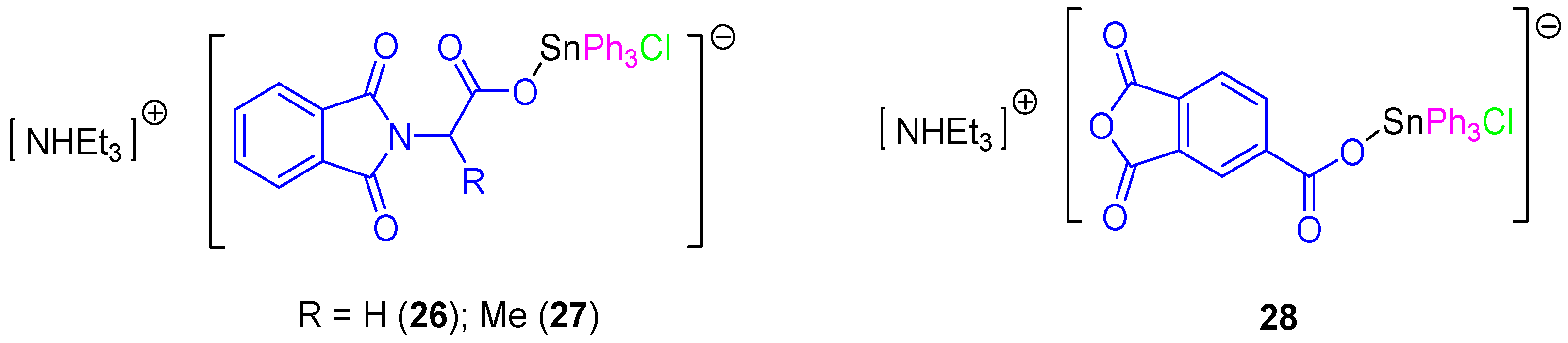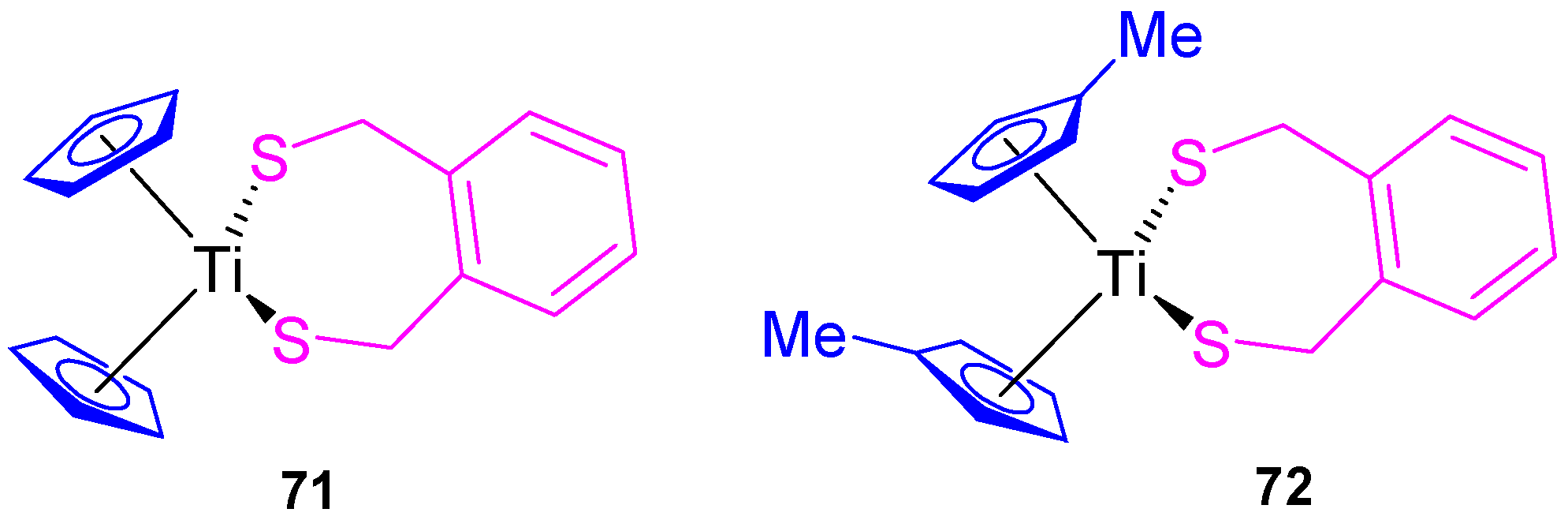Abstract
For more than 100 years metal complexes have been extensively used in therapy and since the discovery of cisplatin the research in this field has expanded exponentially. The scientific community is always in search of new alternatives to platinum compounds and a wide variety of metallodrugs based on other metals have been reported with excellent therapeutic results. This short review focuses on the work that our research group has carried out since 2007 in collaboration with others and centers on the preparation of organogallium(III) compounds, organotin(IV) derivatives, and titanocene(IV) complexes together with the study of their cytotoxic anticancer properties.
1. Introduction
Metals have been used in medicinal applications for more than 500 years [1]. For example, the Egyptians used copper to sterilize water, gold was used in a variety of medicines in Arabia and China, and various iron remedies were used in Egypt around 1500 BC. At about the same time zinc was discovered to promote the healing of wounds. In the Renaissance era, mercury chloride was used as a diuretic and the nutritional essentiality of iron was discovered. However, in the last 100 years, the medicinal activity of inorganic compounds has been developed in a rational manner. Thus, in the early 1900s K[Au(CN)2] was used for treating tuberculosis and various Sb compounds for leishmaniasis. In addition, the antibacterial activity of various gold salts and arsenic compounds were used for treating various diseases [2].
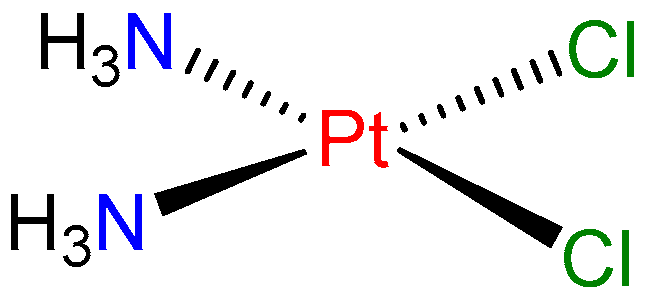
In the twentieth century, a very important therapeutic activity of metal complexes was discovered, namely their application for the treatment of cancer. Rosenberg’s serendipitous discovery of the anti-cancer action of cisplatin (cis-[Pt(NH3)2Cl2], Figure 1) in the 1960s precipitated a widespread search for related complexes with similar or better activity [3]. The observation of the cell division suppression by this compound was crucial for its development.
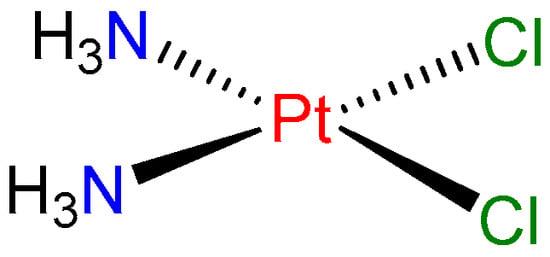
Figure 1.
Structure of cisplatin.
Until the turn of the century, cisplatin had been the most used drug in the world in therapy of cancer, administered alone or combined with other compounds. Researchers still had the expectation to develop alternative drugs to improve the potential and the effectiveness against cancer, and especially to overcome the undesirable effects of cisplatin, such as nephrotoxicity, neurotoxicity, ototoxicity, nausea and vomiting [4].
In this context, an extensive study of other metal complexes with similar anti-cancer action was carried out by the scientific community. Thus, the first non-platinum complex to enter clinical trials was budotitane although its applications were limited due to its low solubility and liver toxicity [5].
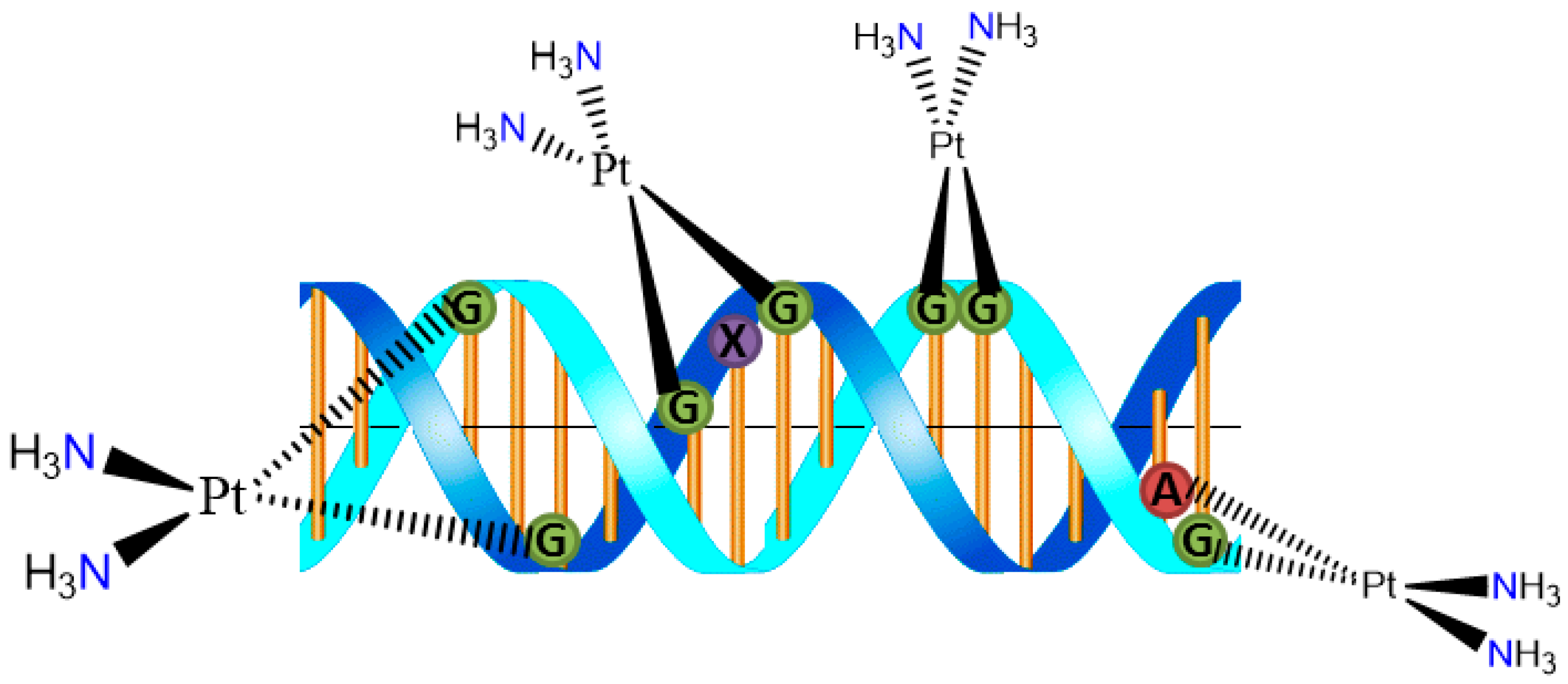
The cytotoxicity of cisplatin originates from its binding to DNA and the formation of covalent cross-links. The 1,2-intrastrand d(GpG) cross-link is the major adduct. Binding of cisplatin to DNA causes significant distortion of the helical structure and results in inhibition of DNA replication and transcription (Figure 2) [6]. The Pt2+ unit covalently binds to deoxyribonucleic acid (DNA), particularly to the N7 of either guanine (G) or adenine (A) in the nucleotide sequences GG and AG to form interstrand cross-links [7] The so-formed cisplatin-DNA unit activates a new cellular pathway which leads to transcription inhibition, cell-cycle arrest, DNA repair, and finally apoptosis [8].
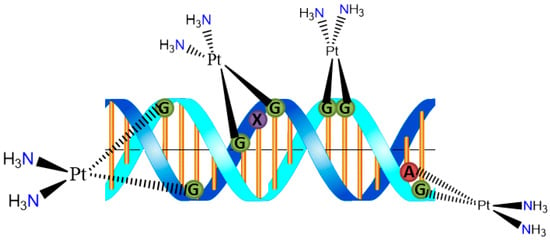
Figure 2.
DNA adduct formation with cisplatin moiety.
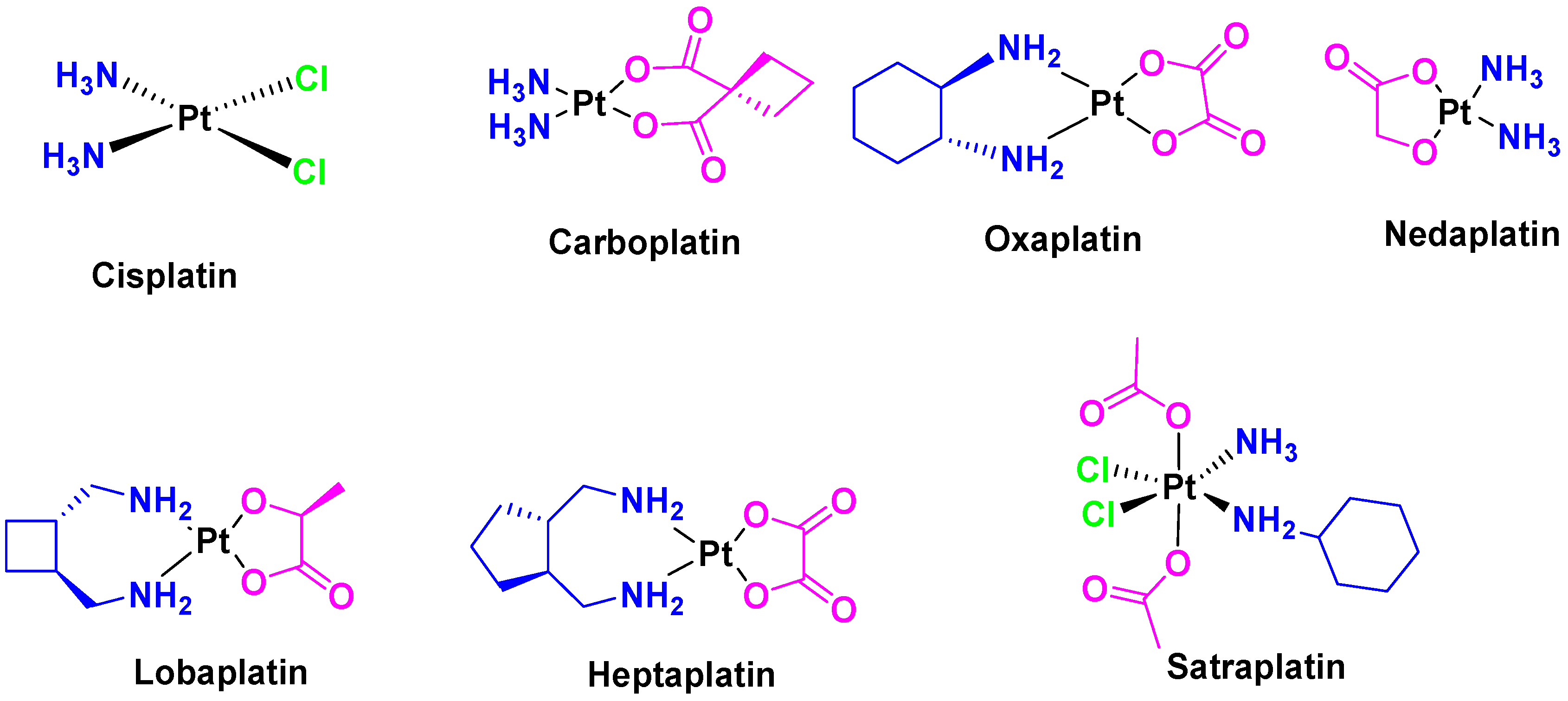
Immediately after the initial elucidation of the cell death mechanism of cisplatin, other platinum analogues such as carboplatin [9] and oxaliplatin [10] (Figure 3) were synthesized and approved by the FDA for use as anticancer drugs. In addition, some other compounds such as nedaplatin, lobaplatin, heptaplatin, and satraplatin (Figure 3) are currently in clinical trial phase [11].
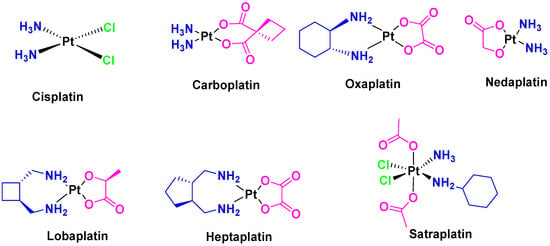
Figure 3.
Anticancer platinum metallodrugs (FDA-approved and investigated in clinical trials).
One of the main disadvantages of cisplatin is that, in many cases, cancer cells acquire resistance to this drug, deactivating its effect against damaged cells. To overcome this, cisplatin can be combined with other chemotherapeutics agents like 5-fluorouracil, for example [12].
Thus, cisplatin and its derivatives have been used for many years as chemotherapy agents in the treatment of cancer with excellent results against a wide variety of cell lines and tumors. However, because of the induction of drug-resistance of the tumors after treatment with cisplatin, the side-effects, the intrinsic toxicity of platinum, the limited bioavailability, and the solubility in physiological media of cisplatin-like compounds, it is of upmost importance to find alternative agents based on non-platinum metals-based systems with fewer side effects and improved cytotoxic and anticancer properties.
Thus, a wide variety of preclinical and clinical studies using anticancer metallodrugs have been reported using different elements such as gallium, titanium, palladium, gold, cobalt, ruthenium, and tin.
In this review, we describe the synthetic methods and preclinical studies in anticancer tests that our research group has carried out in the search for alternatives to cisplatin-like materials. As our work has mainly been based on the use of gallium, tin, and titanium compounds, we have divided this manuscript into three main parts, which cover specifically these metal complexes. In addition, a short section describes the latest results from our group using metallodrugs of other elements.
2. Gallium-Based Metallodrugs
Among the p-block metals, gallium has shown some clinical activity in the treatment of soft tissue tumors. Gallium(III) complexes present a special activity in anticancer therapy due to the analogy of the Ga(III) ion with the Fe(III) ion in ionic radius, electron affinity, electronegativity, coordination geometry, and Lewis base affinity [13,14]. These similarities suggest that the Ga(III) ion may follow an analogous biochemical pathway to that observed in iron metabolism. Gallium(III) is stable under biological conditions, while the oxidation state 2+ in gallium is energetically unfavorable and too reactive under physiological conditions to be stable. Hence, redox chemistry is therefore not possible for Ga(III) in biological media. This phenomenon enables the utilization of gallium(III) as a potential therapeutic agent and facilitates its study in biological conditions [15].
The literature has described some interesting results of gallium(III) compounds in phase II clinical trials in the treatment of lymphomas and bladder carcinoma. In addition, the combination with other agents in the treatment of metastatic carcinoma of the urethelium and cisplatin-resistant ovarian cancer has delivered promising results [16].
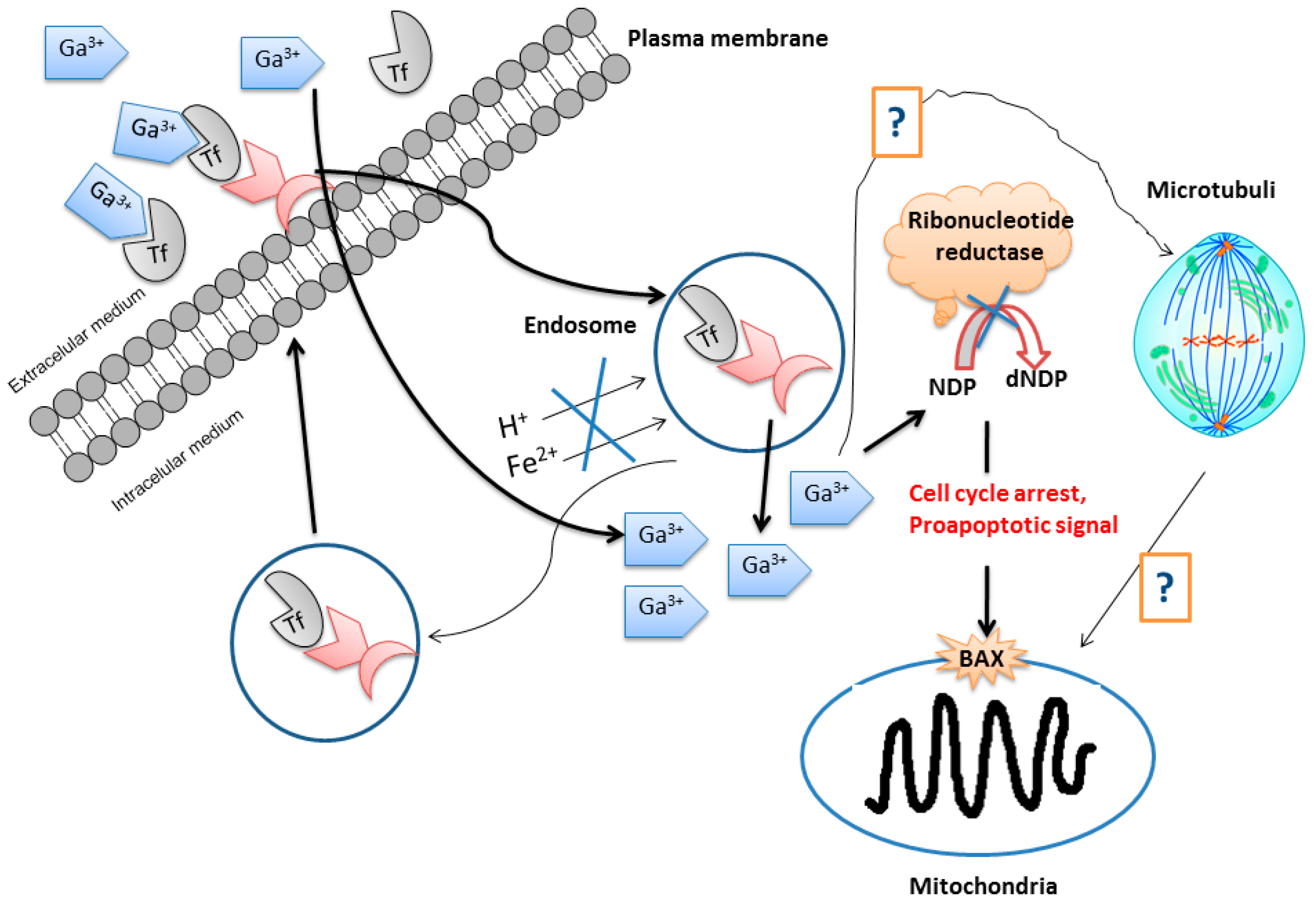
The mechanism of action of gallium(III) complexes in anticancer chemotherapy has been briefly studied. Ga3+ ions usually compete with Fe3+ for binding to transferrin to reach the intracellular medium. In this way, a cellular uptake of large amounts of gallium is achieved [17]. Analyzing all the biochemical pathways of the gallium(III) ion, it seems clear that the enzyme ribonucleotide reductase is its biological target [18]. Furthermore, the induction of apoptosis through activation of the proapoptotic factor BAX and caspase-3 can be considered as a possible mechanism of cell death. However, proteasome inhibition should not be ruled out [19].
The simplest and most familiar gallium compound used as an anticancer drug is gallium nitrate. However, this compound is readily hydrolyzed in biological medium to give non-soluble gallium oxides which are able to block the absorption and membrane permeation of the gallium ion reducing its effectivity in cancer treatments. Other gallium(III) compounds containing caboxylato, thiolato, and alkoxo ligands have been tested as anticancer agents. In general, the cellular acquisition of gallium mainly occurs by transferrin-mediated uptake followed by accumulation in endosomes. After transport into the cytosol, gallium(III) binds to and inhibits the functioning of ribonucleotide reductase (RR, an enzyme recognized as the most significant intracellular target for antiproliferative activity of gallium). DNA replication activates cell cycle arrest and results finally in apoptosis through the mitochondrial pathway, from which gallium is liberated during, or before, passage across the intestinal epithelium to become in part bound to transferrin in blood (Figure 4) [20].
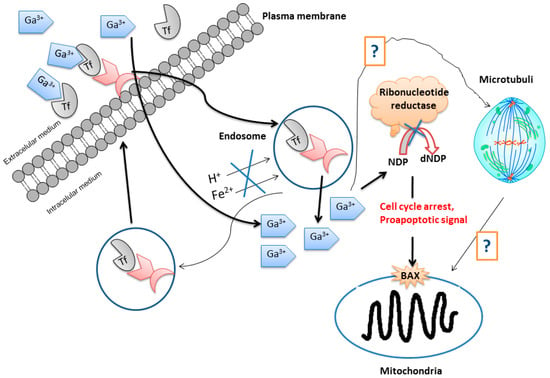
Figure 4.
Schematic representation of the mode of action of gallium. Abbreviations: Tf = transferrin; NDP = nucleoside diphosphate; dNDP = desoxynucleoside diphosphate; BAX = a proapoptotic protein (Adapted from Ref. 20 with permission from The Royal Society of Chemistry).
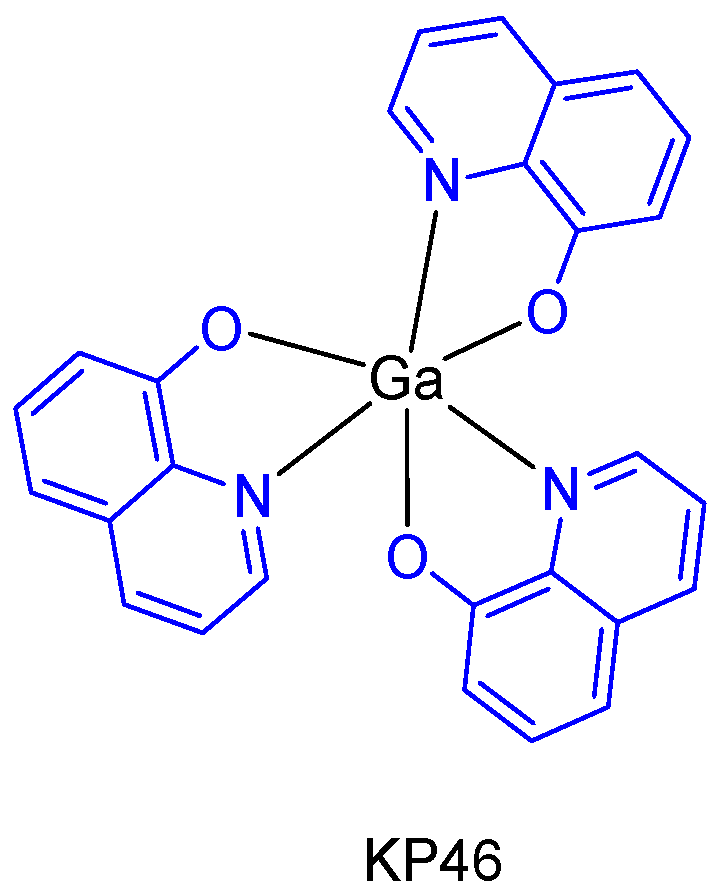
The metallodrug, KP46 (tris(8-hydroxyquinolinato)gallium(III)) (Figure 5), contains the metal chelating agent 8-hydroxyquinoline, which itself has anticancer properties [21]. Due to its well-defined toxicological and pharmacokinetic advantages, KP46 not only enables higher and well tolerable tissue gallium concentrations to be established, but also inhibitory effects on cell growth proliferation in vitro and in vivo superior to gallium salts (with IC50 values typically in the low micromolar range). In addition, an oral formulation of KP46 (IT-235 from the companies Niikipharma and Intezyne Technologies) showed a novel pattern of cytotoxicity with synergism across a broad range of antitumor agents targeting the endoplasmic reticulum in multiple tumor types [22].
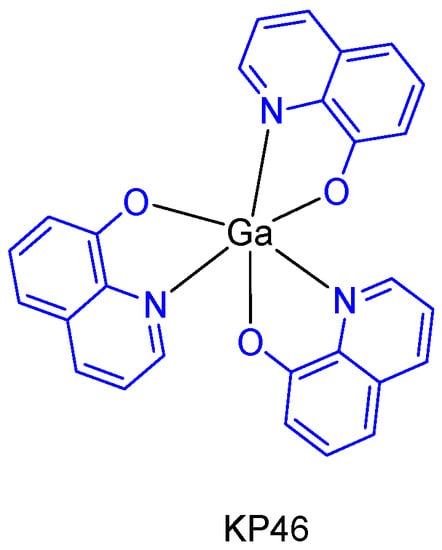
Figure 5.
KP46 which was formulated and used in clinical trials.
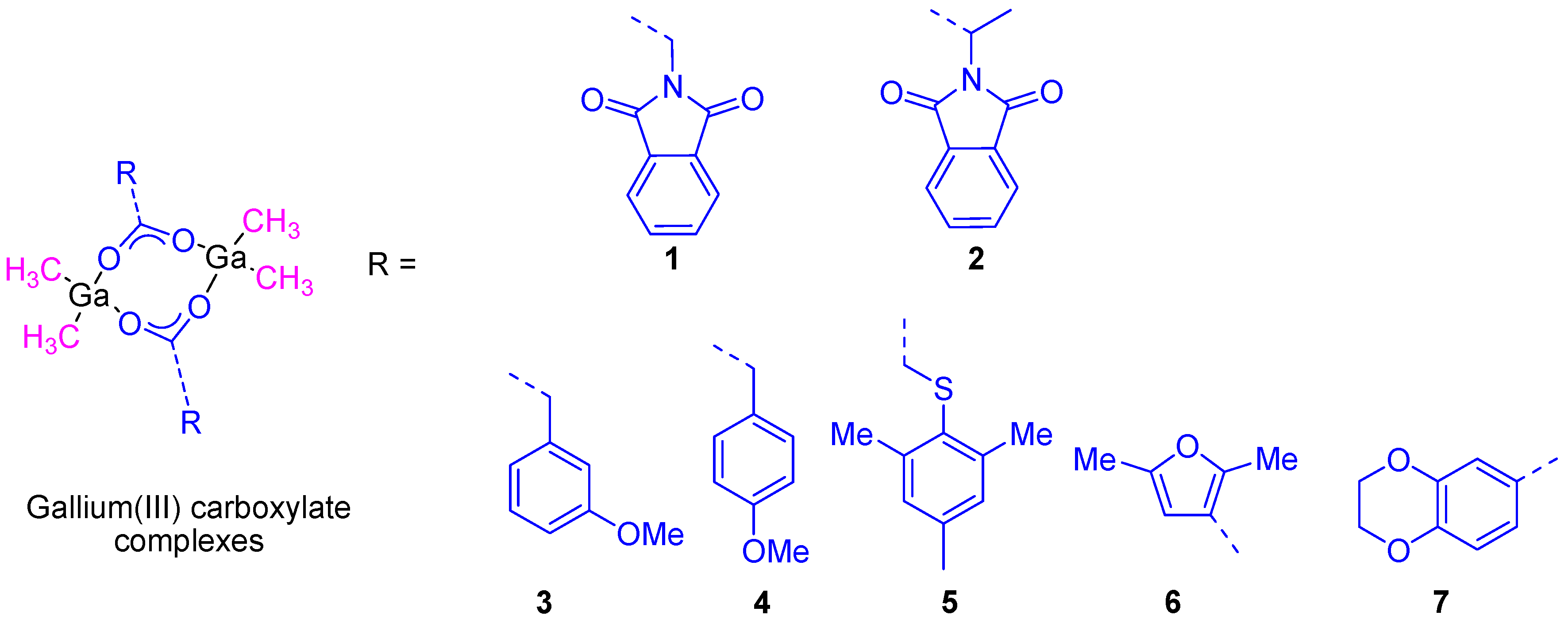
Similar gallium(III) complexes to KP46, including the ligand 7-chloroquinoline, were synthesized by other groups and showed not only a very high cytotoxic activity in vitro but also antimalarial properties [23]. Bearing in mind the promising properties of gallium compounds our group embarked on the preparation of several gallium compounds with different ligands. Thus, as the literature had shown an interesting and synergistic relation between gallium complexes and aminoacid derivatives such as glycine and DL-alanine in cancer cell death, we decided to synthesize two gallium complexes based on N-phthaloyl derivatives of neutral aminoacids, namely [Me2Ga(µ-O2CCH2N(CO)2C6H4)]2 (1) and RS-[Me2Ga(µ-O2CCHMeN(CO)2C6H4)]2 (2) (Figure 6). The formation of a single diastereoisomer RS was observed in the crystal structure determined by X-ray diffraction studies. The high solubility and stability of both compounds in DMSO and mixtures of DMSO/water, make them good candidates for anticancer tests.
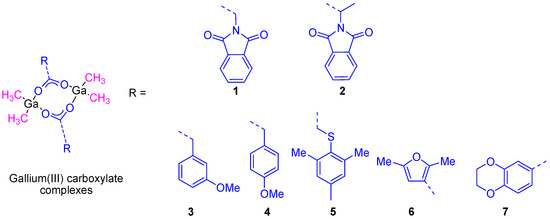
Figure 6.
Gallium(III) carboxylate compounds synthesized by our group.
Compounds 1 and 2 were tested as anticancer agents against four human tumor cell lines: 8505C anaplastic thyroid cancer, A253 head and neck carcinoma, A549 lung carcinoma, A2780 ovarian cancer, and DLD-1 colon carcinoma. Comparing the results of cytotoxicity with gallium(III) nitrate, the compounds 1 and 2 presented a higher antiproliferative effect. Both complexes present similar IC50 values in all the studied cell lines (Table 1) [24].

Table 1.
IC50 (μM) after 96 h of action of gallium compounds, gallium(III) nitrate and cisplatin on different cancer cell lines.
In a second study, additional organometallic gallium(III) compounds (3–7) containing phenyl, thiophenyl, furane, and benzodioxane carboxylato ligands (Figure 6) were synthesized and characterized. The cytotoxic study of all of these compounds showed a dose-dependent antiproliferative effect towards different cancer cell lines such as 8505C, A253, A549, A2780, and DLD-1. The cytotoxic activity of all the studied compounds was much higher than that presented by gallium(III) nitrate. From all the reported complexes, 7 (containing the benzodioxane carboxylate ligand) presented the highest cytotoxicity against A253 cells with the lowest IC50 value of 6.6 ± 0.2 µM [25].
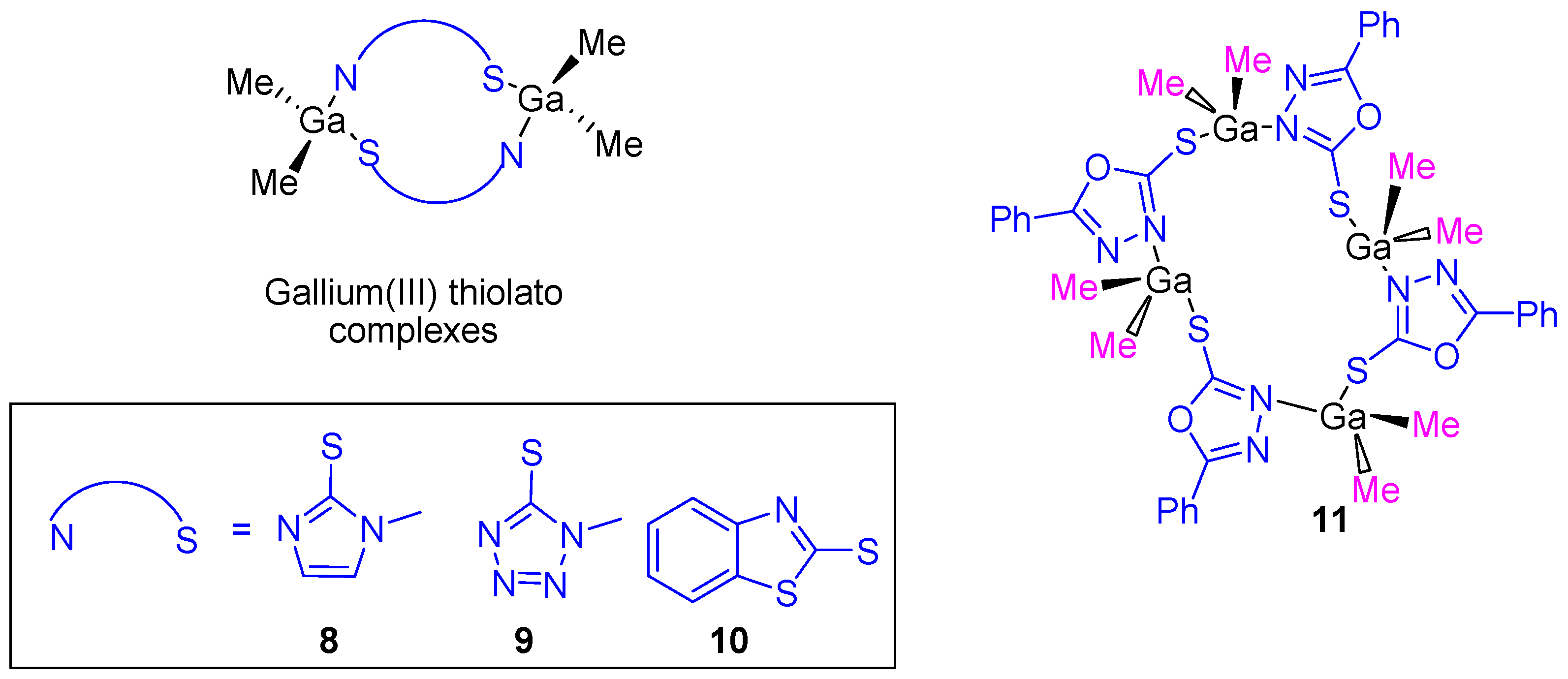
After the cytotoxic studies using the gallium(III) carboxylate complexes, our research group prepared dinuclear and tetranuclear organometallic gallium(III) compounds containing heterocyclic thiolato ligands (Figure 7). These compounds were synthesized by a simple protonolysis reaction of trimethylgallium and the thiol group of mercapto-substituted imidazole, tetrazole, benzothiazole or phenyl-oxadiazol heterocycles (Figure 7). All the compounds were characterized by NMR, IR, and UV–Vis spectroscopy and X-ray diffraction studies confirmed the formation of dinuclear or tetranuclear complexes.
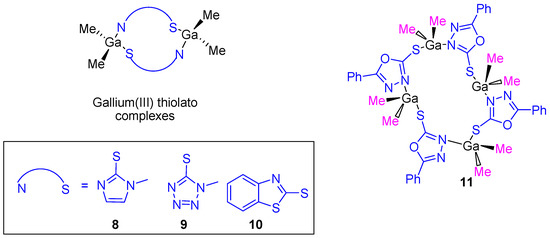
Figure 7.
Heterocyclic thiolate polynuclear gallium(III) derivatives with improved anticancer activity.
Thiolate complexes 8–11 were tested against the same cancer cell lines used on carboxylate gallium compounds (1–7) and again a dose-dependent antiproliferative effect on all cancer cell lines (Table 1) was observed. The cytotoxicity of gallium(III) heterocyclic thiolato complexes is much higher than that of gallium nitrate, while being in the same range as that of cisplatin. An especially high cytotoxic activity was observed for 11 with an IC50 value against DLD-1 of 5.49 ± 0.16 µM which is similar to that observed for cisplatin (5.14 ± 0.12 µM). After selectivity tests of gallium compounds 8–11 and cisplatin on WWO70327 human fibroblasts, gallium(III) complexes were shown to be much more selective to cancer cells than cisplatin, indicating, therefore their potential applicability in anticancer therapy [26]. In the apoptosis studies, after 24 h exposure to IC90 concentrations of compounds 8–11, typical DNA ladders in DLD-1 cell line were observed which indicated the induction of apoptosis promoted by the gallium compounds. In addition, compounds 8–11 showed binding affinity to FS-DNA (confirmed by UV spectroscopy in simulated physiological medium) but not to plasmid pBR322 DNA.
Following the interesting results observed for carboxylate and thiolate gallium(III) complexes (1–11), additional biological studies were carried out on a series of cancer cell lines, HN (soft palate), Cal27 and Cal33 (tongue), FaDu (hypopharynx), and A253 (Submandibular duct) (Table 1) [27]. Gallium(III) complexes 3, 6, and 8 induced cell death mediated apoptosis. Cal27 and FaDu cells were treated for 24 h with IC90 concentration of the complexes and DNA appeared as characteristic ladder-like fragments suggesting an apoptotic cell death promotion. In contrast to the Cal27 cell line, there was a slight translation of FaDu cells from the G1 phase to the apoptotic phase (Sub-G1) after treatment with compounds 3, 6, and 8, which indicates that apoptosis caused by these compounds on FaDu cells may be due to interference caused in the G1 phase of the cell cycle [28].
Finally, gallium(III) complexes 1, 3–8, and 11 were also tested against CT26CL25, HCT116, and SW480 colon cancer cell lines using CV and MTT assays. Compounds 1 and 3–8 affect mitochondrial function, while gallium(III) complex 11 activates different cell death pathways and presents an activity 1.7–3.0 times higher than the other organogallium(III) complexes. In addition, 11 induces caspase independent apoptosis with a strong blockage of first and second division inhibition of CT26CL25 cell proliferation [29].
In view of the biological tests carried out for the organogallium(III) compounds reported by our group, one can envisage that these compounds may be suitable alternatives to KP46 which finished phase I trials with the outcome of promising tolerability and evidence of clinical activity in renal cell carcinoma. However, we have observed that gallium(III) complexes present a limited selectivity on cancer cells. Only in some studies have we observed selectivity when comparing their action against cancer cells with fibroblasts. Thus, the research in this area should be directed to the preparation of new gallium(III) compounds with recognizable fragments to different overexpressed targets in cancer cells to improve the selectivity and cancer cell uptake. In addition, as gallium(III) compounds present water solubility issues, formulation of these compounds with encapsulating agents (such as chitosan or analogues) may increase the solubility or dispersability in water and the cell permeation ability, and should, therefore, be of current interest for the application of these compounds in animal tests. Finally, bearing in mind that our group has not carried out in vivo studies, a complete investigation on the toxicity in animals should be undertaken to determine their potential use in humans.
3. Tin-Based Metallodrugs
The therapeutic properties of triphenyltin acetate in mice tumors was observed in the early 1970s [30], and this discovery triggered a very wide study of other organotin compounds against different cancer cells [31,32]. In this context, a recent study carried out by our group using very simple tricyclohexyltin(IV) compounds demonstrated the potential of tin compounds to overcome multidrug resistance as these metallodrugs are not substrates of the Pgp protein in K562 (leukemia), PANC-1 (pancreatic carcinoma), LN-229 and U87 (multiform glioblastoma) [33].
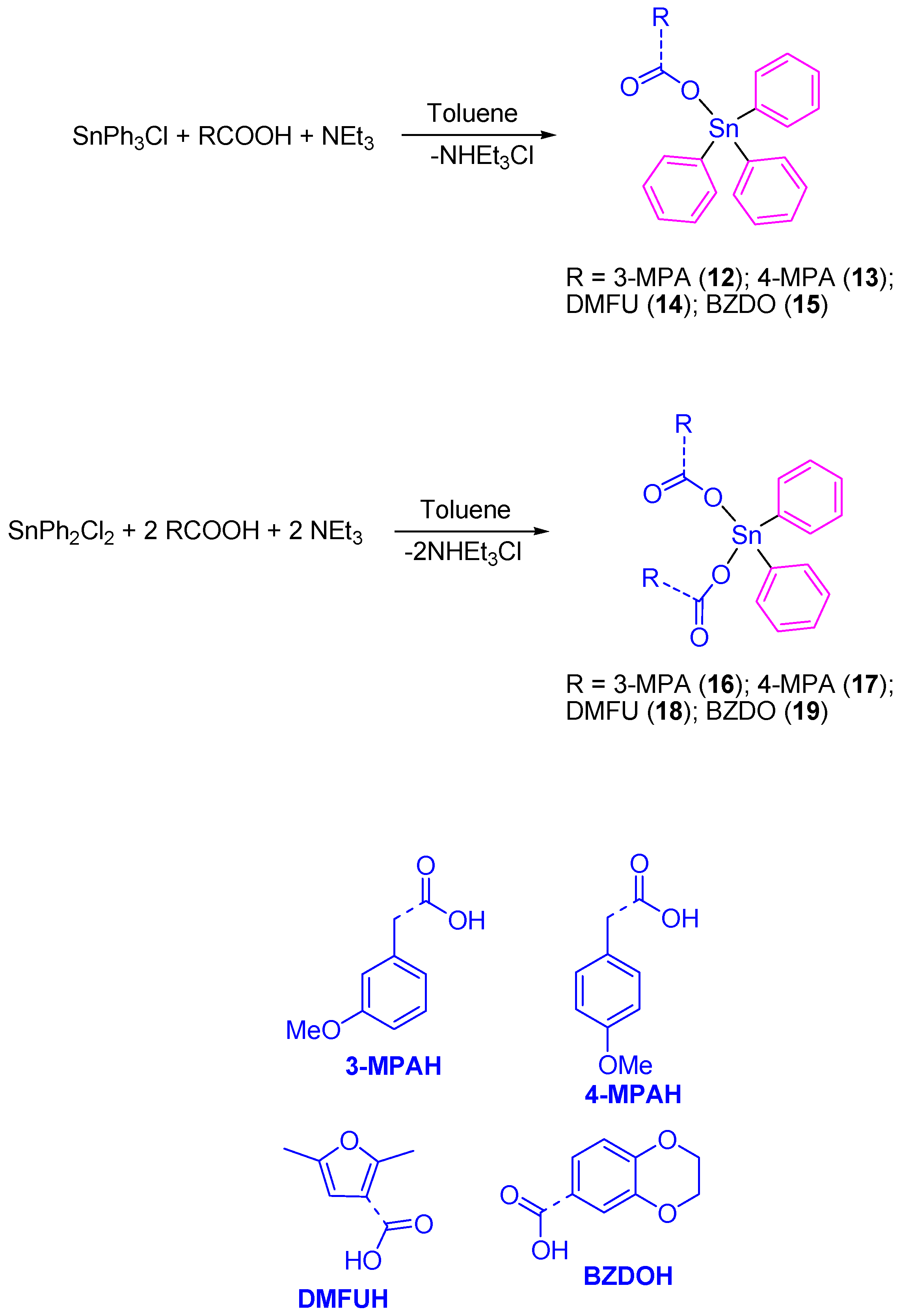
Our research group prepared a series of Sn(IV) compounds namely [SnPh3(3-MPA)] (12), [SnPh3(4-MPA)] (13), [SnPh3(DMFU)] (14), [SnPh3(BZDO)] (15), [SnPh2(3-MPA)2] (16), [SnPh2(4-MPA)2] (17), [SnPh2(DMFU)2] (18), and [SnPh2(BZDO)2] (19) by the reaction of the carboxylic acids 3-methoxyphenylacetic acid (3-MPAH), 4-methoxyphenylacetic acid (4-MPAH), 2,5-dimethyl-3-furoic acid (DMFUH) or 1,4-benzodioxane-6-carboxylic acid (BZDOH) with triphenyltin(IV) chloride or diphenyltin(IV) dichloride, respectively, in the presence of triethylamine (Scheme 1).
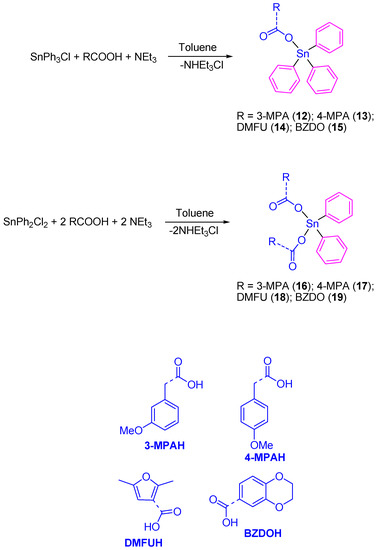
Scheme 1.
Synthesis of tin(IV) complexes 12–19.
All the tin(IV) compounds 12–19 were characterized by multinuclear NMR spectroscopy, mass spectrometry, and IR, and were tested against human adenocarcinoma (HeLa), human myelegenous leukemia (K562), and human malignant melanoma (Fem-x) using MTT-based assays. The carboxylic acids showed no antipoliferative effect under physiological conditions, however, tin(IV) compounds (12–19) showed a dose-dependent antipoliferative effect toward all cell lines and on human PBMC and stimulated PBMC (Table 2). The cytotoxic activity of the compounds was several times higher than that of cisplatin. Notably, compound 14 presented from 30 to 112 times higher activity than that recorded for cisplatin. In this study, we observed that triphenyltin(IV) derivatives presented lower IC50 values against all the studied cancer cell lines than their corresponding diphenyltin(IV) counterparts [34].

Table 2.
IC50 (μM) after 96 h of action of tin compounds 13–21 and cisplatin on different cancer cell lines.
In addition to this study, an analogous triphenyltin(IV) compound containing the 2,6-dimethoxynicotinate ligand was tested against HeLa, K562, Fem-x, and on human peripheral blood mononuclear cells (PMBC) showing a high activity against all evaluated cancer cell lines with a moderate selectivity on K562 compared to unstimulated PBMC [35].
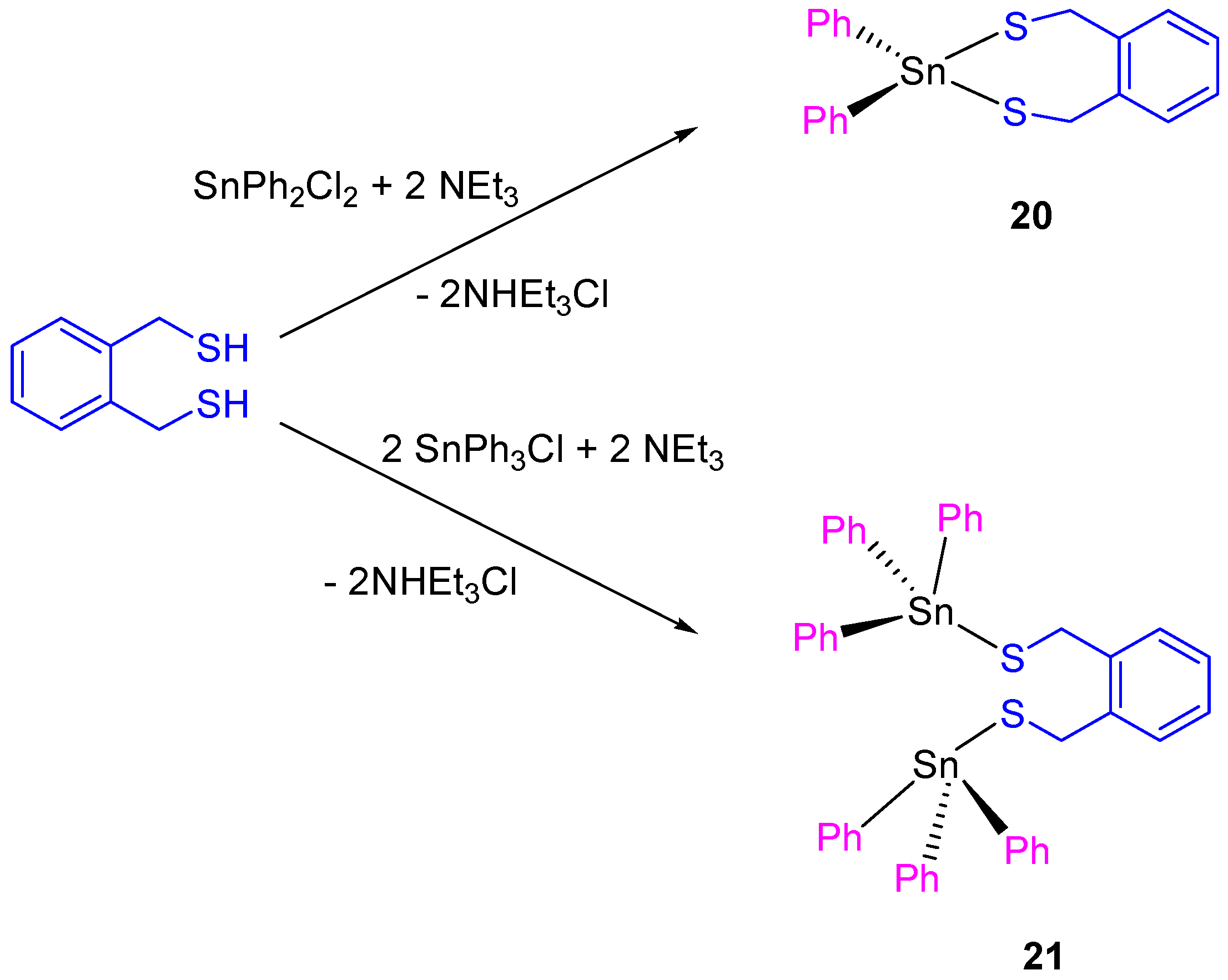
Following the work on tin(IV) compounds, additional thiolate complexes containing α,α’-dimercapto-o-xylene ligand were synthesized (Scheme 2). The compounds 20 and 21 showed a good activity against different cancer cell lines, with IC50 values between 9.7 ± 0.2 and 21.1 ± 1.1 µM (Table 2).
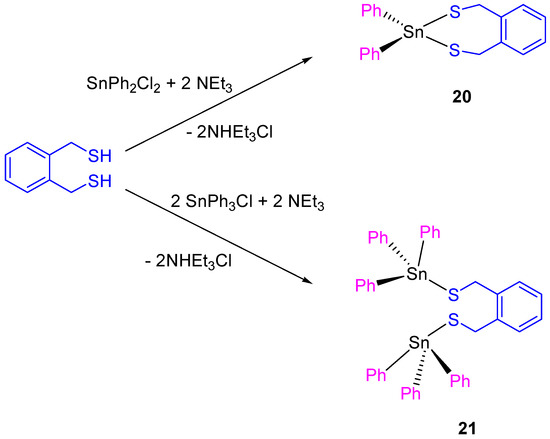
Scheme 2.
Synthesis of Compounds 20 and 21.
The dinuclear tin(IV) compound 21 is more cytotoxic than 20. This result was expected as compound 21 presents two SnPh3 units which are normally associated with increase of cytotoxic activity due to the interaction of SnPh3+ moieties with protein kinases DNA. The cytotoxic activity of 20 and 21 was lower than that reported for carboxylate tin(IV) complexes (12–19) [36]. However, a more in depth study of compound 21 against HeLa and Fem-x cell showed the induction of an apoptotic cell death [37].
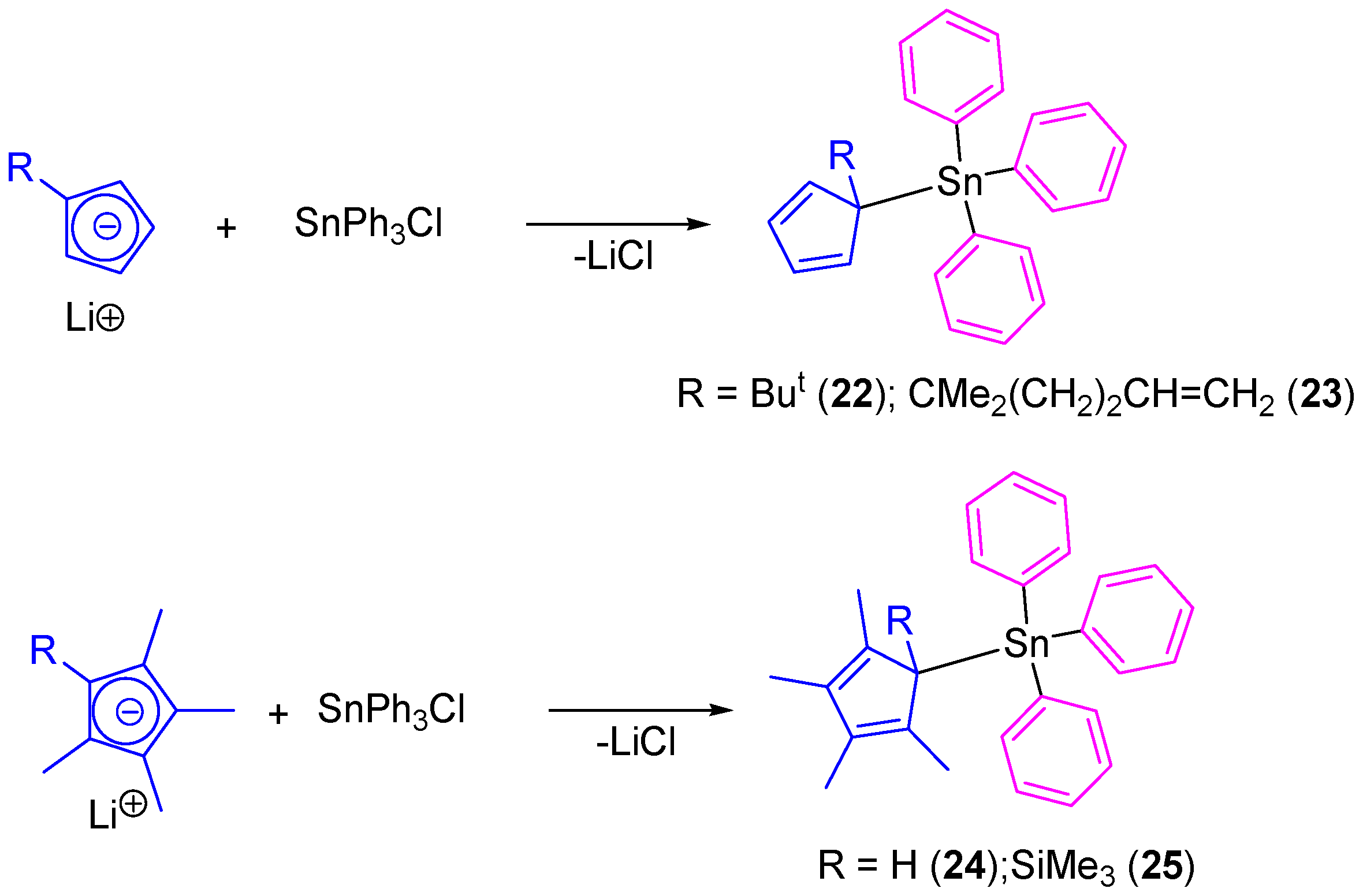
In another study, our group prepared tetraorganotin(IV) compounds containing cyclopentadienyl ligands (22–25) which were prepared by the simple transmetallation reaction of lithium cyclopentadienide derivatives with SnPh3Cl (Scheme 3) [38]. All the compounds were isolated as single isomers even though the position of the double bonds makes possible the formation of a mixture of positional isomers.
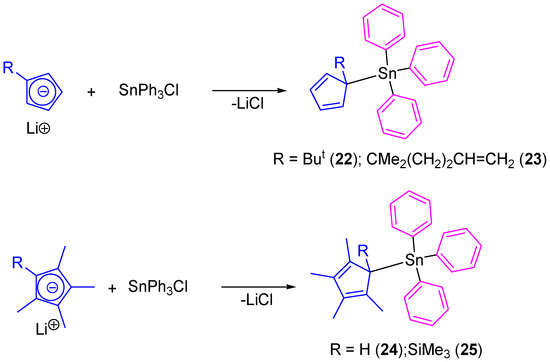
Scheme 3.
Synthesis of cyclopentadienyl-substituted tin(IV) compounds 22–25.
Tin(IV) complexes (22–25) were tested in vitro against 8505C, A253, A549, A2780, and DLD-1 cell lines by using the sulforhodamine-B microculture colorimetric assay (Table 3) [39]. All the compounds showed a dose-dependent antiproliferative effect toward cell lines and presented lower IC50 values than those observed for cisplatin against the same cell lines. From all the series of cyclopentadienyl-substituted tin compounds, 24 (which contains the tetramethylcyclopentadienyl moiety) presented the highest cytotoxic activities against all the studied cancer cell lines with IC50 values between 0.037 and 0.085 µM (from 17 to 104 times higher than cisplatin). Compounds 22 and 23 presented similar activities (0.042–0.103 µM and 0.061–0.119 µM) while 25 had a lower cytotoxicity with IC50 values between 0.163 and 0.384 µM.

Table 3.
IC50 (μM) after 96 h of action of tin compounds 22–30 and cisplatin on different cancer cell lines.
In a subsequent study, a series of rare ionic triphenyltin(IV) chloride carboxylate complexes (26–28, Figure 8) was synthesized and tested against 8505C, A253, A549, A2780, and DLD-1 cell lines (Table 3). All the ionic tin(IV) compounds presented anticancer activities up to 50 times more active than those of cisplatin (for example in DLD-1 complex 26 has an IC50 value of 0.103 µM compared to that of cisplatin of 5.14 µM). Therefore, from this series, 26 seems to be very promising for future applications in clinical trials due to its high solubility, high activity and its capacity to induce a clean apoptotic cell death. This compound affected the G1 and G2/M phases of the cell cycle. Its apoptotic action seems to be related to the interaction between SnPh3+ moieties with protein kinases and DNA [40]. In addition, the apoptotic properties of compound 26 and the interaction with caspases 2, 3, and 8 were studied in DLD-1 cells with only caspase 8 being found to be upregulated after 4 h. However, cells treated for 6 h showed an additional activation of caspase 2 and 8, which was in contrast with the results observed when treating the cells with cisplatin which only showed activation of caspase 8 and 9. These results suggest that 26 promotes a faster activation of apoptosis and that this was achieved in the DLD-1 cell line in a different way to that observed for cisplatin. Respectively, cisplatin promotes apoptosis by both intrinsic (mitochondrial pathway, caspase 9 dependent pathway) and external signals (extrinsic or death receptor pathway), while 26 induces apoptosis only via extrinsic receptor pathway [40].
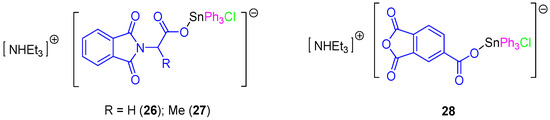
Figure 8.
Ionic triphenyltin(IV) chloride carboxylate complexes with improved cytotoxic activity.
Further studies of the in vitro activity of 26 and 28 against 518A2 (melanoma), FaDu (head and neck carcinoma), HT-29 (colon cancer), MCF-7 (breast carcinoma), and SW1736 (thyroid cancer) cell lines showed the potent cytotoxic activity of 26 and 28 which induce apoptosis. These results were confirmed by the observation of membrane blebbing, translocation of phosphatidylserine, DNA fragmentation, and accumulation of cells in the Sub-G1 phase [41].
Our group prepared two different 1D-polymeric triphenyltin(IV) carboxylate derivatives, based on the reaction of SnPh3Cl with mesitylthioacetic acid and xylythioactic acid. The 1D-chains [{SnPh3(O2CCH2SXyl)}∞] (29) (Xyl = 3,2-Me2C6H3) and [{SnPh3(O2CCH2SMes)}∞] (30) (Mes = 2,4,6-Me3C6H2) were tested in vitro against 8505C, A253, A549, and DLD-1 cell lines observing that they present higher activity (from 8 to 85 times higher) than those of cisplatin (Table 3) and between 285 and 2520 times higher than their gallium(III) and titanocene(IV) analogues, respectively [42]. In addition, these studies showed that compounds 29 and 30 interacted with DNA by classical electrostatic interactions with intrinsic binding constants of 1.68 × 105 and 1.02 × 105 M−1, respectively.
Thus, one can conclude from our results that tin compounds show potential due to the high cytotoxicity that they present in vitro, the possibility of overcoming multidrug resistance [33], and the wide variety of cancer cells that they can treat [43]. In addition, in the age of nanotechnology, their medicinal applications are being enhanced by simple conjugations with silica-based nanomaterials. For example, our group is now working on the support of novel organotin(IV) compounds onto nanostructured silica [44]. Our latest results showed excellent in vitro [45] and in vivo [44] behavior of the new encapsulated systems which have the potential to be used in the future in phase I clinical trials.
4. Titanium-Based Metallodrugs
Although Ti3+ can exist in aqueous media, the aqueous chemistry of titanium is dominated by oxidation state +4 and the tendency of free Ti4+ to hydrolyze and precipitate, ultimately forming insoluble TiO2, is very high. However, hydrolytic reactions can be minimized by surrounding the metal with the appropriate ligands which decreases the rate of the hydrolysis reactions. Thus, the titanium β-diketonate complex, budotitane, was the first non-platinum metal complex to enter clinical trials for treatment of cancer. In this context, cyclopentadienyl ligands are also ideal candidates for improving the hydrolytic stability of titanium(IV) with potential anticancer properties of titanocene dihalide derivatives being observed by Köpf-Maier and Köpf in the 1980s [46].
The preclinical trials of titanium compounds indicated their potential as therapeutic metallodrugs against different tumors [47]. The main biological target of titanium-based metallodrugs is the inhibition of DNA synthesis, triggering apoptosis [48]. Some additional recent studies have reported the inhibition of the enzyme topoisomerase II by titanocene dichloride and this, therefore, may be an alternative cell death induction pathway [49].
Titanocene dichloride was also studied in phase I clinical trials in 1993 [50] and later in phase II clinical trials [51,52] and became very important in the field of antitumor metallodrugs. Although the results of phase II clinical trials were not satisfactory because of the lack of activity against the studied tumors, the excellent research on titanium compounds published by Tacke, Meléndez, McGowan, Baird, Valentine, and Tshuva, reignited the interest in novel titanium compounds with anticancer properties [53,54,55,56,57,58,59,60].
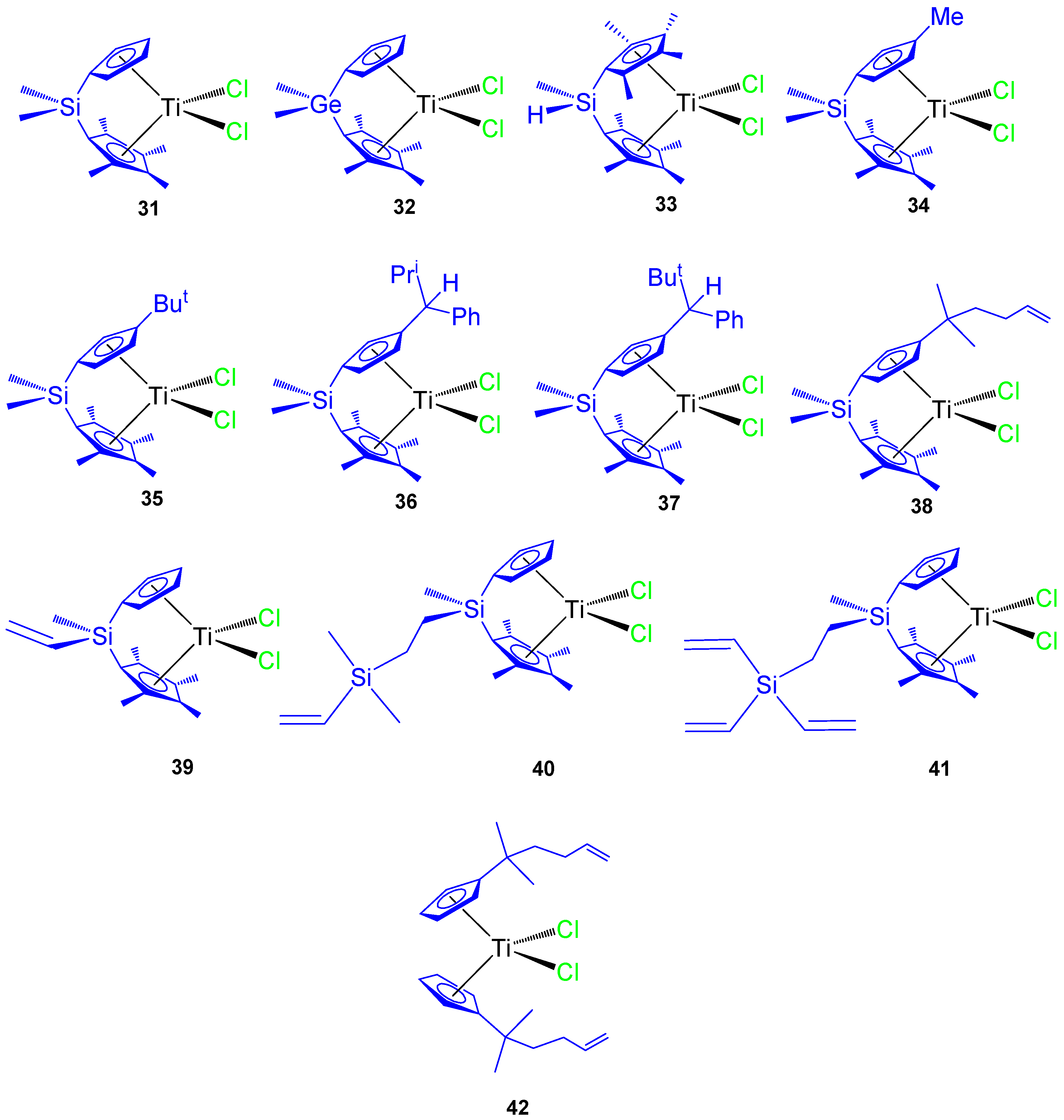
Since 2007 our research group has synthesized different titanocene derivatives which have demonstrated high activity against a series of cancer cell lines. In our first study, titanocene and ansa-titanocene compounds with different alkyl and alkenyl ligands (Figure 9 compounds 31–42) were prepared and characterized. Most of the compounds were active against all the studied cancer cell lines and the activity was dependent on the substituent at the Cp ring or at the ansa-bridge [61,62]. Of special interest were the alkenyl-substituted compounds 38, 39, and 42 which showed improved cytotoxic activity against the studied cell lines HeLa, K562, and Fem-x (Table 4).
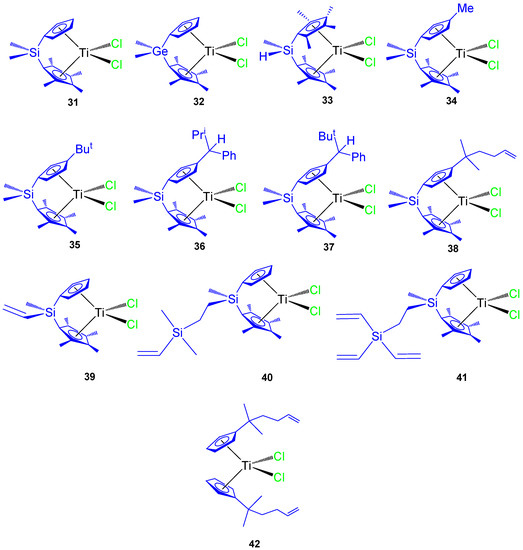
Figure 9.
Titanocene and ansa-titanocene complexes 31–42.

Table 4.
IC50 (μM) of titanium(IV) compounds on different cancer cell lines.
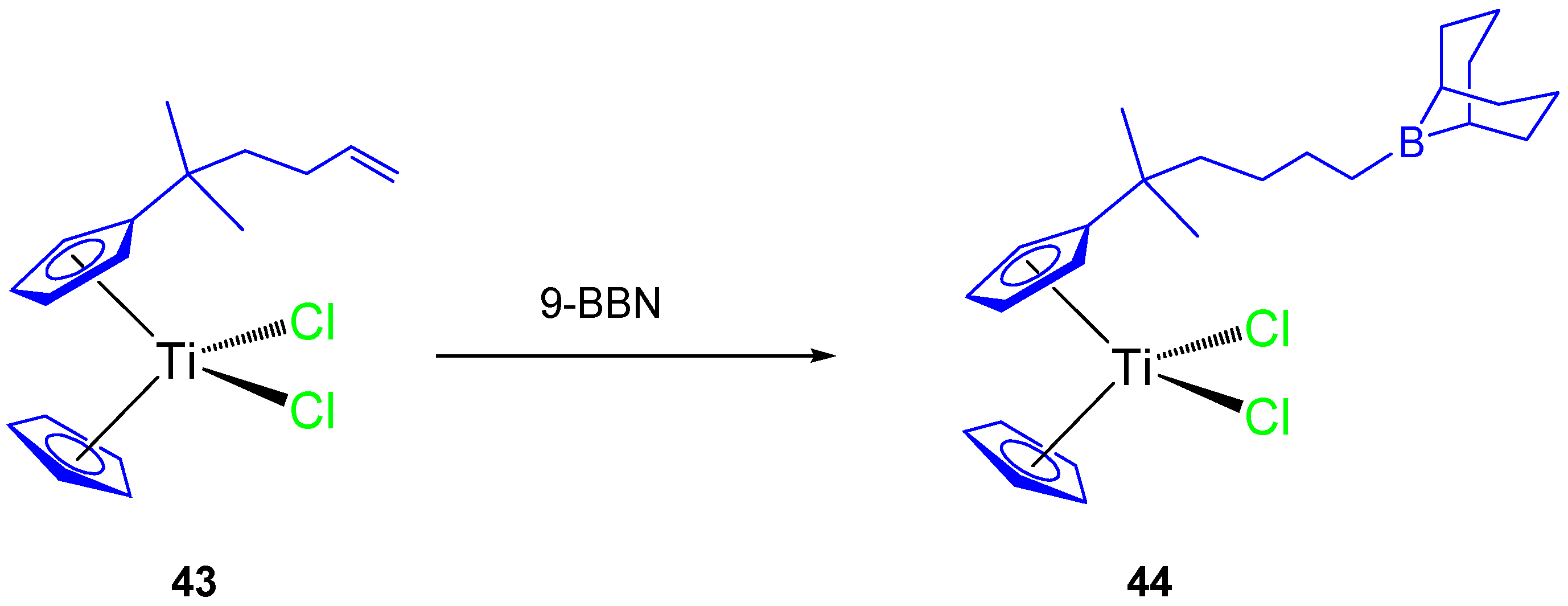
Thus, a subsequent study with an alkenyl monosubstituted titanocene complex (43) and its 9-BBN hydroboration product (44, Scheme 4) were synthesized and characterized. Both compounds were tested against HeLa, K562, and MBA-MB-361 cell lines [63] and showed a dose-dependent antiproliferative effect towards all cell lines and on human PBMC and stimulated PBMC (Table 4).
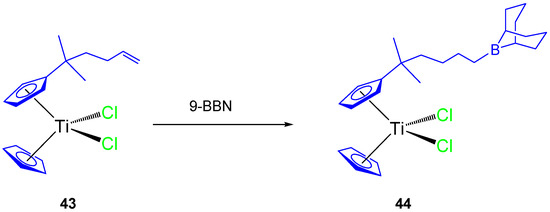
Scheme 4.
Hydroboration reaction of an alkenyl-substituted titanocene derivative.
The alkenyl-substituted complex 44, presented good activity against K562 (IC50 96.6 ± 3.4 µM) and moderate activity on HeLa (IC50 149.2 ± 2.9 µM) and Fem-x (IC50 133.6 ± 9.4 µM), while complex 43 presented only moderate activity on K562, HeLa ,and Fem-x (Table 4).
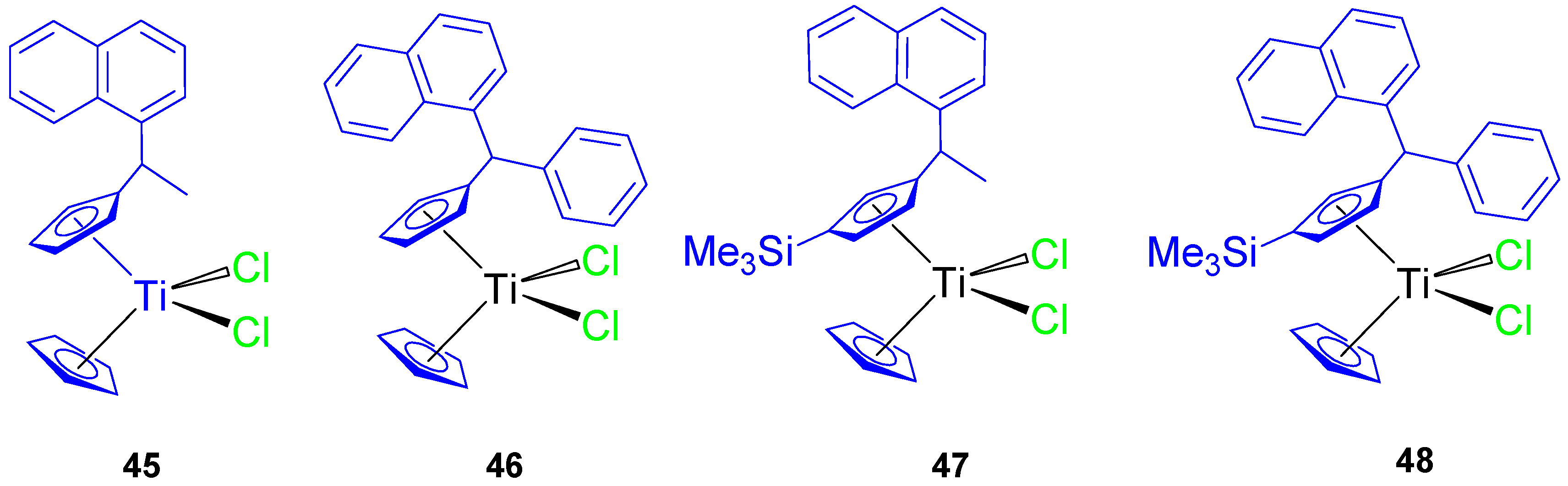
Subsequently, a series of naphthyl-substituted titanocene compounds (45–48) were also synthesized and characterized by our group (Figure 10) [64]. The molecular structure of 46 was established by single-crystal X-ray diffraction studies.
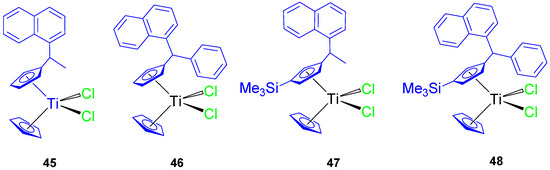
Figure 10.
Naphthyl-substituted titanocene(IV) dichloride complexes.
In anticancer tests against 8505C, A549, A2780, DLD-1, and FaDu, the titanocene(IV) complexes (45–48) showed a significant cytotoxic activity with IC50 values (Table 5) lower than titanocene dichloride. Compound 48 was the most active of all the tested compounds, with IC50 values between 35.65 ± 4.95 and 69.02 ± 1.67 µM. The improvements in cytotoxic activity of 48 were due to the presence of the trimethylsilyl group.

Table 5.
IC50 (μM) of titanocene compounds (43–48 and 59–70) on different cancer cell lines.
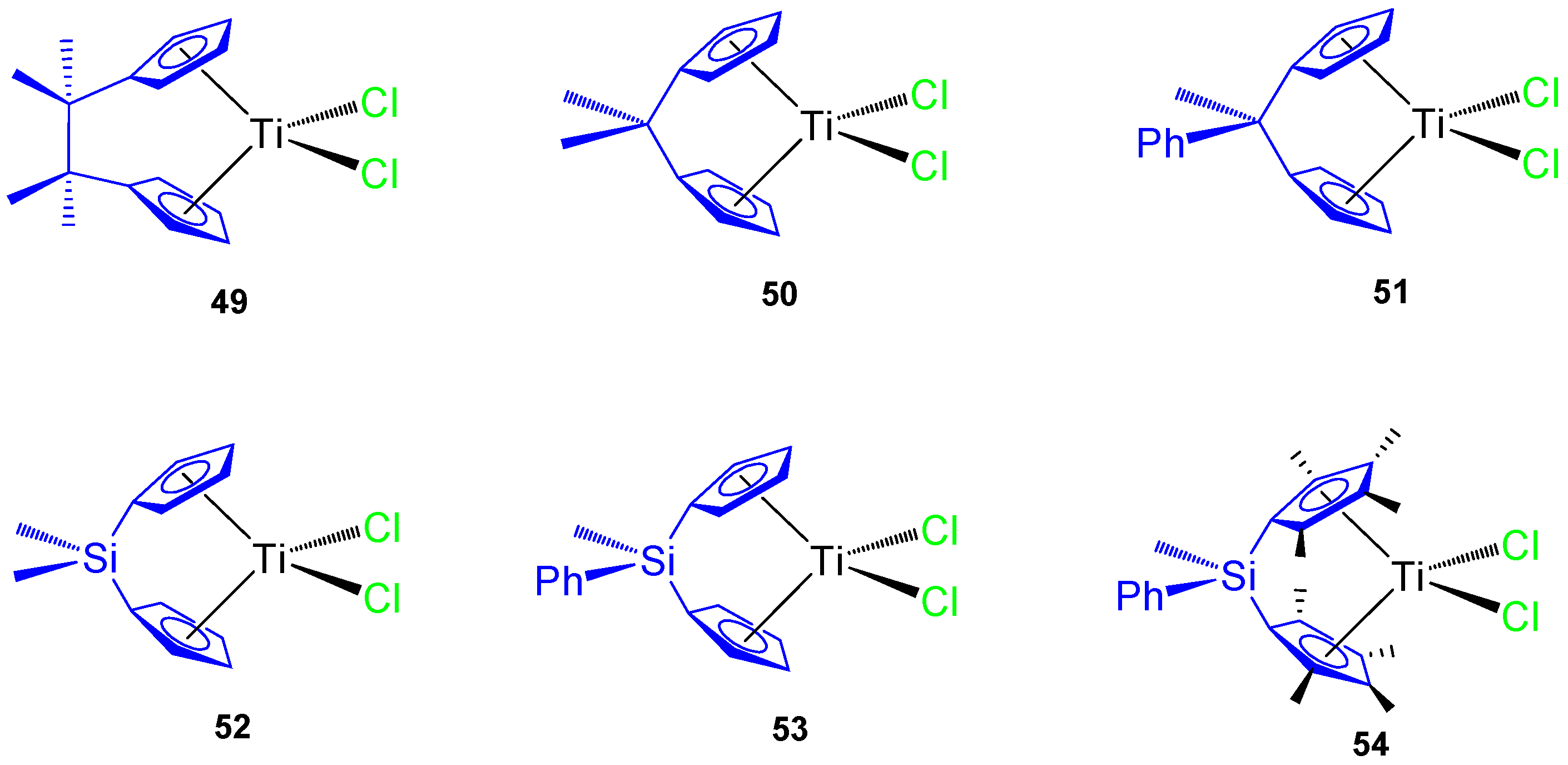
In addition, several carbon and silicon-bridged ansa-titanocene(IV) derivatives were synthesized (Figure 11) and tested against different tumor cell lines, namely murine melanoma B16, human melanoma A375, colon cancer HCT116 and SW620, prostate cancer LNCaP and DU145, and mouse colon cancer CT26CL25.
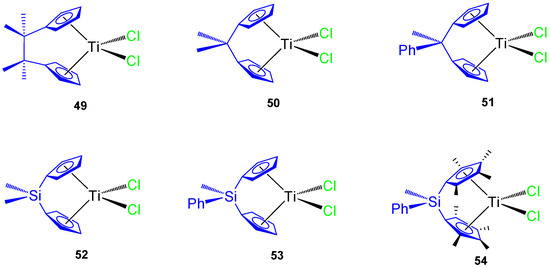
Figure 11.
ansa-Titanocene derivatives.
The C-bridged ansa-titanocene derivatives showed an increase in cytotoxic activity with an ethylene bridge (complex 49) and poor activity with methylene (complexes 50 and 51) while the incorporation of a phenyl ring attached directly to the bridging atom decreases the viability of the cancer cells in carbon-bridged compounds (51), but increases the viability of cancer cells in silicon-bridged systems (53 and 54) (Table 6). The most cytotoxic titanocene complexes 49 and 54 were also tested against primary mouse keratinocynates and lung fibroblasts while observing a large viability of both primary cells and that 54 was nontoxic to primary cells [65]. In addition, 49 and 54 showed accumulation of hypodiploid cells in subG compartment in cisplatin resistant HCT116 and SW620.

Table 6.
IC50 (μM) of ansa-titanocene compounds (49–54) on different cancer cell lines.
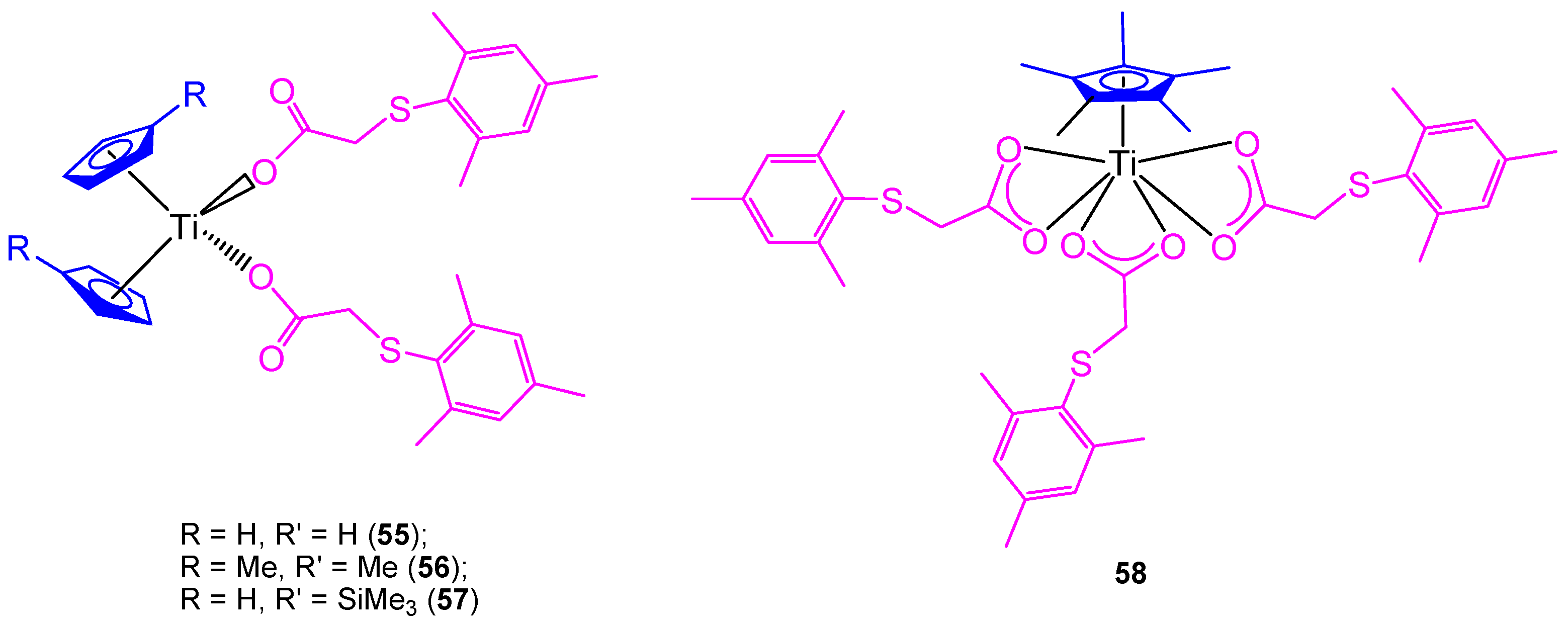
All the previously described titanocene and ansa-titanocene compounds were chloride derivatives, however, a complete study of the substitution of the chlorido by carboxylato ligands was carried out using mesitylthioacetic acid and different cyclopentadienyl ligands (Figure 12) [66].
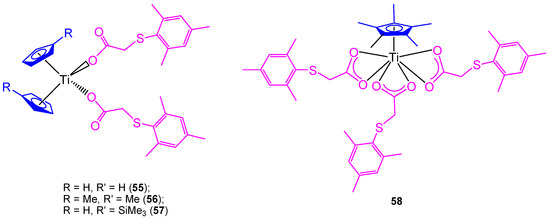
Figure 12.
Titanium carboxylate complexes containing the mesitylthioaceticatato ligand.
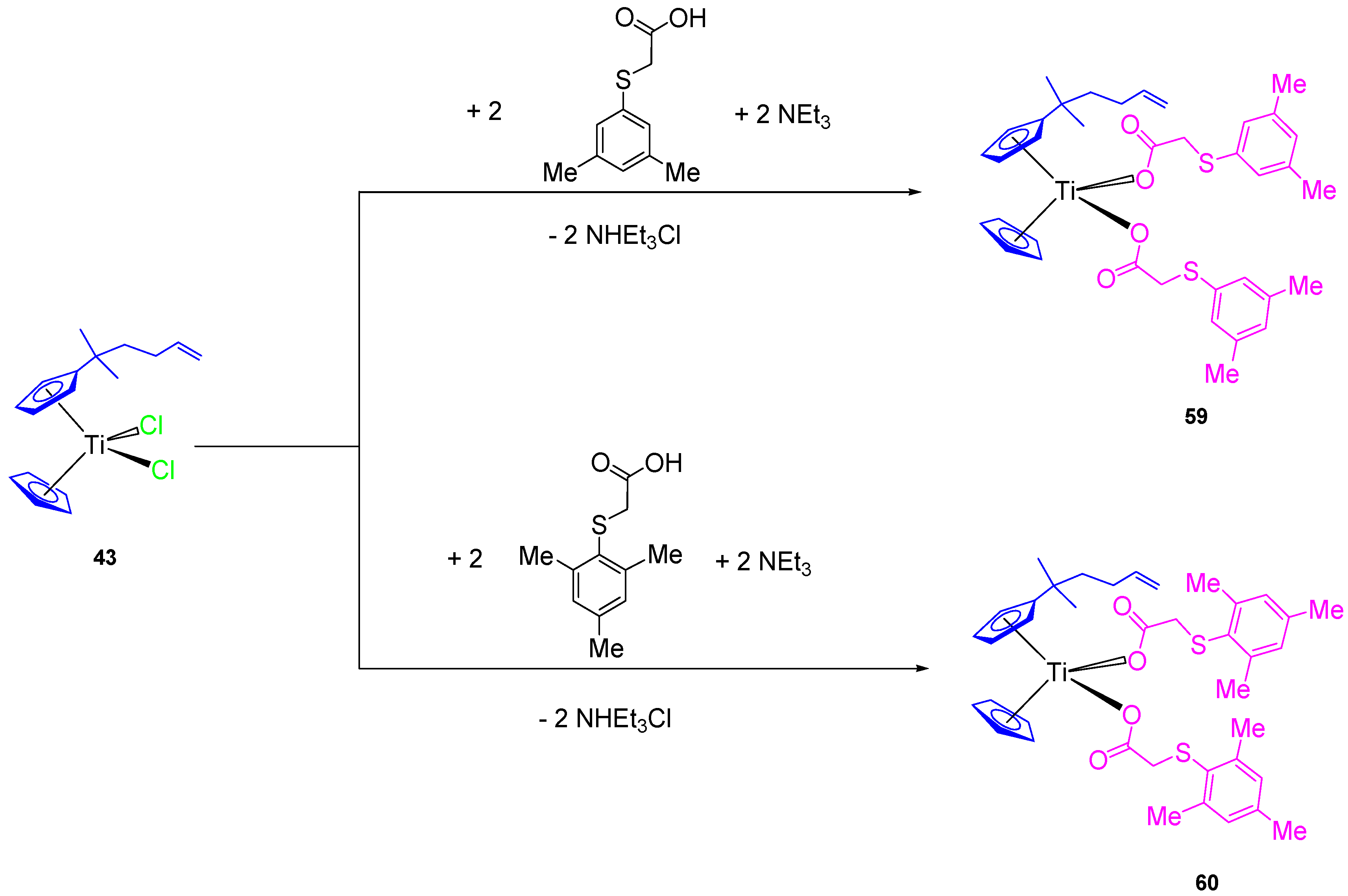
In addition, two new alkenyl-substituted titanocene(IV) carboxylate complexes containing the mesitylthioacetato and the xilylthioacetato ligands (59 and 60, respectively, Scheme 5) were synthesized and characterized. The comparison of cytotoxic activities of the titanocene(IV) carboxylate and titanocene(IV) dichloride against 8505C, A253, A549, and DLD-1, showed that titanocene(IV) carboxylates (59 and 60) are less active against all the studied cells than their corresponding dichloride counterpart (43) (Table 5), indicating that the effect of the carboxylato ligands on the cytotoxicity is not synergistic but negative in the case of alkenyl-substituted titanocene compounds [67]. This was confirmed in the DNA interaction tests, where complex 43 showed a higher intrinsic binding constant than 59 and 60.
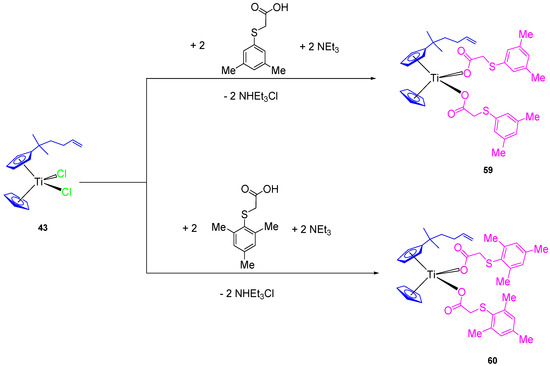
Scheme 5.
Synthesis of alkenyl-substituted titanocene(IV) derivatives with carboxylato ligands.
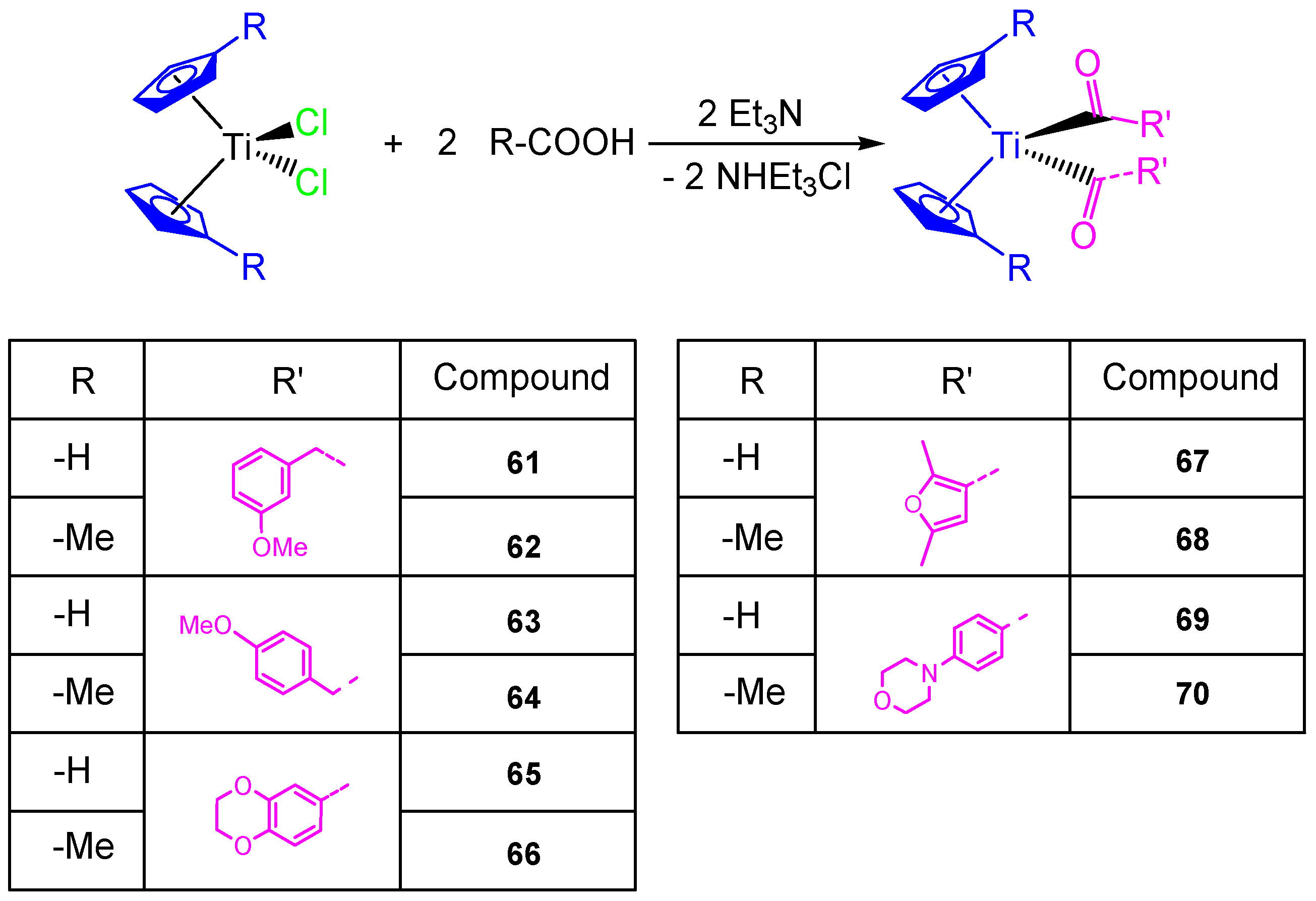
This study on the influence of carboxylato ligands on the cytotoxic activity of titanocene complexes was completed by the preparation of a wide variety of titanocene carboxylate derivatives of the type [Ti(η5-C5H5)2(OOC-L)2] and [Ti(η5-C5H4Me)2(OOC-L)2] with different carboxylato ligands such as 3-methoxyphenylacetato, 4-methoxyphenylacetato, 1,4-benzodioxane-6-carboxylato, 2,5-dimethyl-3-furoato, and 4-(4-morpholinyl)benzoato (Scheme 6). All the carboxylate compounds 61–70 showed a higher cytotoxic activity than [Ti(η5-C5H5)2Cl2] or [Ti(η5-C5H4Me)2Cl2] against ovarian cell line (A2780), with IC50 values from 40.54 ± 4.39 to 82.14 ± 8.95 µM (Table 5). In addition, the DNA binding studies carried out in simulated body fluid showed the weak interaction of the titanocene compounds with DNA [68].
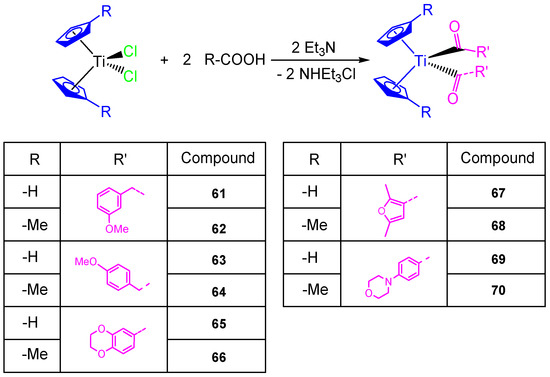
Scheme 6.
Synthesis of alkenyl-substituted titanocene(IV) derivatives with carboxylato ligands.
Our group also synthesized titanocene compounds containing the α,α’-dimercapto-o-xylene as a thiolato ligand (71 and 72, Figure 13) and tested their efficacy against human tumor cell lines HeLa, Fem-x, K562 (Table 4) [36]. When the biological activity was analyzed, an improvement of the cytotoxic activity was observed compared with [Ti(η5-C5H5)2Cl2] and [Ti(η5-C5H4Me)2Cl2] against the cell lines tested K562, HeLa, and Fem-x with a higher cytotoxicity of 72 and a slight preference against K562 (Table 4) [36].
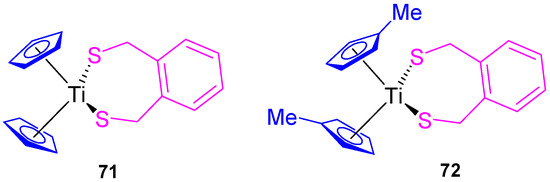
Figure 13.
Titanium(IV) complexes with dmox as ligand.
Our group also synthesized two titanium(IV) complexes anchored by a tripodal diamine bis(phenolate) ligands (73 and 74) which showed hydrolytic stability and a high cytotoxic activity against HeLa, K562, Fem-x and MDA-MB-453 with IC50 values between 22.4 ± 1.2 and 48.9 ± 0.8 µM [69].
After the extensive study of our group on the biological applications of titanocene derivatives with different substituents either at the Cp ring or directly bound to titanium (31–72), we observed that most of the synthesized compounds (especially those containing thiolato or carboxylato ligands) show a low hydrolytic stability. Therefore, the anticancer action of these compounds is usually due to decomposition products which are soluble in water and/or DMSO and that are formed after the elimination of one or more ligands [70]. Thus, we focused on the formulation of these titanium derivatives via functionalization of mesoporous silica-based nanostructured materials such as MCM-41, SBA-15, MSU-2, alumina or hydroxyapatite [71,72,73,74] in order to overcome the problems associated with the low hydrolytic stability of titanocene compounds.
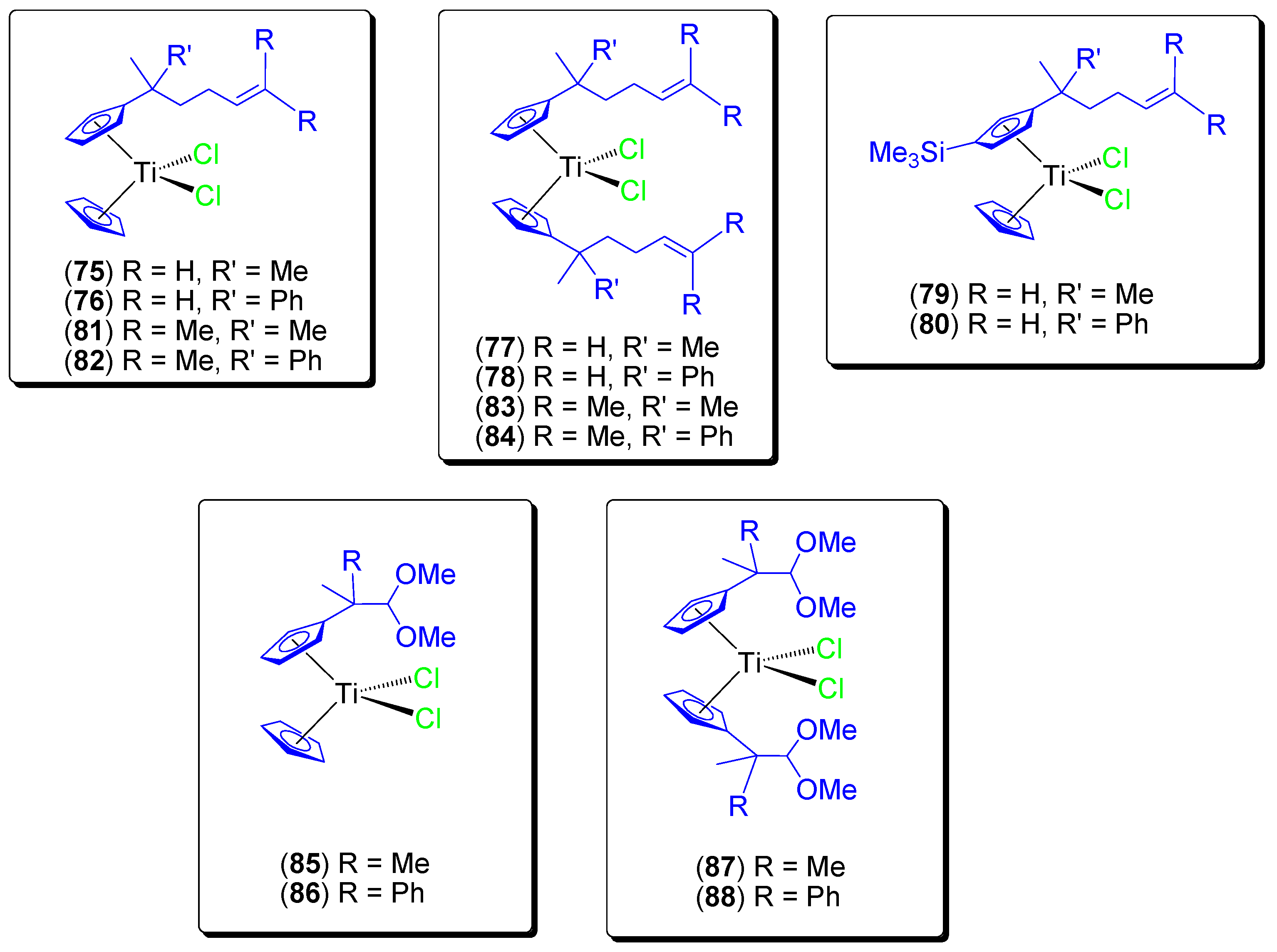
During these studies and in an effort to encapsulate titanocene compounds on KIT-6, additional alkenyl substituted (75–80) [75] and ether-substituted (81–84) [76] titanocene(IV) dichloride compounds were synthesized (Figure 14), characterized and tested in vitro against a wide variety of cancer cell lines (Table 7). We observed a very high cytotoxicity (IC50 values in the range of those described in the literature for the most active cytotoxic titanocene compounds such as titanocene-Y synthesized by Tacke) with high selectivity towards cancer cell lines.
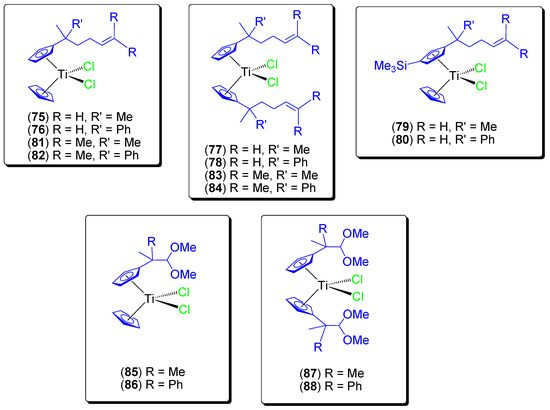
Figure 14.
Titanocene(IV) complexes with alkenyl- or ether-substituents at the Cp rings.

Table 7.
IC50 (μM) of titanocene compounds (75–87) on different cancer cell lines.
Finally, after the incorporation of the compounds in KIT-6, a higher Ti-uptake by the treated cancer cells (from 4% to 23% of the initial amount of Ti) was observed when compared with the “free” titanocene compounds giving clear insights on the positive effect of the encapsulation with nanostructured silica.
5. Metallodrugs Based on Other Metals
As previously explained in the introduction of this review, non-platinum compounds are being considered as an alternative to cisplatin-like compounds because their preclinical trials indicate that they might be capable of reducing the relatively high number of side effects associated with platinum treatments. In recent years, promising results have been obtained using other metallodrugs of main group or transition metal complexes, however, less attention has been paid to lanthanide and actinide compounds [77].
In this context, our research group synthesized a series of metal complexes of Y(III), La(III), Ce(III), Nd(III), Sm(III), and Yb(III) with p-substituted-cinnamate and p-substituted phenylacetate ligands. The toxicity of these compounds against immunocompetent cells (mice macrophages and erythrocytes) was tested. In addition, the cytotoxicity against specific human cancer cell lines such as HL60 (human promyelocytic leukemia), K562 (human erythromyeloblastoid leukemia), and MCF7 (breast cancer) was studied. All the lanthanide compounds tested showed a dose-dependent toxic activity which began to be significant from 400 µM. The cytotoxic activities of all the compounds synthesized were very low with IC50 values between 542.7 and >750 µM. This indicates that the studied lanthanide complexes with cinnamate and/or phenylacetate ligands were not appropriate for cancer therapy [78].
In spite of the discouraging results with lanthanides, recently, our group studied the cytotoxic properties of a novel Dy-based metallodrug [DyNa(ampy)4]n (88), a metalorganic framework prepared from 5-aminopyridine-2-carboxilic acid (Hampy) as ligand [79]. The structure of this compound was determined by X-ray crystallography. [DyNa(ampy)4]n (88) was tested against colon carcinoma cells, HT-29, DLD-1, and Caco-2 (Table 8) showing a moderate cytotoxicity especially against DLD-1. More interestingly, we observed that the combination of treatment with the dysprosium compound with a short exposure to a magnetic field led to a reduction of proliferation in all the cell lines. In addition, after short exposure to a magnetic field the multidrug-resistant properties of this 1D-MOF changed. Thus, our multidisciplinary preliminary study relating magnetic properties with cytotoxicity of MOFs is a very interesting starting point for further studies of different magnetic lanthanides or actinides in cancer therapy.

Table 8.
Cytotoxic results of [DyNa(ampy)4]n (88) on HT-29, DLD-1, and Caco-2.
6. Conclusions
The use of metallodrugs is still a field of upmost interest for the scientific community and a high number of reports on this topic are being published. In this context our group has carried out basic scientific work in the field of metallodrugs of gallium, tin, and titanium and demonstrated a structure activity relationship which may be of interest for drug-design.
It seems clear that the limitations of the metallodrugs regarding side-effects, low solubility, and low bioavailability in the human body due to their low hydrolytic stability are very difficult to address from a monodisciplinary point of view. However, these problems associated with metallodrugs can be overcome by the use of a mixed metallodrug-nanotechnological approaches such as encapsulation of metal-based drugs in different nanostructured materials. For example, the use of liposomes, lipid nanocapsules, human proteins, ceramic materials, carbon nanotubes, and metal or metal oxide nanoparticles with anticancer metallodrugs should be of great interest. Thus, by combining nanomaterials and metallodrugs to obtain more potent and reliable formulations, we can predict that a bright future still lies ahead for metal-based drugs in anticancer chemotherapy.
Acknowledgments
We would like to thank to all the people involved in the work of our group during the last 10 years. In addition, we would like to thank Ministerio de Economía y Competitividad, Spain (Grant No. CTQ2015-66164-R) and the Universidad Rey Juan Carlos-Banco de Santander (Excellence Group QUINANOAP) for their support.
Author Contributions
Younes Ellahioui, Sanjiv Prashar and Santiago Gómez-Ruiz contributed in the search, explanation and discussion of the cited literature references.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sadler, P.J. Inorganic Chemistry and Drug Design. Adv. Inorg. Chem. 1991, 36, 1–48. [Google Scholar]
- Williams, K.J. The introduction of ‘chemotherapy’ using arsphenamine—The first magic bullet. J. R. Soc. Med. 2009, 102, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Camp, L.V. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970, 30, 1799–1802. [Google Scholar] [PubMed]
- Giaccone, G. Clinical perspectives on platinum resistance. Drugs 2000, 59, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Thornback, J.R. Medicinal Applications of Coordination Chemistry; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Gómez-Ruiz, S.; Maksimović, I.D.; Mijatović, S.; Kaluderović, G.N. On the Discovery, Biological Effects, and Use of Cisplatin and Metallocenes in Anticancer Chemotherapy. Bioinorg. Chem. Appl. 2012, 2012, 140284. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, J. Why does Cisplatin reach Guanine-N7 with competing S-donor ligands available in the cell? Chem. Rev. 1999, 99, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S. Cellular processing of platinum anticancer drugs. J. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Cleare, M.J.; Hoeschele, J.D.; Rosenberg, B.; VanCamp, L. Malonato Platinum Anti-Tumor Compounds. U.S. Patent 4,140,707, 19 December 1989. [Google Scholar]
- Kidani, Y.; Masahide, N. Cytostatic Platinum Organic Complexes. U.S. Patent 4,710,577, 1 December 1987. [Google Scholar]
- Boulikas, T. Clinical overview on Lipoplatin: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18, 1197–1218. [Google Scholar] [CrossRef] [PubMed]
- Decatris, M.P.; Sundar, S.; O’Byrne, K.J. Platinum-based chemotherapy in metastatic breast cancer: Current status. Cancer Treat. Rev. 2004, 30, 53–81. [Google Scholar] [CrossRef]
- Bernstein, L.R. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar] [PubMed]
- Green, M.A.; Welch, M.J. Gallium radiopharmaceutical chemistry. Int. J. Radiat. Appl. Instrum. B 1989, 16, 435–443. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Keppler, B.K. Gallium in cancer treatment. Curr. Top. Med. Chem. 2004, 4, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.J.; Zhu, F.; Frasca, D.R. Non-platinum chemotherapeutic metallopharmaceuticals. Chem. Rev. 1999, 99, 2511–2533. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R.; Zivkovic, Z. Uptake of gallium-67 by human leukemic cells: Demonstration of transferrin receptor-dependent and transferrin-independent mechanisms. Cancer. Res. 1987, 47, 3929–3934. [Google Scholar] [PubMed]
- Narasimhan, J.; Antholine, W.E.; Chitambar, C.R. Effect of gallium on the tyrosyl radical of the iron-dependent M2 subunit of ribonucleotide reductase. Biochem. Pharmacol. 1992, 44, 2403–2408. [Google Scholar] [CrossRef]
- Chitambar, C.R.; Wereley, J.P.; Matsuyama, S. Gallium-induced cell death in lymphoma: Role of transferrin receptor cycling, involvement of Bax and the mitochondria, and effects of proteasome inhibition. Mol. Cancer. Ther. 2006, 5, 2834–2843. [Google Scholar] [CrossRef] [PubMed]
- Timerbaev, A.R. Advances in developing tris(8-quinolinolato)gallium(III) as an anticancer drug: Critical appraisal and prospects. Metallomics 2009, 1, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Nordenberg, J.; Novogrodsky, A.; Beery, E.; Patia, M.; Wasserman, L.; Warshawsky, A. Anti-proliferative effects and phenotypic alterations induced by 8-hydroxyquinoline in melanoma cell lines. Eur. J. Cancer 1990, 26, 905–907. [Google Scholar] [CrossRef]
- Baerga, R.; Cobb, J.; Ogden, A.; Sheshbaradaran, H. NKP-2235 exhibits a novel pattern of cytotoxicity with synergism across a broad range of antitumor agents demonstrated in multiple tumor types. In Cancer Research, Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research, Chicago, IL, USA, 31 March–4 April 2012; AACR: Philadelphia, PA, USA, 2012; Volume 72, (Suppl. S8), Abstract nr 3838. [Google Scholar]
- Kumar, K.; Schniper, S.; González-Sarrías, A.; Holder, A.A.; Sanders, N.; Sullivan, D.; Jarrett, W.L.; Davis, K.; Bai, F.; Seeram, N.P.; et al. Highly potent anti-proliferative effects of a gallium(III) complex with 7-chloroquinoline thiosemicarbazone as a ligand: Synthesis, cytotoxic and antimalarial evaluation. Eur. J. Med. Chem. 2014, 86, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, S.; Gallego, B.; Kaluderovic, M.R.; Kommera, H.; Hey-Hawkins, E.; Paschke, R.; Kaluderovic, G.N. Novel gallium(III) complexes containing phthaloyl derivatives of neutral aminoacids with apoptotic activity in cancer cells. J. Organomet. Chem. 2009, 694, 2191–2197. [Google Scholar] [CrossRef]
- Kaluđerović, M.R.; Gómez-Ruiz, S.; Gallego, B.; Hey-Hawkins, E.; Paschke, R.; Kaluđerović, G.N. Anticancer activity of dinuclear gallium(III) carboxylate complexes. Eur. J. Med. Chem. 2010, 45, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Gallego, B.; Kaluđerović, M.R.; Kommera, H.; Paschke, R.; Hey-Hawkins, E.; Remmerbach, W.T.; Kaluđerović, G.N.; Gómez-Ruiz, S. Cytotoxicity, apoptosis and study of the DNA-binding properties of bi- and tetranuclear gallium(III) complexes with heterocyclic thiolato ligands. Investig. New Drugs 2011, 29, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, R.M.; Kaluđerović, N.G.; Gómez-Ruiz, S.; Paschke, R.; Hemprich, A.; Kühling, J.; Remmerbach, W.T. Organogallium(III) complexes as apoptosis promoting anticancer agents for head and neck squamous cell carcinoma (HNSCC) cell lines. J. Inorg. Biochem. 2011, 105, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zanias, S.; Papaefstathiou, G.S.; Raptopoulou, C.P.; Papazisis, K.T.; Vala, V.; Zambouli, D.; Kortsaris, A.H.; Kyriakidis, D.A.; Zafiropoulos, T.F. Synthesis, Structure, and Antiproliferative Activity of Three Gallium(III) Azole Complexes. Bioinorg. Chem. Appl. 2010, 2010, 168030. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, R.M.; Mojić, M.; Gómez-Ruiz, S.; Mijatović, S.; Maximović, I.D. Anticancer Activity of Organogallium(III) Complexes in Colon Cancer Cells. Anticancer Agents Med. Chem. 2016, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.J. The chemotherapeutic properties of tin compounds. Drugs Future 1987, 12, 255–275. [Google Scholar]
- Gielen, M. Tin-Based Antitumor Drugs; Springer: Berlin, Germany, 1990; Volume 37. [Google Scholar]
- Gielen, M.; Tiekink, E.R.T. Tin Compounds and Their Therapeutic Potential Met-Allotherapeutic Drugs and Metal-Based Diagnostic Agents. The Use of Metalsin Medicine; J. Wiley & Sons: New York, NY, USA, 2005; pp. 421–439. [Google Scholar]
- Rocamora-Reverte, L.; Carrasco-García, E.; Ceballos-Torres, J.; Prashar, S.; Kaluđerović, G.N.; Ferragut, J.A.; Gómez-Ruiz, S. Study of the Anticancer Properties of Tin(IV) Carboxylate Complexes on a Panel of Human Tumor Cell Lines. ChemMedChem 2012, 7, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, S.; Kaluđerović, G.N.; Prashar, S.; Hey-Hawkins, E.; Erić, A.; Žižak, Z.; Juranić, D.Z. Study of the cytotoxic activity of di and triphenyltin(IV) carboxylate complexes. J. Inorg. Biochem. 2008, 102, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, S.; Žižak, Z.; Kaluđerović, G.N. A triphenyltin(IV) nicotinate derivative—Synthesis and toxicity towards different tumour and normal cell lines. Lett. Drug Des. Discov. 2012, 9, 737–741. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Stanojković, T.P.; Kaluđerović, G.N. Synthesis, characterization, biological studies and in vitro cytotoxicity on human cancer cell lines of titanium(IV) and tin(IV) derivatives with the α,α’-dimercapto-o-xylene ligand. Appl. Organomet. Chem. 2012, 26, 383–389. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Žižak, Z.; Kaluđerović, G.N. Structural studies and cytotoxic activity against human cancer cell lines of mono and dinuclear tin(IV) complexes with the α,α’-dimercapto-o-xylene ligand. Inorg. Chim. Acta 2014, 423, 117–122. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Prashar, S.; Walther, T.; Fajardo, M.; Steinborn, D.; Paschke, R.; Kaluđerović, G.N. Cyclopentadienyltin (IV) derivatives: Synthesis, characterization and study of their cytotoxic activities. Polyhedron 2010, 29, 16–23. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, G.N.; Kommera, H.; Hey-Hawkins, E.; Paschke, R.; Gómez-Ruiz, S. Synthesis and biological applications of ionic triphenyltin(IV) chloride carboxylate complexes with exceptionally high cytotoxicity. Metallomics 2010, 2, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Hubner, D.; Kaluderovic, M.R.; Gómez-Ruiz, S.; Kaluderovic, G.N. Anionic chlorido(triphenyl)tin(IV) bearing N-phthaloylglycinato or 1,2,4-benzenetricarboxylato 1,2-anhydride ligands: Potential cytotoxic and apoptosis-inducing agents against several types of cancer. Chem. Biol. Drug Des. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, G.N.; Paschke, R.; Prashar, S.; Gómez-Ruiz, S. Synthesis, characterization and biological studies of 1-D polymeric triphenyltin(IV) carboxylates. J. Organomet. Chem. 2010, 695, 1883–1890. [Google Scholar] [CrossRef]
- Williams, J.L.; Lewis-Alleyne, L.C.; Solomon, M.; Nguyen, L.; Johnson, R.; Vital, J.; Ji, P.; Durant, J.; Cooper, C.; Cagle, P.; et al. An in vitro study on the effect of synthesized tin(IV) complexes on glioblastoma, colorectal, and skin cancer cell lines. Biomed. Res. Clin. Pract. 2016, 1, 7–15. [Google Scholar] [CrossRef]
- Bulatović, M.Z.; Maksimović-Ivanić, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korać, A.; Golić, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-Loaded Mesoporous Silica as a Biocompatible Strategy in Cancer Treatment. Angew. Chem. Int. Ed. 2014, 53, 5982–5987. [Google Scholar] [CrossRef] [PubMed]
- Bensing, C.; Mojić, M.; Gómez-Ruiz, S.; Carralero, S.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Evaluation of functionalized mesoporous silica SBA-15 as a carrier system for Ph3Sn(CH2)3OH against the A2780 ovarian carcinoma cell line. Dalton Trans. 2016, 45, 18984–18993. [Google Scholar] [CrossRef] [PubMed]
- Köpf-Maier, P.; Köpf, H. Non-platinum group metal antitumor agents. History, current status, and perspectives. Chem. Rev. 1987, 87, 1137–1152. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M. Antitumour titanium compounds. Mini Rev. Med. Chem. 2004, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kopf-Maier, P. Complexes of metals other than platinum as antitumour agents. Eur. J. Clin. Pharmacol. 1994, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mokdsi, G.; Harding, M.M. Inhibition of human topoisomerase II by the antitumor metallocenes. J. Inorg. Biochem. 2001, 83, 205–209. [Google Scholar] [CrossRef]
- Korfel, A.; Scheulen, M.E.; Schmoll, H.J.; Gründel, O.; Harstrick, A.; Knoche, M.; Fels, L.M.; Skorzec, M.; Bach, F.; Baumgart, J.; et al. Phase I clinical and pharmacokinetic study of titanocene dichloride in adults with advanced solid tumors. Clin. Cancer Res. 1998, 4, 2701–2708. [Google Scholar] [PubMed]
- Kröger, N.; Kleeberg, U.R.; Mross, K.; Edler, L.; Hossfeld, D.K. Phase II Clinical Trial of Titanocene Dichloride in Patients with Metastatic Breast Cancer. Onkologie 2000, 23, 60–62. [Google Scholar] [CrossRef]
- Lümmen, G.; Sperling, H.; Luboldt, H.; Otto, T.; Rübben, H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemother. Pharmacol. 1998, 42, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Melendez, E. Titanium complexes in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 309–315. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M. Metal Ions in Biological System, Vol. 42: Metal Complexes in Tumor Diagnostics and as Anticancer Agents; Sigel, A., Sigel, H., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Dabrowiak, J.C. Metals in Medicine; Wiley: West Sussex, UK, 2009. [Google Scholar]
- Olszewski, U.; Hamilton, G. Mechanisms of Cytotoxicity of Anticancer Titanocenes. Anticancer Agents Med. Chem. 2010, 10, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Strohfeldt, K.; Tacke, M. Bioorganometallic fulvene-derived titanocene anti-cancer drugs. Chem. Soc. Rev. 2008, 37, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- Tshuva, E.Y.; Peri, D. Modern cytotoxic titanium(IV) complexes; insights on the enigmatic involvement of hydrolysis. Coord. Chem. Rev. 2009, 253, 2098–2115. [Google Scholar] [CrossRef]
- Ramos, G.; Loperena, Y.; Ortiz, G.; Szeto, A.; Vera, J.; Velez, J.; Morales, J.; Morrero, D.; Castillo, L.; Dharmawardhane, S.; et al. The addition of Pregnenolone Group Enhances the Anticancer Properties of Titanocene Dichloride in a MCF-7 Xenograft Model. Anticancer Res. 2014, 34, 1609–1615. [Google Scholar] [PubMed]
- Gao, L.M.; Maldonado, W.; Narváez-Pita, X.; Carmona-Negrón, J.A.; Olivero-Verbel, J.; Meléndez, E. Steroid-Functionalized Titanocenes: Docking Studies with Estrogen Receptor Alpha. Inorganics 2016, 4, 38. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Kaluđerović, G.N.; Polo-Cerón, D.; Prashar, S.; Fajardo, M.; Žižak, Z.; Juranić, D.Z.; Sabo, J.T. Study of the cytotoxic activity of alkenyl-substituted ansa-titanocene complexes. Inorg. Chem. Commun. 2007, 10, 748–752. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Kaluđerović, G.N.; Polo-Cerón, D.; Prashar, S.; Fajardo, M.; Žižak, Z.; Sabo, J.T.; Juranić, D.Z. Cytotoxic studies of substituted titanocene and ansa-titanocene anticancer drugs. J. Inorg. Biochem. 2008, 102, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, S.; Kaluđerović, G.N.; Žižak, Z.; Bisu, I.; Juranić, D.Z.; Prashar, S.; Fajardo, M. Anticancer drugs based on alkenyl and boryl substituted titanocene complexes. J. Organomet. Chem. 2009, 694, 1981–1987. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Gómez-Ruiz, S.; Kaluđerović, G.N.; Fajardo, M.; Paschke, R.; Prashar, S. Naphthyl-substituted titanocene dichloride complexes: Synthesis, characterization and in vitro studies. J. Organomet. Chem. 2012, 700, 188–193. [Google Scholar] [CrossRef]
- Mijatović, S.; Bulatović, M.; Mojić, M.; Stošić-Grujičić, S.; Miljković, D.; Maksimović-Ivanić, D.; Gómez-Ruiz, S.; Pinkas, J.; Horáček, Ñ.; Kaluđerović, G.N. Study of the anticancer properties of methyl- and phenyl-substituted carbon- and silicon-bridged ansa-titanocene complexes. J. Organomet. Chem. 2014, 751, 361–367. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Gallego, B.; Žižak, Z.; Hey-Hawkins, E.; Juranić, D.Z.; Kaluđerović, G.N. Titanium(IV) carboxylate complexes: Synthesis, structural characterization and cytotoxic activity. Polyhedron 2010, 29, 354–360. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Tayurskaya, V.; Paschke, R.; Prashar, S.; Fajardo, M.; Gómez-Ruiz, S. Synthesis, characterization and biological studies of alkenyl-substituted titanocene(IV) carboxylate complexes. Appl. Organomet. Chem. 2010, 24, 656–662. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Caballero-Rodríguez, M.; Prashar, S.; Paschke, R.; Steinborn, D.; Kaluđerović, G.N.; Gómez-Ruiz, S. Synthesis, characterization and in vitro biological studies of titanocene(IV) derivatives containing different carboxylato ligands. J. Organomet. Chem. 2012, 716, 201–207. [Google Scholar] [CrossRef]
- Barroso, S.; Coelho, M.A.; Gómez-Ruiz, S.; Calhodra, M.J.; Žižak, Z.; Kaluđerović, G.N.; Martins, A.M. Synthesis, cytotoxic and hydrolytic studies of titanium complexes anchored by a tripodal diamine bis(phenolate) ligand. Dalton. Trans. 2014, 43, 17422–17433. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, P.M.; Harding, M.M. Antitumour bis(cyclopentadienyl) metal complexes: Titanocene and molybdocene dichloride and derivatives. Dalton Trans. 2007, 32, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Žižak, Z.; Sierra, I.; Prashar, S.; Del Hierro, I.; Fajardo, M.; Juranić, D.Z.; Kaluđerović, G.N. A New Generation of Anticancer Drugs: Mesoporous Materials Modified with Titanocene Complexes. Chem. Eur. J. 2009, 15, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Sierra, I.; Prashar, S.; Del Hierro, I.; Žižak, Z.; Juranić, D.Z.; Fajardo, M.; Gómez-Ruiz, S. Study of the influence of the metal complex on the cytotoxic activity of titanocene-functionalized mesoporous materials. J. Mater. Chem. 2010, 20, 806–814. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Žižak, Z.; Juranić, D.Z.; Gómez-Ruiz, S. Improvement of cytotoxicity of titanocene-functionalized mesoporous materials by the increase of the titanium content. Dalton Trans. 2010, 39, 2597–2608. [Google Scholar] [CrossRef] [PubMed]
- García-Peñas, A.; Gómez-Ruiz, S.; Pérez-Quintanilla, D.; Paschke, R.; Sierra, I.; Prashar, S.; Del Hierro, I.; Kaluđerović, G.N. Study of the cytotoxicity and particle action in human cancer cells of titanocene-functionalized materials with potential application against tumors. J. Inorg. Biochem. 2012, 116, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Torres, J.; Virag, P.; Cenariu, M.; Prashar, S.; Fajardo, M.; Fischer-Fodor, E.; Gómez-Ruiz, S. Anticancer Applications of Titanocene-Functionalized Nanostructured Systems: An Insight into Cell Death Mechanisms. Chem. Eur. J. 2014, 20, 10811–10828. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Chicca, A.; Gertsch, J.; Pinar, A.B.; Gómez-Ruiz, S. Ether-substituted group 4 metallocene complexes: Cytostatic effects and applications in ethylene polymerization. Organometallics 2015, 34, 2522–2532. [Google Scholar] [CrossRef]
- Wani, W.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured Materials Functionalized with Metal Complexes: In Search of Alternatives for Administering Anticancer Metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Camprubí-Robles, M.; González-Rey, E.; Salinas-Castillo, A.; Rodríguez-Diéguez, A.; Gómez-Ruiz, S.; Polo-Cerón, D. Dual investigation of lanthanide complexes with cinnamate and phenylacetate ligands: Study of the cytotoxic properties and the catalytic oxidation of styrene. Polyhedron 2014, 80, 117–128. [Google Scholar] [CrossRef]
- Fernandez, B.; Oyarzabal, I.; Fischer-Fodor, E.; Macavei, S.; Sánchez, I.; Seco, J.M.; Gómez-Ruiz, S. Multifunctional Applications of a Dysprosium-Based Metal-Organic Chain with Single-Ion Magnet Behaviour. CrystEngComm 2016, 18, 8718–8721. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).