A γ-Al2O3 and MgO/MgAl2O4 Fabricated via a Facile Pathway as Excellent Dye Eliminators from Water
Abstract
1. Introduction
2. Results and Discussion
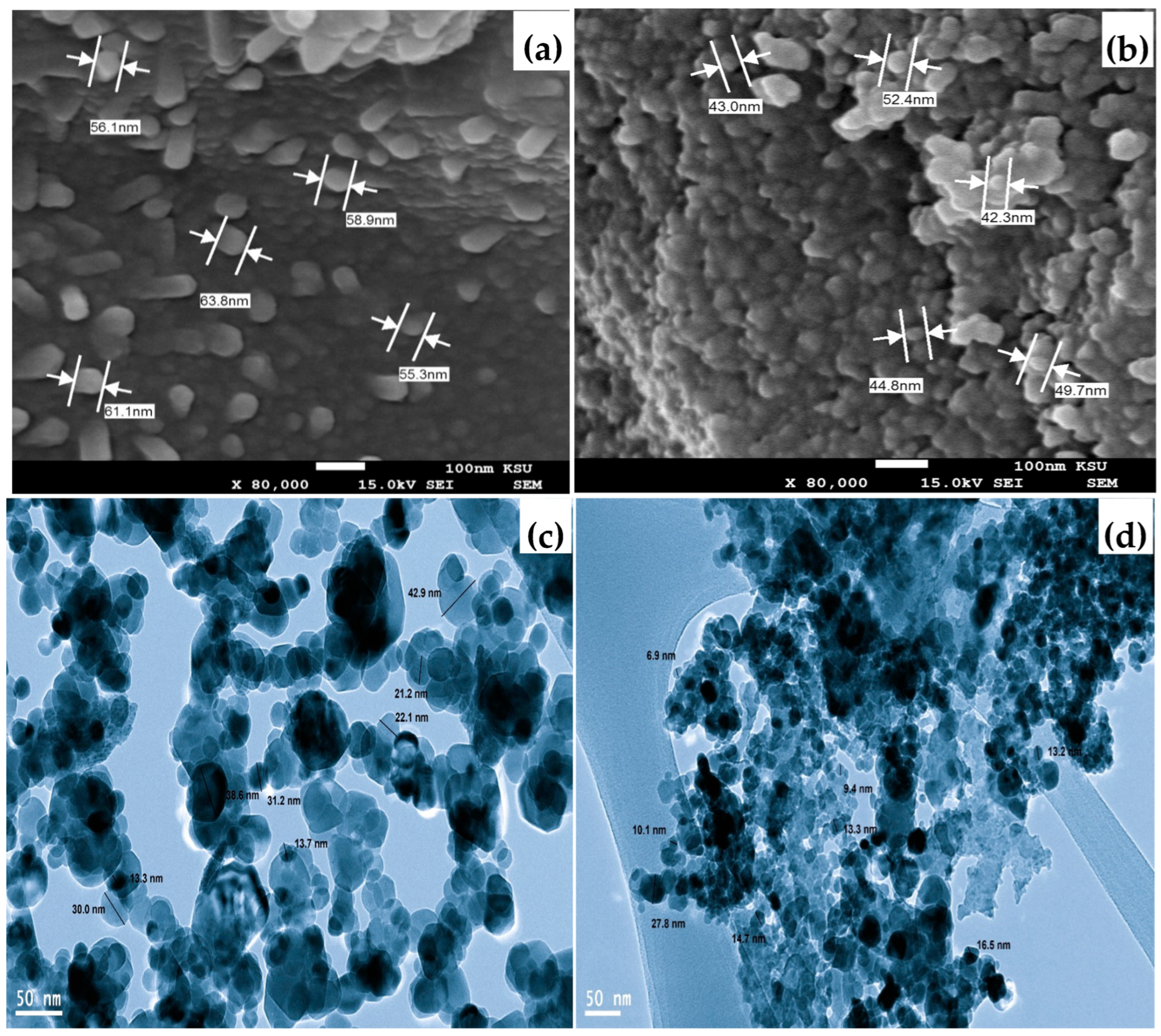
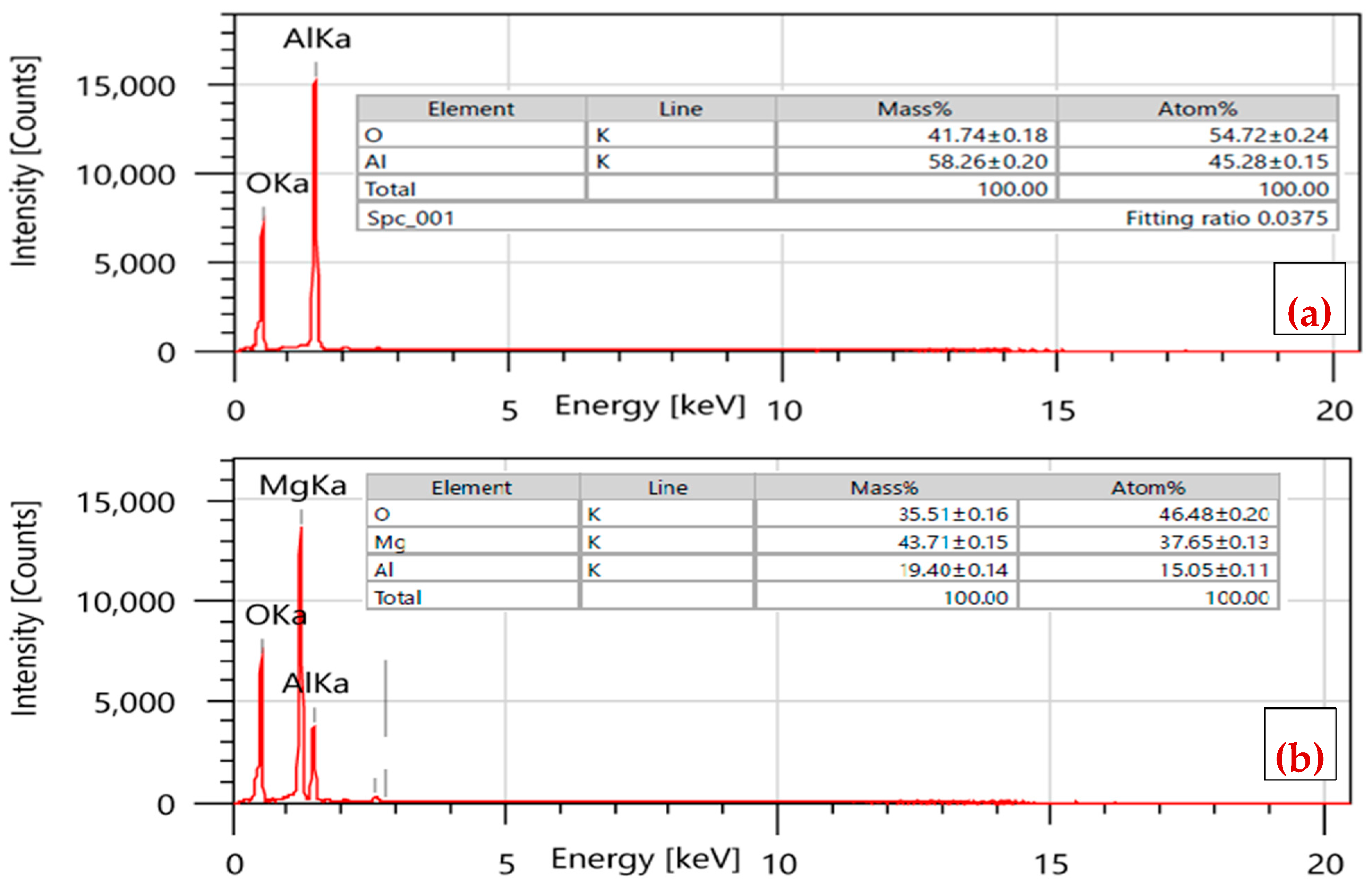
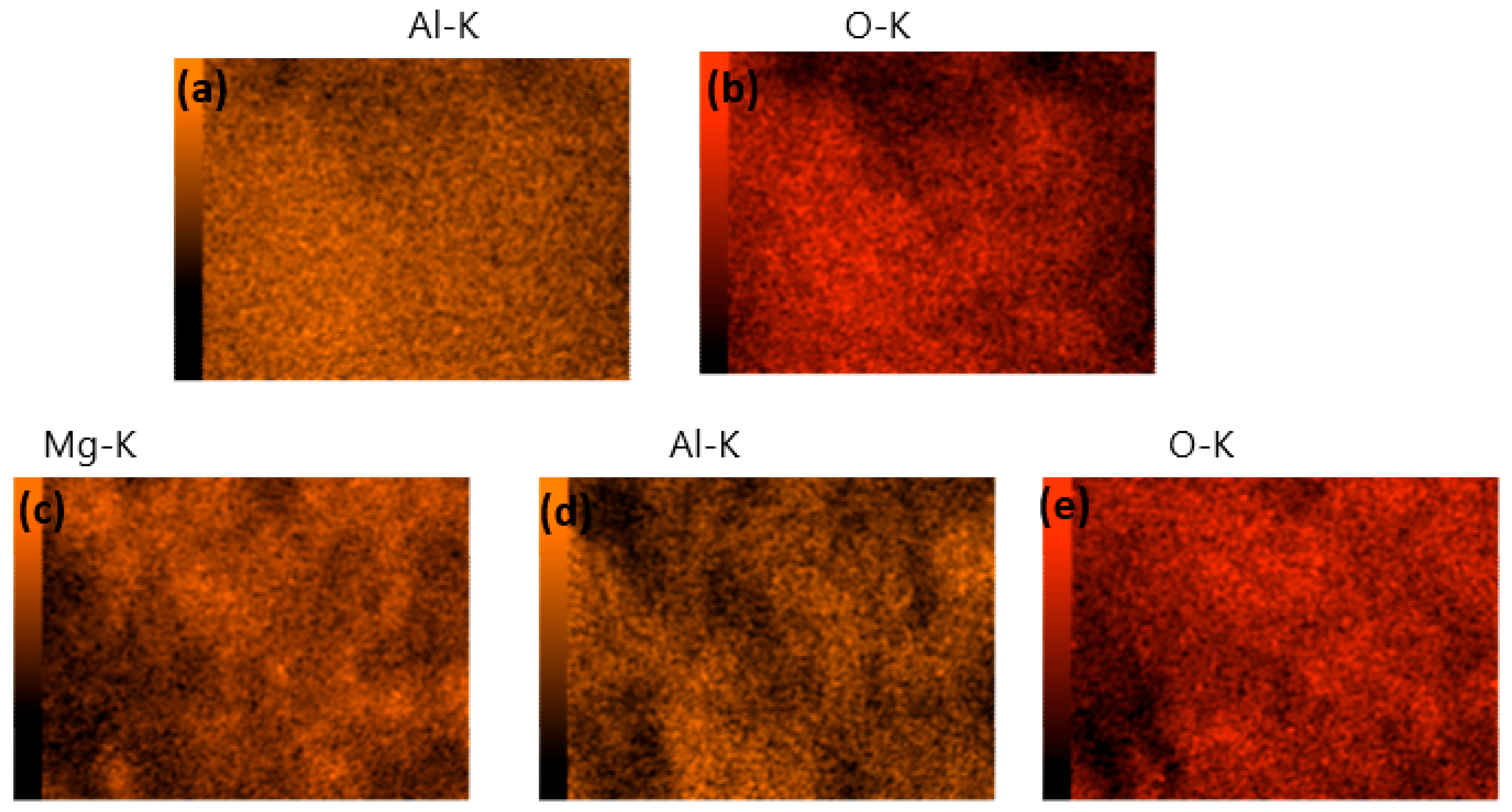
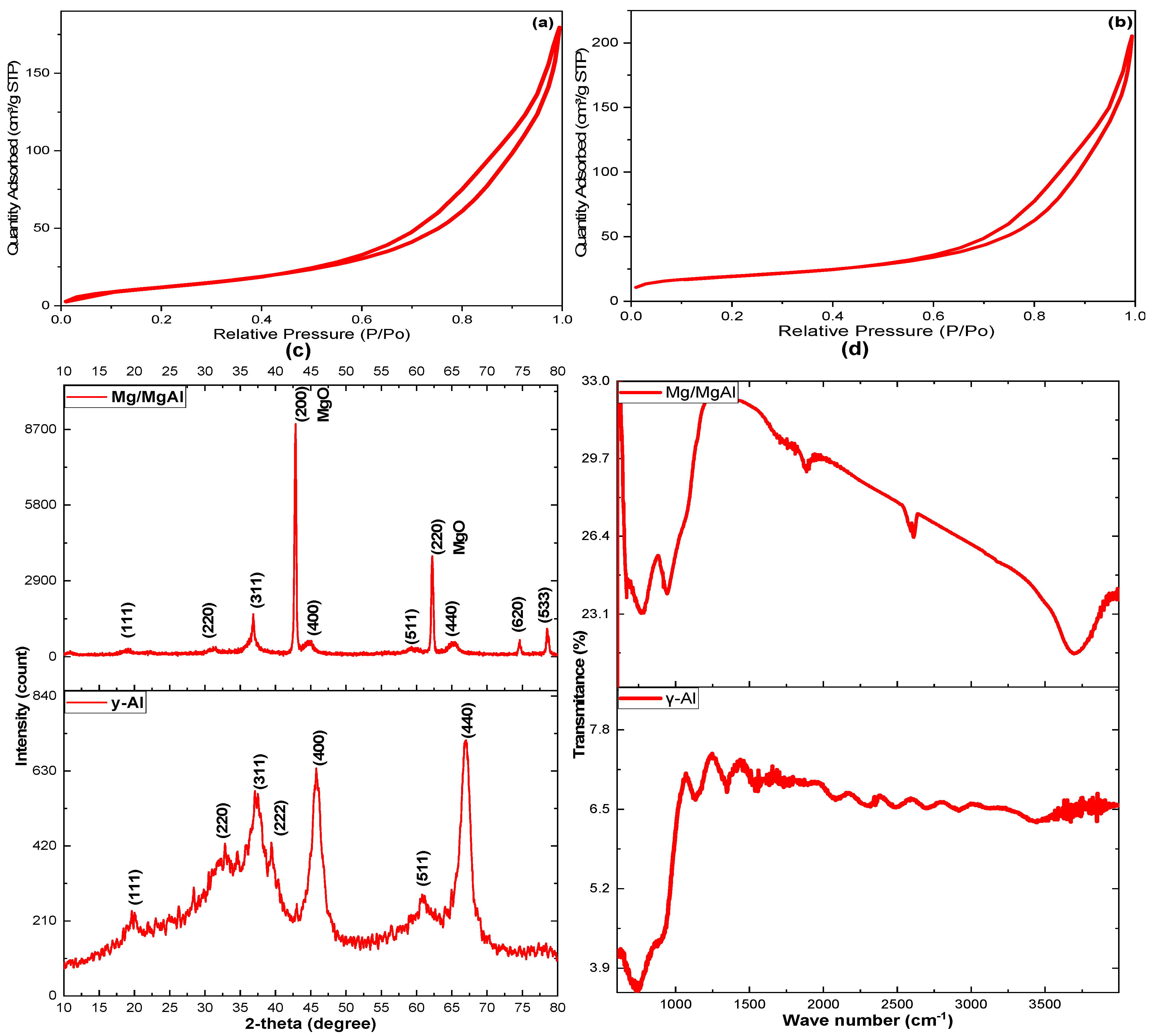
2.1. Characterization of γ-Al and Mg/MgAl
2.2. Contact Time and Kinetic Investigations
2.3. Sorption Equilibria
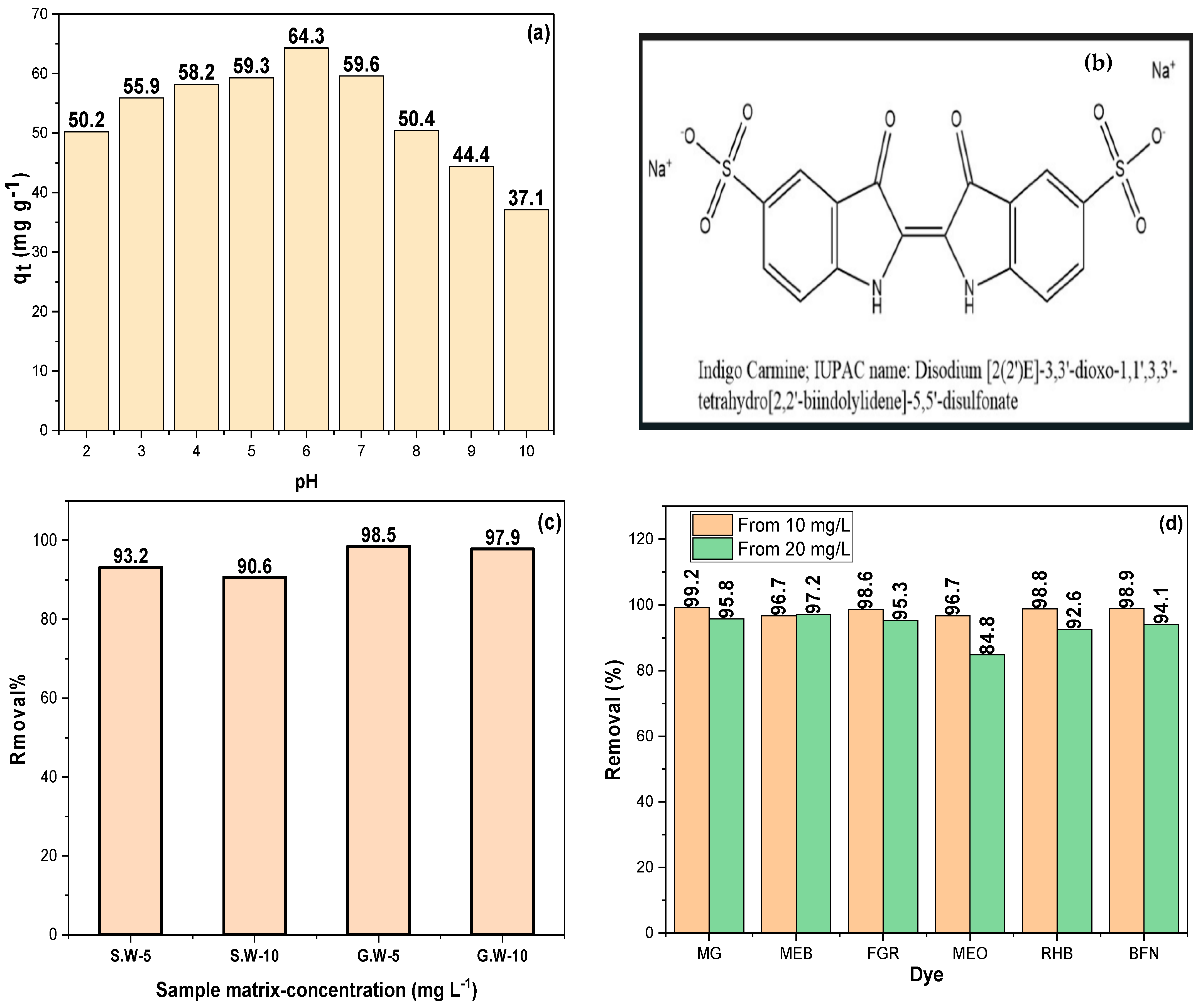
2.4. Effect of pH on IC Sorption by Mg/MgAl
2.5. Application of Sorbents to Natural Water Samples
2.6. Removal of Different Organic Dyes
3. Conclusions
4. Experimental
4.1. Materials
4.2. Preparation of γ-Al and Mg/MgAl
4.3. Characterization of γ-Al and Mg/MgAl
4.4. Adsorption of IC on γ-Al and Mg/MgAl
4.5. Application of γ-Al and Mg/MgAl to Natural Water Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elamin, M.R.; Abdulkhair, B.Y.; Elzupir, A.O. Removal of ciprofloxacin and indigo carmine from water by carbon nanotubes fabricated from a low-cost precursor: Solution parameters and recyclability. Ain Shams Eng. J. 2023, 14, 101844. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Ponce, S.; Chavarria, M.; Norabuena, F.; Chumpitaz, D.; Gutarra, A. Cellulose Microfibres Obtained from Agro-Industrial Tara Waste for Dye Adsorption in Water. Water Air Soil Pollut. 2020, 231, 518. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; Xie, T.; Gao, S.; Qu, Y.; He, L. Rapid identification of artificial and natural food colorants with surface enhanced Raman spectroscopy. Food Control 2018, 92, 267–275. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, S.-P.; Thiruvenkatachari, R.; Shim, W.-G.; Moon, H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dye. Pigment. 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Hajati, S.; Ghaedi, M.; Karimi, F.; Barazesh, B.; Sahraei, R.; Daneshfar, A. Competitive adsorption of Direct Yellow 12 and Reactive Orange 12 on ZnS: Mn nanoparticles loaded on activated carbon as novel adsorbent. J. Ind. Eng. Chem. 2014, 20, 564–571. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Kusumastuti, A.; Anis, S.; Fardhyanti, D.S. Production of natural dyes powder based on chemo-physical technology for textile application. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Lampung Selatan, Indonesia, 19–20 October 2018; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Ayele, M.; Tesfaye, T.; Alemu, D.; Limeneh, M.; Sithole, B. Natural dyeing of cotton fabric with extracts from mango tree: A step towards sustainable dyeing. Sustain. Chem. Pharm. 2020, 17, 100293. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T.J.E.C. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Abouda, N.A.; Jasima, B.E.; Rheimab, A.M. Methylene Orange dye removal in aqueous solution using synthesized CdO-MnO2 nanocomposite: Kinetic and thermodynamic studies. Chalcogenide Lett. 2021, 18, 237–243. [Google Scholar] [CrossRef]
- Ramesh, T.N.; Sreenivasa, V.P.J.S. Removal of indigo carmine dye from aqueous solution using magnesium hydroxide as an adsorbent. J. Mater. 2015, 33, 35. [Google Scholar] [CrossRef]
- Nielsen, I.; Scharff, A.B. The use of cyclododecane for the fixation of bleeding dyes on paper and textiles: A critical evaluation of application methods. In Dyes in History and Archaeology 19: Papers Presented at the 19th Meeting, Held at the Royal Museum, National Museums of Scotland, Edinburgh, UK, 19–20 October 2000; Archetype Publications: London, UK, 2003. [Google Scholar]
- Zhang, X.N.; Mao, G.Y.; Jiao, Y.B.; Shang, Y.; Han, R.P. Adsorption of anionic dye on magnesium hydroxide-coated pyrolytic bio-char and reuse by microwave irradiation. Int. J. Environ. Sci. Technol. 2014, 11, 1439–1448. [Google Scholar] [CrossRef]
- Babu, A.; Reddy, D.; Sharma, P.; Kumar, G.; Ravindhranath, K.; Mohan, G. Removal of hazardous indigo carmine dye from waste water using treated red mud. Mater. Today Proc. 2019, 17, 198–208. [Google Scholar] [CrossRef]
- Debina, B.; Eric, S.N.; Fotio, D.; Arnaud, K.T.; Lemankreo, D.-Y.; Rahman, A.N. Adsorption of Indigo Carmine Dye by Composite Activated Carbons Prepared from Plastic Waste (PET) and Banana Pseudo Stem. J. Mater. Sci. Chem. Eng. 2020, 8, 39–55. [Google Scholar] [CrossRef]
- Mane, V.S.; Mall, I.D.; Srivastava, V.C. Kinetic and equilibrium isotherm studies for the adsorptive removal of Brilliant Green dye from aqueous solution by rice husk ash. J. Environ. Manag. 2007, 84, 390–400. [Google Scholar] [CrossRef]
- Dastgerdi, Z.H.; Meshkat, S.S.; Esrafili, M.D. Enhanced adsorptive removal of Indigo carmine dye performance by functionalized carbon nanotubes based adsorbents from aqueous solution: Equilibrium, kinetic, and DFT study. J. Nanostructure Chem. 2019, 9, 323–334. [Google Scholar] [CrossRef]
- Fatombi, J.K.; Idohou, E.A.; Osseni, S.A.; Agani, I.; Neumeyer, D.; Verelst, M.; Mauricot, R.; Aminou, T. Adsorption of indigo carmine from aqueous solution by chitosan and chitosan/activated carbon composite: Kinetics, isotherms and thermodynamics studies. Fibers Polym. 2019, 20, 1820–1832. [Google Scholar] [CrossRef]
- Ammar, S.; Abdelhedi, R.; Flox, C.; Arias, C.; Brillas, E. Electrochemical degradation of the dye indigo carmine at boron-doped diamond anode for wastewaters remediation. Environ. Chem. Lett. 2006, 4, 229–233. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, Properties, and Selected Technical Applications of Magnesium Oxide Nanoparticles: A Review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Almahmoud, S.A.; Alzahrani, S.S.; Abdulkhair, B.Y. Efficacious Removal of Organic Contaminants from Water Using a Novel CoO–NiO@ MgAl2O4 Nanocomposite. ACS Omega 2025, 10, 11188–11201. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Xiao, F.; Fang, L.; Li, W.; Wang, D. One-step synthesis of aluminum magnesium oxide nanocomposites for simultaneous removal of arsenic and lead ions in water. RSC Adv. 2015, 5, 8190–8193. [Google Scholar] [CrossRef]
- Saied, E.; Eid, A.M.; Hassan, S.E.D.; Salem, S.S.; Radwan, A.A.; Halawa, M. The catalytic activity of biosynthesized magnesium oxide nanoparticles (MgO-NPs) for inhibiting the growth of pathogenic microbes, tanning effluent treatment, and chromium ion removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Siriwardane, I.W.; Udangawa, R.; de Silva, R.M.; Kumarasinghe, A.; Acres, R.G.; Hettiarachchi, A.; Amaratunga, G.A.; de Silva, K.N. Synthesis and characterization of nano magnesium oxide impregnated granular activated carbon composite for H2S removal applications. Mater. Des. 2017, 136, 127–136. [Google Scholar] [CrossRef]
- Romero Toledo, R.; Santoyo, V.R.; Sánchez, C.D.M.; Rosales, M.M. Effect of aluminum precursor on physicochemical properties of Al2O3 by hydrolysis/precipitation method. Nova Sci. 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Barta, J.; Pospisil, M.; Cuba, V. Indirect synthesis of Al2O3 via radiation-or photochemical formation of its hydrated precursors. Mater. Res. Bull. 2014, 49, 633–639. [Google Scholar] [CrossRef]
- Jun-Cheng, L.; Lan, X.; Feng, X.; Zhan-Wen, W.; Fei, W. Effect of hydrothermal treatment on the acidity distribution of γ-Al2O3 support. Appl. Surf. Sci. 2006, 253, 766–770. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, F.; Feng, G.; Wang, S.; Miao, L.; Jiang, W.; Liang, J.; Liu, J. Nonhydrolytic sol-gel in-situ synthesis of high performance MgAl2O4/C adsorbent materials. Arab. J. Chem. 2023, 16, 104393. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Xie, X.; Zhu, C.-G. Self-Propagating High-Temperature Synthesis of Magnesium Aluminate Spinel Using Mg–Al Alloy. ACS Omega 2022, 7, 12617–12623. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wang, W.; Tan, F.; Luo, F.; Chen, J.; Qiao, X. Investigation on the efficiency and mechanism of Cd (II) and Pb (II) removal from aqueous solutions using MgO nanoparticles. J. Hazard. Mater. 2015, 299, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Oladoja, N.; Aboluwoye, C.; Oladimeji, Y. Kinetics and Isotherm Studies on Methylene Blue Adsorption onto Ground Palm Kernel Coat. Turk. J. Eng. Environ. Sci. 2008, 32, 303–312. [Google Scholar]
- Ibrahim, T.G.; Almufarij, R.S.; Abdulkhair, B.Y.; Elaziz, M.E.A. Eliminating Manifold Pharmaceutical Pollutants with Carbon Nanoparticles Driven via a Short-Duration Ball-Milling Process. Surfaces 2024, 7, 493–507. [Google Scholar] [CrossRef]
- Adam, F.A.; Ghoniem, M.G.; Diawara, M.; Rahali, S.; Abdulkhair, B.Y.; Elamin, M.R.; Ben Aissa, M.A.; Seydou, M. Enhanced adsorptive removal of indigo carmine dye by bismuth oxide doped MgO based adsorbents from aqueous solution: Equilibrium, kinetic and computational studies. RSC Adv. 2022, 12, 24786–24803. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Algethami, F.K.; Khezami, L. Linear and nonlinear investigations for the adsorption of paracetamol and metformin from water on acid-treated clay. Sci. Rep. 2021, 11, 13606. [Google Scholar] [CrossRef] [PubMed]
- An, B. Cu (II) and As (V) adsorption kinetic characteristic of the multifunctional amino groups in chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Almufarij, R.S.; Abdulkhair, B.Y.; Salih, M. Fast-simplistic fabrication of MoO3@ Al2O3-MgO triple nanocomposites for efficient elimination of pharmaceutical contaminants. Results Chem. 2024, 7, 101281. [Google Scholar] [CrossRef]
- Ibrahim, T.G.; Almufarij, R.S.; Abdulkhair, B.Y.; Ramadan, R.S.; Eltoum, M.S.; Elaziz, M.E.A. A Thorough Examination of the Solution Conditions and the Use of Carbon Nanoparticles Made from Commercial Mesquite Charcoal as a Successful Sorbent for Water Remediation. Nanomaterials 2023, 13, 1485. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Sillanpää, M. Optimizing the removal of pharmaceutical drugs Carbamazepine and Dorzolamide from aqueous solutions using mesoporous activated carbons and multi-walled carbon nanotubes. J. Mol. Liq. 2017, 238, 379–388. [Google Scholar] [CrossRef]
- Ismail, B.; Hussain, S.T.; Akram, S. Adsorption of methylene blue onto spinel magnesium aluminate nanoparticles: Adsorption isotherms, kinetic and thermodynamic studies. Chem. Eng. J. 2013, 219, 395–402. [Google Scholar] [CrossRef]
- Treviño, P.; Garcia-Castro, A.C.; López-Moreno, S.; Bautista-Hernández, A.; Bobocioiu, E.; Reynard, B.; Caracas, R.; Romero, A.H. Anharmonic contribution to the stabilization of Mg(OH)2 from first principles. Phys. Chem. Chem. Phys. 2018, 20, 17799–17808. [Google Scholar] [CrossRef] [PubMed]
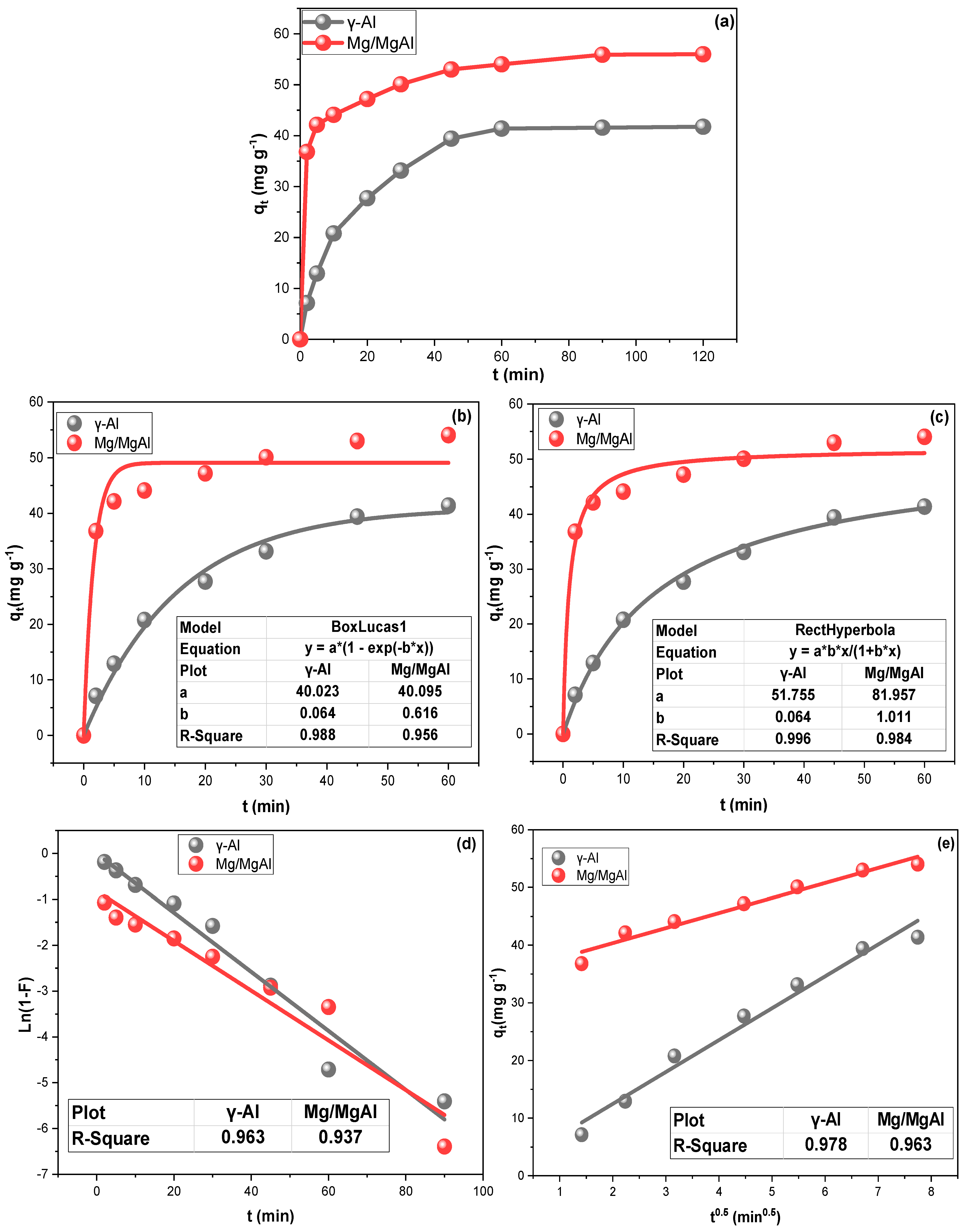

| Adsorption Rate Order | |||||||||||
| Sorbent | qmax exp (mg g−1) | PFO | PSO | ||||||||
| qe (mg·g−1) | K1 | R2 | X2 | RSS | qe (mg·g−1) | K2 | R2 | X2 | RSS | ||
| γ-Al | 41.57 | 40.022 | 0.064 | 0.988 | 3.110 | 18.661 | 51.755 | 0.064 | 0.996 | 0.952 | 5.714 |
| Mg/MgAl | 55.90 | 49.095 | 0.062 | 0.956 | 15.715 | 94.951 | 51.957 | 1.011 | 0.984 | 5.721 | 34.327 |
| Adsorption Rate Mechanism | |||||||||||
| Sorbent | IDM | LDM | |||||||||
| KIP (mg·g−1 min1/2) | C (mg·g−1) | R2 | X2 | RSS | KLF (min−1) | R2 | X2 | RSS | |||
| γ-Al | 5.531 | 1.404 | 0.963 | 12.448 | 22.361 | 0.074 | 0.978 | 0.067 | 1.0457 | ||
| Mg/MgAl | 2.603 | 35.154 | 0.937 | 13.498 | 8.444 | 0.038 | 0.963 | 0.066 | 1.3057 | ||
| Adsorption Isotherms | |||||||
|---|---|---|---|---|---|---|---|
| Isotherm Model → | Langmuir | Freundlich | |||||
| Temperature | R2 | KL (L·g−1) | qm (mg·g−1) | R2 | Kf (L·g−1) | 1/n (a.u) | |
| 293 K | 0.997 | 0.012 | 188.774 | 0.956 | 9.446 | 0.502 | |
| Thermodynamic Results | |||||||
| Conc. (mg L−1) | ΔH° | ΔS° | ΔG° (293 K) | ΔG° (303 K) | ΔG° (313 K) | ΔG° (323 K) | R2 |
| 25.0 | 35.692 | 0.131 | −2.695 | −4.005 | −5.315 | −6.625 | 0.921 |
| 50.0 | 3.610 | 0.020 | −2.210 | −2.409 | −2.608 | −2.806 | 0.626 |
| 100.0 | 39.644 | 0.136 | −0.165 | −1.524 | −2.883 | −4.241 | 0.994 |
| 200.0 | 19.799 | 0.068 | −0.033 | −0.710 | −1.387 | −2.064 | 0.974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhory, S.H.; Elamin, M.R.; Ibrahim, T.G.; Salih, M.; Algethami, F.K.; Eltoum, M.S.; Abdulkhair, B.Y. A γ-Al2O3 and MgO/MgAl2O4 Fabricated via a Facile Pathway as Excellent Dye Eliminators from Water. Inorganics 2025, 13, 284. https://doi.org/10.3390/inorganics13090284
Elhory SH, Elamin MR, Ibrahim TG, Salih M, Algethami FK, Eltoum MS, Abdulkhair BY. A γ-Al2O3 and MgO/MgAl2O4 Fabricated via a Facile Pathway as Excellent Dye Eliminators from Water. Inorganics. 2025; 13(9):284. https://doi.org/10.3390/inorganics13090284
Chicago/Turabian StyleElhory, Salah H., Mohamed R. Elamin, Tarig G. Ibrahim, Mutaz Salih, Faisal K. Algethami, Mohamed S. Eltoum, and Babiker Y. Abdulkhair. 2025. "A γ-Al2O3 and MgO/MgAl2O4 Fabricated via a Facile Pathway as Excellent Dye Eliminators from Water" Inorganics 13, no. 9: 284. https://doi.org/10.3390/inorganics13090284
APA StyleElhory, S. H., Elamin, M. R., Ibrahim, T. G., Salih, M., Algethami, F. K., Eltoum, M. S., & Abdulkhair, B. Y. (2025). A γ-Al2O3 and MgO/MgAl2O4 Fabricated via a Facile Pathway as Excellent Dye Eliminators from Water. Inorganics, 13(9), 284. https://doi.org/10.3390/inorganics13090284







