First-Principles Calculations for the H Adsorption of Monolayer MoTe2 for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results
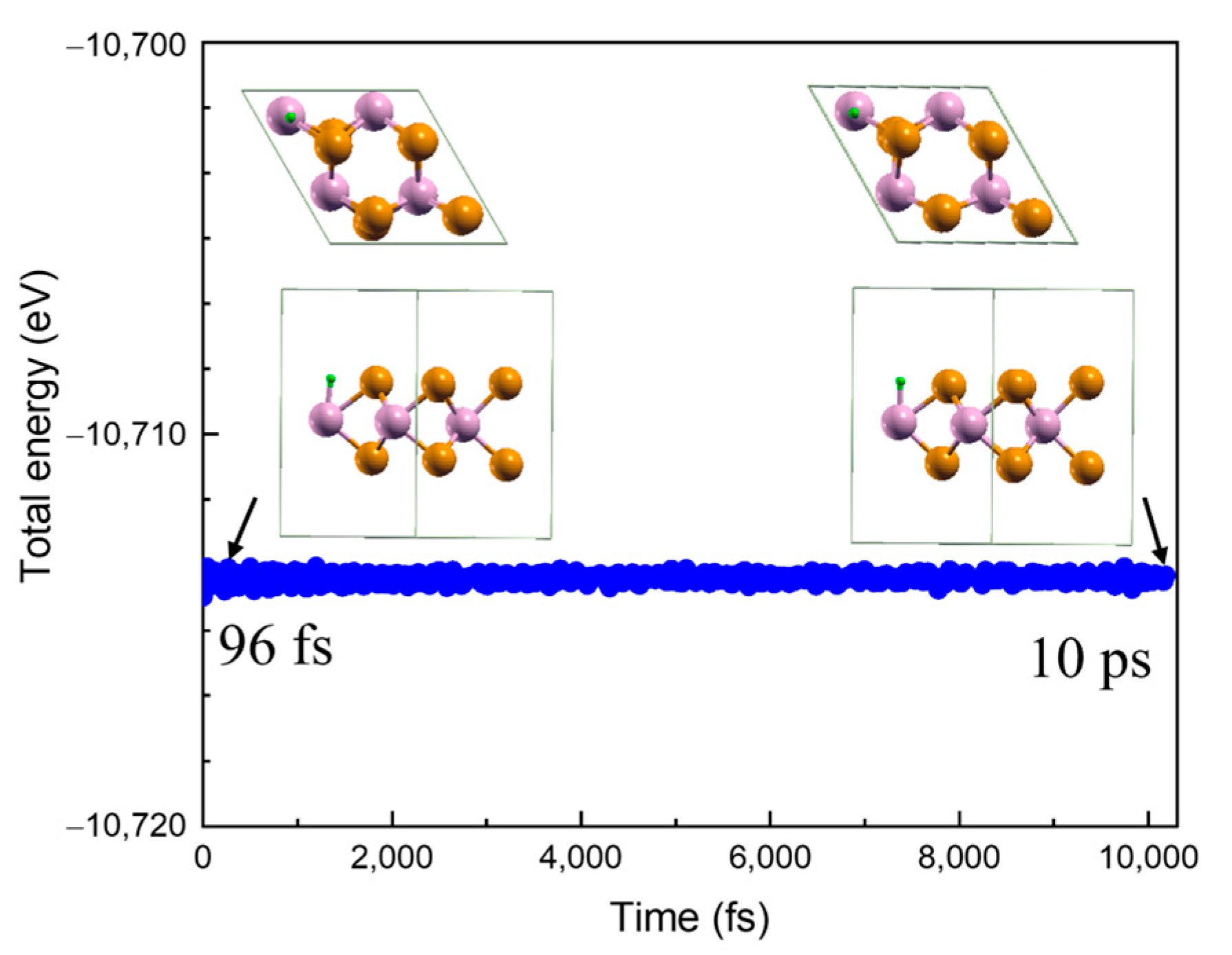
2.1. Structure
2.2. Gibbs Free Energy (∆GH) of H Adsorption Sites and Coverage
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On hydrogen and hydrogen energy strategies: I: Current status and needs. Renew. Sustain. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Macdonald, T.J.; Sobrido, A.J.; Liu, L.; Feng, J.; He, G. Recent Advances in Ultralow-Pt-Loading Electrocatalysts for the Efficient Hydrogen Evolution. Adv. Sci. 2023, 10, 2301098. [Google Scholar] [CrossRef] [PubMed]
- Saji, V.S.; Pillai, V.K.; Sotiropoulos, S. Recent advances in electrochemical water splitting. Electrochim. Acta 2024, 475, 143645. [Google Scholar] [CrossRef]
- Mao, J.; Ta, Q.T.H.; Tri, N.N.; Shou, L.; Seo, S.; Xu, W. 2D MoTe2 nanomesh with a large surface area and uniform pores for highly active hydrogen evolution catalysis. Appl. Mater. Today 2023, 35, 101939. [Google Scholar] [CrossRef]
- Xiao, Z.; Gan, X.; Zhu, T.; Lei, D.; Zhao, H.; Wang, P. Activating the Basal Planes in 2H-MoTe2 Monolayers by Incorporating Single-Atom Dispersed N or P for Enhanced Electrocatalytic Overall Water Splitting. Adv. Sustain. Syst. 2022, 6, 2100515. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Wen, Q.; Mi, Y.; Liu, Y.; Li, H.; Zhai, T. Single MoTe2 sheet electrocatalytic microdevice for in situ revealing the activated basal plane sites by vacancies engineering. Nano Res. 2021, 14, 4814–4821. [Google Scholar] [CrossRef]
- Mao, J.; Zhou, L.; Li, Y.; Tao, Y.; Chai, K.; Shi, Y.; Xu, W. Synthesis of MoTe2 nanowire as an efficient hydrogen evolution reaction material. Mater. Lett. 2021, 290, 129471. [Google Scholar] [CrossRef]
- Shinde, P.V.; Mane, P.; Late, D.J.; Chakraborty, B.; Rout, C.S. Promising 2D/2D MoTe2/Ti3C2Tx Hybrid Materials for Boosted Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 11886–11897. [Google Scholar] [CrossRef]
- Lu, D.; Ren, X.; Ren, L.; Xue, W.; Liu, S.; Liu, Y.; Chen, Q.; Qi, X.; Zhong, J. Direct Vapor Deposition Growth of 1T′ MoTe2 on Carbon Cloth for Electrocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2020, 3, 3212–3219. [Google Scholar] [CrossRef]
- Lei, Y.; Xiao, X.; Ma, T.; Li, W.; Zhang, H.; Ma, C. Facile hydrothermal synthesis of layered 1T′ MoTe2 nanotubes as robust hydrogen evolution electrocatalysts. Front. Chem. 2022, 10, 1005782. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.; Zhang, Y.; Zhang, H.; Xiong, K.; Ye, X.; Liu, Q.; Zhu, J. Transition-metal atoms embedded MoTe2 single-atom catalyst for efficient electrocatalytic hydrogen evolution reaction. Appl. Surf. Sci. 2025, 680, 161335. [Google Scholar] [CrossRef]
- Pu, M.; Guo, Y.; Guo, W. Wrinkle facilitated hydrogen evolution reaction of vacancy-defected transition metal dichalcogenide monolayers. Nanoscale 2021, 13, 20576–20582. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S.; Yim, K.; Kim, K.Y.; Jang, H.W.; Kang, Y.; Han, S. Hydrogen Evolution Reaction at Anion Vacancy of Two-Dimensional Transition-Metal Dichalcogenides: Ab Initio Computational Screening. J. Phys. Chem. Lett. 2018, 9, 2049–2055. [Google Scholar] [CrossRef]
- Shen, Y.; Yuan, P.; Yuan, Z.; Cui, Z.; Ma, D.; Cheng, F.; Qin, K.; Wang, H.; Li, E. Modulation of electronic and optical properties of g-CN/XTe2(X=W, Mo) heterojunctions by biaxial strain. Int. J. Hydrogen Energy 2024, 80, 289–297. [Google Scholar] [CrossRef]
- Chen, Y.; Ou, P.; Bie, X.; Song, J. Basal plane activation in monolayer MoTe2 for the hydrogen evolution reaction via phase boundaries. J. Mater. Chem. A 2020, 8, 19522–19532. [Google Scholar] [CrossRef]
- Xu, Z.-W.; Shi, C.-S.; Zhao, G.-H.; Wang, M.-Y.; Liu, G.-W.; Qiao, G.-J. Hydrogen adsorption mechanism on single-layer MoSe2 for hydrogen evolution reaction: First-principles study. Acta Phys. Sin. 2018, 67, 217102. [Google Scholar] [CrossRef]
- Lamba, A.; Kumar, R.; Srivastava, S. Band gap engineering of 2H-MX2 (M = Mo; X = S, Se, Te) monolayers using strain effect. Mater. Today Proc. 2021, 54, 677–681. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.-E. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Yang, T.T.; Patil, R.B.; McKone, J.R.; Saidi, W. Revisiting trends in the exchange for hydrogen evolution. Catal. Sci. Technol. 2021, 11, 6832–6838. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Zhang, F.; Fan, Q.; Jin, H.; Chen, S.; Jin, Y.; Gao, S.; Xiao, Y.; Mwankemwa, N.; et al. The electronic properties of hydrogenated Janus MoSSe monolayer: A first principles investigation. Mater. Res. Express 2019, 6, 105055. [Google Scholar] [CrossRef]
- Li, X.D.; Qian, Y.Y.; Guo, T.S.; Fu, L.J. DFT study on adsorption properties of TM(Ni, Co)Nx-doped graphene for high-temperature sensing of SF6 decomposed gases. Mater. Today Chem. 2024, 35, 101904. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Wang, J.S.; Liu, J.; Zhang, B.; Ji, X.; Xu, K.; Chen, C.; Miao, L.; Jiang, J.J. The mechanism of hydrogen adsorption on transition metal dichalcogenides as hydrogen evolution reaction catalyst. Phys. Chem. Chem. Phys. 2017, 19, 10125–10132. [Google Scholar] [CrossRef]
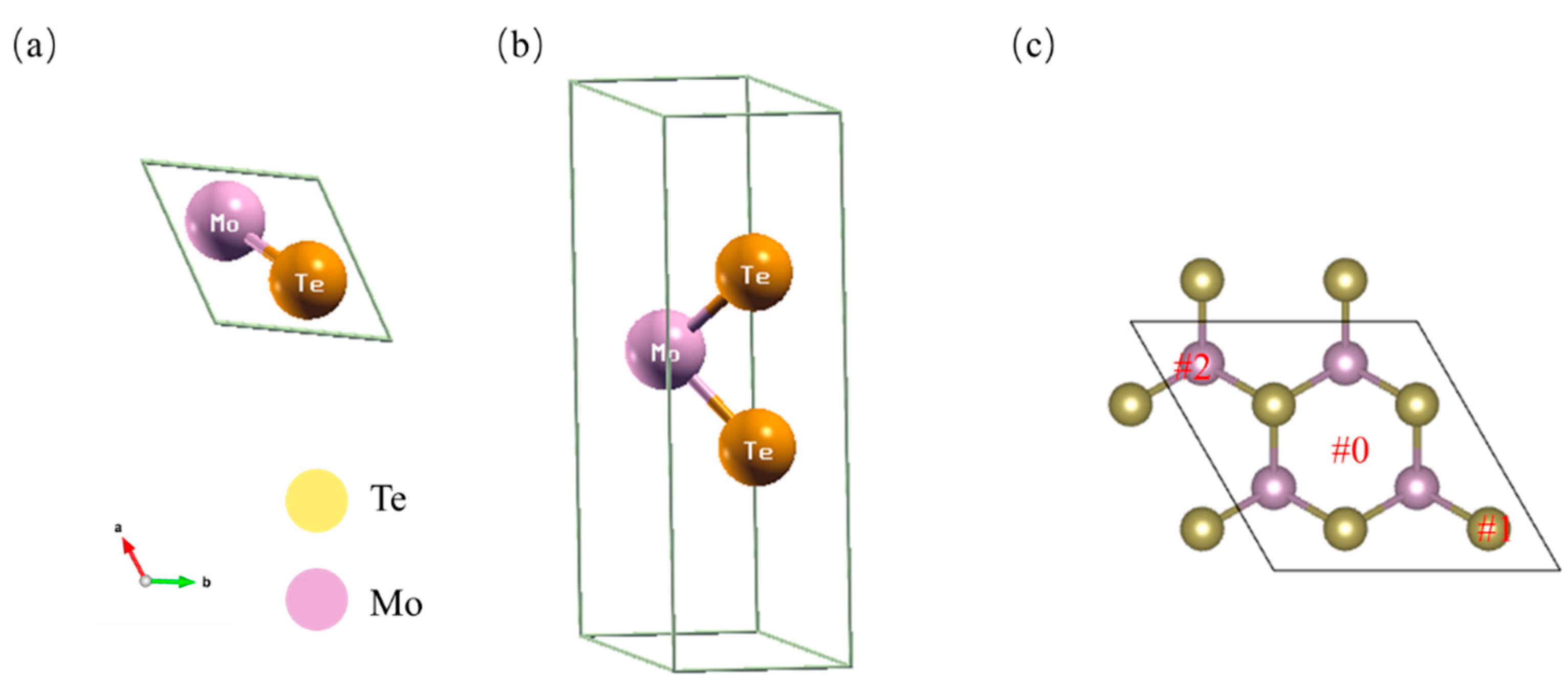
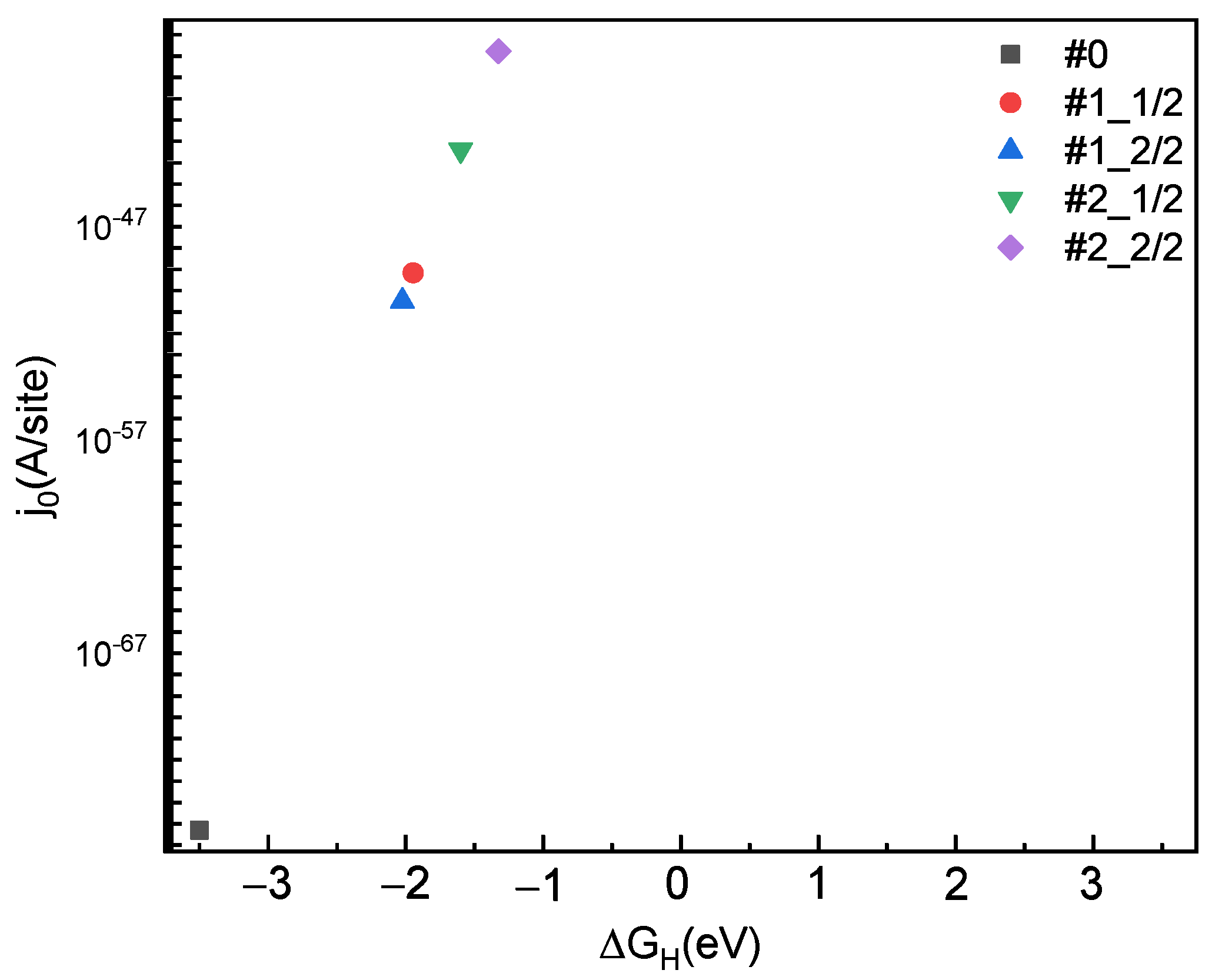
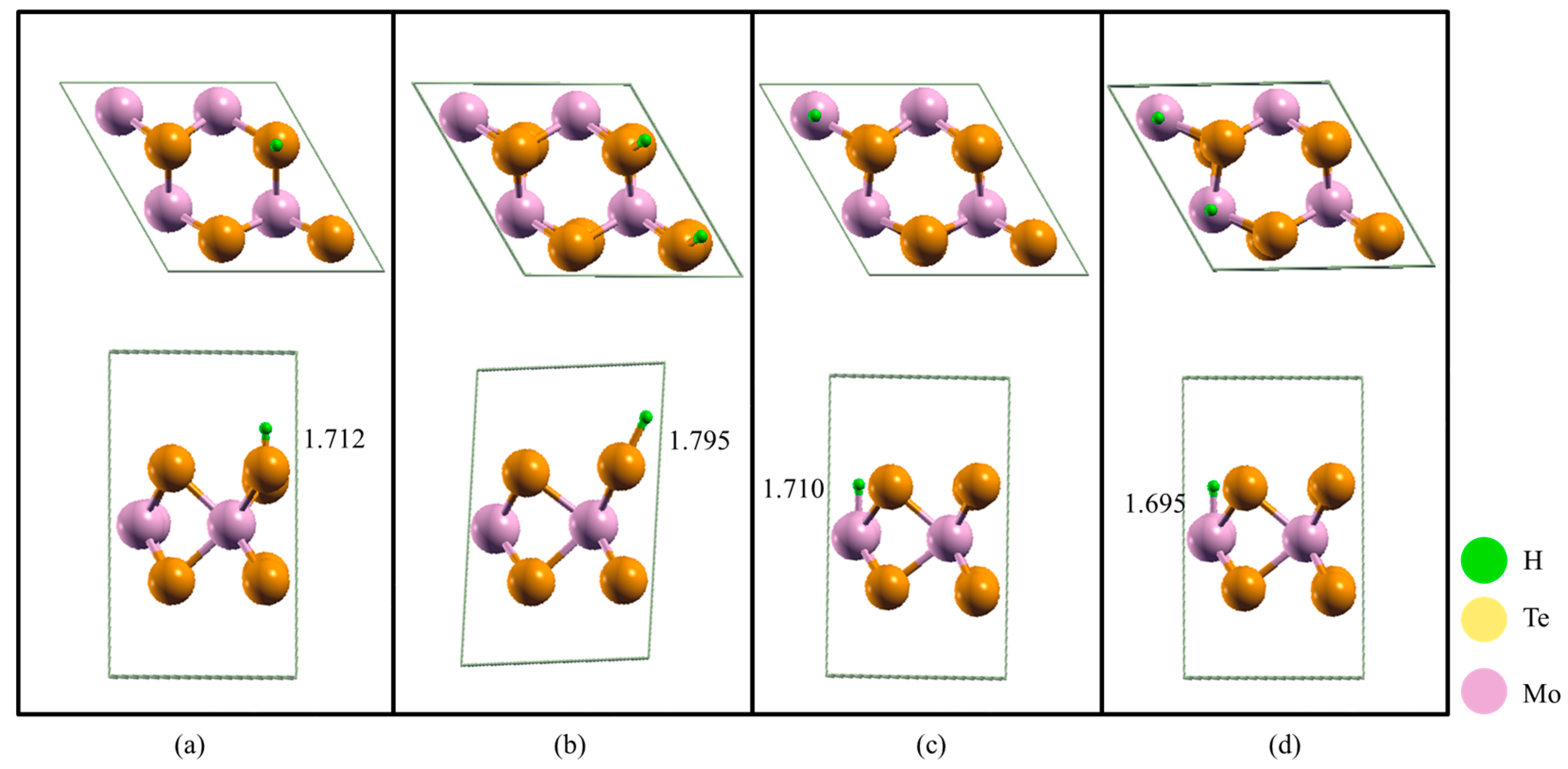
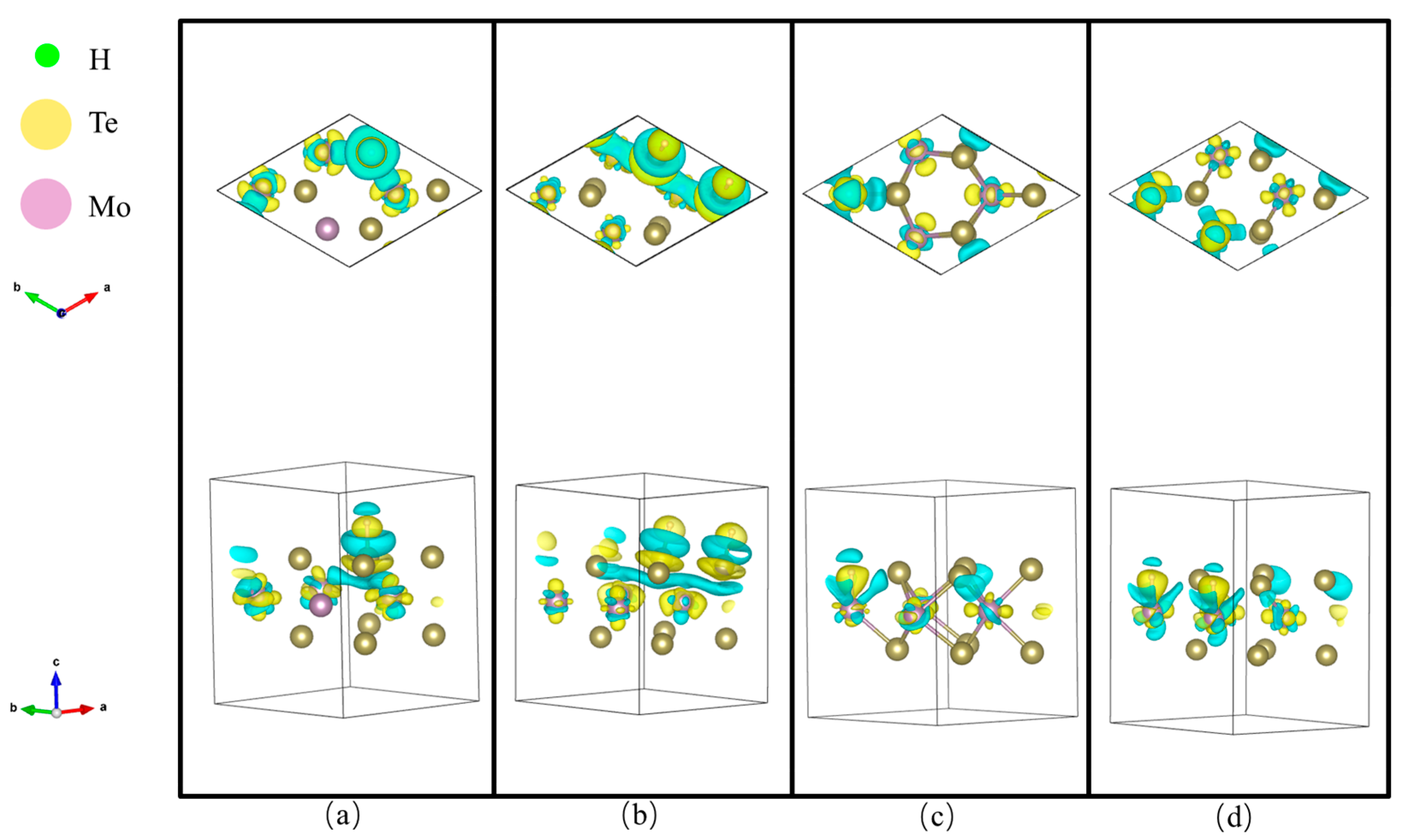
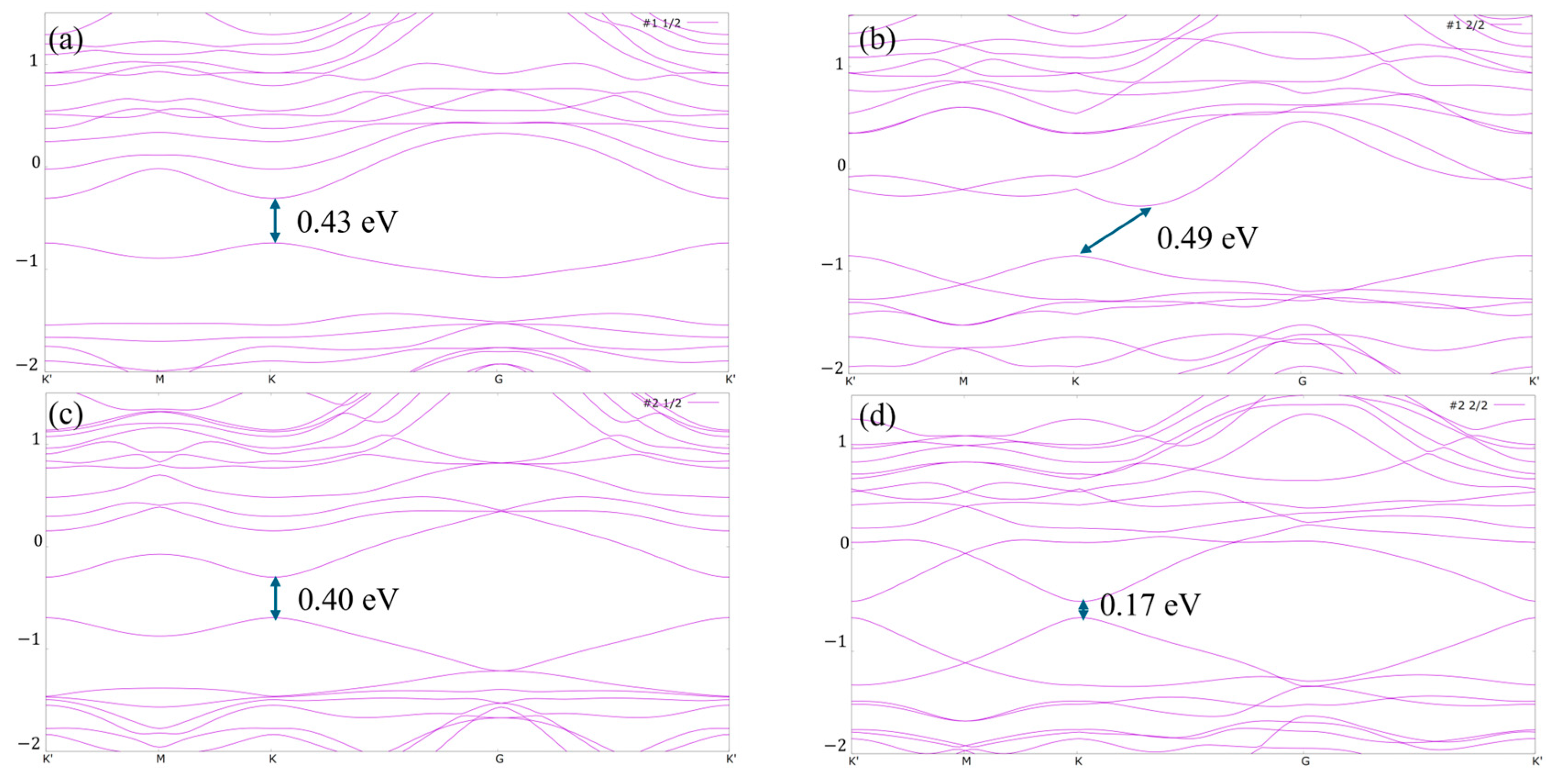
| Adsorption Sites and Coverage | ΔGH(eV) |
|---|---|
| # 0 | 3.501 |
| #1_1/2 | 1.945 |
| #1_2/2 | 2.024 |
| #2_1/2 | 1.601 |
| #2_2/2 | 1.326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Meng, J. First-Principles Calculations for the H Adsorption of Monolayer MoTe2 for Hydrogen Evolution Reaction. Inorganics 2025, 13, 197. https://doi.org/10.3390/inorganics13060197
Gao X, Meng J. First-Principles Calculations for the H Adsorption of Monolayer MoTe2 for Hydrogen Evolution Reaction. Inorganics. 2025; 13(6):197. https://doi.org/10.3390/inorganics13060197
Chicago/Turabian StyleGao, Xujing, and Jianling Meng. 2025. "First-Principles Calculations for the H Adsorption of Monolayer MoTe2 for Hydrogen Evolution Reaction" Inorganics 13, no. 6: 197. https://doi.org/10.3390/inorganics13060197
APA StyleGao, X., & Meng, J. (2025). First-Principles Calculations for the H Adsorption of Monolayer MoTe2 for Hydrogen Evolution Reaction. Inorganics, 13(6), 197. https://doi.org/10.3390/inorganics13060197






