Synthesis, Structure, and Luminescence Properties of Zinc(II) Complex with a Spacer-Armed Tetradentate N2O2-Donor Schiff Base
Abstract
1. Introduction
2. Results
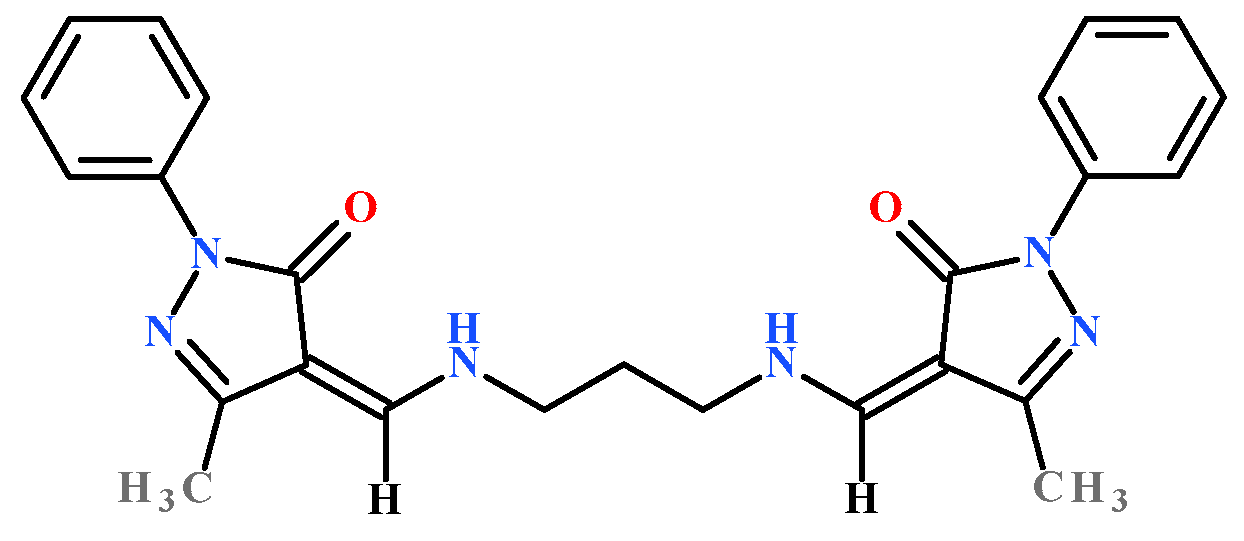
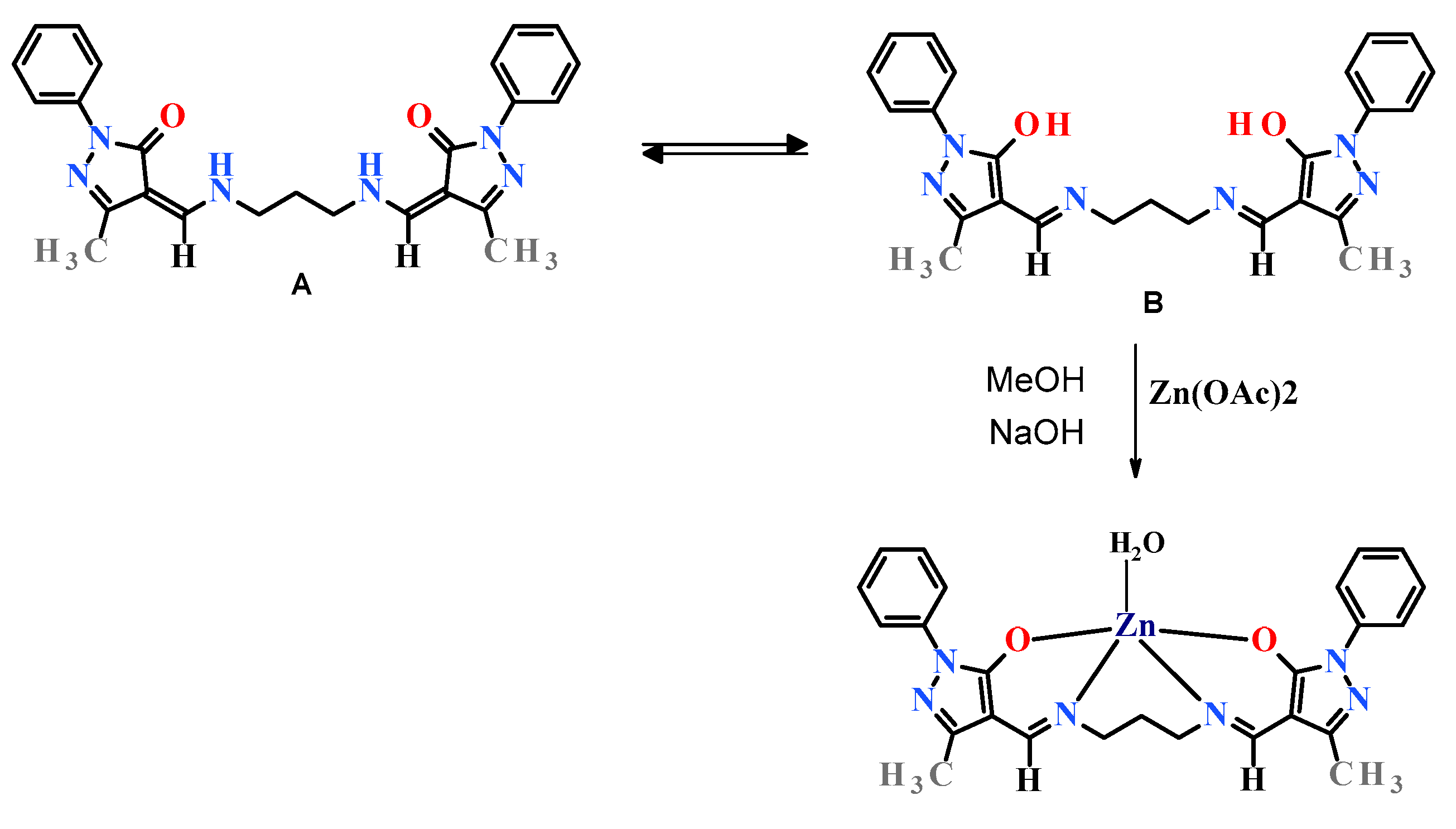
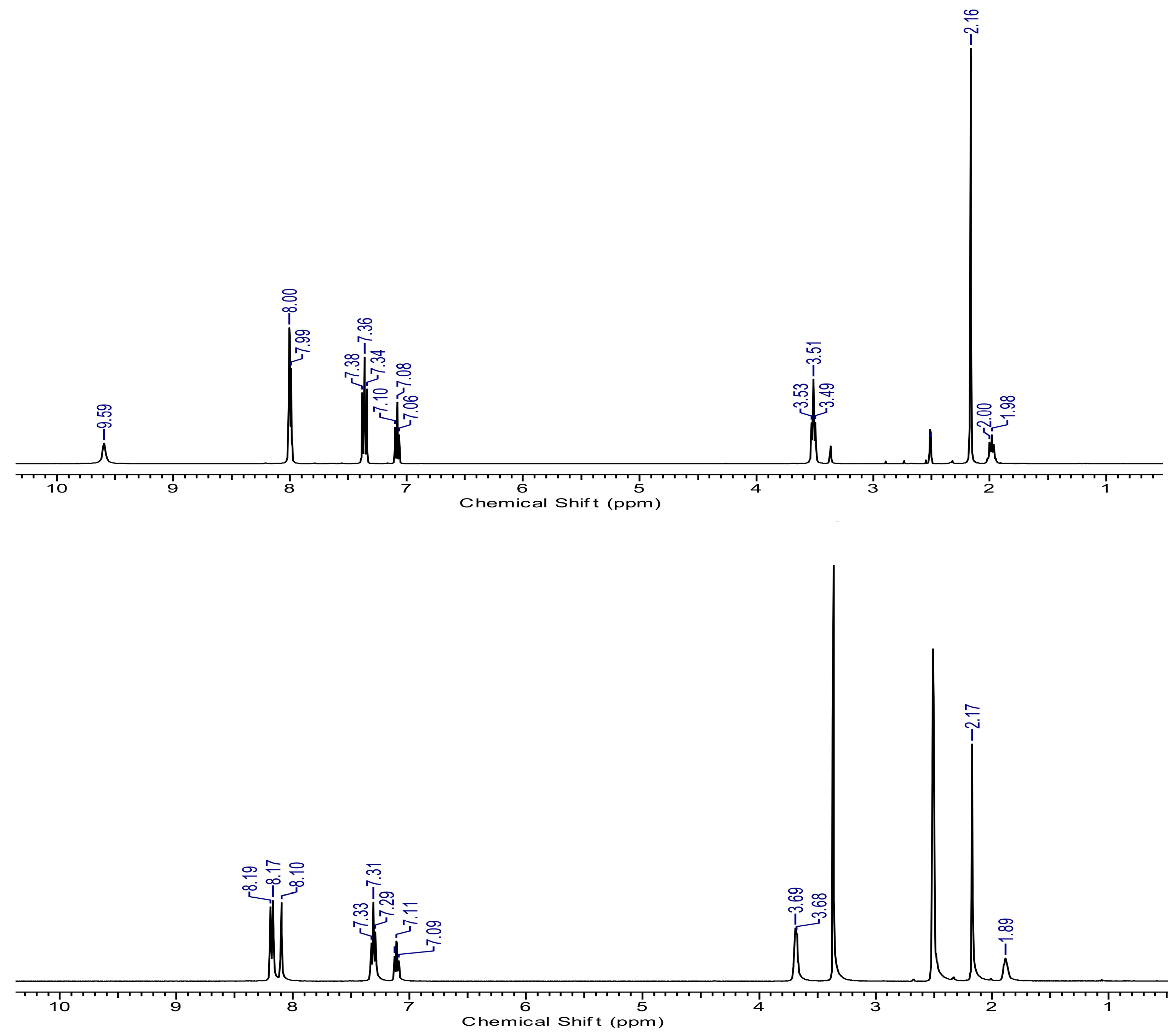
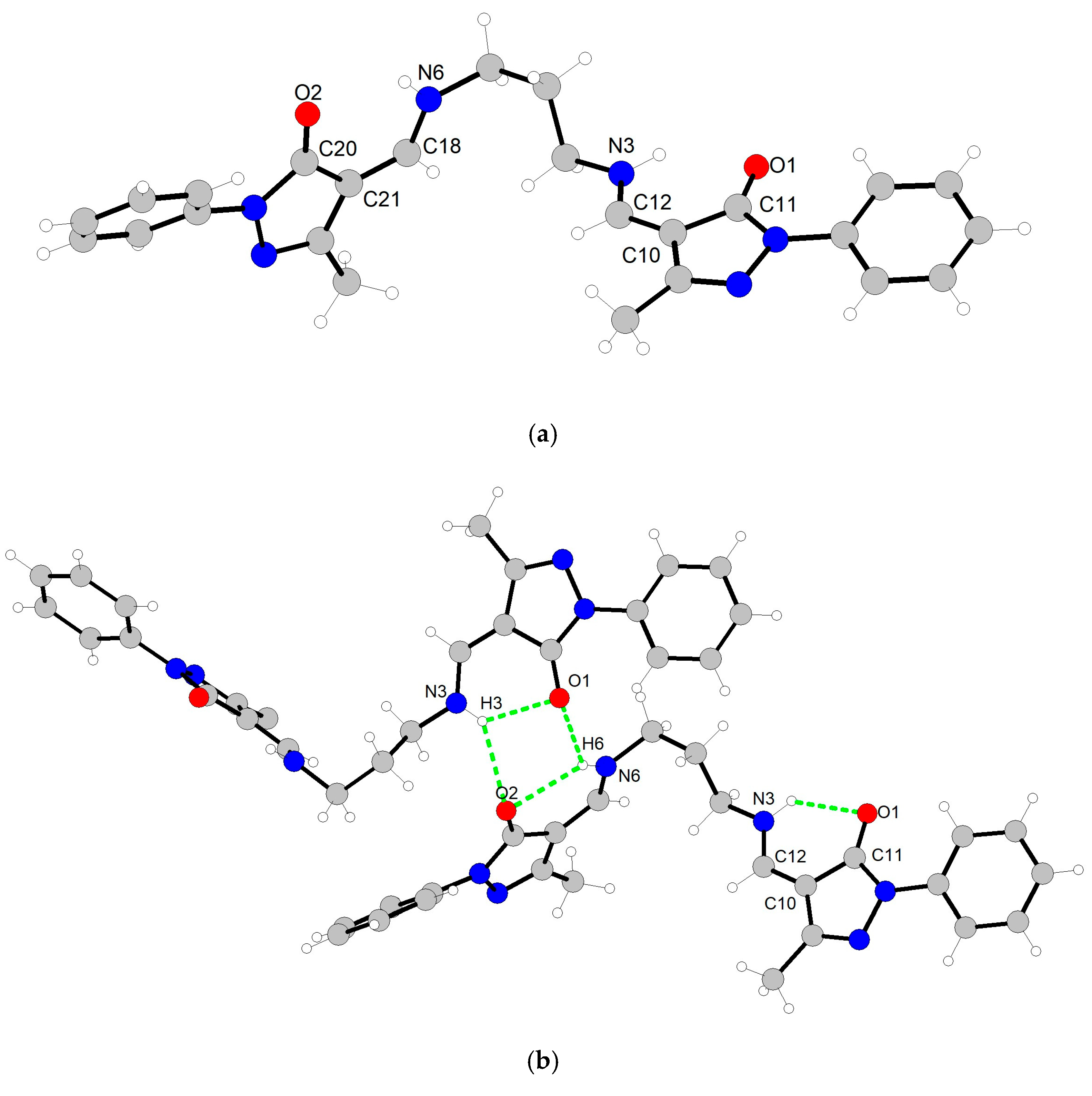
2.1. Structure of Ligand and Zinc Complex
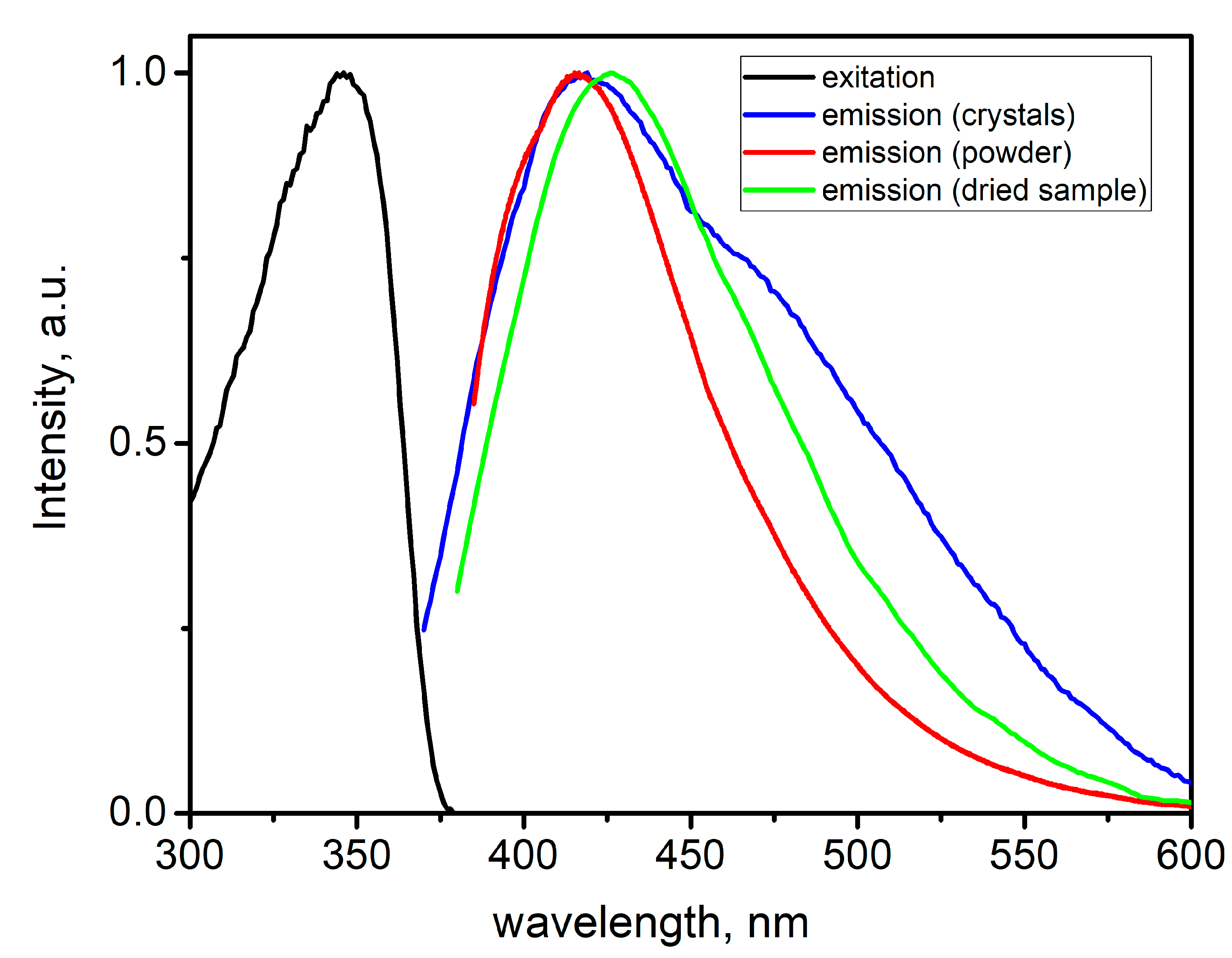
2.2. Photophysical Properties of Zinc Complex
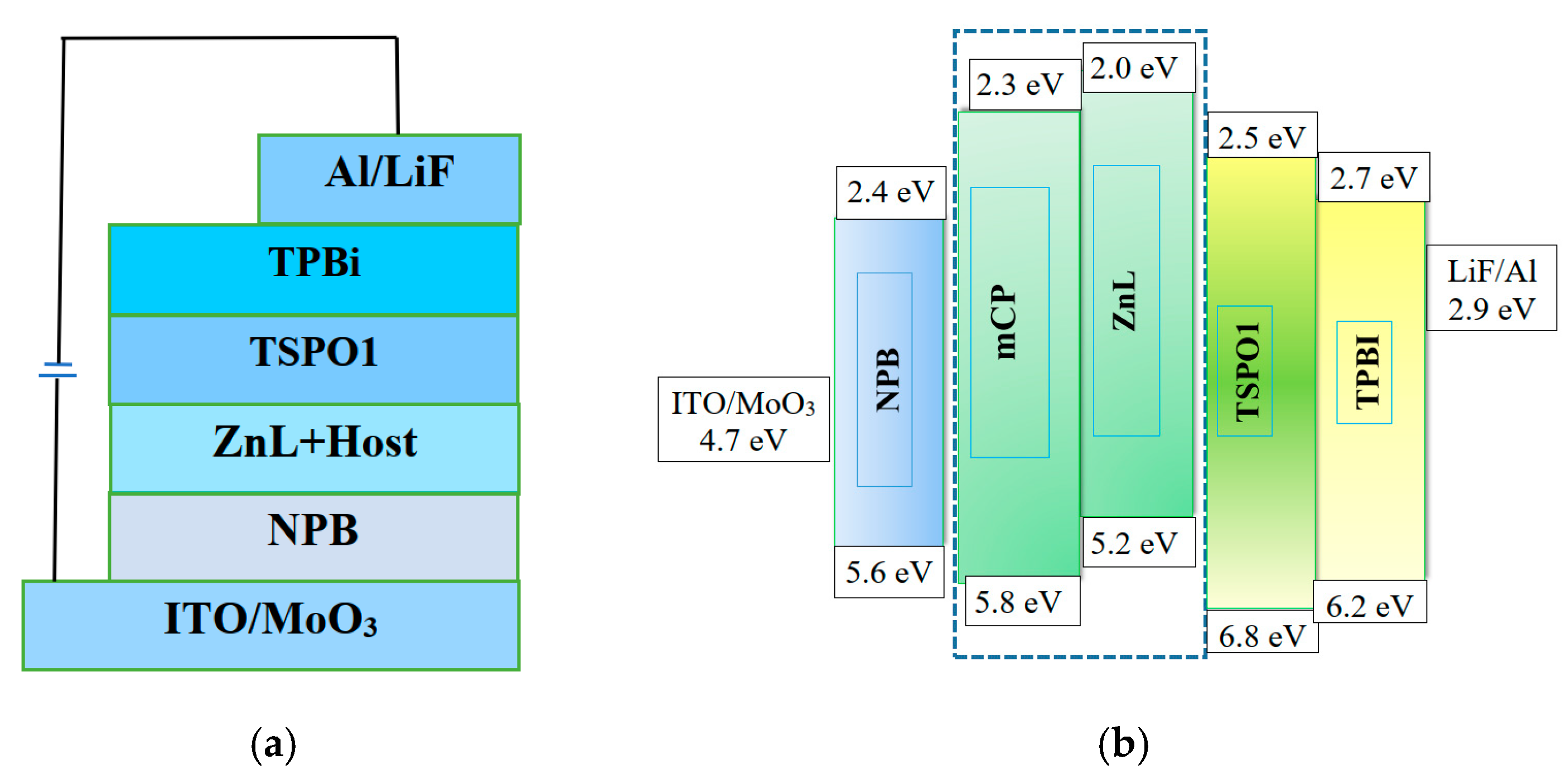
2.3. Electroluminescence Performance
3. Materials and Methods
OLED Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayak, D.; Bilash, R. Choudhary, A survey of the structure, fabrication, and characterization of advanced organic light emitting diodes. Microelectron. Reliab. 2023, 144, 114959. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S.; Hong, G.; Gan, X.; Leonhardt, C.; et al. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, 2005630. [Google Scholar] [CrossRef] [PubMed]
- Tankeleviciūte, E.; Samuel, I.D.W.; Zysman-Colman, E. The Blue Problem: OLED Stability and Degradation Mechanisms. J. Phys. Chem. Lett. 2024, 15, 1034–1047. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, C.-H.; Lee, P.-H.; Lin, H.-Y.; Leung, M.; Chiu, T.-L.; Lin, C.-F. Blue organic light-emitting diodes: Current status, challenges, and future outlook. J. Mater. Chem. C 2019, 7, 5874–5888. [Google Scholar] [CrossRef]
- Xia, S.C.; Kwong, R.C.; Adamovich, V.I.; Weaver, M.S.; Brown, J.J. OLED Device Operational Lifetime: Insights and Challenges. In Proceedings of the 2007 IEEE International Reliability Physics Symposium Proceedings. 45th Annual, Phoenix, AZ, USA, 15–19 April 2007; pp. 253–257. [Google Scholar]
- Iwasaki, H.; Majima, Y.; Izawa, S. Low-voltage turn-on in blue organic light-emitting diodes. Synth. Met. 2024, 309, 117772. [Google Scholar] [CrossRef]
- Marques dos Santos, J.; Hall, D.; Basumatary, B.; Bryden, M.; Chen, D.; Choudhary, P.; Comerford, T.; Crovini, E.; Danos, A.; De, J.; et al. The Golden Age of Thermally Activated Delayed Fluorescence Materials: Design and Exploitation. Chem. Rev. 2024, 124, 13736–14110. [Google Scholar] [CrossRef]
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv. Mater. 2014, 26, 7931–7958. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thanikachalam, V.; Thilagavathy, S. Phosphorescent organic light-emitting devices: Iridium based emitter materials—An overview. Coord. Chem. Rev. 2023, 483, 215100. [Google Scholar] [CrossRef]
- Tao, P.; Lü, X.; Zhou, G.; Wong, W.-Y. Asymmetric Tris-Heteroleptic Cyclometalated Phosphorescent Iridium(III) Complexes: An Emerging Class of Metallophosphors. Acc. Mater. Res. 2022, 3, 830–842. [Google Scholar] [CrossRef]
- Gao, H.; Qian, M.; Yang, X.; Ma, S.; Li, H. Recent advances of the cyclometalated iridium(III) complexes for electrochemiluminescence sensing. Dye. Pigment. 2025, 232, 112493. [Google Scholar] [CrossRef]
- Williams, J.A.G.; Develay, S.; Rochester, D.L.; Murphy, L. Optimising the luminescence of platinum (II) complexes and their application in organic light emitting devices (OLEDs). Coord. Chem. Rev. 2008, 252, 2596–2611. [Google Scholar]
- Tang, M.-C.; Chan, A.K.-W.; Chan, M.-Y.; Yam, V.W.-W. Platinum and Gold Complexes for OLEDs. Top. Curr. Chem. 2016, 374, 46. [Google Scholar] [CrossRef] [PubMed]
- Giobbio, G.; Costa, R.D.; Gaillard, S. Earth Abundant Transition Metals Complexes in Light-emitting Electrochemical Cells: Successes, Challenges and Perspectives. Dalton Trans. 2025, 54, 3573–3580. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Gusev, A.N.; Kiskin, M.A.; Braga, E.V.; Kryukova, M.A.; Baryshnikov, G.V.; Karaush-Karmazin, N.N.; Minaeva, V.A.; Minaev, B.F.; Ivaniuk, K.; Stakhira, P.; et al. Schiff Base Zinc(II) Complexes as Promising Emitters for Blue Organic Light-Emitting Diodes. ACS Appl. Electron. Mater. 2021, 3, 3436–3444. [Google Scholar] [CrossRef]
- Gusev, A.N.; Kiskin, M.A.; Braga, E.V.; Chapran, M.; Wiosna-Salyga, G.; Baryshnikov, G.V.; Minaeva, V.A.; Minaev, B.F.; Ivaniuk, K.; Stakhira, P.; et al. Novel Zinc Complex with an Ethylenediamine Schiff Base for High-Luminance Blue Fluorescent OLED Applications. J. Phys. Chem. C 2019, 123, 11850–11859. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Milutka, M.S.; Koshchienko, Y.V.; Makarova, N.I.; Lazarenko, V.A.; Trigub, A.L.; Kolodina, A.A.; Zubenko, A.A.; Metelitsa, A.V.; et al. Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted N-[2-(Phenyliminomethyl)phenyl]-4-methylbenzenesulfamide and Their Zinc(II) Complexes. Int. J. Mol. Sci. 2022, 23, 15259. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Milutka, M.S.; Koshchienko, Y.V.; Lazarenko, V.A.; Trigub, A.L.; Kolodina, A.A.; Zubenko, A.A.; Braga, E.V.; Gusev, A.N.; et al. Zinc Complexes of Fluorosubstituted N-[2-(Phenyliminomethyl)phenyl]-4-methylbenzenesulfamides: Synthesis, Structure, Luminescent Properties, and Biological Activity. Materials 2024, 17, 438. [Google Scholar] [CrossRef]
- Gusev, A.; Shul’gin, V.; Braga, E.; Zamnius, E.; Kryukova, M.; Linert, W. Luminescent properties of Zn complexes based on tetradentate N2O2-donor pyrazolone schiff bases. Dye. Pigm. 2020, 183, 108626–108632. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, R.; Pettinari, C. Recent advances in acylpyrazolone metal complexes and their potential applications. Coord. Chem. Rev. 2015, 303, 1–31. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(ii) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(ii) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Olennikov, V.E.; Zvereva, V.V.; Kriventsov, V.V.; Konchenko, S.N.; Sukhikh, T.S. Molecular Switches Guided by a Reversible Access to Room-Temperature Phosphorescence and ESIPT Fluorescence. Inorg. Chem. 2025, 64, 6964–6976. [Google Scholar] [CrossRef]
- Massue, J.; Jacquemin, D.; Ulrich, G. Molecular Engineering of Excited-state Intramolecular Proton Transfer (ESIPT) Dual and Triple Emitters. Chem. Lett. 2018, 47, 1083–1089. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef]
- Maillard, J.; Klehs, K.; Rumble, C.; Vauthey, E.; Heilemann, M.; Fürstenberg, A. Universal quenching of common fluorescent probes by water and alcohols. Chem. Sci. 2021, 12, 1352–1362. [Google Scholar] [CrossRef]
- Rashamuse, T.J.; Mohlala, R.L.; Coyanis, E.M.; Magwa, N.P. A Review: Blue Fluorescent Zinc (II) Complexes for OLEDs—A Last Five-Year Recap. Molecules 2023, 28, 5272. [Google Scholar] [CrossRef]
- Kumar, D.; Justin Thomas, K.R.; Ching-Chiao, L.; Joue, J.H. Molecule-based monochromatic and polychromatic OLEDs with wet-process feasibility. Chem. Asian J. 2013, 8, 2111–2124. [Google Scholar] [CrossRef]
- Mondal, A.; Paterson, L.; Kun-Han Lin, J.C.; van der Zee, B.; Wetzelaer, G.-J.A.H.; Stankevych, A.; Vakhnin, A.; Kim, J.-J.; Kadashchuk, A.; Blom Falk May, P.W.M.; et al. Molecular library of OLED host materials—Evaluating the multiscale simulation workflow. Chem. Phys. Rev. 2021, 2, 031304. [Google Scholar] [CrossRef]
- Kempegowda, R.M.; Malavalli, M.K.; Malimath, G.H.; Naik, L.; Manjappa, K.B. Synthesis and Photophysical Properties of Multi-Functional Bisimidazolyl Phenol Zinc (II) Complex: Application in OLED, Anti-Counterfeiting and Latent Finger Print Detection. ChemistrySelect 2021, 6, 3033–3039. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Empirical Absorption Correction Program; Bruker AXS Inc.: Madiso, WI, USA, 1997; p. 27. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]





| Compounds | Turn-On Voltage (V) | Luminance (cd/m2) | CIE Coordinates | CE (cd/A) | EQE (%) |
|---|---|---|---|---|---|
| A | 4.2 | 5430 | 0.1552, 0.1405 | 12.4 | 4 |
| B | 4.4 | 4320 | 0.1578, 0.1540 | 8.9 | 3.1 |
| C | 5.2 | 1020 | 0.1532, 0.2306 | 3.8 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusev, A.; Braga, E.; Mamontov, K.; Kiskin, M.; Linert, W. Synthesis, Structure, and Luminescence Properties of Zinc(II) Complex with a Spacer-Armed Tetradentate N2O2-Donor Schiff Base. Inorganics 2025, 13, 173. https://doi.org/10.3390/inorganics13050173
Gusev A, Braga E, Mamontov K, Kiskin M, Linert W. Synthesis, Structure, and Luminescence Properties of Zinc(II) Complex with a Spacer-Armed Tetradentate N2O2-Donor Schiff Base. Inorganics. 2025; 13(5):173. https://doi.org/10.3390/inorganics13050173
Chicago/Turabian StyleGusev, Alexey, Elena Braga, Kirill Mamontov, Mikhail Kiskin, and Wolfgang Linert. 2025. "Synthesis, Structure, and Luminescence Properties of Zinc(II) Complex with a Spacer-Armed Tetradentate N2O2-Donor Schiff Base" Inorganics 13, no. 5: 173. https://doi.org/10.3390/inorganics13050173
APA StyleGusev, A., Braga, E., Mamontov, K., Kiskin, M., & Linert, W. (2025). Synthesis, Structure, and Luminescence Properties of Zinc(II) Complex with a Spacer-Armed Tetradentate N2O2-Donor Schiff Base. Inorganics, 13(5), 173. https://doi.org/10.3390/inorganics13050173










