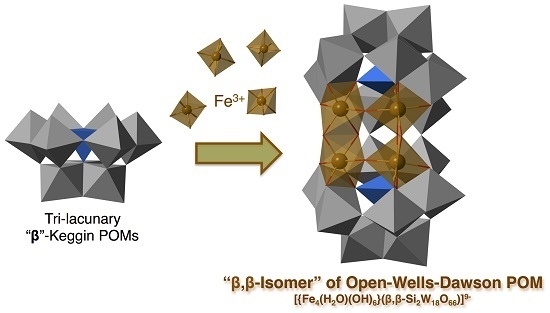
β,β-Isomer of Open-Wells–Dawson Polyoxometalate Containing a Tetra-Iron(III) Hydroxide Cluster: [{Fe4(H2O)(OH)5}(β,β-Si2W18O66)]9−
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
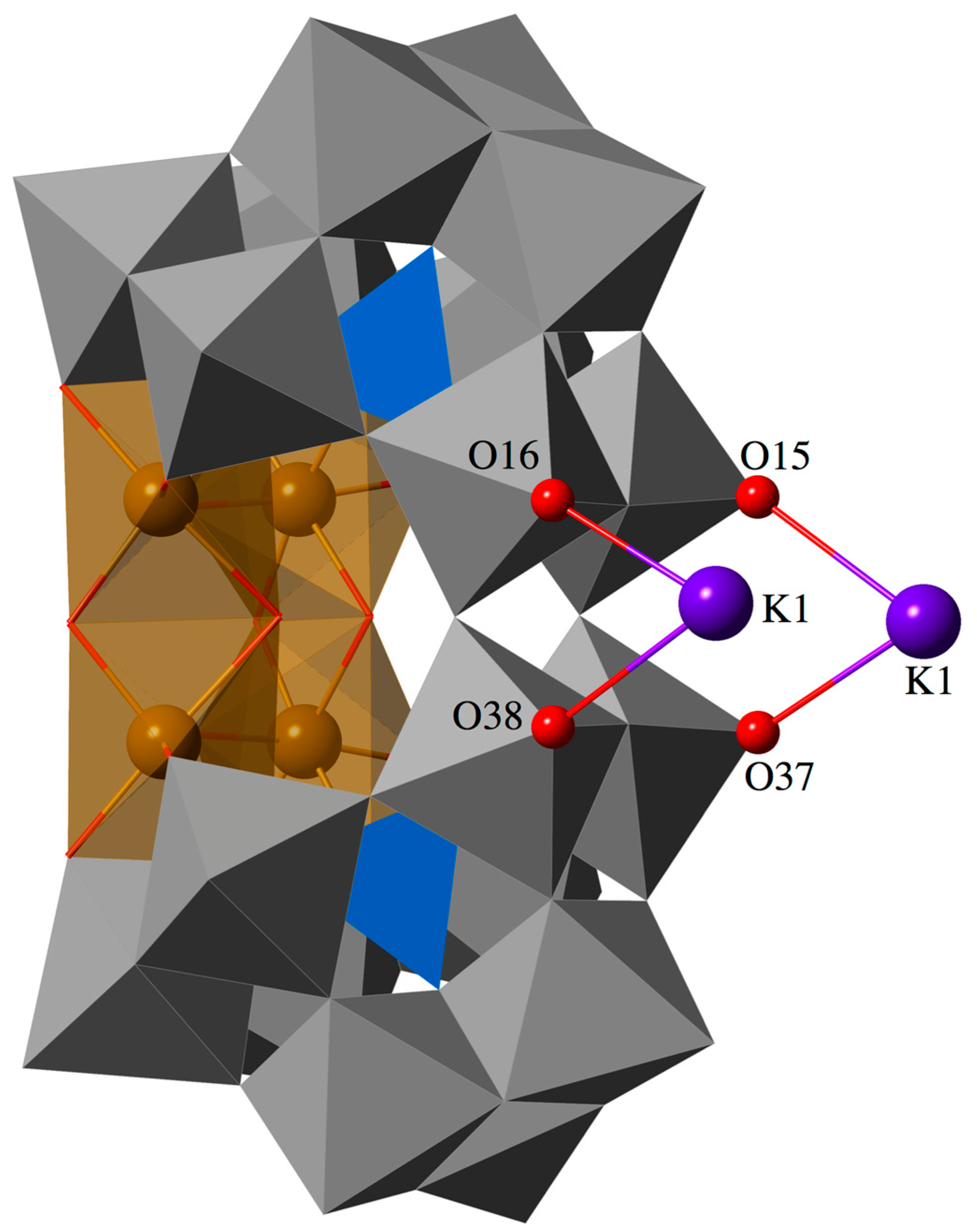
2.2. Molecular Structure
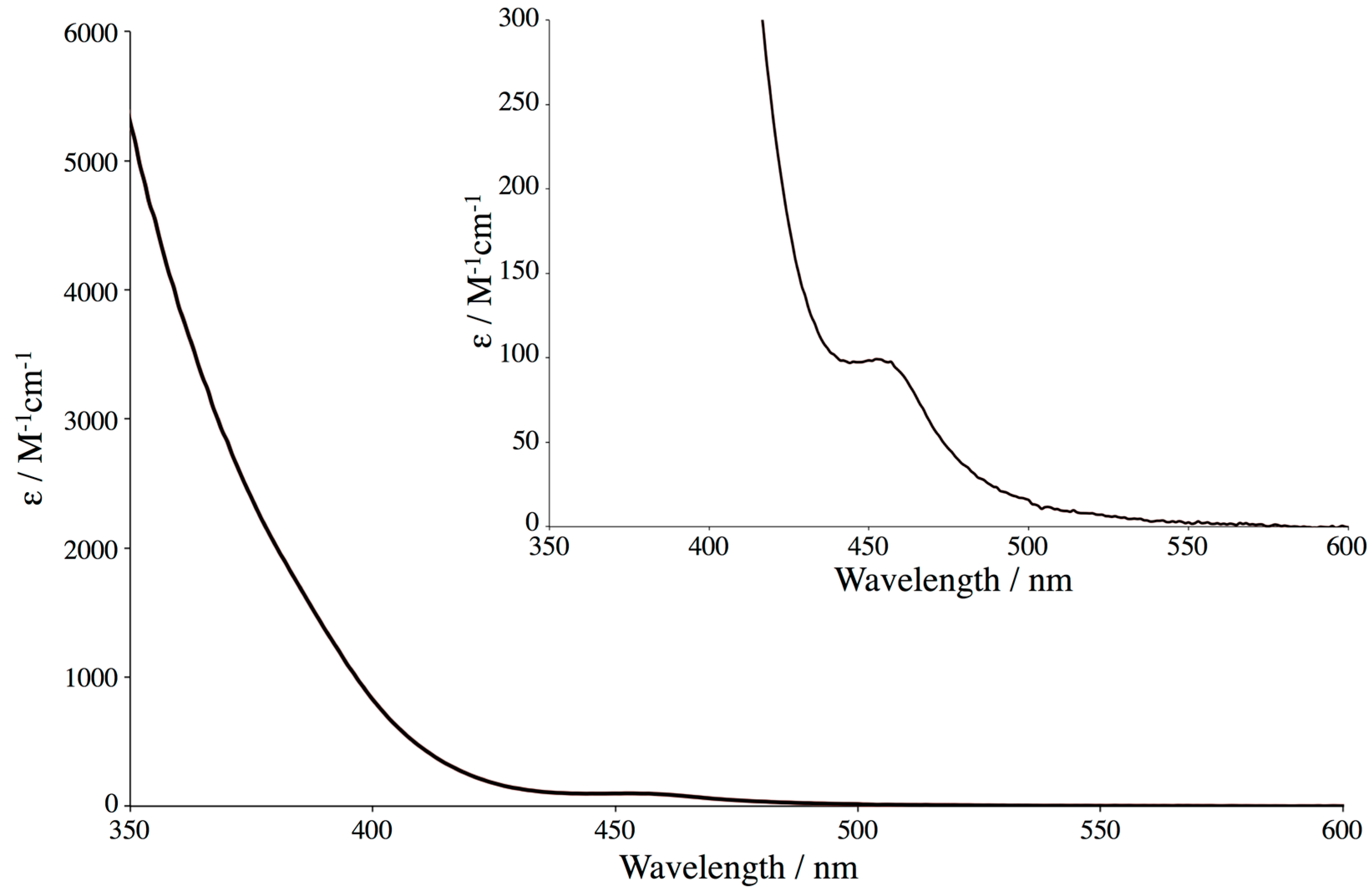
2.3. Absorption Spectrum
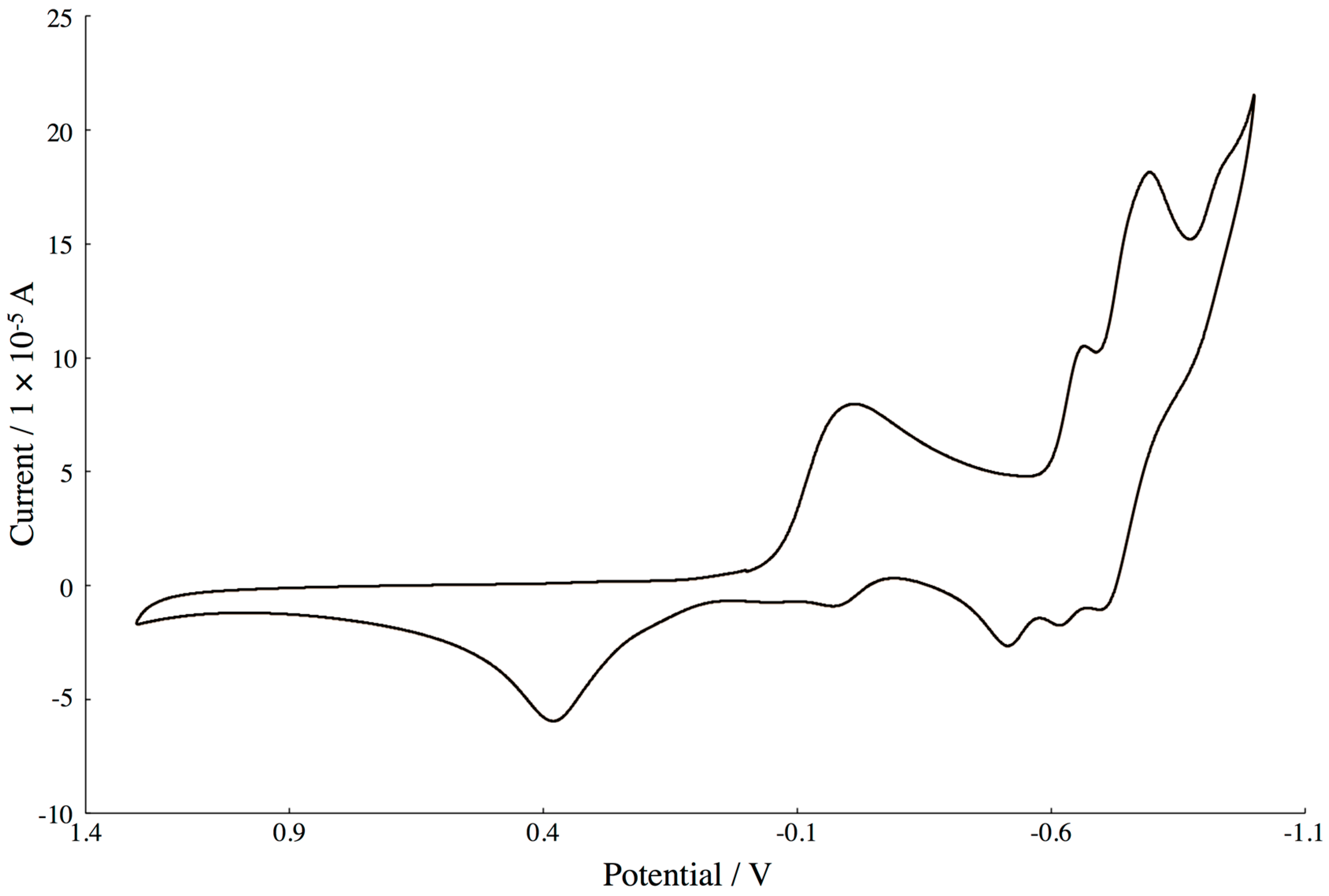
2.4. Electrochemistry
3. Experimental Section
3.1. Materials
3.2. Instrumentation and Analytical Procedures
3.3. Synthesis of K9[{Fe4(H2O)(OH)5}(β,β-Si2W18O66)]·17H2O (Potassium Salt of β,β-Fe4-Open)
3.4. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| POM | Polyoxometalate |
| TG/DTA | Thermogravimetric/differential thermal analyses |
| BVS | Bond valence sum |
References
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Ed. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopolyoxometalates; Springer-Verlag: New York, NY, USA, 1983. [Google Scholar]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Neumann, R. Polyoxometalate complexes in organic oxidation chemistry. Prog. Inorg. Chem. 1998, 47, 317–370. [Google Scholar]
- Proust, A.; Thouvenot, R.; Gouzerh, P. Functionalization of polyoxometalates: Towards advanced applications in catalysis and materials science. Chem. Commun. 2008, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- Hasenknopf, B.; Micoine, K.; Lacôte, E.; Thorimbert, S.; Malacria, M.; Thouvenot, R. Chirality in polyoxometalate chemistry. Eur. J. Inorg. Chem. 2008, 5001–5013. [Google Scholar] [CrossRef]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, K.; Sakai, Y.; Matsunaga, S. Chemistry of group IV metal ion-containing polyoxometalates. Eur. J. Inorg. Chem. 2011, 179–196. [Google Scholar] [CrossRef]
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble metals in polyoxometalates. Angew. Chem. Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Rompel, A. The use of polyoxometalates in protein crystallography—An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 2015, 299, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, A.; Rompel, A. The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Laronze, N.; Marrot, J.; Hervé, G. Synthesis, molecular structure and chemical properties of a new tungstosilicate with an open Wells–Dawson structure, α-[Si2W18O66]16−. Chem. Commun. 2003, 2360–2361. [Google Scholar] [CrossRef]
- Bi, L.-H.; Kortz, U. Synthesis and structure of the pentacopper(II) substituted tungstosilicate [Cu5(OH)4(H2O)2(A-α-SiW9O33)2]10−. Inorg. Chem. 2004, 43, 7961–7962. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Marrot, J.; Hervé, G. Cation-directed synthesis of tungstosilicates. 2. Synthesis, structure, and characterization of the open Wells–Dawson anion α-[{K(H2O)2}(Si2W18O66)]15− and its transiton-metal derivatives [{M(H2O)}(μ-H2O)2K(Si2W18O66)]13− and [{M(H2O)}(μ-H2O)2K{M(H2O)4}(Si2W18O66)]11−. Inorg. Chem. 2005, 44, 1275–1281. [Google Scholar] [PubMed]
- Nellutla, S.; Tol, J.V.; Dalal, N.S.; Bi, L.-H.; Kortz, U.; Keita, B.; Nadjo, L.; Khitrov, G.A.; Marshall, A.G. Magnetism, electron paramagnetic resonance, electrochemistry, and mass spectrometry of the pentacopper(II)-substituted tungstosilicate [Cu5(OH)4(H2O)2(A-α-SiW9O33)2]10−, A model five-spin frustrated cluster. Inorg. Chem. 2005, 44, 9795–9806. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Haouas, M.; Marrot, J.; Taulelle, F.; Hervé, G. Step-by-step assembly of trivacant tungstosilicates: Synthesis and characterization of tetrameric anions. Angew. Chem. Int. Ed. 2006, 45, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Marrot, J.; Hervé, G. Dinuclear vanadium and tetranuclear iron complexes obtained with the open Wells–Dawson [Si2W18O66]16− tungstosilicate. C. R. Chim. 2006, 9, 1467–1471. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Liu, S.-X.; Wang, C.-L.; Xie, L.-H.; Zhang, C.-D.; Gao, B.; Su, Z.-M.; Jia, H.-Q. Synthesis, structure and characterization of a new cobalt-containing germanotungstate with open Wells–Dawson structure: K13[{Co(H2O)}(μ-H2O)2K(Ge2W18O66)]. J. Mol. Struct. 2006, 785, 170–175. [Google Scholar] [CrossRef]
- Wang, C.-L.; Liu, S.-X.; Sun, C.-Y.; Xie, L.-H.; Ren, Y.-H.; Liang, D.-D.; Cheng, H.-Y. Bimetals substituted germanotungstate complexes with open Wells–Dawson structure: Synthesis, structure, and electrochemical behavior of [{M(H2O)}(μ-H2O)2K{M(H2O)4}(Ge2W18O66)]11− (M = Co, Ni, Mn). J. Mol. Struct. 2007, 841, 88–95. [Google Scholar] [CrossRef]
- Ni, L.; Hussain, F.; Spingler, B.; Weyeneth, S.; Patzke, G.R. Lanthanoid-containing open Wells–Dawson silicotungstates: Synthesis, crystal structures, and properties. Inorg. Chem. 2011, 50, 4944–4955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Geletii, Y.V.; Zhao, C.; Musaev, D.G.; Song, J.; Hill, C.L. A dodecanuclear Zn cluster sandwiched by polyoxometalate ligands. Dalton Trans. 2012, 41, 9908–9913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Glass, E.N.; Zhao, C.; Lv, H.; Vickers, J.W.; Geletii, Y.V.; Musaev, D.G.; Song, J.; Hill, C.L. A nickel containing polyoxometalate water oxidation catalyst. Dalton Trans. 2012, 41, 13043–13049. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Geletii, Y.V.; Song, J.; Zhao, C.; Glass, E.N.; Bacsa, J.; Hill, C.L. Di- and tri-cobalt silicotungstates: Synthesis, characterization, and stability studies. Inorg. Chem. 2013, 52, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Spingler, B.; Weyeneth, S.; Patzke, G.R. Trilacunary Keggin-type POMs as versatile building blocks for lanthanoid silicotungstates. Eur. J. Inorg. Chem. 2013, 1681–1692. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, D.; Chen, L.; Song, Y.; Zhu, D.; Xu, Y. Syntheses, structures and magnetic properties of two unprecedented hybrid compounds constructed from open Wells–Dawson anions and high-nuclear transition metal clusters. Dalton Trans. 2013, 42, 8454–8459. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Inoue, Y.; Otaki, T.; Osada, H.; Nomiya, K. Aluminum- and Gallium-Containing Open-Dawson Polyoxometalates. Z. Anorg. Allg. Chem. 2016, 642, 539–545. [Google Scholar] [CrossRef]
- Minato, T.; Suzuki, K.; Kamata, K.; Mizuno, N. Synthesis of α-Dawson-type silicotungstate [α-Si2W18O62]8− and protonation and deprotonation inside the aperture through intramolecular hydrogen bonds. Chem. Eur. J. 2014, 20, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-Q.; Guan, W.; Yan, L.-K.; Zhang, Y.-T.; Xu, M.-T.; Hayfron-Benjamin, E.; Su, Z.-M. On the origin of the relative stability of Wells–Dawson isomers: A DFT study of α-, β-, γ-, α*-, β*-, and γ*-[(PO4)2W18O54]6− anions. Inorg. Chem. 2011, 50, 4967–4977. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.C.W.; Figgis, J.S. New fundamental type of inorganic complex: Hybrid between heteropoly and conventional coordination complexes. Possibilities for geometrical isomerisms in 11-, 12-, 17-, and 18-heteropoly derivatives. J. Am. Chem. Soc. 1970, 92, 3794–3797. [Google Scholar] [CrossRef]
- Dawson, B. The structure of the 9(18)-heteropoly anion in potassium 9(18)-tungstophosphate, K6(P2W18O62)·14H2O. Acta Crystalligr. 1953, 6, 113–126. [Google Scholar] [CrossRef]
- Neubert, H.; Fuchs, J. Crystal structures and vibrational spectra of two isomers of octadecatungsto-diarsenate (NH4)6As2W18O62·nH2O. Z. Naturforsch. 1987, 42b, 951–958. [Google Scholar] [CrossRef]
- Contant, R.; Thouvenot, R. A reinvestigation of isomerism in the Dawson structure: Syntheses and 183W NMR structural characterization of three new polyoxotungstates [X2W18O62]6− (X = PV, AsV). Inorg. Chim. Acta 1993, 212, 41–50. [Google Scholar] [CrossRef]
- Richardt, P.J.S.; Gable, R.W.; Bond, A.M.; Wedd, A.G. Synthesis and redox characterization of the polyoxo Anion, γ*-[S2W18O62]4−: A unique fast oxidation pathway determines the characteristic reversible electrochemical behavior of polyoxometalate anions in acidic media. Inorg. Chem. 2001, 40, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bond, A.M. Voltammetric reduction of α- and γ*-[S2W18O62]4− and α-, β-, and γ-[SiW12O40]4−: Isomeric dependence of reversible potentials of polyoxometalate anions using data obtained by novel dissolution and conventional solution-phase processes. Inorg. Chem. 2004, 43, 8263–8271. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Zhang, Z.-B.; Sun, Q.; Xu, Y. Syntheses, characterization and catalytic properties of two new Wells–Dawson molybdosulfates. Chin. J. Inorg. Chem. 2011, 27, 556–560. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Zonnevijlle, F.; Tourné, C.M.; Tourné, G.F. Preparation and Characterization of Iron(III)- and Rhodium(III)-Containing Heteropolytungstates. Identification of Novel Oxo-Bridged Iron(III) Dimers. Inorg. Chem. 1982, 21, 2751–2757. [Google Scholar] [CrossRef]
- Tézé, A.; Hervé, G. α-, β-, and γ-Dodecatungstosilicic acids: Isomers and related lacunary compounds. Inorg. Synth. 1990, 27, 85–96. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, S.; Miyamae, E.; Inoue, Y.; Nomiya, K. β,β-Isomer of Open-Wells–Dawson Polyoxometalate Containing a Tetra-Iron(III) Hydroxide Cluster: [{Fe4(H2O)(OH)5}(β,β-Si2W18O66)]9−. Inorganics 2016, 4, 15. https://doi.org/10.3390/inorganics4020015
Matsunaga S, Miyamae E, Inoue Y, Nomiya K. β,β-Isomer of Open-Wells–Dawson Polyoxometalate Containing a Tetra-Iron(III) Hydroxide Cluster: [{Fe4(H2O)(OH)5}(β,β-Si2W18O66)]9−. Inorganics. 2016; 4(2):15. https://doi.org/10.3390/inorganics4020015
Chicago/Turabian StyleMatsunaga, Satoshi, Eriko Miyamae, Yusuke Inoue, and Kenji Nomiya. 2016. "β,β-Isomer of Open-Wells–Dawson Polyoxometalate Containing a Tetra-Iron(III) Hydroxide Cluster: [{Fe4(H2O)(OH)5}(β,β-Si2W18O66)]9−" Inorganics 4, no. 2: 15. https://doi.org/10.3390/inorganics4020015
APA StyleMatsunaga, S., Miyamae, E., Inoue, Y., & Nomiya, K. (2016). β,β-Isomer of Open-Wells–Dawson Polyoxometalate Containing a Tetra-Iron(III) Hydroxide Cluster: [{Fe4(H2O)(OH)5}(β,β-Si2W18O66)]9−. Inorganics, 4(2), 15. https://doi.org/10.3390/inorganics4020015