Mixed-Ligand Copper(II) Complexes Derived from Pyridinecarbonitrile Precursors: Structural Features and Thermal Behavior
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and General Aspects
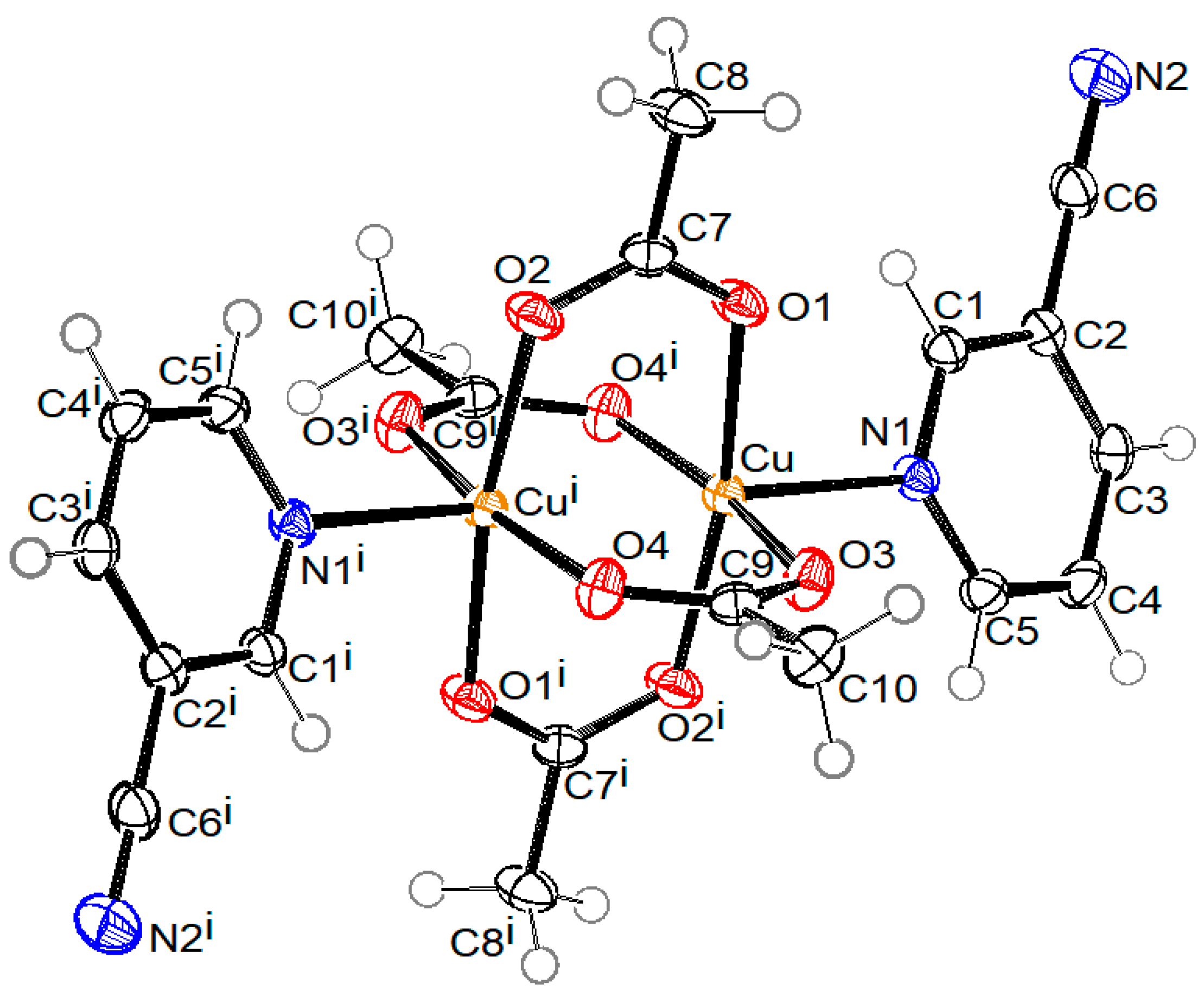
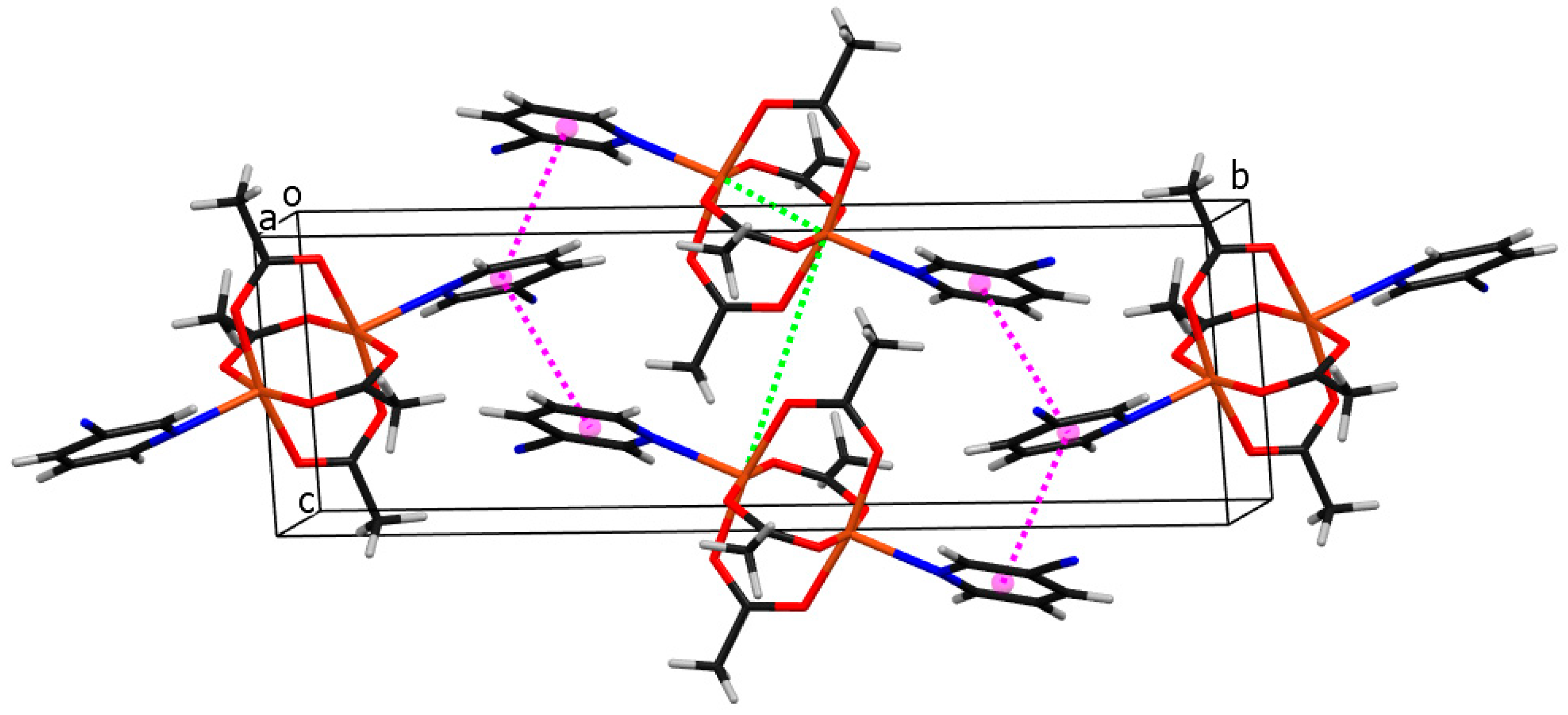
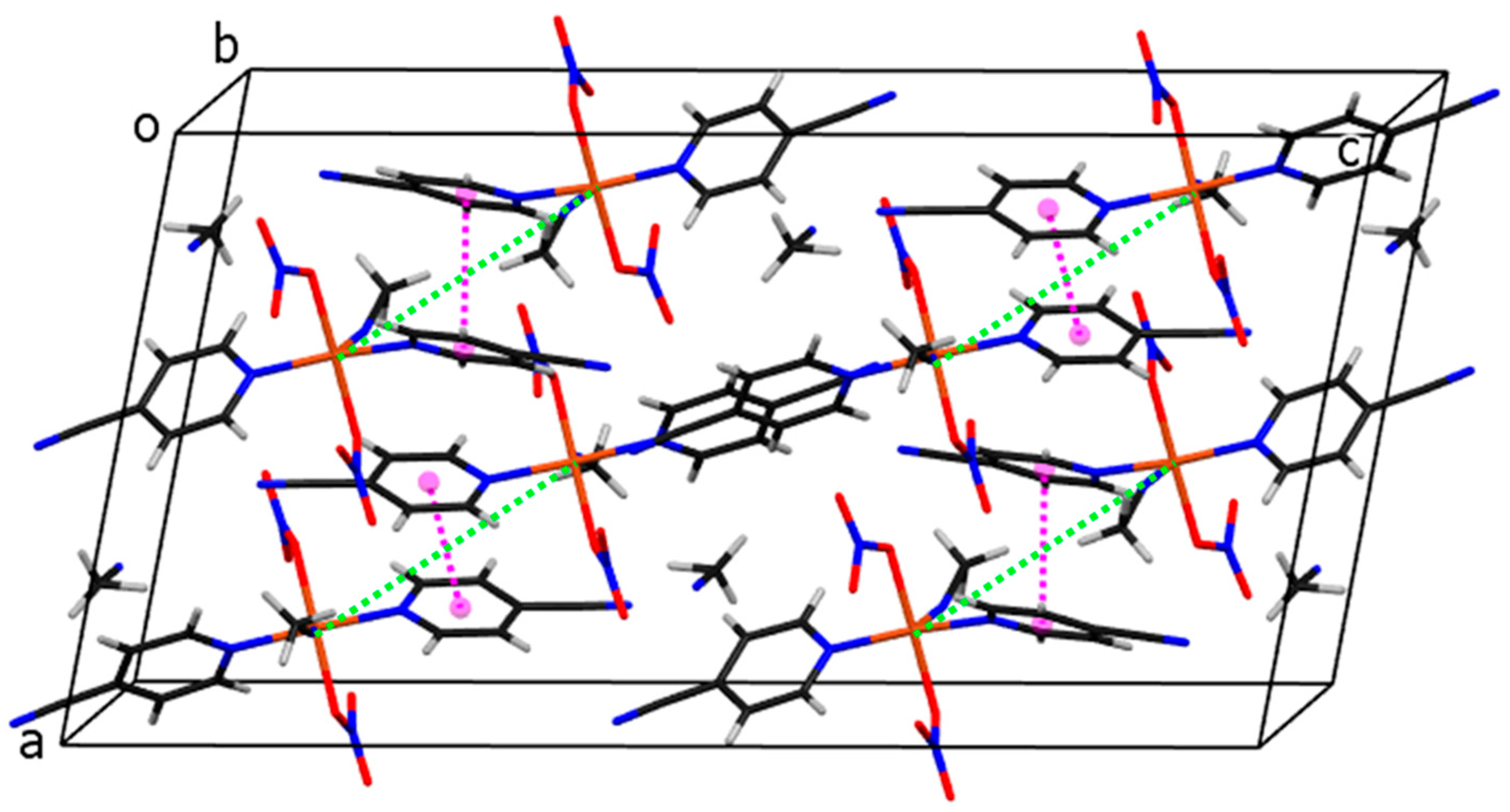
2.2. Description of Structures
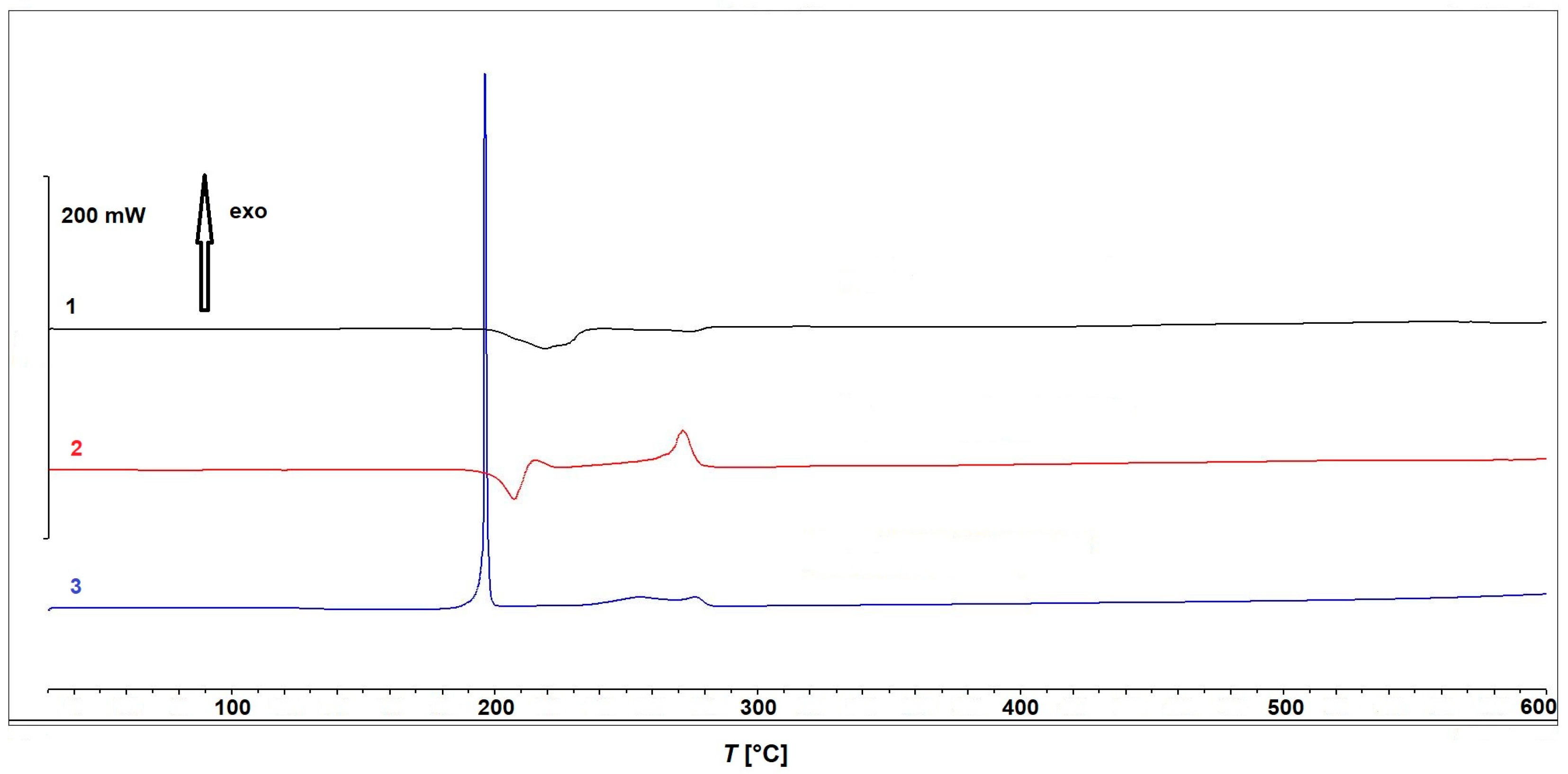
2.3. Thermal Analysis
2.4. FT-IR Spectra
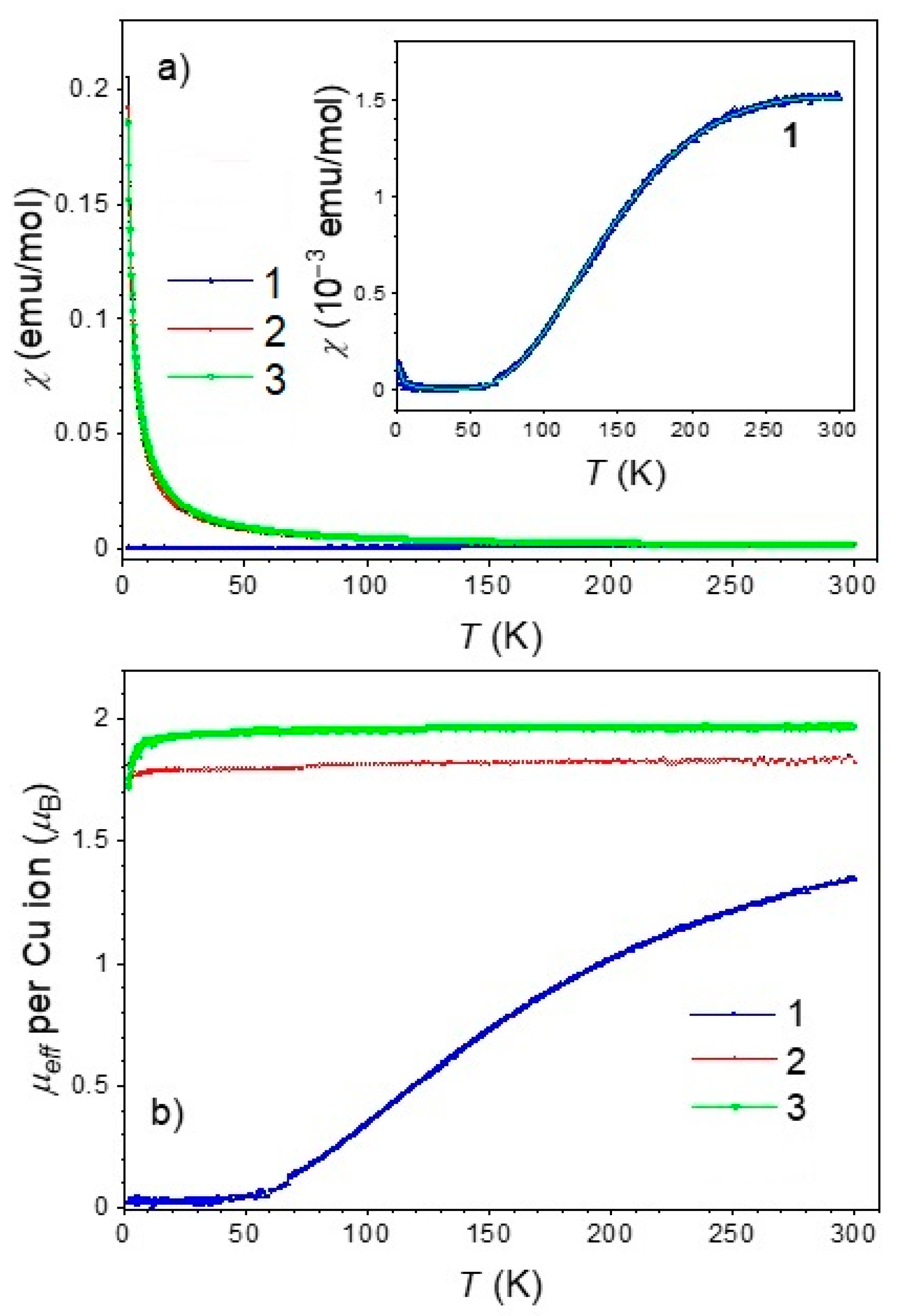
2.5. Magnetic Properties
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of [Cu2(μ-Ac)4(3-pyCN)2] (1)
3.1.2. Synthesis of [Cu(H2O)2(2-EtPIC)2]NO3 (2)
3.1.3. Synthesis of [Cu(NO3)2(CH3CN)(4-pyCN)2]·CH3CN (3)
3.2. Crystallographic Data Collection and Refinement
3.3. IR Spectroscopy
3.4. Magnetic Measurements
3.5. Thermal Analysis and X-Ray Powder Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-pyCN | 2-pyridinecarbonitrile |
| 3-pyCN | 3-pyridinecarbonitrile |
| 4-pyCN | 4-pyridinecarbonitrile |
| EtOH | Ethanol |
| MeCN | Acetonitrile |
| Etpic | Ethyl Picolinimidate |
| RT | Room Temperature |
| TGA | Thermogravimetric analysis |
| DSC | Differential Scanning Calorimetry |
| FTIR | Fourier Transform Infrared Spectroscopy |
References
- Bakhtiar, R.; Ochiai, E.-I. Pharmacological applications of inorganic complexes. Gen. Pharmacol. Vasc. System 1999, 32, 525–540. [Google Scholar] [CrossRef]
- Marzano , C.; Pellei , M.; Tisato, F.; Santini , C. Copper complexes as anticancer agents. Anticancer Agents Med Chem. 2009, 9, 185–211. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; Romero-Pérez, A.I.; Álvarez, A.; Platero-Prats, A.E. Copper Coordination Complexes for Energy-Relevant Applications. Energies 2020, 13, 2198. [Google Scholar] [CrossRef]
- Wenzel, J.; Malz, S.; Rau, S.; Yersin, H. TADF: Enabling Luminescent Copper(I) Coordination Compounds for Light-Emitting Electrochemical Cells. J. Mater. Chem. C 2022, 10, 709–722. [Google Scholar] [CrossRef]
- Ullah, S.; Sirajuddin, M.; Ullah, Z.; Mushtaq, A.; Naz, S.; Zubair, M.; Haider, A.; Ali, S.; Kubicki, M.; Wani, T.A.; et al. Synthesis, Structural Elucidation and Pharmacological Applications of Cu(II) Heteroleptic Carboxylates. Pharmaceuticals 2023, 16, 693. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Noor, A.; Muhammad, M.; Ullah, F.; Tahir, M.N.; Khan, G.S.; Khan, E. Complexes of 2-Amino-3-methylpyridine and 2-Amino-4-methylbenzothiazole with Ag(I) and Cu(II): Structure and Biological Applications. Inorganics 2023, 11, 152. [Google Scholar] [CrossRef]
- Moreno-Narváez, M.E.; González-Sebastián, L.; Colorado-Peralta, R.; Reyes-Márquez, V.; Franco-Sandoval, L.O.; Romo-Pérez, A.; Cruz-Navarro, J.A.; Mañozca-Dosman, I.V.; Aragón-Muriel, A.; Morales-Morales, D. Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review. Inorganics 2025, 13, 38. [Google Scholar] [CrossRef]
- Turomsha, I.S.; Gvozdev, M.Y.; Osipovich, N.P.; Staravoitava, V.A.; Shiman, D.I.; Loginova, N.V. Copper(II) complexes of sterically hindered phenolic Schiff bases: Synthesis, characterization, interaction with biomolecules, and antioxidant and antimicrobial activity. New J. Chem. 2024, 48, 7134–7147. [Google Scholar] [CrossRef]
- Ngece, K.; Khwaza, V.; Paca, A.M.; Aderibigbe, B.A. The Antimicrobial Efficacy of Copper Complexes: A Review. Antibiotics 2025, 14, 516. [Google Scholar] [CrossRef]
- Rani, J.J.; Roy, S. Recent Development of Copper(II) Complexes of Polypyridyl Ligands in Chemotherapy and Photodynamic Therapy. ChemMedChem 2023, 18, e202200652. [Google Scholar] [CrossRef]
- Silva, C.M.F.; Lino, R.C.; de Moura, M.C.T.; de Sá Borges, A.P.; de Oliveira Jún, R.J. Innovative Approaches in the Synthesis and Optimization of Copper Complexes for Antitumor Therapies: A Comprehensive Review. Molecules 2025, 30, 2104. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Rajeshwari; Barman, T.R.; Fernandes, A.R.; Paradinha, F.; Roma-Rodrigues, C.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Mixed ligand aroylhydrazone and N-donor heterocyclic Lewis base Cu(II) complexes as potential antiproliferative agents. J. Inorg. Biochem. 2017, 175, 267–275. [Google Scholar] [CrossRef]
- Devonport, J.; Bodnár, N.; McGown, A.; Bukar Maina, M.; Serpell, L.C.; Kállay, C.; Spencer, J.; Kostakis, G.E. A Multipurpose Metallophore and Its Copper Complexes with Diverse Catalytic Antioxidant Properties to Deal with Metal and Oxidative Stress Disorders: A Combined Experimental, Theoretical, and In Vitro Study. Inorg. Chem. 2024, 63, 14827–14850. [Google Scholar] [CrossRef]
- Abdalla, E.M.; Aly, S.A.-H. Using Coordination Compounds as Antioxidants. In The Power of Antioxidants—Unleashing Nature’s Defense Against Oxidative Stress; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Kong, Y.-J.; Chai, W.-X.; Wang, Z.-X.; Hong, J.-Q.; Song, L. Multi-stimuli circular dichroism responses, UV-enhanced emission in solution and circularly polarized luminescence of a pair of chiral cuprous complexes. J. Mol. Struct. 2025, 1331, 141613. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Dutta, K.K.; Banik, S.; Gomila, R.M.; Barceló-Oliver, M.; Frontera, A. Supramolecular assembly in Cu(II) and Zn(II) compounds with pyridine and anthraquinone-1,5-disulfonate: Experimental and theoretical analysis. Inorg. Chim. Acta 2024, 567, 122042. [Google Scholar] [CrossRef]
- Kotakommula, H.; Chintala, V.; Nannapaneni, S.S.; Kumar Katari, N.; Kapavarapu, R.; Pal, M. Wang resin catalyzed sonochemical synthesis of 2-amino-3,5-dicarbonitrile-6-thio-pyridines as potential inhibitors of SIRT1. J. Mol. Struct. 2024, 1295, 136756. [Google Scholar] [CrossRef]
- Dubois, R.J.; Landee, C.P.; Rademeyer, M.; Turnbull, M.M. Pyridine-based complexes of copper(II) chloride and bromide: Ligand conformation effects on crystal structure. Synthesis, structure and magnetic behavior of Cu(2-Cl-3-X′py)2X2 [X, X′ = Cl, Br]. J. Coord. Chem. 2019, 72, 1785–1809. [Google Scholar] [CrossRef]
- Amani, V.; Ahmadi, R.; Naseh, M.; Ebadi, A. Synthesis, spectroscopic characterization, crystal structure and thermal analyses of two zinc(II) complexes with methanolysis of 2-pyridinecarbonitrile as a chelating ligand. J. Iran. Chem. Soc. 2017, 14, 635–642. [Google Scholar] [CrossRef]
- Das, B.K.; Barman, R.K. Tetrakis(μ-acetato-O,O′)bis[(4-cyanopyridine-N)copper(II)]. Acta Cryst. 2001, C57, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Borysova, K.V.; Sorg, J.R.; Mikhalyova, E.A.; Oberst, K.; Würtele, C.; Müller-Buschbaum, K. Bismuth trihalide based coordination polymers with the N-donor cyanopyridine as source for charge transfer based luminescence. Z. Anorg. Allg. Chem. 2023, 649, e202300131. [Google Scholar] [CrossRef]
- Heine, M.; Fink, L.; Schmidt, M.U. 3-Cyanopyridine as a bridging and terminal ligand in coordination polymers. CrystEngComm 2018, 20, 7556–7566. [Google Scholar] [CrossRef]
- Bertini, V.; Lucchesini, F.; Pocci, M.; De Munno, A. 3,5-dichloro-4-pyridinecarmonitrile as a key reagent in a synthesis of copper containing amine oxidase inhibitors. Heterocycles 1995, 41, 675–688. [Google Scholar] [CrossRef]
- Yano, T.; Yamada, T.; Isida, H.; Ohashi, N.; Itoh, T. 2-cyanopyridine derivatives enable N-terminal cysteine bioconjugation and peptide bond cleavage of glutathione under aqueous and mild conditions. RSC Adv. 2024, 14, 6542–6547. [Google Scholar] [CrossRef]
- Yıldız, R.; Döner, A.; Doğan, T.; Dehri, İ. Experimental studies of 2-pyridinecarbonitrile as corrosion inhibitor for mild steel in hydrochloric acid solution. Corr. Sci. 2014, 82, 125–132. [Google Scholar] [CrossRef]
- Slafer, B.W.; Dessoy, M.A.; De Oliveira, R.G.; Mollo, M.C.; Lee, E.; Matheeussen, A.; Maes, L.; Caljon, G.; Ferreira, L.L.G.; Krogh, R.; et al. Synthesis and Anti-Trypanosoma cruzi Activity of 3-Cyanopyridine Derivatives. ACS Omega 2024, 9, 22360–22370. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-E.; Zhu, Y.-S.; Zhang, X.-C.; Tao, Y.-L.; Liu, X. A copper(I) coordination polymer with 3-cyanopyridine ligands. Z. Naturforsch. B 2023, 78, 435–439. [Google Scholar] [CrossRef]
- Adam, F.A.; Abou El-Reash, Y.G.; Ghoniem, M.G.; Zaky, R.R. Green processing and characterization of Novel HETERO-ligand Co(II) complexes with cyanopyridine derivatives: Quantitative structure, Computational assessment, and Biological activity relationship. J. Mol. Struct. 2024, 1308, 137947. [Google Scholar] [CrossRef]
- Näther, C.; Jess, I. Synthesis, crystal structure and thermal behavior of tetrakis(3-cyanopyridine N-oxide-κO)bis(thiocyanato-κN)cobalt(II), which shows strong pseudosymmetry. Acta Cryst. 2023, E79, 867–871. [Google Scholar] [CrossRef]
- Banik, S.; Boro, M.; Gomila, R.M.; Barcelo-Oliver, M.; Frontera, A.; Bhattacharyya, M.K. Supramolecular assemblies involving unusual N(nitrile)∙∙∙π(fum) and nitrile-nitrile non-covalent contacts in fumarato and succinato bridged polymers of Co(II) and Ni(II): Experimental and theoretical studies. J. Mol. Struct. 2024, 1314, 138781. [Google Scholar] [CrossRef]
- Banik, S.; Baishya, T.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Das, J.; Bhattacharyya, M.K. ‘Charge Reverse’ Halogen Bonding Contacts in Metal-Organic Multi-Component Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorganics 2024, 12, 111. [Google Scholar] [CrossRef]
- Müller-Meinhard, A.; Jess, I.; Nähter, C. Synthesis, crystal structure and properties of chloridotetrakis(pyridine-3-carbonitrile) thiocyanatoiron(II). Acta Cryst. 2023, E79, 1173–1178. [Google Scholar] [CrossRef]
- Iqbal, M.; Haleem, M.A.; Ali, S.; Shahid, K.; Abbas, S.M.; Tahir, M.N.; Rehman, M. Centro-symmetric paddlewheel copper(II) carboxylates: Synthesis, structural description, DNA-binding and molecular docking studies. Polyhedron 2021, 208, 115407. [Google Scholar] [CrossRef]
- Viola, M.N.; Khan, I.N.; Ali, Z.; Ibrahim, M.; Shujah, S.; Ali, S.; Ikram, M.; Rehman, S.; Khan, G.S.; Wadood, A.; et al. Synthesis, characterization, antioxidant, antileishmanial, anticancer, DNA and theoretical SARS-CoV-2 interaction studies of copper(II) carboxylate complexes. J. Mol. Struct. 2022, 1253, 132308. [Google Scholar] [CrossRef]
- Muhammad, N.; Viola, V.; Khan, I.N.; Kubicki, M.; Ikram, M.; Rehman, S.; Samad, A.; Shujah, S.; Noor, A.; Qayyum, S. Syntheses, crystal structure description, anticancer, antioxidant, enzyme inhibition and DNA interaction potential of neutral hetero- and anionic homoleptic paddle-wheel copper(II) carboxylates. J. Mol. Struct. 2025, 1339, 142430. [Google Scholar] [CrossRef]
- Sanchiz, J.; Kremer, C.; Torre, M.H.; Facchin, G.; Kremer, E.; Castellano, E.E.; Ellena, J. Magnetic properties of copper(II) complexes containing peptides. Crystal structure of [Cu(phe-leu)]. J. Mol. Struct. 2006, 797, 179–183. [Google Scholar] [CrossRef]
- Rojas, O.; Mirzoyan, G.; Adamyan, Z.; Papoyan, V.V.; Amatuni, G.; Ananikian, N. Magnetic properties and entanglement in antiferromagnetic interactions in copper(II) dinuclear and trinuclear complexes. Sci. Rep. 2025, 15, 11758. [Google Scholar] [CrossRef]
- Hazra, S.; Majumdar, D.; Das, D.; Roy, S.; Dalai, S. A comprehensive review of synthesis, characterization, single-crystal X-ray diffraction, and applications of transition metal complexes with tricyanomethane anions. J. Mol. Struct. 2025, 1344, 142992. [Google Scholar] [CrossRef]
- Kristl, M.; Šturm, J.; Golobič, A.; Jagličić, Z.; Dojer, B. New copper(II) complexes with hydroxypyridines: Synthesis, structural, thermal, and magnetic properties. Inorg. Chim. Acta 2023, 556, 121670. [Google Scholar] [CrossRef]
- Golobič, A.; Dojer, B.; Jagodič, M.; Siher, A.; Pegan, A.; Kristl, M. Synthesis and Characterization of New Copper(II) Coordination Compounds with Methylammonium Cations. Inorganics 2024, 12, 261. [Google Scholar] [CrossRef]
- Sieron, L.; Bukowska-Strzyzewska, M. Trans-Diaquabis(pyridine-2-carboxamide-N1,O)copper(II)Dichloride and Dibromide. Acta Cryst. 1997, C53, 296–298. [Google Scholar] [CrossRef]
- Siddiqui, K.A. 1-D Hydrogen bonded water in Cu(II)-picolinate coordination polymer: Synthesis, crystal structure, and thermogravimetric analysis. J. Coord. Chem. 2012, 65, 4168–4176. [Google Scholar] [CrossRef]
- Dutta, D.; Chetry, S.; Gogoi, A.; Choudhury, B.; Guha, A.K.; Bhattacharyya, M.K. Supramolecular association involving anion–π interactions in Cu(II) coordination solids: Experimental and theoretical studies. Polyhedron 2018, 151, 381–393. [Google Scholar] [CrossRef]
- Gayfullina, R.; Jääskeläinen, S.; Koshevoy, I.O.; Hirva, P. Activation of the Cyano Group at Imidazole via Copper Stimulated Alcoholysis. Inorganics 2019, 7, 87. [Google Scholar] [CrossRef]
- Sánchez Costa, J.; Gonzales Castro, A.; Pievo, R.; Robeau, O.; Modec, B.; Kozlevčar, B.; Teat, S.J.; Gamez, P.; Reedijk, J. Proficiency of the electron-deficient 1,3,5-triazine ring to generate anion–π and lone pair–π interactions. CrystEngComm 2010, 12, 3057–3064. [Google Scholar] [CrossRef]
- Singh, G.; Singh, C.P.; Mannan, S.M. Thermolysis of some transition metal nitrate complexes with 1,4-diamino butane ligand. J. Hazard. Mater. 2005, 122, 111–117. [Google Scholar] [CrossRef]
- Ramos, M.C.; De Oliveira Neto, J.G.; Nogueira, C.E.S.; Reis, A.S.; De Sousa, F.F.; Da Silva, L.M.; Dos Santos, A.O. Structural, Vibrational, Thermal, and Cytotoxic Characterization of Aqua(1,10-Phenanthroline)(L-Serinato)Copper(II) Nitrate Complex Combined with DFT Calculations. Cryst. Res. Technol. 2023, 58, 2300240. [Google Scholar] [CrossRef]
- Al-Matarneh, M.C.; Nicolescu, A.; Dascalu, I.-A.; Shova, S.; Varganici, C.-D.; Fifere, A.; Danac, R.; Marinas, I.-C. Synthesis of New Zinc and Copper Coordination Polymers Derived from Bis (Triazole) Ligands. Crystals 2024, 14, 144. [Google Scholar] [CrossRef]
- Xia, L.-H.; Wang, Y.-N.; Yang, X.-M.; Liang, L.-N.; Li, Z.-M.; Zhang, T.-L. Cupric coordination compounds with multiple anions: A promising strategy for the regulation of energetic materials. RSC Adv. 2023, 13, 22549. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall: London, UK, 1975. [Google Scholar] [CrossRef]
- Rochon, F.D.; Melanson, R.; Howard-Lock, H.E.; Lock, C.J.L.; Turner, G. The vibrational spectra, crystal and molecular structure of bis(acetonitrile)dichloroplatinum(II). Can. J. Chem. 1984, 62, 860–869. [Google Scholar] [CrossRef]
- Farha, F.; Iwamoto, R.T. The Preparation and Infrared Examination of the 2-, 3-, and 4-Cyanopyridine Complexes of Copper(I), Silver(I), and Gold (I) Perchlorates. Inorg. Chem. 1965, 4, 844–848. [Google Scholar] [CrossRef]
- Martini, D.; Pellei, M.; Pettinari, C.; Skelton, B.W.; White, A.H. Synthesis, spectroscopic and structural characterization of Cu(II) derivatives of tris(pyrazol-1-yl)methanes. Inorg. Chim. Acta 2002, 333, 72–82. [Google Scholar] [CrossRef]
- Vargová, Z.; Zenlenák, V.; Cisarová, I.; Györyová, K. Correlation of thermal and spectral properties of zinc(II) complexes of pyridinecarboxylic acids with their crystal structures. Thermochim. Acta 2004, 423, 149–157. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Bleaney, B.; Bowers, K.D. Anomalous Paramagnetism of Copper Acetate. Proc. R. Soc. A 1952, 214, 451–465. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishing: Weinheim, Germany; New York, NY, USA, 1993. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Saunders College Publishing: Philadelphia, PA, USA, 1976. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlisPro; Version 1.171.42.90a; Rigaku Corporation: Oxford, UK, 2023. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, J.L. ORTEP-3 for Windows—A version of ORTEP-3 with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]





| 1 | |||
| Cu—O3 | 1.9586(15) | O1—Cu—O2i | 169.52(6) |
| Cu—O1 | 1.9651(14) | O3—Cu—O4i | 169.53(6) |
| Cu—O2i | 1.9699(15) | O1—Cu—O4i | 89.49(6) |
| Cu—O4i | 1.9752(15) | O2i—Cu—O4i | 87.78(7) |
| Cu—N1 | 2.1862(16) | O3—Cu—N1 | 99.98(6) |
| Cu···Cui | 2.5912(6) | O1—Cu—N1 | 96.57(6) |
| Cu···Cuii | 6.4520(6) | O2i—Cu—N1 | 93.56(6) |
| O3—Cu—O1 | 90.12(6) | O4i—Cu—N1 | 90.46(6) |
| O3—Cu—O2i | 90.74(7) | ||
| (i) −x + 2, −y, −z + 1; (ii) −x + 2, −y, -z; | |||
| 2 | |||
| Cu—N2 | 1.9897(17) | C6—O1 | 1.323 (2) |
| Cu—N2i | 1.9897(17) | Cu···O2i | 2.467(17) |
| Cu—N1i | 2.0339(17) | Cu···Cuii | 7.3037(5) |
| Cu—N1 | 2.0340(17) | N2—Cu—N1i | 98.64(7) |
| Cu···O2 | 2.4670(17) | N1—Cu—N1i | 180.0 |
| C6—N2 | 1.276(3) | N2—Cu—N2i | 180.0 |
| (i) −x + 1, −y + 1, −z; (ii) x, y + 1, z; | |||
| 3 | |||
| Cu—O1 | 1.9881(11) | O1—Cu—O4 | 178.93(5) |
| Cu—O4 | 2.0009(11) | O1—Cu—N1 | 90.01(5) |
| Cu—N1 | 2.0139(13) | O4—Cu—N1 | 89.32(5) |
| Cu—N2 | 2.0164(12) | O1—Cu—N2 | 92.18(5) |
| Cu—N3 | 2.2416(14) | O4—Cu—N2 | 88.29(5) |
| Cu···O2 | 2.7170(11) | N1—Cu—N2 | 167.00(5) |
| Cu···O5 | 2.7830(11) | O1—Cu—N3 | 91.86(5) |
| Cu···Cui | 7.2600(6) | O4—Cu—N3 | 89.04(5) |
| N2—Cu—N3 | 96.70(5) | N1—Cu—N3 | 96.03(5) |
| (i): −x + 1/2, −y + 1/2; −z + 3/2; | |||
| D-H···A | d(D-H) [Å] | d(H···A) [Å] | d(D···A) [Å] | <(DHA) [°] |
|---|---|---|---|---|
| N2—H2···O5i | 0.85(3) | 2.24(3) | 3.075(3) | 167(3) |
| O2—H111···O4ii | 0.91(2) | 1.98(2) | 2.863(3) | 161(3) |
| O2—H112···O4iii | 0.89(2) | 2.08(2) | 2.956(2) | 170(3) |
| (i) −x + 1, −y + 1, −z; (ii) −x, −y + 1, −z + 1; | (iii) x, y, z − 1; |
| Compound | Assignment | ||
|---|---|---|---|
| 1, cm–1 | 2, cm–1 | 3, cm–1 | |
| 3247s, 2987m | νas(N–H) νs(N–H) | ||
| 2885m | ν(O–H) | ||
| 3064m | 3081m | 3016m, 2940m | ν(C–H) |
| 2236s | 2301m, 2273m, 2250m | ν(C≡N) | |
| 1633s | 1680m | ν(C–O) | |
| 1611s | 1643s | 1615s | ν(C=N) |
| 1600s | ν(N–H) deformation | ||
| 1594s, 1568s 1428s, 1415s | νas(COO−) νs(COO−) | ||
| 1031s, 1044s | 1032s, 1026s | 1031m, 1018s | ν(C–N) |
| 1059s 1368s | 1018s 1280s | νs(NO3−) νas(NO3−) | |
| 812s | 785s | ρ(NO3−) | |
| 705m | 742m 710m | δs(NO3−) δas(NO3−) | |
| 760s | δ(C–H), orto-substituted rings | ||
| 819s, 790m | δ(C–H), meta-substituted rings | ||
| 837s, 811m | δ(C–H), para-substituted rings |
| 1 | 2 | 3 | |
|---|---|---|---|
| Formula Mr crystal system space group a (Å) b (Å) c (Å) α (°) β (°) γ (°) V (Å3) Z Calc. density (g cm−3) MoKα radiation (Å) μ (mm−1) crystal color and form crystal dimensions reflections measured θ range (°) h, k, l range measured no. of independent refl. Rint observed r, F2 > 2σ(F2) R[F2 > 2σ(F2)] wR(F2), all data goodness-of-fit, S no. of contrib. reflect. no. of parameters Δρmax, Δρmin (eÅ−3) | C20H20Cu2N4O8 571.48 monoclinic P21/c, no.14 8.1939(5) 20.8089(9) 7.4371(5) 90.00 115.293(8) 90.00 1146.51(13) 2 1.655 0.71073 1.909 blue plate 0.08 × 0.26 × 0.32 10,253 2.7–30.4 −10→10; −28→29; −8→10 3145 0.033 2635 0.032 0.077 1.041 3145 156 0.358, −0.654 | C16H24CuN6O10 523.95 triclinic P-1, no.2 7.3037(5) 8.4784(6) 9.0311(7) 83.723(6) 71.745(6) 80.736(6) 523.13(7) 1 1.663 0.71073 1.113 blue plate 0.07 × 0.25 × 0.33 4734 2.4–30.2 −10→10; −12→10;−11→10 2719 0.037 2428 0.039 0.094 1.065 2719 164 0.470, −0.521 | C30H25Cu2N15O12 914.73 monoclinic I2/a, no.15 14.4721(7) 9.7406(5) 27.8533(13) 90 102.053(4) 90 3839.8(3) 4 1.582 0.71073 1.188 blue plate 0.09 × 0.20 × 0.23 16762 2.5–30.4 −19→20; −10→13;−36→30 5228 0.032 4463 0.029 0.082 1.030 5228 272 0.361, −0.497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golobič, A.; Kristl, M.; Podnar, T.M.; Jagličić, Z.; Dojer, B. Mixed-Ligand Copper(II) Complexes Derived from Pyridinecarbonitrile Precursors: Structural Features and Thermal Behavior. Inorganics 2025, 13, 287. https://doi.org/10.3390/inorganics13090287
Golobič A, Kristl M, Podnar TM, Jagličić Z, Dojer B. Mixed-Ligand Copper(II) Complexes Derived from Pyridinecarbonitrile Precursors: Structural Features and Thermal Behavior. Inorganics. 2025; 13(9):287. https://doi.org/10.3390/inorganics13090287
Chicago/Turabian StyleGolobič, Amalija, Matjaž Kristl, Tinkara Marija Podnar, Zvonko Jagličić, and Brina Dojer. 2025. "Mixed-Ligand Copper(II) Complexes Derived from Pyridinecarbonitrile Precursors: Structural Features and Thermal Behavior" Inorganics 13, no. 9: 287. https://doi.org/10.3390/inorganics13090287
APA StyleGolobič, A., Kristl, M., Podnar, T. M., Jagličić, Z., & Dojer, B. (2025). Mixed-Ligand Copper(II) Complexes Derived from Pyridinecarbonitrile Precursors: Structural Features and Thermal Behavior. Inorganics, 13(9), 287. https://doi.org/10.3390/inorganics13090287






