Redox Mediators for Li2CO3 Decomposition
Abstract
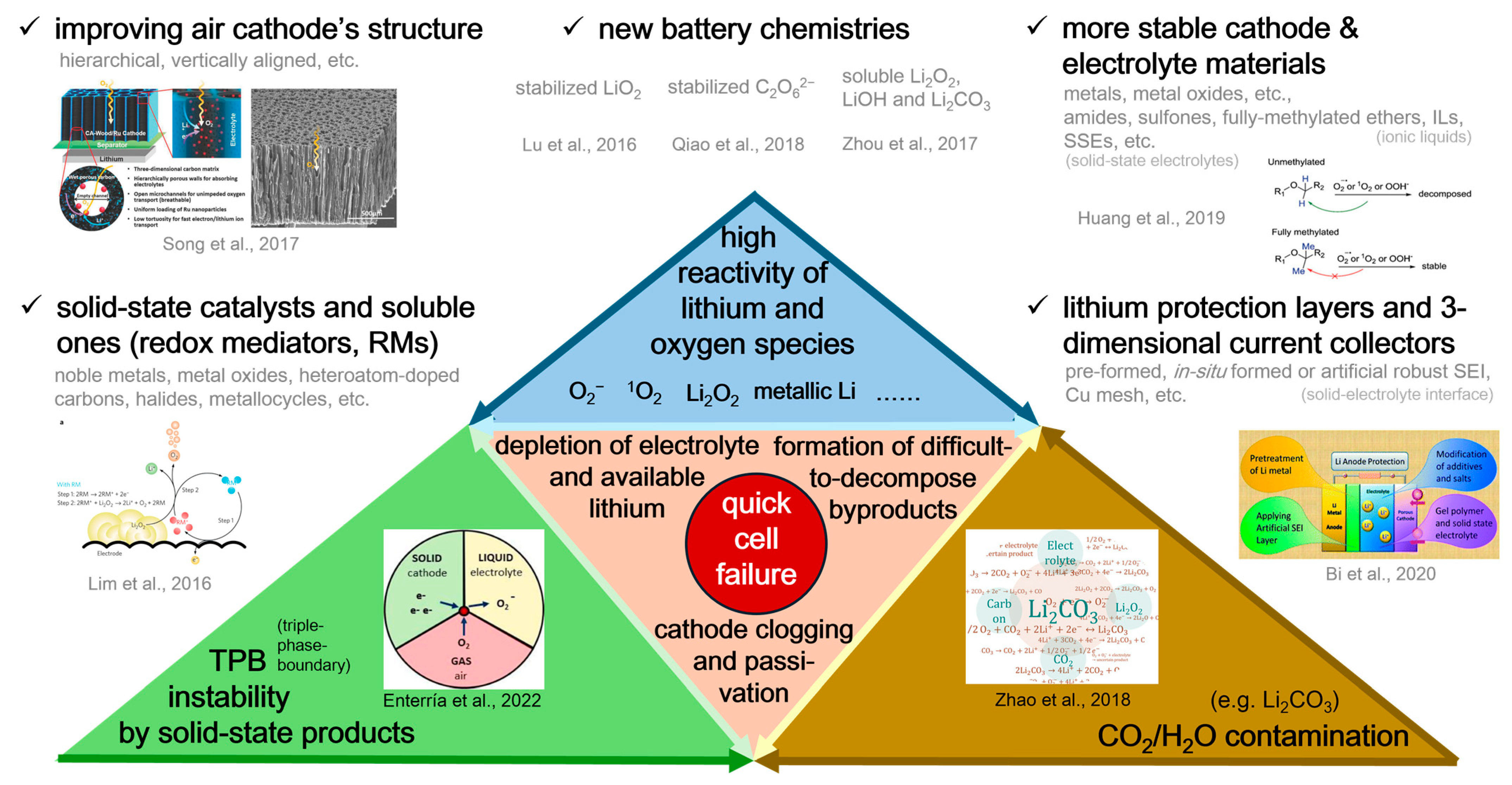
1. Introduction
- (1)
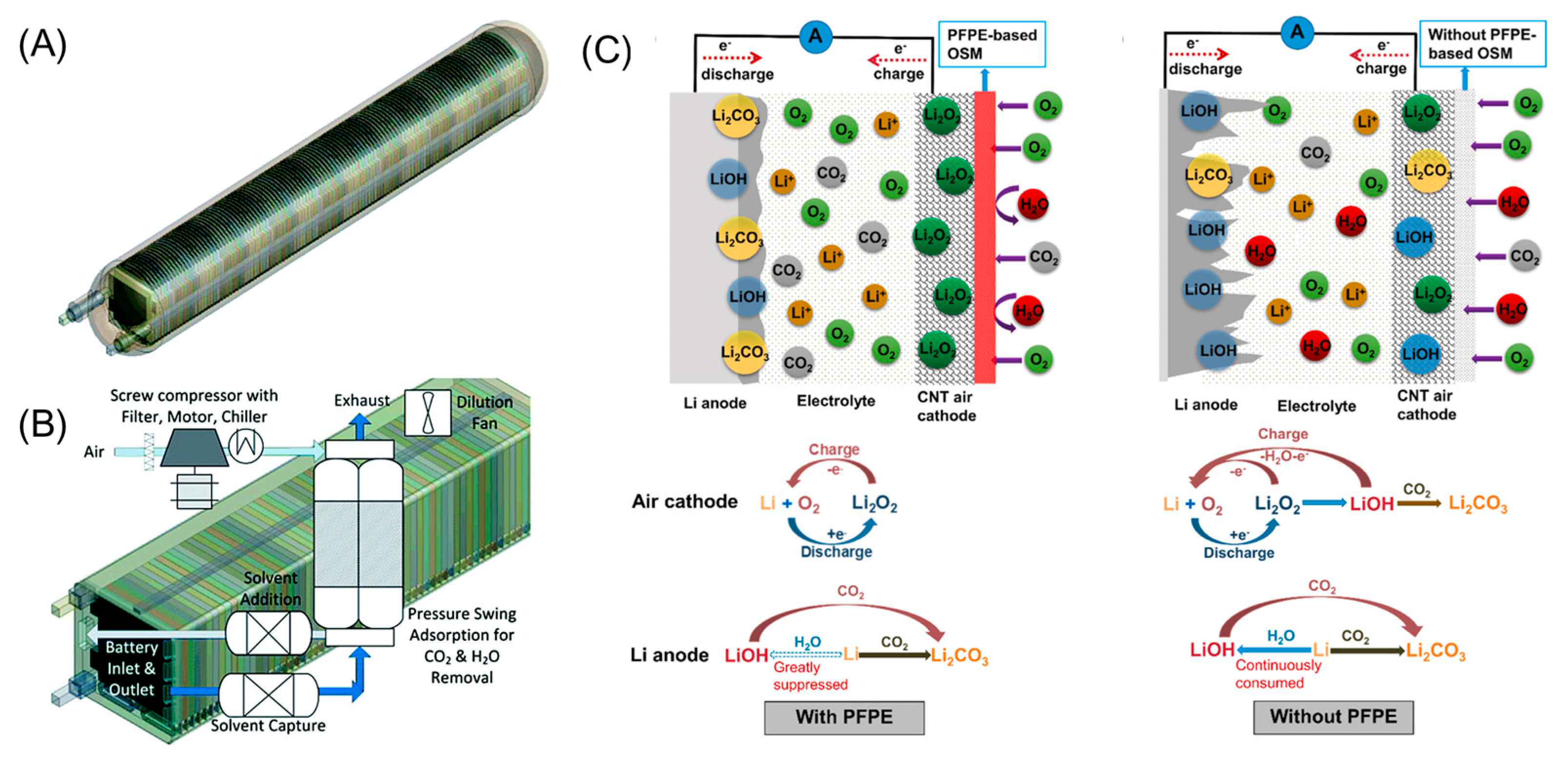
- Using H2O and/or CO2 absorbents/adsorbents (usually with -OH and/or -NH2 groups, etc.) [55,56,57] to entrap them before entering the cell. This approach is only feasible for large-scale LAB modules due to the apparently increased heft (including the regeneration apparatus for prolonged operation), as shown in Figure 3B.
- (2)
- Using oxygen selective membranes (OSMs) to exclusively allow O2 permeation. Since O2 has a larger kinetic diameter (0.346 nm, based on Knudsen diffusion values) than H2O (0.289 nm) and CO2 (0.330 nm) [5,58], CO2 and H2O cannot be filtered out by sheer pore size adjustment, but should be repelled by polarity difference. Recent investigations demonstrated the successful blocking of H2O by various polymer membranes, such as polyester (PET) [2,3], high-density polyethylene (HDPE) [2], low-density polyethylene (LDPE) [24], polytetrafluoroethylene (PTFE) [59,60,61], perfluoropolyether (PFPE) [54,62], polysiloxanes [63], silicone oil [30,64,65], etc. However, the development of CO2-repelling (rather than adsorbing/absorbing, which has a limited lifetime) and O2-permeable membranes is still challenging, and there has been no such literature to the authors’ knowledge. Fortunately, the good news is that Li2CO3 formation can also be greatly suppressed by these H2O-repelling membranes [24,54,62,66], as illustrated in Figure 3C.
- (3)
- Shifting the CO2-involved reaction routes to prevent Li2CO3 deposition. For example, in 2018, Zhou’s group [15] reported a Li2CO3-free Li–O2/CO2 battery with a [Li(DMSO)3]+–[TFSI−] contact ion pair (CIP) as the electrolyte. The positive charge of highly solvated Li+ cations is efficiently neutralized by the DMSO sheath, preventing its combination with the peroxydicarbonate (C2O62−) anion to form solid-state Li2CO3; thus, C2O62− becomes a soluble discharge product, rather than intermediate. This is demonstrated by the presence of CO42− and C2O62− and the absence of Li2CO3 signals in in situ Raman and infrared spectroscopy tests. As a result, the charge voltage plateau was significantly reduced from ~4.2 V to ~3.5 V. In 2021, Wang’s group [67] discovered tris(2,2′-bipyridyl)-dichloro-ruthenium(II) (Ru(bpy)3Cl2) as a bi-functional redox mediator (RM) for Li-CO2 batteries. Through X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) characterizations, they found that the cell with Ru(bpy)3Cl2 contained little Li2CO3 after a shallow discharge to 1000 mAh g−1, in sharp contrast to that without Ru(bpy)3Cl2. They speculated that the shallow discharge product was lithium oxalate, and the RuII center could interact with dissolved CO2 molecules to promote the formation and stabilization of oxalate, inhibiting the transformation from oxalate to carbonate. During recharge, the cell with Ru(bpy)3Cl2 exhibited a much lower voltage plateau at 3.7~3.8 V than the blank control (4.2~4.3 V), attributed to the easier decomposition of soluble oxalate than solid-state Li2CO3. This approach is highly desirable because the soluble nature of C2O62− and oxalate significantly reduces the charge potential. However, to date there have been only a few pieces of literature reported [15,67,68,69,70,71,72,73,74,75], and more investigations should be made in the future.
- (4)
- Developing Li2CO3-decomposing catalysts. Aside from addressing the CO2-induced Li2CO3 issue, this approach can also handle Li2CO3 produced via internal parasitic reactions. Due to the sluggish kinetics of the ORR and oxygen evolution reaction (OER), cathode catalysts are usually used to improve LABs/LOBs’ performance, which constitutes a large portion of research in the LABs/LOBs field [5]. Despite the primary goals of ORR catalysis and Li2O2/LiOH decomposition, some of them are also found to be capable of decomposing Li2CO3, such as Ru [76] and its compounds (RuO2 [77], RuP2 [78]), Au [79], NiO [80,81], Ir/B4C [82], LiCoO2 [83], etc. Aside from this, some catalysts are found to be capable of catalyzing the co-decomposition reaction of 2Li2CO3 + C (Equation (2)), such as MoS2 [84], Ti3C2Tx MXene [85], etc., while their effects on Li2CO3 decomposition need to be further investigated.
2. Halides
3. Metal–Chelate Complexes
4. Metal-Free Organic Compounds
5. Conclusions and Prospects
- (1)
- To address the 1O2 issue in Li2CO3 decomposition and enable true reversible cell chemistry, stable and efficient 1O2 quenchers are urgently needed. Despite many recent investigations for 1O2 quenchers in Li2O2 decomposition [139,140,141,142,143], to the authors’ knowledge there has been only one study conducted on Li2CO3 decomposition by Fruenberger’s group [121]. In that research, the 3O2 evolution rate is 2~3 orders of magnitude higher when using a 1O2 quencher, 1,4-diazabicyclo[2.2.2]octane (DABCO), than that without a quencher; nevertheless, only ~1% of the produced 1O2 was quenched even when using the quencher. The reason for such unsatisfactory quenching efficiency may be attributed to three aspects. First, the potential required to decompose Li2CO3 (3.71~3.82 V vs. Li/Li+) is beyond the electrochemical window of many quenchers, such as azides and aliphatic amines (with upper limit to 3.5~3.6 V). Second, amine-based quenchers have logarithmical correlations between quenching efficiency and ionization potential (and hence oxidation potential). The quenching efficiency drops by four orders of magnitude when the oxidation potential increases by 1 volt [140,144]. Third, the Lewis basicity of amide- and azide-based quenchers can impair quenching [144], which is even exacerbated in Li2CO3 decomposition by adsorbing CO2. Therefore, finding efficient approaches to address the 1O2 issue remains a highly challenging task.
- (2)
- More efforts should be devoted to ascertaining the mechanisms of Li2CO3 decomposition, as well as the corresponding catalysis processes. As discussed above, there have been contradicting experimental results. On the one hand, many Li2CO3-decomposing RMs, such as Br2 (or the Br2…Br3− complex) [95], I2 (in TMP) [105,106], bi-/multi-nuclear metal phthalocyanines [22,114,115], and porphyrins [116], can be categorized into two-electron (or multi-electron) RMs, which is supported by potentiostatic charging tests (with Li2CO3-preloaded carbon paper insulated from electrodes, as shown in Figure 5B) and DFT calculations [75,114]. On the other hand, some recently reported one-electron RMs, such as MPTZ [135], TMPD [135], DMPZ [135], DBDMB [136], PHX [137], Ru(aca)3 [124], and the salen-Co(II) complex [125], can also catalyze Li2CO3 decomposition. Other one-electron RMs (Ruc [123] and OPD [119]) and two-electron RMs (SPC− [134] and I2 in ethereal electrolytes [31]) have demonstrated their capability in promoting the co-decomposition of 2Li2CO3 + C, but their efficacy in Li2CO3 decomposition should be further investigated.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.C.; Park, J.O.; Kim, M.; Kwon, H.J.; Kim, J.-H.; Choi, K.H.; Kim, K.; Im, D. High-Energy-Density Li-O2 Battery at Cell Scale with Folded Cell Structure. Joule 2019, 3, 542–556. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Wang, D.; Xu, W.; Xiao, J.; Williford, R.E. Ambient operation of Li/Air batteries. J. Power Sources 2010, 195, 4332–4337. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, J.; Xu, W.; Zhang, J.-G. High Capacity Pouch-Type Li–Air Batteries. J. Electrochem. Soc. 2010, 157, A760–A764. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, Y.; Li, K.; Yang, S.; Zhou, H.; He, P. Boosting the Li-O2 pouch cell beyond 860 Wh kg−1 with an O2-enriched localized high-concentration electrolyte. Natl. Sci. Rev. 2025, 12, nwaf059. [Google Scholar] [CrossRef]
- Kwak, W.-J.; Rosy; Sharon, D.; Xia, C.; Kim, H.; Johnson, L.R.; Bruce, P.G.; Nazar, L.F.; Sun, Y.-K.; Frimer, A.A.; et al. Lithium-Oxygen Batteries and Related Systems: Potential, Status, and Future. Chem. Rev. 2020, 120, 6626–6683. [Google Scholar] [CrossRef] [PubMed]
- Trahan, M.J.; Jia, Q.; Mukerjee, S.; Plichta, E.J.; Hendrickson, M.A.; Abraham, K.M. Cobalt Phthalocyanine Catalyzed Lithium-Air Batteries. J. Electrochem. Soc. 2013, 160, A1577–A1586. [Google Scholar] [CrossRef]
- Lu, J.; Lee, Y.J.; Luo, X.; Lau, K.C.; Asadi, M.; Wang, H.-H.; Brombosz, S.; Wen, J.; Zhai, D.; Chen, Z.; et al. A lithium-oxygen battery based on lithium superoxide. Nature 2016, 529, 377–382. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Yang, T.; Shan, N.; Singh, S.K.; Jaradat, A.; Ncube, M.K.; Redfern, P.; Subramanian, A.; Huang, Z.; et al. Lithium superoxide-based high rate Li-Air batteries enabled by Di-iridium sulfur bridge active sites. Energy Storage Mater. 2023, 60, 102844. [Google Scholar] [CrossRef]
- Shi, L.; Xu, A.; Zhao, T.S. Formation of Li3O4 nano particles in the discharge products of non-aqueous lithium-oxygen batteries leads to lower charge overvoltage. Phys. Chem. Chem. Phys. 2015, 17, 29859–29866. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Ma, Y. A Stable, Magnetic, and Metallic Li3O4 Compound as a Discharge Product in a Li-Air Battery. J. Phys. Chem. Lett. 2014, 5, 2516–2521. [Google Scholar] [CrossRef]
- Schwenke, K.U.; Metzger, M.; Restle, T.; Piana, M.; Gasteiger, H.A. The Influence of Water and Protons on Li2O2 Crystal Growth in Aprotic Li-O2 Cells. J. Electrochem. Soc. 2015, 162, A573–A584. [Google Scholar] [CrossRef]
- Liu, T.; Kim, G.; Jónsson, E.; Castillo-Martinez, E.; Temprano, I.; Shao, Y.; Carretero-González, J.; Kerber, R.N.; Grey, C.P. Understanding LiOH Formation in a Li-O2 Battery with LiI and H2O Additives. ACS Catal. 2018, 9, 66–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, Z.; Zheng, J.; Wang, S.; Liu, Z.; Bi, Y.; Chen, Y.; Wu, G.; Li, H.; Cui, P.; et al. The long life-span of a Li-metal anode enabled by a protective layer based on the pyrolyzed N-doped binder network. J. Mater. Chem. A 2017, 5, 9339–9349. [Google Scholar] [CrossRef]
- Song, H.; Xu, S.; Li, Y.; Dai, J.; Gong, A.; Zhu, M.; Zhu, C.; Chen, C.; Chen, Y.; Yao, Y.; et al. Hierarchically Porous, Ultrathick, “Breathable” Wood-Derived Cathode for Lithium-Oxygen Batteries. Adv. Energy Mater. 2018, 8, 1701203. [Google Scholar] [CrossRef]
- Qiao, Y.; Yi, J.; Guo, S.; Sun, Y.; Wu, S.; Liu, X.; Yang, S.; He, P.; Zhou, H. Li2CO3-free Li–O2/CO2 battery with peroxide discharge product. Energy Environ. Sci. 2018, 11, 1211–1217. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, L.; Zhang, Y.; Wang, J.; Ma, L.; Zhang, W.-H.; Fu, Z.; Peng, Z. A High-Performance Li-O2 Battery with a Strongly Solvating Hexamethylphosphoramide Electrolyte and a LiPON-Protected Lithium Anode. Adv. Mater. 2017, 29, 1701568. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, H.; Xie, M.; Lin, X.; Huang, Z.; Shen, Y.; Huang, Y. A Stable Lithium–Oxygen Battery Electrolyte Based on Fully Methylated Cyclic Ether. Angew. Chem. Int. Ed. 2018, 58, 2345–2349. [Google Scholar] [CrossRef]
- Lim, H.-D.; Lee, B.; Zheng, Y.; Hong, J.; Kim, J.; Gwon, H.; Ko, Y.; Lee, M.; Cho, K.; Kang, K. Rational design of redox mediators for advanced Li-O2 batteries. Nat. Energy 2016, 1, 16066. [Google Scholar] [CrossRef]
- Enterría, M.; Letona-Elizburu, A.; Medinilla, L.; Echeverría, M.; Ortiz-Vitoriano, N. Controlling the triple phase boundary on Na-O2 battery cathodes with perfluorinated polymers. Electrochim. Acta 2022, 435, 141375. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, J.; Peng, Z. Achilles’ Heel of Lithium–Air Batteries: Lithium Carbonate. Angew. Chem. Int. Ed. 2017, 57, 3874–3886. [Google Scholar] [CrossRef]
- Bi, X.; Amine, K.; Lu, J. The importance of anode protection towards lithium oxygen batteries. J. Mater. Chem. A 2020, 8, 3563–3573. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Jia, C.; Wan, H.; Peng, Z.; Bi, Y.; Liu, Y.; Peng, Z.; Wang, Q.; Li, H.; et al. Decomposing lithium carbonate with a mobile catalyst. Nano Energy 2017, 36, 390–397. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, H. A reversible long-life lithium-air battery in ambient air. Nat. Commun. 2013, 4, 1817. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.; Zhang, Y.; Cheng, X.; Liu, L.; Peng, H. A Li-Air Battery with Ultralong Cycle Life in Ambient Air. Adv. Mater. 2018, 30, 1704378. [Google Scholar] [CrossRef]
- Huang, S.; Cui, Z.; Zhao, N.; Sun, J.; Guo, X. Influence of Ambient Air on Cell Reactions of Li-air Batteries. Electrochim. Acta 2016, 191, 473–478. [Google Scholar] [CrossRef]
- Tan, P.; Shyy, W.; Zhao, T.S.; Zhang, R.H.; Zhu, X.B. Effects of moist air on the cycling performance of non-aqueous lithium-air batteries. Appl. Energy 2016, 182, 569–575. [Google Scholar] [CrossRef]
- Wang, N.; Fu, J.; Cao, X.; Tang, L.; Meng, X.; Han, Z.; Sun, L.; Qi, S.; Xiong, D. Hydrophobic RuO2/Graphene/N-doped porous carbon hybrid catalyst for Li-air batteries operating in ambient air. Electrochim. Acta 2022, 428, 140894. [Google Scholar] [CrossRef]
- Chi, X.; Li, M.; Di, J.; Bai, P.; Song, L.; Wang, X.; Li, F.; Liang, S.; Xu, J.; Yu, J. A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 2021, 592, 551–557. [Google Scholar] [CrossRef]
- Li, C.; Wei, J.; Qiu, K.; Wang, Y. Li-air Battery with a Superhydrophobic Li-Protective Layer. ACS Appl. Mater. Interfaces 2020, 12, 23010–23016. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhao, T.S.; Wei, Z.H.; Tan, P.; An, L. A high-rate and long cycle life solid-state lithium–air battery. Energy Environ. Sci. 2015, 8, 3745–3754. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Liu, J.; Wang, Y.; Xia, Y. A Long-Life Lithium-Air Battery in Ambient Air with a Polymer Electrolyte Containing a Redox Mediator. Angew. Chem. Int. Ed. 2017, 56, 7505–7509. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Liu, X.; Ma, W.; Cao, Z.; Wang, Y.; Ding, Y. Flexible Lithium-Air Battery in Ambient Air with an In Situ Formed Gel Electrolyte. Angew. Chem. Int. Ed. 2018, 57, 16131–16135. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Gao, Z.; Tang, L.; Zhong, L.; Li, J.; Zhang, Y.; Liu, T. Coupling Water-Proof Li Anodes with LiOH-Based Cathodes Enables Highly Rechargeable Lithium-Air Batteries Operating in Ambient Air. Adv. Sci. 2022, 9, e2103760. [Google Scholar] [CrossRef]
- Cheng, J.; Jiang, Y.; Zhang, M.; Sun, Y.; Zou, L.; Chi, B.; Pu, J.; Jian, L. Aprotic Lithium–Air Batteries Tested in Ambient Air with a High-Performance and Low-Cost Bifunctional Perovskite Catalyst. ChemCatChem 2018, 10, 1635–1642. [Google Scholar] [CrossRef]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A lithium–oxygen battery with a long cycle life in an air-like atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Guo, Z.; Xu, Y.; Wang, Y.; Peng, H. High-Performance Lithium-Air Battery with a Coaxial-Fiber Architecture. Angew. Chem. Int. Ed. 2016, 55, 4487–4491. [Google Scholar] [CrossRef]
- Meini, S.; Tsiouvaras, N.; Schwenke, K.U.; Piana, M.; Beyer, H.; Lange, L.; Gasteiger, H.A. Rechargeability of Li-air cathodes pre-filled with discharge products using an ether-based electrolyte solution: Implications for cycle-life of Li-air cells. Phys. Chem. Chem. Phys. 2013, 15, 11478–11493. [Google Scholar] [CrossRef]
- Liu, T.; Leskes, M.; Yu, W.; Moore, A.J.; Zhou, L.; Bayley, P.M.; Kim, G.; Grey, C.P. Cycling Li-O2 batteries via LiOH formation and decomposition. Science 2015, 350, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, K.; Viswanathan, V.V.; Towne, S.A.; Hardy, J.S.; Xiao, J.; Nie, Z.; Hu, D.; Wang, D.; Zhang, J.-G. Reaction mechanisms for the limited reversibility of Li–O2 chemistry in organic carbonate electrolytes. J. Power Sources 2011, 196, 9631–9639. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Chen, Y.; Peng, Z.; Griffin, J.M.; Hardwick, L.J.; Bardé, F.; Novák, P.; Bruce, P.G. Reactions in the Rechargeable Lithium–O2 Battery with Alkyl Carbonate Electrolytes. J. Am. Chem. Soc. 2011, 133, 8040–8047. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Chen, Y.; Drewett, N.E.; Hardwick, L.J.; Bardé, F.; Bruce, P.G. The Lithium-Oxygen Battery with Ether-Based Electrolytes. Angew. Chem. Int. Ed. 2011, 50, 8609–8613. [Google Scholar] [CrossRef] [PubMed]
- Ottakam Thotiyl, M.M.; Freunberger, S.A.; Peng, Z.; Bruce, P.G. The Carbon Electrode in Nonaqueous Li–O2 Cells. J. Am. Chem. Soc. 2013, 135, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Itkis, D.M.; Semenenko, D.A.; Kataev, E.Y.; Belova, A.I.; Neudachina, V.S.; Sirotina, A.P.; Hävecker, M.; Teschner, D.; Knop-Gericke, A.; Dudin, P.; et al. Reactivity of Carbon in Lithium–Oxygen Battery Positive Electrodes. Nano Lett. 2013, 13, 4697–4701. [Google Scholar] [CrossRef]
- Takechi, K.; Shiga, T.; Asaoka, T. A Li-O2/CO2 battery. Chem. Commun. 2011, 47, 3463–3465. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Lyu, Y.; Li, H.; Chen, L. Rechargeable Li/CO2–O2 (2 : 1) battery and Li/CO2 battery. Energy Environ. Sci. 2014, 7, 677–681. [Google Scholar] [CrossRef]
- Lim, H.-K.; Lim, H.-D.; Park, K.-Y.; Seo, D.-H.; Gwon, H.; Hong, J.; Goddard, W.A.; Kim, H.; Kang, K. Toward a Lithium–“Air” Battery: The Effect of CO2 on the Chemistry of a Lithium–Oxygen Cell. J. Am. Chem. Soc. 2013, 135, 9733–9742. [Google Scholar] [CrossRef]
- Yin, W.; Grimaud, A.; Lepoivre, F.; Yang, C.; Tarascon, J.M. Chemical vs Electrochemical Formation of Li2CO3 as a Discharge Product in Li-O2/CO2 Batteries by Controlling the Superoxide Intermediate. J. Phys. Chem. Lett. 2017, 8, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Das, S.K.; Archer, L.A. The Li–CO2 battery: A novel method for CO2 capture and utilization. RSC Adv. 2013, 3, 6656–6660. [Google Scholar] [CrossRef]
- Mu, X.; Pan, H.; He, P.; Zhou, H. Li-CO2 and Na-CO2 Batteries: Toward Greener and Sustainable Electrical Energy Storage. Adv. Mater. 2020, 32, e1903790. [Google Scholar] [CrossRef]
- Wang, L.; Dai, W.; Ma, L.; Gong, L.; Lyu, Z.; Zhou, Y.; Liu, J.; Lin, M.; Lai, M.; Peng, Z.; et al. Monodispersed Ru Nanoparticles Functionalized Graphene Nanosheets as Efficient Cathode Catalysts for O2-Assisted Li–CO2 Battery. ACS Omega 2017, 2, 9280–9286. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Sheng, T.; Zhou, Y.; Wu, Y.-J.; Xiang, C.-C.; Lin, J.-X.; Li, Y.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. Li-CO2/O2 battery operating at ultra-low overpotential and low O2 content on Pt/CNT catalyst. Chem. Eng. J. 2022, 448, 137541. [Google Scholar] [CrossRef]
- Cao, D.; Tan, C.; Chen, Y. Oxidative decomposition mechanisms of lithium carbonate on carbon substrates in lithium battery chemistries. Nat. Commun. 2022, 13, 4908. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.G.; Goebel, S.; Greszler, T.; Mathias, M.; Oelerich, W.; Eroglu, D.; Srinivasan, V. Quantifying the promise of lithium–air batteries for electric vehicles. Energy Environ. Sci. 2014, 7, 1555–1563. [Google Scholar] [CrossRef]
- Xie, M.; Huang, Z.; Lin, X.; Li, Y.; Huang, Z.; Yuan, L.; Shen, Y.; Huang, Y. Oxygen selective membrane based on perfluoropolyether for Li-Air battery with long cycle life. Energy Storage Mater. 2019, 20, 307–314. [Google Scholar] [CrossRef]
- Amici, J.; Alidoost, M.; Francia, C.; Bodoardo, S.; Martinez Crespiera, S.; Amantia, D.; Biasizzo, M.; Caldera, F.; Trotta, F. O2 selective membranes based on a dextrin-nanosponge (NS) in a PVDF-HFP polymer matrix for Li-air cells. Chem. Commun. 2016, 52, 13683–13686. [Google Scholar] [CrossRef]
- Cao, L.; Lv, F.; Liu, Y.; Wang, W.; Huo, Y.; Fu, X.; Sun, R.; Lu, Z. A high performance O2 selective membrane based on CAU-1-NH2@polydopamine and the PMMA polymer for Li-air batteries. Chem. Commun. 2015, 51, 4364–4367. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhao, Y.; Yao, C.; Liu, Y.; Liu, X. CO2 Capture Membrane for Long-Cycle Lithium-Air Battery. Molecules 2023, 28, 2024. [Google Scholar] [CrossRef]
- Kang, J.-H.; Lee, J.; Jung, J.-W.; Park, J.; Jang, T.; Kim, H.-S.; Nam, J.-S.; Lim, H.; Yoon, K.R.; Ryu, W.-H.; et al. Lithium–Air Batteries: Air-Breathing Challenges and Perspective. ACS Nano 2020, 14, 14549–14578. [Google Scholar] [CrossRef]
- Crowther, O.; Keeny, D.; Moureau, D.M.; Meyer, B.; Salomon, M.; Hendrickson, M. Electrolyte optimization for the primary lithium metal air battery using an oxygen selective membrane. J. Power Sources 2012, 202, 347–351. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.; Li, X.; Liu, W. Air Dehydration Membranes for Nonaqueous Lithium–Air Batteries. J. Electrochem. Soc. 2010, 157, A940–A946. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.; Liu, W. Oxygen-selective immobilized liquid membranes for operation of lithium-air batteries in ambient air. J. Power Sources 2010, 195, 7438–7444. [Google Scholar] [CrossRef]
- Wen, X.; Zhu, X.; Wu, Y.; Wang, Y.; Man, Z.; Lv, Z.; Wang, X. A hydrophobic membrane to enable lithium-air batteries to operate in ambient air with a long cycle life. Electrochim. Acta 2022, 421, 140517. [Google Scholar] [CrossRef]
- Zou, X.; Liao, K.; Wang, D.; Lu, Q.; Zhou, C.; He, P.; Ran, R.; Zhou, W.; Jin, W.; Shao, Z. Water-proof, electrolyte-nonvolatile, and flexible Li-Air batteries via O2-Permeable silica-aerogel-reinforced polydimethylsiloxane external membranes. Energy Storage Mater. 2020, 27, 297–306. [Google Scholar] [CrossRef]
- Amici, J.; Francia, C.; Zeng, J.; Bodoardo, S.; Penazzi, N. Protective PVDF-HFP-based membranes for air de-hydration at the cathode of the rechargeable Li–air cell. J. Appl. Electrochem. 2016, 46, 617–626. [Google Scholar] [CrossRef]
- Ruan, Y.; Sun, J.; Song, S.; Yu, L.; Chen, B.; Li, W.; Qin, X. A perfluorocarbon–silicone oil oxygen–selective membrane for ambient operation of aprotic Li–air batteries. Electrochem. Commun. 2018, 96, 93–97. [Google Scholar] [CrossRef]
- Leal Silva, J.F.; Policano, M.C.; Tonon, G.C.; Anchieta, C.G.; Doubek, G.; Maciel Filho, R. The potential of hydrophobic membranes in enabling the operation of lithium-air batteries with ambient air. Chem. Eng. J. Adv. 2022, 11, 100336. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, W.-L.; Cai, Z.-P.; Cheng, J.-H.; Kuang, H.-Y.; Dong, B.-X.; Wang, Y.-B.; Wang, K.-X.; Chen, J.-S. Enhanced Electrochemical Performance of Aprotic Li-CO2 Batteries with a Ruthenium-Complex-Based Mobile Catalyst. Angew. Chem. Int. Ed. 2021, 60, 16404–16408. [Google Scholar] [CrossRef]
- Li, J.; Dai, A.; Amine, K.; Lu, J. Correlating Catalyst Design and Discharged Product to Reduce Overpotential in Li-CO2 Batteries. Small 2021, 17, 2007760. [Google Scholar] [CrossRef]
- Wu, C.; Qi, G.; Zhang, J.; Cheng, J.; Wang, B. Porous Mo3P/Mo Nanorods as Efficient Mott-Schottky Cathode Catalysts for Low Polarization Li-CO2 Battery. Small 2023, 19, 2302078. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, Z.; Chen, J.; Fang, Y.; Lin, S.; Zhang, J.; Xiang, S.; Zhou, Y.; Zhang, Z. Mo2N-ZrO2 Heterostructure Engineering in Freestanding Carbon Nanofibers for Upgrading Cycling Stability and Energy Efficiency of Li-CO2 Batteries. Small 2023, 19, 2301685. [Google Scholar] [CrossRef]
- Sun, X.; Mu, X.; Zheng, W.; Wang, L.; Yang, S.; Sheng, C.; Pan, H.; Li, W.; Li, C.-H.; He, P.; et al. Binuclear Cu complex catalysis enabling Li-CO2 battery with a high discharge voltage above 3.0 V. Nat. Commun. 2023, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Zhang, J.; Chen, L.; Wang, B.; Cheng, J. Binder-Free MoN Nanofibers Catalysts for Flexible 2-Electron Oxalate-Based Li-CO2 Batteries with High Energy Efficiency. Adv. Funct. Mater. 2022, 32, 2112501. [Google Scholar] [CrossRef]
- Yang, C.; Guo, K.; Yuan, D.; Cheng, J.; Wang, B. Unraveling Reaction Mechanisms of Mo2C as Cathode Catalyst in a Li-CO2 Battery. J. Am. Chem. Soc. 2020, 142, 6983–6990. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Liu, L.; Liu, Y.; Chou, S.; Shi, D.; Liu, H.; Wu, Y.; Zhang, W.; Chen, J. Mo2C/CNT: An Efficient Catalyst for Rechargeable Li-CO2 Batteries. Adv. Funct. Mater. 2017, 27, 1700564. [Google Scholar] [CrossRef]
- Xiong, L.; Su, N.Q. From Li2CO3 to Li2C2O4: Understanding Discharge Product Decomposition in Li-CO2 Batteries. Inorg. Chem. 2025, 64, 8376–8385. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Zhang, J.-Y.; Sheng, T.; Lu, Y.-Q.; Yin, Z.-W.; Li, Y.-Y.; Peng, X.-X.; Zhou, Y.; Li, J.-T.; Wu, Y.-J.; et al. Synergetic Effect of Ru and NiO in the Electrocatalytic Decomposition of Li2CO3 to Enhance the Performance of a Li-CO2/O2 Battery. ACS Catal. 2019, 10, 1640–1651. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Hu, Y.-Y.; Cui, X.-Y.; Li, J.-H.; Zeng, S.-Y.; Li, J.; Hao, H.-G.; Kong, X.-J.; Zhou, Y.; Li, J.-T. Interfacial engineering of RuO2 for electrocatalytic decomposition of Li2CO3 in Li–CO2/O2 battery. Mater. Today Phys. 2024, 40, 101307. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Qi, H.; Sun, X.; Li, H.; Tamirat, A.G.; Liu, J.; Wang, Y.; Wang, L. A Highly Reversible Long-Life Li-CO2 Battery with a RuP2-Based Catalytic Cathode. Small 2019, 15, e1803246. [Google Scholar] [CrossRef]
- Fan, W.; Guo, X.; Xiao, D.; Gu, L. Influence of Gold Nanoparticles Anchored to Carbon Nanotubes on Formation and Decomposition of Li2O2 in Nonaqueous Li–O2 Batteries. J. Phys. Chem. C 2014, 118, 7344–7350. [Google Scholar] [CrossRef]
- Hong, M.; Choi, H.C.; Byon, H.R. Nanoporous NiO Plates with a Unique Role for Promoted Oxidation of Carbonate and Carboxylate Species in the Li–O2 Battery. Chem. Mater. 2015, 27, 2234–2241. [Google Scholar] [CrossRef]
- Wang, R.; Yu, X.; Bai, J.; Li, H.; Huang, X.; Chen, L.; Yang, X. Electrochemical decomposition of Li2CO3 in NiO–Li2CO3 nanocomposite thin film and powder electrodes. J. Power Sources 2012, 218, 113–118. [Google Scholar] [CrossRef]
- Song, S.; Xu, W.; Zheng, J.; Luo, L.; Engelhard, M.H.; Bowden, M.E.; Liu, B.; Wang, C.-M.; Zhang, J.-G. Complete Decomposition of Li2CO3 in Li-O2 Batteries Using Ir/B4C as Noncarbon-Based Oxygen Electrode. Nano Lett. 2017, 17, 1417–1424. [Google Scholar] [CrossRef]
- Fan, L.; Tang, D.; Wang, D.; Wang, Z.; Chen, L. LiCoO2-catalyzed electrochemical oxidation of Li2CO3. Nano Res. 2016, 9, 3903–3913. [Google Scholar] [CrossRef]
- Pipes, R.; He, J.; Bhargav, A.; Manthiram, A. Efficient Li–CO2 Batteries with Molybdenum Disulfide Nanosheets on Carbon Nanotubes as a Catalyst. ACS Appl. Energy Mater. 2019, 2, 8685–8694. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, Y.; Yu, D.; Liu, Q.; Zhou, L.; Zhang, K.; Li, P.; Hu, F.; Li, L.; Chou, S.; et al. Hierarchical Ti3C2Tx MXene/Carbon Nanotubes for Low Overpotential and Long-Life Li-CO2 Batteries. ACS Nano 2021, 15, 8407–8417. [Google Scholar] [CrossRef]
- Lacey, M.J.; Frith, J.T.; Owen, J.R. A redox shuttle to facilitate oxygen reduction in the lithium air battery. Electrochem. Commun. 2013, 26, 74–76. [Google Scholar] [CrossRef]
- Chen, Y.; Freunberger, S.A.; Peng, Z.; Fontaine, O.; Bruce, P.G. Charging a Li–O2 battery using a redox mediator. Nat. Chem. 2013, 5, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Lu, Z.; Dai, S.; Fei, H. Recent Progress of Halide Redox Mediators in Lithium-Oxygen Batteries: Functions, Challenges, and Perspectives. Chem. Bio. Eng. 2024, 1, 737–756. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, K.; Yoo, J.; Kwon, G.; Park, H.; Kim, J.; Lee, B.; Song, J.-H.; Kim, J.; Kang, K. Redox mediators for oxygen reduction reactions in lithium–oxygen batteries: Governing kinetics and its implications. Energy Environ. Sci. 2023, 16, 5525–5533. [Google Scholar] [CrossRef]
- Dou, Y.; Xie, Z.; Wei, Y.; Peng, Z.; Zhou, Z. Redox mediators for high-performance lithium-oxygen batteries. Natl. Sci. Rev. 2022, 9, nwac040. [Google Scholar] [CrossRef]
- Arrechea, P.L.; Knudsen, K.B.; Mullinax, J.W.; Haskins, J.B.; Bauschlicher, C.W.; Lawson, J.W.; McCloskey, B.D. Suppression of Parasitic Chemistry in Li–O2 Batteries Incorporating Thianthrene-Based Proposed Redox Mediators. ACS Appl. Energy Mater. 2020, 3, 8812–8821. [Google Scholar] [CrossRef]
- Liang, Z.; Lu, Y.C. Critical role of redox mediator in suppressing charging instabilities of lithium-oxygen batteries. J. Am. Chem. Soc. 2016, 138, 7574–7583. [Google Scholar] [CrossRef]
- Bergner, B.J.; Schürmann, A.; Peppler, K.; Garsuch, A.; Janek, J. TEMPO: A Mobile Catalyst for Rechargeable Li-O2 Batteries. J. Am. Chem. Soc. 2014, 136, 15054–15064. [Google Scholar] [CrossRef]
- Wang, X.-G.; Wang, C.; Xie, Z.; Zhang, X.; Chen, Y.; Wu, D.; Zhou, Z. Improving Electrochemical Performances of Rechargeable Li−CO2 Batteries with an Electrolyte Redox Mediator. ChemElectroChem 2017, 4, 2145–2149. [Google Scholar] [CrossRef]
- Marques Mota, F.; Kang, J.-H.; Jung, Y.; Park, J.; Na, M.; Kim, D.H.; Byon, H.R. Mechanistic Study Revealing the Role of the Br3−/Br2 Redox Couple in CO2-Assisted Li–O2 Batteries. Adv. Energy Mater. 2020, 10, 1903486. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, Y.J. Effect of multi-catalysts on rechargeable Li–air batteries. J. Alloys Compd. 2014, 591, 164–169. [Google Scholar] [CrossRef]
- Lim, H.-D.; Song, H.; Kim, J.; Gwon, H.; Bae, Y.; Park, K.-Y.; Hong, J.; Kim, H.; Kim, T.; Kim, Y.H.; et al. Superior Rechargeability and Efficiency of Lithium-Oxygen Batteries: Hierarchical Air Electrode Architecture Combined with a Soluble Catalyst. Angew. Chem. Int. Ed. 2014, 53, 3926–3931. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, J.; Zhang, J.; Wang, D.; Zhang, J.-G. Optimization of Nonaqueous Electrolytes for Primary Lithium/Air Batteries Operated in Ambient Environment. J. Electrochem. Soc. 2009, 156, A773–A779. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, J.; Wang, D.; Zhang, J.; Zhang, J.-G. Effects of Nonaqueous Electrolytes on the Performance of Lithium/Air Batteries. J. Electrochem. Soc. 2009, 157, A219–A224. [Google Scholar] [CrossRef]
- Kwak, W.-J.; Hirshberg, D.; Sharon, D.; Afri, M.; Frimer, A.A.; Jung, H.-G.; Aurbach, D.; Sun, Y.-K. Li–O2 cells with LiBr as an electrolyte and a redox mediator. Energy Environ. Sci. 2016, 9, 2334–2345. [Google Scholar] [CrossRef]
- Bi, X.; Li, J.; Dahbi, M.; Alami, J.; Amine, K.; Lu, J. Understanding the Role of Lithium Iodide in Lithium-Oxygen Batteries. Adv. Mater. 2022, 34, e2106148. [Google Scholar] [CrossRef]
- Petrongari, A.; Piacentini, V.; Pierini, A.; Fattibene, P.; De Angelis, C.; Bodo, E.; Brutti, S. Insights into the LiI Redox Mediation in Aprotic Li-O(2) Batteries: Solvation Effects and Singlet Oxygen Evolution. ACS Appl. Mater. Interfaces 2023, 15, 59348–59357. [Google Scholar] [CrossRef]
- Leverick, G.; Tułodziecki, M.; Tatara, R.; Bardé, F.; Shao-Horn, Y. Solvent-Dependent Oxidizing Power of LiI Redox Couples for Li-O2 Batteries. Joule 2019, 3, 1106–1126. [Google Scholar] [CrossRef]
- Shiga, T.; Kondo, H.; Kato, Y.; Hase, Y. Iodine Mediator with Anomalously High Redox Potential and Its Application to a Catalytic Cycle for Lithium Carbonate Decomposition toward Future Lithium Reproduction. J. Phys. Chem. C 2019, 123, 3944–3950. [Google Scholar] [CrossRef]
- Shiga, T.; Kato, Y.; Inoue, M.; Hase, Y. Bifunctional Catalytic Activity of Iodine Species for Lithium–Carbon Dioxide Battery. ACS Sustain. Chem. Eng. 2019, 7, 14280–14287. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, B.; Zhao, L.; Li, B.-L.; Su, Z.-M.; Guan, W. Theoretical insights into H2S desulfurization: Catalysis by binuclear cobalt phthalocyanine. Mol. Catal. 2025, 572, 114734. [Google Scholar] [CrossRef]
- Bernstein, P.A.; Lever, A.B.P. Two-electron oxidation of cobalt phthalocyanines by thionyl chloride. Implications for lithium/thionyl chloride batteries. Inorg. Chem. 1990, 29, 608–616. [Google Scholar] [CrossRef]
- Makarov, S.G.; Ketkov, S.Y.; Wöhrle, D. A planar binuclear cobalt(II) phthalocyanine as a highly efficient catalyst for the oxidation of a mercaptan. Chem. Commun. 2020, 56, 5653–5656. [Google Scholar] [CrossRef]
- Li, B.; Zhou, X.; Wang, X.; Liu, B.; Li, B. Hybrid binuclear-cobalt-phthalocyanine as oxygen reduction reaction catalyst in single chamber microbial fuel cells. J. Power Sources 2014, 272, 320–327. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, K.; Tan, A.; Yang, Y. Enhanced Electrocatalytic Performance for Oxygen Reduction Reaction of Porous Carbon Materials Functionalized by Binuclear Ball-Type Metallophthalocyanines. Appl. Organomet. Chem. 2025, 39, e7916. [Google Scholar] [CrossRef]
- Hu, X.; Xia, D.; Zhang, L.; Zhang, J. High crystallinity binuclear iron phthalocyanine catalyst with enhanced performance for oxygen reduction reaction. J. Power Sources 2013, 231, 91–96. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, J.; Luan, P.; Ding, C.; Li, C. Understanding the factors governing the water oxidation reaction pathway of mononuclear and binuclear cobalt phthalocyanine catalysts. Chem. Sci. 2022, 13, 8797–8803. [Google Scholar] [CrossRef]
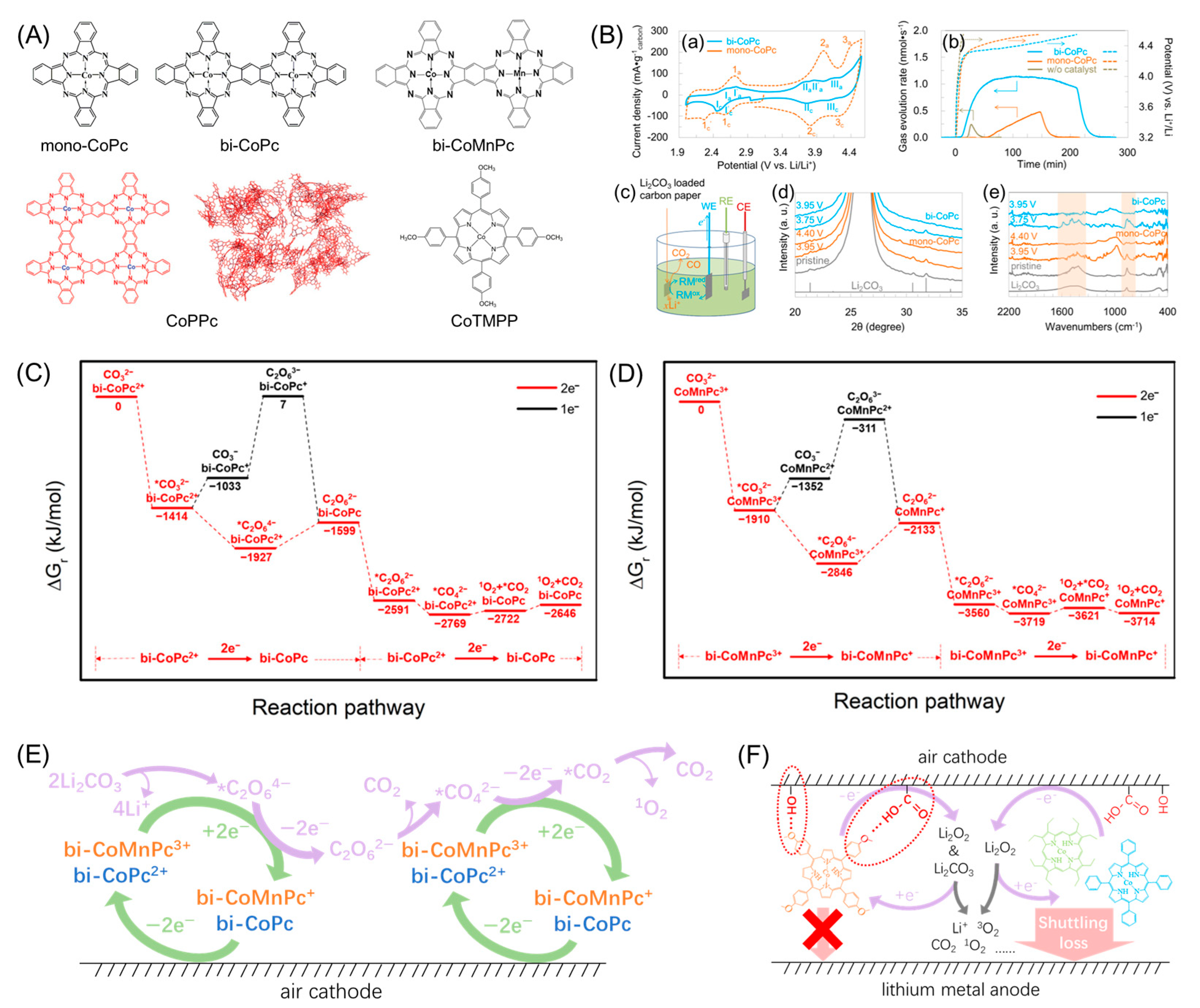
- Yan, Q.; Yan, L.; Huang, H.; Chen, Z.; Liu, Z.; Zhou, S.; He, H. Effects of Central Metal Ion on Binuclear Metal Phthalocyanine-Based Redox Mediator for Lithium Carbonate Decomposition. Molecules 2024, 29, 2034. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, K.; Ding, P.; Deng, J.; Zha, C.; Hu, Y.; Zhao, X.; Wu, J.; Fan, J.; Li, Y. Conjugated Cobalt Polyphthalocyanine as the Elastic and Reprocessable Catalyst for Flexible Li-CO2 Batteries. Adv. Mater. 2019, 31, e1805484. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Liu, Z.; Yan, Q.; Ma, B.; Wang, D.; Wei, F.; Chen, Z.; He, H. Surface Enrichment of Redox Mediator for Long-Cyclable Lithium–Air Batteries. Energy Fuels 2023, 37, 11465–11471. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Qi, H.; Sun, X.; Song, X.; Guo, Z.; Tamirat, A.G.; Liu, J.; Wang, L.; Feng, S. Drawing a Pencil-Trace Cathode for a High-Performance Polymer-Based Li-CO2 Battery with Redox Mediator. Adv. Funct. Mater. 2019, 29, 1806863. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Feng, Y.; Wang, D.; Yin, H.; Chi, Z.; Li, L.; Liu, J.; Li, S.; Huang, J.; et al. Protecting Li-metal anode with ethylenediamine-based layer and in-situ formed gel polymer electrolyte to construct the high-performance Li–CO2 battery. J. Power Sources 2021, 506, 230226. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.; Johnson, L.R.; Bruce, P.G. Kinetics of lithium peroxide oxidation by redox mediators and consequences for the lithium-oxygen cell. Nat. Commun. 2018, 9, 767. [Google Scholar] [CrossRef]
- Ahn, S.; Zor, C.; Yang, S.; Lagnoni, M.; Dewar, D.; Nimmo, T.; Chau, C.; Jenkins, M.; Kibler, A.J.; Pateman, A.; et al. Why charging Li-air batteries with current low-voltage mediators is slow and singlet oxygen does not explain degradation. Nat. Chem. 2023, 15, 1022–1029. [Google Scholar] [CrossRef]
- Mahne, N.; Renfrew, S.E.; McCloskey, B.D.; Freunberger, S.A. Electrochemical Oxidation of Lithium Carbonate Generates Singlet Oxygen. Angew. Chem. Int. Ed. 2018, 57, 5529–5533. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hong, H.; Feng, Q.; Wang, X.; Zhao, X.; Xu, M.; Wu, X.; Li, H.; Zhi, C.; Han, C. Conjugated cobalt polyphthalocyanine with defective π-π extended structure for enhanced rechargeable li-oxygen batteries. Chem. Eng. J. 2022, 444, 136544. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Shuai, L.; Tang, Y.; Qiu, M.; Xie, J.; Liu, J.; Wen, W.; Chen, H.; Nan, S.; et al. Remarkable improvement of cyclic stability in Li–O2 batteries using ruthenocene as a redox mediator. Chin. Chem. Lett. 2020, 31, 1997–2002. [Google Scholar] [CrossRef]
- Lian, Z.; Lu, Y.; Ma, S.; Wang, L.; Li, Z.; Liu, Q. An integrated strategy for upgrading Li-CO2 batteries: Redox mediator and separator modification. Chem. Eng. J. 2022, 450, 138400. [Google Scholar] [CrossRef]
- Wan, H.; Sun, Y.; Yu, J.; Shi, Q.; Zhu, Y.; Qian, Y. A chiral salen-Co(II) complex as soluble redox mediator for promoting the electrochemical performance of Li-O2 batteries. Nano Res. 2022, 15, 8101–8108. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Z.; Wang, D.; Wang, M.; Peng, Z.; Liu, Z.; He, H.; Wang, M.; Li, H. High-performance Li-air battery after limiting inter-electrode crosstalk. Energy Storage Mater. 2021, 39, 225–231. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, Z.; Wang, C.; Gao, M.; Lin, X.; Duan, H.; Wang, Y.; Sun, X. Design of multifunctional interfaces on ceramic solid electrolytes for high-performance lithium-air batteries. Green Energy Environ. 2025, 10, 183–192. [Google Scholar] [CrossRef]
- Jung, J.-W.; Nam, J.S.; Klyukin, K.; Youn, D.-Y.; Kim, I.-D. Straightforward strategy toward a shape-deformable carbon-free cathode for flexible Li–air batteries in ambient air. Nano Energy 2021, 83, 105821. [Google Scholar] [CrossRef]
- Jia, C.; Pan, F.; Zhu, Y.G.; Huang, Q.; Lu, L.; Wang, Q. High–energy density nonaqueous all redox flow lithium battery enabled with a polymeric membrane. Sci. Adv. 2015, 1, e1500886. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.-N.; Wang, Y.-F.; Li, F.; Wang, X.-X.; Wang, H.-F.; Xu, J.-J. A TEMPO-grafted multi-functional cathode with strong anchoring ability towards redox mediators for high energy efficiency Li-O2 batteries. Energy Storage Mater. 2022, 45, 191–200. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Ševčík, A. Oscillographic polarography with periodical triangular voltage. Collect. Czechoslov. Chem. Commun. 1948, 13, 349–377. [Google Scholar] [CrossRef]
- Brown, A.P.; Anson, F.C. Cyclic and Differential Pulse Voltammetric Behavior of Reactants Confined to the Electrode Surface. Anal. Chem. 1977, 49, 1589–1595. [Google Scholar] [CrossRef]
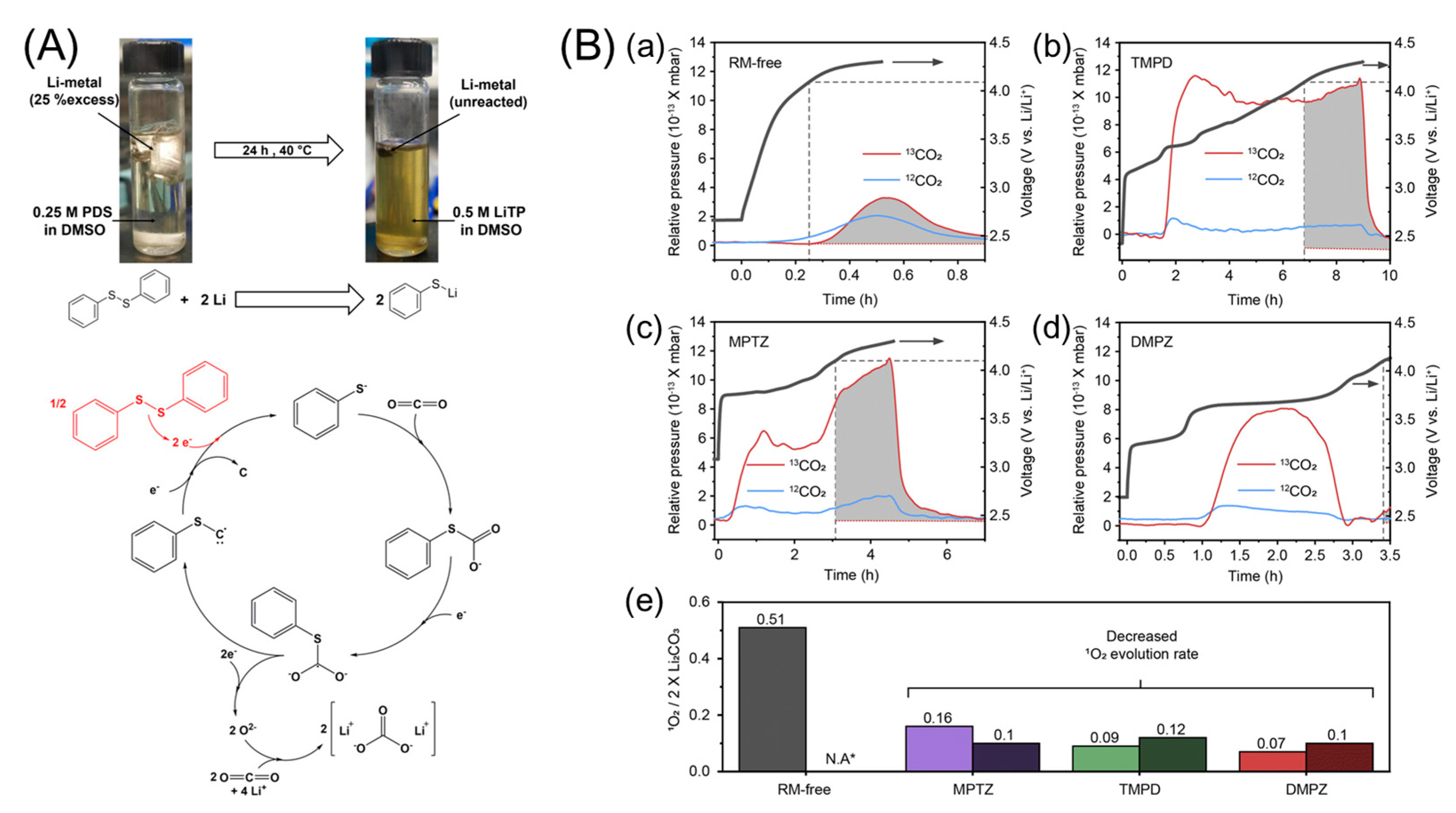
- Pipes, R.; Bhargav, A.; Manthiram, A. Phenyl Disulfide Additive for Solution-Mediated Carbon Dioxide Utilization in Li–CO2 Batteries. Adv. Energy Mater. 2019, 9, 1900453. [Google Scholar] [CrossRef]
- Ko, S.; Yoo, Y.; Choi, J.; Lim, H.-D.; Park, C.B.; Lee, M. Discovery of organic catalysts boosting lithium carbonate decomposition toward ambient air operational lithium–air batteries. J. Mater. Chem. A 2022, 10, 20464–20472. [Google Scholar] [CrossRef]
- Xiong, Q.; Huang, G.; Zhang, X.B. High-Capacity and Stable Li-O2 Batteries Enabled by a Trifunctional Soluble Redox Mediator. Angew. Chem. Int. Ed. 2020, 59, 19311–19319. [Google Scholar] [CrossRef]
- Cao, D.; Liu, X.; Yuan, X.; Yu, F.; Chen, Y. Redox Mediator-Enhanced Performance and Generation of Singlet Oxygen in Li-CO2 Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39341–39346. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Ma, S.; Lian, Z.; Gu, X.; Li, J.; Li, Z.; Liu, Q. Optimizing CO2 reduction and evolution reaction mediated by o-phenylenediamine toward high performance Li-CO2 battery. Electrochimica Acta 2022, 419, 140424. [Google Scholar] [CrossRef]
- Liang, Z.; Zou, Q.; Xie, J.; Lu, Y.-C. Suppressing singlet oxygen generation in lithium–oxygen batteries with redox mediators. Energy Environ. Sci. 2020, 13, 2870–2877. [Google Scholar] [CrossRef]
- Petit, Y.K.; Leypold, C.; Mahne, N.; Mourad, E.; Schafzahl, L.; Slugovc, C.; Borisov, S.M.; Freunberger, S.A. DABCOnium: An Efficient and High-Voltage Stable Singlet Oxygen Quencher for Metal-O2 Cells. Angew. Chem. Int. Ed. 2019, 58, 6535–6539. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, Y.; Zhu, Z.; Gao, S.; Lv, Q.; Li, F. Quenching singlet oxygen via intersystem crossing for a stable Li-O2 battery. Proc. Natl. Acad. Sci. USA 2022, 119, e2202835119. [Google Scholar] [CrossRef] [PubMed]
- Kwak, W.-J.; Freunberger, S.A.; Kim, H.; Park, J.; Nguyen, T.T.; Jung, H.-G.; Byon, H.R.; Sun, Y.-K. Mutual Conservation of Redox Mediator and Singlet Oxygen Quencher in Lithium–Oxygen Batteries. ACS Catal. 2019, 9, 9914–9922. [Google Scholar] [CrossRef]
- Lee, H.-W.; Kim, J.-Y.; Kim, J.-E.; Jo, Y.-J.; Dewar, D.; Yang, S.; Gao, X.; Bruce, P.G.; Kwak, W.-J. Effect of singlet oxygen on redox mediators in lithium–oxygen batteries. J. Mater. Chem. A 2023, 11, 16003–16008. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yoo, J.; Kang, L. Predicting the chemical reactivity of organic materials using a machine-learning approach. Chem. Sci. 2020, 11, 7813–7822. [Google Scholar] [CrossRef]
- Mo, P.; Li, C.; Zhao, D.; Zhang, Y.; Shi, M.; Li, J.; Liu, J. Accurate and efficient molecular dynamics based on machine learning and non von Neumann architecture. Npj Comput. Mater. 2022, 8, 107. [Google Scholar] [CrossRef]
- Wang, Y.; Lamim Ribeiro, J.M.; Tiwary, P. Machine learning approaches for analyzing and enhancing molecular dynamics simulations. Curr. Opin. Struct. Biol. 2020, 61, 139–145. [Google Scholar] [CrossRef]
- Ruth, M.; Gerbig, D.; Schreiner, P.R. Machine Learning for Bridging the Gap between Density Functional Theory and Coupled Cluster Energies. J. Chem. Theory Comput. 2023, 19, 4912–4920. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Freunberger, S.A.; Chen, Y.; Bruce, P.G. A reversible and higher-rate Li-O2 battery. Science 2012, 337, 563–566. [Google Scholar] [CrossRef]
- Ottakam Thotiyl, M.M.; Freunberger, S.A.; Peng, Z.; Chen, Y.; Liu, Z.; Bruce, P.G. A stable cathode for the aprotic Li–O2 battery. Nat. Mater. 2013, 12, 1050–1056. [Google Scholar] [CrossRef]
- Zhao, G.; Lv, J.; Xu, Z.; Zhang, L.; Sun, K. Carbon and binder free rechargeable Li–O2 battery cathode with Pt/Co3O4 flake arrays as catalyst. J. Power Sources 2014, 248, 1270–1274. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Liu, Q.; Rong, Y.; Chen, H.; Yang, J.; Jia, C.; Yu, L.J.; Karton, A.; Ren, Y.; Xu, X.; et al. Proton enhanced dynamic battery chemistry for aprotic lithium-oxygen batteries. Nat. Commun. 2017, 8, 14308. [Google Scholar] [CrossRef] [PubMed]
| Discharge Product Species | Possible Decomposition Reaction | ΔGr0 (kJ∙mol−1) | E0 (VLi) | Practical Decomposition Potential in Carbon Electrode (VLi) | Practical Decomposition Potential in Carbon Electrode W/Catalyst (VLi) |
|---|---|---|---|---|---|
| Li2O2 | Li2O2 → 2Li+ + O2 + 2e− | −15.67 | 2.96 | ~4.1 [37] | ~3.9 (Pt) [37] |
| Li2O | 2Li2O → 4Li+ + O2 + 4e− | −50.84 | 2.91 | ~5 [37] | ~4.0 (Pt) [37] |
| Li2CO3 | 2Li2CO3 → 4Li+ + 2CO2 + O2 + 4e− | 302.16 | 3.82 | ~4.8 [37] | ~4.2 (Pt) [37] |
| LiOH | 4LiOH → 4Li+ + 2H2O(l) + O2 + 4e− | 118.56 | 3.35 | ~5 [37] | ~3.8 (Pt) [37] ~3.0 (LiI) [38] |
| HCOOLi (a) | 2HCOOLi → 2Li+ + H2 + 2CO2 + 2e− | −112.81 | 2.46 | N/A | N/A |
| CH3COOLi (a) | 2CH3COOLi → 2Li+ + 3H2O(l) + 3C + CO + 2e− | −157.91 | 2.22 | N/A | N/A |
| R(OCOOLi)2 | R(OCOOLi)2 + 2H2O → R(OH)2 + 2CO2 + 2OH• + 2Li+ + 2e− | N/A | N/A | ~3.9 [39] | ~3.9 (Fe3O4) [39] |
| R(OCOOLi)2 + xO2 → yCO2 + zH2O + 2Li+ + 2e− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Huang, H.; Chen, Z.; He, H.; Wang, D.; Li, Z. Redox Mediators for Li2CO3 Decomposition. Inorganics 2025, 13, 192. https://doi.org/10.3390/inorganics13060192
Liu Z, Huang H, Chen Z, He H, Wang D, Li Z. Redox Mediators for Li2CO3 Decomposition. Inorganics. 2025; 13(6):192. https://doi.org/10.3390/inorganics13060192
Chicago/Turabian StyleLiu, Zixuan, Haoshen Huang, Zhengfei Chen, Haiyong He, Deyu Wang, and Zhoupeng Li. 2025. "Redox Mediators for Li2CO3 Decomposition" Inorganics 13, no. 6: 192. https://doi.org/10.3390/inorganics13060192
APA StyleLiu, Z., Huang, H., Chen, Z., He, H., Wang, D., & Li, Z. (2025). Redox Mediators for Li2CO3 Decomposition. Inorganics, 13(6), 192. https://doi.org/10.3390/inorganics13060192










