1. Introduction
Metal-containing polymers offer properties and opportunities not available to other polymers because of the presence of the metal site. Group 4 metals represent the chemical family with the closest properties, making them difficult to separate. Even so, they are among the most important elements and their metallocenes show wide application in a number of diverse areas. For instance, the metallocene dichlorides are widely employed as steroregulating agents in the production of many important polymers including polypropylene and polystyrene and a variety of linear polyethylene materials [
1,
2]. Here, our focus is on their potential as incorporated units in polymers.
2. Synthesis and Naming
A variety of Group 4 condensation metallocene polymers have been synthesized employing the interfacial polycondensation process. The Group 4 metallocene dichlorides are commercially available at a reasonable cost. This has allowed the rapid (often less than 30 s) synthesis of high molecular weight polymers. These products are relatively nontoxic.
Table 1 contains the repeat backbone for many of these polymers, illustrating the variety of linear products possible along with the general polymer name. The polymers are named with the metallocene portion treated as a methylene unit.
Our initial reported synthesis of titanocene polymers was in 1971 [
3,
4]. Since then we found that metallocene-containing polymers offered a number of useful properties including spontaneously forming fibers [
5], use as control release agent for plant growth hormones, PGHs [
6,
7,
8,
9,
10,
11], and moderate laser emissions when present in ppm. Later we will briefly discuss these results as well as other important applications [
12].
3. Solubility
Polymer solubility, compared to the solubility of smaller molecules, is more difficult in terms of broadness, amount, and time [
1,
2,
13]. Metallocene polymers have an even greater problem with respect to solubility. This has discouraged advancement in some important areas. Biologically, two metal-containing polymers have undergone human testing as anticancer agents. One is the platinum-containing small molecules, including one of the most widely employed cisplatin groups. The other is titanocene dichloride, which has undergone testing. Researchers noted the difficulty of achieving solubility of the titanocene materials, which has been a discouragement. Essentially, all of the metallocene-containing polymers made by us are soluble to an extent that allows for molecular weight and biological determination. In general, compared to the zirconocene and hafnocene polymers, the titanocene polymers have the poorest solubilities. Recently, we reported the synthesis of water-soluble polymers based on the reaction of various polyethylene oxides with the metallocene dichlorides. We are currently involved in the synthesis of copolymers that incorporate Lewis bases, which offer outstanding biological activities and polyethylene oxide as the second Lewis base. This has been reviewed recently [
13].
4. Fiber Formation
We initially observed the fiber forming properties of metallocene polymers in 1971 and since then noted it as a side comment in a number of our publications [
14]. The following briefly describes some of these observations.
Fibers are found for both organotin and metallocene polymers of various structures. These fibers are large enough to be seen with the naked eye. They may be embedded in the polymer as they are originally recovered or may form when they are removed for storage. For instance, products precipitate from reaction and are collected using a Buchner filter, washed with the organic solvent and water removing unreacted materials and salts, and subsequently washed into a glass Petri dish. The product is allowed to dry. In some cases the fibers are present on evaporation of the acetone. In other cases, fibers are spontaneously produced when they are scraped, using a flat-ended steel spatula, from the glass Petri dish. It appears that the mechanical agitation is sufficient to induce fiber formation.
Typical fibers are colorless and transparent. They generally have a somewhat smooth stem, with short branching radiating from the stem. The number of fibers visible generally varies from 1 to greater than 15% by weight of the total product. Some fibers are 1 mm in length with a diameter of 0.0005 to 0.0008, giving an aspect ratio (length to diameter) in excess of 100.
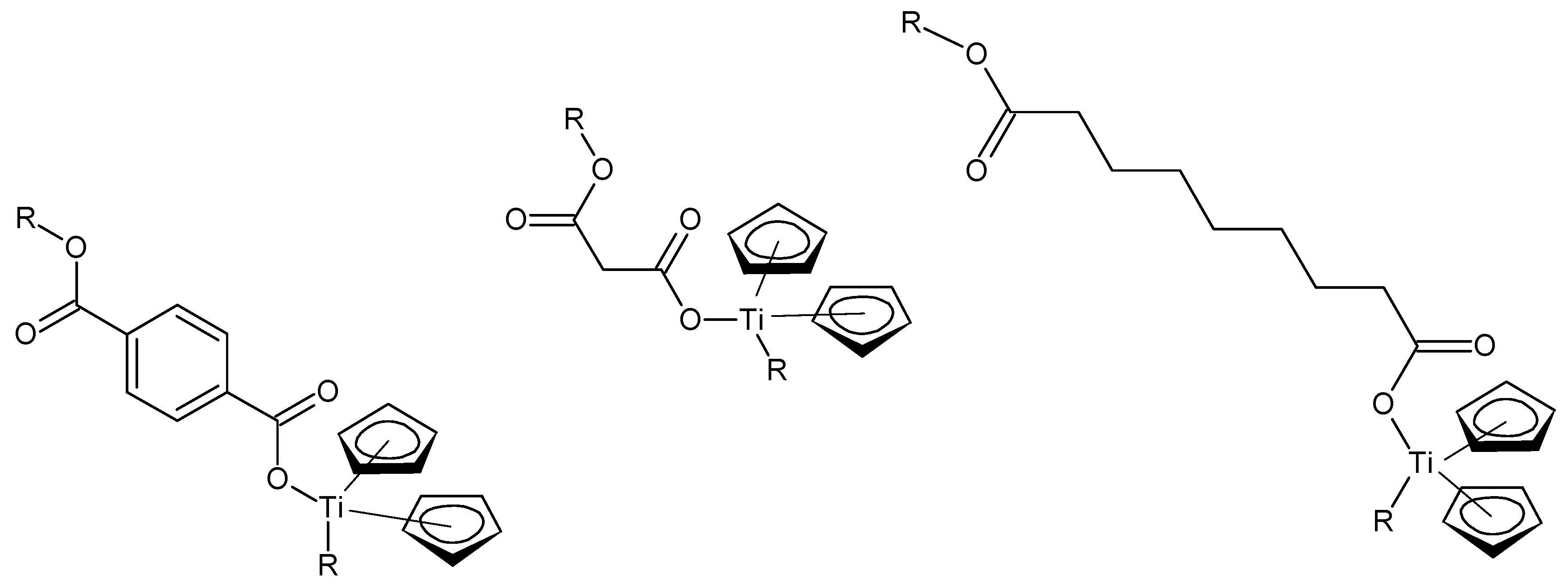
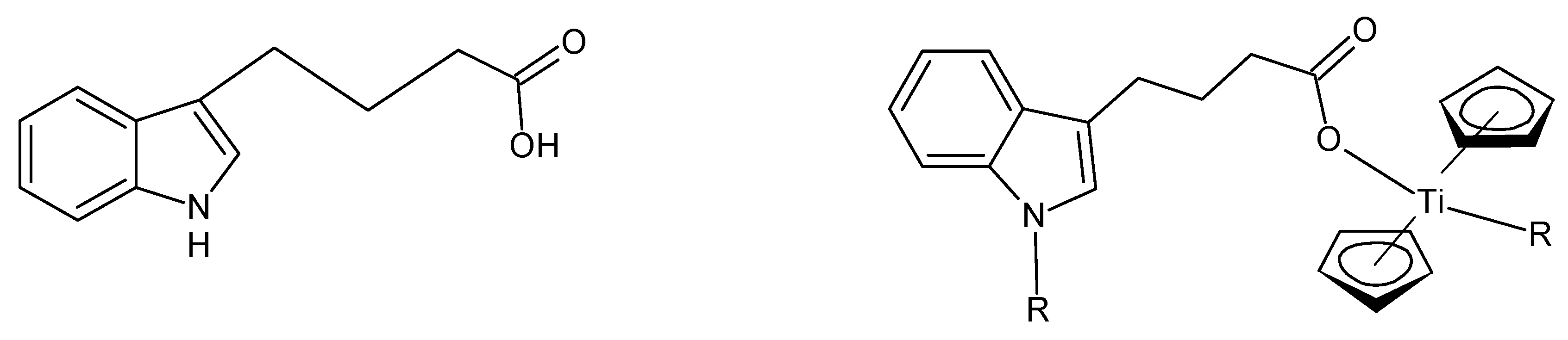
Repeat units for some of the metallocene fiber-forming metallocene polymers are given in
Figure 1. Most of the fiber-forming structures contain rigid backbones, as is the case with the terephthalic acid-derived products shown in
Figure 1 (left), but may be semi-rigid as with the acid portion derived from itaconic acid (methylene succinic acid) (middle) or flexible as with azelatic acud (nonanediolic acid) (right). While the representative structures are given for titanocene products, the analogous zirconocene and hafnocene products also form fibers.
While the structures are all drawn for M = Ti, products from Zr and Hf also gave fibers. It is important to note that while the metallocenes are tetrahedral, the cyclopentadenyl moiety faces the metal similar to a distorted ferrocene structure.
The structures and physical properties of these fibers were studied and compared with analogous non-fibrous portions of the product. Phase transitions observed using differential scanning calorimetry, DSC, appear at the same locations and magnitude. (DSC is the measurement of changes in energy of a heated sample, here polymer, and the reference standard based on power addition.) Furthermore, DSC thermograms are the same in air or nitrogen, consistent with degradation being similar in both air and nitrogen. Loss of weight begins for many of the products at about 1000 °C, with a lower weight reduction occurring in nitrogen in comparison to air. Infrared spectra of the fibrous and non-fibrous solids prior to heating and after heating are similar. Finally, few noticeable differences are found in the infrared spectra of the fibrous and non-fibrous portions of the same polymer in the range of 3000 to 200 cm−1. One difference observed for some products was that the non-fibrous products generally exhibited a sharp band at 3130 cm−1 associated with the Cp and terphthalate moieties for terephthalic acid-derived products. This peak is less prominent in the fibrous product, being somewhat covered by bands between 3480 and 3100 cm−1. This increased intensity of the bands may be associated with increased hydrogen bonding.
The fibers are flexible, with some flexibility retained up to 500 °C. These fibers retained their original flexibility and other properties for over 30 years.
Since that time we have noticed fiber formation for products but generally only note it in passing when presenting the synthesis of new polymers, or may simply not mention it.
5. Electrical Conductivity
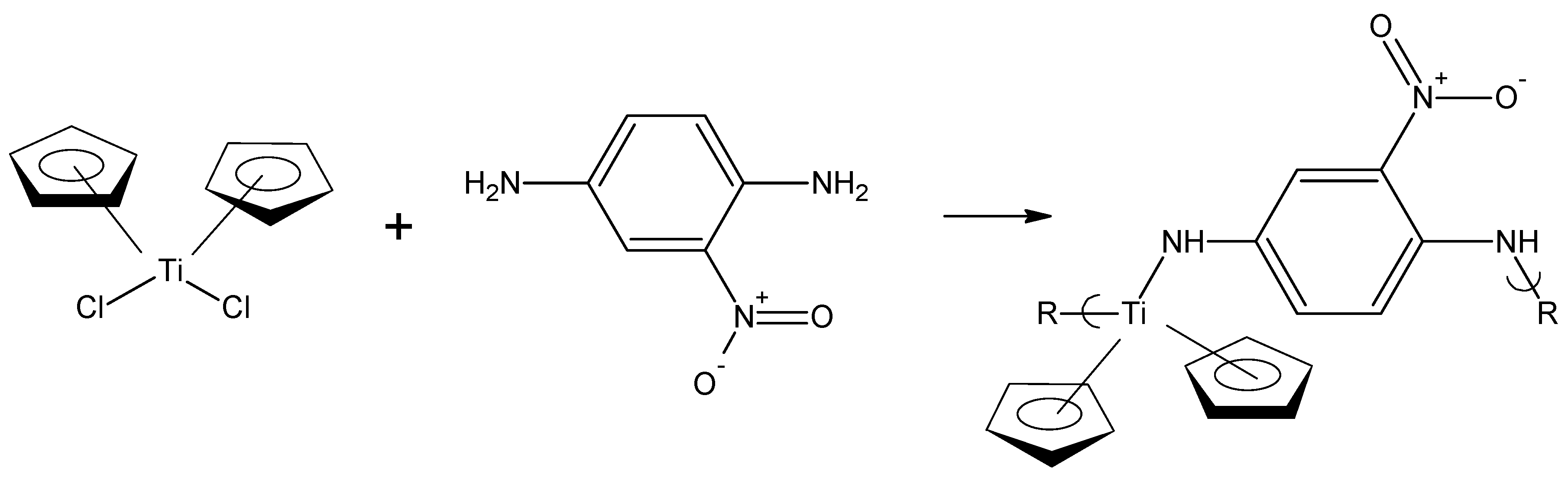
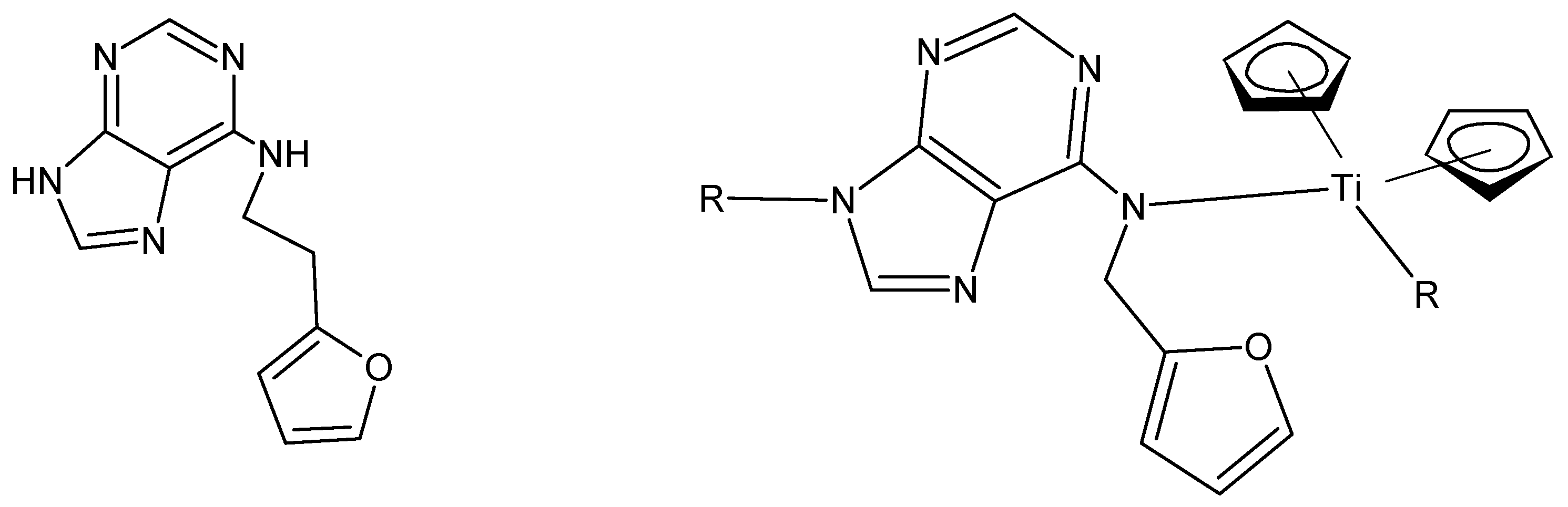
Many of the metallocene polymers synthesized by us are semiconductors. We studied the ability to increase the conductivity of about 150 metal-containing polymers through iodine doping. The process is simple, involving mixing the polymer with small amounts of solid iodine or exposing the polymer pellet to iodine vapors and measuring the conductivity. While small increases, up to 10-fold, in conductivity were common, larger increases were not. Eventually, we found that the polymer from titanocene dichloride and 2-nitro-1,4-phenhylenediamine (
Figure 2) did allow decent increases in bulk conductivity [
15]. Our initial efforts focused on simple exposure of the polymer pellet to iodine vapor and found up to a 10
5 increase in bulk conductivity, moving the products from being semiconductors to low conductors.
We next mixed iodine into the polymer and formed a pellet [
16]. Again, there was a good increase in conductivity to 10
4.
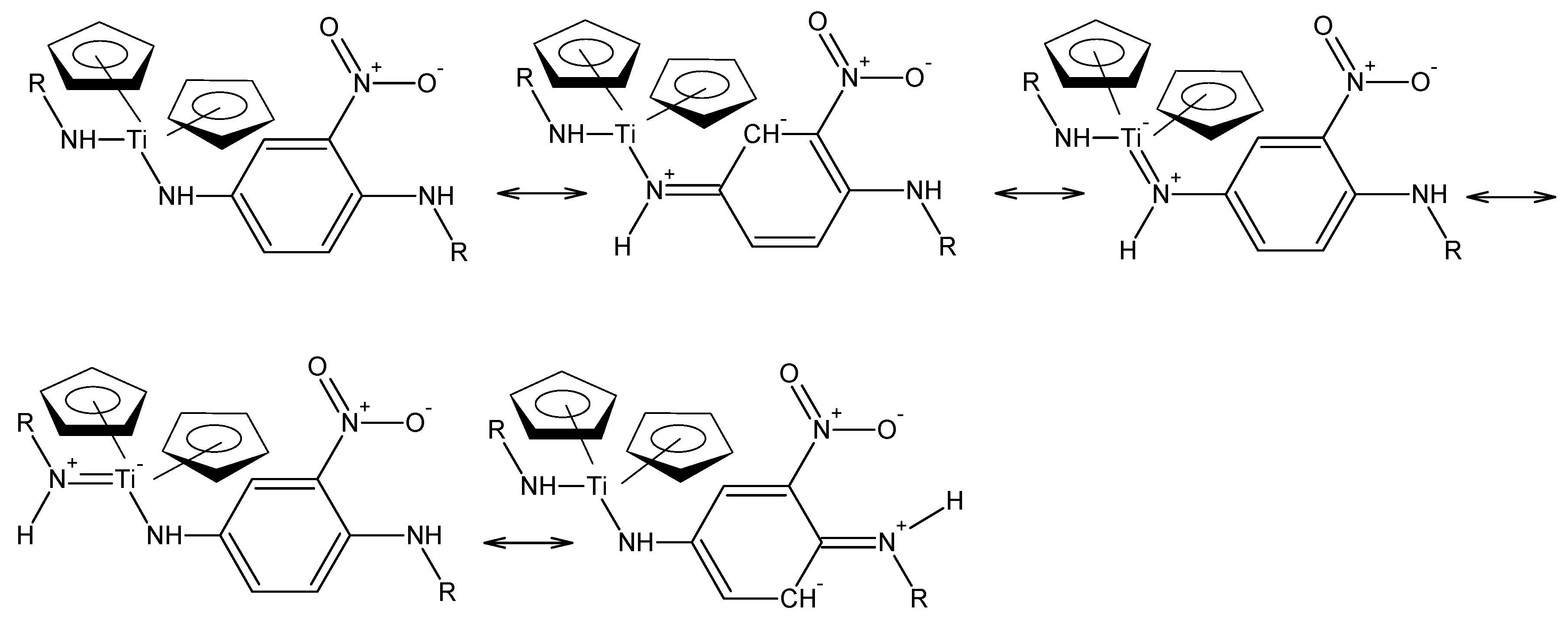
One of our reasons for choosing certain polymers to investigate doping is that the polymer chain could be considered to have whole-chain resonance. The 2-nitro-1,4-phenylenediamne polymer can show this resonance as shown below,
Figure 3, consistent with this idea.
We then synthesized other structurally similar polymers using zirconocene and hafnocene dichlorides [
17]. The largest increase of 10
6 occurred for the hafnocene product.
This was followed by looking at phenylenediamines of various structures. None of these products exhibited more than a 10-fold increase. Thus, the conductivity increase was specific and real.
6. Plant Growth Hormones
People need food. Many individuals grow food from damaged and aged seeds. In the Florida Everglades sawgrass is being displaced by cattails, threatening the existence of the Everglades. These problems revolve around the ability to grow plants. Our approach is to synthesize polymers that contain agents that assist with growth. These agents are referred to under different names including plant growth hormones, PGHs, which is the term employed here. We will briefly describe several PGHs we studied [
6,
7,
8,
9,
10,
11].
Gibberellins [
7,
9,
11] are cyclic diterenes. They have the ability to induce a number of plant responses including cell elongation and cell division. While various gibberellins are widespread in nature, the major commercially employed gibberellin is called gibberellic acid, GA3 (
Figure 4). GA3 is the most active of the naturally occurring gibberellins and is the most widely utilized in research and commercially to increase plant and food production. GA3 is the gibberellin we used. GA3 is relatively non-toxic, with a LD50 of 6300 mg/kg.
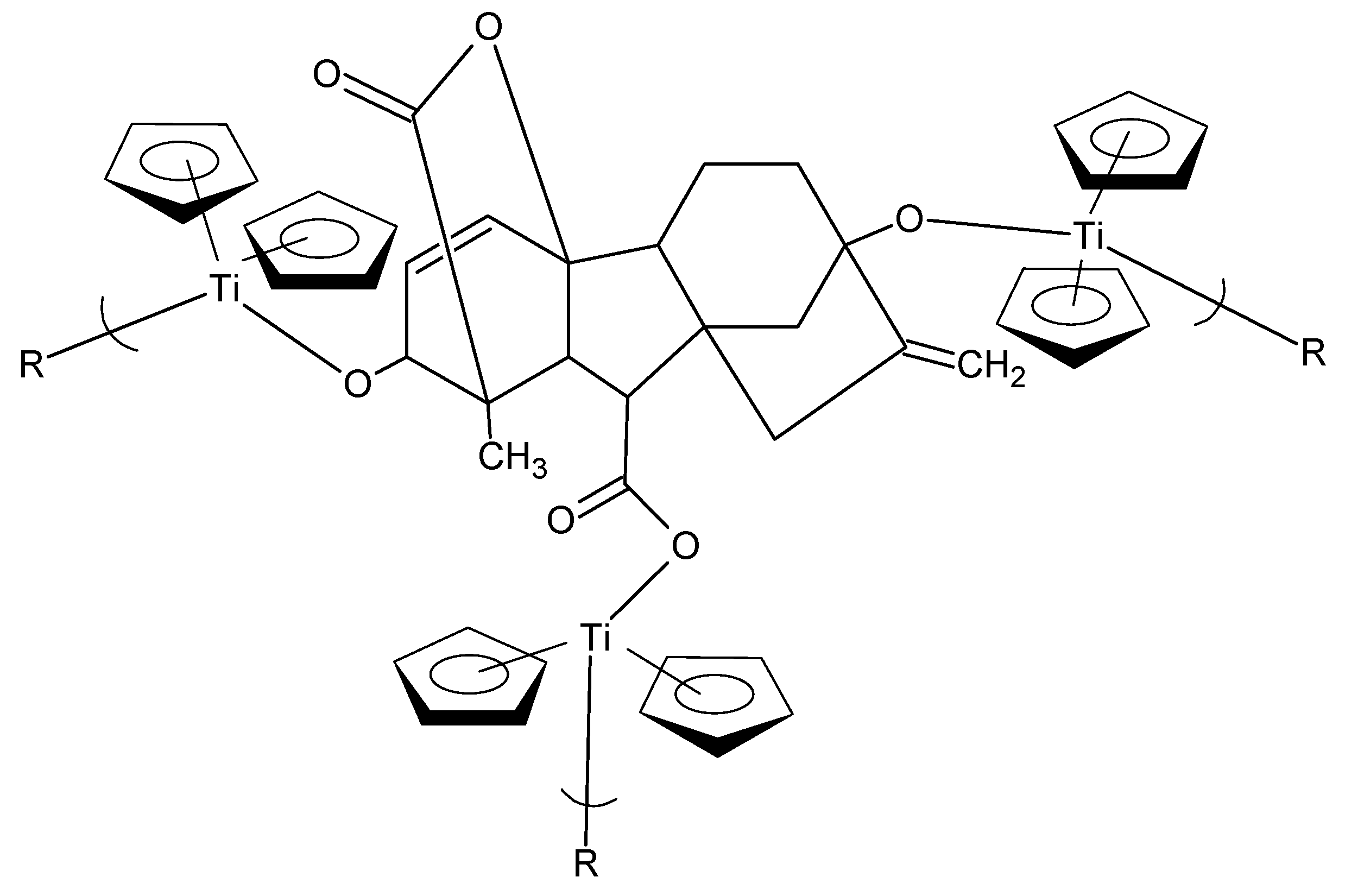
GA3 has three functional groups, two alcohols and one acid functional group, which can react in a condensation reaction. The resulting products are crosslinked and consequently insoluble (
Figure 5—possible unit for product of the metallocene dichlorides and GA3). Any release of the GA3 occurs through the breakdown or degradation of the polymer chain, releasing the GA3. This probably occurs through physical hydrolysis as the polymer comes into contact with water. The following is a brief description of some of our efforts with GA3.
A number of food-producing seeds were studied including soybeans, purple top turnips, Little Marvel peas, Jagger wheat, Florida Broad Leaf mustard, and Calabrese broccoli. These seeds were four years old and contained mold, acting as stand-ins for many seeds from so-called Third World countries.
Table 2 contains results for GA3 and polymer treatment for four of the seeds.
For Jagger wheat the germination percentages for all of the GA3-containing mixtures were larger than for the control. The best germination was for GA3/10 ppm, followed by the hafnocene treatments. For the Purple Top turnip seeds, the highest germination was for the hafnocene treatments. For the Florida Broad Leaf mustard seeds the highest germination was for the titanocene and zirconocene treatments. For the Little Marvel peas the highest treatments were for titanocene, GA3/1000, and hafnocene/GA3/10.
Related to the seed germination studies employing food seeds was an effort to increase the seed germination of sawgrass seeds. The single most prominent aspect of the Florida Everglades, and many wetlands around the world, is the presence of sawgrass. Sawgrass is not a grass but rather a sedge. It is responsible for trapping sediments and pollutants, holding rain water, decreasing the tendency for flooding, helping recharge groundwater supplies, filtering out toxins and other water pollutants, and helping provide water for some 10 million people in south Florida. The main competitor is cattail (Typa domingenesis), which is pushing out the sawgrass and reproducing through rhizomes and seeds. Cattail and weed seeds germinate rapidly and in high percentages compared to sawgrass seeds. It has been said by some naturalists that they had not seen a sawgrass seedling. Thus, the seed germination of sawgrass seeds is only about 1%. Furthermore, cattails produce more seeds per plant (sawgrass about 3000 seeds/plant and cattail about 300,000 seeds/plant).
In studies employing both GA3 and kinetin-containing polymers, we were able to increase the germination of wild, non-selected sawgrass seeds to above 60% through simple exposure of the sawgrass seeds to talc mixtures containing the PGH-containing polymers. This will allow the damaged part of the Everglades to be replenished (reseeded) through application of treated seeds from an airboat or airplane. In some cases, the applied polymeric PGH actually inhibited cattail growth while increasing sawgrass germination. Thus, in some cases polymeric materials are biased towards the germination of sawgrass while inhibiting cattail germination.
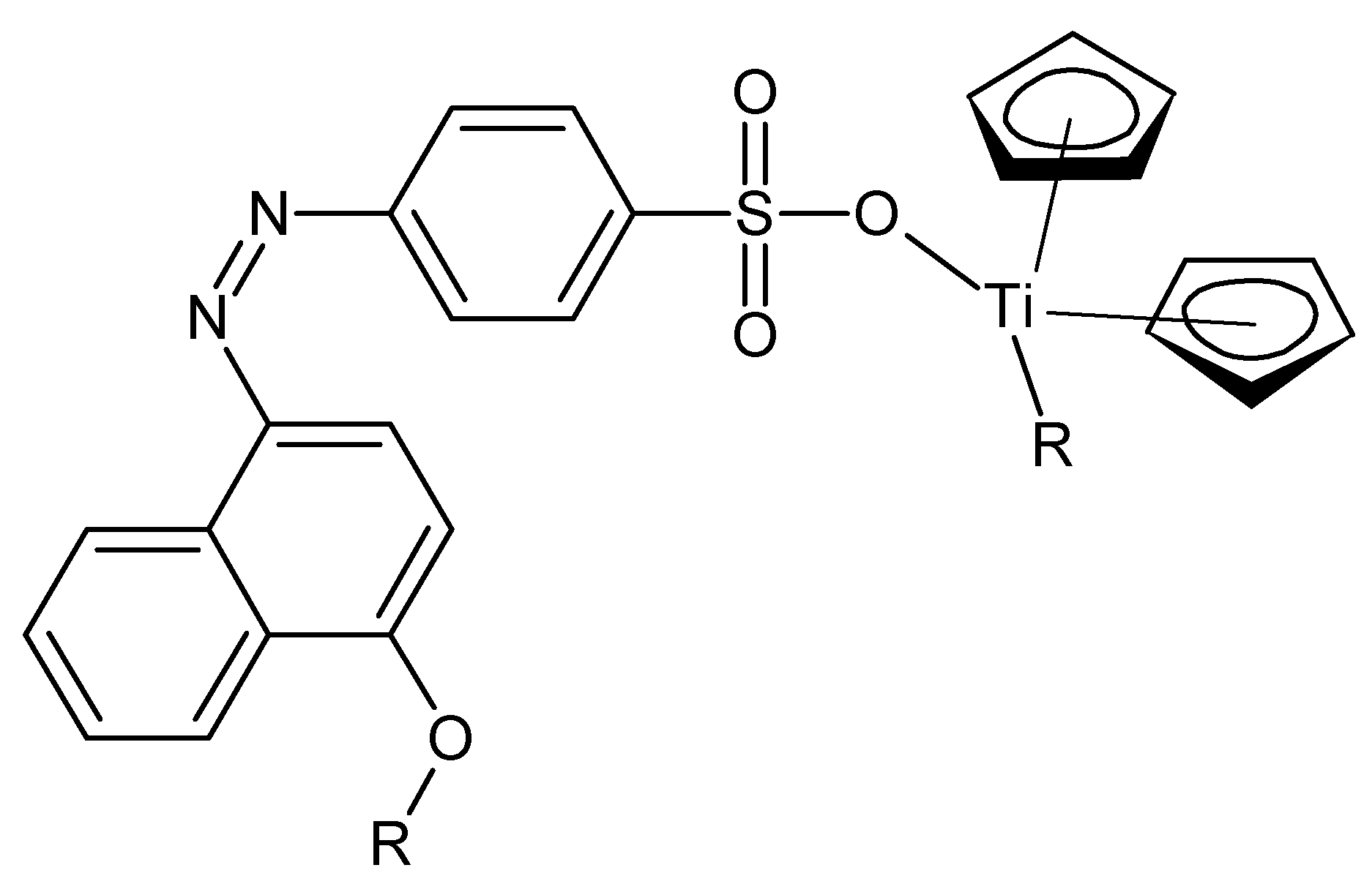
Auxin is a generic name for compounds with the ability to induce elongation in shoot cells [
6,
7,
9,
11]. The major commercially employed auxin is indole-3-butyric acid, IBA (
Figure 6, left). It is present in almost all commercial rooting mixtures. Formulations generally contain on the order of 100 ppm IBA. Commercially, it is mainly employed to promote and accelerate the rooting of plant cuttings.
Much of the recent research involving IBA has focused on the ability of IBA and IBA-containing polymers (
Figure 6, right) to promote the rooting and development of woody plants. Studies were carried out employing titanocene derivatives of IBA. In these studies the effect of the polymers containing IBA was studied employing
Hibiscus rosa-sinenesis, variety
Albo Lacinatus (Anderson’s crepe pink). Unlike seeds, which show great variation between species, the effect on one woody plant should more closely follow the effect on other woody plants.
Two indicators of plant propagation were monitored. These were leaf formation and root formation.
Table 3 contains the leaf formation after 10 and 20 days and root formation after 20 days for hibiscus stocks treated with various applications including monomers and polymers. They are listed in decreasing effectiveness of leaf formation.
After 20 days, the polymer treatments showed the greatest percentage of stocks with leaves, consistent with the incorporation of IBA into the polymer encouraging leave formation. Furthermore, even with no treatment, about 60% of the stock showed leaf formation, consistent with the vitality of the Albo Lacinatus as a rooting material.
Polymer treatments generally produced greater and more rapid rooting compared to IBA alone and the controls. Further, the average number of roots formed was greater for the polymer treatments in comparison to IBA itself, which in turn was greater than no treatment. The least rooting activity was found for the monomer titanocene dichloride. The number of roots for the polymers at concentrations of 0.02% to 1% was almost double that for IBA and three times that of the control and the metal-containing monomer. Thus, for this study, incorporation of the IBA into a polymer structure had an overall favorable effect on the rooting and production of side roots.
The next group of PGHs discussed here is the cytokinetins. While there are a number of natural and synthetic kinetins available, we worked with cytokinetin kinetin (6-furfurylaminopurine) itself (
Figure 7, left). It is the most widely studied cytokinetin and is naturally found in plants.
Here we briefly describe a study related to the ability of the associated polymer (
Figure 7, right) to influence plant growth, specifically seed germination. Results for seed germination using the same seeds we studied previously for GA3 were obtained. Results for three of the seeds appear in
Table 4 [
7,
8,
9].
For the kinetin-containing treatments, significant increases in germination were found for many of the treatments. The increases were modest for mustard, with the largest germination rates found for the higher concentrations (1000 ppm) for the metallocene-containing polymers, with averages being 32% (Cp2Ti), 34% (Cp2Zr), and 40% (Cp2Hf) in comparison to the control value of 23%. For Jagger Wheat, the highest yields were found for kinetin, 10 ppm itself (25%), and Cp2Ti/K, 1000 ppm (20%; compared to a control value of 9%). These increases represent a doubling in the percentage germination in comparison to the control. The largest differences were found for turnip seeds, where all of the treatments gave at least a doubling in the germination rate with several giving about a 6–8-fold increase. The highest increase was Cp2Zr/K, 1000 ppm (27%). There appeared to be little correlation between the treatment concentration and percentage germination.
In summary, incorporation of PGHs into metallocene-containing condensation polymers generally has a positive effect—increasing the germination of seeds, including food seeds, and the rooting of woody plants. Increased germination rates should allow older seeds a better chance at producing food crops. Increased rooting of woody plants should likewise allow greater success at propagating such plants.
7. Interaction with Laser Radiation
We have described the synthesis of a number of metallocene polymers that show an unusual ability to interact with laser radiation [
12]. Much of this involves the synthesis and testing of what we call polydyes. These are actually our usual condensation polymers, except with the Lewis base is derived from a dye. These dyes vary in family structure but include any dye that contains the usual Lewis base functional groups employed in our other studies. Typical dyes involved in the study included Phloxine B, Rose Bengel, Eosin Y, Mercurochrome, Orange I, Acid Red 88, and Crocein Orange G.
For our studies related to laser stability, a Sylvania, Model 971 CO
2 laser was used (1.06 nm). In one study ppm amounts of polymer are added to a paint, forming a film. The film is subjected to laser radiation. Compared to paint films not containing the polydye, the dye-containing films showed better stability, as shown by greater “burn-through” times and less damage to the film. In another set of studies, plastic plugs were made from polyacrylamide. As the plugs were drying, several drops of a dimethylsulfoxide (DMSO) solution containing polydye were added, representing a polydye concentration of 2 × 10
−6 g of polydye per gram of plastic. Again, the burn-through times increased. It is interesting that such a small amount of polydye can affect the ability to resist laser damage. It is believed that the polydye acts to scatter the laser radiation before major polymer damage occurs. The structure for the titanocene product from orange I is given in
Figure 8.
Polydye solutions have been used to impregnate a variety of paper products, adhesives, caulks, and paints. The polymeric nature makes them more fixed since the polymeric nature inhibits chain migration.
8. Treatment of Cancer
The following focuses on the use of metallocene polymers in the treatment of cancers. Our approach is to select Lewis bases that themselves have some biological activity, not necessarily anticancer, in hopes that the combination will have a synergetic effect. All of the Group 4 metallocene dichlorides exhibit anticancer activity [
18,
19,
20,
21,
22]. In fact, only two metal-containing molecules have undergone human cancer treatment. Cisplatin and related compounds [
23,
24] are the most widely used drugs to treat solid cancers. The second metal is titanium in the form of titanocene dichloride.
Titanocene dichloride, in preclinical studies, was found to inhibit the ovarian A2780 CP3 cancer cell line. This cell line was 20-fold resistant to cisplatin while the cell line was only about two and a half resistant to titanocene dichloride. This is consistent with an absence of cross-resistance between the two metal-containing drugs [
25]. This is consistent with in vivo studies where titanocene dichloride showed a much greater ability to inhibit cisplatin-resistant human ovarian cancer xenografts compared to cisplatin. Further, titanocene dichloride largely overcame cisplatin resistance for the A2780CP and CH1cisR ovarian cancer cell lines in bcl-2 and p53 transfectants of A2780 cells [
26]. Also, this difference indicates that cancer treatments may profit from the use of both the metallocene and platinum drugs since they act differently.
Studies are consistent with DNA–metallocene interactions, including titanocene dichloride, zirconocene dichloride, and hafnocene dichloride, being a major determinant in the anticancer activity of these materials [
27].
Phase I clinical trials were undertaken with titanocene dichloride. These studies were consistent with titanocene dichloride showing a dose-limiting side effect associated with nephrotoxicity with unwanted physical side effects including nausea, reversible metallic taste, pain during infusion, and hypoglycemia. Such side effects are unwanted and similar to those found for platinum-containing drugs. On the positive side, the absence of an effect on proliferative activity of the bone marrow, generally a dose-limiting side effect, was positive. Some phase II clinical trials were undertaken with patients with breast metastatic carcinoma [
28] and advanced renal cell carcinoma [
29]. Unfortunately, further clinical study was stopped because of the overall low activity observed.
We have studied about 250 different metallocene polymers and over 500 organotin polymers with respect to their ability to inhibit cancer growth. The polymer drugs are cytotoxic and cell death is by necrosis [
23,
30,
31,
32]. Anticancer activity occurs with the intact polymer without polymer degradation [
23,
30,
32]. This is consistent with the observation that polymers do not degrade in DMSO with half-chain lives, the time for the polymer chain length to halve, greater than 30 weeks [
23,
30,
31]. The tested compounds are initially dissolved in dimethylsulfoxide (DMSO) and then water added such that the DMSO content is generally 1% or less. Most organometallic compounds associate with polar solvents such as DMSO. This association can influence the observed biological results. We find that this is generally not the case [
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36] for polymers with a similar structure to those reported here with the influence less than 20% [
30,
32].
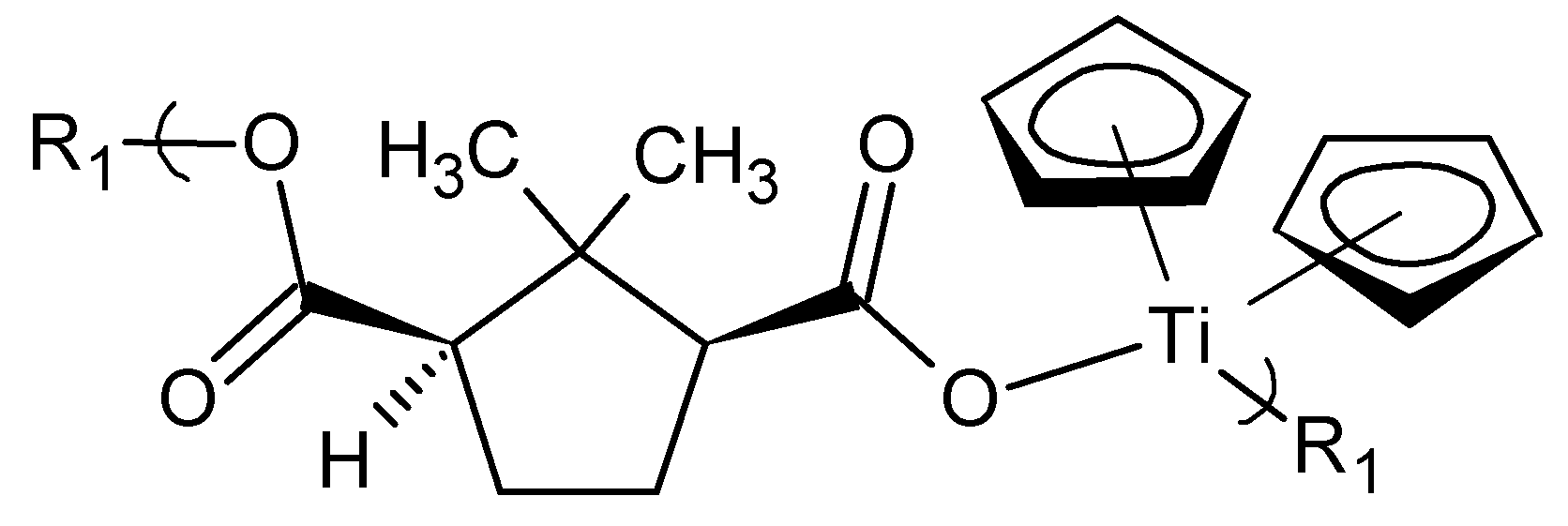
Here we will describe one group of polymers derived from the reaction of camphoric acid and the group 4 metallocene dichlorides (
Figure 9) [
24]. As in essentially all cases in our research, the reactants are commercially available, allowing for ready synthesis by others including in industry [
13,
24,
25,
26,
27,
28,
29]. The technique we employed is called the interfacial reaction or interfacial polycondensation process [
1,
2,
37,
38,
39]. In industry, this technique produces aramid fibers and polycarbonates so it is widely used and allows for ready production for the polymers from a microgram to industrially required amounts [
1,
2,
37,
38,
39]. Polymerization is rapid—generally less than 15 s. The reaction occurs in a one-quart Kimax emulsifying jar. The jar is fitted on top of a Waring Blender (model 1120; no load speed of about 18,000 rpm; reactions were carried out at about 25 °C).
A brief description of the human cell lines employed in the current study is given in
Table 5. They represent a broad range of solid cancer cell lines.
Two measures are usually employed for the evaluation of the effectiveness for test material to inhibit cell growth. The first measure is the amount of test material required to reduce the growth of a particular cell line. This concentration is often called the effective concentration (EC) and most often reported as EC50, which is the concentration of the test material inducing a response halfway between the baseline and maximum after a specified exposure time—called the 50% response concentration, EC50. WI-38 human normal embryonic lung cells are most widely used as the standard cell line in such testing.
Recently, a focus of much of our effort is finding drugs that exhibit good inhibition of pancreatic cancer since it does not have an accepted cure and is an important cancer with respect to the number of individuals affected by it. The set of tested cells includes two widely employed human pancreatic cell lines. These cell lines are AsPC-1, which is an adenocarcinoma pancreatic cell line, and PANC-1, which is an epithelioid carcinoma pancreatic cell line. Combined, they account for about 90% of pancreatic cancers. We also include a matched pair of breast cancer cell lines. The MDA-MB-231 (strain number 7233) cells are estrogen-independent and estrogen-receptor-negative, while the MCF-7 (strain line 7259) cells are estrogen receptor (ER)-positive.
Table 6 presents EC
50 values for the monomers and polymers. Values for cisplatin are also given. Cisplatin and related platinum drugs are among the anticancer drugs most widely employed for the treatment of solid cancers. Cisplatin is highly toxic, with many unwanted side effects. Many of the tested compounds have low toxicity towards the tested cell lines. Camphoric acid itself shows no ability to inhibit any of the cell lines at the concentration limits employed.
For the metallocene dichlorides, the EC50 values are generally a decade higher than for cisplatin for all of the cancer cell lines. By comparison, for the standard healthy cell line standard W38 the difference is much greater. The polymers show values that are between 100 and 1000 times less compared with cisplatin and the metallocene dichlorides themselves, with some having EC50 values in the nanogram/mL range. Since the Lewis base itself shows no inhibition towards any of the cells tested (at the concentration limits employed), it is the combination that is responsible for the activity.
There does not appear to be a difference between the metallocenes themselves and the polymers in terms of the ability to inhibit growth for the two pancreatic cancer cell lines as well as the cell lines of the other cancer-associated cells. For the pancreatic cancer cell lines, this may indicate that the materials will show good inhibition of other pancreatic cancer cell lines.
The second common measure of the potential use of compounds to inhibit cell line growth is the chemotherapeutic index, CI. The CI50 is then the ratio of the EC50 for the standard WI-38 cells divided by the EC50 for the particular compound.
The CI
50 values for the monomers and polymers are given in
Table 7. For comparison, values for cisplatin are also given [
31].
Significance is considered to be when CI50 values of 2 and greater are found. Thus, high CI50 are preferred, consistent with the test compound preferentially inhibiting the growth of the cancer cells compared with the standard healthy cells. For the present case, with the exception of the pancreatic cancer cell lines, the CI50 values for cisplatin are higher than for the metallocene dichlorides. However, the CI50 values for the polymers are larger compared with the metallocene dichlorides and cisplatin, with the difference in some cases being over 1000-fold.
Another important general trend involves the EC50 and CI50 values and the nature of the metals for the polymers. In the current study, and in most of our studies, the EC50 values follow the order Ti > Zr > Hf and consequently the CI50 values follow the order Hf > Zr > Ti. The Zr and Hf values are often similar, while the values for Ti are considerably less favorable. Thus, while titanocene dichloride was chosen for clinical studies, from our systems it would be the Zr- and Hf-associated monomers and polymers that should be chosen for study.
9. Conclusions
As noted in the introduction, the presence of metal-containing moieties allows the polymers to exhibit behavior not possible without the presence, in this case, of the group 4 metallocenes. Thus, doping of the polymer, where the Lewis base is 2-nitro-1,4-phenhylenediamine containing the group 4 metallocene, allows an increase in electrical conductivity to 105–106. Fibers are formed from a variety of group 4 metallocene products. Polymers containing plant growth hormones allow for increased seed germination and formation of roots for woody plants. Finally, a wide variety of metallocene-containing polymers show good inhibition of a variety of solid tumor cancers.