Advances in Synthesis and Applications of Bismuth Vanadate-Based Structures
Abstract
1. Introduction
2. Discussion
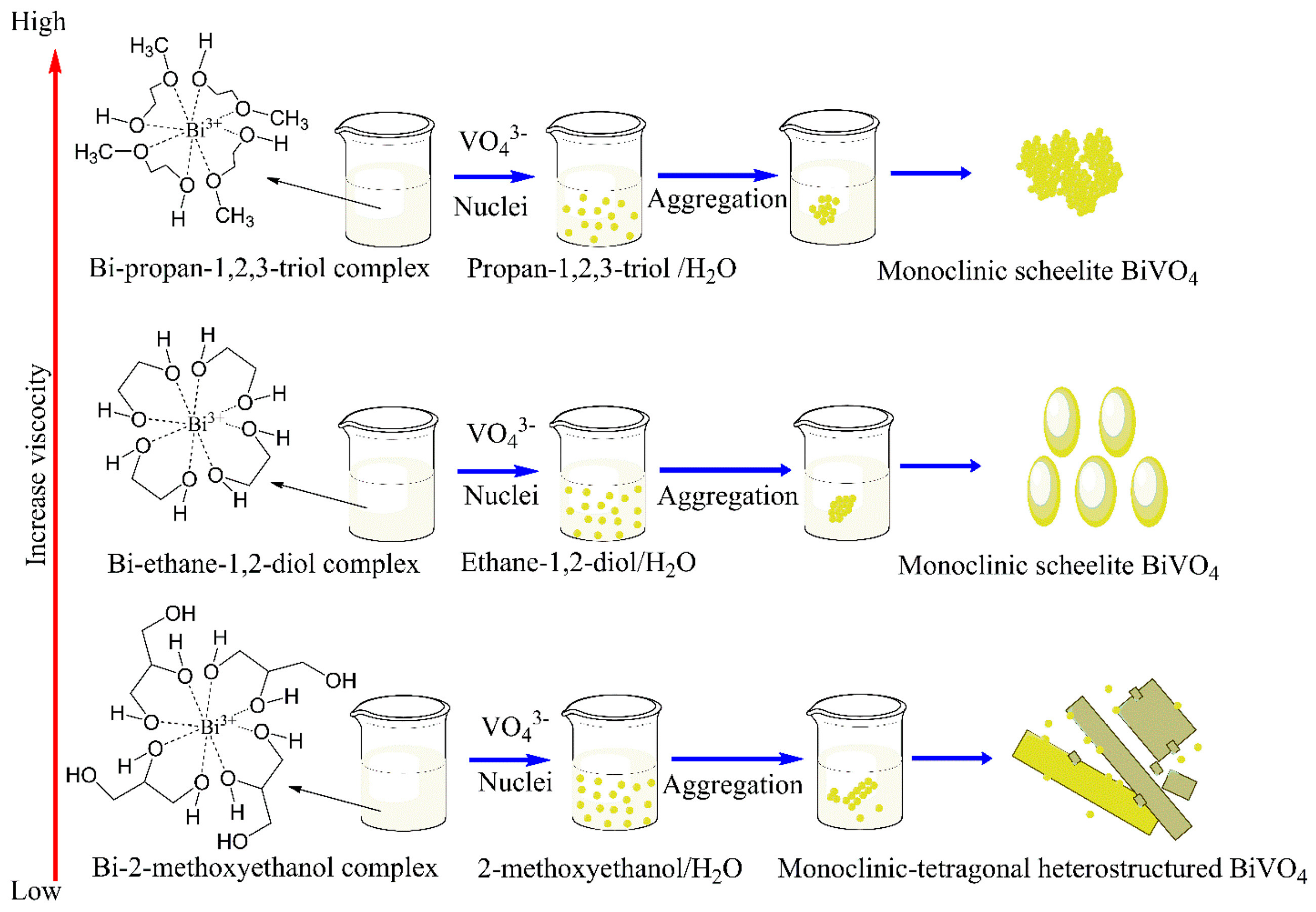
2.1. Advances in Synthesis of BiVO4-Based Structures
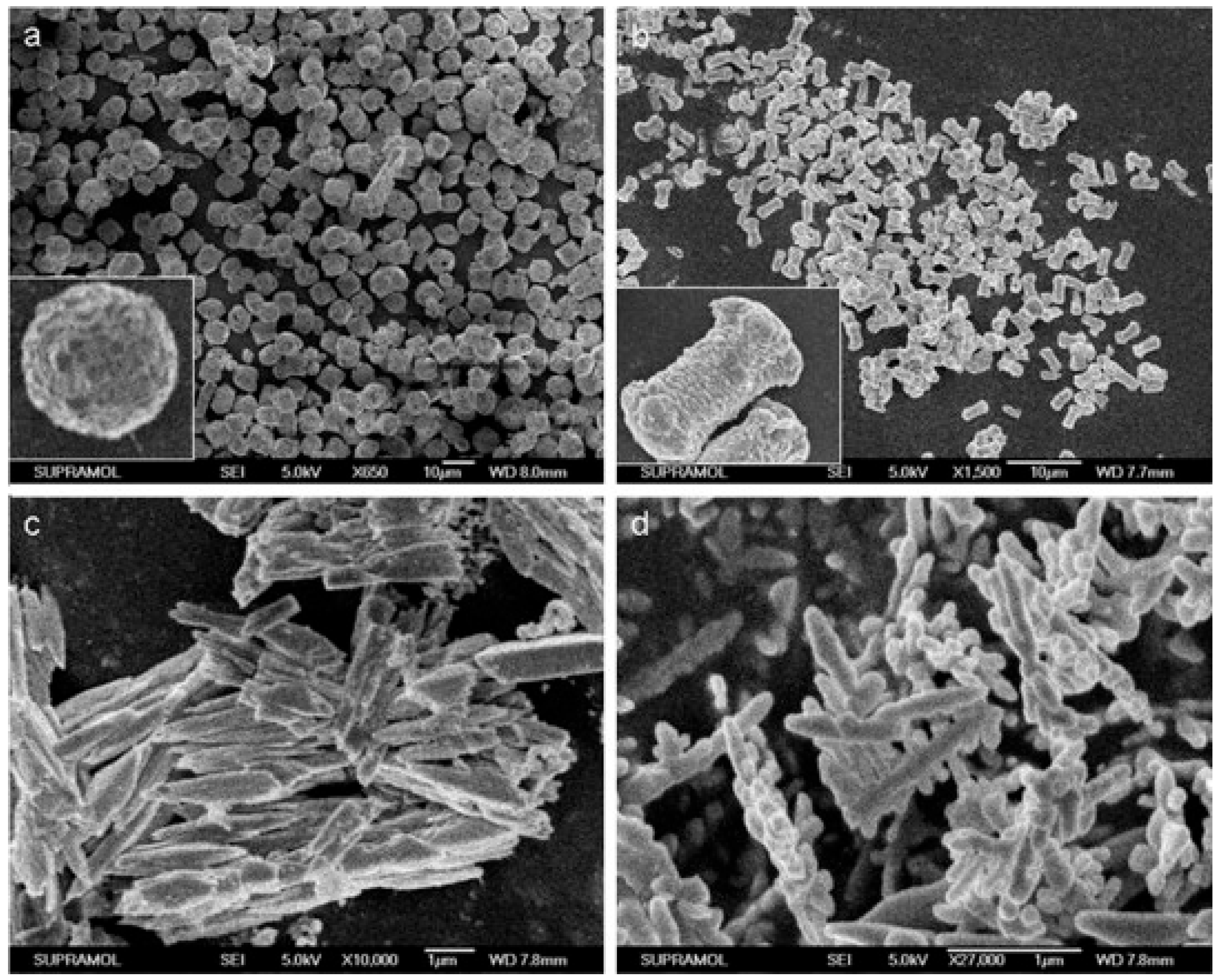
2.2. Advances in the Study of Morphologies and Sizes of BiVO4-Based Structures
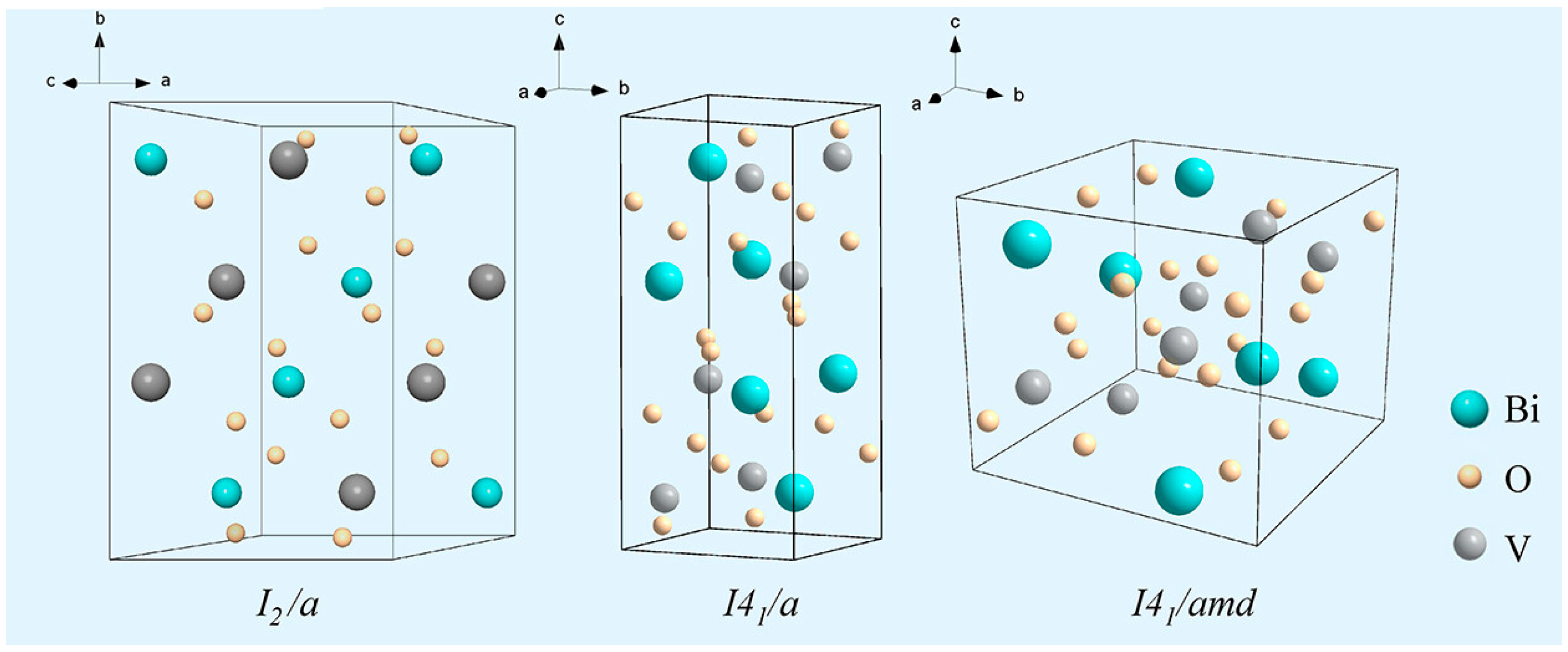
2.3. Crystal and Electronic Structure of BiVO4
3. Advances in Applications of BiVO4-Based Structures
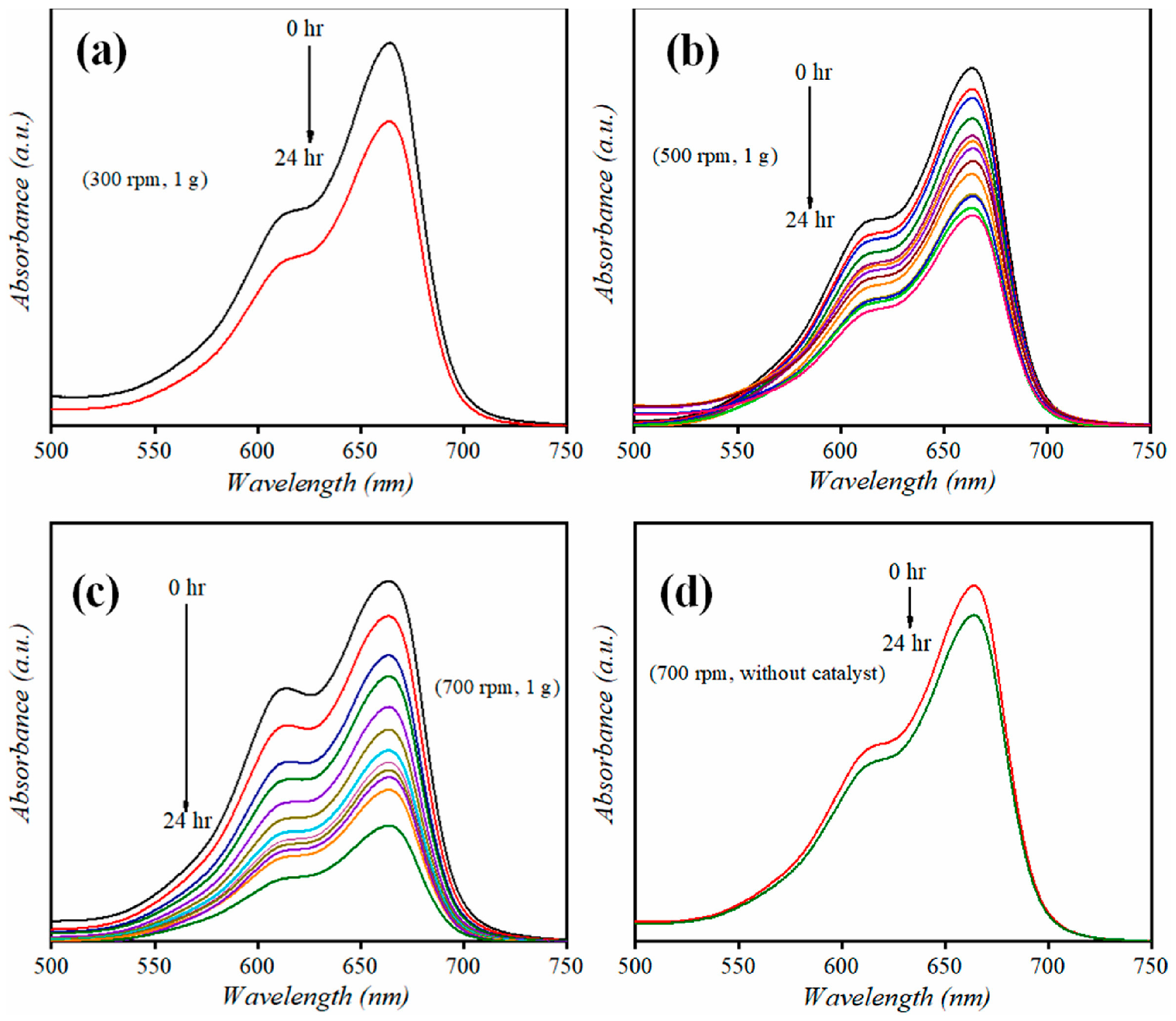
3.1. Degradation of Organic Compounds: Role of BiVO4-Based Composite Photocatalysts
3.2. Antibacterial Activity of the BiVO4-Based Structures
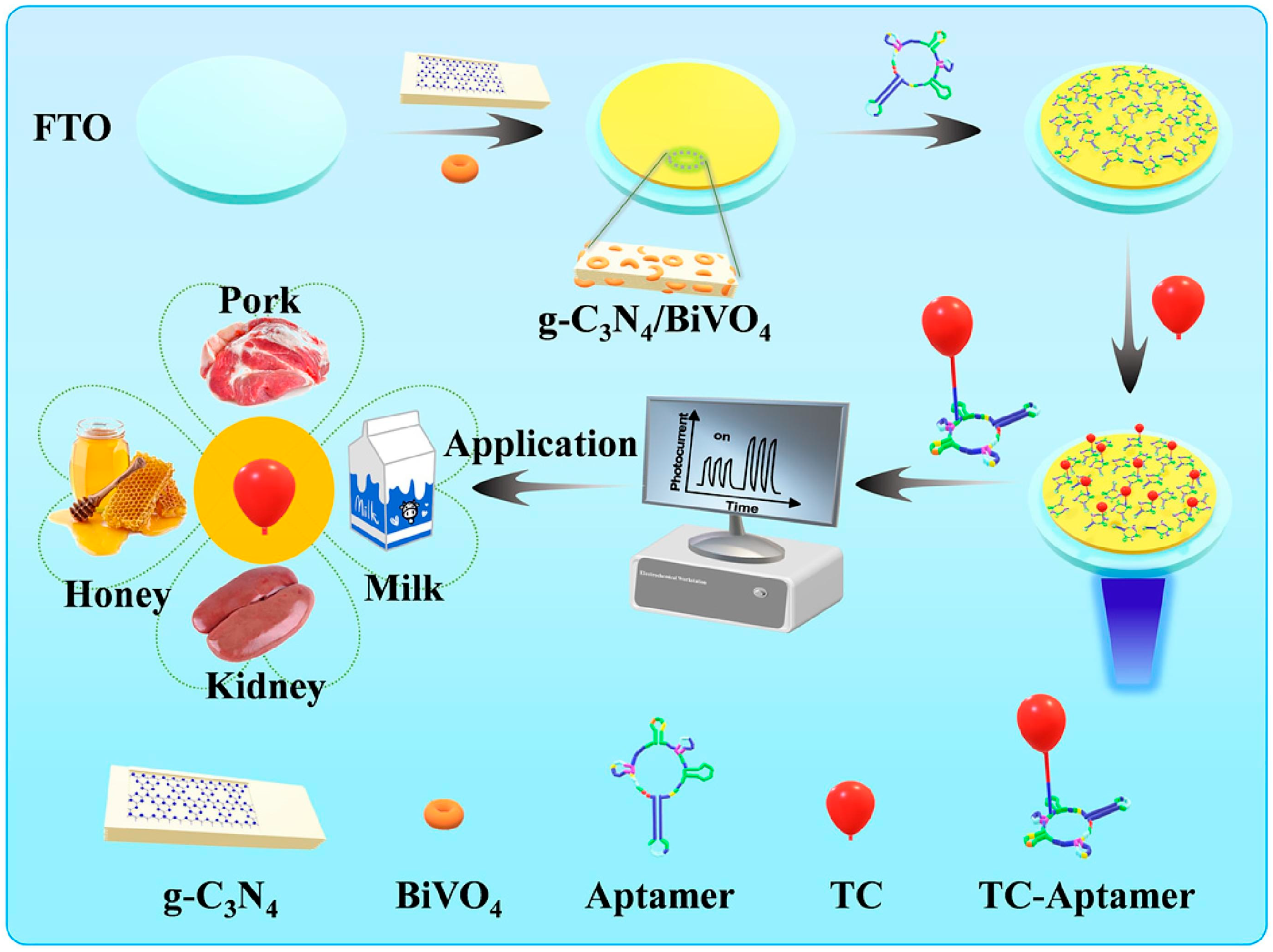
3.3. Application of the BiVO4-Based Structures in the Food Industry
3.4. Applications of BiVO4-Based Structures as Photoelectrodes in Water Splitting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khader, E.H.; Muslim, S.A.; Saady, N.M.C.N.; Ali, S.; Salih, I.K.; Mohammed, T.J.; Albayati, T.M.; Zendehboudi, S. Recent advances in photocatalytic advanced oxidation processes for organic compound degradation: A review. Desalination Water Treat. 2024, 318, 100384. [Google Scholar] [CrossRef]
- Yang, F.; Yu, X.; Wang, K.; Liu, Z.; Gao, Z.; Zhang, T.; Niu, J.; Zhao, J.; Yao, B. Photocatalytic degradation of methylene blue over BiVO4/BiPO4/rGO heterojunctions and their artificial neural network model. J. Alloys Compd. 2023, 960, 170716. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, V.H.; Nanda, S.; Vo, D.-V.N.; Nguyen, V.H.; Tran, T.V.; Nong, L.X.; Nguyen, T.T.; Bach, L.-G.; Abdullah, B.; et al. BiVO4 photocatalysis design and applications to oxygen production and degradation of organic compounds: A review. Environ. Chem. Lett. 2020, 18, 1779–1801. [Google Scholar] [CrossRef]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Suarez, C.M.; Hernández, S.; Russo, N. BiVO4 as photocatalyst for solar fuels production through water splitting: A short review. Appl. Catal. A Gen. 2015, 504, 158–170. [Google Scholar] [CrossRef]
- Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z. Photoelectrochemical cells for solar hydrogen production: Current state of promissing photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347–370. [Google Scholar] [CrossRef]
- Qiao, R.; Mao, M.; Hu, E.; Zhong, Y.; Ning, J.; Hu, Y. Facile formation of mesoporous BiVO4/Ag/AgCl heterostructured microspheres with enhanced visible-light photoactivity. Inorg. Chem. 2015, 54, 9033–9039. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Hernández, S.; Bensaid, S.; Saracco, G.; Russo, N. Green-synthesized W- and Mo-doped BiVO4 oriented along the {0 4 0} facet with enhanced activity for the sun-driven water oxidation. Appl. Catal. B Environ. 2016, 180, 630–636. [Google Scholar] [CrossRef]
- Nagabhushana, G.P.; Tavakoli, A.H.; Navrotsky, A. Energetics of bismuth vanadate. J. Solid State Chem. 2015, 225, 187–192. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chanéac, C.; Durupthy, O.; Mariey, L.; Maugé, F.; Brezová, V.; Jolivet, J.-P. New insights into BiVO4 properties as visible light photocatalyst. J. Phys. Chem. C. 2015, 119, 12967–12977. [Google Scholar] [CrossRef]
- Iwase, A.; Kato, H.; Kudo, A. A simple preparation method of visible-light-driven BiVO4 photocatalysts from oxide starting materials (Bi2O3 and V2O5) and their photocatalytic activities. J. Sol. Energy Eng. 2010, 132, 021106. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Y.; Wu, H.; Xie, R. Recent progress in BiVO4-based heterojunction nanomaterials for photocatalytic applications. Mater. Sci. Eng. B 2023, 289, 116278. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Wei, Z.; Yang, S.; He, H.; Sun, C. Fabrication of a novel p–n heterojunction photocatalyst n-BiVO4@p-MoS2 with core–shell structure and its excellent visible-light photocatalytic reduction and oxidation activities. Appl. Catal. B-Environ. 2016, 185, 242–252. [Google Scholar] [CrossRef]
- Srinivasan, N.; Sakai, E.; Miyauchi, M. Balanced excitation between two semiconductors in bulk heterojunction Z-scheme system for overall water splitting. ACS Catal. 2016, 6, 2197–2200. [Google Scholar] [CrossRef]
- Xie, M.; Feng, Y.; Fu, X.; Luan, P.; Jing, L. Phosphate-bridged TiO2-BiVO4 nanocomposites with exceptional visible activities for photocatalytic water splitting. J. Alloys Compd. 2015, 631, 120–124. [Google Scholar] [CrossRef]
- Lv, Y.-R.; Liu, C.-J.; He, R.-K.; Li, X.; Xu, Y.-H. BiVO4/TiO2 heterojunction with enhanced photocatalytic activities and photoelectochemistry performances under visible light illumination. Mater. Res. Bull. 2019, 117, 35–40. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Mao, H.; Lu, Y.; Lu, J.; Huang, J.; Ye, Z.; Lu, B. Electrostatic self-assembly of BiVO4-reduced graphene oxide nanocomposites for highly efficient visible light photocatalytic activities. ACS Appl. Mater. Interfaces 2014, 6, 12698–12706. [Google Scholar] [CrossRef]
- Phu, N.D.; Hoang, L.H.; Guo, P.-C.; Chen, X.-B.; Chou, W.C. Study of photocatalytic activities of Bi2WO6/BiVO4 nanocomposites. J. Sol-Gel Sci. Technol. 2017, 83, 640–646. [Google Scholar] [CrossRef]
- Cai, L.; Kisch, H. Visible light induced photoelectrochemical properties of n-BiVO4 and n-BiVO4/p-Co3O4. Phys. Chem. C 2008, 112, 548–554. [Google Scholar] [CrossRef]
- Li, J.; Lu, P.; Deng, W.; Zeng, Z.; Lin, L.; Zhao, G. Facile synthesis of sheet-like BiVO4/Bi4V2O11 composite for enhanced photocatalytic properties. Mater. Chem. Phys. 2020, 254, 123489. [Google Scholar] [CrossRef]
- Qi, X.; Gu, M.; Zhu, X.; Wu, J.; Wu, Q.; Long, H.; He, K. Controlled synthesis of Ag3PO4/BiVO4 composites with enhanced visible-light photocatalytic performance for the degradation of RhB and 2, 4-DCP. Mater. Res. Bull. 2016, 80, 215–222. [Google Scholar] [CrossRef]
- Xiong, B.; Wu, Y.; Du, J.; Li, J.; Liu, B.; Ke, G.; He, H.; Zhou, Y. Cu3Mo2O9/BiVO4 heterojunction films with integrated thermodynamic and kinetic advantages for solar water oxidation. ACS Sustain. Chem. Eng. 2020, 8, 14082–14090. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Sun, Z.; Liang, Q.; Zhou, M.; Xu, S.; Li, Z.; Sun, D. Fabrication and efficient photocatalytic dye degradation over Z-scheme-based BiVO4/CdS heterojunction under visible-light irradiation. J. Mater. Sci. Mater. Electron. 2020, 31, 15742–15750. [Google Scholar] [CrossRef]
- Guo, Y.; Ao, Y.; Wang, P.; Wang, C. Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocatalysts with enhanced visible-light-driven performance towards carbamazepine degradation. Appl. Catal. B-Environ. 2019, 254, 479–490. [Google Scholar] [CrossRef]
- Zhao, Z.; Bian, J.; Zhao, L.; Wu, H.; Xu, S.; Sun, L.; Li, Z.; Zhang, Z.; Jing, L. Construction of 2D Zn-MOF/BiVO4 S-scheme heterojunction for efficient photocatalytic CO2 conversion under visible light irradiation. Chin. J. Catal. 2022, 43, 1331–1340. [Google Scholar] [CrossRef]
- Song, A.; Zhang, Y.; Li, Z.; Hu, J. BiVO4/CuBi2O4 heterojunction photoanodes with enhanced charge separation for efficient photoelectrochemical water splitting. Mater. Sci. Eng. B 2024, 302, 117241. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.; Nan, C.; Zhou, W.; Gao, N.; Jia, Y.; Zhang, Y.; Chen, R. Efficient CO2 reduction to formate using a Cu-doped BiVO4 electrocathode in a WO3 photoanode-assisted photoelectrocatalytic system. J. Electroanal. Chem. 2023, 930, 117146. [Google Scholar] [CrossRef]
- Ghaware, R.C.; Birajdar, N.B.; Kamble, G.S.; Kolekar, S.S. Degradation of organic Pollutant by using of BiVO4–NiFe2O4 heterostructure photocatalyst under visible light irradiation: Assessment of detoxicity study using Cirrhinus mrigala. Langmuir 2024, 40, 14426–14439. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, C.; Li, H.; Chu, J.; Sun, G.; Xu, Y.; Yan, Y. Synthesis, characterization and photocatalytic activities of rare earth-loaded BiVO4 catalysts. Appl. Surf. Sci. 2009, 256, 597–602. [Google Scholar] [CrossRef]
- Wang, M.; Wu, L.; Zhang, F.; Gao, L.; Geng, L.; Ge, J.; Tian, K.; Chai, H.; Niu, H.; Liu, Y.; et al. Doping with rare earth el-ements and loading cocatalysts to improve the solar water splitting performance of BiVO4. Inorganics 2023, 11, 203. [Google Scholar] [CrossRef]
- Guardiano, M.G.; Gonzaga, I.M.D.; Ribeiro, L.K.; da Silva, A.C.V.; Mascaro, L.H. Gd-BiVO4: An efficient photoanode for pharmaceuticals degradation in contaminated waters via solar photoelectrocatalysis. J. Chem. Eng. 2025, 503, 158463. [Google Scholar] [CrossRef]
- Liu, B.; Yan, X.; Yan, H.; Yao, Y.; Cai, Y.; Wei, J.; Chen, S.; Xu, X.; Li, L. Preparation and characterization of Mo doped in BiVO4 with enhanced photocatalytic properties. Materials 2017, 10, 976. [Google Scholar] [CrossRef]
- Su, W.; Lu, Z.; Shi, Q.; Cheng, C.; Liu, C.; Lu, C.; Xie, H.; Lu, B.; Huang, K.; Xu, M.; et al. Surface states of Mo-doped BiVO4 nanoparticle-based photoanodes for photoelectrochemical degradation of chloramphenicol. ACS Appl. Nano Mater. 2024, 7, 14232–14241. [Google Scholar] [CrossRef]
- Wang, G.-L.; Shan, L.-W.; Wu, Z.; Dong, L.-M. Enhanced photocatalytic properties of molybdenum-doped BiVO4 prepared by sol–gel method. Rare Met. 2017, 36, 129–133. [Google Scholar] [CrossRef]
- Cen, J.; Li, S.; Zheng, J.; Pan, F. Electron polarons in the subsurface layer of Mo/W-doped BiVO4 surfaces. RSC Adv. 2019, 9, 819–823. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Chung, R.-J.; Yougbaré, S.; Lin, L.-Y. Rational design of W-doped BiVO4 photoanode coupled with FeOOH for highly efficient photoelectrochemical catalyzing water oxidation. Int. J. Hydrogen Energy 2022, 47, 27012–27022. [Google Scholar] [CrossRef]
- Lalrindiki, F.; Singh, N.P.; Singh, N.M. A review of synthesis, photocatalytic, photoluminescence and antibacterial properties of bismuth vanadate-based nanomaterial. Inorg. Chem. Commun. 2024, 168, 112846. [Google Scholar] [CrossRef]
- Fan, H.; Jiang, T.; Li, H.; Wang, D.; Wang, L.; Zhai, J.; He, D.; Wang, P.; Xie, T. Effect of BiVO4 crystalline phases on the photoinduced carriers behavior and photocatalytic activity. J. Phys. Chem. C. 2012, 116, 2425–2430. [Google Scholar] [CrossRef]
- Cao, X.; Gu, Y.; Tian, H.; Fang, Y.; Johnson, D.; Ren, Z.; Chen, C.; Huang, Y. Microemulsion synthesis of ms/tz-BiVO4 composites: The effect of pH on crystal structure and photocatalytic performance. Ceram. Int. 2020, 46, 20788–20797. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Selective synthesis and visible-light photocatalytic activities of BiVO4 with different crystalline phases. Mater. Chem. Phys. 2007, 103, 162–167. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Kudo, A. Synthesis and photocatalytic performances of BiVO4 by ammonia co-precipitation process. J. Solid State Chem. 2009, 182, 223–228. [Google Scholar] [CrossRef]
- de la Cruz, A.M.; Pérez, U.M.G. Photocatalytic properties of BiVO4 prepared by the co-precipitation method: Degradation of rhodamine B and possible reaction mechanisms under visible irradiation. Mater. Res. Bull. 2010, 45, 135–141. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, S.; Luo, W.; Zhao, L. Impact of preparative pH on the morphology and photocatalytic activity of BiVO4. Int. J. Photoenergy 2012, 2012, 392865. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Wu, Y.-S.; Lu, C.-S.; Lin, P.-F.; Wu, T.-Y. Photodegradation of alachlor using BiVO4 photocatalyst under visible light irradiation. Water Air Soil Pollut. 2015, 226, 194. [Google Scholar] [CrossRef]
- Hazarika, M.; Lutukurthi, D.N.V.V.K.; Ra, V.K. Photocatalytic activity of Ho3+-Yb3+ activated BiVO4 upconverting phosphors. J. Alloys Compd. 2024, 1002, 175453. [Google Scholar] [CrossRef]
- Ahmed, T.; Zhang, H.; Gao, Y.-Y.; Xu, H.; Zhang, Y. Surfactant-free synthesis of m-BiVO4 nanoribbons and enhanced visible-light photocatalytic properties. Mater. Res. Bull. 2018, 99, 298–305. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, R.; Li, X.; Wang, Y.; Tian, J.; Zhu, M.; Gong, X. Surfactants modulating of BiVO4 on photocatalytic property as a regulation of surface free energy. Langmuir 2024, 40, 26540–26550. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; El-Sheikh, S.M.; Yu, J.; Eid, A.I.; El-Haka, S.A.; Samra, S.E. Novel synthesis of BiVO4 using homogeneous precipitation and its enhanced photocatalytic activity. J. Nanopart. Res. 2020, 22, 132. [Google Scholar] [CrossRef]
- Montañés, L.; Mesa, C.A.; Gutiérrez-Blanco, A.; Robles, C.; Julián-López, B.; Giménez, S. Facile surfactant-assisted synthesis of BiVO4 nanoparticulate films for solar water splitting. Catalysts 2021, 11, 1244. [Google Scholar] [CrossRef]
- Zhansheng, W.; Xue, Y.; He, X.; Li, Y.; Yang, X.; Wu, Z.; Cravotto, G. Surfactants-assisted preparation of BiVO4 with novel morphologies via microwave method and CdS decoration for enhanced photocatalytic properties. J. Hazard. Mater. 2020, 387, 122019. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Kong, L.; Xiao, Q.; Huang, S. Surfactants-assisted morphological regulation of BiVO4 nanostructures for photocatalytic degradation of organic pollutants in wastewater. J. Phys. Chem. Solids 2023, 172, 111079. [Google Scholar] [CrossRef]
- Cheng, C.; Tan, H.; Zhu, W.; Liu, L.; Chen, K.; Yan, J. The transition of tetragonal to monoclinic phase in BiVO4 coupled with peroxymonosulfate for photocatalytic degradation of tetracycline hydrochloride. Environ. Res. 2025, 267, 120631. [Google Scholar] [CrossRef]
- Polo, A.; Dozzi, M.V.; Marra, G.; Sivula, K.; Selli, E. Improving the photoelectrocatalytic efficiency of CuWO4 through molybdenum for tungsten substitution and coupling with BiVO4. Sustain. Energy Fuels 2024, 8, 3182–3191. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Tang, Y.; Liu, Y.; Wang, X. A rapid microwave synthesis of nanoscale BiVO4/Bi2O3@SiO2 with large specific surface area and excellent visible-light-driven activity. Desalin. Water Treat. 2019, 152, 99–107. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Tang, Y.; Wang, X.; Shu, K. Facile and rapid synthesis of a novel spindle-like heterojunction BiVO4 showing enhanced visible-light-driven photoactivity. RSC Adv. 2020, 10, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martınez, D.; Hernandez-Uresti, D.B.; Torres-Martinez, L.; Mejia-Rosales, M.S. Photocatalytic properties of BiVO4 synthesized by microwave-assisted hydrothermal method under simulated sunlight irradiation. Res. Chem. Intermed. 2015, 41, 8839–8854. [Google Scholar] [CrossRef]
- Tan, G.; Zhang, L.; Ren, H.; Wei, S.; Huang, J.; Xia, A. Effects of pH on the hierarchical structures and photocatalytic performance of BiVO4 powders prepared via the microwave hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 5186–5193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, G.; Wei, S.; Ren, H.; Xia, A.; Luo, Y. Microwave hydrothermal synthesis and photocatalytic properties of TiO2/BiVO4 composite photocatalysts. Ceram. Int. 2013, 39, 8597–8604. [Google Scholar] [CrossRef]
- Chen, S.-H.; Jiang, Y.-S.; Lin, H. Easy synthesis of BiVO4 for photocatalytic overall water splitting. ACS Omega 2020, 5, 8927–8933. [Google Scholar] [CrossRef]
- Kshetri, Y.K.; Regmi, C.; Dhakal, D.R.; Kim, T.-H.; Kim, S.H.; Kim, H.-S.; Lee, S.W. Microwave hydrothermal synthesis and upconversion properties of BiVO4 nanoparticles. Nanotechnology 2020, 31, 244001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ding, J.; Zhou, F.; Zhao, Q.; Wang, K.; Chen, X.; Gao, Q. Insight into a novel microwave-assisted W doped BiVO4 self-assembled sphere with rich oxygen vacancies oriented on rGO (W-BiVO4-x/rGO) photocatalyst for efficient contaminants removal. Sep. Purif. Technol. 2021, 277, 119610. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Wang, J.; Chang, L.; Song, N.; Yan, Z.; Wan, X. Additive-free solvothermal preparation, characterization, and photocatalytic activity of 3D butterfly-like BiVO4. Res. Chem. Intermed. 2015, 41, 2465–2477. [Google Scholar] [CrossRef]
- Kamble, G.S.; Ling, Y.C. Solvothermal synthesis of facet-dependent BiVO4 photocatalyst with enhanced visible-light-driven photocatalytic degradation of organic pollutant: Assessment of toxicity by zebrafish embryo. Sci. Rep. 2020, 10, 12993. [Google Scholar] [CrossRef]
- Pham, M.Q.; Ngo, T.M.; Nguyen, V.H.; Nong, L.X.; Vo, D.-V.N.; Tran, T.V.; Nguyen, T.-D.; Bui, X.-T.; Nguyen, T.D. Facile solvothermal synthesis of highly active monoclinic scheelite BiVO4 for photocatalytic degradation of methylene blue under white LED light irradiation. Arab. J. Chem. 2020, 13, 8388–8394. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Huang, W.; Zhao, H.; Chen, J.; Cai, Q.; Jiang, X.; Lu, C.; Shi, W. Solvothermal synthesis of CoO/BiVO4 p-n heterojunction with micro-nano spherical structure for enhanced visible light photocatalytic activity towards degradation of tetracycline. Mater. Res. Bull. 2021, 135, 111161. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Y.; Wang, Z.; Cui, H.; Sun, T.; Tang, Y. Simple glycerol-assisted and morphology controllable solvothermal synthesis of CeVO4/BiVO4 hierarchical hollow microspheres with enhanced photocatalytic activities. Mater. Chem. Front. 2021, 5, 6522–6529. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Shi, J.; Zhang, Y.; Liu, X.; Dong, P.; Xi, X. Development of Z-scheme Bi5O7I/BiVO4 heterostructure for efficient photocatalytic water splitting activity. Colloids Surf. A Physicochem. Eng. Asp. 2025, 713, 136472. [Google Scholar] [CrossRef]
- Yu, S.; Su, C.; Xiao, Z.; Kuang, Y.; Gong, X.; He, X.; Liu, J.; Jin, Q.; Sun, Z. Tuning surface hydrophilicity of a BiVO4 photoanode through interface engineering for efficient PEC water splitting. RSC Adv. 2025, 15, 815–823. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Cao, V.D.; Nguyen, V.H.; Nong, L.X.; Luu, T.D.; Vo, D.-V.N.; Do, S.T.; Lam, T.D. Synthesized BiVO4 was by the co-precipitation method for Rhodamine B degradation under visible light. IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 012058. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Sangili, A.; Chen, S.-M.; Abinaya, M. Additive-free synthesis of BiVO4 microspheres as an electrochemical sensor for determination of antituberculosis drug rifampicin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126849. [Google Scholar] [CrossRef]
- Riapanitra, A.; Setyaningtyas, T.; Haryanto, M.J.; Haryadinaru, G.H. Hydrothermal and coprecipitation synthesis design of BiVO4 for Methylene blue degradation. J. Ris. Kim. 2025, 16, 20–32. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, H.; Luo, J.; Zhang, S.; Wang, L.; Wang, X.; Ma, Y.; Ning, P. Phase transition guided V2O5/β-Bi2O3 Z-scheme heterojunctions for efficient photocatalytic Hg0 oxidation. Sep. Purif. Technol. 2024, 330, 125318. [Google Scholar] [CrossRef]
- Phu, N.D.; Hoang, L.H.; Vu, P.K.; Kong, M.-H.; Chen, X.-B.; Wen, H.C.W.; Chou, C. Control of crystal phase of BiVO4 nanoparticles synthesized by microwave assisted method. J. Mater. Sci. Mater. Electron. 2016, 27, 6452–6456. [Google Scholar] [CrossRef]
- Sun, Q.; Qiao, F.; Zhou, T. CTAB assisted hydrothermal synthesis of oxygen vacancy enriched BiVO4 for enhanced photocatalytic hydrogen production. CrystEngComm 2025, 27, 948–955. [Google Scholar] [CrossRef]
- Yan, M.; Yan, Y.; Wu, Y.; Shi, W.; Hua, Y. Microwave-assisted synthesis of monoclinic–tetragonal BiVO4 heterojunctions with enhanced visible-light-driven photocatalytic degradation of tetracycline. RSC Adv. 2015, 5, 90255–90264. [Google Scholar] [CrossRef]
- Shafi, I.; Liang, E.; Li, B. Self-assembled BiVO4 nanorods: A fascinating electrode material for highly efficient pseudocapacitors and electrochemical nitrite sensors. J. Phys. Chem. Solids 2022, 162, 110517. [Google Scholar] [CrossRef]
- Heckel, S.; Wittmann, M.; Reid, M.; Villa, K.; Simmchen, J. An account on BiVO4 as photocatalytic active matter. Acc. Mater. Res. 2024, 5, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Ribeiro, F.W.P.; Gromboni, M.F.; Marken, F.; Mascaro, L.H. Photoelectrocatalytic properties of BiVO4 prepared with different alcohol solvents. Int. J. Hydrog. Energy 2016, 41, 17380–17389. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Liu, Z.; Dai, C.; Song, Z.; Sun, Y.; Fang, J.; Zhao, J. Low-temperature synthesis of BiVO4 crystallites in molten salt medium and their UV–vis absorption. Ceram. Int. 2010, 36, 2073–2077. [Google Scholar] [CrossRef]
- Cheng, B.; Lou, H.; Zeng, Z.; Liu, Y.; Zeng, Q. Structural phase transition in BiVO4 nanosheets under high pressure. J. Phys. Chem. C 2024, 128, 12267–12273. [Google Scholar] [CrossRef]
- Zhao, Z.; Ling, Q.; Deng, S.; Li, Z.; Lv, J.; Yang, L.; Cao, C.; Sun, Z.; Zhang, M. Photocatalytic degradation of tetracycline hydrochloride using a BiVO4/MIL-88B(Fe) heterojunction. New J. Chem. 2024, 48, 9442–9456. [Google Scholar] [CrossRef]
- Miersch, L.; Rüffer, T.; Schlesinger, M.; Lang, H.; Mehring, M. Hydrolysis studies on bismuth nitrate: Synthesis and crystallization of four novel polynuclear basic bismuth nitrates. Inorg. Chem. 2012, 51, 9376–9384. [Google Scholar] [CrossRef]
- Pang, J.; Han, Q.; Liu, W.; Shen, Z.; Wang, X.; Zhu, J. Two basic bismuth nitrates: [Bi6O6(OH)2](NO3)4 2H2O with superior photodegradation activity for rhodamine B and [Bi6O5(OH)3](NO3)5·3H2O with ultrahigh adsorption capacity for methyl orange. App. Surf. Sci. 2017, 422, 283–294. [Google Scholar] [CrossRef]
- Dolić, S.D.; Jovanović, D.J.; Smits, K.; Babić, B.; Marinović-Cincović, M.; Porobić, S.; Dramićanin, M.D. A comparative study of photocatalytically active nanocrystalline tetragonal zyrcon-type and monoclinic scheelite-type bismuth vanadate. Ceram. Int. 2018, 44, 17953–17961. [Google Scholar] [CrossRef]
- Dolić, S.D.; Jovanović, D.J.; Štrbac, D.; Đačanin Far, L.J.; Dramićanin, M.D. Improved coloristic properties and high NIR reflectance of environment-friendly yellow pigments based on bismuth vanadate. Ceram. Int. 2018, 44, 22731–22737. [Google Scholar] [CrossRef]
- Marinković, D.; Righini, G.C.; Ferrari, M. Synthesis, optical, and photocatalytic properties of the BiVO4 semiconductor nanoparticles with tetragonal zircon-type structure. Photonics 2025, 12, 438. [Google Scholar] [CrossRef]
- Nguyena, T.D.; Hong, S.-S. Facile solvothermal synthesis of monoclinic-tetragonal heterostructured BiVO4 for photodegradation of rhodamine B. Catal. Commun. 2020, 136, 105920. [Google Scholar] [CrossRef]
- Esmaili, Z.; Sadeghian, Z.; Ashrafizadeh, S.N. Tailoring of BiVO4 morphology for efficient antifouling of visible-light-driven photocatalytic ceramic membranes for oily wastewater treatment. J. Water Process Eng. 2024, 67, 106145. [Google Scholar] [CrossRef]
- Li, J.-Q.; Guo, Z.-Y.; Wang, D.-F.; Lui, H.; Du, J.; Zhu, Z.-F. Effects of pH value on the surface morphology of BiVO4 microspheres and removal of methylene blue under visible light. J. Exp. Nanosci. 2014, 9, 616–624. [Google Scholar] [CrossRef]
- Huang, J.G.; Wang, B.; Pang, S.; Zhang, X.Y.; Yang, X.Y.; Guo, X.T.; Wang, X.S. Effects of pH value and hydrothermal time on the structure and photocatalytic activity of monoclinic-scheelite BiVO4. Optoelectron. Adv. Mater. Rapid Commun. 2015, 9, 1273–1279. [Google Scholar]
- Dong, S.; Xia, L.; Zhang, F.; Li, F.; Wang, Y.; Cui, L.; Feng, J.; Sun, J. Effects of pH value and hydrothermal treatment on the microstructure and natural-sunlight photocatalytic performance of ZnSn(OH)6 photocatalyst. J. Alloys Compd. 2019, 810, 151955. [Google Scholar] [CrossRef]
- Sun, M.; Guo, P.; Wang, M.; Ren, F. The effect of pH on the photocatalytic performance of BiVO4 for phenol mine sewage degradation under visible light. Optik 2019, 179, 672–679. [Google Scholar] [CrossRef]
- Kim, M.-W.; Samuel, E.; Kim, K.; Yoon, H.; Joshi, B.; Swihart, M.T.; Yoon, S.S. Tuning the morphology of electrosprayed BiVO4 from nanopillars to nanoferns via pH control for solar water splitting. J. Alloys Compd. 2018, 769, 193–200. [Google Scholar] [CrossRef]
- Wang, L.; Su, J.; Guo, L. Self-assembly synthesis of monodisperse BiVO4 nanosphere via a hybrid strategy for photoelectrochemical water splitting. ChemCatChem 2020, 12, 5269–5275. [Google Scholar] [CrossRef]
- Li, F.; Yang, C.; Li, Q.; Cao, W.; Li, T. The pH-controlled morphology transition of BiVO4 photocatalysts from microparticles to hollow microspheres. Mater. Lett. 2015, 145, 52–55. [Google Scholar] [CrossRef]
- Sun, W.; Xie, M.; Jing, L.; Luan, Y.; Fu, H. Synthesis of large surface area nano-sized BiVO4 by an EDTA-modified hydrothermal process and its enhanced visible photocatalytic activity. J. Solid State Chem. 2011, 184, 3050–3054. [Google Scholar] [CrossRef]
- Mao, J.; Wu, Q.; Tao, F.; Xu, W.; Hong, T.; Dong, Y. Facile fabrication of porous BiVO4 hollow spheres with improved visible-light photocatalytic properties. RSC Adv. 2020, 10, 6395–6404. [Google Scholar] [CrossRef]
- Yin, W.; Wang, W.; Shang, M.; Zhou, L.; Sun, S.; Wang, L. BiVO4 hollow nanospheres: Anchoring synthesis, growth mechanism, and their application in photocatalysis. EurJIC 2009, 2009, 4379–4384. [Google Scholar] [CrossRef]
- Dong, L.; Guo, S.; Zhu, S.; Xu, D.; Zhang, L.; Huo, M.; Yang, X. Sunlight responsive BiVO4 photocatalyst: Effects of pH on L-cysteine-assisted hydrothermal treatment and enhanced degradation of ofloxacin. Catal. Commun. 2011, 16, 250–254. [Google Scholar] [CrossRef]
- Xia, G.; Ye, J. Synthesis of bismuth vanadate nanoplates with exposed {001} facets and enhanced visible-light photocatalytic properties. Chem. Commun. 2010, 46, 1893–1895. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiang, H.; Zhang, X.; Xing, J.; Guan, Y. Synthesis of hierarchical m-BiVO4 particles via hydro-solvothermal method and their photocatalytic properties. Ceram. Int. 2014, 4, 16485–16493. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Meng, D.; Xing, Y.; Tian, X.; Yu, X.; Xu, K.; Wu, X. Effects of citric acid and urea on the structural and morphological characteristics of BiVO4 synthesized by the sol–gel combustion method. J. Sol-Gel Sci. Technol. 2015, 76, 562–571. [Google Scholar] [CrossRef]
- Adán, C.; Marugán, J.; Obregón, S.; Colón, G. Photocatalytic activity of bismuth vanadates under UV-A and visible light irradiation: Inactivation of Escherichia coli vs oxidation of methanol. Catal. Today 2015, 240, 93–99. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Wang, W.; Jiang, Y.; Cao, K.; Jiao, Z. F-regulate the preparation of polyhedral BiVO4 enclosed by high-index facet and enhance its photocatalytic activity. J. Colloid Interface Sci. 2022, 606, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ravidhas, C.; Josephine, A.J.; Sudhagar, P.; Devadoss, A.; Terashima, C.; Nakata, K.; Fujishima, A.; Raj, A.M.E.; Sanjeeviraja, C.; Pitchaimuthu, S. Facile synthesis of nanostructured monoclinic bismuth vanadate by a co-precipitation method: Structural, optical and photocatalytic properties. Mat. Sci. Semicon. Proc. 2015, 30, 343–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Yang, X.; Yang, H.; Lu, Z.; Chen, R. Monoclinic BiVO4 micro-/nanostructures: Microwave and ultrasonic wave combined synthesis and their visible-light photocatalytic activities. J. Alloys Compd. 2013, 551, 544–550. [Google Scholar] [CrossRef]
- Lin, X.; Yu, L.; Yan, L.; Li, H.; Yan, Y.; Liu, C.; Zhai, H. Visible light photocatalytic activity of BiVO4 particles with different morphologies. Solid State Sci. 2014, 32, 61–66. [Google Scholar] [CrossRef]
- Zhang, H.M.; Liu, J.B.; Wang, H.; Zhang, W.X.; Yan, H. Rapid microwave-assisted synthesis of phase controlled BiVO4 nanocrystals and research on photocatalytic properties under visible light irradiation. J. Nanopart. Res. 2008, 10, 767–774. [Google Scholar] [CrossRef]
- Trinh, D.T.T.; Khanitchaidecha, W.; Channei, D.; Nakaruk, A. Synthesis, characterization and environmental applications of bismuth vanadate. Res. Chem. Intermed. 2019, 45, 5217–5259. [Google Scholar] [CrossRef]
- Wu, S.M.; Jing, Q.; Feng, X.; Chen, L. BiVO4 microstructures with various morphologies: Synthesis and characterization. Appl. Surf. Sci. 2018, 427, 525–532. [Google Scholar] [CrossRef]
- Qurashi, M.M.; Barnes, W.H.A. Preliminary structure for pucherite, BiVO4. Am. Mineral. 1952, 37, 423–426. [Google Scholar]
- Zhou, D.; Pang, L.X.; Wang, D.W.; Reaney, I.M. BiVO4 based high K microwave dielectric materials: A review. J. Mater. Chem. C 2018, 6, 9290–9313. [Google Scholar] [CrossRef]
- Jovanović, D.J.; Chiappini, A.; Zur, L.; Gavrilović, T.V.; Tran, T.N.L.; Chiasera, A.; Lukowiak, A.; Smits, K.; Dramićanin, M.D.; Ferrari, M. Synthesis, structure and spectroscopic properties of luminescent GdVO4:Dy3+ and DyVO4 particles. Opt. Mater. 2018, 76, 308–316. [Google Scholar] [CrossRef]
- Sleight, A.W.; Chen, H.; Ferretti, A.; Cox, D.E. Crystal growth and structure of BiVO4. Mater. Res. Bull. 1979, 14, 1571–1581. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Gea, J.; Wu, L.; Gab, L.; Niu, H.; Liu, M.; Zou, Y.; Wang, J.; Jin, J. Green light all the way: Triple modification synergistic modification effect to enhance the photoelectrochemical water oxidation performance of BiVO4 photoanode. J. Colloid Interface Sci. 2025, 677, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kweon, K.E.; Ye, H.; Paek, E.; Hwang, G.S.; Bard, A.J. Factors in the metal doping of BiVO4 for improved photoelectrocatalytic activity as studied by scanning electrochemical microscopy and first-principles density-functional calculation. J. Phys. Chem. C 2011, 115, 1787017879. [Google Scholar] [CrossRef]
- Abdellaoui, I.; Islam, M.M.; Remeika, M.; Higuchi, Y.; Kawaguchi, T.; Harada, T.; Budich, C.; Maeda, T.; Wada, T.; Ikeda, S.; et al. Photocarrier recombination dynamics in BiVO4 for visible light-driven water oxidation. J. Phys. Chem. C 2020, 124, 39623972. [Google Scholar] [CrossRef]
- Ravensbergen, J.; Abdi, F.F.; van Santen, J.H.; Frese, R.N.; Dam, B.; van de Krol, R.; Kennis, J.T.M. Unraveling the Carrier Dynamics of BiVO4: A femtosecond to microsecond transient absorption study. J. Phys. Chem. C 2014, 118, 2779327800. [Google Scholar] [CrossRef]
- Suzuki, Y.; Murthy, D.H.K.; Matsuzaki, H.; Furube, A.; Wang, Q.; Hisatomi, T.; Domen, K.; Seki, K. Rational interpretation of correlated kinetics of mobile and trapped charge carriers: Analysis of ultrafast carrier dynamics in BiVO4. J. Phys. Chem. C 2017, 121, 1904419052. [Google Scholar] [CrossRef]
- Ma, Y.; Pendlebury, S.R.; Reynal, A.; Formal, L.F.; Durrant, J.R. Dynamics of photo generated holes in undoped BiVO4 photoanodes for solar water oxidation. Chem. Sci. 2014, 5, 29642973. [Google Scholar] [CrossRef]
- Merupo, V.; Velumani, S.; Oza, G.; Makowska-Janusik, M.; Kassiba, A. Structural, electronic and optical features of molybdenum-doped bismuth vanadium oxide. Mater. Sci. Semicond. Process. 2015, 31, 618623. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seo, H. An experimental and density functional theory studies of Nb-doped BiVO4 photoanodes for enhanced solar water splitting. J. Catal. 2022, 410, 144–155. [Google Scholar] [CrossRef]
- Kunioku, H.; Higashi, M.; Tomita, O.; Yabuuchi, M.; Kato, D.; Fujito, H.; Kageyama, H.; Abe, R. Strong hybridization between Bi-6s and O-2p orbitals in Sillén–Aurivillius perovskite Bi4MO8X (M = Nb, Ta; X = Cl, Br), visible light photocatalysts enabling stable water oxidation. J. Mater. Chem. A 2018, 6, 3100–3107. [Google Scholar] [CrossRef]
- Tolod, K.R.; Hernández, S.; Russo, N. Recent advances in the BiVO4 photocatalyst for sun-driven water oxidation: Top-performing photoanodes and scale-up challenges. Catalysts 2017, 7, 13. [Google Scholar] [CrossRef]
- Pramila, S.; Mallikarjunaswamy, C.; Ranganatha, V.L.; Nagaraju, G. BiVO4 nanoballs: A simple precipitation pathway, promising electrochemical sensor, and photodegradation under visible light. Ionics 2024, 30, 2819–2838. [Google Scholar] [CrossRef]
- Aghakhaninejad, S.; Rahimi, R.; Zargari, S. Application of BiVO4 nanocomposite for photodegradation of methyl orange. Proceedings 2019, 9, 52. [Google Scholar] [CrossRef]
- Sobahi, T.R. Application of BiVO4-MWCNT nanocomposites for boosted photocatalytic oxidation of atrazine under visible light. Appl. Nanosci. 2021, 11, 2811–2822. [Google Scholar] [CrossRef]
- Chopade, A.S.; Kolhe, N.D.; Walekar, L.S.; Kadam, A.N.; Patil, V.A.; Al-Enizi, A.M.; Tamboli, M.S.; Mhamane, D.S.; Mali, M.G. Chemical bath assisted construction of CuO/BiVO4 p-n heterojunction photocatalyst for visible light driven rapid removal of MB and Cr(VI). Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134665. [Google Scholar] [CrossRef]
- Liapun, V.; Hanif, M.B.; Sihor, M.; Vislocka, X.; Pandiaraj, S.; Unnikrishnan, V.K.; Thirunavukkarasu, G.K.; Edelmannová, M.F.; Reli, M.; Monfort, O.; et al. Versatile application of BiVO4/TiO2 S-scheme photocatalyst: Photocatalytic CO2 and Cr(VI) reduction. Chemosphere 2023, 337, 139397. [Google Scholar] [CrossRef]
- Jing, Q.; Feng, X.; Zhao, X.; Duan, Z.; Pan, J.; Chen, L.; Liu, Y. Bi/BiVO4 chainlike hollow microstructures: Synthesis, characterization, and application as visible-light-active photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2653–2661. [Google Scholar] [CrossRef]
- Shi, H.; Li, C.; Zheng, R.; Wang, L.; Wang, W.; Bian, J.; Meng, X. Synergistic effect of oxygen vacancies and built-in electric field in GdCrO3/BiVO4 composites for boosted photocatalytic reduction of nitrate in water. J. Clean. Prod. 2023, 407, 137088. [Google Scholar] [CrossRef]
- Jeon, H.C.; Kumar, G.M.; Lee, D.J.; Sekar, S.; Kim, D.Y.; Ilanchezhiyan, P. Fabrication of 2D/2D InVO4/BiVO4 heterojunction with synergistic effects for enhanced photocatalytic degradation and photoelectrochemical applications. Int. J. Energy Res. 2024, 1, 1–12. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. Towards visible light driven photoelectrocatalysis for water treatment: Application of a FTO/BiVO4/Ag2S heterojunction anode for the removal of emerging pharmaceutical pollutants. Sci. Rep. 2020, 10, 5348. [Google Scholar] [CrossRef]
- Alhaddad, M.; Amin, M.S. Removal of ciprofloxacin applying Pt@BiVO4-g-C3N4 nanocomposite under visible light. Opt. Mater. 2022, 124, 111976. [Google Scholar] [CrossRef]
- Kumar, M.; Gaur, A.; Chauhan, V.S.; Vaish, R.; Kebaili, I. Tribocatalytic dye degradation using BiVO4. Ceram. Int. 2024, 50, 8360–8369. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, S.; Wang, D.; Hogan, J.; Sadrzadeh, M. CTAC-assisted monoclinic BiVO4 with oxygen defects for efficient photocatalytic performances: A combined experimental and DFT study. J. Alloys Compd. 2024, 990, 174404. [Google Scholar] [CrossRef]
- Ge, Y.-D.; Xing, M.-Y.; Zhang, H.-S.; Zhou, X.-Y.; Zhang, L.; Wang, X.; Xu, L. Study on the development of an innovative type-II sonocatalyst of BiVO4/FeVO4 and its application in sonocatalytic removal of tetracycline. J. Alloys Compd. 2025, 1018, 179089. [Google Scholar] [CrossRef]
- Obregón, S.; Caballero, A.; Colón, G. Hydrothermal synthesis of BiVO4: Structural and morphological influence on the photocatalytic activity. Appl. Catal. B Environ. 2012, 117–118, 59–66. [Google Scholar] [CrossRef]
- Ying, Y.; Tao, F.; Hong, T.; Wang, L. Controlled fabrication of bismuth vanadium oxide hierarchical microtubes with enhanced visible light photocatalytic activity. Mat. Sci. Semicon. Proc. 2015, 32, 82–89. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Y.-S.; Xiao, H.-M.; Fu, S.-Y. Novel core–shell structured BiVO4 hollow spheres with an ultra-high surface area as visible-light-driven catalyst. CrystEngComm 2014, 16, 6059–6065. [Google Scholar] [CrossRef]
- Cai, H.; Cheng, L.; Chen, H.; Dou, R.; Chen, J.; Zhao, Y.; Li, F.; Fang, Z. Facile phase control and photocatalytic performance of BiVO4 crystals for methylene blue degradation. Int. J. Environ. Res. Public Health 2023, 20, 3093. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Roškarič, M.; Žerjav, G.; Finšgar, M.; Zavašnik, J.; Pintar, A. Influence of the calcination duration of g-C3N4/TiO2 “veggie-toast-like” photocatalyst on the visible-light triggered photocatalytic oxidation of bisphenol A. J. Alloys Compd. 2023, 947, 169585. [Google Scholar] [CrossRef]
- Vasilić, R.; Stojadinović, S.; Radić, N.; Stefanov, P.; Dohčević-Mitrović, Z.; Grbić, B. One-step preparation and photocatalytic performance of vanadium doped TiO2 coatings. Mater. Chem. Phys. 2015, 151, 337–344. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Yu, K.; Song, K.; Wang, X.; Liu, W.; Hou, J.; Liang, C. Enhanced photocatalytic performance of Nd3+-doped TiO2 nanosphere under visible light. Chem. Phys. 2020, 528, 110538. [Google Scholar] [CrossRef]
- Ücker, C.L.; Goetzke, V.; Almeida, S.R.; Moreira, E.C.; Ferrer, M.M.; Jardim, P.L.G.; Moreira, M.L.; Raubach, C.W.; Cava, S. Photocatalytic degradation of rhodamine B using Nb2O5 synthesized with different niobium precursors: Factorial design of experiments. Ceram. Int. 2021, 47, 20570–20578. [Google Scholar] [CrossRef]
- An, G.W.; Mahadik, M.A.; Piao, G.; Chae, W.-S.; Park, H.; Cho, M.; Chung, H.-S.; Jang, J.S. Hierarchical TiO2@In2O3 heteroarchitecture photoanodes: Mechanistic study on interfacial charge carrier dynamics through water splitting and organic decomposition. Appl. Surf. Sci. 2019, 480, 1–12. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO: Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Ekthammathat, N.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Synthesis, characterization and antibacterial activity of BiVO4 microstructure. Russ. J. Phys. Chem. 2018, 92, 1036–1040. [Google Scholar] [CrossRef]
- Sharma, R.; Uma; Singh, S.; Verma, A.; Khanuja, M. Visible light induced bactericidal and photocatalytic activity of hydrothermally synthesized BiVO4 nano-octahedrals. J. Photochem. Photobiol. B Biol. 2016, 162, 266–272. [Google Scholar] [CrossRef]
- Saleem, A.; Ahmed, T.; Ammar, M.; Zhang, H.; Xu, H.; Tabassum, R. Direct growth of m-BiVO4@carbon fibers for highly efficient and recyclable photocatalytic and antibacterial applications. J. Photochem. Photobiol. B Biol. 2020, 213, 112070. [Google Scholar] [CrossRef]
- Iqbal, T.; Qureshi, M.T.; Shahzad, R.; Afsheen, S.; Kausar, S.; Yunus, G.; Mansha, M.S.; Aamir, L.; Munir, R.M.; El-Serehy, H.A.; et al. Synergistic impacts of novel tantalum doping in BiVO4 for effective photocatalytic applications, antimicrobial activity, and antioxidant aspect. Inorg. Chem. Commun. 2024, 170, 113365. [Google Scholar] [CrossRef]
- Bulut, D.T. Exploring the dual role of BiVO4 nanoparticles: Unveiling enhanced antimicrobial efficacy and photocatalytic performance. J. Sol-Gel Sci. Technol. 2025, 114, 198–222. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Kannan, N.; Venkatesh, P.S.; Paulraj, G.; Jeganathan, K.; Ali, D.M. Synthesis and characterization of BiVO4 nanoparticles for environmental applications. RSC Adv. 2020, 10, 18315–18322. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Larumbe, S.; Gil, A.; Muñoz, D.; Fernández, L.R.; Barquín, L.F.; García-Prieto, A.; Fdez-Gubieda, M.L.; Muela, A. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surf. Interfac. 2021, 22, 100867. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Pósfai, M.; Makó, É.; Juzsakova, T.; Fónagy, O. The photocatalytic and antibacterial performance of nitrogen-doped TiO2: Surface-structure dependence and silver-deposition effect. Nanomaterials 2020, 10, 2261. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, B.; Reddy, A.S.S.; Prasad, P.S.; Prasad, A.; Devi, P.P.K.; Kumar, V.R.; Veeraiah, N. The impact of Nb2O5 on in-vitro bioactivity and antibacterial activity of CaF2–CaO–B2O3–P2O5–SrO glass system. Ceram. Int. 2021, 47, 28328–28337. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Pandey, S.; Jeong, D.; Lee, C.-T.; Do, J.Y.; Park, S.-M.; Kang, M. Effective antibacterial/photocatalytic activity of ZnO nanomaterials synthesized under low temperature and alkaline conditions. Nanomaterials 2022, 12, 4417. [Google Scholar] [CrossRef]
- Abid, H.N.; Ahmed, D.S.; Al-keisy, A.H. Constructed p-2D/n-2D BiOCl/BiVO4 nanoheterostructure for photocatalytic antibacterial activity. ECS Trans. 2022, 107, 2283. [Google Scholar] [CrossRef]
- Li, B.; Gao, X.; Qu, J.; Xiong, F.; Xuan, H.; Jin, Y.; Yuan, H. Visible-light-driven antimicrobial activity and mechanism of polydopamine-reduced graphene oxide/BiVO4 composite. Int. J. Mol. Sci. 2022, 23, 7712. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Chen, H.; Bai, X.; Bi, S.; Jiang, H.; Cai, G.; Cheng, D.; Wang, X. Immobilizing CuO/BiVO4 nanocomposite on PDA-templated cotton fabric for visible light photocatalysis, antimicrobial activity and UV protection. Appl. Surf. Sci. 2019, 493, 1167–1176. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Z.; Chen, X.; Cheng, B.; Ou, W. Water purification using a BiVO4/graphene oxide multifunctional hydrogel based on interfacial adsorption-enrichment and photocatalytic antibacterial activity. Ceram. Int. 2023, 49, 9657–9671. [Google Scholar] [CrossRef]
- Pramila, S.; Nagaraju, G.; Mallikarjunaswamy, C.; Latha, K.C.; Chandan, S.; Ramu, R.; Rashmi, V.; Ranganatha, V.L. Green synthesis of BiVO4 nanoparticles by microwave method using Aegle Marmelos juice as a fuel: Photocatalytic and antimicrobial study. Anal. Chem. Lett. 2020, 10, 298–306. [Google Scholar] [CrossRef]
- Wang, L.; Han, D.; Ni, S.; Ma, W.; Wang, W.; Niu, L. Photoelectrochemical device based on Mo-doped BiVO4 enables smart analysis of the global antioxidant capacity in food. Chem. Sci. 2015, 6, 6632–6638. [Google Scholar] [CrossRef]
- Han, F.; Luo, S.; Wu, Z.; Liang, Z.; Yang, W.; Han, D.; Sun, Z.; Liu, Z.; Niu, L. A label-free photoelectrochemical sensor based on BiVO4@graphene oxide hybrid for analysis of the antioxidant capacity in food. J. Electroanal. Chem. 2023, 946, 117713. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; He, L.; Bu, Y. Crystal-reconstructed BiVO4 semiconductor photoelectrochemical sensor for ultra-sensitive tumor biomarker detection. J. Mater. Chem. B 2022, 10, 870–879. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Savickaja, I.; Kovger-Jarosevic, J.; Skruodiene, M.; Juodkazyte, J.; Ramanavicius, S.; Ramanavicius, A. BiVO4-based photoelectrochemical sensors for the detection of diclofenac: The role of doping, electrolytes and applied potentials. Chemosensors 2024, 12, 249. [Google Scholar] [CrossRef]
- Wu, W.; Tan, Z.; Chen, X.; Chen, X.; Cheng, L.; Wu, H.; Li, P.; Zhang, Z. Carnation-like morphology of BiVO4-7 enables sensitive photoelectrochemical determination of Cr(VI) in the food and environment. Biosensors 2022, 12, 130. [Google Scholar] [CrossRef]
- Ye, C.; Xu, S.; Wu, Z.; Wang, M. Cu3(PO4)2/BiVO4 photoelectrochemical sensor for sensitive and selective determination of synthetic antioxidant propyl gallate. Anal. Bioanal. Chem. 2022, 414, 4139–4147. [Google Scholar] [CrossRef]
- Murugan, E.; Poongan, A. A new sensitive electrochemical sensor based on BiVO4/ZrO2@graphene modified GCE for concurrent sensing of acetaminophen, phenylephrine hydrochloride and cytosine in medications and human serum samples. Diam. Relat. Mater. 2022, 126, 109117. [Google Scholar] [CrossRef]
- Sarikaya, I.; Kaleoglu, E.; Çakar, S.; Soykan, C.; Özacar, M. An enhanced photosensitive sensor based on ITO/MWCNTs@polymercomposite@BiVO4 for quercetin detection. Biosensors 2023, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.B.; Cao, Q.; Huang, L.; Zhou, C.H.; Ling, J.; Hu, R.; Yang, T. Wavelength-dependent photoelectrochemical sensor array based on Bi2WO6/TiO2 electrospun nanoheterojunction for multiple antioxidants identification and total antioxidant capacity analysis in natural tea. Microchem. J. 2024, 206, 111568. [Google Scholar] [CrossRef]
- Nurdin, M.; Maulidiyah, M.; Salim, L.O.A.; Muzakkar, M.Z.; Umar, A.A. High performance cypermethrin pesticide detection using anatase TiO2-carbon paste nanocomposites electrode. Microchem. J. 2019, 145, 756–761. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of tartrazine based on TiO2-electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef]
- Mai, Q.-D.; Thanh, D.C.; Anh, N.T.; Manh, T.V.; Bach, T.N.; Nguyen, H.-A.; Pham, A.-T.; Le, A.-T. Smart 3D Ag-decorated TiO2 nanostructure: An advanced synergistic SERS substrate for trace detection of analytes with diverse natures. Sens. Actuators B Chem. 2024, 410, 135651. [Google Scholar] [CrossRef]
- Bruce, J.; Bosnick, K.; Heidari, E.K. Pd-decorated ZnO nanoflowers as a promising gas sensor for the detection of meat spoilage. Sens. Actuators B Chem. 2022, 355, 131316. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Panamaldeniya, S.A.; Maraloiu, V.A.; Dassanayake, B.S.; Gunawardhana, N.; Comini, E. Nb2O5 microcolumns for ethanol sensing. Sensors 2024, 24, 1851. [Google Scholar] [CrossRef] [PubMed]
- Mokrushin, A.S.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Kadyrov, N.C.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Chemoresistive gas-sensing properties of highly dispersed Nb2O5 obtained by programmable precipitation. J. Alloys Compd. 2021, 868, 159090. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Z.; Lin, X.; Han, F.; Liang, Z.; Huang, L.; Dai, M.; Han, D.; Han, L.; Niu, L. A label-free PEC aptasensor platform based on g-C3N4/BiVO4 heterojunction for tetracycline detection in food analysis. Food Chem. 2023, 402, 134258. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Qi, Y.; Zhang, J.; Wang, N.; Liu, M.; Zhang, B.; Cai, X.; Zhang, H.; Wei, S.; et al. Etched BiVO4 photocatalyst with charge separation efficiency exceeding 90%. Nat. Commun. 2025, 16, 3776. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef]
- Wang, S.; Wan, K.; Feng, J.; Yang, Y.; Wang, S. BiVO4 photoanodes with enhanced photoelectrochemical performance: Preparation, modification and emerging applications. J. Mater. Sci. Tech. 2025, 217, 182–220. [Google Scholar] [CrossRef]
- Huang, J.; Lin, T.; Lin, L.; Ma, G.; Zhang, Z.; Handschuh-Wang, S.; Meng, A.; Han, P.; He, B. Boosted charge transport efficiency for bismuth and oxygen dual vacancy-engineered BiVO4 photoanodes. ACS Appl. Energy Mater. 2024, 7, 10710–10720. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Zhang, Y.; Wan, K.; Xu, L.; Ma, S.; Zhao, R.; Wang, S.; Huang, W. Bismuth vacancies induced lattice strain in BiVO4 photoanodes boosting charge separation for water oxidation. Adv. Energy Mater. 2025, 15, 2403835. [Google Scholar] [CrossRef]
- Yi, Q.; Wang, H.; Lee, J.-M. BiVO4-based photoelectrochemical water splitting. ChemElectroChem 2025, 12, e202400600. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Nasir, J.A.; Munir, A.; Ahmad, N.; Haq, T.; Khan, Z.; Rehman, Z. Photocatalytic Z-scheme overall water splitting: Recent advances in theory and experiments. Adv. Mater. 2021, 33, 2105195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Jeong, Y.J.; Tan, R.; Saravanan, I.; Han, H.S.; Kim, D.H.; Cho, I.S. Texture development and surface reconstruction of BiVO4 photoanode via one-pot hydrothermal reaction for enhanced photoelectrochemical water splitting. J. Adv. Ceram. 2025, 14, 9221043. [Google Scholar] [CrossRef]
- Chi, J.; Wei, Z.; Fang, W.; Yan, J.; Wang, J.; Zu, H.; Cheng, J.; Luo, H.; Ye, Z.; Liu, J.; et al. Octadecahedral BiVO4 with exposed high-reactivity {121} facets for enhanced photoelectrochemical water splitting. Appl. Catal. B Environ. 2025, 365, 124973. [Google Scholar] [CrossRef]
- Xiao, C.; Assavachin, S.; Hahn, W.; Wang, L.; van Benthem, K.; Osterloh, F.E. Flux synthesis of single crystal bismuth vanadate (BiVO4) nanowires and their visible light driven photocatalytic water oxidation properties. J. Mater. Chem. A 2025, 13, 7834–7844. [Google Scholar] [CrossRef]
- Veetil, S.P.; Vattakkoval, N.; Veedu, S.K.; Vijayan, B.K. Supercapacitor applications of cerium doped BiVO4 nanosheets as electrode materials. AIP Conf. Proc. 2025, 3198, 020131. [Google Scholar] [CrossRef]
- Wan, X.; Luo, X.; Lu, D.; Liu, G.; Fu, Y.; Cai, L.; Hu, C.; Wan, H. Controllable fabrication of Cu: BiVO4 nanostructures via a two-step electrodeposition strategy for efficient photoelectrochemical water splitting. J. Alloys Compd. 2025, 1010, 177903. [Google Scholar] [CrossRef]
- Wang, H.; Bai, Y.; Wang, R.; Fu, Y.; Mei, Q.; Bai, B.; Wang, Q. Boosting photoelectrochemical water splitting: Enhanced hole transport in BiVO4 photoanodes via interfacial coupling. Catal. Sci. Technol. 2025, 15, 405–415. [Google Scholar] [CrossRef]
- Li, Q.; Cui, X.; Liu, X.; Wang, W. Ti3C2 quantum dots decorated BiVO4 photoelectrode for both photoelectrochemical water splitting and H2O2 determination. J. Alloys Compd. 2025, 1011, 178399. [Google Scholar] [CrossRef]
- Li, H.; Lyu, M.; Lai, Y.; Cheng, X.; Dong, Z. For effective water splitting by inserting a p-type copper thiocyanate hole transfer layer between BiVO4 and Ni-doped FeOOH cocatalyst. Int. J. Hydrogen Energy 2025, 106, 1006–1015. [Google Scholar] [CrossRef]
- Saad, A.M.; Mady, A.H.; Sayed, M.S.; Kim, G.; Kim, M.; Kim, W.K. Boosting water oxidation kinetics of BiVO4 through a metal-organic co-catalyst enriched with phosphate groups (Co,Fe-NTMP): Insights from LMCT mechanism and DFT study. Appl. Catal. B Environ. Energy 2025, 370, 125163. [Google Scholar] [CrossRef]
- Kavitha, T.; Rojviroon, O.; Rajendran, R.; Rojviroon, T. Construction of hybrid 2D g-C3N4/BiVO4 photocatalyst decorated with RGO for enhancing the H2 production and photocatalytic degradation of antibiotics. J. Porous Mater. 2025, 32, 1457–1469. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, R.; Li, X.; Sun, F.; Ge, M.; Huang, N.; Zhao, Y.; Chang, Z.; Wang, H. Photoreduced Ag nanoparticles-decorated BiVO4 nanoplates as photoanode boosting photoelectrochemical H2O2 fuel cell performance. J. Power Sources 2025, 629, 235998. [Google Scholar] [CrossRef]
- Andrei, V.; Chiang, Y.-H.; Rahaman, M.; Anaya, M.; Kang, T.; Ruggeri, E.; Stranks, S.D.; Reisner, E. Modular perovskite-BiVO4 artificial leaves towards syngas synthesis on a m2 scale. Energy Environ. Sci. 2025, 18, 3623–3632. [Google Scholar] [CrossRef]
- Fu, H.; Wu, Y.; Guo, Y.; Sakurai, T.; Zhang, Q.; Liu, Y.; Zheng, Z.; Cheng, H.; Wang, Z.; Huang, B.; et al. A scalable solar-driven photocatalytic system for separated H2 and O2 production from water. Nat. Commun. 2025, 16, 990. [Google Scholar] [CrossRef] [PubMed]

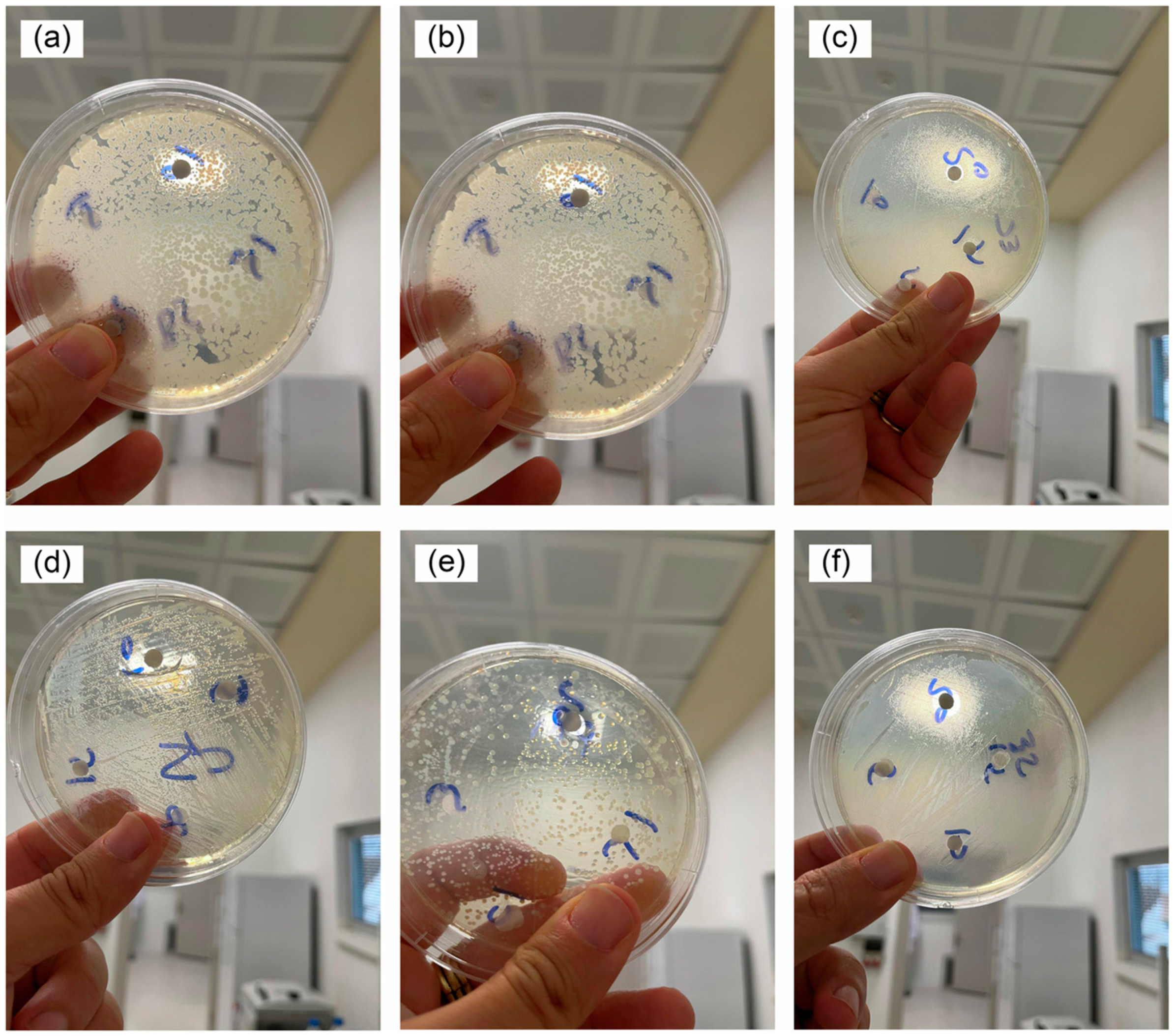

| Catalysts/Dose/Morphology | (Photocatalytic Parameters) Dye */Concentration/Light Source | Degradation Efficiency/Time | Ref. |
|---|---|---|---|
| BiVO4/1.0 mgmL−1/peanut | MB/10 mgL−1/Xenon lamp with 35 W/m2 | 40%/120 min | [141] |
| BiVO4/1.0 mgmL−1/microtube | MO/20 mgL−1/250 W Xenon arc lamp | 95%/180 min | [142] |
| BiVO4/0.5 mgmL−1/spheres | RhB/10−5 molL−1/500 W Xenon lamp | 27%/150 min | [143] |
| BiVO4/0.5 mgmL−1/biscuits | RhB/10−5 molL−1/500 W Xenon lamp | 44%/150 min | [143] |
| tz-BiVO4/1.0 mgmL−1/spherical particles | MB/20 mgL−1/550 W Xenon lamp | 20%/150 min | [144] |
| ms-BiVO4/1.0 mgmL−1/spherical particles | MB/20 mgL−1/550 W Xenon lamp | 45%/150 min | [144] |
| tz-BiVO4/1.0 gL−1/nanoparticles | MO/5 mgL−1/300 W Osram Ultra-Vitalux lamp | 100%/240 min | [86] |
| ms-BiVO4/1.0 gL−1/nanoparticles | MO/5 mgL−1/300 W Osram Ultra-Vitalux lamp | 30–35%/240 min | [86] |
| BiVO4/1 mgmL−1/hollow nanosphere | RhB/10−5 M/500 W Xe lamp | 100%/70 min | [145] |
| BiVO4/1 mgmL−1/microtubes | MO/20 mgL−1/250 W Xe lamp | 95%/180 min | [145] |
| TiO2 (Commercially)/0.125 mgL−1/- | BPA/10mgL−1/150 W Hmminminmininaminlogen lamp | 9%/120 min | [146] |
| g-C3N4-TiO2/0.125 mgL−1/veggie-toast-like | BPA/10mgL−1/150 W Halogen lamp | 21.5%/120 min | [146] |
| g-C3N4/0.125 mgL−1/“cheese” | BPA/10mgL−1/150 W Halogen lamp | 11.4%/120 min | [146] |
| V2O5/TiO2 coatings (anatase phase)/15 mm × 10 mm (active surface)/microdischarges | MO/8 mgL−1/Sunlight | 35%/480 min | [147] |
| Nd3+-TiO2/0.05 g/nanosphere | MB/20 mgL−1/Visible light | 91.83%/120 min | [148] |
| Nd3+-TiO2/0.05 g/nanosphere | MB/20 mgL−1/Sunlight | 99.14%/80 min | [148] |
| Nb2O5/50 mg/particle clusters | RhB/1 ×10 −5 M/6 UVC lamps (15 W each lamp totaling the power of 90 W-TUV Philips) | 98.99%/60 min | [149] |
| In2O3-TiO2/1 × 1 cm2 area/nanorods | MO/10 µM/Sunlight | 86%/360 min | [150] |
| TiO2-Degussa (P25)/1 mgmL−1/- | MB MO | 35%/60 min 35%/60 min | [151] |
| ZnO:Eu(10%)/1 mgmL−1/nanoparticles | MO | 100%/60 min | [151] |
| Material BiVO4 | |||
|---|---|---|---|
| Crystal Forms/Band Gap (Eg) | Ref. | ||
| monoclinic-scheelite (ms)/2.40 eV space group: I2/b; a = 5.1935, b = 5.0898, c = 11.6972 Å, β = 90.3871° | tetragonal-zircon (tz)/2.90 eV space group: I41/amd a = b = 7.303 and c = 6.584 Å | tetragonal-scheelite (ts)/2.34 eV space group: I41/a a = b = 5.1470, c = 11.7216 Å | [3,4] |
| Properties | |||
| stability, physicochemical, dielectric, ferroelasticity, semiconductivity, photocatalytic, antibacterial | [86,87,114,125,141,142,143,144,145,146,147,148,149,150,151,156,189,190] | ||
| Method of synthesis | Morphology | ||
| Co-precipitation; micro-emulsion; hydrothermal synthesis with and without surfactant or template; rapid microwave-assisted process; microwave-assisted hydrothermal method and solvothermal approach. | Ellipsoidal; highly uniform monodisperse nanospheres; irregular spheres; hollow spheres; nanorods; needles; irregular dog-bone; butterfly, leaf peanut roundish aggregates; polyhedral; decagonal shape rods; potato and broccoli-like; bowknot; dumbbell-like; spherical nanoparticles. | [11,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] [45,56,57,63,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] | |
| Advantages/Applications | Disadvantages/Future work | ||
| Narrow band gap; yellow color of powder; pigmentation; cost-effectiveness; ease of synthesis; photocatalytic water splitting; photocatalytic degradation of air/water pollutants; reduction in CO2 and heavy metal ions; antibacterial agents; antioxidant, food safety monitoring; gas sensor; PEC sensor; H2 production; electrodes. | Fast recombination of photogenerated electron–hole pairs; developing novel synthesis techniques; industrial-scale production; improving the charge separation efficiency | [6,15,16,27,28,29,31,50,60,71,77,167,168,204] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinković, D.; Righini, G.C.; Ferrari, M. Advances in Synthesis and Applications of Bismuth Vanadate-Based Structures. Inorganics 2025, 13, 268. https://doi.org/10.3390/inorganics13080268
Marinković D, Righini GC, Ferrari M. Advances in Synthesis and Applications of Bismuth Vanadate-Based Structures. Inorganics. 2025; 13(8):268. https://doi.org/10.3390/inorganics13080268
Chicago/Turabian StyleMarinković, Dragana, Giancarlo C. Righini, and Maurizio Ferrari. 2025. "Advances in Synthesis and Applications of Bismuth Vanadate-Based Structures" Inorganics 13, no. 8: 268. https://doi.org/10.3390/inorganics13080268
APA StyleMarinković, D., Righini, G. C., & Ferrari, M. (2025). Advances in Synthesis and Applications of Bismuth Vanadate-Based Structures. Inorganics, 13(8), 268. https://doi.org/10.3390/inorganics13080268








