The Melt of Sodium Nitrate as a Medium for the Synthesis of Fluorides
Abstract
:1. Introduction
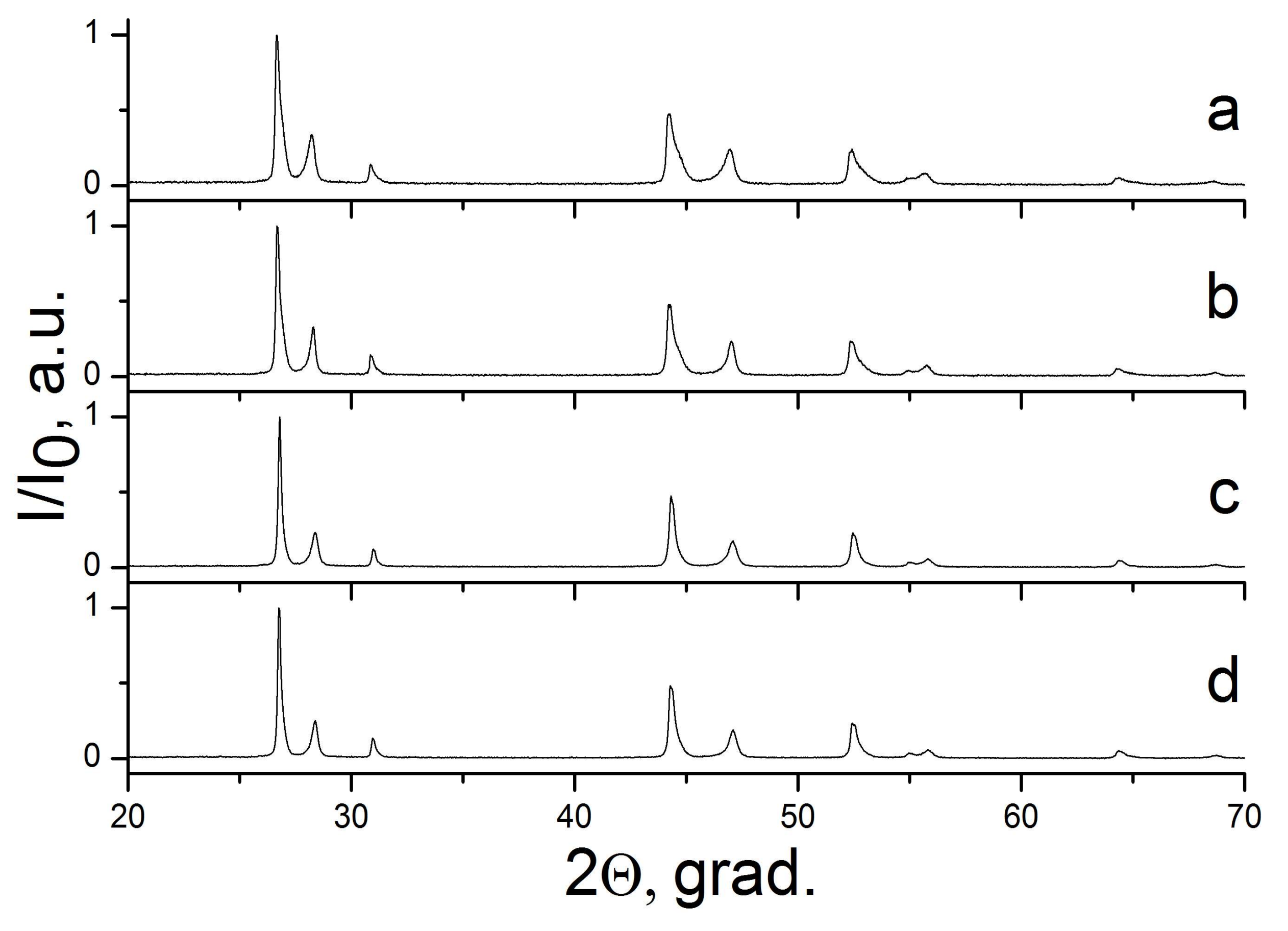
2. Results
2.1. The NaF–YF3 System
2.2. The NaF–LaF3 and NaF–CeF3 Systems
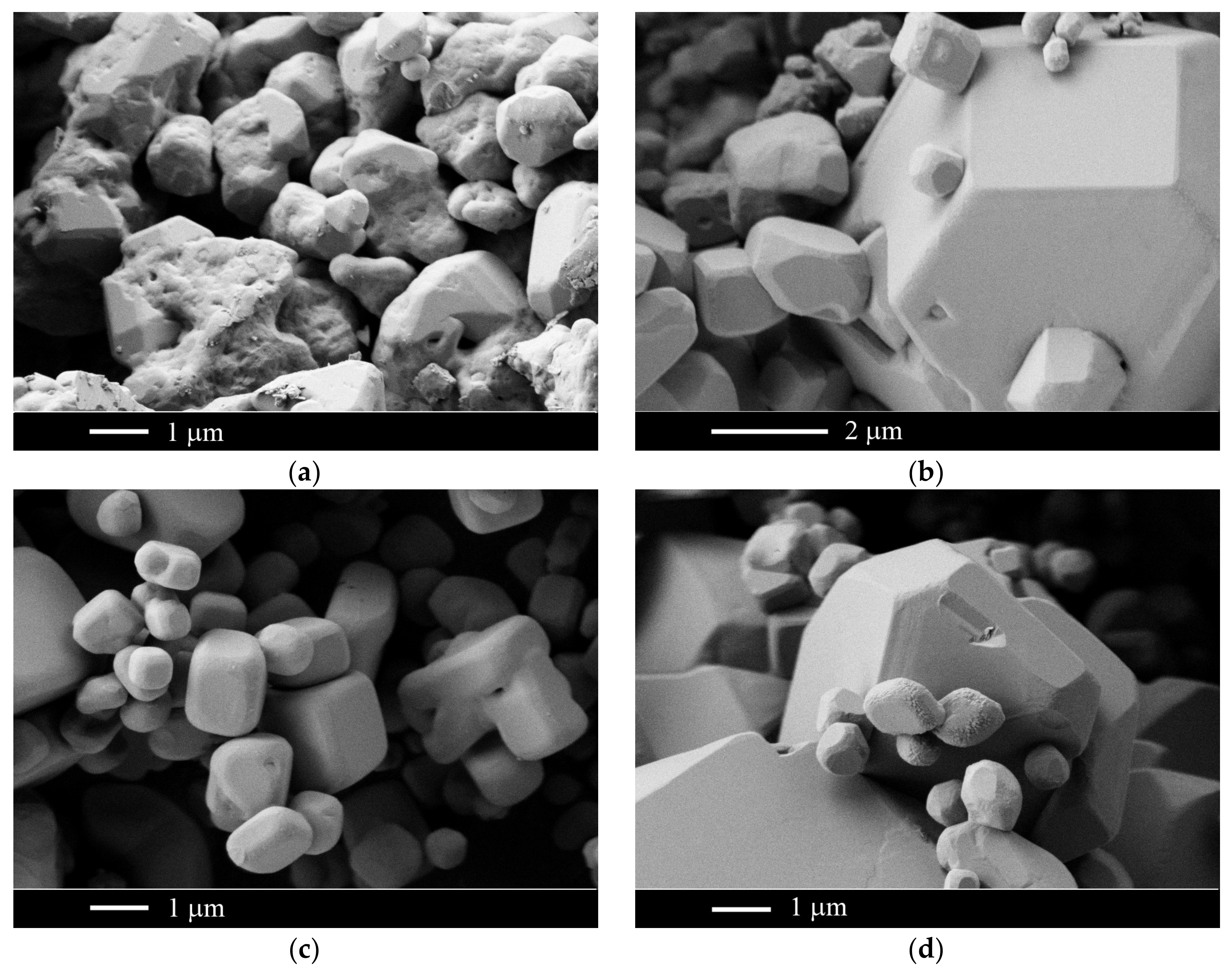
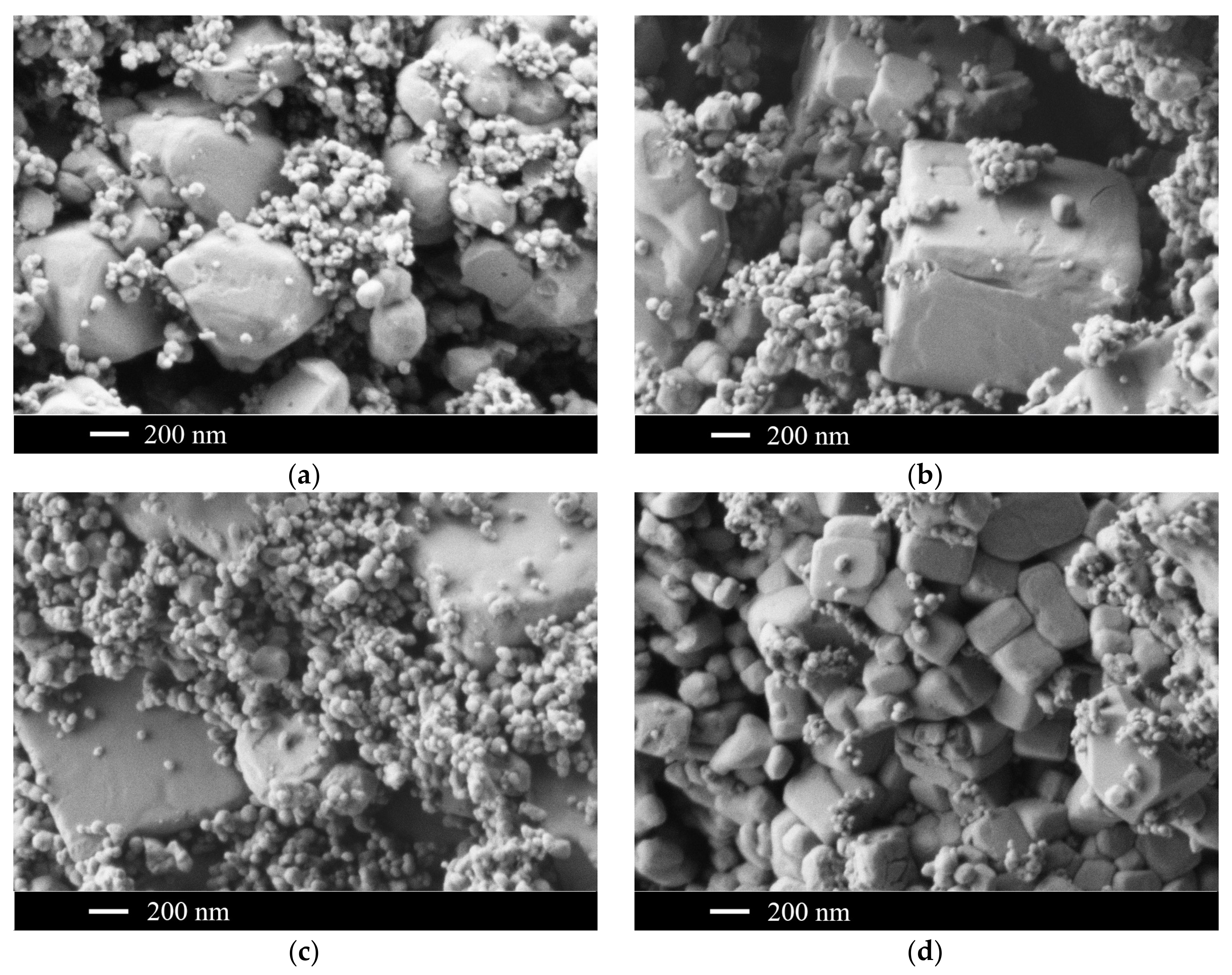
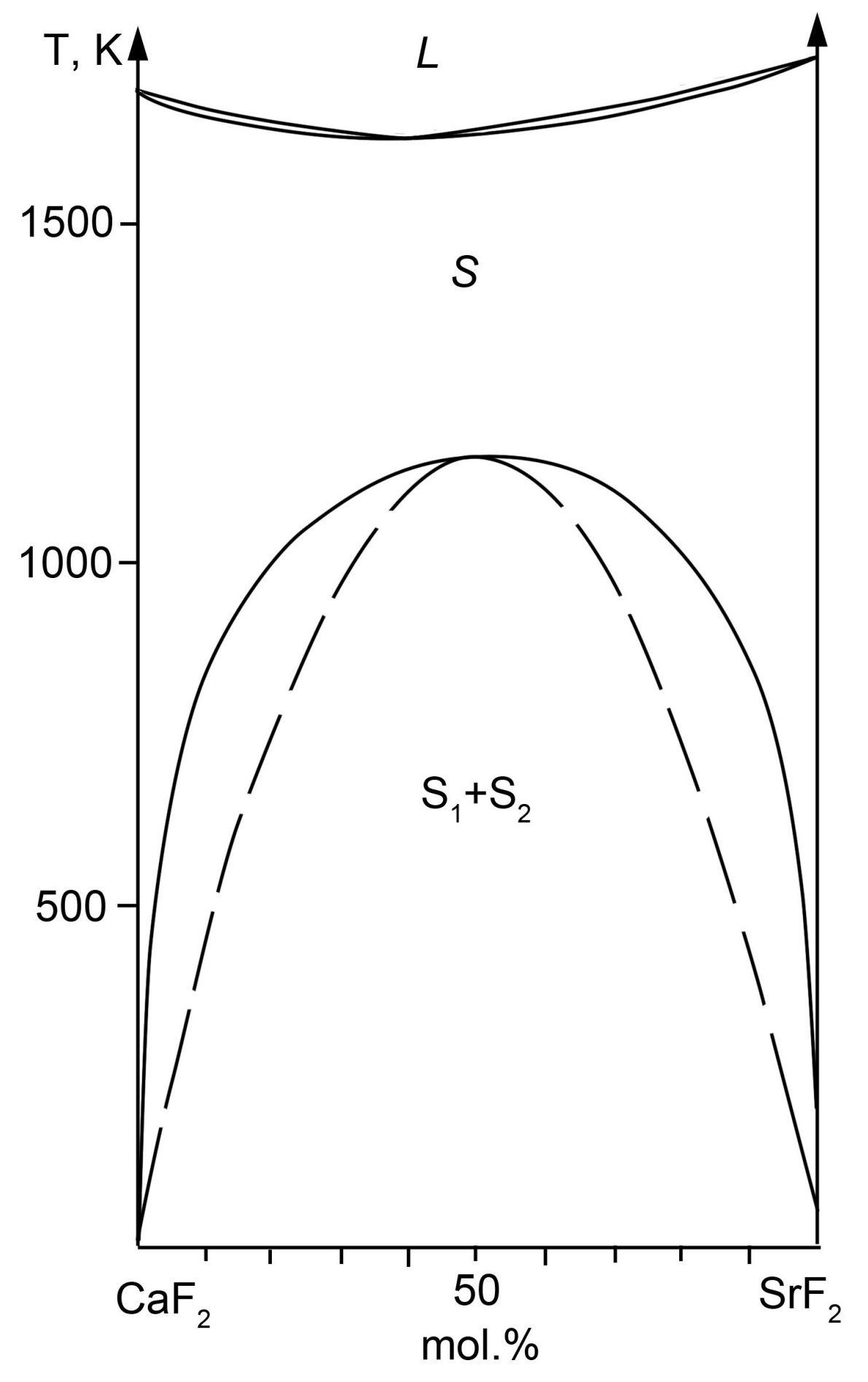
2.3. The CaF2–SrF2 System
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Paper Sample Number | Laboratory Sample Number |
|---|---|
| 1 | F1065 |
| 2 | F1068 |
| 3 | F1064а |
| 4 | F1064б |
| 5 | F1711 |
| 6 | F1084 |
| 7 | F1731 |
| 8 | F1804 |
| 9 | F1812 |
| 10 | F1798 |
| 11 | F1802 |
| 12 | F1805 |
| 13 | F1811 |
| 14 | F1799 |
| 15 | F1797 |
| 16 | F1803 |
| 17 | F1807 |
| 18 | F1808 |
| 19 | F1809 |
| 20 | F1810 |
| Paper Sample Number | Mass Theor., g | Mass Exp., g | Yield, % |
|---|---|---|---|
| 8 | 5 | 4.35 | 87.0 |
| 9 | 5 | 4.59 | 91.8 |
| 10 | 5 | 3.90 | 78.0 |
| 12 | 5 | 4.32 | 86.4 |
| 13 | 5 | 3.77 | 75.4 |
| 14 | 5 | 2.99 | 59.8 |
| 15 | 5 | 4.18 | 83.6 |
| 17 | 5 | 4.34 | 86.8 |
| 18 | 5 | 4.55 | 91.0 |
| 19 | 5 | 3.98 | 79.6 |
| 20 | 5 | 3.84 | 76.8 |
References
- Bergmann, H. Sc, Y, La and lanthanide: Fluoride, oxifluoride und zugehogige alkalidoppelverbindungen. In Gmelin Handbuch der Anorganischen Chemie; Springer: Berlin, Germany, 1976; ISBN 978-3-540-93321-2. [Google Scholar]
- Sobolev, B.P. The Rare Earth Trifluorides: The High Temperature Chemistry of the Rare Earth Trifluorides; Institut d’Estudis Catalans: Barcelona, Spain, 2000; ISBN 84-7283-518-9. [Google Scholar]
- Kuznetzov, S.V.; Osiko, V.V.; Tkatchenko, E.A.; Fedorov, P.P. Inorganic nanofluorides and related nanocomposites. Russ. Chem. Rev. 2006, 7, 1065–1082. [Google Scholar] [CrossRef]
- Fedorov, Р.P.; Luginina, A.A.; Kuznetsov, S.V.; Osiko, V.V. Nanofluorides. J. Fluor. Chem. 2011, 132, 1012–1039. [Google Scholar] [CrossRef]
- Stosiek, C.; Scholz, G.; Schroeder, S.L.M.; Kemnitz, E. Structure and properties of noncrystalline aluminum oxide-hydroxide fluorides. Chem. Mater. 2010, 22, 2347–2356. [Google Scholar] [CrossRef]
- Wilkening, M.; Duvel, A.; Preishuber-Pflugl, F.; Da Silva, K.; Breuer, S.; Sepelak, V.; Heitjans, P. Structure and ion dynamics of mechanosynthesized oxides and fluorides. Z. Kristallog. 2016, 232, 107–127. [Google Scholar] [CrossRef]
- Warf, J.C.; Cline, W.D.; Tevebaugh, R.D. Pyrohydrolysis in the determination of fluoride and other halids. Anal. Chem. 1954, 26, 342–346. [Google Scholar] [CrossRef]
- Banks, C.V.; Burke, K.E.; O’Laughlin, J.W. The determination of fluorine in rare earth fluorides by high temperature hydrolysis. Anal. Chim. Acta 1958, 19, 239–243. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Fedorov, P.P.; Steynberg, D.B.; Sinitsyn, B.V.; Shakhkalanian, G.S. On the problem of polymorphism and fusion of lanthanide trifluorides. I. Influence of oxygen on phase transition temperatures. J. Solid State Chem. 1976, 17, 191–199. [Google Scholar] [CrossRef]
- Bamberger, C.E. Experimental Techniques in Molten Fluoride Chemistry. In Advances in Molt Salt Chemistry; Braunstein, J., Mamantov, G., Smith, G.P., Eds.; Plenum Press: New York, NY, USA, 1975; Volume 3, pp. 177–248. [Google Scholar]
- Fedorov, P.P.; Osiko, V.V. Crystal growth of fluorides. In Bulk Crystal Growth of Electronic, Optical and Optoelectronic Materials; Capper, P., Ed.; John Wiley & Son, Ltd.: Chichester, UK, 2005; pp. 339–356. [Google Scholar]
- Sobolev, B.P.; Fedorov, P.P. Phase diagramms of the CaF2–(Y,Ln)F3 systems. I. Experimental. J. Less Common Met. 1978, 60, 33–46. [Google Scholar] [CrossRef]
- Fedorov, P.P. Anneal time determined by studying phase transitions in solid binary systems. Russ. J. Inorg. Chem. 1992, 37, 973–975. [Google Scholar]
- Kuznetsov, S.V.; Fedorov, P.P. Morphological stability of solid–liquid interface during melt crystallization of solid solutions M1−xRxF2+x. Inorg. Mater. 2008, 44, 1434–1458. [Google Scholar] [CrossRef]
- Druon, F.; Ricaud, S.; Papadopoulos, D.N.; Pellegrina, A.; Camy, P.; Doualan, J.L.; Moncorge, R.; Courjaud, A.; Mattay, E.; Georges, P. On Yb:CaF2 and Yb:SrF2: Review of spectroscopic and thermal properties and their impact on femtosecond and high power laser performance. Opt. Mater. Express. 2011, 1, 489–502. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Butashin, A.V.; Sul’yanov, S.N. Crystallization and some spectroscopic properties of CsBi2F7–Nd3+. Neorg. Mater. 1996, 32, 110–112. (In Russian) [Google Scholar]
- Matar, S.; Reau, J.-M.; Grannec, J.; Rabardel, L. On a low-temperature form of KBiF4. J. Solid State Chem. 1983, 50, 1–6. [Google Scholar] [CrossRef]
- Dombrovski, E.N.; Serov, T.V.; Abakumov, A.M.; Ardashnikova, E.I.; Dolgikh, V.A.; Van Tendeloo, G. The structural investigation of Ba4Bi3F17. J. Solid State Chem. 2004, 177, 312–318. [Google Scholar] [CrossRef]
- Ratnikova, I.D.; Korenev, Y.M.; Fedorov, P.P.; Sobolev, B.P. Phase diagrams of the systems BaF2–RF4 (R = Zr, Hf). Zh. Neorgan. Khimii 1997, 42, 302–307. (In Russian) [Google Scholar]
- Popov, A.I.; Scharabarin, A.V.; Sukhoverkhov, V.F.; Tchumaevsky, N.A. Synthesis and properties of pentavalent antimony and bismuth fluorides. Z. Anorg. Allgem. Chem. 1989, 576, 242–254. [Google Scholar] [CrossRef]
- Popov, A.I.; Valkovskii, M.D.; Kiselev, Y.M. Structure of MII(AuVF6)2 of alkali-earth elements. Zh. Neorgan. Khimii 1990, 35, 1970–1977. (In Russian) [Google Scholar]
- Buchinskaya, I.I.; Fedorov, P.P. Lead difluoride and related systems. Rus. Chem. Rev. 2004, 73, 371–400. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Zibrov, I.P.; Tarasova, E.V.; Polunina, S.M.; Sobolev, B.P.; Fedorov, P.I. Transition from peritectics to eutectics in PbF2–RF3 systems. Zh. Neorgan. Khimii 1988, 33, 3222–3225. (In Russian) [Google Scholar]
- Fedorov, P.P. Third law of thermodynamics as applied to phase diagrams. Rus. J. Inorg. Chem. 2010, 55, 1722–1739. [Google Scholar] [CrossRef]
- Heise, M.; Scholz, G.; Düvel, A.; Heitjans, P.; Kemnitz, E. Mechanochemical synthesis, structure and properties of lead containing alkaline earth metal fluoride solid solutions MxPb1−xF2 (M = Ca, Sr, Ba). Solid State Sci. 2018, 77, 45–53. [Google Scholar] [CrossRef]
- Ritter, B.; Krahl, T.; Rurack, K.; Kemnitz, E. Nanoscale CaF2 doped with Eu3+ and Tb3+ through fluorolytic sol–gel Synthesis. J. Mater. Chem. C 2014, 2, 8607–8613. [Google Scholar] [CrossRef]
- Glazunova, T.Y.; Boltalin, A.I.; Fedorov, P.P. Synthesis of calcium, strontium, and barium fluorides by thermal decomposition of trifluoroacetates. Russ. J. Inorg. Chem. 2006, 51, 983–987. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Kuznetsov, S.V.; Mayakova, M.N.; Voronov, V.V.; Ermakov, R.P.; Baranchikov, A.E.; Osiko, V.V. Coprecipitation from aqueous solutions to prepare binary fluorides. Russ. J. Inorg. Chem. 2011, 56, 1525–1531. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Mayakova, M.N.; Kuznetsov, S.V.; Voronov, V.V.; Osiko, V.V.; Ermakov, R.P.; Gontar’, I.V.; Timofeev, A.A.; Iskhakova, L.D. Coprecipitation of barium–bismuth fluorides from aqueous solutions: Nanochemical effects. Nanotechnol. Russ. 2011, 6, 203–210. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Mayakova, M.N.; Kuznetsov, S.V.; Voronov, V.V.; Ermakov, R.P.; Samarina, K.S.; Popov, A.I.; Osiko, V.V. Co-precipitation of yttrium and barium fluorides from aqueous solutions. Mater. Res. Bull. 2012, 47, 1794–1799. [Google Scholar] [CrossRef]
- Mayakova, M.N.; Luginina, A.A.; Kuznetsov, S.V.; Voronov, V.V.; Ermakov, R.P.; Baranchikov, A.E.; Ivanov, V.K.; Karban, O.V.; Fedorov, P.P. Synthesis of SrF2–YF3 nanopowders by co-precipitation from aqueous solutions. Mendeleev Commun. 2014, 24, 360–362. [Google Scholar] [CrossRef]
- Kuznetsov, S.V.; Ovsyannikova, A.A.; Tupitsyna, E.A.; Yasyrkina, D.S.; Voronov, V.V.; Fedorov, P.P.; Batyrev, N.I.; Iskhakova, L.D.; Osiko, V.V. Phase formation in LaF3-NaGdF4, NaGdF4-NaLuF4, NaYF4-NaLuF4 systems: Synthesis of powders by co-precipitation from aqueous solutions. J. Fluor. Chem. 2014, 161, 95–101. [Google Scholar] [CrossRef]
- Mayakova, M.N.; Voronov, V.V.; Iskhakova, L.D.; Kuznetsov, S.V.; Fedorov, P.P. Low-temperature phase formation in the BаF2-CeF3 system. J. Fluor. Chem. 2016, 187, 33–39. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Kuznetsov, S.V.; Osiko, V.V. Elaboration of nanofluorides and ceramics for optical and laser applications. In Photonic and Electronic Properties of Fluoride Materials; Tressaud, A., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 7–31. ISBN 9780128016398. [Google Scholar]
- Fedorov, P.P.; Luginina, A.A.; Ermakova, Y.А.; Kuznetsov, S.V.; Voronov, V.V.; Uvarov, O.V.; Pynenkov, A.A.; Nischev, K.N. Preparation of nanodispersed fluorite-type Sr1−xRxF2+x (R = Er, Yb, Ho) phases from citrate solutions. J. Fluor. Chem. 2017, 194, 8–15. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Mayakova, M.N.; Kuznetsov, S.V.; Voronov, V.V.; Ermakova, Y.A.; Baranchikov, A.E. Synthesis of СаF2–YF3 nanopowders by co-precipitation from aqueos solutions. Nanosystems 2017, 8, 462–470. [Google Scholar] [CrossRef]
- Luginina, A.A.; Baranchikov, A.E.; Popov, A.I.; Fedorov, P.P. Preparation of barium monohydrofluoride BaF2·HF from nitrate aqueous solutions. Mater. Res. Bull. 2014, 49, 199–205. [Google Scholar] [CrossRef]
- Rozhnova, Y.A.; Luginina, A.A.; Voronov, V.V.; Ermakov, R.P.; Kuznetsov, S.V.; Ryabova, A.V.; Pominova, D.V.; Arbenina, V.V.; Osiko, V.V.; Fedorov, P.P. White light luminophores based on Yb3+/Er3+/Tm3+-Coactivated strontium Fluoride Powders. Mater. Chem. Phys. 2014, 148, 201–207. [Google Scholar] [CrossRef]
- Rozhnova, Y.A.; Kuznetsov, S.V.; Luginina, A.A.; Voronov, V.V.; Ryabova, A.V.; Pominova, D.V.; Ermakov, R.P.; Usachev, V.A.; Kononenko, N.E.; Baranchikov, A.E.; et al. New Sr1−x−yRx(NH4)yF2+x−y (R = Yb, Er) solid solution as precursor for high efficiency up-conversion luminophor and optical ceramics on the base of strontium fluoride. Mater. Chem. Phys. 2016, 172, 150–157. [Google Scholar] [CrossRef]
- Ivanov, V.K.; Fedorov, P.P.; Baranchikov, A.Y.; Osiko, V.V. Oriented aggregation of particles: 100 years of investigations of non-classical crystal growth. Russ. Chem. Rev. 2014, 83, 1204–1222. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Kuznetsov, S.V.; Voronov, V.V.; Yarotskaya, I.V.; Arbenina, V.V. Soft сhemical synthesis of NaYF4 nanopowders. Russ. J. Inorg. Chem. 2008, 53, 1681–1685. [Google Scholar] [CrossRef]
- Lucier, B.E.G.; Johnston, K.E.; Arnold, D.C.; Lemyre, J.-L.; Beaupre, A.; Blanchette, M.; Ritcey, A.M.; Schurko, R.W. Comprehensive solid-state characterization of rare earth fluoride nanoparticles. J. Phys. Chem. C 2014, 118, 1213–1228. [Google Scholar] [CrossRef]
- Khaidukov, N.M.; Fedorov, P.P.; Dem’yanrts, L.D.; Zibrov, I.P.; Malyusov, V.A. K2LnF5 compounds. Russ. J. Inorg. Chem. 1990, 35, 383–384. (In Russian) [Google Scholar]
- Khaidukov, N.M.; Fedorov, P.P.; Abramov, N.A. Thermal and crystallographic charasteristics of KLnF4 synthesized under hydrothermal conditions. Inorg. Mater. 1991, 27, 2243–2246. (In Russian) [Google Scholar]
- Khaidukov, N.M.; Filatova, T.G.; Ikrami, M.B.; Fedorov, P.P. Morphotropy in lanthanide fluoride series. Inorg. Mater. 1993, 29, 1152–1156. (In Russian) [Google Scholar]
- Fedorov, P.P. Systems of alcali and rare-earth metal fluorides. Russ. J. Inorg. Chem. 1999, 44, 1703–1727. [Google Scholar]
- Cao, J.; Yuan, L.; Hu, S.; Tang, J.; Zhou, X.; Yang, J. Tuning the phase, morphology and size of monodisperse ScF3 and NaScF4 crystals through lanthanide doping. CrystEngComm 2016. [Google Scholar] [CrossRef]
- Kostiv, U.; Rajsiglov, L.; Luptáková, D.; Pluháček, T.; Vannucci, L.; Havliček, V.; Engstová, H.; Jirák, D.; Šlouf, M.; Makovicky, P.; et al. Biodistribution of upconversion/magnetic silicacoated NaGdF4:Yb3+/Er3+ nanoparticles in mouse models. RSC Adv. 2017, 7, 45997–46006. [Google Scholar] [CrossRef]
- Hyppänen, I.; Perälä, N.; Arppe, R.; Schäferling, M.; Soukka, T. Environmental and excitation power effects on the ratiometric upconversion luminescence based temperature sensing using nanocrystalline NaYF4:Yb3+,Er3+. ChemPhysChem 2017, 18, 692–701. [Google Scholar] [CrossRef]
- Timofeeva, V.A. Physical-Chemical and Metodical Bases of Melt-Solution Design of New Technical Crystals; VINITI: Moscow, Russia, 1990; p. 498. (in Russian) [Google Scholar]
- Pavlova, L.N.; Fedorov, P.P.; Ol’khovaya, L.A.; Ikrami, D.D.; Sobolev, B.P. Ordering of heterovalent solid solution with the fluorite structure in the NaF–BaF2–GdF3 system. Crystallogr. Rep. 1993, 38, 221–224. [Google Scholar]
- Garton, G.; Wanklyn, B.M. Growth of complex fluoride single crystals by the flux method. J. Cryst. Growth 1967, 1, 49–51. [Google Scholar] [CrossRef]
- Hoppe, R. Novel routs to the synthesis of metal fluorides. J. Fluor. Chem. 1985, 29, 38. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zhang, J.; Ji, Y.; Jiang, N.; Ma, B.; Wang, X.; Liu, L. A facile way to synthesis KMgF3 and its luminescent property with Eu doping. Inorg. Chem. Commun. 2013, 33, 165–169. [Google Scholar] [CrossRef]
- Courbion, G.; Randrianohavy, J.V.; Rousseau, J.J. ESR Study of Cr3+ and Fe3+ Ions in KGaF4 Single Crystals. J. Solid State Chem. 1989, 81, 285–292. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Mayakova, M.N.; Kuznetsov, S.V.; Maslov, V.А.; Sorokin, N.I.; Baranchikov, А.Е.; Ivanov, V.K.; Pynenkov, A.A.; Uslamina, М.А.; Nishchev, К.N. Phase diagram of the NaF–CaF2 system and the electrical conductivity of a CaF2-based solid solution. Russ. J. Inorg. Chem. 2016, 61, 1472–1478. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Mayakova, M.N.; Maslov, V.А.; Baranchikov, А.Е.; Ivanov, V.K.; Pynenkov, А.А.; Uslamina, М.А.; Nishchev, К.N. The solubility of sodium and potassium fluorides in the strontium fluoride. Nanosystems 2017, 8, 830–834. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Luginina, A.A.; Popov, A.I. Transparent oxyfluoride glass ceramics. J. Fluor. Chem. 2015, 172, 22–50. [Google Scholar] [CrossRef]
- Morris, E.; Groy, T.; Leinenweber, K. Crystal structure and bonding in the high-temperature form of fluorite (CaF2). J. Phys. Chem. Solids 2001, 62, 1117–1122. [Google Scholar] [CrossRef]
- Batsanova, L.R. Rare-earth fluorides. Russ. Chem. Rev. 1971, 40, 465–484. [Google Scholar] [CrossRef]
- Batsanova, L.R.; Kupriyanova, А.К.; Doroshenko, V.I. Study of the interaction of the rare-earth nitrates with sodium fluorides in molten NaNO3. Inorg. Mater. 1971, 7, 1876–1877. (In Russian) [Google Scholar]
- Ding, M.; Lu, C.; Cao, L.; Huang, W.; Ni, Y.; Xu, Z. Molten salt synthesis of tetragonal LiYF4:Yb3+/Ln3+ (Ln = Er, Tm, Ho) microcrystals with multicolor upconversion luminescence. CrystEngComm 2013, 15, 6015–6021. [Google Scholar] [CrossRef]
- Huang, X.Y.; Hu, G.H.; Xu, Q.J.; Li, X.X.; Yu, Q.M. Molten-salt synthesis and upconversion of hexagonal NaYF4:Er3+:Yb3+ micro-/nano-crystals. J. Alloys Compd. 2014, 616, 652–661. [Google Scholar] [CrossRef]
- Huang, X.Y. Synthesis in NH4NO3 flux and abnormal upconversion of LiYF4:Er3+ /Yb3+ microcrystals. Opt. Mater. Exp. 2014, 4, 2381–2391. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, L.; Xu, Q.; Li, X.; He, A. Low-temperature molten-salt synthesis and upconversion of novel hexagonal NaBiF4:Er3++/Yb3+ micro-/nanocrystals. RSC Adv. 2017, 7, 41190–41203. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, L.; Li, X.; He, A. Manipulating upconversion emission of cubic BaGdF5:Ce3+/Er3+/Yb3+ nanocrystals through controlling Ce3+ doping. J. Alloys Compd. 2017, 721, 374–382. [Google Scholar] [CrossRef]
- Sadovskiy, A.; Sukhanova, E.; Belov, S.; Kostikov, V.; Zykova, M.; Artyushenko, M.; Zharikov, E.; Avetissov, I. Axial vibration control of melt structure of sodium nitrate in crystal growth process. J. Cryst. Growth 2015, 417, 16–24. [Google Scholar] [CrossRef]
- Nyankovskaya, R.N.; Bergman, A.G. Singular irreversible-resiprocal system from nitrates and fluorides of sodium and potassium. Izv. Sektora Phys. Khim Anal. 1952, 21, 250–258. (In Russian) [Google Scholar]
- Kruglov, A.I.; Kochergin, V.P. Onset of thermal decomposition of mixtures of sodium and potassium nitrates with its halogenides. Izv. VUZov Khimiya Khim Tekhnol. 1971, 14, 1429–1430. (In Russian) [Google Scholar]
- Voskresenskaya, N.К.; Evseeva, N.N.; Berul, S.I.; Vereschetina, I.P. Handbook on the Melting of Salt Systems; Izd. Akad. Nauk: Moscow, Russia, 1961; p. 848. (In Russian) [Google Scholar]
- Greis, O.; Bahamdam, K.M.; Uwais, B.M. The phase diagram of the system NaNO3–KNO3 studied by differential scanning calorimetry. Thermochim. Acta 1985, 86, 343–350. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Ushakov, S.N.; Uslamina, M.A.; Chernova, E.V.; Kuznetsov, S.V.; Voronov, V.V.; Düvel, A.; Heitjans, P.; Pynenkov, A.A.; Nishchev, K.N.; et al. Morphological stability of the solid‒liquid interface during melt crystallization of Ca1–xSrxF2 solid solution. Crystallogr. Rep. 2018, 63, 512–516. [Google Scholar]
- Heise, M.; Scholz, G.; Düvel, A.; Heitjans, P.; Kemnitz, E. Mechanochemical synthesis, structure, and properties of solid solutions of alkaline earth metal fluorides: Ma1−xMbxF2 (M: Ca, Sr, Ba). Solid State Sci. 2016, 60, 65–74. [Google Scholar] [CrossRef]
- Ol’khovaya, L.A.; Karpenko, G.A.; Ikrami, D.D.; Fedorov, P.P. The CaF2–SrF2–MnF2 ternary system. Russ. J. Inorg. Chem. 1991, 36, 1639–1642. [Google Scholar]
- Fedorov, P.P.; Izotova, O.E.; Alexandrov, V.B.; Sobolev, B.P. New phases with fluorite-derived structure in CaF2–(Y, Ln)F3 systems. J. Solid State Chem. 1974, 9, 368–374. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Sobolev, B.P.; Belov, S.F. Fusibility diagram of the system NaF–YF3, and the cross-section Na0.4Y0.6F2.2–YOF. Inorg. Mater. 1979, 15, 640–643. [Google Scholar]
- Galwey, A.K.; Brown, M.E. Thermal Decomposition of Ionic Solids; Elsevier Science: Amsterdam, The Netherlands, 1999; ISBN 0-444-82437-5. [Google Scholar]
- Zhang, C.; Chen, J. Facile EG/ionic liquid interfacial synthesis of uniform RE3+ doped NaYF4 nanocubes. Chem. Commun. 2010, 46, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, T.; Qiao, Y.M.; Zhao, L.H.; Li, Z.Q. Ionic liquid-based approach to monodisperse luminescent LaF3:Ce, Tb nanodiskettes: Synthesis, structural and photoluminescent properties. J. Nanosci. Nanotechnol. 2010, 10, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Deng, Y.; Chen, J.; Zou, H. Ionic liquid-assisted hydrothermal synthesis of rare earth luminescence materials. In Application of Ionic Liquids on Rare Earth Green Separation and Utilization, Green Chemistry and Sustainable Technology; Chen, J., Ed.; Springer: Heidelberg/Berlin, Germany, 2016; pp. 207–257. [Google Scholar]
- Liu, S.; Hui, Y.; Zhu, L.; Fan, X.; Zou, B.; Cao, X. Synthesis and luminescence properties of CeF3:Tb3+ nanodisks via ultrasound assisted ionic liquid method. J. Rare Earths 2014, 32, 508–513. [Google Scholar] [CrossRef]
- Bartůněk, V.; Pinc, J.; Ulbrich, P.; Rak, J.; Pelánková, B.; Král, V.; Kuchař, M.; Ježek, P.; Engstová, H.; Smolková, K. Tunable rapid microwave synthesis of up-converting hexagonal NaYxGdyYbzEr(1−x−y−z)F4 nanocrystals in large quantity. J. Fluor. Chem. 2015, 178, 56–60. [Google Scholar] [CrossRef]
- Mun, J.; Sim, H. (Eds.) Handbook of Ionic Liquids: Properties, Applications and Hazards; NOVA Science Publishers, Inc.: New York, NY, USA, 2012; ISBN 978-1-62100-349-6. [Google Scholar]
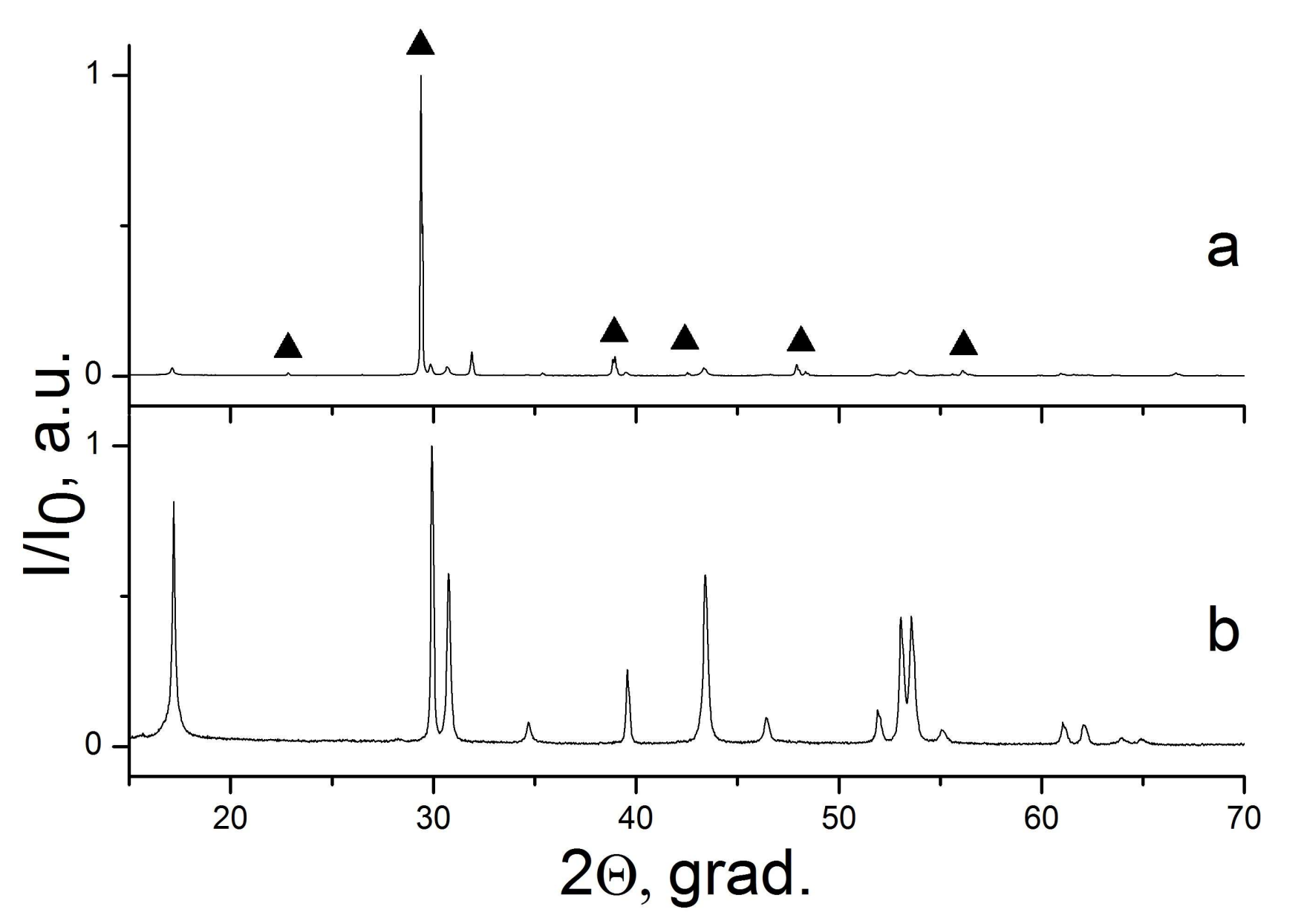
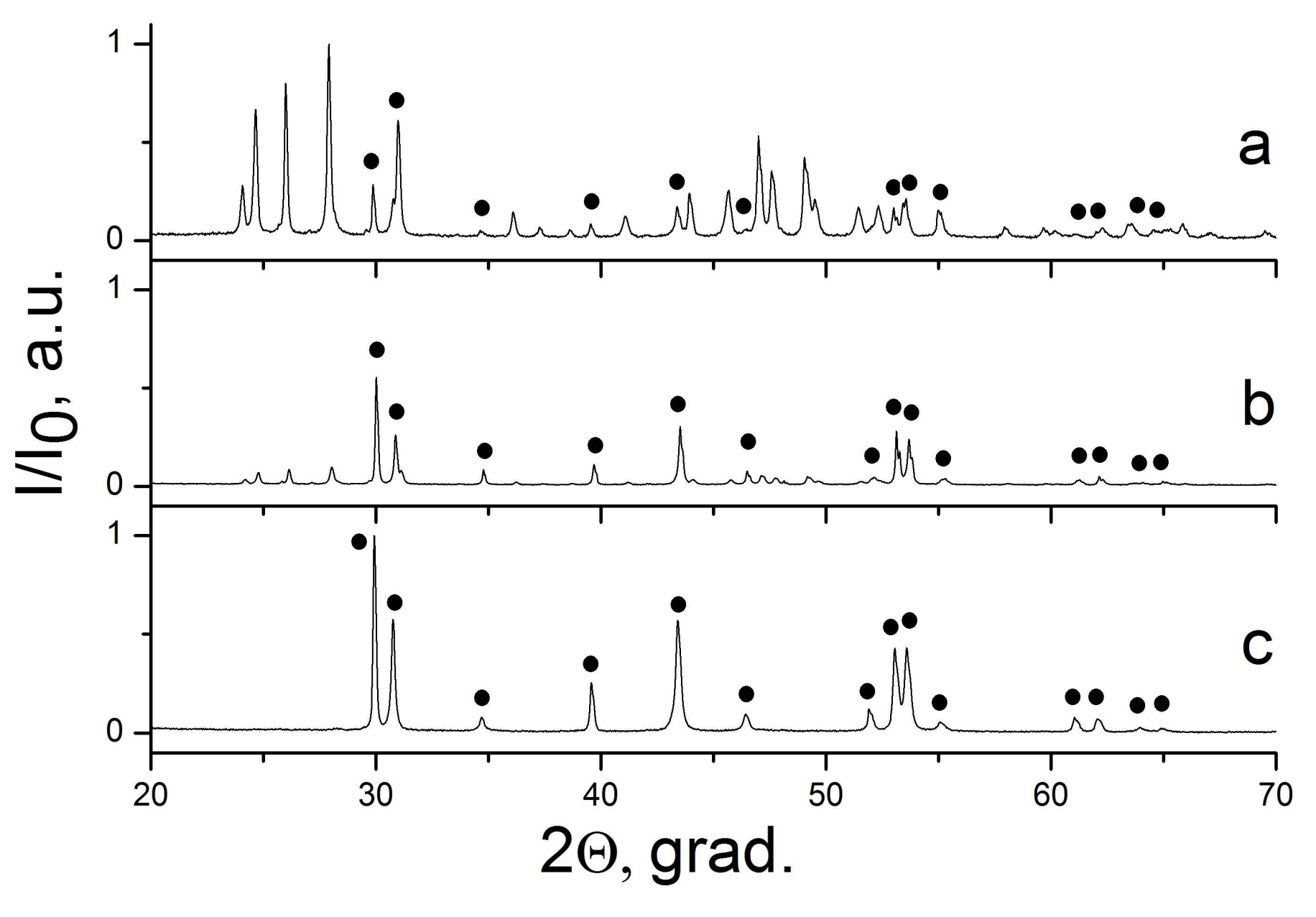
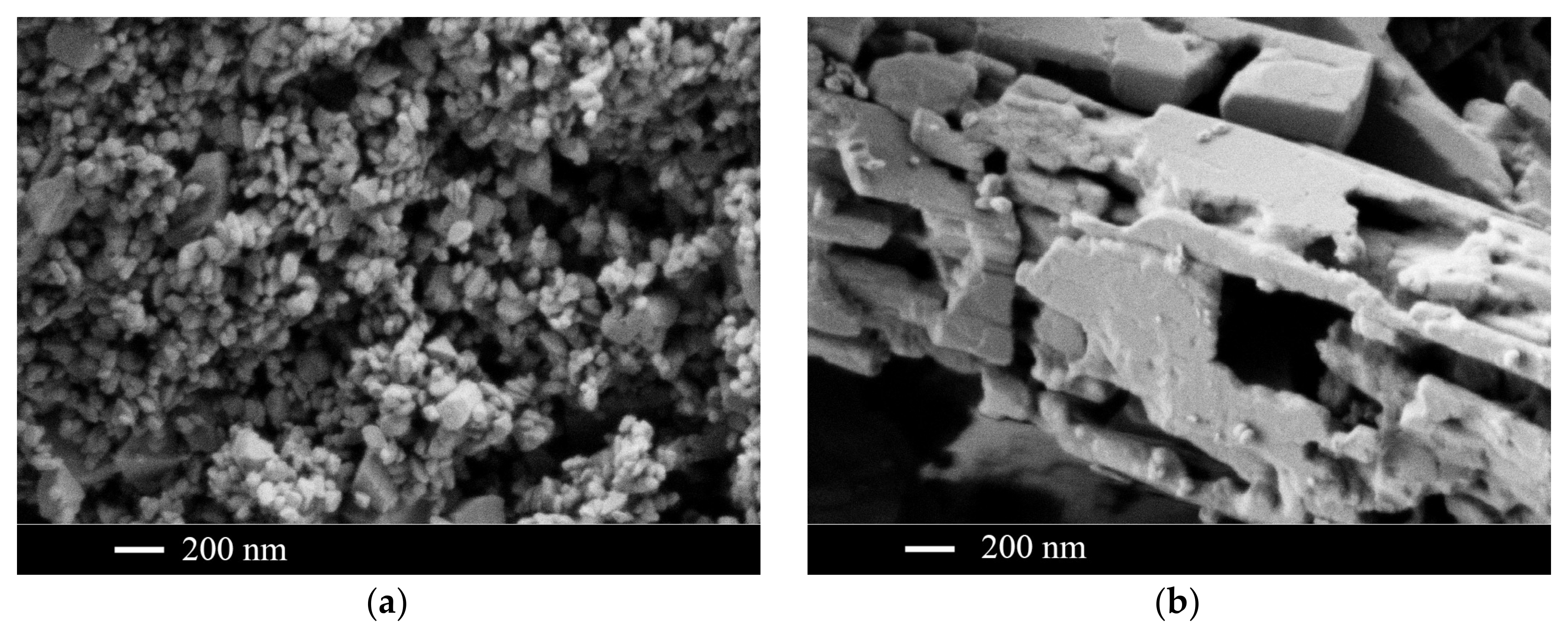

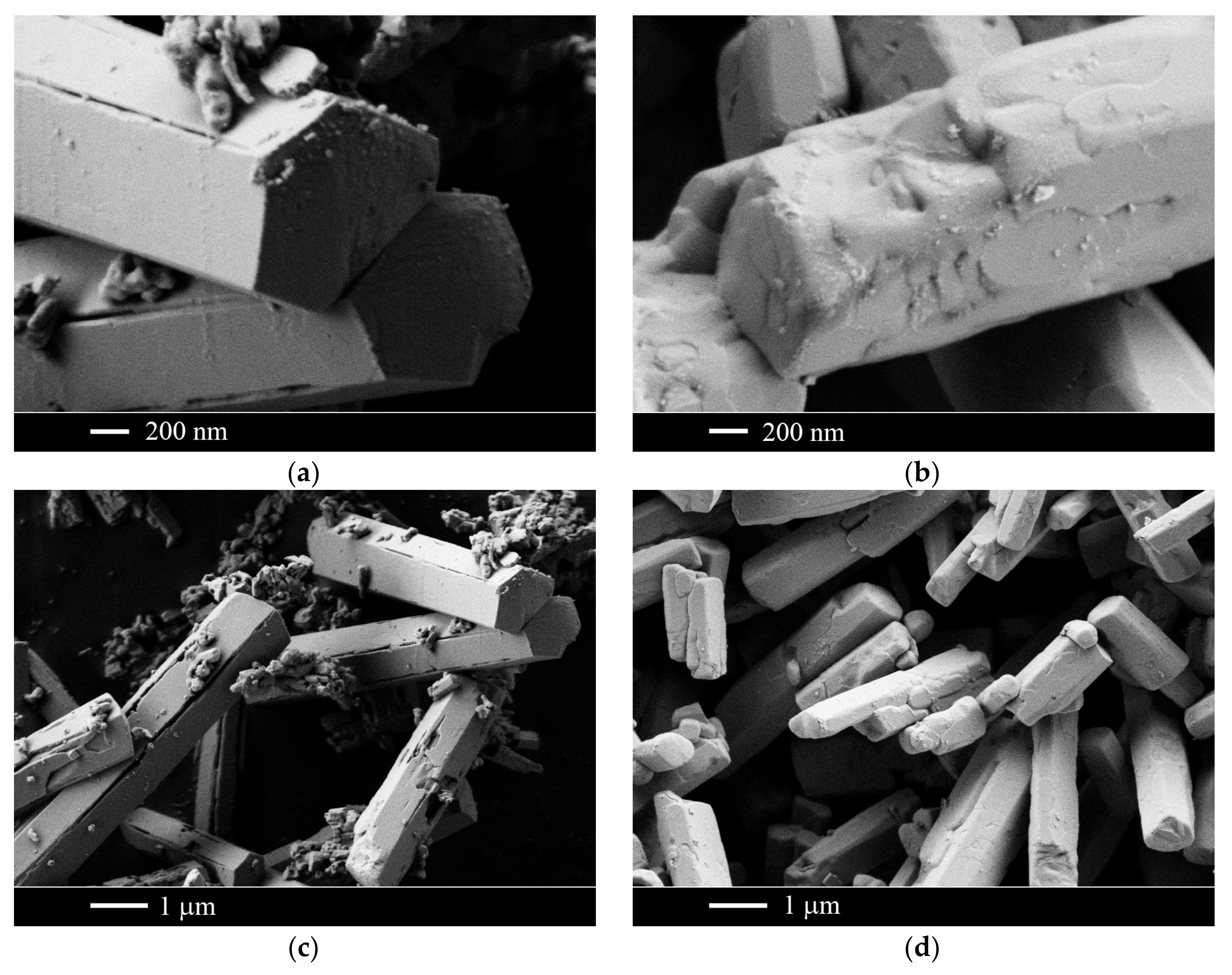
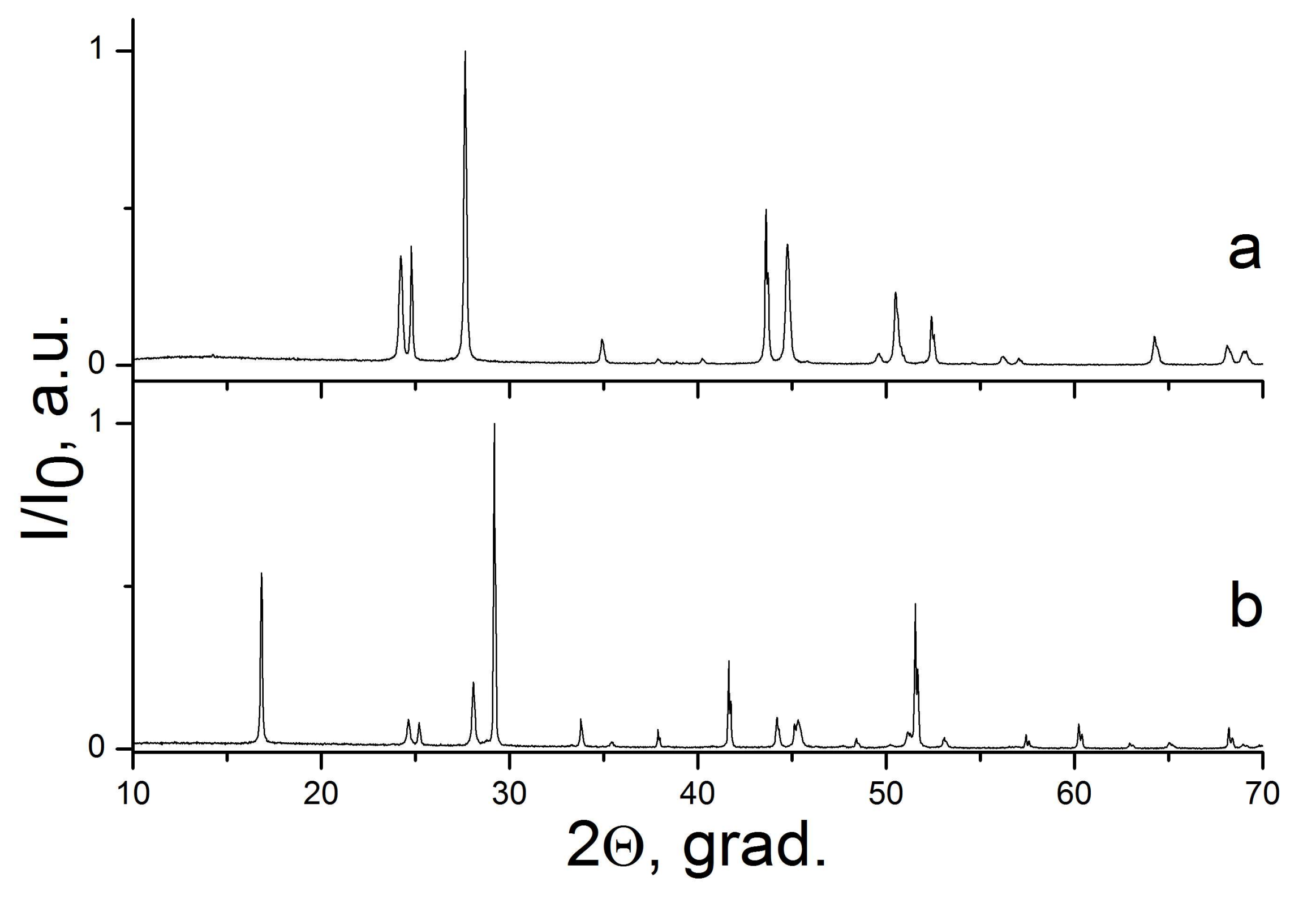
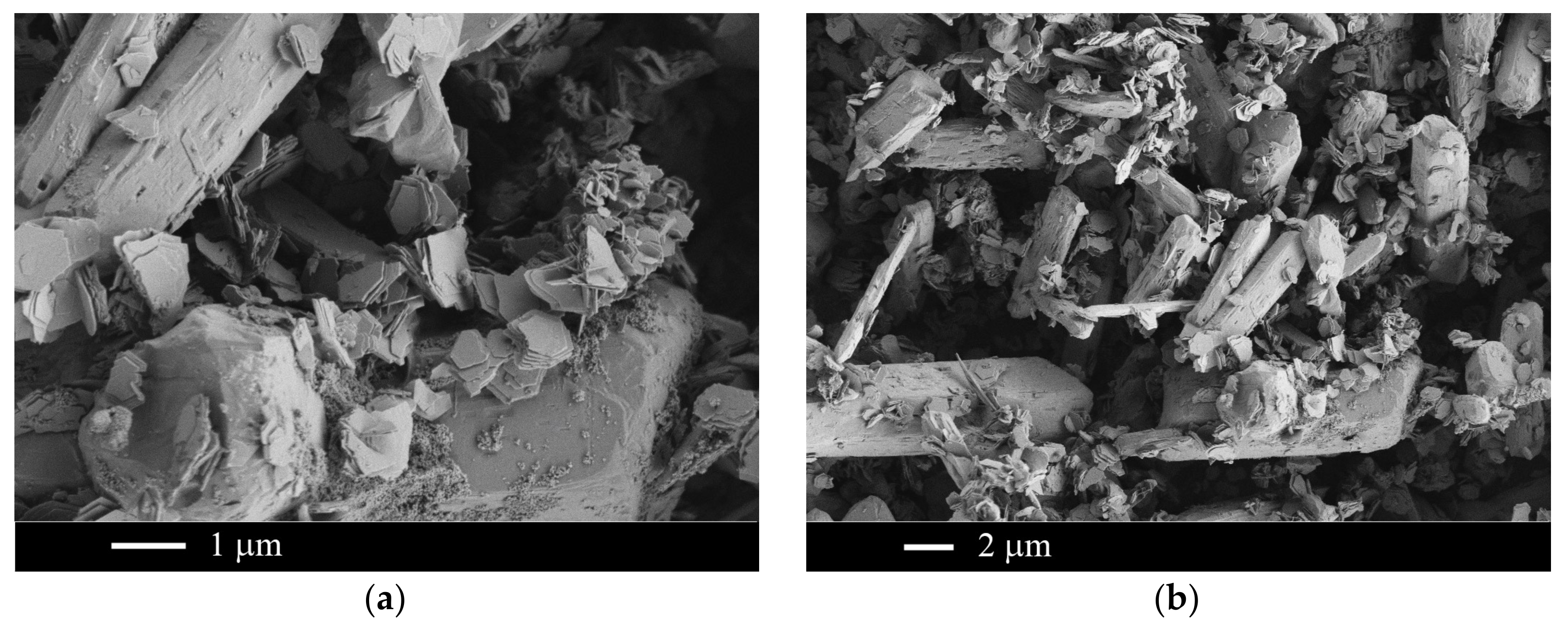
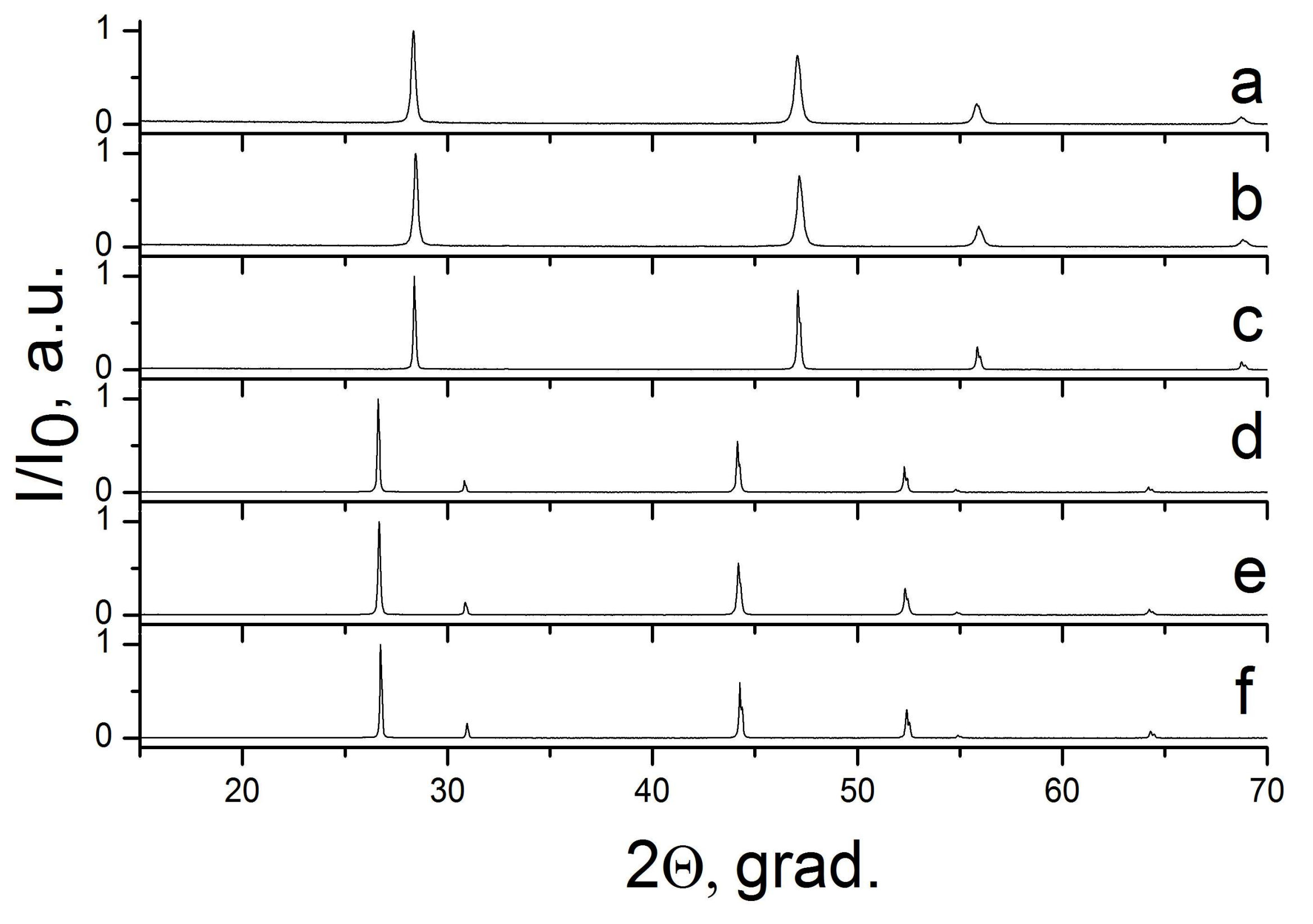
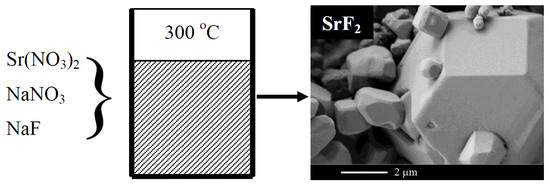
| Sample Number * | Ratios of Starting Materials, mol %(M,Ln) (NO3)x:NaF:NaNO3 | Temp. (°C) | Phase Composition | Space Symmetry Group | Crystal Lattice Parameters, Å |
|---|---|---|---|---|---|
| 1 | 11:30:59 | 320 | NaYF4 + YF3 | P63/m Pnma | a1 = 5.982(1) c1 = 3.525(1) a2 = 6.388(1) b = 6.846(1) c2 = 4.244(1) |
| 2 | 9:39:52 | 320 | NaYF4 + YF3 | P63/m | a = 5.982(1) c = 3.525(1) |
| 3 | 7:52:41 | 320 | NaYF4 | P63/m | a = 5.984(1) c = 3.525(1) |
| 4 | 7:52:41 | 435 | NaYF4 | P63/m | а = 5.974(2) c = 3.529(1) |
| 5 | 3:6:91 | 400 | LaF3 | P−3c1 | a1 = 7.1815(3) c1 = 7.3365(2) |
| 6 | 90:4:5 | 330 | LaF3 | P−3c1 | a = 7.1841(1) c = 7.3522(1) |
| 7 | 4:53:43 | 400 | CeF3 + NaCeF4 | P−3c1 P321 | a1 = 7.0901(1) c1 = 7.2481(1) a2 = 6.1367(2) c1 = 3.7377(2) |
| 8 | 11:67:22 | 300 | CaF2 | Fm−3m | a = 5.4654(1) |
| 9 | 6:35:59 | 300 | CaF2 | Fm−3m | a = 5.4655(1) |
| 10 | 9:55:36 | 400 | CaF2 | Fm−3m | a = 5.4648(1) |
| 11 | 14:29:57 | 400 | CaF2 | Fm−3m | a = 5.4648(1) |
| 12 | 11:67:22 | 300 | SrF2 | Fm−3m | a = 5.8010(1) |
| 13 | 6:35:59 | 300 | SrF2 | Fm−3m | a = 5.8001(1) |
| 14 | 9:55:36 | 400 | SrF2 | Fm−3m | a = 5.8001(1) |
| 15 | 20:40:40 | 400 | SrF2 | Fm−3m | a = 5.8001(1) |
| 16 | 14:29:57 | 400 | SrF2 | Fm−3m | a = 5.7992(1) |
| 17 | 22:33:45 | 300 | “CaF2” + “SrF2” | Fm−3m | a1 = 5.483(1) a2 = 5.775(1) |
| 18 | 8:12:80 | 300 | “CaF2” + “SrF2” | Fm−3m | a1 = 5.476(1) a2 = 5.784(1) |
| 19 | 17:50:33 | 300 | “CaF2” + “SrF2” | Fm−3m | a1 = 5,479(1) a2 = 5.792(1) |
| 20 | 7:21:72 | 300 | “CaF2” + “SrF2” | Fm−3m | a1 = 5.473(1) a2 = 5.789(1) |
| Property | Symbol (Units) | Value |
|---|---|---|
| Melting point | t (°С) | 305 |
| Density | ρ (kg/m3) | 1903 |
| Thermal conductivity | λ (W/m·K) | 2.5 × 10−3 |
| Specific heat | Cp (J/kg·K) | 2836 |
| Thermal expansivity | β (K−1) | 4.5 × 10−4 |
| Viscosity | m (kg/m·s) | 2.2 × 10−3 |
| Sample Number | Composition |
|---|---|
| 17 | Ca0.94Sr0.06F2 + Sr0.93Ca0.07F2 |
| 18 | Ca0.96Sr0.04F2 + Sr0.95Ca0.05F2 |
| 19 | Ca0.95Sr0.05F2 + Sr0.97Ca0.03F2 |
| 20 | Ca0.97Sr0.03F2 + Sr0.97Ca0.03F2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorov, P.; Mayakova, M.; Alexandrov, A.; Voronov, V.; Kuznetsov, S.; Baranchikov, A.; Ivanov, V. The Melt of Sodium Nitrate as a Medium for the Synthesis of Fluorides. Inorganics 2018, 6, 38. https://doi.org/10.3390/inorganics6020038
Fedorov P, Mayakova M, Alexandrov A, Voronov V, Kuznetsov S, Baranchikov A, Ivanov V. The Melt of Sodium Nitrate as a Medium for the Synthesis of Fluorides. Inorganics. 2018; 6(2):38. https://doi.org/10.3390/inorganics6020038
Chicago/Turabian StyleFedorov, Pavel, Mariya Mayakova, Alexander Alexandrov, Valery Voronov, Sergey Kuznetsov, Alexander Baranchikov, and Vladimir Ivanov. 2018. "The Melt of Sodium Nitrate as a Medium for the Synthesis of Fluorides" Inorganics 6, no. 2: 38. https://doi.org/10.3390/inorganics6020038
APA StyleFedorov, P., Mayakova, M., Alexandrov, A., Voronov, V., Kuznetsov, S., Baranchikov, A., & Ivanov, V. (2018). The Melt of Sodium Nitrate as a Medium for the Synthesis of Fluorides. Inorganics, 6(2), 38. https://doi.org/10.3390/inorganics6020038