral coordination. After 1 h of milling (
Figure 10e) only two signals are present. The tetrahedral coordination remains as the dominant state indicated by the sharp line at 1.15 ppm. The band at 1.04 ppm, probably caused by cubic Li
2TiO
3 with Li and Ti in octahedral coordination and random ordering, indicates an additional new coordination environment. The signal at 1.15 ppm may be caused by Li
2CO
3 that is formed after the milling process. However, PXRD refinements show a portion of only 19% Li
2CO
3 after milling for 1 h, while the NMR signal is clearly caused by the main fraction in the sample. The tetrahedral coordination in Li
2TiO
3 is therefore assumed to be a transition state for the formation of the cubic state. This new broad signal at ca. 1 ppm becomes the dominant state after milling for 6 h (
Figure 10f). The
6Li SPE MAS NMR spectrum of Li
2TiO
3 from milling of LiOH and rutile (WC tools, 6 h milling time) is nearly identical (not shown). This underlines the assumption that this broad signal is caused by octahedrally-coordinated Li in the cubic phase. Parts of the tetrahedra signal remain. This is most probably caused by occupation of interstitial positions and by Li
2CO
3, but it was not possible to separate the contributions of these two states.
The
6Li SPE MAS NMR spectra of Li
4Ti
5O
12 (
Supplementary Materials, Figure S8) show a slightly different behavior compared to the transformation of β-Li
2TiO
3. A broad signal at δ = 1.15 ppm is visible after milling for 1 h, indicating a purely tetrahedral coordination environment. No signal of an octahedral coordination at ca. 1 ppm is visible. Additionally, a small signal at 1.57 ppm can be distinguished. This signal indicates a very short Li-O bonding distance or a coordination number lower than four. This signal may either be attributed to an interstitial position or the unidentified phase seen in PXRD, but no further identification was possible.
In conclusion to the finding that a tetrahedral coordination environment plays a major role in the structure after mechanochemical treatment, a revised structure model for the Rietveld refinement of α-Li
2TiO
3 was constructed. In the ideal NaCl structure model the octahedral 4
a site is occupied by 1/3 Ti and 2/3 Li (model A). In the revised model, the tetrahedral 8
c site was added and the corresponding site occupancy factors (
sof) of Li on the 4
a site (Li_O) and 8
c site (Li_T) were refined. To keep the overall stoichiometry of Li
2TiO
3, a constraint refinement in the form [
sof(Li_T) = ⅓ − ½·
sof(Li_O)] was defined, while the occupation of Ti was kept constant (model B). The resulting structure parameters are shown in
Table 1.
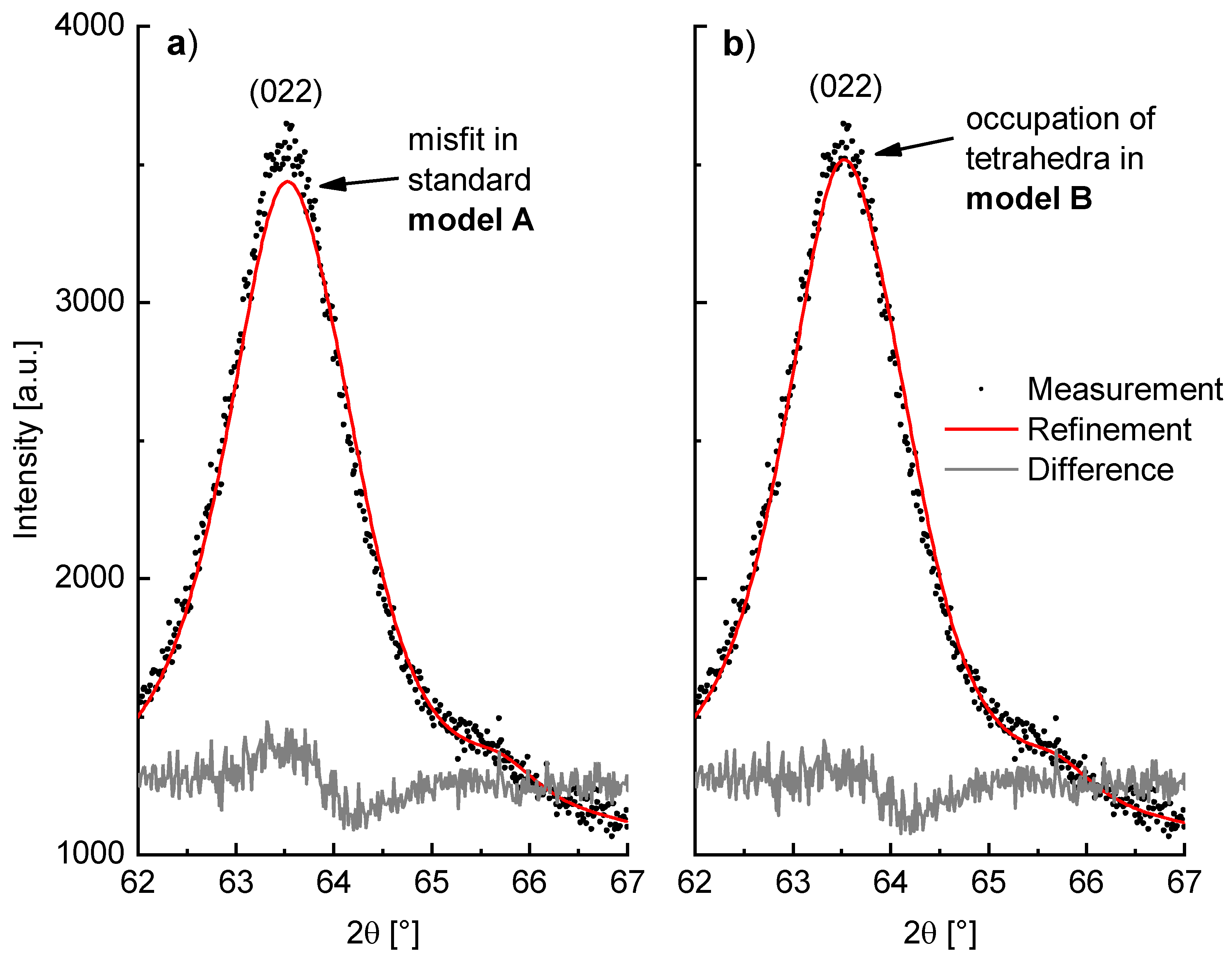
As a consequence of this adapted model, the Rietveld refinement of milled β-Li
2TiO
3 (WC tools, 600 rpm, 6h) is improved. This can be seen particularly in the description of the 022 reflection, which is depicted in
Figure 11 with a fit using the (a) standard model A and (b) the adjusted model B. To better accommodate the shape of the amorphous background, a polynomial of 25th degree was used in these both cases, instead of the elsewise-used polynomial of 15th degree. Both the
RBragg value of the Li
2TiO
3 phase and the overall fit are improved in model B. The
GOF is reduced from 1.13 in model A to 1.11 in model B, which is only slightly better but nonetheless indicating an improvement. Beyond that, the
RBragg value, which only dependents on the agreement between observed and calculated reflection intensities of the phase, is significantly lowered from 0.60% in model A to 0.28% in model B. However, since Li has a very low X-ray scattering factor the obtained values for the site occupancies in
Table 1 are just an indication of an occupation of the tetrahedral site and cannot be regarded as proof. Nevertheless, this model helps to harmonize the results of the Rietveld refinement with the fitted areas of the signals of tetrahedral and octahedral environments in the
6Li SPE MAS NMR spectrum of milled β-Li
2TiO
3 (WC tools, 600 rpm, 6 h). The areas in the
6Li spectrum sum up to a proportion of 0.59 to 1 for tetrahedral- and octahedral-coordinated lithium, which corresponds to 37% tetrahedrally-coordinated lithium. The phase fraction from Rietveld refinement with model A (7% Li
2CO
3, 90% α-Li
2TiO
3 or 10.2 mol % Li
2CO
3 and 88.3 mol % α-Li
2TiO
3) corresponds to only 10% tetrahedrally coordinated lithium, solely arising from Li
2CO
3. This is far below the 37% from the
6Li spectrum. If tetrahedrally-coordinated lithium in α-Li
2TiO
3 from model B (6.2% Li
2CO
3, 91.1% α-Li
2TiO
3 or 9.0 mol % Li
2CO
3 and 89.5 mol % α-Li
2TiO
3) is factored in, a portion of 30% tetrahedrally-coordinated lithium is obtained, which fits the portion of the Li NMR spectrum. Here, a
sof of 0.07 for Li on the 8
c site (Li_T) corresponds to 21% of tetrahedrally coordinated lithium in α-Li
2TiO
3.
2.5. Impedance Spectroscopy
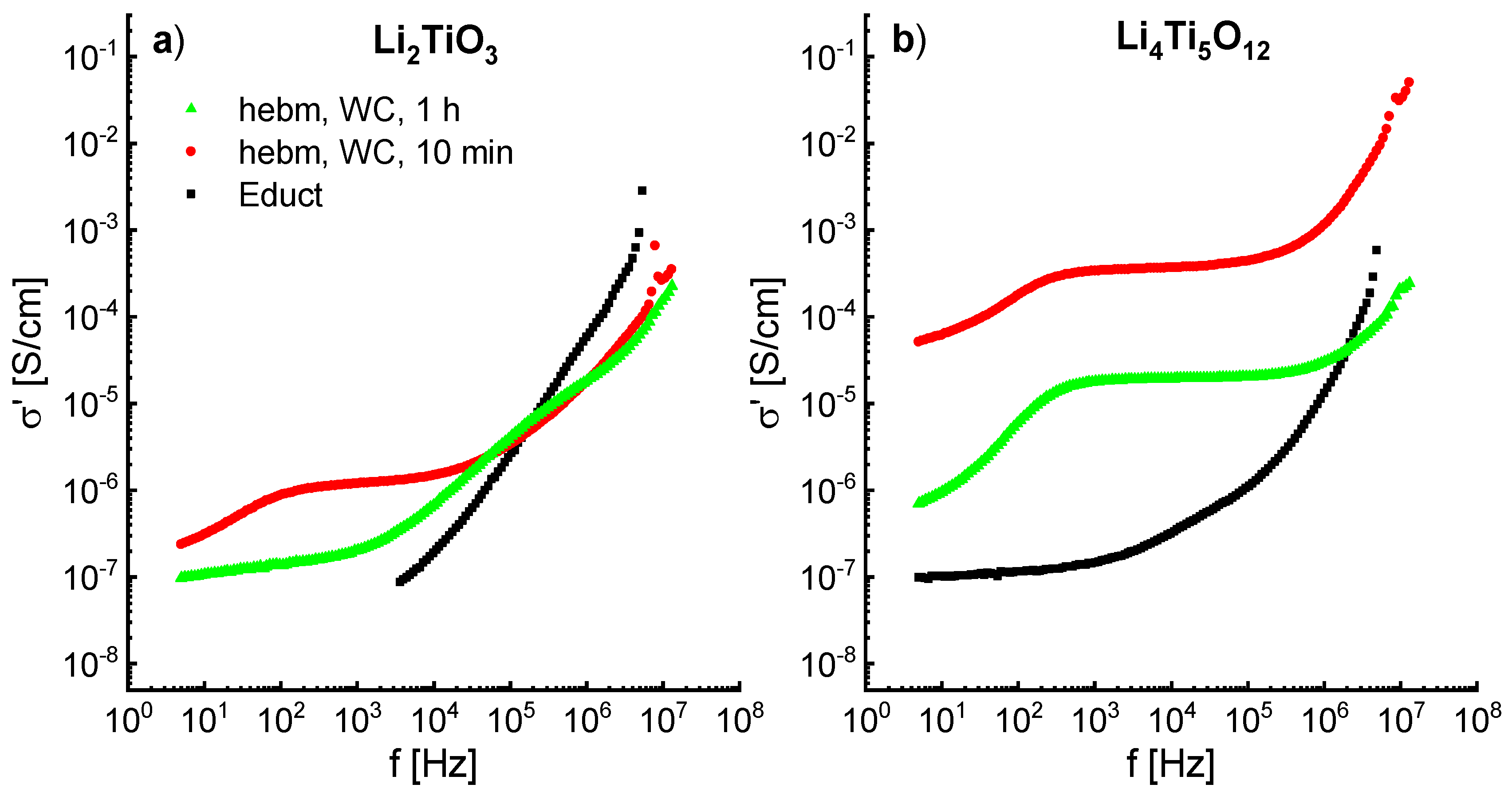
Since ball milling leads to the formation of defects, structure deformations and amorphization, an influence on the ion conductivity can be expected. The impedance spectra of ball-milled Li
2TiO
3 (600 rpm) and Li
4Ti
5O
12 (400 rpm) show an increase in conductivity of up to three orders of magnitude. The frequency dependent real part of the conductivity is shown in
Figure 12a,b. The spectra were recorded at 298K.
The conductivity of Li
2TiO
3 from the solid-state reaction is rather poor and is immeasurable at frequencies below 3 kHz. After milling for 10 min an increase of one order of magnitude can be observed, with a typical dc plateau (σ
dc ≈ 10
−6 S/cm) at lower frequencies and a dispersive regime at higher frequencies. Similar results are shown by Brandstätter et al. [
16], however, only a conductivity σ
dc of about 10
−9 S/cm is reported at room temperature for a milled sample. Milling of Li
2TiO
3 for 1 h decreases the conductivity to ≈ 10
−7 S/cm, indicating a blocking of the Li ion transport. This may be due to the phase transformation from β- to α-Li
2TiO
3. As already shown by
6Li MAS NMR in
Figure 10, milling for 10 min displaces Li to tetrahedrally-coordinated sites, which may be responsible for the increase in conductivity. Longer milling times promote a new octahedral coordinated state, which may be associated to the decrease in conductivity.
Li
4Ti
5O
12 shows a similar behavior to Li
2TiO
3, with a dc plateau at lower frequencies and a dispersive region at higher frequencies and a decrease of the conductivity after longer milling times. However, Li
4Ti
5O
12 from solid state reaction already exhibits a dc plateau at lower frequencies with σ
dc ≈ 10
−7 S/cm at 298 K. Milling for 10 min increases the conductivity by three orders of magnitude to >10
−4 S/cm at 298 K. The observed conductivities are the highest shown yet for ball-milled Li
4Ti
5O
12 at room temperature [
18].