Synthesis, Crystal Structure, and Chemical-Bonding Analysis of BaZn(NCN)2
Abstract
:1. Introduction
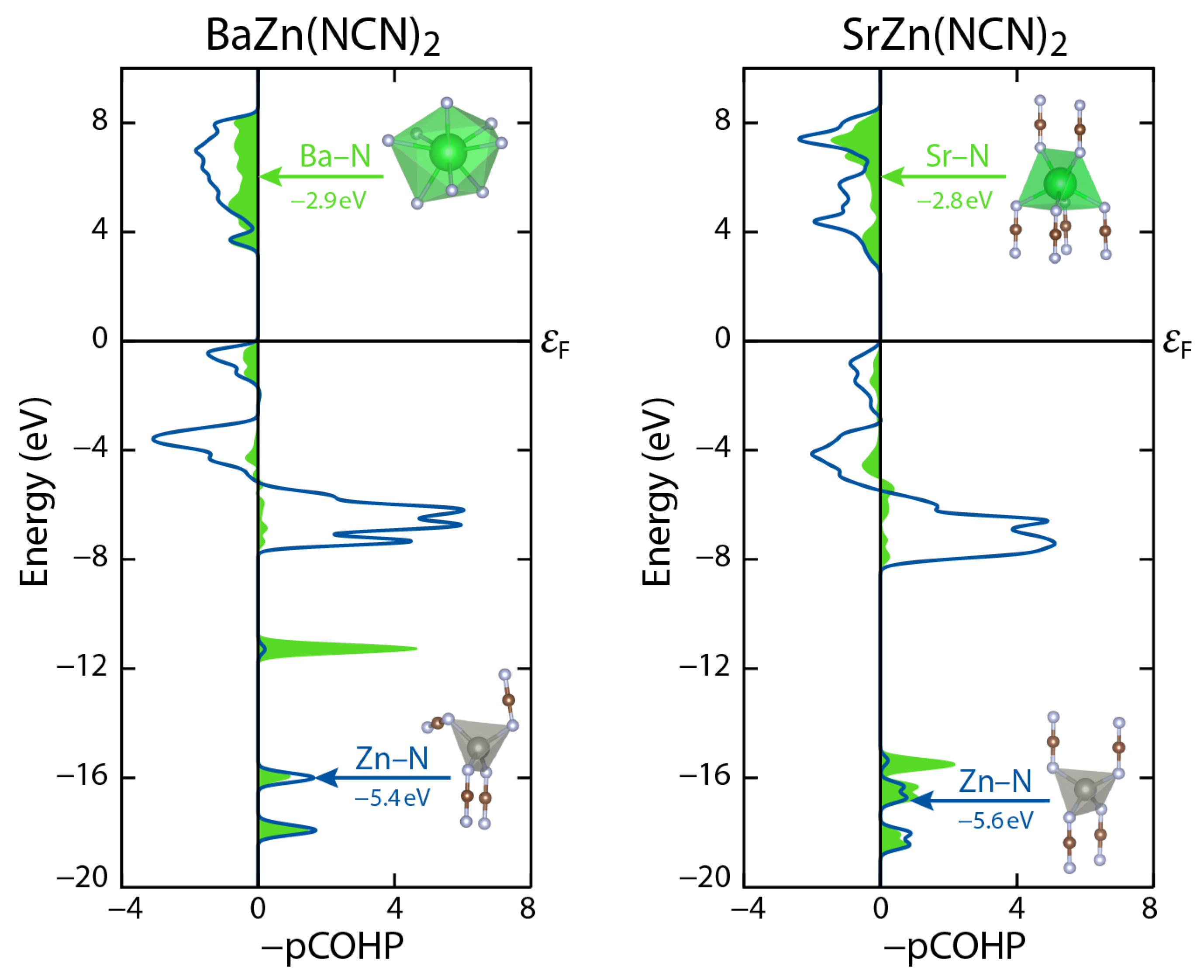
2. Results
3. Discussion
4. Experimental and Computational Details
4.1. Synthesis
4.2. PXRD Analysis
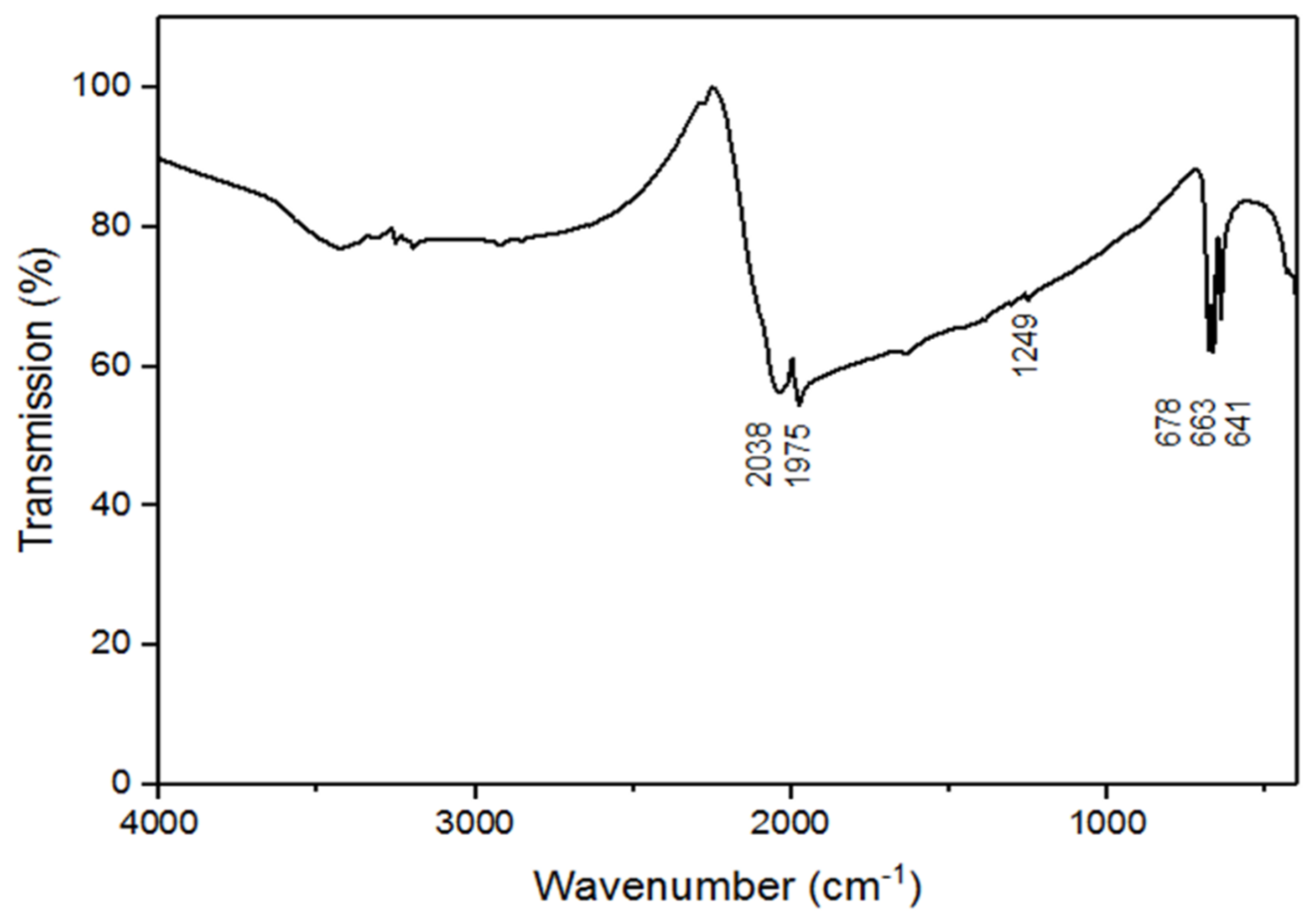
4.3. IR Measurements
4.4. Computational Details
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frank, A.; Garo, N. Verfahren zur Darstellung von Cyanverbindungen aus Carbiden. Ger. Pat. 8363, 1895. [Google Scholar]
- Down, M.G.; Haley, M.J.; Hubberstey, P.; Pulham, R.J.; Thunder, A.E. Synthesis of the dilithium salt of cyanamide in liquid lithium; X-ray crystal structure of Li2NCN. J. Chem. Soc. Chem. Commun. 1978, 52–53. [Google Scholar] [CrossRef]
- Krings, M.; Wessel, M.; Wilsmann, W.; Müller, P.; Dronskowski, R. Temperature-Dependent Synthetic Routes to and Thermochemical Ranking of α- and β-SrNCN. Inorg. Chem. 2010, 49, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Neukirch, M.; Tragl, S.; Meyer, H.J. Syntheses and Structural Properties of Rare Earth Carbodiimides. Inorg. Chem. 2006, 45, 8188–8193. [Google Scholar] [CrossRef] [PubMed]
- Kubus, M.; Heinicke, R.; Ströbele, M.; Enseling, D.; Jüstel, T.; Meyer, H.J. Synthesis of new structurally related cyanamide compounds LiM(CN2)2 where M is Al3+, In3+ or Yb3+. Mater. Res. Bull. 2015, 62, 37–41. [Google Scholar] [CrossRef]
- Dolabdjian, K.; Schedel, C.; Enseling, D.; Jüstel, T.; Meyer, H.-J. Synthesis, Luminescence and Nonlinear Optical Properties of Homoleptic Tetracyanamidogermanates ARE[Ge(CN2)4] (A = K, Cs, and RE = La, Ce, Pr, Nd, Sm, Eu, Gd). Z. Anorg. Allg. Chem. 2017, 643, 488–494. [Google Scholar] [CrossRef]
- Tang, X.; Xiang, H.; Liu, X.; Speldrich, M.; Dronskowski, R. A Ferromagnetic Carbodiimide: Cr2(NCN)3. Angew. Chem. Int. Ed. 2010, 49, 4738–4742. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Krott, M.; Müller, P.; Hu, C.; Lueken, H.; Dronskowski, R. Synthesis, Crystal Structure, and Properties of MnNCN, the First Carbodiimide of a Magnetic Transition Metal. Inorg. Chem. 2005, 44, 3001–3003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Stork, L.; Speldrich, M.; Lueken, H.; Dronskowski, R. FeNCN and Fe(NCNH)2: Synthesis, Structure, and Magnetic Properties of a Nitrogen-Based Pseudo-Oxide and -Hydroxide of Divalent Iron. Chem. Eur. J. 2009, 15, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Krott, M.; Liu, X.; Fokwa, B.P.T.; Speldrich, M.; Lueken, H.; Dronskowski, R. Synthesis, Crystal-Structure Determination and Magnetic Properties of Two New Transition-Metal Carbodiimides: CoNCN and NiNCN. Inorg. Chem. 2007, 46, 2204–2207. [Google Scholar] [CrossRef] [PubMed]
- Ressnig, D.; Shalom, M.; Patscheider, J.; More, R.; Evangelisti, F.; Antonietti, M.; Patzke, G.R. Photochemical and electrocatalytic water oxidation activity of cobalt carbodiimide. J. Mater. Chem. A 2015, 3, 5072–5082. [Google Scholar] [CrossRef] [Green Version]
- Eguia-Barrio, A.; Castillo-Martinez, E.; Liu, X.; Dronskowski, R.; Armand, M.; Rojo, T. Carbodiimides: New materials applied as anode electrodes for sodium and lithium ion batteries. J. Mater. Chem. A 2016, 4, 1608–1611. [Google Scholar] [CrossRef]
- Sougrati, M.T.; Darwiche, A.; Liu, X.; Mahmoud, A.; Hermann, R.P.; Jouen, S.; Monconduit, L.; Dronskowski, R.; Stievano, L. Transition-Metal Carbodiimides as Molecular Negative Electrode Materials for Lithium- and Sodium-Ion Batteries with Excellent Cycling Properties. Angew. Chem. Int. Ed. 2016, 55, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Corkett, A.J.; Konze, P.M.; Dronskowski, R. The Ternary Post-transition Metal Carbodiimide SrZn(NCN)2. Z. Anorg. Allg. Chem. 2017, 643, 1456–1461. [Google Scholar] [CrossRef]
- Unverfehrt, L.; Kalmutzki, M.; Strobele, M.; Meyer, H.J. Solid state synthesis of homoleptic tetracyanamidoaluminates. Dalton Trans. 2011, 40, 9921–9924. [Google Scholar] [CrossRef] [PubMed]
- Kalmutzki, M.; Ströbele, M.; Kroeker, S.; Wren, J.E.C.; Meyer, H.J. Synthesis and Characterization of the First Tetracyanamidogallate. Eur. J. Inorg. Chem. 2013, 2013, 6091–6096. [Google Scholar] [CrossRef]
- Broadley, S.; Gál, Z.A.; Corà, F.; Smura, C.F.; Clarke, S.J. Vertex-Linked ZnO2S2 Tetrahedra in the Oxysulfide BaZnOS: A New Coordination Environment for Zinc in a Condensed Solid. Inorg. Chem. 2005, 44, 9092–9096. [Google Scholar] [CrossRef] [PubMed]
- Salter, E.J.T.; Blandy, J.N.; Clarke, S.J. Crystal and Magnetic Structures of the Oxide Sulfides CaCoSO and BaCoSO. Inorg. Chem. 2016, 55, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Unverfehrt, L.; Ströbele, M.; Meyer, H.J. The New Homoleptic Tetracyanamidoaluminate LiBa2[Al(CN2)4]. Z. Anorg. Allg. Chem. 2013, 639, 1722–1725. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Liu, X.; Müller, P.; Kroll, P.; Dronskowski, R.; Wilsmann, W.; Conradt, R. Experimental and Quantum-Chemical Studies on the Thermochemical Stabilities of Mercury Carbodiimide and Mercury Cyanamide. ChemPhysChem 2003, 4, 789. [Google Scholar] [CrossRef]
- Liu, X.; Müller, P.; Kroll, P.; Dronskowski, R. Synthesis, Structure Determination, and Quantum-Chemical Characterization of an Alternate HgNCN Polymorph. Inorg. Chem. 2002, 41, 4259–4265. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Decker, A.; Schmitz, D.; Dronskowski, R. Crystal Structure Refinement of Lead Cyanamide and the Stiffness of the Cyanamide Anion. Z. Anorg. Allg. Chem. 2000, 626, 103–105. [Google Scholar] [CrossRef]
- Becker, M.; Jansen, M. Zinc cyanamide, Zn(CN2). Acta Crystallogr. Sect. C 2001, 57, 347–348. [Google Scholar] [CrossRef]
- Berger, U.; Schnick, W. Syntheses, crystal structures, and vibrational spectroscopic properties of MgCN2, SrCN2, and BaCN2. J. Alloys Compd. 1994, 206, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Spitsbergen, U. The crystal structures of BaZnO2, BaCoO2 and BaMnO2. Acta Crystallogr. 1960, 13, 197–198. [Google Scholar] [CrossRef]
- Nelson, R.; Konze, P.M.; Dronskowski, R. First-Principles Chemical Bonding Study of Manganese Carbodiimide, MnNCN, As Compared to Manganese Oxide, MnO. J. Phys. Chem. A 2017, 121, 7778–7786. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Bettentrup, H.; Jüstel, T.; Meyer, H.J. Synthesis and Properties of Tetracyanamidosilicates ARE[Si(CN2)4]. Inorg. Chem. 2010, 49, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Dronskowski, R.; Blöchl, P.E. Crystal Orbital Hamilton Populations (COHP). Energy-Resolved Visualization of Chemical Bonding in Solids Based on Density-Functional Calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Analytic Projection from Plane-Wave and PAW Wavefunctions and Application to Chemical-Bonding Analysis in Solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-Wave Based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
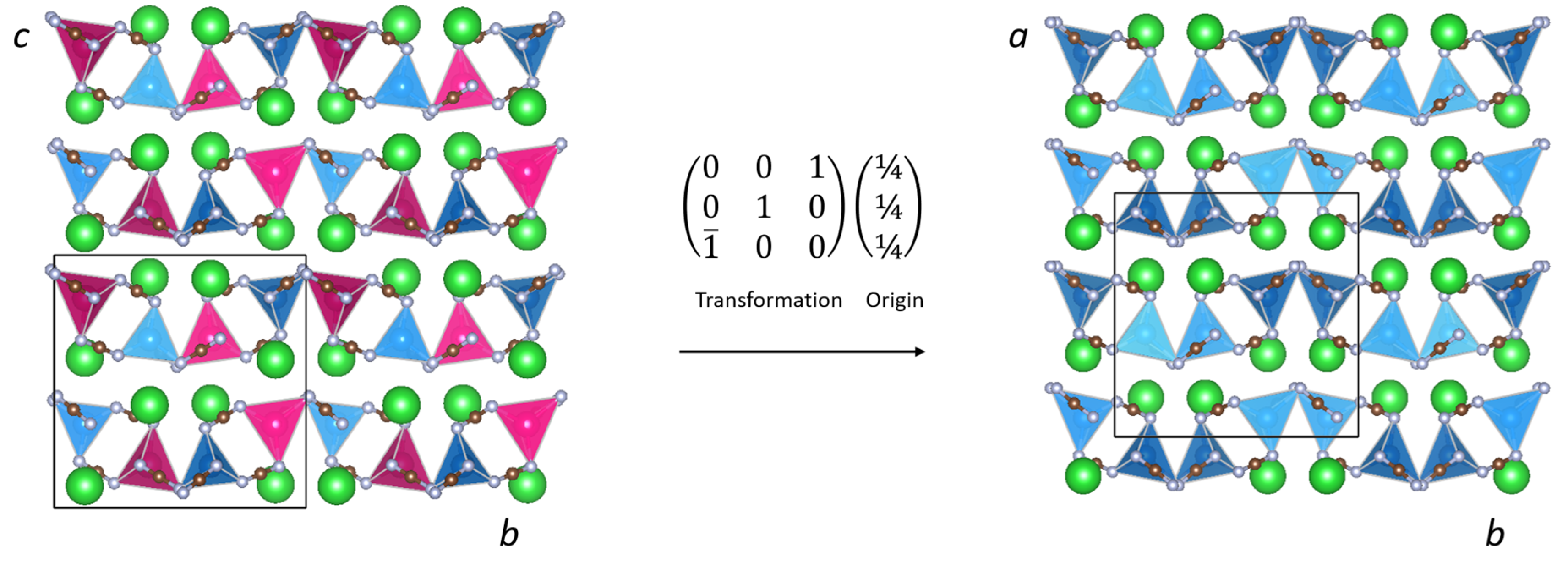
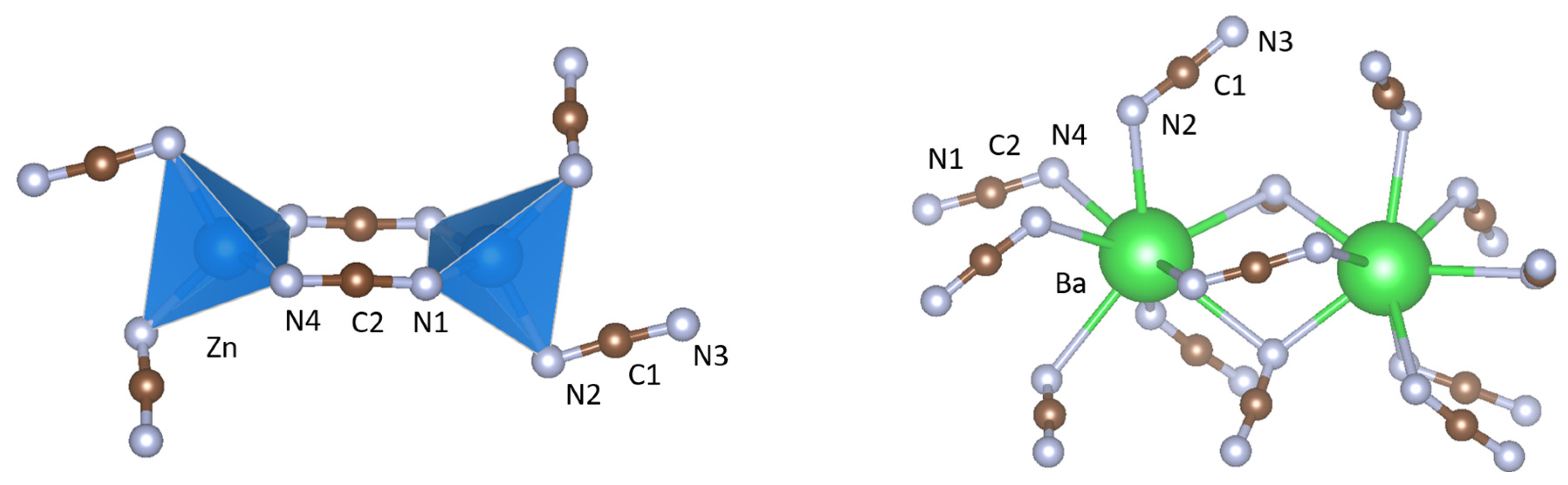

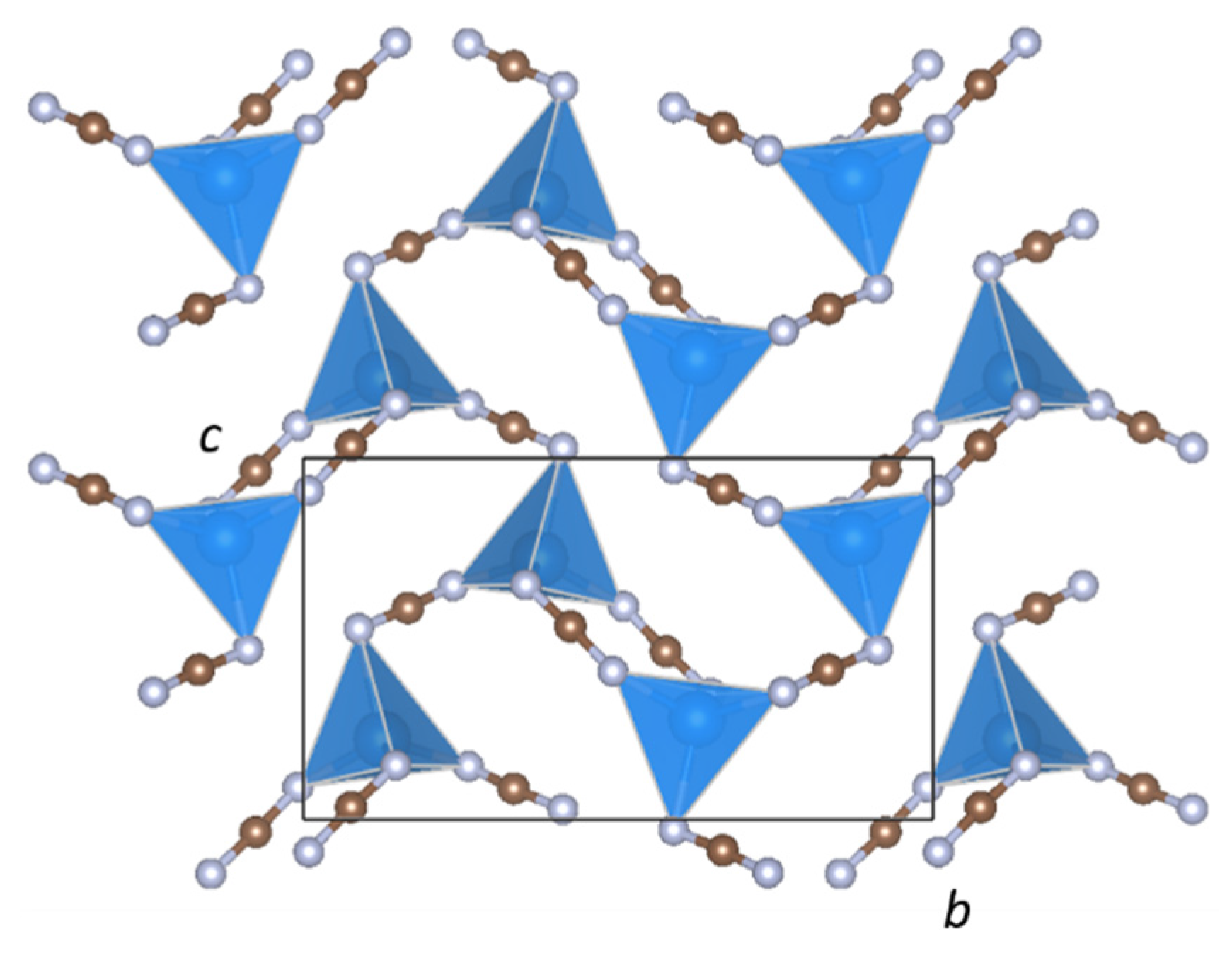
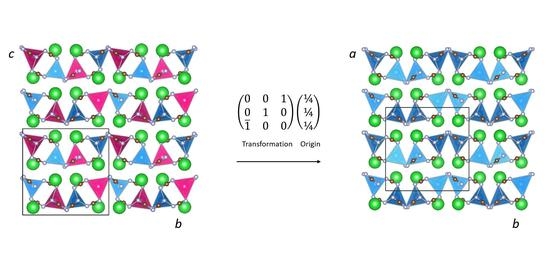
| dobs(unres) (Å) | dobs(res) (Å) | dcalc (Å) | |
|---|---|---|---|
| C1–N2 | 1.209(18) | 1.213(7) | 1.233 |
| C1–N3 | 1.146(18) | 1.212(8) | 1.236 |
| C2–N1 | 1.355(18) | 1.226(7) | 1.233 |
| C2–N4 | 1.213(18) | 1.228(8) | 1.239 |
| θobs(unres) (°) | θobs(res) (°) | θcalc (°) | |
| N2–C1–N3 | 165.1(13) | 174.7(5) | 176.7 |
| N1–C2–N4 | 153.4(14) | 176.1(5) | 179.2 |
| Atom | x | y | z | Uiso (102 × Å2) |
|---|---|---|---|---|
| Ba | 0.83999(9) | 0.87151(9) | 0.0404(1) | 1.54(1) |
| Zn | 0.9226(2) | 0.1252(2) | 0.2781(3) | 1.94(5) |
| C1 | 0.3632(4) | 0.1660(4) | 0.585(1) | 1.8(2) |
| C2 | 0.6117(7) | 0.4240(4) | 0.5343(7) | “ |
| N1 | 0.6432(7) | 0.4912(5) | 0.4103(8) | “ |
| N2 | 0.4196(5) | 0.0878(4) | 0.529(1) | “ |
| N3 | 0.3009(5) | 0.2379(5) | 0.648(1) | “ |
| N4 | 0.5771(7) | 0.3531(6) | 0.6495(9) | “ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corkett, A.J.; Konze, P.M.; Dronskowski, R. Synthesis, Crystal Structure, and Chemical-Bonding Analysis of BaZn(NCN)2. Inorganics 2018, 6, 1. https://doi.org/10.3390/inorganics6010001
Corkett AJ, Konze PM, Dronskowski R. Synthesis, Crystal Structure, and Chemical-Bonding Analysis of BaZn(NCN)2. Inorganics. 2018; 6(1):1. https://doi.org/10.3390/inorganics6010001
Chicago/Turabian StyleCorkett, Alex J., Philipp M. Konze, and Richard Dronskowski. 2018. "Synthesis, Crystal Structure, and Chemical-Bonding Analysis of BaZn(NCN)2" Inorganics 6, no. 1: 1. https://doi.org/10.3390/inorganics6010001
APA StyleCorkett, A. J., Konze, P. M., & Dronskowski, R. (2018). Synthesis, Crystal Structure, and Chemical-Bonding Analysis of BaZn(NCN)2. Inorganics, 6(1), 1. https://doi.org/10.3390/inorganics6010001