Abstract
Eskebornite (CuFeSe2), a member of the I–III–VI2 ternary semiconductor family, was explored in this study as a potential thermoelectric material, offering new insights into its synthesis, structural characteristics, and transport behavior. Structurally analogous to chalcopyrite (CuFeS2)—an extensively studied antiferromagnetic semiconductor—eskebornite remains relatively underexplored, particularly regarding its solid-state synthesis and thermoelectric performance. To address this gap, pure eskebornite was synthesized via mechanical alloying followed by hot pressing, a method that enables the fine control of its phase composition and microstructural features. The synthesized undoped CuFeSe2 exhibited p-type nondegenerate semiconducting behavior, with electrical conductivity increasing monotonically over the temperature range of 323–623 K, indicative of thermally activated carrier transport. Simultaneously, a decreasing trend in thermal conductivity with temperature was observed, likely resulting from intensified phonon scattering, which serves to suppress heat transport and enhance the thermoelectric efficiency by maintaining a thermal gradient across the material. A peak in the Seebeck coefficient occurred between 473 and 523 K, suggesting the onset of intrinsic carrier excitation and a transition in dominant carrier transport mechanisms. The material exhibited a maximum power factor of 1.55 μWm−1K−2, while the dimensionless thermoelectric figure of merit (ZT) reached a peak value of 0.37 × 10−3 at 523 K. Although the ZT remains low, these results underscore the potential of eskebornite as a thermoelectric candidate, with substantial room for optimization through chemical doping, microstructural engineering, or nanostructuring approaches to enhance the carrier mobility and reduce the lattice thermal conductivity.
1. Introduction
The conversion of waste heat into electrical energy via thermoelectric power generation, based on the Seebeck effect, has emerged as a sustainable and scalable technology, particularly relevant to energy recovery in the automotive and industrial sectors [1]. The efficiency of thermoelectric materials is governed by the dimensionless figure of merit, ZT = σα2T/(κE + κL), where σ is the electrical conductivity, α is the Seebeck coefficient, and κE and κL represent the electronic and lattice components of thermal conductivity, respectively [2,3]. Achieving a high thermoelectric performance necessitates the simultaneous optimization of these inherently interdependent properties: high electrical conductivity and a high Seebeck coefficient, along with low thermal conductivity. This trade-off presents a fundamental challenge in thermoelectric research, as improving one parameter often adversely affects another [4,5,6]. To circumvent these limitations, several strategies have been developed, including band engineering via chemical doping to optimize carrier concentration and phonon engineering through nanostructuring or defect modulation to reduce lattice thermal conductivity. These approaches have led to significant enhancements in ZT for state-of-the-art thermoelectric materials such as Bi2Te3 [7,8], PbTe [9,10,11,12], and SnSe [13], particularly near room temperature and in the mid-temperature range.
More recently, I–III–VI2 ternary chalcogenides have garnered growing interest as alternative thermoelectric candidates due to their Earth abundance, tunable band structures, and relatively low toxicity. Within this class, CuFeSe2—commonly referred to as eskebornite—crystallizes in a tetragonal chalcopyrite-type structure (space group ) with the reported lattice parameters a = 0.5518 nm and c = 1.105 nm and a tetragonality (c/a ratio) of 2.003 [14]. Its narrow band gap of ~0.16 eV places it within the regime of semiconductors suitable for thermoelectric applications, comparable to traditional high-performance materials [15,16,17,18]. Despite its potential, CuFeSe2 has been largely overlooked in thermoelectric studies due to its intrinsic metallic-like electrical behavior and low Seebeck coefficient under standard synthesis conditions [1,14,19,20,21,22,23,24,25].
Nonetheless, several recent studies have demonstrated that modifying the synthesis route and composition can significantly alter the thermoelectric properties of CuFeSe2. Moorthy et al. [1] reported that CuFeSe2 nanoparticles synthesized via hydrothermal methods exhibited enhanced Seebeck coefficients compared to bulk counterparts fabricated by vacuum melting. This enhancement was attributed to quantum confinement effects, which widened the band gap, thereby increasing the Seebeck coefficient while simultaneously reducing total thermal conductivity. Similarly, Zhai et al. [14] synthesized Cu1+xFeSe2 alloys with excess Cu using a melting—annealing approach, finding that the additional Cu introduced disorder and secondary phases that enhanced phonon scattering, leading to reduced lattice thermal conductivity and over twofold improvements in ZT.
Building upon these findings, the present study adopted a scalable and efficient solid-state synthesis method combining mechanical alloying (MA) with hot pressing (HP) to fabricate bulk CuFeSe2. This work systematically investigated the effects of MA duration and HP temperature on phase formation, microstructure, and thermoelectric transport properties. By conducting a comparative analysis with previously reported synthesis routes and doping strategies, this study aimed to elucidate the intrinsic thermoelectric potential of eskebornite and explore avenues for further performance enhancement. The insights obtained may inform future efforts to optimize synthesis protocols for CuFeSe2-based thermoelectric materials, thereby expanding the applicability of Earth abundant chalcogenides in energy-harvesting technologies.
2. Results and Discussion
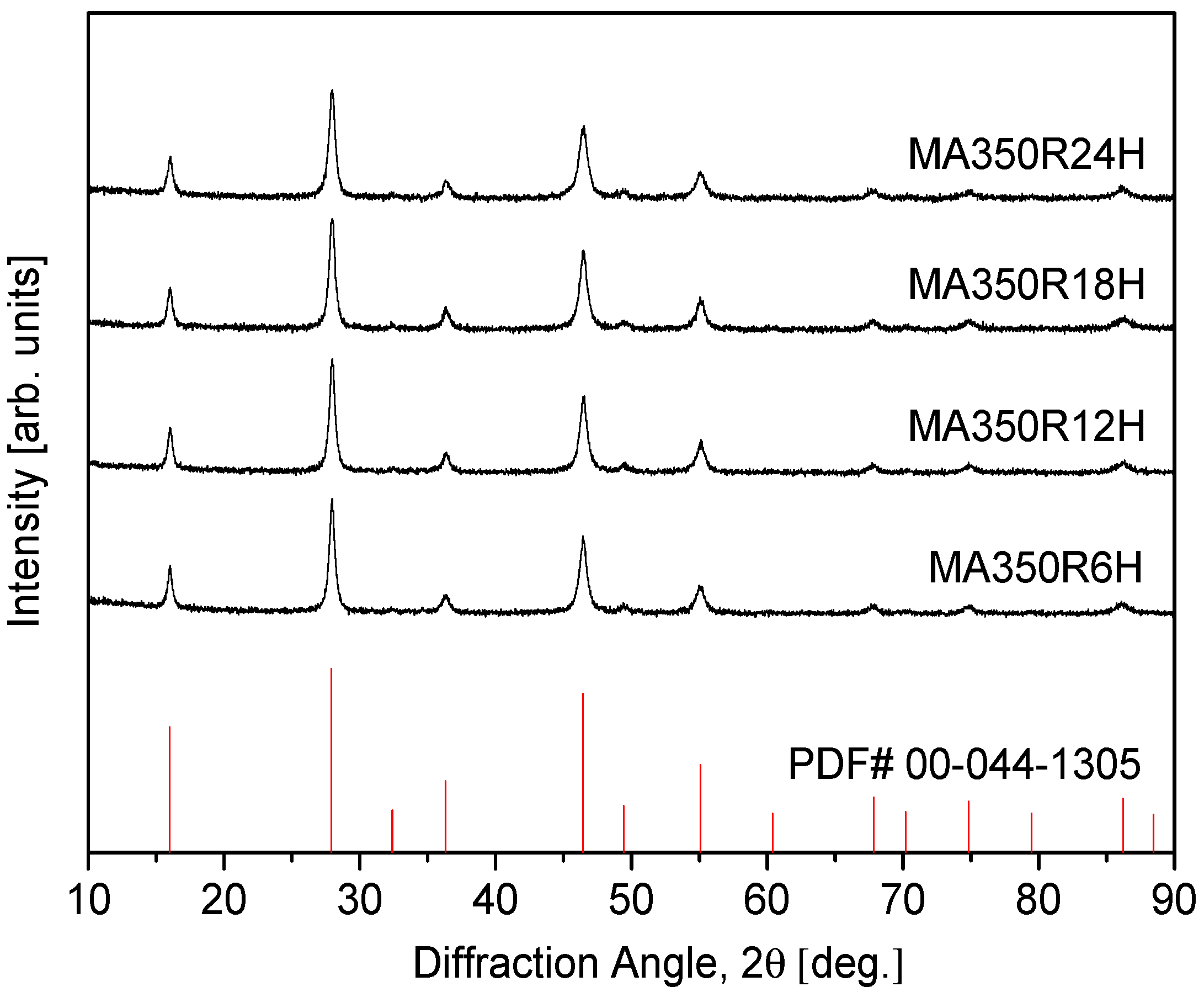
Figure 1 presents the XRD patterns of the synthesized CuFeSe2 powders, illustrating the influence of MA duration on phase formation. The diffraction peaks observed align closely with the reference pattern for tetragonal eskebornite (CuFeSe2; PDF# 00–044–1305), affirming the successful synthesis of the target phase. This structural confirmation is in agreement with earlier findings by Delgado et al. [22], who noted that eskebornite could be considered a derivative of sulvanite (Cu3VS4) due to notable similarities in their diffraction signatures. Specifically, the sample processed via MA for 6 h at 350 rpm (denoted MA350R6H) displayed a well-defined single-phase tetragonal structure, indicating that short-duration milling is sufficient to induce solid-state reactions and phase stabilization. When the milling time was extended to 24 h (MA350R24H), no secondary phases were detected, and the diffraction pattern remained consistent, suggesting that prolonged MA does not compromise phase integrity, nor does it induce additional transformations. This stability under extended milling implies a robust structural resilience in CuFeSe2 against mechanical-induced disorder.
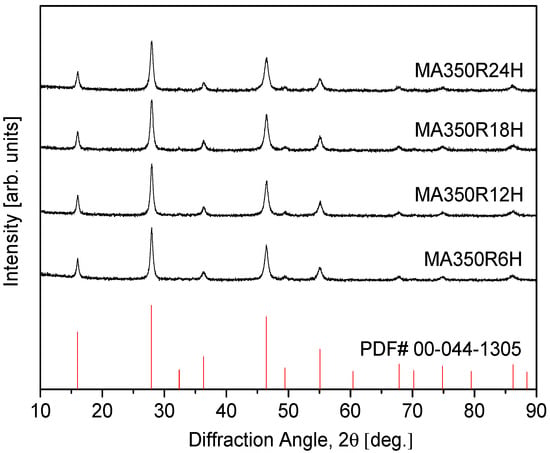
Figure 1.
XRD patterns of synthesized CuFeSe2 powders.
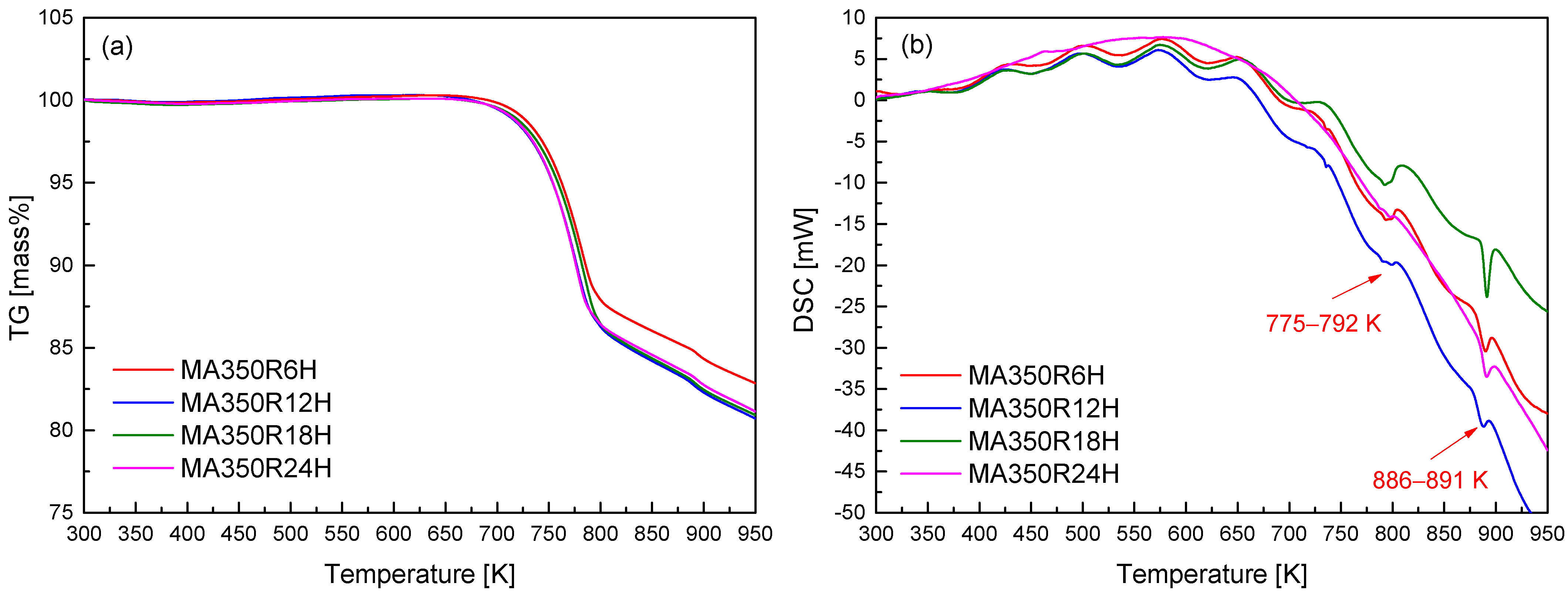
To further elucidate the thermal stability and potential phase transitions of the synthesized CuFeSe2, thermogravimetric (TG) and differential scanning calorimetry (DSC) analyses were conducted, with the results displayed in Figure 2. A noticeable mass loss initiates near 723 K, which is attributed to the volatilization of selenium—a volatile component known to influence the stoichiometry and thermal stability of chalcogenide compounds. The tendency of Se to volatilize upon heating represents a crucial factor affecting the compositional stability and thus the thermoelectric performance of CuFeSe2. The DSC curves exhibit distinct thermal events across all MA powders, characterized by minor endothermic peaks between 775 and 792 K and more pronounced endothermic peaks around 886–891 K. These thermal signatures correspond, respectively, with the onset of decomposition/volatilization and congruent melting of CuFeSe2. Notably, the observed melting behavior near 850 K is in line with previous reports on the congruent melting point of CuFeSe2 [1]. The resolution and reproducibility of these thermal events across samples provide critical information for identifying thermal limits during subsequent sintering or device operation, where phase degradation must be avoided. From the combined XRD and TG–DSC analyses, the optimal MA processing condition was determined to be 350 rpm for 12 h (MA350R12H), balancing effective phase formation with thermal stability. Based on this optimized conditions, further investigations were directed toward exploring how varying HP temperatures influence the microstructural evolution, phase purity, and thermoelectric properties of CuFeSe2.
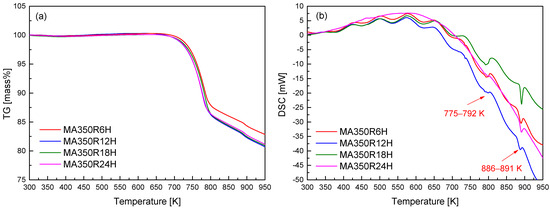
Figure 2.
(a) TG and (b) DSC analyses of mechanically alloyed CuFeSe2.
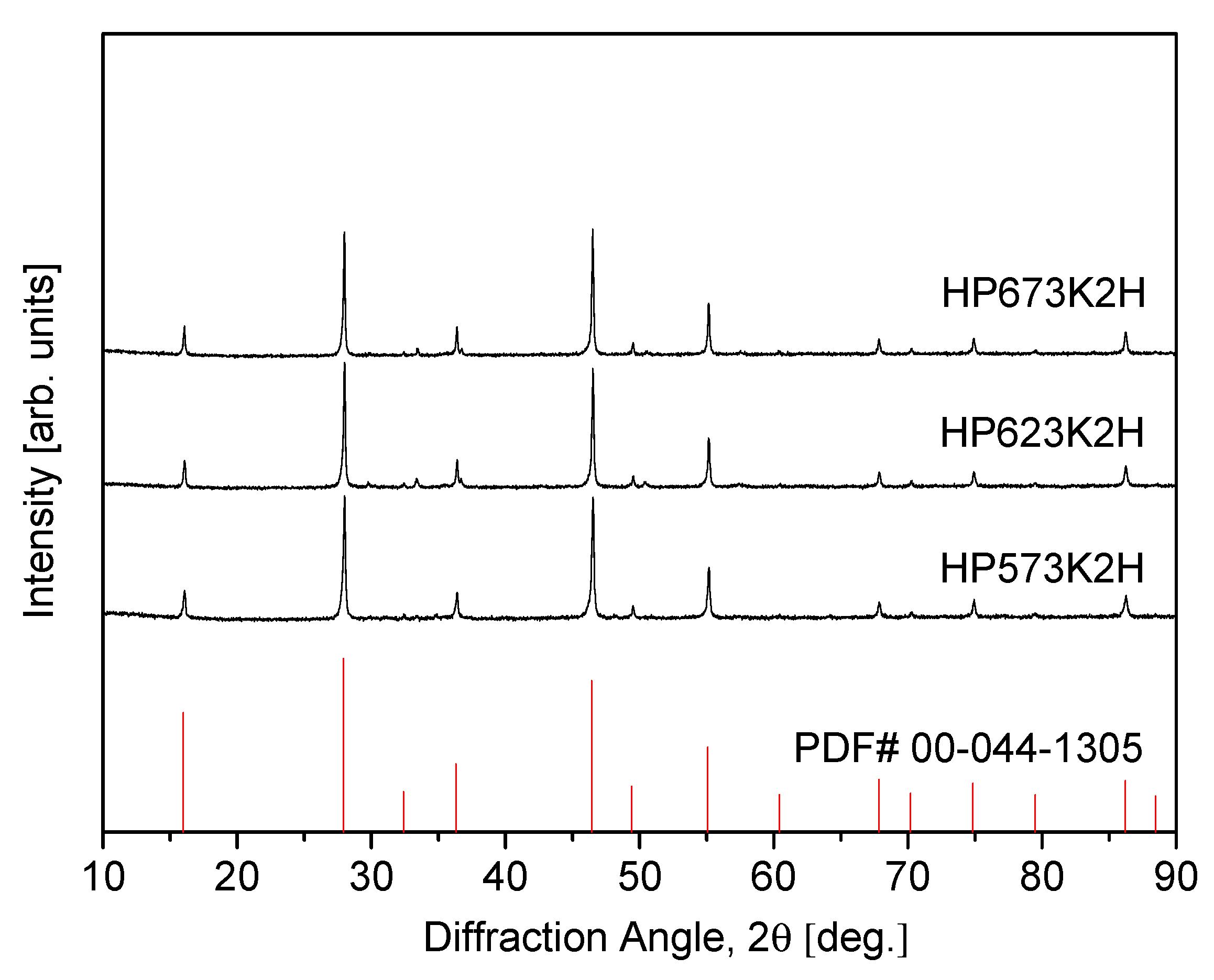
Figure 3 presents the XRD patterns of CuFeSe2 specimens consolidated by HP at various temperatures, utilizing mechanically alloyed powders synthesized under the optimized milling conditions (MA350R12H). The sample labels, such as “HP623K2H”, denote the specific sintering parameters—hot pressing at 623 K for 2 h. The XRD results verify that the eskebornite phase remains structurally stable throughout the HP process, with no observable phase decomposition or formation of secondary phases. This phase retention under thermal and mechanical compaction conditions is of critical importance, as it ensures the preservation of the material’s intrinsic thermoelectric properties, which are strongly dependent on structural integrity. A comparative analysis of the diffraction patterns reveals that the broadened peaks observed in the as-milled powders (Figure 1) are likely attributable to nanocrystalline domains and lattice strain induced by mechanical deformation during ball milling. Following HP, the diffraction peaks become noticeably sharper and more intense, indicative of improved crystallinity. This transformation suggests significant crystallite growth and partial strain recovery facilitated by the elevated temperature and pressure conditions during HP. Such structural refinement is beneficial for optimizing carrier mobility and reducing grain boundary scattering, both of which are essential in enhancing thermoelectric performance.
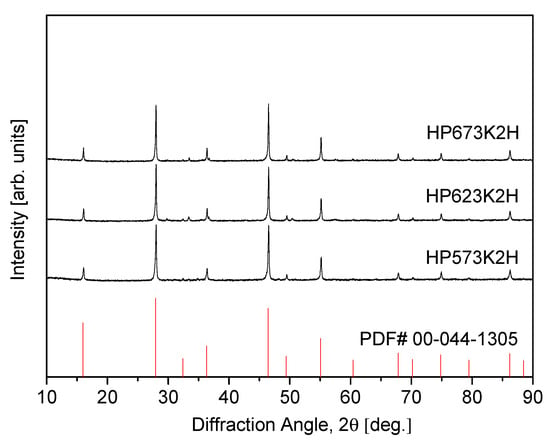
Figure 3.
XRD patterns of sintered CuFeSe2 specimens.
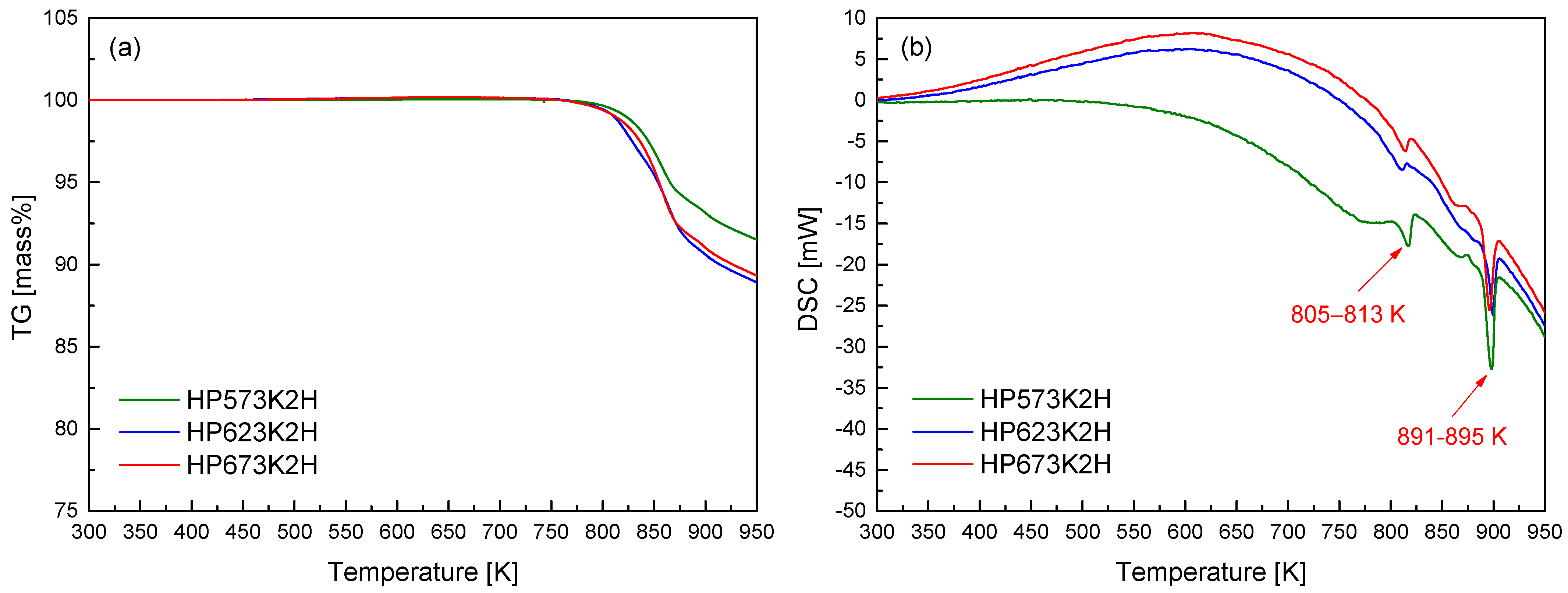
Figure 4 presents the TG–DSC curves for the hot-pressed CuFeSe2 specimens. A pronounced mass loss was detected at temperatures exceeding approximately 823 K. This onset temperature is notably higher than that observed in the TG profiles of the mechanically alloyed powders (Figure 2a), suggesting that hot pressing significantly enhances the thermal stability of the material. In addition to the delayed onset, the rate of mass loss in the hot-pressed samples was markedly reduced. This diminished volatilization can be ascribed to the lower specific surface area and increased densification resulting from the HP process. The compact microstructure produced through sintering acts as a diffusion barrier, particularly impeding the escape of volatile elements such as selenium, which is highly susceptible to sublimation at elevated temperatures. The improved retention of Se is particularly important in preserving phase composition and ensuring the long-term thermoelectric reliability of CuFeSe2 under thermal stress. The DSC thermograms reveal minor endothermic events between 805 and 813 K and more pronounced peaks in the 891–895 K range. These thermal events are associated with phase-related transformations such as incipient volatilization and eventual melting. Importantly, no thermal anomalies were observed below 750 K, indicating that the MA–HP synthesized eskebornite maintains structural stability up to this temperature. Consequently, sintering temperatures for hot pressing should be carefully selected to remain below this thermal threshold in order to prevent undesirable decomposition or phase degradation. This observation aligns with the findings of Zhang et al. [23], who reported that hot-pressed CuFeSe2 exhibited thermal stability up to approximately 723 K, as determined via TG analysis. The elevated stability observed in the present study further corroborates the beneficial impact of optimized MA–HP processing conditions on the high-temperature performance of eskebornite.
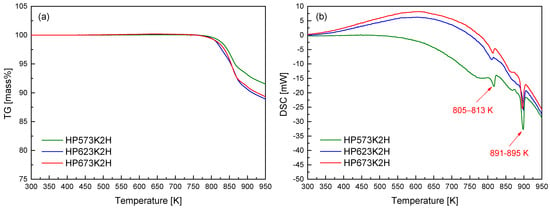
Figure 4.
(a) TG and (b) DSC analyses of hot-pressed CuFeSe2 specimens.
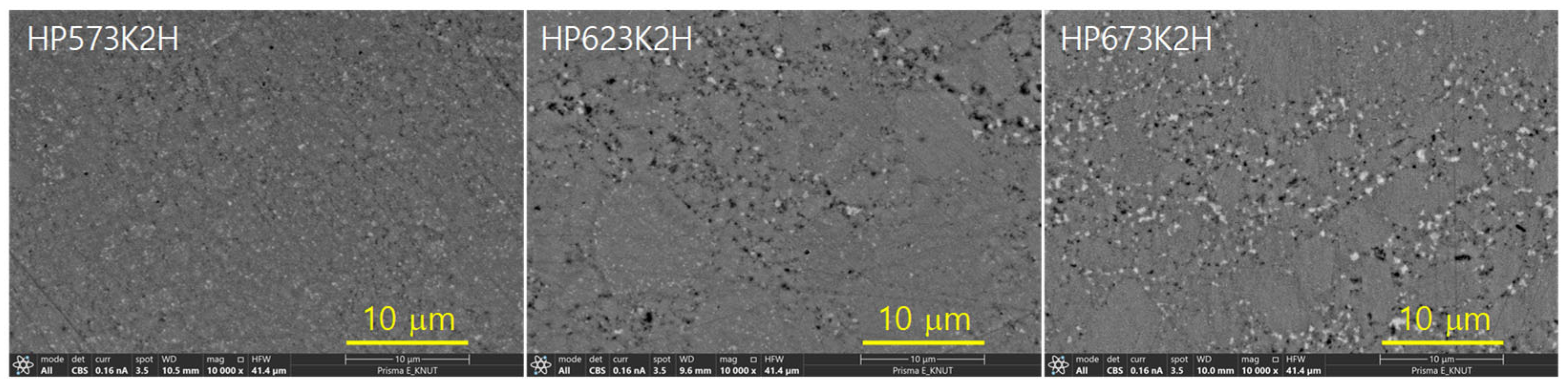
Figure 5 presents the SEM images of sintered CuFeSe2 specimens, elucidating the microstructural evolution under varying HP temperatures. The samples exhibited dense microstructures, with relative densities ranging from 99.1% to 99.6%, as summarized in Table 1, relative to the theoretical density of CuFeSe2 (5.35 gcm−3) [24]. These results confirm the effectiveness of the HP process in promoting substantial densification, which is critical in achieving enhanced mechanical robustness and reduced phonon scattering at grain boundaries—key factors in improving thermoelectric performance. Lattice parameters determined via Rietveld refinement were found to be in the ranges of a = 0.5523–0.5525 nm and c = 1.1040–1.1041 nm, corresponding to a tetragonality (c/a) between 1.9984 and 1.9989. These values closely align with those reported by Moorthy et al. [1] for bulk CuFeSe2 (a = 0.55272 nm, c = 1.10380 nm), confirming the successful formation of the tetragonal eskebornite phase. The minor deviations observed in the lattice constants may stem from differences in synthesis techniques, such as mechanical alloying versus solid-state reaction, or variations in processing parameters like pressure and temperature during HP. Further comparison with the study by Zhai et al. [14], who investigated Cu1+xFeSe2 with varying Cu content (x = 0.00–0.05), revealed that the lattice parameters for stoichiometric CuFeSe2 (x = 0) were slightly lower (a = 0.55136 nm, c = 1.10357 nm), indicating that even minor compositional deviations can subtly affect the unit cell dimensions. This trend emphasizes the sensitivity of the crystal lattice to stoichiometric imbalances, especially in transition metal chalcogenides where bonding environments are composition-dependent. Interestingly, Hamdadou et al. [25] reported relatively invariant lattice constants for CuFeSe2 across a range of Cu/Fe atomic ratios, with a = 0.5500–0.5506 nm and c = 1.1050–1.1064 nm, suggesting a degree of structural robustness against compositional fluctuations. This apparent discrepancy may reflect differences in sample morphology (e.g., polycrystalline vs. nanocrystalline), synthesis kinetics, or thermal history, which can all influence defect densities and lattice strain. Collectively, the high densification and consistent lattice parameters observed in this study indicate that the MA–HP process effectively produces phase-pure, structurally stable eskebornite.

Figure 5.
SEM images of the polished surfaces of CuFeSe2.

Table 1.
Relative densities and lattice parameters of CuFeSe2 prepared via MA–HP process.
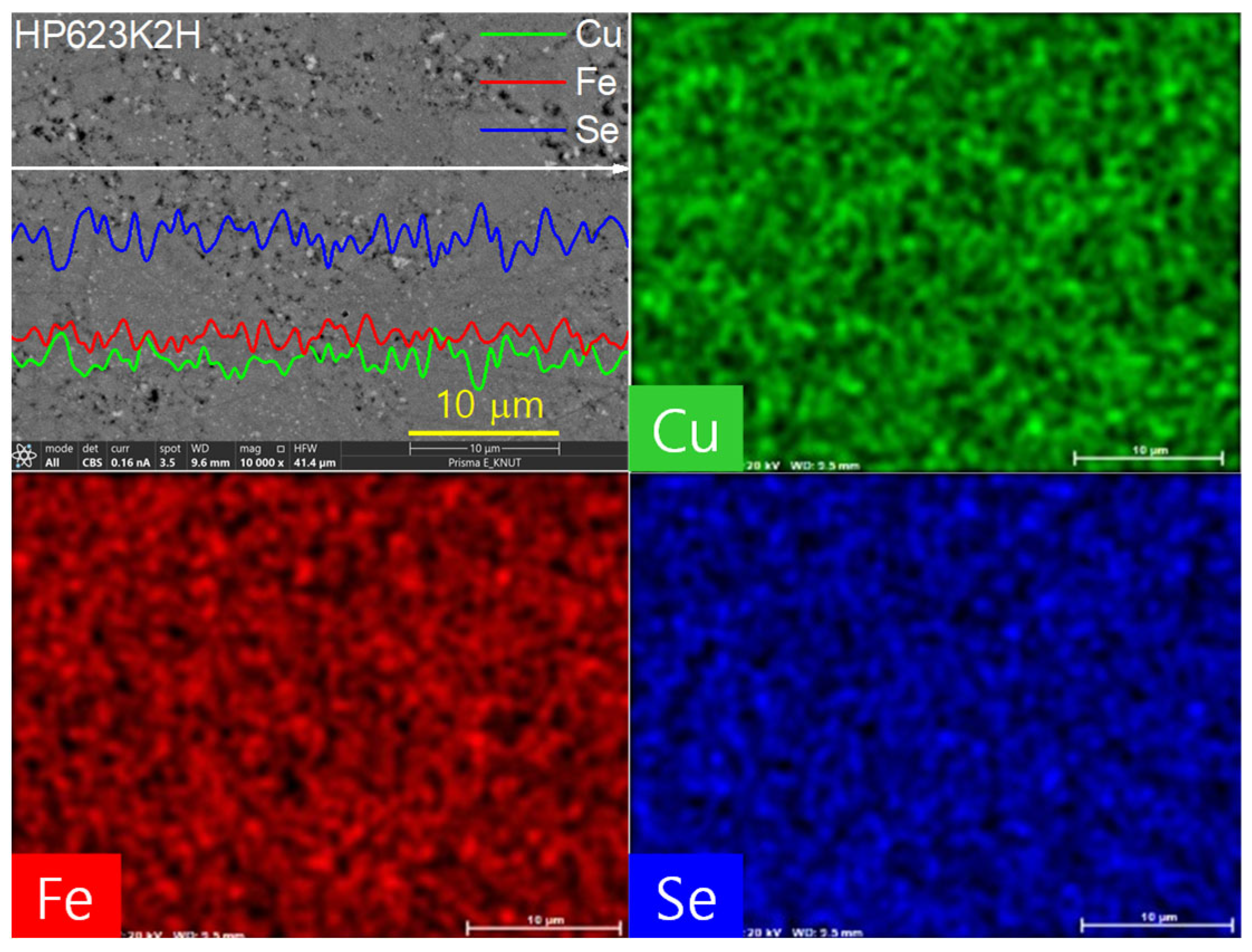
Figure 6 presents a high-resolution examination of the elemental distribution in the CuFeSe2 specimen sintered at 623 K for 2 h (designated HP623K2H), along with the corresponding EDS results. Both the one-dimensional line scan profiles and two-dimensional elemental mapping confirm a uniform spatial distribution of Cu, Fe, and Se throughout the microstructure. This homogeneous distribution of elements is critical in maintaining the intrinsic thermoelectric behavior of the material, as compositional uniformity minimizes the potential for compositional inhomogeneities, secondary phase formation, or localized stoichiometric imbalances. The absence of detectable secondary phases in the EDS spectra strongly supports the preservation of the single-phase tetragonal CuFeSe2 (eskebornite) structure, as corroborated by the XRD results. The microstructural uniformity further indicates that the combination of mechanical alloying and hot pressing successfully facilitated not only intimate mixing at the atomic level but also effective densification, thereby suppressing phase segregation during synthesis and consolidation.
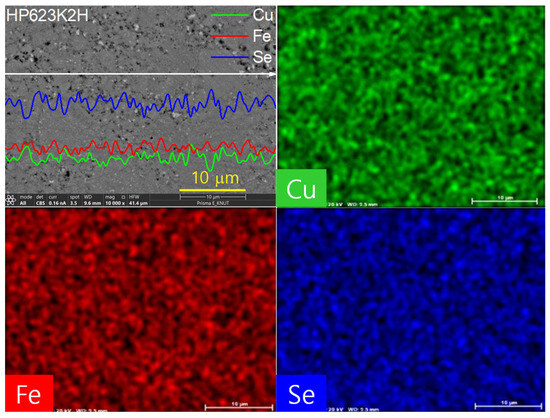
Figure 6.
EDS Analyses for the HP623K2H specimen.
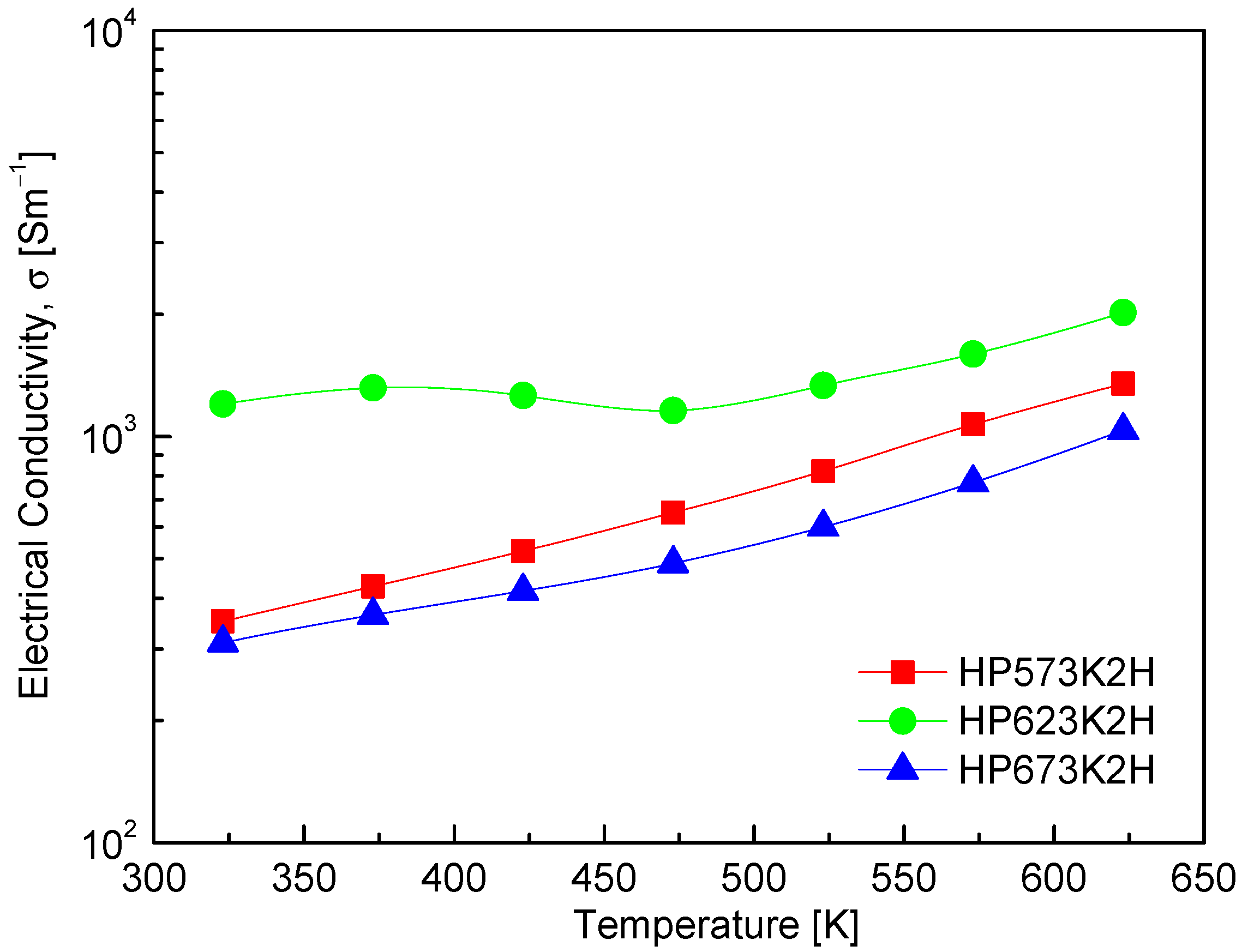
Figure 7 presents the temperature-dependent electrical conductivity of CuFeSe2, clearly illustrating its semiconducting nature. The observed increase in electrical conductivity with rising temperature is characteristic of non-degenerate semiconductors, wherein thermal excitation promotes charge carriers across the band gap, thereby enhancing electrical transport. A detailed comparison of specimens processed under varying HP temperatures reveals a significant influence of sintering conditions on electrical conductivity. Specifically, increasing the HP temperature from 573 to 623 K results in a marked improvement in electrical conductivity, likely due to enhanced densification, improved grain connectivity, and a reduction in grain boundary scattering. However, further increasing the HP temperature to 673 K leads to a decline in electrical conductivity. This deterioration can be attributed to compositional deviations—particularly Se volatilization at elevated temperatures due to its high vapor pressure. The resulting Se vacancies act as compensating donors, effectively reducing the concentration of majority carriers (holes) in this p-type material. Such a decrease in carrier concentration adversely affects electrical conductivity, highlighting the critical importance of maintaining stoichiometric balance during high-temperature processing [26,27]. Among the tested samples, the HP623K2H specimen exhibited the highest electrical conductivity, reaching 1.2 × 103 Sm−1 at 323 K and increasing to 2.0 × 103 Sm−1 at 623 K. This performance indicates that HP at 623 K provides optimal conditions for promoting carrier mobility while minimizing compositional degradation. Comparative insights from previous studies further contextualize these findings. Moorthy et al. [1] reported a decrease in σ from 6.7 × 103 Sm−1 at 300 K to 6.0 × 103 Sm−1 at 500 K, followed by a subsequent increase beyond 500 K, suggesting more complex transport behavior possibly influenced by the synthesis method, the microstructure, or intrinsic defect chemistry. Additionally, Zhai et al. [14] observed that electrical resistance was prohibitively high below 425 K, but conductivity rose sharply to 2.7 × 103 Sm−1 at 723 K, reaffirming the strong temperature dependence of charge transport in CuFeSe2. These variations underscore the sensitivity of electrical conductivity to the synthesis conditions, stoichiometry, and measurement range, reinforcing the significance of fine-tuning processing parameters in thermoelectric optimization.
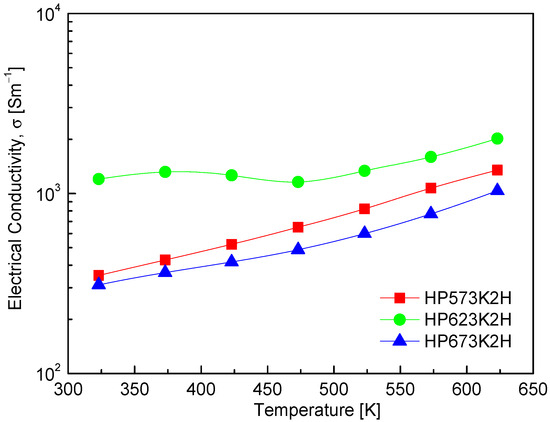
Figure 7.
Temperature dependence of electrical conductivity of CuFeSe2.
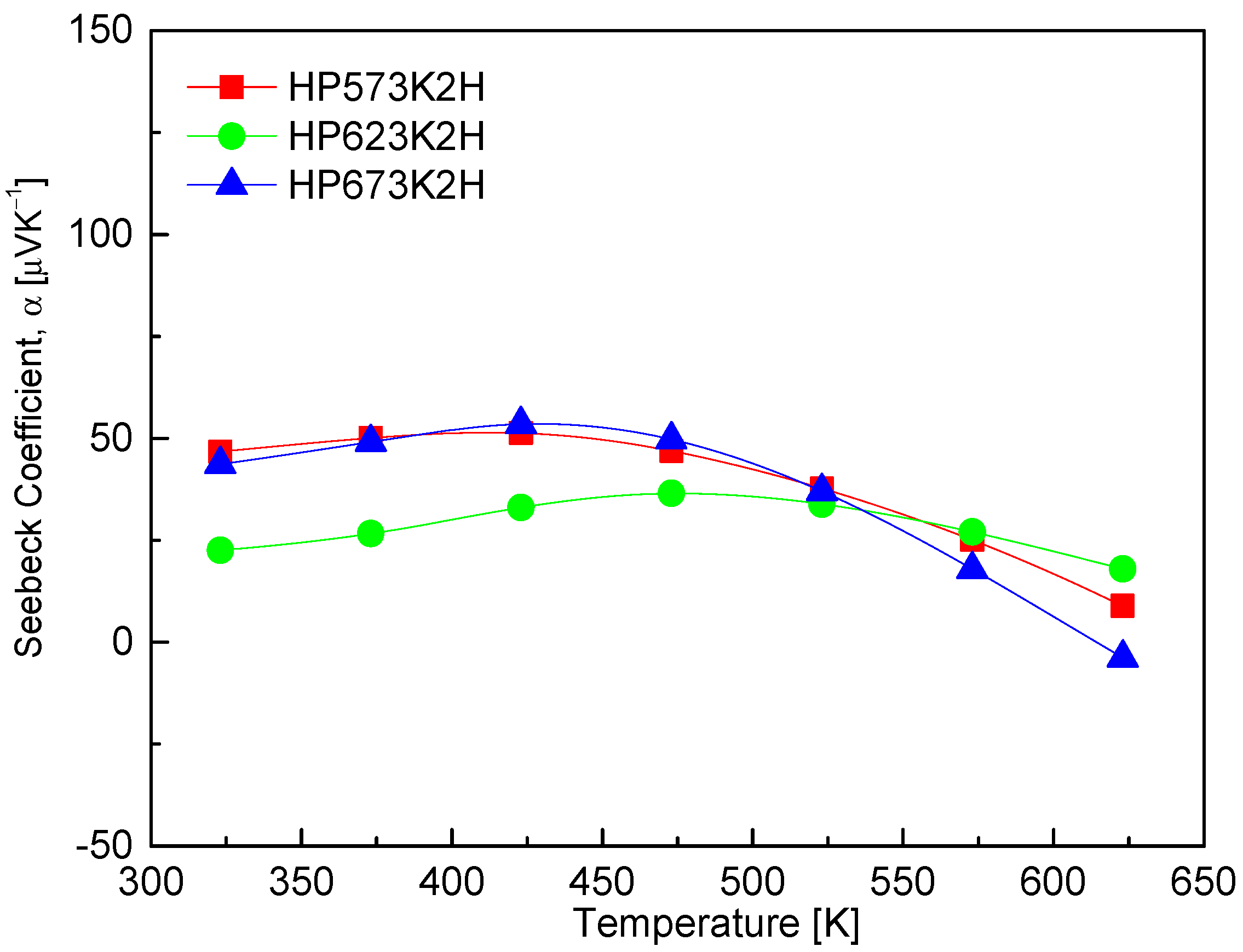
Figure 8 presents the temperature-dependent Seebeck coefficient of CuFeSe2, which reveals positive values across the measured temperature range, thereby confirming dominant p-type conduction and the prevalence of holes as majority charge carriers. The Seebeck coefficient initially increases with temperature, reaching a maximum near an intrinsic transition temperature, beyond which it begins to decrease. This behavior is characteristic of semiconductors and aligns with theoretical models of thermoelectric transport, where the Seebeck coefficient is governed by the interplay between carrier concentration, effective mass, and temperature [28]. At lower temperatures, the increase in the Seebeck coefficient is primarily due to the enhanced thermal excitation of charge carriers, which increases the entropy per carrier and thus the Seebeck coefficient. However, upon reaching the intrinsic transition regime—typically associated with the thermal generation of electron–hole pairs—the rapid rise in carrier concentration leads to bipolar conduction. This phenomenon causes the Seebeck coefficient to decline due to the compensation effect between electrons and holes [29]. In this study, maximum Seebeck coefficient values for CuFeSe2 were observed between 423 and 473 K, with the HP573K2H specimen exhibiting the highest Seebeck coefficient of 51.3 μVK−1 at 473 K. This peak suggests that the sample maintains favorable carrier scattering and a relatively low intrinsic carrier concentration in this temperature window, which is optimal for thermoelectric performance. The comparative literature data reveal significant variability in the Seebeck coefficient values and trends, likely arising from differences in synthesis routes, compositional stoichiometry, and microstructure. Moorthy et al. [1] reported a steady increase in the Seebeck coefficient from 12.2 μVK−1 at 300 K to 16.9 μVK−1 at 645 K, reaffirming hole-dominated transport. In contrast, Zhai et al. [14] observed a sign inversion of the Seebeck coefficient above 323 K, reaching a peak negative value of −175 μVK−1 at 473 K, indicative of a p–n transition and suggesting a shift in majority carrier type. Such a transition may stem from a subtle compositional imbalances or phase evolution during synthesis. Similarly, Berthebaud et al. [30] reported a p–n transition in CuFeSe2 above 325 K, highlighting the sensitivity of the Seebeck coefficient to synthesis conditions and potential phase changes. Conversely, Zhang et al. [23] reported only positive Seebeck coefficients from 300 to 660 K, suggesting stable p-type behavior throughout the measured range.
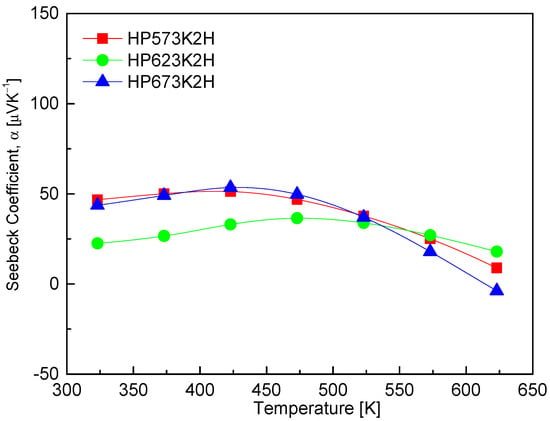
Figure 8.
Temperature dependence of Seebeck coefficient of CuFeSe2.
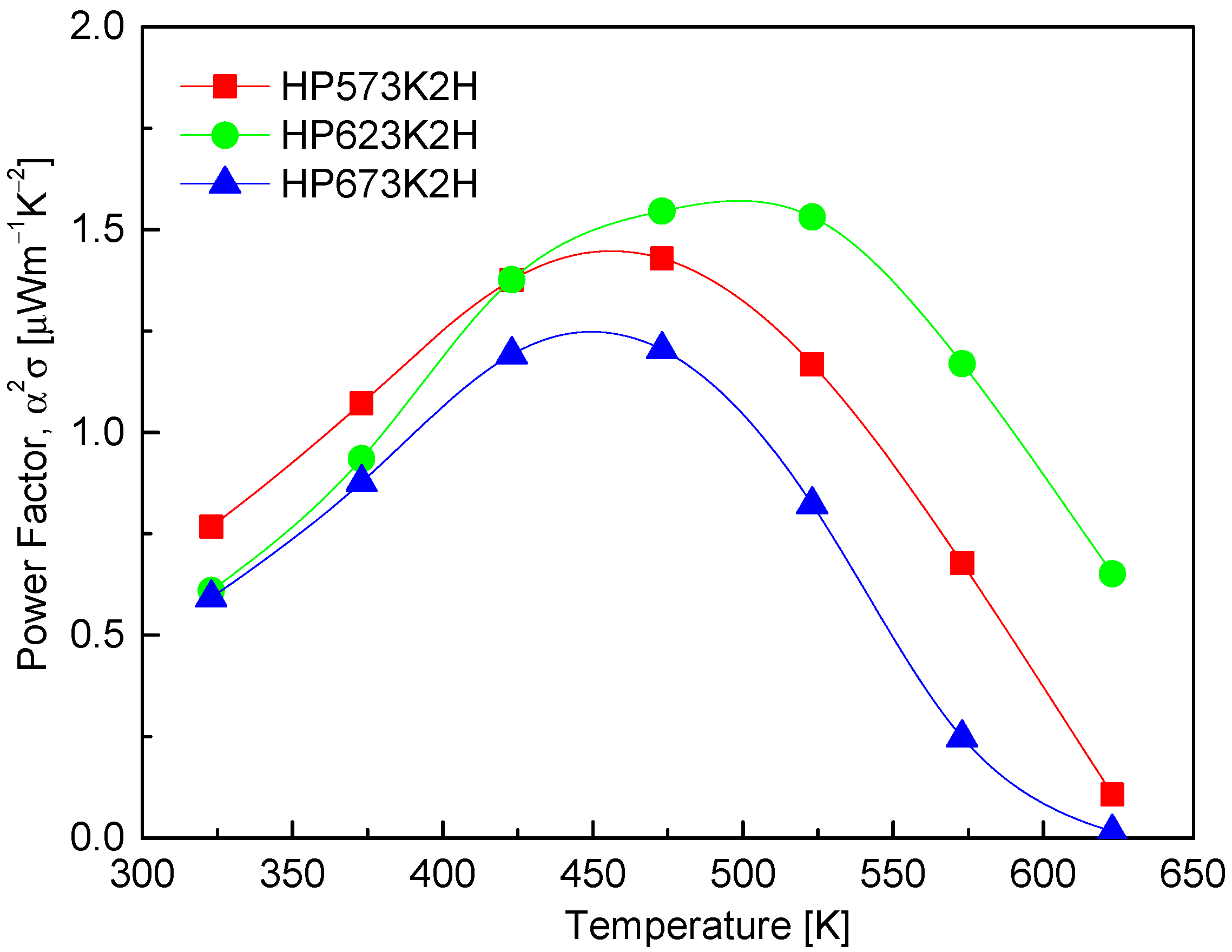
Figure 9 displays the power factor (PF) of CuFeSe2 as a function of temperature, calculated from the measured Seebeck coefficient and electrical conductivity. The results show that the PF reaches its maximum in the temperature range of 473–523 K, which corresponds with the optimal interplay between charge carrier mobility and carrier concentration. Specifically, the HP573K2H and HP623K2H samples exhibit peak PF values of 1.43 μWm−1K−2 at 473 K and 1.55 μWm−1K−2 at 523 K, respectively. These values indicate that the hot-pressing process at moderate temperatures enhances thermoelectric performance by simultaneously improving crystallinity and maintaining a near-stoichiometric composition, which is essential in reducing defect-induced carrier scattering. The enhancement in PF within this temperature window highlights a thermoelectric “sweet spot” where the thermal activation of charge carriers contributes positively to transport properties without inducing significant bipolar conduction or Se volatilization. This suggests that CuFeSe2 synthesized via the MA–HP process exhibits favorable electronic transport properties at intermediate temperatures, making it a promising candidate for mid-temperature thermoelectric applications. When compared to previous studies, the PF values obtained in this work fall within a moderate range. Moorthy et al. [1] reported a higher maximum PF of 2.07 μWm−1K−2 at 645 K for bulk CuFeSe2 synthesized via solid-state reactions, which may reflect improved grain boundary conductivity or lower defect density. On the other hand, Zhang et al. [23] observed a significantly lower PF of 0.37 μWm−1K−2 at 653 K for CuFeSe2 synthesized via a colloidal approach, suggesting that nanostructuring or incomplete densification may have hindered electronic transport in their samples. Remarkably, Zhai et al. [14] reported an exceptionally high n-type PF exceeding 20 μWm−1K−2 at 723 K, which stands in stark contrast with the relatively modest values observed in other reports. This anomalously high performance likely stems from compositional tuning or intrinsic structural differences induced by their specific synthesis route, which may have enhanced charge carrier concentration and mobility. Such discrepancies emphasize the sensitivity of PF to subtle variations in stoichiometry, defect chemistry, and microstructure, reinforcing the importance of processing optimization for high-performance thermoelectric materials.
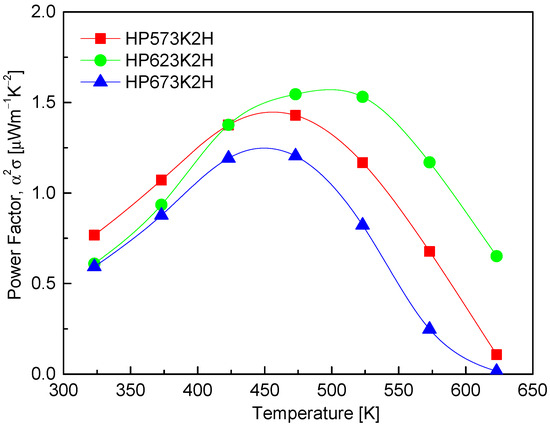
Figure 9.
Temperature dependence of power factor of CuFeSe2.
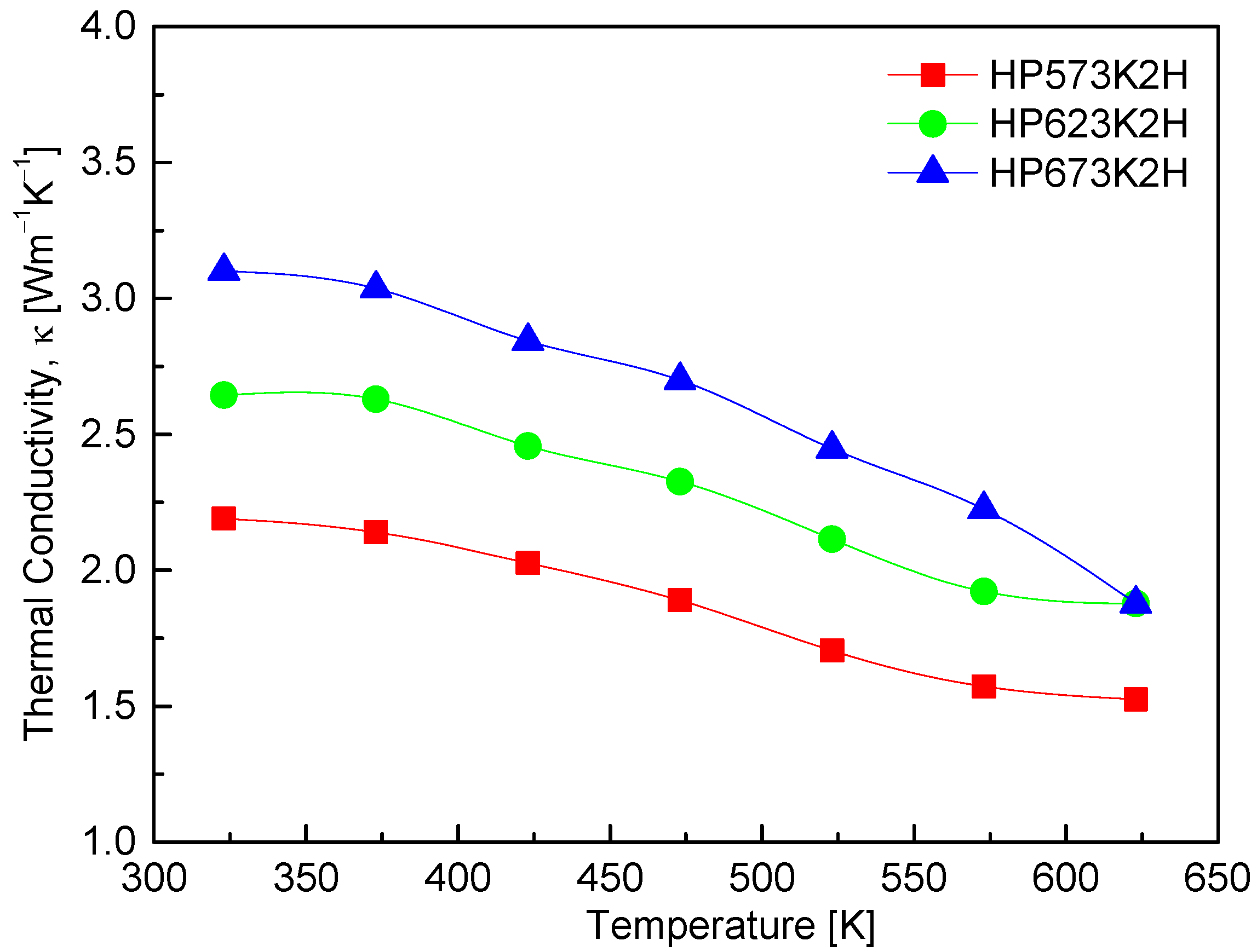
Figure 10 presents the thermal conductivity of CuFeSe2 as a function of the temperature for samples subjected to different HP conditions. The thermal conductivity ranges from 2.19 to 3.10 Wm−1K−1 at 323 K and decreases to between 1.52 and 1.88 Wm−1K−1 at 623 K. This downward trend with increasing temperature is characteristic of many thermoelectric materials and is primarily attributed to enhanced phonon–phonon (Umklapp) scattering, which reduces the phonon mean free path at elevated temperatures. Such behavior supports the intrinsic phonon-dominated heat transport mechanism in CuFeSe2. In addition to temperature effects, the HP temperature also influences thermal conductivity. Higher HP temperatures appear to increase the thermal conductivity across the entire temperature range. For example, the HP573K2H sample shows a reduction in thermal conductivity from 2.19 Wm−1K−1 at 323 K to 1.52 Wm−1K−1 at 623 K, while the HP673K2H sample exhibits a higher thermal conductivity across the board, decreasing from 2.65 to 1.88 Wm−1K−1 over the same temperature range. This increase in thermal conductivity with HP temperature likely results from improved densification and grain growth, which suppress phonon scattering at grain boundaries and increase the effective thermal conductivity. Comparison with the literature further underscores the impact of synthesis conditions on thermal transport. Moorthy et al. [1] reported a significantly higher room-temperature thermal conductivity of 4.96 Wm−1K−1 for bulk CuFeSe2, which decreased to 1.36 Wm−1K−1 at 645 K, reflecting a steep decline in thermal transport likely due to increased phonon scattering at higher temperatures. Zhai et al. [14], on the other hand, reported an even broader range of 9.4 to 2.7 Wm−1K−1 between 323 and 723 K for their nominal CuFeSe2 samples. These variations are attributable to differences in synthesis techniques, microstructural features, and sample stoichiometry, all of which critically influence lattice thermal conductivity. The relatively low thermal conductivity values obtained in this study—particularly in the HP573K2H sample—highlight the effectiveness of mechanical alloying followed by low-to-moderate temperature hot pressing in suppressing phonon transport.
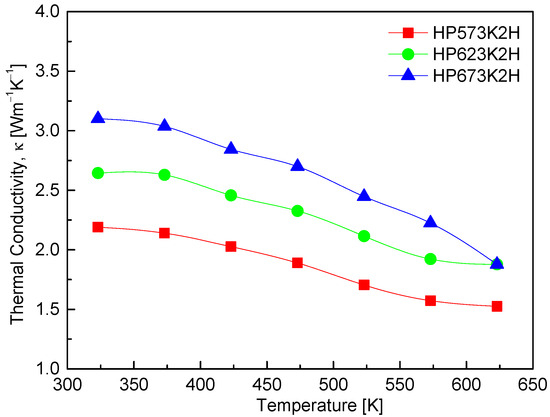
Figure 10.
Temperature dependence of thermal conductivity of CuFeSe2.
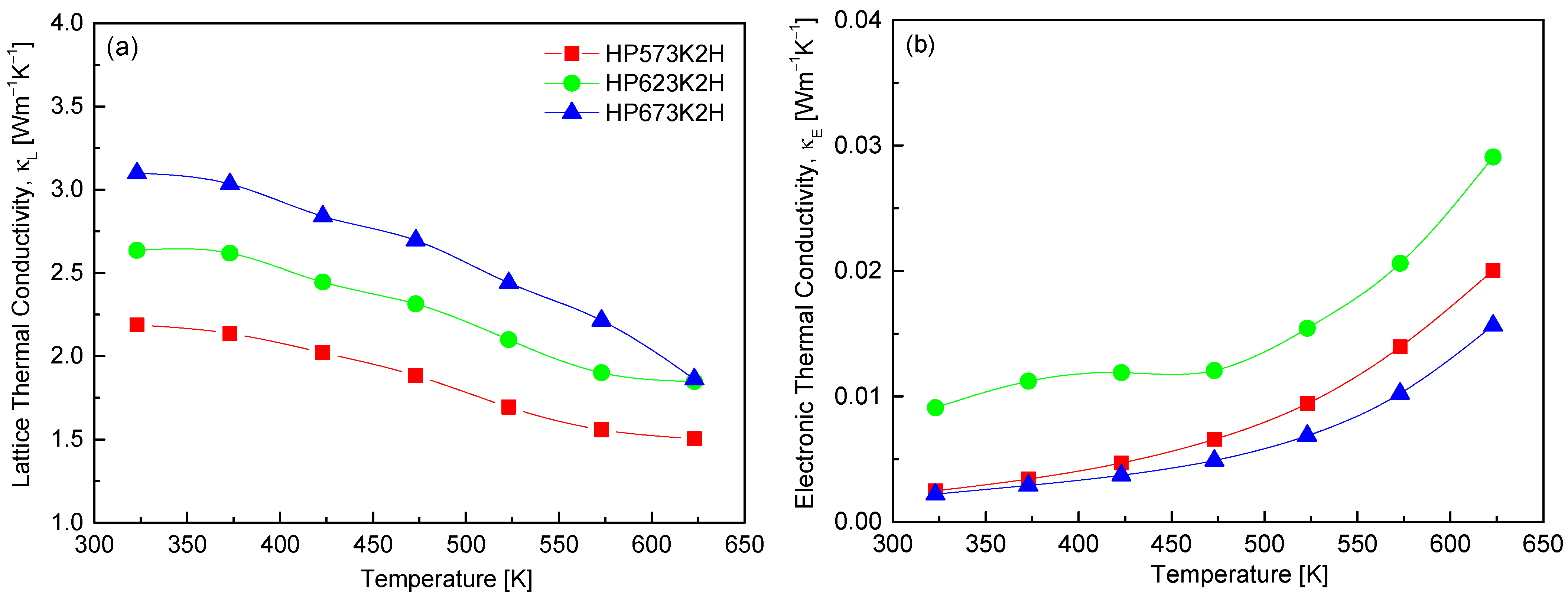
Figure 11 illustrates the separation of the total thermal conductivity into its two primary components, lattice thermal conductivity (κL) and electronic thermal conductivity (κE), for CuFeSe2. The total thermal conductivity in thermoelectric materials is generally expressed as the sum of κL and κE, with negligible contribution from bipolar diffusion in this temperature range. In this study, κE was estimated using the Wiedemann–Franz law, defined as κE = LσT, where σ is the electrical conductivity, T is the absolute temperature, and L is the Lorenz number [31]. The results clearly indicate that κE contributes minimally to thermal conductivity, owing to the intrinsically low electrical conductivity values in the measured samples. As a result, κL dominates the thermal transport, reinforcing the phonon-limited nature of heat conduction in CuFeSe2. For the HP623K2H specimen, κL was found to decrease from 2.64 Wm−1K−1 at 323 K to 1.85 Wm−1K−1 at 623 K. This temperature-dependent decline in κL is typical of crystalline thermoelectric materials, where increased phonon–phonon interactions (Umklapp scattering) at elevated temperatures reduce the phonon mean free path. Comparative analysis with previous reports further supports this trend. Moorthy et al. [1] reported a significantly higher κL of 4.92 Wm−1K−1 at 300 K, which dropped to 1.25 Wm−1K−1 at 645 K. Similarly, Zhai et al. [14] observed a decrease from 7.3 Wm−1K−1 at 423 K to 2.5 Wm−1K−1 at 723 K, attributing this reduction to enhanced phonon scattering induced by intrinsic point defects and selenium vacancies.
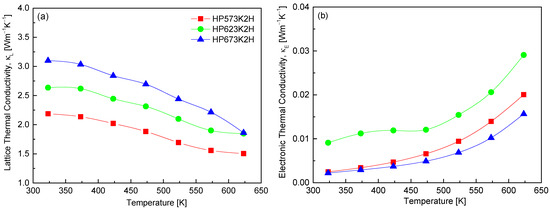
Figure 11.
Temperature dependence of (a) lattice and (b) electronic thermal conductivities of CuFeSe2.
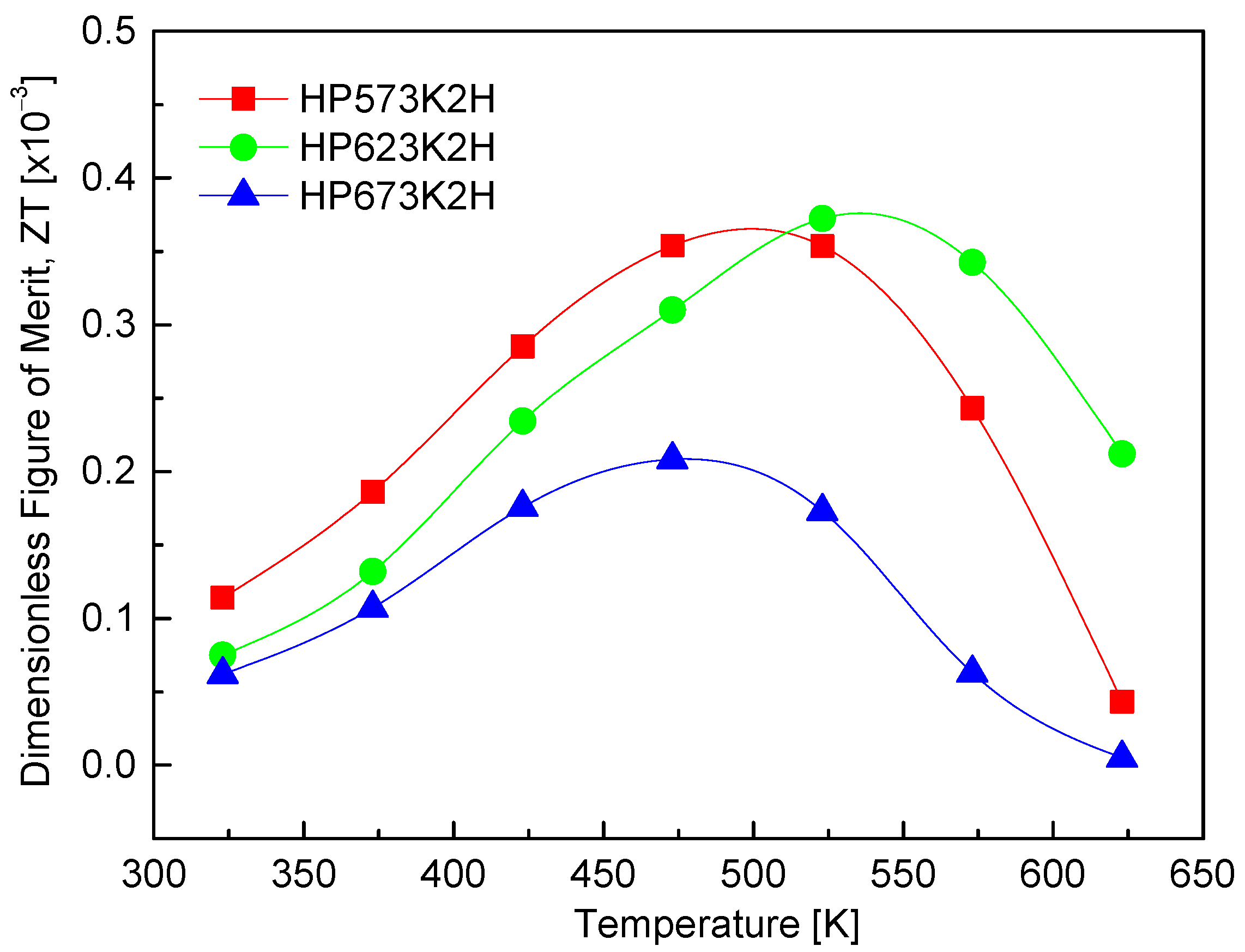
Figure 12 presents the dimensionless figure of merit (ZT) of CuFeSe2 as a function of the temperature. The ZT values exhibit a temperature-dependent increase, peaking in the range of 473–523 K. This trend reflects the interplay of thermoelectric transport parameters—namely, the Seebeck coefficient, electrical conductivity, and thermal conductivity—all of which vary with the temperature. The observed rise in ZT with increasing temperature can be attributed primarily to the enhancement of the Seebeck coefficient and power factor near the intrinsic transition region. Among the samples analyzed, the HP623K2H specimen exhibited the highest ZT value of 0.37 × 10−3 at 523 K, indicating that hot pressing at 623 K provides favorable conditions for thermoelectric optimization. While this value remains relatively low compared to conventional thermoelectric materials, it shows progress in developing CuFeSe2-based compounds. For comparison, Moorthy et al. [1] reported an increase in ZT from 5.9 × 10−5 at 300 K to 9.8 × 10−4 at 645 K by employing a combination of melting, annealing, and hot pressing. Zhai et al. [14] achieved a more substantial enhancement, with ZT reaching 7.0 × 10−3 at 723 K, facilitated by a multistep synthesis approach involving melting, quenching, grinding, annealing, and hot pressing. Although the ZT values reported in this study are lower than those of state-of-the-art thermoelectric materials [32,33], CuFeSe2 remains a promising candidate due to its narrow band gap and potential for property tuning. The current findings highlight 623 K as the optimal hot-pressing temperature, balancing electrical performance and structural integrity. Further improvements in ZT may be achieved through targeted doping strategies to enhance the carrier concentration and power factor or through solid solution formation to further reduce the lattice thermal conductivity by intensifying phonon scattering. These avenues offer a path toward optimizing CuFeSe2 for mid-temperature thermoelectric applications. Table 2 compares the thermoelectric parameters and performance of CuFeSe2 reported in the literature with the data obtained in this study. Since each parameter depends on temperature, the values presented correspond to those measured at the temperatures where the highest ZT value was observed.
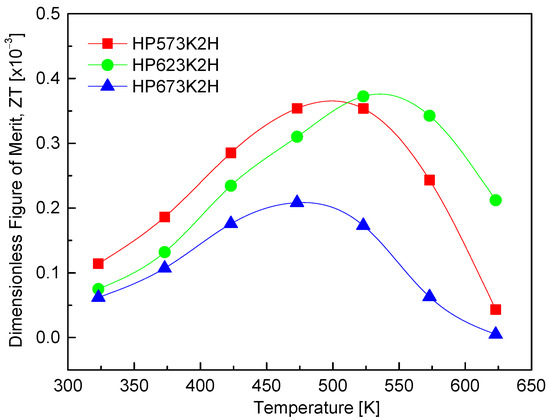
Figure 12.
Dimensionless figure of merit of CuFeSe2.

Table 2.
Comparison of the thermoelectric parameters and performance of CuFeSe2.
3. Experimental Procedure
High-purity elemental powders of copper (Cu, 99.9%, Kojundo, Japan), iron (Fe, 99.99%, Kojundo, Japan), and selenium (Se, 99.999%, Kojundo, Japan) were employed as precursors for synthesizing CuFeSe2 via a solid-state mechanical alloying (MA) approach, aimed at optimizing the synthesis parameters for enhanced phase formation and thermoelectric performance. The MA process was conducted in an argon atmosphere using a planetary ball mill (Pulverisette5, Fritsch, Pittsboro, NC, USA) equipped with stainless steel jars and balls to prevent oxidation and minimize contamination. Milling was performed at a fixed rotational speed of 350 rpm for durations ranging from 6 to 24 h, providing a systematic evaluation of alloying time on phase evolution and homogeneity. Subsequent densification of the mechanically alloyed powders was achieved through hot pressing (HP; JM-HP20, Jungmin, Seoul, Republic of Korea) at temperatures between 573 and 673 K under a uniaxial pressure of 70 MPa for 2 h in a vacuum. This step enabled the fabrication of dense, bulk samples while preserving the refined microstructure generated during MA.
Phase evolution during both the MA and HP stages was monitored using X-ray diffraction (XRD) analysis with a D8-Advance diffractometer (Bruker, Billerica, MA, USA) with Cu Kα radiation. To corroborate the thermal stability and phase transformation behavior, simultaneous thermogravimetric and differential scanning calorimetry (TG–DSC) analyses were conducted using a TC/DSC1 (Mettler Toledo, Columbus, OH, USA) instrument. The measurements were performed in an argon atmosphere with a heating rate of 5 K/min up to 973 K, enabling the detection of endothermic and exothermic events alongside weight changes associated with decomposition or phase transitions. To refine structural parameters and quantify the phase composition, Rietveld refinement was applied to the XRD data using the TOPAS software (v4.1, Bruker). The microstructural features of the sintered specimens were further characterized by scanning electron microscopy (SEM; Prisma E, Thermo Fisher Scientific, Waltham, MA, USA) in backscattered electron (BSE). Elemental distribution and compositional uniformity were assessed using energy-dispersive X-ray spectroscopy (EDS) with a Quantax200 system (Bruker), providing insights into the homogeneity of the alloyed phases.
Electrical transport measurements, including electrical conductivity and the Seebeck coefficient, were conducted simultaneously using a ZEM-3 instrument (Advance Riko, Yokohama, Japan) in a low-pressure helium atmosphere. The power factor (PF = σα2) was calculated directly from these measurements. Thermal diffusivity (D) was determined via laser flash analysis using a TC-9000H system (Advance Riko), and the total thermal conductivity (κ) was calculated using the relation κ = Dcpd, where cp is the specific heat capacity and d is the bulk density of the sample. The dimensionless figure of merit (ZT) was evaluated over the temperature range of 323 to 623 K to assess the thermoelectric efficiency of CuFeSe2 under varying synthesis conditions. This comprehensive methodology, integrating structural, thermal, and electronic characterizations, provides a robust framework for understanding the interrelationships between processing parameters, phase formation, and thermoelectric properties.
4. Conclusions
Tetragonal eskebornite (CuFeSe2) was successfully synthesized via a straightforward solid-state method combining mechanical alloying and hot pressing. The optimal mechanical alloying conditions were identified as 12 h of milling at 350 rpm, based on detailed phase analysis using X-ray diffraction and thermal analysis techniques, including thermogravimetric and differential scanning calorimetry. The resulting powders were consolidated under a uniaxial pressure of 70 MPa for 2 h at hot-pressing temperatures ranging from 573 to 673 K. Near-theoretical relative densities were achieved at hot-pressing temperatures above 623 K, indicating effective densification. All samples exhibited p-type non-degenerate semiconducting behavior, with the electrical conductivity increasing with temperature. However, the electrical conductivity decreased when the hot-pressing temperature reached 673 K, likely due to selenium volatilization at elevated temperatures, which introduces Se vacancies and adversely affects carrier concentration. Among the prepared specimens, the sample hot-pressed at 623 K demonstrated the best thermoelectric performance, achieving a maximum power factor of 1.55 μWm−1K−2 and a peak ZT of 0.37 × 10−3 at 523 K. These results confirm that the MA–HP approach is an efficient and scalable method for synthesizing and sintering CuFeSe2-based thermoelectric materials. The findings underscore the importance of precise control over processing parameters, particularly the hot-pressing temperature, to optimize thermoelectric properties. With further refinement through doping or compositional tuning, eskebornite holds potential as a viable thermoelectric material for mid-temperature applications.
Author Contributions
Conceptualization, S.-H.C. and I.-H.K.; methodology, S.-H.C. and I.-H.K.; software, S.-H.C.; validation, I.-H.K.; formal analysis, S.-H.C.; investigation, S.-H.C.; resources, S.-H.C.; data curation, S.-H.C.; writing—original draft preparation, S.-H.C.; writing—review and editing, I.-H.K.; visualization, S.-H.C.; supervision, I.-H.K.; project administration, I.-H.K.; funding acquisition, I.-H.K. Both authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Small-Medium Enterprise Innovation Growth Support Project of the Korea RIC Association and the Basic Science Research Capacity Enhancement Project (National Research Facilities and Equipment Center) through the Korea Basic Science Institute funded by the Ministry of Education (Grant No. 2019R1A6C1010047).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moorthy, M.; Bhui, A.; Battabyal, M.; Perumal, S. Nanostructured CuFeSe2 eskebornite: An efficient thermoelectric material with ultra-low thermal conductivity. Mater. Sci. Eng. B 2022, 284, 115914. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef]
- Ibanez, M.; Lue, Z.; Genc, A.; Piveteau, L.; Ortega, S.; Cadavid, D.; Dobrozhan, O.; Liu, Y.; Nachtegaal, M.; Zebarjadi, M.; et al. High-performance thermoelectric nanocomposites from nanocrystal building blocks. Nat. Commun. 2016, 7, 10766. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Liu, J.; Wang, C. Recent development of n-type perovskite thermoelectrics. J. Materiom. 2016, 2, 225–236. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, T.; Wang, H.; Su, W.; Wang, X.; Chen, T.; Wang, C. Strategies for optimizing the thermoelectricity of PbTe alloys. Chin. Phys. B 2018, 27, 047306. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, L.D.; Kanatzidis, M.G. Rationally designing high-performance bulk thermoelectric materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Katz, H.E.; Fang, F.; Opila, R.L. Promising thermoelectric properties of commercial PEDOT:PSS materials and their Bi2Te3 powder composites. ACS Appl. Mater. Interf. 2010, 2, 3170–3178. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Thermoelectrics. dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bahk, J.H.; Kang, C.; Hwang, J.; Kim, K.; Kim, J.; Burke, P.; Bowers, J.E.; Gossard, A.C.; Shakouri, A.; et al. Right sizes of nano- and microstructures for high-performance and rigid bulk thermoelectrics. Proc. Natl. Acad. Sci. USA 2014, 111, 10949–10954. [Google Scholar] [CrossRef]
- Wang, H.; Hwang, J.; Snedaker, M.L.; Kim, I.H.; Kang, C.; Kim, J.; Stucky, G.D.; Bowers, J.; Kim, W. High thermoelectric performance of a heterogeneous PbTe nanocomposite. Chem. Mater. 2015, 27, 944–949. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Zhao, L.D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Wang, H.; Su, W.; Wang, T.; Mehmood, F.; Wang, X.; Chen, T.; Huo, T.; Zhang, K.; Wang, C. The p–n transformation and thermoelectric property optimization of Cu1+xFeSe2 (x = 0–0.05) alloys. J. Mater. Chem. C 2019, 7, 9641–9647. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, I.H. Hakite: Solid-state synthesis and thermoelectric performance. J. Korean Phys. Soc. 2024, 84, 708–715. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwak, S.G.; Pi, J.H.; Lee, G.E.; Kim, I.H. Preparation of tetrahedrite Cu12Sb4S13 by mechanical alloying and hot pressing. J. Electron. Mater. 2019, 48, 1857–1863. [Google Scholar] [CrossRef]
- Lee, G.E.; Kim, I.H. Thermoelectric and transport properties of permingeatite Cu3SbSe4 prepared using mechanical alloying and hot pressing. Materials 2021, 14, 1116. [Google Scholar] [CrossRef]
- Lee, G.E.; Pi, J.H.; Kim, I.H. Preparation and thermoelectric properties of famatinite Cu3SbS4. J. Electron. Mater. 2020, 49, 2781–2788. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, J.; Tang, K.; Deng, B.; Qian, Y.; Li, Y. The synthesis of CuFeSe2 through a solventothermal process. J. Cryst. Growth 2000, 217, 271–273. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Lin, Y.G.; Chen, Y.C. One-pot synthesis of CuFeSe2 cuboid nanoparticles. Mater. Res. Bull. 2011, 46, 2117–2119. [Google Scholar] [CrossRef]
- Lee, P.C.; Ou, M.N.; Luo, J.Y.; Wu, M.K.; Chen, Y.Y. Cross-plane Seebeck coefficient and thermal conductivity of CuFeSe2 thin film. AIP Conf. Proc. 2012, 1449, 405. [Google Scholar]
- Delgado, J.M.; de Delgado, G.D.; Quintero, M.; Woolley, J.C. The crystal structure of copper iron selenide, CuFeSe2. Mater. Res. Bull. 1992, 27, 367–373. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Liu, Y.; Zuo, Y.; Chen, J.S.; Song, J.M.; Niu, H.L.; Mao, C.J. Colloidal synthesis and thermoelectric properties of CuFeSe2 nanocrystals. Nanomaterials 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mindat.org/min-1409.html (accessed on 8 May 2025).
- Hamdadou, N.; Morsli, M.; Khelil, A.; Bernede, J.C. Fabrication of n- and p-type doped CuFeSe2 thin films achieved by selenization of metal precursors. J. Phys. D Appl. Phys. 2006, 39, 1042. [Google Scholar] [CrossRef]
- Skoug, E.J.; Cain, J.D.; Majsztrik, P.; Kirkham, M.; Lara-Curzio, E.; Morelli, D.T. Doping effects on the thermoelectric properties of Cu3SbSe4. Sci. Adv. Mater. 2011, 3, 602–606. [Google Scholar] [CrossRef]
- Do, D.T.; Mahanti, S.D. Theoretical study of defects Cu3SbSe4: Search for optimum dopants for enhancing thermoelectric properties. J. Alloys Compd. 2015, 625, 346–354. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Kwak, S.G.; Lee, G.E.; Kim, I.H. Electronic transport and thermoelectric properties of Cu12−xZnxSb4S13 tetrahedrites prepared by mechanical alloying and hot pressing. Korean J. Met. Mater. 2019, 57, 328–333. [Google Scholar] [CrossRef]
- Berthebaud, D.; Lebedev, O.I.; Maignan, A. Thermoelectric properties of n-type cobalt doped chalcopyrite Cu1−xCoxFeS2 and p-type eskebornite CuFeSe2. J. Mater. 2015, 1, 68–74. [Google Scholar] [CrossRef]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.G.; Dargusch, M.S.; Zou, J. High performance thermoelectric materials: Progress and their applications. Adv. Energy Mater. 2018, 8, 1701797. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, Y.; Fu, C.; Heremans, J.P.; Snyder, J.G.; Zhao, X. Compromise and synergy in high-efficiency thermoelectric materials. Adv. Mater. 2017, 29, 1605884. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).