A Comparative Study of Supported Sulfonic Acids Derived from CdO and CaO for the Reactive Adsorption of o-Xylene
Abstract
1. Introduction
2. Results and Discussion
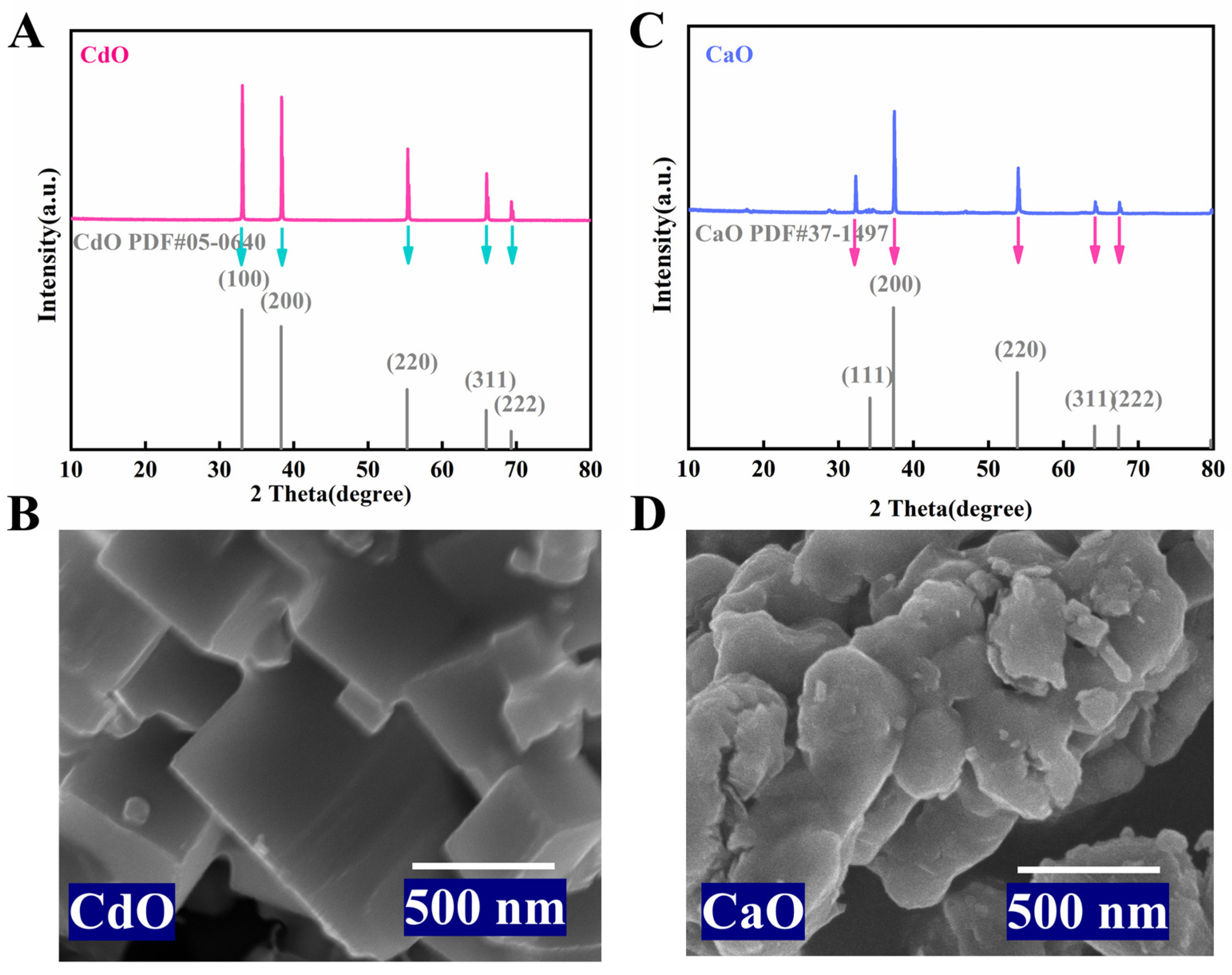
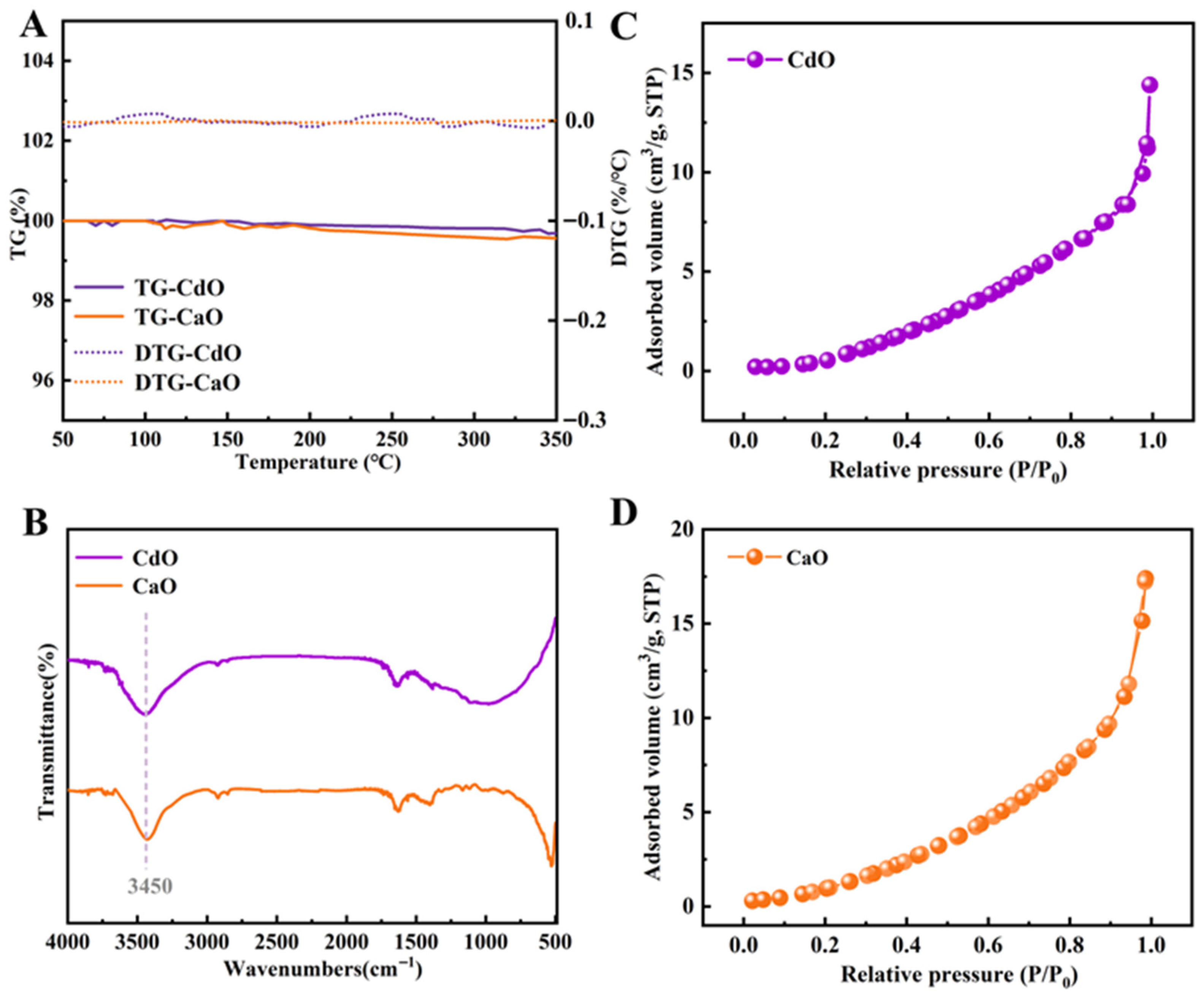
2.1. Basic Characterization of CdO and CaO Support Materials
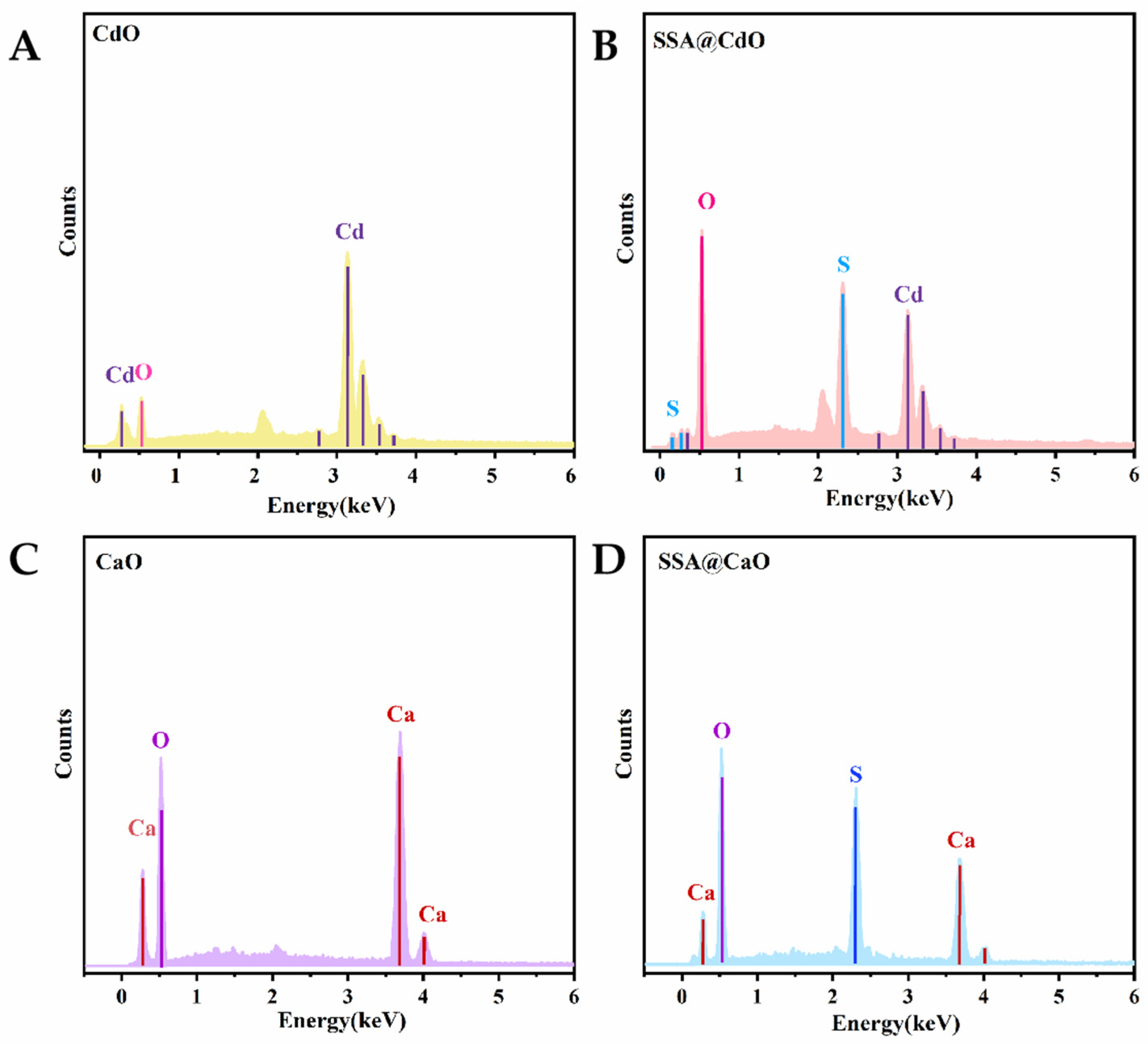
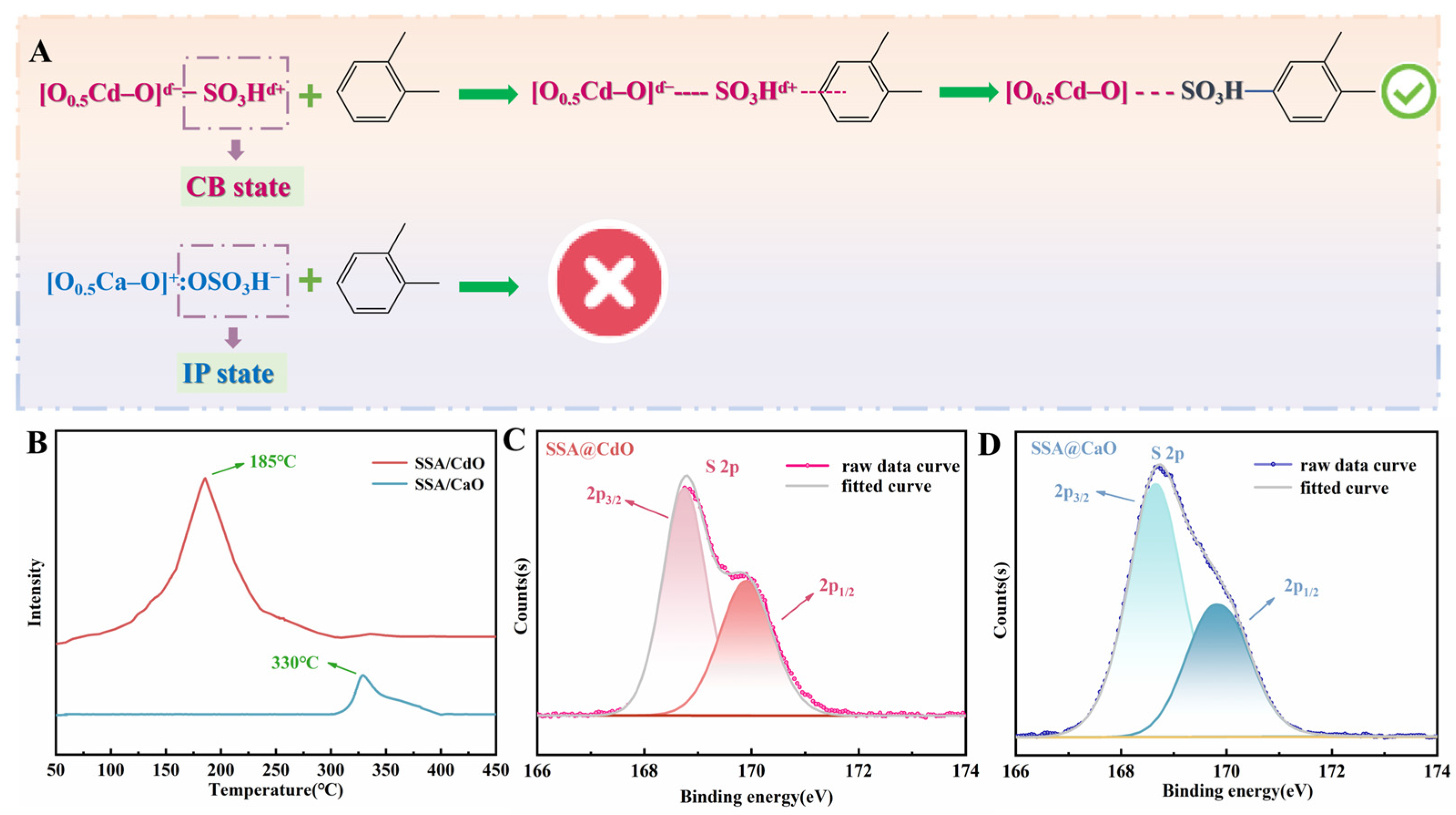
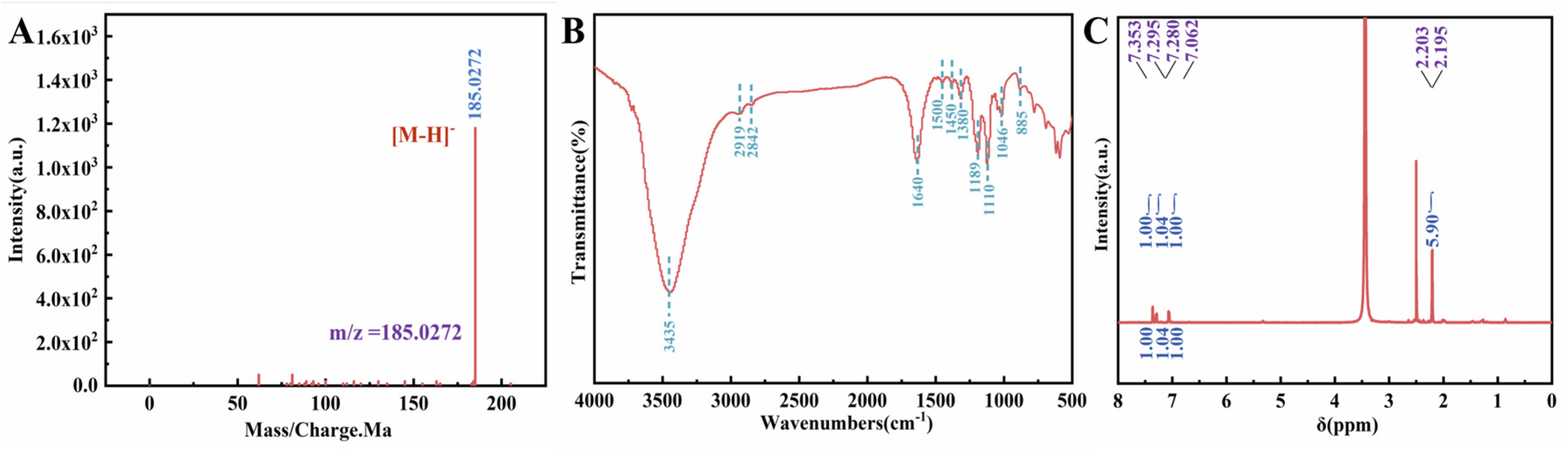
2.2. Comparison of Sulfonic Acid Loading in SSA@CaO and SSA@CdO Materials with Basic Characterization
2.3. Comparison of SSA@CdO and SSA@CaO Reactivity for o-Xylene Removal
2.4. Analysis of Anchoring States of Sulfonic Acid Groups and o-Xylene Removal Mechanism
3. Materials and Methods
3.1. Reagents and Materials
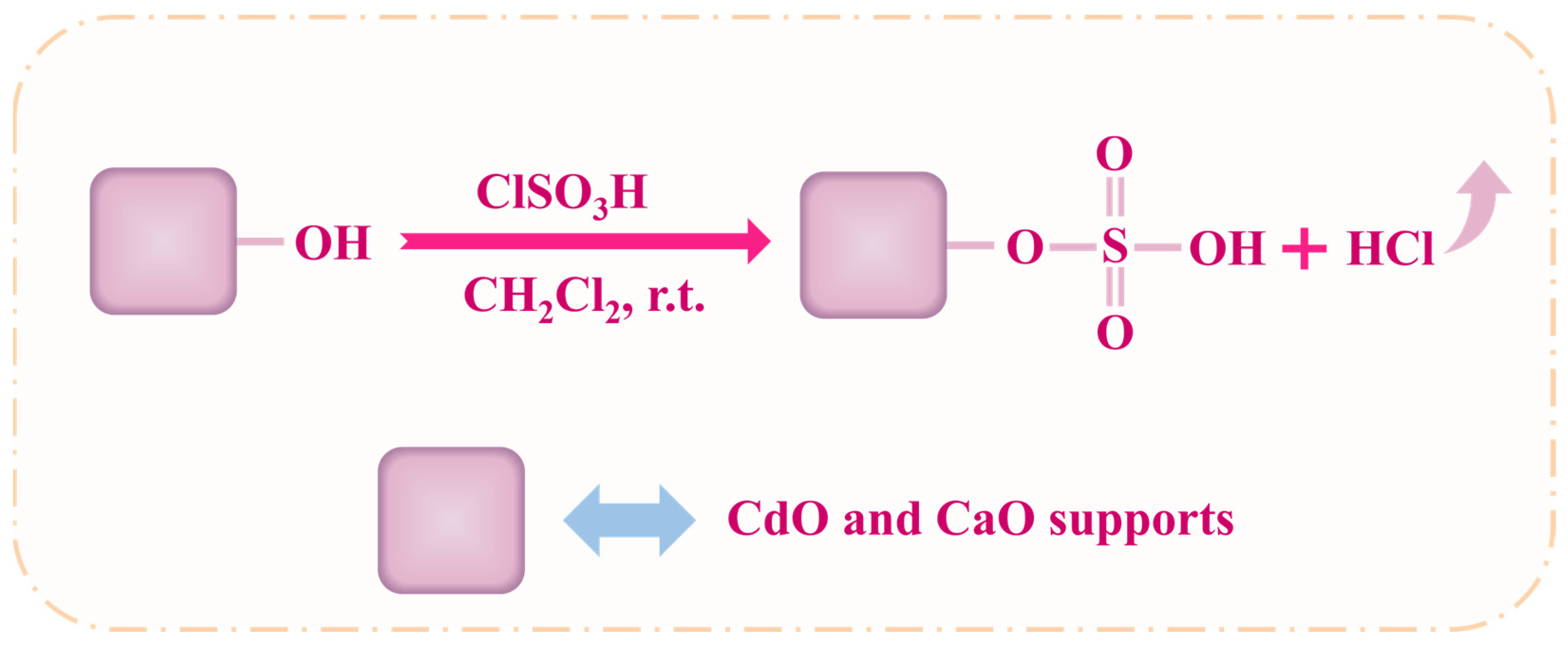
3.2. Preparation of SSA@CdO and SSA@CaO
3.3. Quantification of the Loading Amount of Sulfonic Acid Groups
3.4. Dynamic Adsorption Experiment of o-Xylene
3.5. Materials Analysis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sui, H.; Liu, H.; An, P.; He, L.; Li, X.; Cong, S. Application of silica gel in removing high concentrations toluene vapor by adsorption and desorption process. J. Taiwan Inst. Chem. Eng. 2017, 74, 218–224. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Q.; Wang, F.; Xie, J.; Li, Y.; Li, J.; Wu, S. Emission behavior; environmental impact and priority-controlled pollutants assessment of volatile organic compounds (VOCs) during asphalt pavement construction based on laboratory experiment. J. Hazard. Mater. 2020, 398, 122904. [Google Scholar] [CrossRef]
- Siu, B.; Chowdhury, A.R.; Yan, Z.; Humphrey, S.M.; Hutter, T. Selective adsorption of volatile organic compounds in metal-organic frameworks (MOFs). Coord. Chem. Rev. 2023, 485, 215119. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Chau, D.H.; Bui, K.Q.; Nguyen, N.T.T.; Tran, T.K.N.; Bach, L.G.; Truong, S.N. A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene. Inorganics 2022, 10, 29. [Google Scholar] [CrossRef]
- Ha Chi, N.N.; Kim Oanh, N.T. Photochemical smog modeling of PM2.5 for assessment of associated health impacts in crowded urban area of Southeast Asia. Environ. Technol. Innov. 2020, 21, 101241. [Google Scholar] [CrossRef]
- Hao, X.; Dai, L.; Deng, J.; Liu, Y.; Jing, L.; Wang, J.; Pei, W.; Zhang, X.; Hou, Z.; Dai, H. Nanotubular OMS-2 supported single-atom platinum catalysts highly active for benzene oxidation. J. Phys. Chem. C 2021, 125, 17696–17708. [Google Scholar] [CrossRef]
- Li, Q.; Li, F.-t. Recent advances in surface and interface design of photocatalysts for the degradation of volatile organic compounds. Adv. Colloid Interface Sci. 2020, 284, 102275. [Google Scholar] [CrossRef] [PubMed]
- Kolade, M.A.; Kogelbauer, A.; Alpay, E. Adsorptive reactor technology for VOC abatement. Chem. Eng. Sci. 2009, 64, 1167–1177. [Google Scholar] [CrossRef]
- Velinova, R.; Kaneva, N.; Ivanov, G.; Kovacheva, D.; Spassova, I.; Todorova, S.; Atanasova, G.; Naydenov, A. Synthesis and Characterization of Pd/La2O3/ZnO Catalyst for Complete Oxidation of Methane, Propane and Butane. Inorganics 2025, 13, 17. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.; Liu, G.; Shi, C. Research status of volatile organic compound (VOC) removal technology and prospect of new strategies: A review. Environ. Sci. Process. Impacts 2023, 25, 727–740. [Google Scholar] [CrossRef]
- Baskaran, D.; Dhamodharan, D.; Behera, U.S.; Byun, H.-S. A comprehensive review and perspective research in technology integration for the treatment of gaseous volatile organic compounds. Environ. Res. 2024, 251, 118472. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Teng, Y.-J.; Li, Z.-G.; Liu, W.-H.; Lee, M.-R. Magnetic nanoparticles used in headspace extraction coupled with DSI-GC-IT/MS for analysis of VOCs in dry Traditional Chinese Medicine. Chin. Chem. Lett. 2016, 27, 178–184. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Favre, E. Energy efficiency of a hybrid membrane/condensation process for VOC (Volatile Organic Compounds) recovery from air: A generic approach. Energy 2016, 95, 291–302. [Google Scholar] [CrossRef]
- Lyu, J.; Zhou, L.; Shao, J.; Zhou, Z.; Gao, J.; Dong, Y.; Wang, Z.; Li, J. TiO2 hollow heterophase junction with enhanced pollutant adsorption; light harvesting; and charge separation for photocatalytic degradation of volatile organic compounds. Chem. Eng. J. 2022, 391, 123602. [Google Scholar] [CrossRef]
- Fatima, S.; Govardhan, B.; Kalyani, S.; Sridhar, S. Extraction of volatile organic compounds from water and wastewater by vacuum-driven membrane process: A comprehensive review. Chem. Eng. J. 2022, 434, 134664. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Zong, C.; Li, Y.; Jin, W. Study on membrane performance in vapor permeation of VOC/N2 mixtures via modified constant volume/variable pressure method. Sep. Purif. Technol. 2018, 200, 273–283. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Zhang, L.; Zheng, G.; Guo, L. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation. Chem. Eng. J. 2019, 357, 258–268. [Google Scholar] [CrossRef]
- Soltan, W.B.; Peng, J.; Cao, Z.; Fu, Z.; Liu, H. Bimetallic Fe-Mn loaded H-ZSM-5 zeolites for excellent VOCs catalytic oxidation at low-temperatures: Synergistic effects and catalytic mechanisms. Chem. Eng. J. 2023, 475, 146251. [Google Scholar] [CrossRef]
- Masi, M.; Nissim, W.G.; Pandolfi, C.; Azzarello, E.; Mancuso, S. Modelling botanical biofiltration of indoor air streams contaminated by volatile organic compounds. J. Hazard. Mater. 2022, 422, 126875. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Guo, Y.; Zhu, T.; Xu, W. Adsorption and desorption characteristics of hydrophobic hierarchical zeolites for the removal of volatile organic compounds. Chem. Eng. J. 2021, 411, 128558. [Google Scholar] [CrossRef]
- Dammak, N.; Fakhfakh, N.; Fourmentin, S.; Benzina, M. Treatment of gas containing hydrophobic VOCs by adsorption process on raw and intercalated clays. Res. Chem. Intermed. 2014, 41, 5475–5493. [Google Scholar] [CrossRef]
- Ambrożek, B.; Zwarycz-Makles, K. Theoretical and experimental studies of the recovery of volatile organic compounds from waste air streams in the thermal swing adsorption system with closed-loop regeneration of adsorbent. Energy Convers. Manag. 2014, 85, 646–654. [Google Scholar] [CrossRef]
- Ramalingam, S.G.; Pré, P.; Giraudet, S.; Le Coq, L.; Le Cloirec, P.; Baudouin, O.; Déchelotte, S. Different families of volatile organic compounds pollution control by microporous carbons in temperature swing adsorption processes. J. Hazard. Mater. 2012, 221–222, 242–247. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, Z.; Guo, Y.; Liu, Y.; Zhao, J.; Zhang, C.; Zhu, P. Fabrication of interpenetrating polymer network chitosan/gelatin porous materials and study on dye adsorption properties. Carbohydr. Polym. 2015, 132, 330–337. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Alston, S.; Xiao, Y.; Ajayan, P.; Bu, X.; Feng, P. Multi-Stage optimization of pore size and shape in pore-space-partitioned Metal–Organic Frameworks for highly selective and sensitive benzene capture. Angew. Chem. Int. Ed. 2024, 64, e202415576. [Google Scholar] [CrossRef]
- Cao, M.; Wang, J.; Liu, X.; Pei, Y.; Gao, M.; Wang, W.; Yang, H. Bio-inspired adsorbent with ultra-uniform and abundance sites accelerate breaking the trade-off effect between adsorption capacity and removal efficiency. Chem. Eng. J. 2023, 465, 142790. [Google Scholar] [CrossRef]
- Pak, S.-H.; Jeon, M.-J.; Jeon, Y.-W. Study of sulfuric acid treatment of activated carbon used to enhance mixed VOC removal. Int. Biodeterior. Biodegrad. 2016, 113, 195–200. [Google Scholar] [CrossRef]
- Gao, K.; Ma, M.; Liu, Y.; Ma, Z. A comparative study of the removal of o-xylene from gas streams using mesoporous silicas and their silica supported sulfuric acids. J. Hazard. Mater. 2021, 409, 124965. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Gao, K.; Ma, Z.; Ding, J. Influence of preparation method on the adsorptive performance of silica sulfuric acid for the removal of gaseous o-xylene. Sep. Purif. Technol. 2021, 265, 118484. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, M.; Qian, J.; Wang, Y.; Ma, Z.; Ma, X. Influence of impregnation medium on the adsorptive performance of silica sulfuric acid for the removal of Gaseous o-xylene: Comparison on ethyl acetate and water. Catalysts 2022, 12, 737. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Zolfigol, M.A. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron 2001, 57, 9509–9511. [Google Scholar] [CrossRef]
- Banoqitah, E.M.; Saleh, M.A.; Damoom, M.M.; Alhawsawi, A.M.; Kasmani, R.M.; Al-Hada, N.M. One-step synthesis of bunsenite cadmium oxide nanoparticles. Appl. Sci. 2022, 13, 438. [Google Scholar] [CrossRef]
- Hong, J.; Zhao, Y.; Wu, J.; Xie, X.; Zhao, P.; Li, S.; Yao, H.; Luo, G.; Liu, Z.; Yang, X. Fabrication of Al2O3/CaO with anti-sintering for efficient removal of As2O3 in simulated flue gas: Experimental and DFT study. Fuel 2022, 307, 121812. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Wen, X.; Su, X.; Chu, Y.; Liang, J. Heteroatom Sr doped MCM-41 molecular sieve for VOC adsorption: Study of the surface functionalization and adsorption performance. Chem. Eng. J. 2024, 488, 150924. [Google Scholar] [CrossRef]
- Zhou, G.; Cheng, Y.; Yu, Z.; Liu, X.; Chen, D.; Wang, J.; Hang, Y.; Xu, Y.; Li, C.; Lu, Z. Regulation of coordination and doping environment via target molecular transformation for boosting selective photocatalytic ability. Chem. Commun. 2022, 58, 10036–10039. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.; Guo, X.; Ma, S. Revealing the mechanism of hydration on the CO2 kinetic adsorption of CaO surface via ReaxFF MD simulations with experiments. Appl. Surf. Sci. 2024, 670, 160652. [Google Scholar] [CrossRef]
- Ghotekar, S.; Ravikumar, C.R.; Chauhan, A.; Hikku, G.S.; Lin, K.-Y.A.; Rahdar, A.; Hitler, L.; Jabir, M.S.; Marzban, A.; Oza, R. Eco-friendly fabrication of CdO nanoparticles using Polyalthia longifolia leaves extract for antibacterial and electrochemical sensing studies. J. Sol-Gel Sci. Technol. 2024, 110, 221–232. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, S.; Pal, B. Superior adsorptive removal of brilliant green and phenol red dyes mixture by CaO nanoparticles extracted from egg shells. J. Nanostruct. Chem. 2021, 12, 207–221. [Google Scholar] [CrossRef]
- Li, X.; Lei, Z.; Qu, J.; Zhou, X.; Li, Z.; Zhang, Q. Separation of Cu(ii) from Cd(ii) in sulfate solution using CaCO3 and FeSO4 based on mechanochemical activation. RSC Adv. 2017, 7, 2002–2008. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tuan, W.-H.; Lai, P.-L. Transformation from calcium sulfate to calcium phosphate in biological environment. J. Mater. Sci. Mater. Med. 2021, 32, 146. [Google Scholar] [CrossRef]
- Dacquin, J.-P.; Cross, H.E.; Brown, D.R.; Düren, T.; Williams, J.J.; Lee, A.F.; Wilson, K. Interdependent lateral interactions; hydrophobicity and acid strength and their influence on the catalytic activity of nanoporous sulfonic acid silicas. Green Chem. 2010, 12, 1383–1391. [Google Scholar] [CrossRef]
- Lv, S.; Ma, X.; Wang, Y.; Zheng, Y.; Ma, Z.; Liu, T. Cyclic siloxane removal by ring-opening polymerization on silica gel-supported sulfuric acid. Chem. Eng. J. 2025, 504, 158842. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, R.; He, Y.; Ma, Z.; Ma, X. Efficient reactive adsorption of hexamethyldisiloxane on MCM-41 supported sulfuric acid. Renew. Energy 2024, 224, 120174. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Wang, H.; Zhao, D.; Liu, Y.; Ma, Z. Enhancement of gaseous o-xylene elimination by chlorosulfonic acid-modified H-zeolite socony mobil-5. Molecules 2024, 29, 3507. [Google Scholar] [CrossRef]
- Parreño, R.P. The correlation of sulfonation reaction kinetics with the degree of sulfonation (DS) and its effects on microstructure and morphology of electrospun fibers for the membrane of fuel cells. RSC Adv. 2023, 13, 2523–2529. [Google Scholar] [CrossRef]
- Wang, P.C.; Chen, J.; Lu, M. Electrophilic aromatic nitration: Substituent effects of monosubstituted benzenes. J. Chin. Chem. Soc. 2013, 57, 967–971. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Tan, M.; Jiang, B.; Zheng, J.; Tsubaki, N.; Wu, M. Monodispersed hollow SO3H-functionalized carbon/silica as efficient solid acid catalyst for esterification of oleic acid. ACS Appl. Mater. Interfaces 2015, 7, 26767–26775. [Google Scholar] [CrossRef]
- Karthick, N.K.; Arivazhagan, G.; Kannan, P.P.; Kumbharkhane, A.C.; Joshi, Y.S. Homo/hetero interactions in the binary solutions of toluene with acetonitrile: FTIR spectroscopic; theoretical and dielectric studies. J. Mol. Struct. 2019, 1192, 208–216. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, B.; Fan, S.; Wang, P.; Wang, X.; Long, D.; Zhang, L.; Yang, X.; Li, X. In situ FTIR spectra investigation of the photocatalytic degradation of gaseous toluene over a novel hedgehog-like CaFe2O4 hollow-structured materials. Catal. Commun. 2019, 130, 105754. [Google Scholar] [CrossRef]
- Aripova, S.F.; Abdilalimov, O. Convolacine a new alkaloid from convolvulus subhirsutus. Chem. Nat. Compd. 1993, 29, 74–75. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef] [PubMed]

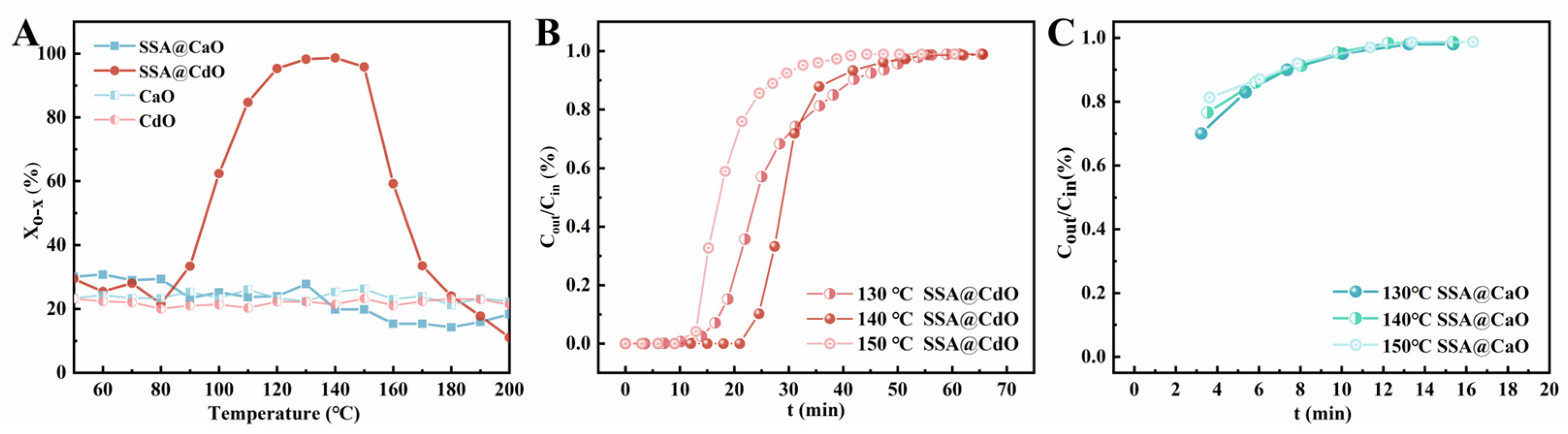
| Sample | T/(°C) | tB/(min) | QB/(mg/g) |
|---|---|---|---|
| SSA@CdO | 130 | 15.25 | 60.99 |
| 140 | 22.90 | 91.59 | |
| 150 | 12.92 | 51.67 | |
| SSA@CaO | 130 | 0 | 0 |
| 140 | 0 | 0 | |
| 150 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, X.; Niu, Y.; Ma, Z. A Comparative Study of Supported Sulfonic Acids Derived from CdO and CaO for the Reactive Adsorption of o-Xylene. Inorganics 2025, 13, 275. https://doi.org/10.3390/inorganics13080275
Wang H, Zhang X, Niu Y, Ma Z. A Comparative Study of Supported Sulfonic Acids Derived from CdO and CaO for the Reactive Adsorption of o-Xylene. Inorganics. 2025; 13(8):275. https://doi.org/10.3390/inorganics13080275
Chicago/Turabian StyleWang, Hongmei, Xiaoxu Zhang, Yifei Niu, and Zichuan Ma. 2025. "A Comparative Study of Supported Sulfonic Acids Derived from CdO and CaO for the Reactive Adsorption of o-Xylene" Inorganics 13, no. 8: 275. https://doi.org/10.3390/inorganics13080275
APA StyleWang, H., Zhang, X., Niu, Y., & Ma, Z. (2025). A Comparative Study of Supported Sulfonic Acids Derived from CdO and CaO for the Reactive Adsorption of o-Xylene. Inorganics, 13(8), 275. https://doi.org/10.3390/inorganics13080275








