Mixed-Valence Pentadecavanadate with Ca2+-ATPase Inhibition Potential and Anti-Breast Cancer Activity
Abstract
1. Introduction
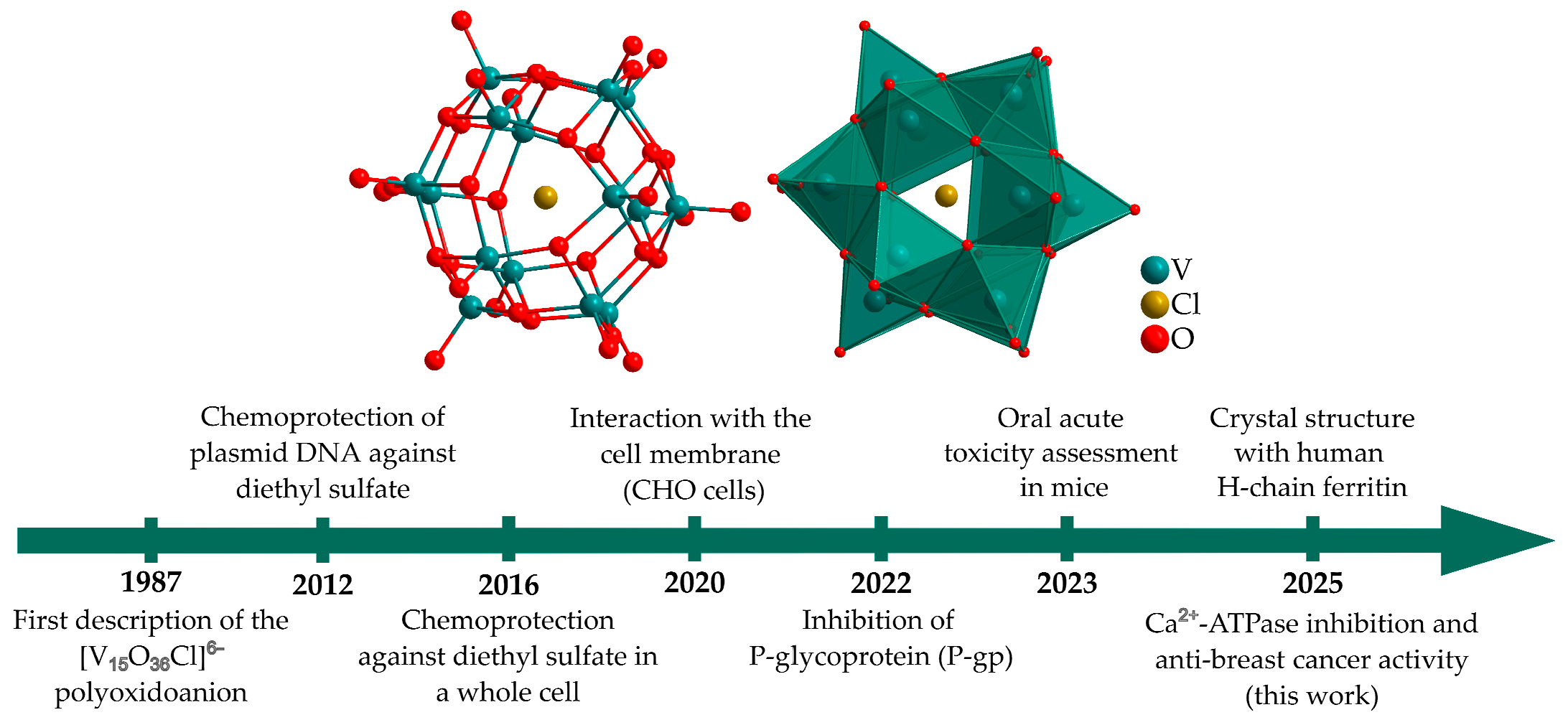
2. Results and Discussion
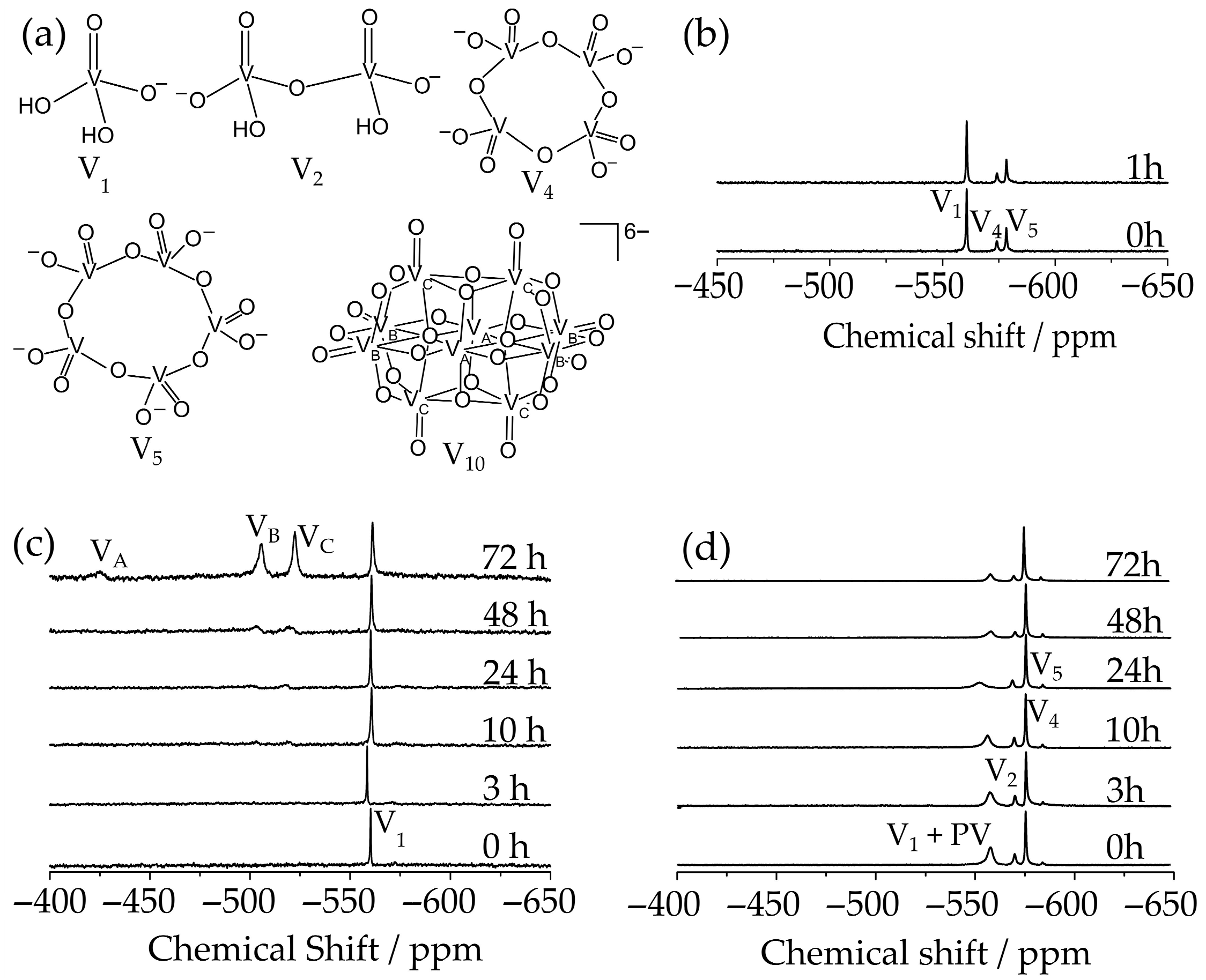
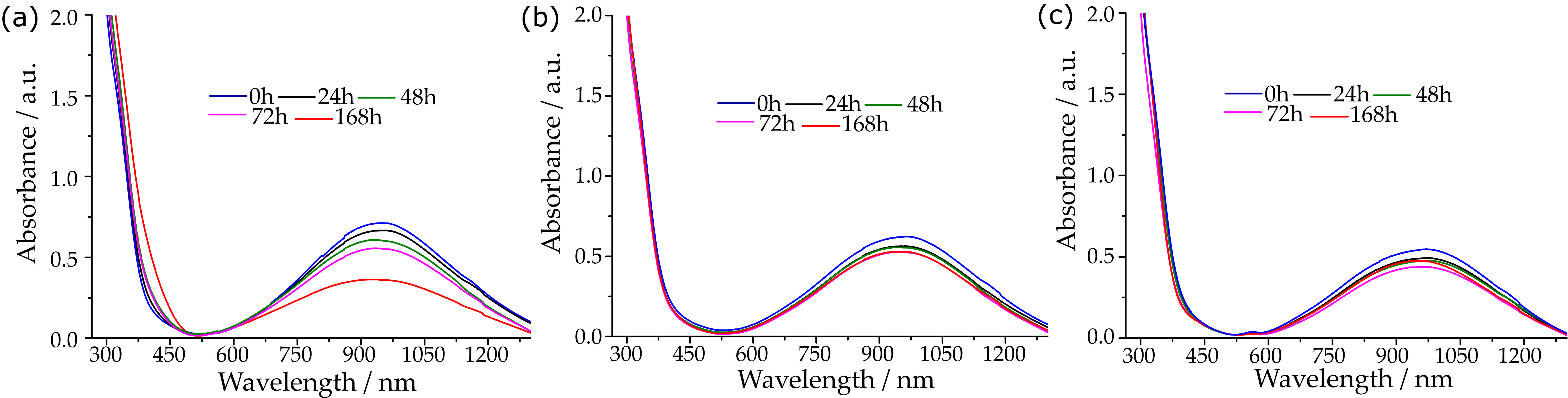
2.1. Stability Studies
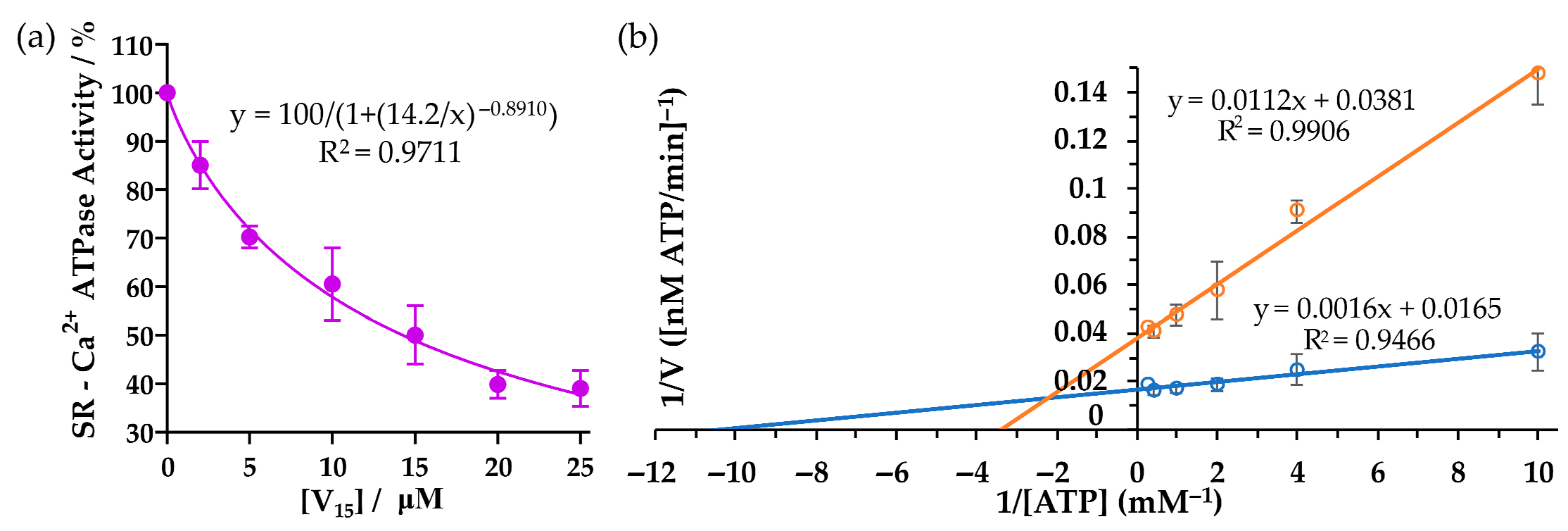
2.2. Inhibition of Ca2+-ATPase
Electrostatic Potential (ESP) Map
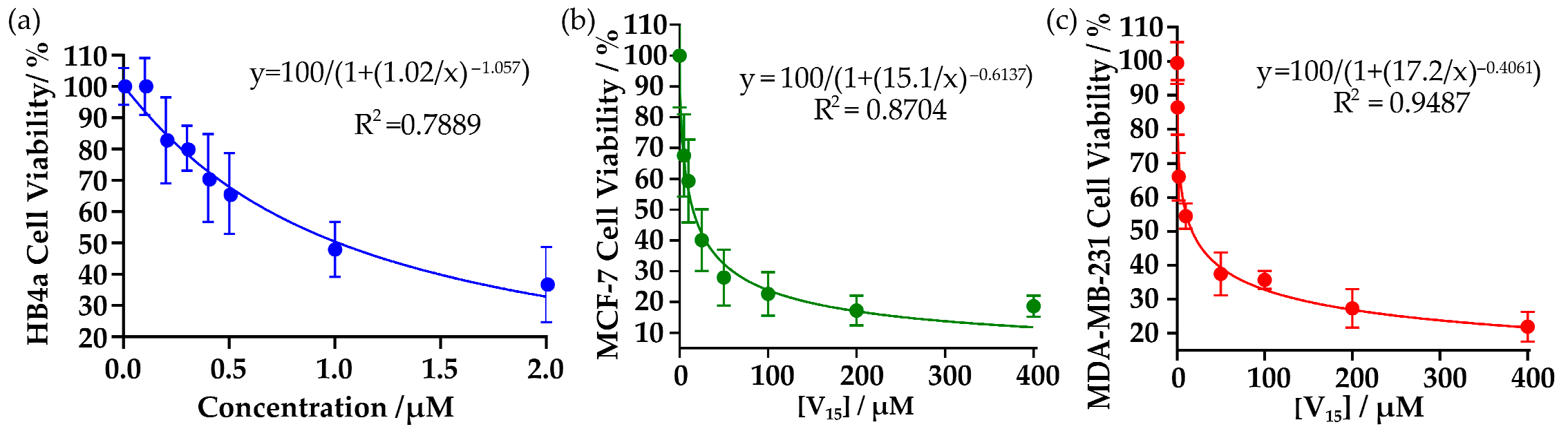
2.3. Inhibition of Cell Viability in Breast Cancer Cells
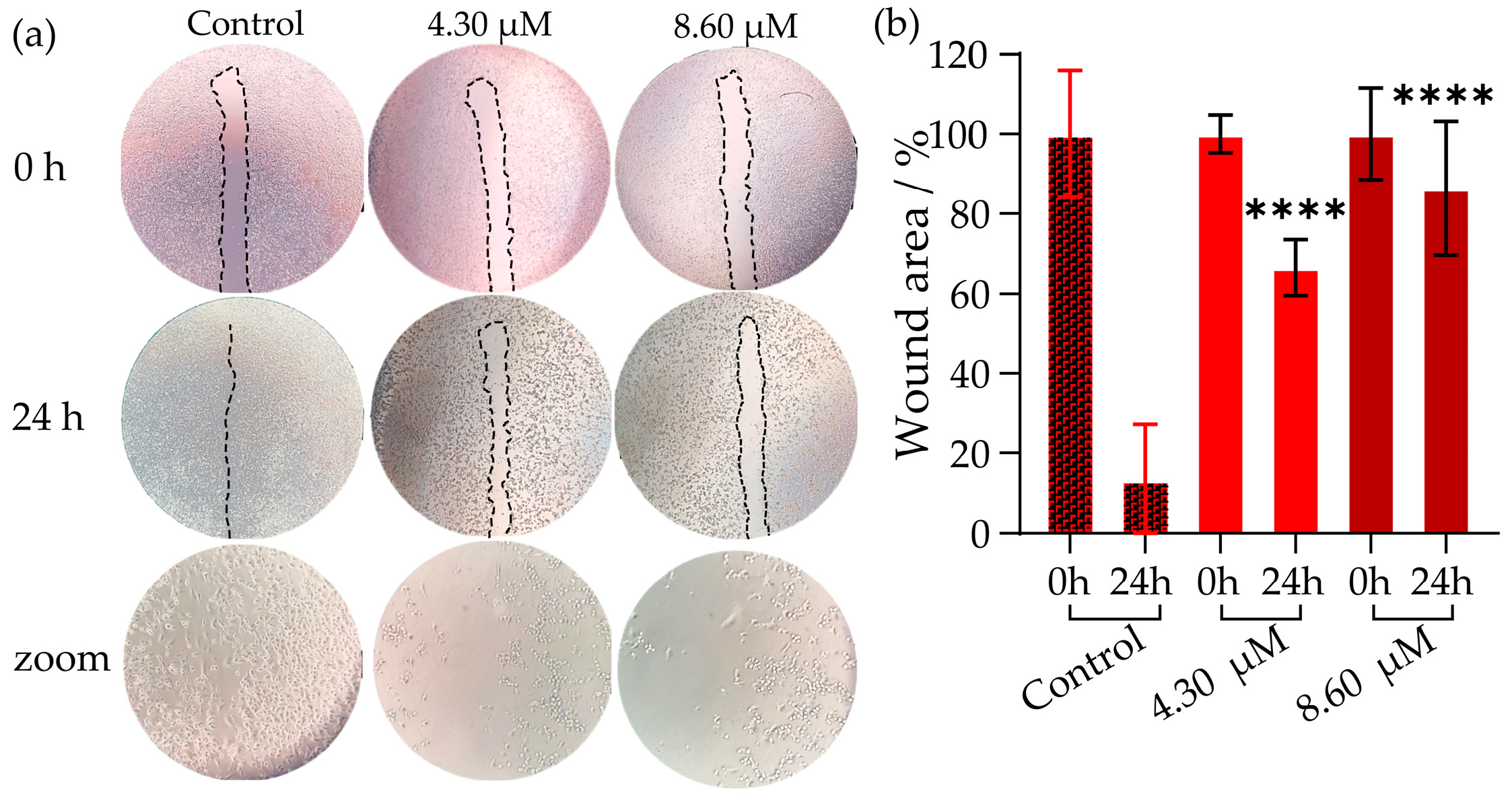
2.4. Inhibition of Cell Migration Using the Wound Healing Assay in MDA-MB-231 Cell Line
2.5. Scanning Electron Microscopy (SEM Images) in MDA-MB-231 Cell Line
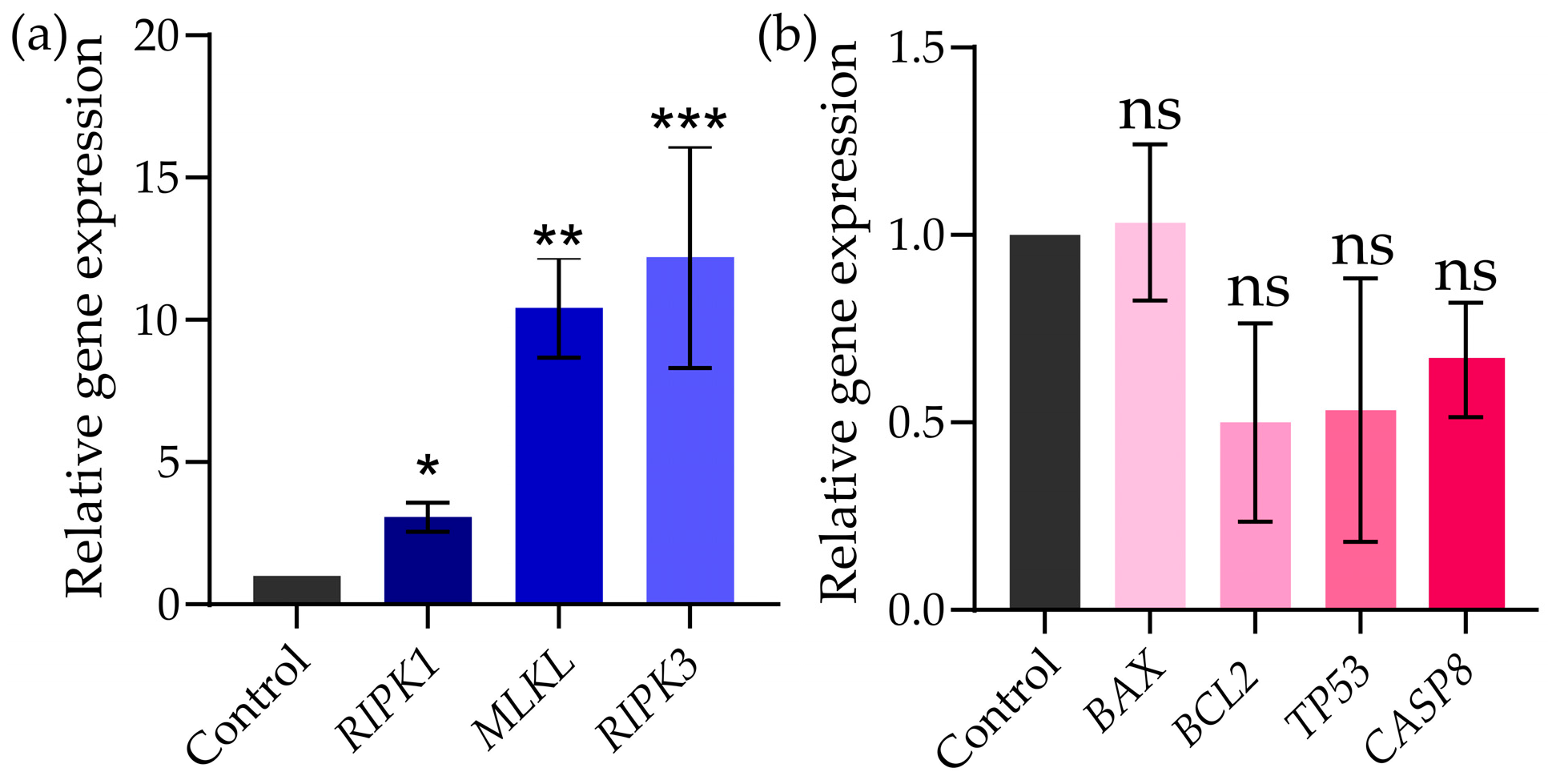
2.6. Gene Expression in MDA-MB-231 Cell Line
3. Materials and Methods
3.1. Generalities
Synthesis and Stock Solution of V15
3.2. Spectroscopic Studies in Solution
3.3. Computational Methods
3.4. Inhibition of Ca2+-ATPase Activity by V15
3.5. Cell Culture
3.6. MTT Assay
3.7. Cell Migration by Wound Healing Assay
3.8. Scanning Electron Microscopy (SEM) Images
3.9. Gene Expression Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCF-7 | Michigan Cancer Foundantion-7 |
| MDA-MB-231 | Monroe Dunaway Anderson Cancer Center-Mammary/Breast-231 |
| FBS | Fetal Bovine Serum |
| RPMI | Roswell Park Memorial Institute |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| HEPES | 4-(2-hidroxyethyl)1-piperazine ethane sulfonic acid |
| PBS | Phosphate-Buffered Saline |
| PIPE | Piperazine-n,n’-Bis(2-ethanesulphonicAcid) |
| MTT | 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| POMs | Polyoxometalates |
| POVs | Polyoxovanadates |
| MV-POVs | Mixed-Valence Polyoxovanadates |
| acac | Acetylacetonate |
| 5-Fu | 5-Fluorouracyl |
| ROS | Reactive Oxygen Species |
| 51V-NMR | Nuclear Magnetic Resonance of Vanadium 51 |
| UV-Vis/NIR | Ultraviolet-Visible/Near-Infrared spectroscopy |
| SEM | Scanning Electron Microscopy |
| ESP | Electrostatic Surface Potential |
| LHR | Luteinizing Hormone Receptor |
| ATP | Adenosine Triphosphate |
| ABC | ATP-Binding Cassette |
| CHO | Chinese Hamster Ovary |
| P-gp | P-glycoprotein |
| EMT | Epithelial–Mesenchymal Transition |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| RIPK1 | Receptor-Interacting Serine/Threonine-Protein Kinase 1 |
| RIPK2 | Receptor-Interacting Serine/Threonine-Protein Kinase 2 |
| RIPK3 | Receptor-Interacting Serine/Threonine-Protein Kinase 3 |
| BAX | Bcl-2-Associated X Protein; |
| BCL2 | B-Cell Lymphoma 2 |
| TP53 | Tumor Protein p53 |
| CASP8 | Caspase-8 |
| MLKL | Mixed Lineage Kinase Domain-Like Protein |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| SR | Sarcoplasmic Reticulum |
| SERCA | Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase |
| IC50 | Half Maximal Inhibitory Concentration |
| LB | Luria–Bertani |
| TNG | Triple-Negative |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
References
- Aureliano, M. Decavanadate: A Journey in a Search of a Role. Dalton Trans. 2009, 42, 9093–9100. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrão, G.; Gonçalves, J.; Sánchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and Metformin-Decavanadate Effects in Human Melanoma Cells. J. Inorg. Biochem. 2022, 235, 111915. [Google Scholar] [CrossRef]
- Soman, S.K.; Woodruff, M.R.J.; Dagda, R.K. The Mitochondrial Foundations of Parkinson’s Disease: Therapeutic Implications. Aging Dis. 2025, 16, 2695–2720. [Google Scholar] [CrossRef]
- Rathi, K.; Bhole, R.; Bansode, P.; Motghare, N. Navigating the Landscape of Alzheimer’s Disease: From Epidemiology to Drug Re-Purposing. Adv. Pharmacol. Pharm. 2024, 12, 135–148. [Google Scholar] [CrossRef]
- Hu, H.; Liang, W.; Ding, G. Ion Homeostasis in Diabetic Kidney Disease. Trends Endocrinol. Metab. 2024, 35, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, S.V.; McGrath, K.M.; Caraveo, G. The Role of Ca2+ Signaling in Parkinson’s Disease. Dis. Model Mech. 2017, 10, 519–535. [Google Scholar] [CrossRef]
- Dowling, P.; Swandulla, D.; Ohlendieck, K. Biochemical and Proteomic Insights into Sarcoplasmic Reticulum Ca2+-ATPase Complexes in Skeletal Muscles. Expert Rev. Proteom. 2023, 20, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Fraqueza, G.; Ohlin, C.A. Ion Pumps as Biological Targets for Decavanadate. Dalton Trans. 2013, 42, 11770–11777. [Google Scholar] [CrossRef]
- Sukumaran, P.; Nascimento Da Conceicao, V.; Sun, Y.; Ahamad, N.; Saraiva, L.R.; Selvaraj, S.; Singh, B.B. Calcium Signaling Regulates Autophagy and Apoptosis. Cells 2021, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef]
- Kumari, N.; Pullaguri, N.; Rath, S.N.; Bajaj, A.; Sahu, V.; Ealla, K.K.R. Dysregulation of Calcium Homeostasis in Cancer and Its Role in Chemoresistance. Cancer Drug Resist. 2024, 7, 11. [Google Scholar] [CrossRef]
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+-and Ca2+-ATPase Activities by Phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700. [Google Scholar] [CrossRef]
- Poejo, J.; Gumerova, N.I.; Rompel, A.; Mata, A.M.; Aureliano, M.; Gutierrez-Merino, C. Unveiling the Agonistic Properties of Preyssler-Type Polyoxotungstates on Purinergic P2 Receptors. J. Inorg. Biochem. 2024, 259, 112640. [Google Scholar] [CrossRef]
- Ferraro, G.; Garribba, E.; Merlino, A. Exploring Polyoxidovanadate–Protein Interaction. Trends Chem. 2025, 7, 3–6. [Google Scholar] [CrossRef]
- Aronica, C.; Chastanet, G.; Zueva, E.; Borshch, S.A.; Clemente-Juan, J.M.; Luneau, D. A Mixed-Valence Polyoxovanadate(III, IV) Cluster with a Calixarene Cap Exhibiting Ferromagnetic V(III)−V(IV) Interactions. J. Am. Chem. Soc. 2008, 130, 2365–2371. [Google Scholar] [CrossRef]
- Solé-Daura, A.; Notario-Estévez, A.; Carbó, J.J.; Poblet, J.M.; de Graaf, C.; Monakhov, K.Y.; López, X. How Does the Redox State of Polyoxovanadates Influence the Collective Behavior in Solution? A Case Study with [I@V 18 O 42] q−(q = 3, 5, 7, 11, and 13). Inorg. Chem. 2019, 58, 3881–3894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, W.; Wang, Y.; Wang, J.; Liu, C.; Ye, F.; Liu, B. An Ecofriendly and Efficient Wood-Based Polyoxovanadate Solar Evaporation Generator. Sci. China Mater. 2023, 66, 3292–3299. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with Emerging Biomedical Activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; Penk, M.; Walberg, H.; Bögge, H. Spherical Mixed-Valence [V15O36]5–, an Example from an Unusual Cluster Family. Angew. Chem. Int. Ed. 1987, 26, 1045–1046. [Google Scholar] [CrossRef]
- Postal, K.; Maluf, D.F.; Valdameri, G.; Rüdiger, A.L.; Hughes, D.L.; de Sá, E.L.; Ribeiro, R.R.; de Souza, E.M.; Soares, J.F.; Nunes, G.G. Chemoprotective Activity of Mixed Valence Polyoxovanadates against Diethylsulphate in E. Coli Cultures: Insights from Solution Speciation Studies. RSC Adv. 2016, 6, 114955–114968. [Google Scholar] [CrossRef]
- Kita, D.H.; Andrade, G.A.; Missina, J.M.; Postal, K.; Boell, V.K.; Santana, F.S.; Zattoni, I.F.; Zanzarini, I.D.S.; Moure, V.R.; Rego, F.G.D.M.; et al. Polyoxovanadates as New P-glycoprotein Inhibitors: Insights into the Mechanism of Inhibition. FEBS Lett. 2022, 596, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Althumairy, D.; Postal, K.; Barisas, B.G.; Nunes, G.G.; Roess, D.A.; Crans, D.C. Polyoxometalates Function as Indirect Activators of a G Protein-Coupled Receptor. Metallomics 2020, 12, 1044–1061. [Google Scholar] [CrossRef]
- Tito, G.; Ferraro, G.; Garribba, E.; Merlino, A. Formation of Mixed-Valence Cage-Like Polyoxidovanadates at 37 °C Upon Reaction of VIVO(Acetylacetonato)2 with Lysozyme. Chem. A Eur. J. 2025, 31, e202500488. [Google Scholar] [CrossRef]
- Lucignano, R.; Tito, G.; Ferraro, G.; Picone, D.; Pisanu, F.; Garribba, E.; Merlino, A. Spherical Mixed-Valence Pentadecavanadate Binding to Human H-Chain Ferritin. Inorg. Chem. Front. 2025. [Google Scholar] [CrossRef]
- Essid, A.; Elbini, I.; Ksiksi, R.; Harrab, N.; Moslah, W.; Jendoubi, I.; Doghri, R.; Zid, M.-F.; Luis, J.; Srairi-Abid, N. Decavanadate Compound Displays In Vitro and In Vivo Antitumor Effect on Melanoma Models. Bioinorg. Chem. Appl. 2025, 2025, 6680022. [Google Scholar] [CrossRef]
- Yao, C.; Zhao, Z.; Tan, T.; Yan, J.; Chen, Z.; Xiong, J.; Li, H.; Wei, Y.; Hu, K. Lindqvist-Type Polyoxometalates Act as Anti-Breast Cancer Drugs via Mitophagy-Induced Apoptosis. Curr. Med. Sci. 2024, 44, 809–819. [Google Scholar] [CrossRef]
- Soares, S.S.; Martins, H.; Duarte, R.O.; Moura, J.J.G.; Coucelo, J.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium Distribution, Lipid Peroxidation and Oxidative Stress Markers upon Decavanadate in Vivo Administration. J. Inorg. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef]
- Barbosa, M.D.M.; de Lima, L.M.A.; Alves, W.A.D.S.; de Lima, E.K.B.; da Silva, L.A.; da Silva, T.D.; Postal, K.; Ramadan, M.; Kostenkova, K.; Gomes, D.A.; et al. In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster. Pharmaceuticals 2023, 16, 1232. [Google Scholar] [CrossRef]
- Nunes, G.G.; Bonatto, A.C.; de Albuquerque, C.G.; Barison, A.; Ribeiro, R.R.; Back, D.F.; Andrade, A.V.C.; de Sá, E.L.; Pedrosa, F.D.O.; Soares, J.F.; et al. Synthesis, Characterization and Chemoprotective Activity of Polyoxovanadates against DNA Alkylation. J. Inorg. Biochem. 2012, 108, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Exon Publications: Brisbane, Australia, 2022; pp. 31–42. [Google Scholar]
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of Outcome in Metastatic Breast Cancer: Insights From a Real-World Scenario. Oncologist 2014, 19, 608–615. [Google Scholar] [CrossRef]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2018, 54, 407–419. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.-G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer Targets Ther. 2023, 15, 721–730. [Google Scholar] [CrossRef]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers 2020, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Liu, Z.; Wang, M.; Zhao, Y.; Liu, X.; Hou, Y.; Ma, W.; Han, J.; Zhou, X.; Ren, D.; et al. Neoadjuvant Apatinib Addition to Sintilimab and Carboplatin-Taxane Based Chemotherapy in Patients with Early Triple-Negative Breast Cancer: The Phase 2 NeoSAC Trial. Signal Transduct. Target. Ther. 2025, 10, 41. [Google Scholar] [CrossRef]
- Bishayee, A.; Waghray, A.; Patel, M.A.; Chatterjee, M. Vanadium in the Detection, Prevention and Treatment of Cancer: The in Vivo Evidence. Cancer Lett. 2010, 294, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Camilo, H.S.; Brito, B.R.; Ribeiro, R.R.; Fraqueza, G.; Klassen, G.; Nunes, G.G. Polyoxovanadates with Anti-breastCancer Activity and Ca2+-ATPase Inhibition Potential. Eur. Assoc. Cancer Res. 2025, 19, 511–512. [Google Scholar] [CrossRef]
- Wang, C.; Dai, Z.; Zhang, Q.; Li, X.; Ma, M.; Shi, Z.; Zhang, J.; Liu, Q.; Chen, H. A Bifunctional Biomineralized Polyoxometalate Enabling Efficient Non-Inflammatory NIR-II Photothermal Tumor Therapy. Chem. Eng. J. 2024, 490, 151601. [Google Scholar] [CrossRef]
- Misra, A.; Kozma, K.; Streb, C.; Nyman, M. Beyond Charge Balance: Counter-Cations in Polyoxometalate Chemistry. Angew. Chem. Int. Ed. 2020, 59, 596–612. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Speciation Atlas of Polyoxometalates in Aqueous Solutions. Sci. Adv. 2023, 9, eadi0814. [Google Scholar] [CrossRef]
- Tito, G.; Ferraro, G.; Pisanu, F.; Garribba, E.; Merlino, A. Non-Covalent and Covalent Binding of New Mixed-Valence Cage-like Polyoxidovanadate Clusters to Lysozyme. Angew. Chem. Int. Ed. 2024, 63, e202406669. [Google Scholar] [CrossRef] [PubMed]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in Solution: Speciation under Spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef]
- Day, P.; Hush, N.S.; Clark, R.J.H. Mixed Valence: Origins and Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 5–14. [Google Scholar] [CrossRef]
- Aureliano, M. Recent Perspectives into Biochemistry of Decavanadate. World J. Biol. Chem. 2011, 2, 215–225. [Google Scholar] [CrossRef]
- Fraqueza, G.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Sarcoplasmic Reticulum Calcium ATPase Interactions with Decaniobate, Decavanadate, Vanadate, Tungstate and Molybdate. J. Inorg. Biochem. 2012, 107, 82–89. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Fraqueza, G.; Lagoa, R.; Vannathan, A.A.; Mal, S.S.; Aureliano, M. Polyoxovanadate Inhibition of Escherichia Coli Growth Shows a Reverse Correlation with Ca2+-ATPase Inhibition. New J. Chem. 2019, 43, 17577–17587. [Google Scholar] [CrossRef]
- Gumerova, N.; Krivosudský, L.; Fraqueza, G.; Breibeck, J.; Al-Sayed, E.; Tanuhadi, E.; Bijelic, A.; Fuentes, J.; Aureliano, M.; Rompel, A. The P-Type ATPase Inhibiting Potential of Polyoxotungstates. Metallomics 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Fraqueza, G.; Batista de Carvalho, L.A.E.; Marques, M.P.M.; Maia, L.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Decavanadate, Decaniobate, Tungstate and Molybdate Interactions with Sarcoplasmic Reticulum Ca2+-ATPase: Quercetin Prevents Cysteine Oxidation by Vanadate but Does Not Reverse ATPase Inhibition. Dalton Trans. 2012, 41, 12749–12758. [Google Scholar] [CrossRef]
- Aureliano, M.; Fraqueza, G.; Berrocal, M.; Cordoba-Granados, J.J.; Gumerova, N.I.; Rompel, A.; Gutierrez-Merino, C.; Mata, A.M. Inhibition of SERCA and PMCA Ca2+-ATPase Activities by Polyoxotungstates. J. Inorg. Biochem. 2022, 236, 111952. [Google Scholar] [CrossRef]
- Sciortino, G.; Aureliano, M.; Garribba, E. Rationalizing the Decavanadate(V) and Oxidovanadium(IV) Binding to G-Actin and the Competition with Decaniobate(V) and ATP. Inorg. Chem. 2021, 60, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal Structure of the Calcium Pump of Sarcoplasmic Reticulum at 2.6 Å Resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Inesi, G.; Toyoshima, C. Distinct Topologies of Mono- and Decavanadate Binding and Photo-Oxidative Cleavage in the Sarcoplasmic Reticulum ATPase. J. Biol. Chem. 2000, 275, 30546–30550. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ Interactions with Proteins: An Overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Zarroug, R.; Artetxe, B.; Ayed, B.; López, X.; Ribeiro, N.; Correia, I.; Pessoa, J.C. New Phosphotetradecavanadate Hybrids: Crystal Structure, DFT Analysis, Stability and Binding Interactions with Bio-Macromolecules. Dalton Trans. 2022, 51, 8303–8317. [Google Scholar] [CrossRef] [PubMed]
- Solé-Daura, A.; Poblet, J.M.; Carbó, J.J. Structure–Activity Relationships for the Affinity of Chaotropic Polyoxometalate Anions towards Proteins. Chem. A Eur. J. 2020, 26, 5799–5809. [Google Scholar] [CrossRef] [PubMed]
- Aybek, H.; Temel, Y.; Ahmed, B.M.; Ağca, C.A.; Çiftci, M. Deciphering of The Effect of Chemotherapeutic Agents on Human Glutathione S-Transferase Enzyme and MCF-7 Cell Line. Protein Pept. Lett. 2020, 27, 888–894. [Google Scholar] [CrossRef]
- Hu, X.; Wang, H.; Huang, B.; Li, N.; Hu, K.; Wu, B.; Xiao, Z.; Wei, Y.; Wu, P. A New Scheme for Rational Design and Synthesis of Polyoxovanadate Hybrids with High Antitumor Activities. J. Inorg. Biochem. 2019, 193, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Kioseoglou, E.; Gabriel, C.; Petanidis, S.; Psycharis, V.; Raptopoulou, C.P.; Terzis, A.; Salifoglou, A. Binary Decavanadate-Betaine Composite Materials of Potential Anticarcinogenic Activity. Z. Anorg. Allg. Chem. 2013, 639, 1407–1416. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, B.; Qi, Y.; Guo, S.; Tian, R.; Sun, J.; Zhao, M. The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)]∙6H2O on Breast Cancer Cell Lines. Molecules 2017, 22, 1535. [Google Scholar] [CrossRef]
- Liu, H.; Zang, C.; Fenner, M.H.; Possinger, K.; Elstner, E. PPARγ Ligands and ATRA Inhibit the Invasion of Human Breast Cancer Cells in Vitro. Breast Cancer Res. Treat. 2003, 79, 63–74. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple Negative Breast Cancer Cell Lines: One Tool in the Search for Better Treatment of Triple Negative Breast Cancer. Breast Dis. 2011, 32, 35–48. [Google Scholar] [CrossRef]
- Ksiksi, R.; Essid, A.; Kouka, S.; Boujelbane, F.; Daoudi, M.; Srairi-Abid, N.; Zid, M.F. Synthesis and Characterization of a Tetra-(Benzylammonium) Dihydrogen Decavanadate Dihydrate Compound Inhibiting MDA-MB-231 Human Breast Cancer Cells Proliferation and Migration. J. Mol. Struct. 2022, 1250, 131929. [Google Scholar] [CrossRef]
- Image, J. JS Software. Available online: https://Ij.Imjoy.Io/ (accessed on 16 June 2025).
- Ghafoor, S.; Garcia, E.; Jay, D.J.; Persad, S. Molecular Mechanisms Regulating Epithelial Mesenchymal Transition (EMT) to Promote Cancer Progression. Int. J. Mol. Sci. 2025, 26, 4364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins & Cotran Patologia: Bases Patológicas Das Doenças, 9th ed.; Elsevier: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Liu, X.; Zhou, M.; Mei, L.; Ruan, J.; Hu, Q.; Peng, J.; Su, H.; Liao, H.; Liu, S.; Liu, W.; et al. Key Roles of Necroptotic Factors in Promoting Tumor Growth. Oncotarget 2016, 7, 22219–22233. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, L.; Liu, Y.; Qin, X.; Zhou, Y.; Zhou, Y.; Liu, J. Mo Polyoxometalate Nanoparticles Inhibit Tumor Growth and Vascular Endothelial Growth Factor Induced Angiogenesis. Sci. Technol. Adv. Mater. 2014, 15, 035010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dianat, S.; Bordbar, A.K.; Tangestaninejad, S.; Zarkesh-Esfahani, S.H.; Habibi, P.; Abbasi Kajani, A. CtDNA Interaction of Co-Containing Keggin Polyoxomolybdate and in Vitro Antitumor Activity of Free and Its Nano-Encapsulated Derivatives. J. Iran. Chem. Soc. 2016, 13, 1895–1904. [Google Scholar] [CrossRef]
- Dianat, S.; Bordbar, A.-K.; Tangestaninejad, S.; Yadollahi, B.; Amiri, R.; Zarkesh-Esfahani, S.-H.; Habibi, P. In Vitro Antitumor Activity of Free and Nano-Encapsulated Na5[PMo10V2O40]·nH2O and Its Binding Properties with CtDNA by Using Combined Spectroscopic Methods. J. Inorg. Biochem. 2015, 152, 74–81. [Google Scholar] [CrossRef]
- Dianat, S.; Bordbar, A.K.; Tangestaninejad, S.; Yadollahi, B.; Zarkesh-Esfahani, S.H.; Habibi, P. In Vitro Antitumor Activity of Parent and Nano-Encapsulated Mono Cobalt-Substituted Keggin Polyoxotungstate and Its CtDNA Binding Properties. Chem. Biol. Interact. 2014, 215, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Geisberger, G.; Gyenge, E.B.; Maake, C.; Patzke, G.R. Trimethyl and Carboxymethyl Chitosan Carriers for Bio-Active Polymer–Inorganic Nanocomposites. Carbohydr. Polym. 2013, 91, 58–67. [Google Scholar] [CrossRef]
- Dianat, S.; Bordbar, A.K.; Tangestaninejad, S.; Yadollahi, B.; Zarkesh-Esfahani, S.H.; Habibi, P. CtDNA Binding Affinity and in Vitro Antitumor Activity of Three Keggin Type Polyoxotungestates. J. Photochem. Photobiol. B Biol. 2013, 124, 27–33. [Google Scholar] [CrossRef]
- Yamase, T. Polyoxometalates for Molecular Devices: Antitumor Activity and Luminescence. Mol. Eng. 1994, 3, 241–262. [Google Scholar] [CrossRef]
- Carvalho, F.; Aureliano, M. Polyoxometalates Impact as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 5043. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.I.; Ahmad, T. Altered Mitochondrial Calcium Handling and Cell Death by Necroptosis: An Emerging Paradigm. Mitochondrion 2021, 57, 47–62. [Google Scholar] [CrossRef]
- Ohlin, C.A.; Villa, E.M.; Fettinger, J.C.; Casey, W.H. A New Titanoniobate Ion—Completing the Series [Nb10O28]6−, [TiNb9O28]7− and [Ti2Nb8O28]8−. Dalton Trans. 2009, 15, 2677–2678. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Sun, C.; Xie, L.; Wang, E.; Hu, N.; Jia, H. A Novel 2D Layered Network Based on 13-Vanadomanganate(IV): K3(HABOB)4[MnV13O38]·9H2O (ABOB=N-Amidino-4-Morpholincarboxamidine). Inorg. Chem. Commun. 2005, 8, 433–436. [Google Scholar] [CrossRef]
- Missina, J.M.; Gavinho, B.; Postal, K.; Santana, F.S.; Valdameri, G.; de Souza, E.M.; Hughes, D.L.; Ramirez, M.I.; Soares, J.F.; Nunes, G.G. Effects of Decavanadate Salts with Organic and Inorganic Cations on Escherichia coli, Giardia intestinalis, and Vero Cells. Inorg. Chem. 2018, 57, 11930–11941. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative Analysis of Molecular Surface Based on Improved Marching Tetrahedra Algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Chen, X.-Y.; Landis, C.R.; Neese, F. All-Electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms. J. Chem. Theory Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M.A.C. A Tetrazolium-Based Colorimetric MTT Assay to Quantitate Human Monocyte Mediated Cytotoxicity against Leukemic Cells from Cell Lines and Patients with Acute Myeloid Leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- GraphPad Software, Inc. GraphPad Prism—Version 7.0 for Windows; GraphPad Prism: Boston, MA, USA, 2018. [Google Scholar]
- Serio, F.; da Cruz, A.F.; Chandra, A.; Nobile, C.; Rossi, G.R.; D’Amone, E.; Gigli, G.; del Mercato, L.L.; de Oliveira, C.C. Electrospun Polyvinyl-Alcohol/Gum Arabic Nanofibers: Biomimetic Platform for in Vitro Cell Growth and Cancer Nanomedicine Delivery. Int. J. Biol. Macromol. 2021, 188, 764–773. [Google Scholar] [CrossRef]
- Gonçalves, J.P.; de Oliveira, C.C.; da Silva Trindade, E.; Riegel-Vidotti, I.C.; Vidotti, M.; Simas, F.F. In Vitro Biocompatibility Screening of a Colloidal Gum Arabic-Polyaniline Conducting Nanocomposite. Int. J. Biol. Macromol. 2021, 173, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Aureliano, M.; Ma, B. Feature Papers in BioChem. BioChem 2025, 5, 17. [Google Scholar] [CrossRef]
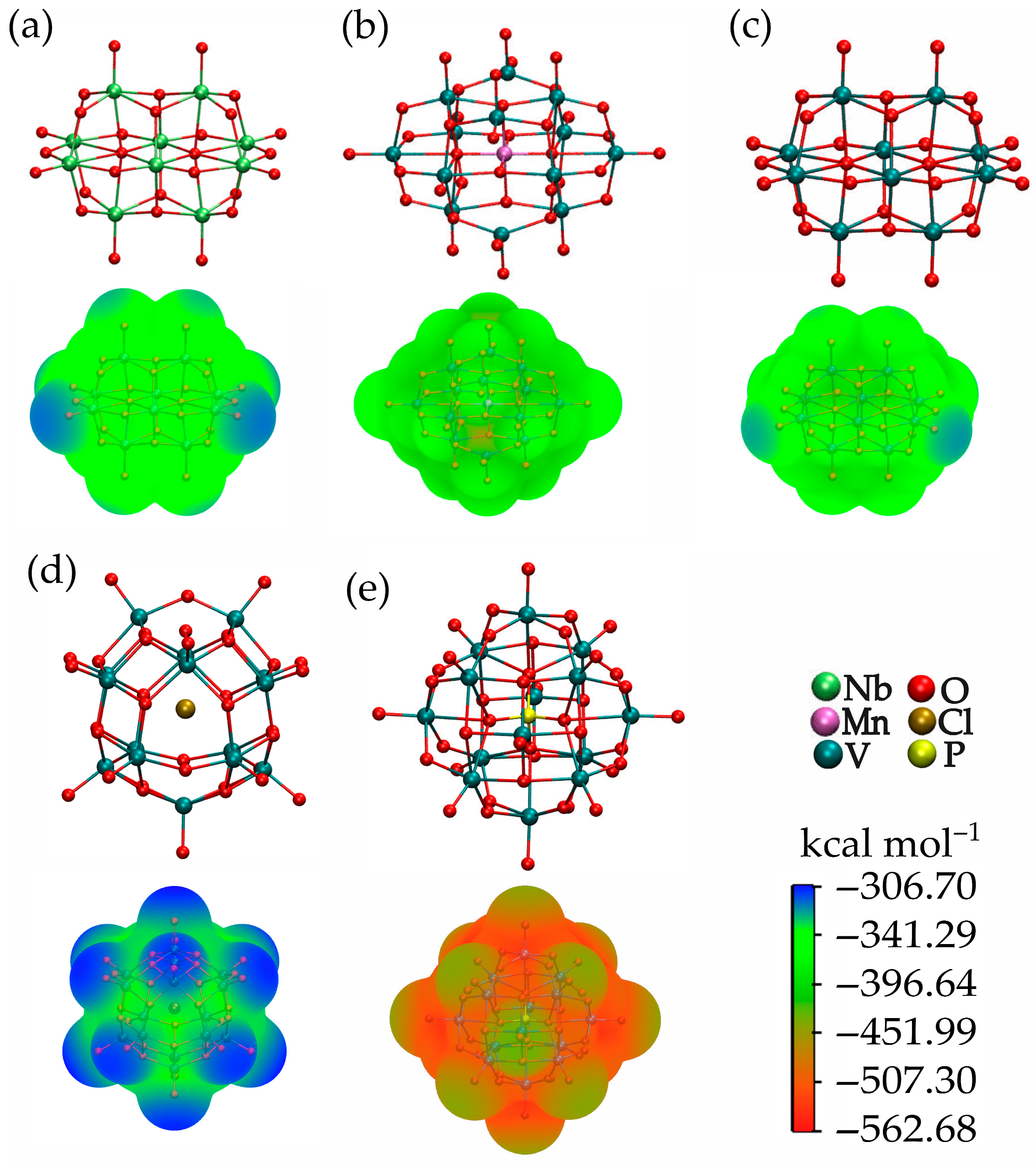


| Parameter | Nb10 | MnV13 | V10 | V15 | PV14 ox |
|---|---|---|---|---|---|
| IC50 μM | 35 | 31 | 15 | 14.2 | 5 |
| Emax/kcal mol−1 | −323.31 | −350.29 | −329.76 | −306.01 | −439.53 |
| Emin/kcal mol−1 | −382.80 | −468.82 | −412.55 | −354.20 | −562.68 |
| ΔE/kcal mol−1 | 59.5 | 118.5 | 82.79 | 48.19 | 123.1 |
| Visosurface/Å3 | 629.94 | 558.32 | 744.23 | 790.49 | 835.19 |
| q/m | 0.6 | 0.54 | 0.6 | 0.4 | 0.64 |
| (q/m)/Visosurface/10–4 | 9.524 | 9.644 | 8.062 | 5.060 | 7.697 |
| Assay | IC50 (µM) |
|---|---|
| Ca2+-ATPase | 14.2 |
| HB4a | 1.02 |
| MCF-7 | 15.1 |
| MDA-MB-231 | 17.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, B.R.; Camilo, H.d.S.; Cruz, A.F.d.; Ribeiro, R.R.; de Sá, E.L.; Camargo de Oliveira, C.; Fraqueza, G.; Klassen, G.; Aureliano, M.; Nunes, G.G. Mixed-Valence Pentadecavanadate with Ca2+-ATPase Inhibition Potential and Anti-Breast Cancer Activity. Inorganics 2025, 13, 306. https://doi.org/10.3390/inorganics13090306
Brito BR, Camilo HdS, Cruz AFd, Ribeiro RR, de Sá EL, Camargo de Oliveira C, Fraqueza G, Klassen G, Aureliano M, Nunes GG. Mixed-Valence Pentadecavanadate with Ca2+-ATPase Inhibition Potential and Anti-Breast Cancer Activity. Inorganics. 2025; 13(9):306. https://doi.org/10.3390/inorganics13090306
Chicago/Turabian StyleBrito, Bianca R., Heloísa de S. Camilo, Anderson F. da Cruz, Ronny R. Ribeiro, Eduardo L. de Sá, Carolina Camargo de Oliveira, Gil Fraqueza, Giseli Klassen, Manuel Aureliano, and Giovana G. Nunes. 2025. "Mixed-Valence Pentadecavanadate with Ca2+-ATPase Inhibition Potential and Anti-Breast Cancer Activity" Inorganics 13, no. 9: 306. https://doi.org/10.3390/inorganics13090306
APA StyleBrito, B. R., Camilo, H. d. S., Cruz, A. F. d., Ribeiro, R. R., de Sá, E. L., Camargo de Oliveira, C., Fraqueza, G., Klassen, G., Aureliano, M., & Nunes, G. G. (2025). Mixed-Valence Pentadecavanadate with Ca2+-ATPase Inhibition Potential and Anti-Breast Cancer Activity. Inorganics, 13(9), 306. https://doi.org/10.3390/inorganics13090306









