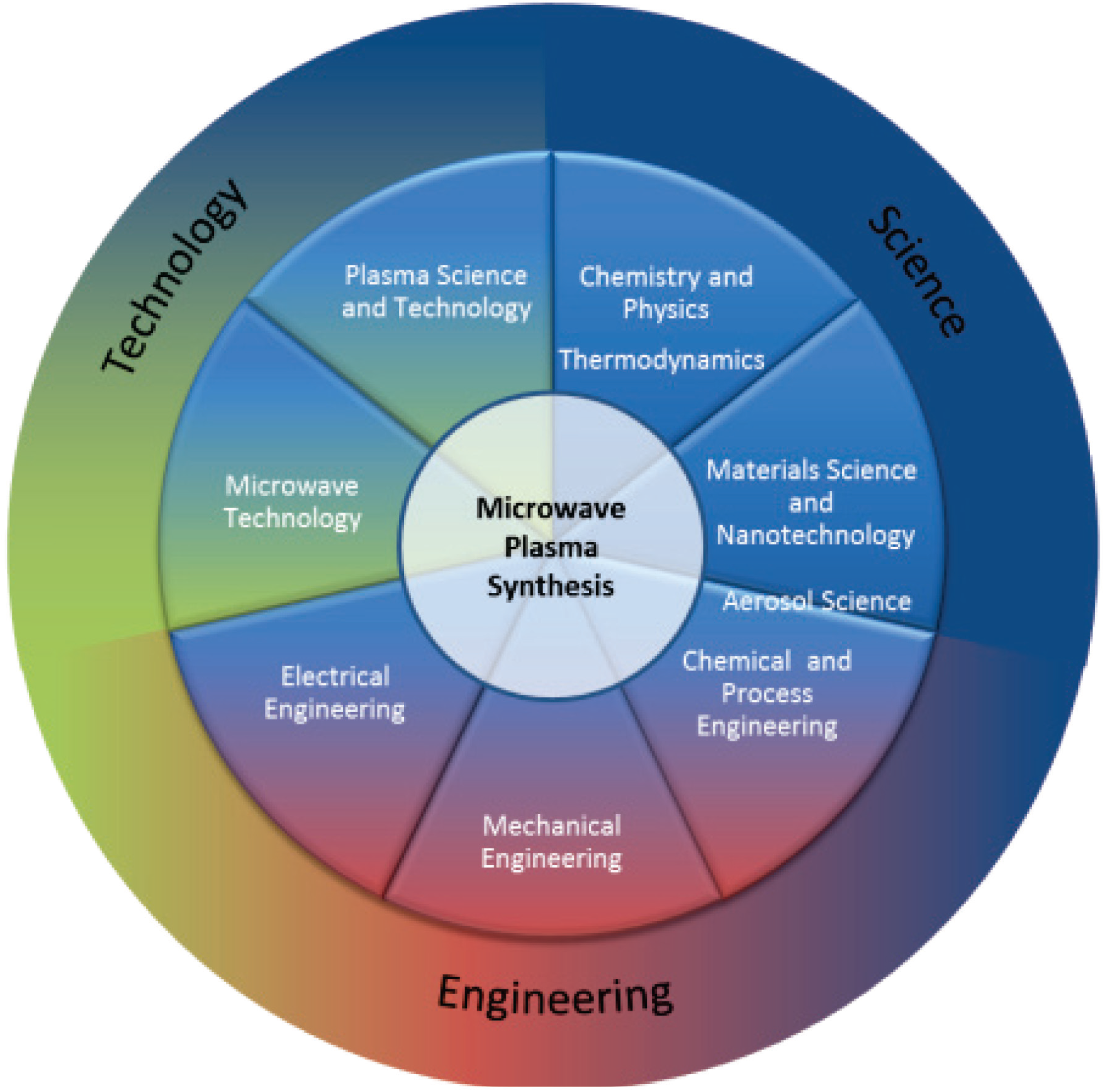
Microwave Plasma Synthesis of Materials—From Physics and Chemistry to Nanoparticles: A Materials Scientist’s Viewpoint
Abstract
:1. Introduction
2. Definition of Terms
- Microwaves are electromagnetic waves in the frequency range from 300 GHz to 300 MHz. The corresponding wavelengths range from 1 mm to 1 m. Industrially used microwave frequencies are 0.915 GHz with wavelength λ~ 32 cm (mobile phone, food processing), and 2.45 GHz with λ~12 cm (kitchen microwave, microwave sterilization).
- In physics and chemistry, plasma is typically an ionized gas [17]. It contains neutral as well as charged elementary particles (electrons, ions, molecules). Plasma is considered to be a distinct state of matter because of its unique properties. The term “ionized” refers to the presence of one or more free electrons, which are not bound to an atom or molecule. The free electric charges make the plasma electrically conductive so that it responds strongly to electromagnetic fields.
- Plasma, containing particles (in the sense of particulate matter), is called “dusty plasma”. This type of plasma is used for particles syntheses [18]. In the context of this paper, “particle” has the meaning of particulate matter.
- Thermal plasmas are characterized by a thermodynamic equilibrium, meaning that all species (electrons, ions, and neutral species) have the same temperature (energy). An example for thermal plasma is arc plasma. Its temperature may be around 10,000 K. This type of plasma is mainly not part of this article.
- Non-thermal plasmas are characterized by a thermal non-equilibrium between the temperature of the electrons and the ions. The temperature of the electrons ranges between several electron volt (eV), whereas the temperature of the positively charged ions and neutral species is significantly colder (around room temperature) [19,20], leading to a quite low overall temperature. Therefore, non-thermal plasmas, also called non-equilibrium plasmas, are favorable for the synthesis of nanoparticles at low temperatures. They can be ignited by microwaves, but also by, e.g., RF or by DBD [9,21]. The temperature of the plasma is around 300–1000 K. Therefore, this type of plasma is also called “cold plasma”.
- Atmospheric pressure plasmas are operated at atmospheric pressure. They are favorable for industrial processes, as they need smaller experimental efforts and thus lower costs. Ignition is possible by DC, by RF, and by microwaves. Atmospheric plasmas may be thermal plasmas or non-thermal plasmas [22].
- Low-pressure plasmas are operated under vacuum conditions (several 100 Pa to 10,000 Pa). They require more expensive vacuum-components and more sophisticated set-ups. They are usually “cold plasmas”.
- Nanomaterials, respectively nanoparticles, are particulate matter and generally defined as materials with grain sizes below 100 nm in at least one dimension. A more stringent definition is to specify nanomaterials or nanoparticles as materials with particles size dependent properties.
- Gas-phase processes are chemical reactions, where gaseous components react in a gaseous environment to form solid reaction products (e.g., particles). All plasma reactions in the context of this paper are gas-phase reactions.
3. Microwave Plasma Processes for Materials Synthesis in Literature
3.1. Finding the Key Players
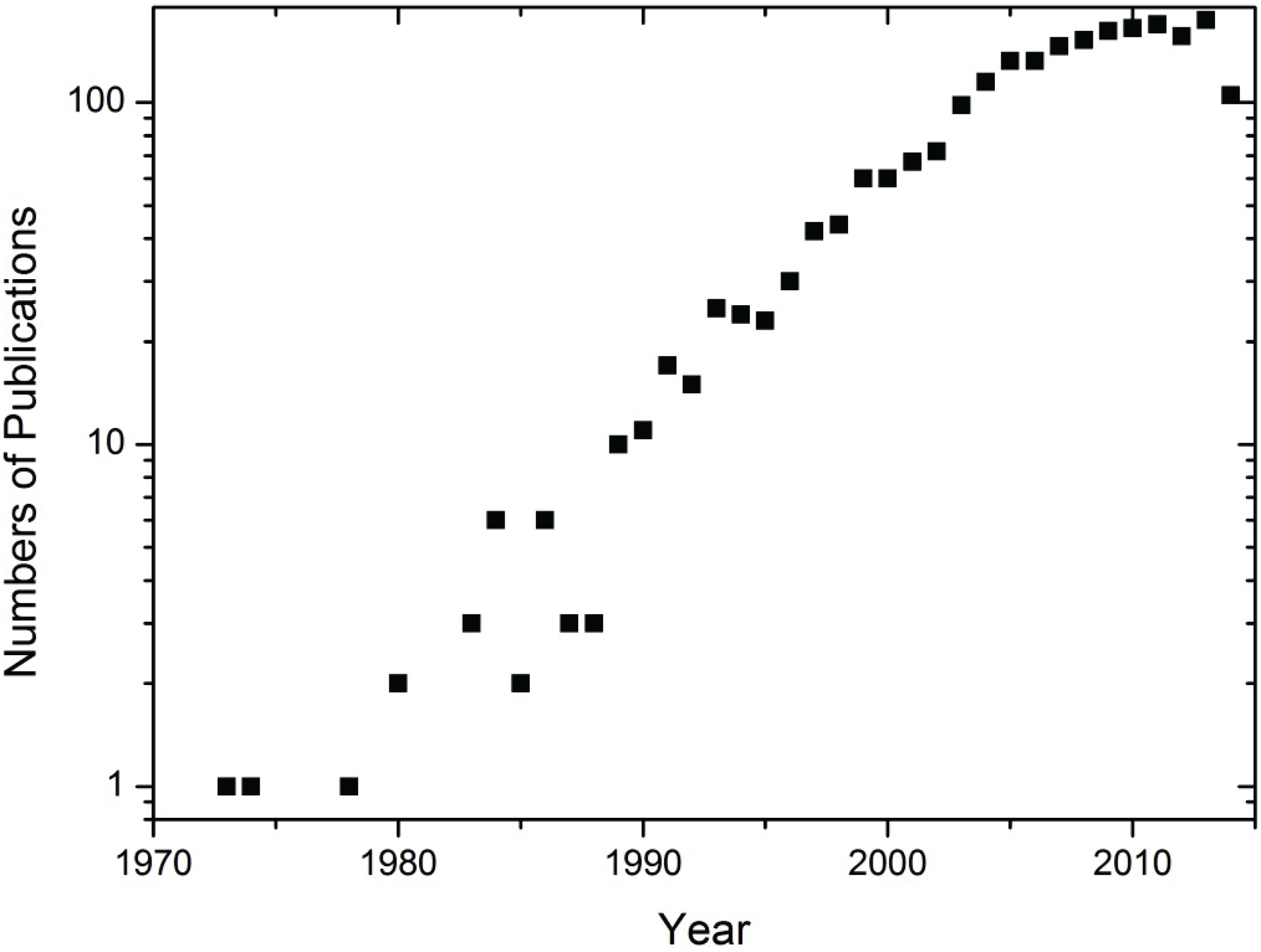
3.2. Microwave Plasma Synthesis of Particulate Matter in the Historical Context
4. Physical Background of the Microwave Plasma Process and Technical Equipment
- operating pressure
- ○
- low pressure plasma
- ○
- atmospheric pressure plasma
- thermodynamic equilibrium
- ○
- thermal or equilibrium plasma (Telectron ≈ Tion≈ Tgas)
- ○
- non-thermal plasma or non-equilibrium plasma (Telectron >> Tion ≈ Tgas)
- temperature
- ○
- low temperature plasma (Tgas < 2000 K)
- ○
- high temperature plasma (Tgas > 2000 K).
- plasma generation
- ○
- microwave discharge (300 MHz ≤ f ≤ 300 GHz)
- ○
- radio frequency discharge (450 kHz–3.0 MHz; 13.56 MHz)
- ○
- DC discharge
- ○
- dielectric barrier discharge
- ○
- corona discharge
- ○
- electric arc
- ○
- hollow cathode discharge
- ○
- electron beam
- ○
- plasma torch
- ○
- alternating current
- type of coupling
- ○
- inductive coupling
- ○
- capacitive coupling
4.1. Energy Transfer in a Microwave Plasma

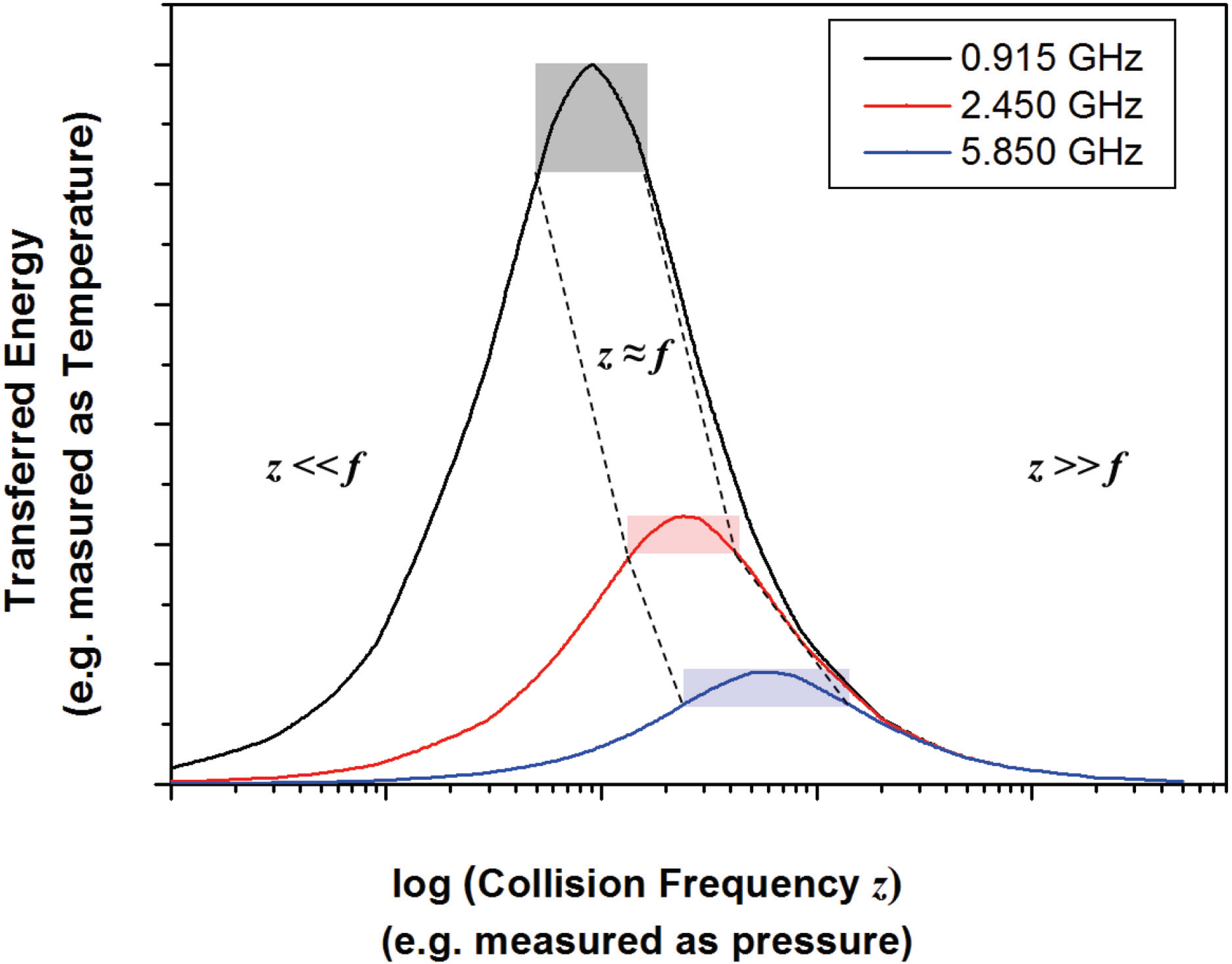
4.2. Impact of Microwave Plasma on Particle Formation

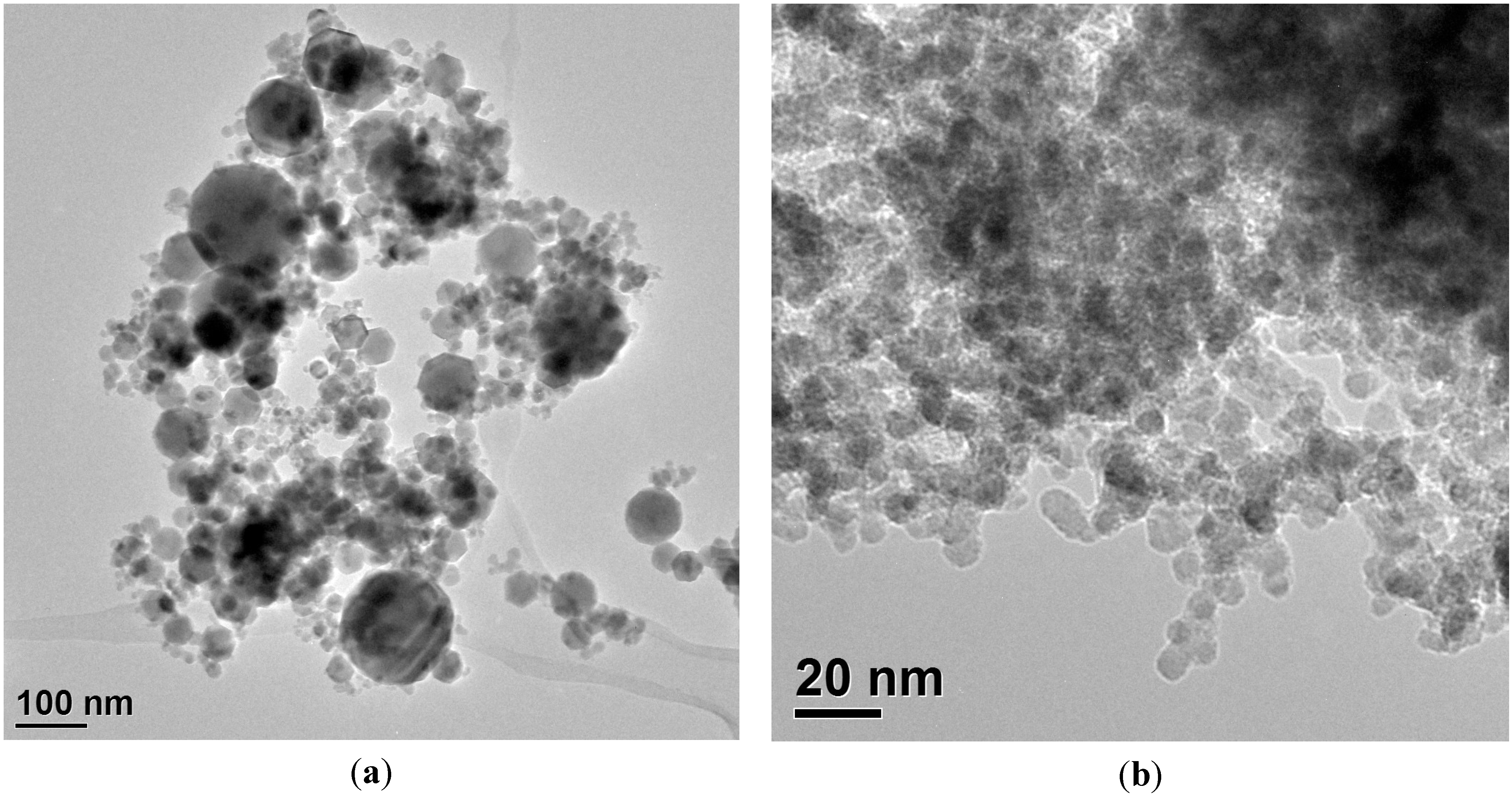
4.3. Influence of Process Parameters on the Resulting Product
4.3.1. Criteria How to Choose a Precursor
| No. | Chemical formula [CAS-Number] | Name/synonym | Melting point/boiling point | Aggregate state | Remarks and hazards |
|---|---|---|---|---|---|
| 1 | FeCl3 [7705-08-0] | Iron(III)chloride | 304 °C/319 °C 120 °C sublimation | solid | Corrosive; hygroscopic; harmful; releases Cl |
| 2 | SiCl4 [10026-04-7] | Silicon(IV) chloride Silicon-tetrachloride | −70 °C/57 °C | liquid | Moisture sensitive; corrosive to metals and tissues; irritant; releases Cl |
| 3 | SnCl4 [7646-78-8] | Tin(IV) chloride Tin-tetrachloride | −33 °C/114 °C | liquid | Moisture and air sensitive; corrosive to metals and tissues; harmful; irritant; releases Cl |
| 4 | TiCl4 [7550-45-0] | Titanium(IV)chloride Titanium-tetrachloride | −25 °C/136 °C | liquid | Moisture sensitive; corrosive to metals and tissues; irritant; releases Cl |
| 5 | Fe(CO)5 [13463-40-6] | Ironpentacarbonyl | −20 °C/103 °C | liquid | Air sensitive; highly flammable; very toxic |
| 6 | SiH4 [7803-62-5] | Mono-Silane Silicon-tetrahydride | −187 °C/−112 °C | gaseous | Extremely flammable; pyrophoric in air |
| 7 | Sn(C4H9)4 [1461-25-2] | Tetra-n-butyltin | −97 °C/145 °C @ 10 mm Hg pressure | liquid | Harmful; toxic |
| 8 | Ti(OC4H9)4 [5593-70-4] | Titanium(IV)-n-butoxide | −55 °C/206 °C @ 10 mm Hg pressure | liquid | Moisture sensitive; flammable; irritant |
| 9 | Zr(OC4H9)4 [2081-12-1] | Zirconium(IV)-t-butoxide | 3 °C/90 °C @ 5 mm Hg pressure | liquid | Moisture sensitive; irritant |
| 10 | HC≡CH [74-86-2] | Acetylene | −82 °C (sublimation) | gaseous | Flammable; may cause fire flash |
| 11 | CH4 [78-82-8] | Methane | −182 °C/−164 °C | gaseous | Flammable; explosive |
| 12 | H2C=CH2 [78-85-1] | Ethylene | −169 °C/−103 °C | gaseous | Highly flammable; may form explosive mixtures with air |
4.3.2. Influence of Precursor Concentration
4.3.3. Influence of Microwave Power, Pressure, Temperature, and Residence Time
4.4. Microwave Components and Experimental Set-Up
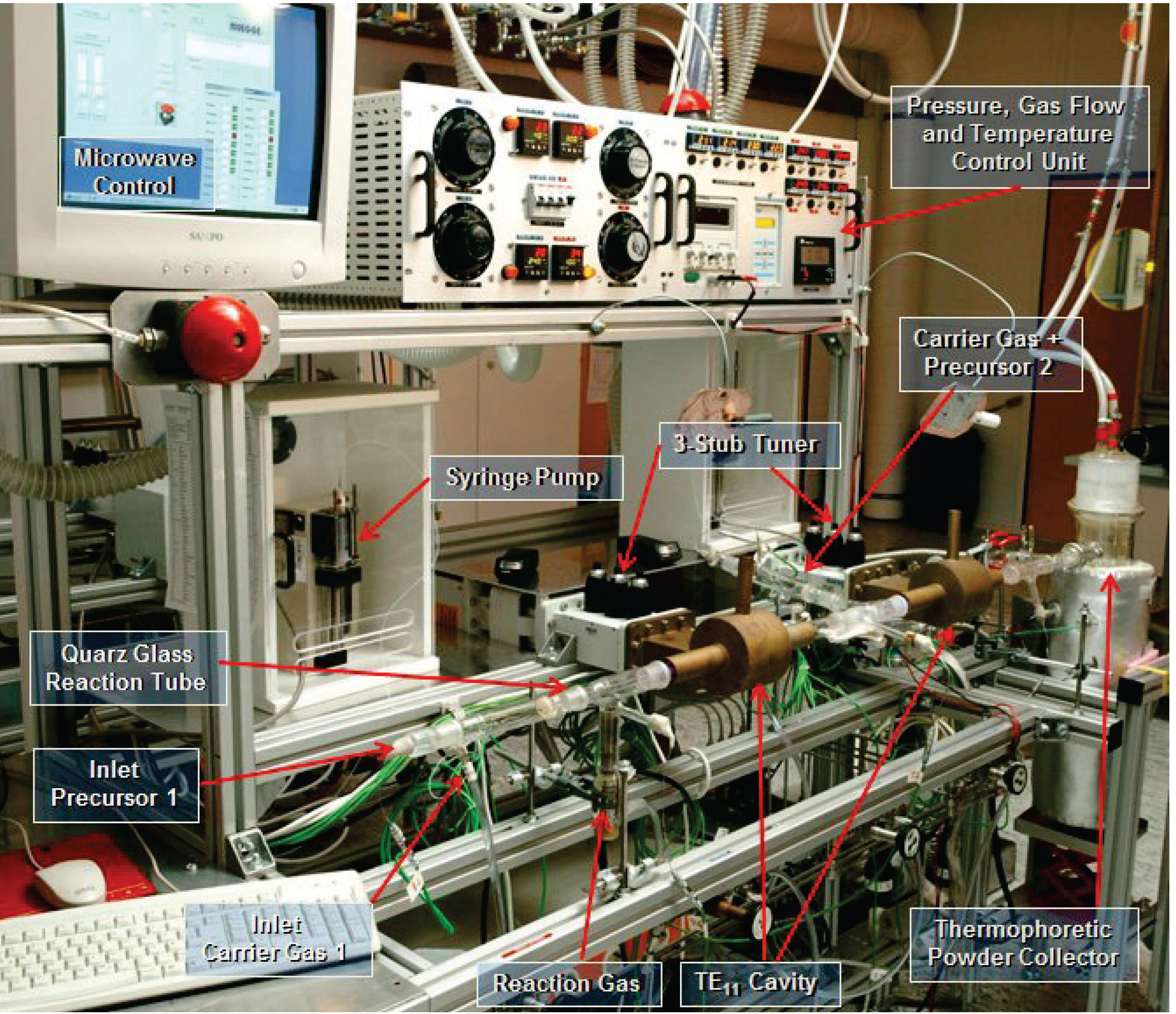
5. Materials Synthesized in Microwave Plasma
5.1. Tabular Overview on Literature Data
| Oxides | MW low pressure plasma | MW atmospheric pressure | RF plasma | DC thermal plasma | |
|---|---|---|---|---|---|
| 2.45 GHz | 0.915 GHz | ||||
| Fe2O3 | [40,72,102,105,106] | [107,108] | [52,102,103,109,110,111] | [112] | [113,114,115] |
| ZrO2 | [74,116,117,118,119,120] | [41,42] | |||
| Al2O3 | [42,43,85,118] | [41] | [121] | ||
| SnO2 | [54,60,83,122] | [123] | |||
| TiO2 | [120,124] | [41] | [55,56,125,126] | [127] | |
| ZnO | [48,73,128] | [129,130] | [131,132] | ||
| Cr2O3 | [133] | ||||
| TeO2 | [134] | ||||
| MgO | [135] | ||||
| V2O5 | [130,136] | ||||
| WO3 | [137] | [138] | |||
| GeO2 | [48] | ||||
| HfO2 | [119] | ||||
| Materials | MW low pressure plasma | MW atmospheric pressure | MW not specified | RF plasma | DC thermal plasma | |
|---|---|---|---|---|---|---|
| 2.45 GHz | 0.915 GHz | |||||
| Nitrides | ||||||
| GaN | [139] | [82,140] | ||||
| TiN | [141,142,143] | [144] | [145] | |||
| ZrN | [146] | [144] | ||||
| BN | [147] | |||||
| VN | [142,148] | |||||
| Si3N4 | [142] | [149] | ||||
| AlN | [150,151] | |||||
| other | [152] | |||||
| Carbides | ||||||
| SiC | [86,153] | [84] | [154,155,156,157] | [156,158,159,160,161] | ||
| B4C | [162] | |||||
| Fe-carbides | [163] | |||||
| Carbon Materials | ||||||
| amorphous C | [164,165] | |||||
| carbonaceous | [166] | |||||
| graphite | [165] | [167] | ||||
| graphene | [63] | |||||
| diamond | [33,35,165] | [168] | ||||
| fullerenes | [169] | |||||
| Chalcogenides | MW low pressure plasma | |
|---|---|---|
| 2.45 GHz | 0.915 GHz | |
| MoS2 | [170] | [170] |
| WS2 | [170,171,172] | [170,171] |
| ZrS2 | [172] | |
| HfS2 | [172] | |
| ZrSe2 | [171] | |
| SnS2 | [171] | |
| MoSe2 | [171] | |
| WSe2 | [171] | [171] |
| Metals | Low pressure plasma 2.45 GHz | MW atmospheric pressure | MW not specified | RF plasma | DC thermal plasma |
|---|---|---|---|---|---|
| Fe | [40,45,101,173] | [174] | [175] | [95,176] | [95] |
| Al | [177] | ||||
| Si | [49,59,81,104] | [143] | [178,179,180,181,182] | ||
| Ge | [183,184,185] | ||||
| In | [186] | ||||
| Zn | |||||
| Cu | [187] | ||||
| Mo | [187] | ||||
| W | [187] | ||||
| Ag | [188] | [189] | |||
| Co | [45] | [175,190] | |||
| Ni | [191] | ||||
| bimetallic particles | [45] | [187] | [175,191] | [192,193] |
| Composite materials | MW low pressure plasma | MW atmospheric pressure | MW not specified | RF plasma | DC thermal plasma | |
|---|---|---|---|---|---|---|
| 2.45 GHz | 0.915 GHz | |||||
| Coated or Core/Shell Nanoparticles | [51,81,97,194,195,196,197,198,199,200,201,202] | [44,117,203] | [63,204] | [190] | [205] | |
| Binary Oxides | [206] | |||||
| Doped Particles | [57,116,207] | [208] | [209,210,211,212] | [213] | ||
| Complex/Composite Particles | [214] | [63,111,215] | [216] | |||
5.2. Materials and Properties
5.2.1. Superparamagnetic Iron Oxide Nanoparticles
| Author | Material and precursor | Plasma type | Particle size | Magnetic properties |
|---|---|---|---|---|
| Li et al. [52] | γ-Fe2O3 from Fe(CO)5 | 2.45 GHz, atmospheric pressure plasma jet, 1 kW | 900 K: Ø 45 nm | not given |
| spread 20–100 nm | ||||
| 1100 K: Ø 26 nm
| ||||
| spread 15–40 nm | ||||
| David et al. [102,109] | γ-Fe2O3 from Fe(CO)5 | 2.45 GHz, atmospheric pressure torch 140 W | 30–100 nm | 293 K(1 T) σs = 66.6 Am2/kg 4 K(1 T): σs = 77.0 Am2/kg |
| Synek et al. [110] | γ-Fe2O3 from Fe(CO)5 | 2.45 GHz, atmospheric pressure torch 180 W | Ø 12 nm spread 5.5–20 nm | not given |
| David et al. [103] | ε-Fe2O3 from Fe(CO)5 | 2.45 GHz atmospheric pressure torch 230 W | 10–100 nm | not given |
| Synek et al. [111] | Fe3O4 and/or γ-Fe2O3 from Fe(CO)5 | 2.45 GHz atmospheric pressure torch 310 W | 5–21 nm | not given |
| Chou & Phillips [40] | Fe2O3 from Ferrocene | 2.45 GHz low pressure plasma 3000 Pa, 200 W | 10–100 nm | not given |
| Janzen et al. [72] | γ-Fe2O3 from Fe(CO)5 | 2.45 GHz low pressure plasma 3000 Pa | 80 W: 5.3 nm 160 W: 4.1 nm | not given |
| David et al. [102] | γ-Fe2O3 from Fe(CO)5 | 2.45 GHz low pressure plasma 4000 Pa, 650 W | 20–80 nm | 293 K(1 T) σs = 59.9 Am2/kg 4 K(1 T) σs = 69.3 Am2/kg |
| Nadeem et al. [105] | γ-Fe2O3 from Fe(CO)5 | 2.45 GHz low pressure plasma | Ø 6 nm spread 3–10 nm | 300 K(5 T) σs = 42 Am2/kg |
| 4.2 K(5 T) σs = 51 Am2/kg | ||||
| Vollath et al. [107] | γ-Fe2O3 from FeCl3 | 0.915 GHz low pressure plasma | 4–5 nm | 300 K(1 T) σs = 2.7 Am2/kg |
| 10 K(1 T) σs = 4.3 Am2/kg | ||||
| Vollath et al. [108] | γ-Fe2O3 from FeCl3 | 0.915 GHz low pressure plasma 3000 Pa | 6–8 nm | 300 K(1 T) σs = 3.4 Am2/kg 10 K(1 T) σs = 6.4 Am2/kg |
| Vollath et al. [108] | γ-Fe2O3 from Fe3(CO)12 | 0.915 GHz low pressure plasma 3000 Pa | 4–6 nm | 300 K(1 T) σs = 16.0 Am2/kg 10 K(1 T) σs = 18.9 Am2/kg |
| Banerjee et al. [114] | γ-Fe2O3 from Fe-metal | DC thermal plasma | 10–100 nm | 300 K(1.5 T) σs = 79 Am2/kg |
| Lei et al. [115] | Fe3O4 and/or γ-Fe2O3 from Ferrocene | DC thermal plasma torch | 8–9 nm | 293 K(1.5 T) σs = 40 Am2/kg 10 K(1.5 T) σs = 43 Am2/kg |
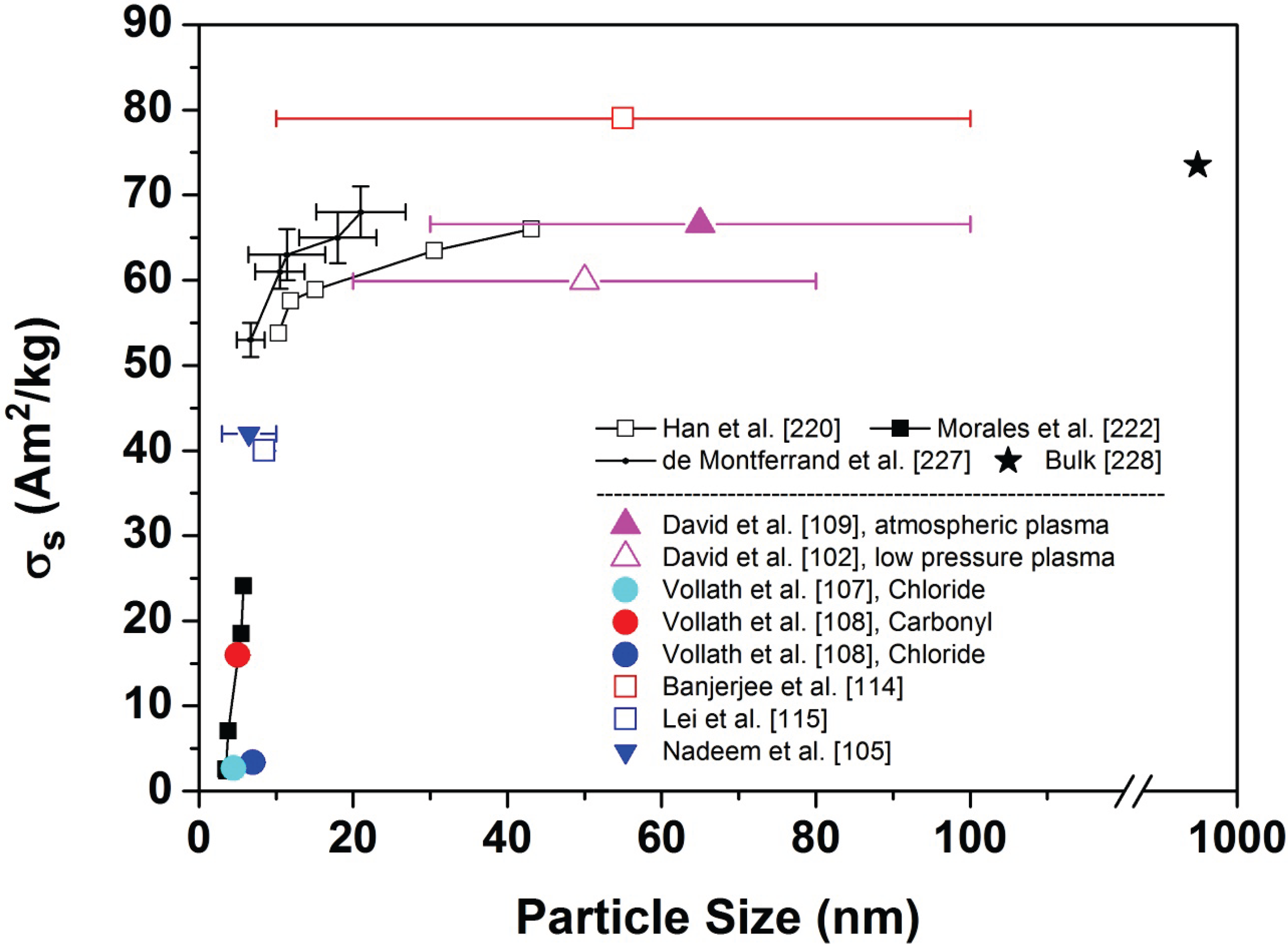
5.2.2. Luminescence Properties and Bifunctional Nanoparticles
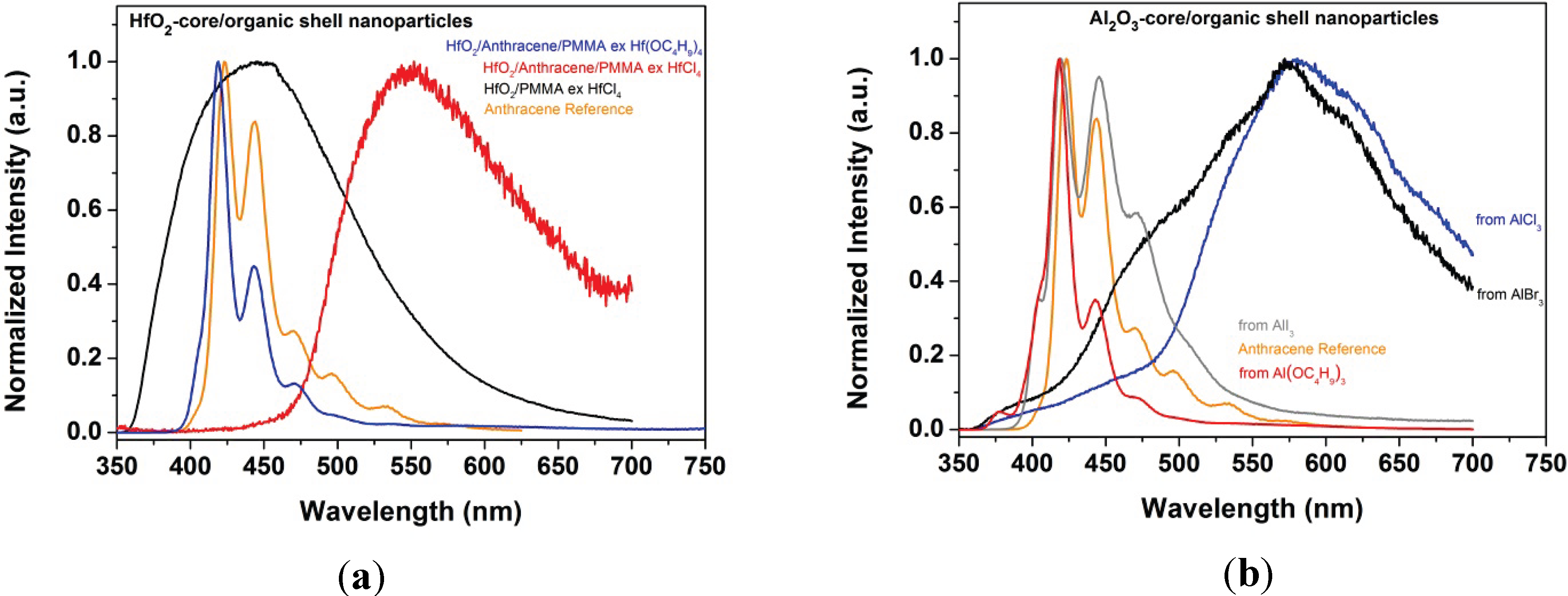
| Core/Shell | Precursor | Spectrum type | Peak positions (nm) |
|---|---|---|---|
| Al2O3/PMMA | AlCl3 | Excimer | 418.5 (very broad) |
| ZrO2/PMMA | ZrCl4 | Excimer | 445 (very broad) |
| ZrO2/PMMA | Zr(OC4H9)4 | Excimer | 408.5 (broad) |
| HfO2/PMMA | HfCl4 | Excimer | 445 (very broad) |
| Anthracene (reference) | Molecule | 423 (M); 444; 470; 496; 533 | |
| Al2O3/Anthracene/PMMA | AlCl3 | Excimer | 580.5 (very broad) |
| ZrO2/Anthracene/PMMA | ZrCl4 | Excimer | 552 (very broad) |
| HfO2/Anthracene/PMMA | HfCl4 | Excimer | 546 (very broad) |
| Al2O3/Anthracene/PMMA | AlBr3 | Excimer | 576 (very broad) |
| Al2O3/Anthracene/PMMA | AlI3 | Molecule | 404 (S); 419 (M); 445.5; 470.5; |
| Al2O3/Anthracene/PMMA | Al(OC4H9)3 | Molecule | 377; 405 (S); 418.5 (M); 443; 471 |
| ZrO2/Anthracene/PMMA | Zr(OC4H9)4 | Molecule | 405 (S); 418.5 (M); 443; 471.5 |
| HfO2/Anthracene/PMMA | Hf(OC4H9)4 | Molecule | 405 (S); 419 (M); 443; 471 |
5.2.3. Sn-Based Nanocomposites for Li-Ion Battery Application
| Material/precursor/gas | Specific capacity [mA h g−1] | References/comments | ||
|---|---|---|---|---|
| 2nd cycle | After 50 cycles | After 100 cycles (% of 2nd cycle) | ||
| SnO2/C core/shell | 706 | 238 | 173 (24.5%) | [100]; no drying step before battery assembly |
| SnCl4/C10H12 | ||||
| Ar/20%O2 | ||||
| SnO2(CxHy) composite | 1186 | 468 | 404 (34.1%) | [244]; improved battery assembly |
| Sn(C4H9)4 | ||||
| Ar/20%O2 | ||||
| SnO2(CxHy) composite | 1137 | 640 | 558 (49.1%) | [244]; vinylene carbonate (VC) addition into electrolyte |
| Sn(C4H9)4 | ||||
| Ar/20%O2 | ||||
| Sn(O)-CxHy | 1132 | 799 | 750 (66.3%) | [244] |
| Sn(C4H9)4 | ||||
| Ar | ||||
| Sn(O)-CxHy | 1553 | 918 | 783 (50.4%) | [214,244]; VC addition into electrolyte |
| Sn(C4H9)4 | ||||
| Ar | ||||
6. Summary
Conflicts of Interest
Author Contributions
References
- Siegel, R.W.; Ramasamy, S.; Hahn, H.; Zongquan, L.; Ting, L.; Gronsky, R. Synthesis, characterization, and properties of nanophase TiO2. J. Mater. Res. 1988, 3, 1367–1372. [Google Scholar] [CrossRef]
- Gurav, A.; Kodas, T.; Pluym, T.; Xiong, Y. Aerosol Processing of Materials. Aerosol Sci. Tech. 1993, 19, 411–452. [Google Scholar] [CrossRef]
- Pratsinis, S.E.; Vemury, S. Particle formation in gases: A review. Powder Technol. 1996, 88, 267–273. [Google Scholar] [CrossRef]
- Hahn, H. Gas phase synthesis of nanocrystalline materials. Nanostruct. Mater. 1997, 9, 3–12. [Google Scholar] [CrossRef]
- Chen, Y.; Glumac, N.; Kear, B.H.; Skandan, G. High rate synthesis of nanophase materials. Nanostruct. Mater. 1997, 9, 101–104. [Google Scholar] [CrossRef]
- Kear, B.H.; Glumac, N.G.; Chen, Y.J.; Skandan, G. Flame Synthesis of Nanophase Oxide Powders. Part. Sci. Technol. 1997, 15, 174. [Google Scholar] [CrossRef]
- Skandan, G.; Chen, Y.J.; Glumac, N.; Kear, B.H. Synthesis of oxide nanoparticles in low pressure flames. Nanostruct. Mater. 1999, 11, 149–158. [Google Scholar] [CrossRef]
- Rellinghaus, B.; Lindackers, D.; Köckerling, M.; Roth, P.; Wassermann, E.F. The Process of Particle Formation in the Flame Synthesis of Tin Oxide Nanoparticles. Phase Transit. 2003, 76, 347–354. [Google Scholar] [CrossRef]
- Vollath, D. Plasma synthesis of nanopowders. J. Nanopart. Res. 2008, 10, 39–57. [Google Scholar] [CrossRef]
- Wiggers, H. Novel Material Properties Based on Flame-synthesized Nanomaterials. KONA 2009, 186–194. [Google Scholar] [CrossRef]
- Kumfer, B.M.; Shinoda, K.; Jeyadevan, B.; Kennedy, I.M. Gas-phase flame synthesis and properties of magnetic iron oxide nanoparticles with reduced oxidation state. J. Aerosol. Sci. 2010, 41, 257–265. [Google Scholar] [CrossRef]
- Pratsinis, S.E. Aerosol-based technologies in nanoscale manufacturing: from functional materials to devices through core chemical engineering. AiChE J. 2010, 56, 3028–3035. [Google Scholar] [CrossRef]
- Schwade, B.; Roth, P. Simulation of nano-particle formation in a wall-heated aerosol reactor including coalescence. J. Aerosol. Sci. 2003, 34, 339–357. [Google Scholar] [CrossRef]
- Giesen, B.; Orthner, H.R.; Kowalik, A.; Roth, P. On the interaction of coagulation and coalescence during gas-phase synthesis of Fe-nanoparticle agglomerates. Chem. Eng. Sci. 2004, 59, 2201–2211. [Google Scholar] [CrossRef]
- Paur, H.R.; Baumann, W.; Mätzing, H.; Seifert, H. Formation of nanoparticles in flames; measurement by particle mass spectrometry and numerical simulation. Nanotechnology 2005, 16, S354–S361. [Google Scholar] [CrossRef]
- Bosisio, R.G.; Wertheimer, M.R.; Weissfloch, C.F. Generation of large volume microwave plasmas. J. Phys. E 1973, 6, 628–630. [Google Scholar] [CrossRef]
- Tonks, L.; Langmuir, I. Oscillations in Ionized Gases. Phys. Rev. 1929, 33, 195–210. [Google Scholar] [CrossRef]
- Seiji, S.; Masaru, H.; Shahid, R.; Kunihide, T.; Peter, B.; Gerrit, K.; Whitehead, J.C.; Anthony, B.M.; Alexander, F.G.; Svetlana, S.; et al. The 2012 Plasma Roadmap. J. Phys. D 2012, 45, 253001. [Google Scholar] [CrossRef]
- Mac Donald, A.D. Microwave Breakdown in Gases; John Wiley and Sons: New York, NY, USA, 1966. [Google Scholar]
- Kortshagen, U. Nonthermal plasma synthesis of semiconductor nanocrystals. J. Phys. D 2009, 42, 113001. [Google Scholar] [CrossRef]
- Szabó, D.V. Microwave Plasma Synthesis of Nanoparticles: From Theoretical Background and Experimental Realization to Nanoparticles with Special Properties. In Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Horikoshi, S., Serpone, N., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 271–309. [Google Scholar]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectroc. Acta Part B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Suzuki, K.; Okudaira, S.; Sakudo, N.; Kanomata, I. Microwave Plasma Etching. Jpn. J. Appl. Phys. 1977, 16, 1979–1984. [Google Scholar] [CrossRef]
- Suzuki, K.; Okudaira, S.; Kanomata, I. The Roles of Ions and Neutral Active Species in Microwave Plasma Etching. J. Electrochem. Soc. 1979, 126, 1024–1028. [Google Scholar] [CrossRef]
- Suzuki, K.; Okudaira, S.; Nishimatsu, S.; Usami, K.; Kanomata, I. Microwave Plasma Etching of Si with CF4 and SF6 Gas. J. Electrochem. Soc. 1982, 129, 2764–2769. [Google Scholar] [CrossRef]
- Eddy Jr, C.R.; Sartwell, B.D.; Youchison, D.L. Diamond thin film growth on silicon at temperatures between 500 and 600 °C using an electron cyclotron resonance microwave plasma source. Surf. Coat. Technol. 1991, 48, 69–79. [Google Scholar] [CrossRef]
- Szekely, J. Overview of Plasma Processing. Mat. Res. Soc. Symp. Proc. 1984, 30, 1–11. [Google Scholar] [CrossRef]
- Young, R.M.; Pfender, E. Generation and behavior of fine particles in thermal plasmas—A review. Plasma Chem. Plasma Process. 1985, 5, 1–37. [Google Scholar] [CrossRef]
- Amouroux, J.; Morvan, D.; Gicquel, A. Plasma for special applications. Fresen. Z. Anal. Chem. 1986, 324, 384–396. [Google Scholar] [CrossRef]
- Chang, Y.; Pfender, E. Thermochemistry of thermal plasma chemical reactions. Part 1. General rules for the prediction of products. Plasma Chem. Plasma Process. 1987, 7, 275–297. [Google Scholar] [CrossRef]
- Chang, Y.; Young, R.M.; Pfender, E. Thermochemistry of thermal plasma chemical reactions. Part 2. A survey of synthesis routes for silicon nitride production. Plasma Chem. Plasma Process. 1987, 7, 299–316. [Google Scholar] [CrossRef]
- Moisan, M.; Wertheimer, M.R. Comparison of Microwave and r.f. Plasmas: Fundamentals and Applications. Surf. Coat. Technol. 1993, 59, 1–13. [Google Scholar] [CrossRef]
- Kamo, M.; Sato, Y.; Matsumoto, S.; Setaka, N. Diamond synthesis from gas phase in microwave plasma. J. Cryst. Growth 1983, 62, 642–644. [Google Scholar] [CrossRef]
- Saito, Y.; Matsuda, S.; Nogita, S. Synthesis of diamond by decomposition of methane in microwave plasma. J. Mater. Sci. Lett. 1986, 5, 565–568. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, K.; Tanaka, H.; Fujita, K.; Matuda, S. Diamond synthesis from methane-hydrogen-water mixed gas using a microwave plasma. J. Mater. Sci. 1988, 23, 842–846. [Google Scholar] [CrossRef]
- Takagi, H.; Ogawa, H.; Yamazaki, Y.; Ishizaki, A.; Nakagiri, T. Quantum size effects on photoluminescence in ultrafine Si particles. Appl. Phys. Lett. 1990, 56, 2379–2380. [Google Scholar] [CrossRef]
- Mehta, P.; Singh, A.K.; Kingon, A.I. Nonthermal Microwave Plasma Synthesis of Crystalline Titanium Oxide and Titanium Nitride Nanoparticles. Mat. Res. Soc. Symp. Proc. 1992, 249, 153–159. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Vollath, D.; Varma, R. Electron-Microscopy Study on Zirconia and Alumina Ceramic Powders Synthesized by Microwave Plasma Pyrolysis. Mat. Res. Soc. Symp. Proc. 1992, 269, 363–369. [Google Scholar] [CrossRef]
- Vollath, D.; Varma, R.; Sickafus, K.E. Synthesis of Nanocrystalline Powders for Oxide Ceramics by Microwave Plasma Pyrolysis. Mat. Res. Soc. Symp. Proc. 1992, 269, 379–384. [Google Scholar] [CrossRef]
- Chou, C.H.; Phillips, J. Plasma production of metallic nanoparticles. J. Mater. Res. 1992, 7, 2107–2113. [Google Scholar] [CrossRef]
- Vollath, D.; Sickafus, K.E. Synthesis of nanosized ceramic oxide powders by microwave plasma reactions. Nanostruct. Mater. 1992, 1, 427–437. [Google Scholar] [CrossRef]
- Vollath, D.; Sickafus, K.E. Synthesis of Ceramic Oxide Powders by Microwave Plasma Pyrolysis. J. Mater. Sci. 1993, 28, 5943–5948. [Google Scholar] [CrossRef]
- Vollath, D.; Sickafus, K.E. Synthesis of Ceramic Oxide Powders in a Microwave Plasma-Device. J. Mater. Res. 1993, 8, 2978–2984. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Nanocoated particles: A special type of ceramic powder. Nanostruct. Mater. 1994, 4, 927–938. [Google Scholar] [CrossRef]
- Brenner, J.R.; Harkness, J.B.L.; Knickelbein, M.B.; Krumdick, G.K.; Marshall, C.L. Microwave plasma synthesis of carbon-supported ultrafine metal particles. Nanostruct. Mater. 1997, 8, 1–17. [Google Scholar] [CrossRef]
- Aliev, Y.M.; Maximov, A.V.; Kortshagen, U.; Schlüter, H.; Shivarova, A. Modeling of microwave discharges in the presence of plasma resonances. Phys. Rev. E 1995, 51, 6091–6103. [Google Scholar] [CrossRef]
- Kortshagen, U.; Bhandarkar, U. Modeling of particulate coagulation in low pressure plasmas. Phys. Rev. E 1999, 60, 887–898. [Google Scholar] [CrossRef]
- Janzen, C.; Kleinwechter, H.; Knipping, J.; Wiggers, H.; Roth, P. Size analysis in low-pressure nanoparticle reactors: comparison of particle mass spectrometry with in situ probing transmission electron microscopy. J. Aerosol. Sci. 2002, 33, 833–841. [Google Scholar] [CrossRef]
- Giesen, B.; Wiggers, H.; Kowalik, A.; Roth, P. Formation of Si-nanoparticles in a microwave reactor: Comparison between experiments and modelling. J. Nanopart. Res. 2005, 7, 29–41. [Google Scholar] [CrossRef]
- Gatti, M.; Kortshagen, U. Analytical model of particle charging in plasmas over a wide range of collisionality. Phys. Rev. E 2008, 78, 046402. [Google Scholar] [CrossRef]
- Vollath, D.; Lamparth, I.; Szabó, D.V. Fluorescence from coated oxide nanoparticles. Mat. Res. Soc. Symp. Proc. 2002, 703, 03–308. [Google Scholar]
- Li, S.-Z.; Hong, Y.C.; Uhm, H.S.; Li, Z.-K. Synthesis of Nanocrystalline Iron Oxide Particles by Microwave Plasma Jet at Atmospheric Pressure. Jpn. J. Appl. Phys. 2004, 43, 7714–7717. [Google Scholar] [CrossRef]
- Schumacher, B.; Ochs, R.; Tröße, H.; Schlabach, S.; Bruns, M.; Szabo, D.V.; Haußelt, J. Electronic micro nose equipped with nano-structured gas sensitive SnO2. MST News 2006, 6, 10–12. [Google Scholar]
- Schumacher, B.; Ochs, R.; Tröße, H.; Schlabach, S.; Bruns, M.; Szabó, D.V.; Haußelt, J. Nanogranular SnO2 Layers for Gas Sensing Applications by In Situ Deposition of Nanoparticles Produced by the Karlsruhe Microwave Plasma Process. Plasma Process. Polym. 2007, 4, S865–S870. [Google Scholar] [CrossRef]
- Mahendra Kumar, S.; Deshpande, P.A.; Krishna, M.; Krupashankara, M.S.; Madras, G. Photocatalytic Activity of Microwave Plasma-Synthesized TiO2 Nanopowder. Plasma Chem. Plasma Process. 2010, 30, 461–470. [Google Scholar] [CrossRef]
- Hong, Y.C.; Lho, T.; Lee, B.J.; Uhm, H.S.; Kwon, O.P.; Lee, S.H. Synthesis of titanium dioxide in O2/Ar/SO2/TiCl4 microwave torch plasma and its band gap narrowing. Curr. Appl. Phys. 2011, 11, 517–520. [Google Scholar] [CrossRef]
- Kautsch, A.; Brossmann, U.; Krenn, H.; Hofer, F.; Szabó, D.V.; Würschum, R. Structural and optical properties of nanoparticulate Y2O3:Eu2O3 made by microwave plasma synthesis. Appl. Phys. A 2011, 105, 709–712. [Google Scholar] [CrossRef]
- Nadeem, K.; Krenn, H.; Traussnig, T.; Würschum, R.; Szabó, D.V.; Letofsky-Papst, I. Effect of dipolar and exchange interactions on magnetic blocking of maghemite nanoparticles. J. Magn. Magn. Mat. 2011, 323, 1998–2004. [Google Scholar] [CrossRef]
- Petermann, N.; Stein, N.; Schierning, G.; Theissmann, R.; Stoib, B.; Brandt, M.S.; Hecht, C.; Schulz, C.; Wiggers, H. Plasma synthesis of nanostructures for improved thermoelectric properties. J. Phys. D 2011, 44, 174034. [Google Scholar] [CrossRef]
- Szabó, D.V.; Kilibarda, G.; Schlabach, S.; Trouillet, V.; Bruns, M. Structural and chemical characterization of SnO2-based nanoparticles as electrode material in Li-ion batteries. J. Mater. Sci. 2012, 47, 4383–4391. [Google Scholar] [CrossRef]
- Ishigaki, T. Synthesis of ceramic nanoparticles with non-equilibrium crystal structures and chemical compositions by controlled thermal plasma processing. J. Ceram. Soc. Jpn. 2008, 116, 462–470. [Google Scholar] [CrossRef]
- Schütze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Phillips, J.; Luhrs, C.C.; Richard, M. Review: Engineering Particles Using the Aerosol-Through-Plasma Method. IEEE Trans. Plasma Sci. 2009, 37, 726–739. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Belmonte, T.; Arnoult, G.; Henrion, G.; Gries, T. Nanoscience with non-equilibrium plasmas at atmospheric pressure. J. Phys. D 2011, 44, 363001. [Google Scholar] [CrossRef]
- Vollath, D. Nanomaterials: An Introduction to Synthesis, Properties and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Vollath, D. Estimation of particle size distributions obtained by gas phase processes. J. Nanopart. Res. 2011, 13, 3899–3909. [Google Scholar] [CrossRef]
- Schweigert, V.A.; Schweigert, I.V. Coagulation in a low-temperature plasma. J. Phys. D 1996, 29, 655–659. [Google Scholar] [CrossRef]
- Kortshagen, U.R.; Bhandarkar, U.V.; Swihart, M.T.; Girshick, S.L. Generation and growth of nanoparticles in low-pressure plasmas. Pure Appl. Chem. 1999, 71, 1871–1877. [Google Scholar]
- Mangolini, L.; Kortshagen, U. Selective nanoparticle heating: Another form of nonequilibrium in dusty plasmas. Phys. Rev. E 2009, 79, 026405. [Google Scholar] [CrossRef]
- Galli, F.; Kortshagen, U.R. Charging, Coagulation, and Heating Model of Nanoparticles in a Low-Pressure Plasma Accounting for Ion-Neutral Collisions. IEEE Trans. Plasma Sci. 2010, 38, 803–809. [Google Scholar] [CrossRef]
- Janzen, C.; Wiggers, H.; Knipping, J.; Roth, P. Formation and in situ sizing of gamma-Fe2O3 nanoparticles in a microwave flow reactor. J. Nanosci. Nanotech. 2001, 1, 221–225. [Google Scholar] [CrossRef]
- Kleinwechter, H.; Janzen, C.; Knipping, J.; Wiggers, H.; Roth, P. Formation and properties of ZnO nanoparticles from gas-phase synthesis processes. J. Mater. Sci. 2002, 37, 4349–4360. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. The Microwave plasma process—A versatile process to synthesise nanoparticulate materials. J. Nanopart. Res. 2006, 8, 417–428. [Google Scholar] [CrossRef]
- Baumann, W.; Thekedar, B.; Paur, H.R.; Seifert, H. Characterization of nanoparticles synthesized in the microwave plasma discharge process by particle mass spectrometry and transmission electron microscopy. In Proceedings of AiChE Fall and Annual Meeting, San Francisco, CA, USA, 12–17 November 2006.
- Baumann, W.; Thekedar, B.; Paur, H.R.; Seifert, H. Comparison of size distribution of iron oxide nanoparticles measured with particle mass spectrometer and transmission electron microscopy. In Proceedings of International Congress on Particle Technology (PARTEC 2007), Nürnberg, Germany, 27–29 March 2007.
- Gozum, J.E.; Pollina, D.M.; Jensen, J.A.; Girolami, G.S. “Tailored” organometallics as precursors for the chemical vapor deposition of high-purity palladium and platinum thin films. J. Am. Chem. Soc. 1988, 110, 2688–2689. [Google Scholar] [CrossRef]
- Hampden-Smith, M.J.; Kodas, T.T. Chemical vapor deposition of metals: Part 1. An overview of CVD processes. Chem. Vap. Depos. 1995, 1, 8–23. [Google Scholar] [CrossRef]
- Rossetto, G.; Zanella, P.; Carta, G.; Bertani, R.; Favretto, D.; Ingo, G.M. Synthesis and characterization of methylcyclopentadienyl-(η3-allyl)platinum and its use as a metallo-organic chemical vapour deposition precursor of platinum. Appl. Organomet. Chem. 1999, 13, 509–513. [Google Scholar] [CrossRef]
- Thekedar, B. Size characterization of nanoparticles synthesized in microwave plasma discharge process. Master Thesis, University of Magdeburg, Chemical and Process Engineering, Magdeburg, Germany, February 2006. [Google Scholar]
- Knipping, J.; Wiggers, H.; Rellinghaus, B.; Roth, P.; Konjhodzic, D.; Meier, C. Synthesis of high purity silicon nanoparticles in a low pressure microwave reactor. J. Nanosci. Nanotech. 2004, 4, 1039–1044. [Google Scholar] [CrossRef]
- Shimada, M.; Wang, W.-N.; Okuyama, K. Synthesis of Gallium Nitride Nanoparticles by Microwave Plasma-Enhanced CVD. Chem. Vap. Depos. 2010, 16, 151–156. [Google Scholar] [CrossRef]
- Szabó, D.V.; Schlabach, S.; Ochs, R. Analytical TEM investigations of size effects in SnO2 nanoparticles produced by microwave plasma synthesis. Microsc. Microanal. 2007, 13, 430–431. [Google Scholar]
- Vennekamp, M.; Bauer, I.; Groh, M.; Sperling, E.; Ueberlein, S.; Myndyk, M.; Mäder, G.; Kaskel, S. Formation of SiC nanoparticles in an atmospheric microwave plasma. Beilstein J. Nanotechnol. 2011, 2, 665–673. [Google Scholar] [CrossRef]
- Fu, L.; Johnson, D.L.; Zheng, J.G.; Dravid, V.P. Microwave Plasma Synthesis of Nanostructured γ-Al2O3 Powders. J. Am. Ceram. Soc. 2003, 86, 1635–1637. [Google Scholar] [CrossRef]
- Schlabach, S.; Szabó, D.V.; Shi, Z.; Wang, D.; Vollath, D. Synthesis of nanoparticulate SiC in a low temperature microwave plasma process. In Nanofair 2004: New Ideas for Industry; VDI-Berichte: Karlsruhe, Germany, 2004; pp. 167–170. [Google Scholar]
- Vollath, D.; Szabó, D.V.; Hausselt, J. Synthesis and properties of ceramic nanoparticles and nanocomposites. J. Europ. Ceram. Soc. 1997, 17, 1317–1324. [Google Scholar] [CrossRef]
- Mühleisen, M.; Möbius, A. Vorrichtung zur reflektionsarmen Absorption von Mikrowellen. Patent DE 195 28 343 C2, May 1997. [Google Scholar]
- Vollath, D.; Szabó, D.V. Synthesis of nanopowders by the microwave plasma process—basic considerations and perspectives for scaling up. In Innovative Processing of Films and Nanocrystalline Powders, 1st ed.; Choy, K.-L., Ed.; Imperial College Press: London, UK, 2002; pp. 219–251. [Google Scholar]
- Zheng, F. Thermophoresis of spherical and non-spherical particles: A review of theories and experiments. Adv. Colloid Interface Sci. 2002, 97, 255–278. [Google Scholar] [CrossRef]
- Piazza, R. Thermophoresis: Moving particles with thermal gradients. Soft Matter 2008, 4, 1740–1744. [Google Scholar] [CrossRef]
- Abdali, A.; Moritz, B.; Gupta, A.; Wiggers, H.; Schulz, C. Hybrid microwave-plasma hot-wall reactor for synthesis of silica nanoparticles under well-controlled conditions. J. Optoelectron. Adv. Mater. 2010, 12, 440–444. [Google Scholar]
- Tsai, C.-J.; Chen, S.-C.; Przekop, R.; Moskal, A. Study of an Axial Flow Cyclone to Remove Nanoparticles in Vacuum. Environ. Sci. Technol. 2007, 41, 1689–1695. [Google Scholar] [CrossRef]
- Chen, S.-C.; Tsai, C.-J. An axial flow cyclone to remove nanoparticles at low pressure conditions. J. Nanopart. Res. 2007, 9, 71–83. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C.P.; Muno, R.; Wu, C.Y.; Yang, L.; Singh, S.K.; McMurry, P.H. Thermal plasma synthesis of ultrafine iron particles. J. Aerosol. Sci. 1993, 24, 367–382. [Google Scholar] [CrossRef]
- Krinke, T.J.; Deppert, K.; Magnusson, M.H.; Schmidt, F.; Fissan, H. Microscopic aspects of the deposition of nanoparticles from the gas phase. J. Aerosol. Sci. 2002, 33, 1341–1359. [Google Scholar] [CrossRef]
- Sagmeister, M.; Brossmann, U.; List, E.J.W.; Ochs, R.; Szabó, D.V.; Würschum, R. In-situ dispersion of ZrO2 nano-particles coated with pentacene. Phys. Status Solidi RRL 2008, 2, 203–205. [Google Scholar] [CrossRef]
- Schlabach, S.; Ochs, R.; Hanemann, T.; Szabó, D.V. Nanoparticles in polymer-matrix composites. Microsyst. Technol. 2011, 17, 183–193. [Google Scholar] [CrossRef]
- Schumacher, B.; Szabó, D.V.; Schlabach, S.; Ochs, R.; Müller, H.; Bruns, M. Nanoparticle SnO2 films as gas sensitive membranes. Mat. Res. Soc. Symp. Proc. 2006, 900E, O08 06.01–O08 06.06. [Google Scholar]
- Ochs, R.; Szabó, D.V.; Schlabach, S.; Becker, S.; Indris, S. Development of nanocomposites for anode materials in Li-ion batteries. Phys. Status Solidi A 2011, 208, 471–473. [Google Scholar] [CrossRef]
- Kalyanaraman, R.; Yoo, S.; Krupashankara, M.S.; Sudarshan, T.S.; Dowding, R.J. Synthesis and consolidation of iron nanopowders. Nanostruct. Mater. 1998, 10, 1379–1392. [Google Scholar] [CrossRef]
- David, B.; Pizúrová, N.; Schneeweiss, O.; Šantavá, E.; Kudrle, V.; Jašek, O. γ-Fe2O3 nanopowders synthesized in microwave plasma and extraordinarily strong temperature influence on their Mössbauer spectra. J. Nanosci. Nanotech. 2012, 12, 9277–9285. [Google Scholar] [CrossRef]
- David, B.; Pizúrová, N.; Synek, P.; Kudrle, V.; Jašek, O.; Schneeweiss, O. ε-Fe2O3 nanoparticles synthesized in atmospheric-pressure microwave torch. Mater. Lett. 2014, 116, 370–373. [Google Scholar] [CrossRef]
- Bywalez, R.; Karacuban, H.; Nienhaus, H.; Schulz, C.; Wiggers, H. Stabilization of mid-sized silicon nanoparticles by functionalization with acrylic acid. Nanoscale Res. Lett. 2012, 7, 76. [Google Scholar] [CrossRef]
- Nadeem, K.; Krenn, H.; Traußnig, T.; Würschum, R.; Szabó, D.V.; Letofsky-Papst, I. Spin-glass freezing of maghemite nanoparticles prepared by microwave plasma synthesis. J. Appl. Phys. 2012, 111, 113911. [Google Scholar]
- Vollath, D.; Szabó, D.V. Microwave plasma synthesis of ceramic nanapowders. J. Aerosol. Sci. 1997, 28, S685–S688. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V.; Taylor, R.D.; Willis, J.O.; Sickafus, K.E. Synthesis and properties of nanocrystalline superparamagnetic gamma-Fe2O3. Nanostruct. Mater. 1995, 6, 941–944. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V.; Taylor, R.D.; Willis, J.O. Synthesis and magnetic properties of nanostructured maghemite. J. Mater. Res. 1997, 12, 2175–2182. [Google Scholar] [CrossRef]
- David, B.; Schneeweiss, O.; Šantavá, E.; Jašek, O. Magnetic properties of γ-Fe2O3 nanopowder synthesized by atmospheric microwave torch discharge. Acta Phys. Pol. A 2012, 122, 9–11. [Google Scholar]
- Synek, P.; Jašek, O.; Zajíčková, L.; David, B.; Kudrle, V.; Pizúrová, N. Plasmachemical synthesis of maghemite nanoparticles in atmospheric pressure microwave torch. Mater. Lett. 2011, 65, 982–984. [Google Scholar] [CrossRef]
- Synek, P.; Jašek, O.; Zajíčková, L. Study of Microwave Torch Plasmachemical Synthesis of Iron Oxide Nanoparticles Focused on the Analysis of Phase Composition. Plasma Chem. Plasma Process. 2014, 34, 327–341. [Google Scholar] [CrossRef]
- Son, S.; Taheri, M.; Carpenter, E.; Harris, V.G.; McHenry, M.E. Synthesis of ferrite and nickel ferrite nanoparticles using radio-frequency thermal plasma torch. J. Appl. Phys. 2002, 91, 7589–7591. [Google Scholar] [CrossRef]
- Vissokov, G.P.; Pirgov, P.S. Plasma-chemical synthesis of ultradispersed iron oxides with pigment qualification. J. Mater. Sci. 1996, 31, 4007–4016. [Google Scholar] [CrossRef]
- Banerjee, I.; Khollam, Y.B.; Balasubramanian, C.; Pasricha, R.; Bakare, P.P.; Patil, K.R.; Das, A.K.; Bhoraskar, S.V. Preparation of γ-Fe2O3 nanoparticles using DC thermal arc-plasma route, their characterization and magnetic properties. Scr. Mater. 2006, 54, 1235–1240. [Google Scholar] [CrossRef]
- Lei, P.; Boies, A.; Calder, S.; Girshick, S. Thermal Plasma Synthesis of Superparamagnetic Iron Oxide Nanoparticles. Plasma Chem. Plasma Process. 2012, 32, 519–531. [Google Scholar] [CrossRef]
- Brossmann, U.; Sagmeister, M.; Polt, P.; Kothleitner, G.; Letofsky-Papst, I.; Szabó, D.V.; Würschum, R. Microwave plasma synthesis of nano-crystalline YSZ. Phys. Status Solidi RRL 2007, 1, 107–109. [Google Scholar] [CrossRef]
- Forker, M.; Schmidberger, J.; Szabó, D.V.; Vollath, D. Perturbed-angular-correlation study of phase transformations in nanoscaled Al2O3-coated and noncoated ZrO2 particles synthesized in a microwave plasma. Phys. Rev. B 2000, 61, 1014–1025. [Google Scholar] [CrossRef]
- Schlabach, S.; Szabó, V.; Vollath, D.; Braun, A.; Clasen, R. Structure of alumina and zirconia nanoparticles synthesized by the Karlsruhe Microwave Plasma Process. Solid State Phenom. 2004, 99–100, 191–196. [Google Scholar] [CrossRef]
- Forker, M.; de la Presa, P.; Hoffbauer, W.; Schlabach, S.; Bruns, M.; Szabó, D.V. Structure, phase transformations, and defects of HfO2 and ZrO2 nanoparticles studied by Ta-181 and Cd-111 perturbed angular correlations, H-1 magic-angle spinning NMR, XPS, and X-ray and electron diffraction. Phys. Rev. B 2008, 77, 054108. [Google Scholar] [CrossRef]
- Schlabach, S.; Szabó, D.V.; Vollath, D.; de la Presa, P.; Forker, M. Zirconia and titania nanoparticles studied by electric hyperfine interactions, XRD and TEM. J. Alloy. Compd. 2007, 434, 590–593. [Google Scholar]
- Ye, R.; Li, J.G.; Ishigaki, T. Controlled synthesis of alumina nanoparticles using inductively coupled thermal plasma with enhanced quenching. Thin Solid Films 2007, 515, 4251–4257. [Google Scholar] [CrossRef]
- Szabó, D.V.; Kilibarda, G.; Schlabach, S. Microwave plasma synthesis of nanomaterials for Li-ion battery application. In 13th International Conference on Microwave and High Frequency Heating (AMPERE 2011); Tao, J., Ed.; CEPAD: Toulouse, France, 2011; pp. 425–428. [Google Scholar]
- Im, J.-H.; Lee, J.-H.; Park, D.-W. Synthesis of nano-sized tin oxide powder by argon plasma jet at atmospheric pressure. Surf. Coat. Technol. 2008, 202, 5471–5475. [Google Scholar] [CrossRef]
- Schlabach, S.; Szabó, D.V.; Vollath, D.; de la Presa, P.; Forker, M. Structure and grain growth of TiO2 nanoparticles investigated by electron and X-ray diffractions and Ta-181 perturbed angular correlations. J. Appl. Phys. 2006, 100, 024305. [Google Scholar]
- Hong, Y.C.; Bang, C.U.; Shin, D.H.; Uhm, H.S. Band gap narrowing of TiO2 by nitrogen doping in atmospheric microwave plasma. Chem. Phys. Lett. 2005, 413, 454–457. [Google Scholar]
- Hong, Y.C.; Uhm, H.S. Production of nanocrystalline TiO2 powder by a microwave plasma-torch and its characterization. Jpn. J. Appl. Phys. 2007, 46, 6027–6031. [Google Scholar] [CrossRef]
- Oh, S.-M.; Li, J.-G.; Ishigaki, T. Nanocrystalline TiO2 powders synthesized by in-flight oxidation of TiN in thermal plasma: Mechanisms of phase selection and particle morphology evolution. J. Mater. Res. 2005, 20, 529–537. [Google Scholar] [CrossRef]
- Wangensteen, T.; Dhakal, T.; Merlak, M.; Mukherjee, P.; Phan, M.H.; Chandra, S.; Srikanth, H.; Witanachchi, S. Growth of uniform ZnO nanoparticles by a microwave plasma process. J. Alloy. Compd. 2011, 509, 6859–6863. [Google Scholar] [CrossRef]
- Hong, Y.C.; Kim, J.H.; Cho, S.C.; Uhm, H.S. ZnO nanocrystals synthesized by evaporation of Zn in microwave plasma torch in terms of mixture ratio of N2 to O2. Phys. Plasmas 2006, 13, 063506. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, Y.C.; Uhm, H.S. Synthesis of oxide nanoparticles via microwave plasma decomposition of initial materials. Surf. Coat. Technol. 2007, 201, 5114–5120. [Google Scholar] [CrossRef]
- Sato, T.; Tanigaki, T.; Suzuki, H.; Saito, Y.; Kido, O.; Kimura, Y.; Kaito, C.; Takeda, A.; Kaneko, S. Structure and optical spectrum of ZnO nanoparticles produced in RF plasma. J. Cryst. Growth 2003, 255, 313–316. [Google Scholar] [CrossRef]
- Felbier, P.; Yang, J.; Theis, J.; Liptak, R.W.; Wagner, A.; Lorke, A.; Bacher, G.; Kortshagen, U. Highly Luminescent ZnO Quantum Dots Made in a Nonthermal Plasma. Adv. Funct. Mater. 2014, 24, 1988–1993. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V.; Willis, J.O. Magnetic properties of nanocrystalline Cr2O3 synthesized in a microwave plasma. Mater. Lett. 1996, 29, 271–279. [Google Scholar] [CrossRef]
- Cho, S.C.; Hong, Y.C.; Uhm, H.S. TeO2 nanoparticles synthesized by evaporation of tellurium in atmospheric microwave-plasma torch-flame. Chem. Phys. Lett. 2006, 429, 214–218. [Google Scholar]
- Hong, Y.C.; Uhm, H.S. Synthesis of MgO nanopowder in atmospheric microwave plasma torch. Chem. Phys. Lett. 2006, 422, 174–178. [Google Scholar]
- Shin, D.H.; Bang, C.U.; Hong, Y.C.; Uhm, H.S. Preparation of vanadium pentoxide powders by microwave plasma-torch at atmospheric pressure. Mater. Chem. Phys. 2006, 99, 269–275. [Google Scholar]
- Sagmeister, M.; Postl, M.; Brossmann, U.; List, E.J.E.; Klug, A.; Letofsky-Papst, I.; Szabó, D.V.; Würschum, R. Structure and electrical properties of nanoparticulate tungsten oxide prepared by microwave plasma synthesis. J. Phys. 2011, 23, 334206. [Google Scholar]
- Su, C.-Y.; Lin, C.-K.; Yang, T.-K.; Lin, H.-C.; Pan, C.-T. Oxygen partial pressure effect on the preparation of nanocrystalline tungsten oxide powders by a plasma arc gas condensation technique. Int. J. Refract. Met. Hard Mat. 2008, 26, 423–428. [Google Scholar] [CrossRef]
- Anthony, R.; Thimsen, E.; Johnson, J.; Campbell, S.; Kortshagen, U. A non-thermal plasma reactor for the synthesis of Gallium Nitride nanocrystals. Mat. Res. Soc. Symp. Proc. 2006, 892, 221–224. [Google Scholar]
- Shimada, M.; Azuma, Y.; Okuyama, K.; Hayashi, Y.; Tanabe, E. Plasma synthesis of light emitting gallium nitride nanoparticles using a novel microwave-resonant cavity. Jpn. J. Appl. Phys. 2006, 45, 328–332. [Google Scholar] [CrossRef]
- Shin, D.H.; Hong, Y.C.; Uhm, H.S. Production of Nanocrystalline Titanium Nitride Powder by Atmospheric Microwave Plasma Torch in Hydrogen/Nitrogen Gas. J. Am. Ceram. Soc. 2005, 88, 2736–2739. [Google Scholar] [CrossRef]
- Bang, C.U.; Hong, Y.C.; Uhm, H.S. Synthesis and characterization of nano-sized nitride particles by using an atmospheric microwave plasma technique. Surf. Coat. Technol. 2007, 201, 5007–5011. [Google Scholar] [CrossRef]
- Kumar, S.; Murugan, K.; Chandrasekhar, S.B.; Hebalkar, N.; Krishna, M.; Satyanarayana, B.S.; Madras, G. Synthesis and characterization of nano silicon and titanium nitride powders using atmospheric microwave plasma technique. J. Chem. Sci. 2012, 124, 557–563. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Kao, C.C. Microwave plasma synthesis of TiN and ZrN nanopowders. Mater. Lett. 2007, 61, 1583–1587. [Google Scholar] [CrossRef]
- Yoshida, T.; Kawasaki, A.; Nakagawa, K.; Akashi, K. The synthesis of ultrafine titanium nitride in an r.f. plasma. J. Mater. Sci. 1979, 14, 1624–1630. [Google Scholar] [CrossRef]
- Vollath, D.; Sickafus, K.E. Synthesis of nanosized ceramic nitride powders by microwave supported plasma reactions. Nanostruct. Mater. 1993, 2, 451–456. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Nanoparticles from compounds with layered structures. Acta Mater. 2000, 48, 953–967. [Google Scholar] [CrossRef]
- Hong, Y.C.; Shin, D.H.; Uhm, H.S. Production of vanadium nitride nanopowders from gas-phase VOCl3 by making use of microwave plasma torch. Mater. Chem. Phys. 2007, 101, 35–40. [Google Scholar]
- Szepvolgyi, J.; Mohai-Toth, I.; Bertoti, I.; Gilbart, E.; Riley, F.L. Synthesis of silicon nitride powders in an RF plasma torch, and studies of their sintering behaviour. Key Eng. Mat. 1994, 89–91, 35–40. [Google Scholar]
- Baba, K.; Shohata, N.; Yonezawa, M. Synthesis and properties of ultrafine AlN powder by rf plasma. Appl. Phys. Lett. 1989, 54, 2309–2311. [Google Scholar] [CrossRef]
- Etemadi, K. Formation of aluminum nitrides in thermal plasmas. Plasma Chem. Plasma Process. 1991, 11, 41–56. [Google Scholar] [CrossRef]
- Troitskiy, V.N.; Domashnev, I.A.; Kurkin, E.N.; Grebtsova, O.M.; Berestenko, V.I.; Balikhin, I.L.; Gurov, S.V. Synthesis and Characteristics of Ultra-Fine Superconducting Powders in the Nb–N, Nb–N–C, Nb–Ti–N–C systems. J. Nanopart. Res. 2003, 5, 521–528. [Google Scholar] [CrossRef]
- Lin, H.; Gerbec, J.A.; Sushchikh, M.; McFarland, E.W. Synthesis of amorphous silicon carbide nanoparticles in a low temperature low pressure plasma reactor. Nanotechnology 2008, 19, 325601. [Google Scholar] [CrossRef]
- Viera, G.; Costa, J.; Roura, P.; Bertran, E. High nucleation rate in pure SiC nanometric powder by a combination of room temperature plasmas and post-thermal treatments. Diam. Relat. Mat. 1999, 8, 364–368. [Google Scholar] [CrossRef]
- Andújar, J.L.; Viera, G.; Polo, M.C.; Maniette, Y.; Bertran, E. Synthesis of nanosize Si-C-N powder in low pressure plasmas. Vacuum 1999, 52, 153–156. [Google Scholar] [CrossRef]
- Ko, S.M.; Koo, S.M.; Kim, J.H.; Cho, W.S.; Hwang, K.T. Synthesis of silicon carbide nano-powder from a silicon-organic precursor by RF inductive thermal plasma. J. Korean Ceram. Soc. 2012, 49, 523–527. [Google Scholar] [CrossRef]
- Hollabaugh, C.M.; Hull, D.E.; Newkirk, L.R.; Petrovic, J.J. RF-plasma system for the production of ultrafine, ultrapure silicon carbide powder. J. Mater. Sci. 1983, 18, 3190–3194. [Google Scholar] [CrossRef]
- Kong, P.; Huang, T.T.; Pfender, E. Synthesis of Ultrafine Silicon Carbide Powders in Thermal Arc Plasmas. IEEE Trans. Plasma Sci. 1986, 14, 357–369. [Google Scholar] [CrossRef]
- Kong, P.C.; Pfender, E. Formation of ultrafine β-silicon carbide powders in an argon thermal plasma jet. Langmuir 1987, 3, 259–265. [Google Scholar] [CrossRef]
- Guo, J.Y.; Gitzhofer, F.; Boulos, M.I. Induction plasma synthesis of ultrafine SiC powders from silicon and CH4. J. Mater. Sci. 1995, 30, 5589–5599. [Google Scholar] [CrossRef]
- Allaire, F.; Parent, L.; Dallaire, S. Production of submicron SiC particles by D.C. thermal plasma: A systematic approach based on injection parameters. J. Mater. Sci. 1991, 26, 4160–4165. [Google Scholar] [CrossRef]
- Du, S.W.; Tok, A.I.Y.; Boey, F.Y.C. RF plasma synthesis of boron carbide nanoparticles. Solid State Phenom. 2008, 136, 23–38. [Google Scholar] [CrossRef]
- Kouprine, A.; Gitzhofer, F.; Boulos, M.; Veres, T. Synthesis of ferromagnetic nanopowders from iron pentacarbonyl in capacitively coupled RF plasma. Carbon 2006, 44, 2593–2601. [Google Scholar] [CrossRef]
- Gurentsov, E.; Priemchenko, K.; Grimm, H.; Orthner, H.; Wiggers, H.; Borchers, C.; Jander, H.; Eremin, A.; Schulz, C. Synthesis of Small Carbon Nanoparticles in a Microwave Plasma Flow Reactor. Z. Phys. Chem. 2013, 227, 357–370. [Google Scholar]
- Gries, T.; Vandenbulcke, L.; Rouzaud, J.N.; De Persis, S. Diagnostics in dusty C-H-O plasmas with diamond and graphitic nanoparticle generation. Plasma Sources Sci. Technol. 2010, 19, 025015. [Google Scholar] [CrossRef]
- Kovacevic, E.; Berndt, J.; Strunskus, T.; Boufendi, L. Size dependent characteristics of plasma synthesized carbonaceous nanoparticles. J. Appl. Phys. 2012, 112, 013303. [Google Scholar] [CrossRef]
- Tian, M.; Batty, S.; Shang, C. Synthesis of nanostructured carbons by the microwave plasma cracking of methane. Carbon 2013, 51, 243–248. [Google Scholar] [CrossRef]
- Ting, C.C.; Young, T.F.; Jwo, C.S. Fabrication of diamond nanopowder using microwave plasma torch technique. Int. J. Adv. Manuf. Tech. 2007, 34, 316–322. [Google Scholar] [CrossRef]
- Fu, D.J.; Liu, X.G.; Du, A.B.; Han, P.D.; Jia, H.S.; Xu, B.S. Synthesis of nano-structured onion-like fullerenes by MW plasma. J. Inorg. Mater. 2006, 21, 576–582. [Google Scholar]
- Vollath, D.; Szabo, D.V. Synthesis of nanocrystalline MoS2 and WS2 in a microwave plasma. Mater. Lett. 1998, 35, 236–244. [Google Scholar] [CrossRef]
- Szabó, D.V.; Vollath, D. Morphological characterisation of nanocrystals with layered structures. Nanostruct. Mater. 1999, 12, 597–600. [Google Scholar] [CrossRef]
- Brooks, D.J.; Douthwaite, R.E.; Brydson, R.; Calvert, C.; Measures, M.G.; Watson, A. Synthesis of inorganic fullerene (MS2, M = Zr, Hf and W) phases using H2S and N2/H2 microwave-induced plasmas. Nanotechnology 2006, 17, 1245–1250. [Google Scholar]
- David, B.; Pizúrová, N.; Schneeweiss, O.; Šantavá, E.; Jašek, O.; Kudrle, V. α-Fe nanopowder synthesised in low-pressure microwave plasma and studied by Mössbauer spectroscopy. J. Phys. Conf. Ser. 2011, 303, 012090. [Google Scholar] [CrossRef]
- David, B.; Pizúrová, N.; Schneeweiss, O.; Kudrle, V.; Jašek, O.; Synek, P. Iron-based nanopowders containing α-Fe, Fe3C, and γ-Fe particles synthesised in microwave torch plasma and investigated with Mössbauer spectroscopy. Jpn. J. Appl. Phys. 2011, 50, 08JF11. [Google Scholar]
- Poddar, P.; Wilson, J.L.; Srikanth, H.; Ravi, B.G.; Wachsmuth, J.; Sudarshan, T.S. Grain size influence on soft ferromagnetic properties in Fe–Co nanoparticles. Mater. Sci. Eng. B 2004, 106, 95–100. [Google Scholar] [CrossRef]
- Panchal, V.; Lahoti, G.; Bhandarkar, U.; Neergat, M. The effects of process parameters on yield and properties of iron nanoparticles from ferrocene in a low-pressure plasma. J. Phys. D 2011, 44, 345205. [Google Scholar] [CrossRef]
- Weigle, J.C.; Luhrs, C.C.; Chen, C.K.; Perry, W.L.; Mang, J.T.; Nemer, M.B.; Lopez, G.P.; Phillips, J. Generation of Aluminum Nanoparticles Using an Atmospheric Pressure Plasma Torch. J. Phys. Chem. B 2004, 108, 18601–18607. [Google Scholar]
- Bertran, E.; Costa, J.; Viera, G.; Zhang, R.Q. Production of nanometric particles in radio frequency glow discharges in mixtures of silane and methane. J. Vac. Sci. Technol. A 1996, 14, 567–571. [Google Scholar] [CrossRef]
- Bapat, A.; Perrey, C.R.; Campbell, S.A.; Carter, C.B.; Kortshagen, U. Synthesis of highly oriented, single-crystal silicon nanoparticles in a low-pressure, inductively coupled plasma. J. Appl. Phys. 2003, 94, 1969–1974. [Google Scholar] [CrossRef]
- Mangolini, L.; Thimsen, E.; Kortshagen, U. High-Yield Plasma Synthesis of Luminescent Silicon Nanocrystals. Nano Lett. 2005, 5, 655–659. [Google Scholar] [CrossRef]
- Bapat, A.; Gatti, M.; Ding, Y.-P.; Campbell, S.A.; Kortshagen, U. A plasma process for the synthesis of cubic-shaped silicon nanocrystals for nanoelectronic devices. J. Phys. D 2007, 40, 2247–2257. [Google Scholar] [CrossRef]
- Kendrick, C.; Klafehn, G.; Guan, T.; Anderson, I.; Shen, H.; Redwing, J.; Collins, R. Controlled growth of SiNPs by plasma synthesis. Sol. Energy Mater. Sol. Cells 2014, 124, 1–9. [Google Scholar] [CrossRef]
- Cernetti, P.; Gresback, R.; Campbell, S.A.; Kortshagen, U. Nonthermal Plasma Synthesis of Faceted Germanium Nanocrystals. Chem. Vap. Depos. 2007, 13, 345–350. [Google Scholar] [CrossRef]
- Gresback, R.; Holman, Z.; Kortshagen, U. Nonthermal plasma synthesis of size-controlled, monodisperse, freestanding germanium nanocrystals. Appl. Phys. Lett. 2007, 91, 093119. [Google Scholar] [CrossRef]
- Pi, X.D.; Kortshagen, U. Nonthermal plasma synthesized freestanding silicon-germanium alloy nanocrystals. Nanotechnology 2009, 20, 295602. [Google Scholar] [CrossRef]
- Hitzbleck, K.; Wiggers, H.; Roth, P. Controlled formation and size-selected deposition of indium nanoparticles from a microwave flow reactor on semiconductor surfaces. Appl. Phys. Lett. 2005, 87, 093105. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Yang, C.-C.; Shih, H.-H. Microwave Plasma Production of Metal Nanopowders. Inorganics 2014, 2, 278–290. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Hsu, M.-K.; Hsieh, C.-C.; Kao, C.-C. Microwave plasma synthesis of silver nanopowders. Mater. Lett. 2005, 59, 905–908. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, S.-M.; Park, D.-W. Preparation of silver nanopowder by thermal plasma. Mater. Sci. Eng. C 2007, 27, 1286–1290. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Hsu, M.-K.; Kao, C.-C. Microwave plasma synthesis of Co and SiC-coated Co nanopowders. Mater. Lett. 2006, 60, 947–951. [Google Scholar] [CrossRef]
- Chau, J.L.H. Synthesis of Ni and bimetallic FeNi nanopowders by microwave plasma method. Mater. Lett. 2007, 61, 2753–2756. [Google Scholar] [CrossRef]
- Yasar-Inceoglu, O.; Mangolini, L. Characterization of Si-Ge alloy nanocrystals produced in a non-thermal plasma reactor. Mater. Lett. 2013, 101, 76–79. [Google Scholar] [CrossRef]
- Scott, J.H.J.; Turgut, Z.; Chowdary, K.; McHenry, M.E.; Majetich, S.A. Thermal plasma synthesis of Fe-Co alloy nanoparticles. Mat. Res. Soc. Symp. Proc. 1998, 501, 121–126. [Google Scholar]
- Vollath, D.; Szabó, D.V.; Fuchs, J. Polymer coated nanoparticulate ferrite powders: Properties and application. Mat. Res. Soc. Symp. Proc. 1999, 557, 443–448. [Google Scholar] [CrossRef]
- Szabó, D.V.; Lamparth, I.; Vollath, D. Complex high frequency properties of ceramic-polymer nanocomposites: Comparison of fluoro-polymers and acrylic-based compounds. Macromol. Symp. 2002, 181, 393–398. [Google Scholar] [CrossRef]
- Lamparth, I.; Szabó, D.V.; Vollath, D. Ceramic nanoparticles coated with polymers based on acrylic derivatives. Macromol. Symp. 2002, 181, 107–112. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V.; Schlabach, S. Oxide/polymer nanocomposites as new luminescent materials. J. Nanopart. Res. 2004, 6, 181–191. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Synthesis and properties of nanocomposites. Adv. Eng. Mater. 2004, 6, 117–127. [Google Scholar] [CrossRef]
- Szabó, D.V.; Reuter, H.; Schlabach, S.; Lellig, C.; Vollath, D. Influence of halides on the luminescence of oxide/anthracene/polymer nanocomposites. Mat. Res. Soc. Symp. Proc. 2005, 846, 77–182. [Google Scholar]
- Fuchs, M.; Breitenstein, D.; Fartmann, M.; Grehl, T.; Kayser, S.; Koester, R.; Ochs, R.; Schlabach, S.; Szabó, D.V.; Bruns, M. Characterization of core/shell nanoparticle thin films for gas analytical applications. Surf. Interface Anal. 2010, 42, 1131–1134. [Google Scholar] [CrossRef]
- Sagmeister, M.; Brossmann, U.; List, E.; Ochs, R.; Szabó, D.V.; Saf, R.; Grogger, W.; Tchernychova, E.; Würschum, R. Synthesis and optical properties of organic semiconductor/zirconia nanocomposites. J. Nanopart. Res. 2010, 12, 2541–2551. [Google Scholar] [CrossRef]
- Vollath, D. Bifunctional nanocomposites with magnetic and luminescence properties. Adv. Mater. 2010, 22, 4410–4415. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Synthesis of coated nanoparticulate ceramic powders. Mat. Res. Soc. Symp. Proc. 1998, 520, 43–148. [Google Scholar]
- Chau, J.L.H.; Yang, C.C. Surface modification of silica nanopowders in microwave plasma. J. Exp. Nanosci. 2014, 9, 357–361. [Google Scholar] [CrossRef]
- Panchal, V.; Neergat, M.; Bhandarkar, U. Synthesis and characterization of carbon coated nanoparticles produced by a continuous low-pressure plasma process. J. Nanopart. Res. 2011, 13, 3825–3833. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, Y.C.; Uhm, H.S. Binary oxide material made from a mixture of Zn and Cd in a microwave plasma. Chem. Phys. Lett. 2007, 443, 122–126. [Google Scholar]
- Gupta, A.; Schulz, C.; Wiggers, H. Influence of etching and surface functionalization on the optical property of luminescing phosphorus doped silicon nanoparticles. J. Optoelectron. Adv. Mater. 2010, 12, 518–522. [Google Scholar]
- Cho, S.C.; Uhm, H.S.; Bang, C.U.; Lee, D.K.; Han, C.S. Production of nanocrystalline Y2O3:Eu powder by microwave plasma-torch and its characterization. Thin Solid Films 2009, 517, 4052–4055. [Google Scholar]
- Sato, T.; Suzuki, H.; Kido, O.; Kurumada, M.; Kamitsuji, K.; Kimura, Y.; Kawasaki, H.; Kaneko, S.; Saito, Y.; Kaito, C. Production of transition metal-doped ZnO nanoparticles by using RF plasma field. J. Cryst. Growth 2005, 275, e983–e987. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, J.G.; Kamiyama, H.; Katada, M.; Ohashi, N.; Moriyoshi, Y.; Ishigaki, T. Pyrogenic Iron(III)-Doped TiO2 Nanopowders Synthesized in RF Thermal Plasma: Phase Formation, Defect Structure, Band Gap, and Magnetic Properties. J. Am. Chem. Soc. 2005, 127, 10982–10990. [Google Scholar]
- Li, J.-G.; Wang, X.-H.; Kamiyama, H.; Ishigaki, T.; Sekiguchi, T. RF plasma processing of Er-doped TiO2 luminescent nanoparticles. Thin Solid Films 2006, 506–507, 292–296. [Google Scholar] [CrossRef]
- Li, J.-G.; Büchel, R.; Isobe, M.; Mori, T.; Ishigaki, T. Cobalt-Doped TiO2 Nanocrystallites: Radio-Frequency Thermal Plasma Processing, Phase Structure, and Magnetic Properties. J. Phys. Chem. C 2009, 113, 8009–8015. [Google Scholar]
- Jung, D.-W.; Park, D.-W. Synthesis of nano-sized antimony-doped tin oxide (ATO) particles using a DC arc plasma jet. Appl. Surf. Sci. 2009, 255, 5409–5413. [Google Scholar]
- Kilibarda, G.; Schlabach, S.; Winkler, V.; Bruns, M.; Hanemann, T.; Szabó, D.V. Electrochemical performance of Tin-based nano-composite electrodes using a vinylene carbonate-containing electrolyte for Li-ion cells. J. Power Sources 2014, 263, 145–153. [Google Scholar] [CrossRef]
- Luhrs, C.; Phillips, J.; Fanson, P.T. Production of complex cerium-Aluminum oxides using an atmospheric pressure plasma torch. Langmuir 2007, 23, 7055–7064. [Google Scholar] [CrossRef]
- Choi, S.; Lee, M.-S.; Park, D.-W. Photocatalytic performance of TiO2/V2O5 nanocomposite powder prepared by DC arc plasma. Curr. Appl. Phys. 2014, 14, 433–438. [Google Scholar] [CrossRef]
- Néel, L. Some theoretical aspects on rock-magnetism. Adv. Phys. 1955, 4, 191–243. [Google Scholar] [CrossRef]
- Néel, L. Influence des fluctuations thermiques sur l'aimantation de grains ferromagnétiques très fins. C. R. Hébdo. Acad. Sci. 1949, 228, 664–666. (In French) [Google Scholar]
- Tang, Z.X.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Size-Dependent Magnetic-Properties of Manganese Ferrite Fine Particles. J. Appl. Phys. 1991, 69, 5279–5281. [Google Scholar]
- Han, D.H.; Wang, J.P.; Luo, H.L. Crystallite Size Effect on Saturation Magnetization of Fine Ferrimagnetic Particles. J. Magn. Magn. Mat. 1994, 136, 176–182. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.J. Size-dependent superparamagnetic properties of MgFe2O4 spinel ferrite nanocrystallites. Appl. Phys. Lett. 1998, 73, 3156–3158. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Serna, C.J. Magnetic properties of uniform γ-Fe2O3 nanoparticles smaller than 5 nm prepared by laser pyrolysis. J. Mater. Res. 1999, 14, 3066–3072. [Google Scholar] [CrossRef]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: magnetic and transport properties. J. Phys. D 2002, 35, R15–R42. [Google Scholar] [CrossRef]
- Goya, G.F.; Leite, E.R. Ferrimagnetism and spin canting of Zn57Fe2O4 nanoparticles embedded in ZnO matrix. J. Phys. 2003, 15, 641–651. [Google Scholar] [CrossRef]
- Witvrouwen, T.; Paulussen, S.; Sels, B. The Use of Non-Equilibrium Plasmas for the Synthesis of Heterogeneous Catalysts. Plasma Process. Polym. 2012, 9, 750–760. [Google Scholar] [CrossRef]
- Sun, S.-N.; Wei, C.; Zhu, Z.-Z.; Hou, Y.-L.; Subbu, S.V.; Xu, Z.-C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- de Montferrand, C.; Lalatonne, Y.; Bonnin, D.; Lièvre, N.; Lecouvey, M.; Monod, P.; Russier, V.; Motte, L. Size-Dependent Nonlinear Weak-Field Magnetic Behavior of Maghemite Nanoparticles. Small 2012, 8, 1945–1956. [Google Scholar] [CrossRef]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic Press/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Millers, D.; Grigorjeva, L.; Lojkowski, W.; Opalinska, A. Luminescence of ZrO2 Nanocrystals. Solid State Phenom. 2005, 106, 103–108. [Google Scholar] [CrossRef]
- Chen, H.; Wu, X.; Xiong, S.; Zhang, W.; Zhu, J. Red photoluminescence mechanism in SnO2 nanostructures. Appl. Phys. A 2009, 97, 365–368. [Google Scholar] [CrossRef]
- Yang, Y.; Leppert, V.J.; Risbud, S.H.; Twamley, B.; Power, P.P.; Lee, H.W.H. Blue luminescence from amorphous GaN nanoparticles synthesized in situ in a polymer. Appl. Phys. Lett. 1999, 74, 2262–2264. [Google Scholar]
- Nienhaus, H.; Kravets, V.; Koutouzov, S.; Meier, C.; Lorke, A.; Wiggers, H.; Kennedy, M.K.; Kruis, F.E. Quantum size effect of valence band plasmon energies in Si and SnOx nanoparticles. J. Vac. Sci. Technol. B 2006, 24, 1156–1161. [Google Scholar]
- Zhou, J.; Wang, Y.; Zhao, F.; Wang, Y.; Zhang, Y.; Yang, L. Photoluminescence of ZnO nanoparticles prepared by a novel gel-template combustion process. J. Lumines. 2006, 119–120, 248–252. [Google Scholar] [CrossRef]
- Corr, S.A.; O’Byrne, A.; Gun’ko, Y.K.; Ghosh, S.; Brougham, D.F.; Mitchell, S.; Volkov, Y.; Prina-Mello, A. Magnetic-fluorescent nanocomposites for biomedical multitasking. Chem. Commun. 2006, 4474–4476. [Google Scholar]
- Chen, D.; Jiang, M.; Li, N.; Gu, H.; Xu, Q.; Ge, J.; Xia, X.; Lu, J. Modification of magnetic silica/iron oxide nanocomposites with fluorescent polymethacrylic acid for cancer targeting and drug delivery. J. Mater. Chem. 2010, 20, 6422–6429. [Google Scholar]
- Gu, Z.; Chen, X.-Y.; Shen, Q.-D.; Ge, H.-X.; Xu, H.-H. Hybrid nanocomposites of semiconductor nanoparticles and conjugated polyelectrolytes and their application as fluorescence biosensors. Polymer 2010, 51, 902–907. [Google Scholar] [CrossRef]
- Cho, H.-S.; Dong, Z.; Pauletti, G.M.; Zhang, J.; Xu, H.; Gu, H.; Wang, L.; Ewing, R.C.; Huth, C.; Wang, F.; et al. Fluorescent, Superparamagnetic Nanospheres for Drug Storage, Targeting, and Imaging: A Multifunctional Nanocarrier System for Cancer Diagnosis and Treatment. ACS Nano 2010, 4, 5398–5404. [Google Scholar] [CrossRef]
- Di Corato, R.; Bigall, N.C.; Ragusa, A.; Dorfs, D.; Genovese, A.; Marotta, R.; Manna, L.; Pellegrino, T. Multifunctional Nanobeads Based on Quantum Dots and Magnetic Nanoparticles: Synthesis and Cancer Cell Targeting and Sorting. ACS Nano 2011, 5, 1109–1121. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Kilibarda, G.; Schlabach, S.; Hanemann, T.; Szabó, D.V. Influence of Environmental Temperature on the Electrochemical Performance of a Tin-Based Nano-Electrode in Lithium Ion Cells. Int. J. Electrochem. Sci. 2013, 8, 6212–6219. [Google Scholar]
- Kilibarda, G.; Szabó, D.V.; Schlabach, S.; Winkler, V.; Bruns, M.; Hanemann, T. Investigation of the degradation of SnO2 electrodes for use in Li-ion cells. J. Power Sources 2013, 233, 139–147. [Google Scholar] [CrossRef]
- Kilibarda, G. Nanopartikel als Anodenmaterialien: Entwicklung von nanoskaligen Schichtstrukturen auf der Basis von Zinn und Kohlenwasserstoffen für die Anwendung als Anodenmaterial in Li-Ionen-Zellen. PhD Thesis, University Freiburg, Germany, June 2014. [Google Scholar]
- Sato, K.; Noguchi, M.; Demachi, A.; Oki, N.; Endo, M. A Mechanism of Lithium Storage in Disordered Carbons. Science 1994, 264, 556–558. [Google Scholar]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for Lithium Insertion in Carbonaceous Materials. Science 1995, 270, 590–593. [Google Scholar]
- Zheng, T.; Liu, Y.; Fuller, E.W.; Tseng, S.; von Sacken, U.; Dahn, J.R. Lithium Insertion in High Capacity Carbonaceous Materials. J. Electrochem. Soc. 1995, 142, 2581–2590. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, J.S.; Zheng, T.; Dahn, J.R. Mechanism of lithium insertion in hard carbons prepared by pyrolysis of epoxy resins. Carbon 1996, 34, 193–200. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Szabó, D.V.; Schlabach, S. Microwave Plasma Synthesis of Materials—From Physics and Chemistry to Nanoparticles: A Materials Scientist’s Viewpoint. Inorganics 2014, 2, 468-507. https://doi.org/10.3390/inorganics2030468
Szabó DV, Schlabach S. Microwave Plasma Synthesis of Materials—From Physics and Chemistry to Nanoparticles: A Materials Scientist’s Viewpoint. Inorganics. 2014; 2(3):468-507. https://doi.org/10.3390/inorganics2030468
Chicago/Turabian StyleSzabó, Dorothée Vinga, and Sabine Schlabach. 2014. "Microwave Plasma Synthesis of Materials—From Physics and Chemistry to Nanoparticles: A Materials Scientist’s Viewpoint" Inorganics 2, no. 3: 468-507. https://doi.org/10.3390/inorganics2030468
APA StyleSzabó, D. V., & Schlabach, S. (2014). Microwave Plasma Synthesis of Materials—From Physics and Chemistry to Nanoparticles: A Materials Scientist’s Viewpoint. Inorganics, 2(3), 468-507. https://doi.org/10.3390/inorganics2030468




