Abstract
In this century, cancer is one of the most important causes of death worldwide, and the need for the development of new treatment options is imperative. The use of metal-based compounds in cancer treatment has increased significantly due to certain properties of these elements, and vanadium has been one of the most studied transition metals in recent decades. Vanadium compounds are being explored as an option for cancer treatment because of their wide range of action mechanisms such as the induction of oxidative stress, DNA damage, cell cycle arrest, induction of apoptosis and regulation of the autophagy process, among the most important mechanisms. Their compounds have been demonstrated to be effective against the cancer types with the highest incidence and mortality rates worldwide, such as lung and breast cancer, with promising results. This review discusses a variety of new vanadium compounds, indicating their mechanisms of action and the neoplasms in which they have shown effectiveness.
Keywords:
metals; metallic compounds; vanadium; oxidovanadium; vanadate; cancer; antineoplastic; anticarcinogenic 1. Introduction
Various factors act as risk factors for developing cancer. In this century, cancer is the cause of one out of every four non-communicable disease-related deaths worldwide (22.8%). When age is considered a risk factor for premature death, cancer occurs in three out of 10 subjects aged 30 to 69 years in 177 of 183 countries. Cancer mortality among women is disproportionately high, primarily due to breast or cervical cancer. Overall, lung cancer is the leading cause of morbidity and mortality, according to the most recent report from 2022 [1]. This problem has other consequences, such as the need for specialized health professionals and more oncology centers with the necessary equipment and medications to properly treat patients. However, this places a significant economic burden on health systems worldwide [2].
One option for cancer treatment is chemotherapy, which is expensive; furthermore, the heterogeneity that can be found in cancer requires the use of more toxic drugs and those to which resistance can develop [3]. For these reasons, the search for less expensive effective antitumor drugs with high selectivity, good bioavailability and low toxicity options is ongoing. Metal compounds are a source of anticancer molecules, such as platinum compounds, which have demonstrated effectiveness in various cancer treatments. Cisplatin was the first metallic compound used against cancer and throughout history, since 1978 when it was approved by the FDA to be used as a chemotherapy agent against ovarian and testicular cancer, it has been used against numerous types of cancer such as bladder, lung, cervical, gastric cancer, melanoma, among others [4].
The rise in the use of platinum (Pt) as an anticancer agent has spurred the search for other metal-based compounds with similar effects but lower toxicity and fewer side effects. Cisplatin has been widely used, but it is important to mention that it produces numerous undesirable adverse effects, such as nephrotoxicity, neurotoxicity, ototoxicity, vomiting, anemia, myelosuppression, and cytotoxicity in normal cells [5,6]. Furthermore, cancer cells acquire resistance relatively quickly [4,5]. Platinum compounds are capable of increasing ROS production and binding to genomic or mitochondrial DNA, causing damage that blocks replication, transcription, and translation, as well as activating signaling pathways that lead to apoptosis or necrosis [4]. The toxicity of cisplatin led to the development of subsequent generations of drugs based on the same metal, aimed at reducing adverse effects; however, these have not shown substantial advantages compared to cisplatin.
Some of the most widely used have been copper (Cu), gold (Au), iridium (Ir), iron (Fe), rhenium (Re), ruthenium (Ru), osmium (Os), Zinc (Zn), vanadium (V), among others [5,7,8,9,10]. The use of metal-based compounds in cancer treatment has increased significantly due to certain properties of these elements: multiple oxidation states and coordination numbers, accessible redox states and kinetic and thermodynamic characteristics that allow them to exert antiproliferative, proapoptotic and antiangiogenic effects, acting as modulators of key signaling pathways [8,9,10,11].
Table 1 includes some of the metals that have been used to develop anticancer drugs. These metals show various advantages compared to Pt compounds; however, there are also some limitations in their use, which are mentioned below.

Table 1.
Advantages and limitations in the use of metal based anticancer complexes compared to platinum drugs.
In the field of cancer research, various V compounds have been synthesized to achieve better tolerance, higher selectivity and reduced toxicity in cancer treatment.
Vanadium compounds are being explored as an option because of their wide range of action mechanisms, such as disrupting cancer cell metabolism, producing reactive oxygen species (ROS), interfering with the epithelial–mesenchymal transition signaling pathway, and inhibiting focal adhesion kinase (FAK) or Notch-1 signaling pathways [22,23,24,25,26]. To reduce the toxicity of V compounds and increase their lipophilicity and transport across cell membranes, a variety of ligands have been incorporated, including bisphosphonates, non-steroidal anti-inflammatory drugs, metformin, cetirizine, and imidazole derivatives, with promising results [24,26,27].
This review discusses a variety of vanadium compounds, indicating their mechanisms of action and the neoplasms in which they have shown effectiveness. Our focus remains exclusively on research conducted from 2015 onwards.
2. Vanadium and Its Antineoplastic Potential
Vanadium is a transition metal from the 3d series of the periodic table, which means it exhibits several oxidation states: the most common being +2, +3, +4, and +5 [28,29,30]. It is used in alloys to enhance strength when combined with other metals [30,31] and is commonly found in soil, bedrock, groundwater, fossil fuels like petroleum, and in living organisms [32,33]. It has been described as an essential micronutrient in some living organisms, such as fungi and worms. However, in humans, it is found in concentrations of up to 100 μg without any reported deficiency symptoms; therefore, it is not considered a micronutrient [29,31,32].
This metal has shown antidiabetic, anticancer, antiparasitic, and antituberculosis properties. Its therapeutic properties are due to the metal’s chemistry; its species and complexes are easily interconverted [29,34]. In the last decade, it has gained relevance in anticancer applications due to its ability to inhibit and induce various pathways and enzymatic pathways and complexes associated with different types of cancer [26,28,32,34,35]. The first vanadium compounds tested as anticancer agents include salts such as: ammonium metavanadate (NH4VO3), sodium metavanadate (NaVO3), and potassium metavanadate (KVO3), and vanadyl sulfate (VO4SO4). Additionally, four commercially available forms of vanadium have been used in studies: sodium decavanadate (Na6V10O28), vanadium pentoxide (V2O5), vanadyl dichloride (VOCl2), and vanadium(III) chloride (VCl3). In recent decades, hundreds of compounds of this metal have been developed, including nanoparticles and a wide range of organic and inorganic complexes, which are intended to induce desirable biological effects for therapeutic use. The most promising compounds have been tested in both in vitro and in vivo biological models to demonstrate low toxicity and higher efficacy [26,35,36].
The therapeutic effects of vanadium depend on several factors, such as oxidation state, dose, and route of administration. Its mechanism can be cytotoxic, but in this case, which is advantageous when targeting tumor cells [26]. Kostenkova and colleagues [35] and Singh and colleagues [26], reviewed the following anticancer properties:
- -
- Inhibition of enzymes, such as tyrosine phosphatase, promoting the activation of tyrosine kinases and oncogene suppressors, which induces apoptosis in cancer cells.
- -
- DNA interaction with vanadium complexes inserting between DNA strands, causing cell cycle breaks or alterations. Some other compounds, such as ‘Metvan’, which has an extensive aromatic system strongly coordinated with V, can also interact with DNA through aromatic π-stacking, which interferes with DNA replication and uncontrolled cell division.
- -
- Generation of reactive oxygen species (ROS) increasing the production of superoxide and hydrogen peroxide, leading to a state of oxidative stress and apoptosis of cancer cells.
- -
- Differentiation between normal and cancer cells, with some vanadium complexes such as VO(maltol)2, which showed selectivity in affecting cancer cells while sparing normal cells.
- -
- Inhibition of specific demethylases, through the vanadium complex bearing tridentate shiff base ligands, which showed potential to inhibit lysine-specific demethylase (LSD1), an important target in cancer treatment.
The first study describing the antineoplastic activity of a vanadium compound, vanadocene dichloride (C5H5)2VCl2, dates back to 1965 [37]. Since then, research on vanadium and its relationship with cancer has experienced significant growth. Among the cancer types in which vanadium’s anticancer activity has been observed are breast, liver, colon, and hematopoietic tissue cancer, among others [36,38]. In recent years, its effect on other types of cancer, such as skin, bone, and lung cancer, to name a few, has also been explored. In recent decades, the use of V complexes, such as those with flavonoids, has achieved significant progress in cancer research due to their potential for action on various cancer cell lines, in addition to the in vivo effects that have been described. This review presents the results of in vivo and in vitro studies, with special emphasis on those conducted in the last decade. Please note that we have selected a number of publications, and as such, our review does not aim to address all studies that have been documented.
3. In Vivo and in Vitro Evidence of Vanadium’s Anticancer Activity
As previously mentioned, the anticancer activity of vanadium has been widely explored in recent decades, and its compounds have emerged as alternative antitumor agents to platinum. In a comprehensive review conducted by Bishayee and colleagues in 2010, the findings up to that point regarding the use of vanadium compounds in in vivo preclinical models were summarized. Among the most common cancer types are breast, colorectal, liver, and hematologic cancers [38]. Cancer treatment is a complex process that faces significant challenges. Conventional clinical treatments include surgery, radiotherapy, and chemotherapy. Currently, oncology is seeking to develop new, safer, and more effective drugs, and vanadium compounds have been proposed as promising therapeutic strategies.
New evidence from the last decade regarding the antineoplastic effects of vanadium in both in vitro studies and in vivo models, along with the mechanisms associated with them, is described below.
3.1. Breast Cancer
According to current data from the Global Cancer Observatory (GLOBOCAN), breast cancer is the leading cause of death from malignant neoplasms in women [39] and ranks second in cancer mortality, just after lung cancer. In vivo evidence has shown that in 1-methyl-1-nitrosourea (MNU)-induced breast cancer models, VOSO4 reduces the incidence and number of mammary tumors in rats. Likewise, in 7,12-dimethylbenz(a) anthrancene (DMBA)-induced mammary cancer models, NH4VO3 suppresses hyperplasia and reduces the incidence, multiplicity, and size of breast tumors. In addition, vanadocene dichloride (C5H5)2VCl2 has proven effective in the treatment of preexisting breast tumors [38].
Over the last decade, additional evidence has emerged confirming the anticancer effect of vanadium in vivo. A study by Roy and colleagues demonstrated the antineoplastic activity of a vanadium compound coupled with quercetin (flavonoid) in a DMBA-induced mammary carcinogenesis model in female Sprague–Dawley rats. The animals treated with the complex exhibited the same histological characteristics as controls without developing hyperplasia or neoplastic lesions. Furthermore, the treatment increased cell death by apoptosis and reduced cellular proliferation. In the same study, the authors observed pro-apoptotic effects in MCF-7 human breast cancer cells treated with the compound, including an increase in p53, caspases 3 and 9, DNA fragmentation, and a decrease in Akt, mTOR, and VEGF [40].
In another study, Tian and colleagues investigated the effects of NH4VO3 in a subcutaneous xenograft model using 4T1 murine breast cancer cells in female BALB/c mice. Their findings demonstrated a reduction in tumor volume, lower development of microvasculature, and an increase in apoptosis. In vitro, the treatment of 4T1 cells showed inhibition of cell proliferation, increased reactive oxygen species (ROS), arrest of the cell cycle in G2/M phase, decreased mitochondrial membrane potential, and induction of apoptosis [41]. Other compounds tested against breast cancer include oxidovanadium complexes with other molecules. In vitro, Kalındemirtaş and colleagues analyzed the effect of two vanadium oxide compounds (VO1a and VO1b) on the MDA-MB-231 and MCF-7 cell lines. The authors reported a decrease in the activity of the multidrug resistance transporter ABCG2 and an increase in apoptosis, but not in necrosis [42]. In another study, an IV- oxidovanadium imidazole complex (VOL) was used in MCF-7 cells; VOL administration induced apoptosis, ROS production, an imbalance in the activity of the antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD), lipid peroxidation, and a reduction in mitochondrial membrane potential [43].
The same research group evaluated the in vivo and in vitro effects of the 1-methylimidazole oxidovanadium complex (OVMI). In vivo, in breast tumors derived from 4T1 cells in female BALB/c mice, the compound reduced tumor volume by decreasing proliferation (as shown by lower Ki67 expression), triggered apoptosis, and exhibited anti-metastatic effects by reducing metastasis markers (E-cadherin and N-cadherin) in tumor tissues. In vitro, treatment of MDA-MB-231 cells with the complex led to delayed proliferation, apoptosis, mitochondrial membrane potential alteration, and increased ROS production. The authors also reported that OVMI was biocompatible and non-toxic in normal cells in vivo [44].
In contrast, a study evaluating nitrilotriacetate-based N-heterocyclic vanadium (IV) salts in MCF-7 cells found that the compound induced DNA fragmentation and arrested the cell cycle in G0/G1 phase. However, because the compound also affected normal cells, the authors ruled out its therapeutic potential for in vivo use [45].
Finally, in recent years, vanadium nanoparticles have been tested against different types of cancer, including breast cancer. In MDA-MB-231 cells treated with nanoparticles of this metal (VnNp), increased ROS production and decreased SOD levels, cell cycle arrest in the G2/M phase, and apoptosis were reported. Furthermore, ultrastructural observation showed the presence of autophagosomes and VnNp in the cytoplasm and mitochondria of these cells [46].
3.2. Liver Cancer
Liver cancer is characterized globally by its high prevalence and mortality. According to GLOBOCAN data, it ranks sixth in both incidence and mortality, as it is one of the most common types of digestive malignancy worldwide [39]. Most deaths from this disease are attributed to hepatocellular carcinoma (HCC), which ranks third among causes of death from digestive system cancer [47].
The review by Bishayee and colleagues includes in vivo evidence demonstrating the anticancer activity of vanadium in models of hepatocellular carcinoma induction with diethylnitrosamine (DENA). In these models, the administration of NH4VO3 in drinking water was able to inhibit the growth of preneoplastic lesions, significantly reduced the incidence, number, size, and multiplicity of hepatic neoplastic nodules; in other studies, it even prevented the formation of nodules and hepatocarcinoma. Moreover, the chemopreventive activity of vanadium has also been demonstrated in models of liver carcinogenesis induction with 2-acetylaminofluorene (2-AAF) [38].
Another compound tested both in vitro and in vivo against hepatocarcinoma is sodium orthovanadate (SOV). Suppression of proliferation, cell cycle arrest, apoptosis, and inhibition of autophagy were demonstrated in the human hepatocellular carcinoma cell lines HepG2, SK-Hep-1, and Hep3B. Using the same compound in vivo in an orthotopic xenograft model with BALB/C nude mice injected with HepG2 cells, the authors reported decreased tumor size, decreased proliferation index and microcirculatory density, and increased apoptosis due to mitochondrial damage [48].
The effect of oxidovanadium compounds on liver cancer cell lines has been demonstrated in vitro. In HepG2 cells independently exposed to NaVO3, bis(maltolate)- oxidovanadium(IV) (VO(ma)2) and bis(acetylacetonate)- oxidovanadium(IV) (VO(acac)2) demonstrated inhibition of cell progression in the G1/S phase by regulating the ERK signaling pathway. Furthermore, it was found that these compounds exhibit greater selectivity toward cancer cells compared to normal hepatocytes of the L02 cell line [49]. On the other hand, Aliabad and colleagues confirmed that the vanadium complex 4-bromo-2-(((5-chloro-2-hydroxyphenyl) imino) methyl) phenol ([IV(L)] complex) induced early apoptosis and late necrosis/apoptosis in both HepG2 cells and L929 normal fibroblast cells, with a higher death rate in cancer cells and a lesser effect on normal cells [50].
The efficacy of vanadium nanoparticles has recently been tested in in vitro studies with liver cancer cells. Yang and colleagues demonstrated that vanadium-doped iron oxide (VIO) nanoparticles induce the death of murine hepatoma Hepa1-6 cells, in addition to having potential antiangiogenic activity since it was shown that human endothelial (HUVEC) and murine endothelial (C166) cell lines are also targeted by these nanoparticles [51]. In another study, the efficacy of cellulose-coated V2O5 nanoparticles on human hepatocellular carcinoma cell line (HuH-7.0) was verified by inducing morphological changes and cell death [52].
3.3. Colorectal Cancer
Currently, according to global data from GLOBOCAN, colorectal cancer is the third most diagnosed type and the second leading cause of cancer-related death worldwide [39]. Several studies have shown that vanadium compounds are used as antineoplastic agents in colon cancer, both in vitro and in vivo models.
In preclinical in vivo studies of 1,2-dimethylhydrazine (DMH)-induced colorectal cancer in rodents (rats and mice), NH4VO3 has demonstrated its anticancer activity by reducing the number and size of aberrant crypt foci, which are considered one of the first observable preneoplastic alterations in the development of colonic mucosal neoplasms. It also reduced the formation of adenomas and carcinomas [38].
Regarding in vitro studies, Sinha and colleagues reported the effect of the vanadium complex vanadyl N-(2-hydroxyacetophenone) glycinate [VO(NG)2] on several cancer cell lines, including the human colorectal carcinoma cell line HCT-116. This compound induced cell cycle arrest in G2/M and apoptosis, without affecting peripheral blood mononuclear cells used as a normal control. The authors also reported glutathione (GSH) depletion and a subsequent increase in ROS levels [53]. Another compound tested is Metvan (4,7-dimethyl-1,10-phenathroline 7 sulphate oxidovanadium(IV)). León and colleagues demonstrated that this compound inhibits mitochondrial metabolism and reduces cell viability in the HT-29 human colon adenocarcinoma cell line [54].
In the last decade, oxidovanadium complexes coupled to flavonoids have also been tested in vitro on colon cancer cell lines. León and colleagues reported the action of the complex Na2[VO(silibinin)2]·6H2O (VOsil) coupled with silibinin, and the complex [VO(chrysin)2EtOH]2 (VOcrys) coupled with chrysin, in HT-29 cells. Both compounds inhibited cell viability. It was identified that, upon exposure to these complexes, the proportion of oxidized and reduced glutathione was altered, which may be related to the effects found. Additionally, exposure to VOcrys induced cell cycle arrest at G2/M, while VOsil activated caspase-3, leading to apoptosis of the cells [55]. In another study, the luteolin complex [VO(lut)(H2O)2]Na3·H2O (VOlut) exhibited cytotoxicity against the CT26 colon carcinoma cell line, and at low concentrations the effect on the viability of normal colon epithelial cells was minimal. In the same study, an in vivo model with BALB/c mice was used to evaluate the effect of the VOlut complex on the metastatic capacity of CT26 cells (injected into the mice’s spleen capsule), from the spleen to the liver. VOlut behaved as a powerful antimetastatic agent, which was confirmed by observing the liver of mice treated with the complex in which no metastatic nodules appeared [56]. The mechanisms of action activated by vanadium were not explored in this study.
Roy and Chakraborty, on the other hand, demonstrated that the V-luteolin complex reduces cell proliferation in the HT-29 cell line and induces apoptosis. In an in vivo model using male Wistar rats, treatment with the compound at the highest dose exhibited the greatest chemotherapeutic potential by significantly reducing the formation of aberrant crypt foci and inducing apoptosis, in addition to increasing the antioxidant function of SOD, CAT, and glutathione (GSH) enzymes. The reported apoptotic effects are attributed to the activation of key proteins such as p53, Bax, and caspase-3, and the downregulation of Bcl2 and mTOR/Akt [57].
Regarding the use of nanoparticles, Xu reported reduced cell viability in several human colorectal cancer cell lines—Caco-2, COLO 320, DLD-1, HCT-15, HCT-116, and HT-29—when treated with vanadium-coupled Salvia officinalis nanoparticles (VNPs@Salvia officinalis). The author suggested that the observed effects might be due to the antioxidant activity of the nanoparticles, although this requires further investigation [58]. Additionally, zinc-chromium-vanadate nanoparticles (VCrZnO4 NPs) exhibited anticancer effects through cytotoxicity against HCT-116 cells [59].
Finally, in another interesting study, Cheng and colleagues used VCl3 to test its effect on glucose metabolism in murine colorectal cancer cells CT26 and MC38, given that tumor cells are known to develop various metabolic strategies that support malignancy progression. The results indicated that VCl3 inhibited the activity of several enzymes (glucose-6-phosphate dehydrogenase [G6PD], lactate dehydrogenase [LDH], hexokinase [HK], and pyruvate kinase [PK]), thereby blocking glucose metabolism and leading to inhibited proliferation. The same study analyzed the effect of a nanosystem composed of glutaminase inhibitors (bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide, BPTES) and vanadium, resulting in dual suppression of energy metabolism in both cell lines. This nanoplatform inhibited glucose metabolism and ATP synthesis, leading to suppressed proliferation, reduced viability, and apoptosis. The experiment was also replicated in vivo in BALB/c mice with tumors derived from CT26 and M38 cells, where results were consistent with the in vitro findings. Additionally, the study demonstrated that the nanoparticles enhance the vulnerability of colorectal cancer cells to chemotherapy and immunotherapy [60].
3.4. Hematological Neoplasms
Hematologic neoplasms involve the abnormal development of blood cells and their persistence in bone marrow, lymphomas, and lymph nodes. These diseases include leukemia, lymphomas, and myeloma [61]. Despite the development of targeted chemotherapy agents for some types of these neoplasms, others are still treated with highly toxic chemotherapeutic agents. Moreover, drug resistance remains a challenge, which is why potential treatments, some of them metal-based, continue to be studied.
In the field of hematological cancers, arsenic trioxide was approved in 2000 for patients with acute promyelocytic leukemia refractory to other treatments. Darinarsine, a glutathione derivative containing arsenic, is used in the treatment of refractory lymphomas, and cisplatin has been used in treatment-resistant leukemia [62]. The effect of vanadium compounds on hematological neoplasms has been studied in recent years, mainly through in vitro models, with promising results, some of which are presented below.
In vivo evidence has shown that in preclinical xenograft models using murine leukemia and Dalton’s lymphoma cells, (C5H5)2VCl2, NH4VO3, and other vanadium complexes increased survival in tumor-bearing animals [38], although the mechanisms by which they exert their action have only been explored in vitro.
In vitro studies in the last decade have revealed interesting results. In one study using vanadium and molybdenum (Mo) compounds, it was found that their cytotoxicity and efficacy against leukemia cells were similar to those of cisplatin. The vanadium compound [(η5-C5H5)2V(5-NH2-phen)]OTf (V1) was used on human lymphoblastic leukemia MOLT-4 cells and human T-cell leukemia Jurkat cells, showing cytotoxic effects, increased apoptosis through activation of caspases 8 and 9, and elevated levels of p53 and its phosphorylated form [63].
Additionally, in 2019, Ta and colleagues synthesized an amide-imine conjugate compound called PTANAP ((E)-N′-((2-hydroxy naphthalene-1-yl)methylene)-4-methylbenzohydrazide), which was used to make metal compounds with vanadium, copper (Cu), and molybdenum (Mo). These compounds were tested on human leukemia K-562 cells and murine lymphoma 2PK3 cells. The PTANAP- oxidovanadium complex showed the greatest antineoplastic effect, as measured by growth inhibition and cytotoxicity in both cell types. The authors propose that ROS generation and oxidative stress led to DNA damage and apoptosis [64].
Sodium Orthovanadate (SOV) has also been studied for its anticancer effects on hematologic neoplasms. In an in vitro study on acute promyelocytic leukemia cell lines HL-60 and HL-60/A, SOV was shown to inhibit cell proliferation, induce cell cycle arrest at G2/M, and trigger apoptosis. The reported mechanisms included activation of poly-ADP-ribose polymerase (PARP), activation of caspases 3 and 9, disruption of mitochondrial membrane potential, and inhibition of autophagy as promoters of tumor cell death [65]. Another oxidovanadium compound associated with a Schiff base was also tested on HL-60 cells. While administration of either this compound or arsenic trioxide alone-demonstrated cytotoxicity, their co-administration resulted in a greater decrease in cell viability, higher expression of p53 and p21 proteins, and a greater rate of apoptosis. Additionally, co-administration made it possible to reduce the dose of arsenic trioxide, thereby also decreasing its toxic effects, which suggests this strategy as a treatment for acute promyelocytic leukemia [66].
Finally, a vanadium compound derived from 8-formyl-7-hydroxy-4-methyl coumarin was synthesized and functionalized with copper nanoparticles. The vanadyl complex with a tridentate coumarin ligand [VO(CUAP)SO4] and this complex functionalized with copper nanoparticles [nano Cu-VO (IV)] were tested on breast cancer and leukemia cells. In the case of K-562 leukemia cells, the vanadyl complex had the greatest anticancer effect, inducing cytotoxicity, inhibiting proliferation, and DNA damage [67].
3.5. Skin Cancer
Skin cancer is one of the most prevalent cancers worldwide, with both its incidence and mortality steadily increasing, particularly in regions with a predominantly white population [68]. Among the types of cancers that develop in the skin is melanoma, which, although not the most common form, has increased in global prevalence and is associated with a high mortality rate. It is estimated that 75% of skin cancer-related deaths are due to melanoma, which has shown higher incidence among adolescents and young adults [69]. This cancer type has increased globally at a faster rate than any other malignant neoplasm [70]. This explains why most research on the effects of vanadium has focused on in vivo and in vitro melanoma cell models rather than other types of skin cancers.
In an in vitro study conducted by Rozzo and colleagues, the activity of various vanadium compounds was reported on A375 and CN-mel malignant melanoma cells: NaVO3 and three oxidovanadium complexes: 1,2-dimethyl-3-hydroxy-4 (1H)-pyridinonate (VS2), 1-methyl-3-hydroxy-4 (1H)-pyridinonate (VS3), and 1-phenyl-2-methyl-3-hydroxy-4 (1H)-pyridinonate (VS4). Evidence indicates that all compounds exhibited antiproliferative activity, while VN and VS2 induced apoptosis and cell cycle arrest [69]. Additionally, the same research group demonstrated that the pro-apoptotic activity of VN and VS2 in these melanoma cells is related to increased ROS production and the consequent inactivation of the MAP kinase (MAPK) pathway [71]. Notably, these compounds were toxic to cancer cells but not to normal fibroblasts under the same conditions.
On the other hand, Das and colleagues evaluated the cytotoxic potential of V2O5 nanoparticles in normal cell lines CHO, HEK-293, and NRK-49F and found that cell viability was not affected, suggesting the biocompatibility of these nanoparticles. In some cancer cell lines, such as the murine melanoma B16F10, antiproliferative effects were observed [72]. These findings support the potential of vanadium for therapeutic use without causing harm to non-malignant cells, a result observed in this and other in vitro studies. In the same melanoma cell line, Nivetha and colleagues demonstrated the antiproliferative activity of V2O5 nanoparticles and V2O5 nanoparticles doped with nickel (Ni@V2O5). In the same study, an in vivo model using BALB/c mice showed that intraperitoneal administration of both types of nanoparticles reduced the volume of melanomas generated by B16F10 cells. These findings were associated with vanadium’s interference in the Akt and PI3K signaling pathways [73].
With regard to the Akt pathway, decavanadates have also been tested against melanoma cells. De Sousa-Coelho and colleagues showed that decavanadate (V10) and the metformin–decavanadate complex (Metf-V10) induce dose-dependent antiproliferative activity, reduce cell viability, arrest the cell cycle in G2/M, and increase phosphorylation of signaling proteins ERK and AKT [70].
In 2021, Amante and colleagues conducted a systematic review on vanadium and melanoma. This review includes vanadium compounds and materials such as oxidovanadium(IV), vanadium carbides (MXenes), vanadium pentoxide, vanadyl sulfate, pyridinone-based oxidovanadium(IV) compounds, vanadate, vanadium(IV/V) polysaccharide complexes, binuclear ruthenium(II)-vanadium(IV) complexes, pyridoxal-based oxidovanadium(IV) complexes, and europium-doped yttrium vanadate nanoparticles. This diversity of vanadium-containing molecules was tested on various cancer cell lines and in vivo models (mice), evaluating effects on cell viability, morphology, apoptosis, cell cycle, ROS production, mitochondrial function, protein expression, tumor regression, and survival rate. In general, the tested compounds showed desirable effects against melanoma to varying degrees, both in vitro and in vivo [74].
3.6. Lung Cancer
Lung cancer is the most common type of cancer and the leading cause of cancer-related death worldwide. In 2022, nearly 2.5 million people were diagnosed with lung cancer, with over 1.8 million deaths reported [39].
Numerous in vitro studies using the A549 cell line, derived from pulmonary adenocarcinoma, have demonstrated the anticancer activity of several vanadium compounds. These studies evaluated the cytotoxic effects of V2O5 complexes [75], vanadium complexes with pyridoxal-derived ligands [76], as well as complexes synthesized from VOSO4 with hydrolyzed Cicer arietinum L. protein [77], all of which negatively affected A549 cell viability. In addition to reduced viability, other studies using the same cells and vanadocene complexes reported the induction of apoptosis, cell cycle arrest, and altered expression of proteins involved in regulating these processes [78]. Ribeiro and colleagues observed that oxidovanadium complexes synthesized from benzohydrazones and vanadyl acetylacetonate inhibited cell proliferation, increased cell death, and raised ROS production [79]. Oxidative stress was also elevated following treatment with accessible compounds such as VOSO4 and NaVO3, along with decreased viability and induced cell death [80]
Regarding vanadium nanoparticles tested in vitro against lung cancer cells, Liu and colleagues demonstrated that exposing several lung cancer cell lines (EBC-1, LK-2, LU65, LU99, STC 1, and RERF-LC-MA) to vanadium nanoparticles synthesized using Salvia officinalis extract (VNPs@Salvia officinalis) significantly reduced viability, an effect associated with their antioxidant properties [81].
As for in vivo studies, López-Valdez and colleagues [82] evaluated the effect of V2O5 inhalation in a model using male CD-1 mice with urethane-induced lung tumors. They observed reduced bronchiolar and alveolar epithelial hyperplasia, smaller and fewer tumors, and a higher apoptosis index in the tumors.
3.7. Nervous System Cancer
According to GLOBOCAN statistics, nervous system cancers rank twelfth in terms of global mortality; however, in children under 14, they are the second leading cause of cancer-related death [39]. Glioblastoma and neuroblastoma are among the most common tumors.
It has been shown that exposing SN56 and SH-SY5Y human neuroblastoma-derived cells to various vanadium compounds produces anticancer effects. On one hand, exposure of SN56 cells to NaVO3 decreased their viability [83], while exposure of SH-SY5Y cells to SOV [84] and VO(acac)2 [85] reduced viability, induced apoptotic cell death, and arrested the cell cycle in G2/M and S phases. The authors associated these effects with changes in the expression levels of proteins regulating these processes and increased ROS production. In 2025, Bates and colleagues evaluated the effects of vanadium complexes on T98G human glioblastoma cells, showing impacts on cell proliferation [86].
Other researchers studied the effects of VO(acac)2 both in vitro and in vivo. In SH-SY5Y neuroblastoma cells and human U251 and murine GL261 glioblastoma cells, Xu and colleagues reported cell cycle changes, energy deficiency, morphological alterations, and activation of methuosis—a form of non-apoptotic cell death. This process was confirmed in vivo in male C57BL/6J mice inoculated with GL261 glioblastoma cells. The authors also reported anti-angiogenic effects through suppression of the HIF-1α factor and tumor growth inhibition in treated mice [87].
3.8. Bone Cancer
Bone cancer is a proliferation of bone cells that appear in any of them, and includes osteosarcoma, Ewing’s sarcoma, and chondrosarcoma. Less than 1% are diagnosed each year and they are associated with significant morbidity and mortality. Osteosarcoma is a type of cancer that begins in the cells that form bones. It usually appears more frequently in children and older adults, and the highest incidence is in the long bones of the legs and also in the arms. It is the most common bone cancer, and because of this it has been the most studied [88].
Studies using vanadium compounds against bone cancer are relatively recent and have demonstrated their effectiveness especially in vitro. The total vanadium content in adults is about 100–200 μg, half of this amount is located in the bones, which are the main long-term storage site, and where vanadate can substitute phosphate in hydroxyapatite [Ca10(PO4)6(OH)2]. Because large amounts of V are detected in bones, the antitumor properties of many compounds of this metal have been widely studied in osteosarcoma [89].
In recent decades, 3D culture models (Multicellular tumor spheroids or 3D bioprinted) have been used to study anticancer effects in bone, both in vitro and in vivo. Thus, León and colleagues reported the effect of the [VO(chrysin)2 EtOH]2 (VOchrys) complex, used in other studies, in a mouse osteosarcoma xenograft model. In this study, the human osteosarcoma cell line MG-63 was used; the cells were seeded in 3D multicellular spheroids, and their shape and viability were measured. For the in vivo model, male and female mice (N:NIH(S)-Fox1) were subcutaneously inoculated with MG-63 cells. The results demonstrated antitumor actions of VOchrys in the 3D model, as the shape of the spheroids was altered and their viability decreased; in the in vivo model a significant reduction in tumor volume and suppression of tumor growth were observed, without any symptoms affecting the health or behavior of the animals [90].
The same group investigated the interaction of the oxidovanadium(IV)-clioquinol complex (VO(CQ)2) with Focal Adhesion Kinase (FAK), which is a tyrosine kinase that plays an important role in adhesion, survival, motility, angiogenesis, and metastasis of cancer cells; this to elucidate the relationship with the regulation of this kinase and the activity of metalloproteinases (MMP), and therefore, to know the antimetastatic effects in the 3D spheroid model of human osteosarcoma cells. For this, the MG-63 cell line was treated with VO(CQ)2, which was then seeded in 3D multicellular spheroids. Viability and migration of the treated spheroids were determined. The results showed that VO(CQ)2 reduced migration in the 3D model of human bone cancer cells, as well as the activity of MMP-2 and MMP-9 in a dose-dependent manner [91].
On the other hand, the effectiveness of vanadium nanoparticles has also been tested in vitro. Kalnina and colleagues reported that hydroxyapatite nanoparticles doped with V (V-doped HAPs), prepared by partial substitution of phosphate by vanadate, produced cytotoxic effects in human bone cancer cells SW1353. The authors suggest that this cytotoxicity would be related to the entry of nanoparticles into cancer cells, which requires further studies [92]. Another group explored the effect of nanostructured lipid carriers (NLCs) loaded with the Metvan complex and observed decreased cell viability in the MG-63 line, cytotoxic effects, and apoptosis [93]. These studies demonstrate that vanadium nanoparticles are a feasible option to continue exploring in vivo.
Finally, it is interesting to mention that the use of scaffolds containing vanadium has been explored to examine their anticancer effect, because patients with osteosarcoma present bone defects in the surgical excision zone, which is associated with a high tumor recurrence rate and the survival percentage is only 10%. In an in vivo study with a rat femur with bone defects caused by surgical resection of a tumor, the effect of a V-doped scaffold was explored to simultaneously inhibit osteosarcoma recurrence and promote regeneration of the surgical bone defects. The results showed that tumor cell growth was inhibited and at the same time bone growth in that area was accelerated promoting regeneration [94]. The previous studies demonstrate that there are different strategies using vanadium compounds that are capable of reducing the size of a bone tumor or repairing bone in surgical excision zones, so this research line should be exploited for the benefit of osteosarcoma patients.
Table 2 and Table 3 summarize the findings on the antineoplastic effects found in the most common and most widely studied types of cancer. However, other cell lines from less common neoplasias have been studied, which are discussed further on.

Table 2.
Biological effects and mechanisms of action exerted by vanadium compounds in vitro.

Table 3.
Biological effects and mechanisms of action exerted by vanadium compounds in vivo.
4. Anticancer Effects in Other Types of Cancer
The effects of V compounds as anticancer agents have been tested in vitro in other types of cancer that have not been previously addressed due to limited studies; these include stomach, pancreatic, cervical, and ovarian cancers, among others.
Regarding stomach cancer, vanadium compounds have been tested in different human gastric cancer cell lines. Ling and colleagues explored the effect of 19 vanadium complexes on four cancer cell lines: MGC-803 human gastric cancer, EC109 esophageal adenocarcinoma, MCF7 breast cancer, and HepG2 hepatic carcinoma. The authors reported the antiproliferative and pro-apoptotic activity of the complexes against these cells, with a preference for MGC-803; in these cells, the expression of Bax, PARP, and caspases 3/9 increased while Bcl2 decreased [95]. In another study, it was demonstrated that the oxidovanadium complex with the Schiff base phenylenediamine ([N,N’-bis (3-methoxy-salicylidene) -1,2-phenylenediamine] V(IV)) in the MKN95 cell line reduced proliferation and cell migration and increased cell death. When analyzing the effect on the cell cycle, the authors observed that the complex arrested the cycle in G2/M, which could be explained by the increase in the expression of p53 and GADD45 and the decrease in CDC25 [96]. On the other hand, Wang and colleagues analyzed the effects of exposure to SOV in vitro and in vivo. They demonstrated that exposure to this compound inhibits cell proliferation, increases the sensitivity to radiotherapy of SGC-7901 and MGC-803 human gastric cancer cells, and tumors induced in a xenograft model in female mice subcutaneously injected with SGC-7901 cells. Another effect observed in this model was that SOV inhibits radiation-induced autophagy, preventing cells from becoming resistant to radiotherapy [97].
Another type of cancer whose relationship with the anticancer effect of vanadium compounds has been little explored is pancreatic cancer. This type of cancer is one of the most difficult to treat, so new therapeutic agent alternatives, such as vanadium compounds, are continuously sought because current options have shown little effectiveness. Wu and colleagues investigated the effect of NaVO3 and VO(acac)2 on the human pancreatic cancer cell line AsPC-1, demonstrating that both compounds induce cell cycle arrest in G2/M by activation of the PI3K/AKT and MAPK/ERK pathways, increase in production and reduced glutathione (GSH) [98]. In another study, similar results were found in the PANC-1 pancreatic ductal adenocarcinoma cell line when independently exposed to 7 V compounds: C1-VOSO4, C2- oxydiacetate-VO complex, C3 and C4 with coupled organic ligands (phenanthroline and bipyridine), C5-C7 with organic ligands phenanthroline, bipyridine, and 4-amino-2-methylquinoline bound as counterions. The results demonstrated cytotoxicity, inhibition of autophagy, cell cycle arrest in G2/M associated with mitotic catastrophe induction, and elevation in ROS levels [99]. Other results from the same group showed the effect of three oxidovanadium(IV) complexes with 2-amino-3-hydroxypyridine against the PANC-1 and MIA PaCa2 cell lines; cytotoxicity, increased ROS production, disruption of mitochondrial membrane potential, and apoptosis were reported [100]. Finally, Griffin and colleagues reported the antiproliferative and cytotoxic activity (by apoptosis or necrosis) of the complex (NH4)[V(dtbc)3] on PANC-1 cells [101].
Regarding cervical cancer, anticancer effects have also been shown. This type of cancer has high incidence and mortality worldwide in women, so it is necessary to explore more therapeutic options to treat it. One study reported the effect of two vanadium complexes coupled with nicotyl hydrazone, OVK89 and OVK49, on human cervical adenocarcinoma cells SiHa and HeLa, which are also positive for Papilloma virus. The results showed that both compounds are capable of inducing cell death by apoptosis by altering the mitochondrial membrane potential and inducing p53, especially in SiHa cells. It is important to mention that this is the first study demonstrating that vanadium compounds can induce p53-dependent apoptosis in Papilloma virus-positive cancers [102]. In another work, on the same cells, it was shown that other vanadium complexes coupled to diketones exhibit cytotoxic activity by activating apoptosis. In silico data provided in this work show the ability of these complexes to bind to DNA and fragment it, behaving as powerful cytotoxics [103]. On the other hand, the bis(triethylammonium) tris [1,1-bis(indol-3-yl)-1-(3,4-catecholate) methane] vanadate (IV) complex has shown promising results; significant antiproliferative activity, a strong capacity to bind DNA, mitochondrial damage, increased ROS formation, and cell cycle arrest in G2/M in ACC-149 cells derived from HeLa [104].
In vivo, the effect of vanadium complexes in cervical cancer has been little studied. Bai and colleagues demonstrated that the oxidovanadium complex coupled to thiosemicarbazones VO-2-hydroxy-1-naphthaldehyde thiosemicarbazone (hntdtsc) 2-(4-nitrophenyl)-imidazo [4,5-f]1,10-phenanthroline (NPIP) exerted significant antiproliferative effect on HeLa cell cultures by arresting the cycle in G0/G1 phase with increased expression of p16 and decreased cyclin D1, CDK4, and p-Rb, increased ROS and apoptosis measured by mitochondrial damage. In vivo the results coincided with those found in vitro: the complex suppressed growth and inhibited tumor progression, which is related to the detected decrease in Ki67 nuclei staining; it also induced apoptosis and increased caspase 3 expression in a xenograft model in nude mice [105].
To conclude this section, ovarian cancer is addressed. Reytman and colleagues demonstrated the cytotoxic activity of six oxidovanadium complexes coupled with phenolate (LpVO) on various cancer cell lines, including A2780 and OVCAR-3 ovarian carcinoma [106]. On the other hand, it has been found that treatment with V2O5 triggers a potent antiproliferative effect in different cell lines including A2780 [107]. In both cases, more studies are required to elucidate their mechanisms of action. Below, the main mechanisms of action induced by vanadium compounds that have been identified are summarized.
5. Conclusions
Vanadium is a metal capable of regulating various biological functions, as evidenced in numerous studies, for which it has been attributed pharmacological properties such as insulin-mimetic, antihyperlipidemic, antihypertensive, and, of course, antineoplastic properties [22,36]. In recent decades, studies exploring the anticancer effect of vanadium have increased exponentially, since the coordination capacity of this metal has allowed the development of a diversity of compounds, which is important given that it opens the possibility of numerous treatment options against the cancer types with the highest incidence and mortality rates worldwide. Vanadium compounds have proven to be viable and useful options for the elimination of cancer cells in in vitro studies, while in vivo models have reaffirmed the possibility of using them as treatments, although these latter studies are scarce.
Experimental evidence has demonstrated that vanadium compounds have desirable effects to combat numerous types of cancer by activating diverse mechanisms of action through which they exert their antitumor action, as summarized in Figure 1 and Figure 2. These include the induction of oxidative stress, DNA damage, cell cycle arrest, induction of apoptosis, and regulation of the autophagy process, among the most important mechanisms.
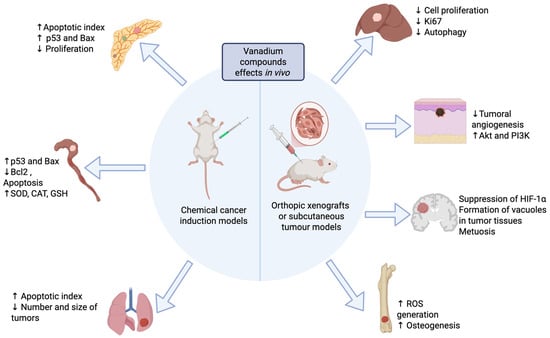
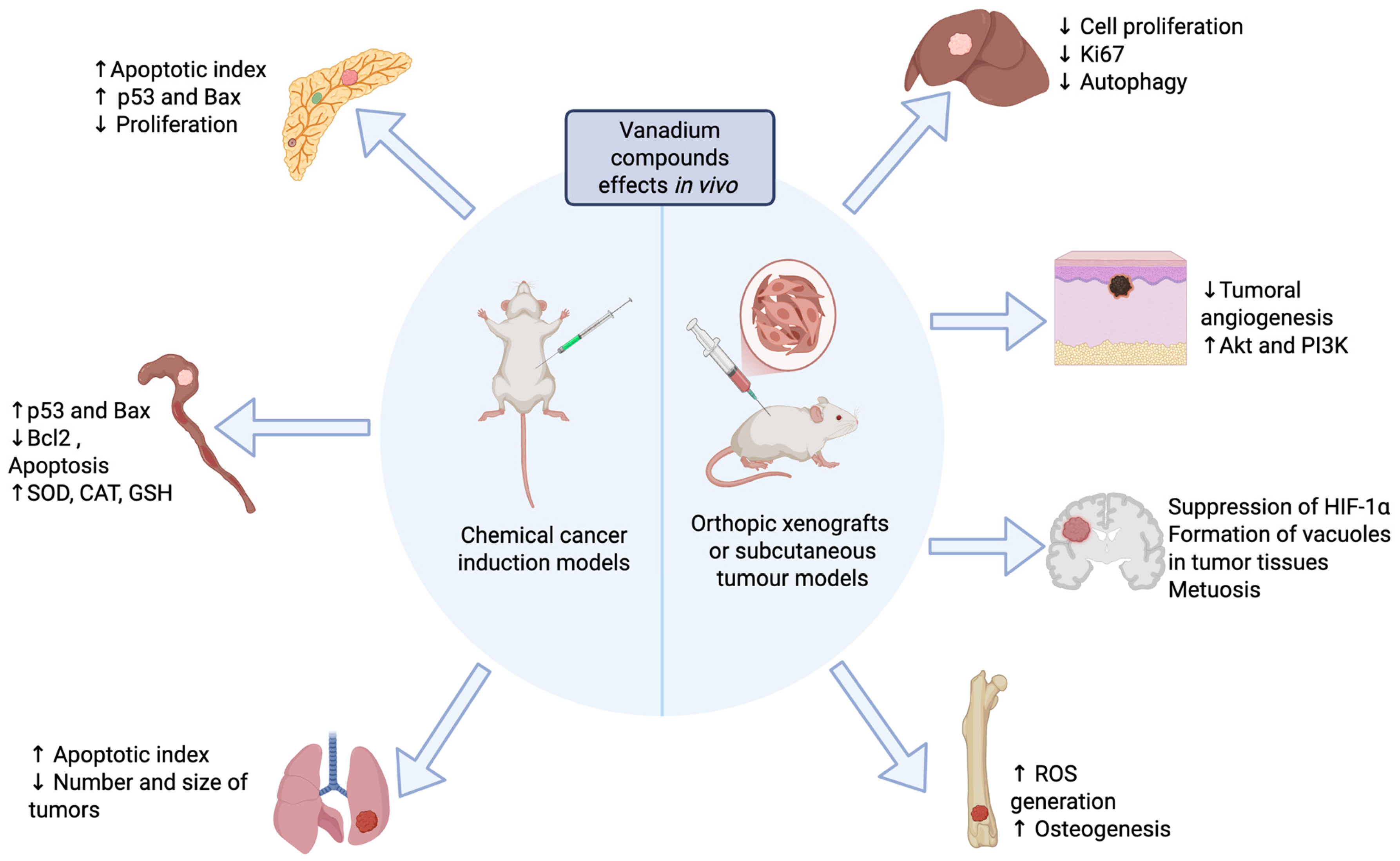
Figure 1.
Antineoplastic effects of vanadium compounds reported in animal models. Created in Biorender. López-Valdez N. (2025). https://app.biorender.com/illustrations/685eb2fa941b8c26dd7a70be?slideId=49f98399-980b-4c34-9b41-d91e37a32caf.
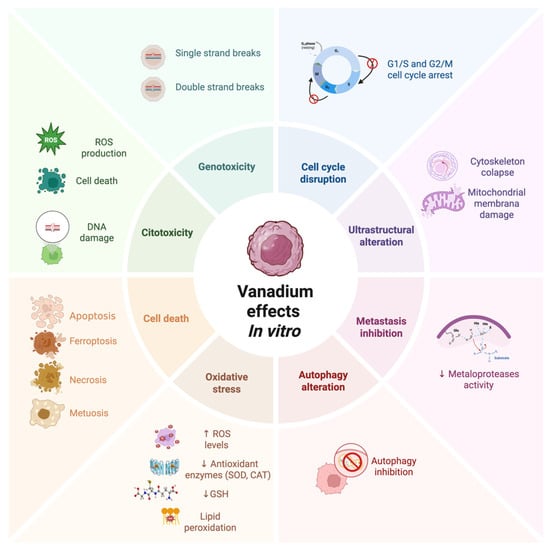
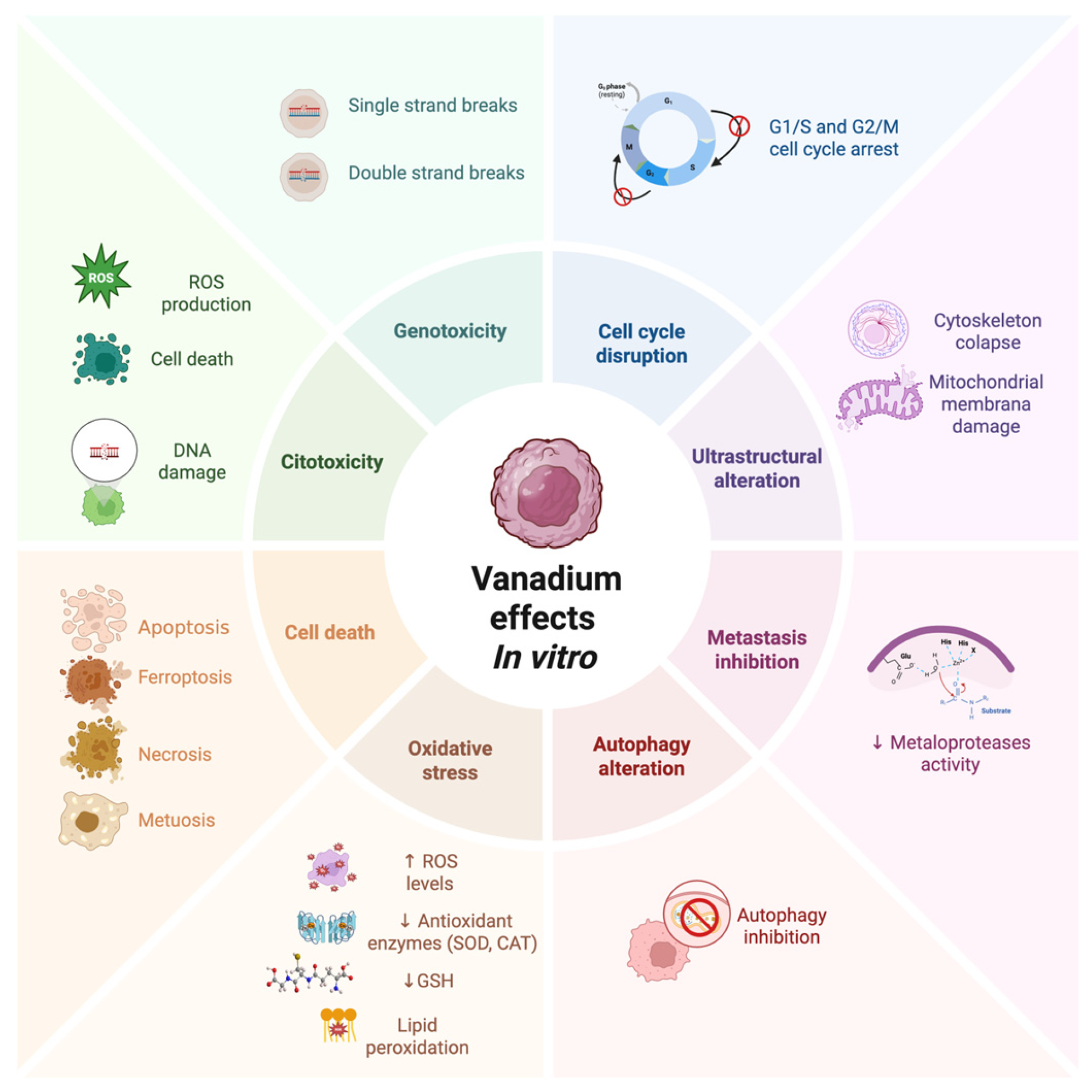
Figure 2.
Antineoplastic effects of vanadium compounds reported in vitro. Created in Biorender. López-Valdez N. (2025). https://app.biorender.com/illustrations/685eeacab1837564afd061d0?slideId=18feee72-70c3-412c-8468-6d4b5d57c220.
As outlined in Table 1, there are several advantages to utilizing alternative metal complexes to Pt as anticancer agents, some of which are analogous to those exhibited by V compounds, including their respective mechanisms of action. In comparison to platinum, they are less toxic and tend to be more selective in their action, targeting cancer cells with greater precision. In comparison to V, it offers the advantage that its mechanisms of action have been the subject of more extensive study, and a limited number of studies have examined the in vivo applicability of these metals’ compounds.
An important point to consider when analyzing the results of different studies to determine the action mechanisms is that compounds with vanadium ions are quite labile, and interconversion between VIV and VV occurs easily. The V compounds, once dissolved in water containing solvents and at low concentrations (as those typically used in therapeutic applications) almost never maintain their integrity. In vitro, it is well known that the reactions of V complexes with components of the culture medium influence the observed biological activity; however, only a few studies have documented the speciation of V complexes once incorporated into the incubation medium [108].
In an interesting study conducted by Nunes and colleagues, it was demonstrated that the three VIVO complexes used as cytotoxic agents—formulated as [VIVO(OSO3)(phen)2] (1) (phen = 1,10-phenanthroline), [VIVO(OSO3)(Me2phen)2] (2) (Me2phen = 4,7-dimethyl-1,10-phenanthroline), and [VIVO(OSO3)(amphen)2] (3) (amphen = 5-amino-1,10-phenanthroline)—underwent hydrolysis, and most of the VIV was oxidized to different VV species. The authors further indicated that when lower concentrations of the complexes were added to the culture medium, hydrolysis occurred, and the VIV species and those containing phenanthroline (phen) remain bound primarily to serum albumin in the medium, whereas at higher concentrations, the complexes persisted for longer periods. Therefore, the mechanisms of action exerted are closely related to the concentration of the complexes used [109].
In another review, Pessoa and Correia provide an in-depth discussion of the chemical changes (hydrolysis, ligand exchange, and redox reactions) undergone by V complexes, emphasizing the importance of evaluating speciation to better understand their mechanisms of action and to attribute them to the correct species [110]. A particularly relevant point highlighted in this work is the significance of these chemical changes in the compounds when used at low concentrations, such as those employed in biological assays, making their consideration essential.
On the other hand, in vivo studies are expected to show behavior similar to what has been reported in vitro; however, this outcome is not guaranteed. A specific compound will undergo various chemical transformations both before administration and afterward, until it reaches its target organ. It is therefore of vital importance to understand these transformations before proposing a compound as a treatment and advancing to preclinical phases [111,112]. Dinda and colleagues provide a comprehensive description of these changes in V complexes used in different studies, detailing the compounds formed in solution as well as the transformations that would occur under experimental conditions, aiming to guide the reader toward determining the speciation of their compounds and thereby more accurately proposing those whose biological activity could be potentially beneficial [112].
One approach currently under consideration to address changes in the speciation of metal complexes is the use of nanoparticles or encapsulation methods. An example is provided by the studies conducted by Sakurai and colleagues, who reported that ECC encapsulation of VIVOSO4 for oral administration to experimental rats increased the bioavailability and absorption of the complex. Their findings indicated that these capsules passed through the digestive tract intact, reaching the small intestine, where they released VIVOSO4 directly into this organ [113,114,115].
It is important to mention that most of the studies reported in the literature on the anticancer potential of V complexes do not include an analysis regarding these aspects of speciation, which represents an important challenge that should be considered in future research.
Figure 1 and Figure 2 provide a general summary of the mechanisms of action exerted by V complexes, both in animal models and in vitro studies. ROS production and the resulting oxidative stress are thought to be one of the main ways in which the metal acts. ROS can function as intracellular messengers that have the ability to participate in different signaling pathways, such as proliferation, differentiation, apoptosis, etc., promoting the elimination of cancer cells. It is interesting to note that both figures show the activation of apoptosis, as well as alterations in the mechanisms of autophagy and proliferation, which ultimately lead to the death of transformed cells [26,28,32,34,35].
As highlighted above, especially in the context of in vitro studies, several of the compounds used exert their cytotoxic actions particularly on cancer cells and not on normal cells; in in vivo models, the compounds did not show toxic effects in the experimental organisms. These aspects stand as advantages of vanadium compounds as anticancer agents due to their preference for malignant cells, which could reduce adverse effects generated by chemotherapeutic treatment, and represent an excellent alternative to platinum compounds, which remain the most widely used.
It is important to highlight that most vanadium compounds with anticancer potential have been tested, especially in vitro; their study in animal models still needs to be expanded, and, if the benefit is considered greater than toxicity, clinical trials could allow their use as treatments in the future. For the moment, the results appear promising.
Finally, another benefit is that it has also been seen that vanadium compounds exert cytotoxic mechanisms in cells that have been identified as resistant to platinum treatment, so in the near future, it is necessary to explore the effects of vanadium compounds, especially in vivo, to have a broader perspective of what could occur in the whole organism.
Author Contributions
N.L.-V. and T.I.F. were responsible for the conceptualization of this review, as well as for the writing and drafting of the manuscript. A.G.-V., M.R.-L., P.B.-N., B.C.-T., M.E.C.-V., M.U.-C., G.G.-P., G.M.-R., and J.Á.S.-H., contributed to the literature search and data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by grants of Proyectos de Investigación e Innovación Tecnológica PAPIIT IN201324 and the APC was funded by División de Investigación, Facultad de Medicina, Universidad Nacional Autónoma de México.
Acknowledgments
The authors are grateful to Armando Zepeda-Rodríguez and Brenda Medina Rodríguez for their technical support. The authors also thank Alejandra Núñez Fortoul for editing the final English version of this manuscript. During the preparation of this manuscript the authors used BioRender Scientific Illustration app to create publication-quality Graphical abstract, Figure 1 and Figure 2, with pre-made icons for the purposes of illustrating the review. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the conceptualization, literature search or data analysis included in this review.
Abbreviations
| NH4VO3 | Ammonium metavanadate |
| NaVO3 | Sodium metavanadate |
| KVO3 | Potassium metavanadate |
| VO4SO4 | Vanadyl sulfate |
| Na6V10O28 | Sodium decavanadate |
| V2O5 | Vanadium pentoxide |
| VOCl2 | Vanadyl dichloride |
| VCl3 | Vanadium(III) chloride |
| (C5H5)2VCl2 | Vanadocene dichloride |
| VO1a | Vanadium oxide compound 1a |
| VO1b | Vanadium oxide compounds 1b |
| VOL | IV- oxidovanadium imidazole complex |
| OVMI | 1-methylimidazole oxidovanadium complex |
| VnNp | Vanadium nanoparticles |
| SOV | Sodium orthovanadate |
| VO(ma)2 | Bis(maltolate)- oxidovanadium(IV) |
| VO(acac)2 | Bis(acetylacetonate)- oxidovanadium(IV) |
| ([IV(L)] complex) | 4-bromo-2-(((5-chloro-2-hydroxyphenyl) imino) methyl) phenol ([IV(L)] complex |
| VIO nanoparticles | Vanadium-doped iron oxide (VIO) nanoparticles |
| VO(NG)2 | Vanadyl N-(2-hydroxyacetophenone) glycinate |
| Metvan | 4,7-dimethyl-1,10-phenathroline 7 sulphate oxidovanadium(IV) |
| VOsil Na2[VO(silibinin)2]·6H2O | Oxidovanadium complex coupled with silibinin |
| VOchrys [VO(chrysin)2EtOH]2 | Oxidovanadium complex coupled with chrysin |
| VOlut [VO(lut)(H2O)2]Na3·H2O | Oxidovanadium complex coupled with luteolin |
| VCrZnO4 NPs | Zinc-chromium-vanadate nanoparticles |
| PTANAP- VO | ((E)-N′-((2-hydroxy naphthalene -1-yl)methylene)-4-methylbenzohydrazide) PTANAP oxidovanadium complex |
| [VO(CUAP)SO4] | Vanadyl complex with a tridentate coumarin ligand |
| Nano Cu-VO (IV) | Vanadyl complex with a tridentate coumarin ligand functionalized with copper nanoparticles |
| VS2 | 1,2-dimethyl-3-hydroxy-4 (1H)-pyridinonate |
| VS3 | 1-methyl-3-hydroxy-4 (1H)-pyridinonate |
| VS4 | 1-phenyl-2-methyl-3-hydroxy-4 (1H)-pyridinonate |
| V2O5 NP | V2O5 nanoparticles |
| Ni@V2O5 NP | V2O5 nanoparticles doped with nickel |
| VNPs@Salvia officinalis | Vanadium nanoparticles synthesized using Salvia officinalis extract |
| VO(CQ)2 | Oxidovanadium(IV)-clioquinol complex |
| V-doped HAPs | Hydroxyapatite nanoparticles doped with V |
| NPIP | VO-2-hydroxy-1-naphthaldehyde thiosemicarbazone (hntdtsc) 2-(4-nitrophenyl)-,10-phenanthroline |
| LpVO | Oxidovanadium complexes coupled with phenolate |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-drugs in cancer therapy: Past, present and future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Sastry, J.; Kellie, S.J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr. Hematol. Oncol. 2005, 22, 441–445. [Google Scholar] [CrossRef]
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in cancer nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Freisinger, E.; Sigel, R.K.O. (Eds.) Preface to Volume 18. In Metallo-Drugs: Development and Action of Anticancer Agents; Metal Ions in Life Sciences; Walter de Gruyter GmbH: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A review on metal complexes and its anti-cancer activities: Recent updates from in vivo studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Leon, I.E.; Cadavid-Vargas, J.F.; Di Virgilio, A.L.; Etcheverry, S.B. Vanadium, ruthenium and copper compounds: A new class of nonplatinum metallodrugs with anticancer activity. Curr. Med. Chem. 2017, 24, 112–148. [Google Scholar] [CrossRef]
- Yeo, C.I.; Ooi, K.K.; Tiekink, E.R. Gold-based medicine: A paradigm shifts in anti-cancer therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An approach against invasion and metastasis in cancer treatment. FEBS Open Bio 2022, 12, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent development of gold (I) and gold (III) complexes as therapeutic agents for cancer diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef]
- Sharma, A.; Sudhindra, P.; Roy, N.; Paira, P. Advances in novel iridium (III) based complexes for anticancer applications: A review. Inorg. Chim. Acta 2020, 513, 119925. [Google Scholar] [CrossRef]
- Bouché, M.; Hognon, C.; Grandemange, S.; Monari, A.; Gros, P.C. Recent advances in iron-complexes as drug candidates for cancer therapy: Reactivity, mechanism of action and metabolites. Dalton Trans. 2020, 49, 11451–11466. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Marker, S.C.; Knopf, K.M.; Wilson, J.J. Anticancer activity of complexes of the third row transition metals, rhenium, osmium, and iridium. Dalton Trans. 2018, 47, 9934–9974. [Google Scholar] [CrossRef]
- Li, X.; Gorle, A.K.; Sundaraneedi, M.K.; Keene, F.R.; Collins, J.G. Kinetically inert polypyridylruthenium(II) complexes as therapeutic agents. Coord. Chem. Rev. 2018, 375, 134–147. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H. Future potential of osmium complexes as anticancer drug candidates, photosensitizers and organelle-targeted probes. Dalton Trans. 2018, 47, 14841–14854. [Google Scholar] [CrossRef]
- Dasgupta, S.; Karim, S.; Banerjee, S.; Saha, M.; Saha, K.D.; Das, D. Designing of novel zinc (II) Schiff base complexes having acyl hydrazone linkage: Study of phosphatase and anti-cancer activities. Dalton Trans. 2020, 49, 1232–1240. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Pathak, P.; Grishina, M.; Yadav, J.P.; Verma, A.; Kumar, P. Metal Complexes in cancer treatment: Journey so far. Chem. Biod. 2023, 20, e202300061. [Google Scholar] [CrossRef]
- Ferretti, V.A.; León, I.E. An overview of vanadium and cell signaling in potential cancer treatments. Inorganics 2022, 10, 47. [Google Scholar] [CrossRef]
- Rehder, D. Perspectives for vanadium in health issues. Future Med. Chem. 2016, 8, 325–338. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Emwas, A.H. Therapeutic properties of vanadium complexes. Inorganics 2022, 10, 244. [Google Scholar] [CrossRef]
- Satya, H.K.; Gupta, S.; Siddique, A.; Joshi, S. Vanadium complexes as potential anticancer agents. Eng. Proc. 2023, 56, 91. [Google Scholar] [CrossRef]
- Singh, A.P.; Roy, S.; Maurya, I.C. Vanadium complexes: Potential candidates for therapeutic applications. Transit. Met. Chem. 2024, 49, 101–119. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Fraqueza, G.; Aureliano, M. Repurposing Therapeutic Drugs Complexed to Vanadium in Cancer. Pharmaceuticals 2023, 17, 12. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301, 87–105. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in biological action: Chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumari, S.; Karan, R.; Kumar, A.; Rawal, R.K.; Gupta, P.K. Anticancer perspectives of vanadium complexes. Inorg. Chem. Commun. 2024, 161, 112014. [Google Scholar] [CrossRef]
- Barceloux, D.G. Vanadium. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Wiszniewska, A.; Kamińska, I. A dual role of vanadium in environmental systems—Beneficial and detrimental effects on terrestrial plants and humans. Plants 2021, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing vanadium as an antidiabetic or anticancer drug: A clinical and historical perspective. Met. Ions Life Sci. 2019, 19, 203–230. [Google Scholar]
- Kostenkova, K.; Klugh, K.; Crans, D.C. Recent Advances of Medicinal Properties of Vanadium Compounds: Cancer and Other Diseases in Metal-Containing Molecules and Nanomaterials: From Diagnosis to Therapy, 1st ed.; CRC Press: Oxford, UK, 2024; pp. 19–50. [Google Scholar]
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Health benefits of vanadium and its potential as an anticancer agent. Met. Ions Life Sci. 2018, 18, 251–279. [Google Scholar] [CrossRef]
- Kieler, J.; Gromek, A.; Nissen, N.I. Studies on the antineoplastic effect of vanadium salts. Acta Chir. Scand. Suppl. 1965, 343, 154–164. [Google Scholar] [PubMed]
- Bishayee, A.; Waghray, A.; Patel, M.A.; Chatterjee, M. Vanadium in the detection, prevention and treatment of cancer: The in vivo evidence. Cancer Lett. 2010, 294, 1–12. [Google Scholar] [CrossRef]
- Global Cancer Observator; IARC. Cancer TODAY. Globocan. 2025. Available online: https://gco.iarc.fr/today/en/dataviz/bars?mode=cancer&group_populations=1&multiple_populations=1 (accessed on 26 May 2025).
- Roy, S.; Banerjee, S.; Chakraborty, T. Vanadium quercetin complex attenuates mammary cancer by regulating the P53, Akt/mTOR pathway and downregulates cellular proliferation correlated with increased apoptotic events. Biometals 2018, 31, 647–671. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, H.; Wang, G.; Li, L.; Zhou, D. Anticancer effect of sodium metavanadate on murine breast cancer both in vitro and in vivo. Biometals 2021, 34, 557–571. [Google Scholar] [CrossRef]
- Kalındemirtaş, F.D.; Kaya, B.; Sert, E.; Şahin, O.; Kuruca, S.E.; Ülküseven, B. New oxovanadium (IV) complexes overcame drug resistance and increased in vitro cytotoxicity by an apoptotic pathway in breast cancer cells. Chem. Biol. Interact. 2022, 363, 109997. [Google Scholar] [CrossRef]
- Ghosh, N.; Chatterjee, S.; Biswal, D.; Pramanik, N.R.; Chakrabarti, S.; Sil, P.C. Oxidative stress imposed in vivo anticancer therapeutic efficacy of novel imidazole-based oxidovanadium(IV) complex in solid tumor. Life Sci. 2022, 301, 120606. [Google Scholar] [CrossRef]
- Ghosh, N.; De, S.; Pramanik, N.R.; Sil, P.C. Multifaceted antineoplastic curative potency of novel water-soluble methylimidazole-based oxidovanadium(IV) complex against triple negative mammary carcinoma. Cell Signal. 2024, 117, 111089. [Google Scholar] [CrossRef] [PubMed]
- Chmur, K.; Tesmar, A.; Zdrowowicz, M.; Rosiak, D.; Chojnacki, J.; Wyrzykowski, D. Exploring the antitumor efficacy of N-heterocyclic nitrilotriacetate oxidovanadium(IV) salts on prostate and breast cancer cells. Molecules 2024, 29, 2924. [Google Scholar] [CrossRef] [PubMed]
- Suma, P.R.; Padmanabhan, R.A.; Telukutla, S.R.; Ravindran, R.; Velikkakath, A.K.G.; Dekiwadia, C.D.; Paul, W.; Laloraya, M.; Srinivasula, S.M.; Bhosale, S.V.; et al. Vanadium pentoxide nanoparticle mediated perturbations in cellular redox balance and the paradigm of autophagy to apoptosis. Free Radic. Biol. Med. 2020, 161, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Meltzer, S.J. The Role of DNA Methylation in Gastrointestinal Disease: An Expanded Review of Malignant and Nonmalignant Gastrointestinal Diseases. Gastroenterology 2024, 168, 245–266. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Xu, Z.; Wang, D.; Zhao, B.; Pan, H.; Wang, J.; Xu, D.; Zhao, X.; Pan, S.; et al. Sodium orthovanadate inhibits growth of human hepatocellular carcinoma cells in vitro and in an orthotopic model in vivo. Cancer Lett. 2014, 351, 108–116. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, T.T.; Fu, Y.; Wang, K.; Yang, X.G. Vanadium compounds discriminate hepatoma and normal hepatic cells by differential regulation of reactive oxygen species. J. Biol. Inorg. Chem. 2010, 15, 1087–1097. [Google Scholar] [CrossRef]
- Aliabad, H.B.; Falahati-pour, S.K.; Ahmadirad, H.; Mohamadi, M.; Hajizadeh, M.R.; Mahmoodi, M. Vanadium complex: An appropriate candidate for killing hepatocellular carcinoma cancerous cells. BioMetals 2018, 31, 981–990. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, J.; Jiang, L.; Ran, L.; Fan, Y.; Zhang, M.; Xu, Y.; Yao, C.; An, B.; Yang, Y.; et al. A Multifunctional Vanadium-Iron-Oxide Nanoparticle Eradicates Hepatocellular Carcinoma via Targeting Tumor and Endothelial Cells. ACS Appl. Mater. Interfaces 2022, 14, 28514–28526. [Google Scholar] [CrossRef]
- Sarwar, Z.; Abbas, M.K.; Shad, N.A.; Akhtar, K.; Mobeen, A.; Abbas, W.; Abd El-Aziz, K.; Tabassum, M.R.; Zulqarnain, M.; Ali, H.T.; et al. Anticancer and acute toxicity studies of cellulose-coated Vanadium oxide nanomaterials. J. Mol. Struct. 2025, 1322, 140633. [Google Scholar] [CrossRef]
- Sinha, A.; Banerjee, K.; Banerjee, A.; Sarkar, A.; Ahir, M.; Adhikary, A.; Chatterjee, M.; Choudhuri, S.K. Induction of apoptosis in human colorectal cancer cell line, HCT-116 by a vanadium-Schiff base complex. Biomed. Pharmacother. 2017, 92, 509–518. [Google Scholar] [CrossRef]
- León, I.E.; Ruiz, M.C.; Franca, C.A.; Parajón-Costa, B.S.; Baran, E.J. Metvan, bis (4,7-Dimethyl-1,10-phenanthroline) sulfatooxidovanadium(IV): DFT and spectroscopic study—Antitumor action on human bone and colorectal cancer cell lines. Biol. Trace Elem. Res. 2019, 191, 81–87. [Google Scholar] [CrossRef]
- Leon, I.E.; Cadavid-Vargas, J.F.; Tiscornia, I.; Porro, V.; Castelli, S.; Katkar, P.; Desideri, A.; Bollati-Fogolin, M.; Etcheverry, S.B. Oxidovanadium(IV) complexes with chrysin and silibinin: Anticancer activity and mechanisms of action in a human colon adenocarcinoma model. J. Biol. Inorg. Chem. 2015, 20, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Naso, L.G.; Badiola, I.; Clavijo, J.M.; Valcarcel, M.; Salado, C.; Ferrer, E.G.; Williams, P.A. Inhibition of the metastatic progression of breast and colorectal cancer in vitro and in vivo in murine model by the oxidovanadium(IV) complex with luteolin. Bioorg. Med. Chem. 2016, 24, 6004–6011. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chakraborty, T. Deciphering the molecular mechanism and apoptosis underlying the in vitro and in vivo chemotherapeutic efficacy of vanadium luteolin complex in colon cancer. Cell Biochem. Funct. 2018, 36, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, R. Salvia officinalis-based green-mediated vanadium nanoparticles: Describing a modern chemotherapeutic drug for the treatment of colorectal carcinoma. Authorea Prepr. 2022. [Google Scholar] [CrossRef]
- Rehman, S.; Alahmari, F.; Aldossary, L.; Alhout, M.; Aljameel, S.S.; Ali, S.M.; Sabir, J.S.M.; Khan, F.A.; Rather, I.A. Nano-sized warriors: Zinc chromium vanadate nanoparticles as a dual solution for eradicating waterborne enterobacteriaceae and fighting cancer. Front. Pharmacol. 2023, 14, 1213824. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, Y.; Zou, D.; Li, Q.; Shi, X.; Qin, Q.; Liu, M.; Wang, L.; Wang, Z. Targeting Metabolic Adaptation of Colorectal Cancer with Vanadium-Doped Nanosystem to Enhance Chemotherapy and Immunotherapy. Adv. Sci. 2025, 12, 2409329. [Google Scholar] [CrossRef]
- Sochacka-Ćwikła, A.; Mączyński, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies—The Last Decade Review. Cancers 2022, 14, 87. [Google Scholar] [CrossRef]
- Milaeva, E.R. Application of Metal Compounds in Medicine. Russ. J. Coord. Chem. 2024, 50, 1043–1123. [Google Scholar] [CrossRef]
- Šebestová, L.; Havelek, R.; Řezáčová, M.; Honzíček, J.; Kročová, Z.; Vinklárek, J. Study of antitumor effect of selected vanadium and molybdenum organometallic complexes in human leukemic T-cells. Chem. Biol. Interact. 2015, 242, 61–70. [Google Scholar] [CrossRef]
- Ta, S.; Ghosh, M.; Ghosh, K.; Brandão, P.; Félix, V.; Hira, S.K.; Manna, P.P.; Das, D. Exploring Anticancer and (Bio)catalytic Activities of New Oxovanadium(V), Dioxomolybdenum(VI), and Copper(II) Complexes of Amide-Imine Conjugates. ACS Appl. Bio Mater. 2019, 2, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, N.; Guan, G.; Song, T.; Xu, Y.; Wang, S.; Zhou, J. Sodium orthovanadate inhibits growth of acute leukemia HL60 cells and HL60/A cells in vitro. Biosci. Rep. 2020, 40, BSR20201918. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, S.; Khaleghian, A.; Kalalinia, F. Effects of co-administration of arsenic trioxide and Schiff base oxovanadium complex on the induction of apoptosis in acute promyelocytic leukemia cells. Biometals 2021, 34, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, N.; Isac Sobana, R.C.; Sindhu, K.B. Development of nanofunctionalized oxovanadium(IV) complex and its anticancer, antidiabetic, DNA cleavage and cell imaging studies. Int. J. Pharm. 2023, 644, 123339. [Google Scholar] [CrossRef]
- Roky, A.H.; Islam, M.M.; Ahasan, A.M.F.; Mostaq, M.S.; Mahmud, M.Z.; Amin, M.N.; Mahmud, M.A. Overview of skin cancer types and prevalence rates across continents. Cancer Pathog. Ther. 2025, 3, 89–100. [Google Scholar] [CrossRef]
- Rozzo, C.; Sanna, D.; Garribba, E.; Serra, M.; Cantara, A.; Palmieri, G.; Pisano, M. Antitumoral effect of vanadium compounds in malignant melanoma cell lines. J. Inorg. Biochem. 2017, 174, 14–24. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrão, G.; Gonçalves, J.; Sánchez-Lombardo, I.; Link, W.; Ferreira, B. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915. [Google Scholar] [CrossRef]
- Pisano, M.; Arru, C.; Serra, M.; Galleri, G.; Sanna, D.; Garribba, E.; Palmieri, G.; Rozzo, C. Antiproliferative activity of vanadium compounds: Effects on the major malignant melanoma molecular pathways. Metallomics 2019, 11, 1687–1699. [Google Scholar] [CrossRef]
- Das, S.; Roy, A.; Barui, A.K.; Alabbasi, M.M.A.; Kuncha, M.; Sistla, R.; Patra, C.R. Anti-angiogenic vanadium pentoxide nanoparticles for the treatment of melanoma and their in vivo toxicity study. Nanoscale 2020, 12, 7604–7621. [Google Scholar] [CrossRef]
- Nivetha, S.; Srivalli, T.; Sathya, P.M.; Mohan, H.; Karthi, N.; Muralidharan, K.; Ramalingam, V. Nickel-doped vanadium pentoxide (Ni@ V2O5) nanocomposite induces apoptosis targeting PI3K/AKT/mTOR signaling pathway in skin cancer: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2024, 234, 113763. [Google Scholar] [CrossRef]
- Amante, C.; De Sousa-Coelho, A.L.; Aureliano, M. Vanadium and melanoma: A systematic review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- Biswas, B.K.; Biswas, N.; Saha, S.; Rahaman, A.; Mandal, D.P.; Bhattacharjee, S.; Sepay, N.; Zangrando, E.; Garribba, E.; Roy-Choudhury, C. Interaction with bioligands and in vitro cytotoxicity of a new dinuclear dioxido vanadium(V) complex. J. Inorg. Biochem. 2022, 237, 111980. [Google Scholar] [CrossRef]
- Mato-López, L.; Sar-Rañó, A.; Fernández, M.R.; Díaz-Prado, M.L.; Gil, A.; Sánchez-González, Á.; Fernández-Bertólez, N.; Méndez, J.; Valdiglesias, V.; Avecilla, F. Relationship between structure and cytotoxicity of vanadium and molybdenum complexes with pyridoxal derived ligands. J. Inorg. Biochem. 2022, 235, 111937. [Google Scholar] [CrossRef]
- Mukhamedov, N.; Wubulikasimu, A.; Rustamova, N.; Nuerxiati, R.; Mirzaakhmedov, S.; Ishimov, U.; Ziyavitdinov, J.; Yili, A.; Aisa, H.A. Synthesis and Characterization of Novel Chickpea Protein Hydrolysate-Vanadium Complexes Having Cell Inhibitory Effects on Lung Cancer A549 Cells Lines. Protein J. 2021, 40, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Melounková, L.; Machálková, A.; Havelek, R.; Honzíček, J.; Řezáčová, M.; Císařová, I.; Peterová, E.; Vinklárek, J. Vanadocene complexes bearing N,N’-chelating ligands: Synthesis, structures and in vitro cytotoxic studies on the A549 lung adenocarcinoma cell line. J. Inorg. Biochem. 2019, 195, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Bulut, I.; Pósa, V.; Sergi, B.; Sciortino, G.; Pessoa, J.C.; Maia, L.B.; Ugone, V.; Garribba, E.; Enyedy, É.A.; et al. Solution chemical properties and anticancer potential of 8-hydroxyquinoline hydrazones and their oxidovanadium(IV) complexes. J. Inorg. Biochem. 2022, 235, 111932. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Palomo, G.; Rendón-Huerta, E.P.; Montaño, L.F.; Fortoul, T.I. Vanadium compounds and cellular death mechanisms in the A549 cell line: The relevance of the compound valence. J. Appl. Toxicol. 2019, 39, 540–552. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Du, X.; Liu, Y.; Zhang, Z. Formulation of a novel anti-lung cancer drug: Vanadium nanoparticles containing Salvia officinalis. Inorg. Chem. Commun. 2023, 150, 110520. [Google Scholar] [CrossRef]
- Lopez-Valdez, N.; Rojas-Lemus, M.; Fortoul, T.I. The Effect of Vanadium Inhalation on the Tumor Progression of Urethane-Induced Lung Adenomas in a Mice Model. Inorganics 2021, 9, 78. [Google Scholar] [CrossRef]
- Suwalsky, M.; Fierro, P.; Villena, F.; Gallardo, M.J.; Jemiola-Rzeminska, M.; Strzalka, K.; Gul-Hinc, S.; Ronowska, A.; Zysk, M.; Szutowicz, A. Effects of sodium metavanadate on in vitro neuroblastoma and red blood cells. Arch. Biochem. Biophys. 2013, 535, 248–256. [Google Scholar] [CrossRef]
- Tian, X.; Fan, J.; Hou, W.; Bai, S.; Ao, Q.; Tong, H. Sodium orthovanadate induces the apoptosis of SH-SY5Y cells by inhibiting PIWIL2. Mol. Med. Rep. 2016, 13, 874–880. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Wang, L.; Zeng, K.; Wang, K.; Yang, X. Vanadyl complexes discriminate between neuroblastoma cells and primary neurons by inducing cell-specific apoptotic pathways. J. Inorg. Biochem. 2018, 188, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.C.; Klugh, K.L.; Galaeva, A.O.; Patch, R.A.; Manganaro, J.F.; Markham, S.A.; Scurek, E.; Levina, A.; Lay, P.A.; Crans, D.C. Optimizing Therapeutics for Intratumoral Cancer Treatments: Antiproliferative Vanadium Complexes in Glioblastoma. Int. J. Mol. Sci. 2025, 26, 994. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, H.; Li, X.; Zhao, J.; Wang, J.; Crans, D.C.; Yang, X. Approaches to selective and potent inhibition of glioblastoma by vanadyl complexes: Inducing mitotic catastrophe and methuosis. J. Inorg. Biochem. 2024, 257, 112610. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.L.; Turner, S.P. Bone Cancer: Diagnosis and Treatment Principles. Am. Fam. Physician AFP 2018, 98, 205–213. [Google Scholar]
- Ścibior, A.; Pietrzyk, L.; Plewac, Z.; Skibad, P. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- León, I.; Cadavid-Vargas, J.F.; Resasco, A.; Maschi, F.; Ayala, M.A.; Carbone, C.; Etcheverry, S.B. In vitro and in vivo antitumor effects of the VO-chrysin complex on a new three-dimensional osteosarcoma spheroids model and a xenograft tumor in mice. J. Biol. Inorg. Chem. 2016, 21, 1009–1020. [Google Scholar] [CrossRef]
- Balsa, L.M.; Quispe, P.; Baran, E.J.; Lavecchia, M.J.; LeoÅLn, I.E. In silico and in vitro analysis of FAK/MMP signaling axis inhibition by VO-clioquinol in 2D and 3D human osteosarcoma cancer cells. Metallomics 2020, 12, 1931–1940. [Google Scholar] [CrossRef]
- Kalniņa, D.; Levina, A.; Pei, A.; Gross, K.A.; Lay, P.A. Synthesis, characterization and in vitro anti-cancer activity of vanadium-doped nanocrystalline hydroxyapatite. New J. Chem. NJC 2019, 43, 17891–17901. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Ruiz, M.C.; Scioli-Montoto, S.; Ruiz, M.E.; Fernandez, M.A.; Torres-Sanchez, R.M.; Baran, E.J.; Castro, G.R.; Leon, I.E. Lipid nanoparticles–Metvan: Revealing a novel way to deliver a vanadium compound to bone cancer cells. New J. Chem. NJC 2019, 43, 17726–17734. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xu, M.; Zhao, Z.; Yin, X.; Pu, X.; Wang, J.; Liao, X.; Huang, Z.; Cao, S.; et al. Mixed-valence vanadium-doped mesoporous bioactive glass for treatment of tumor-associated bone defects. J. Mater. Chem. B 2025, 13, 3138–3160. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.P.; Suo, F.Z.; Feng, Y.L.; Song, L.L.; Li, Y.; Li, Y.J.; Wang, K.T. Synthesis and biological evaluation of vanadium complexes as novel anti-tumor agents. Eur. J. Med. Chem. 2019, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, S.; Dejamfekr, M.; Moshtaghian, A.; Salehi, M.; Behzad, M.; Khaleghian, A. Induction of cell cycle arrest in MKN45 cells after schiff base oxovanadium complex treatment using changes in gene expression of CdC25 and P53. J. Drug Res. 2020, 70, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, X.G.; Bai, Y.P.; Li, H.; Gao, L.X.; Zhang, T.; Dong, F.; Ding, D.; Zhang, Y. SOV sensitizes gastric cancer cells to radiation by suppressing radiation-induced autophagy in vitro and in vivo. Tissue Cell 2023, 82, 102109. [Google Scholar] [CrossRef]
- Wu, J.X.; Hong, Y.H.; Yang, X.G. Bis (acetylacetonato)-oxidovanadium(IV) and sodium metavanadate inhibit cell proliferation via ROS-induced sustained MAPK/ERK activation but with elevated AKT activity in human pancreatic cancer AsPC-1 cells. JBIC J. Biol. Inorg. Chem. 2016, 21, 919–929. [Google Scholar] [CrossRef]
- Kowalski, S.; Hać, S.; Wyrzykowski, D.; Zauszkiewicz-Pawlak, A.; Inkielewicz-Stępniak, I. Selective cytotoxicity of vanadium complexes on human pancreatic ductal adenocarcinoma cell line by inducing necroptosis, apoptosis and mitotic catastrophe process. Oncotarget 2017, 8, 60324. [Google Scholar] [CrossRef]
- Kowalski, S.; Tesmar, A.; Sikorski, A.; Inkielewicz-Stepniak, I. Oxidovanadium(IV) complex disrupts mitochondrial membrane potential and induces apoptosis in pancreatic cancer cells. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2021, 21, 71–83. [Google Scholar] [CrossRef]
- Griffin, E.; Levina, A.; Lay, P.A. Vanadium (V) tris-3,5-di-tert-butylcatecholato complex: Links between speciation and anti-proliferative activity in human pancreatic cancer cells. J. Inorg. Biochem. 2019, 201, 110815. [Google Scholar] [CrossRef]
- Nair, R.S.; Kuriakose, M.; Somasundaram, V.; Shenoi, V.; Kurup, M.P.; Srinivas, P. The molecular response of vanadium complexes of nicotinoyl hydrazone in cervical cancers—A possible interference with HPV oncogenic markers. Life Sci. 2014, 116, 90–97. [Google Scholar] [CrossRef]
- Inamdar, P.R.; Sheela, A. Exploration of DNA binding mode, chemical nuclease, cytotoxic and apoptotic potentials of diketone based oxovanadium (IV) complexes. Int. J. Biol. Macromol. 2015, 76, 269–278. [Google Scholar] [CrossRef]
- Dankhoff, K.; Ahmad, A.; Weber, B.; Biersack, B.; Schobert, R. Anticancer properties of a new non-oxido vanadium (IV) complex with a catechol-modified 3,3′-diindolylmethane ligand. J. Inorg. Biochem. 2019, 194, 1–6. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Wang, Y.; Zhu, L.; Shi, T.; Wei, H.; Xiao, J.; Zhang, Y.; Wang, Z. Novel oxovanadium complex VO (hntdtsc)(NPIP): Anticancer activity and mechanism of action on HeLa cells. Front. Pharmacol. 2021, 11, 608218. [Google Scholar] [CrossRef]
- Reytman, L.; Braitbard, O.; Hochman, J.; Tshuva, E.Y. Highly effective and hydrolytically stable vanadium (V) amino phenolato antitumor agents. Inorg. Chem. 2016, 55, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Lujerdean, C.; Zăhan, M.; Dezmirean, D.S.; Ștefan, R.; Simedru, D.; Damian, G.; Vedeanu, N.S. In Vitro Studies Demonstrate Antitumor Activity of Vanadium Ions from a CaO-P2O5-CaF2: V2O5 Glass System in Human Cancer Cell Lines A375, A2780, and Caco-2. Int. J. Mol. Sci. 2023, 24, 1149. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Nunes, P.; Correia, I.; Cavaco, I.; Marques, F.; Pinheiro, T.; Avecilla, F.; Pessoa, J.C. Therapeutic potential of vanadium complexes with 1,10-phenanthroline ligands, quo vadis? Fate of complexes in cell media and cancer cells. J. Inorg. Biochem. 2021, 217, 111350. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Correia, I. Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets. Inorganics 2021, 9, 17. [Google Scholar] [CrossRef]
- Scior, T.; Guevara-Garcia, A.J.; Do, Q.-T.; Bernard, P.; Laufer, S. Why Antidiabetic Vanadium Complexes are Not in the Pipeline of “Big Pharma” Drug Research? A Critical Review. Curr. Med. Chem. 2016, 23, 2874–2891. [Google Scholar] [CrossRef]
- Dinda, R.; Garribba, E.; Sanna, D.; Crans, D.C.; Costa Pessoa, J. Hydrolysis, ligand exchange, and redox properties of vanadium compounds: Implications of solution transformation on biological, therapeutic, and environmental applications. Chem. Rev. 2025, 125, 1468–1603. [Google Scholar] [CrossRef]
- Fugono, J.; Yasui, H.; Sakurai, H. Enteric-coating capsulation of insulinomimetic vanadyl sulfate enhances bioavailability of vanadyl species in rats. J. Pharm. Pharmacol. 2002, 54, 611–615. [Google Scholar] [CrossRef]
- Sakurai, H.; Fugono, J.; Yasui, H. Pharmacokinetic Study and Trial for Preparation of Enteric-Coated Capsule Containing Insulinomimetic Vanadyl Compounds: Implications for Clinical Use. Mini-Rev. Med. Chem. 2004, 4, 41–48. [Google Scholar] [CrossRef]
- Sakurai, H.; Katoh, A.; Yoshikawa, Y. Chemistry and Biochemistry of Insulin-Mimetic Vanadium and Zinc Complexes. Trial for Treatment of Diabetes Mellitus. Bull. Chem. Soc. Jpn. 2006, 79, 1645–1664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).