Comparative Removal Properties of Sodium Magadiite and Its Protonic Form on Basic-Blue 41 from Contaminated Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. X Ray Diffraction Data
2.2. FTIR Data
2.3. 29Si MAS NMR Data
2.4. TGA Data
2.5. SEM Micrographs Data
2.6. Textural Properties
3. Factors Affecting the Removal of BB-41
3.1. Initial Concentration Impact
3.2. Effect of Dosage
3.3. Effect of pH Environment
3.3.1. Effect of BB-41 pH Solution
3.3.2. Effect of H-Mgd pH Suspension
3.4. Effect of Acid Treatment of Na-Mgd
3.5. Adsorption Isotherms Data
3.6. Regeneration Tests
3.7. Single Batch Adsorber Design
4. Materials and Characterization
4.1. Materials
4.2. Na-Mgd and H-Mgd Preparation
4.3. Basic Blue-41 Removal Process
4.4. Regeneration Tests
4.5. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmail, D.; Yusuke, I. Microporous layered silicates: Old but new microporous materials. New J. Chem. 2020, 44, 9957–9968. [Google Scholar] [CrossRef]
- HomHuan, N.; ImwIset, K.; IrInakorn, T.; Bureekae, S.; Ogawa, M. Ion exchange of a layered alkali silicate, magadiite, with metal ions. Clay Sci. 2017, 21, 21–28. [Google Scholar]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Sirinakorn, T.; Imwiset, K.; Bureekaew, S.; Ogawa, M. Inorganic modification of layered silicates toward functional inorganic-inorganic hybrids. Appl. Clay Sci. 2018, 153, 187–197. [Google Scholar] [CrossRef]
- Jing, X.; Zhang, Y.; Dong, X.; Mu, Y.; Liu, X.; Meng, C. Layered silicate magadiite–derived three-dimensional honeycomb-like cobalt–nickel silicates as excellent cathode for hybrid supercapacitors. Mater. Today Chem. 2021, 22, 100550. [Google Scholar] [CrossRef]
- Dos Santos, T.G.; de Assis, G.C.; da Silva, A.O.S.; Meneghetti, S.M.P. Progress in development of magadiite to produce multifunctional lamellar materials. ACS Appl. Mater. Interfaces 2022, 15, 43234–43250. [Google Scholar] [CrossRef]
- Asgari, M.; Sundararaj, U. Pre-exfoliated nanoclay through two consecutive reaction systems: Silane functionalization followed by grafting of amino acid monomers. Appl. Clay Sci. 2018, 151, 81–91. [Google Scholar] [CrossRef]
- Xue, S.; Song, W.; Wang, K.; Zhu, R.; Zhang, X.; Yang, X.; Bae, J.W.; Gao, X.; Ma, Q.; Zhang, J.; et al. Highly efficient synthesis of high-value olefins from syngas over layered Fe-Mn/magadiite catalyst. Chem. Eng. Technol. 2023, 46, 1886–1895. [Google Scholar] [CrossRef]
- Zhang, S.L.; Wang, Y.; Lv, T.M.; Feng, Z.; Liu, X.; Meng, C.G. Hydrothermal conversion of zeolite omega from magadiite with assistance of seed crystals. Mater. Today Chem. 2021, 20, 100440. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, Y.; Ye, M.; Tang, Y.; Liu, X.; Li, C.C. Unique interlayer chemical environment induced stable zinc plating/stripping via a Zn-based magadiite artificial interphase. J. Power Sources 2023, 554, 232262. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Abboudi, M.; Rakass, S.; Hassani, H.O.; Ibrahim, S.M.; Al-Faze, R. Application of organo-magadiites for the removal of eosin dye from aqueous solutions: Thermal treatment and regeneration. Molecules 2018, 23, 2280. [Google Scholar] [CrossRef]
- Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Yang, Y.; Hu, G.; Jamal, L.; Alam, S.M.J. Adsorption process and properties analyses of a pure magadiite and a modified magadiite on rhodamine-B from an aqueous solution. Processes 2019, 7, 565. [Google Scholar] [CrossRef]
- Attar, K.; Bouazza, D.; Miloudi, H.; Tayeb, A.; Boos, A.; Sastre, A.M.; Demey-Cedeno, H. Cadmium removal by a low-cost magadiite-based material: Characterization and sorption applications. J. Environ. Chem. Eng. 2018, 6, 5351–5360. [Google Scholar] [CrossRef]
- Dinga, H.; Chen, Y.; Fu, T.; Peng, B.; Guo, X. Nanosheet-based magadiite: A controllable two-dimensional trap for selective capture of heavy metals. J. Mater. Chem. A 2018, 6, 13624–13632. [Google Scholar] [CrossRef]
- Ge, M.; Tang, W.; Du, M.; Liang, G.; Hu, G.; Alam, S.M.J. Research on 5-fluorouracil as a drug carrier materials with its in vitro release properties on organic modified magadiite. Eur. J. Pharm. Sci. 2019, 130, 44–53. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Al Dmour, H.; Popoola, S.A.; Hassani, H.O.; Rakass, S.; Al-Faze, R.; Kooli, F. Parameters synthesis of Na-magadiite materials for water treatment and removal of basic blue-41: Properties and single-batch design adsorber. Inorganics 2023, 11, 423. [Google Scholar] [CrossRef]
- Mokhtar, M. Application of synthetic layered sodium silicate magadiite nanosheets for environmental remediation of methylene blue dye in water. Materials 2017, 10, 760. [Google Scholar] [CrossRef]
- Ge, M.; Cao, L.; Du, M.; Hu, G.; Alam, S.M.J. Adsorptive characterization of a pure magadiite and an organic modified magadiite on removal of methylene blue from related aqueous solution. Mater. Chem. Phys. 2018, 217, 533–540. [Google Scholar] [CrossRef]
- Pinnavaia, T.J.; Johnson, I.D.; Max Lipsicas, M. A 29Si MAS NMR study of tetrahedral site distributions in the layered silicic acid H+-magadiite (H2Si14O29 · nH2O) and in Na+-magadiite (Na2Si14O29 · nH2O). J. Solid State Chem. 1986, 63, 18–121. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Macedo, T.R.; Airoldi, C. Sodic and acidic crystalline lamellar magadiite adsorbents for the removal of methylene blue from aqueous solutions: Kinetic and equilibrium studies. Sep. Sci. Technol. 2009, 45, 129–141. [Google Scholar] [CrossRef]
- Amari, A.; Gannouni, H.; Khan, M.I.; Almesfer, M.K.; Abubakr, M.; Elkhaleefa, A.M.; Gannouni, A. Effect of structure and chemical activation on the adsorption properties of green clay minerals for the removal of cationic dye. Appl. Sci. 2018, 8, 2302. [Google Scholar] [CrossRef]
- Hamdi, L.; Boumehdi, L.; Salem, Z. Basic blue 41 dye removal from aqueous solution using lignocellulosic material: Kinetics, equilibrium and statistical design optimization. Int. J. Environ. Sci. Technol. 2023, 20, 3275–3294. [Google Scholar] [CrossRef]
- Kass, L. Basic Blue 41: A New Panoptic Stain for Blood and Bone Marrow Cells. J. Histotechnol. 1998, 11, 10–15. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.A.; Kuo, H.W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized carbon nanotubes (CNTs) for water and wastewater treatment: Preparation to application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Slama, H.B.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Gkika, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Why reuse spent adsorbents? The latest challenges and limitations. Sci. Total Environ. 2022, 822, 153612. [Google Scholar] [CrossRef]
- Marler, B.; Krysiak, Y.; Grosskreuz, I.; Gies, H.; Kolb, U. The crystal structure of mineral magadiite, Na2Si14O28(OH)2∙8H2O. Am. Miner. 2022, 107, 2101–2110. [Google Scholar] [CrossRef]
- Kooli, F.; Mianhui, L.; Alshahateet, S.F.; Chen, F.; Yinghuai, Z. Characterization and thermal stability properties of intercalated Na-magadiite with cetyltrimethylammonium (C16TMA) surfactants. J. Phys. Chem. Solids 2006, 67, 926–931. [Google Scholar] [CrossRef]
- Fletcher, R.A.; Bibby, D.M. Synthesis of kenyaite and magadiite in the presence of various anions. Clays Clay Miner. 1987, 35, 318–320. [Google Scholar] [CrossRef]
- Wang, S.F.; Lin, M.L.; Shieh, Y.N.; Wang, Y.R.; Wang, S.J. Organic modification of synthesized clay-magadiite. Ceram. Int. 2007, 33, 681–685. [Google Scholar] [CrossRef]
- Mokhtar, A.; Abdelkrim, S.; Djelad, A.; Bengueddach, A.; Sassi, M. Preparation of biopolymer/CuO-magadiite biocomposite beads by chemical reduction method and their antibacterial activity. Alger. J. Mater. Chem. 2019, 2, 20–32. [Google Scholar]
- Eypert-Blaison, C.; Michot, L.J.; Humbert, B.; Pelletier, M.; Villieras, F.; Caillerie, J.B.E. Hydration water and swelling behavior of magadiite. The H+, Na+, K+, Mg2+, and Ca2+ exchanged forms. J. Phys. Chem. B 2002, 106, 730–742. [Google Scholar] [CrossRef]
- Silva, B.N.N.; Santos, M.F.; Leitao, A.A.; Pastore, H.O. New DFT insights into silicalite formation from layered H-magadiite. Surf. Interfaces 2025, 69, 106754. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Fernandes, M.M.; Fonseca, M.G.; Filho, E.C.S.; Souza, A.G.; Gaslain, F.; Jaber, M. Direct grafting of ethylene sulfide onto silicic acid magadiite. Microporous Mesoporous Mater. 2014, 196, 292–299. [Google Scholar] [CrossRef]
- Alraddadi, T.S.; Al-Faze, R.; Popoola, S.A.; Alam, M.G.; Rakass, S.; Al Dmour, H.; Kooli, F. Combination of acid and base activation of montmorillonite clay and its impact on the basic blue-41 removal properties: Regeneration and single batch design. Inorganics 2025, 13, 228. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zheng, J.; Hu, T.; Meng, C. Synthesis, structure, optical and magnetic properties of interlamellar decoration of magadiite using vanadium oxide species. Microporous Mesoporous Mater. 2017, 244, 264–277. [Google Scholar] [CrossRef]
- Steudel, A.; Batenburg, L.F.; Fischer, H.R.; Weidler, P.G.; Emmerich, K. Alteration of non-swelling clay minerals and magadiite by acid activation. Appl. Clay Sci. 2009, 44, 95–104. [Google Scholar] [CrossRef]
- Eypert-Blaison, C.; Humbert, B.; Michot, L.J.; Pelletier, M.; Sauzeat, E.; Villieras, F. Structural role of hydration water in Na- and H-magadiite: A spectroscopic study. Chem. Mater. 2001, 13, 4439–4446. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, Z.; Schwieger, W. Vibrational spectroscopic studies of layered silicates. Chem. Mater. 1999, 11, 1210–1217. [Google Scholar] [CrossRef]
- Macedo, T.R.; Airoldi, C. Host lamellar silicic acid magadiite for some heterocyclic amine inclusions and quantitative calorimetric data. Microporous Mesoporous Mater. 2006, 94, 81–88. [Google Scholar] [CrossRef]
- Vieira, R.B.; Pastore, H.O. Polyethylenimine-magadiite layered silicate sorbent for CO2 capture. Environ. Sci. Technol. 2014, 48, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.M.; Sanz, J.; Ruiz-Hitzky, E.; Serratosa, J.M. 29Si MAS-N.M.R. spectra of lamellar silicic acid H-magadiite and its trimethylsilyl derivative. Z. Anorg. Und Allg. Chem. 1986, 540, 227–233. [Google Scholar] [CrossRef]
- Nunes, A.R.; Araújo, K.R.O.; Moura, A.O.; Prado, A.G.S. Magadiite as a support for the controlled release of herbicides. Chem. Pap. 2018, 72, 479–486. [Google Scholar] [CrossRef]
- Moura, H.M.; Pastore, H.O. Functionalized mesoporous solids based on magadiite and [Al]-magadiite. Dalton Trans. 2014, 43, 10471–10483. [Google Scholar] [CrossRef]
- e Silva, J.M.D.S.; Paul, G.; Bendall, J.; Bisio, C.; Marchese, L.; Pastore, H.O. Novel insights on magadiite disaggregation: A multitechnique study on thermal stability. Phys. Chem. Chem. Phys. 2013, 15, 13434–13445. [Google Scholar] [CrossRef]
- Ide, Y.; Tominaka, S.; Kono, H.; Ram, R.; Machida, A.; Tsunoji, N. Zeolitic intralayer microchannels of magadiite, a natural layered silicate, to boost green organic synthesis. Chem. Sci. 2018, 9, 8637–8643. [Google Scholar] [CrossRef]
- Rápó, E.; Tonk, S. Factors affecting synthetic dye adsorption; desorption studies: A review of results from the last five years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef]
- Lellou, S.; Kadi, S.; Ouadjenia, F.; Benhebal, H.; Schott, J.; Marouf, R. Synthesis and application of montmorillonite nanocomposites/phenolic resins for the elimination of Basic Blue 41. Desalin. Water Treat. 2021, 218, 389–400. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Abboudi, M.; Hassani, H.O.; Rakass, S.; Ibrahim, S.M.; Al Wadaani, F. Waste bricks applied as removal agent of Basic Blue 41 from aqueous solutions: Base treatment and their regeneration efficiency. Appl. Sci. 2019, 9, 1237. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Al-Madanat, O.Y.; Popoola, S.A.; Al Dmour, H.; Al-Faze, R.; Kooli, F. Na-Kenyaite as Efficient Basic Blue-41 Dye Removal: Synthesis and Regeneration Studies. Water 2024, 16, 2056. [Google Scholar] [CrossRef]
- Gougazeh, M.; Kooli, F.; Buhl, J.C. Removal efficiency of basic blue 41 by three zeolites prepared from natural jordanian kaolin. Clays Clay Miner. 2019, 67, 143–153. [Google Scholar] [CrossRef]
- Zhao, Z.; Yanhui, L.; Du, Q.; Li, Q. Adsorption of Congo red from aqueous solutions by porous soybean curd Xerogels. Pol. J. Chem. Technol. 2018, 20, 95–102. [Google Scholar] [CrossRef]
- Ercag, A.; Demircivi, P.; Hizal, J. Kinetic, isotherm and pH dependency investigation and environmental application of cationic dye adsorption on montmorillonite. Desalination Water Treat. 2015, 56, 2447–2456. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution, by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, L.; Al-Faze, R.; Al Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116–117, 23–30. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Hamdi, N.; Srasra, E. Preparation and characterization of activated carbon from date pits by chemical activation with zinc chloride for methyl orange adsorption. J. Mater. Environ. Sci. 2014, 5, 1758–1769. [Google Scholar]
- Kooli, F.; Kiyozumi, Y.; Mizukami, F. Effect of alkali cations on the conversion of H-magadiite in tetramethylammonium hydroxide water 1,4 dioxane system. Mater. Chem. Phys. 2002, 77, 134–140. [Google Scholar] [CrossRef]
- González-López, M.E.; Laureano-Anzaldo, C.M.; Pérez-Fonseca, A.A.; Arellano, M.; Robledo-Ortíz, J.R. A critical overview of adsorption models linearization: Methodological and statistical inconsistencies. Sep. Purif. Rev. 2022, 51, 358–372. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Roulia, M.; Vassiliadis, A.A. Interactions between C.I. basic blue 41 and aluminosilicate sorbents. J. Colloid Interface Sci. 2005, 291, 37–44. [Google Scholar] [CrossRef]
- Kul, A.R.; Aldemir, A.; Koyuncu, H. An investigation of natural and modified diatomite performance for adsorption of Basic Blue 41: Isotherm, kinetic, and thermodynamic studies. Desalination Water Treat. 2021, 229, 384–394. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Shahadat, M.M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, E.K.; Metzger, J.W.; Fischer, K.; Hassani, A.H. Design and cost analysis of batch adsorber systems for removal of dyes from contaminated groundwater using natural low-cost adsorbents. Int. J. Ind. Chem. 2020, 11, 101–110. [Google Scholar] [CrossRef]
- Mansour, R.A.; Aboeleneen, N.M.; AbdelMonem, N.M. Adsorption of cationic dye from aqueous solutions by date pits: Equilibrium, kinetic, thermodynamic studies, and batch adsorber design. Int. J. Phytoreme. 2018, 20, 1062–1074. [Google Scholar] [CrossRef]
- Alyasi, H.; Mackey, H.R.; Loganathan, K.; McKay, G. Adsorbent minimisation in a two-stage batch adsorber for cadmium removal. J. Ind. Eng. Chem. 2020, 81, 153–160. [Google Scholar] [CrossRef]

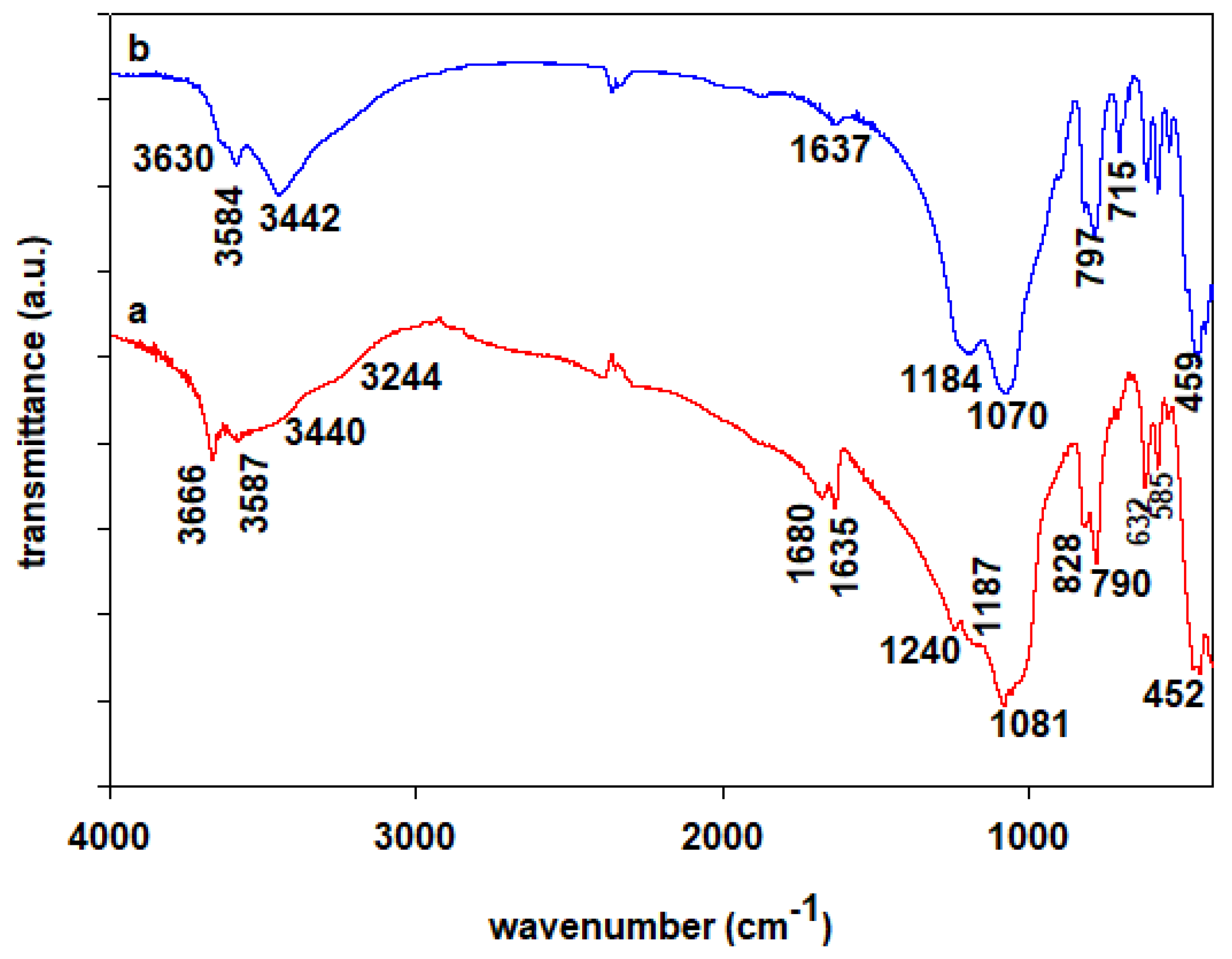



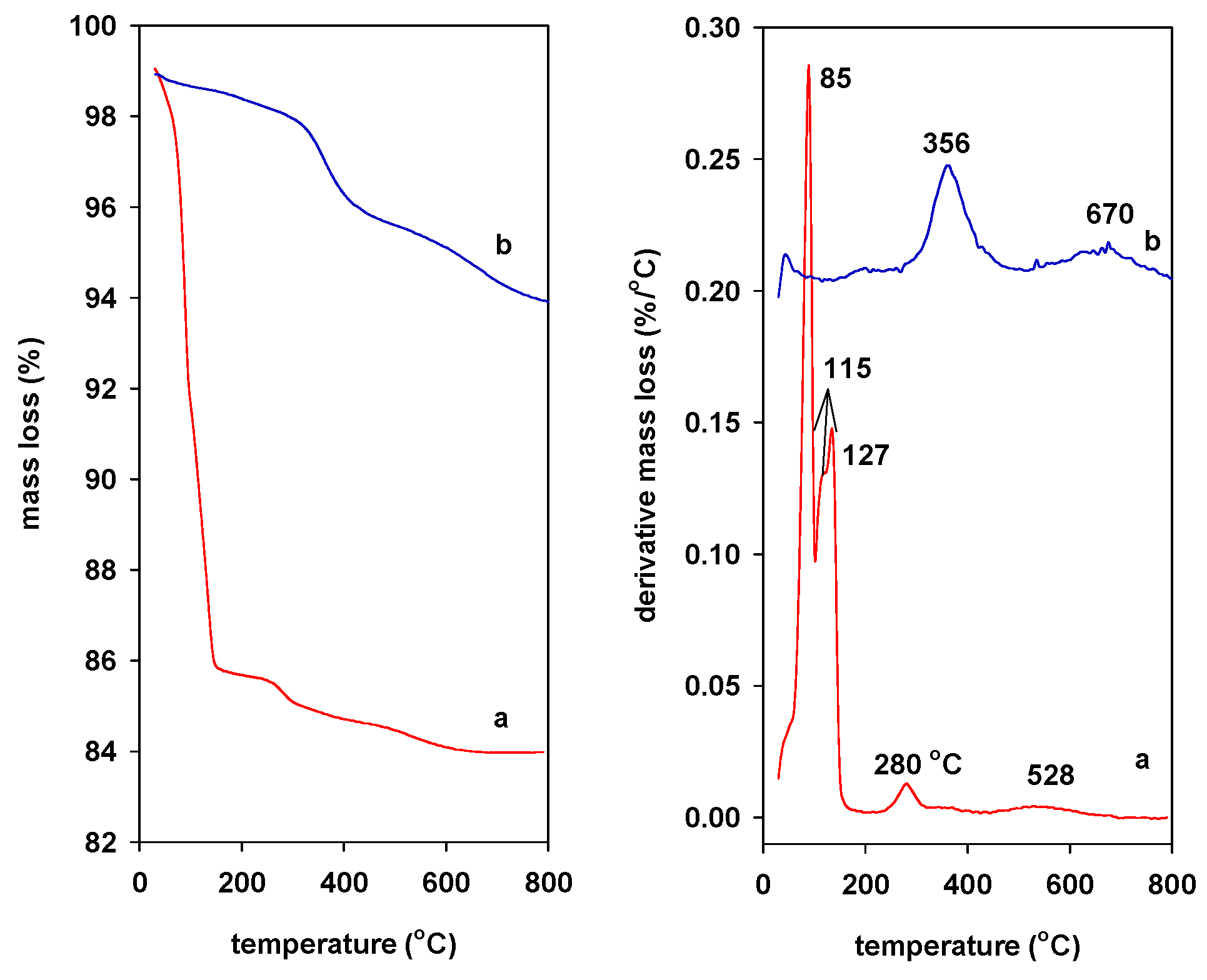
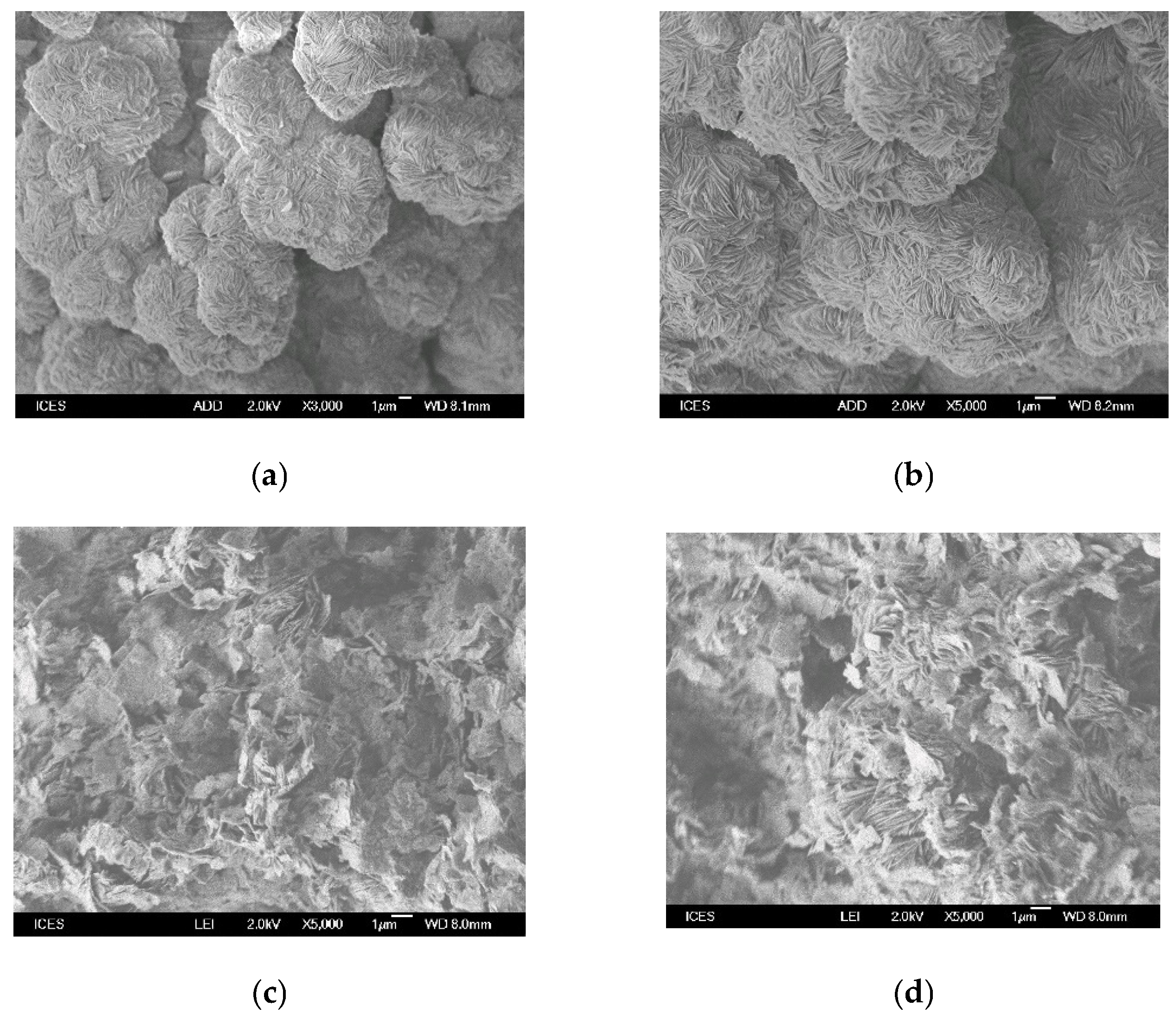
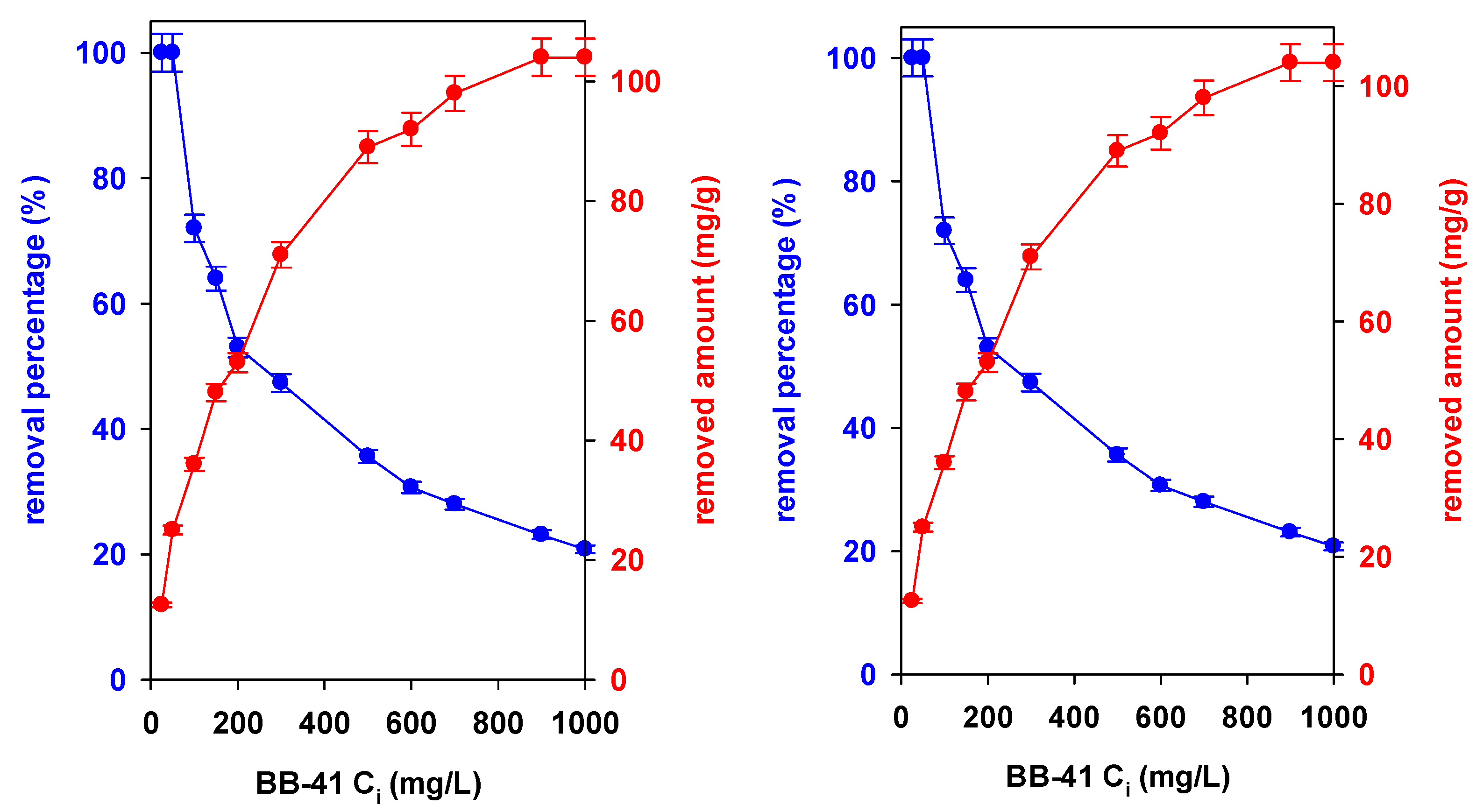
 ) Na-Mgd and (
) Na-Mgd and ( ) H-Mgd samples.
) H-Mgd samples.
 ) Na-Mgd and (
) Na-Mgd and ( ) H-Mgd samples.
) H-Mgd samples.
 ) Na-Mgd and (
) Na-Mgd and ( ) H-Mgd samples.
) H-Mgd samples.
 ) linear Freundlich and (
) linear Freundlich and ( ) Langmuir models.
) Langmuir models.
 ) linear Freundlich and (
) linear Freundlich and ( ) Langmuir models.
) Langmuir models.
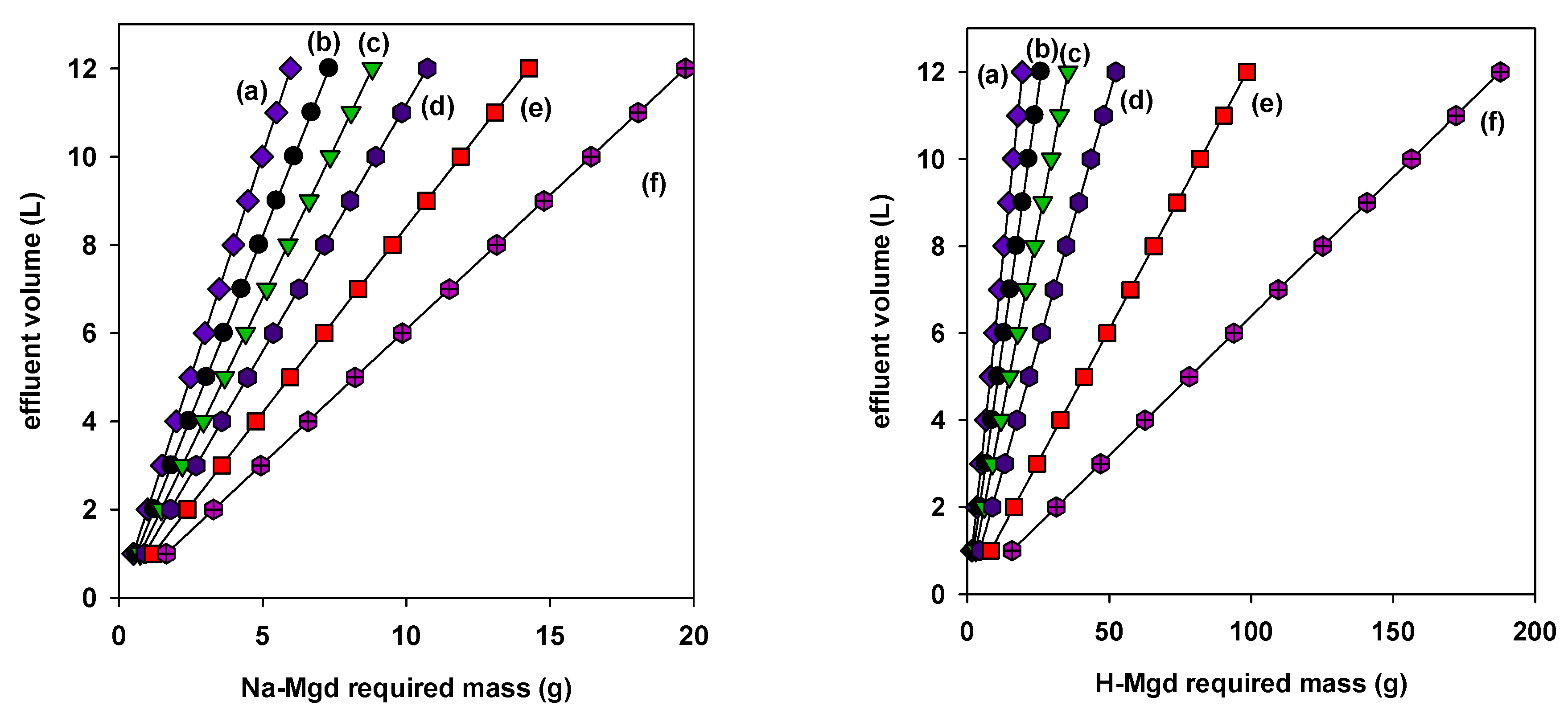
 ) Na-Mgd and (
) Na-Mgd and ( ) H-Mgd to treat 10 L of BB-41 solution with different Ci (mg/L) targeting a removal percentage of 95%.
) H-Mgd to treat 10 L of BB-41 solution with different Ci (mg/L) targeting a removal percentage of 95%.
 ) Na-Mgd and (
) Na-Mgd and ( ) H-Mgd to treat 10 L of BB-41 solution with different Ci (mg/L) targeting a removal percentage of 95%.
) H-Mgd to treat 10 L of BB-41 solution with different Ci (mg/L) targeting a removal percentage of 95%.
| Sample | Na2O (wt%) | SiO2 (wt%) | Total Mass Loss (%) * |
|---|---|---|---|
| Na-Mgd | 5.41 | 94.21 | 14.6 |
| H-Mgd | ~0.00 | 99.52 | 5.14 |
| Sample | A.P.D (nm) | T.P.V. (cm3/g) | SBET (m2/g) |
|---|---|---|---|
| Na-Mgd | 29 | 0.196 | 26 |
| H-Mgd | 25.8 | 0.263 | 40 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | 1/n | KF (L/mg) | R2 | |
| Na-Mgd | 219 | 0.112 | 0.9998 | 0.1866 | 66.68 | 0.9819 |
| (222) * | (0.0681) | (0.9172) | (0.2149) | (57.59) | (0.9387) | |
| H-Mgd | 114 | 0.0123 | 0.9891 | 0.3193 | 12.97 | 0.9891 |
| (113.3) | (0.0119) | (0.9878) | (0.3045) | (14.36) | (0.9878) | |
| Samples | qmax (mg/g) | References |
|---|---|---|
| Na-Mgd | 219 | This study |
| H-Magd | 114 | This study |
| Montmorillonite (Mt) | 55 | [37] |
| Saudi Local clays | 50–70 | [58] |
| Brick wastes | 60–70 | [51] |
| zeolite | 39–70 | [54] |
| Mn modified diatomite | 77 | [64] |
| Acid-activated PGs * | 43–88 | [37] |
| Base acid-activated PGs + | 92–110 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alraddadi, T.S.; Alam, M.G.; Al-Faze, R.; Popoola, S.A.; Rakass, S.; Oudghiri Hassani, H.; Kooli, F. Comparative Removal Properties of Sodium Magadiite and Its Protonic Form on Basic-Blue 41 from Contaminated Aqueous Solution. Inorganics 2025, 13, 303. https://doi.org/10.3390/inorganics13090303
Alraddadi TS, Alam MG, Al-Faze R, Popoola SA, Rakass S, Oudghiri Hassani H, Kooli F. Comparative Removal Properties of Sodium Magadiite and Its Protonic Form on Basic-Blue 41 from Contaminated Aqueous Solution. Inorganics. 2025; 13(9):303. https://doi.org/10.3390/inorganics13090303
Chicago/Turabian StyleAlraddadi, Thamer S., Mohd Gulfam Alam, Rawan Al-Faze, Saheed A. Popoola, Souad Rakass, Hicham Oudghiri Hassani, and Fethi Kooli. 2025. "Comparative Removal Properties of Sodium Magadiite and Its Protonic Form on Basic-Blue 41 from Contaminated Aqueous Solution" Inorganics 13, no. 9: 303. https://doi.org/10.3390/inorganics13090303
APA StyleAlraddadi, T. S., Alam, M. G., Al-Faze, R., Popoola, S. A., Rakass, S., Oudghiri Hassani, H., & Kooli, F. (2025). Comparative Removal Properties of Sodium Magadiite and Its Protonic Form on Basic-Blue 41 from Contaminated Aqueous Solution. Inorganics, 13(9), 303. https://doi.org/10.3390/inorganics13090303








