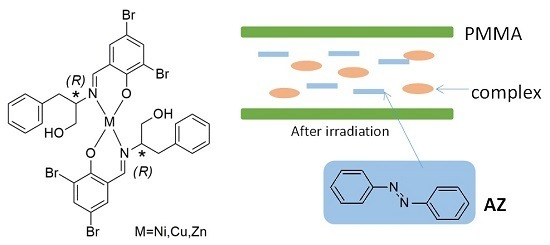
The Viscosity and Intermolecular Interaction of Organic and Inorganic Hybrid Systems Composed of Chiral Schiff Base Ni(II), Cu(II), and Zn(II) Complexes with Long Ligands, Azobenzene, and PMMA
Abstract
:1. Introduction
2. Results
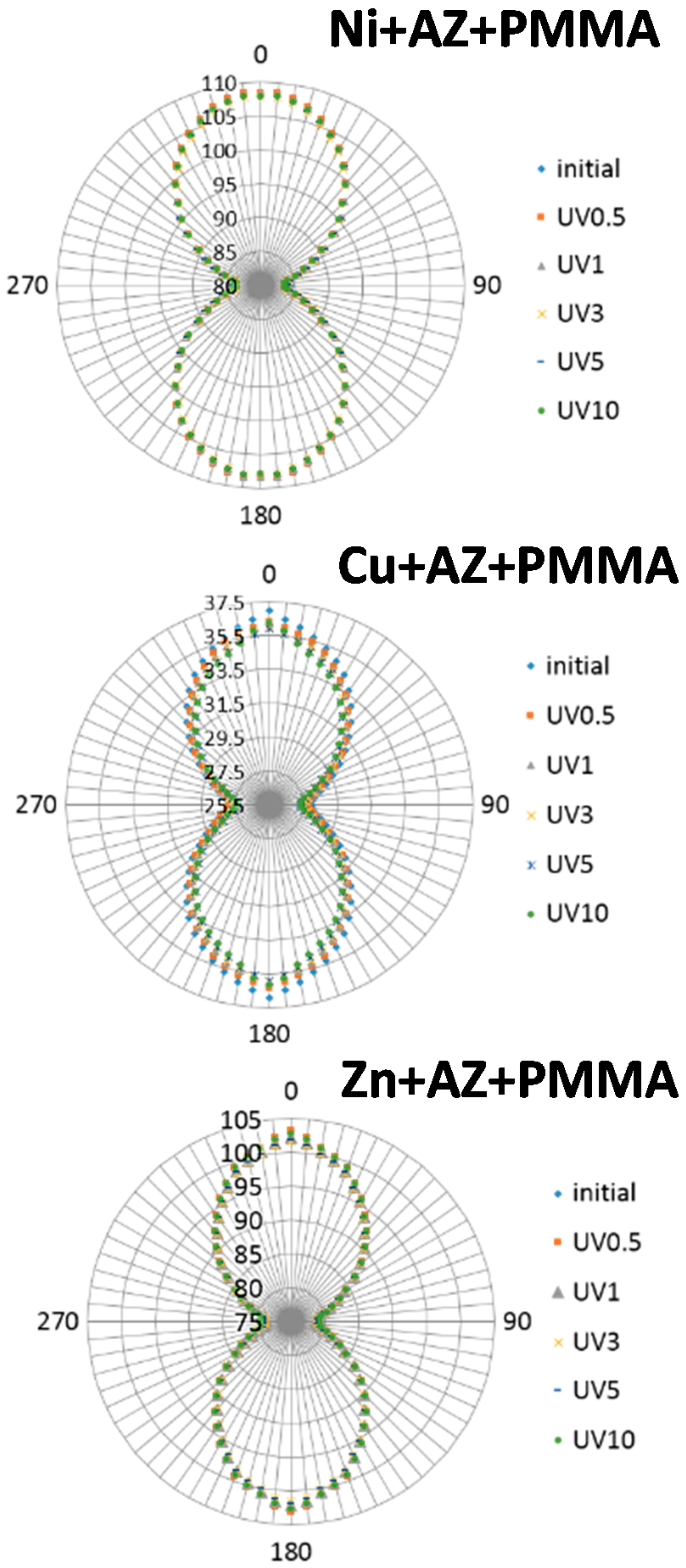
2.1. Polarized IR Spectra
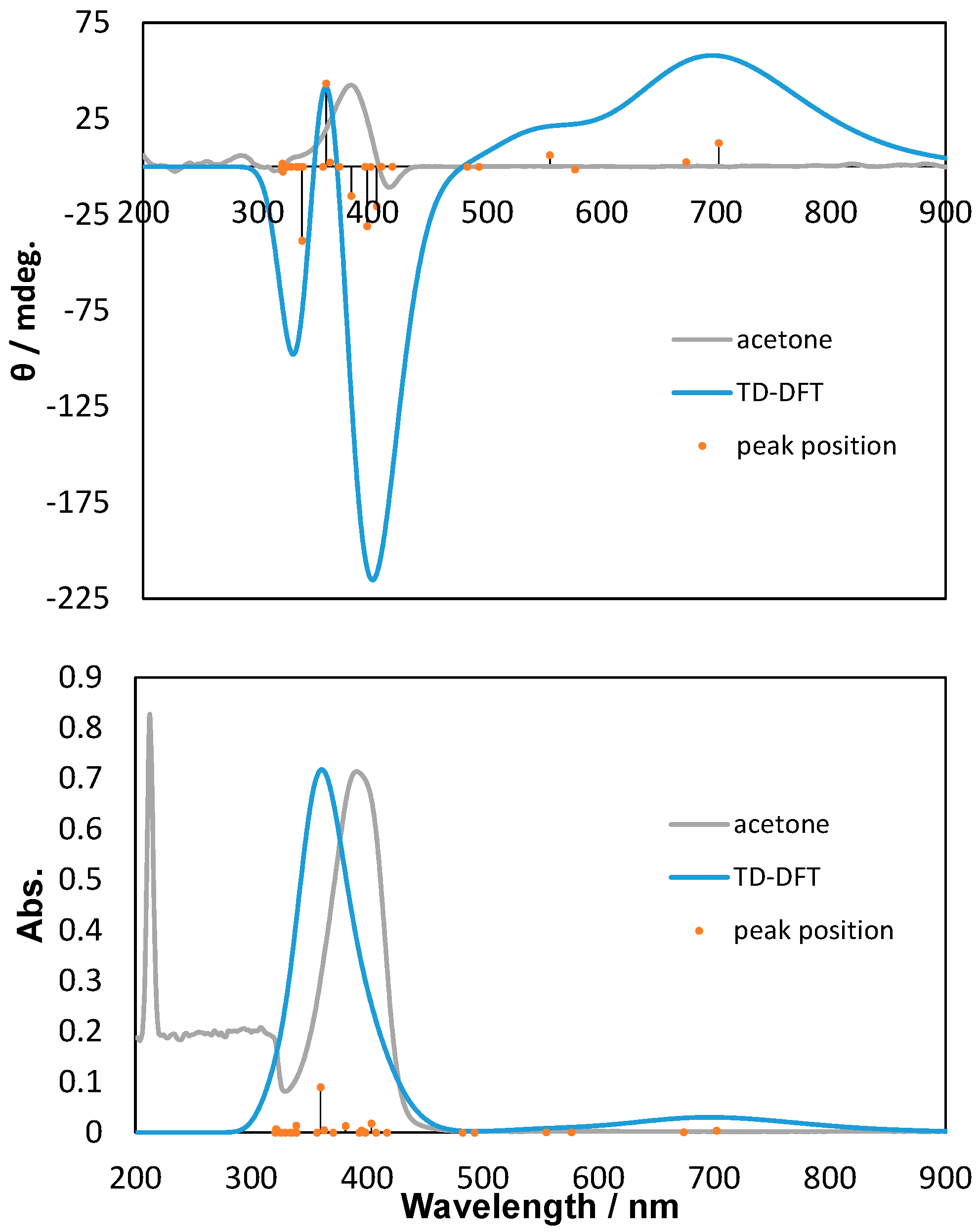
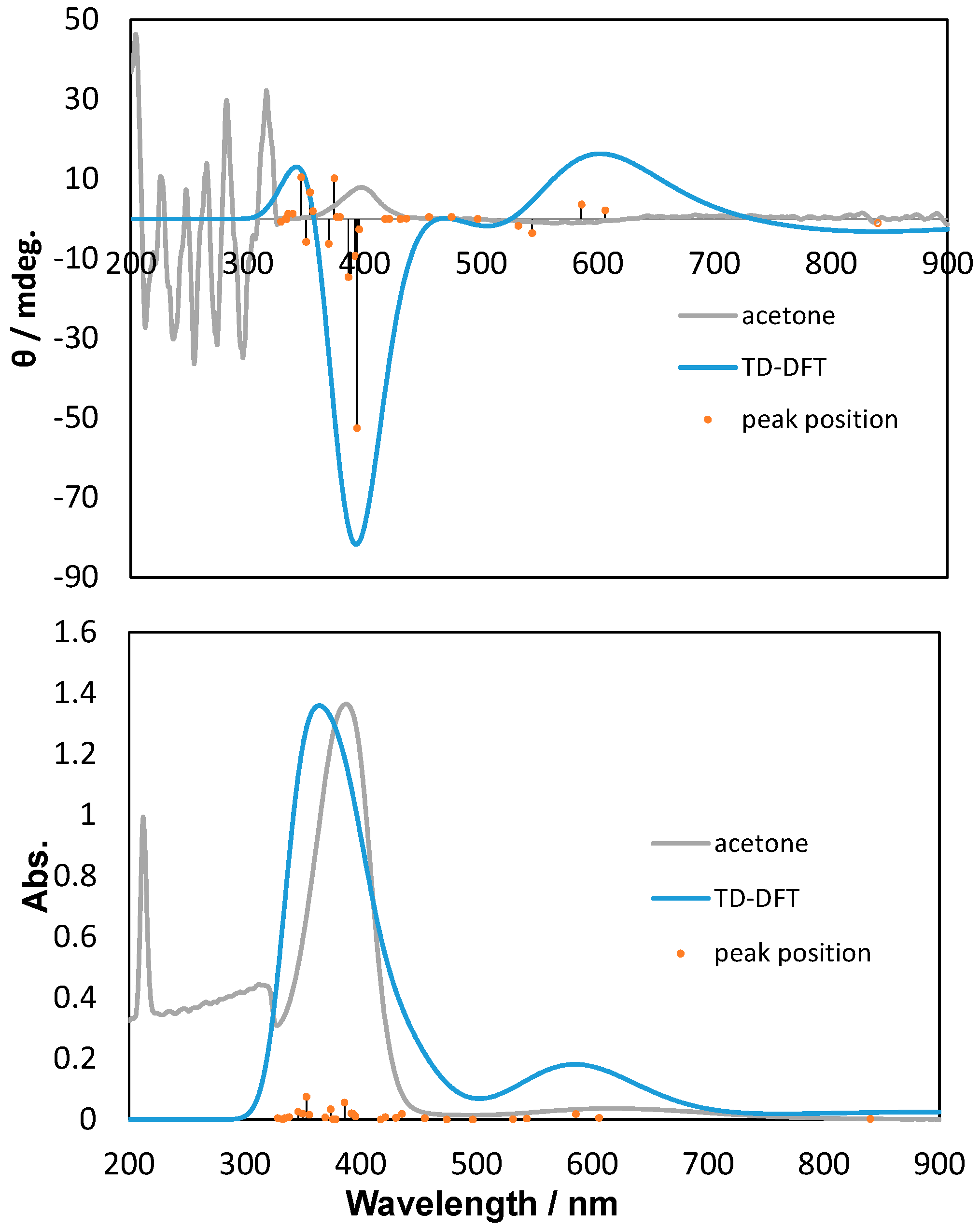
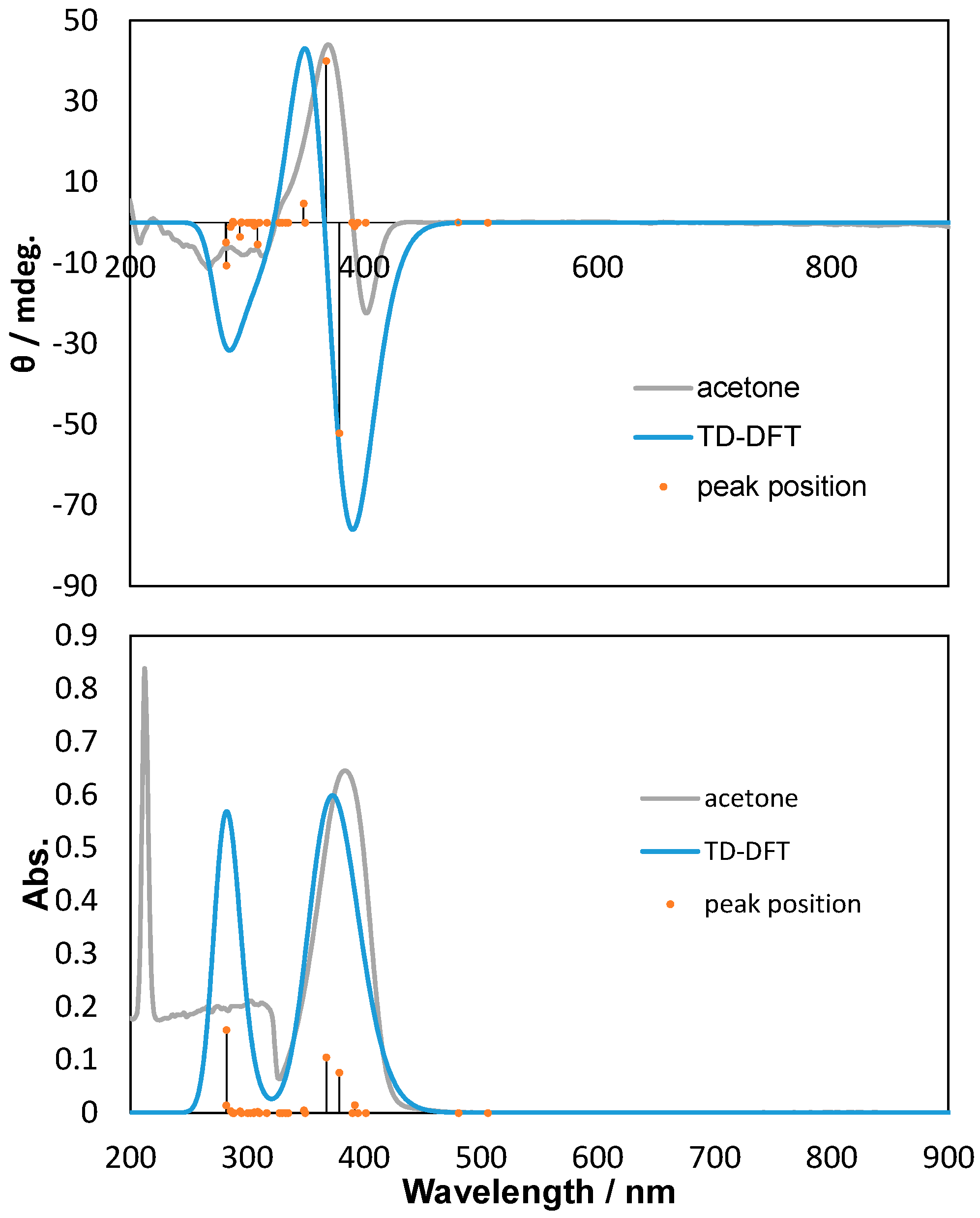
2.2. Polarized UV-Vis and CD Spectra with Thoretical Calculations
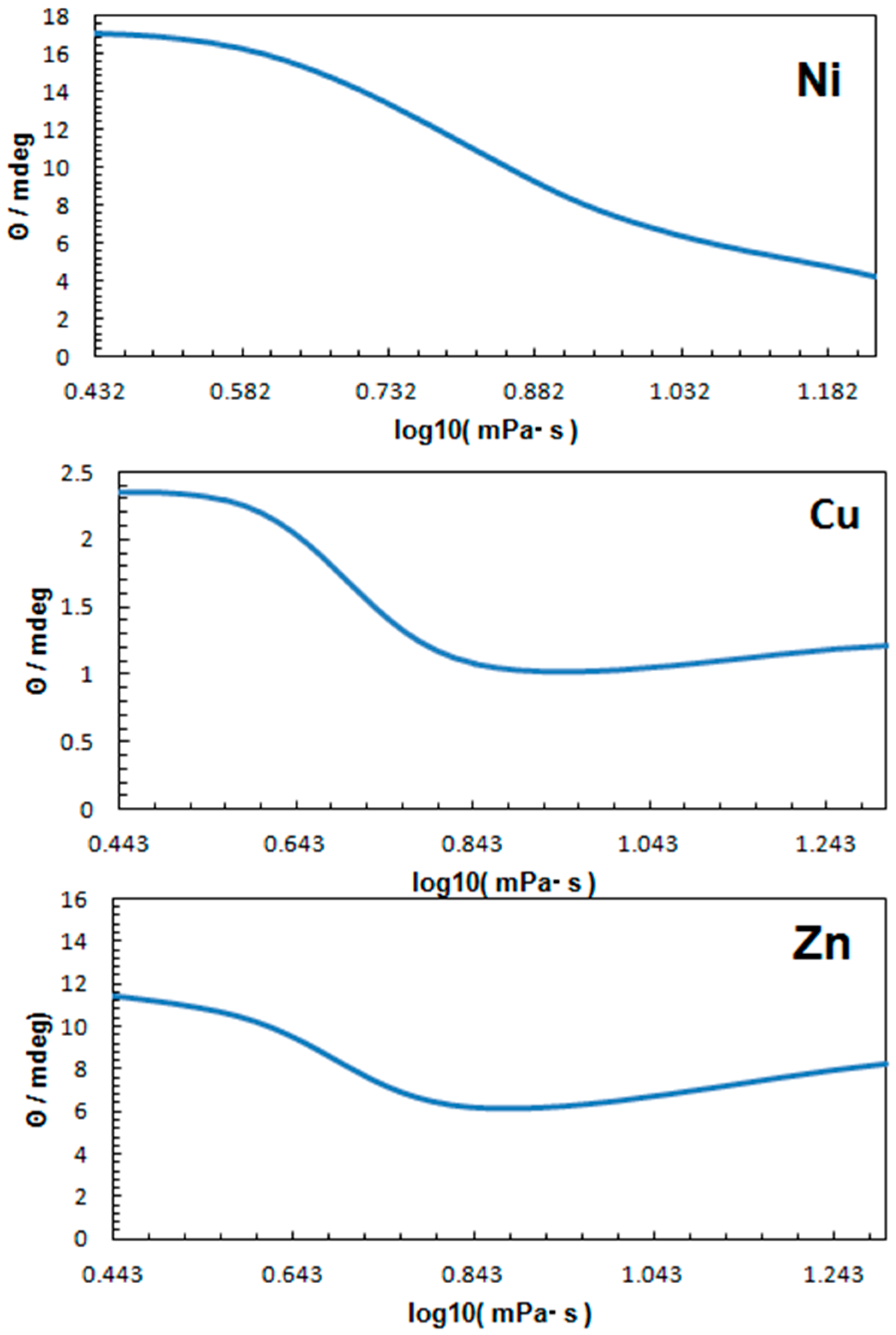
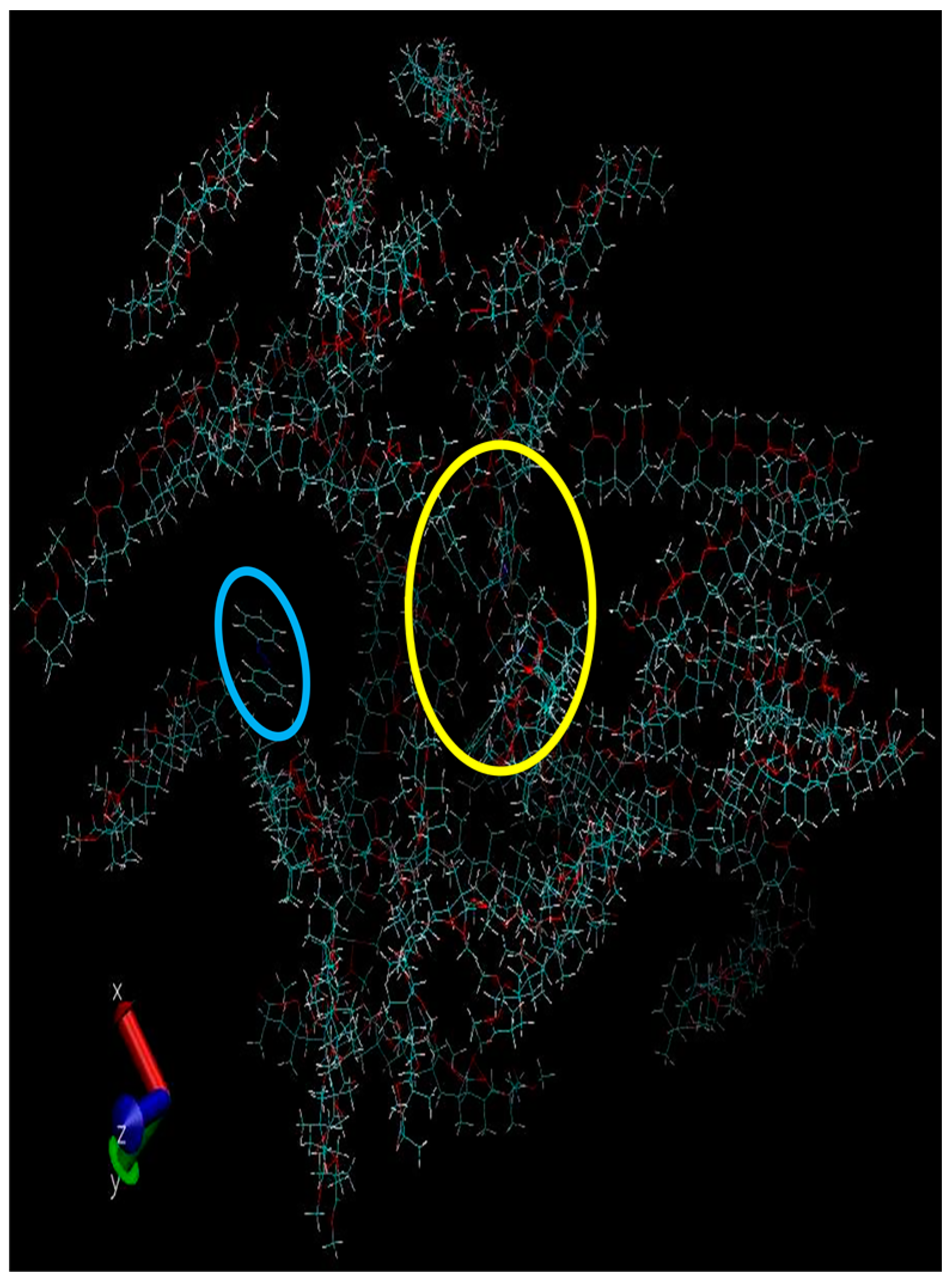
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AZ | azobenzene |
| CD | circular dichroism |
| IR | infrared |
| PMMA | polymethyl methacrylate |
| TD-DFT | time-dependent density functional theory |
| UV | ultraviolet |
| Vis | visible |
References
- Zhang, Z.; Feng, W.; Su, P.; Lu, X.; Song, J.; Fan, D.; Wong, W.-K.; Jones, R.A.; Su, C. Near-Infrared Luminescent PMMA-Supported Metallopolymers Based on Zn−Nd Schiff-Base Complexes. Inorg. Chem. 2014, 53, 5950–5960. [Google Scholar] [CrossRef] [PubMed]
- Takase, M.; Akitsu, T. Linearly polarized light-induced anisotropic orientation of binuclear Ni(II), Cu(II), and Zn(II) Schiff base complexes including or without methyl orange in PVA. In Polymer Science Book Series—Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Centre: Badajoz, Spain, 2016; Volume 1, In press. [Google Scholar]
- Tsuda, E.; Mitsumoto, Y.; Takakura, K.; Sunaga, N.; Akitsu, T.; Konomi, T.; Katoh, M. Electrochemical Tuning by Polarized UV Light Induced Molecular Orientation of Chiral Salen-Type Mn(II) and Co(II) Complexes in an Albumin Matrix. J. Chem. Chem. Eng. 2016, 2, 53–59. [Google Scholar]
- Kominato, C.; Akitsu, T. Photoinduced molecular orientation of catalytic-like chiral azo-Schiff base complexes in PMMA or laccase matrices. Lett. Appl. NanoBioSci. 2015, 2, 264–270. [Google Scholar]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef] [PubMed]
- Akitsu, T.; Itoh, T. Polarized spectroscopy of hybrid materials of chiral Schiff base cobalt(II), nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes in PMMA films. Polyhedron 2010, 29, 477–487. [Google Scholar] [CrossRef]
- Aritake, Y.; Takanashi, T.; Yamazaki, A.; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni(II), Cu(II), Zn(II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894. [Google Scholar] [CrossRef]
- Aritake, Y.; Akitsu, T. The role of chiral dopants in organic/inorganic hybrid materials containing chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes. Polyhedron 2012, 31, 278–284. [Google Scholar] [CrossRef]
- Yamazaki, A.; Akitsu, T. Polarized spectroscopy and polarized UV light-induced molecular orientation of chiral diphenyl Schiff base Ni(II) and Cu(II) complexes and azobenzene in a PMMA film. RSC Adv. 2012, 2, 2975–2980. [Google Scholar] [CrossRef]
- Ito, M.; Akitsu, T.; Palafox, M.A. Theoretical interpretation of polarized light-induced supramolecular orientation on the basis of normal mode analysis of azobenzene as hybrid materials in PMMA with chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes. J. Appl. Solut. Chem. Model. 2016, 5, 30–47. [Google Scholar]
- Akitsu, T.; Tanaka, R. Polarized Electronic and IR Spectra of Hybrid Materials of Chiral Mn(II) Complexes and Different Types of Photochromic Dyes Showing Photoisomerization or Weigert Effect. Curr. Phys. Chem. 2011, 1, 82–89. [Google Scholar] [CrossRef]
- Svechkarev, D.; Kolodezny, D.; Mosquera-Vázquez, S.; Vauthey, E. Complementary Surface Second Harmonic Generation and Molecular Dynamics Investigation of the Orientation of Organic Dyes at a Liquid/Liquid Interface. Langmuir 2014, 30, 13869–13878. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.R.; Kowalski, J.; Rodger, A. LD spectroscopy of natural and synthetic biomaterials. Chem. Soc. Rev. 2010, 39, 3380–3393. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Akitsu, T.; Kominato, C. Molecular Recognition of Trans-Chiral Schiff Base Metal Complexes for Induced CD. In An Integrated View of the Molecular Recognition and Toxinology—From Analytical Procedures to Biomedical Applications; InTech: Rijeka, Croatia, 2013; pp. 515–532. [Google Scholar]
- Ito, M.; Akitsu, T. Polarized UV light induced molecular arrangement depending on flexibility of chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes by azobenzene in PMMA matrix. Comtemp. Eng. Sci. 2014, 7, 869–877. [Google Scholar]
- Takase, M.; Ito, M.; Takano, H.; Akitsu, T. Crystal Structure of a Chiral Schiff Base Zinc(II) Complex, [Zn(C15H12Br2NO2)2]. Struct. Chem. Cryst. Commun. In press.
- Hariu, N.; Ito, M.; Akitsu, T. Linearly, Circularly, or Non-polarized Light Induced Supramolecular Arrangement of Diastereomer Schiff Base Ni(II), Cu(II), and Zn(II) Complexes by Azobenzene in PMMA Matrix. Comtemp. Eng. Sci. 2015, 8, 57–70. [Google Scholar]
- Okamoto, Y.; Nidaira, K.; Akitsu, T. Environmental Dependence of Artifact CD Peaks of Chiral Schiff Base 3d–4f Complexes in Softmater PMMA Matrix. Int. J. Mol. Sci. 2011, 12, 6966–6979. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Okamoto, Y.; Kominato, C.; Akitsu, T. PMMA matirx viscosity dependence of CD bands of flexible chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes. Contemp. Eng. Sci. 2014, 7, 853–859. [Google Scholar]
- Okamoto, Y.; Akitsu, T. Viscosity Dependence of Solid-State CD Peaks of Chiral Schiff Base Ni(II) and Cu(II) Complexes in PMMA Soft Mater Matrices. J. Chem. Chem. Eng. 2013, 7, 487–494. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, H.; Takase, M.; Sunaga, N.; Ito, M.; Akitsu, T. The Viscosity and Intermolecular Interaction of Organic and Inorganic Hybrid Systems Composed of Chiral Schiff Base Ni(II), Cu(II), and Zn(II) Complexes with Long Ligands, Azobenzene, and PMMA. Inorganics 2016, 4, 20. https://doi.org/10.3390/inorganics4030020
Takano H, Takase M, Sunaga N, Ito M, Akitsu T. The Viscosity and Intermolecular Interaction of Organic and Inorganic Hybrid Systems Composed of Chiral Schiff Base Ni(II), Cu(II), and Zn(II) Complexes with Long Ligands, Azobenzene, and PMMA. Inorganics. 2016; 4(3):20. https://doi.org/10.3390/inorganics4030020
Chicago/Turabian StyleTakano, Hiroshi, Masahiro Takase, Nobumitsu Sunaga, Maiko Ito, and Takashiro Akitsu. 2016. "The Viscosity and Intermolecular Interaction of Organic and Inorganic Hybrid Systems Composed of Chiral Schiff Base Ni(II), Cu(II), and Zn(II) Complexes with Long Ligands, Azobenzene, and PMMA" Inorganics 4, no. 3: 20. https://doi.org/10.3390/inorganics4030020
APA StyleTakano, H., Takase, M., Sunaga, N., Ito, M., & Akitsu, T. (2016). The Viscosity and Intermolecular Interaction of Organic and Inorganic Hybrid Systems Composed of Chiral Schiff Base Ni(II), Cu(II), and Zn(II) Complexes with Long Ligands, Azobenzene, and PMMA. Inorganics, 4(3), 20. https://doi.org/10.3390/inorganics4030020