Experimental Attempts at and Theoretical Study of the Thermal Generation of o-Carborane-Supported N-Heterocyclic Carbenes
Abstract
1. Introduction
2. Results and Discussion
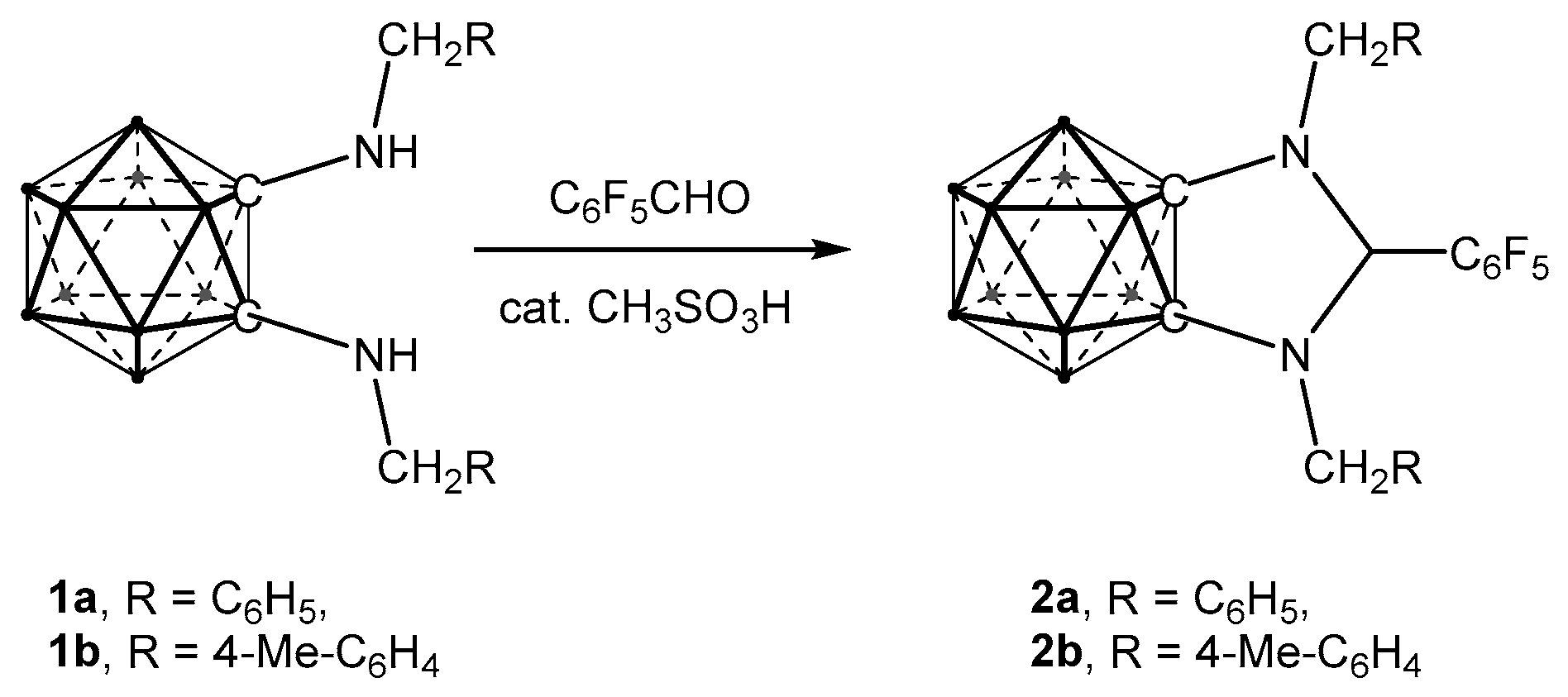
2.1. Synthesis
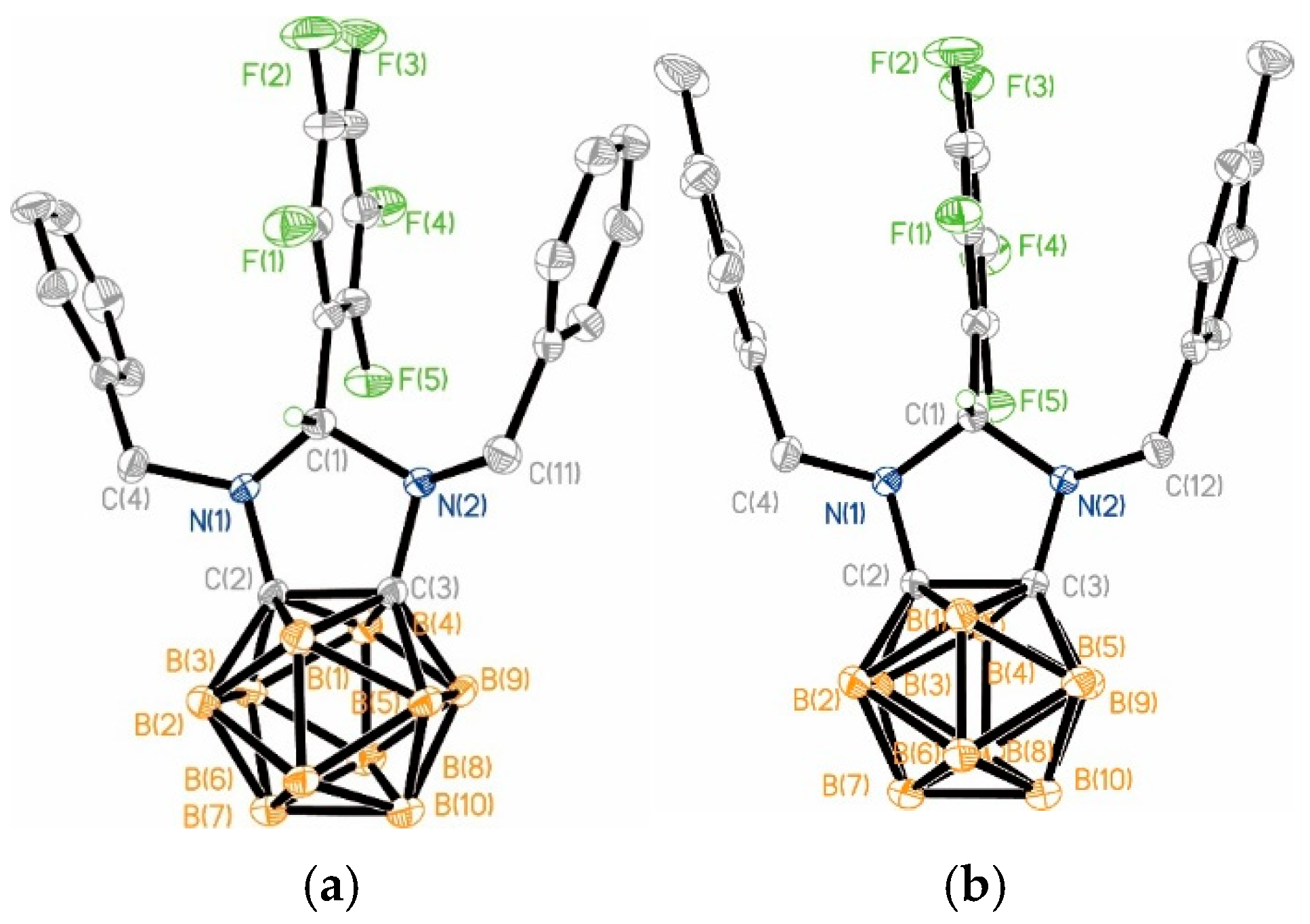
2.2. X-Ray Structures
2.3. Calculations
3. Materials and Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arduengo, A.J.; Kline, M.; Calabrese, J.C.; Davidson, F. Synthesis of a reverse ylide from a nucleophilic carbene. J. Am. Chem. Soc. 1991, 113, 9704–9705. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Lin, I.J.B.; Vasam, C.S. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord. Chem. Rev. 2007, 251, 642–670. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Peris, E. Complexes with poly(N-heterocyclic carbene) ligands: Structural features and catalytic applications. Chem. Rev. 2009, 109, 3677–3707. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; Cesar, V. Synthetic routes to N-heterocyclic carbene precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef]
- Sinha, N.; Hahn, F.E. Metallosupramolecular Architectures Obtained from Poly-N-heterocyclic Carbene Ligands. Acc. Chem. Res. 2017, 50, 2167–2184. [Google Scholar] [CrossRef]
- Huynh, H.V. Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef]
- Gan, M.-M.; Liu, J.-Q.; Zhang, L.; Wang, Y.-Y.; Hahn, F.E.; Han, Y.-F. Preparation and Post-Assembly Modification of Metallosupramolecular Assemblies from Poly(N-Heterocyclic Carbene) Ligands. Chem. Rev. 2018, 118, 9587–9641. [Google Scholar] [CrossRef]
- Hu, C.Y.; Wang, X.F.; Wei, R.; Hu, C.P.; Ruiz, D.A.; Chang, X.Y.; Liu, L.L. Crystalline monometal-substituted free carbenes. Chem 2022, 8, 2278–2289. [Google Scholar] [CrossRef]
- Bai, S.; Han, Y.F. Metal-N-Heterocyclic Carbene Chemistry Directed toward Metallosupramolecular Synthesis and Beyond. Acc. Chem. Res. 2023, 56, 1213–1227. [Google Scholar] [CrossRef]
- Hu, C.P.; Wang, X.F.; Li, J.C.; Chang, X.Y.; Liu, L.L. A stable rhodium-coordinated carbene with a σ0π2 electronic configuration. Science 2024, 383, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H. Luminescent Complexes of Platinum, Iridium, and Coinage Metals Containing N-Heterocyclic Carbene Ligands: Design, Structural Diversity, and Photophysical Properties. Chem. Rev. 2023, 123, 230–270. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Highly Efficient Ring-Opening Metathesis Polymerization (ROMP) Using New Ruthenium Catalysts Containing N-Heterocyclic Carbene Ligands. Angew. Chem. Int. Ed. 2000, 39, 2903–2906. [Google Scholar] [CrossRef]
- Ohishi, T.; Nishiura, M.; Hou, Z. Carboxylation of Organoboronic Esters Catalyzed by N-Heterocyclic Carbene Copper(I) Complexes. Angew. Chem. Int. Ed. 2008, 47, 5792–5795. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. N-heterocyclic carbenes in gold catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [Google Scholar] [CrossRef]
- Riener, K.; Haslinger, S.; Raba, A.; Hogerl, M.P.; Cokoja, M.; Herrmann, W.A.; Kuhn, F.E. Chemistry of iron N-heterocyclic carbene complexes: Syntheses, structures, reactivities, and catalytic applications. Chem. Rev. 2014, 114, 5215–5272. [Google Scholar] [CrossRef]
- Gou, X.X.; Liu, T.; Wang, Y.Y.; Han, Y.F. Ultrastable and Highly Catalytically Active N-Heterocyclic-Carbene-Stabilized Gold Nanoparticles in Confined Spaces. Angew. Chem. Int. Ed. 2020, 59, 16683–16689. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Si, D.H.; Huang, Y.B.; Cao, R. A CO-Masked Carbene Functionalized Covalent Organic Framework for Highly Efficient Carbon Dioxide Conversion. Angew. Chem. Int. Ed. 2022, 61, e202207478. [Google Scholar] [CrossRef]
- Lan, X.; Wang, H.; Liang, Q.; Liu, L.L. A Crystalline Mesoionic Diazasilole Featuring Low-Valent Silicon. Angew. Chem. Int. Ed. 2025, 64, e202415246. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; Präsang, C.; Donnadieu, B.; Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angew. Chem. Int. Ed. 2005, 44, 5705–5709. [Google Scholar] [CrossRef]
- Khramov, D.M.; Rosen, E.L.; Lynch, V.M.; Bielawski, C.W. Diaminocarbene[3]ferrocenophanes and their transition-metal complexes. Angew. Chem. Int. Ed. 2008, 47, 2267–2270. [Google Scholar] [CrossRef] [PubMed]
- Kronig, S.; Theuergarten, E.; Daniliuc, C.G.; Jones, P.G.; Tamm, M. Anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety. Angew. Chem. Int. Ed. 2012, 51, 3240–3244. [Google Scholar] [CrossRef]
- El-Hellani, A.; Lavallo, V. Fusing N-Heterocyclic Carbenes with Carborane Anions. Angew. Chem. Int. Ed. 2014, 53, 4489–4493. [Google Scholar] [CrossRef] [PubMed]
- Teator, A.J.; Tian, Y.; Chen, M.; Lee, J.K.; Bielawski, C.W. An Isolable, Photoswitchable N-Heterocyclic Carbene: On-Demand Reversible Ammonia Activation. Angew. Chem. Int. Ed. 2015, 54, 11559–11563. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.; Lavallo, V. Fusing Dicarbollide Ions with N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2017, 56, 9906–9909. [Google Scholar] [CrossRef]
- Kuhn, N.; Kratz, T. Synthesis of Imidazol-2-ylidenes by Reduction of Imidazole-2(3H)-thiones. Synthesis 1993, 1993, 561–562. [Google Scholar] [CrossRef]
- Denk, K.; Sirsch, P.; Herrmann, W.A. The first metal complexes of bis(diisopropylamino)carbene: Synthesis, structure and ligand properties. J. Organomet. Chem. 2002, 649, 219–224. [Google Scholar] [CrossRef]
- Trnka, T.M.; Morgan, J.P.; Sanford, M.S.; Wilhelm, T.E.; Scholl, M.; Choi, T.L.; Ding, S.; Day, M.W.; Grubbs, R.H. Synthesis and activity of ruthenium alkylidene complexes coordinated with phosphine and N-heterocyclic carbene ligands. J. Am. Chem. Soc. 2003, 125, 2546–2558. [Google Scholar] [CrossRef]
- Nyce, G.W.; Csihony, S.; Waymouth, R.M.; Hedrick, J.L. A general and versatile approach to thermally generated N-heterocyclic carbenes. Chem. Eur. J. 2004, 10, 4073–4079. [Google Scholar] [CrossRef]
- Bedford, R.B.; Betham, M.; Blake, M.E.; Frost, R.M.; Horton, P.N.; Hursthouse, M.B.; Lopez-Nicolas, R.-M. N-Heterocyclic carbene adducts of orthopalladated triarylphosphite complexes. Dalton Trans. 2005, 2774–2779. [Google Scholar] [CrossRef]
- Bedford, R.B.; Betham, M.; Bruce, D.W.; Danopoulos, A.A.; Frost, R.M.; Hird, M. Iron-phosphine, -phosphite, -arsine, and -carbene catalysts for the coupling of primary and secondary alkyl halides with aryl Grignard reagents. J. Org. Chem. 2006, 71, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Bedford, R.B.; Dumycz, H.; Haddow, M.F.; Pilarski, L.T.; Orpen, A.G.; Pringle, P.G.; Wingad, R.L. Chiral triaryl phosphite-based palladacycles and platinacycles: Synthesis and application to asymmetric Lewis acid catalysis. Dalton Trans. 2009, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.P.; Ritter, T.; Grubbs, R.H. Synthesis of N-Heterocylic Carbene-Containing Metal Complexes from 2-(Pentafluorophenyl)imidazolidines. Organometallics 2007, 26, 2122–2124. [Google Scholar] [CrossRef]
- Li, J.; Stewart, I.C.; Grubbs, R.H. Synthesis and Structure of Fused N-Heterocylic Carbenes and Their Rhodium Complexes. Organometallics 2010, 29, 3765–3768. [Google Scholar] [CrossRef]
- Bittermann, A.; Haerter, P.; Herdtweck, E.; Hoffmann, S.D.; Herrmann, W.A. Acceptor substituted N-heterocyclic carbenes and their Rh(I) complexes: Synthesis, structure and properties. J. Organomet. Chem. 2008, 693, 2079–2090. [Google Scholar] [CrossRef]
- Holmes, J.; Pask, C.M.; Fox, M.A.; Willans, C.E. Tethered N-heterocyclic carbene-carboranes: Unique ligands that exhibit unprecedented and versatile coordination modes at rhodium. Chem. Commun. 2016, 52, 6443–6446. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, Y.; Miao, J.; Li, Y.; Zhang, Z. Synthesis and characterization of carboranyl Schiff base compounds from 1-amino-o-carborane. J. Organomet. Chem. 2015, 798, 182–188. [Google Scholar] [CrossRef]
- Nuñez, R.; Tarres, M.; Ferrer-Ugalde, A.; Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Selg, C.; Neumann, W.; Lonnecke, P.; Hey-Hawkins, E.; Zeitler, K. Carboranes as Aryl Mimetics in Catalysis: A Highly Active Zwitterionic NHC-Precatalyst. Chem. Eur. J. 2017, 23, 7932–7937. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, H.; Miao, J.L.; Zhao, X.Q.; Li, Y.X.; Sun, G.X. Synthesis, aggregation-induced emission and mechanochromism of a new carborane-tetraphenylethylene hybrid. J. Organomet. Chem. 2018, 865, 200–205. [Google Scholar] [CrossRef]
- Xu, T.T.; Cao, K.; Zhang, C.Y.; Wu, J.; Ding, L.F.; Yang, J.X. Old Key Opens the Lock in Carborane: The in Situ NHC-Palladium Catalytic System for Selective Arylation of B(3,6)-H Bonds of o-Carboranes via B-H Activation. Org. Lett. 2019, 21, 9276–9279. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Xie, Z. Controlled functionalization of o-carborane via transition metal catalyzed B-H activation. Chem. Soc. Rev. 2019, 48, 3660–3673. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, T.-T.; Wu, J.; Zhang, C.-Y.; Wen, X.-Y.; Yang, J. The in Situ NHC-Palladium Catalyzed Selective Activation of B(3)–H or B(6)–H Bonds of o-Carboranes for Hydroboration of Alkynes: An Efficient Approach to Alkenyl-o-carboranes. Inorg. Chem. 2020, 60, 1080–1085. [Google Scholar] [CrossRef]
- Wang, L.H.; Perveen, S.; Ouyang, Y.Z.; Zhang, S.; Jiao, J.; He, G.; Nie, Y.; Li, P.F. Well-Defined, Versatile and Recyclable Half-Sandwich Nickelacarborane Catalyst for Selective Carbene-Transfer Reactions. Chem. Eur. J. 2021, 27, 5754–5760. [Google Scholar] [CrossRef]
- Cui, P.F.; Liu, X.R.; Lin, Y.J.; Li, Z.H.; Jin, G.X. Highly Selective Separation of Benzene and Cyclohexane in a Spatially Confined Carborane Metallacage. J. Am. Chem. Soc. 2022, 144, 6558–6565. [Google Scholar] [CrossRef]
- Buzsáki, D.; Gál, D.; Szathmári, B.; Holczbauer, T.; Udvardy, A.; Szilágyiné, J.K.; Kargin, D.; Bruhn, C.; Pietschnig, R.; Kelemen, Z. The “chemical tug-of-war” in carborane clusters: Distinct tuning on different sides of the cluster. Inorg. Chem. Front. 2025, 12, 1822–1830. [Google Scholar] [CrossRef]
- Jin, G.-X. Advances in the chemistry of organometallic complexes with 1,2-dichalcogenolato-o-carborane ligands. Coord. Chem. Rev. 2004, 248, 587–602. [Google Scholar] [CrossRef]
- Mandal, N.; Pal, A.K.; Gain, P.; Zohaib, A.; Datta, A. Transition-State-like Planar Structures for Amine Inversion with Ultralong C-C Bonds in Diamino-o-carborane and Diamino-o-dodecahedron. J. Am. Chem. Soc. 2020, 142, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, D.; Kovács, M.B.; Hümpfner, E.; Harcsa-Pintér, Z.; Kelemen, Z. Conjugation between 3D and 2D aromaticity: Does it really exist? The case of carborane-fused heterocycles. Chem. Sci. 2022, 13, 11388–11393. [Google Scholar] [CrossRef]
- Ma, Y.N.; Ren, H.Z.; Wu, Y.X.; Li, N.; Chen, F.J.; Chen, X.N. B(9)-OH-o-Carboranes: Synthesis, Mechanism, and Property Exploration. J. Am. Chem. Soc. 2023, 145, 7331–7342. [Google Scholar] [CrossRef]
- Zhang, H.T.; Gao, Y.; Ma, Y.N.; Chen, X.N. Pd(ii)-catalyzed B(9)-alkynylation of o/m-carboranes. Org. Chem. Front. 2024, 11, 6706–6711. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.G.; Chen, F.J.; Ma, Y.N.; Chen, X.N. HSAB theory guiding electrophilic substitution reactions of o-carborane. Org. Chem. Front. 2024, 12, 76–84. [Google Scholar] [CrossRef]
- Chen, X.-M.; Ge, Y.-W.; Yu, X.-C.; Wang, P.; Jiang, K.; Ma, Y.-N.; Chen, X. Improved Methods for the Synthesis of B10H14 and N-Heterocycle-Coordinated B9H13 (N-Het·B9H13). Inorg. Chem. 2024, 64, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pang, R.; Li, Z.; Lai, G.; Xiao, X.Q.; Müller, T. Exceptionally Long C-C Single Bonds in Diamino-o-carborane as Induced by Negative Hyperconjugation. Angew. Chem. Int. Ed. 2019, 58, 1397–1401. [Google Scholar] [CrossRef]
- Pang, R.; Li, J.; Cui, Z.; Zheng, C.; Li, Z.; Chen, W.; Qi, F.; Su, L.; Xiao, X.Q. Synthesis, structure and DFT calculations of 1,2-N-substituted o-carboranes. Dalton Trans. 2019, 48, 7242–7248. [Google Scholar] [CrossRef]
- Kang, Y.R.; Wang, B.N.; Nan, R.X.; Li, Y.W.; Zhu, Z.L.; Xiao, X.Q. Cyclic Carbonate Synthesis from Epoxides and CO Catalyzed by Aluminum-Salen Complexes Bearing a nido-C2B9 Carborane Ligand. Inorg. Chem. 2022, 61, 8806–8814. [Google Scholar] [CrossRef]
- Wang, B.N.; Zhu, Z.L.; Liang, M.J.; Ren, Y.K.; Xue, J.B.; Zhang, J.Y.; Qi, F.; Xiao, X.Q. A 12-Vertex Metallacarborane of Silver(I). Inorg. Chem. 2024, 63, 5481–5486. [Google Scholar] [CrossRef]
- Xue, J.-B.; Wang, J.-N.; Chen, K.-C.; Cheng, Q.; Zhang, J.-Y.; Xiao, X.-Q. A comparative study on nido- and closo-carborane supported zinc-salen catalysts for the ROCOP of epoxides and anhydrides. Dalton Trans. 2025. [Google Scholar] [CrossRef]
- Cui, Z.Z.; Wang, B.N.; Li, J.X.; Pang, R.L.; Kang, Y.R.; Xiao, X.Q. N-Heterocyclic Carbenes with a Nido-C2B9 Carborane Backbone. Chin. J. Chem. 2021, 39, 2410–2416. [Google Scholar] [CrossRef]
- Nan, R.X.; Li, Y.W.; Zhu, Z.L.; Qi, F.; Xiao, X.Q. Nickelacarborane-Supported Bis-N-heterocyclic Carbenes. J. Am. Chem. Soc. 2023, 145, 15538–15546. [Google Scholar] [CrossRef]
- Ren, Y.-K.; Li, Y.; Liang, M.-J.; Ma, J.-W.; Niu, Z.-X.; Xiao, X.-Q. Self-Assembly and Dynamic Equilibrium of Trinuclear and Tetranuclear Cu(I) Supramolecules Featuring nido-Carborane-Supported N-Heterocyclic Carbene Ligands. Inorg. Chem. 2025, 64, 9727–9734. [Google Scholar] [CrossRef]
- Scarborough, C.C.; Guzei, I.A.; Stahl, S.S. Synthesis and isolation of a stable, axially-chiral seven-membered N-heterocyclic carbene. Dalton Trans. 2009, 2284–2286. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Sheldrick, G. Program for Empirical Absorption Correction of Area Detector Data (SADABS); University of Göttingen: Göttingen, Germany, 1994; Volume 90. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bruker. SHELXTL Version 6.22 Program for Solution and Refinement of Crystal Structures; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 2002, 14, 363–368. [Google Scholar] [CrossRef]

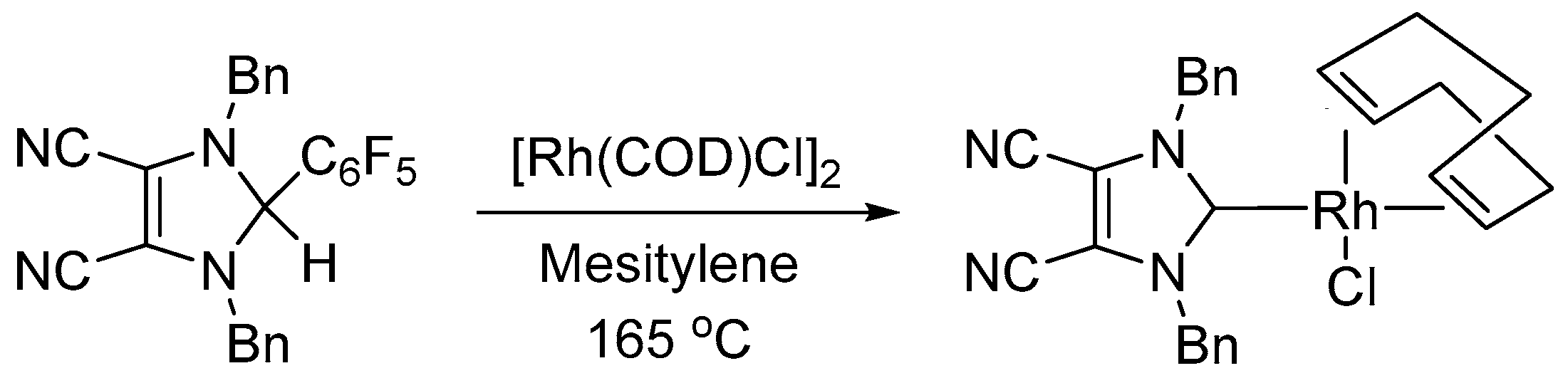


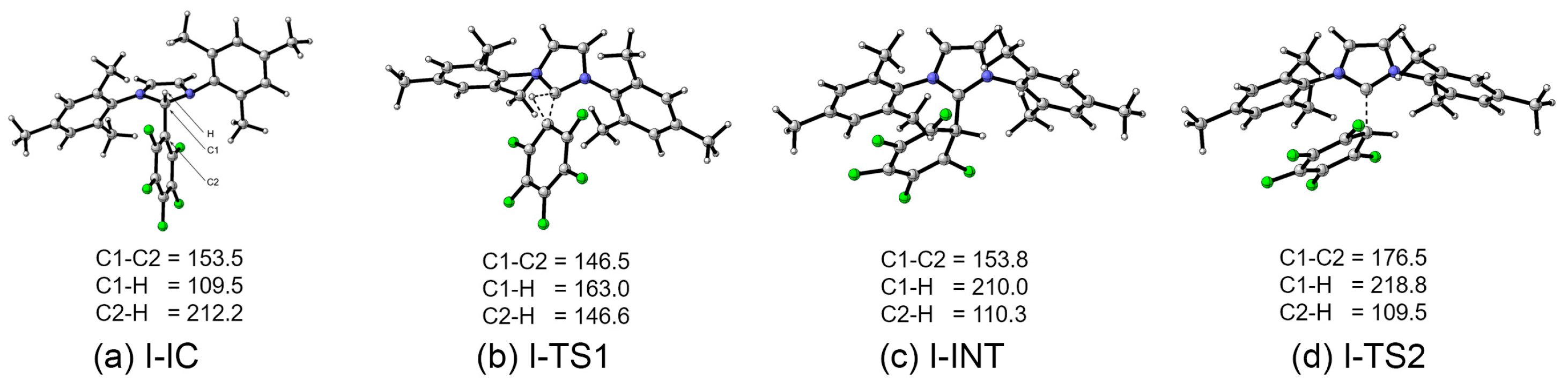
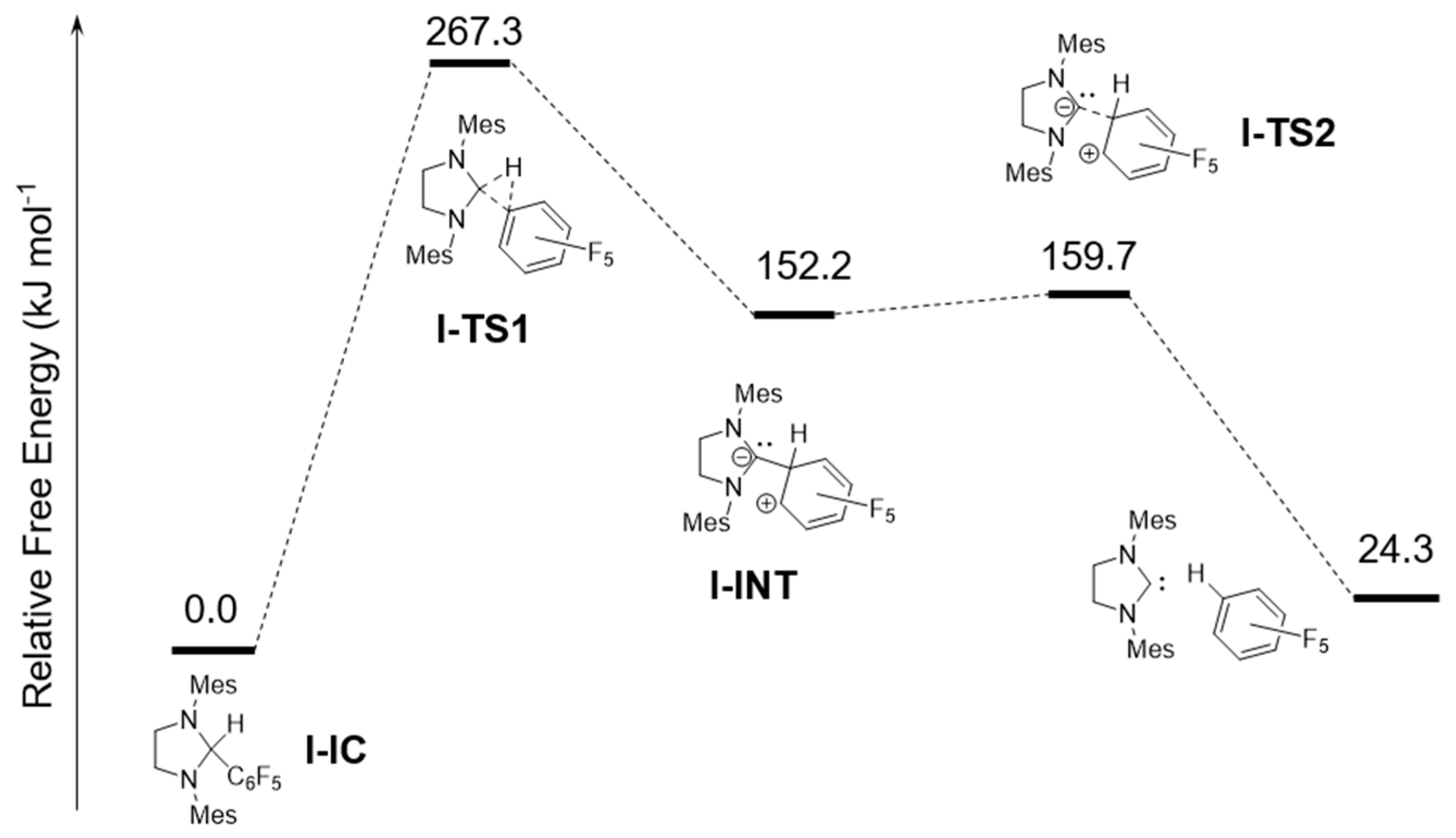
| Models | IC | TS1 | INT | TS2 | PROD |
|---|---|---|---|---|---|
| I | 0.0 | 267.3 | 152.2 | 159.7 | 24.3 |
| II | 0.0 | 246.3 | 97.1 | 109.9 | −29.9 |
| III | 0.0 | 280.6 | 153.0 | 153.8 | −9.6 |
| IV | 0.0 | 293.6 | 186.2 | 192.7 | −10.2 |
| V | 0.0 | 320.5 | 243.3 | 257.4 | 69.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.-J.; Chen, K.-C.; Cui, Z.; Zhou, Y.-C.; Wang, Y.; Qi, F.; Xiao, X.-Q. Experimental Attempts at and Theoretical Study of the Thermal Generation of o-Carborane-Supported N-Heterocyclic Carbenes. Inorganics 2025, 13, 179. https://doi.org/10.3390/inorganics13060179
Liang M-J, Chen K-C, Cui Z, Zhou Y-C, Wang Y, Qi F, Xiao X-Q. Experimental Attempts at and Theoretical Study of the Thermal Generation of o-Carborane-Supported N-Heterocyclic Carbenes. Inorganics. 2025; 13(6):179. https://doi.org/10.3390/inorganics13060179
Chicago/Turabian StyleLiang, Mei-Juan, Ke-Cheng Chen, Zhongzheng Cui, Yan-Chang Zhou, Yan Wang, Fan Qi, and Xu-Qiong Xiao. 2025. "Experimental Attempts at and Theoretical Study of the Thermal Generation of o-Carborane-Supported N-Heterocyclic Carbenes" Inorganics 13, no. 6: 179. https://doi.org/10.3390/inorganics13060179
APA StyleLiang, M.-J., Chen, K.-C., Cui, Z., Zhou, Y.-C., Wang, Y., Qi, F., & Xiao, X.-Q. (2025). Experimental Attempts at and Theoretical Study of the Thermal Generation of o-Carborane-Supported N-Heterocyclic Carbenes. Inorganics, 13(6), 179. https://doi.org/10.3390/inorganics13060179









