Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is responsible for many infections, primarily due to its ability to form biofilms. Nanotechnology has recently been proposed as an alternative for controlling MRSA. In the present work, we evaluated the antimicrobial and antibiofilm activities of silver nanoparticles synthesized with Stenocereus queretaroensis peel extract (SAgNPs). The biosynthesis process was optimized using a response surface design. The results showed antimicrobial activity against MRSA bacteria, with a minimum inhibitory concentration and minimum bactericidal concentration of 0.15 and 0.31 µg/mL, respectively. SAgNPs inhibited biofilm formation in multi-well plates and Congo red agar. Molecular docking analysis revealed that the presence of quercetin, one of the chemical components of S. queretaroensis peel, forms hydrogen bonds with six interacting amino acids. This suggests that quercetin presents a stable binding to this site, which in turn suggests that the mechanism of action of SAgNPs is related to their binding to PBP2a. Therefore, these findings suggest a promising, environmentally friendly approach to combating antibiotic-resistant infections, potentially reducing the reliance on traditional antibiotics.
1. Introduction
The discovery and use of antibiotics for treating infectious diseases is considered one of the main medical advances of the 20th century; millions of human lives have been saved. The significant increase in the life expectancy of the world population in the last 100 years is largely due to the introduction of antibiotics into clinical practice [1]. However, the evolutionary nature of microorganisms and inappropriate use of antibiotics have led to the development of genetic mutations as a mechanism against antimicrobial agents, resulting in resistance [2].
Antimicrobial resistance was observed just a few years after antibiotics started being used commercially. By 1960, methicillin-resistant Staphylococcus aureus (MRSA) strains had already been identified. Currently, more than 70% of pathogenic bacteria are considered to have antimicrobial resistance to at least one antibiotic; in addition, it has been reported that MRSA strains are responsible for 13 to 74% of S. aureus infections worldwide [3]. MRSA strains are characterized by their ability to form biofilms on both biotic and abiotic surfaces [4]. MRSA biofilms protect cells from deleterious environmental factors, including antibiotics and the host immune system. They are primarily composed of polysaccharides, proteins, and extracellular DNA [5].
Antimicrobial resistance in MRSA strains has become a public health problem that, if not adequately resolved, will cause a worldwide increase in mortality [6]. Antimicrobial resistance requires different scientific approaches, including nanotechnology, which has aided in developing new antimicrobial agents [7]. Silver nanoparticles have been shown to have antimicrobial properties against a wide variety of pathogens, and the mechanisms by which they demonstrate these effects are related to alterations in the cell membrane and damage at the intracellular level, among others [8].
Among the different nanoparticle synthesis methods included in bottom-up approaches, biosynthesis with extracts from agricultural waste stands out as an environmentally friendly technique that does not require the use of toxic substances, is cost-effective, and can easily be scaled up. Among the main affecting parameters for this type of biosynthesis are the concentration of the extract, temperature, metal salt, pH, and contact time [9]. Recently, the use of silver nanoparticles synthesized with plants and their parts has reportedly been used to evaluate biofilm formation inhibition through in vitro studies [10]. On the other hand, it has been shown that the antibiotic resistance of MRSA is associated with the synthesis of penicillin-binding protein 2a (PBP2a). As such, through in silico studies, it is possible to predict the molecular binding of chemical components of silver nanoparticles synthesized with S. queretaroensis peel extract (SAgNPs) [11].
Despite the promising advancements in nanotechnology-based antimicrobial strategies, the need for environmentally sustainable and highly effective antibiofilm agents remains critical. This study addresses this gap by exploring the synthesis and antibiofilm activity of SAgNPs, an innovative approach that leverages agricultural waste for medical applications. Previous research has primarily focused on conventional plant extracts or chemically synthesized nanoparticles for combating MRSA. In contrast, our work builds upon these foundations by introducing a novel biosynthesis method that not only offers a green solution but also utilizes a previously underexplored natural resource for nanoparticle production. In this study, we investigated the antibiofilm activity of SAgNPs against MRSA bacteria through in vitro studies and molecular docking.
2. Results
2.1. Nanoparticle Synthesis for Optimization
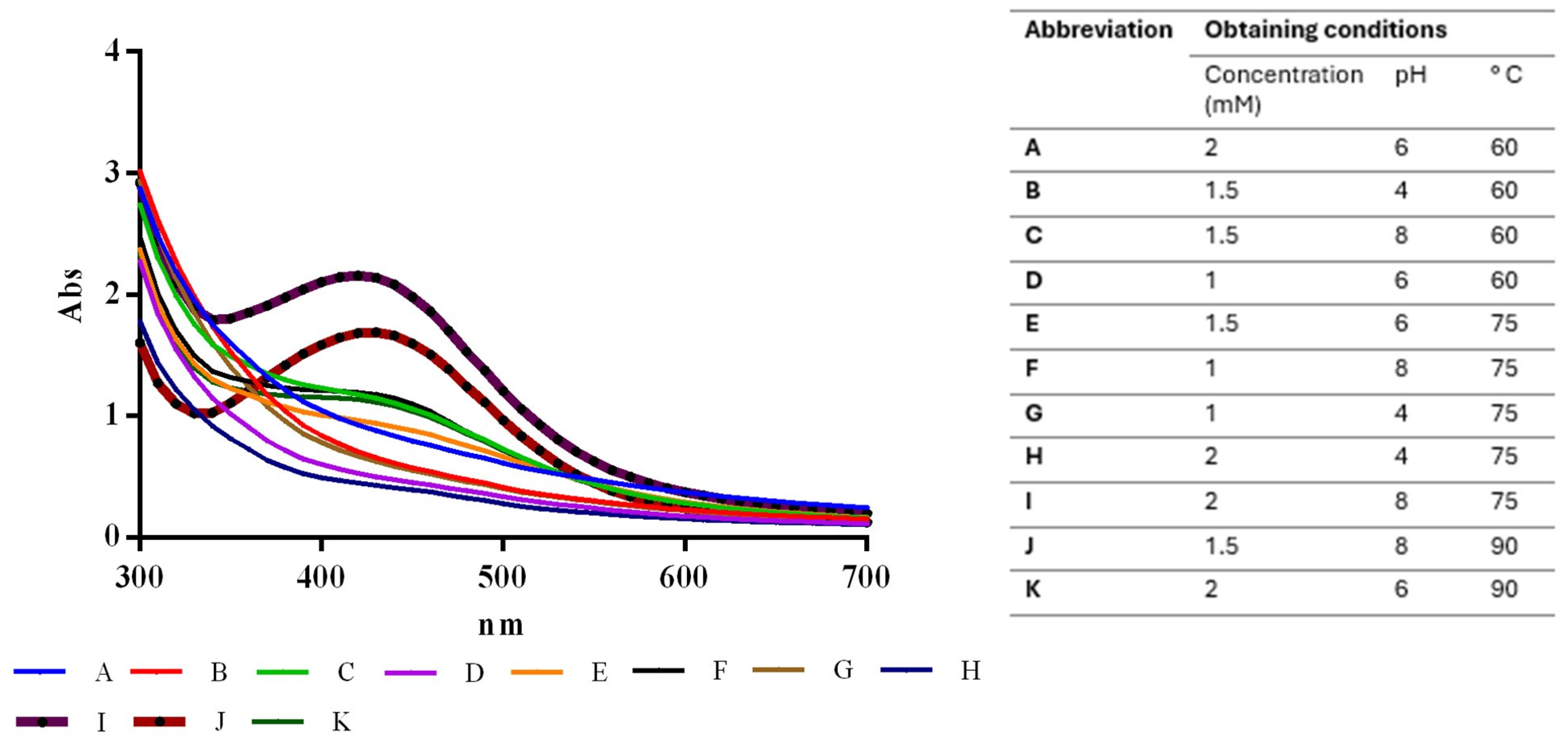
In the initial stage of nanoparticle synthesis, the silver nitrate was transparent, and the extract had a slightly orange color. Throughout incubation, the color of the reaction mixture changed to dark brown, indicating the formation of silver nanoparticles. Different experimental conditions, with the factors previously selected to optimize the nanoparticle synthesis process, resulted in different absorbances, as shown in Figure 1.
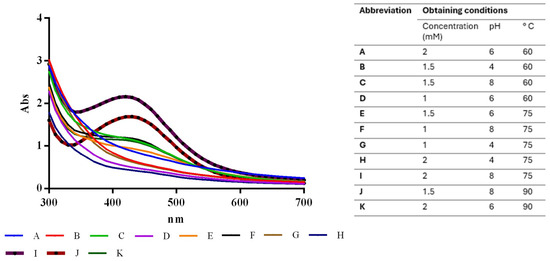
Figure 1.
Absorbance values under optimized conditions for SAgNPs synthesis, highlighting impacts of varying pH and AgNO3 concentrations.
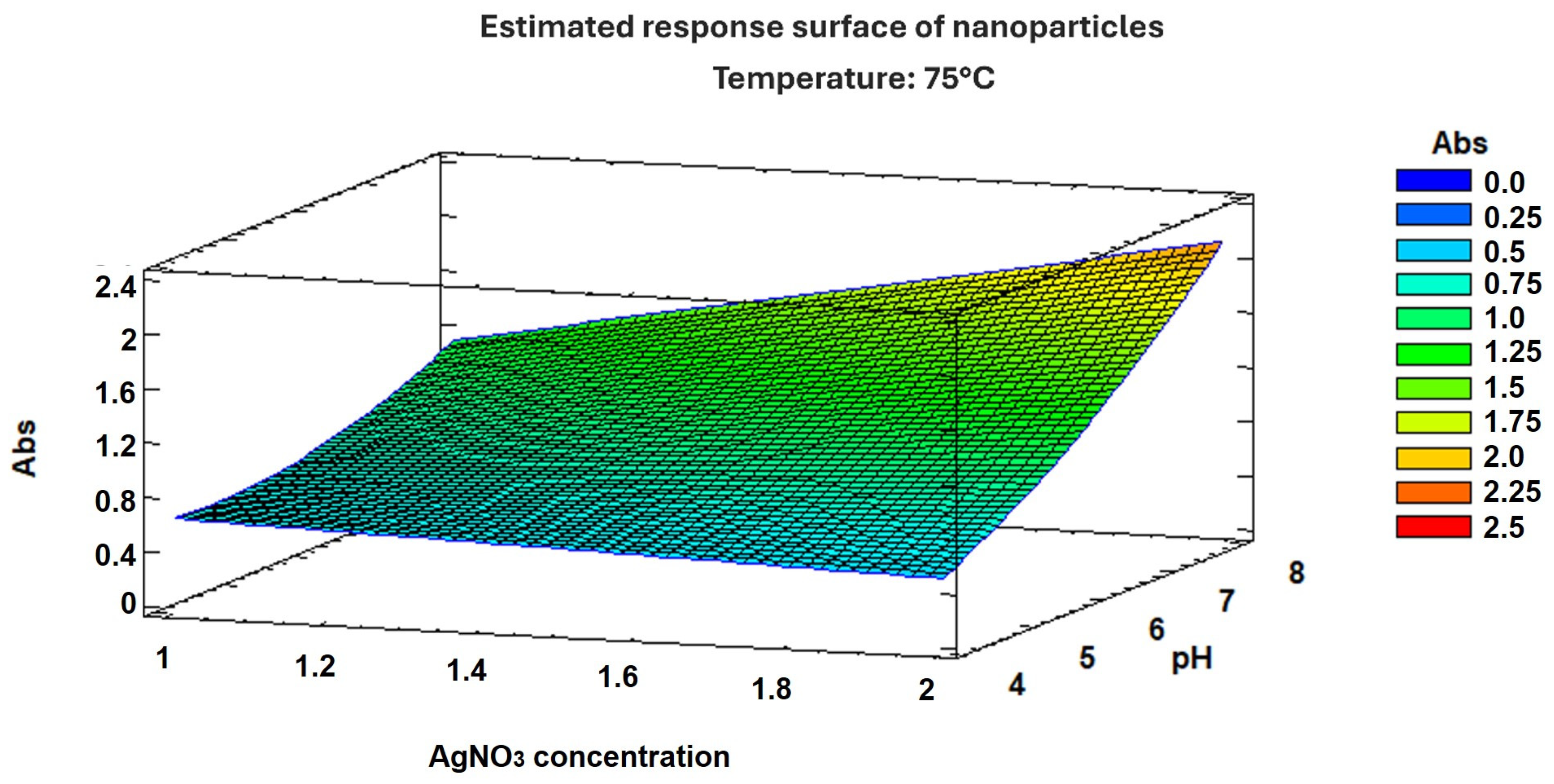
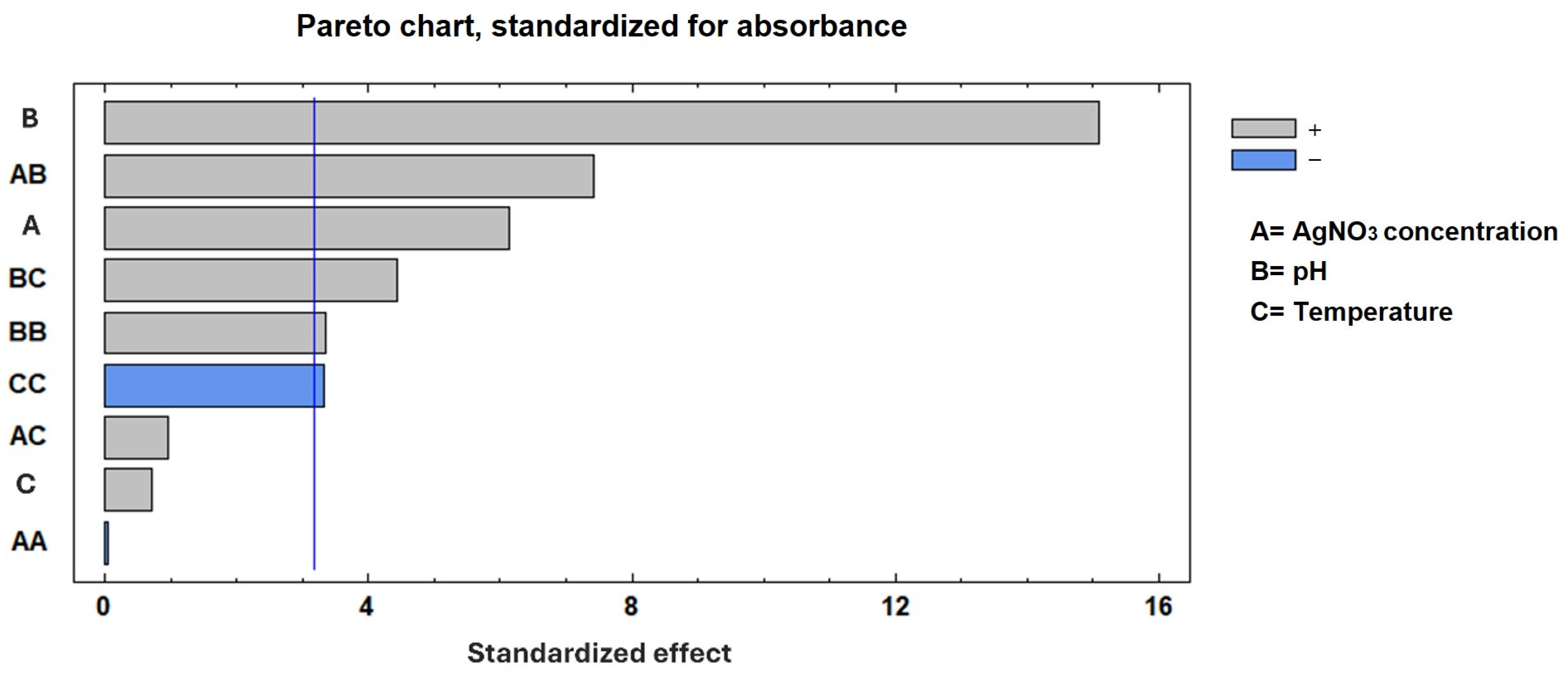
The experimental factor optimization results in Figure 2 show how interactions between AgNO3 concentration and pH increase the absorbance units to 2.25. A Pareto chart confirmed that the pH, as well as the combination of pH and AgNO3 concentration, was the most important factor in increasing absorbance, as shown in Figure 3. These results support the conclusion that the conditions previously reported [12]—namely 2 mM AgNO3 and pH 8—are indeed optimal for the biosynthesis of AgNPs using S. queretaroensis peel extract.
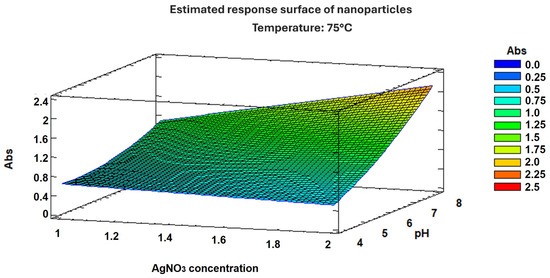
Figure 2.
Response surface graph of nanoparticle optimization.
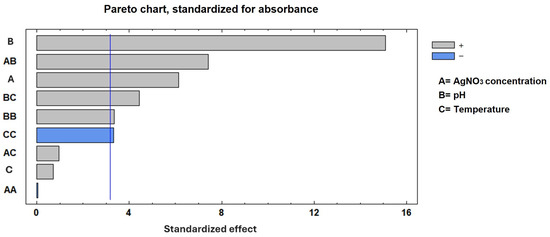
Figure 3.
A Pareto chart of the standardized effects. The Pareto chart indicates the variables that were significant in the process for maximum absorbance. The blue line represents the relative cumulative frequency.
2.2. Antimicrobial Activity of SAgNPs Against MRSA
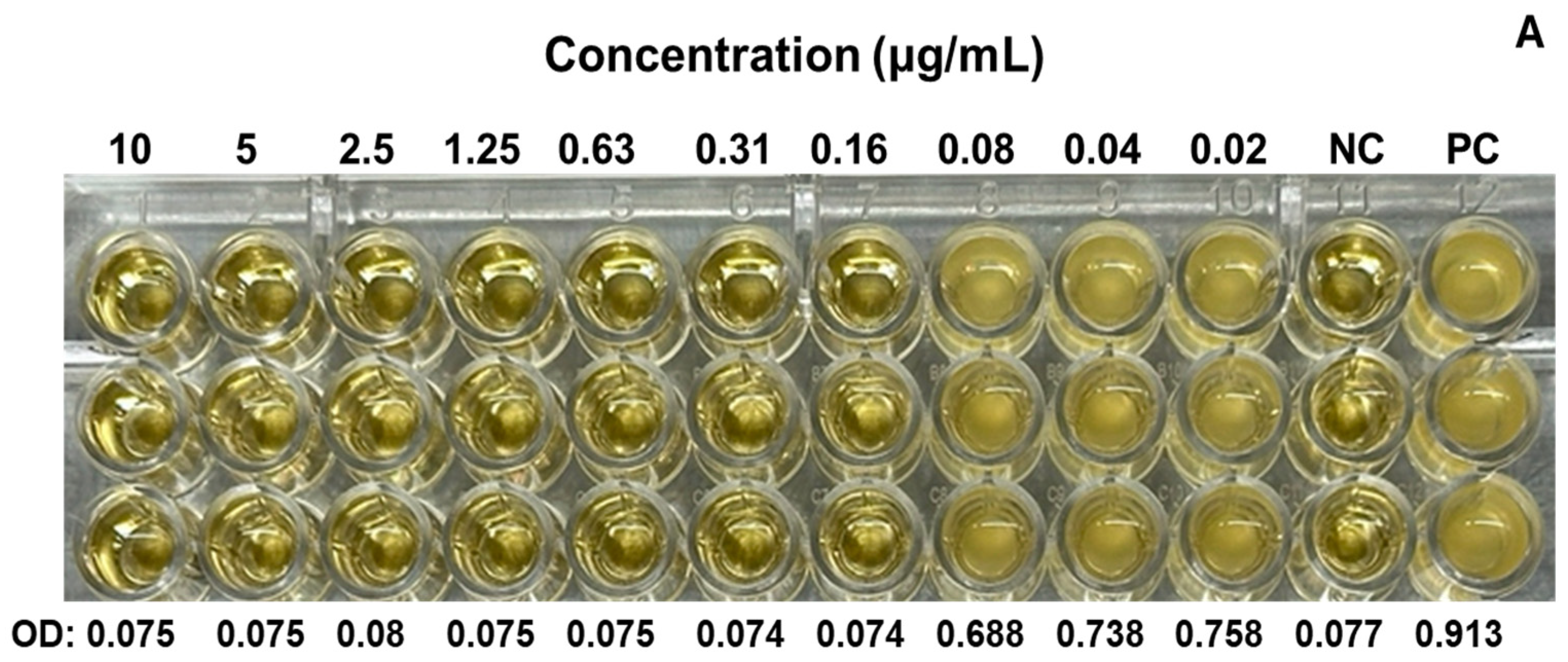
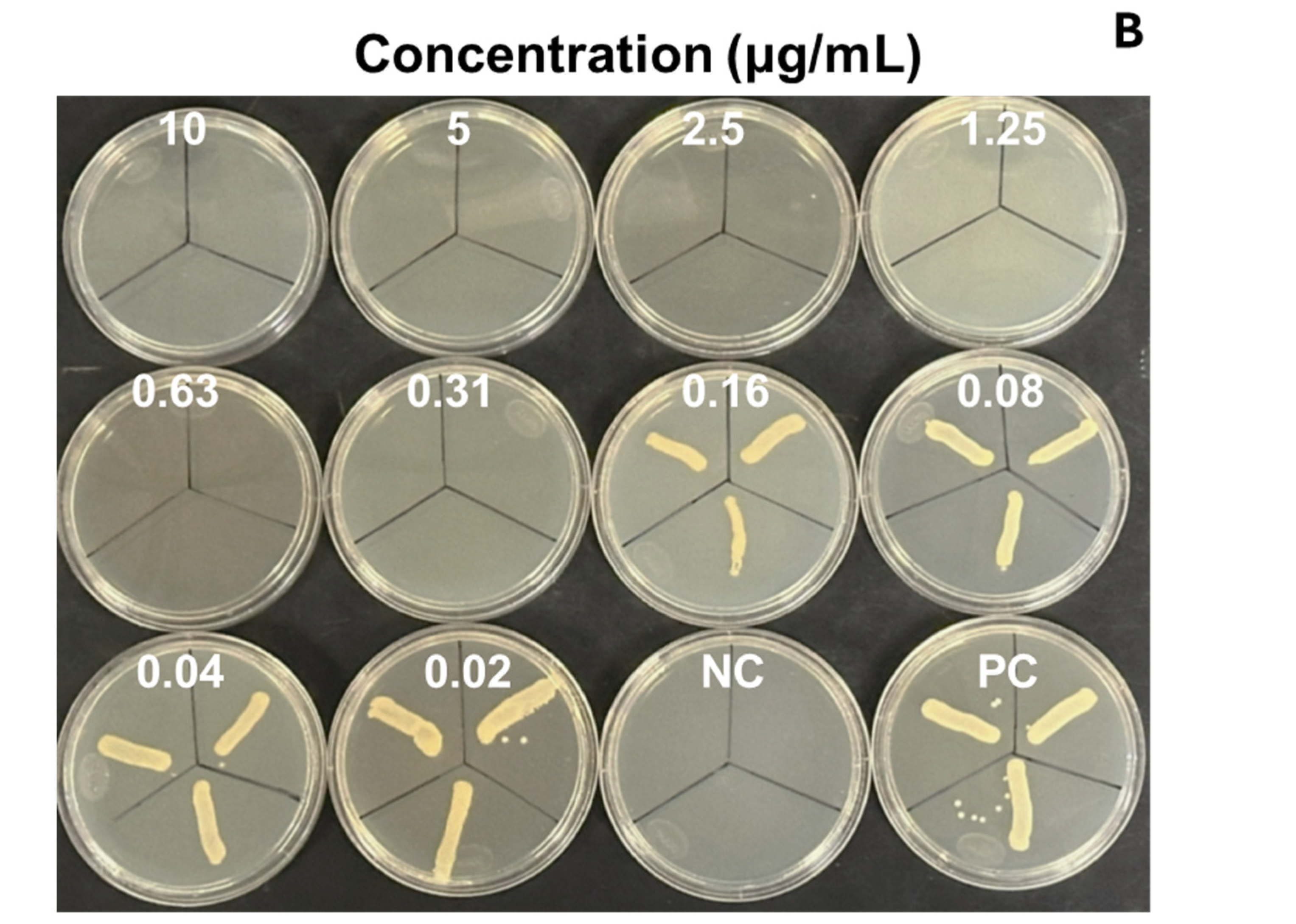
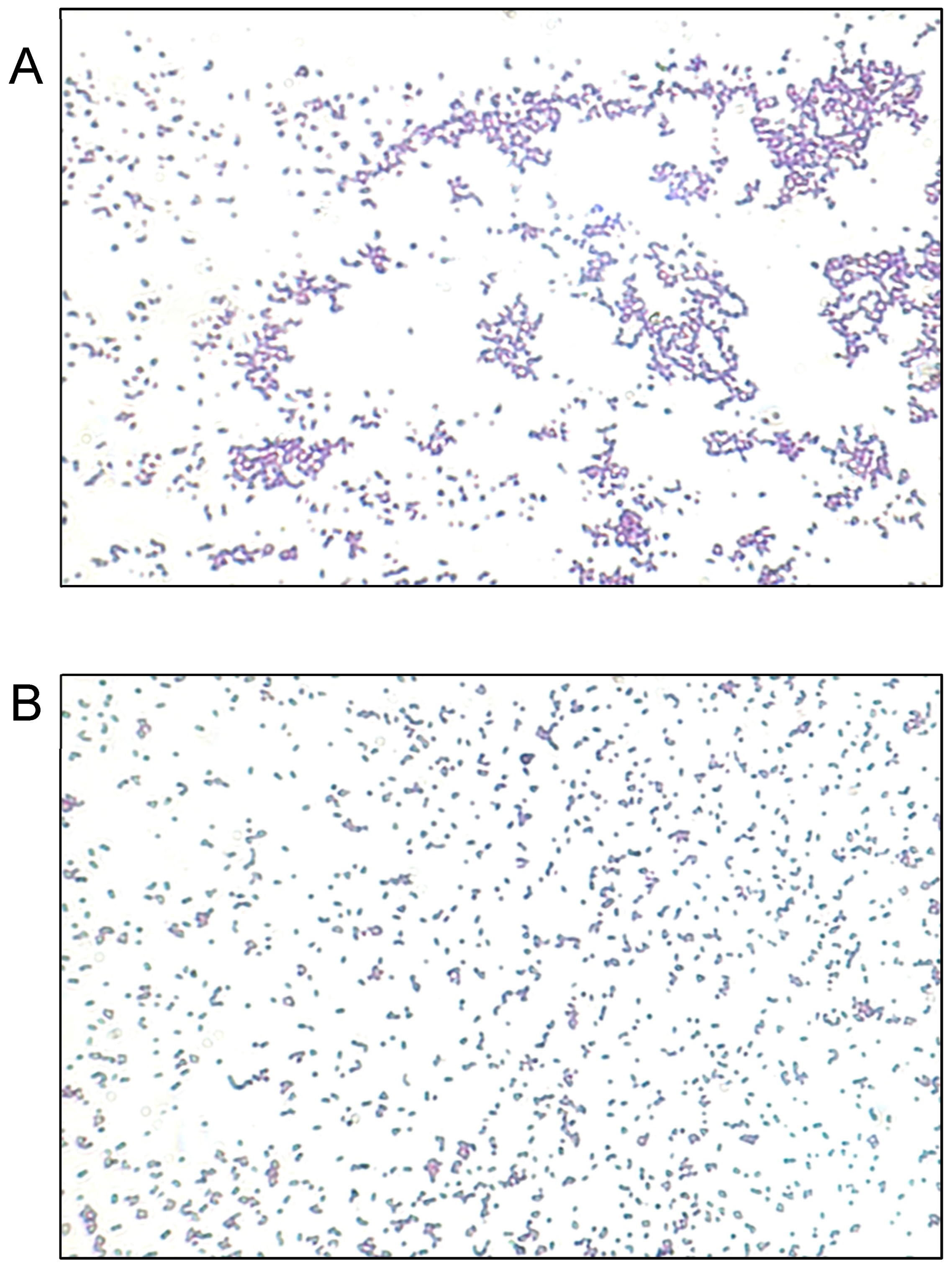
We evaluated the antimicrobial activity against the MRSA ATCC 43300 strain, starting at a concentration of 10 µg/mL of SAgNPs and with two-fold dilutions. The obtained results were consistent because the control with only the culture medium did not show turbidity (Figure 4A), while in the inoculated control, abundant growth was observed, as evidenced by the turbidity. The MIC determined in the test was 0.16 µg/mL, while the MBC (Figure 4B) was 0.31 µg/mL. Figure 5 represents an image of MRSA cells exposed to SAgNPs. A clear morphological change is observed after exposure to the treatment.
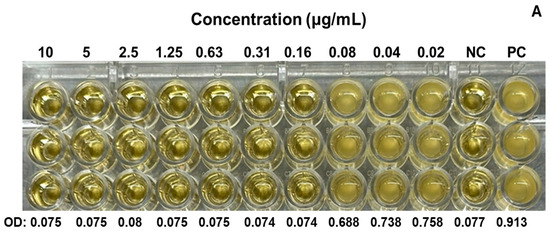
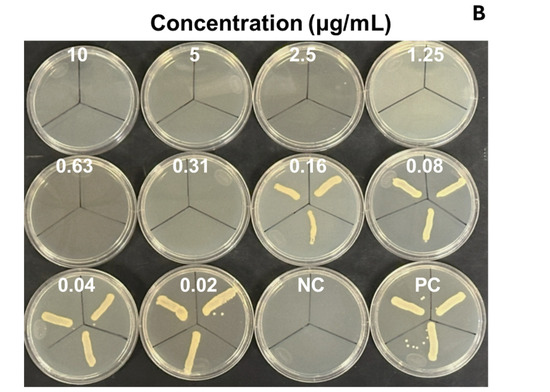
Figure 4.
Antimicrobial activity of SAgNPs for determination of MIC in microplate (A) and growth confirmation in agar plates, for determination of MBC (B). Mean optical density at 600 nm (OD) of triplicates. Negative control without microorganism (NC), positive control without sample (PC).
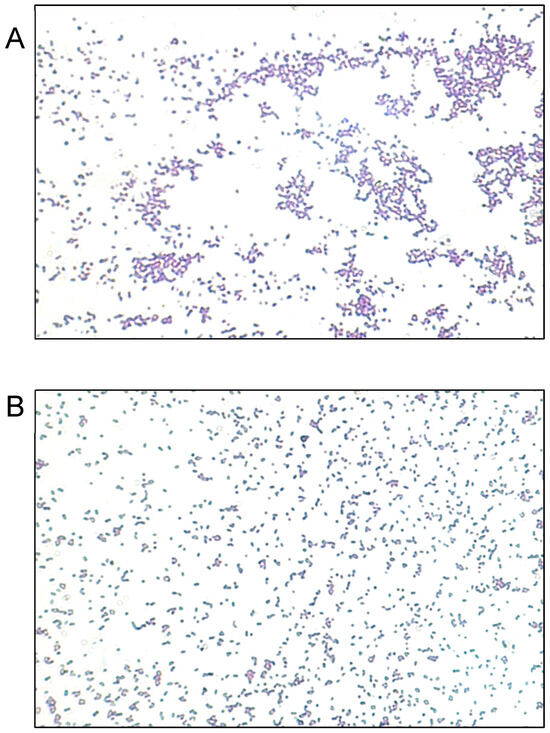
Figure 5.
Representative images of Gram staining of MRSA strain observed at 400×. (A) Untreated cells. (B) Cells exposed to SAgNPs.
2.3. Quantitative Antibiofilm Activity on Plates
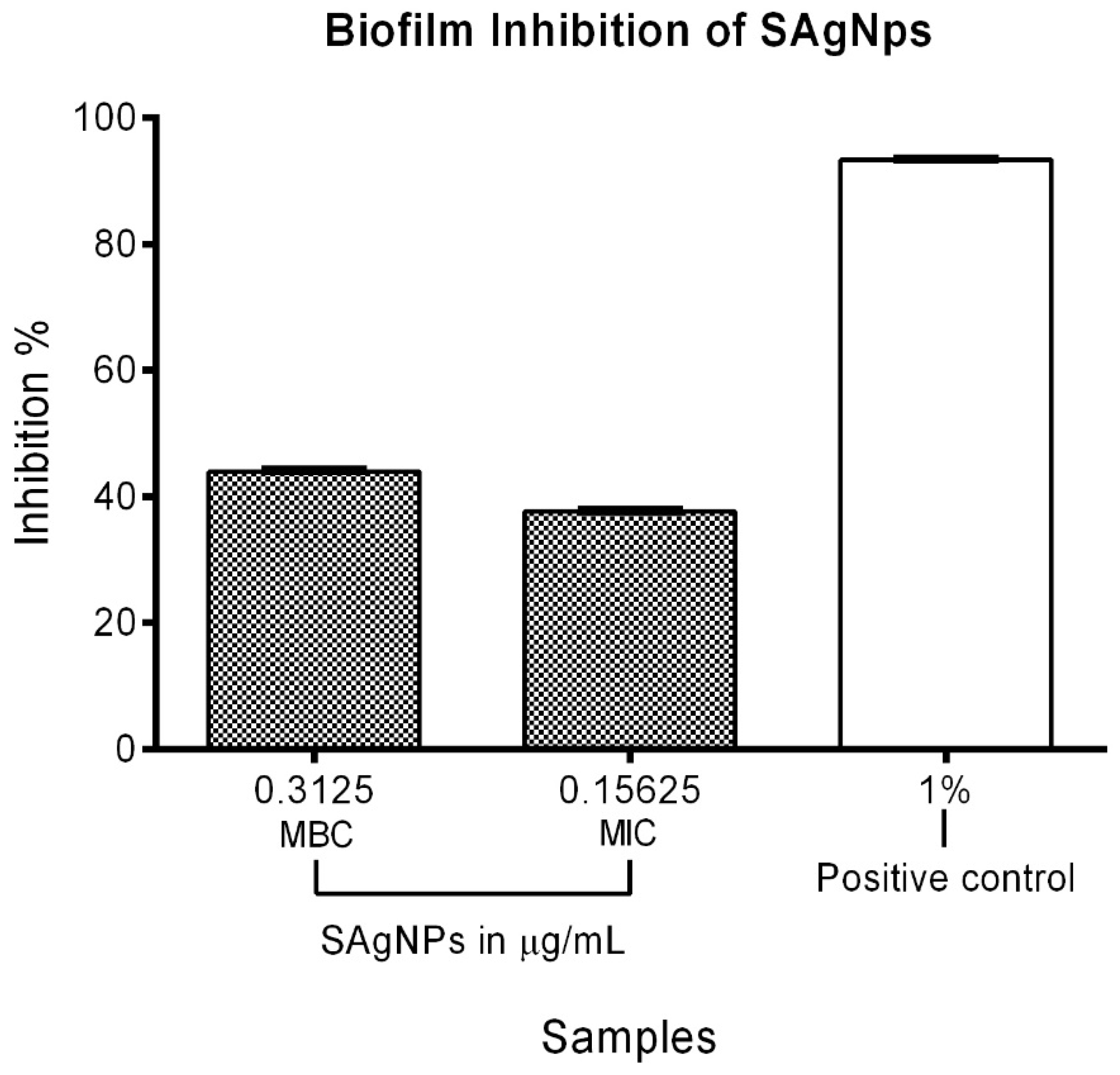
The MIC and MBC of the SAgNPs were used to evaluate their quantitative antibiofilm activity on MRSA. The highest concentration (0.31 µg/mL) showed a 43.96% inhibition, while the MIC (0.16 µg/mL) led to a 37.65% inhibition of biofilm formation. The positive control presented a value of 93.32%, as shown in Figure 6.
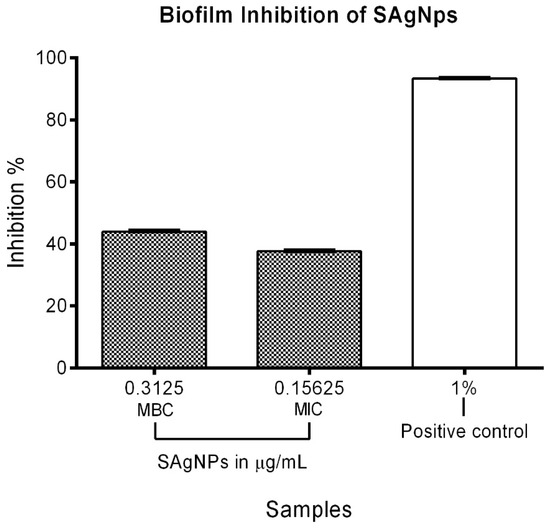
Figure 6.
Inhibitory effect of SAgNPs on MRSA biofilm formation using MIC and MBC.
2.4. Antibiofilm Activity in Congo Red Agar
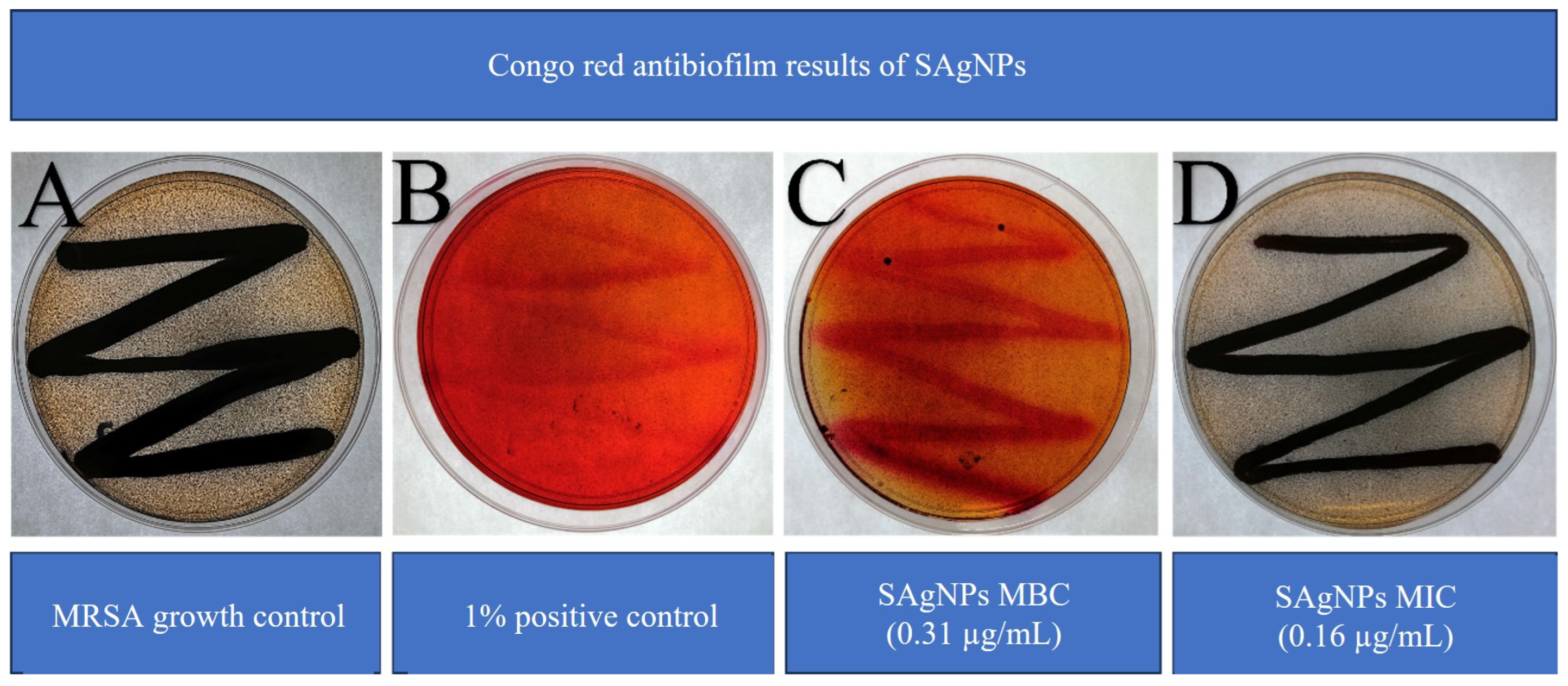
The formation of biofilms was verified by the presence of shiny three-dimensional black colonies due to the development of certain exopolysaccharides. A general color change from red to gray was also observed because, as part of the above-mentioned process, the biofilm-forming strain metabolized the Congo red in the medium. Figure 7 shows typical MRSA growth on Congo red agar (A) and the positive control (detergent 1%), which demonstrates effective biofilm inhibition formation when compared with the growth control (B). The SAgNPs show differences in terms of color and colony growth, with a strong red tone observed, without the presence of 3D colonies. For SAgNPs the MBC value inhibits the formation of biofilm (C), whereas the MIC value does not (D).
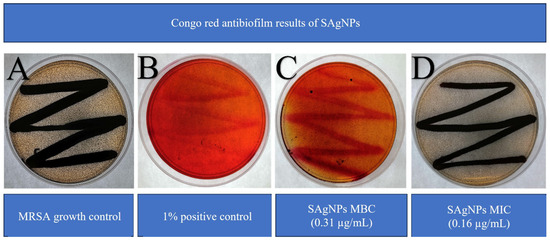
Figure 7.
Inhibition of biofilm formation of MRSA using SAgNPs: (A) MRSA growth control, (B) multi-enzyme detergent as positive control, (C) MBC, and (D) MIC of SAgNPs.
2.5. Molecular Docking Results
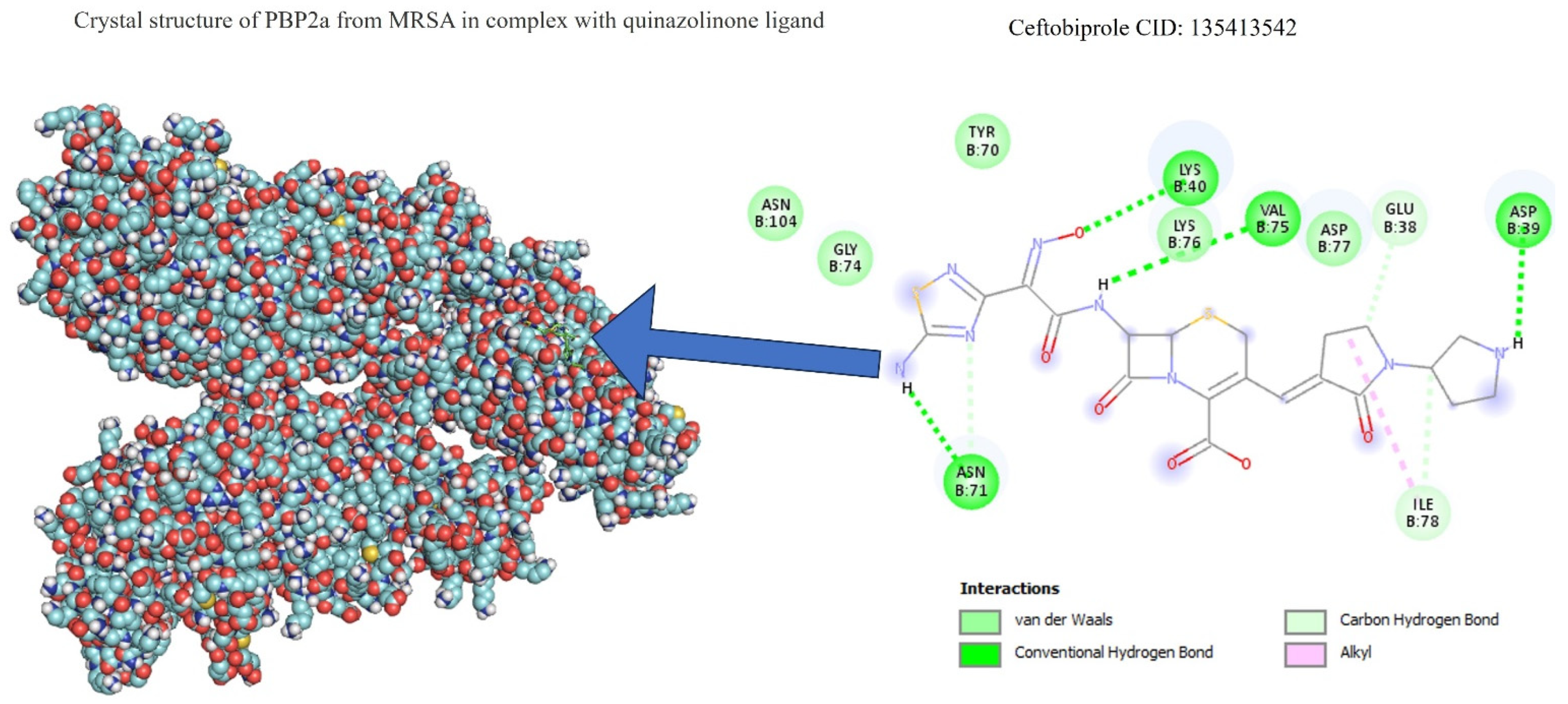
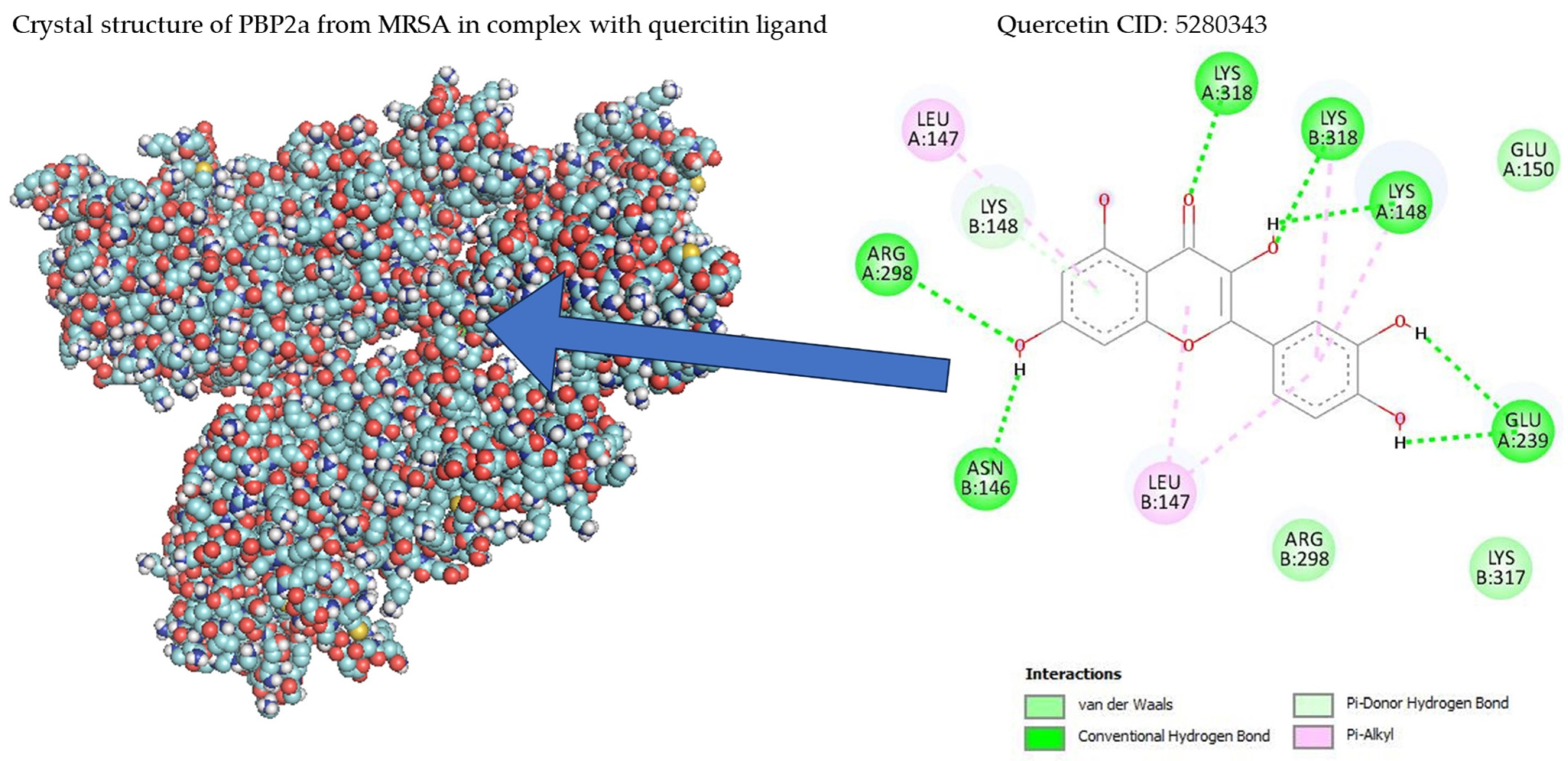
Table 1 shows the results concerning the binding energy and interacting amino acids for PBP2a of ceftobiprole and those of quercetin. Firstly, the binding energy of quercetin is slightly lower than that of ceftobiprole. Additionally, quercetin interacts with a greater number of amino acids (six) compared to the control compound (four), and forms more hydrogen bonds. In addition, we can see that the docking site is different in both cases; quercetin docks at most points on the A chain, while ceftobiprole binds at all points to the B chain.

Table 1.
Docking with PBP2a.
Figure 8 shows the PBP2a binding sites in the ceftobiprole structure, while Figure 9 shows all the quercetin interactions.
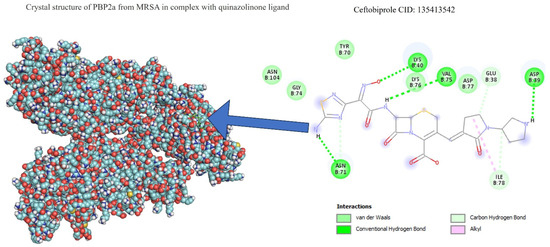
Figure 8.
PBP2a–ceftobiprole interactions.
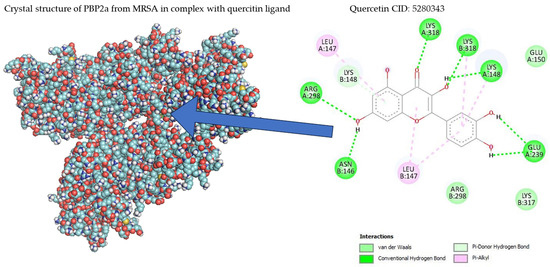
Figure 9.
PBP2a–quercetin interactions.
3. Discussion
In this work, the antimicrobial activity of SAgNPs was evaluated against the MRSA ATCC 43300 strain, and their ability to inhibit biofilm formation was demonstrated using both qualitative and quantitative assays. Docking analysis suggest a potential mechanism of action involving the interaction of quercetin and a penicillin-binding protein. These findings provide valuable insights for the development of novel antibiofilm strategies aimed at combating MRSA and other emerging multidrug-resistant pathogens posing significant threats to public health.
SAgNPs synthesis was possible via an environmentally friendly biological method using S. queretaroensis (also known as pitaya) peel. Since it is not edible, this peel is often discarded in the field, but it contains chemical compounds, such as saponins and flavonoids, that can act as silver nitrate reducing agents to obtain nanoparticles [13]. SAgNPs have previously been characterized [14] through different methods, such as dynamic light scattering, spectroscopy, X-ray diffraction (XRD), and microscopy, and the resulting particle shapes and sizes are similar to those reported by other authors [15].
Antibiotic resistance is a growing concern worldwide. MRSA infections are responsible for many deaths around the world; in 2019, there were 100,000 such deaths globally, more than 11,000 of which were reported in the United States [16]. Given this situation, the World Health Organization has placed MRSA on the list of bacteria for which new and efficient antibiotics are urgently needed [17]. Nanoparticles have been positioned as an alternative for controlling antibiotic-resistant bacteria due to their large surface area per volume, providing them with a broad spectrum of antimicrobial action [18].
The nanoparticle synthesis process is influenced by several factors, such as plant extract and silver nitrate concentrations, temperature, pH, and reaction time; therefore, it was necessary to conduct an experiment that allowed for optimal biosynthesis parameter determination. One of the most used methods for optimization is the Box–Behnken method, which allows interactions between different factors involved in nanoparticle synthesis to be established [19]. This analysis allowed us to confirm the optimal synthesis parameters: a concentration of 2 mM silver nitrate, a pH of 8, and a temperature of 90 °C.
The in vitro antimicrobial activity of the SAgNPs was first evaluated by determining their MIC and MBC, which were 0.16 and 0.31 µg/mL, respectively. These results show that these SAgNPs are more effective than other silver nanoparticles, for which MIC values between 8.12 and 11.25 µg/mL have been reported [20,21]. Different mechanisms of action have been proposed to explain the effect of silver nanoparticles, some of which involve alterations in the bacterial membrane, the production of reactive oxygen species, and some tyrosine phosphorylation, which alter cellular processes and destroy cells [22].
The MRSA strain antibiofilm activity results showed that SAgNPs are capable of inhibiting biofilm formation at concentrations corresponding to the MBC. In the quantitative plate test, an inhibition of close to 40% was observed, while in the qualitative test with Congo red agar, biofilm inhibition was evident. These results are in line with those reported by other authors who used bacterial biomass to synthesize silver nanoparticles that exhibited antibiofilm activity against Pseudomonas aeruginosa and Staphylococcus epidermidis during 24 h treatments [23]. Hunaiza and colleagues synthesized silver nanoparticles with Foeniculum vulgare extract and reported antibiofilm activity against clinical isolates of MRSA both in vitro and in an infected mouse skin model [24].
When comparing our results using a MIC of 0.16 µg/mL and an inhibition percentage of 37.65% with those reported by other authors, such as Barabadi and collaborators (2021), we can find that, at 2 µg/mL, they obtained a percentage inhibition of 62.86% [25]. In another study, by Ansari and collaborators, from 2021, the authors used lower concentrations of AgNPs (0.95 µg/mL), with an inhibition percentage very close to 60% [26]. Recently, in 2023, da-Cunha and collaborators determined the MIC (7.5 µg/mL) for MRSA antibiofilm activity and reported a percentage inhibition value of 88–90% [27]. Additionally, in 2020, Almatroudi and collaborators, who tested AgNPs in combination with ZnO, achieved a 10% inhibition at the lowest concentration of 3.41 µg/mL [28]. Finally, Shahba and collaborators proved that silver nanoparticles at 10 µg/mL inhibited 71.99% of bacteria growth [29].
In all the above-mentioned cases, the concentration used for biofilm inhibition was greater than that tested in the present study, despite better inhibition values. These differences may be due to slight variations in methodology, such as the wavelength used in this study (570 nm) compared to that used by Ansari (595 nm), as well as differences in the medium used (Müller–Hinton broth, trypticasein soy broth) and in the percentage at which crystal violet was used, affecting AgNP effectiveness [24,26]. On the other hand, the changes in medium coloration, as well as in the observable colony characteristics in the qualitative biofilm test, are similar to those observed by Ansari and collaborators in 2015 [21].
These changes in the amount of biofilm produced may be due to the decrease in the expression of the ica genes, as has already been suggested by authors such as Moulavi and collaborators in 2019, who were able to relate silver nanoparticle antibiofilm activity to decreased ica A and ica R gene expression [30]. These genes could be measured in a future investigation of SagNPs, since they are involved in biofilm production and the pathogenesis of MRSA infection. The use of PCR for detecting ica genes, the use of laser scanning analysis with a focal electron microscope (CLSM), and scanning electron microscopy (SEM) are all important in being able to observe SAgNPs’ adhesion to films and their interactions with bacteria.
Some studies suggest that the main mechanism of biofilm destruction occurs through binding in the exopolysaccharide matrix, or their suppressive effect on the expression of related genes [31].
In this work, the inhibition of biofilm formation was not observed at the MIC; other studies have shown biofilm inhibition at concentrations equal to and lower than the MIC [32]. However, the MBC of SAgNPs did inhibit the formation of MRSA biofilm.
In addition, the mechanism of action of SAgNPs could be more closely associated with bacterial cell mortality prior to biofilm establishment, rather than following biofilm maturation, since in antibiofilm activity tests, the period necessary for film formation is 24 to 48 h; therefore, the MIC is not useful for biofilm inhibition. To inhibit the apparent and incomplete growth of the strain, after 24 or 48 h, greater bacterial growth would be expected even at the MIC, which would reduce the percentage of biofilm inhibition in the qualitative tests and perhaps affect the results of the quantitative tests [11,24]. A cell viability assay could be conducted in future work, since this study focused on the quantity and quality of MRSA biofilm.
There are many mechanisms associated with biofilm inhibition by nanoparticles, such as the presence of enzymes produced in the MRSA membrane that help anchor secreted proteins in order to promote bacterial adhesion, such as sortase A. Other mechanisms have also been proposed in the literature. Authors have noted that interactions between size and shape can facilitate bacterial entry into biofilms and allow agents to act directly on the strain through other mechanisms, such as changes in surface chemistry, charge, hydrophobicity, and decreased ica gene expression [24,33]. Therefore, in our study, we showed that SAgNPs inhibit the growth of MRSA prior to biofilm formation.
Regarding docking analyses, the bacterial cell wall, which is composed of peptidoglycans, is mainly responsible for bacterial survival, and the reticular structure of the cell wall (an important part of its function) is made up of penicillin-binding proteins (PBPs), which is why antibiotics acting against PBPs have been sought [34]. S. aureus has four such proteins (PBP1-PBP4), and MRSA produces a different PBP (PBP2a), which is the cause of its antibiotic resistance. Appropriate binding site access for this protein has been studied previously; ten active sites and one allosteric site were identified in PBP2a [35,36]. Within these 10 action sites, the amino acid residue SER403 was identified as specifically preventing antibiotic resistance in this bacterium. The results showed that none of the amino acid interactions for ceftobiprole or quercetin generated strong hydrogen bonds with the SER403 residue. When matching the amino acid residues in the 10 identified sites of action, none were found. Despite this, there were similarities in the binding sites identified as allosteric at amino acid residues ASN146 and LYS148, as well as proximity to amino acids LYS316 and LYS319 at the allosteric site with LYS318, with PBP2a bound to quercetin [36]. Furthermore, as shown in another study with alpha-mangostin, both exhibit a polar interaction at LYS148 and another interaction at GLU239 [37].
In the case of ceftobiprole, no similarities were found with the study by Tabassum et al., 2023 [36]. The latter evaluated 284 antimicrobial phytochemicals in silico, their most repeated binding site, and the resulting coupling energy [36].
Although it has been shown in other studies that ceftobiprole binds to the amino acids LYS406, SER403, GLU447, ASN464, GLU602, and SER462 [38], of which SER403 is one of the main ones responsible for resistance in MRSA and is found at the primary binding site [36], its coupling with and functioning on PBP2a can be seen to occur since a binding energy value is observed at −6.66 (in our work). This is not far from the value obtained for the same antibiotic of −8.8 in the study by Mutie and collaborators, in 2025, that evaluated ceftobiprole as a control against modeled pharmacophores [39], in which coupling did not occur at the same binding site, nor with the same interacting amino acids, as Rani and collaborators mentioned in their study from 2014 [38]. The above suggests that, although the interaction does not occur at the same specific binding site, the binding energy and the hydrogen bonds formed are valuable for the comparison of the results obtained for our molecule of interest, in this case quercetin, present in nanoparticles. It has also been seen that ceftobiprole, even without acting directly on PBP2a, can bind to other membrane proteins such as PBP2, which is why future studies could review the molecular coupling of quercetin present in AgNPs in normal PBP2 [40].
Overall, while the application of silver nanoparticles in biomedical fields holds significant potential for advancing medical technologies, it is essential to acknowledge and address key limitations, including issues related to the stability and scalability of their production. Particular concerns also pertain to their interactions within biological systems, potential cytotoxicity to human cells, and possible environmental repercussions [41].
4. Materials and Methods
4.1. Nanoparticle Synthesis and Optimization
SAgNP synthesis was carried out using a biological method previously established by our research group [12]. In brief, an aqueous extract was prepared with 1g of dried S. queretaroensis peel in 100 mL distilled water, kept boiling for 5 min, and filtered.
Biosynthesis was carried out by mixing the peel extract, adjusted to pH 8, with 2 mM silver nitrate at a proportion of 1/20 and a temperature of 90 °C for 30 min; subsequently, the SAgNPs were centrifuged at 15,000 rpm for 20 min and the supernatant was discarded; finally, the solution was dried at 90 °C for 24 h. The nanoparticles were refrigerated at 4 °C and protected from light until use. For the analyses, nanoparticles were prepared at the time of testing. It is worth mentioning that Baqer et al., in 2024, showed that flavonoids like quercetin can be obtained from different plant sources despite the polarity of the solvent, including water [42].
A Box–Behnken response surface design was used to evaluate the selected parameters of the biosynthesis of SAgNPs, including 15 combinations and a center point of 3 repetitions [13]. For the design, the absorbance at which the highest peak was obtained in the initial synthesis was taken as the response variable. It was measured in a microplate spectrophotometer (xMark, Bio-Rad, Hercules, CA, USA). The pH of the extract (2 to 8), the concentration of AgNO3 (1 to 2 mM) and the temperature (60 to 90 °C) were used as experimental factors for designing the mixtures. Using Statgraphics software version XVI, the response surface design yielded 15 combinations of experimental factors that were tested and evaluated, with 1 combination exhibiting the best theoretical absorbance. Once the synthesis process was complete, the optimal conditions were tested and compared with the absorbance curve to validate the results.
4.2. Nanoparticle Characteristics
As reported previously by Padilla-Camberos, the SAgNPs utilized in this study exhibited a maximum absorbance at a wavelength of 420 nm; the presence of OH groups related to the reduction of silver was previously reported, evidenced by a band at 3450 cm−1 with Fourier Transform Infrared Spectroscopy (FTIR), the presence of silver and silver chloride was evidenced by XRD, a roughly spherical and uniform shape was determined by transmission electron microscopy (TEM), and an average size of 48.8 nm was determined by dynamic light scattering (DLS) [14]. Regarding biocompatibility, both in vitro and in vivo studies on SAgNPs have reported no significant toxicological effects, indicating their low risk to biological systems [43]. The characteristics of SAgNPs are summarized in Table 2.

Table 2.
Physicochemical characteristics of nanoparticles.
4.3. Antimicrobial Activity
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MRSA ATCC 43300 strain was acquired from the American Type Culture Collection Prior to testing; the strain was reactivated in Müller–Hinton broth at 37 °C at 200 rpm for 24 h, after which a microbial suspension was made by adjusting the bacterial concentration to 0.5 McFarland standard (108 colony-forming units per milliliter). The microdilution method was used in the range of 10 to 0.91 µg/mL of SAgNPs in Müller–Hinton broth within a multi-well plate. All the wells were inoculated with a 1% volume of the MRSA strain; the plate was then incubated for 24 h at 37 °C. Control wells contained medium with or without inoculum. The MIC was determined at the lowest concentration where no microbial-growth-induced turbidity was observed and spectrophotometrically confirmed at 600 nm, while the MBC was determined by plating a sample from each well on Müller–Hinton agar plates [44].
4.4. Antibiofilm Activity
4.4.1. Quantitative Antibiofilm Activity
The quantitative antibiofilm activity was evaluated using the crystal violet assay with slight modifications [45,46]. Briefly, 100 µL of SAgNPs at final concentrations of 0.16 and 0.31 µg/mL were added in triplicate to a 96-well microplate (Costar 3590) and mixed with nutrient broth. Subsequently, 20 µL of MRSA suspension was added to each well. The above-mentioned concentrations correspond to the MIC (0.16 µg/mL) and MBC (0.31 µg/mL) concentrations of SAgNPs determined in the present study. The plates were subsequently incubated at 37 °C for 48 h. After incubation, the wells were washed 3 times with phosphate-buffered saline (PBS) at pH 7.2 to eliminate all non-adherent cells. The plates were subsequently dried at 60 °C after 100 µL of 0.1% crystal violet was added for staining. The plate was left for 90 min at room temperature, after which the remaining dye was removed with two more washes with PBS. The biofilm formed was dissolved in 100 µL of 96% ethanol solution per well. After this, the plate was shaken at 180 rpm for 10 min and the absorbance at 570 nm was measured with a spectrophotometer (xMark, Bio-Rad, Hercules, CA, USA). The percentage of biofilm inhibition was calculated using the following formula:
where AM and AC are the absorbances with and without treatment, respectively. Multi-enzymatic detergent (1% w/v) and nutrient broth (growth control) were used for the positive control.
4.4.2. Qualitative Antibiofilm Activity in Agar
We determined the qualitative antibiofilm activity using the Congo red method previously described, with adjustments [23,47]. For this purpose, brain–heart infusion broth (BHI) was prepared, with the addition of 0.08% w/v Congo red, 5% w/v sucrose, 1.5% w/v sodium chloride, and 2% w/v glucose. The medium was mixed and distributed in 50 mL tubes (20 mL per tube). Subsequently, the MIC (0.16 µg/mL) and MBC (0.31 µg/mL) of the SAgNPs (determined in the present study) were added, along with 1% multi-enzymatic detergent as a positive control. Sample-less medium was used as a negative control. The above-mentioned medium was added to 90 × 15 mm sterile plates (Sym laboratorios), seeded with a sterile swab of the MRSA suspension, and incubated for 72 h at 37 °C.
After incubation, the growth of the strain and changes in colony color and morphology in the positive and negative controls were evaluated. The presence of shiny, three-dimensional black and brown colonies was interpreted as a positive result for biofilm formation, indicating exopolysaccharide production by the strains. In contrast, the appearance of dark or light red colonies was considered indicative of negative biofilm formation [21].
4.5. Molecular Docking
In Silico Study
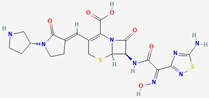
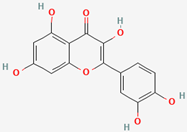
PBP2a is a penicillin-binding protein encoded by the mecA gene. It confers methicillin resistance to MRSA (S. aureus ATCC 43300), which is why it is a direct target in the development of new antimicrobial agents [48]. One of the major phenolic compounds present in S. queretaroensis is quercetin. Notably, the redox properties of such a compound enable it to disrupt oxygen balance, chelate metal ions, and exhibit other bioactive properties [49]. Ceftobiprole is a broad-spectrum cephalosporin used specifically against MRSA due to its effective binding to PBP2a, which prevents cell wall formation in this resistant strain [50]. Therefore, it was used as a positive control in the test. The selected docking compounds (ligands) were obtained from PubChem (pubchem.ncbi.nlm.nih.gov accessed on 21 February 2024 and 23 April 2025) (see Table 3). The crystal structure of the methicillin-resistant S. aureus PBP2a protein in a quinazolinone complex (PDB:4CJN) was obtained from the Protein Data Bank (PDB) (www.rcsb.org). Autodock Tools software (version 1.5.7) was used to remove water, ligands/inhibitors, and Kollman charges from the protein. Gasteiger charges and rotatable bonds were added to the ligands; nonpolar hydrogens were then added before converting the protein and ligand files to pdbqt. Autodock (version 4.2) was used to perform molecular docking with the ligands. The interacting amino acids, the type of bonds, and the distances, in Å, were visualized with the Biovia Discovery Studio 2021 Client (version 21.1.0.20298) [51].

Table 3.
Ligands.
5. Conclusions
In the present work, silver nanoparticle biosynthesis was achieved using S. queretaroensis peel extract. The process was optimized to achieve the maximum number of nanoparticles. The SAgNPs showed antimicrobial activity against MRSA bacteria, with bacteriostatic and bactericidal effects. We evaluated the antibiofilm activity of the SAgNPs using both qualitative and quantitative methods. We conducted an in silico analysis of the antimicrobial effect of SAgNPs on MRSA bacteria by predicting the molecular binding of the main chemical components of the S. queretaroensis peel. The novelty of this research lies in our utilization of a waste material for nanoparticle synthesis, which could offer an eco-friendly and cost-effective alternative to conventional methods. It is suggested to expand the tests with other multidrug-resistant microorganisms. The results of our study show that SAgNPs can be used as antimicrobial agents in the manufacture of medical devices and in the formulation of sanitizers for medical facilities. In conclusion, we advise evaluating SAgNP stability and studying possible interactions with the genes involved in MRSA biofilm formation.
Author Contributions
Conception, E.P.-C. and I.M.S.-H.; experimental design, O.R.T.-G., I.M.S.-H. and A.A.V.-A.; measurements and manuscript composition, E.P.-C. and A.S.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Dodds, D.R. Antibiotic resistance: A current epilogue. Biochem. Pharmacol. 2017, 134, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.; Waugh, C.; Perry, M.R.; Nair, H. Global disease burden due to antibiotic resistance—state of the evidence. J. Glob. Health 2016, 6, 010306. [Google Scholar] [CrossRef]
- Silva, V.; Almeida, L.; Gaio, V.; Cerca, N.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Biofilm formation of multidrug-resistant MRSA strains isolated from different types of human infections. Pathogens 2021, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Asadipour, E.; Asgari, M.; Mousavi, P.; Piri-Gharaghie, T.; Ghajari, G.; Mirzaie, A. Nano-biotechnology and challenges of drug delivery system in cancer treatment pathway. Chem. Biodivers. 2023, 20, e202201072. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Das, P.; Ghosh, S.; Nayak, B. Phyto-fabricated nanoparticles and their anti-biofilm activity: Progress and current status. Front. Nanotechnol. 2021, 3, 739286. [Google Scholar] [CrossRef]
- Hosseini, M.; Shapouri Moghaddam, A.; Derakhshan, S.; Hashemipour, S.M.A.; Hadadi-Fishani, M.; Pirouzi, A.; Khaledi, A. Correlation between biofilm formation and antibiotic resistance in MRSA and MSSA isolated from clinical samples in Iran: A systematic review and meta-analysis. Microb. Drug Resist. 2020, 26, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Camberos, E.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Ramirez-Rodriguez, P.; Diaz, E.; Wille, H.; Flores-Fernandez, J.M. Biosynthesis of silver nanoparticles using Stenocereus queretaroensis fruit peel extract: Study of antimicrobial activity. Materials 2021, 14, 4543. [Google Scholar] [CrossRef] [PubMed]
- Kleijnen, J.P. Response surface methodology for constrained simulation optimization: An overview. Simul. Model. Pract. Theory 2008, 16, 50–64. [Google Scholar] [CrossRef]
- González-Garibay, A.S.; Vallejo-Cardona, A.A.; Villarreal-Amézquita, A.A.; Sánchez-Hernández, I.M.; Torres-González, O.R.; Padilla-Camberos, E. The In Vitro Cytotoxic Potential of Biosynthesized Silver Nanoparticles in MIA PaCa-2 Cells Supported with an In Silico Study. Inorganics 2024, 12, 317. [Google Scholar] [CrossRef]
- Do, B.L.; Bui, T.H.; Ho, T.G.-T.; Duong, N.L.; Nguyen, V.M.; Dang-Bao, T.; Nguyen, T.; Phuong, P.H. Green synthesis of nanosilver and its antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Saudi Chem. Soc. 2023, 27, 101722. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Tasak, N. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Gong, Y.; Yan, X.; Wang, L.; Zheng, W.; Zhao, Y. Recent advances in nanoantibiotics against multidrug-resistant bacteria. Nanoscale Adv. 2023, 5, 6278–6317. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Javed, M.N.; Alam, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Beg, S. Purple heart plant leaves extract-mediated silver nanoparticle synthesis: Optimization by Box–Behnken design. Mater. Sci. Eng. C 2019, 99, 1105–1114. [Google Scholar] [CrossRef]
- Salah, R.; Karmy, M.; Abdelraouf, A.; Kotb, S. Evaluation of the bactericidal effect of silver nanoparticles against methicillin resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains isolated from mastitic milk of small ruminants and their surrounding environment in Aswan, Egypt. J. Vet. Med. Res. 2020, 27, 143–151. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Alzohairy, M.A. Anti-biofilm efficacy of silver nanoparticles against MRSA and MRSE isolated from wounds in a tertiary care hospital. Indian J. Med. Microbiol. 2015, 33, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Masimen, M.A.A.; Harun, N.A.; Maulidiani, M.; Ismail, W.I.W. Overcoming methicillin-resistance Staphylococcus aureus (MRSA) using antimicrobial peptides-silver nanoparticles. Antibiotics 2022, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Hunaiza, F.; Hamdani, S.D.A.; Ahmed, M.; Rajput, T.A.; Gul, A.; Amir, R.; Babar, M.M. Anti-MRSA potential of biogenic silver nanoparticles synthesized from hydroponically grown Foeniculum vulgare. Phytomedicine Plus 2023, 3, 100415. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Ansari, M.A.; Kalam, A.; Al-Sehemi, A.G.; Alomary, M.N.; AlYahya, S.; Aziz, M.K.; Srivastava, S.; Alghamdi, S.; Akhtar, S.; Almalki, H.D.; et al. Counteraction of biofilm formation and antimicrobial potential of Terminalia catappa functionalized silver nanoparticles against Candida albicans and multidrug-resistant gram-negative and gram-positive bacteria. Antibiotics 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, K.F.; Oliveira Garcia, M.; Allend, S.O.; de Albernaz, D.F.T.; Panagio, L.A.; Neto, A.C.P.S.; Oliveira, T.L.; Hartwig, D.D. Biogenic silver nanoparticles: In vitro activity against Staphylococcus aureus methicillin-resistant (MRSA) and multidrug-resistant coagulase-negative Staphylococcus (CoNS). Braz. J. Microbiol. 2023, 54, 2641–2650. [Google Scholar] [CrossRef]
- Almatroudi, A.; Khadri, H.; Azam, M.; Rahmani, A.H.; Al Khaleefah, F.K.; Khateef, R.; Allemailem, K.S. Antibacterial, antibiofilm and anticancer activity of biologically synthesized silver nanoparticles using seed extract of Nigella sativa. Processes 2020, 8, 388. [Google Scholar] [CrossRef]
- Shahba, D.S.A.; Shalaby, M.M.; Ezzand, H.A.; Hussein, M.Z. Inhibitory effect of silver nanoparticles on biofilm production by methicillin resistant staphylococci. Egypt. J. Med. Microbiol. 2017, 26, 49–54. [Google Scholar] [CrossRef]
- Moulavi, P.; Noorbazargan, H.; Dolatabadi, A.; Foroohimanjili, F.; Tavakoli, Z.; Mirzazadeh, S.; Ashrafi, F. Antibiofilm effect of green engineered silver nanoparticles fabricated from Artemisia scoporia extract on the expression of icaA and icaR genes against multidrug-resistant Staphylococcus aureus. J. Basic Microbiol. 2019, 59, 701–712. [Google Scholar] [CrossRef]
- de Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; de Fátima Ramos dos Santos Medeiros, S.M.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Thappeta, K.R.V.; Zhao, L.N.; Nge, C.E.; Crasta, S.; Leong, C.Y.; Ng, V.; Ng, S.B. In-silico identified new natural sortase inhibitors disrupt S. aureus biofilm formation. Int. J. Mol. Sci. 2020, 21, 8601. [Google Scholar] [CrossRef] [PubMed]
- Ambade, S.S.; Gupta, V.K.; Bhole, R.P.; Khedekar, P.B.; Chikhale, R.V. A review on five and six-membered heterocyclic compounds targeting the penicillin-binding protein 2 (PBP2A) of Methicillin-resistant Staphylococcus aureus (MRSA). Molecules 2023, 28, 7008. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Strynadka, N. Structural basis for the β lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Mol. Biol. 2022, 9, 870–876. [Google Scholar] [CrossRef]
- Tabassum, R.; Kousar, S.; Mustafa, G.; Jamil, A.; Attique, S.A. In Silico Method for the Screening of Phytochemicals against methicillin-resistant Staphylococcus aureus. BioMed Res. Int. 2023, 2023, 5100400. [Google Scholar] [CrossRef]
- Anwer, R. Identification of Small Molecule Inhibitors of Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus for the Therapeutics of Bacterial Infection: Inhibitors of PBP2a of Methicillin-Resistant S. aureus. Cell Mol. Biol. 2024, 70, 40–47. [Google Scholar] [CrossRef]
- Rani, N.; Vijayakumar, S.; Velan, L.P.T.; Arunachalam, A. Quercetin 3-O-rutinoside mediated inhibition of PBP2a: Computational and experimental evidence to its anti-MRSA activity. Mol. Biosyst. 2014, 10, 3229–3237. [Google Scholar] [CrossRef]
- Mutie Musila, F.; Gitau, G.W.; Amwayi, P.W.; Kingoo, J.M.; Kinyanyi, D.B.; Njeru, P.N. Pharmacophore modeling, 2D-QSAR, Molecular Docking and ADME studies for the discovery of inhibitors of PBP2a in MRSA. BioRxiv 2025, 2, 636556. [Google Scholar] [CrossRef]
- Alwan, S.M. Computational Calculations of Molecular Properties and Molecular Docking of New and Reference Cephalosporins on Penicillin Binding Proteins and Various β-Lactamases. J. Pharm. Pharmacol. 2016, 4, 212–225. [Google Scholar] [CrossRef][Green Version]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Baqer, S.H.; Al-Younis, Z.K.; Al-Shawi, S.G. Extracting Quercetin from Different Plant Sources, Purifying It Using Different Extraction Methods (Chemical, Physical, and Enzymatic), and Measuring Its Antioxidant Activity. Front. Biosci. (Elite Ed.) 2024, 16, 35. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Juárez-Navarro, K.J.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Flores-Fernandez, J.M. Toxicological Evaluation of Silver Nanoparticles Synthesized with Peel Extract of Stenocereus queretaroensis. Materials 2022, 15, 5700. [Google Scholar] [CrossRef]
- Merghni, A.; Lassoued, M.A.; Noumi, E.; Hadj Lajimi, R.; Adnan, M.; Mastouri, M.; Snoussi, M. Cytotoxic activity and antibiofilm efficacy of biosynthesized silver nanoparticles against methicillin-resistant Staphylococcus aureus strains colonizing cell phones. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 9410024. [Google Scholar] [CrossRef]
- Rahim, K.A.A.A.; Mohamed, A.M.A. Bactericidal and antibiotic synergistic effect of nanosilver against methicillin-resistant Staphylococcus aureus. Jundishapur J. Microbiol. 2015, 8, e25867. [Google Scholar] [CrossRef]
- dos Santos, E.M.P.; Martins, C.C.B.; de Oliveira Santos, J.V.; da Silva, W.R.C.; Silva, S.B.C.; Pelagio-Flores, M.A.; Galembeck, A.; Cavalcanti, I.M.F. Silver nanoparticles-chitosan composites activity against resistant bacteria: Tolerance and biofilm inhibition. J. Nanopart. Res. 2021, 23, 196. [Google Scholar] [CrossRef]
- Kaiser, T.D.L.; Pereira, E.M.; Dos Santos, K.R.N.; Maciel, E.L.N.; Schuenck, R.P.; Nunes, A.P.F. Modification of the congo red agar method to detect biofilm production by Staphylococcus epidermidis. Diagn. Microbiol. Infect. 2013, 75, 235–239. [Google Scholar] [CrossRef]
- Qiu, X.; Janson, C.A.; Smith, W.W.; Green, S.M.; McDevitt, P.; Johanson, K.; Jarvest, R.L. Crystal structure of Staphylococcus aureus tyrosyl-tRNA synthetase in complex with a class of potent and specific inhibitors. Protein Sci. 2001, 10, 2008–2016. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juárez-Onofre, J.E.; Carvajal-Millán, E.; López-Ahumada, G.A.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A.; Rodríguez-Félix, F. Effect of ultrafiltration of pitaya Extract (Stenocereus thurberi) on its phytochemical content, antioxidant capacity, and UPLC-DAD-MS profile. Molecules 2020, 25, 281. [Google Scholar] [CrossRef]
- Morosini, M.I.; Díez-Aguilar, M.; Cantón, R. Mechanisms of action and antimicrobial activity of ceftobiprole. Rev. Esp. Quimioter. 2019, 32, 3–10. [Google Scholar]
- Neupane, N.P.; Kushwaha, A.K.; Karn, A.K.; Khalilullah, H.; Khan, M.M.U.; Kaushik, A.; Verma, A. Antibacterial efficacy of biofabricated silver nanoparticles of aerial part of Moringa oleifera lam: Rapid green synthesis, in-vitro and in-silico screening. Biocatal. Agric. Biotechnol. 2022, 39, 102229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).