Degradation of the Vaccine Additive Thimerosal by L-Glutathione and L-Cysteine at Physiological pH
Abstract
1. Introduction
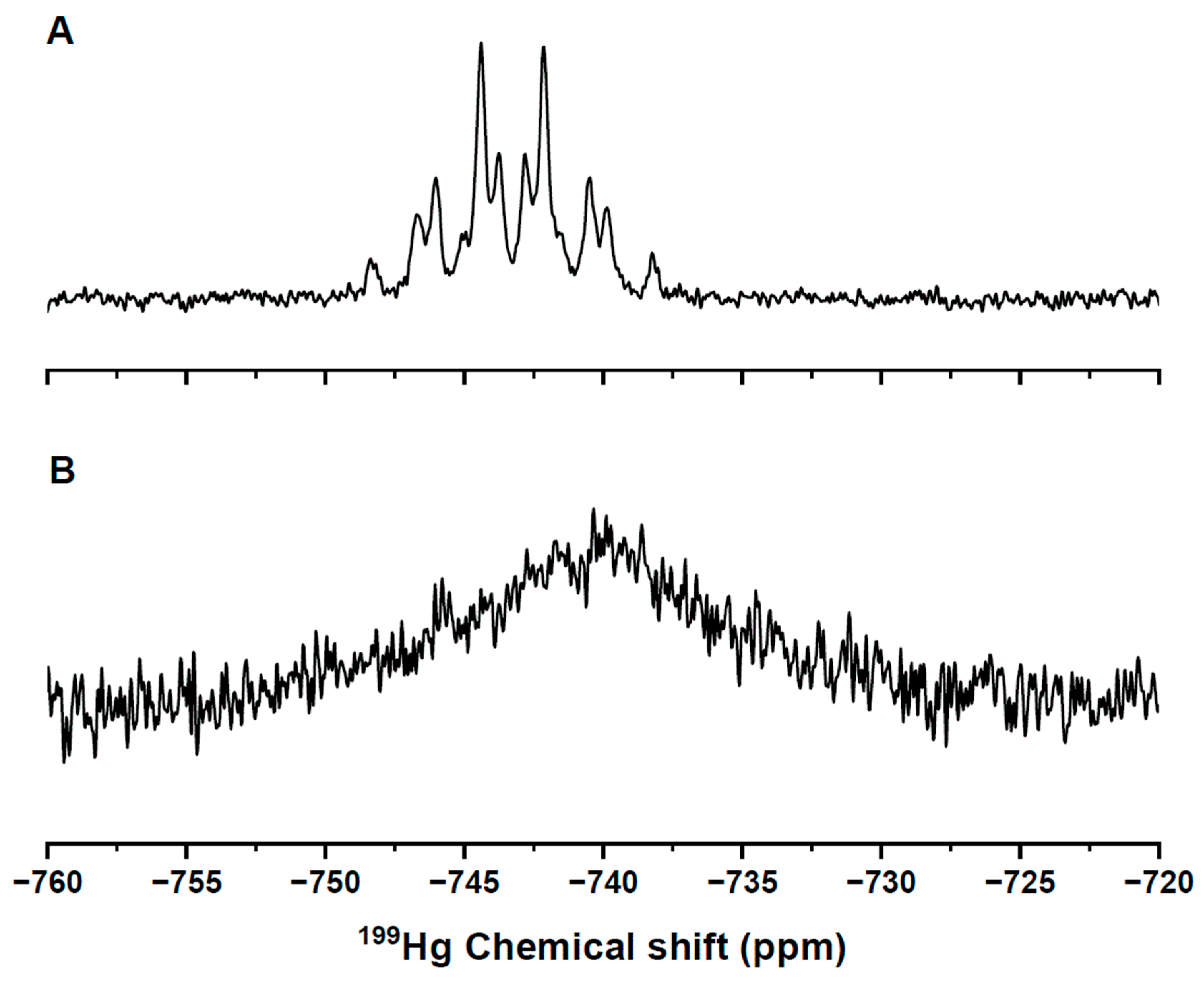
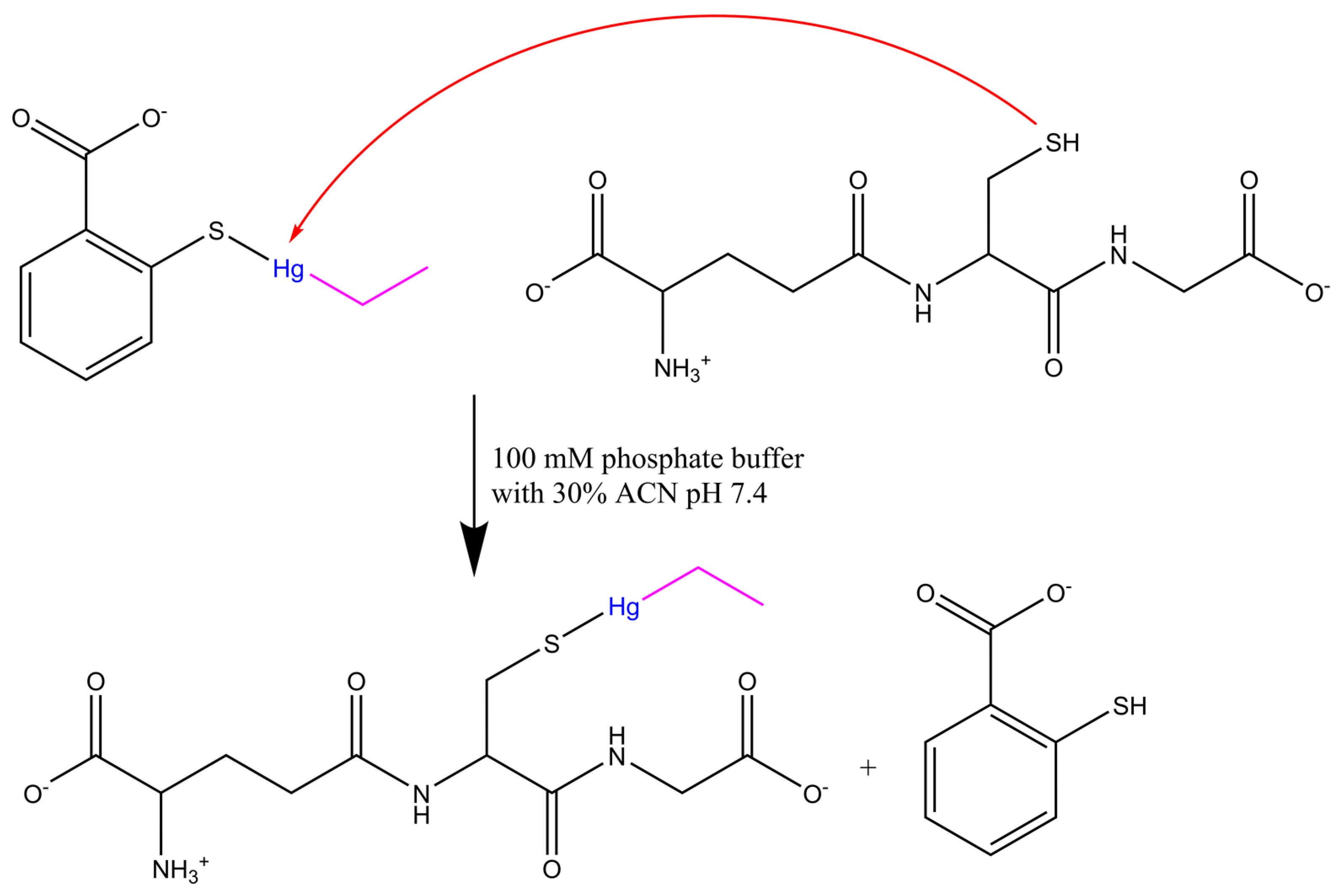
2. Results and Discussion
3. Experimental
3.1. Chemicals and Solutions
3.2. Instrumentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.-G.; Hu, Q.; Zhao, F.J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar]
- Niyogi, S.; Shekh, K.; Amuno, S. Toxicology of trace metals in the environment: A current perspective. In Environmental and Biochemical Toxicology, Concepts, Case Studies and Challenges; Gailer, J., Turner, R.J., Eds.; De Gruyter: Boston, MA, USA, 2022; p. 335. [Google Scholar]
- Charette, T.; Kamisnki, G.; Rosabal, M.; Amyot, M. Effects of speciation, cooking and changes in bioaccessibility on methylmercury exposure assessment for contrasting diets of fish and marine mammals. Int. J. Environ. Res. Public Health 2021, 18, 2565. [Google Scholar] [CrossRef] [PubMed]
- Doroudian, M.; Gailer, J. Integrative metallomics studies of toxic metal(loid) substances at the blood plasma-red blood cell-organ/tumor nexus. Inorganics 2022, 10, 200. [Google Scholar] [CrossRef]
- Carneiro, M.F.H.; Souza, J.M.O.; Grotto, D.; Batista, B.L.; de Oliveira Sousa, V.C.; Barnbosa, F.J. A systematic study of the disposition and metabolism of mercury species in mice after exposure to low levels of thimerosal (ethymercury). Environ. Res. 2014, 134, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Gailer, J. Probing the bioinorganic chemistry of toxic metals in the mammalian bloodstream to advance human health. J. Inorg. Biochem. 2012, 108, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.A.; Sarpong-Kumankomah, S.; Nehzati, S.; George, G.N.; Gailer, J. Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3Hg+ and thimerosal in red blood cell lysate. Metallomics 2017, 9, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- de Magalhanes Silva, M.; de Araujo Dantas, M.D.; de Sila Filho, R.C.; dos Santos Sales, M.V.; de Almeida Xavier, J.; Leite, A.C.R.; Goulart, M.O.F.; Grillo, L.A.M.; de Barros, W.A.; de Fatima, A.; et al. Toxicity of thimerosal in biological systems: Conformational changes in human hemoglobin, decrease of oxygen binding, increase of protein glycation and amyloid formation. Intern. J. Biol. Macromol. 2020, 154, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Zareba, M.; Sanecki, P.T.; Rawski, R. Simultaneous determination of thimerosal and aluminium in vaccines and pharmaceuticals with the use of HPLC Method. Acta Chromatogr. 2016, 28, 299–311. [Google Scholar] [CrossRef]
- Elferink, J.G.R. Thimerosal: A versatile sulfhydryl reagent, calcium mobilizer, and cell function-modulating agent. Gen. Pharmacol. 1999, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Gallo, G.; Campos, C.B.; Hardy, L.; Wuertele, M. Biochemical screening for SARS-CoV-2 main protease inhibitors. PLoS ONE 2020, 15, e0240079. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Haley, B.E.; Geier, M.R. The relationship between mercury and autism: A comprehensive review and discussion. J. Trace Elem. Med. Biol. 2016, 37, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Janzen, R.; Schwarzer, M.; Sperling, M.; Vogel, M.; Schwerdtle, T.; Karst, U. Adduct formation of thimerosal with human and rat hemoglobin: A study using liquid chromatography coupled to electrospray time-of-flight mass spectrometry (LC/ESI-TOF-MS). Metallomics 2011, 3, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.H.; Yates, J.W.; Nicholls, A.W.; Kenna, J.G.; Coen, M.; Ortega, F.; Nicholson, J.K.; Wilson, I.D. Systems toxicology: Modelling biomarkers of glutathione homeostasis and paracetamol metabolism. Drug Discov. Today Technol. 2015, 15, e9–e14. [Google Scholar] [CrossRef]
- Attia, M.I.; Gailer, J. RP-HPLC reveales the L-cysteine induced degradation of phenylmercuric acetate. J. Inorg. Biochem. 2025, 266, 112851. [Google Scholar] [CrossRef] [PubMed]
- Girault, L.; Boudou, A.; Dufourc, E.J. Methyl mercury interactions with phospholipid membranes as reported by fluorescence, 31P and 199Hg NMR. Biochim. Biophys. Acta 1997, 1325, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.W.-H.; Loan, A.; Xu, Y.; Yang, G.; Wang, J.; Chan, H.M. Reduction of glyoxalate 1 expression links fetal methylmercury exposure to autism spectrum disorder pathogenesis. Toxics 2024, 12, 449. [Google Scholar]
- Tan, M.; Parkin, J.E. Route of decomposition of thiomersal (thimerosal). Intern. J. Pharm. 2000, 208, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Reader, M.J.; Lines, C.B. Decomposition of thimerosal in aqueous solution and its determination by high-performance liquid chromatography. J. Pharm. Sci. 1983, 72, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Taylor, C.W.; Berridge, M.J. The thiol reagent, thimerosal, evokes Ca2+ spikes in Hela-cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1992, 267, 25113–25119. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.J.; Zable, A.C.; Favero, T.G.; Salama, G. Thimerosal interacts with the Ca2+ release channel ryanodinereceptor from skeletal-muscle sarcoplasmatic-reticulum. J. Biol. Chem. 1995, 270, 29644–29647. [Google Scholar] [CrossRef] [PubMed]
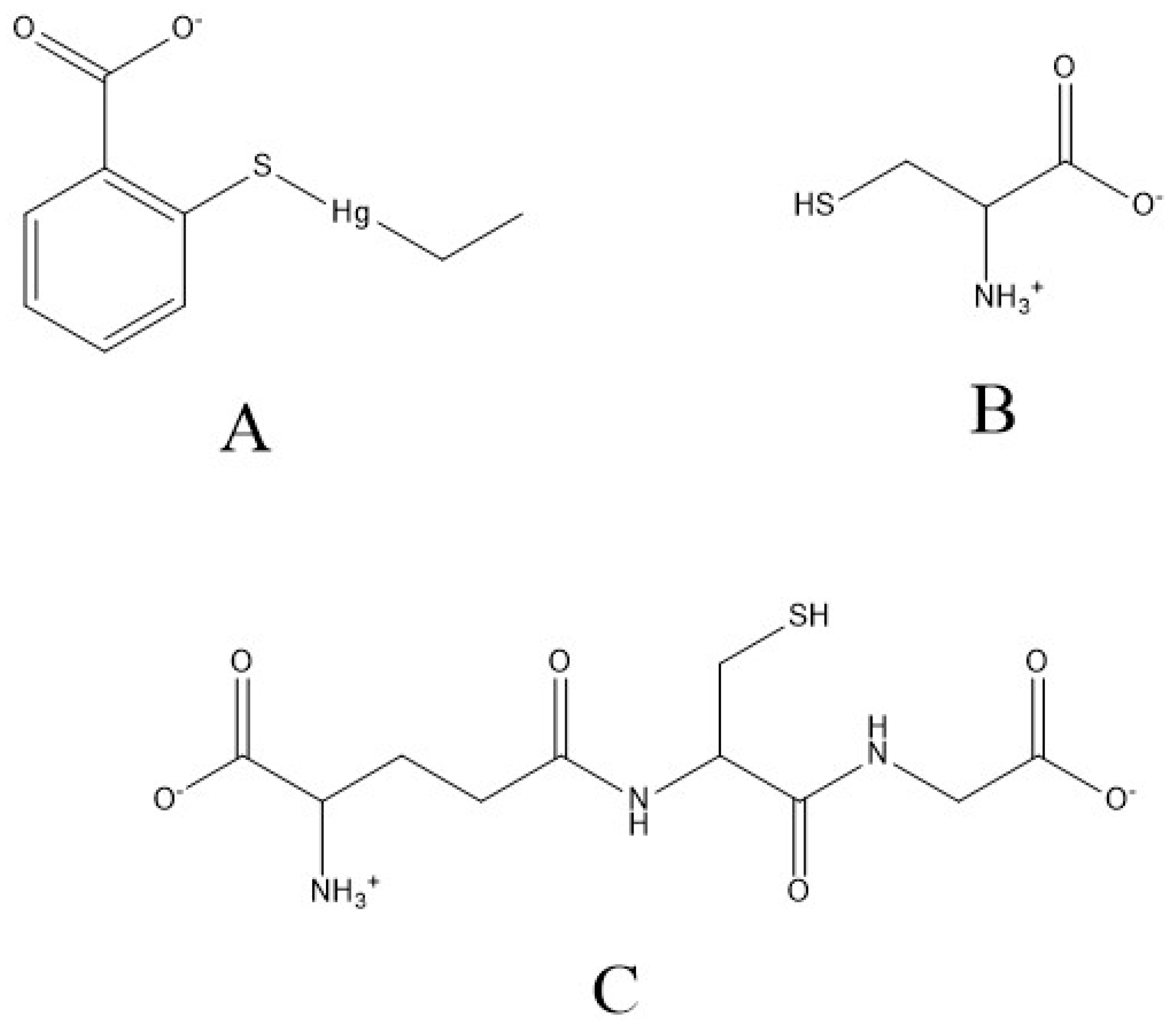
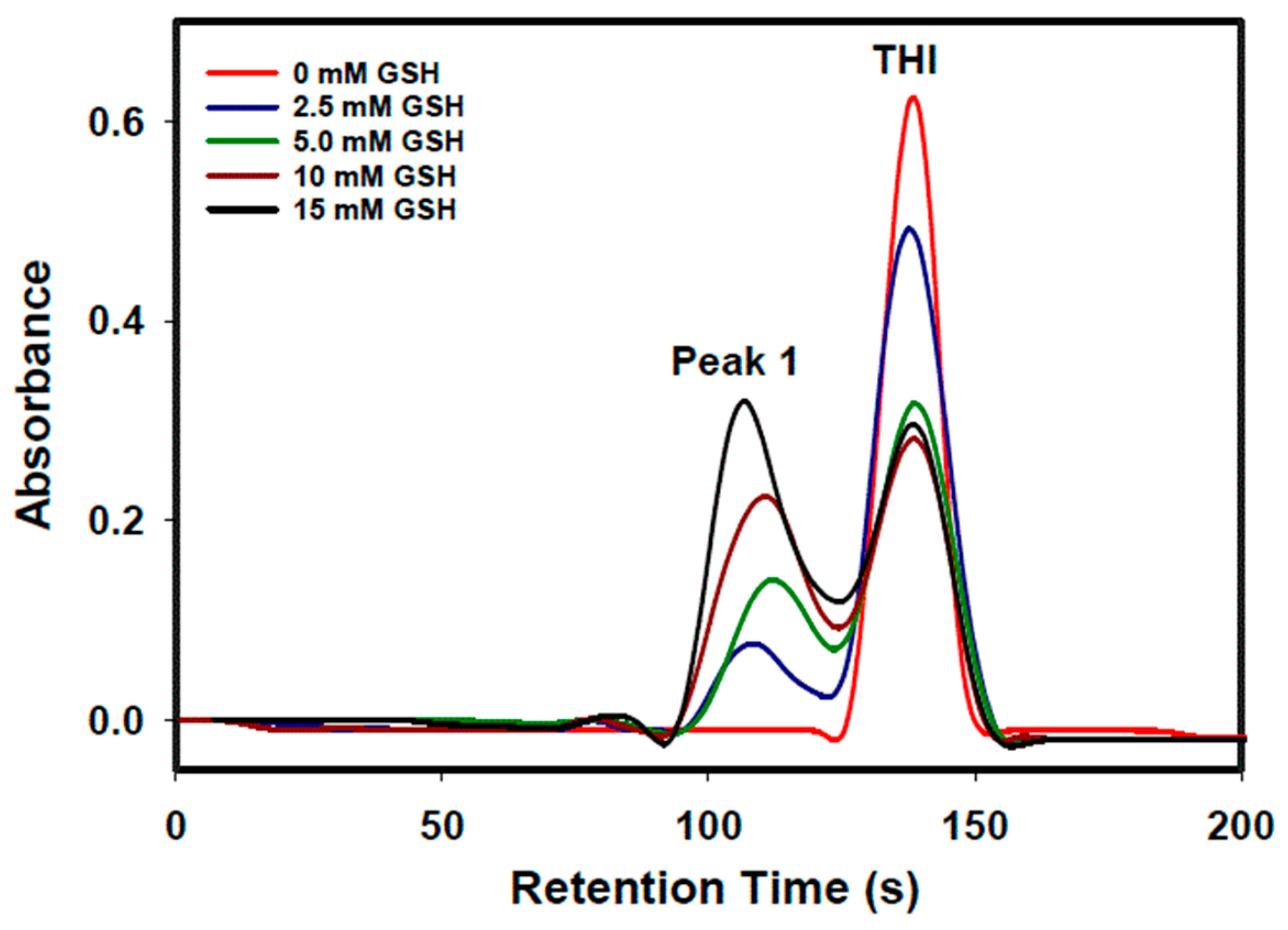
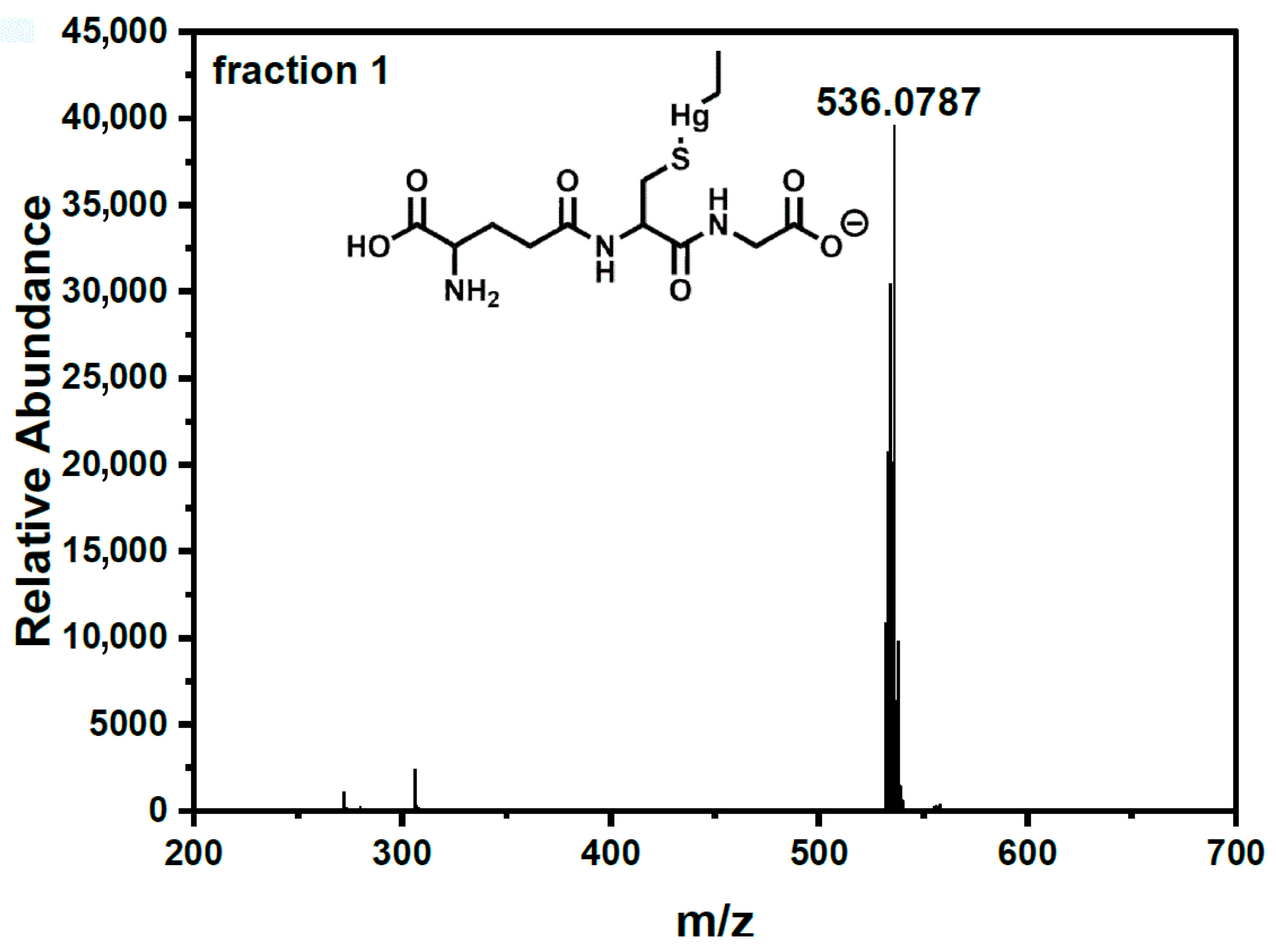
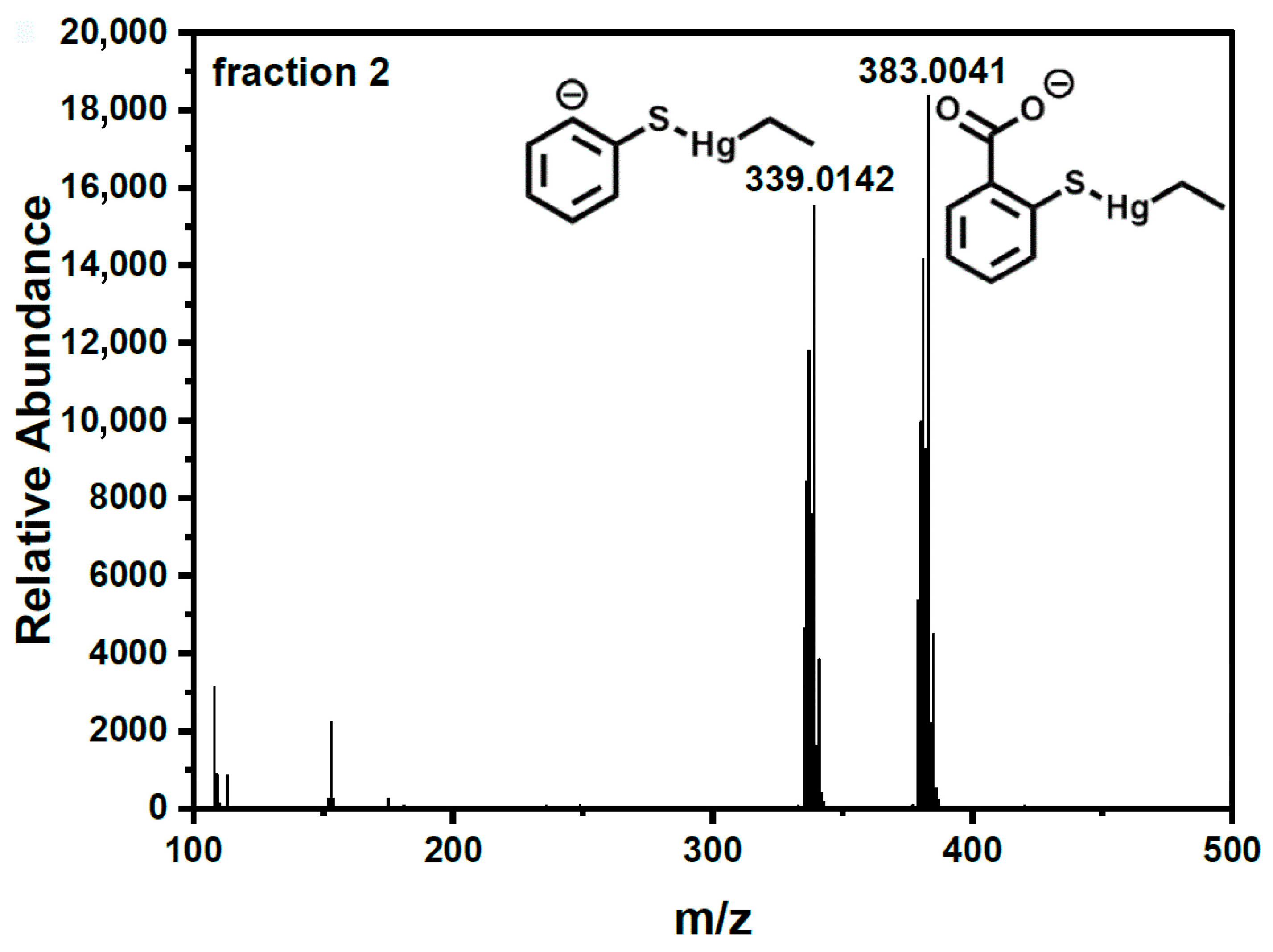
| Concentration of GSH (mM) in Mobile Phase | Retention Time (s) | Peak Area (Area Units) (Area Percentage [%]) | Sum Area (Area Units) (Sum Area Percentage [%]) | ||
|---|---|---|---|---|---|
| Peak 1 | THI | Peak 1 | THI | ||
| 0 | - | 137 ± 1 * | - | 8.43 ± 1.07 (82.2 ± 10.7%) | 8.43 ± 1.07 (82.2 ± 10.7%) |
| 2.5 | 107 ± 2 | 137 ± 1 | 1.71 ± 0.07 (16.7 ± 0.8%) | 8.11 ± 0.37 (79.0 ± 4.3%) | 9.82 ± 0.38 (95.7 ± 4.4%) |
| 5 | 111 ± 1 | 138 ± 1 | 2.79 ± 0.06 (27.2 ± 1.0%) | 6.19 ± 0.20 (60.3 ± 2.6%) | 8.98 ± 0.21 (87.5 ± 2.8%) |
| 10 | 103 ± 1 | 135 ± 1 | 4.75 ± 0.06 (46.3 ± 1.5%) | 5.73 ± 0.37 (55.8 ± 4.0%) | 10.48 ± 0.37 (102.1 ± 4.3%) |
| 15 | 105 ± 1 | 136 ± 1 | 6.23 ± 0.04 (60.7 ± 1.8%) | 5.53 ± 0.02 (53.9 ± 1.6%) | 11.98 ± 0.29 (114.6 ± 2.4%) |
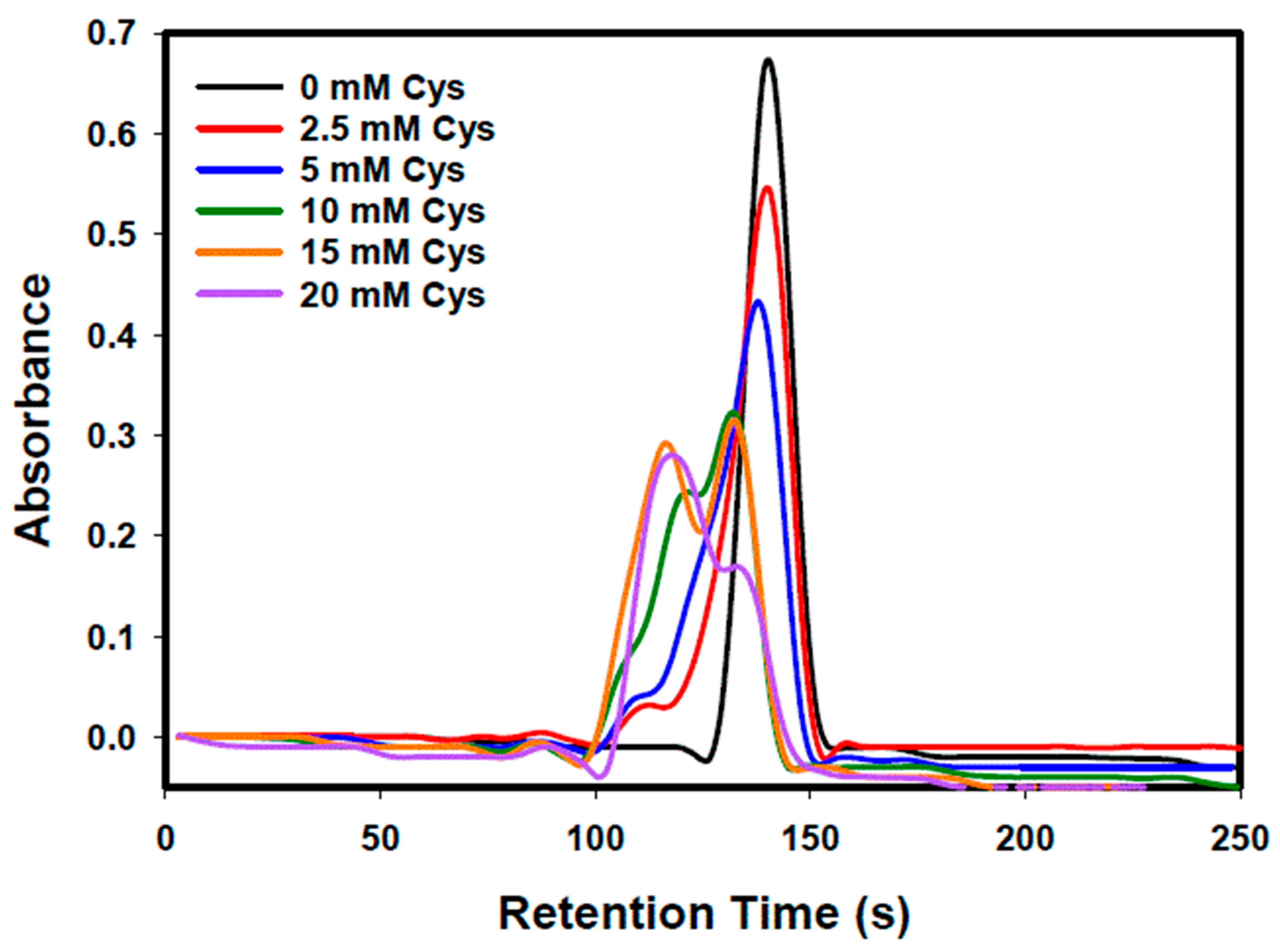
| Concentration of Cys (mM) in Mobile Phase | Retention Time (s) | Peak Area (Area Units) (Area Percentage [%]) | Sum Area (Area Units) (Sum Area Percentage [%]) | ||
|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 1 | Peak 1 | ||
| 0 | - | 140 ± 1 * | - | 8.73 ± 0.30 (85.1 ± 3.8%) | 8.73 ± 0.30 (85.1 ± 3.8%) |
| 2.5 | 115 ± 2 | 140 ± 1 | 0.51 ± 0.01 (4.9 ± 0.2%) | 8.55 ± 0.36 (83.3 ± 4.3%) | 9.06 ± 0.37 (88.3 ± 4.4%) |
| 5 | 111 ± 1 | 139 ± 1 | 0.58 ± 0.06 (5.7 ± 0.6%) | 8.44 ± 0.14 (82.2 ± 2.8%) | 9.02 ± 0.20 (87.9 ± 3.2%) |
| 10 | 121 ± 1 | 132 ± 1 | 3.84 ± 0.31 (37.4 ± 3.2%) | 4.83 ± 0.49 (47.1 ± 4.9%) | 8.67 ± 0.80 (84.5 ± 8.2%) |
| 15 | 116 ± 1 | 133 ± 0 | 5.46 ± 0.39 (53.2 ± 4.1%) | 4.36 ± 0.25 (42.5 ± 2.7%) | 9.82 ± 0.64 (95.7 ± 6.8%) |
| 20 | 117 ± 1 | 134 ± 1 | 5.95 ± 0.17 (57.9 ± 2.4%) | 2.15 ± 0.19 (20.9 ± 1.9%) | 8.10 ± 0.36 (78.9 ± 4.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degorge, M.F.; Mertz, S.; Gailer, J. Degradation of the Vaccine Additive Thimerosal by L-Glutathione and L-Cysteine at Physiological pH. Inorganics 2025, 13, 280. https://doi.org/10.3390/inorganics13090280
Degorge MF, Mertz S, Gailer J. Degradation of the Vaccine Additive Thimerosal by L-Glutathione and L-Cysteine at Physiological pH. Inorganics. 2025; 13(9):280. https://doi.org/10.3390/inorganics13090280
Chicago/Turabian StyleDegorge, Manon Fanny, Silas Mertz, and Jürgen Gailer. 2025. "Degradation of the Vaccine Additive Thimerosal by L-Glutathione and L-Cysteine at Physiological pH" Inorganics 13, no. 9: 280. https://doi.org/10.3390/inorganics13090280
APA StyleDegorge, M. F., Mertz, S., & Gailer, J. (2025). Degradation of the Vaccine Additive Thimerosal by L-Glutathione and L-Cysteine at Physiological pH. Inorganics, 13(9), 280. https://doi.org/10.3390/inorganics13090280







