Abstract
We have successfully synthesized K17{[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}·35H2O (potassium salt of In10-open), an open-Wells–Dawson polyoxometalate (POM) containing ten indium metal atoms. This novel compound was characterized by X-ray crystallography, 29Si NMR, FTIR, complete elemental analysis, and TG/DTA. X-ray crystallography results for {[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}17− (In10-open) revealed two open-Wells–Dawson units containing two In3+ ions and a K+ ion, [{KIn2(μ-OH)2}(α,α-Si2W18O66)]11−, connected by an In6-hydroxide cluster moiety, [In6(μ-OH)13(H2O)8]5+. In10-open is the first example of an open-Wells–Dawson POM containing a fifth-period element. Moreover, to the best of our knowledge, it exhibits the highest nuclearity among the indium-containing POMs reported to date.
1. Introduction
Polyoxometalates (POMs) are discrete metal oxide clusters that are of current interest as soluble metal oxides, as well as for their application in catalysis, medicine, and materials science [,,,,,,,,,,,,]. Recently, open-Wells–Dawson POMs have been reported as an emerging class of POMs [,,,,,,,,,,,,,,]. These compounds are a dimerized species of the trilacunary Keggin POMs, [XW9O34]10− (X = Si, Ge). Standard Wells–Dawson structural POMs are regarded as two trilacunary Keggin POM units assembled together via six W–O–W bonds. However, the electrostatic repulsion between the two units in [XW9O34]10− (X = Si and Ge), induced by the highly charged guest XO44− (X = Si, Ge) ion, is assumed to be so strong that it inhibits the assembly of the standard Wells–Dawson structure in aqueous media. Therefore, when the two trilacunary Keggin units comprise an XO44− (X = Si, Ge) ion, they are linked by only two W–O–W bonds. This results in the formation of an open-Wells–Dawson structural POM []. The open pocket of these POMs can accommodate multiple metal ions (one to six metal ions). Thus, this class of compounds may constitute a promising platform for the development of metal-substituted-POM-based materials and catalysts. To date, many compounds that contain various metal ions in their open pocket, e.g., V5+ [], Mn2+ [,], Fe3+ [], Co2+ [,,,,,], Ni2+ [,,,], Cu2+ [,,], and Zn2+ [] have been reported. Some lanthanoid (Eu3+, Gd3+, Tb3+, Dy3+, and Ho3+)-containing open-Wells–Dawson POMs have also been reported [,]. However, the large ionic radii of these lanthanoid atoms inhibit their complete insertion within the open pocket. This results in a weak coordination, similar to that of the K ions in K-containing open-Wells–Dawson POMs. Recently, we synthesized the Al4- and Ga4-containing open-Wells–Dawson POMs: [{Al4(μ-OH)6}{α,α-Si2W18O66}]10− (Al4-open) and [{Ga4(μ-OH)6}(α,α-Si2W18O66)]10− (Ga4-open), respectively, and successfully determined their molecular structures by single crystal X-ray crystallography []. X-ray structure analyses of Al4- and Ga4-open revealed that the {M4(μ-OH)6}6+ (M = Al3+, Ga3+) clusters are included in the open pocket of the open-Wells–Dawson unit.
In general, trivalent group 13 ions are found as various oligomeric hydroxide species in aqueous solution [,,]. Synthetic and structural studies of group 13 ion-containing POMs provide informative and definitive molecular models of group 13 metal clusters in solution. However, among all the Al-, Ga-, and In-containing POMs, formed by the substitution of several tungsten ions in the parent POMs with trivalent group 13 ions [,,,,,,,,], few well-characterized In-containing POMs have been reported to date [,,]. Thus, In-containing POMs are intriguing target compounds from both a synthetic and a structural point of view.
In this study, we successfully synthesized an open-Wells–Dawson POM containing ten indium metal ions, K17{[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}·35H2O (potassium salt of In10-open), and characterized it by X-ray crystallography, 29Si NMR, FTIR, complete elemental analysis, and thermogravimetric/differential thermal analyses (TG/DTA). In contrast to Al4- and Ga4-open, In10-open showed a dimer structure bridged by a deca-indium-hydroxide cluster.
2. Results and Discussion
2.1. Synthesis
The crystalline sample of potassium salt of In10-open, was afforded in 17.9% yield. This complex was prepared from a 1:5 molar ratio reaction of K13[{K(H2O)3}2{K(H2O)2}(α,α-Si2W18O66)]·19H2O with InCl3. The sample was characterized using complete elemental analysis (H, In, K, O, Si, and W analyses), FTIR, TG/DTA, 29Si NMR in D2O, and X-ray crystallography.
The FTIR spectrum of potassium salt of In10-open (Figure S1) displays peaks at 1000 and 945 cm−1 that correspond to νas(Si–O) and νas(W–Ot), respectively. The characteristic bands at 900–600 cm−1 are associated with ν(W–Oc), ν(W–Ob), and ν(W–O–W). The IR spectrum is very similar to those of the common open-Wells–Dawson POMs.
Before elemental analysis, the sample of In10-open was dried overnight at room temperature under vacuum (10−3–10−4 Torr). All elements (H, In, K, O, Si, and W) were observed for a total analysis of 100.37%. The recorded data were in good accordance with the calculated values for the formula without water of crystallization, K17[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8] (see Experimental section). The weight loss observed during drying, before analysis, was 5.28% corresponding to ca. 35 crystalline water molecules. On the other hand, during the TG/DTA measurements carried out under atmospheric conditions, a weight loss of 6.40%, observed at temperatures below 500 °C, corresponding to a total of ca. 42 water molecules, i.e., 8 coordinated water molecules and 34 molecules of water of crystallization (Figure S2). Thus, the elemental analysis and TG/DTA displayed a presence of a total of 34–35 water molecules for the sample under atmospheric conditions. The formula for potassium salt of In10-open presented herein was determined as K17[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]·35H2O based on the results of the complete elemental analysis.
2.2. Molecular Structure
The molecular structure of the polyoxoanion of potassium salt of In10-open and its polyhedral representation are shown in Figure 1a,b, respectively. X-ray crystallographic data of In10-open reveal that the two open-Wells–Dawson units that include two In3+ ions and a K+ ion, [{KIn2(μ-OH)2}(α,α-Si2W18O66)]11−, are connected by a central In6-hydroxide cluster moiety, [In6(μ-OH)13(H2O)8]5+, to form a dimeric open-Wells–Dawson polyanion, {[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}17− (Figure 1).
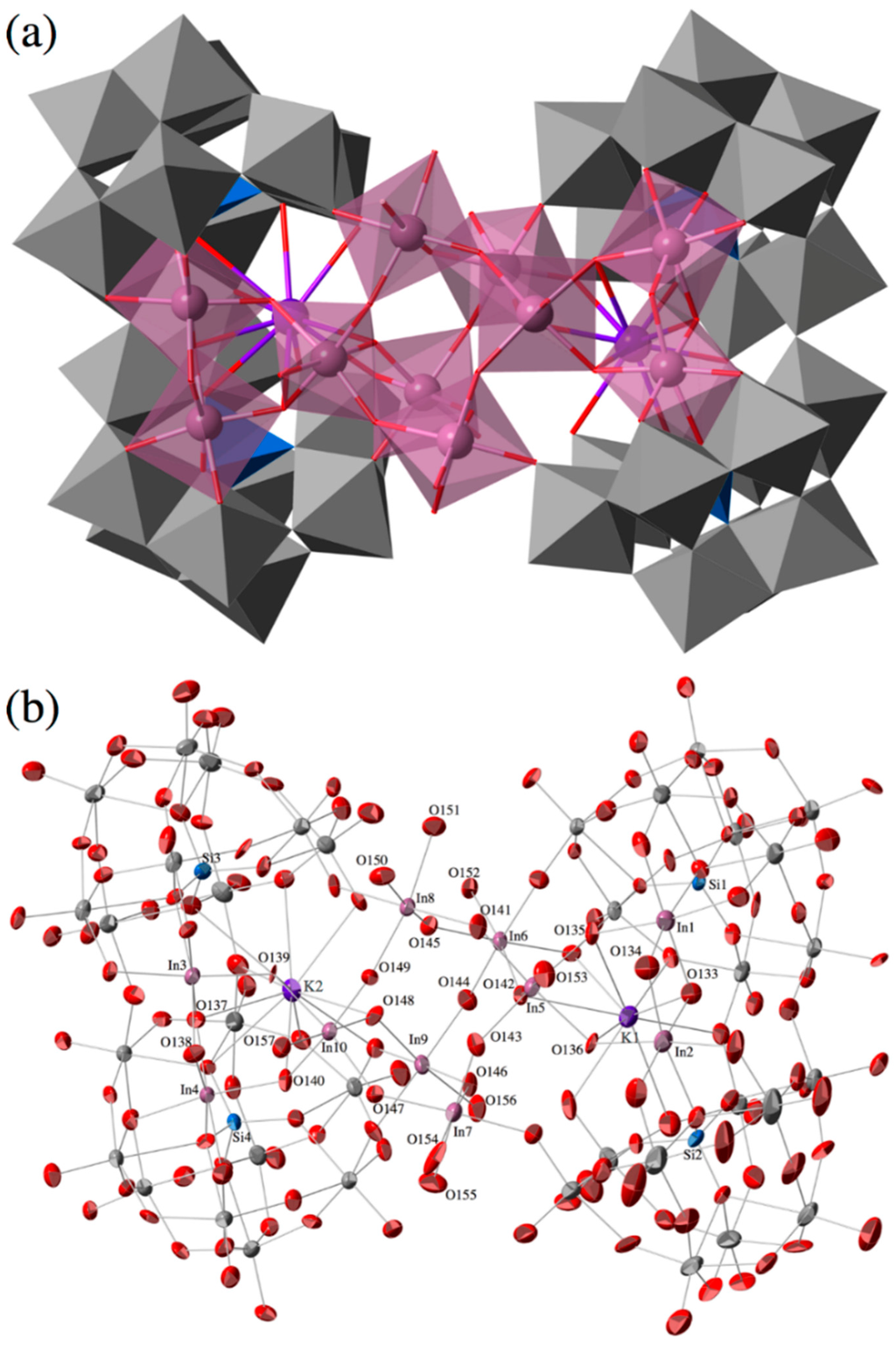
Figure 1.
Molecular structure of the polyoxoanion, {[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13 (H2O)8]}17− of potassium salt of In10-open. (a) Its polyhedral representation; and (b) thermal ellipsoidal plot. Color code: In, pink; K, purple; O, red; Si, blue; W, gray.
The two indium atoms in the open pocket of the open-Wells–Dawson POM units are connected through edge-sharing oxygen atoms (O133, O134 for In1, In2; O137, O138 for In3, In4) with In···In distances of 3.266(2) (In1···In2) and 3.299(2) (In3···In4) (Figure 2a). Each Indium atom in the open pocket is bonded to three oxygen atoms of the lacunary site in the open-Wells–Dawson polyanion [In–O average = 2.2011 Å]. Open-Wells–Dawson POMs that include two metal atoms in the open pocket have been previously reported, e.g., K11[{KV2O3(H2O)2}(Si2W18O66)]·40H2O (V2-open) []. In contrast to our In10-open, the two vanadium atoms in the open pocket of V2-open are bound to only one half {SiW9} of the open-Wells–Dawson unit, and are linked in a corner-sharing fashion (Figure 2b). Therefore, In10-open is the first example of an open-Wells–Dawson POM that includes two metal atoms with edge-sharing fashion.
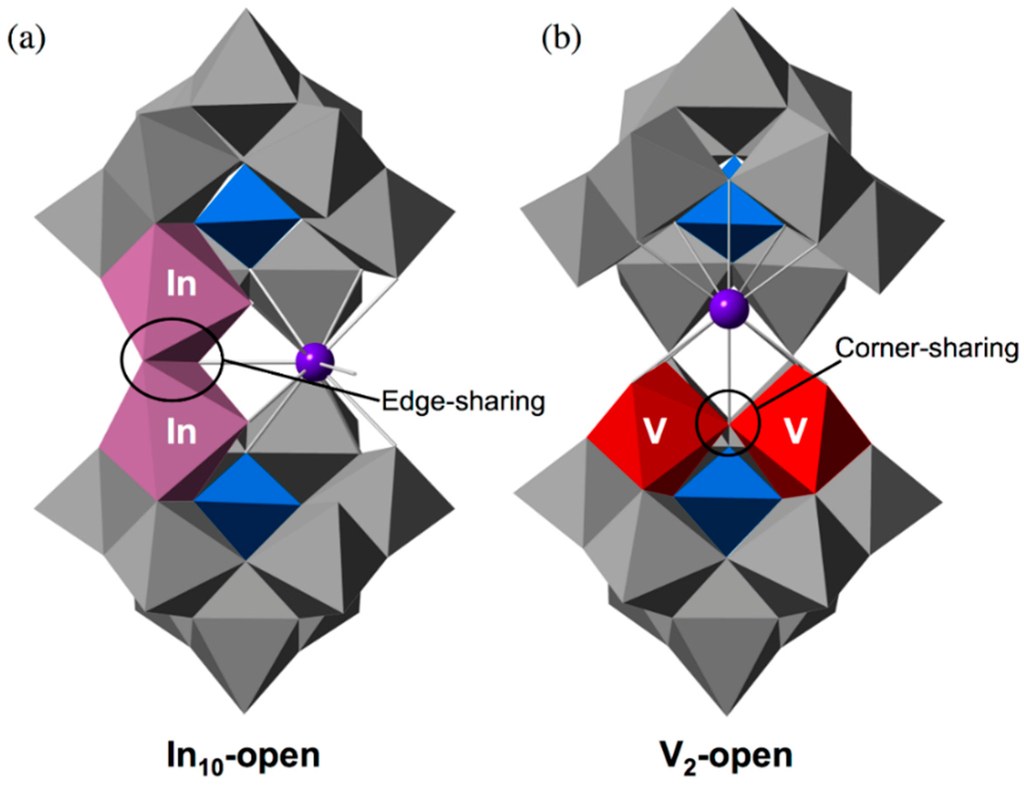
Figure 2.
The metal arrangement of (a) In10-open and (b) V2-open in the open pocket.
In addition to the indium atoms in the open-pocket, the complex contains six indium atoms in the bridging hydroxide cluster. The indium atoms in the bridging cluster are connected to each other in a corner-sharing fashion. Moreover, the In atoms in the open-pocket and the W atoms of the open-Wells–Dawson POM units are also linked to the In atoms of the bridging cluster in a corner-sharing fashion. Thus, all the Indium atoms can be considered to be 6-coordinated. Bond valence sum (BVS) [] calculations suggest that the corner- and edge-sharing oxygen atoms that are linked to the In atoms (corner-sharing: O135, O136, O139, O140, O141, O142, O143, O144, O145, O146, O147, O148, and O149; edge-sharing: O133, O134, O137, and O138) are protonated, i.e., they are ascribed to the hydroxide groups. On the other hand, the terminal oxygen atoms on the indium atoms (O150, O151, O152, O153, O154, O155, O156, and O157) are ascribed to the water groups (Table S1).
In10-open has a dimeric structure composed of two indium-containing open-Wells–Dawson POM moieties bridged by In6 hydroxide clusters. Dimeric open-Wells–Dawson POMs, similar to In10-open, have also been reported for {[Zn6(μ-OH)7(H2O)(α,α-Si2W18O66)]2}22− (Zn12-open) by Hill et al. []. In this complex, the six zinc atoms are included in the open pocket of the open-Wells–Dawson unit, and the two Zn6-containing open-Wells–Dawson units are connected through the two edge-sharing oxygen atoms. The arrangement and the number of metal ions in the open-pocket of the In10-open are different from those of the Zn12-open reported previously.
In open-Wells–Dawson POMs, the bite angle can be defined as the dihedral angle between the planes that pass through the six oxygen atoms of the lacunary site of each trilacunary Keggin unit. The bite angle varies, depending on the metal cluster included in the open pocket of the open-Wells–Dawson unit. The bite angles of In10-open are 64.363° and 65.139° (Figure 3). These values are wider than those of other open-Wells–Dawson POMs, including other group 13 ions, such as Al4- (54.274°) and Ga4-open (56.110°) []. The difference between the bite angles of Al4-, Ga4-, and In10-open is caused by the difference in the ionic radii of the Al (0.53 Å), Ga (0.76 Å), and In (0.94 Å) ions [,]. In10-open displays the widest bite angles when compared to previously reported open-Wells–Dawson POMs, including the Co6 (60.045°) [], Zn6 dimer (60.308°) [], Ni5 (58.925°) [], and Cu5 (61.663°) [,] clusters. The open-Wells–Dawson POM containing a Cu5 cluster (Cu5-open) exhibits a large bite angle (61.663°) due to the long bond lengths between the copper and the edge-sharing oxygen atom, caused by Jahn–Teller distortion [,]. The bite angles of In10-open are ca. 3° wider than that of Cu5-open. This increase appears to be caused by the large ionic radius of the indium ions incorporated in the open pocket.
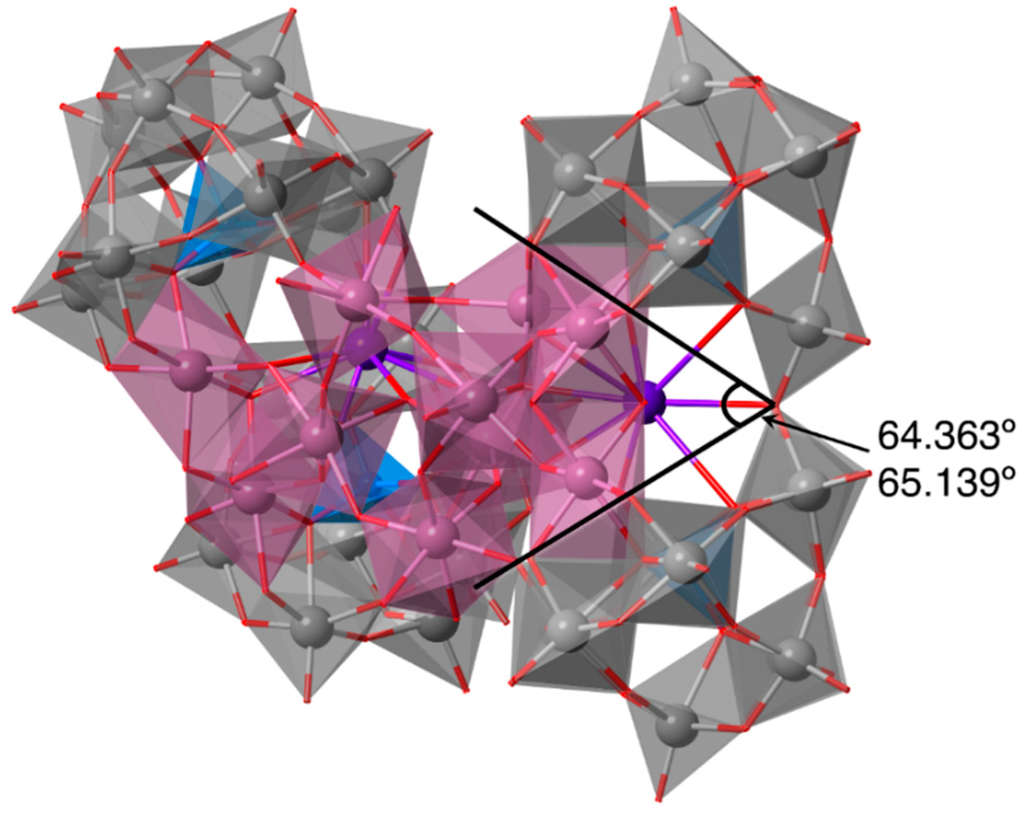
Figure 3.
Bite angles of In10-open.
The previously reported open-Wells–Dawson POMs mainly accommodated the fourth-period elements. Except for the lanthanoid-containing open-Wells–Dawson POMs, whose open-pockets weakly coordinate to the lanthanoid ions, open-Wells–Dawson POMs that accommodate the larger fifth- and sixth-period elements have not been reported to date. Thus, In10-open indicates that elements (such as indium) having large ionic radii (0.94 Å) can be incorporated in the open-pocket of an open-Wells–Dawson unit.
2.3. Solution 29Si NMR
The solution 29Si NMR spectrum of In10-open in D2O displays a two-line spectrum at −82.415 and −83.159 ppm in a 1:1 ratio (Figure 4). The two Si atoms in one open-Wells–Dawson unit are nonequivalent due to the configuration of the other open-Wells–Dawson unit, even though the two units are equivalent. The adjacent two 29Si NMR peaks are consistent with the structure of In10-open observed by X-ray crystallography. This suggests that In10-open exists as a single species and maintains its structure in solution.
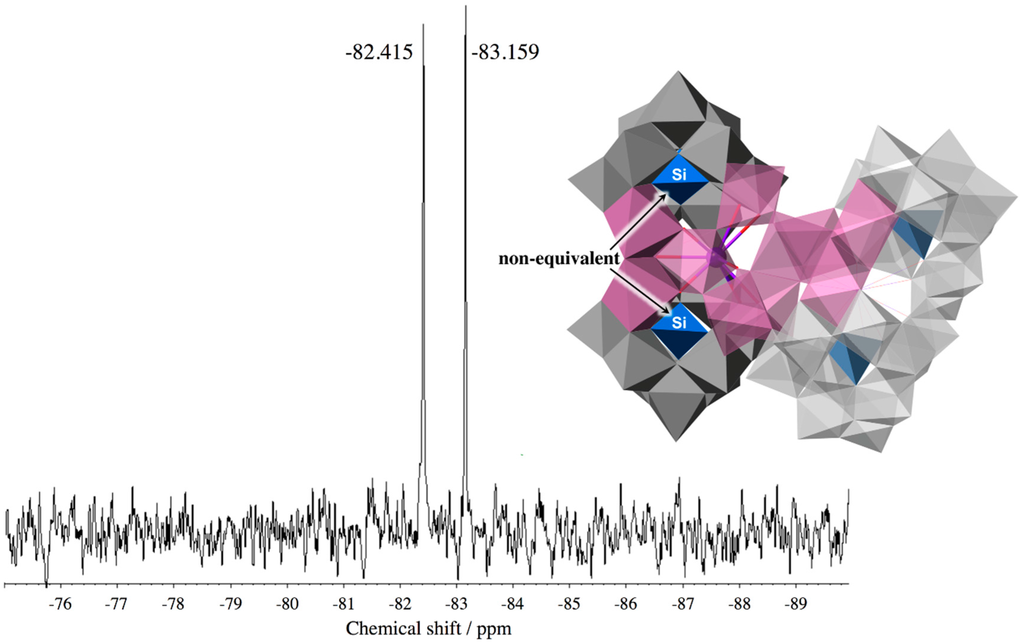
Figure 4.
Solution 29Si NMR spectrum of potassium salt of In10-open dissolved in D2O.
3. Experimental Section
3.1. Materials
The following reagents were used as received: InCl3 (from Sigma-Aldrich Japan, Shinagawa, Japan), KOH, KCl (from Wako Pure Chemical Industries, Osaka, Japan), and D2O (Kanto Chemical, Tokyo, Japan). The K ion-incorporating open-Wells–Dawson POM, K13[{K(H2O)3}2{K(H2O)2}(α,α-Si2W18O66)]·19H2O, was prepared according to literature [], and identified by TG/DTA and FT-IR analysis.
3.2. Instrumentation and Analytical Procedures
A complete elemental analysis was carried out by Mikroanalytisches Labor Pascher (Remagen, Germany). The sample was dried overnight at room temperature under a pressure of 10−3–10−4 Torr before analysis. The 29Si NMR (119.24 MHz) spectra in D2O solution were recorded in 5-mm outer diameter tubes, on a JEOL JNM ECP 500 FTNMR spectrometer with a JEOL ECP-500 NMR data-processing system (JEOL, Akishima, Japan). The 29Si NMR spectrum was referenced to an internal standard of DSS. Infrared spectra were recorded on a Jasco 4100 FTIR spectrometer (Jasco, Hachioji, Japan) by using KBr disks at room temperature. TG/DTA measurements were performed using a Rigaku Thermo Plus 2 series TG8120 instrument (Rigaku, Akishima, Japan), under air flow with a temperature ramp of 4.0 °C per min at a temperature ranging between 26 and 500 °C.
3.3. Synthesis of K17{[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}·35H2O (Potassium Salt of In10-open)
InCl3 (398 mg, 1.80 mmol) was dissolved in water (40 mL). A separate solution of K13[{K(H2O)3}2{K(H2O)2}(α,α-Si2W18O66)]·19H2O (2.00 g, 0.361 mmol) dissolved in 100 mL of distilled water was added dropwise to the resulting solution. The pH of this solution was adjusted to 4.0 using 0.1 M KOHaq. Next, 4 mL of saturated KClaq were added to the solution. The resulting solution was left to stand undisturbed at room temperature for 3 days. The afforded colorless needle crystals were collected on a membrane filter (JG 0.2 μm), and dried in vacuo for 2 h. Yield = 0.381 g (0.0323 mmol, 17.9% based on K13[{K(H2O)3}2{K(H2O)2}(α,α-Si2W18O66)]·19H2O).
The crystalline sample was soluble in water, but insoluble in organic solvents such as methanol, ethanol, and diethyl ether. Elemental analysis (%) calcd. for H33In10K19O157Si4W36 or K17{[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}: H 0.30, In 10.28, K 6.65, O 22.49, Si 1.01, W 59.27; Found: H 0.23, In 10.0, K 6.42, O 23.4, Si 1.02, W 59.3 (total 100.37%). A weight loss of 5.28% (solvated water) was observed during overnight drying at room temperature, at a pressure of 10−3–10−4 Torr before analysis. This suggested the presence of 35 water molecules. From TG/DTA air flow, a weight loss of 6.40% was observed at a temperature below 500 °C; calc. 6.42% for a total of 42 water molecules, i.e., 34 solvated water molecules and 8 coordinated water molecules in K17{[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}·34H2O; IR (KBr, cm−1): 1622 (m), 1000 (w), 945 (m), 890 (vs), 786 (vs), 730 (vs), 649 (s), 548 (m), 523 (m); 29Si NMR (50.0 °C, D2O, DSS, ppm): δ = −82.415, −83.159.
3.4. X-Ray Crystallography
For In10-open, a single crystal with dimensions of 0.21 × 0.06 × 0.05 mm3 was surrounded by liquid paraffin (Paratone-N) and analyzed at 150(2) K. All measurements were performed on a Rigaku MicroMax-007HF with a Saturn CCD diffractometer (Rigaku). The structure was solved by direct methods (SHELXS-97), followed by difference Fourier calculations and refinement by a full-matrix least-squares procedure on F2 (program SHELXL-97) [].
Crystal data: monoclinic, space group P2(1)/a, a = 23.525(5), b = 32.926(7), c = 25.424(6) Å, β = 93.273(2)°, V = 19661(7) Å3, Z = 4, Dcalcd = 3.857 g cm−3, μ(Mo-Kα) = 22.491 mm−1; R1[I > 2.00σ(I)] = 0.0829, R (all data) = 0.1012, wR2 (all data) = 0.2296, GOF = 1.056. Most atoms in the main part of the structure were refined anisotropically, while the rest (as crystallization solvents) were refined isotropically, because of the presence of disorder. The composition and formula of the POM (containing many countercations and many crystalline water molecules) were determined from complete elemental and TG analyses. Similar to structural investigations of other crystals of highly hydrated large POM complexes, it was not possible to locate every countercation and hydrated water molecule in the complex. This frequently encountered situation is attributed to the extensive disorder of the cations and many of the crystalline water molecules. Further details on the crystal structure investigations may be obtained from: Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (Fax: +49-7247-808-666; E-Mail: crysdata@fiz-karlsruhe.de, http://www.fiz-karlsruhe.de/request_for_deposited_data.html?&L=1) on quoting the depository number CSD-430962 (Identification code; 56_11_1).
4. Conclusions
In summary, we prepared and characterized an open-Wells–Dawson structural POM, potassium salt of In10-open, containing ten indium ions, i.e., K17{[{KIn2(μ-OH)2}(α,α-Si2W18O66)]2[In6(μ-OH)13(H2O)8]}·35H2O (potassium salt of In10-open). Single-crystal X-ray analyses revealed that two open-Wells–Dawson units that include two In3+ ions and a K+ ion are connected by an In6-hydroxide cluster moiety to form a dimeric open-Wells–Dawson polyanion. In10-open displayed the widest bite angles among the previously reported open-Wells–Dawson POMs. This is mainly due to the large ionic radius of the indium ion. The solution 29Si spectrum in D2O indicated that In10-open was obtained as a single species and that its structure was maintained in solution. In10-open is the first example of an open-Wells–Dawson POM containing a fifth-period element, and it exhibits the highest nuclearity of any indium-containing POM reported to date. This work can be extended to the future molecular design of novel open-Wells–Dawson POMs containing large fifth- and sixth-period elements, such as Ru, Rh, Pd, Pt. Studies of open-Wells–Dawson structural POMs containing larger metal atoms are in progress.
Supplementary Materials
The following are available online at www.mdpi.com/2304-6740/4/2/16/s1, Figure S1: FT-IR spectrum of potassium salt of In10-open (KBr disk), Figure S2: TG/DTA data of potassium salt of In10-open (from 22 to 500 °C), Table S1: Bond valence sum (BVS) calculations of In and O atoms of the Indium-cluster moieties of In10-open: checkCIF/PLATON report.
Acknowledgments
This study was supported by the Strategic Research Base Development Program for Private Universities of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and also by a grant from the Research Institute for Integrated Science, Kanagawa University (RIIS201505).
Author Contributions
Satoshi Matsunaga and Kenji Nomiya conceived and designed the experiments, and wrote the paper; Takuya Otaki and Yusuke Inoue synthesized and characterized the compound; Kohei Mihara assisted characterization of the compound.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| POM | Polyoxometalate |
| TG/DTA | Thermogravimetric/differential thermal analyses |
| BVS | Bond valence sum |
References
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Ed. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly- and Isopolyoxometalates; Springer-Verlag: New York, NY, USA, 1983. [Google Scholar]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Neumann, R. Polyoxometalate complexes in organic oxidation chemistry. Prog. Inorg. Chem. 1998, 47, 317–370. [Google Scholar]
- Proust, A.; Thouvenot, R.; Gouzerh, P. Functionalization of polyoxometalates: Towards advanced applications in catalysis and materials science. Chem. Commun. 2008, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- Hasenknopf, B.; Micoine, K.; Lacôte, E.; Thorimbert, S.; Malacria, M.; Thouvenot, R. Chirality in polyoxometalate chemistry. Eur. J. Inorg. Chem. 2008, 2008, 5001–5013. [Google Scholar] [CrossRef]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, K.; Sakai, Y.; Matsunaga, S. Chemistry of group IV metal ion-containing polyoxometalates. Eur. J. Inorg. Chem. 2011, 2011, 179–196. [Google Scholar] [CrossRef]
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble metals in polyoxometalates. Angew. Chem. Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Rompel, A. The use of polyoxometalates in protein crystallography—An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 2015, 299, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, A.; Rompel, A. The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Laronze, N.; Marrot, J.; Hervé, G. Synthesis, molecular structure and chemical properties of a new tungstosilicate with an open Wells–Dawson structure, α-[Si2W18O66]16−. Chem. Commun. 2003, 21, 2360–2361. [Google Scholar] [CrossRef]
- Bi, L.-H.; Kortz, U. Synthesis and structure of the pentacopper(II) substituted tungstosilicate [Cu5(OH)4(H2O)2(A-α-SiW9O33)2]10−. Inorg. Chem. 2004, 43, 7961–7962. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Marrot, J.; Hervé, G. Cation-directed synthesis of tungstosilicates. 2. Synthesis, structure, and characterization of the open Wells–Dawson anion α-[{K(H2O)2}(Si2W18O66)]15− and its transiton-metal derivatives [{M(H2O)}(μ-H2O)2K(Si2W18O66)]13− and [{M(H2O)}(μ-H2O)2K{M(H2O)4}(Si2W18O66)]11−. Inorg. Chem. 2005, 44, 1275–1281. [Google Scholar] [PubMed]
- Nellutla, S.; Tol, J.V.; Dalal, N.S.; Bi, L.-H.; Kortz, U.; Keita, B.; Nadjo, L.; Khitrov, G.A.; Marshall, A.G. Magnetism, electron paramagnetic resonance, electrochemistry, and mass spectrometry of the pentacopper(II)-substituted tungstosilicate [Cu5(OH)4(H2O)2(A-α-SiW9O33)2]10−, A model five-spin frustrated cluster. Inorg. Chem. 2005, 44, 9795–9806. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Haouas, M.; Marrot, J.; Taulelle, F.; Hervé, G. Step-by-step assembly of trivacant tungstosilicates: Synthesis and characterization of tetrameric anions. Angew. Chem. Int. Ed. 2006, 45, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Marrot, J.; Hervé, G. Dinuclear vanadium and tetranuclear iron complexes obtained with the open Wells–Dawson [Si2W18O66]16− tungstosilicate. C. R. Chim. 2006, 9, 1467–1471. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Liu, S.-X.; Wang, C.-L.; Xie, L.-H.; Zhang, C.-D.; Gao, B.; Su, Z.-M.; Jia, H.-Q. Synthesis, structure and characterization of a new cobalt-containing germanotungstate with open Wells–Dawson structure: K13[{Co(H2O)}(μ-H2O)2K(Ge2W18O66)]. J. Mol. Struct. 2006, 785, 170–175. [Google Scholar] [CrossRef]
- Wang, C.-L.; Liu, S.-X.; Sun, C.-Y.; Xie, L.-H.; Ren, Y.-H.; Liang, D.-D.; Cheng, H.-Y. Bimetals substituted germanotungstate complexes with open Wells–Dawson structure: Synthesis, structure, and electrochemical behavior of [{M(H2O)}(μ-H2O)2K{M(H2O)4}(Ge2W18O66)]11− (M = Co, Ni, Mn). J. Mol. Struct. 2007, 841, 88–95. [Google Scholar] [CrossRef]
- Ni, L.; Hussain, F.; Spingler, B.; Weyeneth, S.; Patzke, G.R. Lanthanoid-containing open Wells–Dawson silicotungstates: Synthesis, crystal structures, and properties. Inorg. Chem. 2011, 50, 4944–4955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Geletii, Y.V.; Zhao, C.; Musaev, D.G.; Song, J.; Hill, C.L. A dodecanuclear Zn cluster sandwiched by polyoxometalate ligands. Dalton Trans. 2012, 41, 9908–9913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Glass, E.N.; Zhao, C.; Lv, H.; Vickers, J.W.; Geletii, Y.V.; Musaev, D.G.; Song, J.; Hill, C.L. A nickel containing polyoxometalate water oxidation catalyst. Dalton Trans. 2012, 41, 13043–13049. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Geletii, Y.V.; Song, J.; Zhao, C.; Glass, E.N.; Bacsa, J.; Hill, C.L. Di- and tri-cobalt silicotungstates: Synthesis, characterization, and stability studies. Inorg. Chem. 2013, 52, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Spingler, B.; Weyeneth, S.; Patzke, G.R. Trilacunary Keggin-type POMs as versatile building blocks for lanthanoid silicotungstates. Eur. J. Inorg. Chem. 2013, 2013, 1681–1692. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, D.; Chen, L.; Song, Y.; Zhu, D.; Xu, Y. Syntheses, structures and magnetic properties of two unprecedented hybrid compounds constructed from open Wells–Dawson anions and high-nuclear transition metal clusters. Dalton Trans. 2013, 42, 8454–8459. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Inoue, Y.; Otaki, T.; Osada, H.; Nomiya, K. Aluminum-and gallium-containing open-Dawson polyoxometalates. Z. Anorg. Allg. Chem. 2016, 642, 539–545. [Google Scholar] [CrossRef]
- Zhang, F.-Q.; Guan, W.; Yan, L.-K.; Zhang, Y.-T.; Xu, M.-T.; Hayfron-Benjamin, E.; Su, Z.-M. On the origin of the relative stability of Wells–Dawson isomers: A DFT study of α-, β-, γ-, α*-, β*-, and γ*-[(PO4)2W18O54]6− anions. Inorg. Chem. 2011, 50, 4967–4977. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.A.; Clayden, N.J.; Heath, S.L.; Moore, G.R.; Powell, A.K.; Tapparo, A. Defining speciation profiles of Al3+ complexed with small organic ligands: The Al3+-heidi system. Coord. Chem. Rev. 1996, 149, 281–309. [Google Scholar] [CrossRef]
- Casey, W.H. Large aqueous aluminum hydroxide molecules. Chem. Rev. 2006, 106, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mensinger, Z.L.; Wang, W.; Keszler, D.A.; Johnson, D.W. Oligomeric group 13 hydroxide compounds—A rare but varied class of molecules. Chem. Soc. Rev. 2012, 41, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, Y.; Yamaguchi, S.; Nakagawa, Y.; Uehara, K.; Uchida, S.; Yamaguchi, K.; Mizuno, N. Synthesis of a dialuminum-substituted silicotungstate and the diastereoselective cyclization of citronellal derivatives. J. Am. Chem. Soc. 2008, 130, 15872–15878. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Katayama, Y.; Nagami, M.; Kato, M.; Yamasaki, M. A sandwich-type aluminium complex composed of tri-lacunary Keggin-type polyoxotungstate: Synthesis and X-ray crystal structure of [(A-PW9O34)2{W(OH)(OH2)}{Al(OH)(OH2)}{Al(μ-OH)(OH2)2}2]7−. Dalton Trans. 2010, 39, 11469–11474. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, Y.; Yamaguchi, K.; Hibino, M.; Mizuno, N. Layered assemblies of a dialuminum-substituted silicotungstate trimer and the reversible interlayer cation-exchange properties. Inorg. Chem. 2011, 50, 12411–12413. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Bassil, B.S.; Sorarù, A.; Berardi, S.; Suchopar, A.; Kortz, U.; Bonchio, M. A Lewis acid catalytic core sandwiched by inorganic polyoxoanion caps: Selective H2O2-based oxidations with [AlIII4(H2O)10(β-XW9O33H)2]6− (X = AsIII, SbIII). Chem. Commun. 2013, 49, 7914–7916. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Makino, Y.; Unno, W.; Uno, H. Synthesis, molecular structure, and stability of a zirconocene derivative with α-Keggin mono-aluminum substituted polyoxotungstate. Dalton Trans. 2013, 42, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Kashiwagi, T.; Unno, W.; Nakagawa, M.; Uno, H. Syntheses and molecular structures of monomeric and hydrogen-bonded dimeric Dawson-type trialuminum-substituted polyoxotungstates derived under acidic and basic conditions. Inorg. Chem. 2014, 53, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Matsunaga, S.; Nomiya, K. Al16-hydroxide cluster-containing tetrameric polyoxometalate, [{α-Al3SiW9O34(μ-OH)6}4{Al4(μ-OH)6}]22−. Chem. Lett. 2015, 44, 1649–1651. [Google Scholar] [CrossRef]
- Allmen, K.; Car, P.-E.; Blacque, O.; Fox, T.; Müller, R.; Patzke, G.R. Structure and properties of new gallium-containing polyoxotungstates with hexanuclear and tetranuclear cores. Z. Anorg. Allg. Chem. 2014, 640, 781–789. [Google Scholar] [CrossRef]
- Limanski, E.M.; Drewes, D.; Krebs, B. Sandwich-like polyoxotungstates with indium(III) as a heteroatom synthesis and characterization of the first examples of a new type of anions. Z. Anorg. Allg. Chem. 2004, 630, 523–528. [Google Scholar] [CrossRef]
- Hussain, F.; Reicke, M.; Janowski, V.; Silva, S.D.; Futuwi, J.; Kortz, U. Some indium(III)-substituted polyoxotungstates of the Keggin and Dawson types. C. R. Chim. 2005, 8, 1045–1056. [Google Scholar] [CrossRef]
- Zhao, D.; Ye, R.-H. Solvothermal synthesis and structure of a new indium-substituted polyoxotungstate: [(CH3)2NH2]4[In3(H2O)3(NO3)(A-α-H3PW9O34)2]·H2O. J. Clust. Sci. 2011, 22, 563–571. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–761. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).