Abstract
The 2,2′:6′:2″-terpyridine ligand has literally shaped the coordination chemistry of transition metal complexes in a plethora of fields. Expansion of the ligand bite by amine functionalities between the pyridine units in the tridentate N,N’-dimethyl-N,N’-dipyridine-2-yl-pyridine-2,6-diamine ligand (ddpd) modifies the properties of corresponding transition metal complexes, comprising redox chemistry, molecular dynamics, magnetism and luminescence. The origins of these differences between ddpd and tpy complexes will be elucidated and comprehensively summarized with respect to first row transition metal complexes with d2–d10 electron configurations. Emerging applications of these ddpd complexes complementary to those of the well-known terpyridine ligand will be highlighted.
1. Introduction
Pincer-type [1,2] terpyridine ligands (2,2′:6′,2″-terpyridine tpy; pincer ligands are tridentate, 6-electron donor ligands that enforce a meridional coordination geometry on the metal centre) and their manifold complexes [3] play a central role in key fields of coordination chemistry with important applications in supramolecular chemistry [4,5,6,7,8,9,10,11,12,13,14], spin-crossover (SCO) phenomena [15,16,17,18], homogeneous catalysis [19,20,21], biomedical applications [22], redox non-innocence [23,24,25,26,27] or photochemistry [6,7,28], to name just a few.
Variations of the prototypical tpy ligand include substitution at the central and outer pyridines, ring annulation at the outer N-heterocycles, for example, forming quinolines and substitution of pyridines by other heterocycles such as thiophenes or pyrimidines. Expansion of the tpy scaffold by formally inserting a single-atom bridge between the pyridines has been very successfully employed especially in the field of emissive ruthenium(II) complexes and photosensitizers [28,29,30,31,32,33,34,35,36,37,38].
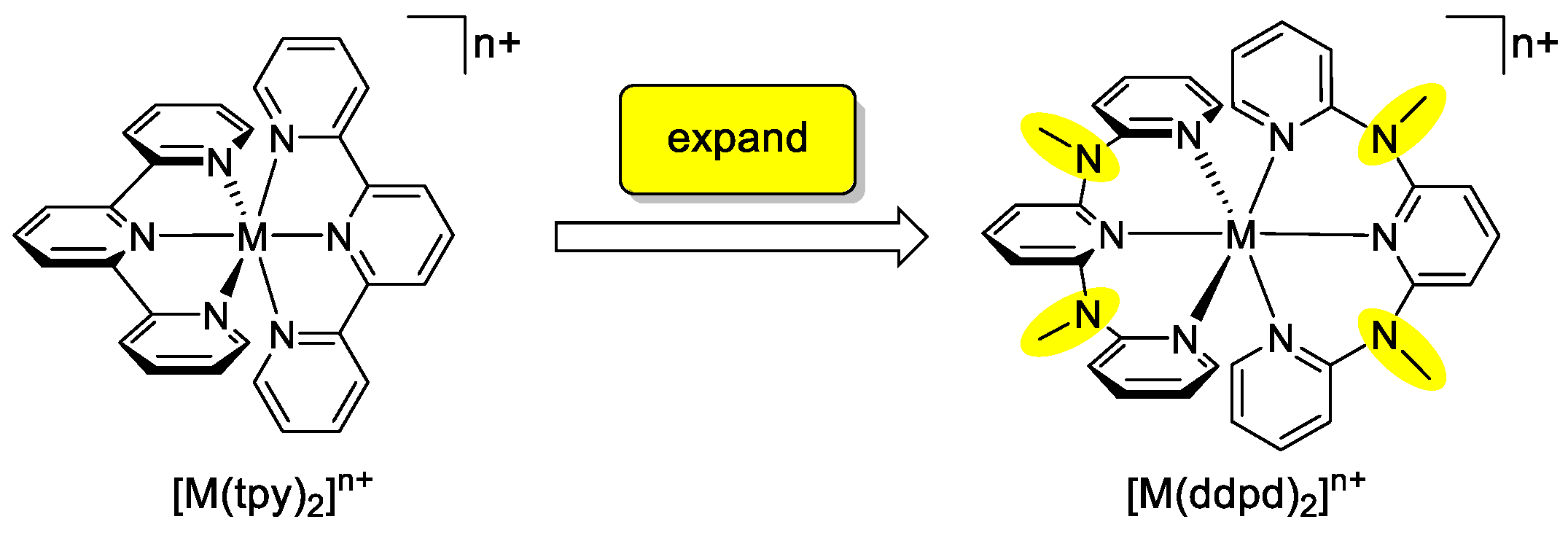
The effect of tpy ligand expansion in other, especially 3d metal complexes has been only scarcely explored and a systematic description and comparison with the prototypical tpy complexes is lacking. This review summarizes all available data on the expanded ligand ddpd (N,N’-dimethyl-N,N’-dipyridine-2-yl-pyridine-2,6-diamine), its homoleptic metal complexes [M(ddpd)2]n+ covering d2–d10 electron configurations of the metal centre and a few heteroleptic complexes MCln(ddpd) and [M(dcpp)(ddpd)]2+ (dcpp = 2,6-bis(2-carbonylpyridyl)pyridine). A special focus will be placed on the similarities and differences with respect to analogous terpyridine complexes (Scheme 1) in the fields of stereochemical, dynamic, magnetic, redox and photophysical phenomena.
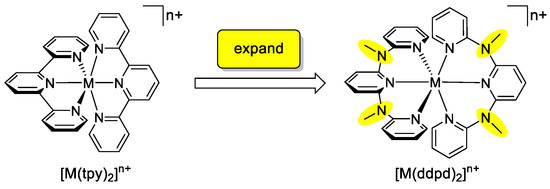
Scheme 1.
Homoleptic metal complexes of the tridentate polypyridine ligands tpy and ddpd.
2. Properties of the Ligand ddpd
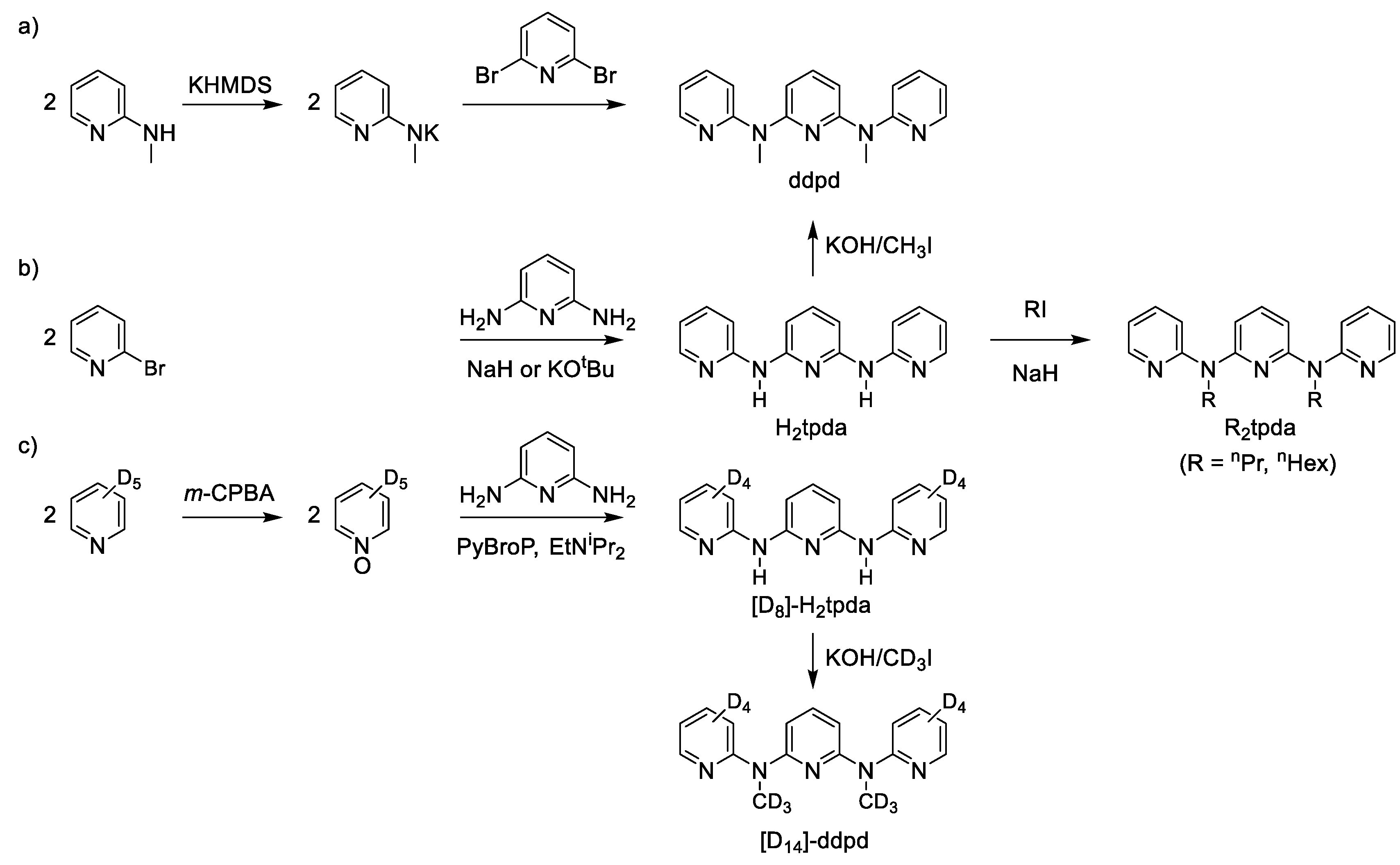
The famous tridentate ligand tpy and its derivatives are typically prepared via copper or palladium mediated C–C coupling reactions or via Kröhnke condensation reactions [28,39,40]. The related expanded terpyridine ligand ddpd and its derivatives (deuterated, N-protonated, N-alkylated) can be prepared by three different routes [35,41,42]. The most direct and convenient way to ddpd is by nucleophilic substitution of 2,6-dibromopyridine with deprotonated 2-(methylamino)pyridine (Scheme 2a) [35]. The inverse substitution of 2-bromopyridine with deprotonated 2,6-diaminopyridine first yields the ligand 2,6 bis(2-pyridylamino)pyridine (H2tpda) [41]. This parent ligand is then N-alkylated by alkyl iodides such as methyl iodide to give ddpd, n-propyl iodide or n-hexyl iodide to give R2tpda (R = nPr, nHex; Scheme 2b) [43]. For deuterated derivatives of ddpd, starting from [D5]-pyridine via its [D5]-N-oxide and coupling with 2,6-diaminopyridine proved to be convenient to yield [D8]-H2tpda. Alkylating [D8]-H2tpda with CD3I gives the deuterated ligand [D14]-ddpd (Scheme 2c) [42].
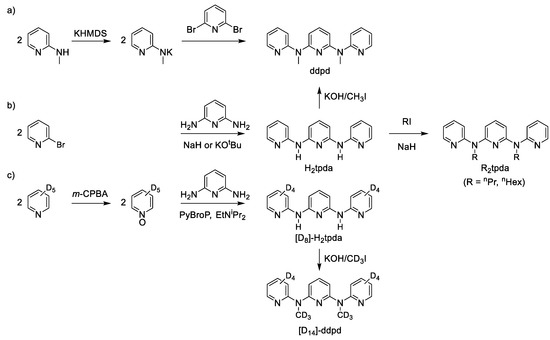
Scheme 2.
Syntheses of (a) ddpd, (b) H2tpda, R2tpda (R = nPr, nHex) and (c) [D8]-H2tpda and [D14]-ddpd; KHMDS = potassium bis(trimethylsilyl)amide, m-CPBA = meta-chloroperoxybenzoic acid, PyBroP = bromotripyrrolidinophosphonium hexafluorophosphate [35,41,42,43].
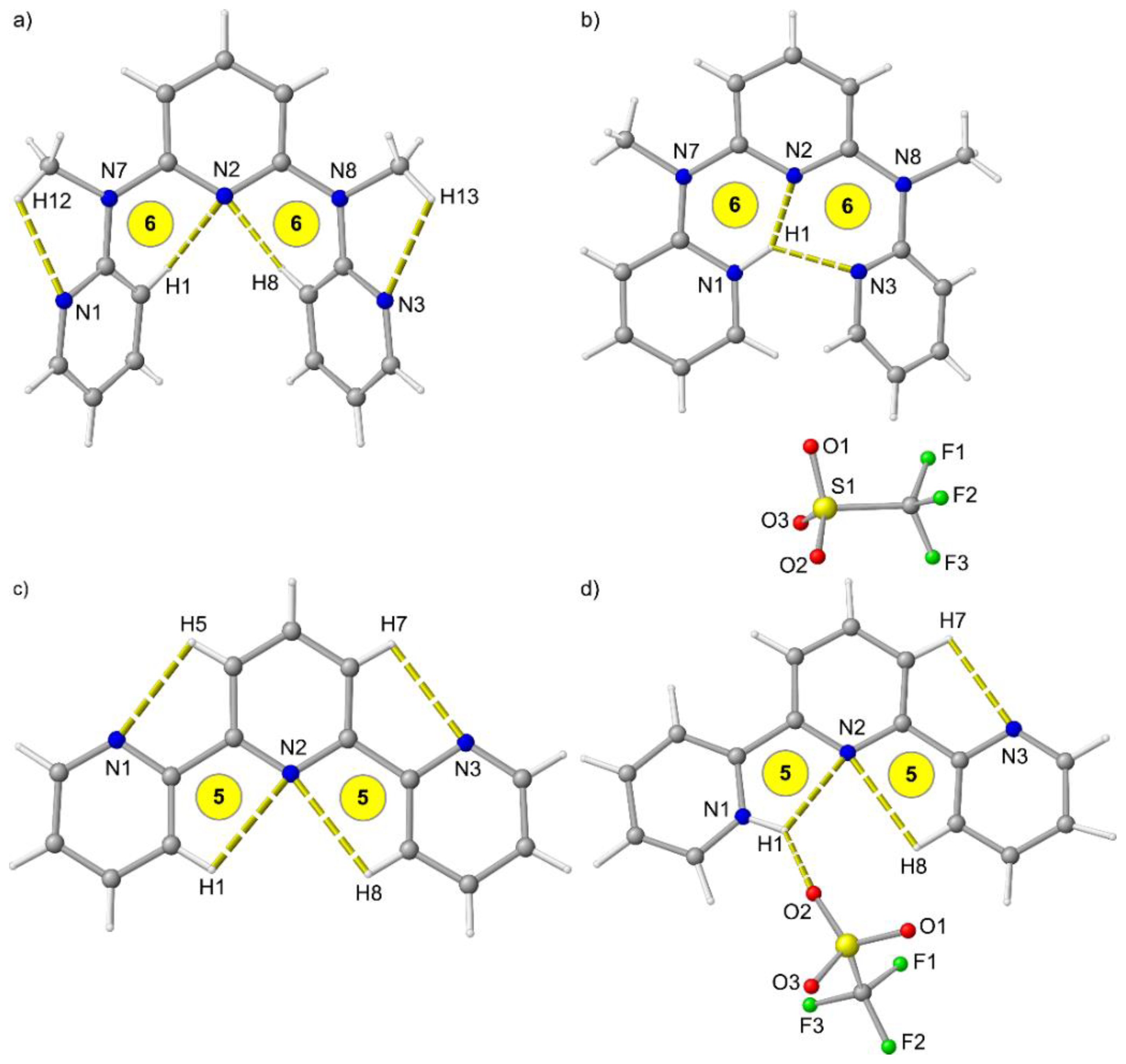
Similar to tpy [44], ddpd is not pre-organized for metal complexation and the terminal pyridines are oriented outward (Figure 1a,c). This allows for CH…N hydrogen bonding interactions and avoids repulsion between the pyridine nitrogen lone pairs and between CH groups (Figure 1a). Terpyridine forms 5-membered rings including a CH…N hydrogen bond with the central pyridine nitrogen atom N2 (Figure 1c), while the additional N–Me groups in ddpd enforce 6-membered rings with N2 (Figure 1a).
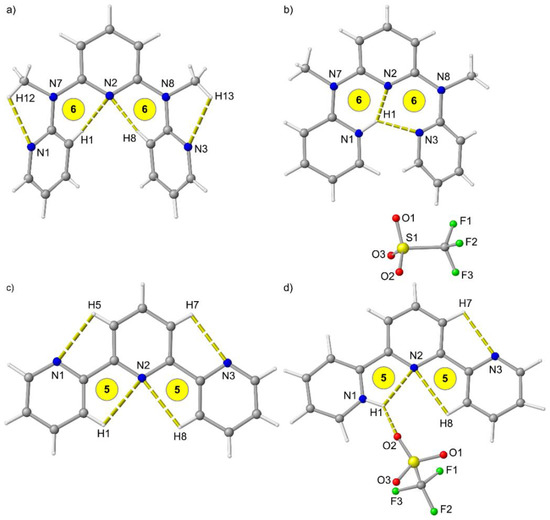
Figure 1.
Molecular structures of (a) ddpd, (b) [H-ddpd][CF3SO3], (c) tpy [44] and (d) [H-tpy][CF3SO3] [48] in the solid state. Hydrogen bonds indicated by yellow dashed lines. Atom numbering unified for clarity. Ring sizes involving the central pyridine highlighted in yellow.
Although the N–Me group of ddpd should be more basic [45,46], addition of acid protonates one terminal pyridine unit (N1) and both terminal pyridines rearrange to point inward allowing for one short and one medium long N1H1…N2/N3 hydrogen bond with N…N distances in the cavity of 2.51, 2.81 and 3.24 Å (Figure 1b). This hydrogen bonding pattern favours the observed pyridine protonation site similar to pre-organized proton sponges with N…N distances between 2.3 and 2.6 Å [47]. The cavity formed by the three pyridines is somewhat too large for the small proton resulting in two different distorted six-membered chelate rings (Figure 1b). On the other hand, this cavity should perfectly fit to accommodate metal ions. Tpy is protonated at N1 as well (Figure 1d) [48]. However, tpy only realizes a single N1H1…N2 hydrogen bond with an N1…N2 distance of 2.65 Å forming a five-membered ring, while the second pyridine (N3) remains oriented outward. The [CF3SO3]− counterion additionally binds to the pyridinium site N1H1.
The conformations and protonation sites already point to key differences between tpy and ddpd, namely the different available ring sizes and the proton sponge-like behaviour of ddpd due to its electron-richness and flexibility.
A computational study of Ni(CO)3(κN1-ddpd), Ni(CO)3(κN2-ddpd) complexes and other Ni(CO)3(L) complexes revealed that the net donor strength (σ + π) of ddpd donor atoms ranks between that of trimethylamine and N-heterocyclic carbene ligands and is larger than that of pyridine, as estimated from the Density Functional Theory (DFT) calculated A1 carbonyl stretching modes of the Ni(CO)3 fragments [35]. Obviously, ddpd is a stronger Lewis base and a stronger Brønsted base than tpy.
The ligand ddpd is fluorescent in solution (λmax = 398 nm; Φ = 8.0%, τ = 3.0 ns in CH3CN; λmax = 410 nm in acid-free CH2Cl2) [49,50]. In acidic CH2Cl2 or in the intentional presence of acid, the emission band shifts to 395 nm (τ = 4.0 ns) [49]. In contrast, protonation of tpy in CH3CN shifts its emission band from 340 nm to 412 nm (25.6 ns) [51].
In line with the electron donating character of the N–Me groups, ddpd is very difficult to reduce to its radical anion ddpd·− (peak potential −3.27 V vs. ferrocene in CH3CN) [49]. On the other hand, tpy is reversibly reduced to tpy·−at −2.55 V versus ferrocene in CH3CN [52]. The relative ease of reduction causes the typical redox non-innocent behaviour of tpy in many first-row transition metal complexes [23,24,25,26,27]. The electron rich ddpd ligand in contrast should behave essentially redox-innocent in transition metal complexes. Oxidation of ddpd’s N–Me groups to the radical cations is irreversible with peak potentials at 0.55 V and 1.06 V versus ferrocene in CH3CN [49].
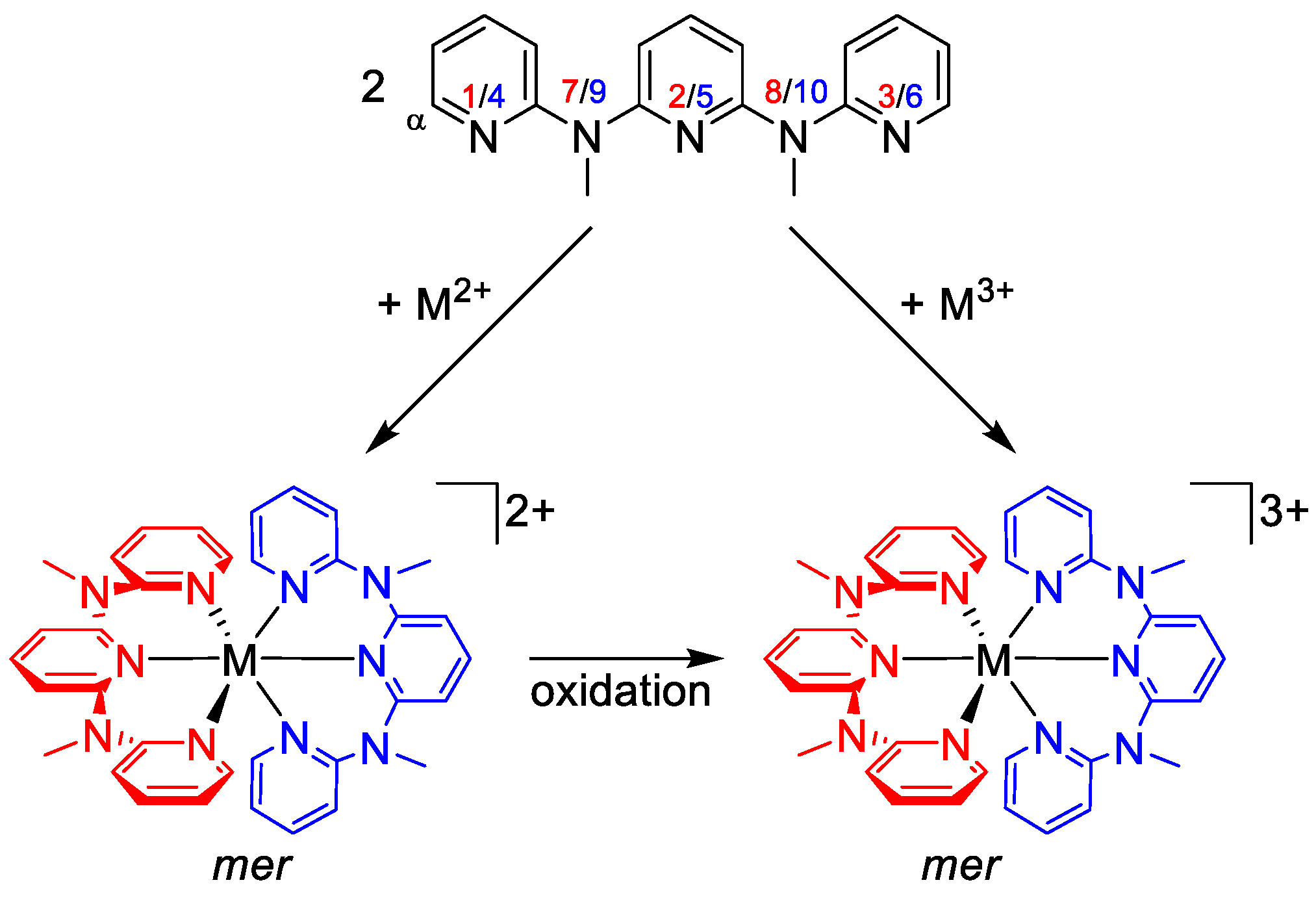
Typical synthetic procedures for homoleptic [M(ddpd)2]2+ complexes employ the tetrafluoroborate salts of the respective metal ions, such as Zn[BF4]2 × 6H2O or Fe[BF4]2 × 6H2O or the respective metal chlorides such as CrCl2 (Scheme 3). Suitable oxidants (oxygen, silver triflate, ceric ammonium nitrate, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone) oxidize [M(ddpd)2]2+ to the +III oxidation state in [M(ddpd)2]3+ (Scheme 3).
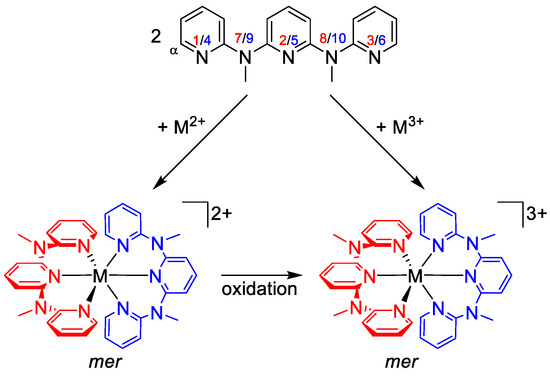
Scheme 3.
General synthetic procedures for [M(ddpd)2]n+ complexes (n = 2, 3) and atom numbering.
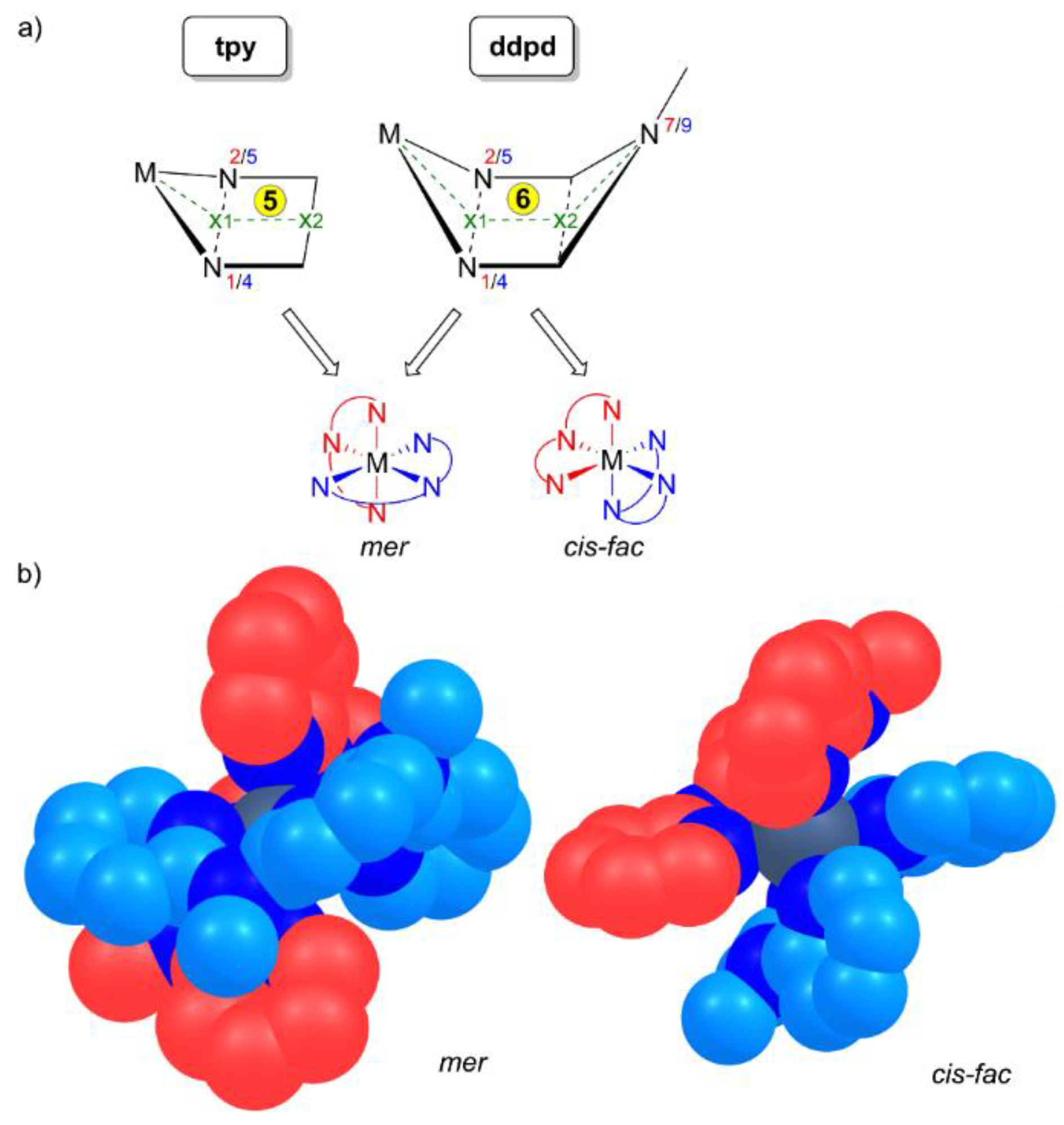
The rigid five-membered C2N2M chelate rings formed by tpy ligands merely allow for a meridional coordination in homoleptic complexes [M(tpy)2]n+. In contrast, the more flexible six-membered C2N3M chelate rings formed by ddpd ligands enable both meridional and facial coordination modes, that is, a pincer-type [1] or a tripodal topology (Scheme 4a). In fact, mer isomers of [M(ddpd)2]n+ complexes appear to be more prevalent but cis-fac isomers have been observed as well. The six-membered chelate rings in the ddpd complexes form boat conformations. The metal centre M and the N–Me group (N7, N9) lie above the mean plane of the ring (Scheme 4a).
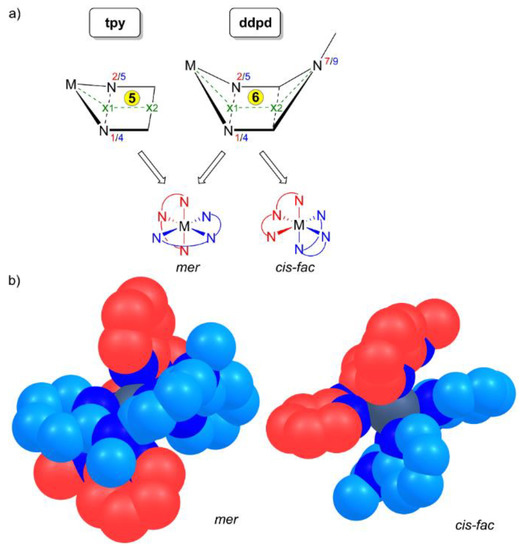
Scheme 4.
(a) Chelate ring size (5 for tpy; 6 for ddpd) and corresponding envelope and boat conformations and the resulting conceivable mer and cis-fac stereoisomers and (b) space-filling models of mer and cis-fac stereoisomers of [M(ddpd)2]n+ with the two ligands color-coded red and blue, respectively. For numbering of nitrogen atoms see Scheme 3. Hydrogen atoms omitted for clarity.
The M–x1–x2 and N7/9–x2–x1 angles vary between 139° and 145°. On the other hand, the chelates formed by tpy are essentially planar with M–x1–x2 angles approaching 180° (Scheme 4a). The N–M–N bite angles of ddpd are significantly larger (80–89°) than that of tpy with 75–82° (Table 1 and Table 2). With the tpy ligand and the five-membered chelates being planar, the point group of [M(tpy)2]n+ complexes is C2v. As ddpd is a non-planar ligand in homo- and heteroleptic complexes, the point group of [M(ddpd)2]n+ complexes is only C2. Consequently, mer-[M(ddpd)2]n+ complexes are chiral with helical chirality (Scheme 4b). Mixed ruthenium(II) complexes [Ru(ddpd)(tpy-X)]2+ form diastereomeric ion pairs with chiral enantiopure counterions [33]. Racemic mer-[Cr(ddpd)2]3+ complexes can be partially resolved into the corresponding P and M enantiomers. These seem to be configurationally stable on the HPLC timescale [53]. The isomeric cis-fac-[M(ddpd)2]n+ complexes are chiral as well (Scheme 4b) and have been isolated only as racemic mixtures so far. In the following, all mer- and cis-fac-[M(ddpd)2]n+ complexes are racemic mixtures and this will not be indicated explicitly.

Table 1.
Relevant metrical data of [M(ddpd)2]n+ ions in the solid state; mer isomers if not indicated otherwise; [BF4]− salts if not indicated otherwise; distances in Å, PL in % and angles in °. Red and blue color code indicates the different ddpd ligands (see Scheme 3).

Table 2.
Relevant metrical data of [M(tpy)2]n+ ions in the solid state; [BF4]− salts if not indicated otherwise, distances in Å and angles in °. Red and blue color code indicates the different tpy ligands.
All homoleptic complexes [M(ddpd)2]n+ and [M(tpy)2]n+ share a [MN6] coordination sphere. The deviation of the experimental coordination geometry from the ideal octahedron can be expressed by a continuous shape measure S(OC-6) as implemented in the program SHAPE 2.1 (free download at http://www.ee.ub.es) [54,55,56,57,58]. For an ideal octahedron, this value will be zero and increases with the structural deviation. The higher flexibility and thus higher adaptability of the six-membered chelate rings of ddpd allow for N–M–N angles closer to 90° and for similar M–N distances and thus enable “more octahedral” coordination geometries. The rigid pincer ligand tpy features smaller N–M–N angles and significantly differing M–N distances to the central and terminal pyridines yielding much more distorted coordination polyhedrons. This is clearly obvious from the significantly larger shape measure S(OC-6) of [M(tpy)2]n+ complexes as compared to analogous [M(ddpd)2]n+ complexes (Table 1 and Table 2). Interestingly, the S(OC-6) values increase along the series FeII/CoIII < CrIII < NiII < CoII < CuII < ZnII in both ligand series, suggesting that this trend is coded in the d electron configuration of the metal ion (low-spin d6, d3, d8, high-spin d7, d9, d10), especially in the occupation of the eg* orbitals and in the presence of Jahn-Teller effects [59]. [Cr(L)2]2+ complexes feature different electron distributions for L = ddpd and tpy as will be discussed below.
Specific to ddpd are the bridging three-coordinate nitrogen atoms N7/N8 and N9/N10 and their respective degree of planarization as defined by PL/% = 100 × {[Σ (X − N − Y)] − 3 × 109.5°]/[3×120.0° − 3 × 109.5°]} [43]. In ddpd as well as in [H-ddpd]+, these N atoms are in a planar environment with PL = 100%, while in the homoleptic complexes mer-[M(ddpd)2]n+ the degree of planarization varies between 81% and 93% (Table 1). This indicates that metal chelation induces some strain in the ligand.
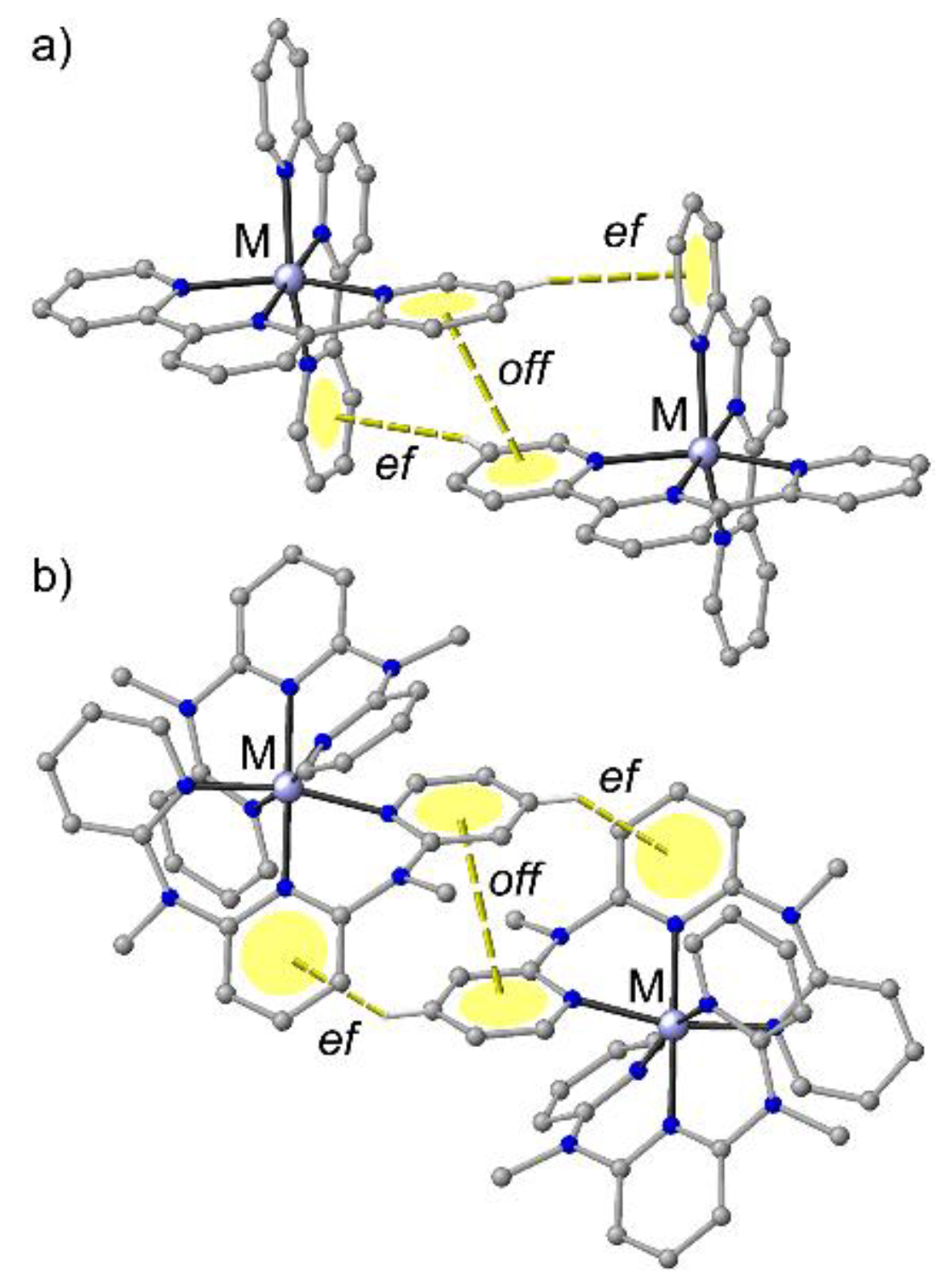
In the solid state, [M(tpy)2]2+ complexes exhibit characteristic edge-to-face (ef) and offset-face-to-face (off) interactions between the outer pyridine rings of the essentially planar aromatic tridentate ligands (terpyridine embrace, Figure 2a) [68,72]. Similarly, [M(ddpd)2]2+ cations pack in the solid state displaying characteristic ef–off–ef interactions between the (non-planar) ddpd ligands of neighbouring complexes. The off interaction occurs between two outer pyridine rings and the two ef interactions between central and outer pyridines (Figure 2b).
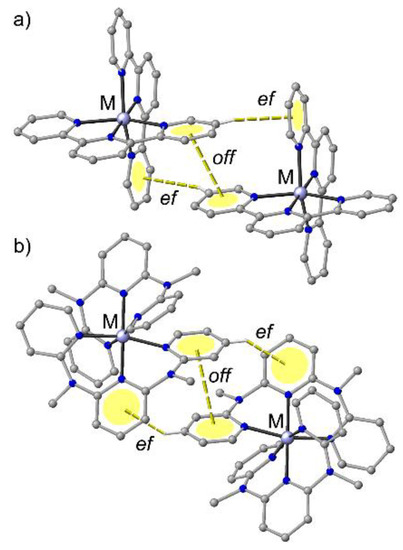
Figure 2.
General motifs of phenyl embraces of (a) [M(tpy)2]2+ and (b) [M(ddpd)2]2+ complexes.
In essence, the main differences between tpy and ddpd are the stronger electron donating and weaker electron accepting character of ddpd in addition to its higher flexibility and larger N–M–N bite angles. In the following chapters, the translation of these key differences of the tridentate oligopyridine ligands into different properties of first row transition metal ddpd and tpy complexes will be elaborated in more detail.
3. mer-[Zn(ddpd)2]2+ and ZnCl2(ddpd) [d10]
The [Zn(L)2]2+ complexes are conveniently prepared from Zn[BF4]2 × 6H2O or Zn[ClO4]2 × 6H2O and the respective tridentate ligand (Scheme 3) [49,73]. Ligand protons experience a coordination shift of the 1H NMR resonances upon complexation to a metal centre. In diamagnetic complexes of ddpd and tpy, this effect is strongest for the α protons of the terminal pyridines. These α protons are directed toward the metal ion in a region of electron density of the metal t2g electrons resulting in additional shielding of the nuclear spin and a corresponding upfield shift. This effect is strong in low-spin d6-[CoIII(L)2]3+ and d6-[FeII(L)2]2+ complexes but less pronounced in the d10-[ZnII(L)2]2+ complexes (L = ddpd, tpy; Table 3). In both diamagnetic series of complexes (L = ddpd, tpy; M = ZnII, CoIII, FeII), the coordination shift correlates roughly with the M…Hα distances estimated from single crystal X-ray diffraction (Table 3). Furthermore, the α protons experience the ring current of pyridines of the second tridentate ligand with H…(pyridine centre) distances of around 3.2 Å (tpy) and 3.3–3.4 Å (ddpd). This effect could account for the slightly less pronounced coordination shift in the ddpd complexes (Table 3).

Table 3.
Coordination shift ∆δ = δ(complex) − δ(ligand) of 1H NMR resonances of Hα in diamagnetic [M(L)2]n+ complexes (L = ddpd, tpy; M = ZnII, CoIII, FeII).
The [Zn(tpy)2]2+ complex can be reversibly reduced twice at the tpy ligands (Table 4) [20]. In contrast, [Zn(ddpd)2]2+ is only irreversibly reduced analogous to the ddpd ligand itself (Table 4). Due to the positive charge of the complex, these potentials are less negative than in the non-coordinated ddpd or tpy ligands (Table 4). Oxidation of the ddpd ligands in [Zn(ddpd)2]2+ is shifted anodically, as compared to ddpd due to its positive charge (Table 4) [49].

Table 4.
Electrochemical data of [M(L)2]n+ complexes (L = ddpd, tpy; M = ZnII, CuII, NiII, CoII, FeII, MnII, CrIII, VII) in V vs. ferrocene/ferrocenium in CH3CN.
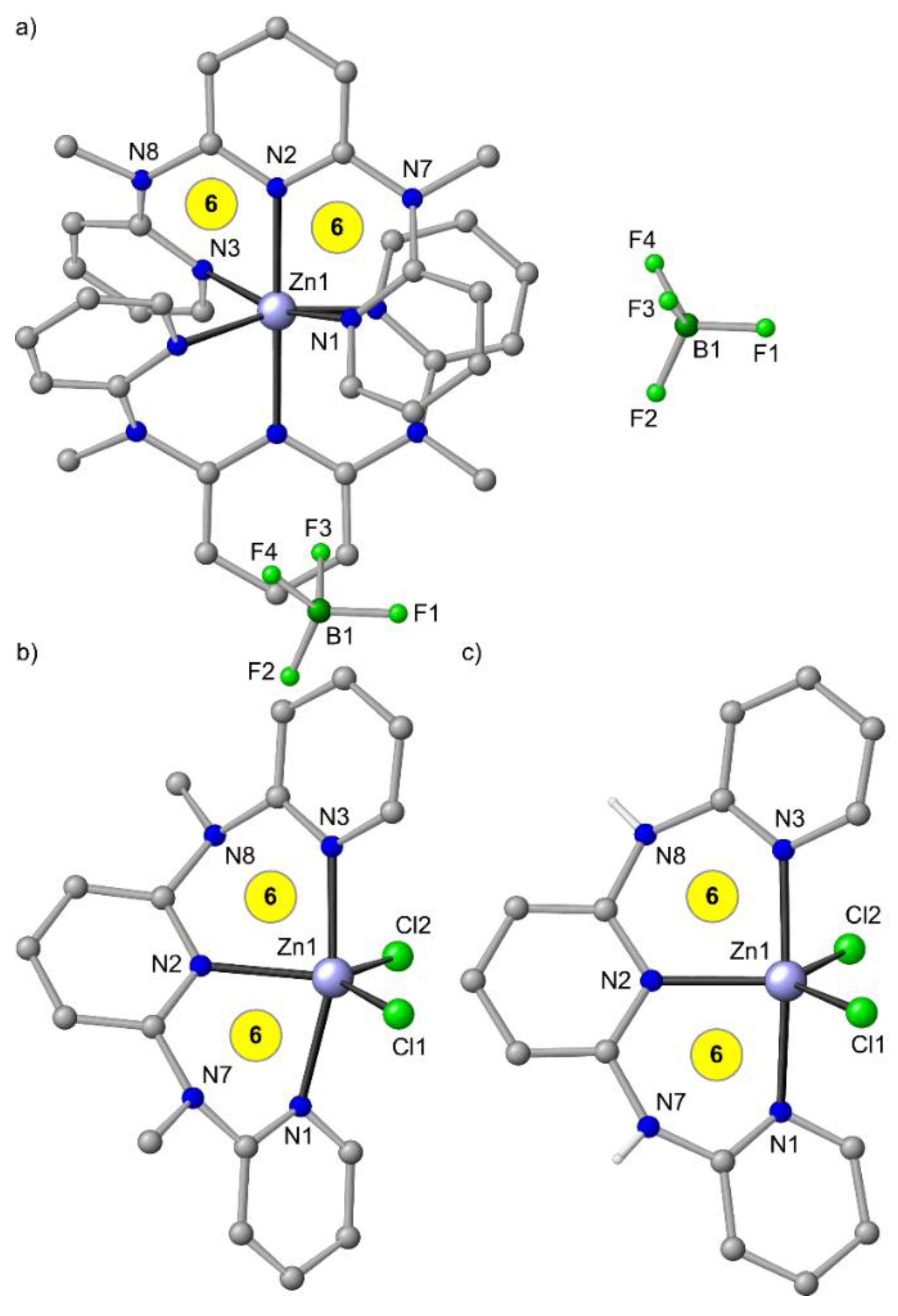
In the solid state, the coordination geometry of [Zn(ddpd)2]2+ deviates appreciably from an idealized octahedron with S(OC-6) = 1.18 when compared to other [M(ddpd)2]n+ complexes (Figure 3a, Table 1). However, the distortion of [Zn(tpy)2]2+ is dramatically larger with S(OC-6) = 4.45, which can be accounted for by the very small N–Zn–N bite angles of 75° (Table 2).
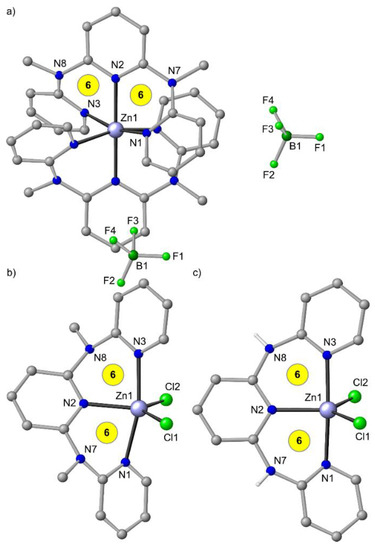
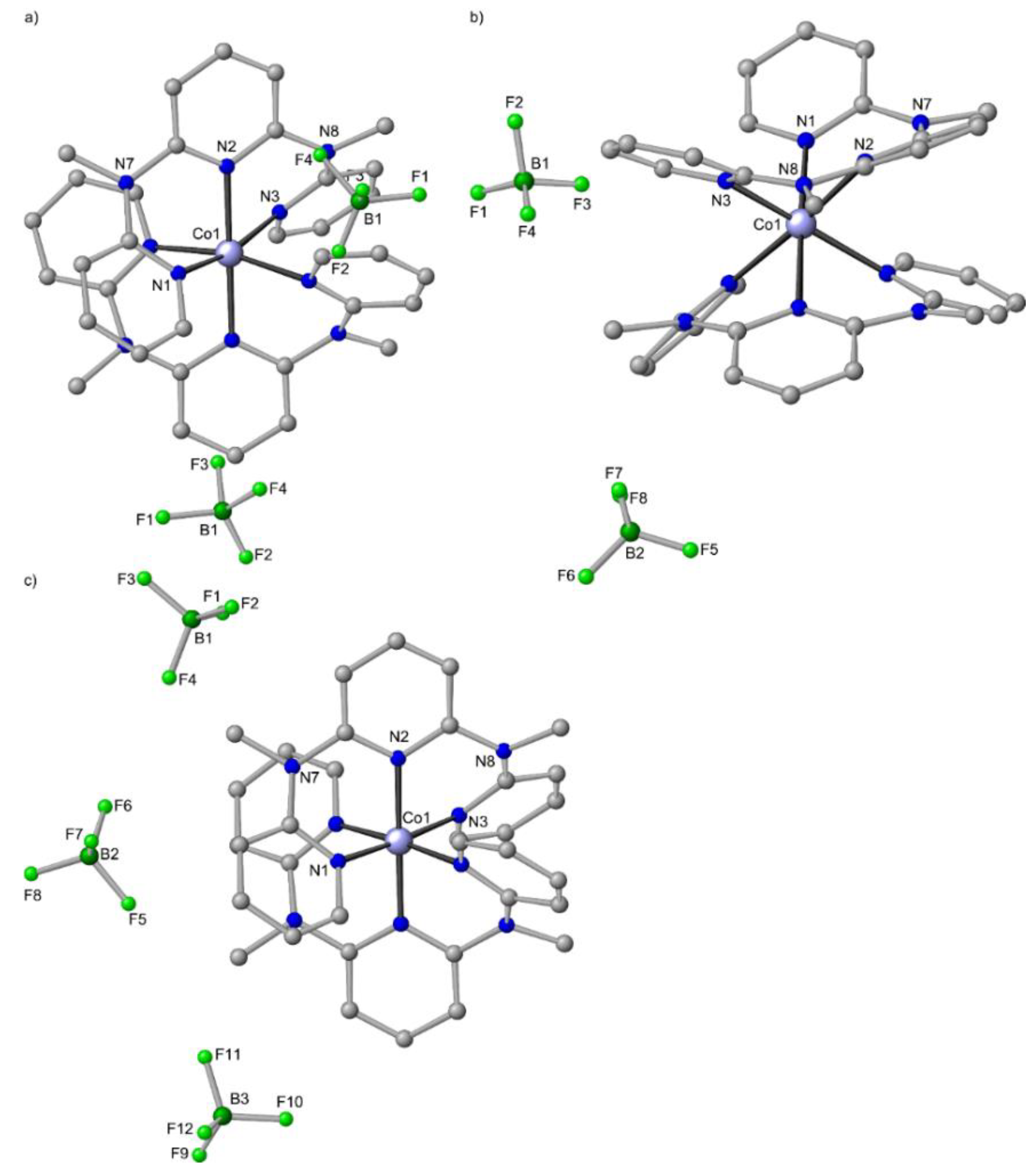
Figure 3.
(a) Molecular structures of mer-[Zn(ddpd)2][BF4]2 [49], (b) ZnCl2(ddpd) from the mer-[Zn(ddpd)2][ZnCl4]/Zn(ddpd)Cl2 co-crystal [49] and (c) ZnCl2(H2tpda) [41] in the solid state. Carbon-bound hydrogen atoms and solvent molecules omitted. Atom numbering unified for clarity. Chelate ring sizes highlighted in yellow.
Coordination of Zn2+ to ddpd shifts the π–π* absorption maxima to higher energy from 274/333 nm to 248/308 nm. In the colourless homoleptic complex, these bands are assigned to ligand-centred transitions essentially from the electron-rich N–Me groups to the pyridine’s π* orbitals. Fluorescence is observed at 373 nm in (acid-free) CH2Cl2 with 6.5% quantum yield and 0.6 ns lifetime [49]. The diminished fluorescence lifetime as compared to ddpd is likely due to the heavy-atom effect of Zn2+, enabling intersystem crossing (ISC) to the triplet state. The tpy-based fluorescence of [Zn(tpy)2]2+ is found at higher energy with significantly higher quantum yield (λem = 353 nm, Φ = 65%) [81]. The N–Me donor groups of ddpd are likely responsible for the slightly lower emission energy of [Zn(ddpd)2]2+. The flexibility of the six-membered chelates possibly allow for enhanced non-radiative relaxation in [Zn(ddpd)2]2+.
With coordinating counter ions such as chloride present, heteroleptic complexes form. ZnCl2 and terpyridine yield the neutral complex ZnCl2(tpy) [82]. With ddpd and ZnCl2 an equilibrium between [Zn(ddpd)2][ZnCl4] and Zn(ddpd)Cl2 is established with a 1:1.8 ratio in CD3CN solution at room temperature according to 1H NMR spectroscopy. From this mixture, co-crystals of [Zn(ddpd)2][ZnCl4]/Zn(ddpd)Cl2 with the complexes in a 1:1 ratio are obtained (Figure 3b) [49]. These findings clearly underscore that the d10 ion zinc(II) is labile and very flexible with respect to coordination numbers (4–6) and geometries (tetrahedral, trigonal-bipyramidal, octahedral). From the CH3CN solution of the equilibrating [Zn(ddpd)2][ZnCl4]/Zn(ddpd)Cl2 mixture, only the emission of the [Zn(ddpd)2]2+ ion is observed and Zn(ddpd)Cl2 appears to be essentially non-emissive.
Interestingly, the analogous five-coordinate complex Zn(H2tpda)Cl2 has been reported (Figure 3c) with a fluorescence emission band peaking at 392 nm in MeOH [41]. The reported UV/Vis absorption spectrum of Zn(H2tpda)Cl2 in MeOH with a strong 311 nm absorption band [41], however, strongly resembles that of [Zn(ddpd)2]2+ ions [49]. Hence, an analogous equilibrium [Zn(H2tpda)2][ZnCl4]/Zn(H2tpda)Cl2 might be present under these conditions as well and the observed fluorescence might be better assigned to the [Zn(H2tpda)2]2+ ion instead to that of the mixed-ligand Zn(H2tpda)Cl2 complex. No photophysical data are reported for ZnCl2(tpy) and consequently a comparison with Zn(ddpd)Cl2 and Zn(H2tpda)Cl2 is unfeasible.
The diamagnetic d10-[ZnL2]2+ complexes with L = ddpd and tpy are distorted, substitutionally labile, optically transparent in the visible spectral region and fluoresce only in the UV spectral region. The tpy complex shows reversible ligand-centred reductions, while the ddpd complex is basically redox-inert between −2.3 V and +1.1 V.
4. mer-[Cu(ddpd)2]2+ [d9]
The green and dark green d9-copper(II) complexes [Cu(ddpd)2]2+ and [Cu(tpy)2]2+ are S = ½ paramagnets (Table 5). As expected for copper(II) ions, the spin-only value of χT = 0.375 cm3 K mol−1 is clearly surpassed [83]. Both copper(II) complexes can be reduced to copper(I) species. However, this process is quasireversible for [Cu(ddpd)2]2+ and irreversible for [Cu(tpy)2]2+ at room temperature (Table 4). This behaviour suggests a reduction of the coordination number in both cases [12]. This phenomenon had been elegantly exploited by Sauvage and co-workers in the design of redox-controlled motions in catenates using redox-active [Cu(tpy)]2+/+ units [12,13,14]. Further irreversible oxidations and reductions are similar to the ones observed for [Zn(ddpd)2]2+ and assigned to ddpd-centred processes (Table 4).

Table 5.
Room temperature magnetic susceptibility and giso data (from SQUID or EPR measurements) of open-shell [M(ddpd)2]n+ and [M(tpy)2]n+ complexes (M = CuII, NiII, CoII, MnII, MnIII, CrII, CrIII, VII).
[Cu(ddpd)2]2+ exhibits two ligand field bands at 653 nm (ε = 25 M−1 cm−1) and 1254 nm (ε = 5 M−1 cm−1) in CH3CN [60]. The latter band arises from the Jahn-Teller [59] distortion of the d9 complex with (t2g)6(eg)3 electron configuration and the resulting splitting of the 2E ground state (term symbol based on idealized O symmetry). From the two bands, the ligand field splitting ∆o and the Jahn-Teller splitting 4δ1 are estimated as 11,325 cm–1 and 7975 cm−1, respectively [60]. For analogous tpy complexes [Cu(tpy)2]2+ with varying counter ions, both ∆o (11,200–11,300 cm−1) and 4δ1 (5900−6500 cm−1) are smaller [91]. This can be traced back to the stronger σ interaction of ddpd with the copper d orbitals and consequently a slightly larger ligand field splitting and furthermore a significantly larger Jahn-Teller [59] splitting. The larger Jahn-Teller splitting is furthermore favoured by the higher flexibility of ddpd.
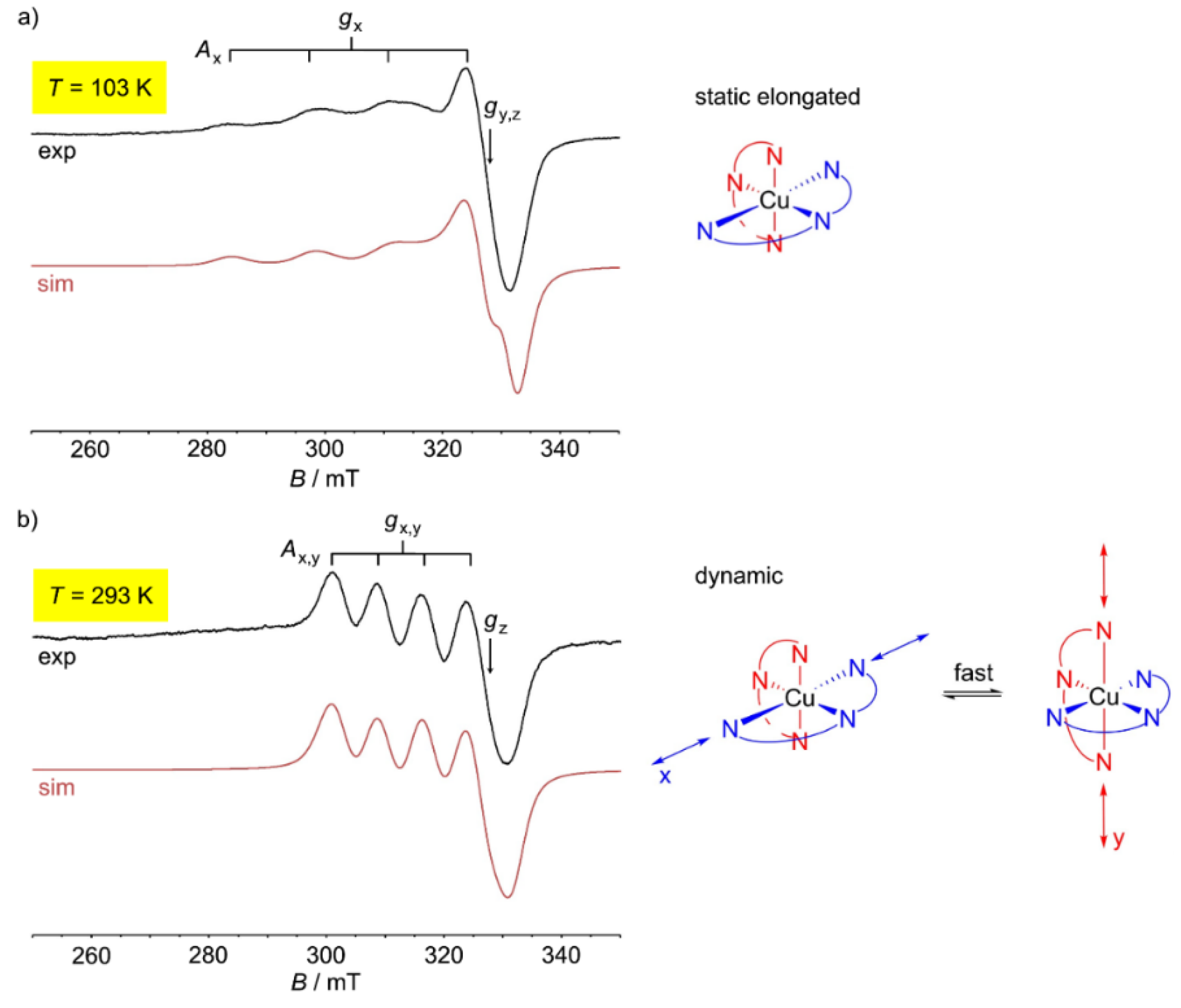
Both copper(II) complexes are fluxional at room temperature. The dynamics slow down at lower temperature. “Freezing-in” occurs below 77 K for the tpy complex and at ≈100 K for the ddpd complex [60]. This suggests a larger Jahn-Teller barrier for [Cu(ddpd)2][BF4]2 between the two degenerate Jahn-Teller isomers (Figure 4b). In the solid state at 123 K, the [CuN6] coordination sphere of [Cu(ddpd)2]2+ corresponds to an elongated octahedron with the Cu–N2/N5 distances (1.989/2.060 Å) fixed due to the chelate ligand, short Cu–N1/N3 (2.0256/2.0256 Å) and long Cu–N4/N6 distances (2.3318/2.3318 Å) [60]. At 263 K, the two possible elongated octahedrons (along x and along y) are dynamically averaged with fixed Cu–N2/N5 distances (2.064/1.979 Å) along z and averaged Cu–N1/N3 (2.149/2.149 Å) and Cu–N4/N6 distances (2.191/2.191 Å) [60]. The combined action of dynamic Jahn-Teller distortion (x/y) and ligand strain (Cu–N2/N5 fixed along z) results in an apparent compressed octahedron. This apparent change from elongated to compressed octahedral upon warming is easily followed in the temperature dependent X-band EPR spectra of [Cu(ddpd)2][BF4]2 (powder), [Cu0.875Fe0.125(ddpd)2][BF4]2 (powder) (Figure 4) or [Cu(ddpd)2][BF4]2 (dissolved in water/glycerol) [60]. For example, copper(II) doped into the diamagnetic [FeII(ddpd)2][BF4]2 host (see Section 7) gives gx = 2.228, gy = gz = 2.063 and Ax(63/65Cu) = 146 G at 103 K, indicating an axially elongated octahedron. At 293 K, gx = gy = 2.156, gz = 2.058 and Ax = Ay(63/65Cu) = 79 G are found, suggesting an axially compressed octahedron (Figure 4). In the intermediate temperature range, the EPR spectra are weighted superpositions of the low- and high-temperature spectra [60]. As this temperature-dependent dynamic is essentially independent of the environment (pure copper(II) crystal, diamagnetic host crystal, frozen solution), cooperative effects are absent and the Jahn-Teller barrier is an intrinsic feature of the [Cu(ddpd)2]2+ cation.
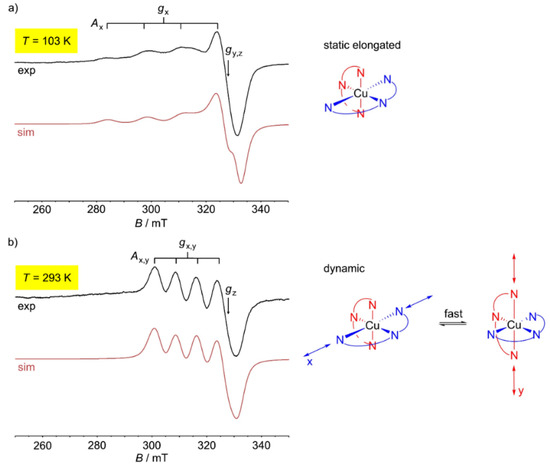
Figure 4.
(a) X-band powder EPR spectrum of [Cu0.875Fe0.125(ddpd)2][BF4]2 at 103 K, simulation in red and illustration of the static elongated octahedron. (b) X-band powder EPR spectrum of [Cu0.875Fe0.125(ddpd)2][BF4]2 at 293 K, simulation in red and illustration of the dynamically elongated octahedrons [60].
Compared to [Cu(tpy)2]2+, [Cu(ddpd)2]2+ is more difficult to reduce to copper(I), features a larger ligand field and Jahn-Teller splitting and a higher barrier for the Jahn-Teller dynamics. All these phenomena can be accounted for by the stronger σ-donating character of ddpd.
5. mer-[Ni(ddpd)2]2+ [d8]
The d electron configuration of nickel(II) in an octahedral ligand field is (t2g)6(eg)2, leading to a 3A2 ground state. The magnetic susceptibility data of χT = 1.20 and 1.11 cm3 K mol−1 for [Ni(tpy)2]2+ and [Ni(ddpd)2]2+ confirm the triplet ground states (Table 5). At low temperature, zero-field splitting is apparent leading to a drop of the χT values in both complexes [61,92]. The ligand-dictated octahedral compression of [Ni(tpy)2]2+ is much more pronounced (S(OC-6) = 3.18; d(Ni–N2)/d(Ni–N1) = 0.94) than that of [Ni(ddpd)2]2+ (S(OC-6) = 0.60; d(Ni–N2)/d(Ni–N1) = 0.98) (Table 1 and Table 2).
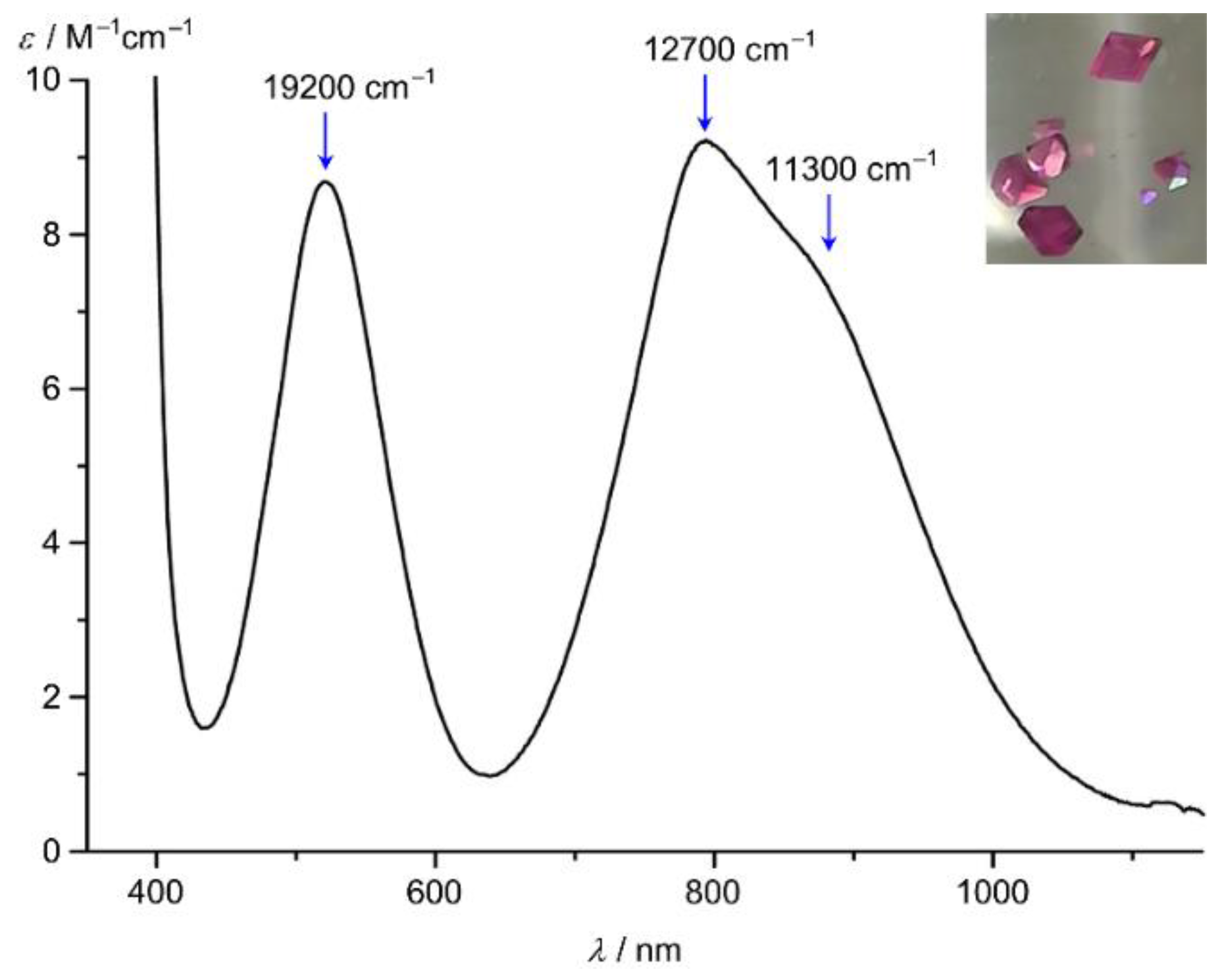
The UV/Vis/NIR absorption spectra of red [Ni(tpy)2]2+ and pink [Ni(ddpd)2]2+ are dominated by the two spin-allowed 3A2 → 3T1 and 3A2 → 3T2 ligand field transitions at 18,500/12,350 and 19,200/12,700 cm–1, respectively (Figure 5) [24,61,91]. The transitions at lowest energy correspond directly to the respective ligand field splittings ∆o = 12,350 and 12,700 cm−1, demonstrating that ddpd induces a stronger ligand field by 350 cm−1. Nickel(II) polypyridine complexes display the spin-forbidden 3A2 → 1E transition close to the low-energy spin-allowed 3A2 → 3T2 transition indicating a comparably strong ligand field in all cases. The intensity of the spin-flip band is rather high due to an intensity borrowing mechanism, which scales directly with the square of the spin-orbit coupling parameter λ and inversely with the square of the energy difference ∆E (Figure 5; shoulder at 11,300 cm–1 for [Ni(ddpd)2]2+) [93]. The energy differences between the 1E and 3T2 spectroscopic terms are quite small and consequently luminescence from the spin-flip 1E states is not observed [61].
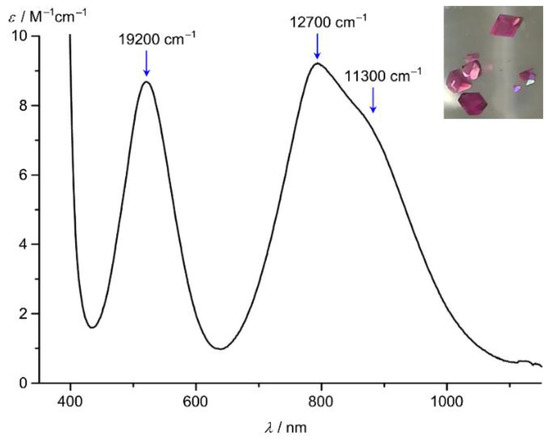
Figure 5.
UV/Vis/NIR spectrum of [Ni(ddpd)2][BF4]2 in CH3CN; ligand field bands and the spin-flip band indicated by blue arrows; photograph of crystals of [Ni(ddpd)2][BF4]2 [61].
Reduction of [Ni(tpy)2]2+ is ligand-based yielding [NiII(tpy)(tpy·−)]+, which slowly releases a tpy ligand [20,94]. The resulting proposed [NiII(tpy·–)]+ species exhibits selectivity for electrochemical CO2 reduction over proton reduction [20]. Oxidation of [Ni(tpy)2]2+ to nickel(III) has been reported at 1.29 V [78]. Similarly, a quasireversible oxidation of [Ni(ddpd)2]2+ to [Ni(ddpd)2]3+ occurs at 1.22 V [61]. Reduction of [Ni(ddpd)2]2+ is irreversible (−2.03 V). Presumably, the irreversible reduction is associated with the formation of [NiI(ddpd)]+ species after ligand loss. In the presence of CO2/acetic acid a catalytic current with an onset potential of ca. −1.1 V versus ferrocene is observed (Figure S2). A detailed comparison of this behaviour and that proposed for [Ni(tpy)2]2+ will be subject to future studies [20].
In summary, the magnetic, optical and redox properties of [Ni(ddpd)2]2+ are very similar to those of [Ni(tpy)2]2+, with the ligand field splitting induced by ddpd being larger by 350 cm−1.
6. mer-[Co(ddpd)2]2+, cis-fac-[Co(ddpd)2]2+ [d7] and mer-[Co(ddpd)2]3+ [d6]
Reaction of Co(BF4)2×6H2O with ddpd in ethanol (Scheme 3) yields a precipitate, which contains two phases according to powder X-ray diffraction and SEM images [63]. Paramagnetic NMR spectroscopy in acetonitrile solution indicates the presence of only a single metal complex isomer [63]. Re-crystallization from CH3CN yields crystals of mer-[Co(ddpd)2][BF4]2×2CH3CN (Figure 6a) [62]. The initial precipitate contains a further microcrystalline phase in addition to crystals of mer-[Co(ddpd)2][BF4]2×2CH3CN. With the aid of electron diffraction tomography, these crystals are identified as cis-fac-[Co(ddpd)2][BF4]2 with the ddpd ligand coordinating in a facial tripodal manner (Figure 6b) [63]. In both stereoisomers, the cobalt(II) centres are in their high-spin state [63]. The cis-fac isomer is the kinetic product, while the mer isomer is the thermodynamically stable one. In contrast, only the mer isomers of [Co(tpy)2]2+ are available due to the rigid nature of tpy.
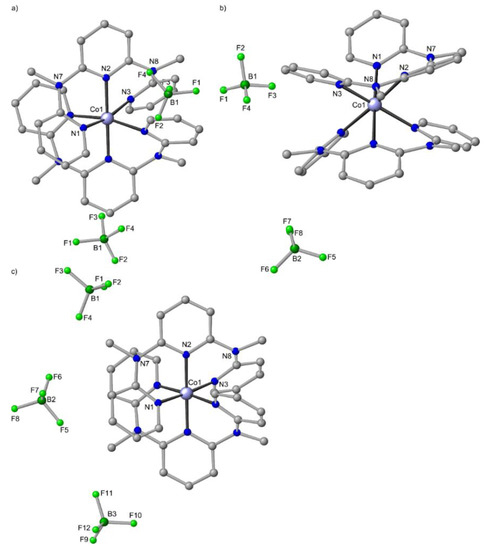
Figure 6.
Molecular structures of (a) mer-[Co(ddpd)2][BF4]2×2CH3CN [62], (b) cis-fac-[Co(ddpd)2][BF4]2 [63] and (c) mer-[Co(ddpd)2][BF4]3×3CH3CN in the solid state; hydrogen atoms and solvent molecules omitted. Atom numbering unified for clarity.
For the d7 electron configuration, the ground state can be either low-spin ((t2g)6(eg)1; 2E; S = 1/2) or high-spin ((t2g)5(eg)2; 4T1; S = 3/2) [15,16]. In fact, the terpyridine complexes [CoII(tpy)2]X2×nH2O (X = halide, pseudohalide, NO3−, ClO4−; n = 0–5) exhibit incomplete and gradual SCO behaviour [95,96]. On the other hand, [Co(tpy)2][PF6]2 remains in the high-spin state down to low temperatures [16]. The entropy change during the SCO due to the spin change amounts to ΔSspin = R[ln (2S + 1)hs − ln(2S + 1)ls] = 5.8 J K–1 mol–1. The ΔSspin of cobalt(II) is smaller than those of d6-iron(II) (13.4 J K–1 mol−1) or d5-iron(III) (9.1 J K–1 mol−1) SCO complexes. Therefore, the SCO phenomenon can be induced in cobalt(II) compounds by smaller external stimuli such as confinement in host lattices (e.g., [Fe0.83Co0.17(tpy)2][PF6]2 displays cobalt-based SCO) [16] or phase transitions enabled by interchain interactions of alkyl substituted tpy ligands 4′-R-tpy [97]. Furthermore, cobalt(II) complexes with certain 4’-substituted terpyridine ligands R-tpy with R = OC14H29, OC16H33 display “reverse SCO” with the low-spin state populated at higher temperature instead of the conventional temperature dependence [97,98].
[Co(tpy)2][BF4]2 has been characterized crystallographically between 30 K and 375 K. The XRD data reveal a Jahn-Teller [59] distorted low-spin complex at 30 K with distances to two terminal pyridines of one tpy ligand elongated and a high-spin fraction >87% at 375 K [99]. Similar to [Co(tpy)2][PF6]2, mer-[Co(ddpd)2][BF4]2×1.5H2O remains in the high-spin state down to low temperature [62]. The crystallographic data of mer-[Co(ddpd)2][BF4]2×2CH3CN at 193 K show no sign for a Jahn-Teller distortion, which would be expected for a low-spin d7 system (Table 1, Figure 6a). The magnetic susceptibility of mer-[Co(ddpd)2][BF4]2×1.5H2O at 300 K amounts to χT = 2.53 cm3 K mol−1 (Table 5), similar to [Co(tpy)2][ClO4]2 at 300 K [95]. mer-[Co(ddpd)2][BF4]2×1.5H2O displays a typical χT versus T profile for a high-spin cobalt(II) ion with spin-orbit coupling in a distorted environment at lower temperature without any sign for SCO [62]. In mixed crystals of [Fe0.83Co0.17(tpy)2][PF6]2 the iron(II) host lattice destabilizes the high-spin state of cobalt(II) to such an extent that the low-spin state prevails up to 250 K and SCO sets in close to room temperature [16]. Furthermore, [Co(tpy)2]2+ appears as a rapidly equilibrating mixture of low- and high-spin species in solution (acetonitrile, methanol, water) [100,101]. Both [Co(tpy)2]2+ and [Co(ddpd)2]2+ exhibit characteristic paramagnetically shifted but sharp 1H NMR spectra [62,68]. In addition, [Co(ddpd)2]2+ displays sharp 13C NMR resonances between δ = +602 ppm and δ = −216 ppm in CD3CN at room temperature [62].
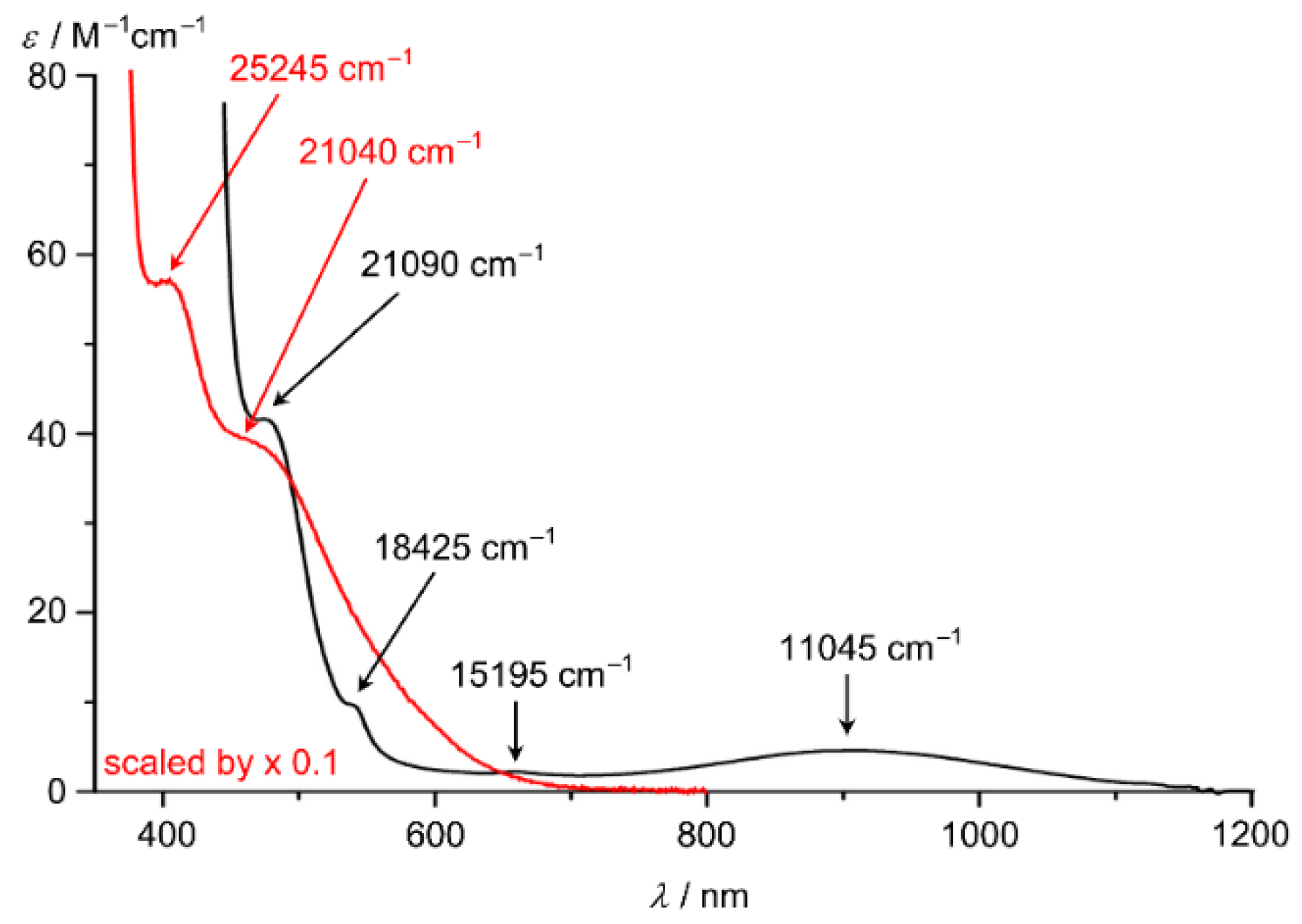
The electronic spectrum of [Co(ddpd)2]2+ in CH3CN displays prominent π–π* and charge transfer bands of the ddpd ligands at 24,330 (980 M−1 cm−1), 32,365 (31,000 M−1 cm−1) and 40,325 (32,000 M−1 cm−1) cm−1 [62]. In addition, four weak ligand field bands of the cobalt(II) centre appear at 11,045 (5 M−1 cm−1), 15,195 (2 M−1 cm−1), 18,425 (9 M−1 cm−1, sh) and 21,090 cm−1 (40 M−1 cm−1; superimposed on a π–π* band) (Figure 7) [62]. Two spin-allowed 4T1 → 4T2 and 4T1 → 4T1(P) ligand-field bands are expected in O symmetry. The lower tetragonal symmetry of [Co(ddpd)2]2+ (compressed octahedron along z, Table 1) splits the t2g and eg levels enabling four transitions. The observed bands can then be interpreted as dxz/yz → dx2−y2, dxy → dx2−y2, dxz/yz → dz2 and dxy → dz2 excitations leading to averaged tetragonal splittings of the t2g and eg levels of 3410 and 6635 cm−1, respectively and an averaged ligand field splitting ∆o of 16,440 cm−1. This value of ∆o is quite high and should enable SCO under appropriate conditions. In single crystals of [Co(tpy)2][PF6]2 (high-spin) two ligand field bands are discernible at 9200 and 11,600 cm−1, assigned to the split components of the 4T1 → 4T2 transition in the distorted octahedron. For [Co(bpy)3][NaRh(ox)3] a band at 11,500 cm–1 has been assigned to the 4T1 → 4T2 transition (without addressing the trigonal splitting). Further transitions of tpy and bpy complexes are masked by MLCT transitions [16].
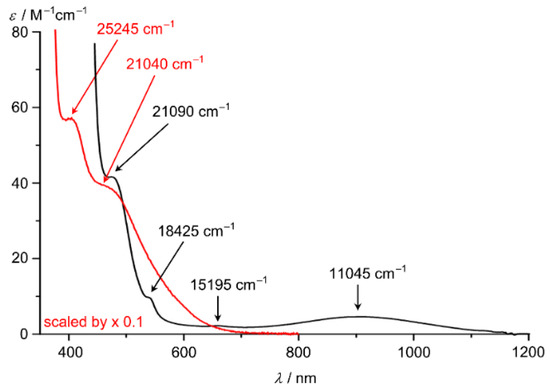
Figure 7.
UV/Vis/NIR spectra of [Co(ddpd)2]2+ (black) and [Co(ddpd)2]3+ (red, scaled by 0.1) in CH3CN; ligand field bands indicated by arrows [62].
Both cobalt(II) complexes undergo a reversible oxidation to the cobalt(III) complexes at low potential (Table 4). [Co(ddpd)2]2+ is easier to oxidize than [Co(tpy)2]2+, likely due to the more electron-rich nature of ddpd (Table 4). Chemical oxidation of [Co(tpy)2][PF6]2 with bromine and of [Co(ddpd)2][BF4]2 with silver triflate, silver tetrafluoroborate or 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (and counter ion exchange) yields the corresponding diamagnetic low-spin cobalt(III) complexes [Co(tpy)2]3+ and [Co(ddpd)2]3+, respectively (Table 3) [62,68,100,102,103]. [Co(tpy)2]3+ displays the lowest value S(OC-6) = 1.22 of the terpyridine series and [Co(ddpd)2]3+ is almost perfectly octahedral with S(OC-6) = 0.10 (Table 1 and Table 2; Figure 6c), underscoring the high electronic symmetry of the (t2g)6 electron configuration.
The low-spin d6 cobalt(III) complex [Co(ddpd)2]3+ displays two absorption bands at 21,040 and 25,245 cm−1 (superimposed onto π–π* and charge transfer bands; maxima fit by Gaussian bands, Figure 7) which can be assigned to the spin-allowed ligand field transitions 1A1 → 1T1 and 1A1 → 1T2, respectively [104]. The lower energy corresponds to the ligand field splitting ∆o = 21,040 cm−1. The ligand field bands of [Co(phen)3]3+ have been reported as 29,240–28,985 cm−1 and 21,786–21,882 cm−1, respectively [105]. The ligand field splitting induced by ddpd is hence smaller than that of phen in the cobalt(III) complexes.
The second order rate constant for the self-exchange reaction [Co(tpy)2]3+/2+ has been determined as kex = 2820–3000 M−1 s−1 (in H2O) between low-spin CoII and low-spin CoIII [106,107]. Much lower rate constants of kex = 41.7 and 17.5 M−1 s−1 have been reported for the [Co(phen)3]3+/2+ and [Co(bpy)3]3+/2+ pairs, respectively [106,107]. This can be traced back to the different spin states of [Co(tpy)2]2+ (mainly low-spin) and [Co(phen)3]2+/[Co(bpy)3]2+ (high-spin) at room temperature in solution. In any case, self-exchange is comparably slow on the 1H NMR timescale and this holds for the [Co(ddpd)2]3+/2+ couple as well [62]. In addition to the spin-barrier for the ddpd complexes, the Co–N bond lengths Co–N2 and Co–N1/3 decrease by 0.148 and 0.166 Å upon oxidation (Table 1). Similarly, the averaged Co–N2 and Co–N1/N3 bond lengths of the tpy complexes shrink by 0.149 and 0.200 Å, respectively (Table 2). Obviously, the large reorganization energies (irrespective of the CoII spin state) mainly account for the comparably slow electron transfer rates. Even smaller self-exchange rate constants are found for CoIII/II complexes of aliphatic amines, such as [Co(en)3]3+/2+ with kex = 2 × 10−5 M−1 s−1 [108]. This has been ascribed to the innocent nature of ethylene diamine in contrast to the redox-activity of bpy or phen ligands. The latter enable a strong electronic coupling via π* orbitals of the ligands in the electron transfer step, for example via the ubiquitous “phenyl embraces” of polypyridine complexes (Figure 2) [72]. As ddpd is a poor electron acceptor, electron transfer to [Co(ddpd)2]3+ is retarded by the high-lying π* orbitals of ddpd.
The slow electron transfer kinetics of the reduction of [Co(ddpd)2]3+ can be beneficial for applications in dye-sensitized solar cells, retarding the undesired back-electron transfer (recombination) at the TiO2 electrode [102]. However, dye-regeneration by [Co(ddpd)2]2+ is also slow [103]. Compared to the standard iodide/triiodide electrolyte, the overall performance of outer sphere cobalt(III/II) electrolytes in dye-sensitized solar cells depends on the dye and the electrode surface treatment among other factors [102,103,109,110].
Reduction of [CoII(tpy)2]2+ leads to [CoI(tpy)2]+ and then to [Co(tpy)2]0 (Table 4) [20]. The latter neutral species has been described either as [CoI(tpy·−)(tpy)]0 [20] or as [CoII(tpy·−)2]0 [24]. After the loss of one tpy ligand, the resulting species catalyses the electroreduction of CO2 to CO and of protons to H2 [20]. The ddpd complex [CoII(ddpd)2]2+ is irreversibly reduced at −1.80 V. This reduction occurs at a potential 0.58 V less negative than the ligand-centred reduction of the [ZnII(ddpd)2]2+ complex, suggesting that the cobalt(II) ion is reduced to cobalt(I) similar to the [Co(tpy)2]2+/+ reduction process (Table 4). The [CoI(tpy)2]+ monocation features some π back-donation into the π* orbitals of tpy [24]. However, the strongly electron donating character of ddpd is incompatible with back-donation from a d8-cobalt(I) centre and consequently the [CoII/I(ddpd)2]2+/+ process becomes irreversible, likely caused by partial or full dissociation of ddpd.
The mer- and cis-fac isomers of [Co(ddpd)2]2+ constitute the first structurally confirmed example for the ability of ddpd to coordinate as pincer and as tripodal ligand. The [Co(L)2]3+/2+ and [Co(L)2]2+/+ redox couples are metal centred for both ligands, yet [Co(ddpd)2]+ is unstable. Electron transfer involving [Co(ddpd)2]2+ or [Co(ddpd)2]3+ is slow. Spin-crossover behaviour is found for several [Co(tpy)2]2+ salts but not yet for [Co(ddpd)2]2+ salts (X = BF4−).
7. mer-[Fe(ddpd)2]2+ [d6] and mer-[Fe(ddpd)2]3+ [d5]
[Fe(ddpd)2]2+ salts are conveniently prepared from Fe[BF4]2×6H2O and ddpd or from FeBr2/ddpd followed by anion exchange with [NH4][PF6] (Scheme 3) [64], similar to the well-known [Fe(tpy)2]2+ complexes [25,111]. Both d6 iron(II) complexes [Fe(tpy)2]2+ and [Fe(ddpd)2]2+ are diamagnetic low-spin complexes with (t2g)6 electron configuration at room temperature. Both complex cations deliver sharp and well-resolved NMR spectra (Table 3 and Table 6). Coordination of iron(II) to tpy and ddpd results in a significant 15N shielding effect of the pyridine nitrogen nuclei [112]. The effect is more pronounced for the terminal pyridines (N1) than for the inner one (N2) in both cases (Table 6). Yet, the relative 15N coordination shift difference between N1 and N2 is much larger for the tpy complex (49 ppm) than for the ddpd derivative (23 ppm), possibly arising from the different symmetry (Table 1 and Table 2). Interestingly, the coordination shift does not correlate with the M–N distances in the solid state (Table 1 and Table 2) [112]. Expectedly, the 15N chemical shift of the bridging N–Me groups of ddpd is hardly affected by the iron(II) coordination (Table 6).

Table 6.
Coordination shift δ = δ(complex) − δ(ligand) of 15N resonances of diamagnetic [Fe(L)2]2+ complexes in CD3CN (L = ddpd, tpy).
In the Mößbauer spectra of the tpy and ddpd iron(II) complexes, the quadrupole doublets appear at δ = 0.28 mm s−1 and δ = 0.39 mm s−1, respectively [25,64]. The quadrupole splitting of ∆EQ = 1.05 mm s−1 of the tpy complex surpasses that of the ddpd complex with ∆EQ = 0.40 mm s−1 suggesting a much higher symmetry of the [Fe(ddpd)2]2+ complex ion [25,64]. This is also reflected in the stronger deviation of the tpy complex from octahedral symmetry (Table 1 and Table 2).
Refluxing a solution of [Fe(ddpd)2]2+ in CH3CN does not lead to spin-crossover (SCO) to the 5T2 high-spin state but to ligand dissociation [64]. Under these conditions, the [Fe(tpy)2]2+ ion is thermally stable but does not undergo SCO either. This suggests a too strong ligand field splitting in both complexes. Substitution of tpy at the sterically unhindered 4,4″ positions retains the low-spin ground state [111]. On the other hand, substituents at the α positions (6,6″) (X = F, Cl, Br) lead to a diminished ligand field strength and high-spin ground states (X = Cl, Br) or SCO behaviour (X = F) [114,115].
Increasing the steric bulk at the bridging nitrogen atom of ddpd in [Fe(nPr2tpda)2]2+ and [Fe(nHex2tpda)2]2+ complexes (see Scheme 2 for R2tpda ligands) increases the hydrolytic lability of the iron(II) complexes, yet the electronic structure remains low-spin iron(II) [43]. The parent complex [Fe(H2tpda)2]2+ is in the low-spin state as well [116]. In acetonitrile solution, deprotonation at the NH groups of [Fe(H2tpda)2]2+ readily occurs for example in the ESI mass spectrometer [43].
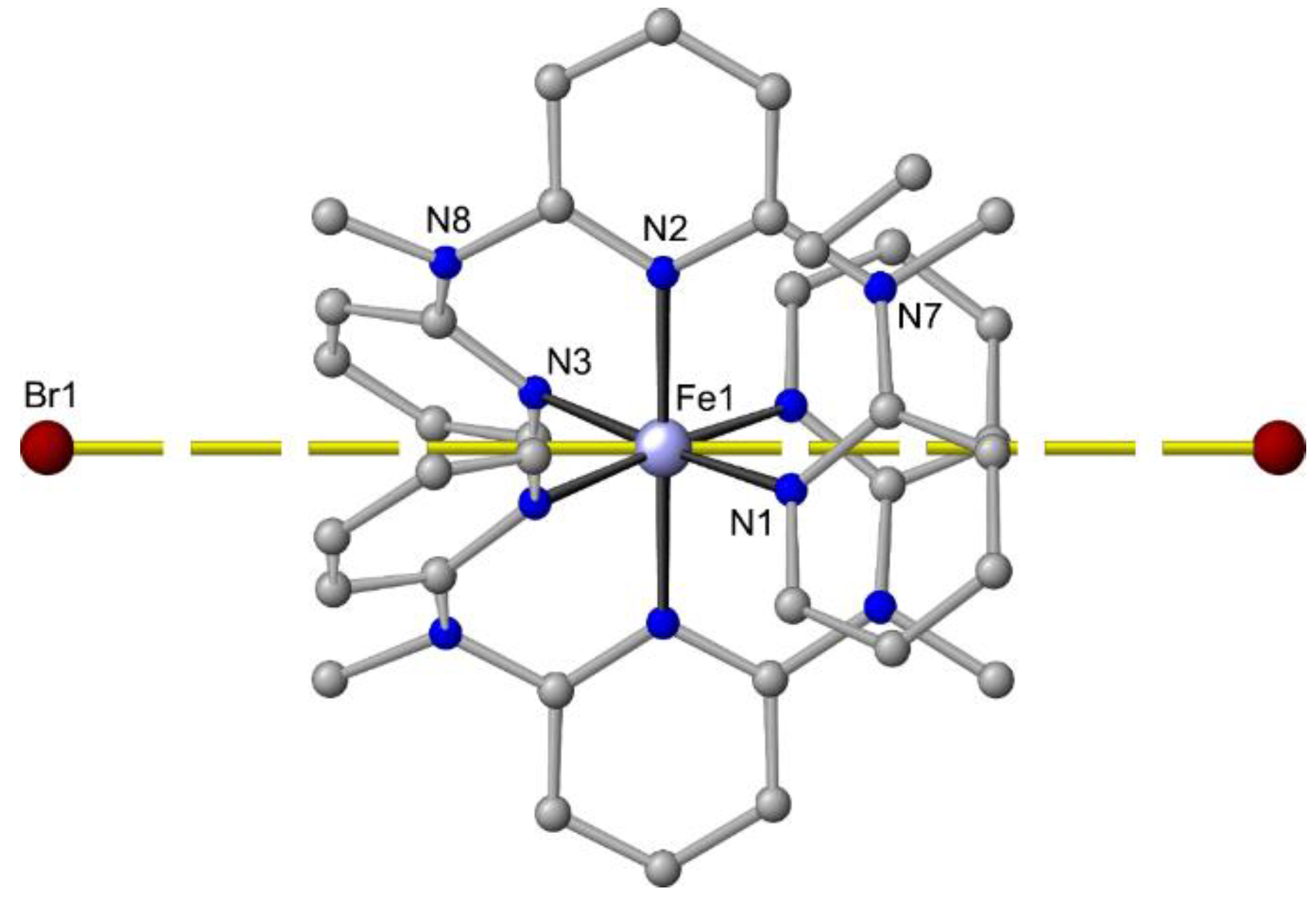
In contrast to the Zn2+/chloride/ddpd system (see above), bromide ions cannot compete with ddpd for iron(II) coordination sites and [Fe(ddpd)2]Br2 can be isolated and crystallized (Figure 8) in addition to the salts with weakly coordinating anions BF4– or PF6– (Table 1) [64]. The Fe…Br distance amounts to 6.6 Å, indicating an electrostatic interaction at best but no coordination. In all [Fe(ddpd)2][X]2 salts, the geometry of the dications is close to octahedral with S(OC-6) < 0.2 (Table 1).
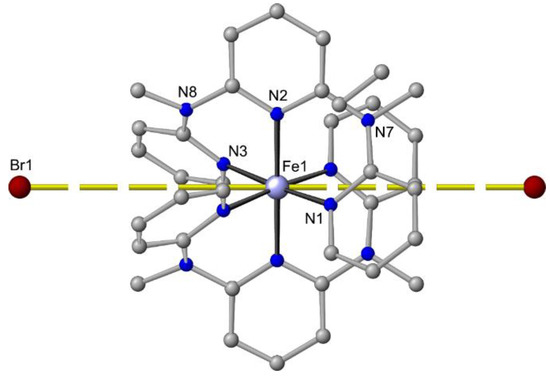
Figure 8.
Molecular structure of mer-[Fe(ddpd)2]Br2 in the solid state; hydrogen atoms and solvent molecules omitted. Atom numbering unified for clarity. Fe…Br distances indicated by yellow dashed lines.
Addition of ddpd to [Fe(tpy)2]2+ does not displace tpy. In contrast, tpy is able to substitute ddpd in [Fe(ddpd)2]2+, as easily judged from the colour change from orange-brown ([Fe(ddpd)2]2+) to purple ([Fe(tpy)2]2+). The characteristic purple colour of [Fe(tpy)2]2+ is commonly assigned to a MLCT transition at 552 nm enabled by the low-energy π* orbitals of tpy [111]. The π* orbitals of ddpd are at higher energy and consequently the MLCT absorption of [Fe(ddpd)2]2+ is strongly hypsochromically shifted to 395 nm [64].
Consistent with the stronger electron donating character of ddpd, the [Fe(ddpd)2]3+/2+ redox process occurs at lower potential than the corresponding [Fe(tpy)2]3+/2+ process by 0.38 V (Table 4). According to Mößbauer spectroscopy, these redox processes are metal centred giving the corresponding low-spin iron(III) complexes with (t2g)5 electron configuration ([Fe(tpy)2]3+: δ = 0.07 mm s−1; [Fe(ddpd)2]3+: δ = 0.11 mm s−1) [25,64]. Similar, to the parent iron(II) complexes, the quadrupole splitting is larger in the tpy complex ([Fe(tpy)2]3+: ∆EQ = 3.43 mm s−1; [Fe(ddpd)2]3+: ∆EQ = 1.97 mm s−1) due to its reduced symmetry. The anisotropic EPR spectra of [Fe(bpy)3]3+ and [Fe(ddpd)2]3+ in frozen solution are typical of low-spin ferric species (S = 1/2) with g = 2.68, 2.60, 2.01 and g = 2.49, 2.30, 1.82, respectively [25,64]. An irreversible ligand-centred oxidation is found for [Fe(ddpd)2]3+ at 1.63 V (Table 4), while no further oxidation process has been reported for [Fe(tpy)2]3+. [Fe(tpy)2]2+ can be reversibly reduced twice to give [FeII(tpy·−)(tpy)]+ and [FeII(tpy·−)2]0, respectively, similar to [Zn(tpy)2]2+ (Table 4) [25]. On the other hand, [Fe(ddpd)2]2+ is only irreversibly reduced at the ddpd ligand, at a similar potential as the [Zn(ddpd)2]2+ complex (Table 4) [64].
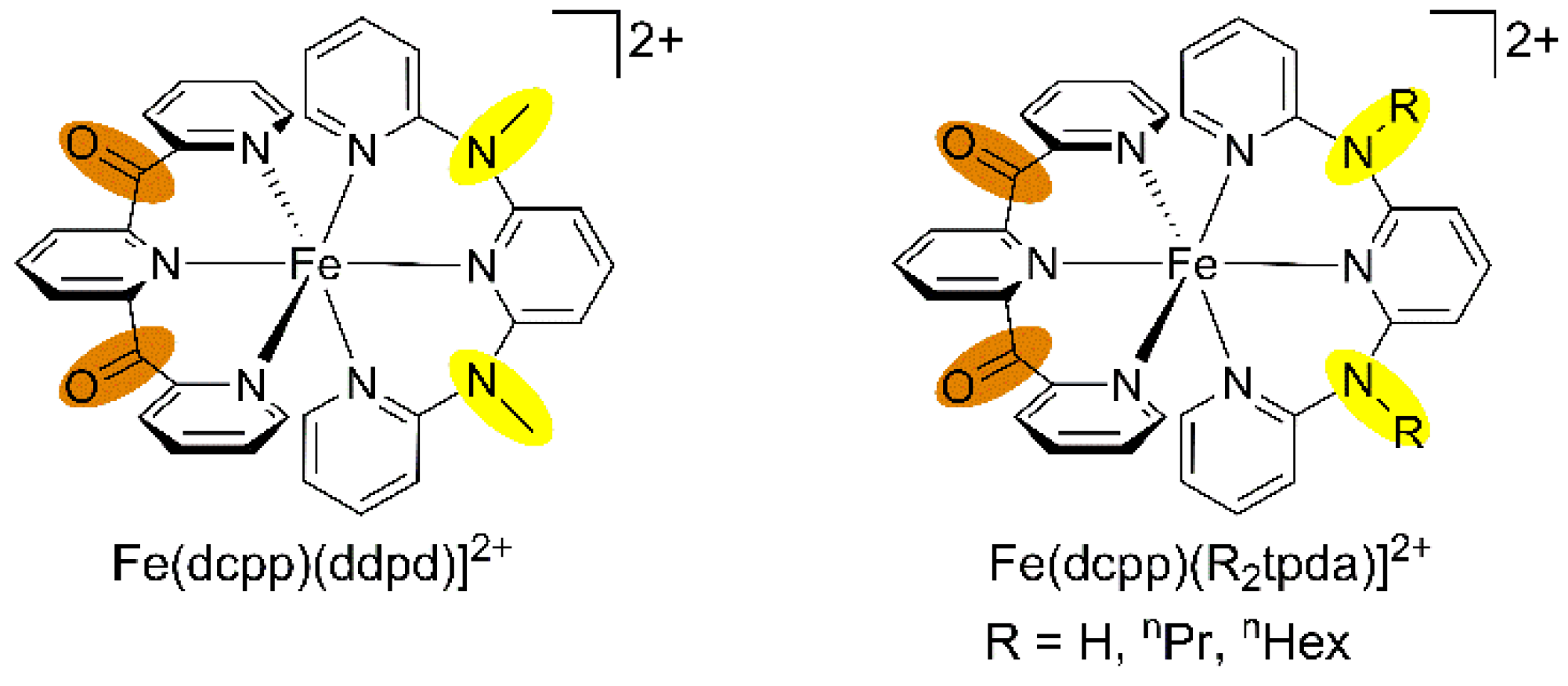
Replacement of a ddpd ligand by the electron accepting tridentate dcpp ligand give the deep blue-coloured heteroleptic push-pull substituted complex [Fe(dcpp)(ddpd)]2+ (Scheme 5) [64]. The colour arises from a strong charge transfer absorption band peaking at 592 nm and tailing into the near-IR. According to time-dependent DFT calculations, the lowest energy components are of mixed MLCT (iron(II) → dcpp) and LL’CT (ddpd → dcpp) character. The homoleptic complex [Fe(dcpp)2]2+ prepared by McCusker features charge transfer bands as well but these are of pure MLCT nature [64,117]. After excitation of the charge transfer bands of [Fe(dcpp)2]2+ and [Fe(dcpp)(ddpd)]2+, transient absorption spectroscopy reveals a rapid recovery of the ground state with τ = 280 and 548 ps, respectively. This unusually fast relaxation in the picosecond range has been tentatively assigned to a shortened relaxation cascade 1MLCT → 3MLCT → 3MC(3T1), omitting the 5MC(5T2) high-spin state with typical lifetimes in the nanosecond range [64,117]. It has been proposed that the very large ligand field splitting in these complexes shifts the 5T2 state close to or even above the 3T1 excited state. However, non-radiative relaxation via the 3T1 states is still too fast to allow for a long-lived 3MLCT state, as has been established recently in polycarbene iron(II) complexes [118,119,120,121,122].
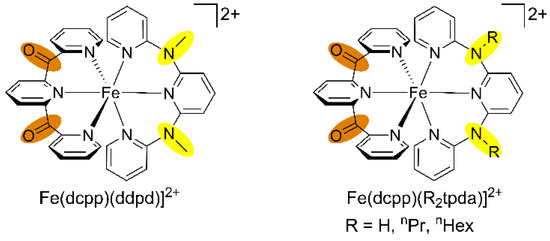
Scheme 5.
Heteroleptic iron(II) complexes of ddpd/R2tpda and dcpp.
The analogous heteroleptic complexes [Fe(dcpp)(R2tpda)]2+ (R = H, nPr, nHex; Scheme 5) feature charge transfer properties similar to those of [Fe(dcpp)(ddpd)]2+ [43]. Furthermore, stepwise deprotonation of [Fe(dcpp)(H2tpda)]2+ at the bridging nitrogen atoms of H2tpda yields the deep green, reasonably stable conjugate bases [Fe(dcpp)(Htpda)]+ and [Fe(dcpp)(tpda)]0. These can be reversibly re-protonated with trifluoroacetic acid to [Fe(dcpp)(H2tpda)]2+ or irreversibly methylated with methyl iodide to [Fe(dcpp)(ddpd)]2+. The charge transfer band maxima of [Fe(dcpp)(Htpda)]+ and [Fe(dcpp)(tpda)]0 are shifted to 613 and 682 nm with respect to that of their conjugate acid [Fe(dcpp)(H2tpda)]2+ (592 nm) [43]. Moreover, additional bands appear at 724 and 872 nm, respectively. TD-DFT calculations suggest that these low-energy bands arise from LL’CT transitions, namely from the amide units localized at the [Htpda]−/[tpda]2− ligands to the dcpp ligand without significant iron participation. The combined MLCT and LL’CT absorptions cover the spectral range from 600 nm to more than 1000 nm [43]. The low-energy 1LL’CT transitions are dipole and spin allowed. However, emission from the corresponding 3LL’CT states is not observed. Possibly, the π-donor character of the pyridyl amide ligands [Htpda]− and [tpda]2− also decrease the ligand field splitting and consequently favour rapid non-radiative relaxation via the low-energy 3MC(3T1) and 5MC(5T2) states.
All iron(II) and iron(III) complexes of the oligopyridine ligands tpy and ddpd are low-spin complexes due to the large ligand field splitting in all cases. SCO is not observed. Ligand-centred reductions are reversible for the tpy complex but irreversible for the ddpd complex. The iron(III/II) couple is reversible for both complex types. MLCT bands appear in the visible spectral region for [Fe(tpy)2]2+ due to the low-energy π* orbitals of tpy but in the UV for the ddpd complex. The heteroleptic complex [Fe(dcpp)(ddpd)]2+ displays MLCT bands with Fe → dcpp and LL’CT bands with ddpd → dcpp character in the Vis-NIR region.
8. [Mn(ddpd)2]n+
Neither homo- nor heteroleptic complexes of manganese and ddpd have been reported so far. In contrast, colourless complexes of the type [Mn(tpy)2]2+ are easily formed from MnCl2×4H2O, tpy and counter ion exchange, for example by perchlorate [87]. The manganese(II) complex cation features a high-spin d5 electron configuration (S = 5/2) with χT = 4.62 cm3 K mol−1 at room temperature (Table 5) [87]. Expectedly, ligand field transitions are Laporte and spin-forbidden and only π–π* transitions appear at 284, 323 and 335 nm in CH3CN [123]. The coordination geometry of the 4’-tolyl derivative [Mn(Tol-tpy)2]2+ is compressed octahedral due to the ligand bite angle [70]. [Mn(tpy)2]2+ displays a rich redox chemistry [26,87,123]. Oxidation to the high-spin d4 manganese(III) complex [Mn(tpy)2]3+ occurs at 0.86 V (Table 4). The 4’-tolyl tpy derivative [Mn(Tol-tpy)2]3+ shows a tetragonally compressed octahedral geometry due to the Jahn-Teller [59] effect and the ligand bite angle [70]. Heteroleptic complexes easily form in higher oxidation states. Binuclear mixed-valent di-μ-oxido terpyridine manganese complexes such as [(H2O)(tpy)MnIII(μ-O)2MnIV(H2O)(tpy)]3+ have been extensively studied as functional models of the oxygen-evolving centre of photosystem II [124,125].
Tpy-centred reductions are found for [Mn(tpy)2]2+ at −1.52, −1.86 and −2.37 V (Table 4) [26]. The manganese centre remains high-spin (SMn = 5/2) throughout this series. The neutral complex MnII(tpy·−)2 has been structurally and magnetically characterized. Its S = 3/2 ground state is attained via antiferromagnetic coupling of the five unpaired electrons of the MnII ion with the unpaired spins of the two tpy·− ligands [26].
A comparison of the structural, optical, electrochemical and magnetic data with those of [Mn(ddpd)2]n+ complexes must await the preparation of the respective manganese ddpd complexes.
9. mer-[Cr(ddpd)2]3+, mer-CrCl3(ddpd) [d3] and mer-[Cr(ddpd)2]2+ [d4]
The heteroleptic complexes CrCl3(tpy) and mer-CrCl3(ddpd) are easily prepared from CrCl3(thf)3 or CrCl3×6H2O and the respective pincer ligand (Figure 9a,b) [71,126]. Recently, Hauser and Piguet realized heteroleptic [Cr(tpy)(L’)]3+ complexes starting from CrCl3(tpy) via Cr(tpy)(CF3SO3)3 and further tridentate polypyridine ligands L’ [126]. The homoleptic chromium(III) complexes [Cr(tpy)2]3+ and [Cr(ddpd)2]3+ are best prepared from more reactive chromium(II) salts such as Cr(CF3SO3)2 or CrCl2 and the respective tridentate ligands, followed by (aerial) oxidation (Figure 9c,d) [50,71]. Surprisingly, [Cr(tpy)2]3+ is quite labile in alkaline aqueous solution, presumably yielding to tpy displacement and [Cr(tpy)(OH)x]n(3−x)n species [71]. On the contrary, [Cr(ddpd)2]3+ is substitutionally inert under these conditions—even under irradiation [50].
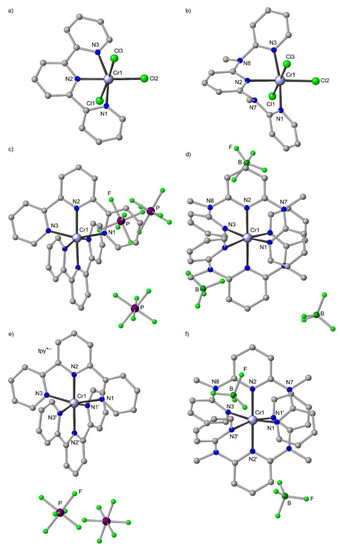
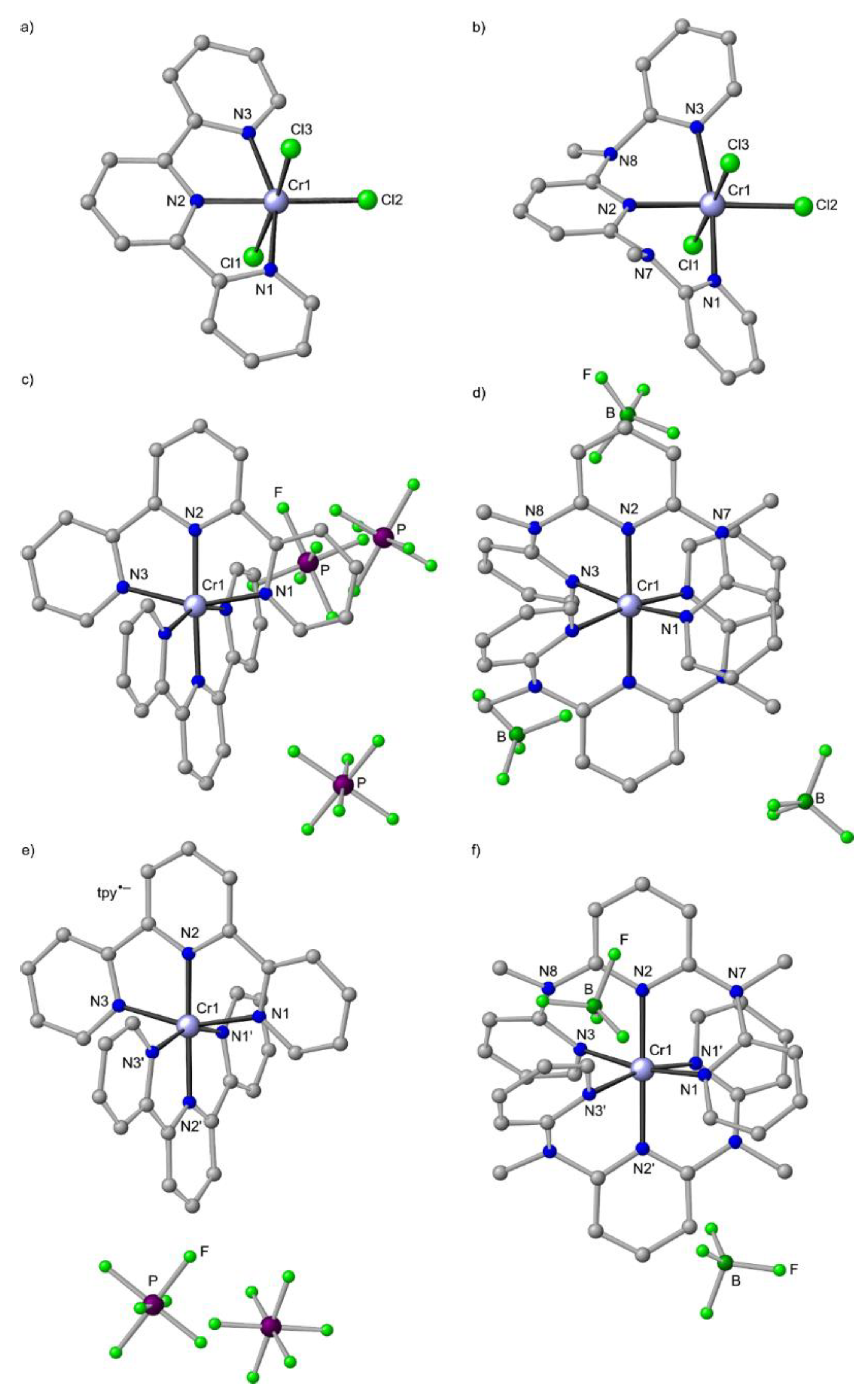
Figure 9.
Molecular structures of (a) CrCl3(tpy)×DMSO [127], (b) mer-CrCl3(ddpd) [126], (c) [Cr(tpy)2][PF6]3×2.5CH3CN [126], (d) mer-[Cr(ddpd)2][BF4]3×3CH3CN [50] and (e) [Cr(tpy)2][PF6]2×2CH3CN [27] and (f) mer-[Cr(ddpd)2][BF4]2×2CH3CN in the solid state; hydrogen atoms and solvent molecules omitted. Atom numbering unified for clarity. In the dications two different tridentate ligands are observed labelled as N1/N2/N3 and N1′/N2′/N3′.
The room temperature magnetic susceptibility of [Cr(tpy)2]3+ and [Cr(ddpd)2]3+ amounts to χT = 1.921 and 1.838 cm3 K mol–1, respectively (Table 5), confirming the expected (t2g)3 electron configuration (4A2 ground state) for both complexes [27,50]. The magnetic moment of [Cr(ddpd)2][BF4]3 is temperature-independent down to 40 K. At 2 K, χT has dropped to 1.788 cm3 K mol−1, suggesting weak zero-field splitting or weak intermolecular antiferromagetic interactions.
The first spin-allowed ligand field transition 4A2 → 4T2 of [Cr(tpy)2]3+ appears at 533 nm [126], that of [Cr(ddpd)2]3+ at 435 nm [50]. These data imply a much larger ligand field splitting ∆o in the latter complex by more than 4200 cm−1.
The non-innocent tpy ligand accounts for the ligand-based reduction chemistry of [CrIII(tpy)2]3+ giving [CrIII(tpy·−)(tpy)]2+, [CrIII(tpy·−)2]+ and [CrIII(tpy2−)(tpy·−)]0, respectively, as demonstrated by Wieghardt and co-workers [27]. On the other hand, [Cr(ddpd)2]3+ is reversibly reduced to the reactive d4 chromium(II) complex [Cr(ddpd)2]2+ at −1.11 V and irreversibly at the ddpd ligand at −1.94 V (Table 4) [50]. The ddpd/ddpd·+ oxidation is observed at +1.71 V, at much higher potential than the oxidation of the ddpd ligand in the dicationic [Zn(tpy)2]2+ complex (+1.17 V, Table 4) due to the +3 charge of [Cr(ddpd)2]3+ [50]. Both the substitutional lability and the electronic structure of the reduced species [Cr(tpy)2]3−n (n = 1–3) are consequences of the π-accepting character and redox non-innocence of terpyridine. In contrast, ddpd is much more electron donating, thus preventing nucleophilic attack of hydroxide ions at the chromium(III) centre in [Cr(ddpd)2]3+.
Single crystals of the green labile, oxygen and moisture sensitive chromium(II) complex [Cr(ddpd)2][BF4]2×2CH3CN could be obtained from [Cr(NCCH3)4][BF4]2 [128] and ddpd (Table 1). The magnetic susceptibility data (in solution) confirm the high-spin state at room temperature (Table 5). According to DFT calculations on the [Cr(ddpd)2]2+ dication, the high-spin d4-chromium(II) complex is Jahn-Teller distorted [59,129] with Cr–N2 = 2.052, Cr–N1 = Cr–N3 = 2.110 and Cr–N2′ = 2.117, Cr–N1′ = 2.311 and Cr–N3′ = 2.312 Å. A similar, yet less pronounced elongated octahedron is observed with Cr–N2 = 2.064(9), Cr–N1 = Cr–N3 = 2.089(6) and Cr–N2′ = 2.070(11), Cr–N1′ = Cr–N3’ = 2.117(7) Å in the crystalline state at 263 K (Table 1). At 263 K, this Jahn-Teller distortion is probably dynamic, as the difference between the short and long equatorial bonds (∆dsl = 0.028 Å) is less than that in [Cr(NCCH3)6][BPh4]2 at 110 K (Cr–N = 2.0655, 2.0918 and 2.4231 Å) [130]. The electronic and geometric situation is similar to that of the d9 Jahn-Teller complex [Cu(ddpd)2][BF4]2×2CH3CN [60] at 263 K (Table 1; vide supra).
In contrast, the tpy analogue [CrIII(tpy·−)(tpy)][PF6]2×2CH3CN features two different tpy ligands, assigned to tpy·– and tpy but no pronounced tetragonal elongation at 100 K (tpy: Cr–N2 = 1.976 Å; Cr–N1/3 = 2.056 Å; tpy·−: Cr–N2 = 1.916 Å; Cr–N1/3 = 2.042 Å; ∆dsl = 0.014 Å; Table 2) [27]. Again, the different electronic description of [Cr(ddpd)2]2+ and [Cr(tpy)2]2+ is rooted in the different electron accepting nature of the oligopyridine ligands.
The neutral complex [Cr(tpy)2]0 is conveniently prepared from Cr(CO)6 and tpy [27]. No heteroleptic intermediate complex Cr(CO)3(tpy) has been reported, probably due to the required meridional configuration of Cr(CO)3(tpy) with trans positioned CO ligands, in contrast to the stable fac-Cr(CO)3(py)3 complex already prepared by Hieber [131,132]. Furthermore, tpy might already acquire π electron density from the low-valent chromium centre in the intermediate complex Cr(CO)3(tpy), which further labilizes the Cr–CO bond. In contrast, neutral [Cr(ddpd)2]0 could not be obtained from Cr(CO)6 and ddpd even under forcing conditions (refluxing mesitylene, b.p. 165 °C). This substitution reaction arrests at the heteroleptic tricarbonyl complex fac-Cr(CO)3(ddpd). The ῦCO IR data of this chromium(0) complex (1891 (s), 1775 (s), 1740 (s) cm−1) suggest a facial ligand arrangement, which is enabled by the flexible ddpd ligand. The ῦCO data are comparable to those of fac-Cr(CO)3[P(2-py)3] with three perfectly identical monodentate pyridine ligands (1901 (s), 1777 (br) cm−1) [133] and to fac-Cr(CO)3[HN(2-py)2](4-pic) with strongly dissimilar pyridine donor ligands (1893 (s), 1780 (s), 1720 (s) cm−1) [134] (cf. Scheme 4 for cis-fac isomers of homoleptic complexes). The similar intensities of the three ῦCO bands also support the facial isomer instead of a mer-isomer, which should show a less intense A1 absorption band. In accordance with this assignment, DFT calculations find the fac-isomer more stable than the mer-isomer by 54 kJ mol−1 (Supplementary Materials). Furthermore, the electron donating character of ddpd stabilize the trans Cr–CO bonds in fac-Cr(CO)3(ddpd) and the three CO ligands resist further substitution by ddpd [135].
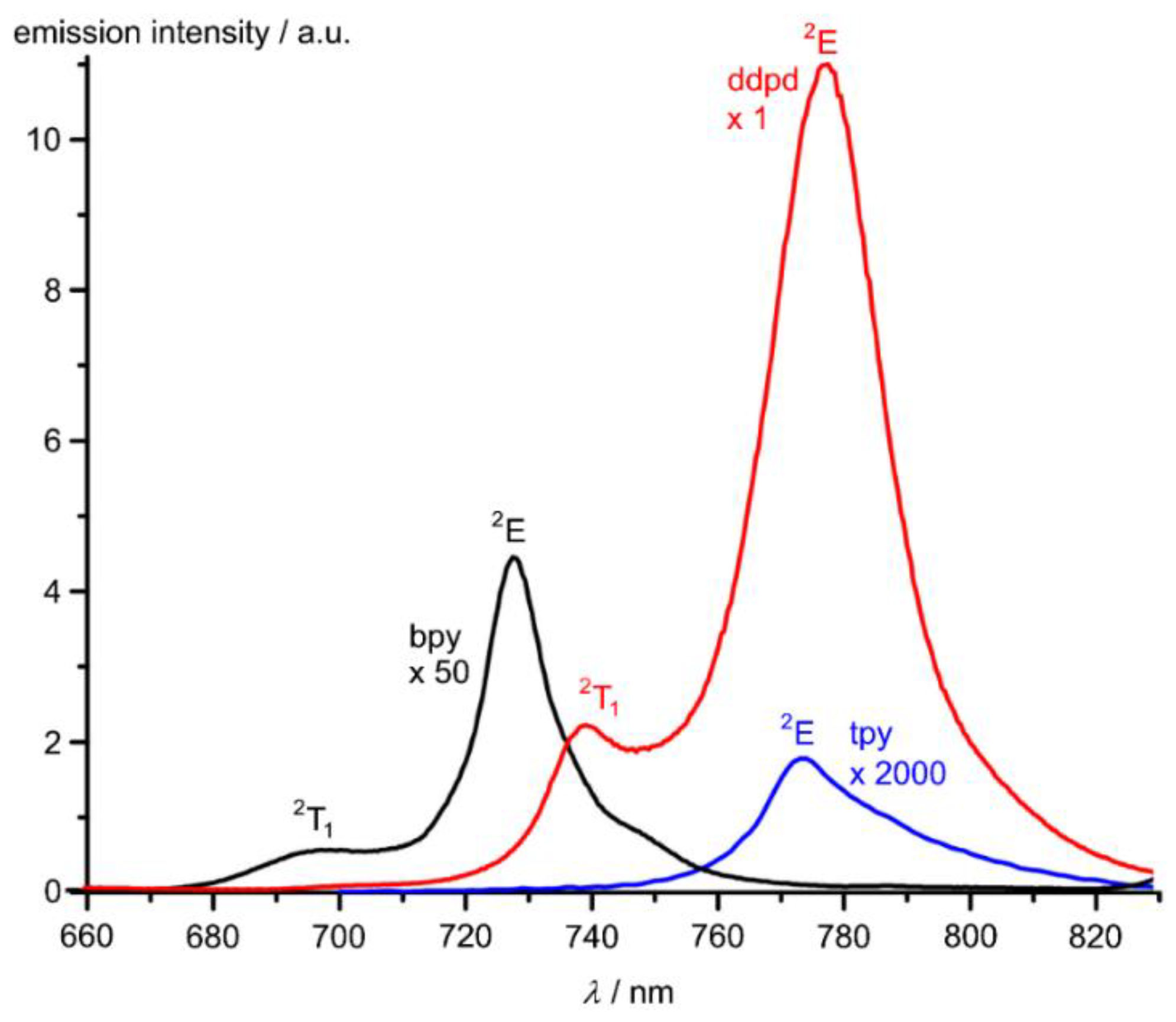
2,2′-Bipyridine (bpy), 1,10-phenanthroline (phen), tpy and ddpd are strong field ligands and consequently, the spin-flip states 2E/2T1 are lower in energy than the 4T2 ligand field state. Indeed, 2E phosphorescence is observed for the corresponding homoleptic chromium(III) complexes at 727, 730, 770 and 775 nm, respectively (Figure 10) [50,136]. However, the photoluminescence quantum yields of the three former complexes are below 0.15% hampering useful optical applications. The very low quantum yield of [Cr(tpy)2]3+ (<0.00089%) has been accounted for by the strong deviation from octahedral symmetry (S(OC-6) > 2.5, Table 2) and consequently a comparably small ligand field splitting with poor chromium-ligand orbital overlap. The small ligand field splitting places the detrimental 4T2 ligand field state close in energy to the luminescent 2E state allowing for thermal back-intersystem crossing and hence non-radiative deactivation. Substituent effects at the 4’-position of tpy slightly improve the brightness of the luminescence of [Cr(R-tpy)2]3+ (R = p-Me-C6H4, p-MeO-C6H4) by increasing both the absorbance in the visible spectral region and the quantum yield by a factor of 4.3 relative to unsubstituted [Cr(tpy)2]3+ [137]. On the other hand, the [Cr(ddpd)2]3+ complex with a geometry close to octahedral (S(OC-6) < 0.5, Table 1) enables record luminescence quantum yields of φ = 12% and 11% in CH3CN and H2O, respectively [50,53]. These values have been further boosted by the combined action of ligand (Scheme 2) and solvent deuteration to φ = 30% and 22% in D2O and CD3CN, respectively [42,53]. These measures reduce the multiphonon relaxation via CH and OH oscillators and consequently increase the luminescence quantum yield [42,53]. Concomitant with the very high quantum yields, the lifetime of the doublet excited state increases to up to 2.3 ms [42]. Similarly, deuteration of the NH groups of the analogous [Cr(H2tpda)2]3+ complex increases the luminescence quantum yield from 8.8% to 12.0% in CH3CN due to the removal of the NH oscillators [138]. The high quantum yields and excited state lifetimes of [Cr(ddpd)2]3+ complexes led to their description as “molecular rubies” [42,50,53].
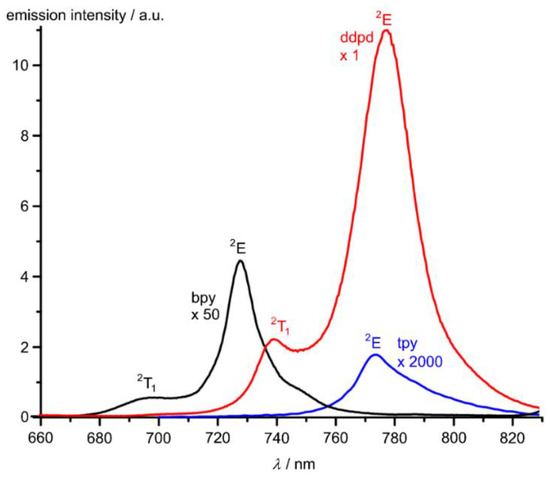
Figure 10.
Luminescence spectra of [Cr(bpy)3][PF6]3 (black) [Cr(tpy)2][PF6]3 (blue) and [Cr(ddpd)2][BF4]3 (red) in CH3CN. The bands are labelled with the respective term symbols. Please note the very different scaling factors.
The reactivity of the excited states of [Cr(ddpd)2]3+ and [Cr(tpy)2]3+/[Cr(bpy)3]3+ differ as well [139]. As the metal-centred [Cr(ddpd)2]3+/2+ redox process occurs at a much more cathodic potential than the tpy-based redox process of [Cr(tpy)2]3+/2+ (Table 4), its oxidative power in the excited state with 0.49 V is much lower than that of [Cr(tpy)2]3+/2+ with 1.08 V [50]. Indeed, [Cr(4’-Tol-tpy)2]3+ photooxidizes the DNA base guanine and hence can cleave DNA [140], while [Cr(ddpd)2]3+ does not damage DNA via guanine photooxidation [50]. The organic photoredox chemistry, using strongly oxidizing [Cr(phen)3]3+ sensitizers, has recently been advanced by Shores and Ferreira [141,142,143]. On the other hand, the very long lifetime and the non-oxidizing character of the 2E state of [Cr(ddpd)2]3+ enables the efficient and selective formation of singlet oxygen via energy transfer to 3O2 [139]. The thus generated 1O2 has been successfully utilized for visible-light-induced oxidative C–H bond functionalization of tertiary amines [139]. The luminescence quenching of the stable and highly luminescent [Cr(ddpd)2]3+ complex by triplet oxygen can be further elaborated into optical oxygen sensors [53].
Furthermore, optical sensing of temperature has been realized with dissolved molecular [Cr(ddpd)2]3+ complexes and with [Cr(ddpd)2]3+ enclosed in polystyrene nanoparticles or micelles [144]. Exploiting the thermal equilibration of the long-lived emissive 2E and 2T1 states allows using the dual emitter [Cr(ddpd)2]3+ as self-referencing dye (Figure 10) [144]. Moreover, the pressure-dependent energy shift of the 2E emission band has been utilized for optical pressure detection both in the solid state and in solution. The pressure sensitivity of [Cr(ddpd)2][BF4]3 (2E state: −14.1 cm−1 kbar−1) is much higher than that of the standard pressure calibrant Al2O3:Cr (ruby, −0.7 cm−1 kbar−1), enabling highly sensitive future applications [145,146]. Interestingly, the pressure shift of the well resolved 2T1 emission band of [Cr(ddpd)2]3+ differs with −7.7 cm−1 kbar−1 significantly from the 2E shift. This phenomenon has been ascribed to the distinct effects of pressure-induced angular distortions on the 2E and 2T1 spin-flip states of chromium(III) ions in [Cr(ddpd)2]3+ [145].
Clearly, the different coordinating ability and electron accepting nature of tpy and ddpd translate into very complementary ground state (substitution, redox) and excited state reactivity (luminescence, electron transfer, energy transfer) of their homoleptic chromium(III) complexes [Cr(ddpd)2]3+ and [Cr(tpy)2]3+.
10. cis-fac-[V(ddpd)2]3+ and mer-VCl3(ddpd) [d2]
The solution chemistry of VCl3 and pyridine is complicated due to complex equilibria and accompanying redox chemistry [147]. Literature reports concerning vanadium(III) complexes with terpyridine are very scarce. The brown heteroleptic complex VCl3(tpy) has been prepared from VCl3 and tpy [80,148,149]. Similarly, yellow mer-VCl3(ddpd) is obtained from VCl3(thf)3 [150] and ddpd displaying an LMCT absorption band at 455 nm (Figure S3). A related VCl3 complex VCl3(L) with L = 2,6-bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine as tridentate nitrogen donor ligand forming 6-membered chelate rings had been reported as pre-catalyst for ethylene polymerization [151]. Yet, structural data of VCl3(tpy) and VCl3(L) are lacking. A few solid-state structures of VCl3(bimpy) (bimpy = 2,6-bis(imino)pyridine) ligands forming five-membered chelate rings) have been reported and these complexes have been employed in radical polymerization catalysis [152]. VCl3(ddpd) crystallised in the monoclinic space group P21/n as a monomeric complex with meridional ddpd coordination (Figure 11a), isostructural to mer-CrCl3(ddpd) [126]. The magnetic susceptibility of VCl3(tpy) (χT = 0.744 cm−1 K mol−1 at 290 K) and mer-VCl3(ddpd) (χT = 0.879 cm−1 K mol−1 at 290 K; Figure S4) is less than expected for a (t2g)2 electron configuration (3T1 ground state) and has been ascribed to the splitting of the 3T1 ground state by the ligand field [148].
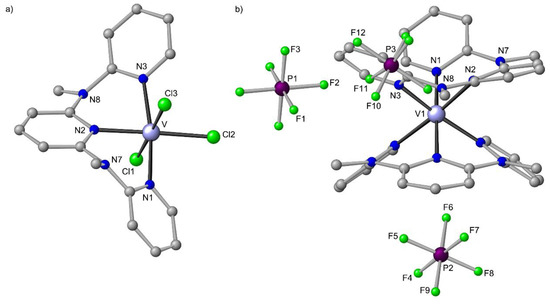
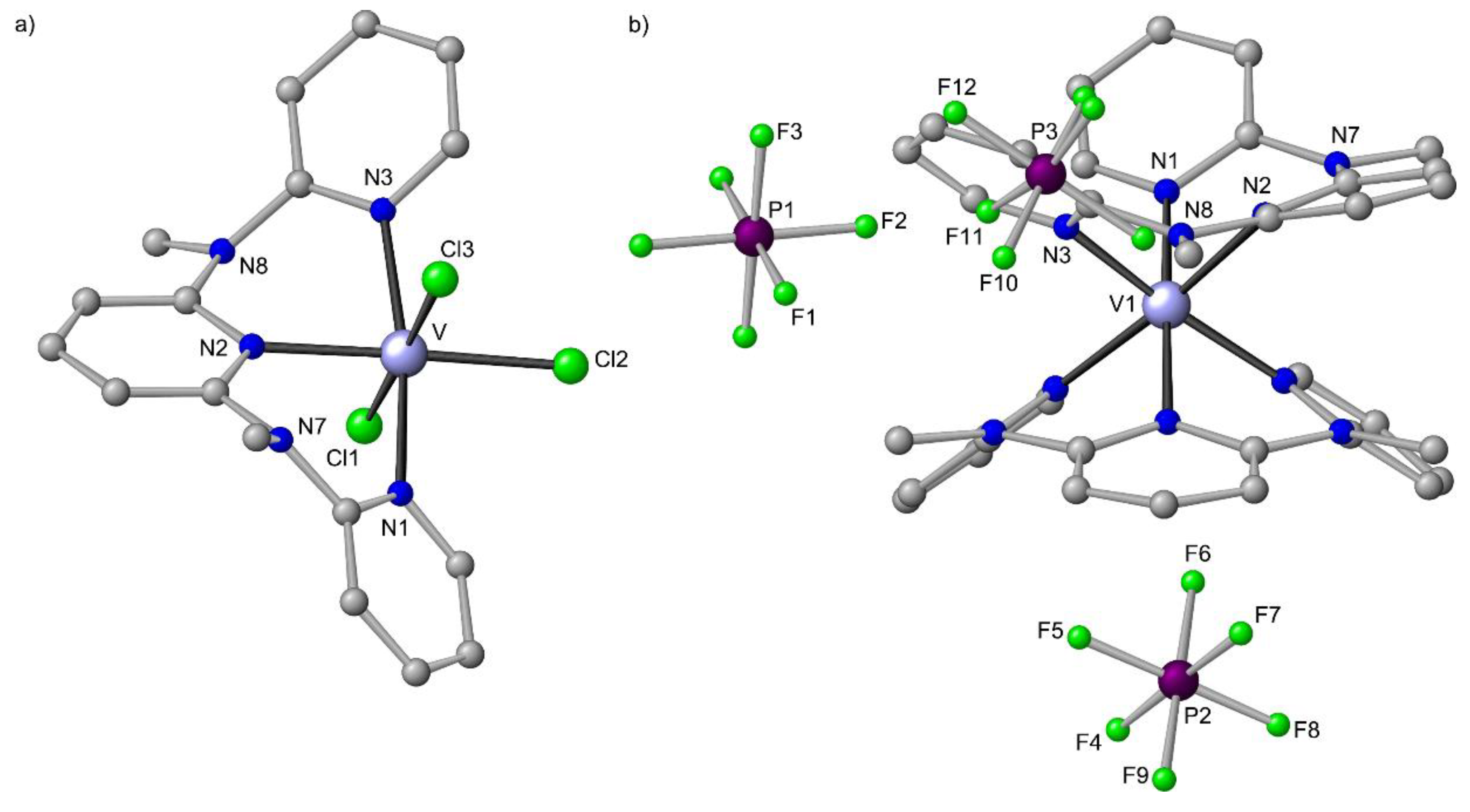
Figure 11.
Molecular structures of (a) mer-VCl3(ddpd) and (b) cis-fac-[V(ddpd)2][PF6]3 in the solid state; hydrogen atoms and solvent molecules omitted. Atom numbering unified for clarity.
In the presence of three equivalents Ag[CF3SO3] and tpy, VCl3(tpy) presumably forms [V(tpy)2]3+ initially. However, this vanadium(III) complex readily disproportionates to [V(tpy)2]2+ and a [VIVO]2+ species, with the oxygen atom likely stemming from traces of water [80]. [V(tpy)2]I2 had been prepared from VSO4, tpy and ion exchange with KI. Its magnetic moment fits to a d3 electron configuration (Table 5) [90]. The dicationic complex [V(tpy)2]2+ exhibits absorption bands at λ = 767 and 402 nm [153]. The intensity of the low-energy band suggests charge transfer character. Two electronic descriptions of the ground state are conceivable, namely [VII(tpy)2]2+ or [VIII(tpy·−)(tpy)]2+ with MLCT or LMCT bands, respectively. However, the electronic structure of [V(tpy)2]2+ has not yet been determined experimentally.
Oxidation of green [V(tpy)2]2+ occurs at −0.09 V (vs. ferrocene) [80] and is reported as quasireversible, again pointing to disproportionation of the initially formed [V(tpy)2]3+ complex (Table 4). Hence, the vanadium(III) complex [V(tpy)2]3+ has not yet been isolated.
In the presence of Tl[PF6] as chloride acceptor, mer-VCl3(ddpd) and ddpd yields [V(ddpd)2]3+ without any signs for disproportionation in VII and [VIVO]2+. In principle, this synthetic procedure could also allow for heteroleptic vanadium(III) complexes with ddpd. The cis-fac stereoisomer of [V(ddpd)2]3+ has been crystallographically characterized (Figure 11b), providing first structural data of a homoleptic polypyridine vanadium(III) complex. Obviously, the electron-rich ddpd ligand stabilizes electron-poor d2-VIII complexes in contrast to the π-accepting tpy ligand.
Ligand substitution of V(η6-C6H6)2, V(CO)4(dppe) or K[V(CO)6] with tpy (dppe = 1,2-bis(diphenylphosphano)ethane) [154], reduction of [V(tpy)2]I2 with Mg in DMF or LiAlH4 in dioxane [90] or sodium amalgam reduction of VCl3 in the presence of tpy yields the black, neutral complex [V(tpy)2]0 [155]. According to single crystal X-ray diffraction and spectroscopic data, [V(tpy)2]0 features a [VIV(tpy2−)2]0 electronic structure with an unpaired electron localized at the vanadium ion (S = ½) [90,154,155]. These data again illustrate the complementary nature of tpy and ddpd, with tpy stabilizing reduced, electron-rich vanadium species by internal redox reactions and ddpd stabilizing electron-poor vanadium(III) complexes.
11. Experimental Section
X-ray diffraction of ddpd, [H-ddpd][CF3SO3], [Co(ddpd)2][BF4]3×3CH3CN, [Fe(ddpd)2]Br2×2CH3CN, VCl3(ddpd), mer-[Cr(ddpd)2][BF4]2×2CH3CN and cis-fac-[V(ddpd)2][PF6]3×3CH3CN:
Intensity data were collected with a STOE IPDS-2T diffractometer with an Oxford cooling system using Mo-Kα radiation (λ = 0.71073 Å). The diffraction frames were integrated using the Bruker SMART software package [156] and most were corrected for absorption with MULABS [157] of the PLATON software package [158]. The structures were solved by direct methods and refined by the full-matrix method based on F2 using the SHELXL software package [159,160]. All non-hydrogen atoms were refined anisotropically, while the positions of all hydrogen atoms were generated with appropriate geometric constraints and allowed to ride on their respective parent atoms with fixed isotropic thermal parameters. Only, the non-hydrogen atoms of co-crystallized acetonitrile in mer-[Fe(ddpd)2]Br2×2CH3CN have been refined isotropically.
Crystallographic data for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no CCDC-1832949 (ddpd), 1832948 ([H-ddpd][CF3SO3]), 1832951 (mer-[Co(ddpd)2][BF4]3×3CH3CN), 1832950 (mer-[Fe(ddpd)2]Br2×2CH3CN), 1844780 (mer-[Cr(ddpd)2][BF4]2×2CH3CN), 1840435 (VCl3(ddpd)) and 1840434 (cis-fac-[V(ddpd)2][PF6]3×3CH3CN). Copies of the data can be obtained free of charge upon application to CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K. [fax (0.44) 1223-336-033; e-mail deposit@ccdc.cam.ac.uk].
Crystallographic Data of ddpd: C17H177N5 (291.35); orthorhombic; Pnma; a = 11.772(2) Å, b = 18.989(4) Å, c = 6.7282(14) Å, V = 1504.0(5) Å3; Z = 4; density, calcd. = 1.287 g cm−3, μ= 0.081 mm−1; F(000) = 616; crystal size 0.910 × 0.590 × 0.090 mm; θ = 3.212 to 28.272 deg.; −15 ≤ h ≤ 13, −20 ≤ k ≤ 25, −7 ≤ l ≤ 8; rfln collected = 5790; rfln unique = 1904 [R(int) = 0.0572]; completeness to θ = 25.242 deg. = 99.6%; semi empirical absorption correction from equivalents; max. and min. transmission 1.09703 and 0.92830; data 1904; restraints 0, parameters 104; goodness-of-fit on F2 = 1.030; final indices [I > 2σ(I)] R1 = 0.0504, wR2 = 0.1255; R indices (all data) R1 = 0.0907, wR2 = 0.1443; largest diff. peak and hole 0.224 and −0.274 e Å−3.
Crystallographic Data of [H-ddpd][CF3SO3]: C18H18F3N5O3S (441.43); monoclinic; P21/n; a = 8.4486(17) Å, b = 14.467(3) Å, c = 15.549(3) Å, β = 97.84(3)°, V = 1882.7(7) Å3; Z = 4; density, calcd. = 1.557 g cm–3, μ = 0.234 mm−1; F(000) = 912; crystal size 0.540 × 0.520 × 0.400 mm; θ = 2.606 to 28.226 deg.; −11 ≤ h ≤ 11, −19 ≤ k ≤ 16, −20 ≤ l ≤ 15; rfln collected = 12534; rfln unique = 4591 [R(int) = 0.0268]; completeness to θ = 25.242 deg. = 99.4%; no absorption correction; data 4591; restraints 0, parameters 277; goodness-of-fit on F2 = 1.040; final indices [I > 2σ(I)] R1 = 0.0349, wR2 = 0.0919; R indices (all data) R1 = 0.0401, wR2 = 0.0958; largest diff. peak and hole 0.437 and −0.340 e Å−3.
Crystallographic Data of mer-[Co(ddpd)2][BF4]3×3CH3CN: C40H43B3CoF12N13 (1025.23); monoclinic; Pn; a = 11.4217(10) Å, b = 16.5963(15) Å, c = 12.9749(11) Å, β= 112.100(2)°, V = 2278.8(3) Å3; Z = 2; density, calcd. = 1.494 g cm−3, μ = 0.473 mm−1; F(000) = 1048; crystal size 0.730 × 0.430 × 0.220 mm; θ = 2.030 to 27.987 deg.; −15 ≤ h ≤ 15, −21 ≤ k ≤ 21, –17 ≤ l ≤ 17; rfln collected = 28339; rfln unique = 10797 [R(int) = 0.0526]; completeness to θ = 25.242 deg. = 100.0%; semi empirical absorption correction from equivalents; max. and min. transmission 1.179 and 0.888; data 10797; restraints 42, parameters 675; goodness-of-fit on F2 = 0.958; final indices [I > 2σ(I)] R1 = 0.0473, wR2 = 0.1047; R indices (all data) R1 = 0.0584, wR2 = 0.1094; largest diff. peak and hole 0.347 and −0.398 e Å−3.
Crystallographic Data of mer-[Fe(ddpd)2]Br2×2CH3CN: C38H40Br2FeN12 (880.49); orthorhombic; Fddd; a = 13.629(3) Å, b = 20.677(4) Å, c = 26.759(5) Å, V = 7541(3) Å3; Z = 8; density, calcd. = 1.551 g cm–3, μ = 2.566 mm−1; F(000) = 3584; crystal size 0.200 × 0.157 × 0.070 mm; θ = 3.355 to 28.037 deg.; −15 ≤ h ≤ 17, −27 ≤ k ≤ 27, −35 ≤ l ≤ 35; rfln collected = 12312; rfln unique = 2273 [R(int) = 0.0514]; completeness to θ = 25.242 deg. = 99.8%; semi empirical absorption correction from equivalents; max. and min. transmission 1.050 and 0.958; data 2273; restraints 3, parameters 122; goodness-of-fit on F2 = 1.059; final indices [I > 2σ(I)] R1 = 0.0547, wR2 = 0.1641; R indices (all data) R1 = 0.0804, wR2 = 0.1828; largest diff. peak and hole 1.726 and −0.823 e Å−3.
Crystallographic Data of mer-[Cr(ddpd)2][BF4]2 × 2CH3CN: C38H40B2F8CrN12 (890.44); T = 263 K; orthorhombic; Fdd2; a = 21.474(4) Å, b = 26.428(5) Å, c = 14.176(3) Å, V = 8045(3) Å3; Z = 8; density, calcd. = 1.470 g cm−3, μ = 0.368 mm−1; F(000) = 3664; crystal size 0.480 × 0.380 × 0.300 mm; θ = 2.883 to 28.236 deg.; −26 ≤ h ≤ 28, −30 ≤ k ≤ 35, −18 ≤ l ≤ 18; rfln collected = 10695; rfln unique = 4596 [R(int) = 0.0591]; completeness to θ = 25.242 deg. = 99.8%; semi empirical absorption correction from equivalents; max. and min. transmission 1.172 and 0.893; data 4596; restraints 122, parameters 357; goodness-of-fit on F2 = 1.032; final indices [I > 2σ(I)] R1 = 0.0455, wR2 = 0.1123; R indices (all data) R1 = 0.0708, wR2 = 0.1394; abs. structure parameter = 0.39(7), largest diff. peak and hole 0.381 and −0.248 e Å−3.
Crystallographic Data of VCl3(ddpd): C17H17Cl3VN5 (448.64); monoclinic; P21/n; a = 9.2520(19) Å, b = 14.874(3) Å, c = 13.647(3) Å, β = 95.99(3)°, V = 1867.8(7) Å3; Z = 4; density, calcd. = 1.595 g cm−3, μ = 0.972 mm−1; F(000) = 912; crystal size 0.600 × 0.270 × 0.080 mm; θ = 2.031 to 28.228 deg.; −12 ≤ h ≤ 12, −17 ≤ k ≤ 19, −18 ≤ l ≤ 18; rfln collected = 12339; rfln unique = 4595 [R(int) = 0.0848]; completeness to θ = 25.242 deg. = 99.7%; semi empirical absorption correction from equivalents; max. and min. transmission 1.282 and 0.864; data 4595; restraints 0, parameters 237; goodness-of-fit on F2 = 1.045; final indices [I > 2σ(I)] R1 = 0.0541, wR2 = 0.1029; R indices (all data) R1 = 0.1047, wR2 = 0.1235; largest diff. peak and hole 0.489 and −0.522 e Å−3.
Crystallographic Data of cis-fac-[V(ddpd)2][PF6]3×3CH3CN: C40H43P3VF18N13 (1191.72); monoclinic; I2/c; a = 22.513(5) Å, b = 12.233(3) Å, c = 37.764(16) Å, β = 102.01(3)°, V = 10173(6) Å3; Z = 8; density, calcd. = 1.556 g cm−3, μ = 0.401 mm−1; F(000) = 4832; crystal size 0.320 × 0.150 × 0.040 mm; θ = 1.904 to 28.483 deg.; −30 ≤ h ≤ 30, −16 ≤ k ≤ 14, −44 ≤ l ≤ 50; rfln collected = 32167; rfln unique = 12717 [R(int) = 0.2772]; completeness to θ = 25.242 deg. = 99.6%; semi empirical absorption correction from equivalents; max. and min. transmission 1.147 and 0.861; data 12717; restraints 84, parameters 780; goodness-of-fit on F2 = 1.039; final indices [I > 2σ(I)] R1 = 0.1357, wR2 = 0.2837; R indices (all data) R1 = 0.3163, wR2 = 0.3895; largest diff. peak and hole 1.151 and −0.540 e Å−3. Unfortunately, only a few needle-shaped crystals were suitable for single-crystal X-ray analysis. The investigated needle-shaped crystal was the one of highest quality. Up to now, no crystals with better diffracting power could be obtained and hence no better reflection data. In spite of the weak diffraction quality, the cis-fac coordination mode of the ddpd ligand in this complex salt is unambiguous.
NMR spectra were recorded with a Bruker Avance DRX 400 spectrometer at 400.31 MHz (1H). All resonances are reported in ppm versus the solvent signal as an internal standard (CD2Cl2: 1H, δ = 5.32 ppm; s = singlet, d = doublet, t = triplet, m = multiplet) [161]. IR spectra were recorded with a BioRad Excalibur FTS 3100 spectrometer as KBr disks or with a Bruker Alpha FT-IR spectrometer with an ATR unit containing a diamond crystal. UV/Vis/near-IR spectra were recorded with a Varian Cary 5000 spectrometer by using 1.0 cm cells (Hellma, Suprasil). ESI+ mass spectra were recorded with a Micromass Q-TOF-Ultima spectrometer. FD mass spectra were recorded on a Thermo Fisher DFS mass spectrometer with a LIFDI upgrade. DC magnetic studies were performed with a Quantum Design MPMS-XL-7 SQUID magnetometer on powdered microcrystalline samples embedded in eicosane to avoid orientation of the crystallites under applied field (1 Tesla). Experimental susceptibility data were corrected for the underlying diamagnetism using Pascal’s constants. The temperature dependent magnetic contribution of the holder and of the embedding matrix eicosane were experimentally determined and subtracted from the measured susceptibility data. Electrochemical experiments were carried out on a BioLogic SP-50 voltammetric analyser using a platinum working electrode, a platinum wire as a counter electrode and a 0.01 M Ag/AgNO3 CH3CN electrode as reference electrode. The measurements were carried out at a scan rate of 100 mV s−1 for cyclic voltammetry experiments using 0.1 M [nBu4N] [PF6] as supporting electrolyte and 0.002 M of the sample in acetonitrile. Potentials are given relative to the ferrocene/ferrocenium couple. Elemental analyses were performed by the microanalytical laboratory of the chemical institutes of the University of Mainz.
DFT calculations were carried out using the ORCA program package (version 4.0.1) [162]. All calculations were performed using the B3LYP functional [163,164,165] and employ the RIJCOSX approximation [166,167]. Relativistic effects were calculated at the zeroth order regular approximation (ZORA) level [168]. The ZORA keyword automatically invokes relativistically adjusted basis sets. To account for solvent effects, a conductor-like screening model (CPCM) modelling acetonitrile was used in all calculations [169]. Geometry optimizations were performed using Ahlrichs’ split-valence double-ξ basis set def2-SVP, which comprises polarization functions for all non-hydrogen atoms [170,171]. Atom-pairwise dispersion correction was performed with the Becke-Johnson damping scheme (D3BJ) [172,173].
Synthesis of fac-Cr(CO)3(ddpd): Under an inert atmosphere, chromiumhexacarbonyl (233 mg, 1.06 mmol) and ddpd (684 mg, 2.34 mmol) were heated under reflux in mesitylene (45 mL) for 7 h. After cooling to room temperature, the red precipitate was collected by filtration and washed with diethylether (3 × 30 mL). The red solid was dissolved in CH2Cl2 and the solvent removed under reduced pressure. After washing with petroleum ether, the red solid was dried under reduced pressure. Yield: 410 mg (0.96 mmol, 90%). Elemental Anal. Calc. for C20H17CrN5O3 (427.37) × 0.2 CH2Cl2 C, 54.60; H, 3.95; N, 15.76. Found: C, 53.78; H, 3.95; N, 16.59. IR (KBr): ῦ = 1891 (vs, CO), 1775 (vs, CO), 1748 (vs, CO), 1589 (s), 1580 (s), 1488 (s), 1444 (s), 1430 (s), 1373 (m), 1338 (m), 1304 (w), 1276 (w), 1237 (w), 1166 (m), 1141 (m), 1131 (m), 1118 (w), 1100 (w), 1088 (w), 1065 (w), 1055 (w), 1005 (w), 841 (w, br), 799 (m), 773 (s), 748 (m), 652 (m) cm−1. MS (ESI+): m/z (%) = 343.1 (100) [M − 3CO]+, 371.1 (2) [M − 2CO]+, 399.1 (6) [M − CO]+, 427.1 (85) [M]+. 1H NMR (CD2Cl2, 400.31 MHz): δ [ppm] = 9.13 (d, 3JHH = 5.6 Hz, 2H, H7), 7.72 (t, 3JHH = 8.2 Hz, 1H, H1), 7.71 (m, 2H, H5), 7.10 (ddd, 3JHH = 7.0 Hz, 3JHH = 5.9 Hz, 4JHH = 0.9 Hz, 2H, H6), 7.02 (d, 3JHH = 8.2 Hz, 2H, H4), 6.66 (d, 3JHH = 8.2 Hz, 2H, H2), 3.62 (s, 6H, H3). UV/Vis/NIR (CH2Cl2): λ (ε/M−1 cm−1) = 445 (7220), 328 (sh, 14100), 298 (17500), 259 (sh, 24700) nm.
Synthesis of crystals of mer-[Cr(ddpd)2][BF4]2×2CH3CN: Under an inert atmosphere, [Cr(NCCH3)4][BF4]2 [128] (13.5 mg, 0.035 mmol) was dissolved in acetonitrile (5 mL) giving a pale blue solution. Upon dropwise addition of ddpd (20 mg, 0.069 mmol) dissolved in acetonitrile (1 mL) the solution turned green. Diffusion of diethyl ether into the solution yielded dark green crystals suitable for single crystals X-ray diffraction and magnetic susceptibility studies. Magnetic susceptibility (Evans NMR method [174,175]): χT (CH3CN) = 2.67 cm3 K mol−1 (294 K).
Synthesis of mer-VCl3(ddpd): Under an inert atmosphere, VCl3(thf)3 [150] (50 mg, 0.13 mmol) was dissolved in acetonitrile (2 mL) giving a green solution. Upon addition of ddpd (47 mg, 0.16 mmol), dissolved in dry acetonitrile (2 mL), the solution turned red and an orange solid started to precipitate. The reaction mixture was heated to reflux for 2 h to give a yellow solution over an orange solid. The solvent was removed under reduced pressure and the orange product was washed under argon with dry petrol ether (40/60) (3 × 5 mL) onto a frit to remove excess ligand. After drying under reduced pressure overnight, a fine-grained orange solid was obtained. Yield: 55 mg (0.12 mmol, 92%). MS (LIFDI): m/z (%) = 291.2 (58) [ddpd]·+, 393.2 (100), 412.2 (34) [M − Cl]+. UV/Vis/NIR (CH2Cl2): λ (ε/M−1 cm−1) = 455 (980), 385 (sh, 2240), 322 (14470), 245 (17080) nm. IR (ATR): ῦ = 2960 (w), 1589 (m), 1566 (w), 1488 (vw), 1472 (w), 1432 (s), 1411 (m), 1389 (w), 1354 (w), 1284 (vw), 1259 (m), 1195 (w), 1171 (w), 1160 (w), 1129 (m), 1094 (s, br), 1082 (s, br), 1014 (s), 964 (m), 888 (sh), 874 (w, br), 795 (s, br), 773 (vs), 752 (m), 746 (m), 727 (m), 707 (w), 663 (w), 630 (vw), 593 (m), 562 (w). Elem. Anal. Calcd. for C17H17Cl3N5V (488.66)×0.9 CH3CN: C, 46.50, H, 4.09, N, 17.02. Found: C, 45.93, H, 3.75, N, 17.59. χT = 0.879 cm−1 K mol−1 (290 K).
12. Conclusions and Future Perspectives
Transition metal complexes of ddpd [M(ddpd)2]n+ with d2 (vanadium(III)) to d10 (zinc(II)) electron configurations are readily available. In all cases, the complex charge corresponds to the metal oxidation state. This is not generally valid for corresponding [M(tpy)2]n+ complexes due to the redox non-innocence of terpyridine allowing [Mn(tpy·−)(tpy)](n−1)+ electronic structures, for example, [CrIII(tpy·−)(tpy)]2+ versus [CrII(ddpd)2]2+. The electron-rich nature of ddpd stabilizes electron-deficient complexes, whereas the π electron accepting tpy favours electron-rich metal ions.
The six-membered chelate rings of ddpd form larger ligand bite angles and geometries closer to an ideal octahedron than the more strained tpy ligand. The increased flexibility of the six-membered rings allow both pincer type (meridional) and tripodal (facial) coordination of ddpd, for example mer- and cis-fac-[Co(ddpd)2]2+ complexes. The meridional isomers [M(ddpd)2]n+ are typically preferred and most often encountered. The bite angles of ddpd close to 90° enable a better metal orbital–pyridine orbital overlap. This results in generally larger ligand field splittings than in the corresponding [M(tpy)2]n+ complexes (except for high-spin [Co(L)2]2+ complexes). The ligand field splitting affects both the ground state multiplicity (low-spin/high-spin) and the excited state level ordering, especially in d3 complexes. Specifically, the very strong ligand field strength of ddpd enables strongly luminescent chromium(III) complexes due to the high energy of the detrimental ligand field states. Electron configurations with degenerate electronic states, especially (eg)1 and (eg)3 configurations, for example high-spin d4-[Cr(ddpd)2]2+, low-spin d7-[Co(tpy)2]2+ and d9-[Cu(L)2]2+ are prone to significant Jahn-Teller distortions. The barrier between the Jahn-Teller isomers of [Cu(ddpd)2]2+ is larger than that of [Cu(tpy)2]2+. Spin-crossover phenomena have been reported for [Co(tpy)2][X]2 and [Fe(R2tpy)2][X]2 salts with substituents R at the 6,6″ positions of tpy. Analogous [Co(ddpd)2][X]2 salts have not yet been discovered with low-spin spin states but might be available with different counter ions or within different matrices. Iron(II) complexes with unsubstituted ddpd ligands are all low-spin. However, lowering the ligand field strength by 6,6″ substitution might be a promising strategy, similar to the tpy complexes.
Modification of ddpd to tune the ligand field strength (higher or lower), to allow for conjugation with functional groups such as light antenna or catalysts, for incorporation into supramolecular arrays or for immobilization on surfaces or in nanoparticles are important future perspectives of transition metal ddpd complexes. The deliberate incorporation of ddpd in heteroleptic chelate complexes will further open plentiful tuning possibilities with respect to optical, magnetic and catalytic properties. These future developments will possibly enable applications of [M(ddpd)(L)m]n+ in magnetic storage devices, optical sensing, catalysis, electrocatalysis, photocatalysis and supramolecular chemistry.
Supplementary Materials
The following are available online at http://www.mdpi.com/2304-6740/6/3/86/s1. Figure S1: Cyclic voltammogram of [Ni(ddpd)2][BF4]2 in CH3CN in the presence of HOAc and CO2, Figure S2: Temperature-dependence of χMT of [Cr(ddpd)2][BF4]3×CH3CN with fit, Figure S3: UV/Vis spectrum of mer-VCl3(ddpd) in CH2Cl2, Figure S4: Temperature-dependence of χMT of VCl3(ddpd) with fit. The CIF and the checkCIF output files of ddpd, [H-ddpd][CF3SO3], mer-[Co(ddpd)2][BF4]3×3CH3CN, mer-[Fe(ddpd)2]Br2×2CH3CN, mer-Cr(ddpd)2][BF4]2×2CH3CN, VCl3(ddpd) and cis-fac-[V(ddpd)2][PF6]3×3CH3CN.
Author Contributions
C.F. solved and refined the X-ray data, M.D. (cis-fac-[V(ddpd)2][PF6]3×3CH3CN, VCl3(ddpd)), T.R. (ddpd), S.O. (fac-Cr(CO)3(ddpd), mer-[Cr(ddpd)2][BF4]2×2CH3CN, mer-[Co(ddpd)2][BF4]3×3CH3CN), G.D. and T.R. ([H-ddpd][CF3SO3]) prepared or crystallized the new compounds, respectively. L.C. and E.R. measured and analysed the magnetic data, K.H. designed the project and wrote the manuscript. All authors have given approval to the final version of the manuscript.
Acknowledgments
We gratefully thank all collaboration partners who have contributed to this interdisciplinary research area. Their names can be found in the respective references. We thank Katharina Mack for crystallizing the complex mer-[Fe(ddpd)2]Br2×2CH3CN. We thank Regine Jung-Pothmann and Dieter Schollmeyer for the collection of the diffraction data. Parts of this research were conducted using the supercomputer Mogon and advisory services offered by the Johannes Gutenberg University Mainz (www.hpc.uni-mainz.de), which is a member of the AHRP and the Gauss Alliance e.V. This work was supported by the Deutsche Forschungsgemeinschaft (GSC 266, Materials Science in Mainz and HE 2778/10−1), the internal university research funds of the Johannes Gutenberg University, Mainz (Germany) and the Center for INnovative and Emerging MAterials (CINEMA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lawrence, M.A.W.; Green, K.-A.; Nelson, P.N.; Lorraine, S.C. Review: Pincer ligands—Tunable, versatile and applicable. Polyhedron 2018, 143, 11–27. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Key factors in pincer ligand design. Chem. Soc. Rev. 2018, 47, 1959–1968. [Google Scholar] [CrossRef]
- Constable, E.C. The Coordination Chemistry of 2,2′:6′,2″-Terpyridine and Higher Oligopyridines. Adv. Inorg. Chem. 1986, 30, 69–121. [Google Scholar] [CrossRef]
- Constable, E.C. 2,2′:6′,2″-terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef]
- Hofmeier, H.; Schubert, U.S. Recent developments in the supramolecular chemistry of terpyridine–metal complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, J.P.; Collin, J.P.; Chambron, J.C.; Guillerez, S.; Coudret, C.; Balzani, V.; Barigelletti, F.; De Cola, L.; Flamigni, L. Ruthenium(II) and Osmium(II) Bis(terpyridine) Complexes in Covalently-Linked Multicomponent Systems: Synthesis, Electrochemical Behavior, Absorption Spectra, and Photochemical and Photophysical Properties. Chem. Rev. 1994, 94, 993–1019. [Google Scholar] [CrossRef]
- Flamigni, L.; Collin, J.P.; Sauvage, J.P. Iridium Terpyridine Complexes as Functional Assembling Units in Arrays for the Conversion of Light Energy. Acc. Chem. Res. 2008, 41, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.-Q.; Wang, H.; Wang, X.-Q.; Song, B.; Chen, L.-J.; Wang, L.; Hao, X.-Q.; Yang, H.-B.; Li, X. Self-assembly of emissive supramolecular rosettes with increasing complexity using multitopic terpyridine ligands. Nat. Commun. 2018, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Hasenknopf, B.; Lehn, J.-M.; Baum, G.; Fenske, D. Self-assembly of a heteroduplex helicate from two different ligand strands and Cu(II) cations. Proc. Natl. Acad. Sci. USA 1996, 93, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Bassani, D.M.; Lehn, J.-M.; Fromm, K.; Fenske, D. Toposelective and Chiroselective Self-Assembly of [2 × 2] Grid-Type Inorganic Arrays Containing Different Octahedral Metallic Centers. Angew. Chem. Int. Ed. 1998, 37, 2364–2367. [Google Scholar] [CrossRef]
- Cárdenas, D.J.; Livoreil, A.; Sauvage, J.-P. Redox Control of the Ring-Gliding Motion in a Cu-Complexed Catenane: A Process Involving Three Distinct Geometries. J. Am. Chem. Soc. 1996, 118, 11980–11981. [Google Scholar] [CrossRef]
- Baumann, F.; Livoreil, A.; Kaim, W.; Sauvage, J.-P. Changeover in a multimodal copper(II) catenate as monitored by EPR spectroscopy. Chem. Commun. 1997, 35–36. [Google Scholar] [CrossRef]
- Livoreil, A.; Sauvage, J.-P.; Armaroli, N.; Balzani, V.; Flamigni, L.; Ventura, B. Electrochemically and Photochemically Driven Ring Motions in a Disymmetrical Copper [2]-Catenate. J. Am. Chem. Soc. 1997, 119, 12114–12124. [Google Scholar] [CrossRef] [PubMed]
- Hayami, S.; Komatsu, Y.; Shimizu, T.; Kamihata, H.; Lee, Y.H. Spin-crossover in cobalt(II) compounds containing terpyridine and its derivatives. Coord. Chem. Rev. 2011, 255, 1981–1990. [Google Scholar] [CrossRef]
- Krivokapic, I.; Zerara, M.; Dakua, M.L.; Vargas, A.; Enachescu, C.; Ambrus, C.; Tregenna-Piggott, P.; Amstutz, N.; Krausz, E.; Hauser, A. Spin-crossover in cobalt(II) imine complexes. Coord. Chem. Rev. 2007, 251, 364–378. [Google Scholar] [CrossRef]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Constable, E.C.; Baum, G.; Bill, E.; Dyson, R.; van Eldik, R.; Fenske, D.; Kaderli, S.; Morris, D.; Neubrand, A.; Neuburger, M.; et al. Control of Iron(II) Spin States in 2,2′:6′,2″-Terpyridine Complexes through Ligand Substitution. Chem. Eur. 1999, 5, 498–508. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Vicic, D.A.; Klein, A. Exploring Mechanisms in Ni Terpyridine Catalyzed C–C Cross-Coupling Reactions—A Review. Inorganics 2018, 6, 18. [Google Scholar] [CrossRef]
- Elgrishi, N.; Chambers, M.B.; Artero, V.; Fontecave, M. Terpyridine complexes of first row transition metals and electrochemical reduction of CO2 to CO. Phys. Chem. Chem. Phys. 2014, 16, 13635–13644. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Newkome, G.R.; Schubert, U.S. Catalytic Applications of Terpyridines and their Transition Metal Complexes. ChemCatChem 2011, 3, 1384–1406. [Google Scholar] [CrossRef]
- Winter, A.; Gottschaldt, M.; Newkome, G.R.; Schubert, U.S. Terpyridines and their complexes with first row transition metal ions: Cytotoxicity, nuclease activity and self-assembly of biomacromolecules. Curr. Top. Med. Chem. 2012, 12, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; England, J.; Weyhermüller, T.; Wieghardt, K. Electronic Structures of “Low-Valent” Neutral Complexes [NiL2]0 (S = 0; L = bpy, phen, tpy)—An Experimental and DFT Computational Study. Eur. J. Inorg. Chem. 2015, 1511–1523. [Google Scholar] [CrossRef]
- England, J.; Bill, E.; Weyhermüller, T.; Neese, F.; Atanasov, M.; Wieghardt, K. Molecular and Electronic Structures of Homoleptic Six-Coordinate Cobalt(I) Complexes of 2,2′:6′,2″-Terpyridine, 2,2′-Bipyridine, and 1,10-Phenanthroline. An Experimental and Computational Study. Inorg. Chem. 2015, 54, 12002–12018. [Google Scholar] [CrossRef] [PubMed]
- England, J.; Scarborough, C.C.; Weyhermüller, T.; Sproules, S.; Wieghardt, K. Electronic Structures of the Electron Transfer Series [M(bpy)3]n, [M(tpy)2]n, and [Fe(tbpy)3]n (M = Fe, Ru; n = 3+, 2+, 1+, 0, 1−): A Mössbauer Spectroscopic and DFT Study. Eur. J. Inorg. Chem. 2012, 4605–4621. [Google Scholar] [CrossRef]
- Wang, M.; England, J.; Weyhermüller, T.; Wieghardt, K. Molecular and Electronic Structures of the Members of the Electron Transfer Series [Mn(bpy)3]n (n = 2+, 1+, 0, 1−) and [Mn(tpy)2]m (m = 4+, 3+, 2+, 1+, 0). An Experimental and Density Functional Theory Study. Inorg. Chem. 2014, 53, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, C.C.; Lancaster, K.M.; DeBeer, S.; Weyhermüller, T.; Sproules, S.; Wieghardt, K. Experimental Fingerprints for Redox-Active Terpyridine in [Cr(tpy)2](PF6)n (n = 3–0), and the Remarkable Electronic Structure of [Cr(tpy)2]1–. Inorg. Chem. 2012, 51, 3718–3732. [Google Scholar] [CrossRef] [PubMed]
- Breivogel, A.; Kreitner, C.; Heinze, K. Redox and Photochemistry of Bis(terpyridine)ruthenium(II) Amino Acids and Their Amide Conjugates–from Understanding to Applications. Eur. J. Inorg. Chem. 2014, 5468–5490. [Google Scholar] [CrossRef]
- Parada, G.A.; Fredin, L.A.; Santoni, M.-P.; Jäger, M.; Lomoth, R.; Hammarström, L.; Johansson, O.; Persson, P.; Ott, S. Tuning the Electronics of Bis(tridentate)ruthenium(II) Complexes with Long-Lived Excited States: Modifications to the Ligand Skeleton beyond Classical Electron Donor or Electron Withdrawing Group Decorations. Inorg. Chem. 2013, 52, 5128–5137. [Google Scholar] [CrossRef] [PubMed]
- Laramée-Milette, B.; Hanan, G.S. Ruthenium bistridentate complexes with non-symmetrical hexahydro-pyrimidopyrimidine ligands: A structural and theoretical investigation of their optical and electrochemical properties. Dalton Trans. 2016, 45, 12507–12517. [Google Scholar] [CrossRef] [PubMed]
- Breivogel, A.; Wooh, S.; Dietrich, J.; Kim, T.Y.; Kang, Y.S.; Char, K.; Heinze, K. Anchor-Functionalized Push-Pull-Substituted Bis(tridentate) Ruthenium(II) Polypyridine Chromophores: Photostability and Evaluation as Photosensitizers. Eur. J. Inorg. Chem. 2014, 2720–2734. [Google Scholar] [CrossRef]
- Breivogel, A.; Park, M.; Lee, D.; Klassen, S.; Kühnle, A.; Lee, C.; Char, K.; Heinze, K. Push-Pull Design of Bis(tridentate) Ruthenium(II) Polypyridine Chromophores as Deep Red Light Emitters in Light-Emitting Electrochemical Cells. Eur. J. Inorg. Chem. 2014, 288–295. [Google Scholar] [CrossRef]
- Breivogel, A.; Meister, M.; Förster, C.; Laquai, F.; Heinze, K. Excited State Tuning of Bis(tridentate) Ruthenium(II) Polypyridine Chromophores by Push–Pull Effects and Bite Angle Optimization: A Comprehensive Experimental and Theoretical Study. Chem. Eur. J. 2013, 19, 13745–13760. [Google Scholar] [CrossRef] [PubMed]
- Friebe, C.; Görls, H.; Jäger, M.; Schubert, U.S. Linear Metallopolymers from Ruthenium(II)-2,6-di(quinolin-8-yl)pyridine Complexes by Electropolymerization–Formation of Redox-Stable and Emissive Films. Eur. J. Inorg. Chem. 2013, 4191–4202. [Google Scholar] [CrossRef]
- Breivogel, A.; Förster, C.; Heinze, K. A Heteroleptic Bis(tridentate)ruthenium(II) Polypyridine Complex with Improved Photophysical Properties and Integrated Functionalizability. Inorg. Chem. 2010, 49, 7052–7056. [Google Scholar] [CrossRef] [PubMed]
- Schramm, F.; Meded, V.; Fliegl, H.; Fink, K.; Fuhr, O.; Qu, Z.; Klopper, W.; Finn, S.; Keyes, T.E.; Ruben, M. Expanding the Coordination Cage: A Ruthenium(II)−Polypyridine Complex Exhibiting High Quantum Yields under Ambient Conditions. Inorg. Chem. 2009, 48, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, M.; Jäger, M.; Österman, T.; Eriksson, L.; Persson, P.; Becker, H.-C.; Johansson, O.; Hammarström, L. A 3.0 μs Room Temperature Excited State Lifetime of a Bistridentate RuII−Polypyridine Complex for Rod-like Molecular Arrays. J. Am. Chem. Soc. 2006, 128, 12616–12617. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, M.; Wolpher, H.; Johansson, O.; Larsson, J.; Kritikos, M.; Eriksson, L.; Norrby, P.-O.; Bergquist, J.; Sun, L.; Åkermark, B.; et al. A New Strategy for the Improvement of Photophysical Properties in Ruthenium(II) Polypyridyl Complexes. Synthesis and Photophysical and Electrochemical Characterization of Six Mononuclear Ruthenium(II) Bisterpyridine-Type Complexes. Inorg. Chem. 2005, 44, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1–24. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of Functionalized 2,2′:,2″-Terpyridines. Eur. J. Org. Chem. 2003, 947–961. [Google Scholar] [CrossRef]
- Ho, K.-Y.; Yu, W.-Y.; Cheung, K.-K.; Che, C.-M. Blue luminescent zinc(II) complexes with polypyridylamine ligands: Crystal structures and luminescence properties. J. Chem. Soc. Dalton Trans. 1999, 1581–1586. [Google Scholar] [CrossRef]
- Wang, C.; Otto, S.; Dorn, M.; Kreidt, E.; Lebon, J.; Sršan, L.; Di Martino-Fumo, P.; Gerhards, M.; Resch-Genger, U.; Seitz, M.; et al. Deuterated Molecular Ruby with Record Luminescence Quantum Yield. Angew. Chem. Int. Ed. 2018, 57, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Mengel, A.K.C.; Bissinger, C.; Dorn, M.; Back, O.; Förster, C.; Heinze, K. Boosting Vis/NIR Charge-Transfer Absorptions of Iron(II) Complexes by N-Alkylation and N-Deprotonation in the Ligand Backbone. Chem. Eur. J. 2017, 23, 7920–7931. [Google Scholar] [CrossRef] [PubMed]
- Bessel, C.A.; See, R.F.; Jameson, D.L.; Churchill, M.R.; Takeuchi, K.J. Structural considerations of terdentate ligands: Crystal structures of 2,2′:6′,2″-terpyridine and 2,6-bis(pyrazol-1-yl)pyridine. J. Chem. Soc. Dalton Trans. 1992, 3223–3228. [Google Scholar] [CrossRef]
- Pearson, R.G.; Williams, F.V. Rates of Ionization of Pseudo Acids.1 V. Steric Effects in the Base-catalyzed Ionization of Nitroethane. J. Am. Chem. Soc. 1953, 75, 3073–3075. [Google Scholar] [CrossRef]
- Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I.A. Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales. J. Org. Chem. 2005, 70, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Staab, H.A.; Saupe, T. “Proton Sponges” and the Geometry of Hydrogen Bonds: Aromatic Nitrogen Bases with Exceptional Basicities. Angew. Chem. Int. Ed. Engl. 1988, 27, 865–879. [Google Scholar] [CrossRef]
- Hergold-Brundić, A.; Popović, Z.; Matković-Calogović, D. 2,2′:6′,2″-Terpyridinium Trifluoromethanesulfonate, [terpyH](CF3SO3). Acta Cryst. 1996, C52, 3154–3157. [Google Scholar] [CrossRef]
- Otto, S.; Moll, J.; Förster, C.; Geißler, D.; Wang, C.; Resch-Genger, U.; Heinze, K. Three-in-One Crystal: The Coordination Diversity of Zinc Polypyridine Complexes. Eur. J. Inorg. Chem. 2017, 5033–5040. [Google Scholar] [CrossRef]
- Otto, S.; Grabolle, M.; Förster, C.; Kreitner, C.; Resch-Genger, U.; Heinze, K. [Cr(ddpd)2]3+: A Molecular, Water-Soluble, Highly NIR-Emissive Ruby Analogue. Angew. Chem. Int. Ed. 2015, 54, 11572–11576. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Yamabe, S.; Kanehisa, N.; Takashima, H.; Tsukahara, K. A metal free blue emission by the protonated 2,2′:6′,2″-terpyridine hexafluorophosphate. J. Phys. Org. Chem. 2009, 22, 410–417. [Google Scholar] [CrossRef]
- Hamacher, C.; Hurkes, N.; Kaiser, A.; Klein, A.; Schüren, A. Electrochemistry and Spectroscopy of Organometallic Terpyridine Nickel Complexes. Inorg. Chem. 2009, 48, 9947–9951. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Dorn, M.; Förster, C.; Bauer, M.; Seitz, M.; Heinze, K. Understanding and exploiting long-lived near-infrared emission of a molecular ruby. Coord. Chem. Rev. 2018, 359, 102–111. [Google Scholar] [CrossRef]
- Alemany, P.; Casanova, D.; Alvarez, S.; Dryzun, C.; Avnir, D. Continuous Symmetry Measures: A New Tool in Quantum Chemistry. In Reviews in Computational Chemistry; Parrill, A.L., Lipkowitz, K.B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 30, pp. 289–352. [Google Scholar]
- Alvarez, S. Distortion Pathways of Transition Metal Coordination Polyhedra Induced by Chelating Topology. Chem. Rev. 2015, 115, 13447–13483. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Zabrodsky, H.; Peleg, S.; Avnir, D. Continuous Symmetry Measures. J. Am. Chem. Soc. 1992, 114, 7843–7851. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states—I—Orbital degeneracy. Proc. R. Soc. Lond. A 1937, 161, 220–235. [Google Scholar] [CrossRef]
- Mack, K.; Wünsche von Leupoldt, A.; Förster, C.; Ezhevskaya, M.; Hinderberger, D.; Klinkhammer, K.W.; Heinze, K. Effect of chelate ring expansion on Jahn-Teller distortion and Jahn-Teller dynamics in copper(II) complexes. Inorg. Chem. 2012, 51, 7851–7858. [Google Scholar] [CrossRef] [PubMed]
- Dorn, M.; Mack, K.; Carrella, L.M.; Rentschler, E.; Förster, C.; Heinze, K. Structure and electronic properties of an expanded terpyridine complex of nickel(II) [Ni(ddpd)2](BF4)2. Z. Anorg. Allg. Chem. 2018, 644, 706–712. [Google Scholar] [CrossRef]
- Förster, C.; Mack, K.; Carrella, L.M.; Ksenofontov, V.; Rentschler, E.; Heinze, K. Coordination of expanded terpyridine ligands to cobalt. Polyhedron 2013, 52, 576–581. [Google Scholar] [CrossRef]
- Förster, C.; Gorelik, T.E.; Kolb, U.; Ksenofontov, V.; Heinze, K. Crystalline Non-Equilibrium Phase of a Cobalt(II) Complex with Tridentate Ligands. Eur. J. Inorg. Chem. 2015, 920–924. [Google Scholar] [CrossRef]
- Mengel, A.K.C.; Förster, C.; Breivogel, A.; Mack, K.; Ochsmann, J.R.; Laquai, F.; Ksenofontov, V.; Heinze, K. A Heteroleptic Push–Pull Substituted Iron(II) Bis(tridentate) Complex with Low-Energy Charge-Transfer States. Chem. Eur. J. 2015, 21, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Skelton, B.W.; Harrowfield, J.M. CCDC 1585922, Experimental Crystal Structure Determination (2017), doi:10.5517/ccdc.csd.cc1q78s0. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc1q78s0&sid=DataCite (accessed on 26 July 2018).
- Docherty, R.; Tuna, F.; Kilner, C.A.; McInnes, E.J.L.; Halcrow, M.A. Suppression of the Jahn–Teller distortion in a six-coordinate copper(II) complex by doping it into a host lattice. Chem. Commun. 2012, 48, 4055–4057. [Google Scholar] [CrossRef] [PubMed]
- Anderer, C.; Näther, C.; Bensch, W. Bis(2,2′:6′,2″-terpyridine-κ3N,N′,N″)nickel(II) bis(perchlorate) hemihydrate. IUCrData 2016, 1, x161009. [Google Scholar] [CrossRef]
- Constable, E.C.; Harris, K.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Turning {M(tpy)2}n+ embraces and CH…π interactions on and off in homoleptic cobalt(II) and cobalt(III) bis(2,2′:6′,2″-terpyridine) complexes. CrystEngComm 2010, 12, 2949–2961. [Google Scholar] [CrossRef]
- Oshio, H.; Spiering, H.; Ksenofontov, V.; Renz, F.; Gütlich, P. Electronic relaxation phenomena following 57Co(EC)57Fe nuclear decay in [Mn(II)(terpy)2](ClO4)2·1/2H2O and in the spin crossover complexes [Co(II)(terpy)2]X2·nH2O (X = Cl and ClO4): A Mössbauer emission spectroscopic study. Inorg. Chem. 2001, 40, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Romain, S.; Duboc, C.; Neese, F.; Rivière, E.; Hanton, L.R.; Blackman, A.G.; Philouze, C.; Leprêtre, J.-C.; Deronzier, A.; Collomb, M. An Unusual Stable Mononuclear MnIII Bis-terpyridine Complex Exhibiting Jahn–Teller Compression: Electrochemical Synthesis, Physical Characterisation and Theoretical Study. Chem. Eur. J. 2009, 15, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Schönle, J.; Zampese, J.A. The surprising lability of bis(2,2′:6′,2″-terpyridine)chromium(III) complexes. Dalton Trans. 2014, 43, 7227–7235. [Google Scholar] [CrossRef] [PubMed]
- Scudder, M.L.; Goodwin, H.A.; Dance, I.G. Crystal supramolecular motifs: Two-dimensional grids of terpy embraces in [ML2]z complexes (L = terpy or aromatic N3-tridentate ligand). New J. Chem. 1999, 23, 695–705. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X. Homoleptic Zinc(II) Complexes Based on 4′-(2-Thienyl)-terpyridine: Preparation, Structures, and Properties. Z. Anorg. Allg. Chem. 2017, 643, 398–402. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Hostettler, N.; Housecroft, C.E.; Schmitt, R.; Schönhofer, E. The d10 route to dye-sensitized solar cells: Step-wise assembly of zinc(II) photosensitizers on TiO2 surfaces. Chem. Commun. 2012, 48, 5727–5729. [Google Scholar] [CrossRef] [PubMed]
- Jameson, D.L.; Guise, L.E. An improved, two-step synthesis of 2,2′:6′,2″-terpyridine. Tetrahedron Lett. 1991, 32, 1999–2002. [Google Scholar] [CrossRef]
- Elsbernd, H.; Beattie, J.K. The NMR spectra of terpyridine and the bis-terpyridine complexes of cobalt(III) and iron(II). J. Inorg. Nucl. Chem. 1972, 34, 771–774. [Google Scholar] [CrossRef]
- Machan, C.W.; Adelhardt, M.; Sarjeant, A.A.; Stern, C.L.; Sutter, J.; Meyer, K.; Mirkin, C.A. One-Pot Synthesis of an Fe(II) Bis-Terpyridine Complex with Allosterically Regulated Electronic Properties. J. Am. Chem. Soc. 2012, 134, 16921–16924. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Scaife, D.B. Electro-oxidation and electro-reduction of some iron(II), cobalt(II) and nickel(II) polypyridyl complexes in acetonitrile. J. Electroanal. Chem. 1977, 84, 373–386. [Google Scholar] [CrossRef]
- Rao, J.M.; Hughes, M.C.; Macero, D.J. Voltammetry of terpyridine and terosine complexes of cobalt(II) and iron(II). Inorg. Chim. Acta 1976, 16, 231–236. [Google Scholar] [CrossRef]
- Dobson, J.C.; Taube, H. Coordination chemistry and redox properties of polypyridyl complexes of vanadium(II). Inorg. Chem. 1989, 28, 1310–1315. [Google Scholar] [CrossRef]
- Albano, G.; Balzani, V.; Constable, E.C.; Maestri, M.; Smith, D.R. Photoinduced processes in 4′-(9-anthryl)-2,2′:6′,2″-terpyridine, its protonated forms and Zn(II), Ru(II) and Os(II) complexes. Inorg. Chim. Acta 1998, 277, 225–231. [Google Scholar] [CrossRef]
- Morgan, G.; Burstall, F.H. Researches on residual affinity and co-ordination. Part XXXVII. Complex metallic salts containing 2:6-di-2′-pyridylpyridine (2:2′:2″-tripyridyl). J. Chem. Soc. 1937, 1649–1655. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993; pp. 9–10. [Google Scholar]
- Saravani, H.; Rezvani, A.R.; Hadadzadeh, H.; Safari, N. An Investigation of Z-in Distortion in Mononuclear Cu(II) Complex with Terpyridine Ligands, [Cu(terpy)2](PF6)2. Iran. J. Chem. Chem. Eng. 2007, 26, 103–110. [Google Scholar]
- Folgado, J.-V.; Henke, W.; Allmann, R.; Stratemeier, H.; Beltrán-Porter, D.; Roja, T.; Reinen, D. Fluxionality in hexacoordinated copper(II) complexes with 2,2′:6′,2″-terpyridine (terpy) and related ligands: Structural and spectroscopic investigations. Inorg. Chem. 1990, 29, 2035–2042. [Google Scholar] [CrossRef]
- Hogg, R.; Wilkins, R.G. Exchange studies of certain chelate compounds of the transitional metals. Part VIII. 2,2′,2″-terpyridine complexes. J. Chem. Soc. 1962, 341–350. [Google Scholar] [CrossRef]
- Rao, J.M.; Hughes, M.C.; Macero, D.J. Redox behavior of aromatic tridentate imine ligand complexes of manganese and chromium. Inorg. Chim. Acta 1976, 18, 127–131. [Google Scholar] [CrossRef]
- Scarborough, C.C.; Sproules, S.; Weyhermüller, T.; DeBeer, S.; Wieghardt, K. Electronic and Molecular Structures of the Members of the Electron Transfer Series [Cr(tbpy)3]n (n = 3+, 2+, 1+, 0): An X-ray Absorption Spectroscopic and Density Functional Theoretical Study. Inorg. Chem. 2011, 50, 12446–12462. [Google Scholar] [CrossRef] [PubMed]
- Casellato, U.; Graziani, R.; Bonomo, R.P.; Di Bilio, A.J. X-ray crystal structures and electron spin resonance spectroscopic characterization of mixed-ligand chromium(III) complexes with L-aspartate or pyridine-2,6-dicarboxylate and 1,10-phenanthroline or 2,2′:6′,2″-terpyridyl. J. Chem. Soc. Dalton Trans. 1991, 23–31. [Google Scholar] [CrossRef]
- Herzog, S.; Aul, H. Über elektronenreiche Komplexe des Vanadins mit 2,2′,2″-Tripyridyl. Zeitschr. Chem. 1966, 6, 343–344. [Google Scholar] [CrossRef]
- Henke, W.; Reinen, D. Spektroskopische Untersuchungen zum Jahn-Teller-Effekt des Cu2+-Ions in Terpyridin-Komplexen Cu(terpy)2X2·nH2O [X = NO3–, ClO4–, Br–]. Z. Anorg. Allg. Chem. 1977, 436, 187–200. [Google Scholar] [CrossRef]
- Waldmann, O.; Hassmann, J.; Müller, P.; Volkmer, D.; Schubert, U.S.; Lehn, J.-M. Magnetism of self-assembled mono- and tetranuclear supramolecular Ni2+ complexes. Phys. Rev. B 1998, 58, 3277–3285. [Google Scholar] [CrossRef]
- González, E.; Rodrigue-Witchel, A.; Reber, C. Absorption spectroscopy of octahedral nickel(II) complexes: A case study of interactions between multiple electronic excited states. Coord. Chem. Rev. 2007, 251, 351–363. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Orchard, K.L.; Dalle, K.E.; Reisner, E. Selective Photocatalytic CO2 Reduction in Water through Anchoring of a Molecular Ni Catalyst on CdS Nanocrystals. J. Am. Chem. Soc. 2017, 139, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Judge, J.S.; Baker, W.A., Jr. On the spin equilibrium in bis(2,2′,2″-terpyridine) cobalt(II) salts. Inorg. Chim. Acta 1967, 1, 68–72. [Google Scholar] [CrossRef]
- Kremer, S.; Henke, W.; Reinen, D. High-spin-low-spin equilibria of cobalt(2+) in the terpyridine complexes Co(terpy)2X2.nH2O. Inorg. Chem. 1982, 21, 3013–3022. [Google Scholar] [CrossRef]
- Komatsu, Y.; Kato, K.; Yamamoto, Y.; Kamihata, H.; Lee, Y.H.; Fuyuhiro, A.; Kawata, S.; Hayami, S. Spin-Crossover Behaviors Based on Intermolecular Interactions for Cobalt(II) Complexes with Long Alkyl Chains. Eur. J. Inorg. Chem. 2012, 2769–2775. [Google Scholar] [CrossRef]
- Hayami, S.; Shigeyoshi, Y.; Akita, M.; Inoue, K.; Kato, K.; Osaka, K.; Takata, M.; Kawajiri, R.; Mitani, T.; Maeda, Y. Reverse Spin Transition Triggered by a Structural Phase Transition. Angew. Chem. Int. Ed. 2005, 44, 4899–4903. [Google Scholar] [CrossRef] [PubMed]
- Kilner, C.A.; Halcrow, M.A. An unusual discontinuity in the thermal spin transition in [Co(terpy)2][BF4]2. Dalton Trans. 2010, 39, 9008–9012. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.S.; Constable, E.C.; Housecroft, C.E.; Kulicke, K.J.; Tao, Y. When electron exchange is chemical exchange–assignment of 1H NMR spectra of paramagnetic cobalt(II)-2,2′:6′,2″-terpyridine complexes. Dalton Trans. 2005, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.K.; Binstead, R.A.; Kelso, M.T.; Del Favero, P.; Dewey, T.G.; Turner, D.H. Dynamics of cobalt(II) spin-equilibrium complexes. Inorg. Chim. Acta 1995, 235, 245–251. [Google Scholar] [CrossRef]
- Mengel, A.K.C.; Cho, W.; Breivogel, A.; Char, K.; Kang, Y.S.; Heinze, K. A Bis(tridentate)cobalt Polypyridine Complex as Mediator in Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2015, 3299–3306. [Google Scholar] [CrossRef]
- Kreitner, C.; Mengel, A.K.C.; Lee, T.K.; Cho, W.; Char, K.; Kang, Y.S.; Heinze, K. Strongly Coupled Cyclometalated Ruthenium Triarylamine Chromophores as Sensitizers for DSSCs. Chem. Eur. J. 2016, 22, 8915–8928. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, R.A.D.; Piper, T.S. A Crystal Field Model for the Spectral Relationships in Monoacidopentaammine and Diacidotetraammine Complexes of Cobalt(III). Inorg. Chem. 1965, 4, 709–714. [Google Scholar] [CrossRef]
- Sharma, R.P.; Singh, A.; Brandão, P.; Felix, V.; Venugopalan, P. Second sphere coordination in binding of fluoroanions: Synthesis, spectroscopic characterization and single crystal X-ray structure determination of [Co(phen)3](BF4)3·H2O and [Co(phen)3](PF6)3·CH3COCH3. J. Mol. Struct. 2009, 920, 119–127. [Google Scholar] [CrossRef]
- Sutin, N.; Brunschwig, B.S.; Creutz, C.; Winkler, J.R. Nuclear reorganization barriers to electron transfer. Pure Appl. Chem. 1988, 60, 1817–1830. [Google Scholar] [CrossRef]
- Cummins, D.; Gray, H.B. Electron-transfer protein reactivities. Kinetic studies of the oxidation of horse heart cytochrome c, Chromatium vinosum high potential iron-sulfur protein, Pseudomonas aeruginosa azurin, bean plastocyanin, and Rhus vernicifera stellacyanin by pentaammminepyridineruthenium(III). J. Am. Chem. Soc. 1977, 99, 5158–5167. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.; Creutz, C.; Sutin, N. Rate constants and activation parameters for outer-sphere electron-transfer reactions and comparisons with the predictions of Marcus theory. J. Am. Chem. Soc. 1977, 99, 5615–5623. [Google Scholar] [CrossRef]
- Ondersma, J.W.; Hamann, T.W. Recombination and redox couples in dye-sensitized solar cells. Coord. Chem. Rev. 2103, 257, 1533–1543. [Google Scholar] [CrossRef]
- Yum, J.-H.; Baranoff, E.; Kessler, F.; Moehl, T.; Ahmad, S.; Bessho, T.; Marchioro, A.; Ghadiri, E.; Moser, J.-E.; Yi, C.; et al. A cobalt complex redox shuttle for dye-sensitized solar cells with high open-circuit potentials. Nat. Commun. 2012, 3, 631. [Google Scholar] [CrossRef] [PubMed]
- Harzmann, G.D.; Neuburger, M.; Mayor, M. 4,4’’-Disubstituted Terpyridines and Their Homoleptic FeII Complexes. Eur. J. Inorg. Chem. 2013, 3334–3347. [Google Scholar] [CrossRef]
- Pazderski, L.; Pawlak, T.; Sitkowski, J.; Kozerski, L.; Szlyk, E. 1H, 13C, 15N NMR coordination shifts in Fe(II), Ru(II) and Os(II) cationic complexes with 2,2′:6′,2″-terpyridine. Magn. Reson. Chem. 2011, 49, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Mack, K. Neue Übergangsmetall-Komplexe des N,N′-dimethyl-N,N′-dipyridin-2-ylpyridin-2,6-diamins-Strukturelle, magnetische und optische Eigenschaften. Diploma Thesis, Johannes Gutenberg University, Mainz, Germany, 2012. [Google Scholar]
- Shepard, S.G.; Fatur, S.M.; Rappé, A.K.; Damrauer, N.H. Highly Strained Iron(II) Polypyridines: Exploiting the Quintet Manifold To Extend the Lifetime of MLCT Excited States. J. Am. Chem. Soc. 2016, 138, 2949–2952. [Google Scholar] [CrossRef] [PubMed]
- Fatur, S.M.; Shepard, S.G.; Higgins, R.F.; Shores, M.P.; Damrauer, N.H. A Synthetically Tunable System To Control MLCT Excited-State Lifetimes and Spin States in Iron(II) Polypyridines. J. Am. Chem. Soc. 2017, 139, 4493–4505. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, T.-W.; Chou, C.-C.; Lee, H.-C.; Chang, H.-C.; Lee, G.-H.; Leung, M.; Peng, S.-P. New oligo-α-pyridylamino ligands and their metal complexes. Chem. Commun. 1997, 2279–2280. [Google Scholar] [CrossRef]
- Jamula, L.L.; Brown, A.M.; Guo, D.; McCusker, J.K. Synthesis and Characterization of a High-Symmetry Ferrous Polypyridyl Complex: Approaching the 5T2/3T1 Crossing Point for FeII. Inorg. Chem. 2014, 53, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Harlang, T.; Canton, S.E.; Chábera, P.; Suárez-Alcántara, K.; Fleckhaus, A.; Vithanage, D.A.; Göransson, E.; Corani, A.; Lomoth, R.; et al. Towards longer-lived metal-to-ligand charge transfer states of iron(II) complexes: An N-heterocyclic carbene approach. Chem. Commun. 2013, 49, 6412–6414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kjær, K.S.; Fredin, L.A.; Chábera, P.; Harlang, T.; Canton, S.E.; Lidin, S.; Zhang, J.; Lomoth, R.; Bergquist, K.-E.; et al. A Heteroleptic Ferrous Complex with Mesoionic Bis(1,2,3-triazol-5-ylidene) Ligands: Taming the MLCT Excited State of Iron(II). Chem. Eur. J. 2015, 21, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Duchanois, T.; Etienne, T.; Monari, A.; Beley, M.; Assfeld, X.; Haacke, S.; Gros, P.C. A new record excited state 3MLCT lifetime for metalorganic iron(II) complexes. Phys. Chem. Chem. Phys. 2016, 18, 12550–12556. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Burkhardt, L.; Friedrich, A.; Steube, J.; Neuba, A.; Schepper, R.; Müller, P.; Flörke, U.; Huber, M.; Lochbrunner, S.; et al. The Connection between NHC Ligand Count and Photophysical Properties in Fe(II) Photosensitizers: An Experimental Study. Inorg. Chem. 2018, 57, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Chábera, P.; Kjaer, K.S.; Prakash, O.; Honarfar, A.; Liu, Y.; Fredin, L.A.; Harlang, T.C.B.; Lidin, S.; Uhlig, J.; Sundström, V.; et al. FeII Hexa N-Heterocyclic Carbene Complex with a 528 ps Metal-to-Ligand Charge-Transfer Excited-State Lifetime. J. Phys. Chem. Lett. 2018, 9, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Romain, S.; Baffert, C.; Duboc, C.; Leprêtre, J.-C.; Deronzier, A.; Collomb, M.-N. Mononuclear MnIII and MnIV Bis-terpyridine Complexes: Electrochemical Formation and Spectroscopic Characterizations. Inorg. Chem. 2009, 48, 3125–3131. [Google Scholar] [CrossRef] [PubMed]
- Limburg, J.; Vrettos, J.S.; Liable-Sands, L.M.; Rheingold, A.L.; Crabtree, R.H.; Brudvig, G.W. A Functional Model for O-O Bond Formation by the O2-Evolving Complex in Photosystem II. Science 1999, 283, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Young, K.J.; Brennan, B.J.; Tagore, R.; Brudvig, G.W. Photosynthetic Water Oxidation: Insights from Manganese Model Chemistry. Acc. Chem. Res. 2015, 48, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zare, D.; Doistau, B.; Nozary, H.; Besnard, C.; Guénée, L.; Suffren, Y.; Pelé, A.-L.; Hauser, A.; Piguet, C. CrIII as an alternative to RuII in metallo-supramolecular chemistry. Dalton Trans. 2017, 46, 8992–9009. [Google Scholar] [CrossRef] [PubMed]
- Cloete, N.; Visser, H.G.; Roodt, A. mer-Trichloro(2,2′,2″-terpyridine)chromium(III) dimethyl sulfoxide solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m45–m47. [Google Scholar] [CrossRef]
- Henriques, R.T.; Herdtweck, E.; Kühn, F.E.; Lopes, A.D.; Mink, J.; Romão, C.C. Synthesis, characterization, and reactions of tetrakis(nitrile)chromium(II) tetrafluoroborate complexes. J. Chem. Soc. Dalton Trans. 1998, 1293–1297. [Google Scholar] [CrossRef]
- Åkesson, R.; Pettersson, L.G.M.; Sandström, M.; Wahlgren, U. Theoretical calculations of the Jahn-Teller effect in the hexahydrated Copper(II), chromium(II), and manganese(III) ions, hexaaquacopper(2+), hexaaquachromium(2+) and hexaaquamanganese(3+), and comparisons with the hexahydrated copper(I), chromium(III), and manganese(II) clusters. J. Phys. Chem. 1992, 96, 150–156. [Google Scholar] [CrossRef]
- Thangavel, A.; Wieliczko, M.; Scarborough, C.; Dittrich, B.; Bacsa, J. An investigation of the electron density of a Jahn–Teller-distorted CrII cation: The crystal structure and charge density of hexakis(acetonitrile-κN)chromium(II) bis(tetraphenylborate) acetonitrile disolvate. Acta Crystallogr. Sect. C 2015, C71, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Hieber, W.; Mühlbauer, F. Über Metallcarbonyle. XII. Reaktionen und Derivate der Hexacarbonyle des Chroms und Molybdäns. Z. Anorg. Allg. Chem. 1935, 221, 337–348. [Google Scholar] [CrossRef]
- Hieber, W.; Abeck, W.; Platzer, H.K. Über Metallcarbonyle. 69. Über Tricarbonyl-triammin-Chrom und seine Derivate. Z. Anorg. Allg. Chem. 1955, 280, 252–263. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Fuh, Y.-S.; Shiue, J.-Y.; Yu, S.J.; Lee, G.-H.; Peng, S.-M. Syntheses and chemistry of Tris(2-pyridyl)phosphine complexes of Group VI transition metals. X-ray structural studies of the molybdenum complexes. J. Organomet. Chem. 1999, 588, 260–267. [Google Scholar] [CrossRef]
- Howie, R.A.; McQuillan, G.P. Trisubstituted Group 6 metal carbonyl complexes with di-2-pyridylamine ligands. Crystal structures of (2,2′-bipyridyl-N,N′) tricarbonyl(di-2-pyridylamine-N′)-molybdenum(0) and -tungsten(0). J. Chem. Soc. Dalton Trans. 1986, 759–764. [Google Scholar] [CrossRef]
- Otto, S. Synthese, experimentelle und theoretische Charakterisierung neuartiger Polypyridyl-Komplexe des Chrom, Mangan und Zink. Diploma Thesis, Johannes Gutenberg University, Mainz, Germany, 2015. [Google Scholar]
- Xiang, H.; Cheng, J.; Ma, X.; Zhou, X.; Chruma, J.J. Near-infrared phosphorescence: Materials and applications. Chem. Soc. Rev. 2013, 42, 6128–6185. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.C.; Kim, A.J.I.; de Vries, E.; Shaner, S.E.; Lovaasen, B.M. Chromium (III) Bis-Arylterpyridyl Complexes with Enhanced Visible Absorption via Incorporation of Intraligand Charge-Transfer Transitions. Inorg. Chem. 2017, 56, 8212–8222. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Förster, C.; Wang, C.; Resch-Genger, U.; Heinze, K. A strongly luminescent chromium(III) complex acid. Chem. Eur. J. 2018, 24. in press. [Google Scholar] [CrossRef]
- Otto, S.; Nauth, A.M.; Ermilov, E.; Scholz, N.; Friedrich, A.; Resch-Genger, U.; Lochbrunner, S.; Opatz, T.; Heinze, K. Photo-Chromium: Sensitizer for Visible-Light-Induced Oxidative C−H Bond Functionalization—Electron or Energy Transfer? ChemPhotoChem 2017, 1, 344–349. [Google Scholar] [CrossRef]
- Vaidyanathan, V.G.; Nair, B.U. Nucleobase Oxidation of DNA by (Terpyridyl)chromium(III) Derivatives. Eur. J. Inorg. Chem. 2004, 1840–1846. [Google Scholar] [CrossRef]
- Stevenson, S.M.; Shores, M.P.; Ferreira, E.M. Photooxidizing Chromium Catalysts for Promoting Radical Cation Cycloadditions. Angew. Chem. Int. Ed. 2015, 54, 6506–6510. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.F.; Fatur, S.M.; Shepard, S.G.; Stevenson, S.M.; Boston, D.J.; Ferreira, E.M.; Damrauer, N.H.; Rappé, A.K.; Shores, M.P. Uncovering the Roles of Oxygen in Cr(III) Photoredox Catalysis. J. Am. Chem. Soc. 2016, 138, 5451–5464. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.M.; Higgins, R.F.; Shores, M.P.; Ferreira, E.M. Chromium photocatalysis: Accessing structural complements to Diels–Alder adducts with electron-deficient dienophiles. Chem. Sci. 2017, 8, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Scholz, N.; Behnke, T.; Resch-Genger, U.; Heinze, K. Thermo-Chromium: A Contactless Optical Molecular Thermometer. Chem. Eur. J. 2017, 23, 12131–12135. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Harris, J.P.; Heinze, K.; Reber, C. Molecular Ruby under Pressure. Angew. Chem. Int. Ed. 2018, 57, 11069–11073. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.A.; Piermarini, G.J.; Barnett, J.D.; Block, S. Pressure measurement made by the utilization of ruby sharp-line luminescence. Science 1972, 176, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Pajdowski, L.; Karwecka, Z.; Fried, K.; Adamczak, H. Complex compounds of vanadium(III) in pyridine solutions. J. Inorg. Nucl. Chem. 1974, 36, 585–589. [Google Scholar] [CrossRef]
- Casey, A.T.; Clark, R.J.H. Preparations and properties of vanadium(III) complexes of bidentate and terdentate nitrogen-donor ligands. Transit. Met. Chem. 1977, 2, 76–80. [Google Scholar] [CrossRef]
- Silverman, L.D.; Dewan, J.C.; Giandomenico, C.M.; Lippard, S.J. Molecular structure and ligand-exchange reactions of trichlorotris(tert-butyl isocyanide)vanadium(III). Synthesis of the hexakis(tert-butyl isocyanide)vanadium(II) cation. Inorg. Chem. 1980, 19, 3379–3383. [Google Scholar] [CrossRef]
- Manzer, L.E.; Deaton, J.; Sharp, P.; Schrock, R.R. Tetrahydrofuran Complexes of Selected Early Transition Metals. Inorg. Synth. 1982, 21, 135–140. [Google Scholar] [CrossRef]
- Abbo, H.S.; Titinchi, S.J.J. A New Vanadium (III) Complex of 2,6-Bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine as a Catalyst for Ethylene Polymerization. Molecules 2013, 18, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.R.; Allan, L.E.N.; Decken, A.; Shaver, M.P. Organometallic mediated radical polymerization of vinyl acetate using bis(imino)pyridine vanadium trichloride complexes. Dalton Trans. 2013, 42, 9157–9165. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.E.; Taube, H. An Investigation of the Vanadium(II)-Vanadium(III) Couple with Polypyridine Ligands. Inorg. Chem. 1968, 7, 254–261. [Google Scholar] [CrossRef]
- Behrens, H.; Brandl, H.; Lutz, K. Über Bis-[tripyridyl(2.2′.2″)]-vanadin(0). Z. Naturforsch. 1967, 22b, 99–100. [Google Scholar] [CrossRef]
- Wang, M.; Weyhermüller, T.; England, J.; Wieghardt, K. Molecular and Electronic Structures of Six-Coordinate “Low-Valent” [M(Mebpy)3]0 (M = Ti, V, Cr, Mo) and [M(tpy)2]0 (M = Ti, V, Cr), and Seven-Coordinate [MoF(Mebpy)3](PF6) and [MX(tpy)2](PF6) (M = Mo, X = Cl and M = W, X = F). Inorg. Chem. 2013, 52, 12763–12776. [Google Scholar] [CrossRef] [PubMed]
- STOE & Cie. X-Red; STOE & Cie: Darmstadt, Germany, 2002. [Google Scholar]
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Crystallogr. A 1995, 51, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXL-2014/7; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, D.A.; Chen, X.-Y.; Landis, C.R.; Neese, F. All-Electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms. J. Chem. Theory Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, S.; Rajendran, A.; Klamt, A.; Diedenhofen, M.; Neese, F. Calculation of Solvent Shifts on Electronic g-Tensors with the Conductor-Like Screening Model (COSMO) and Its Self-Consistent Generalization to Real Solvents (Direct COSMO-RS). J. Phys. Chem. A 2006, 110, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F. The Determination of the Paramagnetic Susceptibility of Substances in Solution by Nuclear Magnetic Resonance. J. Chem. Soc. 1959, 2003–2005. [Google Scholar] [CrossRef]
- Evans, D.F.; Fazakerley, G.V.; Phillips, R.F. Organometallic Compounds of Bivalent Europium, Ytterbium, and Samarium. J. Chem. Soc. A 1971, 1931–1934. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).