Abstract
Mechanical energy is a plentiful, environmentally friendly, and sustainable energy source in the natural world. In this paper, ZnO tribocatalysts were synthesized using the hydrothermal and sol–gel methods. Under magnetic stirring, the catalyst particles and the polytetrafluoroethylene (PTFE)-sealed magnetic bar rub against each other, transferring electrons across the contact interface. While the PTFE absorbs the electrons, holes are left in the catalyst. The holes in the valence band of sol–gel catalysts exhibit strong oxidative ability, allowing for effective oxidation of organic pollutants. Tribocatalytic tests demonstrated that sol–gel ZnO could remove the antibiotic Cefuroxime Axetil (Axetine) more quickly when stirred magnetically in the dark. Sol–gel and hydrothermal ZnO was enhanced by varying the stirring speed (100, 300 and 500 rpm), the length (2, 2.5 and 3 cm) of magnetic rods, and the type of beaker material (glass and polytetrafluoroethylene). This work presents a viable pathway for transforming environmental mechanical energy into chemical energy, which could be utilized in sustainable energy and environmental remediation, in addition to creating a green tribocatalysis method for the oxidative purification of organic pollutants.
1. Introduction
Wastewater poses a significant threat to the global environment and is a major cause of harm to human health due to its complex composition and slow degradation rate [1,2,3]. One promising method for degrading organic compounds in wastewater is the utilization of catalysts and widely dispersed environmental energy sources, such as solar, thermal, and mechanical energy [4,5,6,7]. A catalyst for the photocatalysis (e.g., degradation of pollutants, water splitting, CO2 reduction) needs to meet several conditions: It must absorb light of the desired energy range (usually UV or visible), the conduction band (CB) should be more negative than the reduction potential of the target reaction (e.g., H+→H2). The valence band (VB) should be more positive than the oxidation potential (e.g., OH−→•OH). Photoexcited electrons and holes must not recombine quickly. The material should promote separation and migration to the surface where reactions occur. It must be chemically and thermally stable under irradiation and in the reaction environment (aqueous, oxidative, etc.). Many semiconductors photocorrode. Preferably inexpensive, environmentally safe, and easy to synthesize at scale. Solar energy is a clean and renewable resource that certain photocatalysts can harness to facilitate various photocatalytic reactions, including the breakdown of organic pollutants, the reduction of CO2, and the decomposition of water into H2 [8,9,10]. However, both thermal and solar energy can be unstable, as they depend on environmental conditions. An eco-friendly approach for treating wastewater is the photocatalytic degradation of organic pollutants, which occurs when wastewater is subjected to ultraviolet or visible light. Despite this, the practical applications of photocatalysis technology are limited due to the high rate of photoexcited carrier recombination. Also, not every photocatalyst is effective—only those meeting the electrochemical (if electron–hole recombination is too fast, efficiency will be negligible; If the band gap is too narrow, the CB/VB may not straddle the redox potentials needed for the reaction) and stability criteria are useful.
The introduction of pyroelectric catalysis offers new prospects for energy-driven breakdown of organic pollutants. Most pyroelectric catalysts require a significant rate of temperature change to effectively convert thermal energy into chemical energy. However, achieving the necessary conditions for thermal catalysis during natural diurnal processes can be challenging [11,12]. Therefore, finding a novel catalytic method is crucial for addressing current environmental pollution challenges. Both tribocatalysis and piezocatalysis harness mechanical energy to initiate a series of chemical reactions [13]. Piezocatalysis is based on the deformation of piezoelectric materials subjected to external forces [14]. The charges generated from this deformation fuel various electrochemical catalytic processes, including the breakdown of organic pollutants, water splitting, and CO2 conversion [15,16,17]. Today, most piezocatalysis is performed using ultrasound, as a substantial external force is needed to deform the piezoelectric material. An innovative catalytic technique known as tribocatalysis captures weak mechanical energy from the environment to induce chemical reactions [18]. The tribocatalysis effect has garnered significant attention due to its unique advantages of reproducibility and environmental sustainability [19,20,21]. The triboelectric effect generates triboelectric charges when two materials come into contact with one another [22,23,24]. Utilizing the positive and negative triboelectric charges produced during the friction process to react with hydroxyl ions and oxygen in water, respectively, presents a promising method for reducing environmental pollution through pollutant decomposition [25,26]. The tribocatalysis effect has led to substantial advancements in environmental dye pollution to date. Thus, utilizing the frictional effect of nanomaterials for dye degradation is an effective concept. The drug degradation with nanomaterials with varying morphologies has not been evaluated in these studies. The research object for this work was chosen to be ZnO nanomaterials. Zinc oxide is widely studied because the band gap is approximately 3.2 eV, similar to that of TiO2. It helps reduce electron–hole recombination. It is abundant, inexpensive, non-toxic (relatively green), and chemically stable. It can be synthesized into nanostructures (nanorods, nanoparticles, nanosheets) that increase surface area and reactivity. The large specific surface area is generally considered crucial for materials used in tribocatalysis.
This work examined the tribocatalysis activity of degrading cephalosporin antibiotics under dark conditions using hydrothermal and sol–gel ZnO nanomaterials with varying morphologies. By adjusting the stirring speed, and quantity of magnetic PTFE rods, as well as the types of beaker materials, the degradation efficiency can be improved. All findings demonstrate that the catalytic materials’ larger specific surface area can more effectively promote charge carrier separation and transfer.
2. Results and Discussion
2.1. The Morphology, Specific Area and Structure of Zinc Oxide
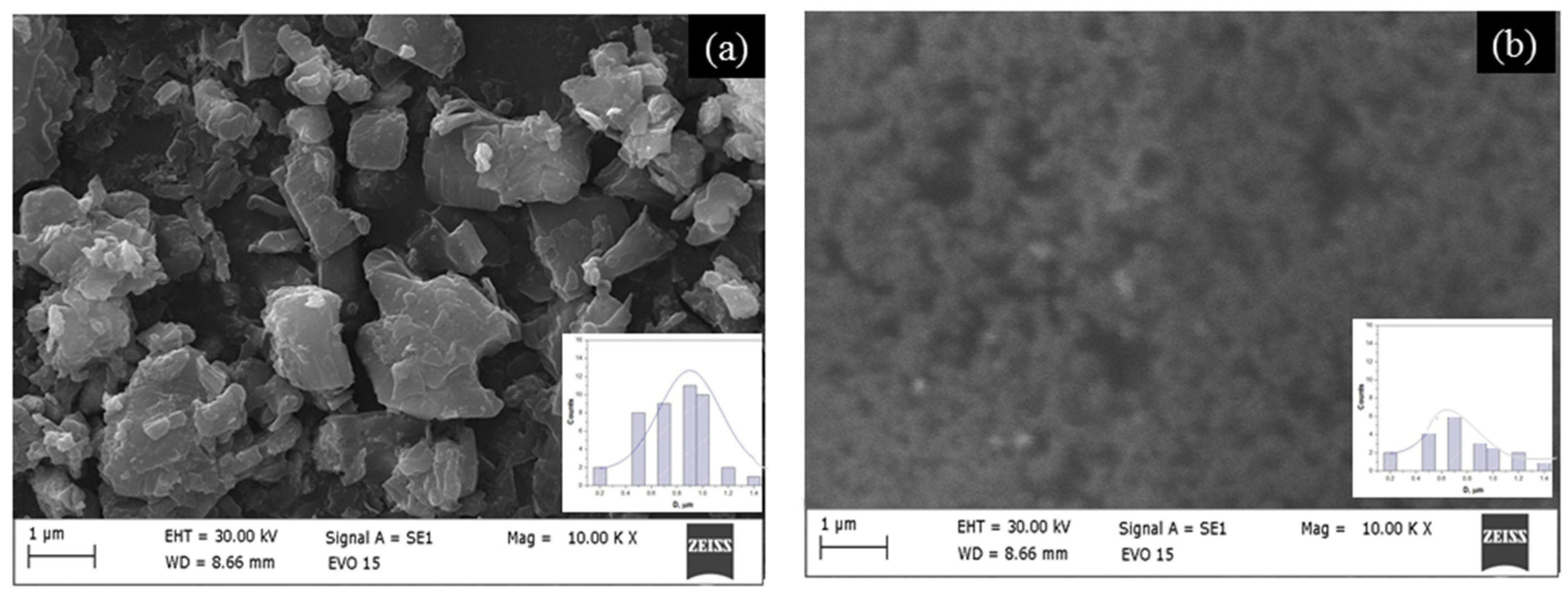
On one hand, the material’s specific surface area is determined by the shape of the catalyst. Conversely, it influences the force conditions during material friction. ZnO is widely used in sensors, batteries, luminescence, and catalysis. Its advantages include non-toxicity, good compatibility, chemical stability, and thermal stability. This study investigates how specific surface area and structural morphology affect the tribocatalytic breakdown of cephalosporin antibiotics using sol–gel and hydrothermal ZnO. The morphology of two types of ZnO is examined with scanning electron microscopy (Figure 1). These micrographs clearly display differences in the surfaces of the samples. The hydrothermal ZnO, shown in Figure 1a, features irregularly shaped particles, varying in size and shape, scattered across a relatively smooth surface. The sol–gel ZnO sample has a homogeneous surface with small particles. The average particle diameter for hydrothermal ZnO is 0.9 ± 0.2 µm, according to an analysis of the SEM images; in the sol–gel ZnO case, this diameter drops to 0.5 ± 0.1 µm. The different method, respectively, the low treatment temperature, does not preserve the morphology of the samples. Test results indicate that these two materials have specific surface areas of 10.3 and 14.5 m2/g, respectively. The findings demonstrate that a higher specific surface area promotes more effective tribocatalysis. This observation aligns with literature data [19,27]. The insets show minimal change in the morphology of ZnO after tribocatalysis. This is also supported by the values of the specific surface area (11.8 and 15.7 m2/g). The magnetic stirring during tribocatalytic tests caused a slight increase in the ZnO surface area, indicating an increase in the number of surface sites. This is probably due to a minor change in surface morphology (insets in Figure 1a,b) resulting from the prolonged degradation of the drug.
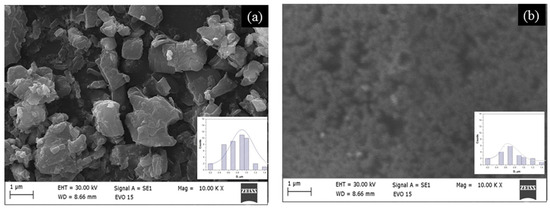
Figure 1.
SEM images of hydrothermal (a) and sol–gel (b) ZnO. The insert is the particle size distribution.
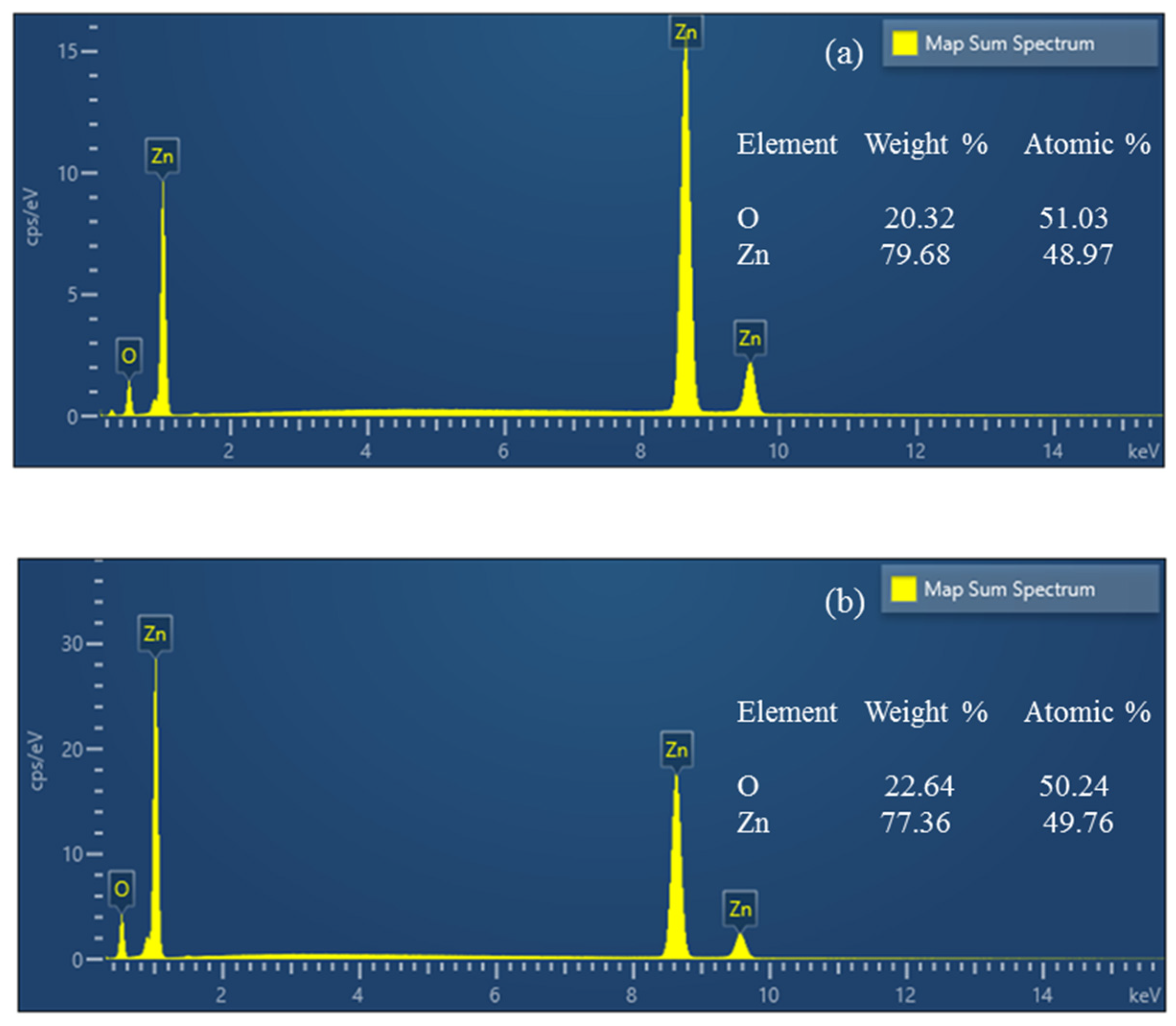
ZnO samples are analyzed using energy-dispersive X-ray spectroscopy (EDS). Hydrothermal and sol–gel ZnO are the two types of tribocatalysts whose atomic percentages of elements are known. There were no additional elemental peaks discovered, confirming the ZnO tribocatalysts’ purity (Figure 2).
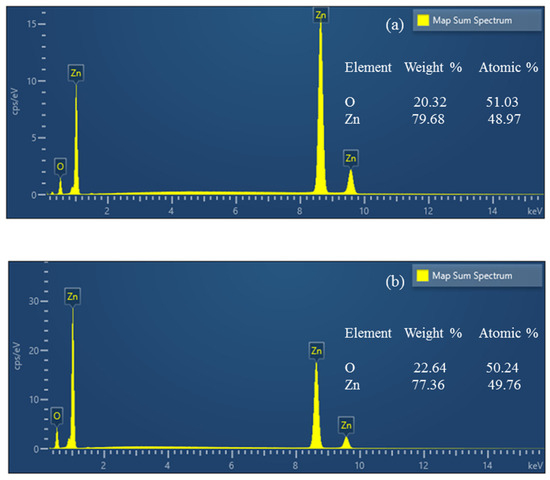
Figure 2.
EDS spectra of hydrothermal (a) and sol–gel (b) ZnO.
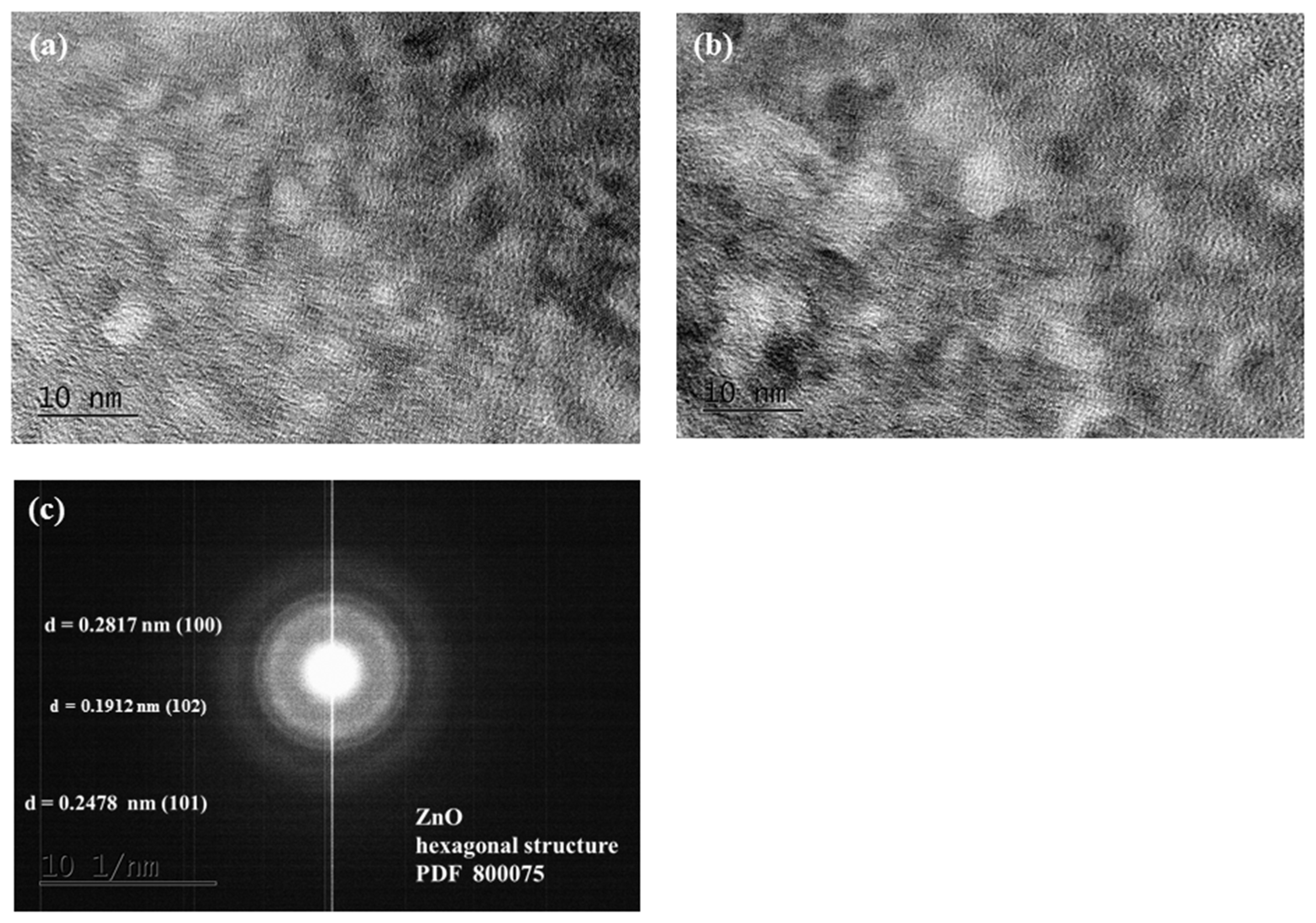
Figure 3 displays TEM images of the hydrothermal and sol–gel ZnO distinct phases. Selected area electron diffraction patterns (SAED, Figure 3c) obtained from TEM images validate the hexagonal wurtzite structure of ZnO and are consistent with the XRD data. The interplanar distances of the crystal lattice of the two varieties of ZnO catalysts (d100–0.2817 nm, d101–0.2478 nm, and d102–0.1807 nm) do not alter, as shown in Figure 3c.
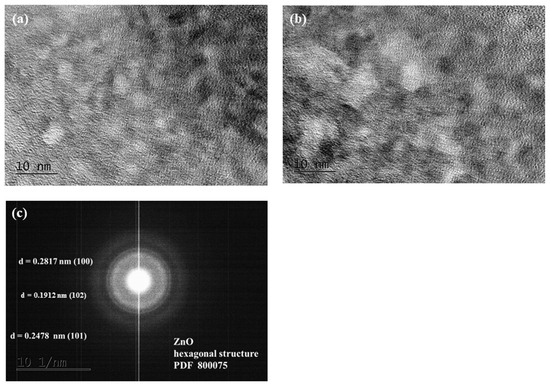
Figure 3.
HRTEM image of (a) hydrothermal and (b) sol–gel ZnO; (c) SAED pattern.
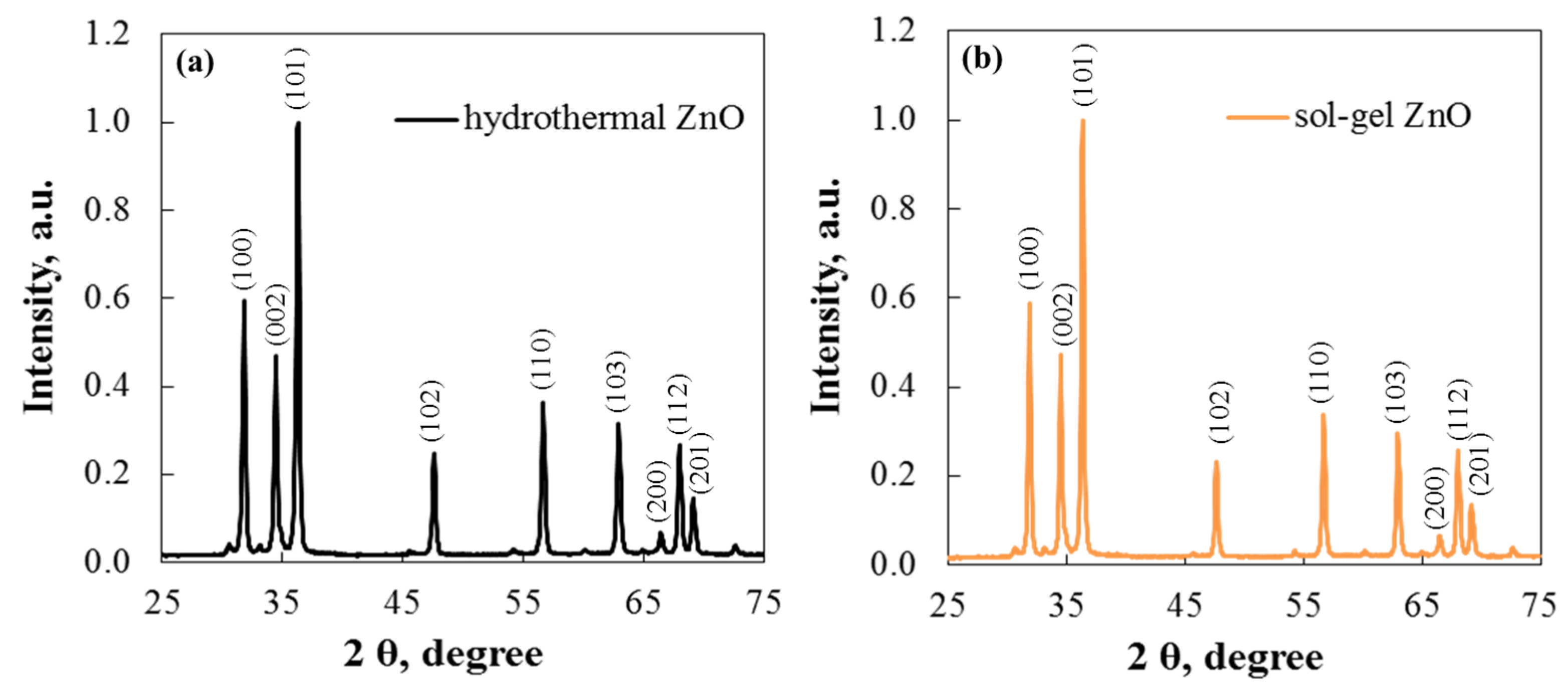
Figure 4 shows the ZnO XRD spectra of the two morphologies. The main diffraction peaks appear at 31.85°, 34.45°, 36.3°, 47.75°, 56.8°, and 63.05°, corresponding to (100), (002), (101), (102), (110), and (103). All peaks confirm that the zinc oxide phase is hexagonal wurtzite and match the standard card, PDF #96-230-0117 [28]. The samples are of high purity and good crystallinity, as indicated by the sharp diffraction peaks and the absence of impurity peaks.
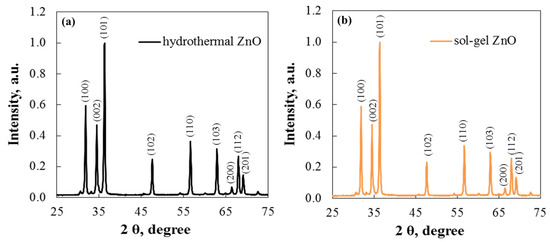
Figure 4.
XRD pattern of (a) hydrothermal and (b) sol–gel ZnO.
Additionally, Table 1 shows the structural parameters that are calculated for sol–gel and hydrothermal tribocatalysts. Indicating that the crystalline lattice stayed comparatively stable, the average crystallite size decreased. The hydrothermal and sol–gel samples’ structural parameters are shown in Table 1. In both samples, it is discovered that the ZnO unit cell parameters are remarkably similar. The calculations used positive values to indicate tensile strain. The sol–gel particles showed a greater magnitude of tensile strain than the pure samples, and the strain in the samples derived from sol–gel is tensile, based on the values in Table 1.

Table 1.
The XRD patterns of the sol–gel and hydrothermal samples were used to calculate the structural parameters.
2.2. Optical Properties of Hydrothermal and Sol–Gel Zinc Oxide
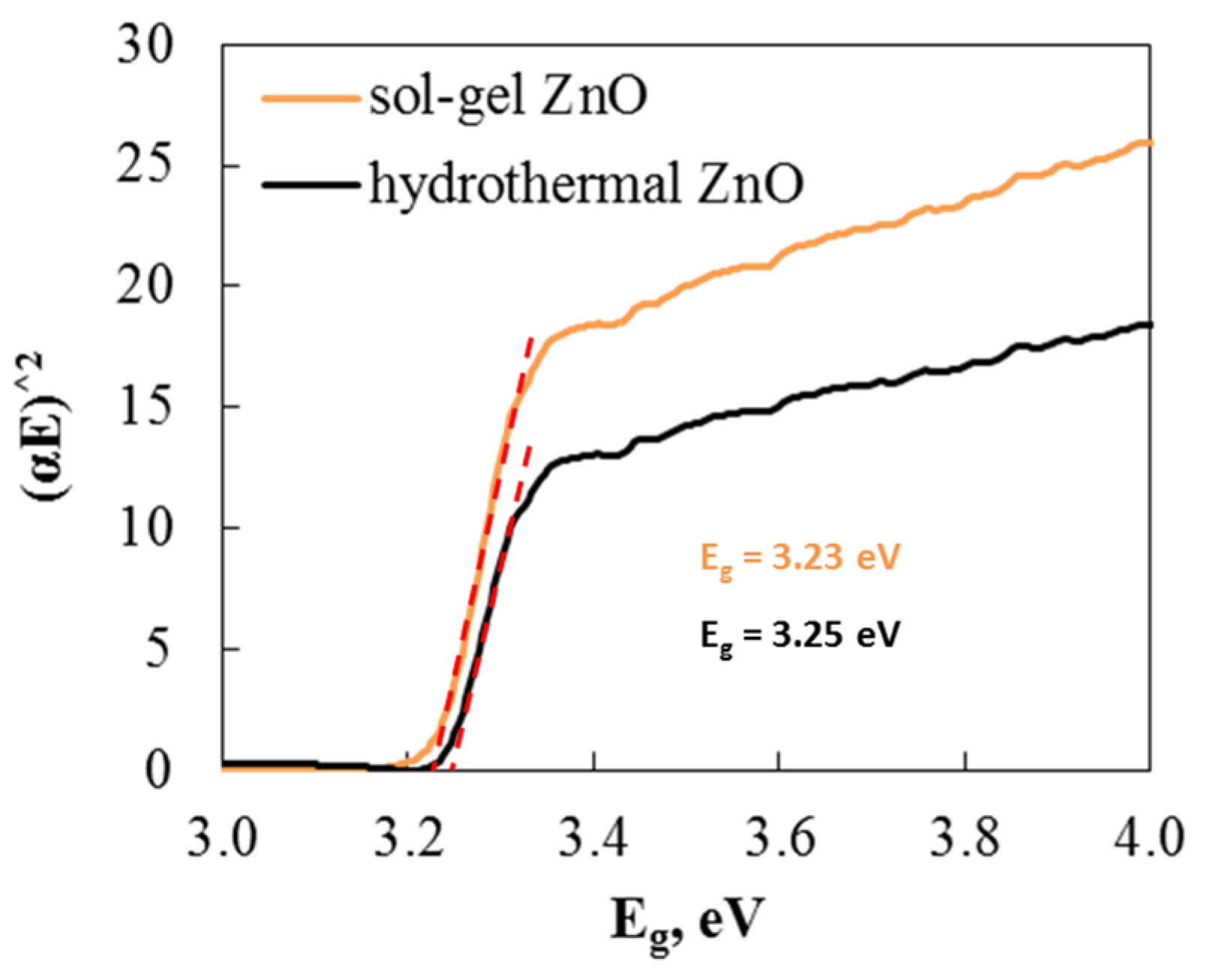
One method commonly used to study the optical properties of materials is UV–visible absorption spectroscopy. Tauc’s equation is used to plot (αE)2 with respect to E and extrapolate as a straight line along the x-axis to determine the bandgap energy values. The values obtained from extrapolation for the ZnO samples’ energy band gap are shown in Figure 5. The band gaps of hydrothermal (3.25 eV) and sol–gel (3.23 eV) ZnO are nearly identical. Therefore, based on these results, there is no difference in the band gap.
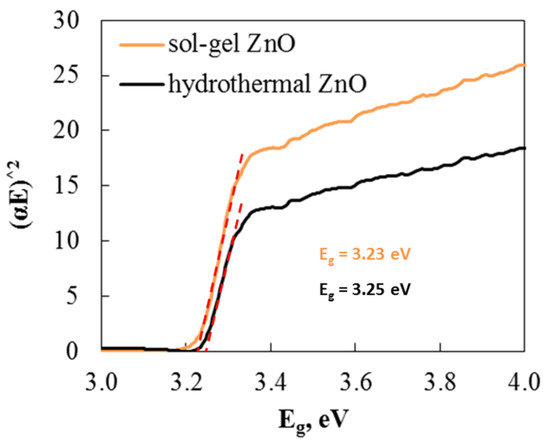
Figure 5.
Energy band gap calculated from Tauc’s equation of hydrothermal and sol–gel ZnO.
2.3. Tribocatalysis of Cefuroxime Axetil—Effect of Stirring Speed (100, 300 and 500 rpm)
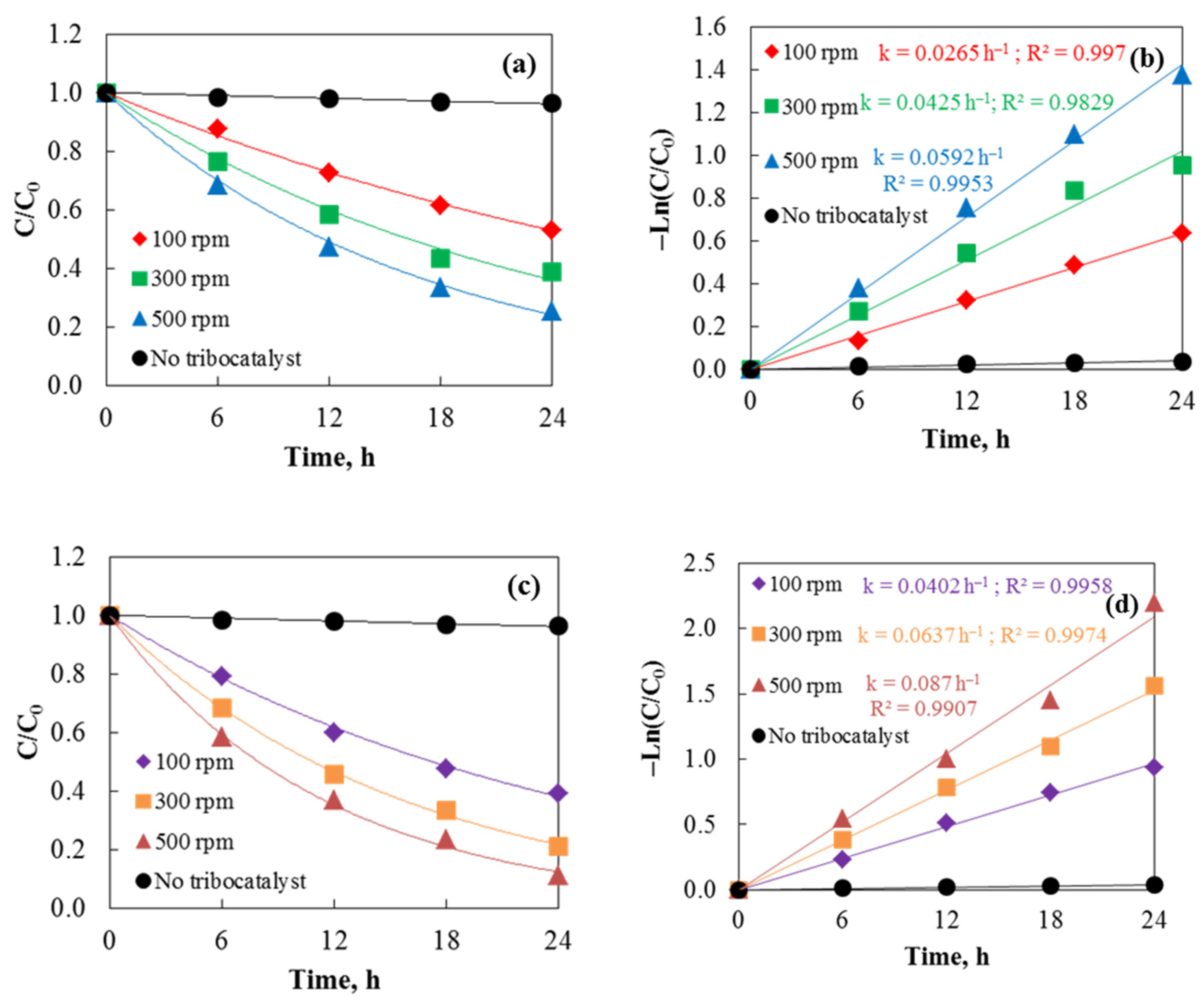
The degradation of the Cefuroxime Axetil under dark conditions was assessed to determine the tribocatalytic efficiency of hydrothermal and sol–gel ZnO powders. Magnetic stirring with a 2.5 cm rod facilitated the tribocatalytic process, and the drug concentration was 75 mg/L in all tests. UV–vis spectroscopy was used to monitor Axetine degradation by tracking the absorption maxima at 291 nm. Sol–gel ZnO powder shows the fastest degradation rate after 24 h of friction, as demonstrated by the tribodegradation results at various stirring speeds in Figure 6. Conversely, a control experiment without a tribocatalyst exhibited very little degradation (about 4%), highlighting the catalyst’s importance in the friction process. Both sample types exhibit catalytic efficiencies following the sequence 500 rpm > 300 rpm > 100 rpm. The higher efficiency of sol–gel ZnO is likely due to its surface morphology. The entire surface is uniform with small particles, which improves carrier participation in redox reactions [27]. The morphology of the hydrothermal samples, where agglomeration of particles is observed, is significantly different. As a result of agglomeration, the active surface area of the catalyst decreases, and the likelihood of recombination of the generated electron-hole pairs increases. The reaction rate constants (k), shown in Figure 6b,d, were determined using the pseudo-first-order kinetics equation −Ln(C/C0) = kt, commonly used for catalytic removal [29]. Sol–gel ZnO exhibited the highest rate constant (k = 0.087 h−1) at 500 rpm, further supporting these results. Degradation efficiencies (D, D (%) = ((C0 − C)/C0) × 100, where C and C0 are at time t and the initial concentration) for the sol–gel ZnO sample were 60.68%, 78.94%, and 88.82% at 100, 300, and 500 rpm, respectively. The tribocatalytic efficiency increases with rotation speed because higher speeds enhance contact between drug molecules and the catalyst, promoting the tribocatalytic reaction. Similarly, Wu et al. [30] observed that in the tribocatalytic degradation of Rhodamine B by Bi2WO6, pollutant degradation efficiency rose with increasing rotation speed. However, excessively high speeds can cause the stirrer to rebound during stirring, splashing the catalyst onto the beaker wall and reducing catalytic efficiency. The aforementioned research demonstrates that magnets are significant in the tribocatalytic process, with friction area, frequency, and charge transfer efficiency between materials all closely linked to the formation of tribocatalytic charges. The percentages of Axetine decomposition and rate constants after the initial tribocatalytic cycle are shown in Table 2.
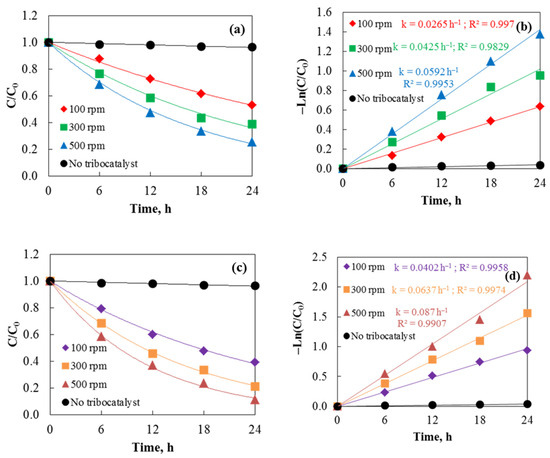
Figure 6.
Tribocatalysis of Cefuroxime Axetil using hydrothermal (a,b) and sol–gel (c,d) ZnO at three different stirring speeds with 2.5 cm magnetic rod.

Table 2.
The values of rate constants and percent of Axetine decomposition using the ZnO.
2.4. Tribocatalysis of Cefuroxime Axetil—Effect of Length of Magnetic Rods
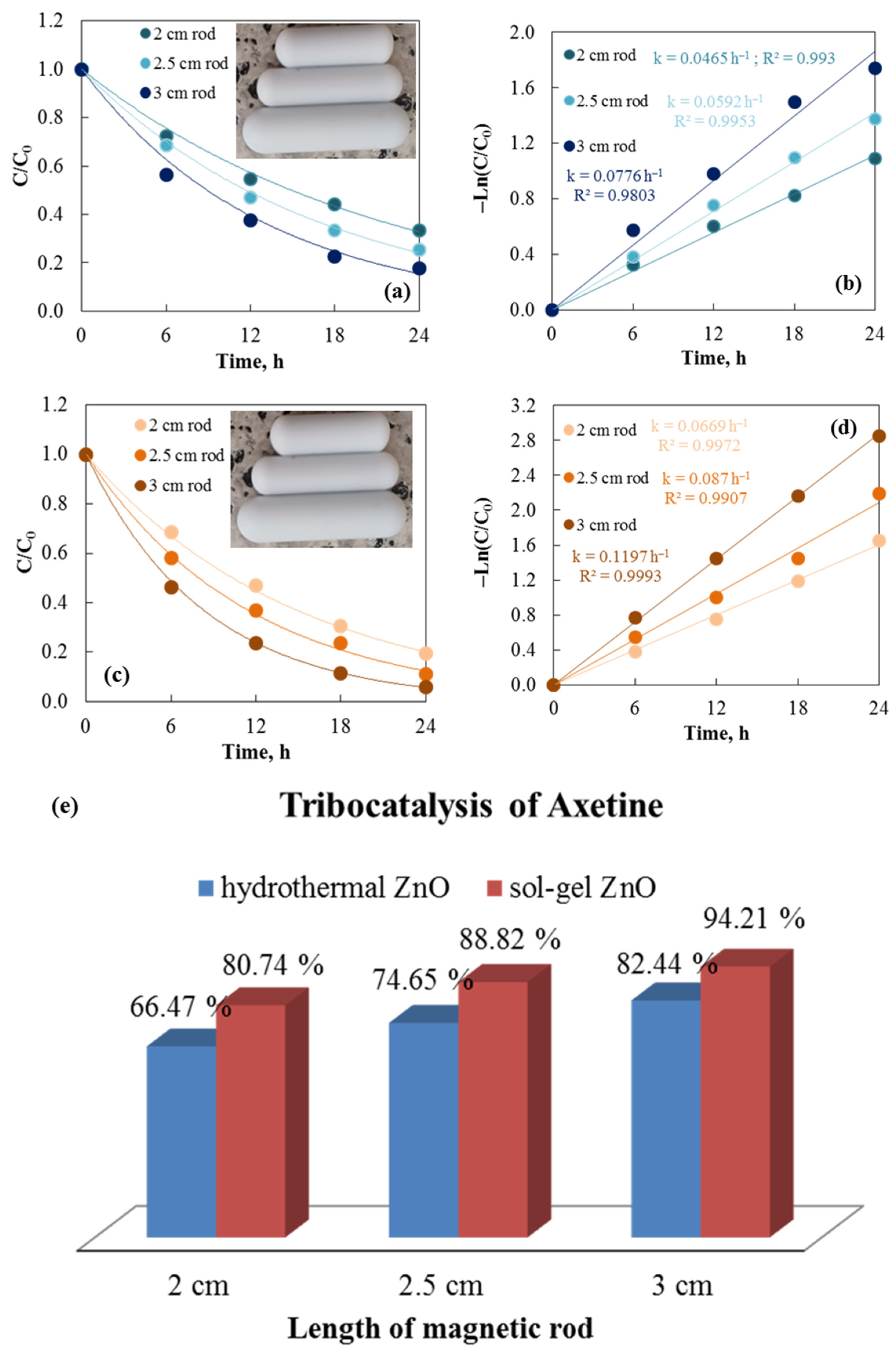
The number, size, shape, length, and speed at which the stirring rods are used all alter the friction efficiency between the materials, which in turn affects the tribocatalytic efficiency. Three magnetic rods of different lengths (2, 2.5, and 3 cm) are used to demonstrate the tribocatalytic efficiencies of hydrothermal and sol–gel ZnO powders for degrading Axetine in Figure 7. It illustrates how the efficiency of all catalysts increases as the rod length increases. Figure 7b,d, which display the corresponding rate constant values for both tribocatalysts, confirm the trend of higher tribocatalytic efficiency at longer lengths. The highest reaction rate was achieved with the longest rod (3 cm): ksol–gel = 0.1197 h−1 and khydrothermal = 0.0776 h−1. The contact area between the rod and the catalyst increases with bar size. As a result, the catalyst’s tribocatalytic efficiency improves as the rod length increases [31,32]. All of these findings have been independently verified from Wu et al. [31], who also studied the effect of stirring rods on the tribocatalytic degradation of Rhodamine B, using rod lengths of 2.0, 2.5, and 3.0 cm. The results show that the degradation rate of Rhodamine B increased as the stirring rod’s length grew.
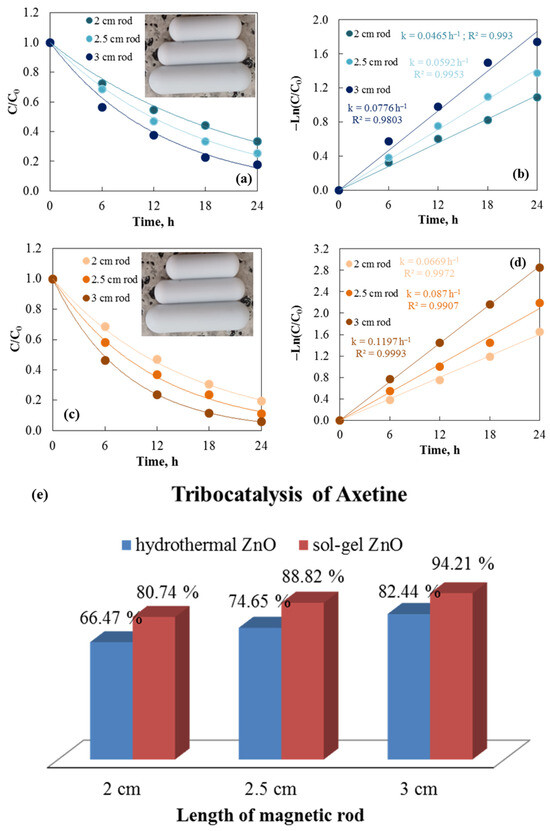
Figure 7.
Tribocatalysis of Cefuroxime Axetil at 500 rpm stirring speed using hydrothermal (a,b) and sol–gel (c,d) ZnO with three different lengths of magnetic rods. Degrees of Axetine degradation versus length of magnetic rods (e).
The tribocatalytic properties of ZnO powders are confirmed and contrasted in Figure 7e for the degradation of Axetine dissolved in distilled water using rods of three distinct lengths. The decomposition of the drug with a 2 cm rod is 66.47% and 80.74% using hydrothermal and sol–gel ZnO, while it increases to 74.65 and 88.82%, 82.44 and 94.21%, at 2.5 and 3 cm, respectively. High gravity is created and the catalyst-stirring rod contact surface is improved by the 3 cm stirring rod’s greater mass compared to the 2.5 and 2.0 cm stirring rods [33].
2.5. Tribocatalysis of Cefuroxime Axetil—Effect of Type Beaker Material
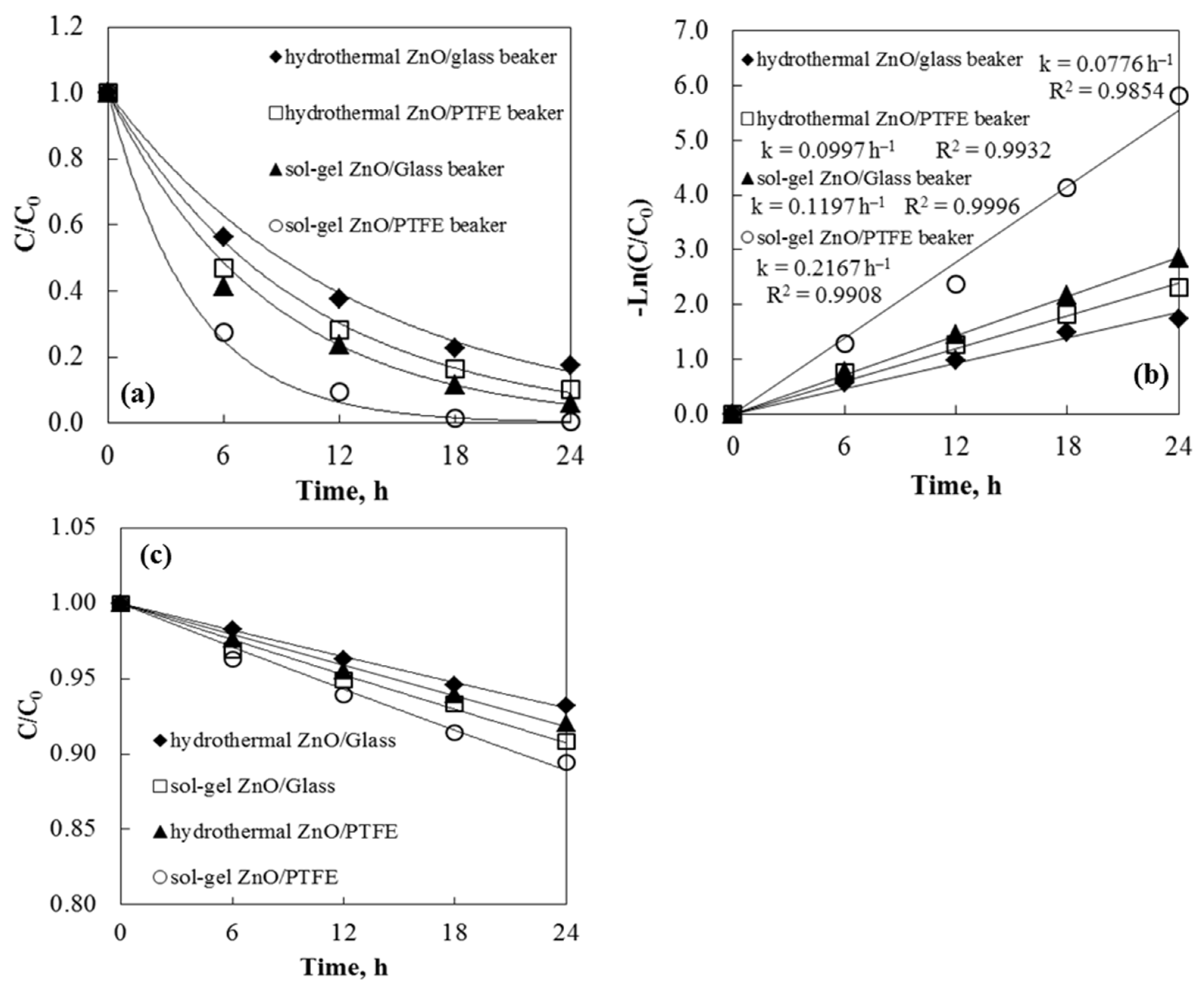
The efficiency of tribocatalysis is greatly affected by electron transfer between the friction pairs. During stirring, electron transfer may occur due to friction between the catalyst and the vessel or between the stirrer and the reactor bottom. Since the vessel significantly influences the tribocatalytic efficiency, the material of the vessel is now considered during tribocatalysis. It was discovered that polytetrafluoroethylene can absorb more electrons than glass during the friction process [34]. Consequently, the catalyst releases more electrons upon contact with the PTFE stirrer and PTFE beaker, resulting in increased tribocatalytic efficiency. To investigate the impact of beaker material on tribocatalysis using ZnO as a catalyst, two different beakers were used. Experimental results showed that the degradation rates of Axetine in the glass and PTFE beakers were 94.2% and 99.7%, respectively, after 24 h of stirring with a PTFE rod (Figure 8). The same trend was observed with hydrothermal tribocatalysts, though with less degradation of the drug. The electron affinity of different materials influences the electron transfer caused by friction [35,36]. A higher tribocatalytic efficiency occurs when the catalyst interacts with the PTFE beaker and stirring rod because PTFE can transfer more electrons during friction than glass. Additionally, a dark phase adsorption experiment was conducted with Axetine in a glass and PTFE beaker in the presence of ZnO tribocatalysts and a 3 cm magnetic rod. In all four cases, the drug concentration decreased by 6–10%. The adsorption data are shown in Figure 8c.
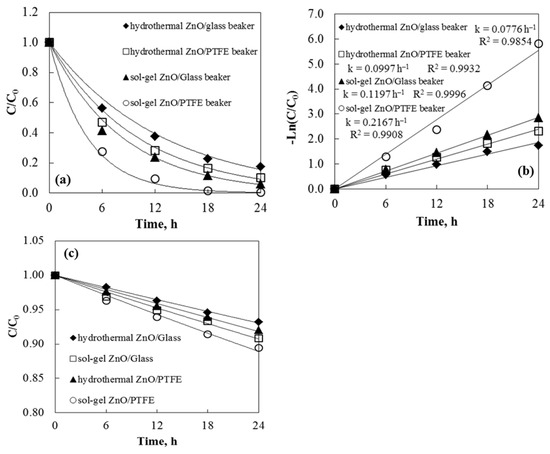
Figure 8.
Tribocatalysis and beaker materials’ effects: (a) degradation of Axetine at 500 rpm stirring speed; (b) kinetic fitting. Adsorption test in the presence of tribocatalyst and magnetic rod, without stirring (c).
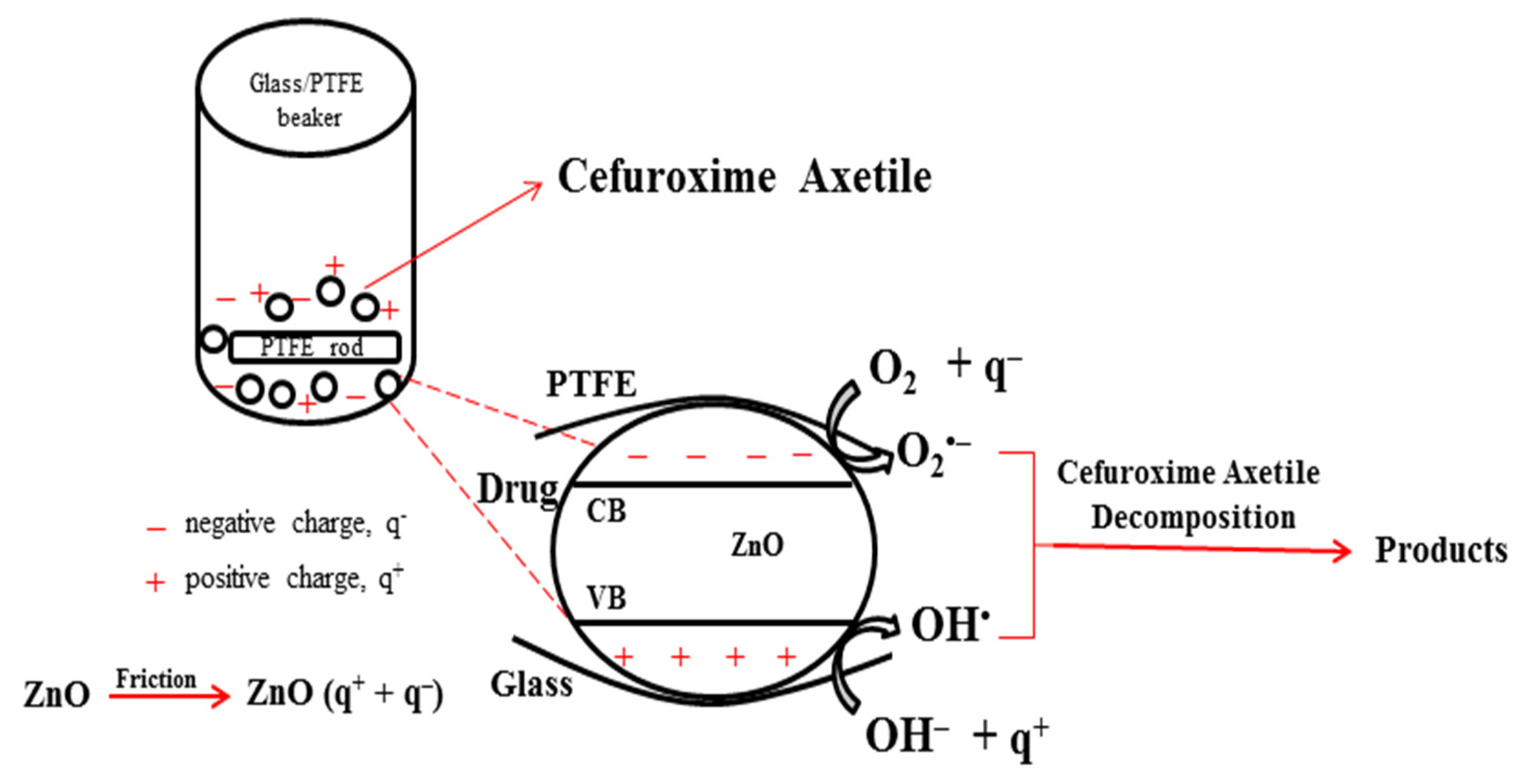
Figure 9 shows a potential tribocatalysis principle diagram. Stirring the ZnO catalysts and applying friction results in the production of positive (q+) and negative (q−) charges on the surfaces of the ZnO powders and the PTFE stirring rod [37]. When ZnO is excited by friction, it produces both positive and negative charges. Like photocatalytic drug decomposition based on photogenerated carriers, Cefuroxime Axetil decomposition can also be accomplished by triboelectrically generated electric charges. Although the positive charges on the ZnO powders’ surface are released into the water, creating active OH• radicals, superoxide radicals (O2•−) are created when oxygen molecules react with negative charges [37,38,39,40]. The antibiotic is further degraded by these radicals into inorganic substances like water and carbon dioxide.
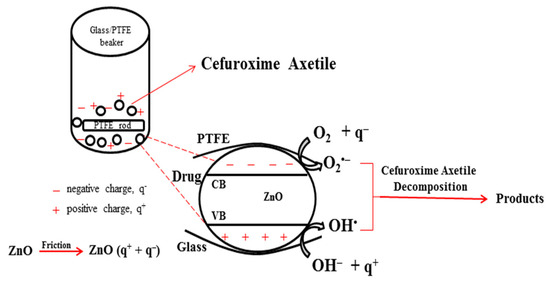
Figure 9.
Diagram of a potential reaction mechanism for the tribocatalytic effect.
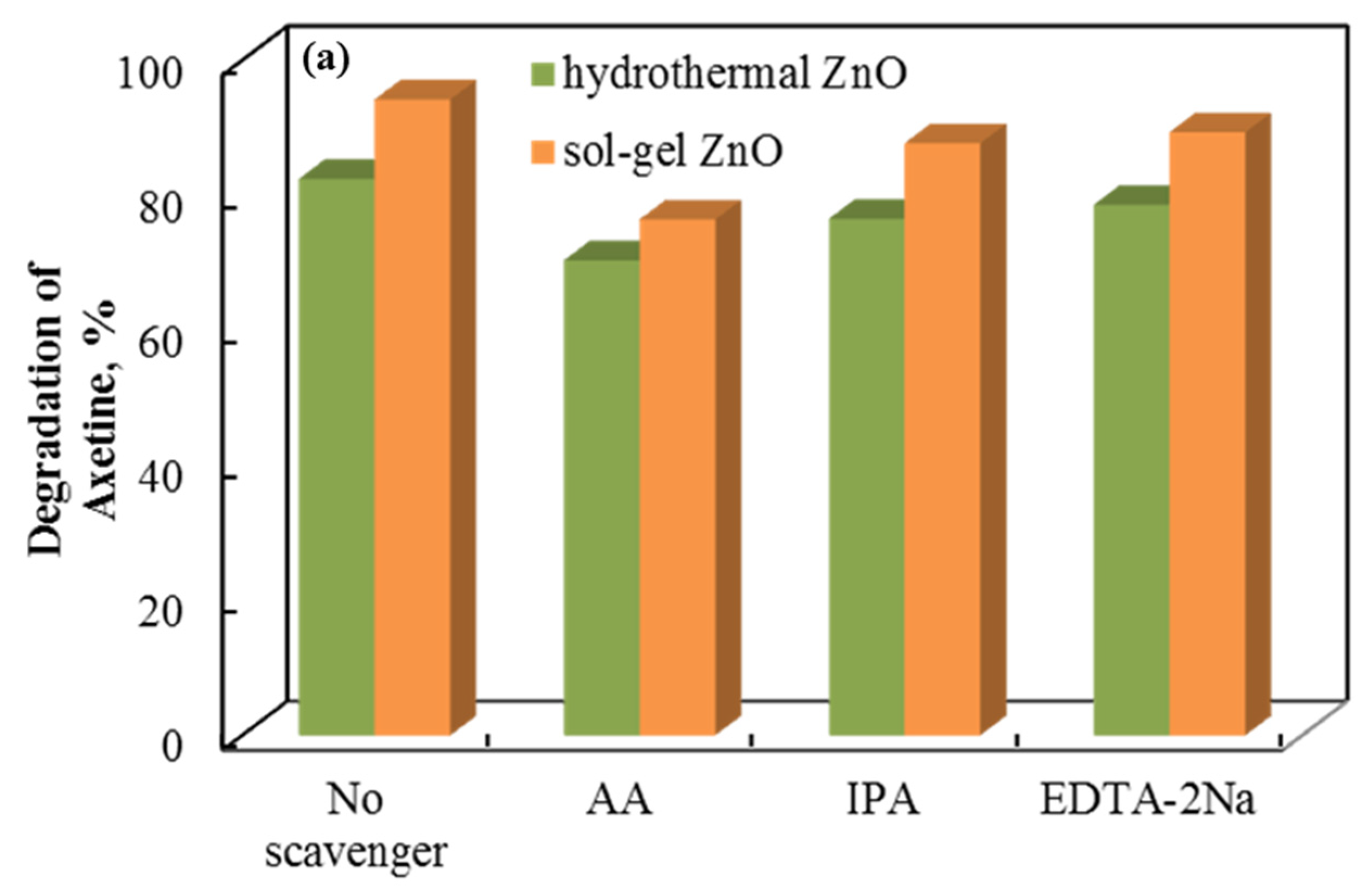
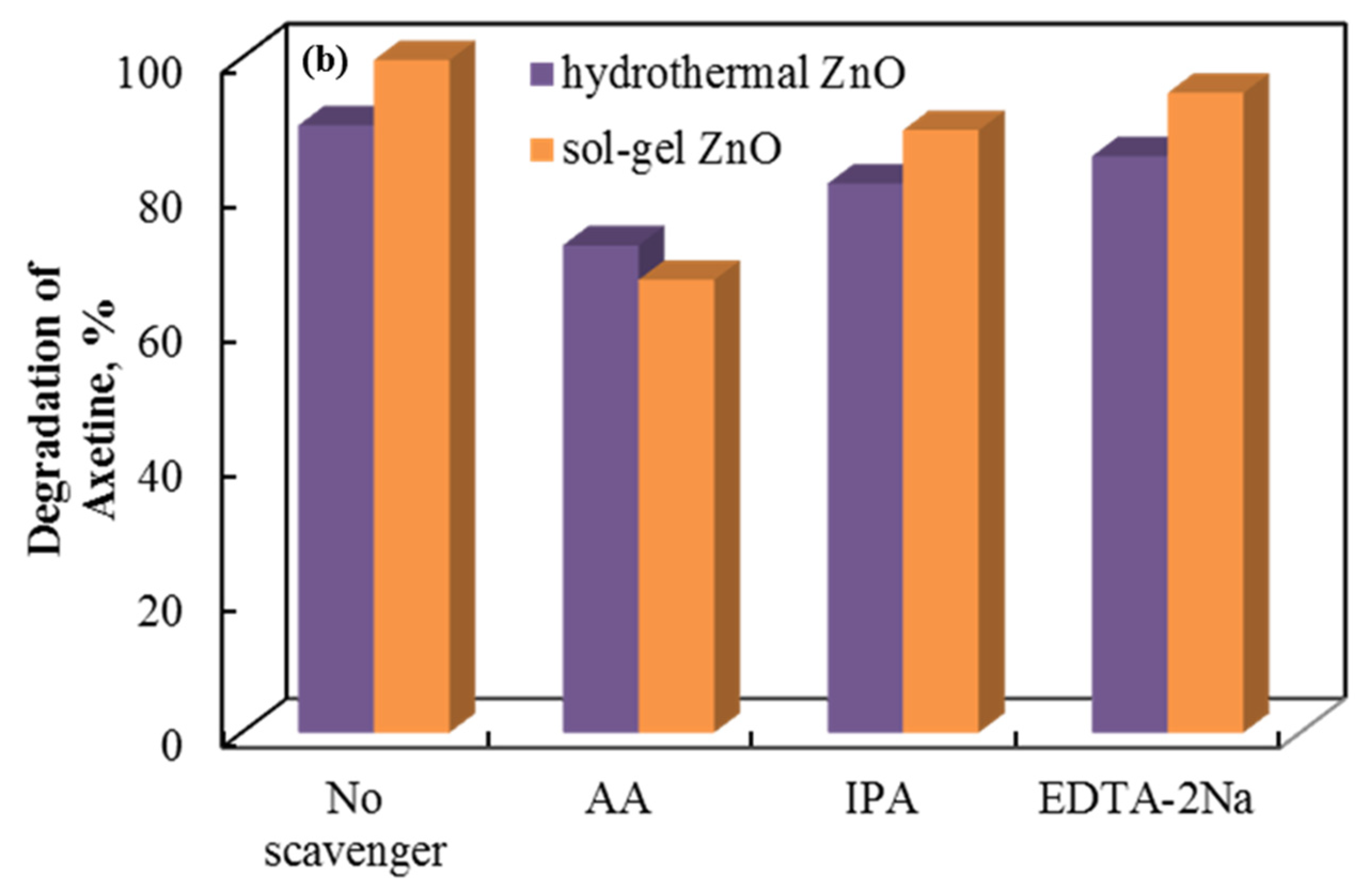
The role of the holes, superoxide, and hydroxyl radicals involved is demonstrated by a radical scavenger assay. Figure 10 shows the data. Axetine’s degradation by holes, superoxide, and hydroxyl radicals was quantified by adding ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), ascorbic acid (AA), and isopropyl alcohol (IPA) scavengers, which capture the corresponding reactive species [38,39,40]. The addition of EDTA-2Na and IPA did not significantly alter the degradation efficiency of Axetine. As a result, h+ plays a negligible part in the drug’s tribodegradation over ZnO tribocatalyst [31,41,42]. But AA showed a stronger inhibition (compared to EDTA decreases by about 8–13% in glass and 13–27% in PTFE beaker), suggesting that the production of superoxide radicals contributes more to the rate of Axetine degradation. As illustrated in Figure 10, the tribocatalysis process of Axetine drug degradation was 67.11%, 94.84%, and 89.32% when AA, EDTA-2Na, and IPA were added. In contrast, a PTFE beaker containing sol–gel ZnO powder achieved 99.7% degradation without the addition of any scavengers. Superoxide radicals were crucial for drug breakdown, as shown in Figure 10, which shows that the AA scavenger caused the least amount of degradation.
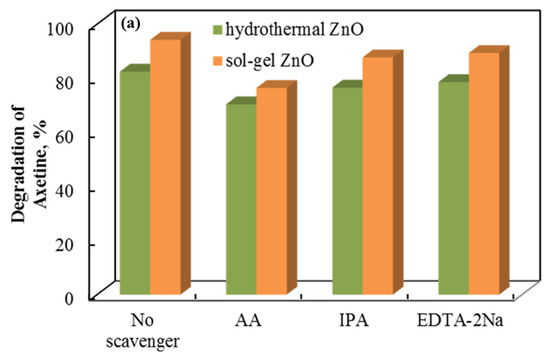
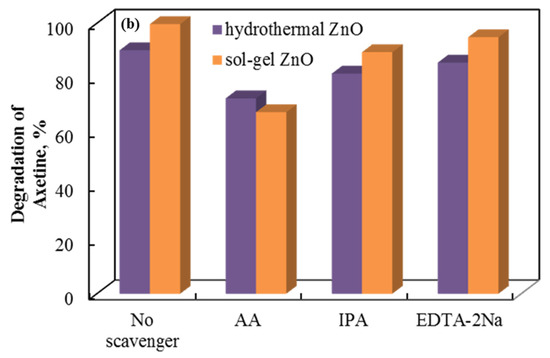
Figure 10.
Radical scavenger assay for the tribocatalytic decomposition of Axetine in (a) glass and (b) PTFE beaker with ZnO.
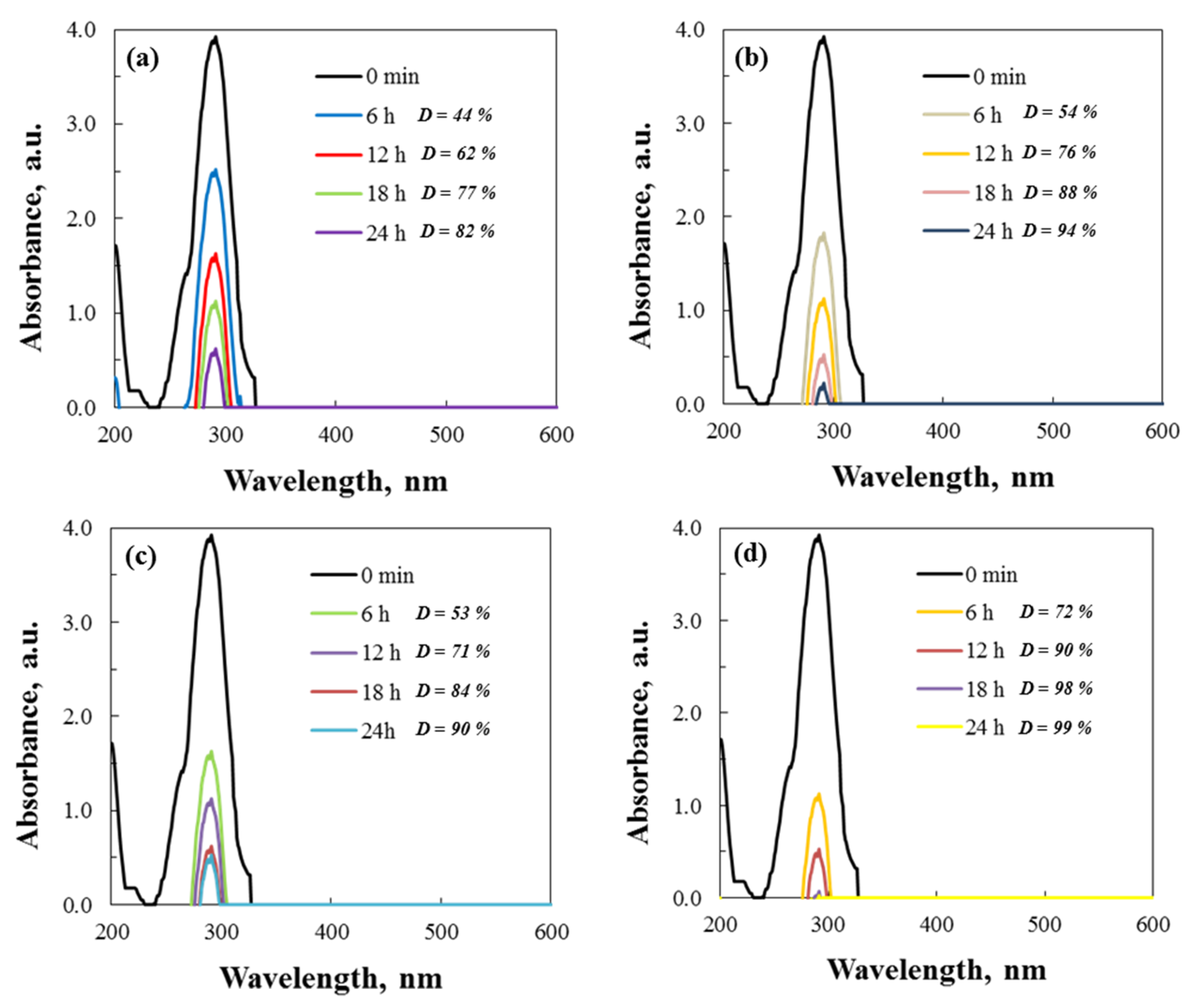
UV–vis spectroscopy was used to monitor the absorption maximum at 291 nm, tracking the degradation of Cefuroxime Axetil at 500 rpm. Spectral changes in the degradation of cephalosporin antibiotic were reviewed to ascertain the activity of the two types of ZnO and the various beaker materials during the tribocatalytic process. Figure 11 presents the UV–vis spectra and the percent degradation of Axetine.
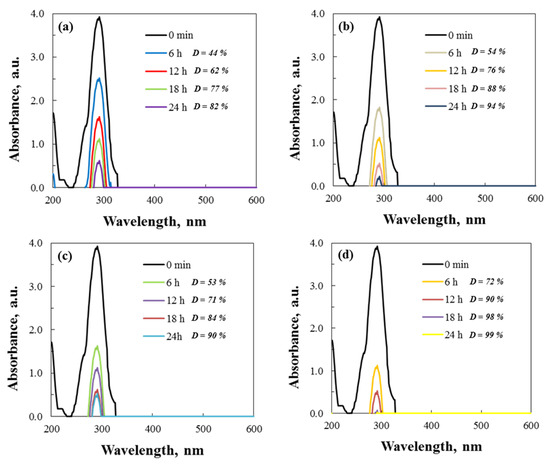
Figure 11.
Spectral data for the tribodegradation of Axetine in glass ((a) hydrothermal and (b) sol–gel ZnO) and PTFE ((c) hydrothermal and (d) sol–gel ZnO) beaker.
A possible mechanism for Cefuroxime Axetil degradation, caused by a reaction with hydroxyl radicals, has been reported in the literature [43]. According to Hernández-Coronado et al. [43], who used titanium dioxide, HO• radicals cleave the N=C double bond in the first step, causing the central heterocyclic molecule to open up. The hydroxyl radicals attack and produce smaller molecules, such as methane, ethanoic, oxalic, propanoic, and 2,4-hexene-1,6-dioic acids. As a result of the subsequent attack, the antibiotic is degraded to carbon dioxide and water. In this article related to the tribocatalysis of Axetin, it is assumed that a similar mechanism is used to degrade the drug. Evidence for this is the straight lines observed in the UV–vis spectrum in Figure 11.
2.6. Tribocatalysis of Cefuroxime Axetil—Effect of Stabillity
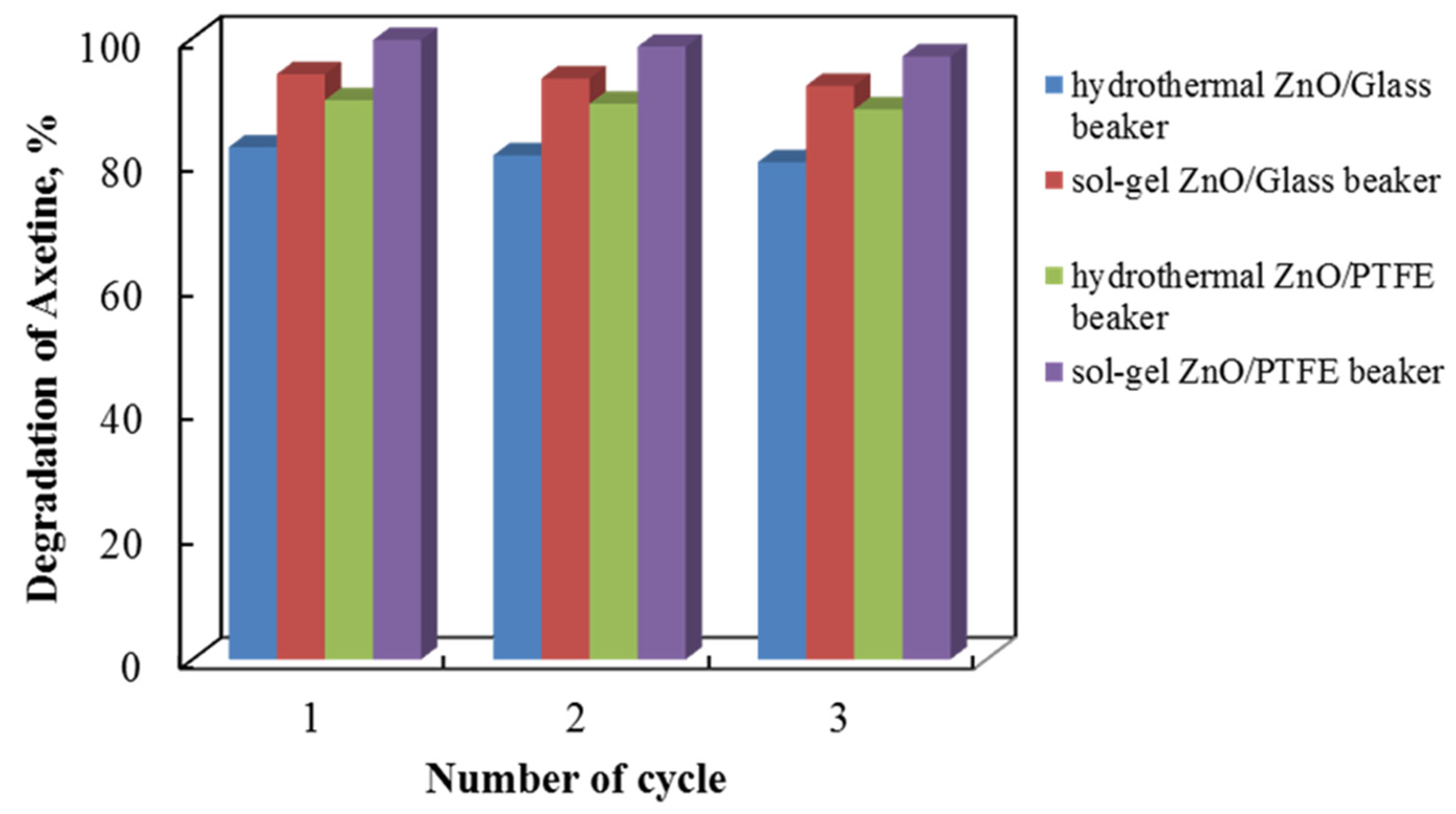
Tribocatalysts used in environmental applications must be able to sustain consistently high tribocatalytic properties over time. Tribocatalytic tests are thus performed to assess the cycling stability of four distinct catalyst types for the degradation of Axetine at 500 rpm. The findings of an investigation into sample regeneration and repurposing are shown in Figure 12, which shows a progressive decline in catalytic quality with each cycle. After three cycles, the tribocatalytic breakdown of Axetine for the hydrothermal ZnO/Glass, sol–gel ZnO/Glass, hydrothermal ZnO/PTFE, and sol–gel ZnO/PTFE catalysts dropped by about 2%. Nonetheless, the nanostructures continued to demonstrate elevated photocatalytic activity following three experimental cycles.
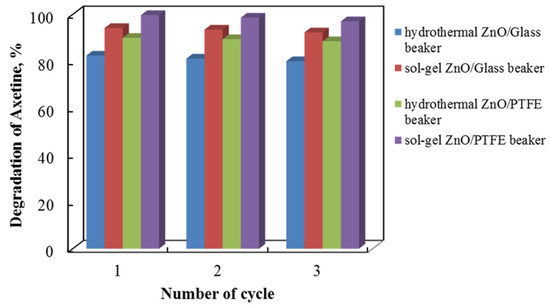
Figure 12.
Tribocatalytic stability of hydrothermal and sol–gel ZnO in both types of beaker material with a 3 cm magnetic rod.
3. Materials and Methods
Zinc acetate dihydrate (Zn(CH3COO)2·x2H2O, >99.5%), 1-propanol (C3H8O, >99.0%), triethylamine (C6H15N, >99.5%), sodium hydroxide (NaOH, >99.0%), and ethylene glycol (C2H6O2, >99.0%) were sourced from Fluka (Buchs, Switzerland). Distilled water was used in all experiments, and all other chemicals and reagents were of analytical reagent grade.
All tribocatalytic tests utilized the commercial drug Axetine (C20H22N4O10S, λmax = 291 nm, 99.0%, cephalosporin antibiotic, antipyretic medication) as a model contaminant. The widespread presence of Cefuroxime Axetil (Axetine) in the environment, particularly in aquatic environments, indicates that it is not a totally safe medication and can have harmful effects because of its widespread use and high production [44]. In addition to increasing liver enzymes, the antibiotic may also decrease platelets, which are cells that aid in blood clotting, and increase eosinophilia, a type of white blood cell [45].
The tribocatalytic tests demonstrated how Axetine breaks down in the presence of various impurities, mimicking natural water systems with distilled water.
To create the hydrothermal ZnO nanoparticles, 50 milliliters of 1-propanol were stirred while preparing Zn(CH3COO)2·2H2O (0.1 M) stock solutions. A NaOH solution made in 1-propanol (25 mL) was added to this stock solution while being constantly stirred (300 rpm), adjusting the reactants’ pH values between 8 and 11. Under autogenous pressure, these solutions were maintained at various temperatures between 100 and 200 °C for six to twelve hours after being transferred to sealed stainless steel autoclaves lined with PTFE (Polytetrafluoroethylene). They were then allowed to cool down naturally to room temperature. Once the reaction was completed, the white solid products were washed with 1-propanol and dried in a laboratory oven set at 80 °C.
The sol–gel method was the second technique employed to produce ZnO particles. In a round-bottom flask equipped with a reflux condenser, 0.5 g of zinc acetate dihydrate, 0.15 mL of ethylene glycol, 20 mL of 1-propanol, and 0.35 mL of triethylamine were combined and stirred at 300 rpm for 15 min at room temperature. The resulting solution was then stirred for 60 min at 80 °C. To produce powders for the tribocatalytic degradation of Axetine, the zinc oxide particles were rinsed twice with pure 1-propanol, and dried in a lab oven at 80 °C.
To investigate the morphology and microstructure of the obtained samples, we used an imaging device a Zeiss Evo 15 microscope (Bruker Resolution 126 eV, Berlin, Germany) and energy-dispersive X-ray spectroscopy (EDS, Tokyo, Japan). At low temperatures (−196 °C), nitrogen adsorption was used to measure the specific surface areas using a Quantachrome Instruments, Anton Paar, NOVA 1200e, Boyton Beach, FL, USA). XRD (Siemens D500 with Cu Kα radiation, Karlsruhe, Germany) was employed to analyze the crystallinity and phase composition of the catalysts. Scherrer’s equation was applied to estimate the average crystallite sizes. PowderCell [46] was used for Rietveld analysis, and the March–Dollase texturing model [47] was utilized to investigate the presence of preferential orientation in the hydrothermal and sol–gel ZnO samples. Thermo Scientific’s Evolution 300 spectrophotometer (Madison, WI, USA), equipped with a DRA-EV-300 Diffuse Reflectance Accessory, was employed to acquire UV–vis spectra. A laboratory dryer with forced convection for hydrothermal syntheses, model: SLW 53 SMART, brand: Pol-Eko, manufacturer: Pol-Eko—Kokoszycka, Poland.
Tribocatalysis was employed to degrade a 50 mL Axetine solution. Using distilled water and a magnetic stirrer, the medication was prepared in a 100 mL beaker. At a constant room temperature of 23 ± 2 °C, the tribocatalytic reaction occurred in the absence of light. Initially, the antibiotic concentration was 75 ppm. Fifty mg of hydrothermal or sol–gel ZnO catalyst was added to a reactor containing the drug solution. The suspension was magnetically stirred with a magnetic bar sealed with polytetrafluoroethylene (PTFE). The resulting mixture was soaked for 30 min without any magnetic stirring to achieve the adsorption equilibrium between the tribocatalysts and the Cefuroxime Axetil solution. The reactor was then switched on, initially spinning at a steady 300 rpm. Aliquots of the reaction solution, each containing two milliliters, were regularly collected. The tribocatalyst was then centrifuged at 6000 rpm. Aside from variations in the length of the magnetic rods, the magnetic stirring conditions (100 and 500 rpm), and the type of beaker material (glass and PTFE), this process was consistent across all other decomposition performance tests. Consequently, the catalyst releases more electrons upon contact with the PTFE stirrer and PTFE beaker, resulting in increased tribocatalytic efficiency. To obtain the UV–vis spectra of Axetine in the 200–600 nm range, Thermo Scientific’s Evolution 300 spectrophotometer (Madison, WI, USA) was utilized. The reactive species that were causing the Cefuroxime Axetil to break down were investigated using a scavenger test. Ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), isopropyl alcohol (IPA) and ascorbic acid (AA) were used as scavengers to absorb holes, superoxide and hydroxyl radicals, respectively. The specific reactive species that led to the tribocatalysis-induced degradation of the organic drug (50 mL) were identified by using six milligrams of each scavenger separately.
4. Conclusions
In summary, by utilizing the friction energy from stirring, two distinct types of zinc oxide (hydrothermal and sol–gel) are used to produce strong tribocatalysis for the breakdown of Axetine. The ZnO sol–gel composite showed a higher specific surface area and more effective degradation. The tribocatalytic drug decomposition ratio reaches approximately 94.21% (in a glass beaker) and 99.70% (in a PTFE beaker) after the drug is agitated for 24 h at 500 rpm using the longest rods. According to the data, mechanical energy absorbed during friction effectively excites the electrons and holes in zinc oxide particles, leading to efficient drug breakdown. The tribocatalytic effect is a promising and eco-friendly way to use environmental mechanical energy to fight pollution. By promoting drug degradation, tribocatalysis opens up new avenues for reducing environmental contamination.
Funding
This research was funded by the Bulgarian NSF project KP-06-N89/07 (KΠ-06-H89/07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Maximillian, J.; Brusseau, M.; Glenn, E.; Matthias, A. Pollution and Environmental Perturbations in the Global System. In Environmental and Pollution Science, 3rd ed.; Academic Press: Boston, MA, USA, 2019; pp. 457–476. [Google Scholar] [CrossRef]
- Gao, X.; Sun, H.; Wang, Y.; Wang, H. Degradation of phenylarsonic acid in phenylarsenic chemical warfare agents by Fe-modified CNT electroactive filter activating peroxymonosulfate: Performance and mechanism. Surf. Interfaces 2024, 44, 103723. [Google Scholar] [CrossRef]
- Gaggero, E.; Cai, W.; Calza, P.; Ohno, T. Enhanced hydrogen peroxide production and organic substrates degradation using atomically dispersed antimony P-doped carbon nitride photocatalysts. Surf. Interfaces 2024, 48, 104143. [Google Scholar] [CrossRef]
- Liu, J.; Qi, W.; Xu, M.; Thomas, T.; Liu, S. Piezocatalytic techniques in environmental remediation Angew. Chem. Int. Edit. 2023, 62, e202213927. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Jia, Y.; Hu, G.; Hu, J. Pyroelectric nanoplates for reduction of CO2 to methanol driven by temperature-variation. Nat. Commun. 2021, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Xie, S.; Wang, G.; Tian, Z. Tribocatalysis: Challenges and perspectives. Sci. China Chem. 2021, 64, 1609–1613. [Google Scholar] [CrossRef]
- He, T.; Cao, Z.; Li, G.; Jia, Y.; Peng, B. High efficiently harvesting visible light and vibration energy in (1−x)AgNbO3–xLiTaO3 solid solution around antiferroelectric–ferroelectric phase boundary for dye degradation. J. Adv. Ceram. 2022, 11, 1641–1653. [Google Scholar] [CrossRef]
- Zhang, Y.; Aljibori, H.; Algarni, Z.; Amari, A.; Mahariq, I.; Zhang, K.; El-Sabban, H. Enhanced photocatalytic organic pollutant degradation, H2 production and N2 fixation via a versatile zinc oxide-based nanocomposite: Synthesis, characterization and mechanism Insight. Chem. Eng. J. 2024, 500, 156725. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.; Verykios, E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Ma, J.; Wu, Z.; Luo, W.; Zheng, Y.; Jia, Y.; Wang, L.; Huang, H. High pyrocatalytic properties of pyroelectric BaTiO3 nanofibers loaded by noble metal under room-temperature thermal cycling. Ceram. Int. 2018, 44, 21835–21841. [Google Scholar] [CrossRef]
- Wang, C.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Pyroelectric catalysis. Nano Energy 2020, 78, 105371. [Google Scholar] [CrossRef]
- Pei, C.; Liu, Z.; Liu, H.; Gao, X.; Liu, J. Highly tribocatalys driven by mechanical friction using micron-sized BaSb2O6 catalyst. Surf. Interfaces 2024, 52, 104920. [Google Scholar] [CrossRef]
- Li, A.; Li, Z.; Liang, Y.; He, Y.; Jiang, X. Optimized piezoelectric bone regeneration through inhibiting sympathetic nerve-bone interaction. Surf. Interfaces 2024, 48, 104380. [Google Scholar] [CrossRef]
- Ma, J.; Xiong, X.; Wu, D.; Wang, Y.; Ban, C.; Feng, Y.; Meng, J.; Gao, X.; Dai, J.-Y.; Han, G.; et al. Band Position-independent piezo-electrocatalysis for ultrahigh CO2 Conversion. Adv. Mater. 2023, 35, 2300027. [Google Scholar] [CrossRef]
- Long, Y.; Xu, H.; He, J.; Li, C.; Zhu, M. Piezoelectric polarization of BiOCl via capturing mechanical energy for catalytic H2 evolution. Surf. Interfaces 2022, 31, 102056. [Google Scholar] [CrossRef]
- Bao, Y.; Xiao, K.; Yue, S.; Zhang, M.; Du, X.; Wang, J.; Oh, W.-D.; Zhou, Y.; Zhan, S. Wastewater decontamination via piezoelectric based technologies: Materials design, applications and prospects. Surf. Interfaces 2023, 40, 103107. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Chen, Y.; Zhang, Y.; An, Q. Tribocatalysis mechanisms: Electron transfer and transition. J. Mater. Chem. A 2023, 11, 4458–4472. [Google Scholar] [CrossRef]
- Lei, H.; Cui, X.; Jia, X.; Qi, J.; Wang, Z.; Chen, W. Enhanced Tribocatalytic degradation of organic pollutants by ZnO nanoparticles of high crystallinity. Nanomaterials 2022, 13, 46. [Google Scholar] [CrossRef]
- Ikeda, S.; Takata, T.; Komoda, M.; Hara, M.; Kondo, J.N.; Domen, K.; Tanaka, A.; Hosono, H.; Kawazoe, H. Mechano-catalysis-a novel method for overall water splitting. Phys. Chem. Chem. Phys. 1999, 1, 4485–4491. [Google Scholar] [CrossRef]
- Zou, H.; Guo, L.; Xue, H.; Zhang, Y.; Shen, X.; Liu, X.; Wang, P.; He, X.; Dai, G.; Jiang, P.; et al. Quantifying and understanding the triboelectric series of inorganic non-metallic materials. Nat. Commun. 2020, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Y.; Yi, Y.; Zhou, B.; Sun, P.; Dong, X. Regulation of friction pair to promote conversion of mechanical energy to chemical energy on Bi2WO6 and realization of enhanced tribocatalytic activity to degrade different pollutants. J. Hazard. Mater. 2023, 459, 132147. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, H.; Lei, H.; Jia, X.; Jiang, Y.; Fei, L.; Jia, Y.; Chen, W. Surprising Tribo-catalytic Conversion of H2O and CO2 into flammable gases utilizing frictions of copper in water. Chem. Sel. 2023, 8, e202204146. [Google Scholar] [CrossRef]
- Hara, M.; Komoda, M.; Hasei, H.; Yashima, M.; Ikeda, S.; Takata, T.; Kondo, J.N.; Domen, K. A study of mechano-catalysts for overall water splitting. J. Phys. Chem. B 2000, 104, 780–785. [Google Scholar] [CrossRef]
- Che, J.; Gao, Y.; Wu, Z.; Ma, J.; Wang, Z.; Liu, C.; Jia, Y.; Wang, X. Review on tribocatalysis through harvesting friction energy for mechanically-driven dye decomposition. J. Alloys Compd. 2024, 1002, 175413. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, N.; Xue, Y.; Shi, H.; Xu, H.; Li, M.; Sun, C.; Xing, Y.; Gao, B.; Ma, B. Challenges and perspectives of tribocatalysis in the treatment for dye wastewater. J. Water Process. Eng. 2024, 63, 105455. [Google Scholar] [CrossRef]
- Chong, J.; Tai, B.; Zhang, Y. Tribocatalysis effect based on ZnO with various specific surface areas for dye degradation. Chem. Phys. Lett. 2024, 835, 140998. [Google Scholar] [CrossRef]
- Ada, K.; Gökgöz, M.; Önal, M.; Sarıkaya, Y. Preparation and characterization of a ZnO powder with the hexagonal plate particles. Powder Technol. 2008, 181, 285–291. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Luo, W.; Li, H.; Wu, Z.; Xu, Z.; Zhang, Y.; Zhang, H.; Yuan, G.; Gao, J. Strong Tribo-Catalysis of Zinc Oxide Nanorods Via Triboelectrically-Harvesting Friction Energy. Ceram. Int. 2020, 46, 25293–25298. [Google Scholar] [CrossRef]
- Wu, M.; Lei, H.; Chen, J.; Dong, X. Friction energy harvesting on bismuth tungstate catalyst for tribocatalytic degradation of organic pollutants. J. Colloid Interface Sci. 2021, 587, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, Y.; He, Q.; Sun, P.; Weng, X.; Dong, X. Tribocatalysis of homogeneous material with multi-size granular distribution for degradation of organic pollutants. J. Colloid Interface Sci. 2022, 622, 602–611. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, S.; Liu, M.; He, G.; Li, X. Enhanced tribocatalytic degradation performance of organic pollutants by Cu1.8S/CuCo2S4 p-n junction. J. Colloid Interface Sci. 2024, 655, 187–198. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Wang, X.; Zhang, L.; Yuan, G.; Wu, Z. Efficient tribocatalysis of magnetically recyclable cobalt ferrite nanoparticles through harvesting friction energy. Sep. Purif. Technol. 2023, 307, 122846. [Google Scholar] [CrossRef]
- Jia, X.; Wanga, H.; Lei, H.; Mao, C.; Cui, X.; Liu, Y.; Jia, Y.; Yao, W.; Chen, W. Boosting tribocatalytic conversion of H2O and CO2 by CO3O4 nanoparticles through metallic coatings in reactors. J. Adv. Ceram. 2023, 12, 1833–1843. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Hu, Y.; Rao, W. Effect of strontium substitution on the tribocatalytic performance of barium titanate. Materials 2023, 16, 3160. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Choi, M.; Jeon, J.; Yoon, H. Rational molecular design of polymeric materials toward efficient triboelectric energy harvesting. Nano Energy 2019, 66, 104158. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, R.; Zhang, Y.; Zhou, B.; Sun, P.; Dong, X. Unveiling the Mechanism of Frictional Catalysis in Water by Bi(12)TiO(20): A Charge Transfer and Contaminant Decomposition Path Study. Langmuir 2022, 38, 14153–14161. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Zhou, C.; Liu, Y.; Qin, T.; Li, D.; Dong, X.; Muddassir, M.; Zhong, A. A new type Co(II)-based photocatalyst for the nitrofurantoin antibiotic degradation. J. Mol. Struct. 2024, 1312, 138501. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Thongtem, S.; Thongtem, T. Synthesis, characterization, and UV light-driven photocatalytic properties of CeVO4 nanoparticles synthesized by sol-gel method. J. Aust. Ceram. Soc. 2021, 57, 597–604. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, M.; Zhang, H.; Gao, J.; Kwok, K.; Kong, L.; Jia, Y.; Liu, L.; Peng, B. Enhanced tribocatalytic degradation of dye pollutants through governing the charge accumulations on the surface of ferroelectric barium zirconium titanate particles. Nano Energy 2022, 100, 107519. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Song, W.; Shi, J.; Zhang, Y. Performance of tribocatalysis and tribo-photocatalysis of pyrite under agitation. J. Cleaner Prod. 2023, 414, 137566. [Google Scholar] [CrossRef]
- Hernández-Coronado, E.; Ruiz-Ruiz, E.; Hinojosa-Reyes, L.; Beltrán, F.; López-Gallego, J.; Gracia-Pinilla, M.; Villanueva-Rodríguez, M. Effective degradation of cefuroxime by heterogeneous photo-Fenton under simulated solar radiation using α-Fe2O3-TiO2. J. Environ. Chem. Eng. 2021, 9, 106822. [Google Scholar] [CrossRef]
- Michelow, I.; McCracken, G. CHAPTER 24—Antibacterial therapeutic agents. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 6th ed.; Feigin, R.D., Cherry, J.D., Demmler-Harrison, G.J., Kaplan, S.L., Eds.; Saunders: Philadelphia, PA, USA, 2009; pp. 3178–3227. [Google Scholar] [CrossRef]
- Brook, I. Use of oral cephalosporins in the treatment of acute otitis media in children. Int. J. Antimicrob. Agents 2004, 24, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Scofield, J. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).