New Complex of Salinomycin with Hg(II)—Synthesis and Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis
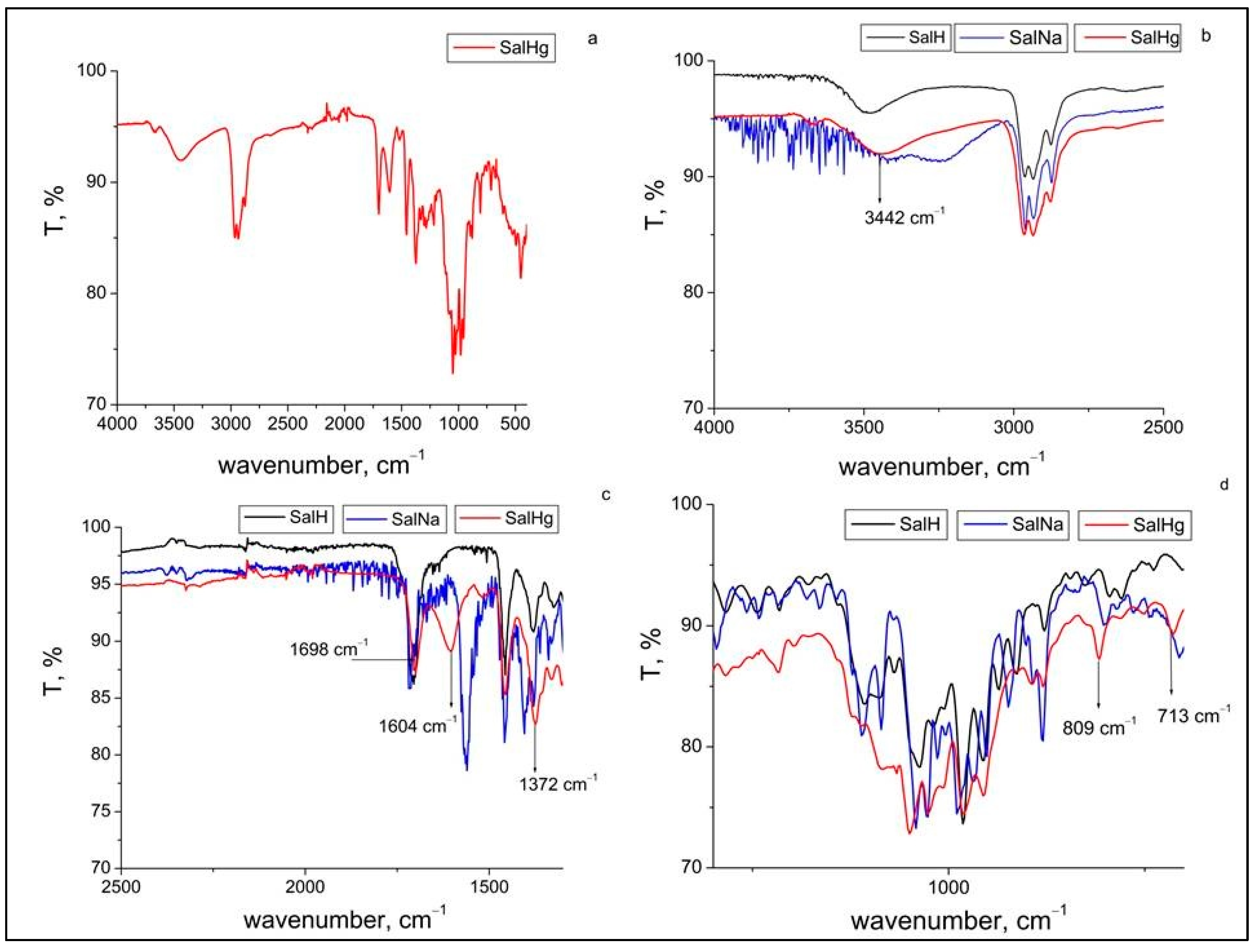
2.2. ATR-FTIR Spectral Analysis
2.3. ESI-MS Analysis
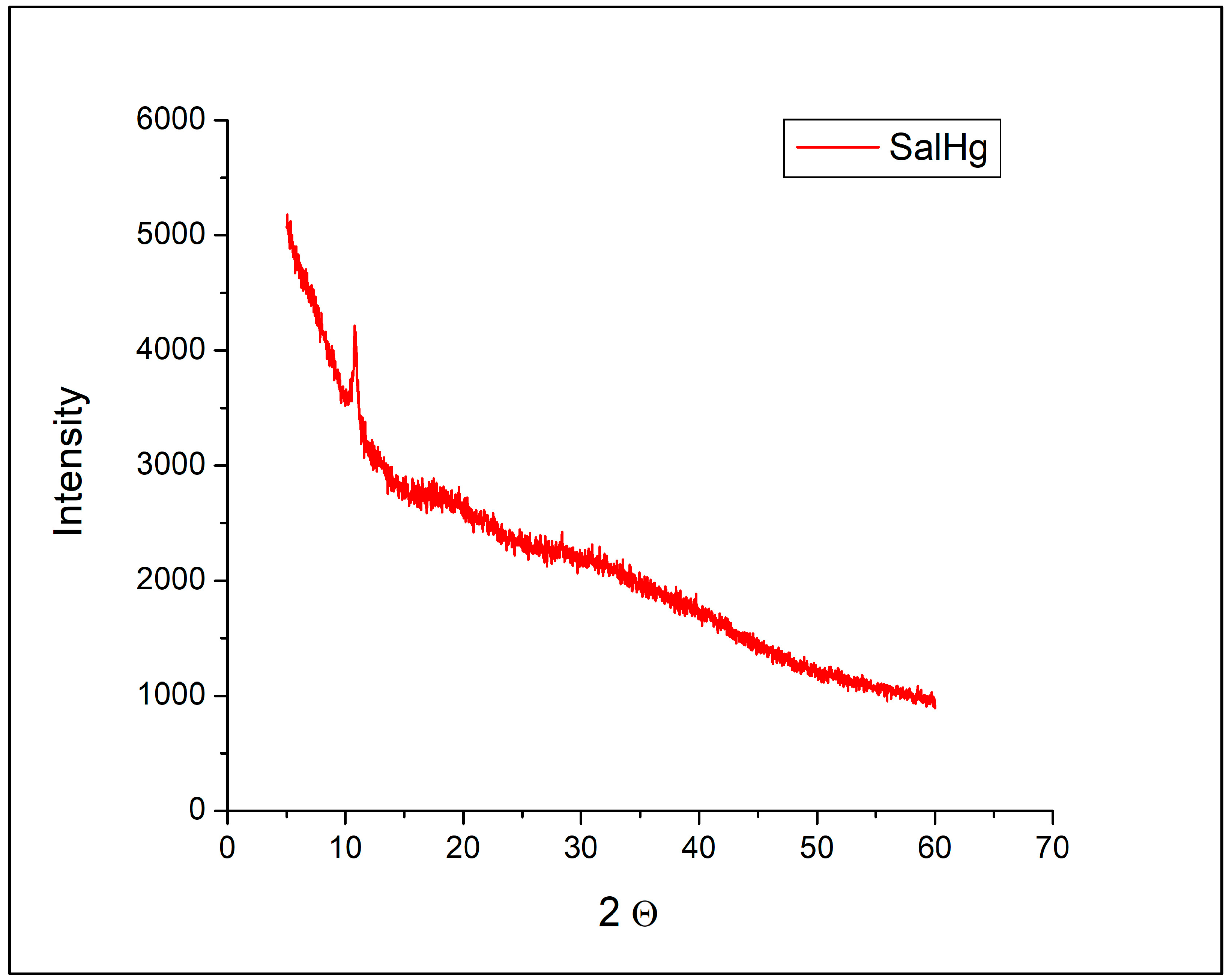
2.4. Powder X-Ray Diffraction
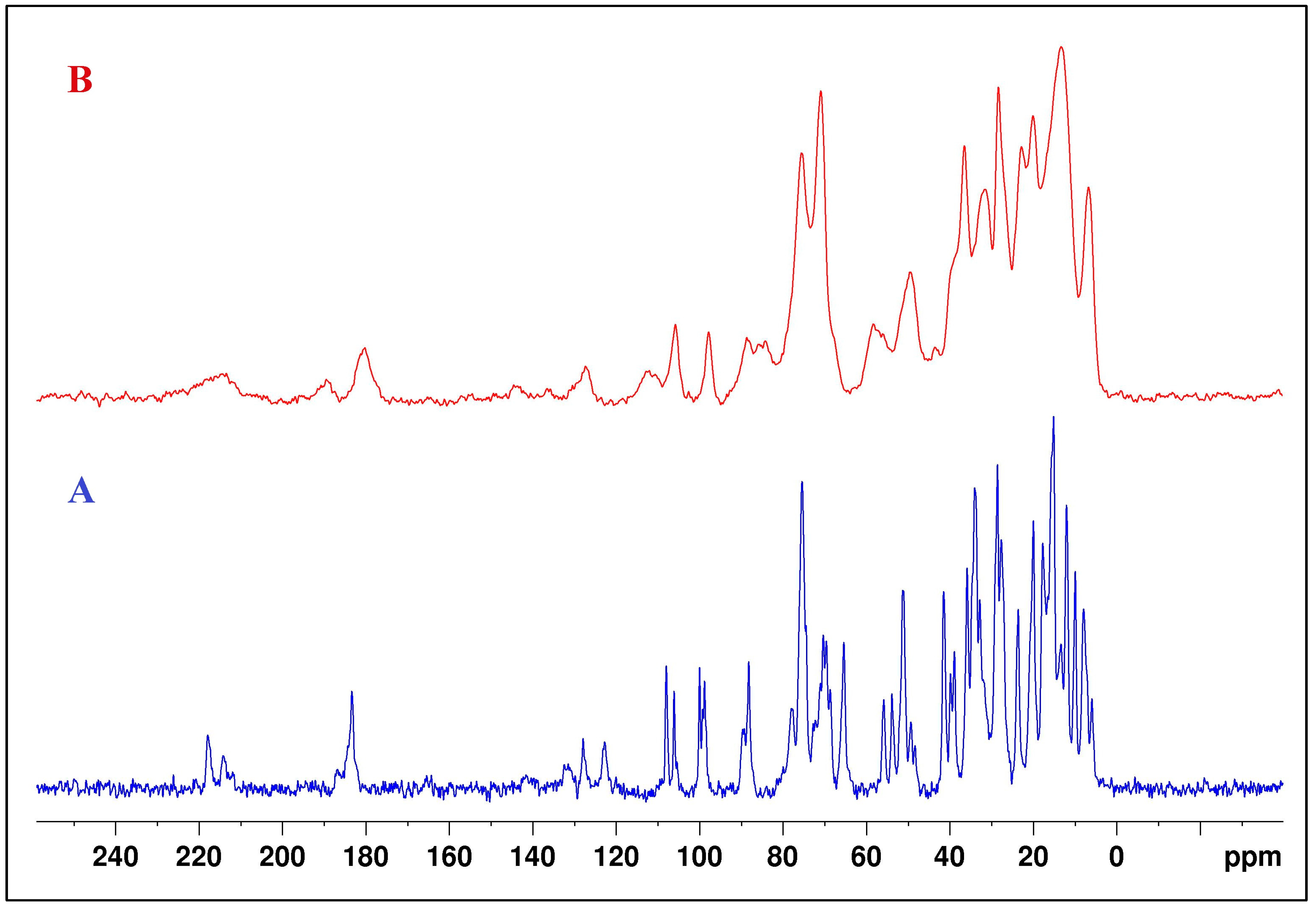
2.5. NMR Studies
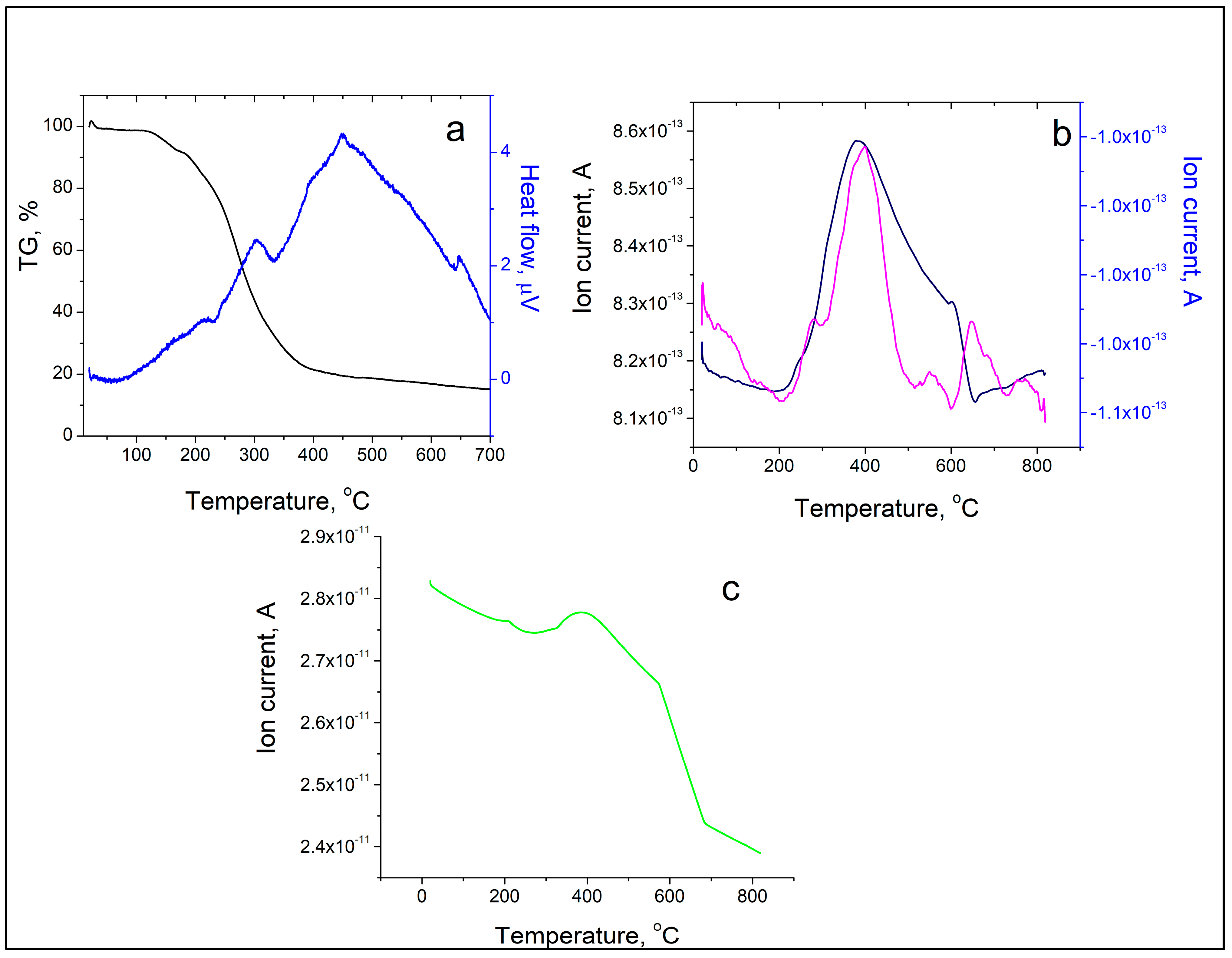
2.6. TG-DTA and TG-MS Analysis
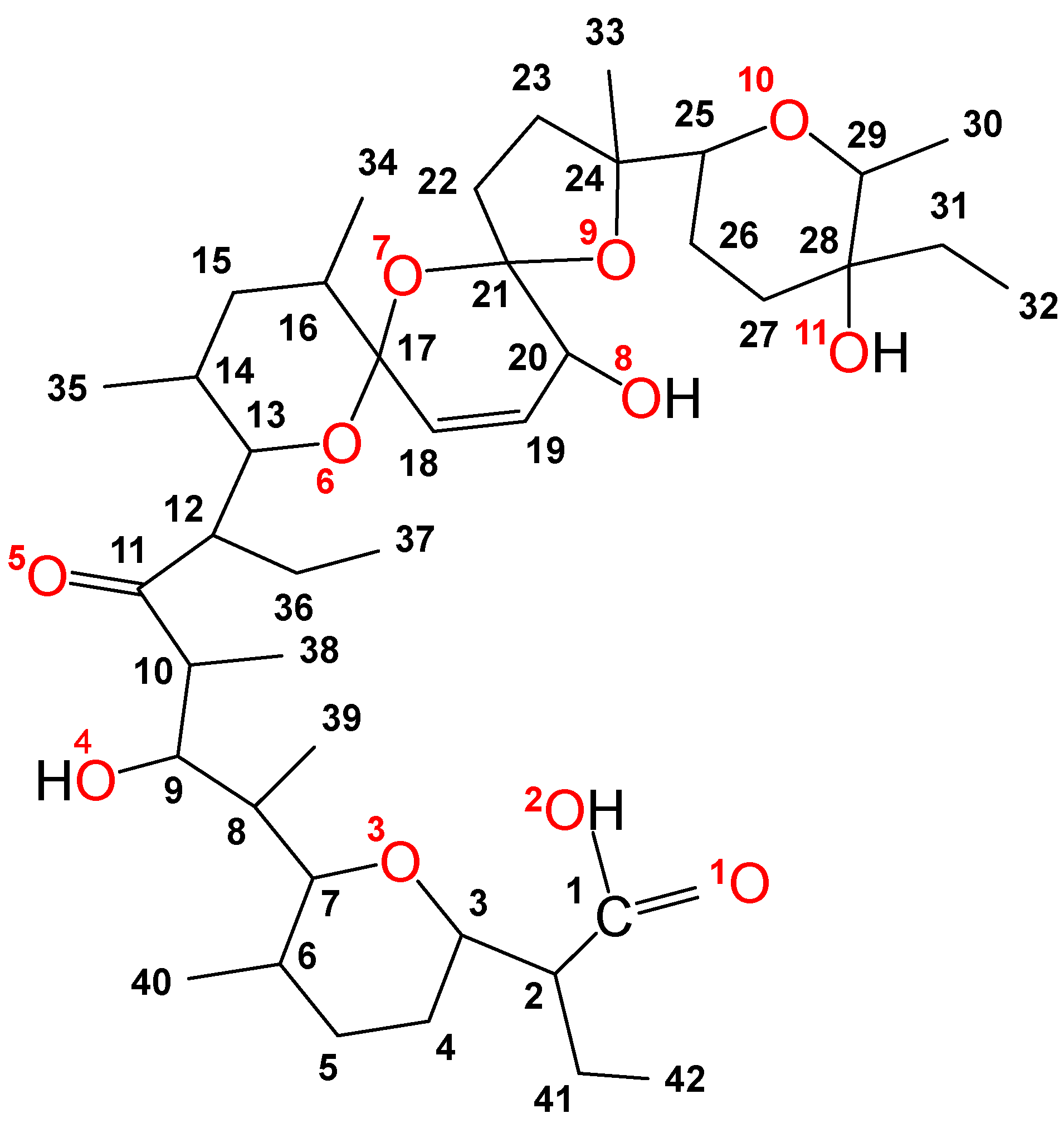
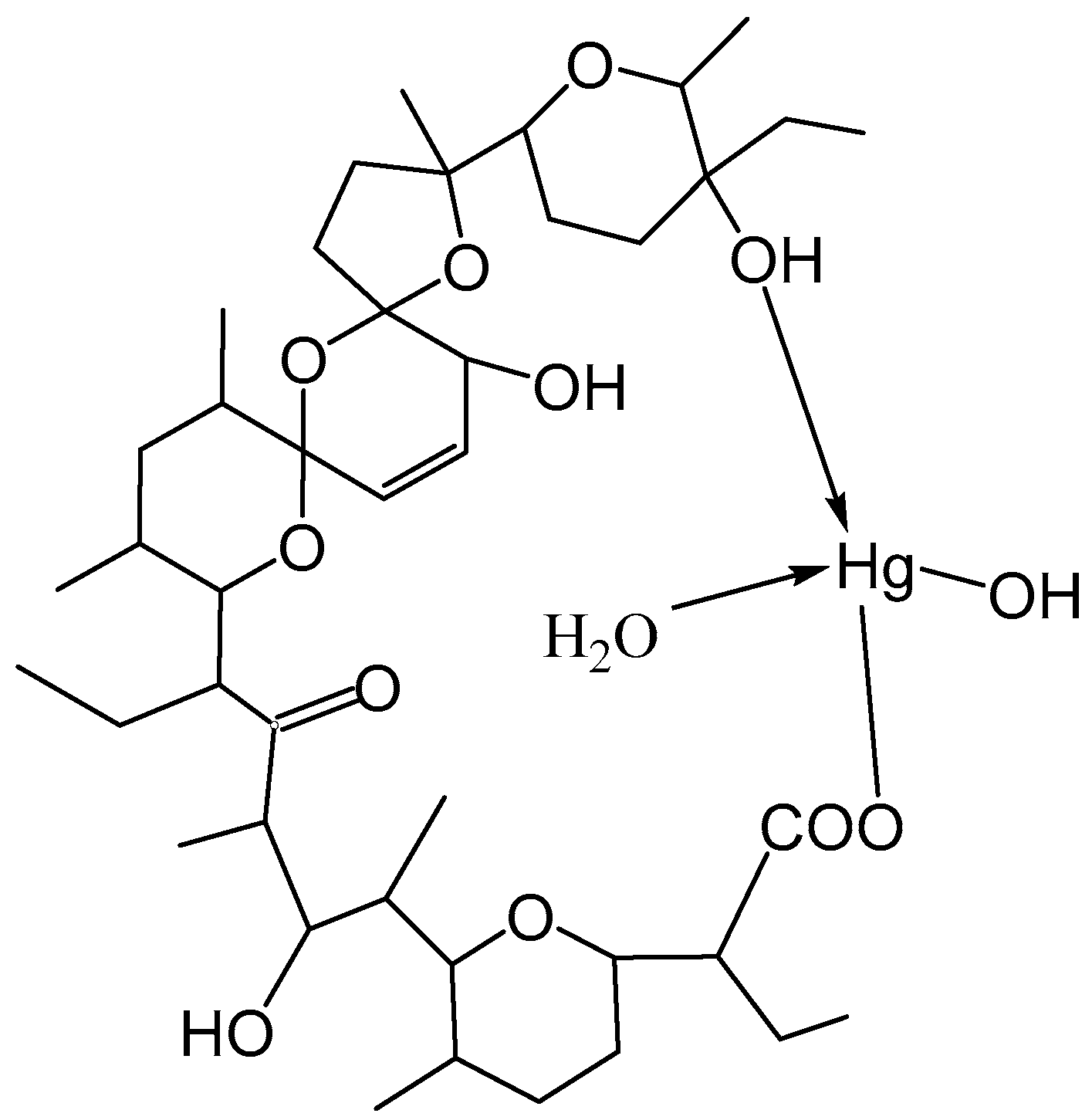
2.7. Proposed Structure of SalHg
3. Materials and Methods
3.1. Chemicals
Synthesis of Complex of Salinomycin with Hg(II)
3.2. Methods
3.2.1. Elemental Analysis
3.2.2. Attenuated Total Reflectance–Fourier Transform Spectroscopy
3.2.3. Electrospray Ionization–Mass Spectrometry (ESI-MS)
3.2.4. Powder X-Ray Diffraction (XRD)
3.2.5. Nuclear Magnetic Spectroscopy
3.2.6. Thermogravimetric Analysis with Differential Thermal Analysis (TG-DTA) and Mass Spectrometry Thermogravimetric Measurements (TG−MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyazaki, Y.; Shibuya, M.; Sugawara, H.; Kawaguchi, O.; Hirose, C.; Nagatsu, J.; Esumi, S. Salinomycin, a new polyether antibiotic. J. Antibiot. 1974, 27, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Westley, J.W. Polyether antibiotics: Versatile carboxylic acid ionophores produced by Streptomyces. Adv. Appl. Microbiol. 1977, 22, 177–223. [Google Scholar] [CrossRef]
- Zhou, S.F.; Wong, E.T.; Fonkem, E.; Hsieh, T.C.; Wu, J.M.; Wu, E. Salinomycin: A novel anti-cancer agent with known anti-coccidial activities. Curr. Med. Chem. 2013, 20, 4095–4101. [Google Scholar] [CrossRef]
- Antoszczak, M.; Steverding, D.; Huczyński, A. Anti-parasitic activity of polyether ionophores. Eur. J. Med. Chem. 2019, 166, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Shin, J.S.; Yoon, Y.S.; Go, Y.Y.; Lee, H.W.; Kwon, O.S.; Park, S.; Park, M.S.; Kim, M. Salinomycin Inhibits Influenza Virus Infection by Disrupting Endosomal Acidification and Viral Matrix Protein 2 Function. J. Virol. 2018, 92, e01441-18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Huang, X.; Zhai, R.; Ma, Y.; Xu, A.; Zhang, P.; Yang, Q. In Vitro Antiviral Activities of Salinomycin on Porcine Epidemic Diarrhea Virus. Viruses 2021, 13, 580. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Chen, S.; Lai, R.; Zheng, C.; Lu, J.; Jiang, X.; He, F.; Yang, C.; Li, K.; et al. Salinomycin alleviates osteoarthritis progression via inhibiting Wnt/β-catenin signaling. Int. Immunopharmacol. 2022, 112, 109225. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Kolar, B.; et al. Safety and efficacy of Sacox® microGranulate (salinomycin sodium) for chickens for fattening and chickens reared for laying. EFSA J. 2017, 15, e04670. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Huczyński, A. Polyether ionophores-promising bioactive molecules for cancer therapy. Bioorg. Med. Chem. Lett. 2012, 22, 7002–7010. [Google Scholar] [CrossRef]
- Naujokat, C.; Steinhart, R. Salinomycin as a drug for targeting human cancer stem cells. J. Biomed. Biotechnol. 2012, 2012, 950658. [Google Scholar] [CrossRef]
- Antoszczak, M.; Huczyński, A. Anticancer Activity of Polyether Ionophore-Salinomycin. Anticancer Agents Med. Chem. 2015, 15, 575–591. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Kaysiewicz, J.; Markowska, J.; Huczyński, A. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg. Med. Chem. Lett. 2019, 29, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, J.; Srivastava, S.; Rath, S.K. Salinomycin: A new paradigm in cancer therapy. Tumour Biol. 2017, 39, 1010428317695035. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Qaed, E.; Zhang, J.; Song, Y.; Wu, R.; Bu, X.; Wang, Q.; Tang, Z. Salinomycin, as an autophagy modulator-- a new avenue to anticancer: A review. J. Exp. Clin. Cancer Res. 2018, 37, 26. [Google Scholar] [CrossRef]
- Antoszczak, M. A comprehensive review of salinomycin derivatives as potent anticancer and anti-CSCs agents. Eur. J. Med. Chem. 2019, 166, 48–64. [Google Scholar] [CrossRef]
- Magrath, J.W.; Kim, Y. Salinomycin’s potential to eliminate glioblastoma stem cells and treat glioblastoma multiforme (Review). Int. J. Oncol. 2017, 51, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Tefas, L.R.; Barbălată, C.; Tefas, C.; Tomuță, I. Salinomycin-Based Drug Delivery Systems: Overcoming the Hurdles in Cancer Therapy. Pharmaceutics 2021, 13, 1120. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.N.; Zhorova, R.; Momekov, G.; Simova, S.; Stoyanova, R.; Zhecheva, E.; Ivanova, S.; Mitewa, M. Synthesis, spectral properties, antibacterial and antitumor activity of salinomycin complexes with Co(II), Ni(II), Cu(II) and Zn(II) transition metal ions. J. Chem. Chem. Eng. 2012, 6, 551–562. [Google Scholar][Green Version]
- Pantcheva, I.; Petkov, N.; Simova, S.; Zhorova, R.; Dorkov, P. 4 Alkaline-earth metal(II) complexes of salinomycin—Spectral properties and antibacterial activity. In Biochemical and Environmental Applications; Ramasami, P., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2022; Volume 2, pp. 65–78. [Google Scholar] [CrossRef]
- Pashkunova-Martic, I.; Kukeva, R.; Stoyanova, R.; Pantcheva, I.; Dorkov, P.; Friske, J.; Hejl, M.; Jakupec, M.; Hohagen, M.; Legin, A.; et al. Novel Salinomycin-Based Paramagnetic Complexes—First Evaluation of Their Potential Theranostic Properties. Pharmaceutics 2022, 14, 2319. [Google Scholar] [CrossRef]
- Pantcheva, I.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W. First solid state alkaline-earth complexes of monensic acid A (MonH): Crystal structure of [M(Mon)2(H2O)2] (M = Mg, Ca), spectral properties and cytotoxicity against aerobic Gram-positive bacteria. Biometals 2010, 3, 59–70. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.N.; Mitewa, M.; Simova, S.; Tanabe, M.; Osakada, K. Cd(II) and Pb(II) complexes of the polyether ionophorous antibiotic salinomycin. Chem. Cent. J. 2011, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Petrova, E.; Kamenova, K.; Gluhcheva, Y. Comparative effects of meso-2,3-dimercaptosuccinic acid, monensin, and salinomycin on cadmium-induced brain dysfunction in cadmium-intoxicated mice. Interdiscip. Toxicol. 2017, 10, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Kamenova, K.; Petrova, E.; Vladov, I.; Gluhcheva, Y.; Dorkov, P. Comparative study on the effects of salinomycin, monensin and meso-2,3-dimercaptosuccinic acid on the concentrations of lead, calcium, copper, iron and zinc in lungs and heart in lead-exposed mice. J. Trace Elements Med. Biol. 2019, 58, 126429. [Google Scholar] [CrossRef]
- Gluhcheva, Y.; Pashkunova-Martic, I.; Schaier, M.; Vladov, I.; Stoykova, S.; Petrova, E.; Pavlova, E.; Dorkov, P.; Helbich, T.H.; Keppler, B.; et al. Comparative effects of deferiprone and salinomycin on lead-induced disturbance in the homeostasis of intrarenal essential elements in mice. Int. J. Mol. Sci. 2022, 23, 4368. [Google Scholar] [CrossRef] [PubMed]
- Ugrina, M.; Čeru, T.; Nuić, I.; Trgo, M. Comparative Study of Mercury(II) Removal from Aqueous Solutions onto Natural and Iron-Modified Clinoptilolite Rich Zeolite. Processes 2020, 8, 1523. [Google Scholar] [CrossRef]
- Hietanen, S.; Sillen, L.G. Studies on the hydrolysis of metal ions. II. The hydrolysis of the mercury (II) ion Hg2+. Acta Chim. Scand. 1952, 6, 747–758. [Google Scholar] [CrossRef]
- Florez, E.; Zapata-Escobar, A.D.; Ferraro, F.; Becerra, C.I.; Chamorro, Y.; Maldonado, A.F. Coordination of Mercury(II) in Water Promoted over Hydrolysis in Solvated Clusters [Hg(H2O)1–6](aq)2+: Insights from Relativistic Effects and Free Energy Analysis. J. Phys. Chem. A 2023, 127, 8032–8049. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectroscopy of Inorganic and Coordination Compounds, 5th ed.; Wiley: Toronto, ON, Canada, 1997. [Google Scholar]
- Nara, M.; Tanokura, M. Infrared spectroscopic study of the metal-coordination structures of calcium-binding proteins. Biochem. Biophys. Res. Commun. 2008, 369, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.C.; da Silva, G.; Franks, G.V. Modeling the IR spectra of aqueous metal carboxylate complexes: Correlation between bonding geometry and stretching mode wavenumber shifts. Chemistry 2015, 21, 6801–6805. [Google Scholar] [CrossRef]
- Justi, M.; Puggina de Freitas, M.; Silla, J.M.; Nunes, C.A.; Silva, C.A. Molecular structure features and fast identification of chemical properties of metal carboxylate complexes by FTIR and partial least square regression. J. Mol. Struct. 2021, 1237, 130405. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J.; Deacon, R.; Phillips, G. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W. Crystal structures and spectral properties of new Cd(II) and Hg(II) complexes of monensic acid with different coordination modes of the ligand. Open Chem. 2010, 8, 852–860. [Google Scholar] [CrossRef]
- McIndoe, J.S.; Vikse, K.L. Assigning the ESI mass spectra of organometallic and coordination compounds. J. Mass. Spectrom. 2019, 54, 466–479. [Google Scholar] [CrossRef]
- Ivanova, J.; Kukeva, R.; Stoyanova, R.; Zhivkova, T.; Abudalleh, A.; Dyakova, L.; Alexandrova, R.; Pashkunova-Martic, I.; Theiner, J.; Dorkov, P.; et al. New Iron(III)-Containing Composite of Salinomycinic Acid with Antitumor Activity—Synthesis and Characterization. Inorganics 2024, 12, 206. [Google Scholar] [CrossRef]
- Kumar, T.V.; Chary, A.S.; Bhardwaj, S.; Awasthi, A.M.; Reddy, S.N. Dielectric Relaxation, Ionic Conduction and Complex Impedance Studies on NaNo3 Fast Ion Conductor. Int. J. Mater. Sci. Appl. 2013, 2, 173–178. [Google Scholar] [CrossRef]
- Thompsett, S.J. A Study of the Effect of Structure and Conformation on the Transport Properties of Ionophores Using NMR. Ph.D. Thesis, University of St Andrews, St Andrews, Scotland, 1992. [Google Scholar]
- Seto, H.; Miyazaki, Y.; Fujita, K.-I.; Otake, N. Studies on the ionophorous antibiotics. X. The assignments of 13C-NMR spectrum of salinomycin. Tetrahedron Lett. 1977, 28, 2417–2420. [Google Scholar] [CrossRef]
- Petkov, N.; Pantcheva, I.; Ivanova, A.; Stoyanova, R.; Kukeva, R.; Alexandrova, R.; Abudalleh, A.; Dorkov, P. Novel Cerium(IV) Coordination Compounds of Monensin and Salinomycin. Molecules 2023, 28, 4676. [Google Scholar] [CrossRef] [PubMed]
- Paulus, E.F.; Kurz, M.; Matter, H.; Vértesy, L. Solid-State and Solution Structure of the Salinomycin–Sodium Complex: Stabilization of Different Conformers for an Ionophore in Different Environments. J. Am. Chem. Soc. 1998, 120, 8209–8221. [Google Scholar] [CrossRef]
- Kandioller, W.; Theiner, J.; Keppler, B.K.; Kowol, C.R. Elemental analysis: An important purity control but prone to manipulations. Inorg. Chem. Front. 2021, 9, 412–416. [Google Scholar] [CrossRef]
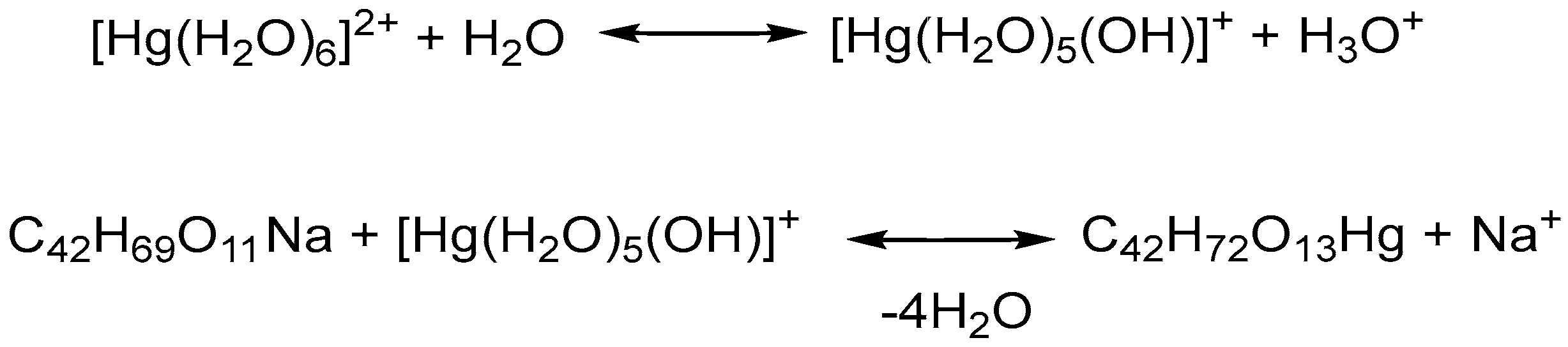
| Element | Experimental Values, % | Molar Ratios | Calculated Value 2 |
|---|---|---|---|
| C | 48.73 ± 0.32 | 51.18 | |
| H | 6.79 ± 0.12 | 7.36 | |
| O | 20.51 ± 0.32 | 21.10 | |
| N 1 | 0.320 ± 0.003 | - | |
| Hg | 19.34 ± 2.50 | C:Hg = 42.27 H:Hg = 70.17 O:Hg = 13.35 | 20.35 |
| Na | <0.05 | - | |
| NO3 | <0.1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, J.; Pashkunova-Martic, I.; Theiner, J.; Burdzhiev, N.; Dorkov, P.; Grabchev, I. New Complex of Salinomycin with Hg(II)—Synthesis and Characterization. Inorganics 2025, 13, 220. https://doi.org/10.3390/inorganics13070220
Ivanova J, Pashkunova-Martic I, Theiner J, Burdzhiev N, Dorkov P, Grabchev I. New Complex of Salinomycin with Hg(II)—Synthesis and Characterization. Inorganics. 2025; 13(7):220. https://doi.org/10.3390/inorganics13070220
Chicago/Turabian StyleIvanova, Juliana, Irena Pashkunova-Martic, Johannes Theiner, Nikola Burdzhiev, Peter Dorkov, and Ivo Grabchev. 2025. "New Complex of Salinomycin with Hg(II)—Synthesis and Characterization" Inorganics 13, no. 7: 220. https://doi.org/10.3390/inorganics13070220
APA StyleIvanova, J., Pashkunova-Martic, I., Theiner, J., Burdzhiev, N., Dorkov, P., & Grabchev, I. (2025). New Complex of Salinomycin with Hg(II)—Synthesis and Characterization. Inorganics, 13(7), 220. https://doi.org/10.3390/inorganics13070220









