Modification Strategies of g-C3N4-Based Materials for Enhanced Photoelectrocatalytic Degradation of Pollutants: A Review
Abstract
1. Introduction
2. Development History and Structures of Graphite Carbon Nitride
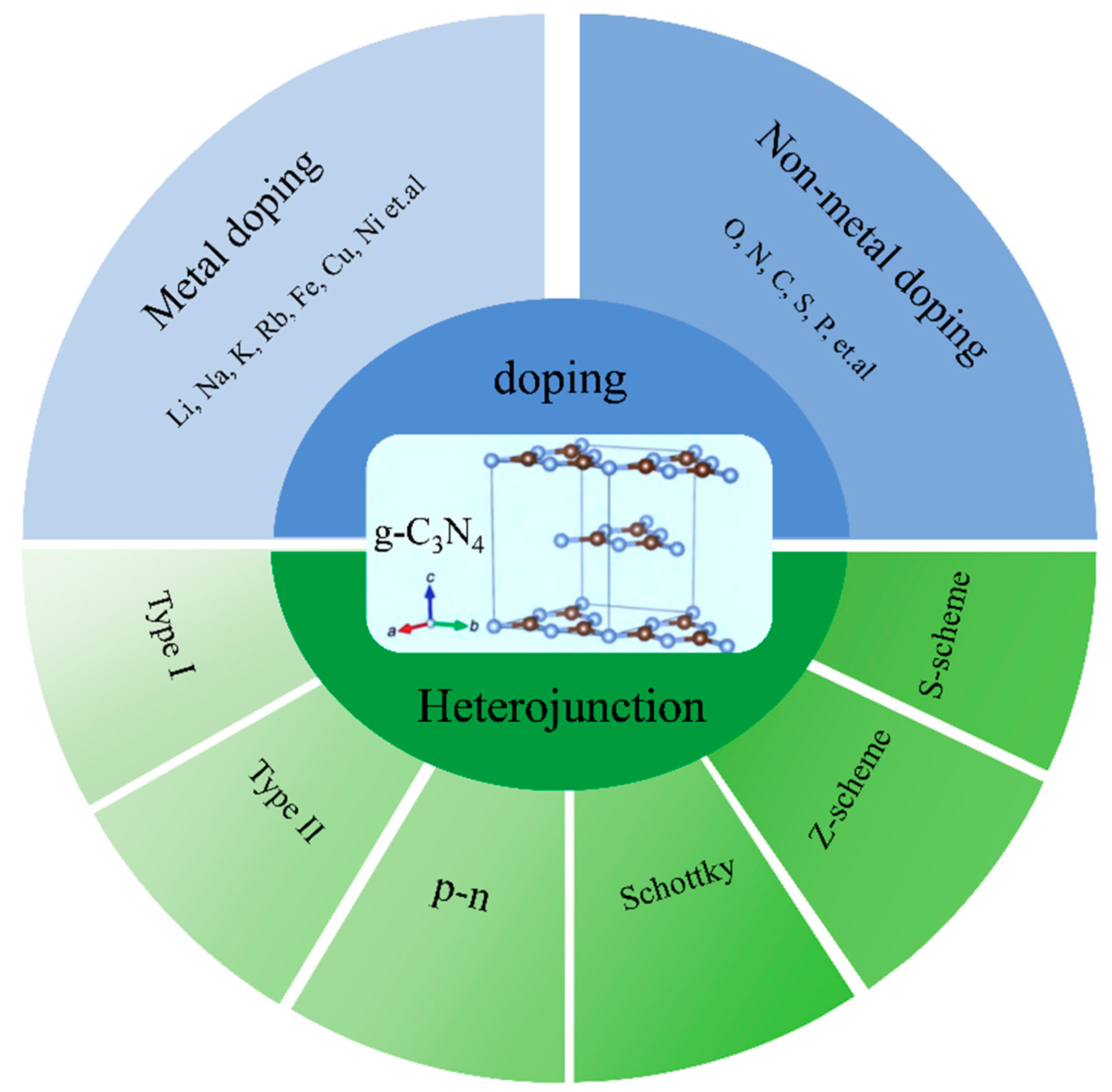
3. Graphitic Carbon Nitride Modification
3.1. Dopant Modification
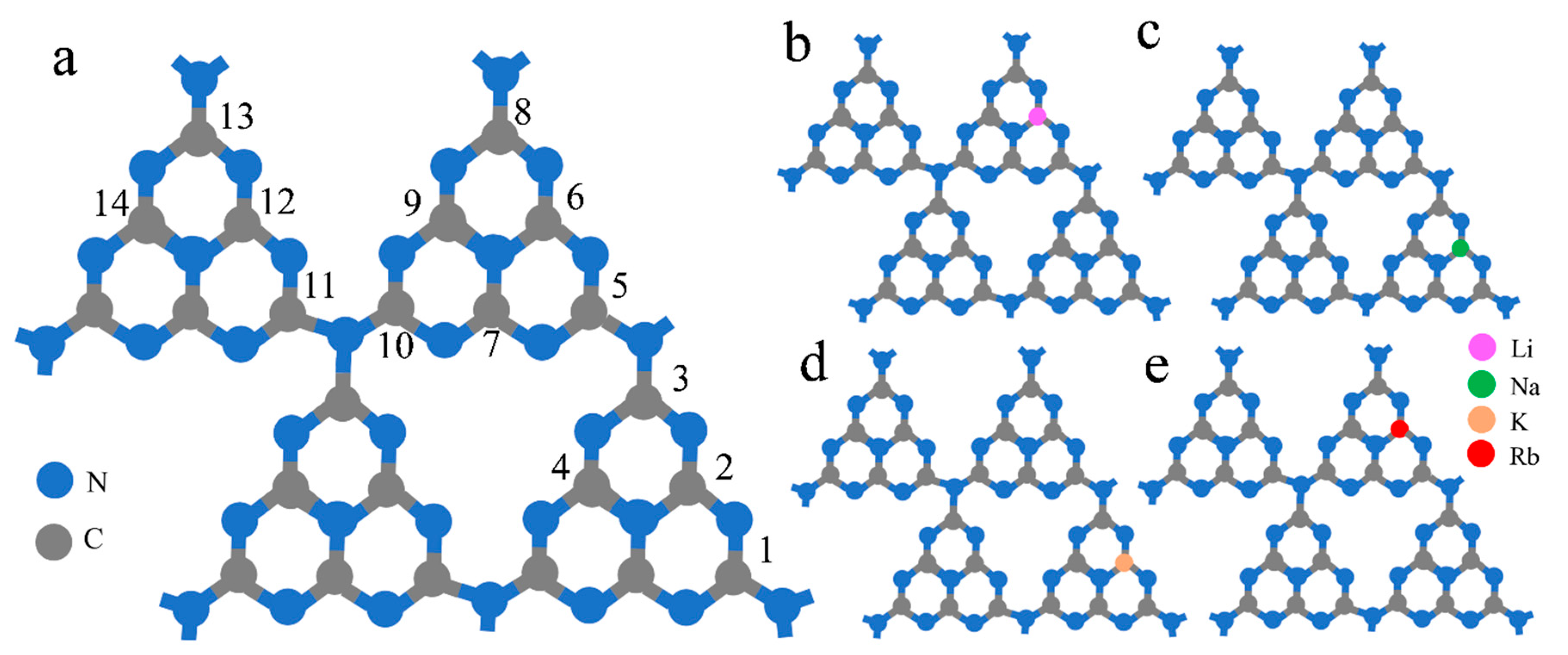
3.1.1. Metal Doping
3.1.2. Non-Metal Doping
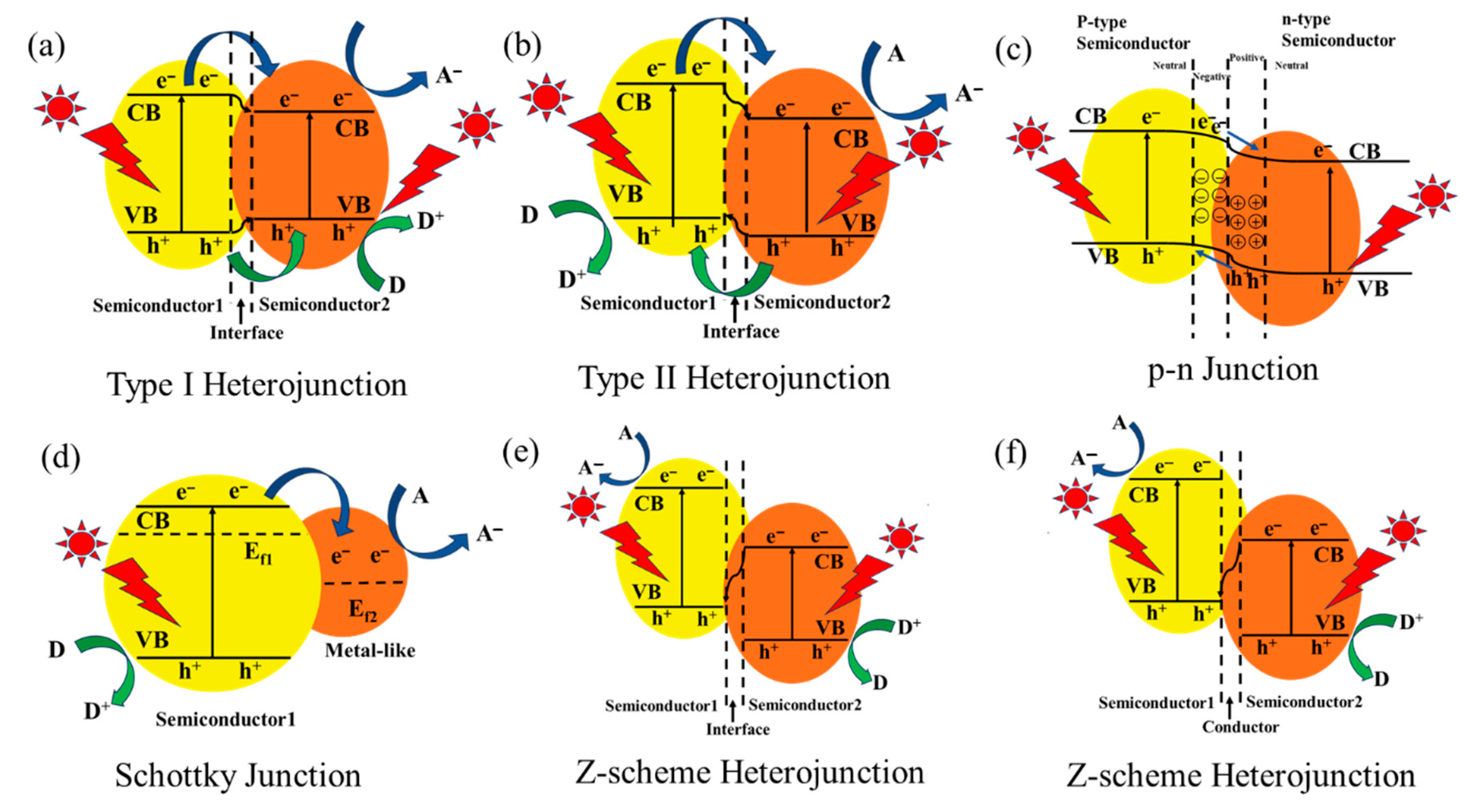
3.2. Constructing Heterojunction Structures
| Classification | Typical Example | Application | |
|---|---|---|---|
| Type I heterojunction | ZnIn2S4/ultrathin-g-C3N4 [85] | Photocatalytic H2 production reaction under visible light | |
| CdIn2S4/g-C3N4 [86] | Photodegradation of RB19 under visible-light irradiation | ||
| Type II heterojunction | g-C3N4/g-C3N4 [87] (with different raw materials) | Enhancement of photocatalytic H2 production and CO2 reduction activity | |
| g-C3N4@Cs2AgBiBr6 (CABB) [88] | CO2 photoreduction process | ||
| p-n junction | Co3O4/n-type g-C3N4 [89] | CO2 reduction | |
| P-group intercalated g-C3N4 (NP–CN)/Bi2WO6 (BWO), NPB [90] | Photodegradation of p-nitrophenol into harmless products | ||
| Schottky junction | Ag-decorated P-doped g-C3N4 nanosheets (Ag-(P/CNNS)) [91] | Water splitting and degradation of rhodamine B (RhB) | |
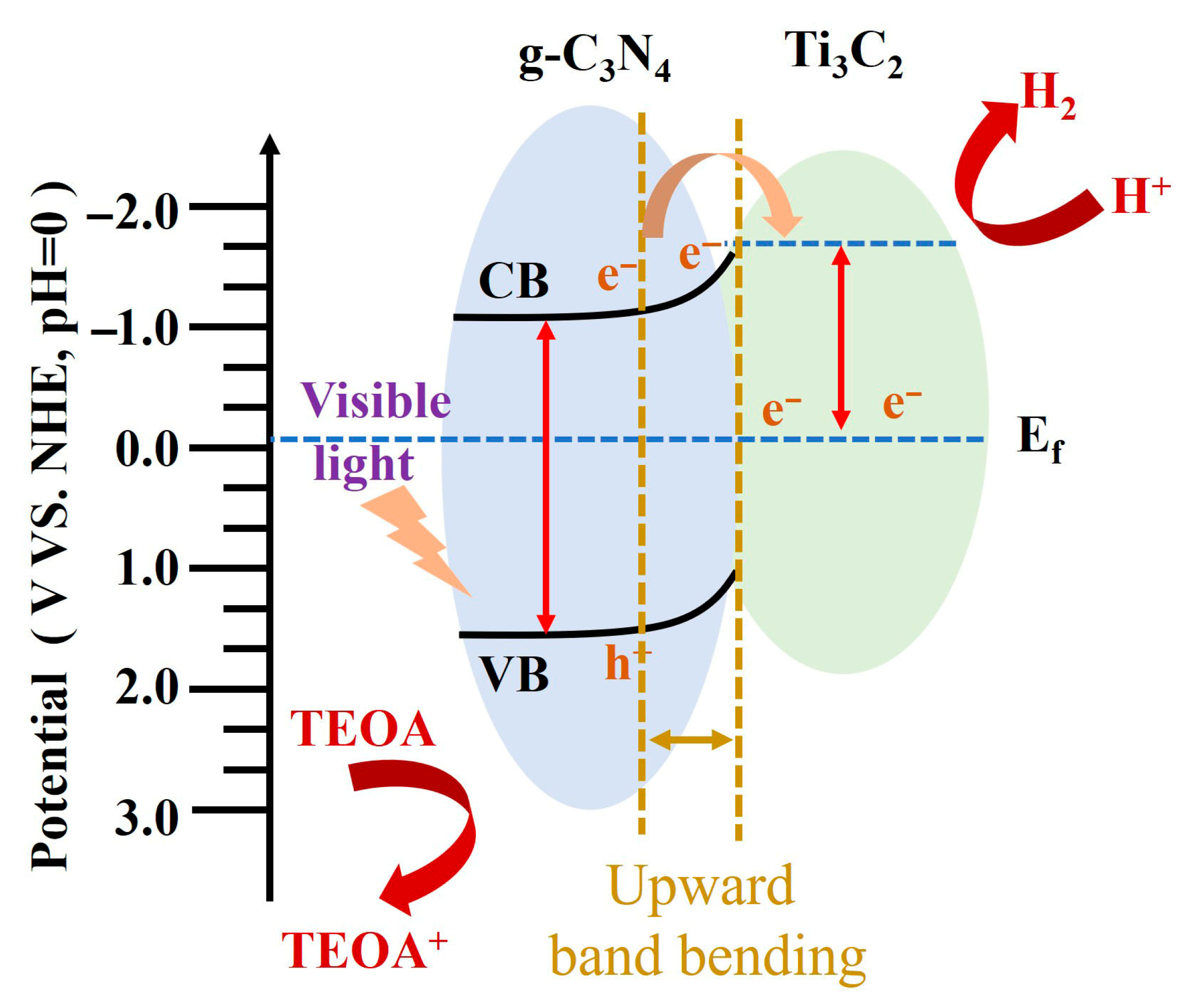
| g-C3N4/Ti3C2 [92] | Efficient visible-light photocatalytic hydrogen | ||
| Z-scheme heterojunction | Direct Z-scheme | BiOI/g-C3N4 [93] | Photocatalytic degradation of phenol |
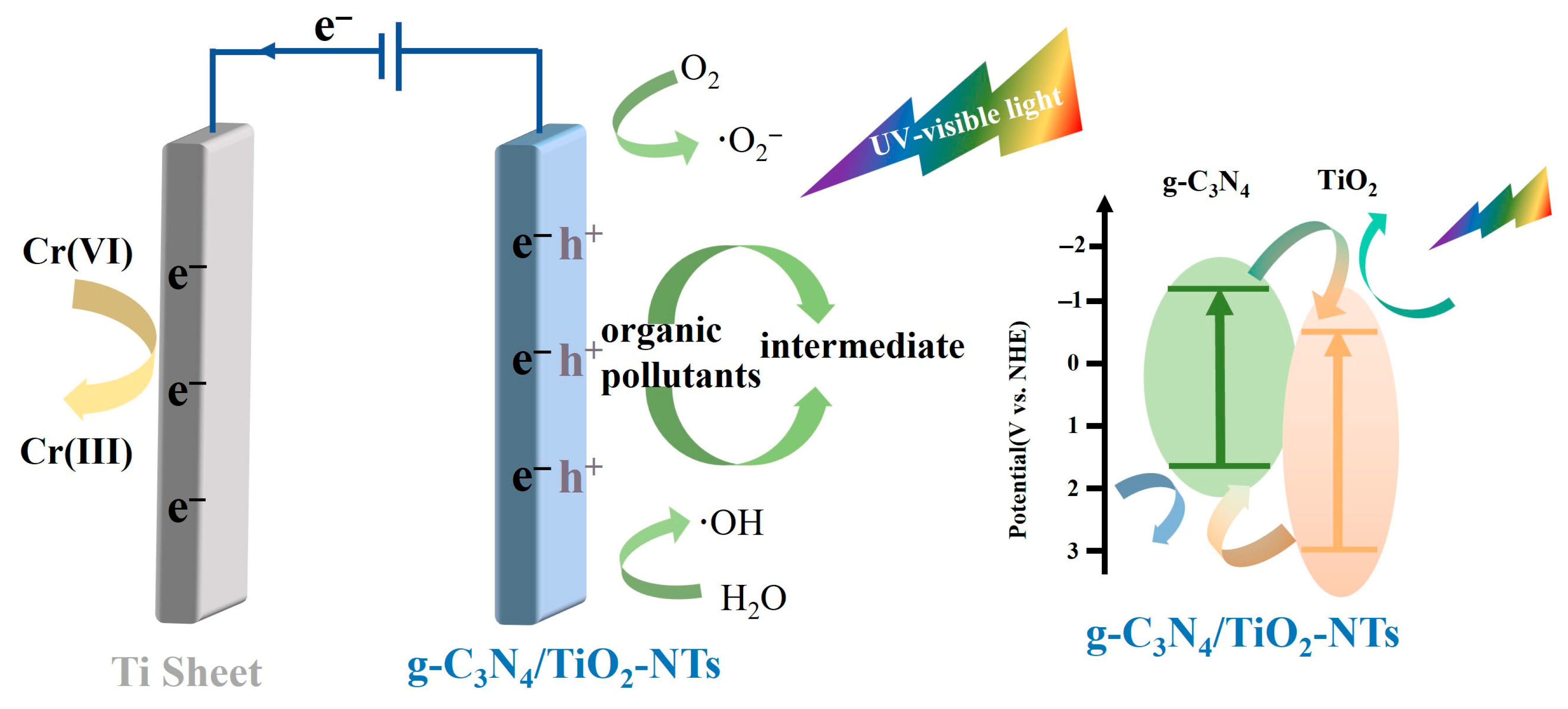
| Indirect Z-scheme | TiO2/BC/g-C3N4 [94] | Photocatalytic reduction of Cr(VI) in aqueous solution | |
| S-scheme heterojunction | C–O-bridged CeO2/g-C3N4 (cCN) [95] | Photofixation of N2, H2 energy generation, and methyl orange photodegradation | |
3.2.1. Type I Heterojunction
3.2.2. Type II Heterojunction
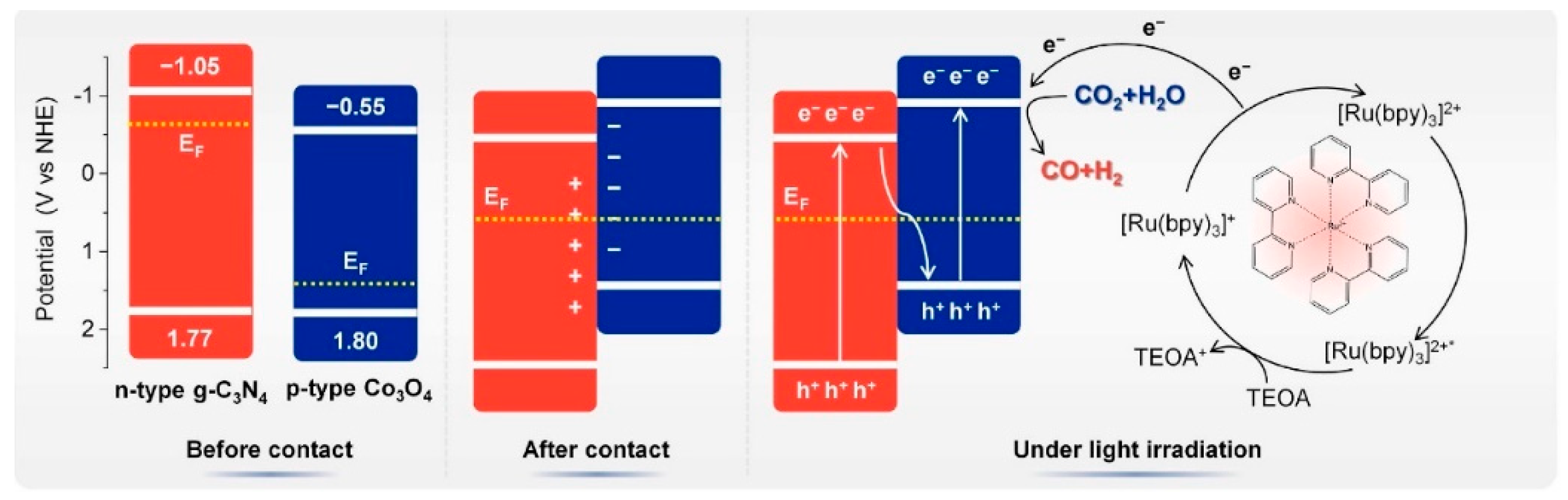
3.2.3. The p-n Junction
3.2.4. Schottky Junction
3.2.5. Z-Scheme Heterojunction
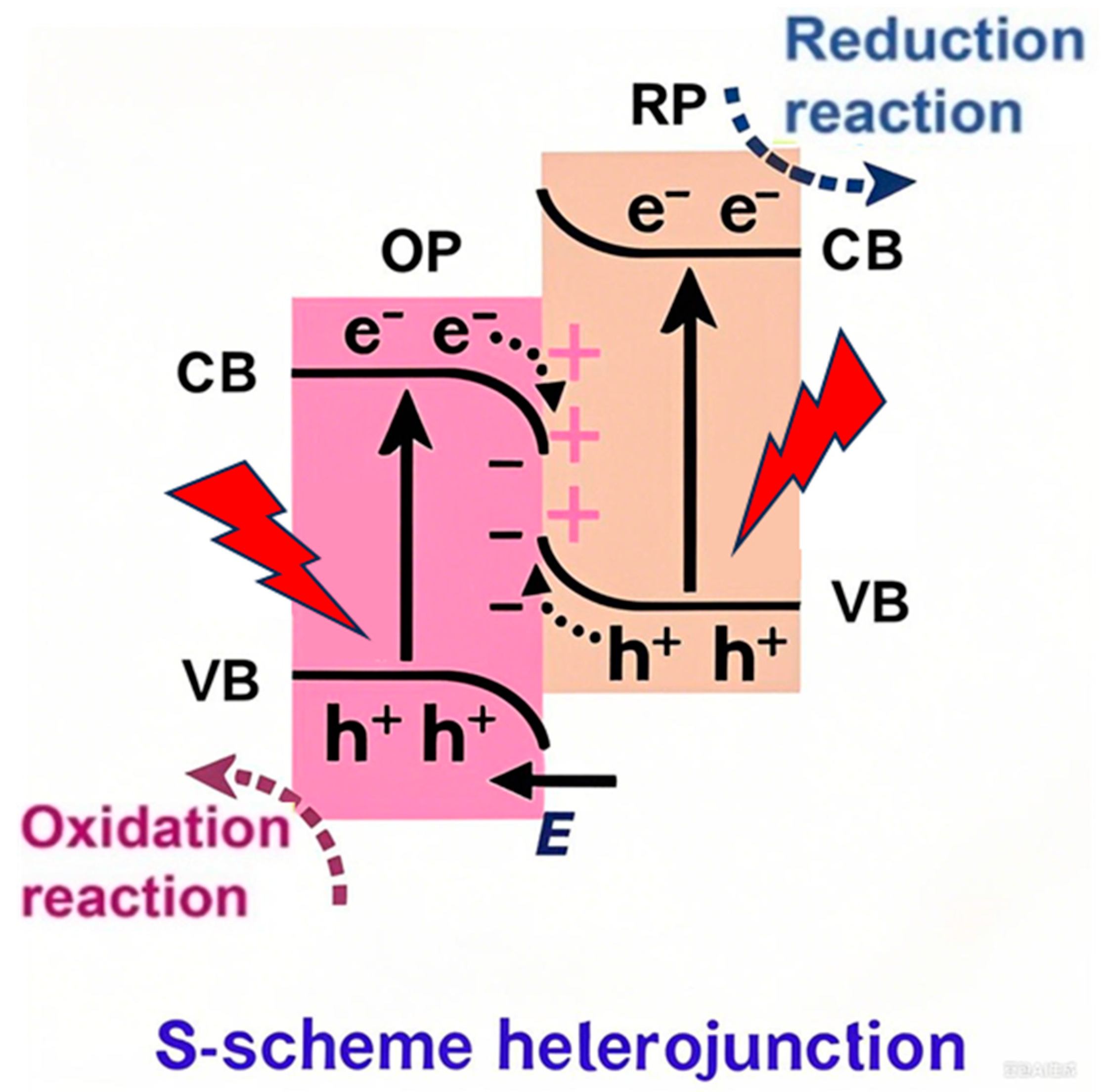
3.2.6. S-Scheme Heterojunction
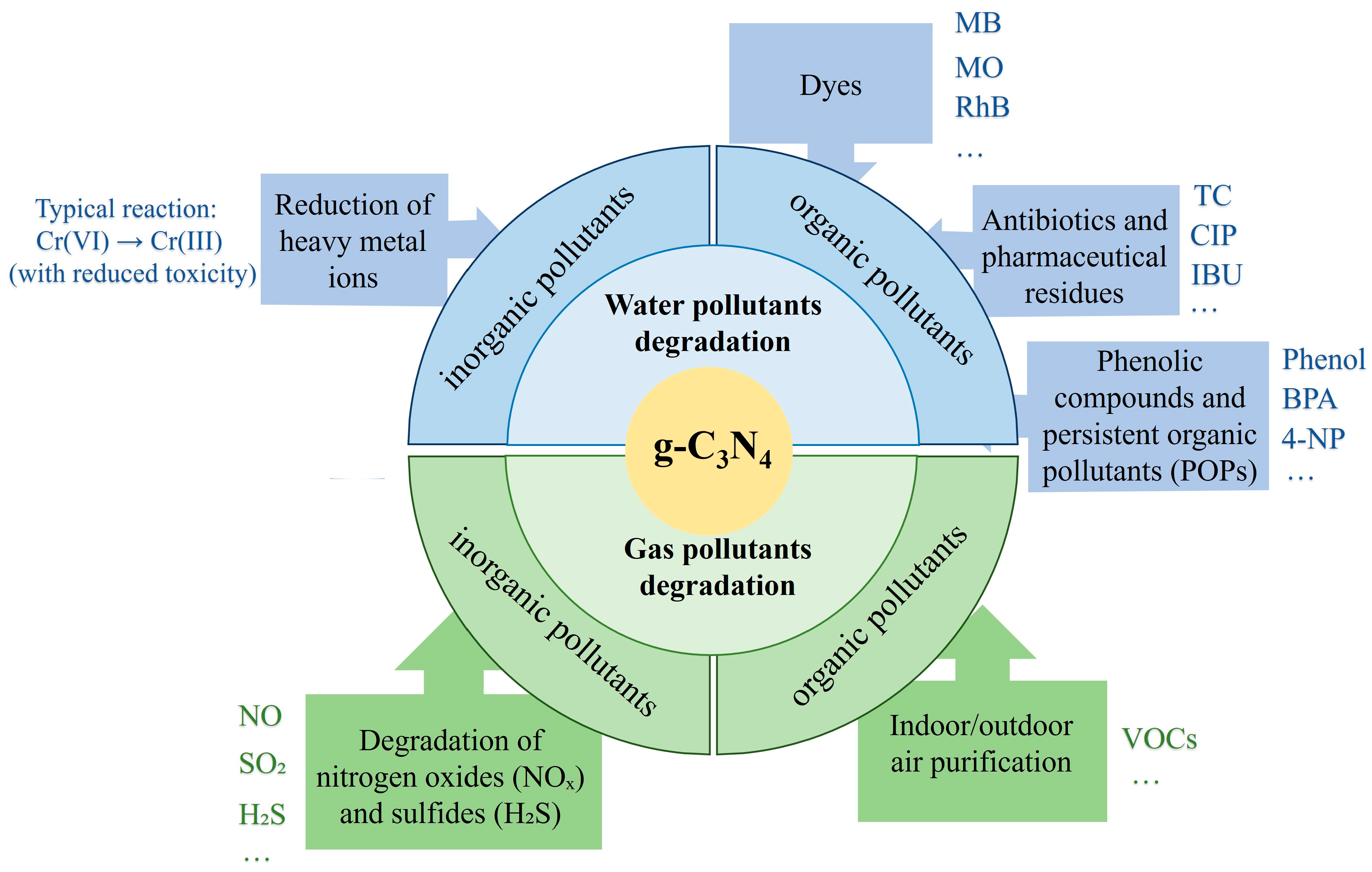
4. Application of Graphitic Carbon Nitride in Photocatalytic and Photoelectrocatalytic Degradation
4.1. Methodological Principles of Photocatalysis and Photoelectrocatalysis
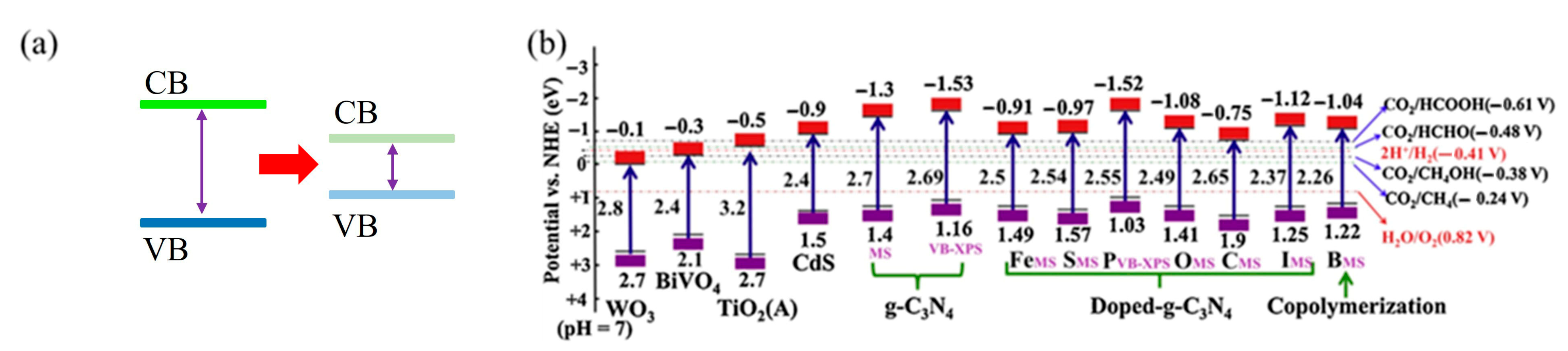
4.2. Relationship Between g-C3N4 Modification and Photo Response
- Being combined with a co-catalyst
- 2.
- Enhanced light absorption
- 3.
- Improvement in transmission efficiency
- 4.
- Good stability
4.3. Photocatalytic Performance of g-C3N4 and Its Modified Material Under Different Light Sources
4.3.1. UV Photocatalytic Degradation
4.3.2. Visible-Light Photocatalytic Degradation
4.3.3. Near-Infrared Photocatalytic Degradation
4.4. Application of g-C3N4 and Its Modified Materials in Photoelectrocatalysis Under Different Light Sources
5. Conclusions and Prospective
- Although the photoelectrocatalytic activity of g-C3N4-based photoelectrocatalytic materials has been significantly improved, it still cannot meet the production demand. For the final application of g-C3N4 photocatalysts in real pollution treatment, research needs to consider the simplicity and environmental safety of the synthesis method, as well as the recovery and recycling of catalysts.
- There is a lack of systematic understanding of how modifying g-C3N4-based photocatalysts affects catalytic activity. Therefore, it is necessary to explore the modification mechanism of g-C3N4, elucidate the electron transfer pathways inside the catalysts, and improve the controllability of the modification of g-C3N4-based materials.
- More kinds of pollutants with different properties were selected for photo- and photoelectrocatalytic experiments, and the effect of the solution injection volume on degradation efficiency should not be neglected so as to provide ideas and references for the development of more efficient g-C3N4-based photo- and photoelectrocatalysts.
Funding
Conflicts of Interest
References
- Li, Z.; Xu, X.; Sheng, X.; Lin, P.; Tang, J.; Pan, L.; Kaneti, Y.V.; Yang, T.; Yamauchi, Y. Solar-Powered Sustainable Water Production: State-of-the-Art Technologies for Sunlight–Energy–Water Nexus. ACS Nano 2021, 15, 12535–12566. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Bui, X.T.; Rakib, M.R.J.; Nguyen, H.L.; Truong, Q.M.; Hoang, H.G.; Tran, H.-T.; Malafaia, G.; Idris, A.M. Occurrence and fate of pharmaceutical pollutants in wastewater: Insights on ecotoxicity, health risk, and state–of–the-art removal. Chemosphere 2024, 354, 141678. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhou, R.; Xiong, L.; Li, H.; Liu, Q.; Zheng, L.; Guo, Z.; Deng, Z. Preparation of a Ti0.7W0.3O2/TiO2 nanocomposite interfacial photocatalyst and its photocatalytic degradation of phenol pollutants in wastewater. Nanoscale Adv. 2020, 2, 425–437. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, Y.X.; Zhang, S. Editorial: Photocatalysis for Environmental Applications. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, W.C.; Guo, Y.J.; Li, S.L.; Chen, Y.R.; Wang, H.; Bian, Z.Y. Recent advances in photoelectrocatalytic advanced oxidation processes: From mechanism understanding to catalyst design and actual applications. Chem. Eng. J. 2023, 455, 140801. [Google Scholar] [CrossRef]
- Li, D.; Qu, J. The progress of catalytic technologies in water purification: A review. J. Environ. Sci. 2009, 21, 713–719. [Google Scholar] [CrossRef]
- Galushchinskiy, A.; González-Gómez, R.; McCarthy, K.; Farràs, P.; Savateev, A. Progress in Development of Photocatalytic Processes for Synthesis of Fuels and Organic Compounds under Outdoor Solar Light. Energy Fuels 2022, 36, 4625–4639. [Google Scholar] [CrossRef]
- He, Y.; Yin, L.; Yuan, N.; Zhang, G. Adsorption and activation, active site and reaction pathway of photocatalytic CO2 reduction: A review. Chem. Eng. J. 2024, 481, 148754. [Google Scholar] [CrossRef]
- Mishra, S.; Acharya, L.; Marandi, B.; Sanjay, K.; Acharya, R. Boosted photocatalytic accomplishment of 3D/2D hierarchical structured Bi4O5I2/g-C3N4 p-n type direct Z-scheme heterojunction towards synchronous elimination of Cr(VI) and tetracycline. Diam. Relat. Mater. 2024, 142, 110834. [Google Scholar] [CrossRef]
- Acharya, R.; Mishra, S. Visible light sensitized graphitic carbon nitride based semiconducting materials for photoelectrochemical water splitting. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Zhu, P.J.; Yan, Y.N.; Zhou, Y.; Qi, Z.J.; Li, Y.F.; Chen, C.M. Thermal Properties of Graphene and Graphene-Based Nanocomposites: A Review. ACS Appl. Nano Mater. 2024, 7, 8377–9815. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, Y. Enhancement of mineralization ability for phenol via synergetic effect of photoelectrocatalysis of g-C3N4 film. Appl. Catal. B Environ. 2016, 180, 324–329. [Google Scholar] [CrossRef]
- Paul, S.; Panja, S.; Hazra, N.; Gayen, K.; Banerjee, A. Carbon Dot as Visible-Light Photoredox Catalysts for a Myriad of Organic Transformations. J. Org. Chem. 2024, 89, 91–100. [Google Scholar] [CrossRef]
- Xie, S.Y.; Li, X.; Zheng, H.W.; Feng, L.; Khan, S. Research Progress of Organic Carbon Nanotubes-Modified Metal-Composite Photocatalytic Materials in Water Treatment. Mini-Rev. Org. Chem. 2022, 19, 898–905. [Google Scholar] [CrossRef]
- Xiangjuan, C.; Huan, W.; Weijia, A.; Li, L.; Wenquan, C. Study on Photoelectrocatalysis of Organic Carbon Materials. Prog. Chem. 2022, 34, 2361–2372. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, H.C.; Liu, M.Q.; Xu, X.J. Metal-Free Graphitic Carbon Nitride Photocatalyst Goes Into Two-Dimensional Time. Front. Chem. 2018, 6, 551. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Zou, X.; Sun, Z.; Hu, Y.H. g-C3N4-based photoelectrodes for photoelectrochemical water splitting: A review. J. Mater. Chem. A 2020, 8, 21474–21502. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 751–790. [Google Scholar] [CrossRef]
- Schwarzer, A.; Saplinova, T.; Kroke, E. Tri-s-triazines (s-heptazines)-From a “mystery molecule” to industrially relevant carbon nitride materials. Coord. Chem. Rev. 2013, 257, 2032–2062. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. New Light on an Old Story: Formation of Melam during Thermal Condensation of Melamine. Chem. Eur. J. 2007, 13, 4956–4968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fugane, K.; Mori, T.; Niu, L.; Ye, J. Wet chemical synthesis of nitrogen-doped graphene towards oxygen reduction electrocatalysts without high-temperature pyrolysis. J. Mater. Chem. 2012, 22, 6575–6580. [Google Scholar] [CrossRef]
- Zhang, Y.; Mori, T.; Ye, J. Polymeric Carbon Nitrides: Semiconducting Properties and Emerging Applications in Photocatalysis and Photoelectrochemical Energy Conversion. Sci. Adv. Mater. 2012, 4, 282–291. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J. ChemInform Abstract: Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as Metal-Free Catalysts. J. Mater. Chem. 2008, 40, 4893–4908. [Google Scholar] [CrossRef]
- Wang, J.B.; Lei, J.L.; Wang, R.H. Diffraction-pattern calculation and phase identification of hypothetical crystalline C3N4. Phys. Rev. B 1998, 58, 11890–11895. [Google Scholar] [CrossRef]
- Yao, H.; Ching, W.Y. Optical properties of beta -C3N4 and its pressure dependence. Phys. Rev. B Condens. Matter 1994, 50, 11231–11234. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wen, B.; Melnik, R. Relative stability of nanosized β-C3N4 and graphitic C3N4 from first principles calculations. Phys. E Low-Dimens. Syst. Nanostructures 2012, 45, 190–193. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Structural properties and electronic structure of low-compressibility materials: Beta -Si3N4 and hypothetical beta-C3N4. Phys. Rev. B Condens. Matter 1990, 41, 10727–10734. [Google Scholar] [CrossRef] [PubMed]
- Mattesini, M.; Matar, S.F.; Etourneau, J. Stability and electronic property investigations of the graphitic C3N4 system showing an orthorhombic unit cell. J. Mater. Chem. 2000, 10, 709–713. [Google Scholar] [CrossRef]
- Sahoo, S.; Acharya, R. An overview on recent developments in synthesis and molecular level structure of visible-light responsive g-C3N4 photocatalyst towards environmental remediation. Mater. Today Proc. 2021, 35, 150–155. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Takanabe, K.; Kamata, K.; Wang, X.C.; Antonietti, M.; Kubota, J.; Domen, K. Photocatalytic hydrogen evolution on dye-sensitized mesoporous carbon nitride photocatalyst with magnesium phthalocyanine. Phys. Chem. Chem. Phys. 2010, 12, 13020–13025. [Google Scholar] [CrossRef]
- Sohail, M.; Anwar, U.; Taha, T.A.; Qazi, H.I.A.; Al-Sehemi, A.G.; Ullah, S.; Algarni, H.; Ahmed, I.M.; Amin, M.A.; Palamanit, A.; et al. Nanostructured materials based on g-C3N4 for enhanced photocatalytic activity and potentials application: A review. Arab. J. Chem. 2022, 15, 104070. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, L.; Liang, S.J.; Wu, W.; Wang, G.; Lee, C.H.; Ong, W.L.; Yang, H.Y.; Ang, L.K.; Yang, S.A.; et al. Efficient Ohmic contacts and built-in atomic sublayer protection in MoSi2N4 and WSi2N4 monolayers. Npj 2D Mater. Appl. 2021, 5, 71. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ji, N.; Wei, L.; Liang, Q.; Li, J.; Tian, Z.; Su, J.; Chen, Q. Modulation of the electronic structure of metallic bismuth catalysts by cerium doping to facilitate electrocatalytic CO2 reduction to formate. J. Mater. Chem. A 2024, 12, 7528–7535. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Z.; Zou, Y.; Chen, J.; Shi, J.-W. The progress of g-C3N4 in photocatalytic H2 evolution: From fabrication to modification. Coord. Chem. Rev. 2024, 500, 215489. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Tyborski, T.; Merschjann, C.; Orthmann, S.; Yang, F.; Lux-Steiner, M.C.; Schedel-Niedrig, T. Crystal structure of polymeric carbon nitride and the determination of its process-temperature-induced modifications. J. Phys. Condens. Matter 2013, 25, 395402. [Google Scholar] [CrossRef] [PubMed]
- Buriak, J.M.; Toro, C.; Choi, K.-S. Chemistry of Materials for Water Splitting Reactions. Chem. Mater. 2018, 30, 7325–7327. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Döblinger, M.; Sehnert, J.; Seyfarth, L.; Senker, J.; Oeckler, O.; Schnick, W. Unmasking Melon by a Complementary Approach Employing Electron Diffraction, Solid-State NMR Spectroscopy, and Theoretical Calculations—Structural Characterization of a Carbon Nitride Polymer. Chem. A Eur. J. 2007, 13, 4969–4980. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, K.; Xu, B.; Yang, C.; Wang, H.; Zhan, P.; Xie, S. Synthesis of a novel Z-scheme Ag/WO3/g-C3N4 nanophotocatalyst for degradation of oxytetracycline hydrochloride under visible light. Mater. Sci. Semicond. Process. 2022, 137, 106168. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Fang, J.; Wu, K.; Feng, W.; Zhu, X.; Fang, Z. Facile synthesis of iron and cerium co-doped g-C3N4 with synergistic effect to enhance visible-light photocatalytic performance. Mater. Res. Bull. 2020, 125, 110812. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, W.; Dang, L.; Mao, Y.; Wu, J.; Xu, K. Recent Progress in Doped g-C3N4 Photocatalyst for Solar Water Splitting: A Review. Front. Chem. 2022, 10, 955065. [Google Scholar] [CrossRef]
- Hu, X.; Guo, R.T.; Lin, Z.D.; Bi, Z.X.; Chen, X.; Wang, J.; Pan, W. Construction of Carbon Dot-Modified g-C3N4/BiOIO3 Z-Scheme Heterojunction for Boosting Photocatalytic CO2 Reduction under Full Spectrum Light. ACS Sustain. Chem. Eng. 2022, 10, 11143–11153. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Li, Y.; Wang, D.H.; Hong, X.D.; Liang, B. Recent Advances in Heteroatom Doped Graphitic Carbon Nitride (g-C3N4) and g-C3N4/Metal Oxide Composite Photocatalysts. Curr. Org. Chem. 2020, 24, 673–693. [Google Scholar] [CrossRef]
- Cui, W.; Chen, P.; Chen, L.C.; Li, J.Y.; Zhou, Y.; Dong, F. Alkali/alkaline-earth metal intercalated g-C3N4 induced charge redistribution and optimized photocatalysis: Status and challenges. J. Phys. Energy 2021, 3, 032008. [Google Scholar] [CrossRef]
- Bai, L.Q.; Huang, H.W.; Yu, S.X.; Zhang, D.Y.; Huang, H.T.; Zhang, Y.H. Role of transition metal oxides in g-C3N4-based heterojunctions for photocatalysis and supercapacitors. J. Energy Chem. 2022, 64, 214–235. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Lee, K.C.; Pan, G.T.; Leo, B.F.; Chong, S.; Pan, K.L. Co-doped, tri-doped, and rare-earth-doped g-C3N4 for photocatalytic applications: State-of-the-Art. Catalysts 2022, 12, 586. [Google Scholar] [CrossRef]
- Arunachalapandi, M.; Roopan, S.M. Environment friendly g-C3N4-Based catalysts and their recent strategy in organic transformations. High. Energy Chem. 2022, 56, 73–90. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.Q.; Liu, Z.X.; Zhu, Z.; Tang, X.; Wang, Y.M. Study on optical properties of alkali metal doped g-C3N4 and their photocatalytic activity for reduction of CO2. Chem. Phys. Lett. 2020, 751, 137467. [Google Scholar] [CrossRef]
- Ye, S.; Qiu, L.G.; Yuan, Y.P.; Zhu, Y.J.; Xia, J.; Zhu, J.F. Facile fabrication of magnetically separable graphitic carbon nitride photocatalysts with enhanced photocatalytic activity under visible light. J. Mater. Chem. A 2013, 1, 3008–3015. [Google Scholar] [CrossRef]
- Mugaka, B.P.; Zhang, S.; Li, R.Q.; Ma, Y.; Wang, B.; Hong, J.; Hu, Y.H.; Ding, Y.; Xia, X.H. One-Pot Preparation of Peptide-Doped Metal-Amino Acid Framework for General Encapsulation and Targeted Delivery. ACS Appl. Mater. Interfaces 2021, 13, 11195–11204. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xiong, J.; Lei, S.; Wang, W.; Ou, X.; Xu, Y.; Xiao, Y.; Cheng, B. Nickel formate induced high-level in situ Ni-doping of g-C3N4 for a tunable band structure and enhanced photocatalytic performance. J. Mater. Chem. A 2019, 7, 22385–22397. [Google Scholar] [CrossRef]
- Ma, J.; Jia, N.; Shen, C.; Liu, W.; Wen, Y. Stable cuprous active sites in Cu+-graphitic carbon nitride: Structure analysis and performance in Fenton-like reactions. J. Hazard. Mater. 2019, 378, 120782. [Google Scholar] [CrossRef]
- Ruan, L.; Xu, G.; Gu, L.; Li, C.; Zhu, Y.; Lu, Y. The physical properties of Li-doped g-C3N4 monolayer sheet investigated by the first-principles. Mater. Res. Bull. 2015, 66, 156–162. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Namgung, G.; Noh, J.S. Facile synthesis of porous metal-doped ZnO/g-C3N4 composites for highly efficient photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 368, 110–119. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Chen, P.; Yao, J.; Dong, X. One-Step Hydrothermal Deposition of Ag-Doped g-C3N4-TiO2 Nanocomposites on Cotton Fabric Surface with Enhanced Photocatalytic Activity. Fibers Polym. 2023, 24, 575–588. [Google Scholar] [CrossRef]
- Ji, S.; Yang, Y.; Zhou, Z.; Li, X.; Liu, Y. Photocatalysis-Fenton of Fe-doped g-C3N4 catalyst and its excellent degradation performance towards RhB. J. Water Process Eng. 2021, 40, 101804. [Google Scholar] [CrossRef]
- Tang, C.S.; Cheng, M.; Lai, C.; Li, L.; Yang, X.F.; Du, L.; Zhang, G.X.; Wang, G.F.; Yang, L. Recent progress in the applications of non-metal modified graphitic carbon nitride in photocatalysis. Coord. Chem. Rev. 2023, 474, 214846. [Google Scholar] [CrossRef]
- Starukh, H.; Praus, P. Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts 2020, 10, 1119. [Google Scholar] [CrossRef]
- Kuchmiy, S.Y.; Stroyuk, O.L. Photocatalytic Fixation of Molecular Nitrogen in Systems Based on Graphite-Like Carbon Nitride: A Review. Theor. Exp. Chem. 2021, 57, 85–112. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, F.; Cao, F.; Zhao, X.; Meng, S.; Cui, Y. Facile synthesis of oxygen doped carbon nitride hollow microsphere for photocatalysis. Appl. Catal. B Environ. 2017, 206, 417–425. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, S. Progress and Prospects of Non-Metal Doped Graphitic Carbon Nitride for Improved Photocatalytic Performances. Acta Phys. Chim. Sin. 2020, 36, 1905080. [Google Scholar] [CrossRef]
- Arumugam, M.; Tahir, M.; Praserthdam, P. Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4. Chemosphere 2022, 286, 131765. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Dangwang Dikdim, J.M.; Gong, Y.; Noumi, G.B.; Sieliechi, J.M.; Zhao, X.; Ma, N.; Yang, M.; Tchatchueng, J.B. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation. Chemosphere 2019, 217, 833–842. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Wang, Y.; Gong, Y.; Cao, D.; Qiao, M. Peroxymonosulfate-enhanced visible light photocatalytic degradation of bisphenol A by perylene imide-modified g-C3N4. Appl. Catal. B Environ. 2018, 237, 976–985. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, H.; Zhang, X.; Zhao, M. Electron spin-polarization and band gap engineering in carbon-modified graphitic carbon nitrides. J. Mater. Chem. C 2015, 3, 10886–10891. [Google Scholar] [CrossRef]
- Ling, F.; Li, W.; Ye, L. The synergistic effect of non-metal doping or defect engineering and interface coupling on the photocatalytic property of g-C3N4: First-principle investigations. Appl. Surf. Sci. 2019, 473, 386–392. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Li, Z.; Raziq, F.; Liu, C.; Bai, L.; Jing, L. Surface-engineering strategies for g-C3N4 as efficient visible-light photocatalyst. Curr. Opin. Green Sustain. Chem. 2017, 6, 57–62. [Google Scholar] [CrossRef]
- Que, M.D.; Cai, W.H.; Chen, J.; Zhu, L.L.; Yang, Y.W. Recent advances in g-C3N4 composites within four types of heterojunctions for photocatalytic CO2 reduction. Nanoscale 2021, 13, 6692–6712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gong, X.; Wang, S.; Jiang, W.; Xuan, S. Shear Stiffening Gels for Intelligent Anti-impact Applications. Cell Rep. Phys. Sci. 2020, 1, 100266. [Google Scholar] [CrossRef]
- Medina-Llamas, M.; Speltini, A.; Profumo, A.; Panzarea, F.; Milella, A.; Fracassi, F.; Listorti, A.; Malavasi, L. Preparation of Heterojunctions Based on Cs3Bi2Br9 Nanocrystals and g-C3N4 Nanosheets for Photocatalytic Hydrogen Evolution. Nanomaterials 2023, 13, 263. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.F.; He, Y.F.; Li, M.; Liu, Y.; Chen, M.M.; Cao, D.W. Charge transfer in photocatalysis of direct Z-scheme g-C3N4-based ferroelectric heterojunction. J. Alloys Compd. 2022, 893, 162270. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, D.; Ong, W.J. Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: A review. Chin. J. Catal. 2019, 40, 289–319. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Acharya, R.; Pati, S.; Parida, K. A review on visible light driven spinel ferrite-g-C3N4 photocatalytic systems with enhanced solar light utilization. J. Mol. Liq. 2022, 357, 119105. [Google Scholar] [CrossRef]
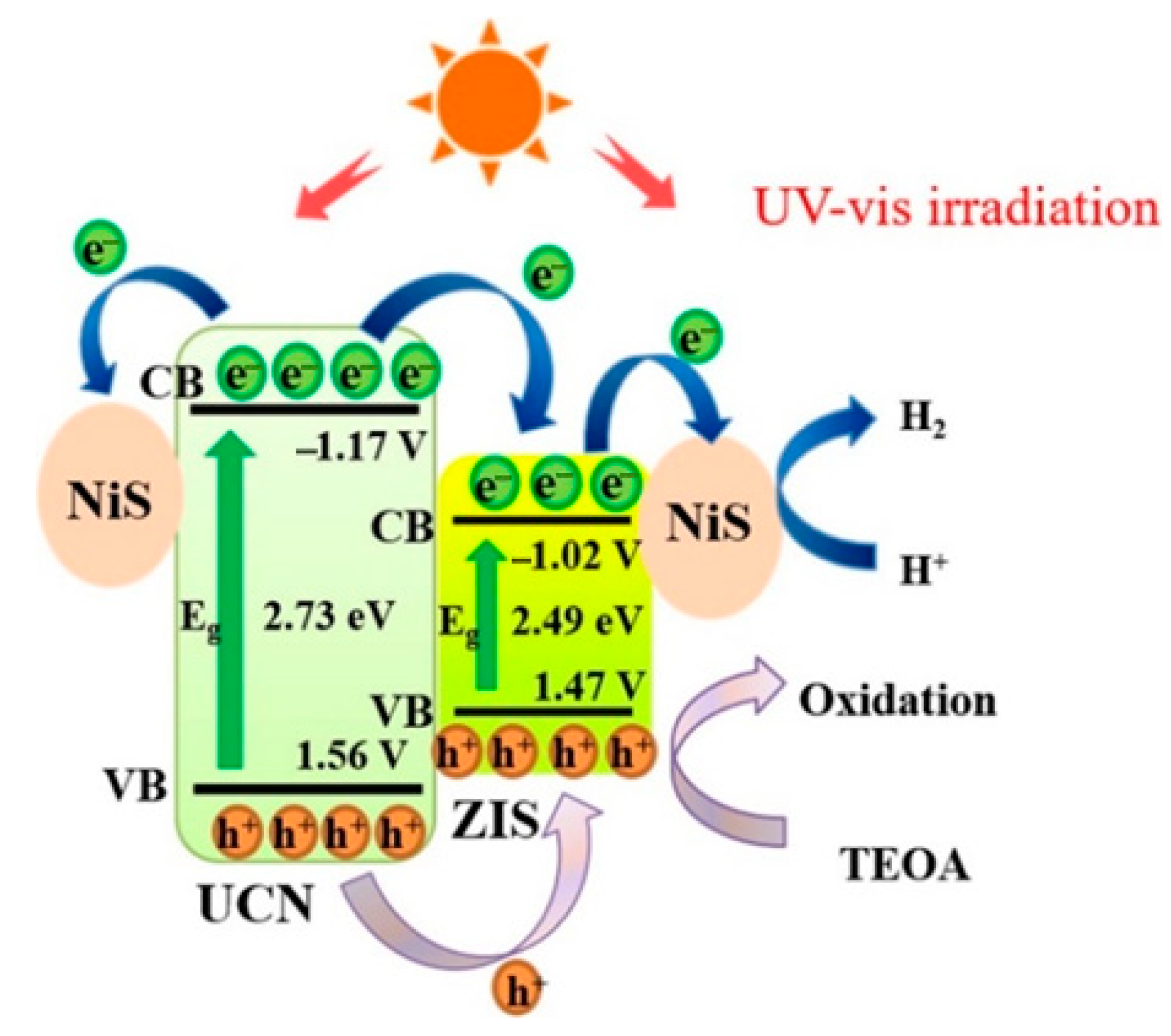
- Ji, X.Y.; Guo, R.T.; Tang, J.Y.; Lin, Z.D.; Yuan, Y.; Hong, L.F.; Pan, W.G. Fabrication of a ternary NiS/ZnIn2S4/g-C3N4 photocatalyst with dual charge transfer channels towards efficient H2 evolution. J. Colloid Interface Sci. 2022, 618, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Huo, H.; Li, Z.; Shi, J. A novel binary visible-light-driven photocatalyst type-I CdIn2S4/g-C3N4 heterojunctions coupling with H2O2: Synthesis, characterization, photocatalytic activity for Reactive Blue 19 degradation and mechanism analysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124322. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Jiang, S.P. The edge-epitaxial growth of yellow g-C3N4 on red g-C3N4 nanosheets with superior photocatalytic activities. Chem. Commun. 2021, 57, 3119–3122. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Dong, Y.; Pan, A. Fabricating a type II heterojunction by growing lead-free perovskite Cs2AgBiBr6in situ on graphite-like g-C3N4 nanosheets for enhanced photocatalytic CO2 reduction. Nanoscale 2023, 15, 15619–15625. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yan, Y.; Wang, Y.; Chen, F.F.; Yu, Y. Dual role of g-C3N4 microtubes in enhancing photocatalytic CO2 reduction of Co3O4 nanoparticles. Carbon 2023, 201, 415–424. [Google Scholar] [CrossRef]
- Hu, S.; Zhai, W.; Chen, F.; He, Q. Improvement of separation, transfer and redox ability arising from P–N heterojuncted composite phosphate-group-intercalated g-C3N4 /Bi2WO6. Mater. Today Phys. 2024, 40, 101311. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, X.; Wang, X.; Tian, Z.; Yang, X.; Lu, J.; Bai, H.; Jiao, T.; Huang, H.; Hu, J. Construction of Ag decorated P-doped g-C3N4 nanosheets Schottky junction via silver mirror reaction for enhanced photocatalytic activities. Int. J. Hydrog. Energy 2022, 47, 250–263. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Wang, S.; Li, J.; Wang, G.; Wang, J. In situ fabrication of 2D/3D g-C3N4/Ti3C2 (MXene) heterojunction for efficient visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 515, 145922. [Google Scholar] [CrossRef]
- He, R.; Cheng, K.; Wei, Z.; Zhang, S.; Xu, D. Room-temperature in situ fabrication and enhanced photocatalytic activity of direct Z-scheme BiOI/g-C3N4 photocatalyst. Appl. Surf. Sci. 2019, 465, 964–972. [Google Scholar] [CrossRef]
- Chen, M.; Wang, G.; Dai, J.; Li, H.; Deng, N. Indirect Z-scheme TiO2/BC/g-C3N4 for efficient photocatalytic reduction of Cr(VI) in aqueous solution. J. Chem. Technol. Biotechnol. 2024, 99, 415–425. [Google Scholar] [CrossRef]
- Bai, K.; Cui, Z.; Li, E.; Ding, Y.; Zheng, J.; Liu, C.; Zheng, Y. Electronic and optical characteristics of GaS/g-C3N4 van der Waals heterostructures: Effects of biaxial strain and vertical electric field. Vacuum 2020, 180, 109562. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Liu, S.; Zhao, M.; Duan, X.; Wu, H.; Liu, L.; Liu, W.; Li, J.; Ren, S.; et al. Constructing C–O bridged CeO2/g-C3N4 S-scheme heterojunction for methyl orange photodegradation: Experimental and theoretical calculation. J. Environ. Manag. 2023, 335, 117608. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Ge, H.; Li, M.; Li, Y.; Liu, B.; Duan, T.; He, R.; Zhu, W. Efficient extraction of uranium in organics-containing wastewater over g-C3N4/GO hybrid nanosheets with type-II band structure. J. Hazard. Mater. 2020, 384, 121383. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of g-C3N4/g-C3N4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zhong, Q.; Li, J.; Wu, H.; Zhang, B.; Jin, L.; Tao, H.B.; Liu, B. Electrostatic self-assembly of a AgI/Bi2Ga4O9 p–n junction photocatalyst for boosting superoxide radical generation. J. Mater. Chem. A 2020, 8, 4083–4090. [Google Scholar] [CrossRef]
- Lei, J.; Gu, X.; Xiao, P.; Ding, G.; Yang, Y.; Fu, X.; Long, B.; Chen, S.; Meng, S. Fabrication of 2D/2D BiOBr/g-C3N4 with efficient photocatalytic activity and clarification of its mechanism. Phys. Chem. Chem. Phys. 2022, 24, 19806–19816. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yang, C.; Fan, L.; Fu, Z.; Yang, X.; Wang, X.; Wang, R. Dyadic promotion of photocatalytic aerobic oxidation via the Mott–Schottky effect enabled by nitrogen-doped carbon from imidazolium-based ionic polymers. Energy Environ. Sci. 2019, 12, 418–426. [Google Scholar] [CrossRef]
- Yang, L.; Charnas, A.; Qiu, G.; Lin, Y.-M.; Lu, C.-C.; Tsai, W.; Paduano, Q.; Snure, M.; Ye, P.D. How Important Is the Metal–Semiconductor Contact for Schottky Barrier Transistors: A Case Study on Few-Layer Black Phosphorus? ACS Omega 2017, 2, 4173–4179. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Zhang, J.; Lin, Z.; Zheng, J.; Huang, F. Schottky or Ohmic Metal–Semiconductor Contact: Influence on Photocatalytic Efficiency of Ag/ZnO and Pt/ZnO Model Systems. ChemSusChem 2014, 7, 101–104. [Google Scholar] [CrossRef]
- Hu, T.; Yang, Y.; Dai, K.; Zhang, J.; Liang, C. A novel Z-scheme Bi2MoO6/BiOBr photocatalyst for enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2018, 456, 473–481. [Google Scholar] [CrossRef]
- Li, H.; Hu, T.; Zhang, R.; Liu, J.; Hou, W. Preparation of solid-state Z-scheme Bi2MoO6/MO (MCu, Co3/4, or Ni) heterojunctions with internal electric field-improved performance in photocatalysis. Appl. Catal. B Environ. 2016, 188, 313–323. [Google Scholar] [CrossRef]
- Mishra, S.; Acharya, R. Recent updates in modification strategies for escalated performance of Graphene/MFe2O4 heterostructured photocatalysts towards energy and environmental applications. J. Alloys Compd. 2023, 960, 170576. [Google Scholar] [CrossRef]
- Ghosh, U.; Pal, A. Graphitic carbon nitride based Z scheme photocatalysts: Design considerations, synthesis, characterization and applications. J. Ind. Eng. Chem. 2019, 79, 383–408. [Google Scholar] [CrossRef]
- Liao, G.F.; Li, C.X.; Li, X.Z.; Fang, B.Z. Emerging polymeric carbon nitride Z-scheme systems for photocatalysis. Cell Rep. Phys. Sci. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Jourshabani, M.; Lee, B.K.; Shariatinia, Z. From Traditional Strategies to Z-scheme Configuration in Graphitic Carbon Nitride Photocatalysts: Recent Progress and Future Challenges. Appl. Catal. B-Environ. 2020, 276, 119157. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Du, Y.; Yang, G.; Ren, T.; Ding, H. In situ ionic-liquid-assisted synthesis of plasmonic photocatalyst Ag/AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. Catal. Today 2015, 258, 41–48. [Google Scholar] [CrossRef]
- Lu, S.; Wu, T.; Liu, Y.; Luo, H.; Jiang, F.; Nie, X.; Chen, H. All-solid Z-scheme Bi/γ-Bi2O3/O-doped g-C3N4 heterojunction with Bi as electron shuttle for visible-light photocatalysis. J. Alloys Compd. 2022, 911, 164980. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, C.; Zhang, X.; Liu, J.; Duan, D.; Fan, C.; Wang, Y. Construction of Pt-decorated g-C3N4/Bi2WO6 Z-scheme composite with superior solar photocatalytic activity toward rhodamine B degradation. Inorg. Chem. Commun. 2019, 100, 81–91. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, L.; Meng, M.; Zhang, Q.; Li, B.; Wu, Y.; Zhang, Y.; Lang, J.; Li, C. Facile preparation of antifouling g-C3N4/Ag3PO4 nanocomposite photocatalytic polyvinylidene fluoride membranes for effective removal of rhodamine B. Korean J. Chem. Eng. 2019, 36, 236–247. [Google Scholar] [CrossRef]
- Zhu, T.; Song, Y.; Ji, H.; Xu, Y.; Song, Y.; Xia, J.; Yin, S.; Li, Y.; Xu, H.; Zhang, Q.; et al. Synthesis of g-C3N4/Ag3VO4 composites with enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2015, 271, 96–105. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y.H. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef]
- Zhang, X.B.; Song, H.Y.; Sun, C.Y.; Chen, C.X.; Han, F.Q.; Li, X.F. Photocatalytic oxidative desulfurization and denitrogenation of fuels over sodium doped graphitic carbon nitride nanosheets under visible light irradiation. Mater. Chem. Phys. 2019, 226, 34–43. [Google Scholar] [CrossRef]
- Sridharan, K.; Shenoy, S.; Kumar, S.G.; Terashima, C.; Fujishima, A.; Pitchaimuthu, S. Advanced Two-Dimensional Heterojunction Photocatalysts of Stoichiometric and Non-Stoichiometric Bismuth Oxyhalides with Graphitic Carbon Nitride for Sustainable Energy and Environmental Applications. Catalysts 2021, 11, 426. [Google Scholar] [CrossRef]
- Li, N.; Gao, H.; Wang, X.; Zhao, S.; Lv, D.; Yang, G.; Gao, X.; Fan, H.; Gao, Y.; Ge, L. Novel indirect Z-scheme g-C3N4/Bi2MoO6/Bi hollow microsphere heterojunctions with SPR-promoted visible absorption and highly enhanced photocatalytic performance. Chin. J. Catal. 2020, 41, 426–434. [Google Scholar] [CrossRef]
- Huang, Z.L.; Liu, J.C.; Zong, S.; Wang, X.Y.; Chen, K.X.; Liu, L.L.; Fang, Y.X. Fabrication of graphitic carbon Nitride/Nonstoichiometric molybdenum oxide nanorod composite with the nonmetal plasma enhanced photocatalytic hydrogen evolution activity. J. Colloid Interface Sci. 2022, 606, 848–859. [Google Scholar] [CrossRef]
- Huang, G.; Liu, S.; Tian, C.; Tao, Y. Construction of S scheme ZnO/g-C3N4 heterojunction for the removal of pyridine from coal chemical wastewater. Opt. Mater. 2024, 150, 115288. [Google Scholar] [CrossRef]
- Alsulmi, A.; Hussein, I.A.; Nasherty, M.; Hesham, M.; Soltan, A.; Messih, M.F.A.; Ahmed, M.A. Sonochemical Fabrication of S-Scheme AgI/g-C3N4 Heterojunction for Efficient Photocatalytic Degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2024, 34, 640–654. [Google Scholar] [CrossRef]
- Mohammad, A.; Ahmad, K.; Qureshi, A.; Tauqeer, M.; Mobin, S.M. Zinc oxide-graphitic carbon nitride nanohybrid as an efficient electrochemical sensor and photocatalyst. Sens. Actuators B Chem. 2018, 277, 467–476. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Ou, H.; Ma, T.; Zhang, Y. Self-sacrifice transformation for fabrication of type-I and type-II heterojunctions in hierarchical BixOyIz/g-C3N4 for efficient visible-light photocatalysis. Appl. Surf. Sci. 2019, 470, 1101–1110. [Google Scholar] [CrossRef]
- Raza, A.; Haidry, A.A.; Amin, T.; Hussain, A.A.; Shah, S.A.M.H.; Ahsan, M. Boosting the water splitting and hydrogen production of S-scheme fabricated porous g-C3N4 modified with CuO. Diam. Relat. Mater. 2024, 141, 110703. [Google Scholar] [CrossRef]
- Kong, X.; Fan, J.; Feng, B.; Li, J.; Yang, G.; Xue, C. Carbon dots-triggered the fabrication of miniature g-C3N4/CDs/WO3 S-scheme heterojunction for efficient CO2 photoreduction. Chem. Eng. J. 2023, 476, 146774. [Google Scholar] [CrossRef]
- Wan, Y.; Du, S.; Lu, C.; Ren, K.; Shi, B.; Liu, S.; Li, C.; Dou, W.; Fang, P.; Ye, N. Metallic CuS decorated CdS nanowires for efficient photocatalytic H2 evolution under visible-light irradiation. J. Alloys Compd. 2021, 871, 159461. [Google Scholar] [CrossRef]
- Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, S.; Fenoll, J. Solar reclamation of agro-wastewater polluted with eight pesticides by heterogeneous photocatalysis using a modular facility. A Case Study. Chemosphere 2020, 249, 126156. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Sun, Y.; Jiang, G.; Chu, Y.; Lee, S.C.; Dong, F. Tailoring the rate-determining step in photocatalysis via localized excess electrons for efficient and safe air cleaning. Appl. Catal. B Environ. 2018, 239, 187–195. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, X.; Xie, X.; Mahmood, A.; Lu, G.; Wang, Y.; Sun, J. Band bending of TiO2 induced by O-xylene and acetaldehyde adsorption and its effect on the generation of active radicals. J. Colloid Interface Sci. 2020, 572, 374–383. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Zhang, Y.; Liu, K.; Huang, Y.; Liu, H. Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminants. Environ. Res. 2020, 185, 109409. [Google Scholar] [CrossRef]
- Prabhu, S.; Cindrella, L.; Kwon, O.J.; Mohanraju, K. Photoelectrochemical and photocatalytic activity of TiO2-WO3 heterostructures boosted by mutual interaction. Mater. Sci. Semicond. Process. 2018, 88, 10–19. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B-Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Arai, S. Chemistry of chromian spinel in volcanic rocks as a potential guide to magma chemistry. Mineral. Mag. 1992, 56, 173–184. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Hou, L.A.A. critical review of g-C3N4-based photocatalytic membrane for water purification. Chem. Eng. J. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Tang, L.; Liu, L.; Yang, F. FeMoO4-graphene oxide photo-electro-catalyst for berberine removal and hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 19755–19761. [Google Scholar] [CrossRef]
- Gao, B.; Peng, C.; Chen, G.Z.; Li Puma, G. Photo-electro-catalysis enhancement on carbon nanotubes/titanium dioxide (CNTs/TiO2) composite prepared by a novel surfactant wrapping sol–gel method. Appl. Catal. B Environ. 2008, 85, 17–23. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Y.; Yao, X.; Zhang, J. Simultaneous removal of organic pollutants and heavy metals in wastewater by photoelectrocatalysis: A review. Chemosphere 2021, 273, 128503. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Yang, Y.; Zeng, G.; Zhang, C.; Zhou, Y.; Yang, J.; Huang, D.; Wang, H.; Xiong, W.; et al. Carbon nitride based photocatalysts for solar photocatalytic disinfection, can we go further? Chem. Eng. J. 2021, 404, 126540. [Google Scholar] [CrossRef]
- Xu, Y.; Song, J.; Chen, F.; Wang, X.F.; Yu, H.G.; Yu, J.G. Amorphous Ti(IV)-modified Bi2WO6 with enhanced photocatalytic performance. RSC Adv. 2016, 6, 65902–65910. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Zhang, L.J.; Qi, J.; Jin, Q.; Lin, K.F.; Wang, D. Graphdiyne with Enhanced Ability for Electron Transfer. Acta Phys.-Chim. Sin. 2018, 34, 1048–1060. [Google Scholar] [CrossRef]
- An, P.F.; Zhu, W.H.; Qiao, L.Y.; Sun, S.C.; Xu, Y.Y.; Jiang, D.L.; Chen, M.; Meng, S.C. 0D ultrafine ruthenium quantum dot decorated 3D porous graphitic carbon nitride with efficient charge separation and appropriate hydrogen adsorption capacity for superior photocatalytic hydrogen evolution. Dalton Trans. 2021, 50, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yao, D.; Yan, J.; Ye, T.; Liu, H.; Zeng, H.; Pan, X.; Zhang, G.; Ding, J. Pressure-Optimized Band Gap and Enhanced Photoelectric Response of Graphitic Carbon Nitride with Nitrogen Vacancies. Phys. Rev. Appl. 2023, 19, 024048. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. One-step synthesis of Mo and S co-doped porous g-C3N4 nanosheets for efficient visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 536, 147743. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, S.; Huang, Y.; Lv, K.; Fang, S.; Wu, X.; Li, Q.; Fan, J. Sharply increasing the visible photoreactivity of g-C3N4 by breaking the intralayered hydrogen bonds. Appl. Surf. Sci. 2020, 505, 144654. [Google Scholar] [CrossRef]
- Ma, W.; Wang, X.; Zhang, F.; Fei, X.; Zhang, X.; Ma, H.; Dong, X. Synergetic effect of Li doping and Ag deposition for enhanced visible light photocatalytic performance of g-C3N4. Mater. Res. Bull. 2017, 86, 72–79. [Google Scholar] [CrossRef]
- Selvaraj, V.; Pandikumar, A. Turning UV Light-Active BiOF into Visible Light-Active BiOF by Forming a Heterojunction with g-C3N4 and Its Photoelectrochemical Water Splitting Performance in Reverse Osmosis-Rejected Wastewater. J. Phys. Chem. C 2022, 126, 79–90. [Google Scholar] [CrossRef]
- Gahlot, S.; Dappozze, F.; Mishra, S.; Guillard, C. High surface area g-C3N4 and g-C3N4-TiO2 photocatalytic activity under UV and Visible light: Impact of individual component. J. Environ. Chem. Eng. 2021, 9, 105587. [Google Scholar] [CrossRef]
- Wu, X.; Tan, Z.; Liu, R.; Liao, Z.; Ou, H. Gaseous products generated from polyethylene and polyethylene terephthalate during ultraviolet irradiation: Mechanism, pathway and toxicological analyses. Sci. Total Environ. 2023, 876, 162717. [Google Scholar] [CrossRef]
- Bezerra, K.C.H.; Fiaschitello, T.R.; Labuto, G.; Freeman, H.S.; Fragoso, W.D.; da Costa, S.M.; da Costa, S.A. Reuse of water from real reactive monochromic and trichromic wastewater for new cotton dyes after efficient treatment using H2O2 catalyzed by UV light. J. Environ. Chem. Eng. 2021, 9, 105731. [Google Scholar] [CrossRef]
- Asaithambi, P.; Govindarajan, R.; Yesuf, M.B.; Alemayehu, E. Removal of color, COD and determination of power consumption from landfill leachate wastewater using an electrochemical advanced oxidation processes. Sep. Purif. Technol. 2020, 233, 115935. [Google Scholar] [CrossRef]
- Hongxia, J.; Yanlin, G.; Longxiang, L.; Xu, W.; Wangjun, P. A new double Z-scheme TiO2/ZnO-g-C3N4 nanocomposite with enhanced photodegradation efficiency for Rhodamine B under sunlight. Environ. Prog. Sustain. Energy 2023, 42, e13968. [Google Scholar] [CrossRef]
- Lu, X.; Chen, F.; Qian, J.; Fu, M.; Jiang, Q.; Zhang, Q. Facile fabrication of CeF3/g-C3N4 heterojunction photocatalysts with upconversion properties for enhanced photocatalytic desulfurization performance. J. Rare Earths 2021, 39, 1204–1210. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, H.B.; Choi, J.H.; Baek, K. Bifunctional iron-modified graphitic carbon nitride (g-C3N4) for simultaneous oxidation and adsorption of arsenic. Environ. Res. 2020, 188, 109832. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.J.; Chen, X.D.; Zhao, J.C. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Liao, G.F.; Gong, Y.; Zhang, L.; Gao, H.Y.; Yang, G.J.; Fang, B.Z. Semiconductor polymeric graphitic carbon nitride photocatalysts: The "holy grail" for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Li, K.; Chen, M.; Chen, L.; Zhao, S.; Xue, W.; Han, Y. Investigating the Effect of Bi2MoO6/g-C3N4 Ratio on Photocatalytic Degradation of Sulfadiazine under Visible Light. Processes 2023, 11, 1059. [Google Scholar] [CrossRef]
- Sheydaei, M.; Ayoubi-Feiz, B.; Abbaszade-Fakhri, G. A visible-light active g-C3N4/Ce–ZnO/Ti nanocomposite for efficient photoelectrocatalytic pharmaceutical degradation: Modelling with artificial neural network. Process Saf. Environ. Prot. 2021, 149, 776–785. [Google Scholar] [CrossRef]
- Li, D.; Zhang, W.; Huang, Y.; Feng, H.; Wang, Z.; Yang, Z.; Chen, J.; Zhang, X.; Zhang, G.; Chen, Y. Visible light-induced catalytic performance of composite photocatalyst synthesized with nanomaterials WO3 and two-dimensional ultrathin g-C3N4. Water Sci. Technol. 2023, 88, 1910–1925. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zuo, S.; Jin, X.; Zheng, B.; Deng, R.; Liu, W.; Wang, J. Synergistic effects of lanthanide surface adhesion and photon-upconversion for enhanced near-infrared responsive photodegradation of organic contaminants in wastewater. Environ. Sci. Nano 2020, 7, 3333–3342. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, D.; Pu, X.; Yao, X.; Han, R.; Yin, J.; Ren, X. A novel Ag2O/g-C3N4 p-n heterojunction photocatalysts with enhanced visible and near-infrared light activity. Sep. Purif. Technol. 2019, 210, 786–797. [Google Scholar] [CrossRef]
- Wu, F.; Ma, Y.; Hu, Y.H. Near Infrared Light-Driven Photoelectrocatalytic Water Splitting over P-Doped g-C3N4. ACS Appl. Energy Mater. 2020, 3, 11223–11230. [Google Scholar] [CrossRef]
- Li, H.; Chen, T.; Wang, Y.; Tang, J.; Wang, Y.; Sang, Y.; Liu, H. Surface-sulfurized Ag2O nanoparticles with stable full-solar-spectrum photocatalytic activity. Chin. J. Catal. 2017, 38, 1063–1071. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, G.; Jun, Y.S.; Park, Y.I. Visible/near-infrared driven highly efficient photocatalyst based on upconversion nanoparticles/g-C3N4 nanocomposite. Appl. Surf. Sci. 2020, 508, 144839. [Google Scholar] [CrossRef]
- Tian, H.; Liu, X.; Liang, Z.; Qiu, P.; Qian, X.; Cui, H.; Tian, J. Gold nanorods/g-C3N4 heterostructures for plasmon-enhanced photocatalytic H2 evolution in visible and near-infrared light. J. Colloid Interface Sci. 2019, 557, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liang, F.; Liu, Y.; Luo, W.; Wang, J.; Yao, W.; Zhu, Y. Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/g-C3N4 hybrid heterostructure thin film. Appl. Catal. B-Environ. 2017, 201, 600–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Lu, J.; Wang, Q.; Cong, Y. Synergistic photoelectrochemical reduction of Cr(VI) and oxidation of organic pollutants by g-C3N4/TiO2-NTs electrodes. Chemosphere 2016, 162, 55–63. [Google Scholar] [CrossRef]
- Rather, R.A.; Lo, I.M.C. Photoelectrochemical sewage treatment by a multifunctional g-C3N4/Ag/AgCl/BiVO4 photoanode for the simultaneous degradation of emerging pollutants and hydrogen production, and the disinfection of E. coli. Water Res. 2020, 168, 115166. [Google Scholar] [CrossRef]
- Murugan, C.; Nataraj, R.A.; Kumar, M.P.; Ravichandran, S.; Pandikumar, A. Enhanced charge transfer process of bismuth vanadate interleaved graphitic carbon nitride nanohybrids in mediator-free direct Z scheme photoelectrocatalytic water splitting. Chemistryselect 2019, 4, 4653–4663. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Chen, S.; Fan, X.; Quan, X.; Yu, H. Integration of membrane filtration and photoelectrocatalysis on g-C3N4/CNTs/Al2O3 membrane with visible-light response for enhanced water treatment. J. Membr. Sci. 2017, 541, 153–161. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Song, X.; Wang, H.; Bian, Z. One-step synthesis of MnOx/g-C3N4 nanocomposites for enhancing the visible light photoelectrochemical oxidation performance. Chem. Eng. J. 2020, 399, 125825. [Google Scholar] [CrossRef]





| Strategy | Principle | Advantages | Disadvantages | |
|---|---|---|---|---|
| Doping | Metal doping | The doped metal ions are positively charged and have strong interactions with the negatively charged C and N, which can form coordination bonds. This changes the lattice structure of g-C3N4. | Alteration of the g-C3N4 lattice structure to reduce the band gap and expand the absorption range of visible light. | Limited resources and high prices. |
| Non-metal doping | The non-metallic elements themselves have high electro-negativity and ionization energies and can form covalent bonds with other compounds during the reaction process. | Non-toxic and harmless, abundant sources, simple preparation process, good thermal and chemical stability, excellent light absorption performance, and adjustable band gap structure. | Performance is not yet up to the performance requirements of noble metal-based catalysts. | |
| Heterojunction | The interface between two regions of different semiconductors with unequal band structures create interfacial band alignments. | The heterojunction structure of g-C3N4 was constructed to be able to effectively inhibit carrier composite. | The method for determining the type of heterojunction is complex and needs to be analyzed on an experimental basis. | |
| Doped Metal | Pollutants | Degradation Efficiency | References | |
|---|---|---|---|---|
| Alkali metal-doped g-C3N4 | Pure g-C3N4 | CO2 | 3.6 μmol g−1 | [55] |
| Li | 5.6 μmol g−1 | |||
| Na | 7.4 μmol g−1 | |||
| K | 9.8 μmol g−1 | |||
| Rb | 12.1 μmol g−1 | |||
| Ni-doping of g-C3N4 | Pure g-C3N4 | MO | 47.8 (140 min) | [58] |
| Ni | 97.3 (90 min) | |||
| Cu-graphitic carbon nitride | Cu | RhB | 99.2 (60 min) | [59] |
| ZnO/g-C3N4 | ZnO | MB | 91 (20 min) | [61] |
| Fe-g-C3N4 | Pure g-C3N4 | RhB | 69 (45 min) | [63] |
| 5% Fe | 92.9 (45 min) | |||
| 10% Fe | 95.5 (45 min) | |||
| 15% Fe | 90.5 (45 min) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lian, P.; Hao, X.; Zhang, L.; Yang, L.; Jiang, L.; Zhang, K.; Liao, L.; Qin, A. Modification Strategies of g-C3N4-Based Materials for Enhanced Photoelectrocatalytic Degradation of Pollutants: A Review. Inorganics 2025, 13, 225. https://doi.org/10.3390/inorganics13070225
Zhang Y, Lian P, Hao X, Zhang L, Yang L, Jiang L, Zhang K, Liao L, Qin A. Modification Strategies of g-C3N4-Based Materials for Enhanced Photoelectrocatalytic Degradation of Pollutants: A Review. Inorganics. 2025; 13(7):225. https://doi.org/10.3390/inorganics13070225
Chicago/Turabian StyleZhang, Yijie, Peng Lian, Xinyu Hao, Li Zhang, Lihua Yang, Li Jiang, Kaiyou Zhang, Lei Liao, and Aimiao Qin. 2025. "Modification Strategies of g-C3N4-Based Materials for Enhanced Photoelectrocatalytic Degradation of Pollutants: A Review" Inorganics 13, no. 7: 225. https://doi.org/10.3390/inorganics13070225
APA StyleZhang, Y., Lian, P., Hao, X., Zhang, L., Yang, L., Jiang, L., Zhang, K., Liao, L., & Qin, A. (2025). Modification Strategies of g-C3N4-Based Materials for Enhanced Photoelectrocatalytic Degradation of Pollutants: A Review. Inorganics, 13(7), 225. https://doi.org/10.3390/inorganics13070225






