Electrochemical and Gas-Solid Hydrogen Storage Properties of a Multi-Metal Magnesium-Based Alloy Obtained by Ball Milling
Abstract
1. Introduction
2. Results and Discussion
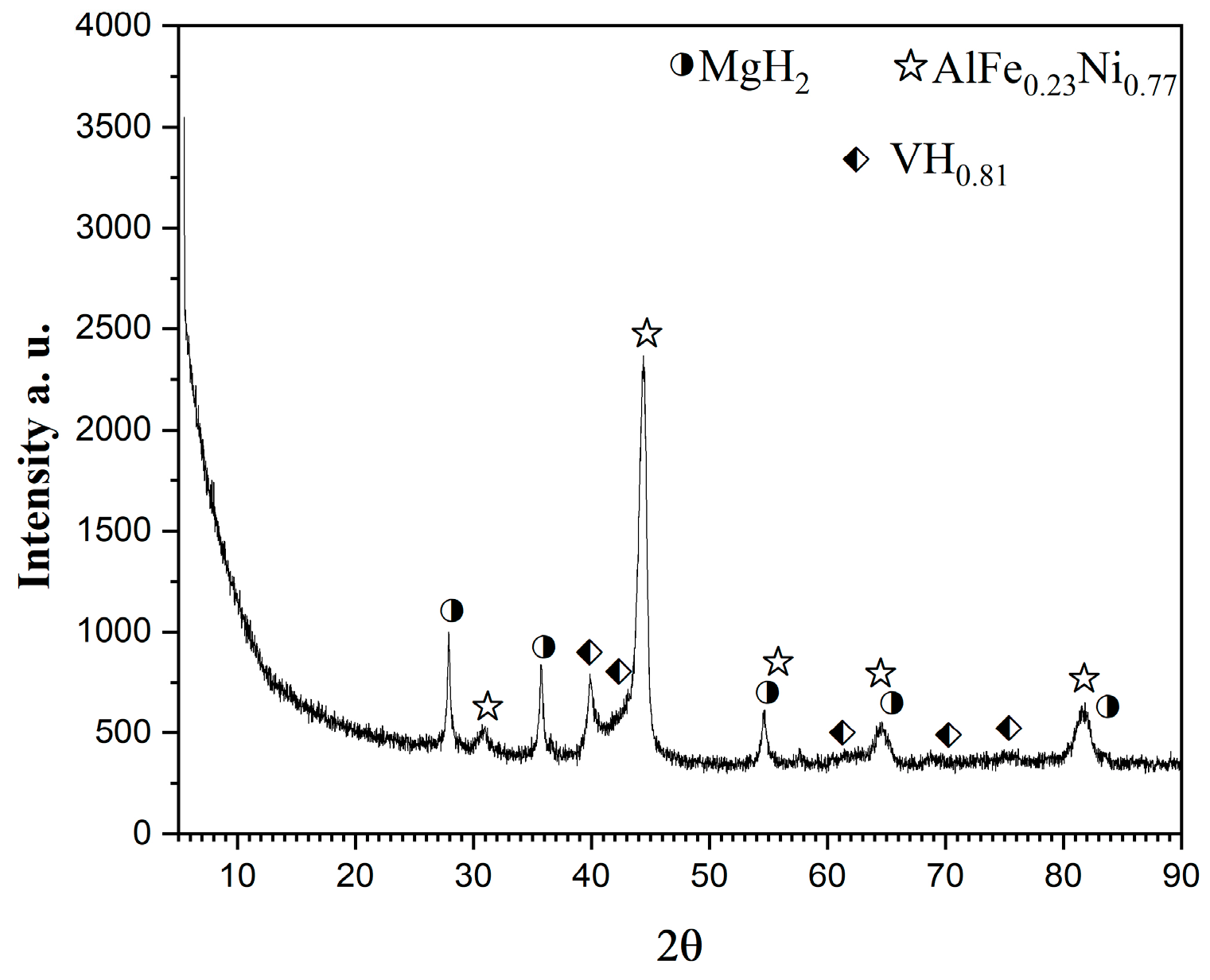
2.1. X-Ray Diffraction Analyses
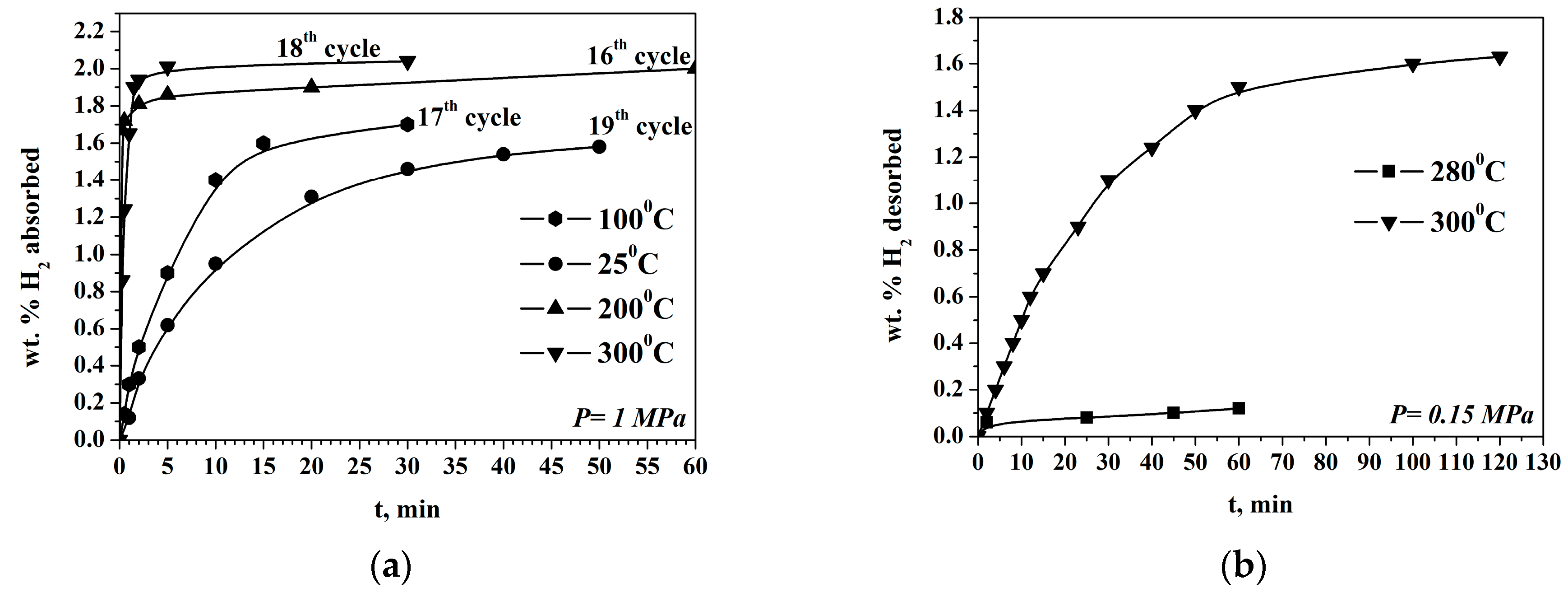
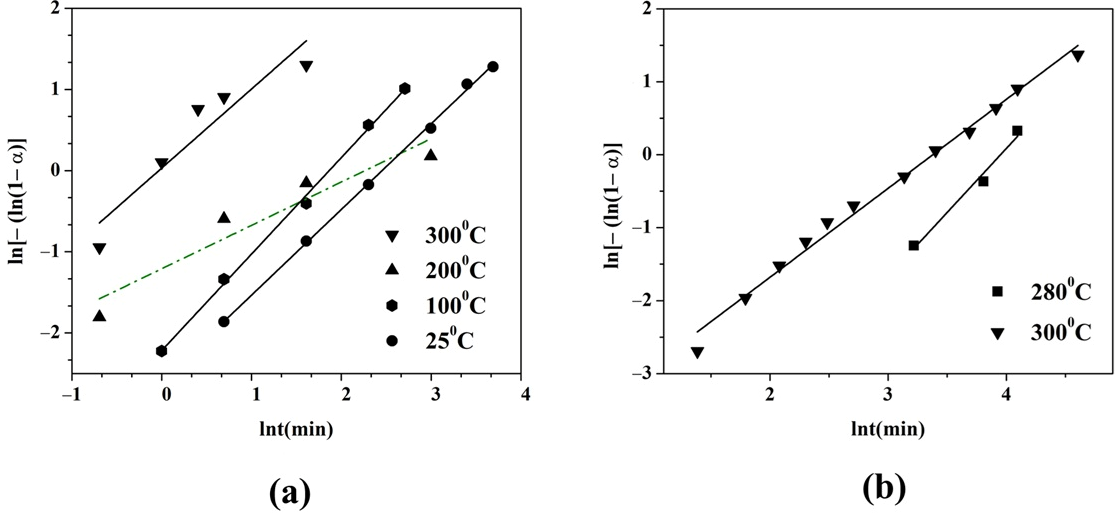
2.2. Gas Hydrogen Storage Properties
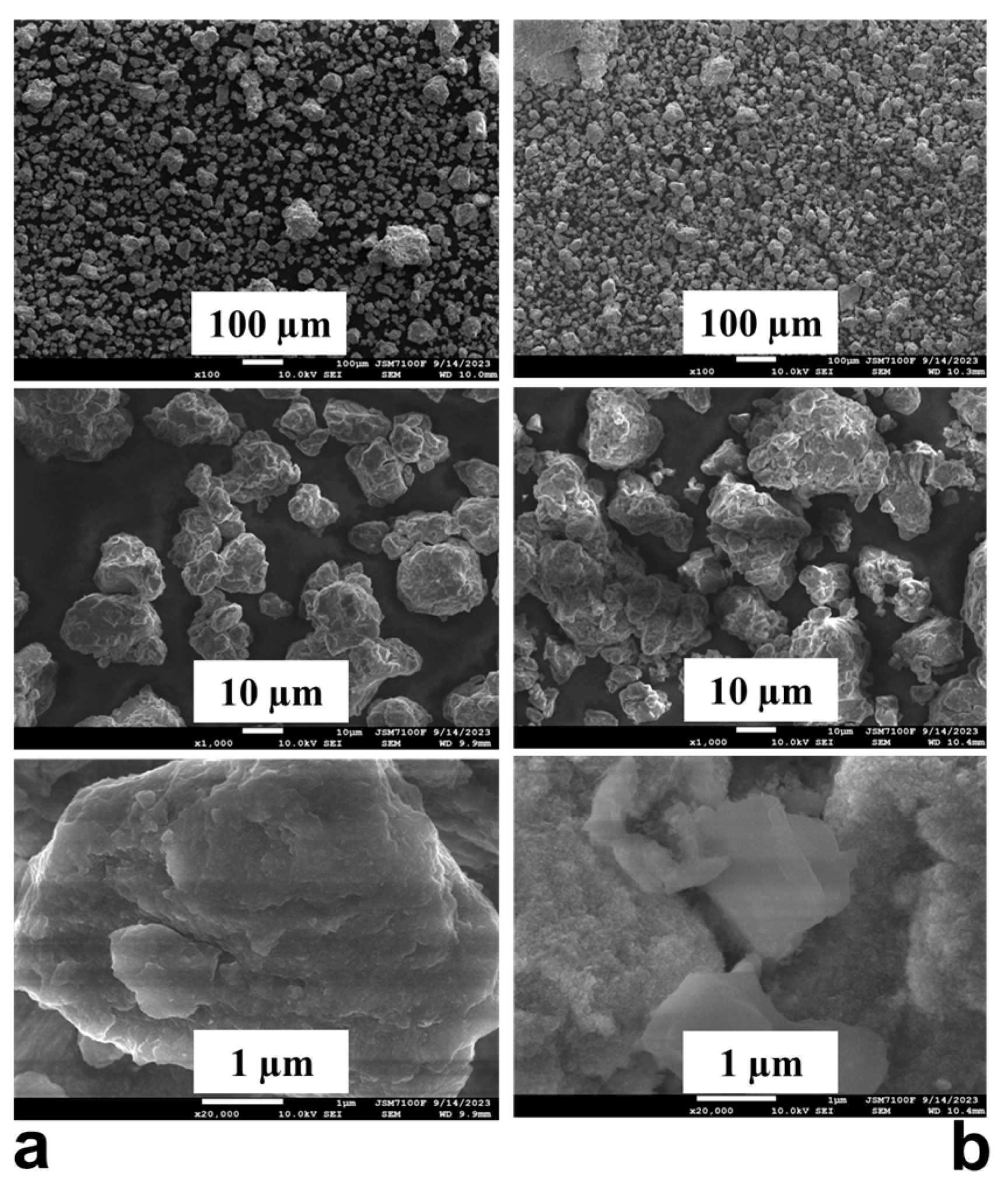
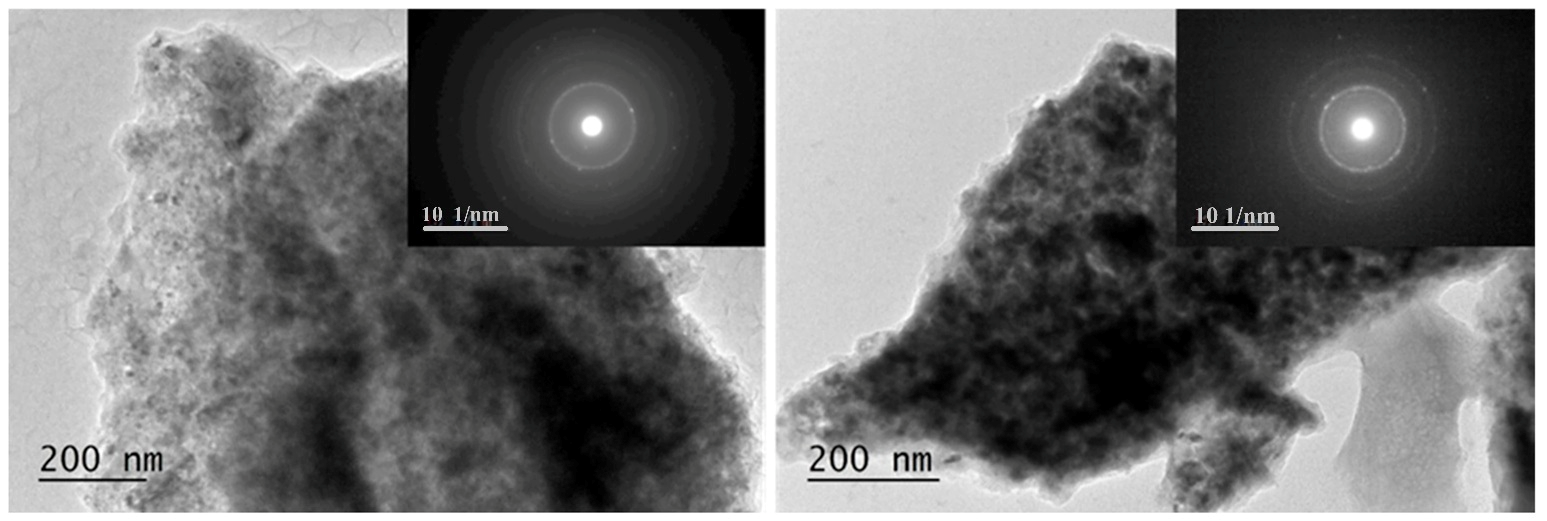
2.3. SEM and TEM Characterization of the Mg50Ni12.5Al12.5V12.5Fe12.5 After Ball Milling and Hydrogenation
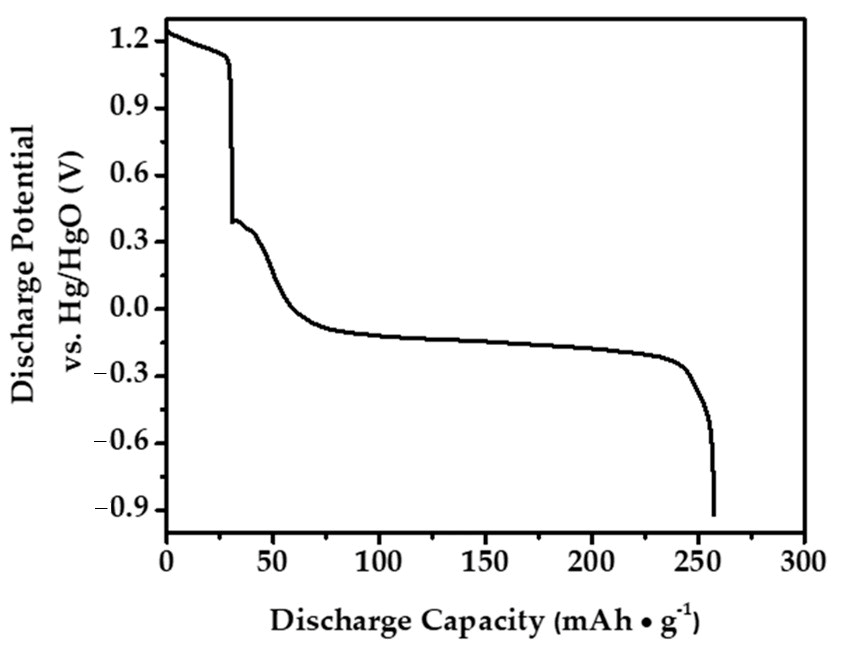
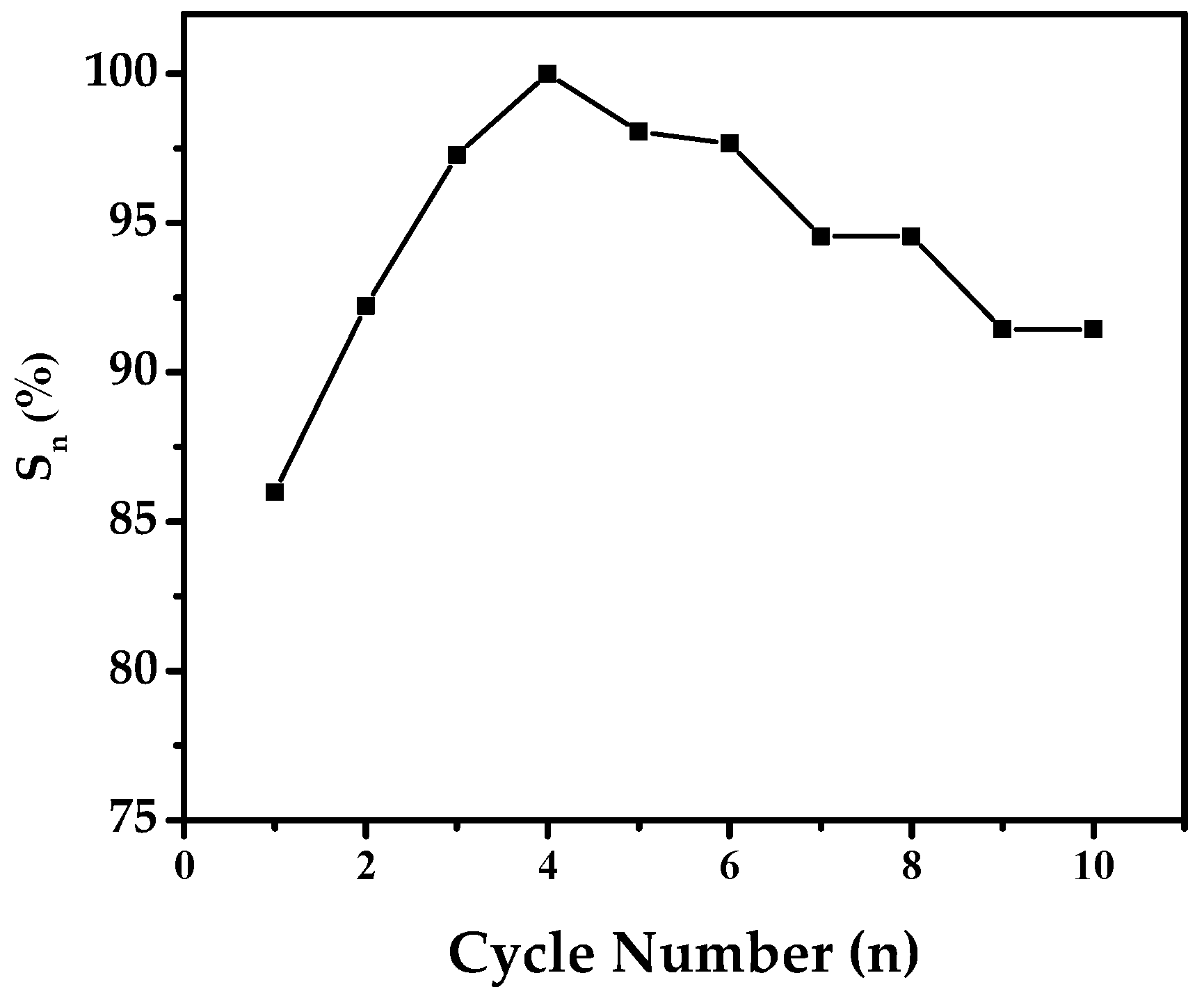
2.4. Electrochemical Properties and Cycle Stability
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rampai, M.M.; Mtshali, C.B.; Seroka, N.S.; Khotseng, L. Hydrogen production, storage, and transportation: Recent advances. RSC Adv. 2024, 14, 6699–6718. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Zhu, M. Magnesium based materials for hydrogenbased energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules 2024, 29, 2525. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Zhang, N.; Li, Z.; Lin, X.; Zhu, W.; Lu, C.; Ding, W.; Zou, J. Nanostructuring of Mg-based hydrogen storage materials: Recent advances for promoting key applications. Nano-Micro Lett. 2023, 15, 93. [Google Scholar] [CrossRef]
- Çolak, A.B. An innovative study on high entropy energy storage Mg-Y-Ni-cu systems: Machine learning-driven optimization of electrical cycling in Ni-MH battery alloys. J. Energy Storage 2025, 107, 114958. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloys 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloys 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Ruggeri, S.; Roue, L.; Huot, J.; Schulz, R.; Aymard, L.; Tarascon, J.M. Properties of mechanically alloyed Mg-Ni-Ti ternary hydrogen storage alloys for Ni-MH batteries. J. Power Sources 2002, 112, 547–556. [Google Scholar] [CrossRef]
- Abe, T.; Inoue, S.; Mu, D.; Hatano, Y.; Watanabe, K. Electrochemical studies of the effect of surface modification of amorphous MgNi electrodes by carbon or Ni. J. Alloys Compd. 2003, 349, 279–283. [Google Scholar] [CrossRef]
- Anik, M. Effect of Titanium additive element on the discharging behavior of MgNi Alloy electrode. Int. J. Hydrogen Energy 2011, 36, 15075–15080. [Google Scholar] [CrossRef]
- Anik, M.; Özdemir, G.; Kücükdeveci, N.; Baksan, B. Effect of Al, B, Ti and Zr additive elements on the electrochemical hydrogen storage performance of MgNi alloy. Int. J. Hydrogen Energy 2011, 36, 1568–1577. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, Y.; Zhu, Y.; Qu, Y.; Zhan, L.; Wan, N.; Li, L. The effects of Pd and/or Zr additives on the structures and cyclic stabilities of Mg50Ni50-based electrode alloys. Int. J. Hydrogen Energy 2015, 40, 2768–2774. [Google Scholar] [CrossRef]
- Küçküdeveci, N.; Erdoğan, I.A.; Aybar, A.B. Effects of Zr addition on electrochemical characteristics of MgTiMnNi hydrogen storage alloys. Int. J. Hydrogen Energy 2023, 48, 18753–18760. [Google Scholar] [CrossRef]
- Li, P.; Wang, H.; Jiang, W.; Liu, J.; Ouyang, L.; Zhu, M. Promoting the cycling stability of amorphous MgNi-based alloy electrodes by mitigating hydrogen-induced crystallization. Int. J. Hydrogen Energy 2021, 46, 6701–6708. [Google Scholar] [CrossRef]
- Huang, J.; Ouyang, L.; Wang, H.; Liu, J.; Zhu, M.; Fang, F.; Sun, D. Hydrogenation and crystallization of amorphous phase: A new mechanism for the electrochemical capacity and its decay in milled MgNi alloys. Electrochim. Acta 2019, 305, 145–154. [Google Scholar] [CrossRef]
- Han, S.-C.; Lee, P.S.; Lee, J.-Y.; Züttel, A.; Schlapbach, L. Effects of Ti on the cycle life of amorphous MgNi-based alloy prepared by ball milling. J. Alloys Compd. 2000, 306, 219–226. [Google Scholar] [CrossRef]
- Küçükdeveci, N.; Akay Erdoğan, I.; Binal Aybar, A.; Anik, M. Electrochemical hydrogen storage properties of mechanically alloyed Mg0.8Ti0.2-xMnxNi (x = 0, 0.025, 0.05, 0.1) type alloys. Int. J. Hydrogen Energy 2022, 47, 2511–2519. [Google Scholar] [CrossRef]
- Wang, J.F.; Ding, P.D.; Gao, S.; Pan, F.S.; Tang, A. Effect of Al content on hydrogen storage properties of amorphous Mg1-xAlxNi (x = 0, 0.1, 0.2, 0.3) alloy. Mater. Sci. Forum 2009, 610–613, 946–950. [Google Scholar] [CrossRef]
- Rongeat, C.; Grosjean, M.H.; Ruggeri, S.; Dehmas, M.; Bourlot, S.; Marcotte, S.; Roué, L. Evaluation of different approaches for improving the cycle life of MgNi-based electrodes for Ni-MH batteries. J. Power Sources 2006, 158, 747–753. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Ouyang, L.; Liu, J.; Zhu, M. Reducing the electrochemical capacity decay of milled Mg-Ni alloys: The role of stabilizing amorphous phase by Ti-substitution. J. Power Sources 2019, 438, 226984. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Cai, Y.; Zhao, S. Enhancing the cycle stability of milled Mg-Ni alloys: The role of Pd substitution on reversible electrochemical hydrogenation/dehydrogenation reactions. Electrochem. Commun. 2025, 170, 107860. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, Y.; Jiao, L.; Yuan, H.; Zhou, X.; Jang, M.-B. Preparation and electrochemical characteristics of MgNi-FeB alloys. Int. J. Hydrogen Energy 2007, 32, 3915–3920. [Google Scholar] [CrossRef]
- Guo, M.; Wang, H.; Liu, J.; Ouyang, L. Improving hydrogen-induced crystallization and electrochemical hydrogen storage properties of MgNi amorphous alloy with CoB addition. J. Non-Cryst. Solids 2022, 588, 121646. [Google Scholar] [CrossRef]
- Chen, W.; Sha, J.; Li, L.; Bao, J.; Yang, Y.; Qiao, M.; Tian, J.; Zhang, Z.J. The effect of Gd on the microstructure and electrochemical properties of Mg-Ni-based alloys. Alloys Compd. 2024, 981, 173638. [Google Scholar] [CrossRef]
- Parente, A.; Nale, A.; Catti, M.; Kopnin, E.; Caracino, P. Hydrogenation properties of Mg2AlNi2 and mechanical alloying in the Mg-Al-Ni system. J. Alloys Compd. 2009, 477, 420–424. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, Y.; Zhang, J.; Zhang, Y.; Liu, Y.; Lin, H.; Wang, T.; Li, L. Effect of partial substitution of Ti for Al on the phase structure and electrochemical hydrogen storage properties of Mg3AlNi2 alloy. J. Alloys Compd. 2018, 746, 421–427. [Google Scholar] [CrossRef]
- Sheng- Long, L.; Fu- Kai, H.; Wen- Chi, C.; Chih- Kuang, L.; Jing- Chie, L. Influence of Mg3AlNi2 content on the cycling stability of Mg2Ni-Mg3AlNi2 hydrogen storage alloy electrodes. Intermetallics 2011, 19, 1953–1958. [Google Scholar]
- Guzmán, D.; Ordoñez, S.; Fernández, J.F.; Sánchez, C.; Serafini, D.; Rojas, P.A.; Aguilar, C.; Tapia, P. Effect of amorphous Mg50Ni50 on hydriding and dehydriding behavior of Mg2Ni alloy. Mater. Charact. 2011, 62, 442–450. [Google Scholar] [CrossRef]
- Shao, H.; Asano, K.; Enoki, H.; Akiba, E. Preparation and hydrogen storage properties of nano-structured Mg-Ni BCC alloys. J. Alloys Compd. 2009, 477, 301–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.-X.; Lei, Y.-Q.; Wang, Q.-D. The reduction of cycling capacity degradation of Mg–Ni-based electrode alloys by Fe substitution. Int. J. Hydrogen Energy 2002, 27, 501–506. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T. Structural, thermodynamic, and electrochemical properties of TixZr1−x(VNiCrMnCoAl)2 C14 Laves phase alloys. J. Alloys Compd. 2008, 464, 238–247. [Google Scholar] [CrossRef]
- Etiemble, A.; Rousselot, S.; Guo, W.; Idrissi, H.; Roué, L. Influence of Pd addition on the electrochemical performance of Mg-Ni-Ti-Al-based metal hydride for Ni-MH batteries. Int. J. Hydrogen Energy 2013, 38, 7169–7177. [Google Scholar] [CrossRef]
- Tan, S.; Shen, Y.; Onur Şahin, E.; Noréus, D.; Öztürk, T. Activation behavior of an AB2 type metal hydride alloy for NiMH batteries. Int. J. Hydrogen Energy 2016, 41, 9948–9953. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; Ouchi, T.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy–Part 1 structural, hydrogen storage and electrochemical properties. J. Alloys Compd. 2016, 660, 407–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, X.; Gao, J.; Hu, F.; Qi, Y.; Zhao, D. Electrochemical hydrogen storage behaviors of as-milled Mg-Ti-Ni-Co-Al-based alloys applied to Ni-MH battery. Electrochim. Acta 2020, 342, 136123. [Google Scholar] [CrossRef]
- Iwakura, H.; Inoue, H.; Matsuoka, M.; Tamura, S. Electrochemical and structural properties of Mg–Ni hydrogen storage alloys. J. Less-Common Met. 1991, 172–174, 1051–1060. [Google Scholar]
- Werwiński, M.; Szajek, A.; Marczyńska, A.; Smardz, L.; Novak, M.; Jurczyk, M. Effect of substitution La by Mg on electrochemical and electronic properties in La2−xMgxNi7 alloys: A combined experimental and ab initio studies. J. Alloys Compd. 2018, 763, 951–959. [Google Scholar] [CrossRef]
- Ash, B.; Nalajala, V.S.; Popuri, A.K.; Subbaiah, T.; Minakshi, M. Perspectives on nickel hydroxide electrodes suitable for rechargeable batteries: Electrolytic vs. chemical synthesis routes. Nanomaterials 2020, 10, 1878. [Google Scholar] [CrossRef] [PubMed]



| Ball Milling, h | Mg | Ni | Al | V |
|---|---|---|---|---|
| 10 | 38.4 | 32.6 | 42.2 | 30.0 |
| 30 | 16.2 | 17.3 | 19.8 | 17.5 |
| 50 | 10.8 | 12.1 | 7.5 | 9.3 |
| Composition | Electrochemical Capacity (mAh/g) | Notes/Cycling Performance | References |
|---|---|---|---|
| Mg50Ni12.5Al12.5V12.5Fe12.5 | 257 | 235 mAh/g, 10 cycles | This work |
| Mg1−xTix+yNiy (x = 0.15, 0.2; y = 0, 0.05, 0.1) | 542–442 | ≈350 mAh/g, 20 cycles | [11] |
| Mg1-xAlxNi (x = 0.1, 0.2) | 404, 350 | 234 and 231 mAh/g, 20 cycles | [12] |
| Mg50Ni50, Mg45Pd5Ni50 | 445, 315.8 | ≈60 and 150 mAh/g, 20 cycles | [13] |
| Mg55Ni45, Mg55Pd3Ni42 and Mg55Pd4Ni41 | 488.65, 543 and 564.6 | ≈110 mAh/g, 13 cycles 181 and 287 mAh/g, 50 cycles | [22] |
| MgNi–FeB 15h MA | 317 | ≈200 mAh/g, 50 cycles | [23] |
| MgNi-CoB | 254.9–440 | 113.1–174.8 mAh/g, 30 cycles | [24] |
| Mg71Ni29 and Mg72.6Ni25.3Gd1.1 | 134.3 and 91.9 | ≈30 mAh/g, 10 cycles | [25] |
| La2−xMgxNi7(x = 0.25, 0.5, 0.75) | 304–220 | 225–150 mAh/g, 30 cycles | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorova, E.; Çakmak, G.; Yüce, H.; Markov, P. Electrochemical and Gas-Solid Hydrogen Storage Properties of a Multi-Metal Magnesium-Based Alloy Obtained by Ball Milling. Inorganics 2025, 13, 299. https://doi.org/10.3390/inorganics13090299
Grigorova E, Çakmak G, Yüce H, Markov P. Electrochemical and Gas-Solid Hydrogen Storage Properties of a Multi-Metal Magnesium-Based Alloy Obtained by Ball Milling. Inorganics. 2025; 13(9):299. https://doi.org/10.3390/inorganics13090299
Chicago/Turabian StyleGrigorova, Eli, Gülhan Çakmak, Hakan Yüce, and Pavel Markov. 2025. "Electrochemical and Gas-Solid Hydrogen Storage Properties of a Multi-Metal Magnesium-Based Alloy Obtained by Ball Milling" Inorganics 13, no. 9: 299. https://doi.org/10.3390/inorganics13090299
APA StyleGrigorova, E., Çakmak, G., Yüce, H., & Markov, P. (2025). Electrochemical and Gas-Solid Hydrogen Storage Properties of a Multi-Metal Magnesium-Based Alloy Obtained by Ball Milling. Inorganics, 13(9), 299. https://doi.org/10.3390/inorganics13090299











