Abstract
Pyridinecarbonitriles (pyCN), also referred to as cyanopyridines, are promising ligands for the formation of pyridine-based coordination compounds due to their two different N-donor atoms, which enable versatile coordination modes. Copper(II) complexes containing pyCN derivatives are of particular interest for their potential applications in medicinal chemistry and materials science. In this study, the synthesis, structural characterization, and thermal and magnetic properties of three new copper(II) complexes with 3-pyCN, 4-pyCN, and ethyl picolinimidate, obtained in situ by means of alcoholysis of 2-pyCN, are reported: [Cu2(μ-Ac)4(3-pyCN)2] (1), [Cu(H2O)2(Etpic)2]NO3 (2), and [Cu(NO3)2(CH3CN)(4-pyCN)2]·CH3CN (3). Single-crystal X-ray diffraction confirmed that complex 1 features a dinuclear paddle-wheel structure with bridging acetato ligands and monodentate 3-pyCN molecules, coordinated through the ring nitrogen, while complexes 2 and 3 are mononuclear. Thermal analysis showed an intense and highly exothermic decomposition of complex 3, containing nitrate ligands. Magnetic measurements revealed strong antiferromagnetic coupling in the dinuclear complex 1, whereas complexes 2 and 3 displayed paramagnetic behavior with effective magnetic moments ranging from 1.8 μB to 2.0 μB, consistent with isolated Cu(II) centers.
1. Introduction
Despite a long history and numerous publications in this field [1], copper coordination compounds remain an active area of research due to their structural diversity, accessible redox states, and functional properties, which make them valuable in areas ranging from catalysis and magnetism to bioinorganic and materials chemistry [2,3,4]. Being an essential trace metal, present in various enzymes in mammalian cells due to its ability to adopt various oxidation states (+1 and +2), Cu is a good choice for the synthesis of complexes with biological and medicinal applications [5]. A large number of novel copper complexes have been reported recently, exhibiting promising antibacterial [6,7,8,9], antifungal [9], anticancer/antitumor [7,10,11,12], antiproliferative [13], and antioxidant [8,14,15] activities. The coordination behavior of copper(I) and copper(II) is notably versatile, enabling the formation of discrete complexes as well as extended architectures, depending on the nature of the ligands and reaction conditions [16].
However, the preparation of metal-based drugs is not an easy task, with the proper selection of ligands being one of the most influential factors contributing to the desired final geometry of the complexes [5]. Among various possible ligands, N-donor ligands play a crucial role in the construction of copper coordination compounds due to their strong affinity for copper ions, enabling the formation of stable complexes with diverse geometries, facilitating applications in catalysis, medicine, and materials science [17]. The usefulness of the pyridine framework in the construction of coordination compounds with biological and medicinal applications and interesting magnetic properties is well known [18,19]. Among the numerous N-donor ligands, pyridinecarbonitriles (pyCN), also known as cyanopyridines, have emerged as promising building blocks for the construction of coordination compounds and networks [20]. These ligands feature both a pyridine nitrogen and a cyano-group, offering multiple coordination modes and geometric flexibility [21]. Their dual character, featuring an electron-withdrawing cyano-group over an electron-accepting pyridine ring [5], allows them to act as bridging or terminal ligands, depending on the metal center and reaction environment [22]. Due to its weaker Lewis-base character, the donating ability of cyano-nitrogen is weaker than that of pyridine nitrogen; hence, structures with bridging cyanopyridine groups are comparatively rare [23]. In addition to their coordination versatility, cyanopyridines are of interest due to their potential applications in medicinal chemistry. Although the first reports on the successful preparation of amine oxidase inhibitors based on 4-cyanopyridine (4-pyCN) ligands already appeared three decades ago [24], this area continues to be the subject of active and ongoing research. For instance, 2-pyCN derivatives have recently been reported to allow cysteine-selective peptide bond cleavage, making them an attractive choice for the treatment of prostate cancer [25]. Cu(II) complexes with 2-pyCN were found to exhibit a fair antioxidant potential and good inhibition properties [5]. Outside of the medicinal chemistry area, 2-pyCN has been reported to have a good inhibition effect for the corrosion of mild steel in acidic conditions [26]. Derivatives of 3-pyCN have recently been under investigation due to their activity against the Trypanosoma cruzi parasite, which affects millions of people, mostly in Latin America [27]. Cu(I) coordination polymers with the 3-pyCN ligand have shown promise as compounds with special structural architectures and excellent photoluminescence properties [28].
Besides copper complexes, pyCN coordination compounds of other Period 4 transition metals have shown potential in various research areas. Co(II) complexes with pyCN derivatives have recently been investigated for their antioxidant and antimicrobial activities [29] and promising magnetic behavior [30]. Banik et al. investigated different coordination compounds of Co(II) and Ni(II) with 3-pyCN, revealing non-covalent interactions, which provide rigidity to the crystal structure, and the possibility to effectively bind with the active sites of anti-apoptotic proteins, which are involved in cancer progression [31,32]. Iron(II) complexes with 3-pyCN and NCS co-ligands have also been reported recently [33].
From a structural perspective, copper(II) complexes with bidentate ligands form a significant and well-studied class of coordination compounds, with carboxylates being among the most prevalent. A notable feature of copper carboxylate compounds is the formation of the paddle-wheel structure, typically described by the general formula [Cu2(RCOO)4L2], where L denotes an apical ligand. These dinuclear units are characterized by their high symmetry and structural stability, leading to discrete molecular assemblies when the apical ligands are monodentate. To date, over 1500 copper(II) paddle-wheel complexes have been reported in the literature [34,35,36]. The magnetic properties of copper(II) complexes remain the subject of active research owing to their significance in biological chemistry [37], with particular attention given to dinuclear copper(II) species. In such systems, the magnetic behavior is governed by spin configuration and exchange coupling between the copper centers. Depending on the nature of the bridging ligands, these complexes may exhibit ferromagnetic, antiferromagnetic, or even spin-frustrated interactions [38,39]. In this context, our study introduces new copper(II) complexes that provide additional insights into structure–property relationships and expand the scope of systems available for magnetic investigation.
As a continuation of our ongoing research in the area of copper coordination compounds with N-donor ligands [40,41], we report the synthesis and structural characterization of three new copper(II) coordination compounds with 3- and 4-pyCN ligands, as well as a ligand, obtained through in situ solvolysis of 2-pyCN, in the present study. The resulting complexes exhibit diverse coordination motifs, including paddle-wheel structures, elongated octahedra, and distorted square pyramids. All compounds were further characterized through elemental analysis, FTIR spectroscopy, thermal analysis (including identification of decomposition products by means of powder X-ray diffraction), and magnetic susceptibility measurements.
2. Results and Discussion
2.1. Synthesis and General Aspects
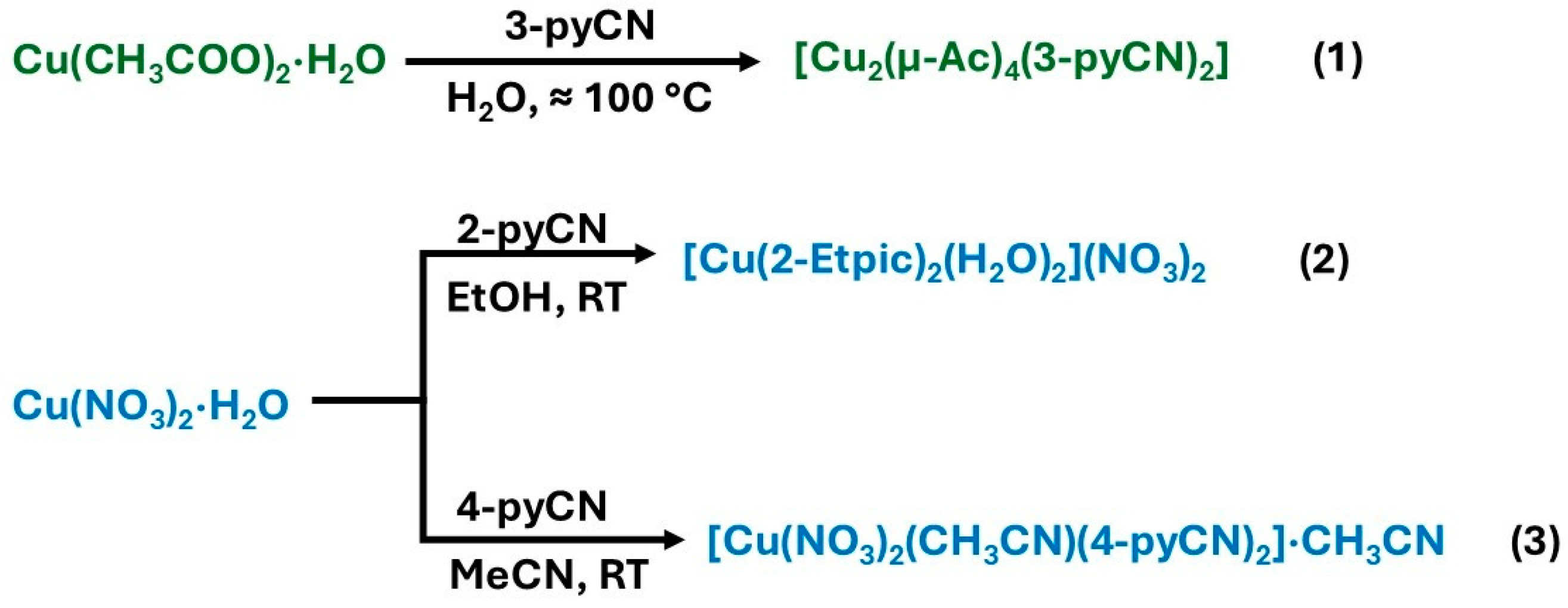
The Cu(II) complexes 1–3 were synthesized in good yield from aqueous (1), ethanolic (2), or acetonitrile (3) solutions. It is noteworthy to mention that a complete series of reactions with three different ligands (2-pyCN, 3-pyCN, and 4-pyCN), two copper(II) salts (acetate and nitrate), and three solvents (i.e., water, ethanol, and acetonitrile) was performed during the study. Unfortunately, the three title compounds are the only new structures that could be obtained. Several experiments yielded well-crystallized copper coordination compounds, which correspond to previously reported structures. For example, the reaction of copper(II) acetate with 4-pyCN in water led to the formation of a complex reported by Das and Barman [21] featuring a paddle-wheel structure with apical 4-pyCN ligands, structurally similar to compound 1. Additionally, the reaction of 2-pyCN with both aforementioned copper(II) salts in water resulted in the formation of known copper complexes featuring picolinamide and picolinate ligands, arising from in situ hydrolysis of 2-pyCN [42,43,44]. The summary of the reactions is presented in Figure 1.
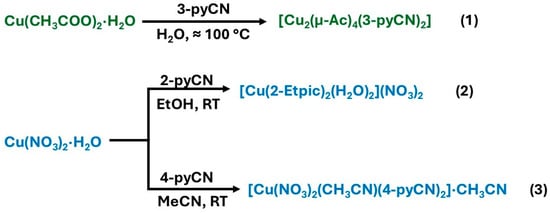
Figure 1.
Synthesis pathways of complexes 1, 2, and 3.
2.2. Description of Structures
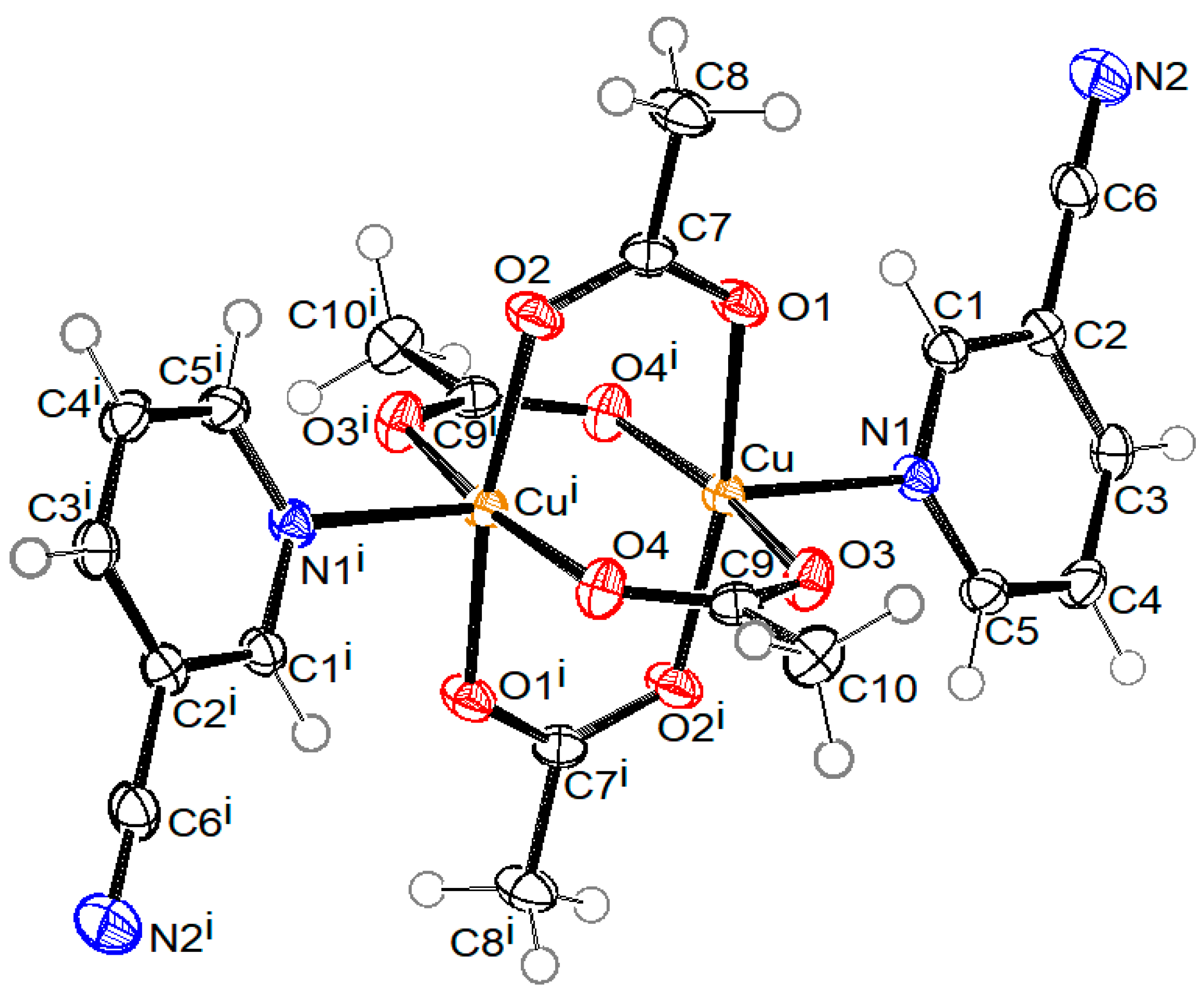
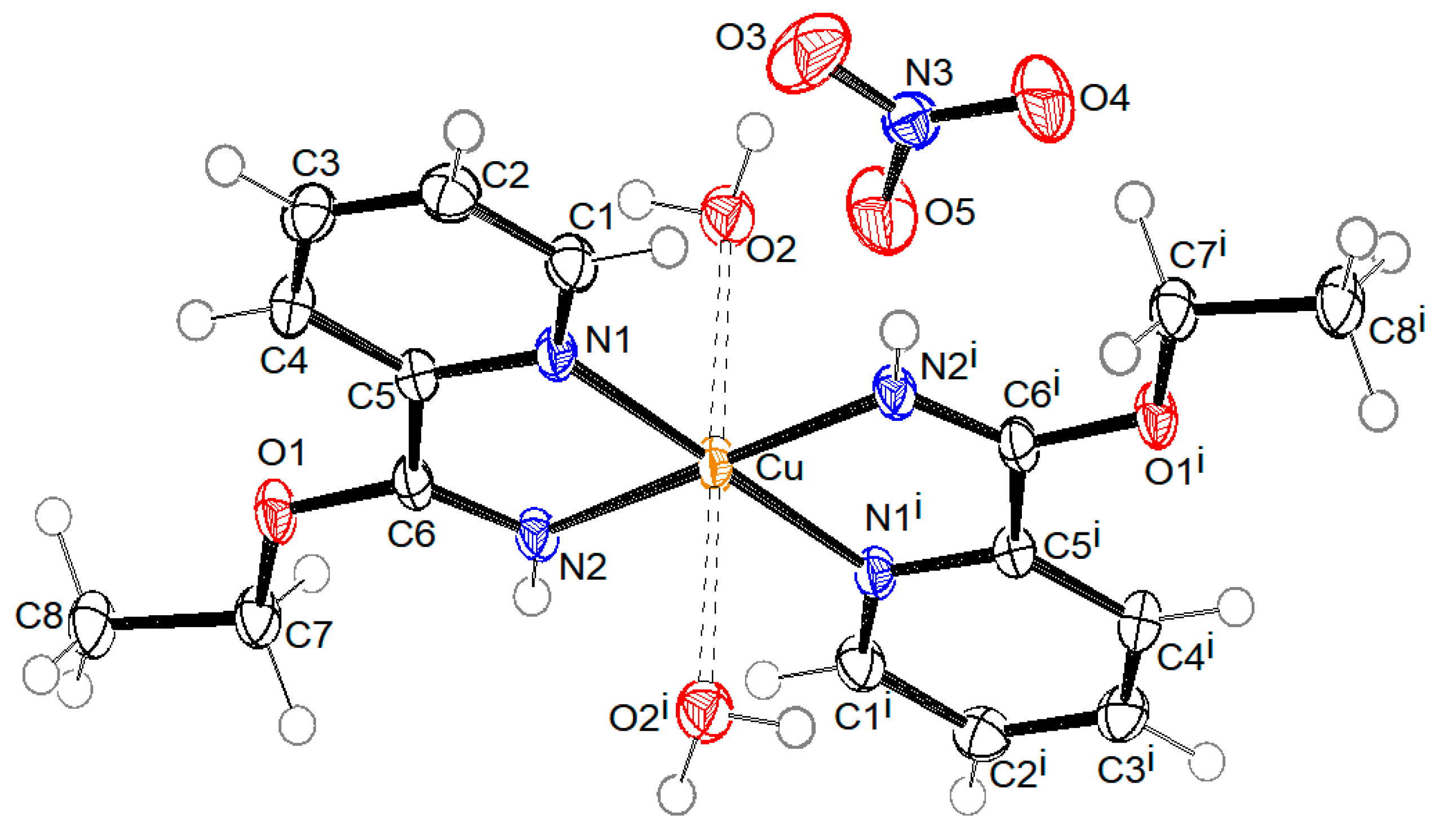
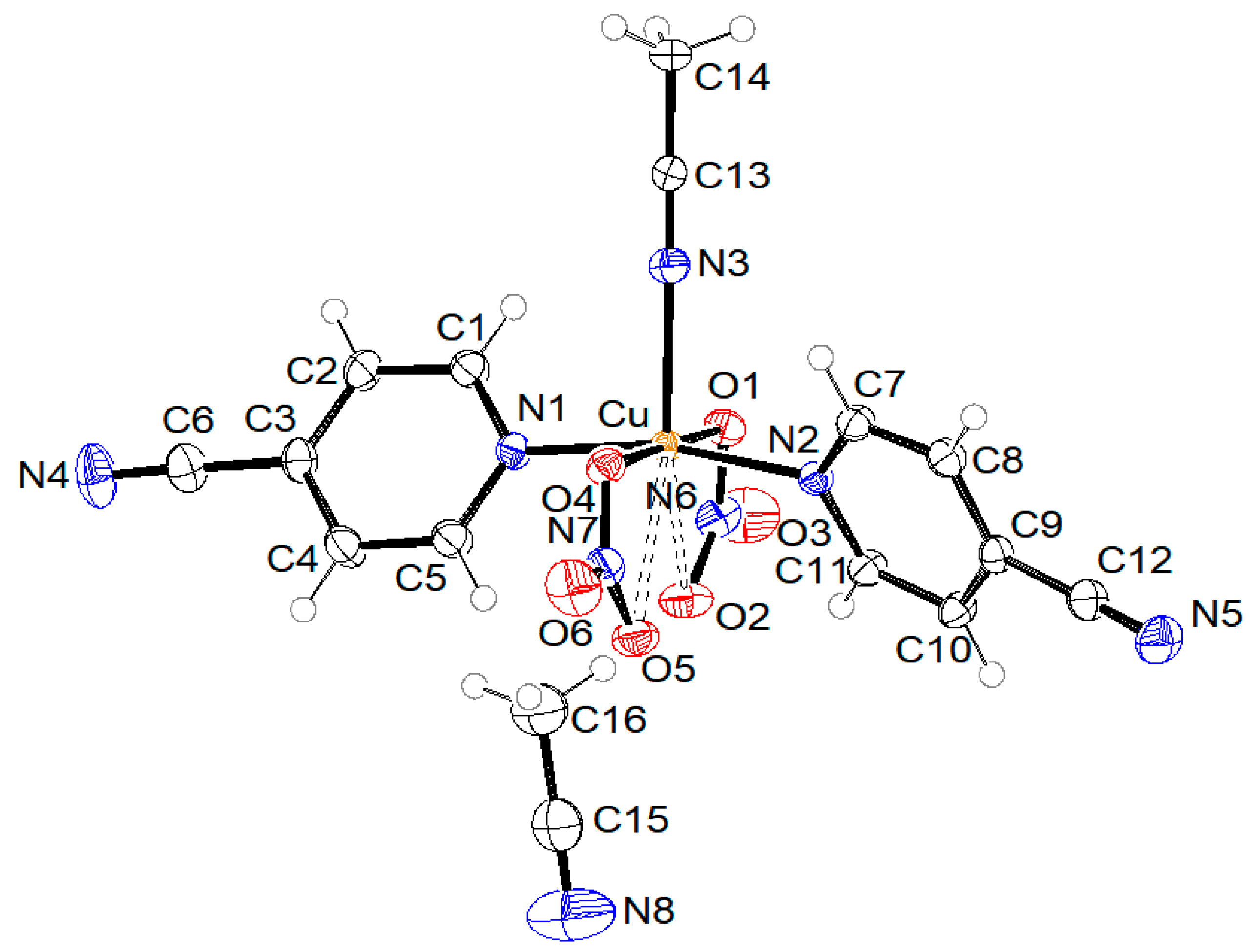
ORTEP drawings of crystal structure of compound 1 with the formula [Cu2(µ-Ac)4(3-pyCN)2], compound 2 with the formula [Cu(2-Etpic)2(H2O)2](NO3)2, and compound 3 with formula [Cu(NO3)2(CH3CN)(4-pyCN)2]·CH3CN are shown in Figure 2, Figure 3, and Figure 4, respectively. Coordination bond lengths and angles in all three compounds are given in Table 1. All bond lengths and angles are available in the Supplementary Materials in Tables S1–S3 for compounds 1, 2, and 3, respectively.
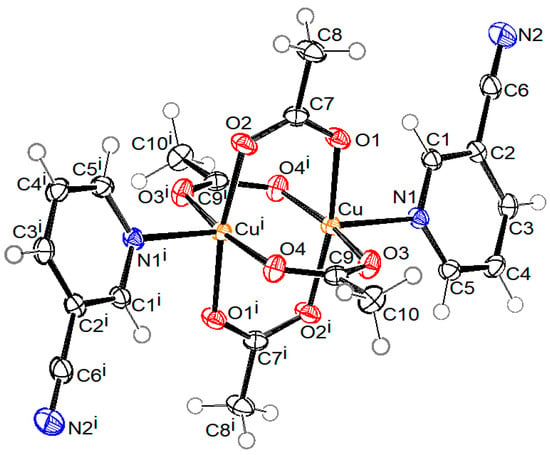
Figure 2.
ORTEP drawing of compound 1 with labelling of non-H atoms. Ellipsoids are drawn at the 50% level. (i: 2 − x, −y, 1 − z).
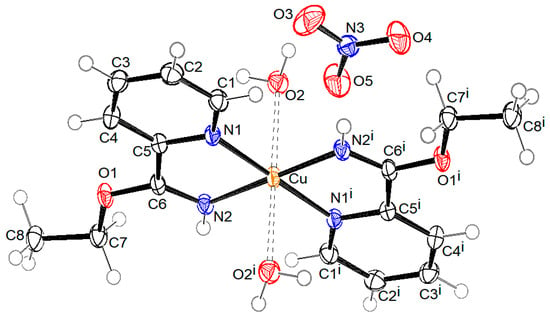
Figure 3.
ORTEP drawing of compound 2 with labelling of non-H atoms. Ellipsoids are drawn at the 50% level. (i: 1 − x, 1 − y, −z).
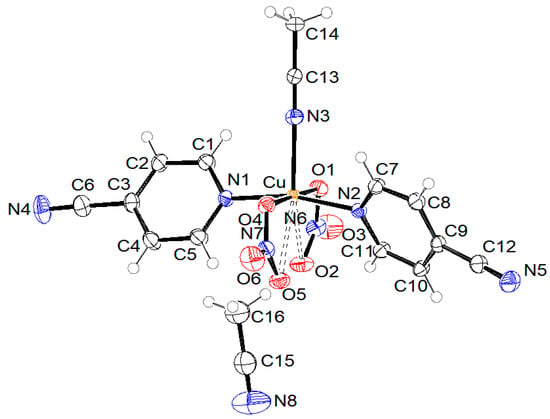
Figure 4.
ORTEP drawing of compound 3 with labelling of non-H atoms. Ellipsoids are drawn at the 50% level.

Table 1.
Selected interatomic distances (Å) and angles (°) for compounds 1–3.
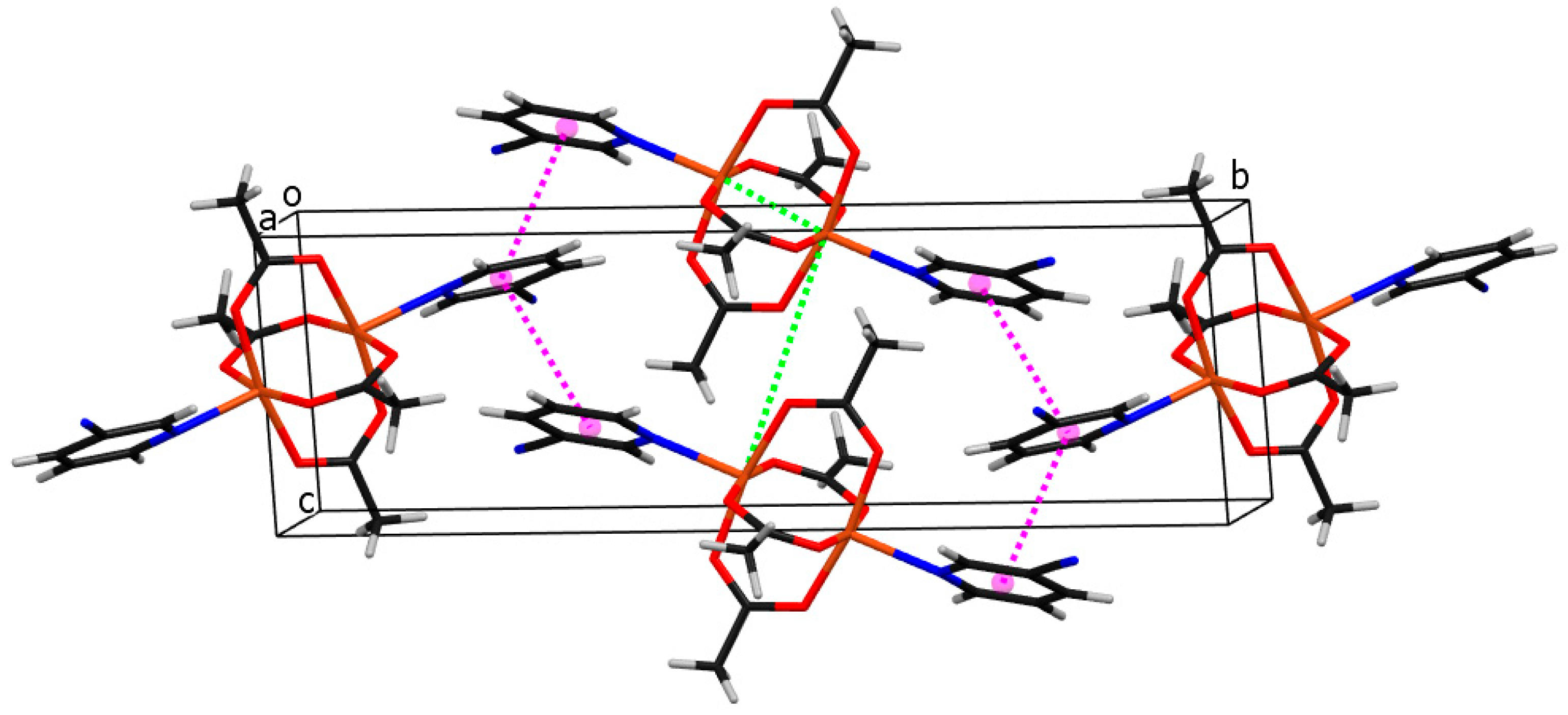
As shown in Figure 2, the crystal structure of compound 1 consists of binuclear centrosymmetric coordination molecules where two copper(II) central atoms are bridged by four acetato bridges in syn-syn mode, forming a paddle-wheel structure type. Each copper atom is coordinated by four acetato oxygen atoms, which form a basal plane of the square pyramid, and by the aromatic nitrogen atom of the 3-pyCN ligand lying on the apex of the aforementioned (distorted) square pyramid. The bond lengths of Cu–O range from 1.9586(15)–1.9752(15) Å. The Cu–N bond is substantially longer (2.1862(16) Å), which can be explained as a consequence of the Jahn–Teller effect. The copper atom lies 0.178(1) Å away from the basal plane. The separation between copper atoms from the same molecule is 2.5912(6) Å. The shortest distance between copper atoms from neighboring molecules is 6.4520(6) Å. Both contacts are shown with a green dotted line in Figure 5, presenting the crystal packing of compound 1. Pink dotted lines represent contacts between ring centroids of heteroaromatic ligands of neighboring molecules, which stabilize the crystal structure of compound 1. The angle and distance between neighboring rings are 25.93(10)° and 4.0840(12) Å, respectively. The structure corresponds to the well-documented class of binuclear Cu(II) complexes with paddle-wheel structures, which typically display Cu-O bond lengths in the range between 1.962 and 1.983 Å, Cu-N axial bond lengths between 2.149 and 2.201 Å, and Cu···Cu separations of 2.600 Å–2.692 Å, which is shorter than the sum of the Van der Waal’s radii of 2.8 Å [5,21,34,35,36,41].
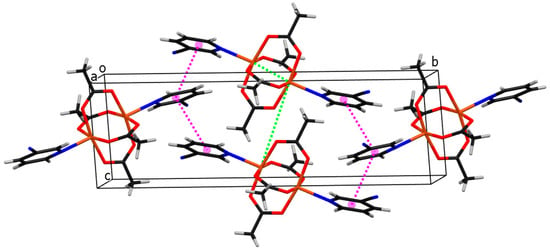
Figure 5.
The crystal packing of molecules of compound 1. Cu, C, H, N, O atoms and ring centroids are drawn in orange, black, white, blue, red, and pink colors, respectively. The closest Cu···Cu separations within and between coordination molecules are marked with green dotted lines.
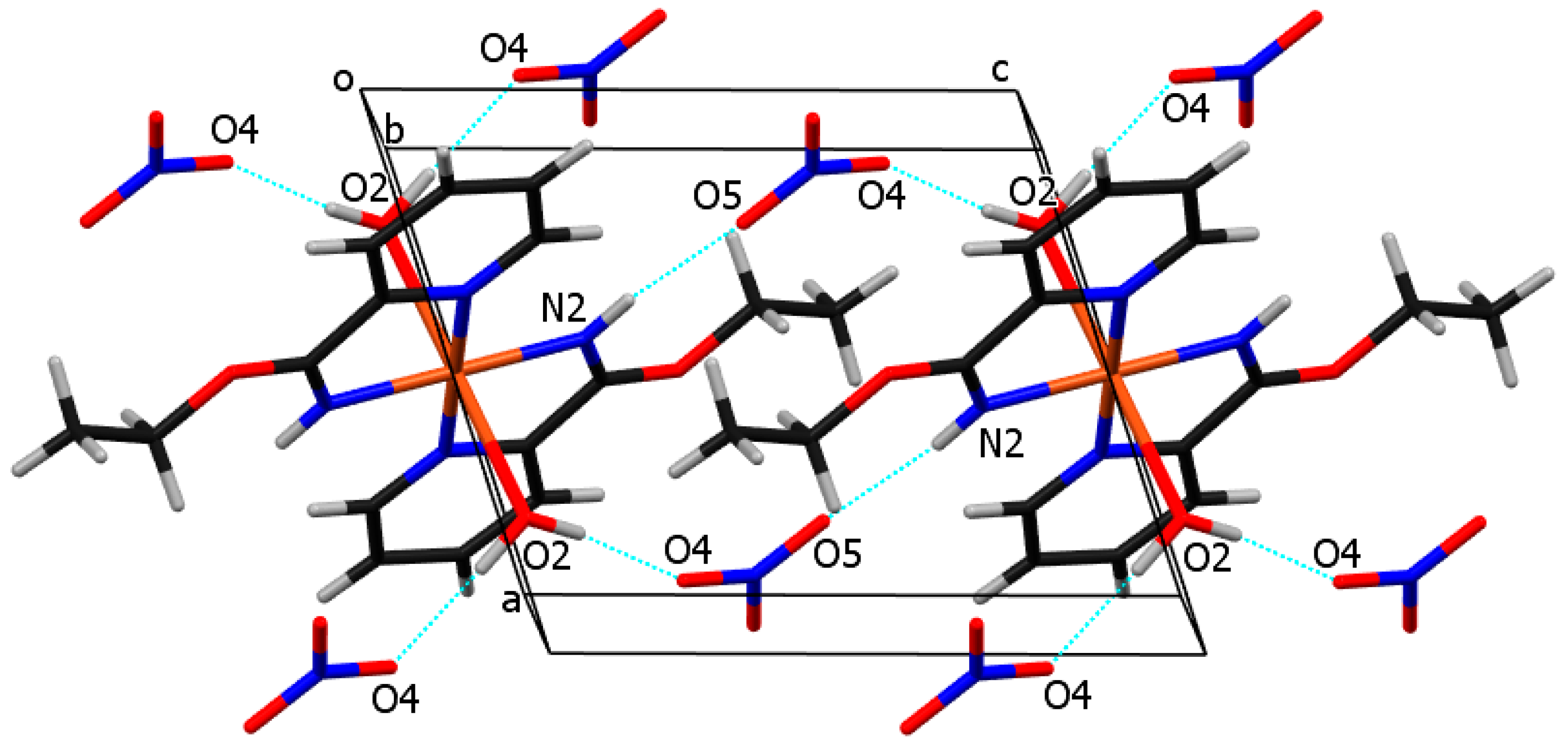
The crystal structure of compound 2 consists of mononuclear centrosymmetric coordination cations and nitrate anions. The copper(II) central ion is six (4 + 2) coordinated in the shape of an elongated distorted octahedron. The equatorial plane is defined by four N atoms of two ethyl picolinimidato (2-Etpic) ligands and the axial positions occupied by two water molecules, as presented in Figure 3. Bond lengths Cu-O2 2.4670(17) Å are significantly longer in comparison to Cu–N 1.9897(17) Å (Cu-N2) and 2.0339(17) Å (Cu-N1) due to the Jahn–Teller effect. The bond distance between C6 and N2 1.276(3) Å is in agreement with the imine double bond. Figure 6 shows the crystal packing in compound 2. Coordination cations and nitrate anions are hydrogen-bonded through O-H···O hydrogen bonds, donated by water molecules, and through N-H···O hydrogen bonds, donated by the imine group. The geometric parameters of hydrogen bonds are given in Table 2. The shortest distance between copper atoms from neighboring molecules is 7.3037(5) Å, corresponding to molecules translated for one period along the b axis.
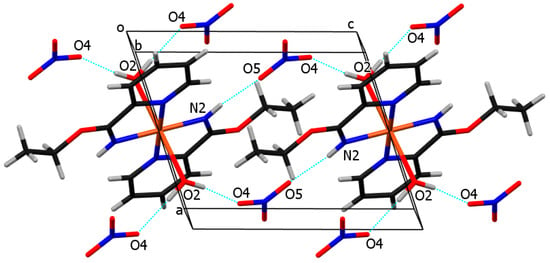
Figure 6.
Crystal structure of compound 2, showing hydrogen bonds (cyan). H atoms from aromatic rings were omitted for clarity. Cu, C, H, N, O atoms are drawn in orange, black, white, blue, and red olors, respectively.

Table 2.
Hydrogen bond geometry for compound 2.
The presence of the unexpected 2-Etpic ligand observed in complex 2 can be attributed to the solvolysis of 2-pyCN in ethanol. The nitrile group is susceptible to nucleophilic attack and, under the reaction conditions, undergoes alcoholysis to form corresponding carboximidates—in our case, ethyl picolinimidate (also referred to as ethyl pyridine-2-carboximidate). Similar copper-catalyzed in situ transformations of nitrile-containing ligands in alcoholic media have been reported in the literature [45], where four related complexes with comparable Cu-N distances of 1.989–2.038 Å and a distinctly longer Cu-O bond of 2.275 Å were described.
The crystal structure of compound 3 is built from mononuclear coordination molecules and solvent acetonitrile molecules. The copper(II) central ion is five-coordinated in the shape of a distorted square pyramid by two O atoms from two nitrate anions and three N atoms from one acetonitrile and two 4-pyCN molecules, as presented in Figure 4. A basal plane is formed by two nitrate O atoms at distances of 1.9881(11) and 2.0009(11) Å and two heteroaromatic N atoms from 4-pyCN ligands at distances of 2.0139(13) and 2.0164(12) Å. The apex is the N3 atom of the acetonitrile molecule at a significantly longer distance of 2.2416(14) Å due to the Jahn–Teller effect. The copper atom lies 0.120(1) Å away from the basal plane. At an even greater distance from the central atom are the oxygen atoms O2 and O5 (2.7170(11) and 2.7830(11) Å). Taking into account the bonding of these two oxygen atoms, we would describe the coordination of nitrate ions to copper(II) as chelating. Half of all acetonitrile molecules are coordinated to the copper(II) ion, and the other half of the solvent molecules are uncoordinated. Non-hydrogen atoms of uncoordinated acetonitrile C15, C16, and N8 lie on a twofold rotation axis. Hydrogen atoms are disordered due to the symmetry at two positions with 50% occupancy, each. When compared to related mononuclear copper(II) complexes containing pyridine derivatives and nitrate ligands, the Cu-N(heteroaromatic) distances in 3 are slightly longer than those observed in the picolinamide complex reported in [44], which are in the range of 1.975 Å. In contrast, the Cu-O(nitrate) bond in 3 is markedly shorter than the 2.820 Å reported in [44], indicating stronger coordination of the nitrate ligand. The Cu-O(nitrate) distances in 3 are consistent with values reported for two analogous copper(II) compounds with triazine and nitrate ligands, which exhibit strongly asymmetric nitrate bonding with two short (1.982–2.040 Å) and two long (2.640–2.875 Å) Cu-O bonds [46].
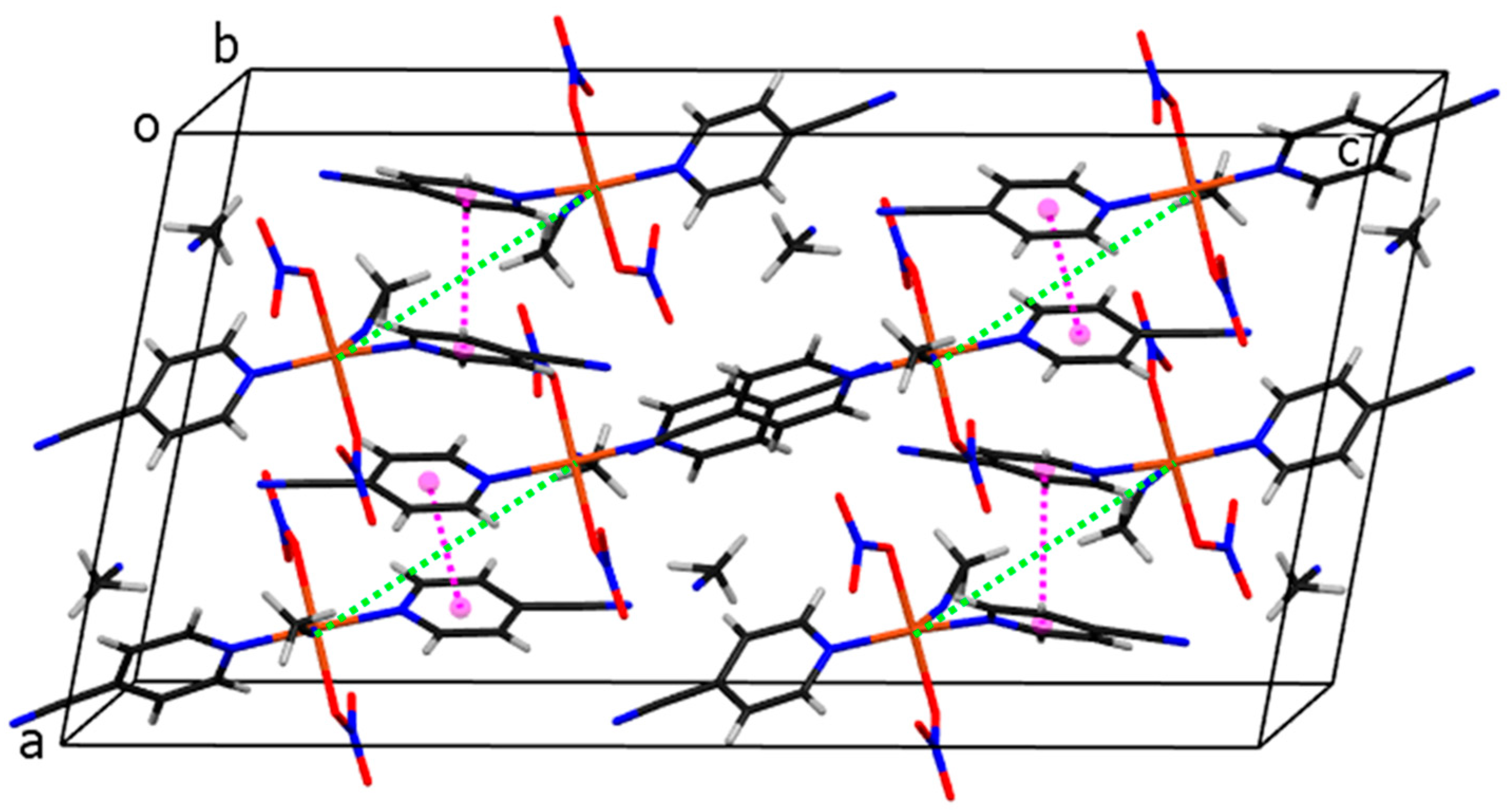
The arrangement of molecules in the unit cell is shown in Figure 7. The shortest distance between copper atoms from neighboring molecules is 7.2600(6) Å (shown with green dotted lines). Pink dotted lines represent contacts between ring centroids of heteroaromatic ligands of neighboring molecules, which stabilize the crystal structure of compound 3. The angle and distance between neighboring rings are 0.03(7)° and 3.9449(9) Å, respectively.
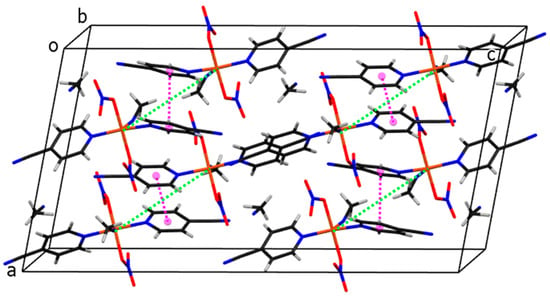
Figure 7.
The crystal packing of molecules of compound 3. Cu, C, H, N, O atoms and ring centroids are drawn in orange, black, white, blue, red, and pink colors, respectively. The closest Cu···Cu separations are marked with green dotted lines.
2.3. Thermal Analysis
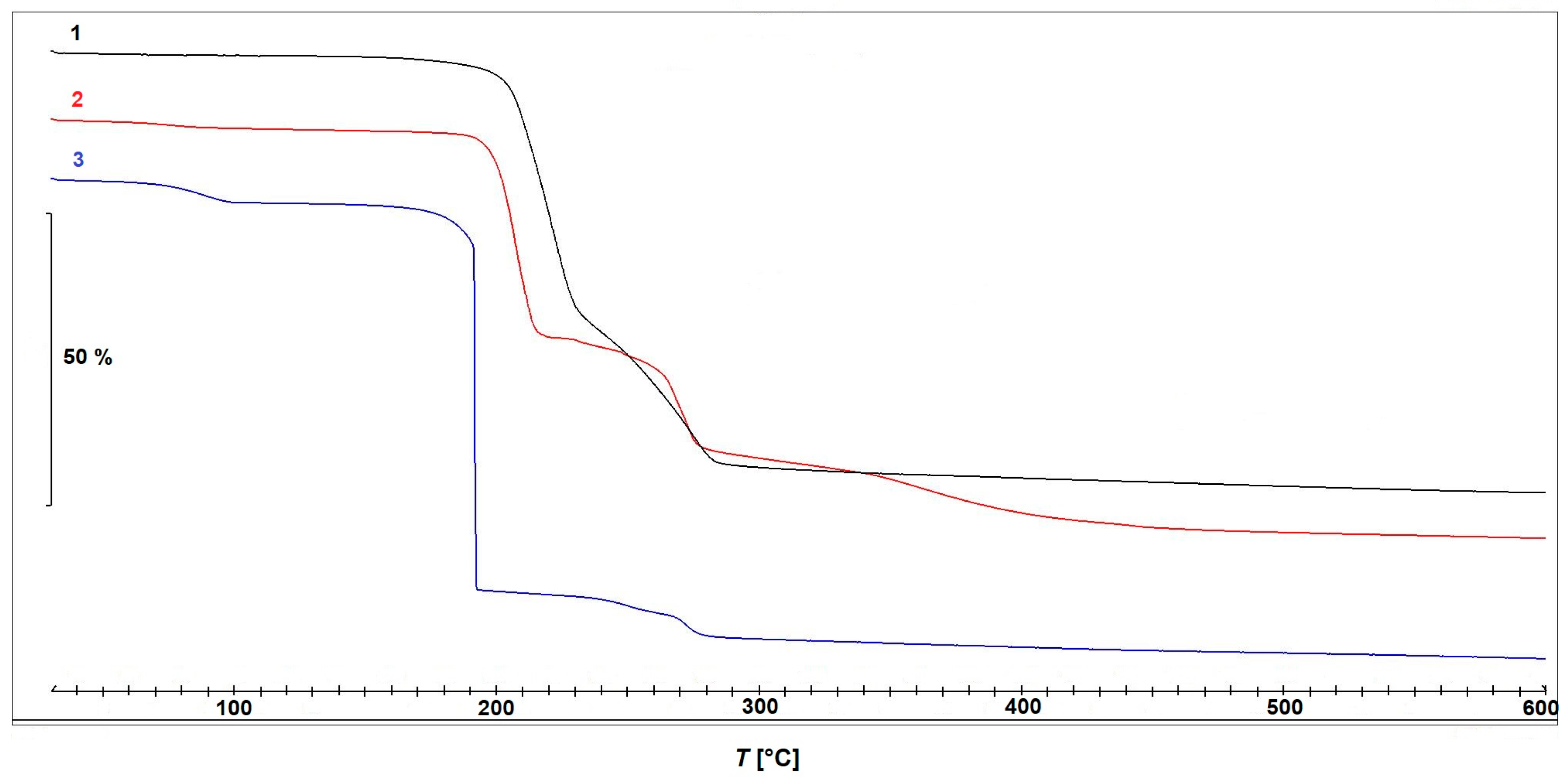
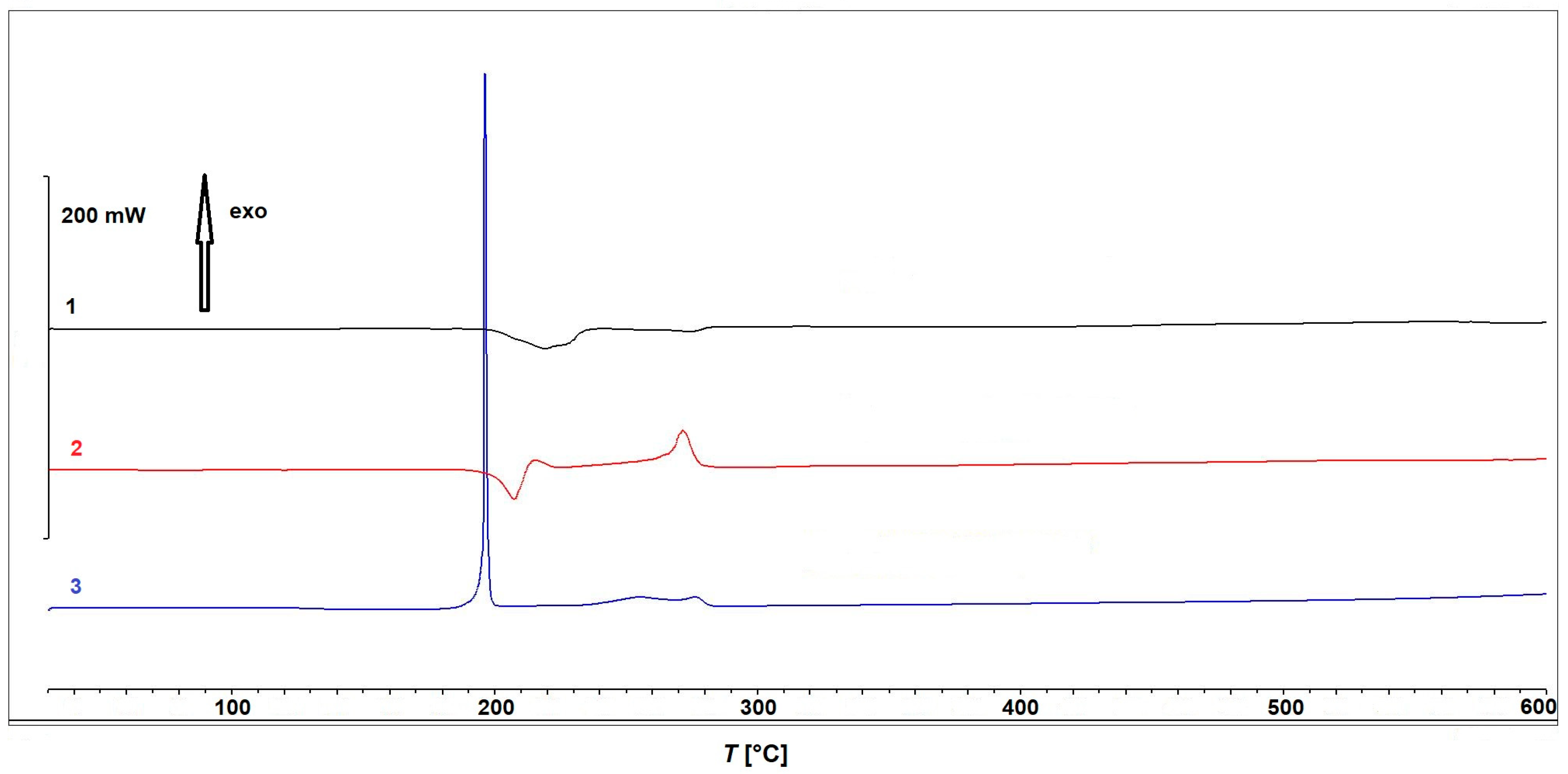
The results of the thermogravimetric measurements of compounds 1–3 are presented in Figure 8.
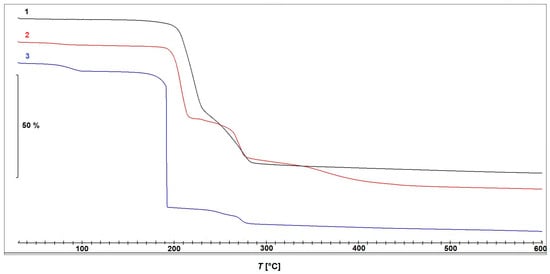
Figure 8.
Thermogravimetric curves obtained during thermal decomposition of compounds 1–3.
Compound 1 is thermally stable up to approximately 150 °C and decomposes in two partially overlapping steps. The first step occurs between 180 °C and 240 °C, with a mass loss of 45.66% and a peak decomposition temperature at 220 °C. This is immediately followed by a second step between 240 °C and 285 °C, exhibiting a mass loss of 25.01% and a peak at 270 °C. Although the decomposition steps are not well separated, making precise assignments difficult, the observed mass losses suggest that the first step can be attributed to the release of the bridging acetate ions, while the second step is likely associated with the elimination of 3-pyCN. The observed total mass loss of approximately 78% between room temperature (RT) and 600 °C aligns well with the calculated value for the decomposition of 1 to elemental copper (Δmcalc = 77.78%).
Compound 2 exhibits a minor mass loss of <2% at approximately 100 °C, which can again be attributed to the loss of the solvent, which was ethanol in this particular case. The complex remains thermally stable up to 180 °C, after which it undergoes three consecutive decomposition steps with peak temperatures at 208 °C, 270 °C, and approximately 360 °C. Based on the mass losses, the first step could be most plausibly assigned to a thermal decomposition of the in situ formed ligand 2-Etpic, consistent with ethanol elimination. The corresponding DSC curve, shown in Figure 9, reveals an endothermic process in the first step, followed by an exothermic event during the second step, while the third step occurs without a distinct DSC signal, suggesting a less energetic or overlapping transformation. Finally, complex 3 decomposes in three steps, with the second one being the most prominent. The first minor mass loss occurring between RT and 100 °C corresponds well with the loss of one uncoordinated acetonitrile molecule (Δmcalc = 4.48%). The compound remains thermally stable up to 160 °C, followed by a rapid and intense decomposition with a mass loss of 66.09% and a sharp peak at 190 °C. This decomposition is clearly dominated by the strongly oxidative nitrate ligands, preventing a clear and stepwise assignment of individual reactions. A third, minor decomposition step with an additional mass loss of 7.23% takes place between 230 °C and 280 °C. The corresponding DSC curve (Figure 9) shows a prominent exothermic peak in the temperature range corresponding to the major mass loss observed on the TGA curve, supporting the occurrence of a highly energetic process likely driven by the presence of nitrate ligands. The observed total mass loss of approximately 85% between RT and 600 °C aligns well with the calculated mass loss for the decomposition of 3 to elemental copper (Δmcalc = 86.12%).
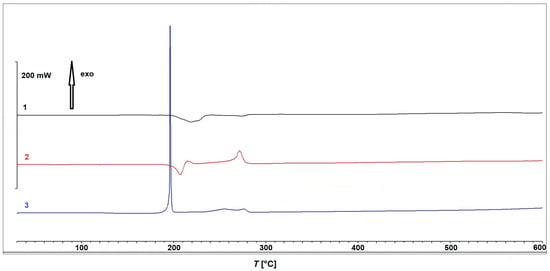
Figure 9.
DSC curves for compounds 1–3.
A comparison of the TGA and DSC data clearly highlights distinct differences in thermal behavior between the investigated compounds. Complex 3, containing coordinated nitrate ions, exhibits intense and highly exothermic decomposition events over narrow temperature ranges. In contrast, nitrate-free complexes 1 and 2 decompose in a more gradual and less energetic manner, lacking pronounced exothermic peaks or abrupt mass losses. These findings are consistent with well-established trends in the thermal chemistry of coordination compounds: nitrate ligands, due to their strong oxidizing character, often promote internal redox reactions upon heating, leading to exothermic decomposition. Similar behavior has been reported in the literature for other complexes containing nitrate ligands and analogous nitrogen-rich units [47,48,49], and such compounds have been investigated for potential use as novel energetic materials [50].
Detailed thermogravimetric curves of all three compounds, including first derivative curves and mass losses for the particular steps, are provided as Supplementary Materials (Figures S1–S3). All residues obtained after thermal decomposition of (1–3) could be identified through powder X-ray diffraction as elemental copper, JCPDS No. 00-004-0836.
2.4. FT-IR Spectra
Characteristic absorption bands in the IR spectra of the title compounds are presented in Table 3. The C–H stretching vibrations in compound 1 appear around 3050 cm−1 and were used as a reference for comparison across compounds 1–3.

Table 3.
Characteristic absorption bands in the IR spectra of the title compounds.
For the determination of the asymmetric and symmetric vibrations of N–H bonds in compound 2, the equations:
or
can be used [51].
Some vibrations around 2700 cm−1 are assigned as O–H stretching vibrations for compound 2.
The stretching vibrations of C≡N can be observed around 2300–2200 cm−1 and were compared for compounds 1 and 3. Due to differences in the bonding modes of the nitrile groups—namely, free acetonitrile, coordinated acetonitrile, and coordinated 4-cyanopyridine—variations in the stretching frequencies are expected. According to literature data, coordinated acetonitrile typically exhibits higher C≡N stretching frequencies, around 2300 cm−1 [52], while values below 2250 cm−1 are characteristic of coordinated 4-cyanopyridine [53]. Bands appearing between 2250 and 2300 cm−1 can be attributed to uncoordinated acetonitrile. Strong C–O stretching bands can be observed in compounds 1 and 2 between 1680 and 1630 cm−1. Additionally, C=N stretching vibrations are observed in the range of 1650–1600 cm−1 and can be compared across all compounds.
For carboxylate groups, the difference between the asymmetric and symmetric carboxylate stretches (Δ = νas(COO−) − νs(COO−)) is often used to distinguish between monodentate, ionic, bridging, or chelating carboxylate groups:
Δ (monodentate) > Δ (ionic) > Δ (bridging bidentate) > Δ (chelating bidentate)
The calculated Δ values for the bridging bidentate acetate in compound 1 are 166 and 153 cm−1, consistent with literature values for bidentate coordination, which are typically below 170 cm−1 [54,55].
Nitrate ions, independent of whether present as ionic, monodentate, or bidentate chelate, typically display two absorption bands in the 1650–1150 cm−1 region. One additional band should be observed around 1000 cm−1, rocking modes around 800 cm−1, and bending modes around 700–800 cm−1 [56].
The IR spectra also reveal differences between the presence of C–H bending of orto-substituted aromatic rings in the range of 790 and 740 cm−1, meta-substituted of 820 and 770 cm−1 and para-substituted of 850 and 790 cm−1.
Figures of the FTIR spectra are provided as Supplementary Materials (Figures S4–S6).
2.5. Magnetic Properties
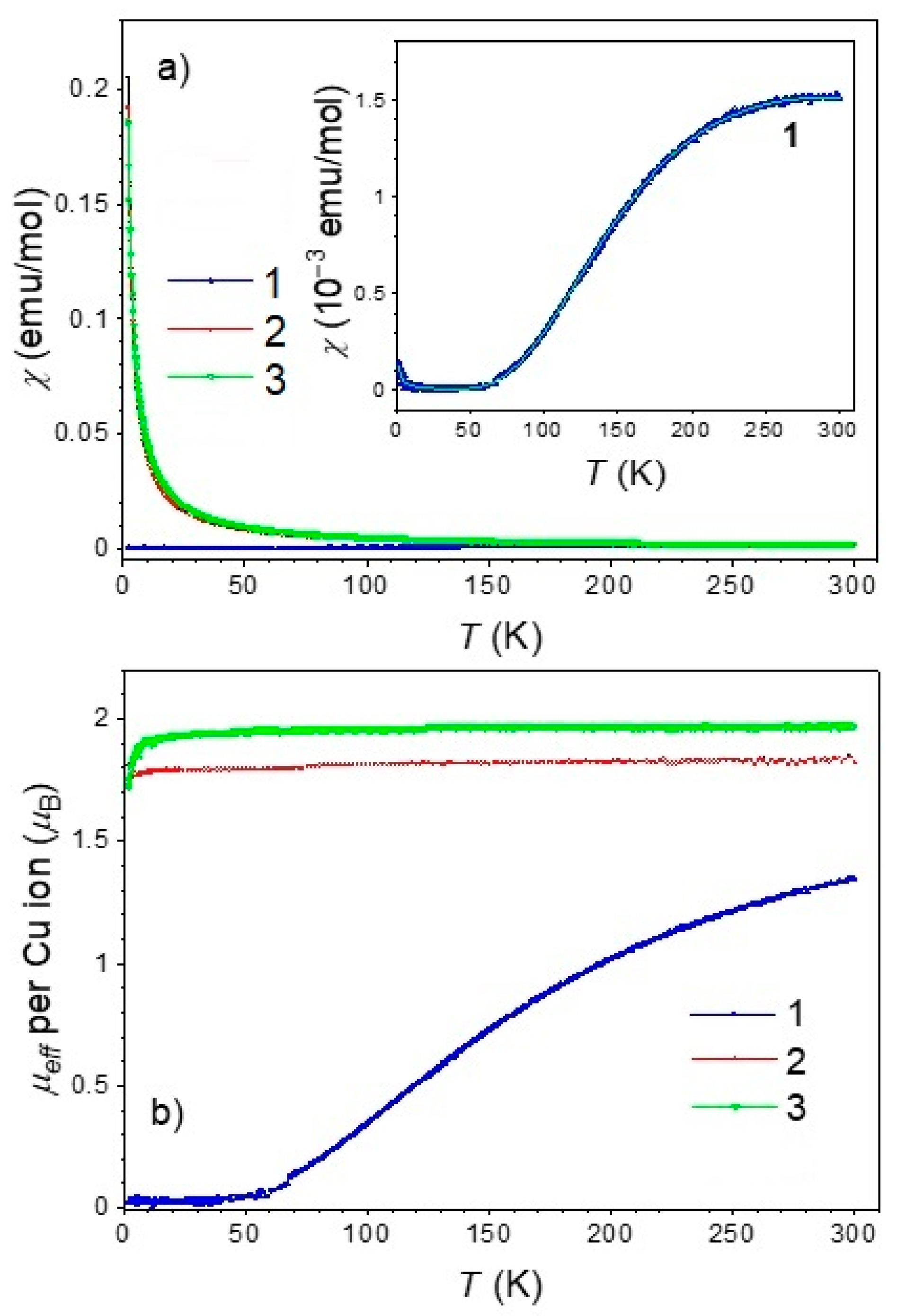
The dinuclear compound 1 exhibits a strong antiferromagnetic interaction between the Cu(II) ions in a molecule. The temperature-dependent susceptibility is described as the sum of the Bleaney-Bowers equation (the corresponding interaction Hamiltonian is H = −2 J S1 · S2) and a paramagnetic part taking into account a small contribution of non-interacting impurities with atomic ratio ρ [57,58]. The result of the fit is shown as a full line in the inset of Figure 10a, and the parameters obtained are J = −238 K, g = 2.2, and ρ = 2.7·10−4.
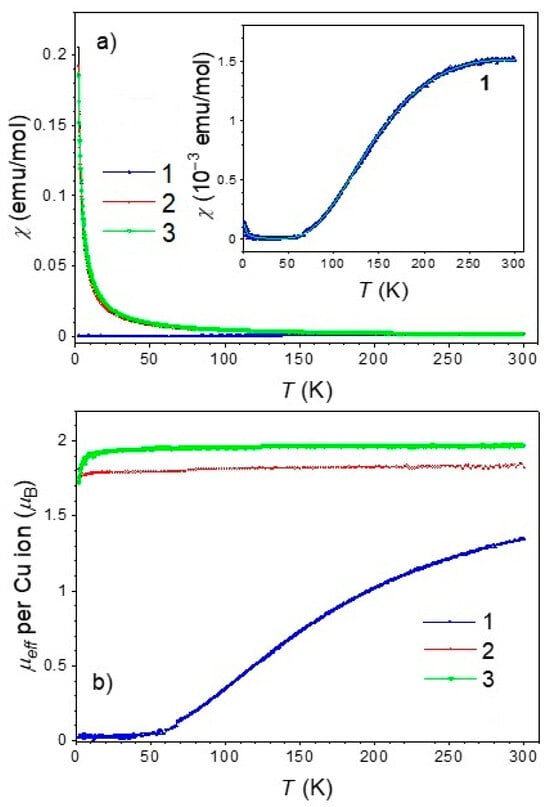
Figure 10.
Magnetic measurements of the title compounds. (a) Temperature dependence of the magnetic susceptibility of compounds 1–3, measured under an applied DC magnetic field of 1 kOe. The inset shows the zoomed-in susceptibility data for compound 1 with the corresponding Bleaney–Bowers best-fit line. (b) Effective magnetic moment for compounds 1–3 as a function of temperature, derived from the susceptibility data using the Curie law.
In contrast, the mononuclear compounds 2 and 3 display paramagnetic behavior consistent with isolated Cu(II) centers. Their effective magnetic moment, shown in Figure 10b, ranges from 1.8 μB to 2.0 μB, in agreement with the expected values for non-interacting Cu(II) ions [59].
3. Materials and Methods
All reagents and solvents used were commercially available and employed without additional purification. Copper salts utilized were copper(II) acetate monohydrate (Merck, Darmstadt, Germany, cryst. extra pure) and copper(II) nitrate trihydrate (Fluka, Buchs, Switzerland, puriss. p.a.). Ligands employed included 2-pyridinecarbonitrile, 3-pyridinecarbonitrile, and 4-pyridinecarbonitrile, all obtained by Sigma–Aldrich. Solvents such as distilled water, ethanol (Supelco, St. Louis, MO, USA, absolute for analysis), and acetonitrile (Supelco, St. Louis, MO, USA, gradient grade for LC) were used according to the specific solubility requirements of each synthesis. Elemental analysis was carried out using a Perkin Elmer CHNS/O 2400 Series II elemental analyzer (PerkinElmer, Inc., Waltham, MA, USA) while the content of copper was measured on a Varian SpectrAA-10 flame atomic spectrometer (AAS; Varian, Palo Alto, CA, USA).
3.1. Synthesis
3.1.1. Synthesis of [Cu2(μ-Ac)4(3-pyCN)2] (1)
Copper(II) acetate monohydrate (0.3991 g, 2 mmol) was mixed with 3-pyridinecarbonitrile (0.4159 g, 4 mmol) and dissolved in 20 mL of distilled water. The solution was stirred magnetically and heated under reflux conditions by employing an oil bath maintained slightly above the solvent’s boiling point for approximately 60 min. Following reflux, the solution was carefully transferred to a clean 50 mL Erlenmeyer flask. Crystallization proceeded slowly at room temperature. Dark-blue crystals suitable for single-crystal X-ray diffraction analysis were obtained after several days. The resulting crystals were isolated through gentle filtration and subsequently air-dried at ambient temperature. Yield: 0.44 g (77%). Anal. calc. for C20H20Cu2N4O8 (Mr = 571.48): C, 42.03%; H, 3.53%; Cu, 22.24%; N, 9.80%; O, 22.40%. Found: C, 41.66%; H, 3.58%; Cu, 22.41%; N, 9.71%; O, 22.23%.
3.1.2. Synthesis of [Cu(H2O)2(2-EtPIC)2]NO3 (2)
Copper(II) nitrate trihydrate (0.4834 g, 2 mmol) and 2-pyridinecarbonitrile (0.4163 g, 4 mmol) were dissolved thoroughly in 20 mL of ethanol at room temperature with vigorous magnetic stirring. The solution was transferred to a 50 mL Erlenmeyer flask, securely covered, and allowed to crystallize at ambient temperature. Large, well-defined blue crystals formed within one day. These crystals were subsequently isolated using filtration, gently rinsed with small amounts of cold ethanol, and air-dried. Crystals suitable for single-crystal X-ray diffraction were thus obtained. Yield: 0.85 g (81%). Anal. calc. for C16H24CuN6O10 (Mr = 523.95): C, 36.68%; H, 4.62%; Cu, 12.13%; N, 16.04%; O, 30.54%. Found: C, 36.73%; H, 4.53%; Cu, 12.14%; N, 15.87%; O, 30.33%.
3.1.3. Synthesis of [Cu(NO3)2(CH3CN)(4-pyCN)2]·CH3CN (3)
Copper(II) nitrate trihydrate (0.4829 g, 2 mmol) was mixed with 4-pyridinecarbonitrile (0.4157 g, 4 mmol) in 20 mL acetonitrile. The mixture was stirred magnetically at room temperature to achieve complete dissolution. The clear solution was then carefully transferred into a 50 mL Erlenmeyer flask and left uncovered under controlled laboratory conditions. Crystallization began after several days, yielding high-quality crystals. The resulting crystals were separated via filtration, gently washed with fresh acetonitrile to remove residual solvent and impurities, and finally air-dried. The obtained crystalline product was suitable for structural determination through single-crystal X-ray diffraction. Yield: 0.78 g (85%). Anal. calc. for C30H25Cu2N15O12 (Mr = 914.73): C, 39.39%; H, 2.75%; Cu, 13.89%; N, 22.97%; O, 20.99%. Found: C, 39.33%; H, 2.68%; Cu, 13.74%; N, 23.02%; O, 20.92%.
3.2. Crystallographic Data Collection and Refinement
Single-crystal diffraction data for all three compounds have been collected on a SuperNova dual source diffractometer with an Atlas detector at 150 K using a mirror monochromator and MoKα radiation with λ = 0.71073 Å. The diffraction data were processed using CrysAlis Pro Version 1.171.42.90a software [60]. Structures were solved with direct methods, using Sir2014 [61]. Full-matrix least-squares refinements on F2 were performed with anisotropic displacement parameters for all non-hydrogen atoms. Hydrogen atoms bonded to O and N atoms were located using a difference Fourier map. Their coordinates were refined together with their isotropic displacement parameter. In water molecules, restraints on the O-H distance were applied. Hydrogen atoms bonded to C atoms of the aromatic ring were placed at calculated positions and treated as a riding model. In compound 3, the hydrogen atoms of coordinated acetonitrile were placed at the calculated position for an idealized methyl group with a torsion angle from electron density. Carbon and nitrogen atoms from a non-coordinated acetonitrile molecule lie on a twofold axis. H atoms from this molecule were located using a difference Fourier map. The occupancy was fixed to 50% due to the symmetry-related disorder. Shelxl-2018/3 software [62] was used for structure refinement and interpretation. Drawings of the structure were produced using Ortep-III and Mercury [63,64]. Crystal data, data collection, and refinement parameters are given in Table 4. Structural and other crystallographic details on data collection and refinement have been deposited with the Cambridge Crystallographic Data Centre as CCDC Deposition Numbers 2476404, 2476406, and 2476530 for 1 to 3, respectively. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: ţ44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).

Table 4.
Experimental data for the X-ray diffraction studies of compounds 1–3.
3.3. IR Spectroscopy
Infrared spectra were taken on a Perkin Elmer FTIR spectrometer (PerkinElmer, Inc., Waltham, MA, USA) at room temperature on solid samples, using ATR in the range of 650–4000 cm−1.
3.4. Magnetic Measurements
The temperature dependence of magnetic susceptibility for samples 1–3 was measured between 2 K and 300 K in a DC magnetic field of 1 kOe using a Quantum Design MPMS XL-5 magnetometer (Quantum Design, San Diego, CA, USA). The data were corrected for the contribution of the sample holder and temperature-independent diamagnetism of inner-shell electrons using Pascal’s tables [51].
3.5. Thermal Analysis and X-Ray Powder Diffraction
The thermal decomposition of compounds 1–3 was studied using a Mettler TGA 2 system and a Mettler DSC 1 Differential Scanning Calorimeter (Mettler-Toledo, Columbus, OH, USA) in the temperature range of 30–600 °C, using 70 μL Al2O3 standard crucibles (TGA) and 40 μL Al crucibles (DSC), a heating range of 10 °C/min, and nitrogen flow atmosphere (50 mL/min). The final products of the decomposition were characterized with an AXS-Bruker/Siemens D5005 diffractometer (Bruker, Billerica, MA, USA), utilizing graphite monochromated CuKα radiation (λ = 1.54178 Å) and a silicon single-crystal holder.
4. Conclusions
In this paper, we report on the successful preparation, characterization, and structural analysis of three new copper coordination compounds with cyanopyridines. The structural analysis revealed that complex 1 features a dinuclear [Cu2(Ac)4(3-pyCN)2] molecule with four acetate ligands arranged in a paddle-wheel conformation and two 3-pyCN ligands in the apical position. On the contrary, complexes 2 and 3 are mononuclear, with copper(II) coordinated by nitrate ligands, coordinated solvent molecules, 4-pyCN molecules (3), and ethyl picolinimidate (2) obtained through in situ reaction of 2-pyCN with ethanol, acting as both the nucleophile and the solvent in this reaction. Crystal structures of complexes 1 and 3 are further stabilized by contacts between heteroatomic rings of ligands, while hydrogen bonding between coordination cations and nitrate anions is present in complex 2.
The thermal, FTIR, and magnetic measurement data are consistent with the structural characterization results. In particular, TGA and DSC measurements revealed significantly different thermal behavior of complex 3, containing nitrate ligands, which exhibit intense exothermic decomposition reactions, and those of complexes 1 and 2, decomposing in a much more gradual manner. FTIR results reveal variations in the stretching frequencies of the nitrile groups, as expected due to differences in their bonding modes. While the dinuclear complex 1 exhibits a strong antiferromagnetic interaction between the Cu(II) ions, mononuclear compounds 2 and 3 display paramagnetic behavior consistent with effective magnetic moments, agreeing with expected values for non-interacting Cu(II) ions. Further studies will be necessary to fully understand the behavior of the ligands used in this work, particularly 2-pyCN, during synthesis, with the goal of minimizing its in situ reactions with solvents.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/inorganics13090287/s1: Tables S1–S3: All bond lengths and angles for compounds 1, 2, and 3. Figures S1–S3: Detailed thermogravimetric curves of compounds 1–3, including first derivative curves and mass losses. Figures S4–S6: FTIR spectra of compounds 1–3. Cif files of compounds 1, 2, and 3.
Author Contributions
Conceptualization, A.G., M.K., and B.D.; methodology, A.G., M.K., Z.J., and B.D.; investigation, A.G., M.K., T.M.P., Z.J., and B.D.; data curation, A.G.; writing—original draft preparation, A.G. and M.K.; writing—review and editing, A.G., M.K., Z.J., and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of the Centre for Research Infrastructure at the University of Ljubljana, Faculty of Chemistry and Chemical Technology, which is part of the Network of Research and Infrastructural Centres UL (MRIC UL) and is financially supported by the Slovenian Research and Innovation Agency (Infrastructure programme No. I0-0022). Z.J. acknowledges the financial support of the Slovenian Research and Innovation Agency (Grant No. P2-0348). The financial support from the grant numbers P2-0006 and P1-0175 of the Slovenian Research and Innovation Agency (ARIS) is also gratefully acknowledged.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge Sabina Vohl for the technical support provided during the investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-pyCN | 2-pyridinecarbonitrile |
| 3-pyCN | 3-pyridinecarbonitrile |
| 4-pyCN | 4-pyridinecarbonitrile |
| EtOH | Ethanol |
| MeCN | Acetonitrile |
| Etpic | Ethyl Picolinimidate |
| RT | Room Temperature |
| TGA | Thermogravimetric analysis |
| DSC | Differential Scanning Calorimetry |
| FTIR | Fourier Transform Infrared Spectroscopy |
References
- Bakhtiar, R.; Ochiai, E.-I. Pharmacological applications of inorganic complexes. Gen. Pharmacol. Vasc. System 1999, 32, 525–540. [Google Scholar] [CrossRef]
- Marzano , C.; Pellei , M.; Tisato, F.; Santini , C. Copper complexes as anticancer agents. Anticancer Agents Med Chem. 2009, 9, 185–211. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; Romero-Pérez, A.I.; Álvarez, A.; Platero-Prats, A.E. Copper Coordination Complexes for Energy-Relevant Applications. Energies 2020, 13, 2198. [Google Scholar] [CrossRef]
- Wenzel, J.; Malz, S.; Rau, S.; Yersin, H. TADF: Enabling Luminescent Copper(I) Coordination Compounds for Light-Emitting Electrochemical Cells. J. Mater. Chem. C 2022, 10, 709–722. [Google Scholar] [CrossRef]
- Ullah, S.; Sirajuddin, M.; Ullah, Z.; Mushtaq, A.; Naz, S.; Zubair, M.; Haider, A.; Ali, S.; Kubicki, M.; Wani, T.A.; et al. Synthesis, Structural Elucidation and Pharmacological Applications of Cu(II) Heteroleptic Carboxylates. Pharmaceuticals 2023, 16, 693. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Noor, A.; Muhammad, M.; Ullah, F.; Tahir, M.N.; Khan, G.S.; Khan, E. Complexes of 2-Amino-3-methylpyridine and 2-Amino-4-methylbenzothiazole with Ag(I) and Cu(II): Structure and Biological Applications. Inorganics 2023, 11, 152. [Google Scholar] [CrossRef]
- Moreno-Narváez, M.E.; González-Sebastián, L.; Colorado-Peralta, R.; Reyes-Márquez, V.; Franco-Sandoval, L.O.; Romo-Pérez, A.; Cruz-Navarro, J.A.; Mañozca-Dosman, I.V.; Aragón-Muriel, A.; Morales-Morales, D. Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review. Inorganics 2025, 13, 38. [Google Scholar] [CrossRef]
- Turomsha, I.S.; Gvozdev, M.Y.; Osipovich, N.P.; Staravoitava, V.A.; Shiman, D.I.; Loginova, N.V. Copper(II) complexes of sterically hindered phenolic Schiff bases: Synthesis, characterization, interaction with biomolecules, and antioxidant and antimicrobial activity. New J. Chem. 2024, 48, 7134–7147. [Google Scholar] [CrossRef]
- Ngece, K.; Khwaza, V.; Paca, A.M.; Aderibigbe, B.A. The Antimicrobial Efficacy of Copper Complexes: A Review. Antibiotics 2025, 14, 516. [Google Scholar] [CrossRef]
- Rani, J.J.; Roy, S. Recent Development of Copper(II) Complexes of Polypyridyl Ligands in Chemotherapy and Photodynamic Therapy. ChemMedChem 2023, 18, e202200652. [Google Scholar] [CrossRef]
- Silva, C.M.F.; Lino, R.C.; de Moura, M.C.T.; de Sá Borges, A.P.; de Oliveira Jún, R.J. Innovative Approaches in the Synthesis and Optimization of Copper Complexes for Antitumor Therapies: A Comprehensive Review. Molecules 2025, 30, 2104. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Rajeshwari; Barman, T.R.; Fernandes, A.R.; Paradinha, F.; Roma-Rodrigues, C.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Mixed ligand aroylhydrazone and N-donor heterocyclic Lewis base Cu(II) complexes as potential antiproliferative agents. J. Inorg. Biochem. 2017, 175, 267–275. [Google Scholar] [CrossRef]
- Devonport, J.; Bodnár, N.; McGown, A.; Bukar Maina, M.; Serpell, L.C.; Kállay, C.; Spencer, J.; Kostakis, G.E. A Multipurpose Metallophore and Its Copper Complexes with Diverse Catalytic Antioxidant Properties to Deal with Metal and Oxidative Stress Disorders: A Combined Experimental, Theoretical, and In Vitro Study. Inorg. Chem. 2024, 63, 14827–14850. [Google Scholar] [CrossRef]
- Abdalla, E.M.; Aly, S.A.-H. Using Coordination Compounds as Antioxidants. In The Power of Antioxidants—Unleashing Nature’s Defense Against Oxidative Stress; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Kong, Y.-J.; Chai, W.-X.; Wang, Z.-X.; Hong, J.-Q.; Song, L. Multi-stimuli circular dichroism responses, UV-enhanced emission in solution and circularly polarized luminescence of a pair of chiral cuprous complexes. J. Mol. Struct. 2025, 1331, 141613. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Dutta, K.K.; Banik, S.; Gomila, R.M.; Barceló-Oliver, M.; Frontera, A. Supramolecular assembly in Cu(II) and Zn(II) compounds with pyridine and anthraquinone-1,5-disulfonate: Experimental and theoretical analysis. Inorg. Chim. Acta 2024, 567, 122042. [Google Scholar] [CrossRef]
- Kotakommula, H.; Chintala, V.; Nannapaneni, S.S.; Kumar Katari, N.; Kapavarapu, R.; Pal, M. Wang resin catalyzed sonochemical synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines as potential inhibitors of SIRT1. J. Mol. Struct. 2024, 1295, 136756. [Google Scholar] [CrossRef]
- Dubois, R.J.; Landee, C.P.; Rademeyer, M.; Turnbull, M.M. Pyridine-based complexes of copper(II) chloride and bromide: Ligand conformation effects on crystal structure. Synthesis, structure and magnetic behavior of Cu(2-Cl-3-X′py)2X2 [X, X′ = Cl, Br]. J. Coord. Chem. 2019, 72, 1785–1809. [Google Scholar] [CrossRef]
- Amani, V.; Ahmadi, R.; Naseh, M.; Ebadi, A. Synthesis, spectroscopic characterization, crystal structure and thermal analyses of two zinc(II) complexes with methanolysis of 2-pyridinecarbonitrile as a chelating ligand. J. Iran. Chem. Soc. 2017, 14, 635–642. [Google Scholar] [CrossRef]
- Das, B.K.; Barman, R.K. Tetrakis(μ-acetato-O,O′)bis[(4-cyanopyridine-N)copper(II)]. Acta Cryst. 2001, C57, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Borysova, K.V.; Sorg, J.R.; Mikhalyova, E.A.; Oberst, K.; Würtele, C.; Müller-Buschbaum, K. Bismuth trihalide based coordination polymers with the N-donor cyanopyridine as source for charge transfer based luminescence. Z. Anorg. Allg. Chem. 2023, 649, e202300131. [Google Scholar] [CrossRef]
- Heine, M.; Fink, L.; Schmidt, M.U. 3-Cyanopyridine as a bridging and terminal ligand in coordination polymers. CrystEngComm 2018, 20, 7556–7566. [Google Scholar] [CrossRef]
- Bertini, V.; Lucchesini, F.; Pocci, M.; De Munno, A. 3,5-dichloro-4-pyridinecarmonitrile as a key reagent in a synthesis of copper containing amine oxidase inhibitors. Heterocycles 1995, 41, 675–688. [Google Scholar] [CrossRef]
- Yano, T.; Yamada, T.; Isida, H.; Ohashi, N.; Itoh, T. 2-cyanopyridine derivatives enable N-terminal cysteine bioconjugation and peptide bond cleavage of glutathione under aqueous and mild conditions. RSC Adv. 2024, 14, 6542–6547. [Google Scholar] [CrossRef]
- Yıldız, R.; Döner, A.; Doğan, T.; Dehri, İ. Experimental studies of 2-pyridinecarbonitrile as corrosion inhibitor for mild steel in hydrochloric acid solution. Corr. Sci. 2014, 82, 125–132. [Google Scholar] [CrossRef]
- Slafer, B.W.; Dessoy, M.A.; De Oliveira, R.G.; Mollo, M.C.; Lee, E.; Matheeussen, A.; Maes, L.; Caljon, G.; Ferreira, L.L.G.; Krogh, R.; et al. Synthesis and Anti-Trypanosoma cruzi Activity of 3-Cyanopyridine Derivatives. ACS Omega 2024, 9, 22360–22370. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-E.; Zhu, Y.-S.; Zhang, X.-C.; Tao, Y.-L.; Liu, X. A copper(I) coordination polymer with 3-cyanopyridine ligands. Z. Naturforsch. B 2023, 78, 435–439. [Google Scholar] [CrossRef]
- Adam, F.A.; Abou El-Reash, Y.G.; Ghoniem, M.G.; Zaky, R.R. Green processing and characterization of Novel HETERO-ligand Co(II) complexes with cyanopyridine derivatives: Quantitative structure, Computational assessment, and Biological activity relationship. J. Mol. Struct. 2024, 1308, 137947. [Google Scholar] [CrossRef]
- Näther, C.; Jess, I. Synthesis, crystal structure and thermal behavior of tetrakis(3-cyanopyridine N-oxide-κO)bis(thiocyanato-κN)cobalt(II), which shows strong pseudosymmetry. Acta Cryst. 2023, E79, 867–871. [Google Scholar] [CrossRef]
- Banik, S.; Boro, M.; Gomila, R.M.; Barcelo-Oliver, M.; Frontera, A.; Bhattacharyya, M.K. Supramolecular assemblies involving unusual N(nitrile)∙∙∙π(fum) and nitrile-nitrile non-covalent contacts in fumarato and succinato bridged polymers of Co(II) and Ni(II): Experimental and theoretical studies. J. Mol. Struct. 2024, 1314, 138781. [Google Scholar] [CrossRef]
- Banik, S.; Baishya, T.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Das, J.; Bhattacharyya, M.K. ‘Charge Reverse’ Halogen Bonding Contacts in Metal-Organic Multi-Component Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorganics 2024, 12, 111. [Google Scholar] [CrossRef]
- Müller-Meinhard, A.; Jess, I.; Nähter, C. Synthesis, crystal structure and properties of chloridotetrakis(pyridine-3-carbonitrile) thiocyanatoiron(II). Acta Cryst. 2023, E79, 1173–1178. [Google Scholar] [CrossRef]
- Iqbal, M.; Haleem, M.A.; Ali, S.; Shahid, K.; Abbas, S.M.; Tahir, M.N.; Rehman, M. Centro-symmetric paddlewheel copper(II) carboxylates: Synthesis, structural description, DNA-binding and molecular docking studies. Polyhedron 2021, 208, 115407. [Google Scholar] [CrossRef]
- Viola, M.N.; Khan, I.N.; Ali, Z.; Ibrahim, M.; Shujah, S.; Ali, S.; Ikram, M.; Rehman, S.; Khan, G.S.; Wadood, A.; et al. Synthesis, characterization, antioxidant, antileishmanial, anticancer, DNA and theoretical SARS-CoV-2 interaction studies of copper(II) carboxylate complexes. J. Mol. Struct. 2022, 1253, 132308. [Google Scholar] [CrossRef]
- Muhammad, N.; Viola, V.; Khan, I.N.; Kubicki, M.; Ikram, M.; Rehman, S.; Samad, A.; Shujah, S.; Noor, A.; Qayyum, S. Syntheses, crystal structure description, anticancer, antioxidant, enzyme inhibition and DNA interaction potential of neutral hetero- and anionic homoleptic paddle-wheel copper(II) carboxylates. J. Mol. Struct. 2025, 1339, 142430. [Google Scholar] [CrossRef]
- Sanchiz, J.; Kremer, C.; Torre, M.H.; Facchin, G.; Kremer, E.; Castellano, E.E.; Ellena, J. Magnetic properties of copper(II) complexes containing peptides. Crystal structure of [Cu(phe-leu)]. J. Mol. Struct. 2006, 797, 179–183. [Google Scholar] [CrossRef]
- Rojas, O.; Mirzoyan, G.; Adamyan, Z.; Papoyan, V.V.; Amatuni, G.; Ananikian, N. Magnetic properties and entanglement in antiferromagnetic interactions in copper(II) dinuclear and trinuclear complexes. Sci. Rep. 2025, 15, 11758. [Google Scholar] [CrossRef]
- Hazra, S.; Majumdar, D.; Das, D.; Roy, S.; Dalai, S. A comprehensive review of synthesis, characterization, single-crystal X-ray diffraction, and applications of transition metal complexes with tricyanomethane anions. J. Mol. Struct. 2025, 1344, 142992. [Google Scholar] [CrossRef]
- Kristl, M.; Šturm, J.; Golobič, A.; Jagličić, Z.; Dojer, B. New copper(II) complexes with hydroxypyridines: Synthesis, structural, thermal, and magnetic properties. Inorg. Chim. Acta 2023, 556, 121670. [Google Scholar] [CrossRef]
- Golobič, A.; Dojer, B.; Jagodič, M.; Siher, A.; Pegan, A.; Kristl, M. Synthesis and Characterization of New Copper(II) Coordination Compounds with Methylammonium Cations. Inorganics 2024, 12, 261. [Google Scholar] [CrossRef]
- Sieron, L.; Bukowska-Strzyzewska, M. Trans-Diaquabis(pyridine-2-carboxamide-N1,O)copper(II)Dichloride and Dibromide. Acta Cryst. 1997, C53, 296–298. [Google Scholar] [CrossRef]
- Siddiqui, K.A. 1-D Hydrogen bonded water in Cu(II)-picolinate coordination polymer: Synthesis, crystal structure, and thermogravimetric analysis. J. Coord. Chem. 2012, 65, 4168–4176. [Google Scholar] [CrossRef]
- Dutta, D.; Chetry, S.; Gogoi, A.; Choudhury, B.; Guha, A.K.; Bhattacharyya, M.K. Supramolecular association involving anion–π interactions in Cu(II) coordination solids: Experimental and theoretical studies. Polyhedron 2018, 151, 381–393. [Google Scholar] [CrossRef]
- Gayfullina, R.; Jääskeläinen, S.; Koshevoy, I.O.; Hirva, P. Activation of the Cyano Group at Imidazole via Copper Stimulated Alcoholysis. Inorganics 2019, 7, 87. [Google Scholar] [CrossRef]
- Sánchez Costa, J.; Gonzales Castro, A.; Pievo, R.; Robeau, O.; Modec, B.; Kozlevčar, B.; Teat, S.J.; Gamez, P.; Reedijk, J. Proficiency of the electron-deficient 1,3,5-triazine ring to generate anion–π and lone pair–π interactions. CrystEngComm 2010, 12, 3057–3064. [Google Scholar] [CrossRef]
- Singh, G.; Singh, C.P.; Mannan, S.M. Thermolysis of some transition metal nitrate complexes with 1,4-diamino butane ligand. J. Hazard. Mater. 2005, 122, 111–117. [Google Scholar] [CrossRef]
- Ramos, M.C.; De Oliveira Neto, J.G.; Nogueira, C.E.S.; Reis, A.S.; De Sousa, F.F.; Da Silva, L.M.; Dos Santos, A.O. Structural, Vibrational, Thermal, and Cytotoxic Characterization of Aqua(1,10-Phenanthroline)(L-Serinato)Copper(II) Nitrate Complex Combined with DFT Calculations. Cryst. Res. Technol. 2023, 58, 2300240. [Google Scholar] [CrossRef]
- Al-Matarneh, M.C.; Nicolescu, A.; Dascalu, I.-A.; Shova, S.; Varganici, C.-D.; Fifere, A.; Danac, R.; Marinas, I.-C. Synthesis of New Zinc and Copper Coordination Polymers Derived from Bis (Triazole) Ligands. Crystals 2024, 14, 144. [Google Scholar] [CrossRef]
- Xia, L.-H.; Wang, Y.-N.; Yang, X.-M.; Liang, L.-N.; Li, Z.-M.; Zhang, T.-L. Cupric coordination compounds with multiple anions: A promising strategy for the regulation of energetic materials. RSC Adv. 2023, 13, 22549. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall: London, UK, 1975. [Google Scholar] [CrossRef]
- Rochon, F.D.; Melanson, R.; Howard-Lock, H.E.; Lock, C.J.L.; Turner, G. The vibrational spectra, crystal and molecular structure of bis(acetonitrile)dichloroplatinum(II). Can. J. Chem. 1984, 62, 860–869. [Google Scholar] [CrossRef]
- Farha, F.; Iwamoto, R.T. The Preparation and Infrared Examination of the 2-, 3-, and 4-Cyanopyridine Complexes of Copper(I), Silver(I), and Gold (I) Perchlorates. Inorg. Chem. 1965, 4, 844–848. [Google Scholar] [CrossRef]
- Martini, D.; Pellei, M.; Pettinari, C.; Skelton, B.W.; White, A.H. Synthesis, spectroscopic and structural characterization of Cu(II) derivatives of tris(pyrazol-1-yl)methanes. Inorg. Chim. Acta 2002, 333, 72–82. [Google Scholar] [CrossRef]
- Vargová, Z.; Zenlenák, V.; Cisarová, I.; Györyová, K. Correlation of thermal and spectral properties of zinc(II) complexes of pyridinecarboxylic acids with their crystal structures. Thermochim. Acta 2004, 423, 149–157. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Bleaney, B.; Bowers, K.D. Anomalous Paramagnetism of Copper Acetate. Proc. R. Soc. A 1952, 214, 451–465. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishing: Weinheim, Germany; New York, NY, USA, 1993. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Saunders College Publishing: Philadelphia, PA, USA, 1976. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlisPro; Version 1.171.42.90a; Rigaku Corporation: Oxford, UK, 2023. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, J.L. ORTEP-3 for Windows—A version of ORTEP-3 with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).