Abstract
The aim of this paper is to obtain ethyl (2-(methylcarbamoyl)phenyl)carbamate and its metal complexes as promising antimicrobial agents. The title compound was synthesized using the ring-opening of isatoic anhydride with methylamine and further acylation with ethyl chloroformate. All metal complexes were successfully obtained after mixing the ligand dissolved in DMSO and water solutions of the corresponding metal salts and sodium hydroxide, in a metal-to-ligand-to base ratio 1:2:2. As a result, mixed ligand complexes of ethyl 2-(methylcarbamoyl)phenyl)carbamate and 3-methylquinazoline-2,4(1H,3H)-dione were obtained. The obtained complexes were characterized by their melting points, FTIR, NMR spectroscopy, and MP-AES. Then, the antimicrobial effect of the compounds against both Gram-negative and Gram-positive bacteria, yeasts, and fungi was studied. Only the Co(II) complex showed antimicrobial activity against almost all Gram-positive and Gram-negative bacteria. The cobalt complex exhibited promising antimicrobial activity against Gram-positive Micrococcus luteus with inhibition zones of 20 mm, Listeria monocytogenes (15 mm), Staphylococcus aureus (13 mm), as well as Gram-negative Klebsiella pneumoniae (13 mm) and Proteus vulgaris (13 mm). Given the potential of metal complexes as antimicrobial agents, understanding their cytotoxic effects is crucial for evaluating their therapeutic safety. To assess the in vitro biocompatibility of the experimental compounds, a range of cell viability assays was conducted using human malignant leukemic cell lines (LAMA-84, K-562) and normal murine fibroblast cells (CCL-1). The Ni(II) complex shows IC50 = 105.1 µM against human malignant leukemic cell lines LAMA-84. Based on the reported results, it may be concluded that the mixed cobalt complex of 2-(methylcarbamoyl)phenyl)carbamate and 3-methylquinazoline-2,4(1H,3H)-dione can be attributed as a promising antimicrobial agent. Future in vivo tests will contribute to establishing the antimicrobial properties of this complex.
1. Introduction
The membrane-like extracellular matrix formed by the attachment of bacterial colonies and extracellular polymeric materials, such as polysaccharides, nucleic acids, and proteins generated by bacteria during growth, is referred to as a biofilm, which is an organized population of bacteria [1]. EPS interacts with bacterial aggregates to give the biofilm cohesiveness and viscoelastic qualities [2]. Bacteria can therefore stick to the surfaces of both living and non-living things. Chronic persistent infections are largely caused by the production of pathogenic biofilms [3]. Researchers currently believe that bacterial biofilms are responsible for more than 80% of persistent illnesses [4].
Staphylococcus aureus, a drug-resistant pathogen, can lead to skin and soft tissue infections, as well as serious conditions like endocarditis, osteomyelitis, pneumonia, and other invasive diseases [5,6,7,8,9,10].
Listeria monocytogenes, a Gram-positive rod-shaped bacterium, is recognized for causing listeriosis upon infection [11]. This pathogen shows an extraordinary ability to withstand various external conditions, such as high salt concentrations and both acidic and alkaline environments [12]. It can multiply within a temperature range of 0–45 °C and remain viable under refrigeration for long periods, earning it the nickname “refrigerator killer” because of its potential danger to human health [13]. In human beings, L. monocytogenes infection can result in serious clinical consequences such as bacteremia, monocytopenia, meningitis, among others [14,15]. L. monocytogenes can penetrate the body’s defense mechanisms, leading to a death rate of 20–40% among those infected [16].
One of the main causes of nosocomial infections, including pneumonia, bacteremia, liver abscesses, and meningitis, is the gram-negative bacteria Klebsiella pneumoniae. Treatment options for K. pneumoniae infections are limited due to the recent rise in antibiotic resistance among isolates and the appearance of highly invasive hypervirulent strains [17,18].
Furthermore, it is thought that bacteria may adapt to severe settings by forming biofilms, which is a protected growth mode [19]. The biofilm protects bacterial cells against harmful factors such as high temperatures, a lack of nutrients, dehydration, and even antibacterial chemicals [20]. It also creates a stable internal environment for bacterial cell activity. The development of drug resistance depends on biofilm formation in addition to the diverse array of secreted virulence factors [21]. The microbial environment can benefit greatly from biofilm, which can enhance strain resistance by up to 1500 times [22].
Consequently, it is crucial to create new antibiofilm agents targeting Klebsiella pneumoniae, L. monocytogenes, and S. aureus.
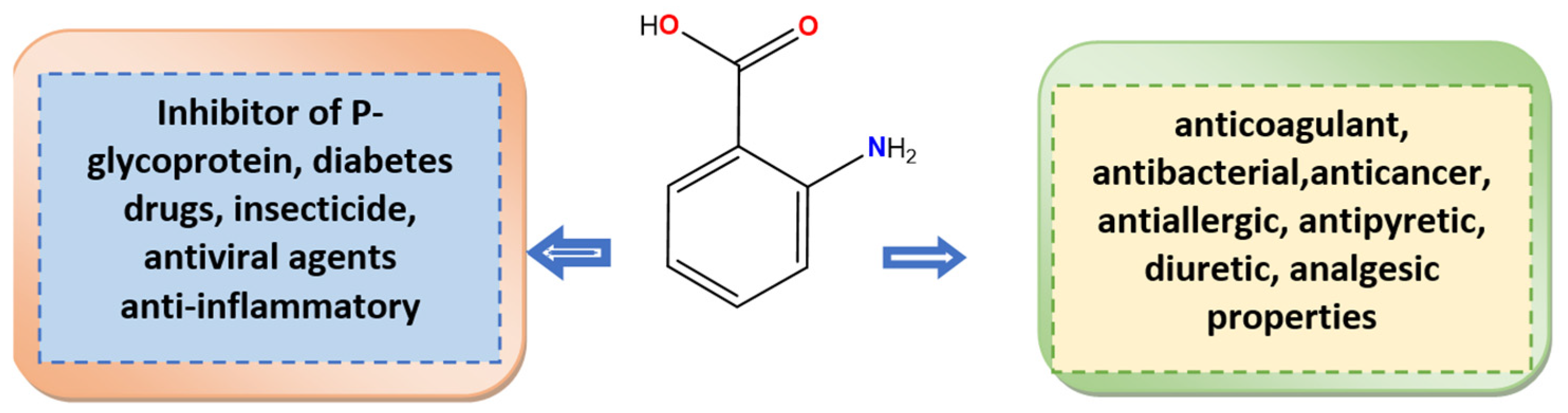
Anthranilic acid analogues and their derivatives hold great promise for developing compounds for targeted antitumor activity, tackling metabolic issues related to diabetes [23,24], acting as antimicrobial and antiviral agents [25], and serving as biologically compatible anti-inflammatory agents [26,27]. Anthranilic acid is also capable of forming coordination complexes with various metals [28,29].
Some of the application areas of anthranilic acid and its metal complexes are highlighted in Figure 1.
Figure 1.
Anthranilic acid, its biological activities, and pharmaceutical applications.
The anthranilic acid complexes with chromium [30], cobalt, nickel, and zinc [31,32] were obtained. Buvaylo et al. have obtained a new neutral CoII complex with dimethylformamide (DMF) solvent molecules [33]. Recently, Cheng Xiao-Xue et al. used an azo-coupling reaction between 4-aminobenzoic acid and anthranilic acid, salicylaldehyde, and 2-naphthol to create nanostructured cobalt(II) metalorganic complexes [34]. Metal complexes of nano-anthranilic acid with various metal ions, including Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Mn(II), Bi(III), Al(III), and Cr(III), were successfully synthesized and characterized, as reported in reference [35]. El-Ajaily and Morad obtained cobalt(II) and copper(II) Schiff base complexes of salicylaldehyde and anthranilic acid [36]. Different variants of chelation [30,31,32,33] or bridge coordination [37] are present in the literature. The oxalate-bridged binuclear metal complexes with copper(II) and nickel(II) were synthesized by Saghatforoush et al. [37]. Preclinical pharmacological screenings for synthetic metal complexes’ antibacterial, antifungal, anti-inflammatory, anticancer, DNA interaction, and antitumor properties have been a goal of researchers over the past few decades [38].
The biological properties and synthesis of new complexes with anthranilic acid were reported recently [39]. Mixed-ligand metal complexes of Co(II), Ni(II), Cu(II), Zn(II), Cd(II), and Hg(II) were synthesized using a Schiff base ligand N,N′-Bis-(4-dimethylamino-benzylidene)-benzene-1,4-diamine in combination with anthranilic acid [40]. The anthranilic acid, its derivatives, and their metal complexes were found to have anti-inflammatory properties [41,42], cytotoxicity [43], and antimicrobial activities [26,35,44,45,46,47,48,49]. They are also inhibitors of aldo-keto reductase enzymes [50].
Therefore, the current study aims to synthesize an anthranilic acid derivative, its nickel and cobalt complexes, and to study their antimicrobial properties.
2. Results and Discussion
Anthranilic acid (Figure 1) and its analogs play a crucial role in numerous bioactive substances and medications, exhibiting diverse biological effects in the treatment of various diseases [51,52,53,54]. Functional groups, such as -COOH, -NH2, -CONH2, -COOCH3, and -NHCOCH3 assist conjugation for interaction with biological targets [55,56,57]. In order to determine the best pharmacophores, the functional groups serve as the sites for molecule customization and the analysis of the structure-activity connection of anthranilic acid-based libraries.
Esters could be found naturally in propolis, which demonstrates important biological activities, such as neuroprotective activity [58]. The ester functional group was also found to possess antiproliferative and antimetastatic effects on human lung adenocarcinoma cells [59]. The design and synthesis of prodrugs for nonsteroidal anti-inflammatory drugs has been a major focus of medicinal chemists, especially in the last ten years. Anti-inflammatory medications are among the most widely used drug classes. To shield the gastrointestinal tract from local irritation, the majority of the work put into creating ester prodrugs was focused on hiding the molecules’ free acidic groups [60]. Some of them, such as salsalate, flurbiprofen axetil, etc., contain the ester functional group. Excellent analgesic and anti-inflammatory prodrugs were shown by ester and amide derivatives of certain acidic anti-inflammatory drugs, including mefenamic acid, ketoprofen, and ibuprofen [61].
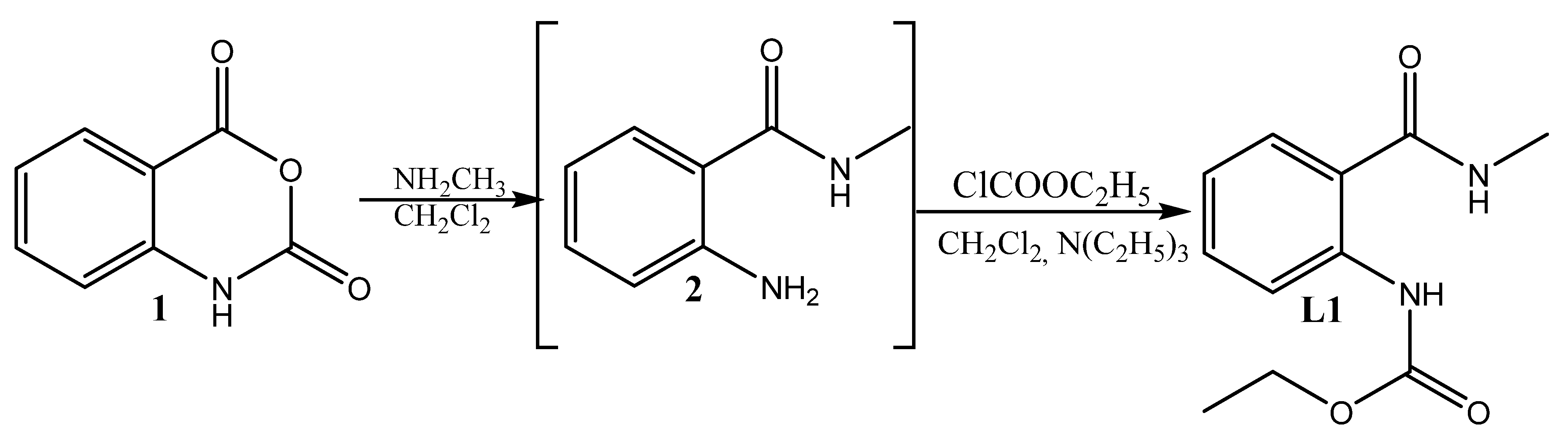
Esterification is regarded as one of the best methods for producing prodrugs of weak acids with a high yield (up to 89%) that does not require complicated setup, hazardous solvents, expensive reaction vessels, or complicated operating conditions [62]. Esters of weak acidic medicinal substances offer an ideal way to avoid the gastric acidic environment that restricts hydrolysis, as well as improving aqueous solubility, transcellular absorption (especially in the small intestine), and rapid metabolism to produce high concentrations of the active drug in the blood to meet desired bioavailability [63]. Thus, we decided to incorporate an ester residue in the anthranilic acid amide. To synthesize it, we used a previously reported procedure [64,65,66,67], which involves the ring-opening of isatoic anhydride 1 with methylamine to amide 2, which further acylates to produce the ester of anthranilamide, (L1) (Scheme 1).
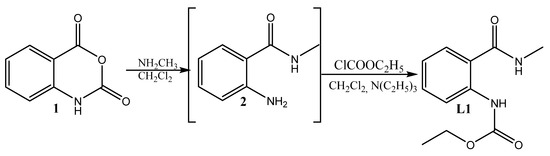
Scheme 1.
Synthesis of the ligand L1.
The resultant compound L1 was characterized by its melting point, FTIR, and 13C{1H} NMR spectra. The structure of the synthesized compound was confirmed through spectral analysis, as detailed in Supplementary Figures S1–S3.
The interaction between the ligand and transition metal ions resulted in the formation of thermodynamically stable coordination compounds, with isolated yields ranging from 31% to 35%. The synthesized Ni(II) and Co(II) complexes exhibit characteristic green and purple colors, respectively. These complexes are air- and moisture-stable under ambient conditions and display limited solubility in common organic solvents such as ethanol, ethyl acetate, and tetrahydrofuran (THF), while being readily soluble in dimethyl sulfoxide (DMSO). The analytical results, including the yield percentages of the mixed ligand Ni(II) and Co(II) complexes, are shown in Table 1.

Table 1.
Analytical and physical characteristics of coordination compounds.
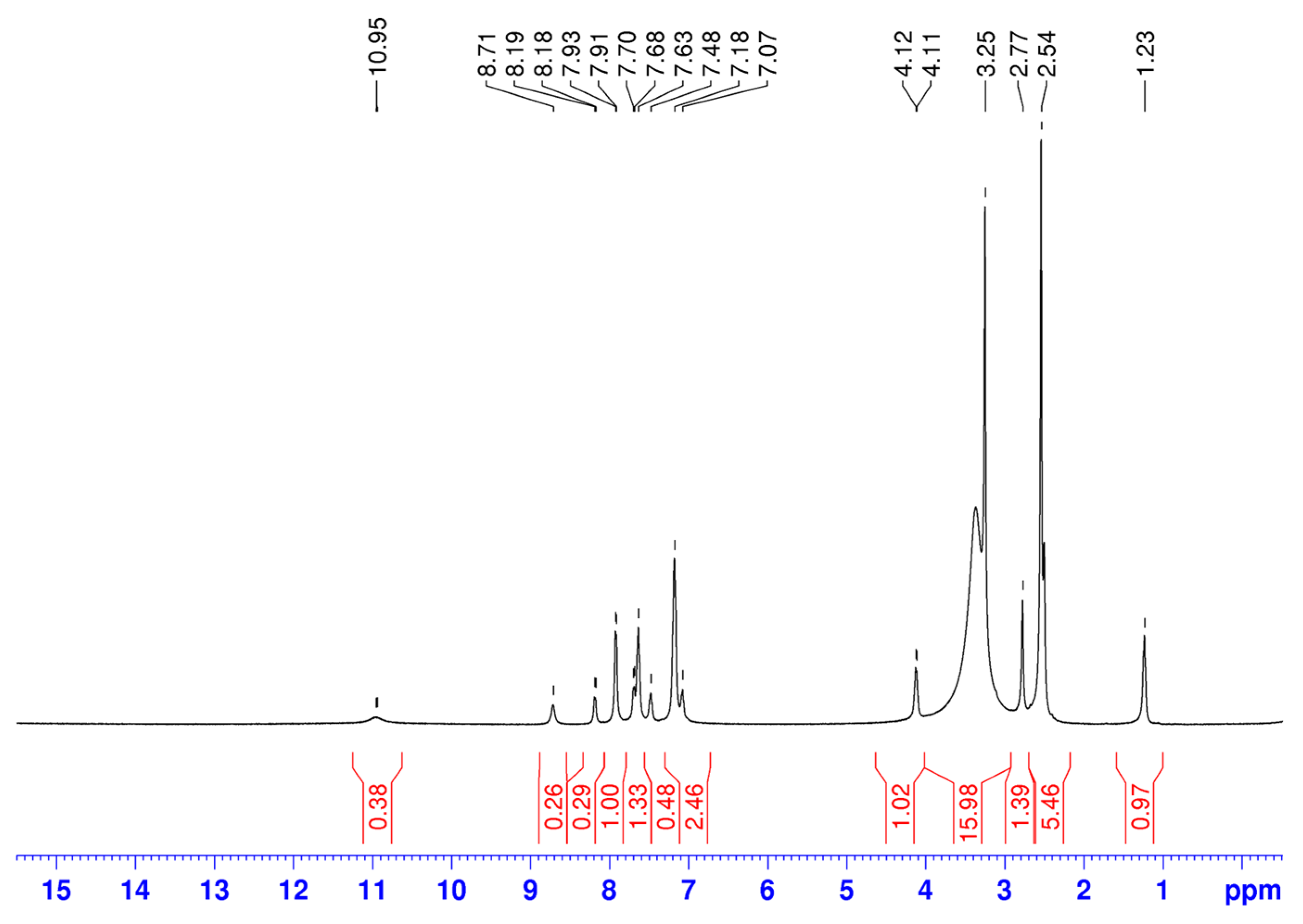
The 1H-NMR data for ethyl (2-(methylcarbamoyl)phenyl)carbamate (L1) and its metal complexes are given in Table 2. However, in both 1H and 13C NMR spectra of the complexes, we observe the presence of additional, stronger signals than the signals of L1 (Figure 2).

Table 2.
1H-NMR data for L1, L2 and their mixed-ligand metal complexes of Ni(II) and Co(II).
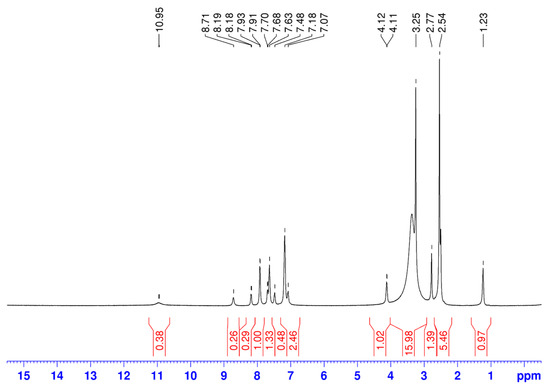
Figure 2.
The 1H NMR spectrum of a Ni(II) coordination compound.
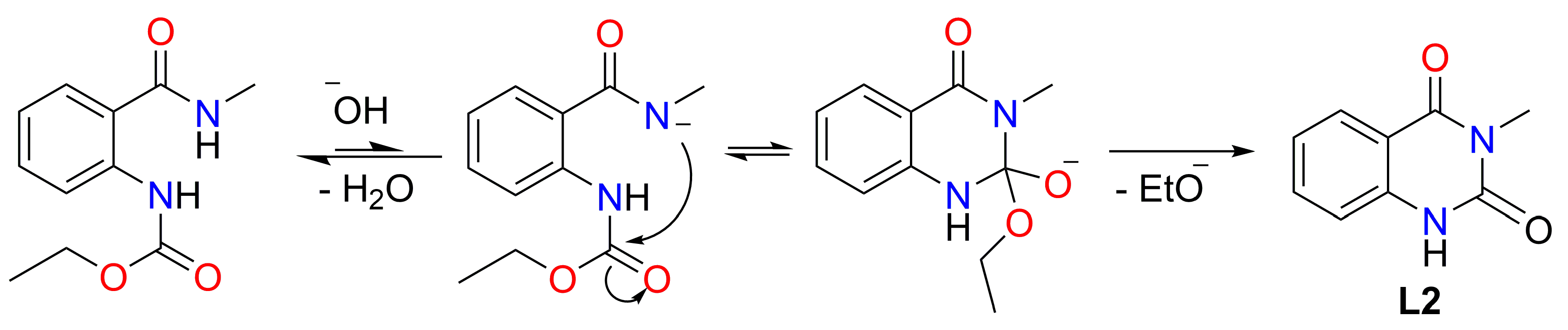
Based on the spectral data, we anticipated that the metals formed a mixed complex with another byproduct ligand (labeled as L2). Roughly, the ratio can be estimated at 1:3 for L1 and L2, respectively. We deduced the structure of L2 from the chemical shift data for the 1H and 13C signals. The signals are completely consistent with 3-methylquinazoline-2,4(1H,3H)-dione [68]. The possible mechanism of L2 formation and the structure formula are given in Scheme 2.
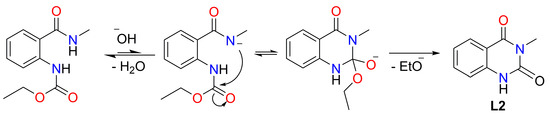
Scheme 2.
Reaction scheme for obtaining the second ligand 3-methylquinazoline-2,4(1H,3H)-dione (L2).
It should be mentioned that mixed ligand complexes of ethyl 2-(methylcarbamoyl)phenyl)carbamate and 3-methylquinazoline-2,4(1H,3H)-dione were obtained for the first time.
The lack of a signal for the proton of the amide group in L2 is observed when comparing the 1H-NMR spectrum of the Ni(II) complex with the spectra of L1 and L2. This suggests that the nitrogen atom from this functional group is involved in the coordination to the metal center. The signal for the proton from the NHCH3 group is moved to lower frequencies with 2.25 ppm in the 1H-NMR spectrum of the Co(II) complex (Table 3). This demonstrates that the N-methyl group is involved in coordination with the metal ion. This fact is consistent with the information included in the infrared spectra.

Table 3.
13C-NMR data for L1, L2 and their coordination compounds with Ni(II) and Co(II).
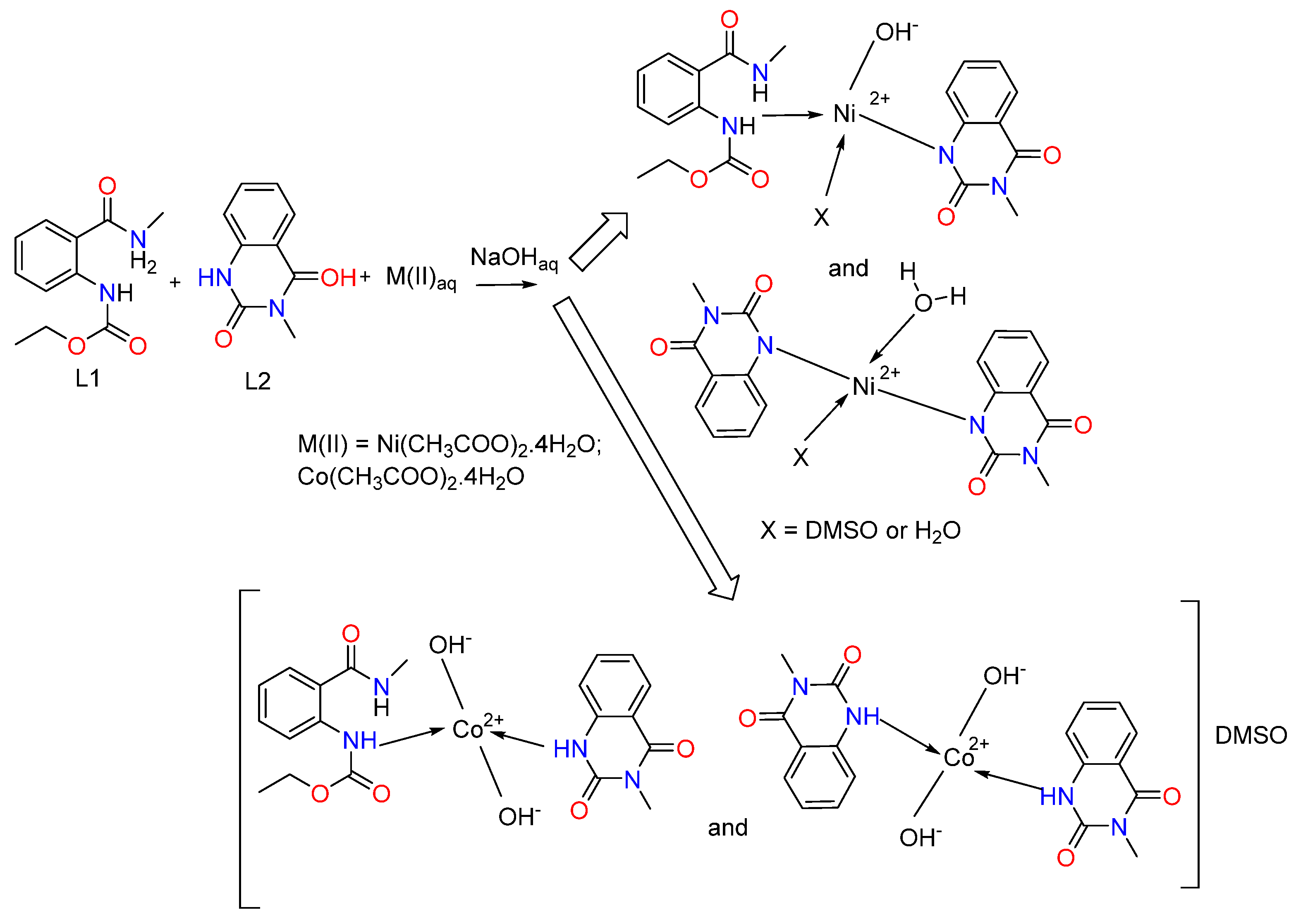
When comparing the 1H-NMR spectrum of Ni(II) complex with L1 and L2’s spectra, it is observed that one signal for the proton from the amide group in L2 is absent, which indicates the participation of the N-atom from this functional group in the coordination to the metal center. In the 1H-NMR spectrum of the Co(II) complex, the signal for the proton from the NHCH3 group is shifted to a lower frequency by 2.25 ppm (Table 3). This is an indication of the involvement of this group in coordination with the metal ion. The presented data is in agreement with the IR spectrum data. The aforementioned gives us reason to propose the following reaction scheme for the synthesis of metal complexes of ethyl (2-(methylcarbamoyl)phenyl)carbamate (L1) and 3-methylquinazoline-2,4(1H,3H)-dione (L2) with Ni(II) and Co(II) (Scheme 3).
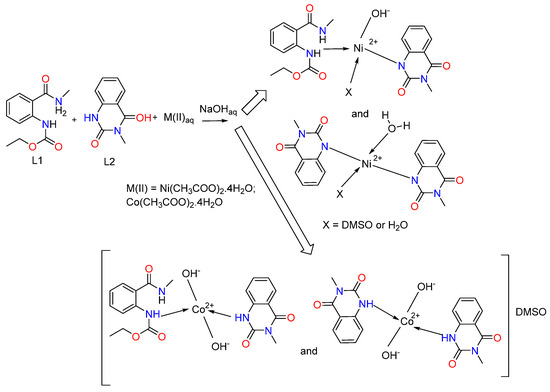
Scheme 3.
Synthetic pathway for the preparation of mixed-metal complexes of ethyl (2-(methylcarbamoyl)phenyl)carbamate (L1) and 3-methylquinazoline-2,4(1H,3H)-dione (L2) with Ni(II) and Co(II).
Furthermore, the presence of a solvent molecule in the Ni(II) and Co(II) complexes was confirmed by the signals observed at 2.54 ppm and 2.53 ppm in the 1H NMR spectra, and at 40.88 ppm and 40.90 ppm in the 13C NMR spectra, respectively.
The 13C-NMR data for ethyl (2-(methylcarbamoyl)phenyl)carbamate (L1), 3-methylquinazoline-2,4(1H,3H)-dione (L2) and their coordination compounds are given in Table 3. The 13C-NMR spectra of the Ni(II) and Co(II) complexes are almost identical.
Selected experimental IR spectral data (cm−1) for the Ni(II)L and Co(II)L complexes, along with the free ligands L1 and L2, are summarized in Table 4. In the infrared spectrum of L1, the bands at 3345 cm−1 and 3258 cm−1 may refer to the stretching vibrations of the N-H groups of L1. In the infrared spectrum of L2, the band at 3165 cm−1 can be attributed to stretching vibrations of the N-H group [69]. The same band in the spectrum of the nickel (II) complex is missing, but for cobalt(II) complexes was shifted to higher frequencies by 23 and 24 cm−1, respectively (the comparison is for L2). This showed that the NH group of the ligand L2 participates in the coordination of all complexes. In the IR spectra of L1 and L2, the bands at 1739 cm−1 and 1715 cm−1 can be attributed to stretching vibrations of C=O groups from the ligand L1 and L2, respectively. Comparing the infrared spectra of the complexes with that of L2 shows that the same band in the IR spectra of all complexes was observed at 1717 cm−1 and is not changed. This showed that the two C=O groups of the ligand L2 do not participate in the coordination with metal ions. In all infrared spectra, a broad band is observed at 3500–3400 cm−1, confirming the presence of a hydroxyl group from a water molecule. That is fully consistent with previously obtained mixed ligand complexes of anthranilic acid [70].

Table 4.
Selected experimental IR spectral data (cm−1) for Ni(II) and Co(II) complexes of ligands L1 and L2.
The nickel and cobalt content in the complexes was determined using microwave plasma–atomic emission spectrometry (MP-AES). Based on the data obtained from 13C{1H} NMR spectroscopy, infrared (IR) spectroscopy, and elemental analysis, tentative average molecular compositions were proposed for the Ni(II) and Co(II) complexes, as presented in Table 5.

Table 5.
Elemental analysis data for the metal ions in the synthesized complexes.
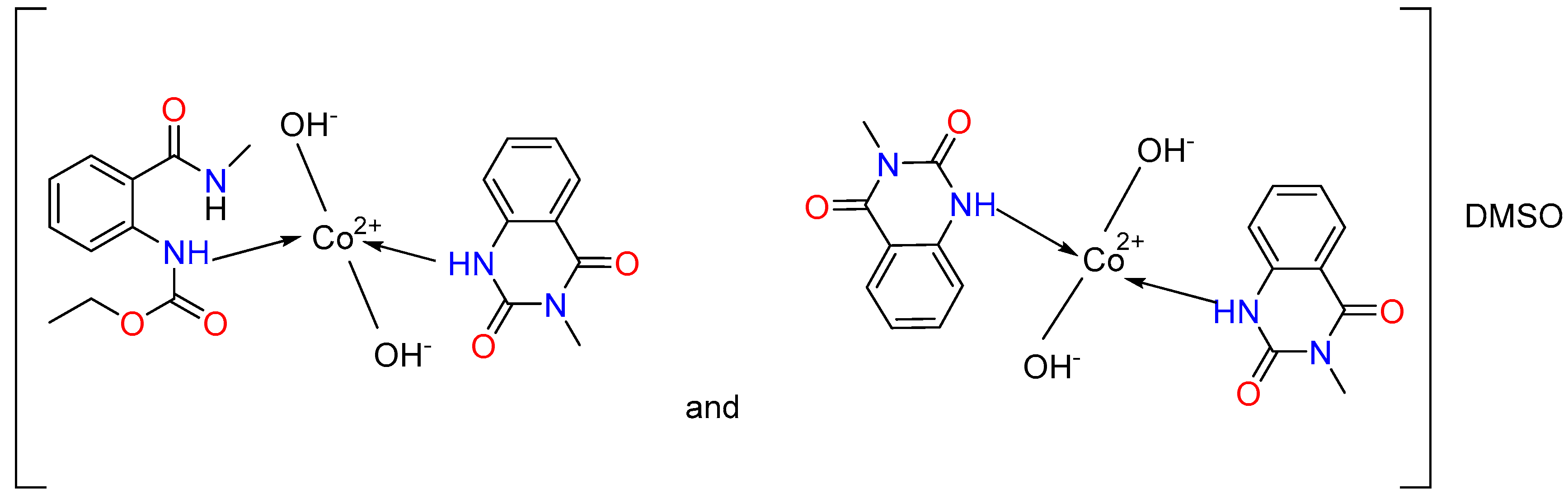
We assumed that the ligand in the complexes coordinates monodentately through the N-atom (Figure 3). We observe a combination of two complexes for the cobalt(II). In the first, two hydroxyl groups have been coordinated by the metal center, and both ligands L1 and L2 are engaged, while only ligand L2 is coordinated in the second one. Two hydroxyl groups are also linked to the cobalt ion. The charge of the metal center is offset by these groups. The complex’s composition includes the solvent molecule DMSO, as indicated by the metal’s elemental analysis data as well as the 13C{1H} NMR spectra. We consider the outer coordination sphere to be the location of DMSO.
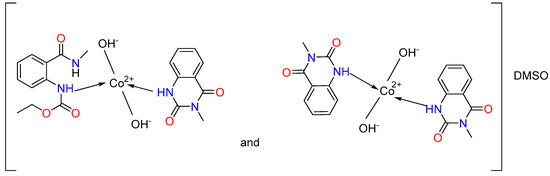
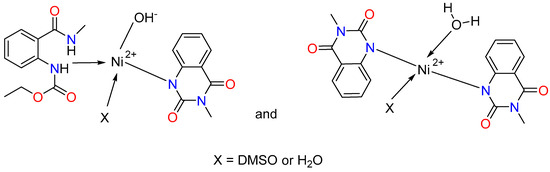
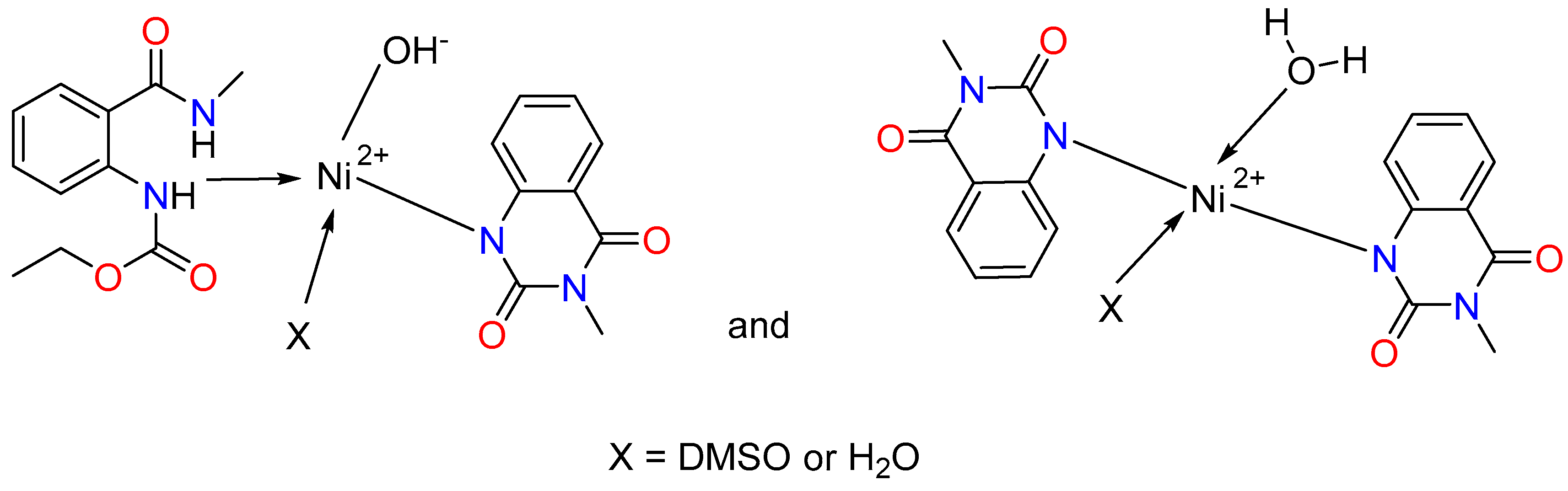
Figure 3.
Proposed coordination modes of ligands L1 and L2 with Co(II) and Ni(II), illustrating the suggested donor binding sites.
We observe again a mixture of two complexes with different structures and compositions for the nickel(II). L1 is coordinated through the nitrogen atom of the ester group, and L2 is deprotonated and coordinated through the N donor atom in the first one. The third coordination site is occupied by a hydroxyl group, and the fourth coordination site is occupied by either a DMSO molecule or a water molecule (Figure 3). The second complex contains two deprotonated ligands, L2, coordinated to the metal ion through the nitrogen donor atoms, and one water molecule. The fourth coordination site can be occupied by either a DMSO solvent molecule or a water molecule. It should be mentioned that the structure of the complexes probably contains two bulky ligands and two smaller ones due to steric reasons.
The structure of the obtained complexes was similar to that presented for the Pd(II) complex of N-(2-benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide [71]. The presented data are also consistent with previously synthesized Mn(II), Ni(II), Co(II), Cu(II), Zn(II), and Cd(II) complexes of anthranilic acid and pyridine-2-aldoxime in an alkaline environment [47]. The authors [47] found that nickel and cobalt complexes have a tetrahedral geometry and coordination number of 4, as we assumed for the Ni(II) and Co(II) complexes. Recently, Alwan reported the synthesis of mixed ligand complexes of Cu(II), Ni(II), Co(II), and Zn(II) with anthranilic acid [72]. Square planar geometry was suggested for the Co(II) and Zn(II) complexes, while we proposed tetrahedral geometries for the Ni(II) and Cu(II) complexes [72]. It should be noted that the complexes are soluble only in DMSO and partially in hot ethanol, similarly to our experiments. The tetrahedral geometry was also confirmed by Al-Noor et al. for Ni(II), Co(II), Fe(II), Zn(II), and Cd(II) mixed-ligands complexes containing anthranilic acid and amino acid L-alanine with Cu(II), Ni(II), Co(II), Mn(II), Fe(II), Zn(II) and Cd(II) [73]. The resulting complexes are insoluble in water but soluble in DMSO and DMF.
The title compound and its metal complexes were synthesized as promising antimicrobial agents. Therefore, our main goal was to investigate their antimicrobial and antifungal activity.
The antibacterial activity against five Gram-positive, five Gram-negative, two yeasts, and three fungi was investigated using the agar diffusion technique. The sensitivity of harmful bacteria is revealed by the excellent diffusion method. As a result, a chemically sensitive organism will not grow close to the well where it was deposited. The area that does not expand is referred to as the clear zone or zone of inhibition. The size of the clear zone corresponds to the tested substance’s inhibition. Table 6 provides a summary of the mean zones of inhibition in millimeters that were generated on the pathogenic bacteria that included L1 and its metal complexes.

Table 6.
Antimicrobial effect of ethyl 2-(methylcarbamoyl)phenyl)carbamate and its metal compounds.
Our results illustrated that only the cobalt complex exhibited promising antimicrobial activity against Gram-positive Staphylococcus aureus, Listeria monocytogenes, Micrococcus luteus, and Gram-negative Klebsiella pneumoniae and Proteus vulgaris. This difference can be explained by different cell membrane permeability for both Gram-positive and Gram-negative bacteria, which is greatly influenced by several intricate factors, such as the membrane’s zeta potential, lipophobicity, the thickness of the membrane and its surrounding layers, and the chemical composition of the antimicrobial drug [74].
The obtained results corresponded to those previously described by Shirode [70]. The author obtained mixed cobalt complexes of anthranilic acid semicarbazone and benzaldehyde and found that the cobalt complexes were moderately active against Bacillus sp. and Staphylococcus sp. In our experiments, mixed cobalt complexes with anthranilic acid ester amide showed similar activity to Bacillus sp. and Staphylococcus sp., but were also sensitive to Listeria monocytogenes and Micrococcus luteus. Results were confirmed by the results from cobalt complexes of a Schiff base ligand 2-ethyl-[3-(hydroxyl propyl)]-3, 1(4H) quinazoline-4-one-4 hydrazone [75] and mixed ligand complexes of anthranillic acid and tributylphosphine [76]. Results were also similar to the antimicrobial activity of bis(2-methoxy-6-{[(2-methylpropyl) imino]methyl}phenolato) nickel (II) complex, which showed significant antibacterial activity against E. coli and S. aureus and a prominent antifungal activity over C. albicans and C. tropicalis [77] and In(III) complex of N-methyl-N–phenyl dithiocarbamate, active against L. monocytogenes (15 mm), B. cereus (16.67 mm), E. faecalis (14.67 mm), and S. enterica (14.67 mm) [78].
The findings of this study on the antimicrobial activity of ethyl 2-(methylcarbamoyl)phenyl)carbamate and its complexes highlight several potential applications in the field of antimicrobial agents. The cobalt complex demonstrated activity against a range of Gram-positive and Gram-negative bacteria, including clinically significant pathogens such as Staphylococcus aureus, Listeria monocytogenes, Micrococcus luteus and Klebsiella pneumoniae. The complex could be a potential candidate for developing novel antibacterial drugs. Activity against Listeria monocytogenes and Staphylococcus aureus suggests potential use in disinfectants or coatings for hospital surfaces and medical devices to control the spread of these pathogens. The selective bacterial activity observed for the cobalt and nickel complexes suggests specific interactions between these metal ions and bacterial cells. Further research into these mechanisms may lead to the design of more effective and specific antimicrobial agents. Given the activity against Salmonella enteritidis and Salmonella typhimurium, these complexes could be investigated for controlling bacterial contamination in the food industry, particularly in poultry and egg production. The observed resistance of fungi and certain bacterial strains provides a basis for understanding limitations and optimizing the structure of these compounds to enhance their antimicrobial spectrum.
The study’s findings on the in vitro biocompatibility of the newly synthesized metal complexes suggest promising potential applications, particularly as anti-infective agents. Below are key avenues for future exploration:
- Development of Safe Antimicrobial Agents
The negligible cytotoxicity and high IC50 values (>100 µM) across diverse cell lines indicate that these compounds are biocompatible, making them ideal candidates for antimicrobial drug development with minimal risk of host toxicity.
- 2.
- Adjunctive Therapies in Infection Management
Given their selective activity against bacterial pathogens and lack of significant cytotoxicity, these compounds could serve as adjunctive agents in treating infections, particularly in immunocompromised individuals, without harming normal cells.
- 3.
- Biomedical Coatings and Materials
The combination of antimicrobial activity (especially in the cobalt complex) and biocompatibility makes these compounds suitable for use in coatings for medical devices, implants, and wound dressings to prevent biofilm formation and infection.
- 4.
- Research into Selectivity Mechanisms
The low cytotoxicity paired with antimicrobial activity opens avenues for studying mechanisms that confer selectivity toward bacterial cells over mammalian cells. Insights from this could inform the design of more targeted antimicrobial therapies.
- 5.
- Applications in Chronic Infections
For chronic myeloid leukemia (CML) or other conditions involving immunosuppression, where infections pose a significant risk, these biocompatible compounds could potentially be integrated into treatment protocols.
- 6.
- Food and Environmental Safety
The safe profile of these compounds supports their application in controlling bacterial contamination in food and water, offering an alternative to traditional disinfectants that may have toxic side effects.
- 7.
- Foundation for Structural Optimization
While the current compounds are biocompatible, their activity could be further enhanced through structural modifications without compromising safety, leading to improved therapeutic potential against infectious agents.
These findings establish a solid foundation for advancing the development of these metal complexes in anti-infective applications while minimizing the risk of adverse effects on host cells. Further in vivo studies and formulation research will be critical to translating these promising results into practical applications.
Given the potential of metal complexes as antimicrobial agents, understanding their cytotoxic effects is crucial for evaluating their therapeutic safety. Finally, the in vitro effect of the metal complexes on cell proliferation and their biocompatibility was assessed in cellular test systems of leukemic (K-562, LAMA-84, bcr-abl+ chronic myeloid leukemia cell lines) and epithelial (CCL-1, normal murine fibroblast cells) origin. The results are presented in Table 7.

Table 7.
In vitro cytotoxicity of the tested compounds [IC50, µM ± SD] against cell lines of different origin.
Both complexes were shown to be devoid of any significant cytotoxic activity and exhibited a negligible effect on cell proliferation. The calculated half-inhibitory concentrations (IC50) in all experimental models consistently exceeded 100 µM, indicating no observable cytotoxicity across the tested concentration range, irrespective of the cell line or the nature of the coordinating metal ion. These findings suggest that the compounds do not induce cellular damage or interfere with vital metabolic functions in mammalian cells, which is a critical prerequisite for their safe application in biomedical settings. Considering the potential application of the newly synthesized compounds as antimicrobial agents, their excellent biocompatibility profiles highlight a favorable therapeutic index. These characteristics position the complexes as promising candidates for further biological investigation, particularly in the development of antimicrobial agents with minimal adverse effects on host tissues.
3. Materials and Methods
3.1. Chemicals
All solvents and reagents were purchased from Merck (Merck KGaA, Darmstadt, Germany). Melting points were measured on a Kruss M5000 melting point meter (A.Krüss Optronic GmbH, Hamburg, Germany). All solvents and reagents were purchased from Merck (Merck KGaA, Darmstadt, Germany). Melting points were determined on a Boetius hot stage apparatus and are uncorrected. All the compounds were characterized by melting point, 1H-NMR, 13C-NMR, IR, and MP-AES. IR spectra were determined on a VERTEX 70 FT-IR spectrometer (Bruker Optics, Ettlingen, Germany). 13C{1H} NMR spectra were recorded on a Bruker Avance III HD 500 spectrometer (Bruker, Billerica, MA, USA) at 500 MHz (1H-NMR) and 125 MHz (13C-NMR), respectively. Chemical shifts are given in ppm and are not referenced additionally in order to facilitate the comparison with the data found in the literature. The NMR spectra were recorded at 298 K.
Microwave Plasma-Atomic Emission Spectrometry (MP-AES) Determination of Co and Ni in the Complexes
A total of 0.0200 g of sample was weighed on an analytical balance and dissolved with 65% nitric acid, p.a. (Chem-Lab NV, Zedelgem, Belgium). Blank solutions were prepared as well. After dilution, the concentration of Co and Ni was determined via MP-AES 4200 (Agilent Technologies, Santa Clara, CA, USA). Calibration standards were prepared from monoelemental standard solutions—1000 mg L−1 (Merck KGaA, Darmstadt, Germany). Conventional MP-AES operating conditions were used. Analytes were measured on three emission lines for estimation of potential spectral interferences, i.e., 341.476 nm, 352.454 nm, and 361.939 nm for Ni and 340.512 nm, 345.351 nm, and 350.228 nm for Co. Five replicates and 5 s measurements were applied for all lines.
3.2. Synthetic Methods Experimental Protocols
3.2.1. Synthesis of the Ligand—Ethyl (2-(Methylcarbamoyl)phenyl)carbamate L1
15 mmol methylamine was added to 10 mmol (1.63 g) isatoic anhydride in dichloromethane (30 mL), and the reaction mixture was stirred overnight at rt. The obtained anthranilamide was treated with ethyl chloroformate (mL, 10 mol) in the presence of 10 mmol of N(C2H5)3. In about 30 min, the reaction mixture was washed consequently with diluted HCl (1:4), Na2CO3, and H2O, then dried with anhydrous Na2SO4, filtered on a short column filled with neutral Al2O3, and concentrated (Supplementary Materials Figures S1–S4).
ethyl (2-(methylcarbamoyl)phenyl)carbamate (L1): white crystals, 80% yield, mp = 136–137 °C. 1H-NMR: 1.24 (t, J = 7.1, 3H, CH2CH3), 2.79 (d, J = 4.4, 3H, NHCH3), 4.13 (d, J = 6.8, 2H, CH2CH3), 7.07–7.11 (m, 1H, Ar), 7.49 (t, J = 7.8, 1H, Ar), 8.2 (d, J = 8.3, 1H, Ar), 8.72 (broad s, 1H, CONHCH3), 10.96 (s, 1H, NHCOO); 13C-NMR: 169.17, 153.38, 139.7, 132.5, 128.4, 122.17, 120.04, 119.06, 61.05, 26.69, 14.86. IR (KBr, cm−1): 3345 (ν(N-H)), 3258 (ν(N-H, -NC(=O)O)), 3116 (ν (Csp2-H, Ph-)), 3072 (ν (Csp2-H, Ph-)), 2985 (νas(Csp3-H, CH3-)), 2951 (νas (Csp3-H, CH3-)), 2913 (νas (Csp3-H, >CH2)), 2880 (νs (Csp3-H, CH3-)), 1739 (ν(C=O)), 1664 (δ(-NH)+ ν(C=O)), 1633 (δ(-NH)+ ν(C=O)), 1605 (ν(C=C-C), Ph-), 1593 (ν(C=C-C), Ph-), 1554 (δ(-NH)), 1530 (ν (C=C-C), Ph-), 1454 (ν(C=C-C), Ph-), 1409, 1390 (δs(-CH3)), 1364 (δs(-CH3)), 1333, 1248, 1218 (νas(N-CO-O-)), 1167, 1149, 1122, 1114, 1063, 1049, 951 (νs(N-CO-O-)), 870, 849, 820, 768, 753 (γ (Csp2-H, Ph-), 721, 669, 651, 536, 510. Raman (cm−1) of 3: 2939, 1730 (C=O), 1641, 1604, 1592, 1555, 1463, 1454, 1392, 1366, 1334, 1305, 1288, 1249, 1219, 1169, 1151, 1123, 1052, 1012, 915, 849, 822, 613, 395, 364.
3.2.2. Synthesis of Ni(II) and Co(II) Complexes of Ethyl (2-(Methylcarbamoyl)phenyl)carbamate L1, M:L:OH− = 1:2:2
The Ni(II) and Co(II) complexes were synthesized by mixing a DMSO solution (5 mL) containing 0.0889 g (0.0004 mol) of the ligand (L1) with water solution of the respective metal salts: Ni(CH3COO)2.4H2O 0.0498 g (0.0002 mol) or Co(CH3COO)2.4H2O 0.0498 g (0.0002 mol). It should be mentioned that the solutions were alkalized with 5 mL of 0.0160 g (0.0004 mol) of NaOH solution. After stirring with an electromagnetic stirrer for 1–2 h, light green (Ni) and purple (Co) precipitates formed. These were filtered, washed with water, air dried, and further dried over silica gel in a desiccator for one week.
3.3. Microbiological Tests
3.3.1. Tested Microorganisms
Antimicrobial Activity Assay
The agar well diffusion method was used to assess AgNPs’ antibacterial properties [25]. A tiny quantity of bacterial/yeast biomass was homogenized in five milliliters of sterile 0.5% NaCl to create the inocula. Five milliliters of sterile 0.5% NaCl were added straight into the cultivation tubes to create the fungal inocula. Before being used, the fungal inocula were filtered and put in fresh tubes after being agitated by vortex V-1 plus (Biosan, Riga, Latvia). A Thoma bacterial counting chamber (Poly-Optik GmbH, Bad Blankenburg, Germany) was used to count the number of fungal spores and viable cells. After that, suspensions were made with roughly 1 × 108 cfu.mL−1 for yeast/bacterial cells and 1 × 105 cfu.mL−1 for fungal spores. The inocula were then divided among LBG/MEA media that had been partially melted and tempered at 45–48 °C. They were then transferred in 18 mL to sterile Petri plates (d = 90 mm) (Gosselin, France). Six wells (d = 6 mm) per Petri plate were drilled after the inoculated agar medium had solidified at room temperature. Duplicates of 60 μL of the extracts were then pipetted into the agar wells. The same conditions were used to incubate the plates.
The antimicrobial activity was determined by measuring the diameter of the inhibition zones around the wells on the 24th and 48th hours of incubation. Tested microorganisms with 18 mm or more inhibition zones were considered sensitive; inhibition zones from 12 to 18 mm were considered moderately sensitive, and inhibition zones up to 12 mm were considered bacteriostatic.
3.3.2. Culture Media
Luria-Bertani agar supplemented with glucose (LBG agar) (Laboratorios Conda S.A., Madrid, Spain) was used to cultivate bacteria. 50 g of LBG agar medium base was dissolved in 1 L of deionized water at pH 7.5 ± 0.2.
To cultivate yeasts and fungi, malt extract agar (MEA) (Scharlab S.L., Barcelona, Spain) was utilized. One liter of deionized water (pH 5.6 ± 0.2) was used to dissolve 48 g of MEA medium base. Before being used, both culture media were prepared per the manufacturer’s instructions and autoclaved for 20 min at 121 °C.
3.4. Cytotoxic Activity
A number of cell viability tests were conducted against normal murine fibroblast cells (CCL-1) and human malignant leukemic cell lines (LAMA-84, K-562) to assess the experimental compounds’ in vitro biocompatibility. The German Collection of Microorganisms and Cell Cultures (DSMZ GmbH, Braunschweig, Germany) was the source of all cell lines. The cell cultures were grown in RPMI 1640, a growth medium supplemented with 10% fetal bovine serum (FBS), 5% L-glutamine, and kept at 37 °C with 5% humidified CO2.
Cell Viability Assay
A standard MTT-based colorimetric test was used to assess cell viability. In 96-well plates, exponential-phase cells were isolated and planted at a density of 100 μL/well for suspension cultures (LAMA-84 and K-562) and 1.5 × 105 for adherent cultures (CCL-1). Different concentrations of the experimental chemicals in the 400–6.25 µM concentration range were applied to cells and cultured with them. A filter-sterilized MTT substrate solution (5 mg/mL in PBS) was applied to each culture plate well following a 72-h exposure period.
The development of purple insoluble formazan precipitates was made in 1–4 h of incubation. The latter were dissolved in an isopropyl alcohol solution with 5% formic acid before the absorbance was measured using a Labexim LMR-1 microplate reader at 550 nm. After being blanked against MTT and isopropanol solution, the collected absorbance values were standardized to the mean of the untreated control (100% cell viability). The screened compounds’ half-inhibitory concentrations against each tested cell line were determined, and semi-logarithmic “dose–response” curves were created. p < 0.05 was regarded as a statistically significant value.
4. Conclusions
Ethyl 2-(methylcarbamoyl)phenyl)carbamate and its novel mixed complexes with 3-methylquinazoline-2,4(1H,3H)-dione were obtained. The novel complexes with Ni(II) and Co(II) were spectrally characterized by their melting points, FTIR, 1H- and 13C-NMR spectra. Based on the spectral data obtained, we could conclude that the nitrogen atom is involved in the coordination with the metal center. From the studies on the antibacterial activity of mixed ligand complexes with Ni(II) and Co(II), we can summarize that the Co(II) complex showed promising antimicrobial activity against all Gram-positive and Gram-negative bacteria investigated. The cytotoxic properties of the novel complexes were also investigated. Based on the obtained results, it may be concluded that the mixed cobalt complex of 2-(methylcarbamoyl)phenyl)carbamate and 3-methylquinazoline-2,4(1H,3H)-dione can be attributed as a promising antimicrobial agent.
By focusing on these applications, future research can tailor these compounds for targeted therapeutic and industrial uses, addressing the growing challenge of antimicrobial resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13080267/s1, Figure S1: 1H-NMR spectrum of the ligand L1, Figure S2: 13C-NMR spectrum of L1, Figure S3: DEPT spectrum of L1, Figure S4: FT-IR spectrum of L1.
Author Contributions
Conceptualization, S.N. and P.M.; methodology, S.N., M.M., N.B., P.M., R.M., E.C., Y.T., and E.V.; investigation, M.M., S.T., Y.T., N.B., E.V., E.C., and R.M.; writing—original draft preparation, S.T., P.M., N.B., and M.M.; writing—review and editing, S.N.; supervision, S.N.; project administration, S.N. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of Scientific Project 466 No KP-06-H73/11 of the National Fund for Scientific Research in Bulgaria, National Program for Basic Research Projects—2023. Research equipment of the Distributed Research Infrastructure INFRAMAT, supported by the Bulgarian Ministry of Education and Science, was used.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular Polymeric Substances, a Key Element in Understanding Biofilm Phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Guilhen, C.; Forestier, C.; Balestrino, D. Biofilm Dispersal: Multiple Elaborate Strategies for Dissemination of Bacteria with Unique Properties. Mol. Microbiol. 2017, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- den Bakker, H.C.; Fortes, E.D.; Wiedmann, M. Multilocus sequence typing of outbreak-associated Listeria monocytogenes isolates to identify epidemic clones. Foodborne Pathog. Dis. 2010, 7, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cheng, J.; Zhang, J.; Chen, Y.; Zeng, H.; Xue, L.; Lei, T.; Pang, R.; Wu, S.; Wu, H.; et al. Isolation, Potential Virulence, and Population Diversity of Listeria monocytogenes From Meat and Meat Products in China. Front. Microbiol. 2019, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Jinneman, K.; Stelma, G.; Smith, B.G.; Lye, D.; Messer, J.; Ulaszek, J.; Evsen, L.; Gendel, S.; Bennett, R.W.; et al. Natural atypical Listeria innocua strains with Listeria monocytogenes pathogenicity island 1 genes. Appl. Environ. Microbiol. 2004, 70, 4256–4266. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Perrodeau, É.; Leclercq, A.; Cazenave, B.; Pilmis, B.; Henry, B.; Lopes, A.; Maury, M.M.; Moura, A.; Goffinet, F.; et al. Clinical features and prognostic factors of listeriosis: The MONALISA national prospective cohort study. Lancet Infect. Dis. 2017, 17, 510–519. [Google Scholar] [CrossRef]
- Shoai-Tehrani, M.; Pilmis, B.; Maury, M.M.; Robineau, O.; Disson, O.; Jouvion, G.; Coulpier, G.; Thouvenot, P.; Bracq-Dieye, H.; Valès, G.; et al. Listeria monocytogenes-associated endovascular infections: A study of 71 consecutive cases. J. Infect. 2019, 79, 322–331. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, X.; Niu, B.; Yang, J.; Chen, Q. Differences in Biofilm Formation of Listeria monocytogenes and Their Effects on Virulence and Drug Resistance of Different Strains. Foods 2024, 13, 1076. [Google Scholar] [CrossRef]
- Kochan, T.J.; Nozick, S.H.; Valdes, A.; Mitra, S.D.; Cheung, B.H.; Lebrun-Corbin, M.; Medernach, R.L.; Vessely, M.B.; Mills, J.O.; Axline, C.M.R.; et al. Klebsiella pneumoniae Clinical Isolates with Features of Both Multi-Drug-Resistance and Hypervirulence Have Unexpectedly Low Virulence. Nat. Commun. 2023, 14, 7962. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Nair, A.V.; Parmar, K.; Rajmani, R.S.; Chakravortty, D.; Das, D. Combating Biofilm-Associated Klebsiella pneumoniae Infections Using a Bovine Microbial Enzyme. NPJ Biofilms Microbiomes 2024, 10, 119. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Guo, H.; Tong, Y.; Cheng, J.; Abbas, Z.; Li, Z.; Wang, J.; Zhou, Y.; Si, D.; Zhang, R. Biofilm and Small Colony Variants—An Update on Staphylococcus aureus Strategies toward Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1241. [Google Scholar] [CrossRef]
- Chen, K.; Peng, C.T.; Chi, F.; Yu, C.D.; Yang, Q.L.; Li, Z.J. Antibacterial and Antibiofilm Activities of Chlorogenic Acid against Yersinia enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef]
- Prosser, B.L.; Taylor, D.; Dix, B.A.; Cleeland, R. Method of Evaluating Effects of Antibiotics on Bacterial Biofilm. Antimicrob. Agents Chemother. 1987, 31, 1502–1506. [Google Scholar] [CrossRef]
- Ihmaid, S. Exploring the Dual Inhibitory Activity of Novel Anthranilic Acid Derivatives towards α-Glucosidase and Glycogen Phosphorylase Antidiabetic Targets: Design, In Vitro Enzyme Assay, and Docking Studies. Molecules 2018, 23, 1304. [Google Scholar] [CrossRef]
- Zheng, J.-W.; Ma, L. Metal Complexes of Anthranilic Acid Derivatives: A New Class of Non-Competitive α-Glucosidase Inhibitors. Chin. Chem. Lett. 2016, 27, 627–630. [Google Scholar] [CrossRef]
- Nasr, T.M.; Aboshanab, A.M.; Abouzid, K.A.M.; Zaghary, W.A. Hands-on Synthetic Approaches and Biological Activities of Anthranilic Acid Derivatives: A Mini-Review. Egypt. J. Chem. 2023, 66, 329–343. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M. Medicinal Chemistry of Anthranilic Acid Derivatives: A Mini Review. Drug Dev. Res. 2021, 82, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Srivastava, V.K.; Kumar, A. Newer N-Substituted Anthranilic Acid Derivatives as Potent Anti-Inflammatory Agents. Eur. J. Med. Chem. 2002, 37, 689–697. [Google Scholar] [CrossRef]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. Acyclic and Cyclic Compartmental Ligands: Recent Results and Perspectives. Coord. Chem. Rev. 2012, 256, 953–1114. [Google Scholar] [CrossRef]
- Al-Noor, L.T.H.; Kakfm, A. Schiff Base and Ligand Metal Complexes of Some Amino Acids and Drug; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2016; ISBN 978-3-659-88556-3. [Google Scholar]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W. Bis{2-[(pyridin-2-yl)methylideneamino]benzoato-κ3N,N′,O}chromium(III) nitrate monohydrate. Acta Crystallogr. 2014, 70, m136. [Google Scholar] [CrossRef]
- Buvaylo, E.; Kokozay, V.; Rubini, K.; Vassilyeva, O.; Skelton, B. Unusual cocrystals made of a Schiff base metal complex and an organic molecule—Close-packing vs. hydrogen bond interactions. J. Mol. Struct. 2014, 1072, 129–136. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Pal, S. Nickel(II) complexes with N,N,O-donor Schiff bases. Self-assembly to two-dimensional network via hydrogen bonding. J. Chem. Crystallogr. 2005, 35, 737–744. [Google Scholar] [CrossRef]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W. Crystal structure of bis(2-{[(pyridin-2-yl)methylidene]amino}benzoato-k3N,N′,O)cobalt(II)N,N-dimethylformamide sesquisolvate. Acta Crystallogr. 2014, 70, 164–166. [Google Scholar] [CrossRef]
- Cheng, X.-X.; Hojaghani, S.; Hu, M.-L.; Sadr, M.H.; Morsali, A. Sonochemical Synthesis and Characterization of New Nanostructures Cobalt(II) Metal-Organic Complexes Derived From the azo-coupling reaction of 4-Amino Benzoic Acid with Anthranilic acid, Salicylaldehyde and 2-Naphtol. Ultrason. Sonochem. 2017, 37, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Abbasi, M.W.; Tariq, M.; Graham, J.P.; Al-Hagri, A.-R.S.; Elkarim, A.A.; Mohamed, M.E.; Nissapatorn, V.; Taha, M.; Hisaindee, S. Synthesis of Metal Anthranilate Complexes: Catalytic and Antipathogenic Studies. BMC Chem. 2022, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- El-Ajaily, M.M.; Morad, F.M. Studies on Cobalt(II) and Copper(II) Schiff Base Complexes. Asian J. Chem. 2007, 19, 4379–4384. Available online: https://asianpubs.org/index.php/ajchem/article/view/19941 (accessed on 6 June 2025).
- Saghatforoush, L.A.; Khalilnezhad, R.; Ershad, S.; Ghammamy, S.; Hasanzadeh, M. Synthesis, Characterization and Electrochemical Properties of μ-Oxalato Copper(II) and Nickel(II) Complexes of Anthranilic Acid Schiff Base Ligands. Asian J. Chem. 2009, 21, 6326–6334. Available online: https://asianpubs.org/index.php/ajchem/article/view/12322/12303 (accessed on 6 June 2025).
- Hossain, M.D.S.; Zakaria, C.M.; Kudrat-E-Zahan, M. Metal Complexes as Potential Antimicrobial Agent: A Review. Am. J. Heterocyc. Chem. 2018, 4, 1–21. [Google Scholar] [CrossRef]
- Marinova, P.; Hristov, M. Synthesis and Biological Activity of Novel Complexes with Anthranilic Acid and Its Analogues. Appl. Sci. 2023, 13, 9426. [Google Scholar] [CrossRef]
- Obaid, S.M.H.; Ghanim, F.H.; Al-Hamdani, A.A.S. Synthesis, Spectroscopic, Characterization and Biological Activities of Schiff Base Ligand and Metal Complexes of Some Metal (II) Salts with Anthranilic Acid. Res. J. Pharm. Biol. Chem. Sci. 2019, 10, 1121–1131. [Google Scholar]
- Prasher, P.; Mudila, H.; Sharma, M.; Khati, B. Developmental Perspectives of the Drugs Targeting Enzyme-Instigated Inflammation: A Mini Review. Med. Chem. Res. 2019, 28, 417–449. [Google Scholar] [CrossRef]
- Singh, P.; Prasher, P.; Dhillon, P.; Bhatti, R. Indole-Based Peptidomimetics as Anti-Inflammatory and Anti-Hyperalgesic Agents: Dual Inhibition of 5-LOX and COX-2 Enzymes. Eur. J. Med. Chem. 2015, 97, 104–123. [Google Scholar] [CrossRef]
- Chuanfeng, N.; Zong, Z.; Zhang, X.; Wu, R.; Li, N.; Wang, H.; Bi, C.; Fan, Y. Synthesis, Structures, and Biological Activity of Novel Complexes with Trifluorinated Anthranilic Acid Derivatives. J. Mol. Struct. 2019, 1194, 42–47. [Google Scholar] [CrossRef]
- Rajalakshmi, R.T.; Bheeter, S.R.; Vasanth, N. Synthesis and Characterization of Biologically Active Metal Complexes of N-Phenyl Anthranilic Acid. ReTeLL 2015, 15, 30–37. [Google Scholar]
- Raza, S.; Iqbal, Y.; Hussian, I.; Raza, M.; Shah, S.U.A.; Khan, A.; Taj, R.; Rauf, A. Synthesis of Anthranilic Acid and Phthalic Anhydride Ligand and Their Metal Complexes. Biochem. Anal. Biochem. 2013, 2, 143–147. [Google Scholar] [CrossRef]
- Ahmad, A.; Abid Obaid-Ur-Rahman; Rehman, W.; Kashif, M.; Zaman, R.; Ali, M.; Mir, S.; Qureshi, M.T. Ultrasonic Assisted Synthesis, Characterization and Bioactivity Assessment of Novel Piperonal Based Schiff Base and Its Metal Complexes. Iran. J. Chem. Chem. Eng. 2020, 39, 105–111. [Google Scholar]
- Oladipupo, O.E.; Ibukun, D.T.; Olalekan, T.E. Synthesis and Characterization of Mixed Ligand Dinuclear Metal(II) Complexes of Anthranilic Acid and Pyridine-2-aldoxime. Niger. J. Chem. Res. 2018, 23, 39–50. [Google Scholar]
- El-Roudi, A.M.; Aly, A.A.M.; Abd El-Gaber, A.A.; El-Shabasy, M. Reactivity of Some Transition Metal Complexes of Anthranilic Acid with Leucine and Monochloroacetic Acid. Croat. Chem. Acta 1988, 61, 775–782. [Google Scholar]
- Lekaak, A.K.; Mahdi, S.H. Spectroscopic, Structural and Antibacterial Activity of Mixed Ligand Complexes from Schiff Base with Anthranilic Acid. IOP Conf. Ser. J. Phys. Conf. Ser. 2019, 1234, 012089. [Google Scholar] [CrossRef]
- Zeng, C.-M.; Chang, L.-L.; Ying, M.-D.; Cao, J.; He, Q.-J.; Zhu, H.; Yang, B. Aldo–Keto Reductase AKR1C1–AKR1C4: Functions, Regulation, and Intervention for Anti-Cancer Therapy. Front. Pharmacol. 2017, 8, 119. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.M.; Janiak, C. Antibacterial susceptibility of new copper(II) N-pyruvoyl anthranilate complexes against marine bacterial strains—In search of new antibiofouling candidate. Arab. J. Chem. 2016, 9, 825–834. [Google Scholar] [CrossRef]
- Jayanthi, M.; Rajakumar, P. Synthesis and Antimicrobial Activity of Unsymmetrical Dendrimers With Indazole, Salicylates and Anthranilates as Surface Units. J. Heterocycl. Chem. 2017, 54, 1963–1973. [Google Scholar] [CrossRef]
- Merk, D.; Lamers, C.; Weber, J.; Flesch, D.; Gabler, M.; Proschak, E.; Zsilavecz, M.S. Anthranilic acid derivatives as nuclear receptor modulators—Development of novel PPAR selective and dual PPAR/FXR ligands. Bioorg. Med. Chem. 2015, 23, 499–514. [Google Scholar] [CrossRef]
- Patrone, J.D.; Pelz, N.F.; Bates, B.S.; Souza-Fagundes, E.M.; Vangamudi, B.; Camper, D.V.; Kuznetsov, A.G.; Browning, C.F.; Feldkamp, M.D.; Frank, A.O.; et al. Identification and optimization of anthranilic acid based inhibitors of replication protein A. Chem. Med. Chem. 2016, 11, 893–899. [Google Scholar] [CrossRef]
- Kwon, I.-S.; Kwak, J.H.; Pyo, S.; Lee, H.-W.; Kim, A.R.; Schmitz, F.J. Oscarellin an anthranilic acid derivative from a Philippine sponge, Oscarella stillans, as an inhibitor of inflammatory cytokines in macrophages. J. Nat. Prod. 2017, 80, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Schrey, H.; Muller, F.J.; Harz, P.; Rupcic, Z.; Stadler, M.; Spiteller, P. Nematicidal anthranilic acid derivatives from Laccaria species. Phytochemistry 2019, 160, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Noumeur, S.R.; Helaly, S.E.; Huttel, S.; Harzallah, D.; Stadler, M. Furanones and anthranilic acid derivatives from the endophytic fungus Dendrothyrium variisporum. Molecules 2017, 22, 1674. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Burgos, V.; Marín, V.; Camins, A.; Olloquequi, J.; González-Chavarría, I.; Ulrich, H.; Wyneken, U.; Luarte, A.; Ortiz, L.; et al. Caffeic Acid Phenethyl Ester (CAPE): Biosynthesis, Derivatives and Formulations with Neuroprotective Activities. Antioxidants 2023, 12, 1500. [Google Scholar] [CrossRef]
- Sampaio, J.G.; Pressete, C.G.; Costa, A.V.; Martins, F.T.; de Almeida Lima, G.D.; Ionta, M.; Teixeira, R.R. Methoxylated Cinnamic Esters with Antiproliferative and Antimetastatic Effects on Human Lung Adenocarcinoma Cells. Life 2023, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Qandil, A.M. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012, 13, 17244–17274. [Google Scholar] [CrossRef]
- Uludağ, M.O.; Ergün, B.Ç.; Alkan, D.A.; Ercan, N.; Özkan, G.Y.; Banoğlu, E. Stable Ester And Amide Conjugates Of Some Nsaids As Analgesic And Antiinflammatory compounds with improved biological activity. Turk. J. Chem. 2011, 35, 7. [Google Scholar] [CrossRef]
- Feng, S. Research Progress in the Synthesis of Esters. IOP Conf. Ser. Earth Environ. Sci. 2020, 440, 022019. [Google Scholar] [CrossRef]
- Kevin, B.; Robert, W.; Iain, G.; Kevin, D. Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: Challenges to the discovery scientist. Curr. Drug Metab. 2003, 4, 461–485. [Google Scholar] [CrossRef]
- Stoyanova, M.; Milusheva, M.; Gledacheva, V.; Stefanova, I.; Todorova, M.; Kircheva, N.; Angelova, S.; Pencheva, M.; Stojnova, K.; Tsoneva, S.; et al. Spasmolytic Activity and Anti-Inflammatory Effect of Novel Mebeverine Derivatives. Biomedicines 2024, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Milusheva, M.; Stoyanova, M.; Gledacheva, V.; Stefanova, I.; Todorova, M.; Pencheva, M.; Stojnova, K.; Tsoneva, S.; Nedialkov, P.; Nikolova, S. 2-Amino-N-Phenethylbenzamides for Irritable Bowel Syndrome Treatment. Molecules 2024, 29, 3375. [Google Scholar] [CrossRef]
- Milusheva, M.; Todorova, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Pencheva, M.; Nedialkov, P.; Tumbarski, Y.; Yanakieva, V.; Tsoneva, S.; et al. Novel Anthranilic Acid Hybrids—An Alternative Weapon against Inflammatory Diseases. Pharmaceuticals 2023, 16, 1660. [Google Scholar] [CrossRef]
- Milusheva, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Mihaylova, R.; Nedialkov, P.; Cherneva, E.; Tumbarski, Y.; Tsoneva, S.; Todorova, M.; et al. Synthesis, Molecular Docking, and Biological Evaluation of Novel Anthranilic Acid Hybrid and Its Diamides as Antispasmodics. Int. J. Mol. Sci. 2023, 24, 13855. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Li, L.; Ma, N.; Tian, J.; Sun, H.; Xu, Q.; Yang, Y.; Li, C. Synthesis of N-Unsubstituted and N3-Substituted Quinazoline-2,4(1H,3H)-diones from o-Aminobenzamides and CO2 at Atmospheric Pressure and Room Temperature. Org. Lett. 2023, 25, 2471–2475. [Google Scholar] [CrossRef]
- Azizian, J.; Mohammadi, A.A.; Karimi, A.R. An Efficient One-Pot Procedure for Preparation of 2,4(1H,3H)-Quinazolinediones and 2-Thioxoquinazolinone Derivatives Under Microwave Irradiation. Synth. Commun. Int. J. Rapid Commun. Synth. Org. Chem. 2003, 33, 415–420. [Google Scholar] [CrossRef]
- Shirode, P.R. A study of mixed ligand complexes of anthranilic acidsemicarbazone and benzaldehyde with Co(II), Ni(II) and Cu(II). World J. Pharm. Res. 2018, 7, 1135–1143. [Google Scholar] [CrossRef]
- Marinova, P.E.; Tsoneva, S.H.; Nikolova, S.A.; Ivanov, I.I. Novel complexes of n-substituted-4,5-dimethoxy-phenylethyl-2-arylketoamides with metal ions. Bulg. Chem. Commun. 2019, 51, 8–11. [Google Scholar]
- Alwan, A.H.M. Spectroscopic and Magnetic Study of Mixed Ligand Complexes with Divalent Transition Metals Using Azo-Azomethine Ligands. Univ. Thi-Qar J. 2025, 20, 1–19. Available online: https://jutq.utq.edu.iq/index.php/main/article/view/397 (accessed on 28 June 2025). [CrossRef]
- Al-Noor, T.H.; AL-Jeboori, A.T.; Ghanim, F.H. Synthesis and characterization of the mixed ligand complexes (L-alanine and anthranilic acid) with some transition Ions. Diyala J. Pure Sci. 2010, 6, 103–110. Available online: https://iasj.rdd.edu.iq/journals/uploads/2024/12/05/ab9ac4b3982c30c3ded242649e575b1f.pdf (accessed on 28 June 2025).
- Mohamed, A.; Dayo, M.; Alahmadi, S.; Ali, S. Anti-Inflammatory and Antimicrobial Activity of Silver Nanoparticles Green-Synthesized Using Extracts of Different Plants. Nanomaterials 2024, 14, 1383. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.K.; Vidyarthi, S.N.; Prakash, O.; Baluni, A. Antimicrobial Activity of Co(II), Ni(II) and Cu(II) Coordination Compounds with Nitrogen, Oxygen Containing Schiff Base. Orient. J. Chem. 2013, 29, 801–806. [Google Scholar] [CrossRef]
- Al-Noor, T.H.; Ali, K.F.; Jarad, A.J.; Kindeel, A.S. Synthesis, Spectral and Antimicrobial Activity of Mixed Ligand Complexes of Co(II), Ni(II), Cu(II) and Zn(II) with Anthranilic Acid and Tributylphosphine. Chem. Mater. Res. 2013, 3, 126–134. [Google Scholar]
- Jayachandiran, K.; Esha, S.; Lakshmi, M.S.; Mahalakshmi, S.; Arockiasamy, S. Synthesis and Structural Insights of Bis(2-Methoxy-6-{[(2-Methylpropyl)imino]methyl}phenolato) Nickel(II) Complex through DFT and Docking Investigations. Sci. Rep. 2025, 15, 1751. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oluwarinde, B.O.; Montso, P.K.; Ateba, C.N.; Onwudiwe, D.C. Antimicrobial Activities of Cu(II), In(III), and Sb(III) Complexes of N-Methyl-N–Phenyl Dithiocarbamate Complexes. Results Chem. 2021, 3, 100241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).