The Influences of π-Conjugated Aliphatic Chains in Ionic Liquids of Antimony Pentachloride with Pyridine Imidazolium Hybrid Salts: A DFT Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Structure Analysis
2.2. Non-Covalent Interaction (NCI)
2.3. Natural Bond Orbital
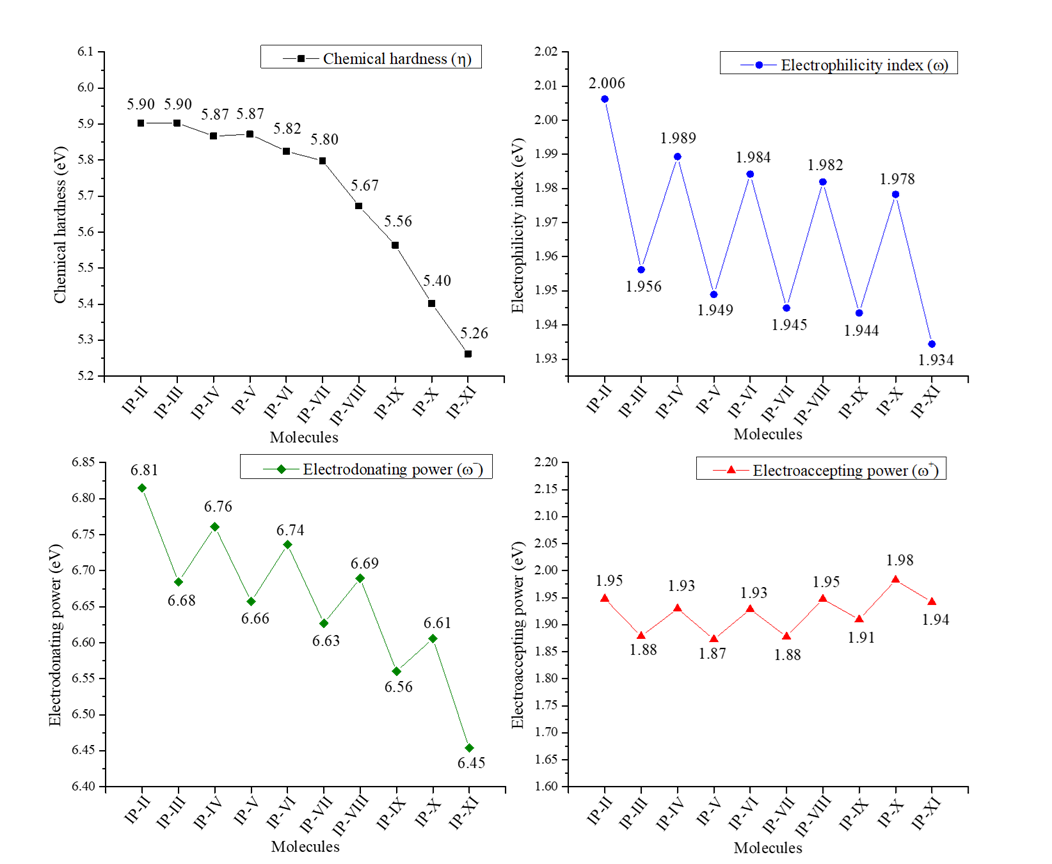
2.4. Chemical Reactivity Parameters
2.5. Frontier Molecular Orbitals
2.6. Energy Gap Analysis


3. Materials and Methods
3.1. Computational Details
3.2. Theoretical Background
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density Functional Theory |
| ECP | Effective Core Potential |
| NBO | Natural Bond Orbital |
| NCI | Non-Covalent Interaction |
| ILs | Ionic Liquids |
| LEECs | Light-Emitting Electrochemical Cells |
| NLO | Nonlinear Optics |
| LANL | Los Alamos National Laboratory |
| DZ | Double Zeta |
| MO | Molecular Orbital |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| Egap | Energy Gap |
| ICT | Intermolecular Charge Transfer |
| RDG | Reduced Density Gradient |
| bcp | Bond Critical Point |
| FMO | Frontier Molecular Orbital |
References
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Hallett, J.; Shiflett, M. Introduction: Ionic Liquids for Diverse Applications. Chem. Rev. 2024, 124, 7533–7535. [Google Scholar] [CrossRef] [PubMed]
- Talip, R.A.A.; Yahya, W.Z.N.; Bustam, M.A. Ionic Liquids Roles and Perspectives in Electrolyte for Dye-Sensitized Solar Cells. Sustainability 2020, 12, 7598. [Google Scholar] [CrossRef]
- Deng, X.; Xie, L.; Wang, S.; Li, C.; Wang, A.; Yuan, Y.; Cao, Z.; Li, T.; Ding, L.; Hao, F. Ionic Liquids Engineering for High-Efficiency and Stable Perovskite Solar Cells. Chem. Eng. J. 2020, 398, 125594. [Google Scholar] [CrossRef]
- Stroppa, P.H.F.; Martins, J.S.; Dias, R.C.; Salla, C.A.M.; Bechtold, I.H.; Legnani, C.; Fragneaud, B.; Quirino, W.G.; David da Silva, A. High Efficient Light-Emitting Electrochemical Cells Based on Ionic Liquids 1,2,3-Triazolium. Org. Electron. 2019, 73, 172–181. [Google Scholar] [CrossRef]
- Mazille, F.; Fei, Z.; Kuang, D.; Zhao, D.; Zakeeruddin, S.M.; Grätzel, M.; Dyson, P.J. Influence of Ionic Liquids Bearing Functional Groups in Dye-Sensitized Solar Cells. Inorg. Chem. 2006, 45, 1585–1590. [Google Scholar] [CrossRef]
- Yang, C.; Sun, Q.; Qiao, T.; Li, Y. Ionic Liquid Doped Polymer Light-Emitting Electrochemical Cells. J. Phys. Chem. B 2003, 107, 12981–12988. [Google Scholar] [CrossRef]
- López, F.I.; Domínguez, J.M.; Miranda, A.D.; Trejo-Durán, M.; Alvarado-Méndez, E.; Vázquez, M.A. Synthesis of Symmetric Ionic Liquids and Their Evaluation of Nonlinear Optical Properties. Opt. Mater. 2019, 96, 109276. [Google Scholar] [CrossRef]
- Boyd, G.T. Applications Requirements for Nonlinear-Optical Devices and the Status of Organic Materials. J. Opt. Soc. Am. B 1989, 6, 685–692. [Google Scholar] [CrossRef]
- Kamath, L.; Manjunatha, K.B.; Shettigar, S.; Umesh, G.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. Investigation of Third-Order Nonlinear and Optical Power Limiting Properties of Terphenyl Derivatives. Opt. Laser Technol. 2014, 56, 425–429. [Google Scholar] [CrossRef]
- Innocenzi, P.; Lebeau, B. Organic-Inorganic Hybrid Materials for Non-Linear Optics. J. Mater. Chem. 2005, 15, 3821–3831. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, Q. A Review of the Relationship between the Structure and Nonlinear Optical Properties of Organic-Inorganic Hybrid Materials. Coord. Chem. Rev. 2024, 498, 215458. [Google Scholar] [CrossRef]
- Tucker, T.G.; Davidowski, S.K.; Angell, C.A. Inorganic vs Organic Cation Ionic Liquids and Their Solutions with Alkali Metal Containing Ionic Liquids. J. Electrochem. Soc. 2017, 164, H153–H158. [Google Scholar] [CrossRef]
- Nishi, T.; Iwahashi, T.; Yamane, H.; Ouchi, Y.; Kanai, K.; Seki, K. Electronic Structures of Ionic Liquids [Cn Mim]+ BF4- and [Cn Mim]+ PF6- Studied by Ultraviolet Photoemission, Inverse Photoemission, and near-Edge X-Ray Absorption Fine Structure Spectroscopies. Chem. Phys. Lett. 2008, 455, 213–217. [Google Scholar] [CrossRef]
- Izgorodina, E.I.; Bernard, U.L.; MacFarlane, D.R. Ion-Pair Binding Energies of Ionic Liquids: Can Dft Compete with Ab Initio-Based Methods? J. Phys. Chem. A 2009, 113, 7064–7072. [Google Scholar] [CrossRef]
- Karu, K.; Ruzanov, A.; Ers, H.; Ivaništšev, V.; Lage-Estebanez, I.; García de la Vega, J. Predictions of Physicochemical Properties of Ionic Liquids with DFT. Computation 2016, 4, 25. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Dong, K.; Fan, M.; Zhang, S. A DFT Study on Lignin Dissolution in Imidazolium-Based Ionic Liquids. RSC Adv. 2017, 7, 12670–12681. [Google Scholar] [CrossRef]
- Seeger, Z.L.; Izgorodina, E.I. A Systematic Study of DFT Performance for Geometry Optimizations of Ionic Liquid Clusters. J. Chem. Theory Comput. 2020, 16, 6735–6753. [Google Scholar] [CrossRef]
- Grimme, S.; Hujo, W.; Kirchner, B. Performance of Dispersion-Corrected Density Functional Theory for the Interactions in Ionic Liquids. Phys. Chem. Chem. Phys. 2012, 14, 4875. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Kolbeck, C.; Lovelock, K.R.J.; Paape, N.; Wölfel, R.; Schulz, P.S.; Wasserscheid, P.; Weber, H.; Thar, J.; Kirchner, B.; et al. Towards a Molecular Understanding of Cation–Anion Interactions—Probing the Electronic Structure of Imidazolium Ionic Liquids by NMR Spectroscopy, X-ray Photoelectron Spectroscopy and Theoretical Calculations. Chem.—A Eur. J. 2010, 16, 9018–9033. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Rocha, M.A.A.; Freire, M.G.; Marrucho, I.M.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Evaluation of Cation-Anion Interaction Strength in Ionic Liquids. J. Phys. Chem. B 2011, 115, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.N.; Inoue, Y.; Nakanishi, I.; Kitaura, K. Cl–π Interactions in Protein–Ligand Complexes. Protein Sci. 2008, 17, 1129–1137. [Google Scholar] [CrossRef]
- Saraogi, I.; Vijay, V.G.; Das, S.; Sekar, K.; Guru Row, T.N. C–Halogen⋯π Interactions in Proteins: A Database Study. Cryst. Eng. 2003, 6, 69–77. [Google Scholar] [CrossRef]
- Thallapally, P.K.; Nangia, A. A Cambridge Structural Database Analysis of the C–H⋯Cl Interaction: C–H⋯Cl− and C–H⋯Cl–M Often Behave as Hydrogen Bonds but C–H⋯Cl–C Is Generally a van Der Waals Interaction. CrystEngComm 2001, 3, 114–119. [Google Scholar] [CrossRef]
- Janiak, C.; Scharmann, T.G. Supramolecular C-H⋯O, C-H⋯N and C-H⋯Cl Interactions in Metal Compounds with Multi-Topic Poly(Pyrazolyl)Borate Ligands. Polyhedron 2003, 22, 1123–1133. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Aulwurm, U.R.; Heinemann, F.W.; Knoch, F.; Kisch, H. Weak M-H⋅⋅⋅H-C and M-Cl⋅⋅⋅H-C Interactions in Orthometalated Iridium and Rhodium Complexes. Chem.—A Eur. J. 1998, 4, 1641–1646. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van Der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Haque, A.; Alenezi, K.M.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Non-Covalent Interactions (NCIs) in π-Conjugated Functional Materials: Advances and Perspectives. Chem. Soc. Rev. 2023, 52, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, J.; Calatayud, M.; Piquemal, J.P.; Recio, J.M. Ionic Interactions: Comparative Topological Approach. Comput. Theor. Chem. 2012, 998, 193–201. [Google Scholar] [CrossRef]
- Contreras-García, J.; Yang, W.; Johnson, E.R. Analysis of Hydrogen-Bond Interaction Potentials from the Electron Density: Integration of Noncovalent Interaction Regions. J. Phys. Chem. A 2011, 115, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, L.; Morera-Boado, C.; López, R.; García De La Vega, J.M. Electron Density Analysis of 1-Butyl-3-Methylimidazolium Chloride Ionic Liquid. J. Mol. Model. 2014, 20, 2175. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural Bond Orbital Methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Nkungli, N.K.; Ghogomu, J.N.; Nogheu, L.N.; Gadre, S.R. DFT and TD-DFT Study of Bis[2-(5-Amino-[1,3,4]-Oxadiazol-2-Yl) Phenol](Diaqua)M(II) Complexes [M = Cu, Ni and Zn]: Electronic Structures, Properties and Analyses. Comput. Chem. 2015, 3, 29–44. [Google Scholar] [CrossRef]
- Martínez, J. Local Reactivity Descriptors from Degenerate Frontier Molecular Orbitals. Chem. Phys. Lett. 2009, 478, 310–322. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute Hardness: Companion Parameter to Absolute Electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.v.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Gázquez, J.L.; Cedillo, A.; Vela, A. Electrodonating and Electroaccepting Powers. J. Phys. Chem. A 2007, 111, 1966–1970. [Google Scholar] [CrossRef]
- Huang, Y.; Rong, C.; Zhang, R.; Liu, S. Evaluating Frontier Orbital Energy and HOMO/LUMO Gap with Descriptors from Density Functional Reactivity Theory. J. Mol. Model. 2017, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Sarala, S.; Geetha, S.K.; Muthu, S.; Irfan, A. Computational Investigation, Comparative Approaches, Molecular Structural, Vibrational Spectral, Non-Covalent Interaction (NCI), and Electron Excitations Analysis of Benzodiazepine Derivatives. J. Mol. Model. 2021, 27, 266. [Google Scholar] [CrossRef] [PubMed]
- Brédas, J.L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-Transfer and Energy-Transfer Processes in π-Conjugated Oligomers and Polymers: A Molecular Picture. Chem. Rev. 2004, 104, 4971–5003. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, B.; Kirkpatrick, J.; Andrienko, D. Density-Functional Based Determination of Intermolecular Charge Transfer Properties for Large-Scale Morphologies. Phys. Chem. Chem. Phys. 2010, 12, 11103–11113. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Vignale, G.; Rasolt, M. Density-Functional Theory in Strong Magnetic Fields. Phys. Rev. Lett. 1987, 59, 2360–2363. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01. 2016. Available online: https://gaussian.com/citation/ (accessed on 10 July 2025).
- Yu, H.S.; He, X.; Li, S.L.; Truhlar, D.G. MN15: A Kohn–Sham Global-Hybrid Exchange—Correlation Density Functional with Broad Accuracy for Multi-Reference and Single-Reference Systems and Noncovalent Interactions. Chem. Sci. 2016, 7, 5032–5051. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-Optimized Gaussian Basis Sets for Molecular Response Calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-row Atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-Type Basis Sets for Local Spin Density Functional Calculations. Part I. Boron through Neon, Optimization Technique and Validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Check, C.E.; Faust, T.O.; Bailey, J.M.; Wright, B.J.; Gilbert, T.M.; Sunderlin, L.S. Addition of Polarization and Diffuse Functions to the LANL2DZ Basis Set for P-Block Elements. J. Phys. Chem. A 2001, 105, 8111–8116. [Google Scholar] [CrossRef]
- Yang, Y.; Weaver, M.N.; Merz, K.M. Assessment of the “6-31+G** + LANL2DZ” Mixed Basis Set Coupled with Density Functional Theory Methods and the Effective Core Potential: Prediction of Heats of Formation and Ionization Potentials for First-Row-Transition-Metal Complexes. J. Phys. Chem. A 2009, 113, 9843–9851. [Google Scholar] [CrossRef] [PubMed]
- Chiodo, S.; Russo, N.; Sicilia, E. LANL2DZ Basis Sets Recontracted in the Framework of Density Functional Theory. J. Chem. Phys. 2006, 125, 104107. [Google Scholar] [CrossRef]
- Gázquez, J.L. A Hardness and Softness Theory of Bond Energies and Chemical Reactivity. Theor. Comput. Chem. 1998, 5, 135–152. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative Analysis of Molecular Surface Based on Improved Marching Tetrahedra Algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Shahab, S.; Sheikhi, M.; Filippovich, L.; Anatol’evich, D.E.; Yahyaei, H. Quantum Chemical Modeling of New Derivatives of (E,E)-Azomethines: Synthesis, Spectroscopic (FT-IR, UV/Vis, Polarization) and Thermophysical Investigations. J. Mol. Struct. 2017, 1137, 335–348. [Google Scholar] [CrossRef]
- Perdew, J.P.; Parr, R.G.; Levy, M.; Balduz, J.L. Density-Functional Theory for Fractional Particle Number: Derivative Discontinuities of the Energy. Phys. Rev. Lett. 1982, 49, 1691–1694. [Google Scholar] [CrossRef]
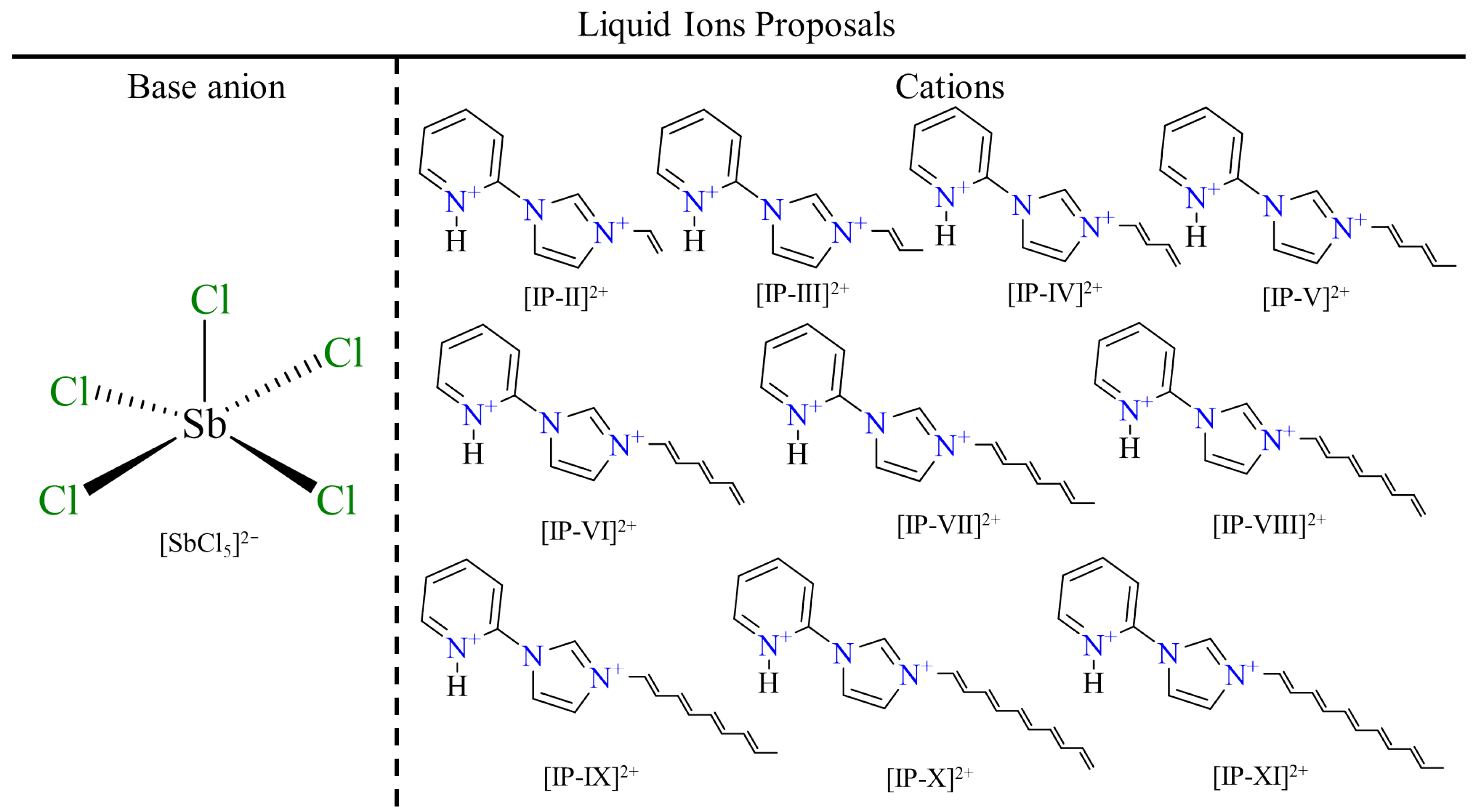

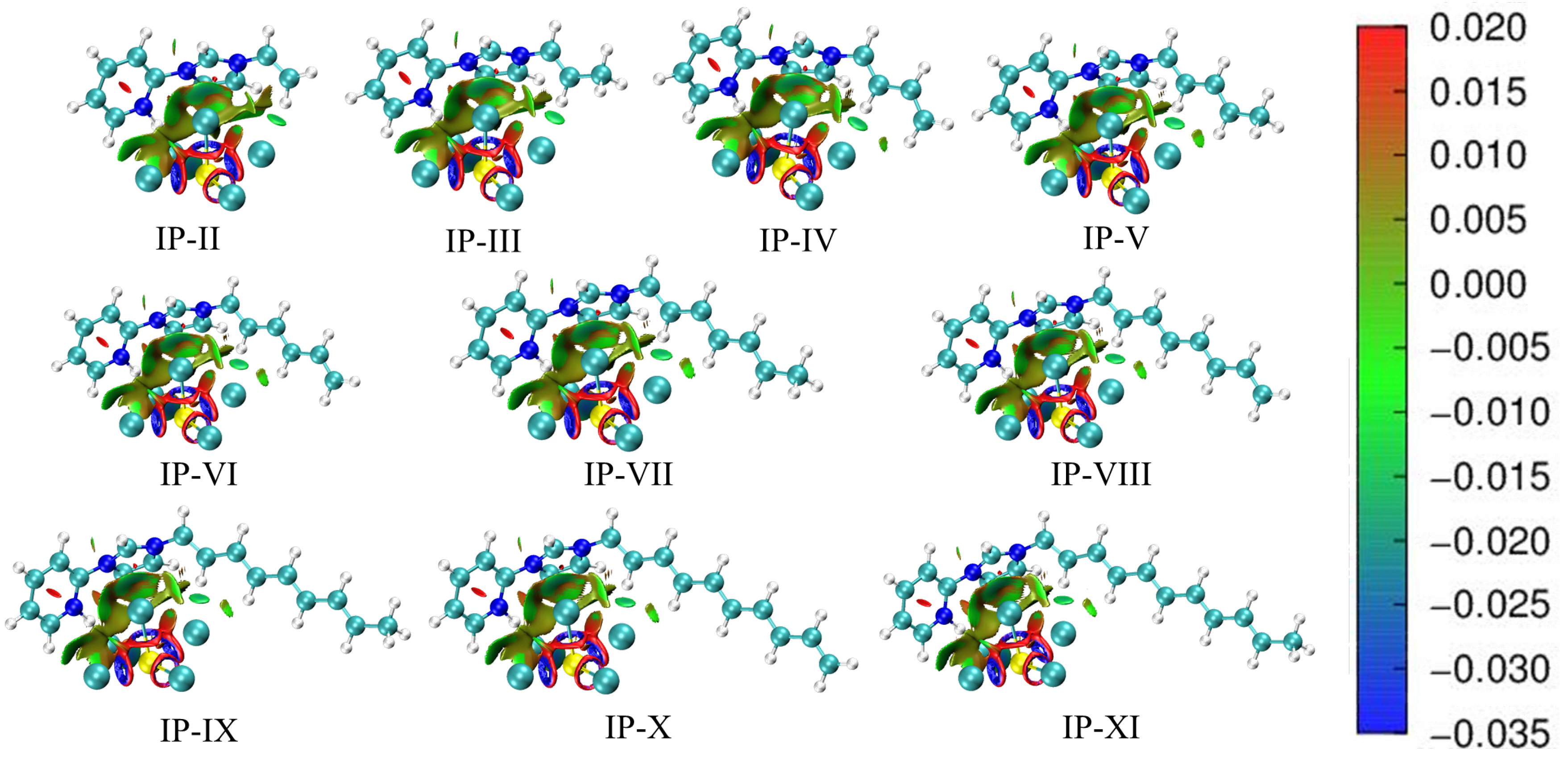

| Molecule | Ha···Cla | Hb···Cla | Hc···Clc | Hd···Clc | He···Clc | Clb···πa | Clb···πb |
|---|---|---|---|---|---|---|---|
| Angstrom (Å) | |||||||
| IP-II | 1.687 | 2.484 | 3.056 | 2.512 | - | 3.816 | 3.048 |
| IP-III | 1.704 | 2.497 | 3.113 | 2.501 | 4.576 | 3.812 | 3.048 |
| IP-IV | 1.708 | 2.508 | 3.203 | 2.452 | 3.409 | 3.850 | 3.028 |
| IP-V | 1.716 | 2.525 | 3.263 | 2.461 | 3.231 | 3.848 | 3.028 |
| IP-VI | 1.719 | 2.526 | 3.290 | 2.453 | 3.538 | 3.855 | 3.025 |
| IP-VII | 1.721 | 2.529 | 3.323 | 2.462 | 3.135 | 3.839 | 3.032 |
| IP-VIII | 1.718 | 2.524 | 3.297 | 2.453 | 3.165 | 3.833 | 3.032 |
| IP-IX | 1.719 | 2.520 | 3.277 | 2.462 | 3.550 | 3.829 | 3.033 |
| IP-X | 1.720 | 2.530 | 3.300 | 2.454 | 3.132 | 3.846 | 3.027 |
| IP-XI | 1.723 | 2.530 | 3.324 | 2.469 | 3.110 | 3.849 | 3.026 |
| Molecules | Ring | Chain | Anion | Ring–Chain (Δq) | Anion–Chain (Δq) |
|---|---|---|---|---|---|
| IP-II | 1.26190 | 0.32449 | −1.58638 | 0.93741 | −1.26189 |
| IP-III | 1.26957 | 0.32885 | −1.59845 | 0.94072 | −1.26960 |
| IP-IV | 1.27139 | 0.32653 | −1.59793 | 0.94486 | −1.27140 |
| IP-V | 1.27058 | 0.33327 | −1.60385 | 0.93731 | −1.27058 |
| IP-VI | 1.27239 | 0.33200 | −1.60437 | 0.94039 | −1.27237 |
| IP-VII | 1.27021 | 0.33736 | −1.60758 | 0.93285 | −1.27022 |
| IP-VIII | 1.26998 | 0.33549 | −1.60545 | 0.93449 | −1.26996 |
| IP-IX | 1.26767 | 0.33994 | −1.60763 | 0.92773 | −1.26769 |
| IP-X | 1.26880 | 0.33756 | −1.60637 | 0.93124 | −1.26881 |
| IP-XI | 1.26802 | 0.34051 | −1.60853 | 0.92751 | −1.26802 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque-Román, M.; Baldenebro-López, J.; Campos-Gaxiola, J.J.; Cruz-Enríquez, A.; Peñuelas, C.A.; Báez-Castro, A.; Soto-Rojo, R.; Delgado-Montiel, T.; Soto-Acosta, S.; Glossman-Mitnik, D. The Influences of π-Conjugated Aliphatic Chains in Ionic Liquids of Antimony Pentachloride with Pyridine Imidazolium Hybrid Salts: A DFT Study. Inorganics 2025, 13, 269. https://doi.org/10.3390/inorganics13080269
Luque-Román M, Baldenebro-López J, Campos-Gaxiola JJ, Cruz-Enríquez A, Peñuelas CA, Báez-Castro A, Soto-Rojo R, Delgado-Montiel T, Soto-Acosta S, Glossman-Mitnik D. The Influences of π-Conjugated Aliphatic Chains in Ionic Liquids of Antimony Pentachloride with Pyridine Imidazolium Hybrid Salts: A DFT Study. Inorganics. 2025; 13(8):269. https://doi.org/10.3390/inorganics13080269
Chicago/Turabian StyleLuque-Román, Manuel, Jesús Baldenebro-López, José J. Campos-Gaxiola, Adriana Cruz-Enríquez, Carlos A. Peñuelas, Alberto Báez-Castro, Rody Soto-Rojo, Tomás Delgado-Montiel, Samuel Soto-Acosta, and Daniel Glossman-Mitnik. 2025. "The Influences of π-Conjugated Aliphatic Chains in Ionic Liquids of Antimony Pentachloride with Pyridine Imidazolium Hybrid Salts: A DFT Study" Inorganics 13, no. 8: 269. https://doi.org/10.3390/inorganics13080269
APA StyleLuque-Román, M., Baldenebro-López, J., Campos-Gaxiola, J. J., Cruz-Enríquez, A., Peñuelas, C. A., Báez-Castro, A., Soto-Rojo, R., Delgado-Montiel, T., Soto-Acosta, S., & Glossman-Mitnik, D. (2025). The Influences of π-Conjugated Aliphatic Chains in Ionic Liquids of Antimony Pentachloride with Pyridine Imidazolium Hybrid Salts: A DFT Study. Inorganics, 13(8), 269. https://doi.org/10.3390/inorganics13080269












