Homo- and Hetero-Multinuclear Iridium(III) Complexes with Cytotoxic Activity
Abstract
1. Introduction
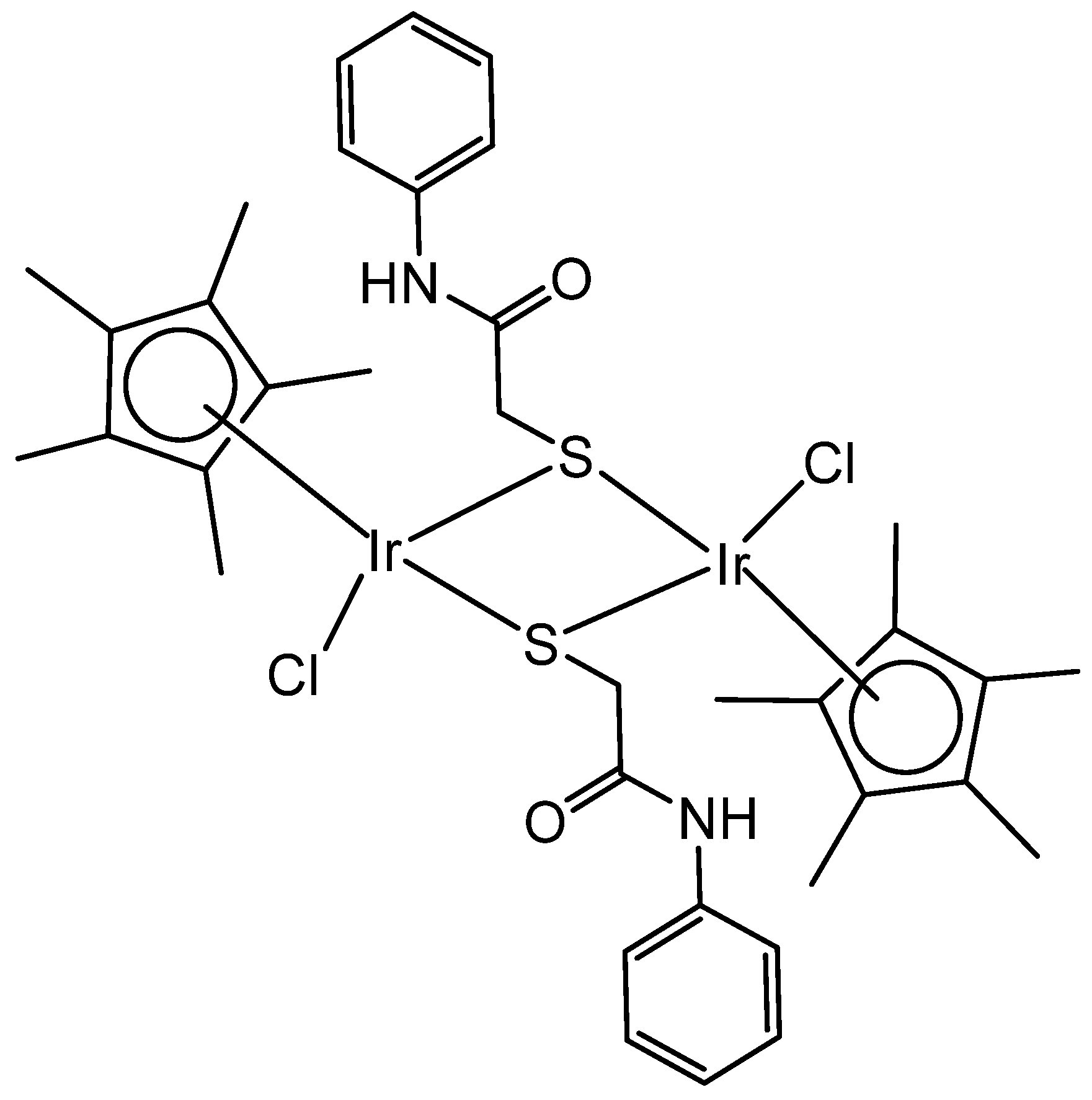
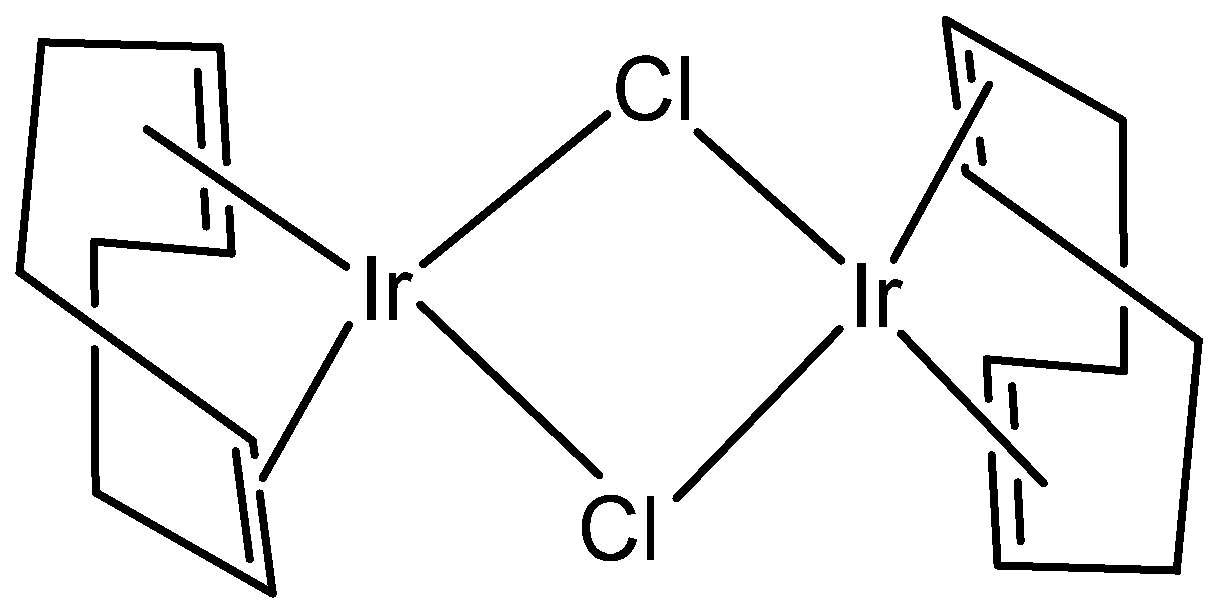
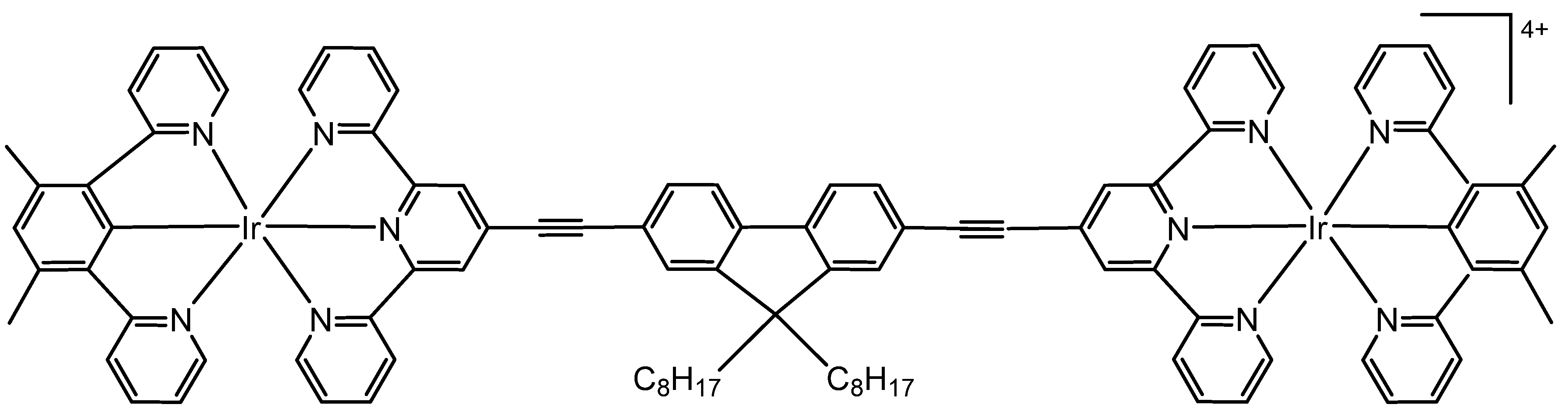
2. Homonuclear Iridium(III) Complexes
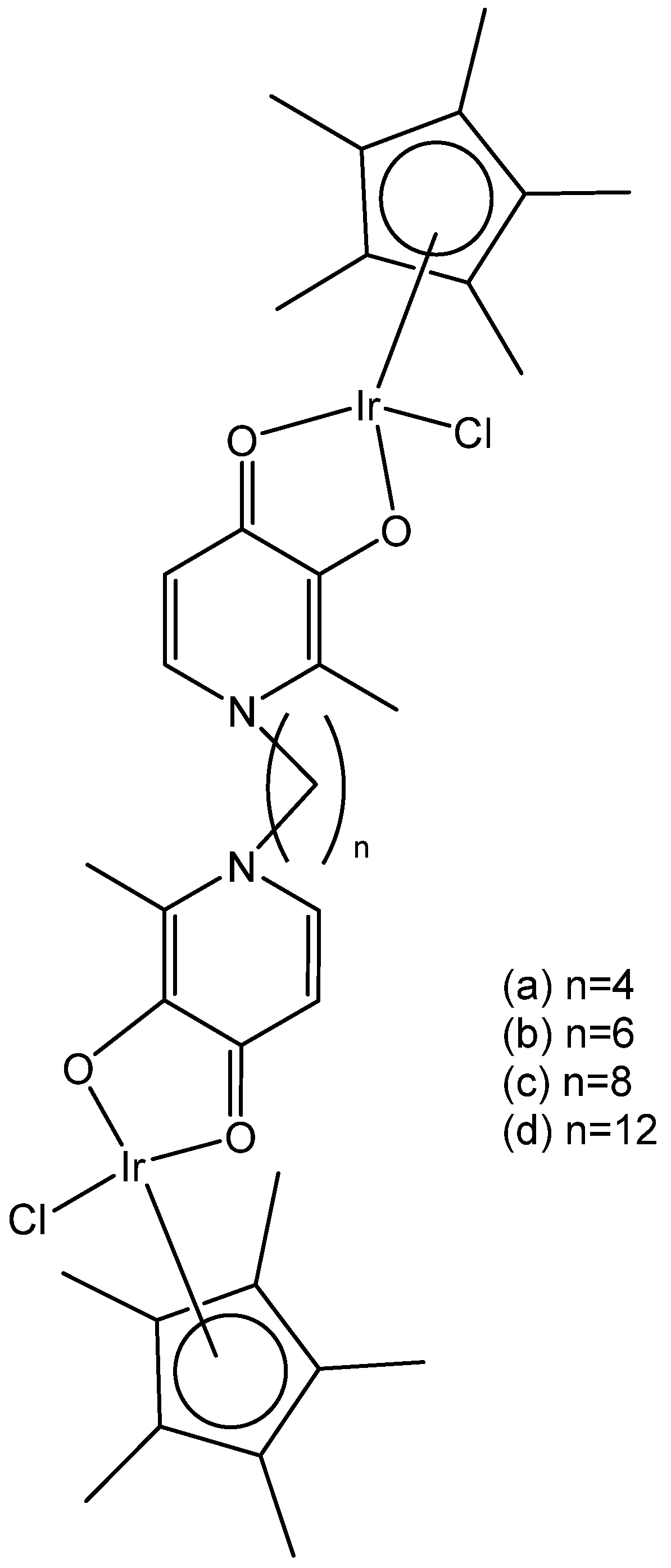
2.1. Iridium(III) Cyclopentadienyl Dinuclear Complexes
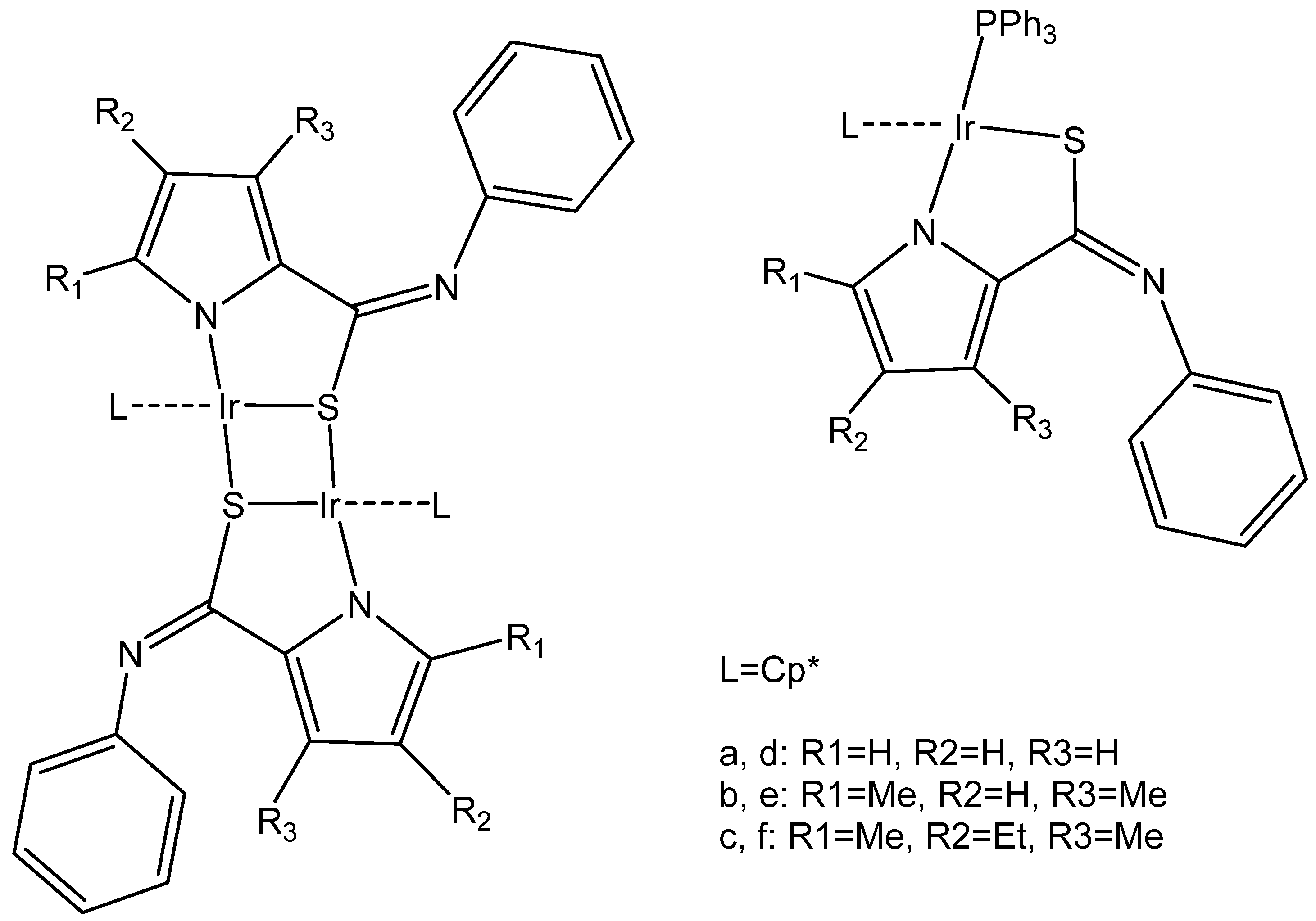
2.2. Iridium Cyclometalated Dinuclear Complexes
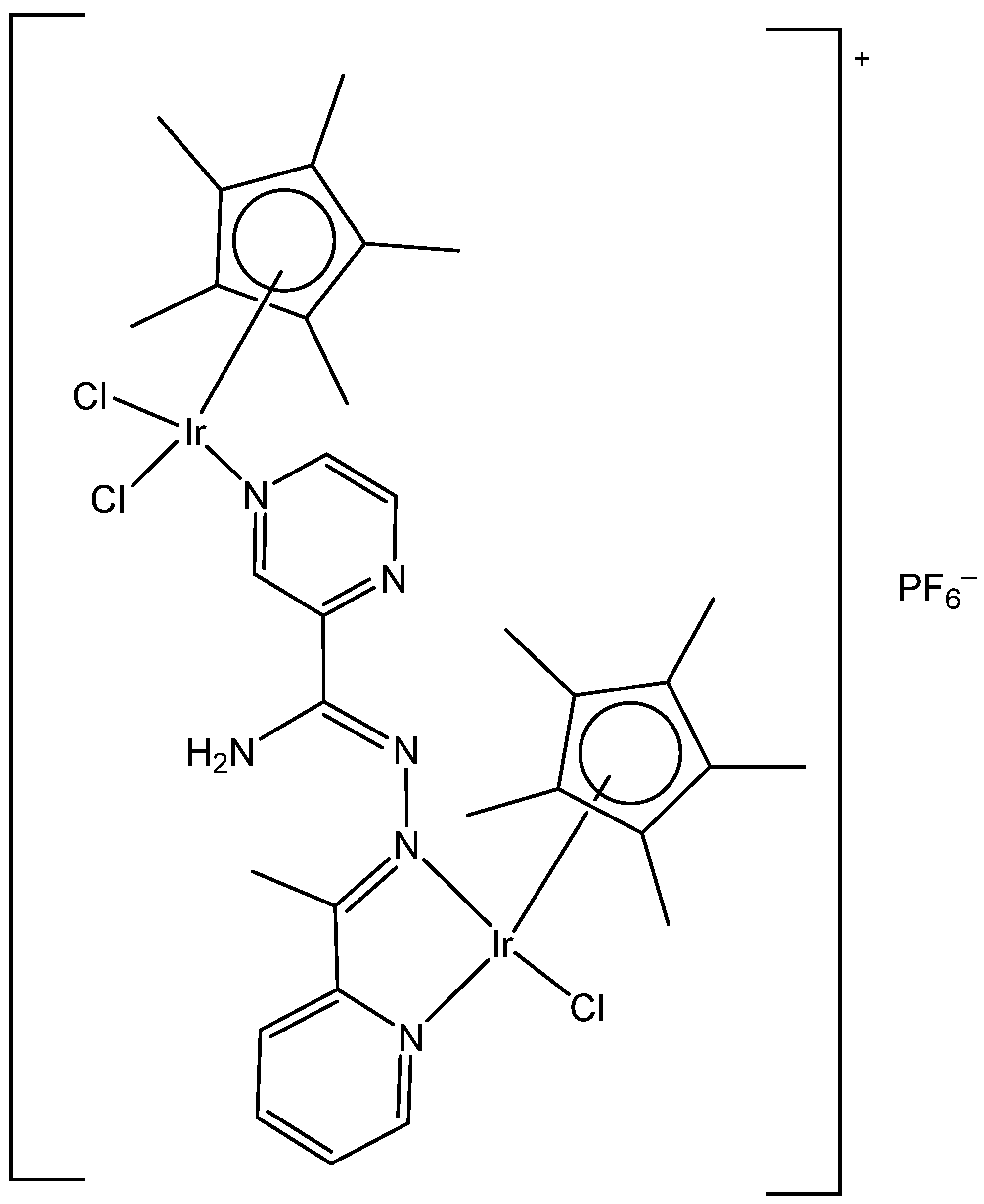
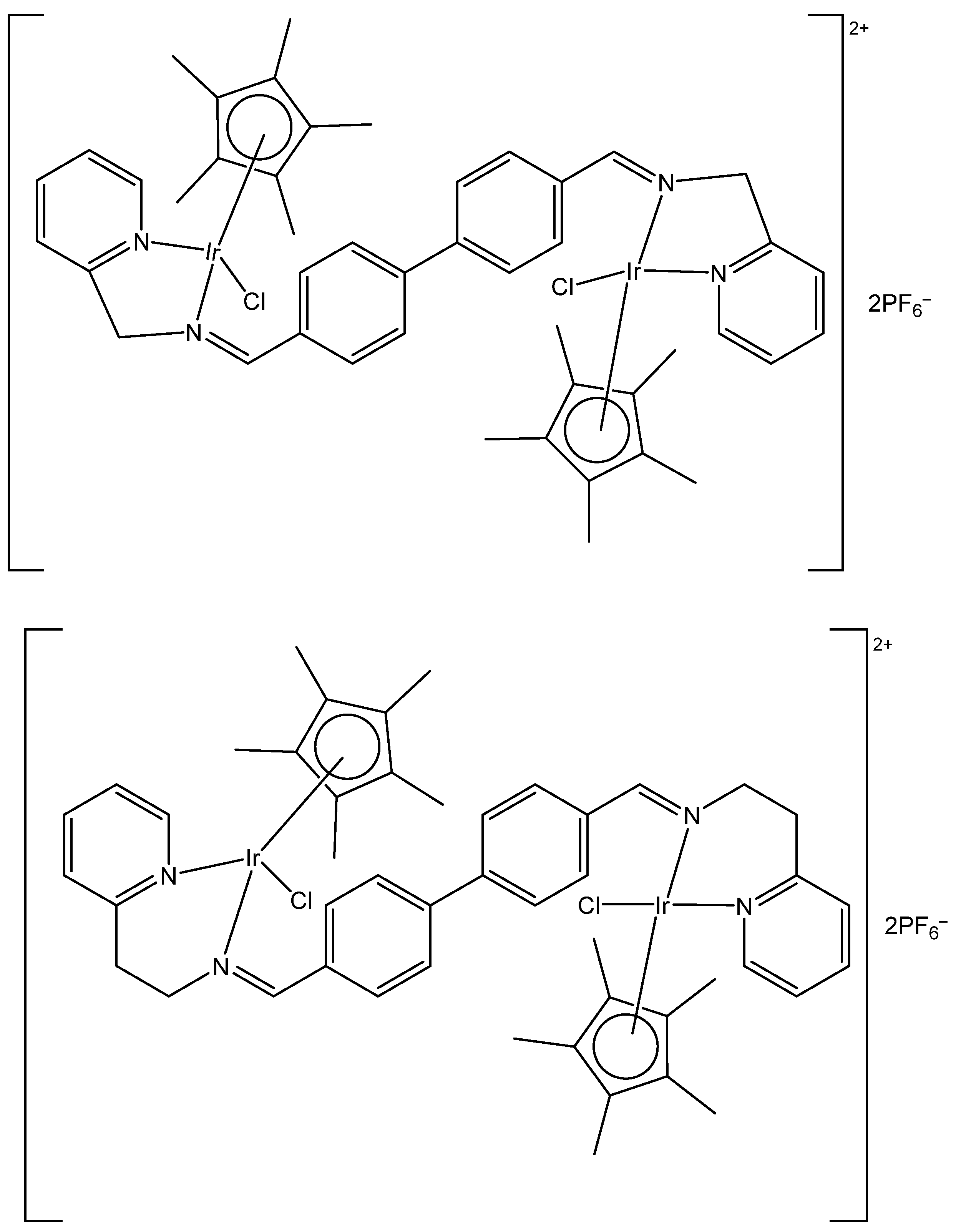
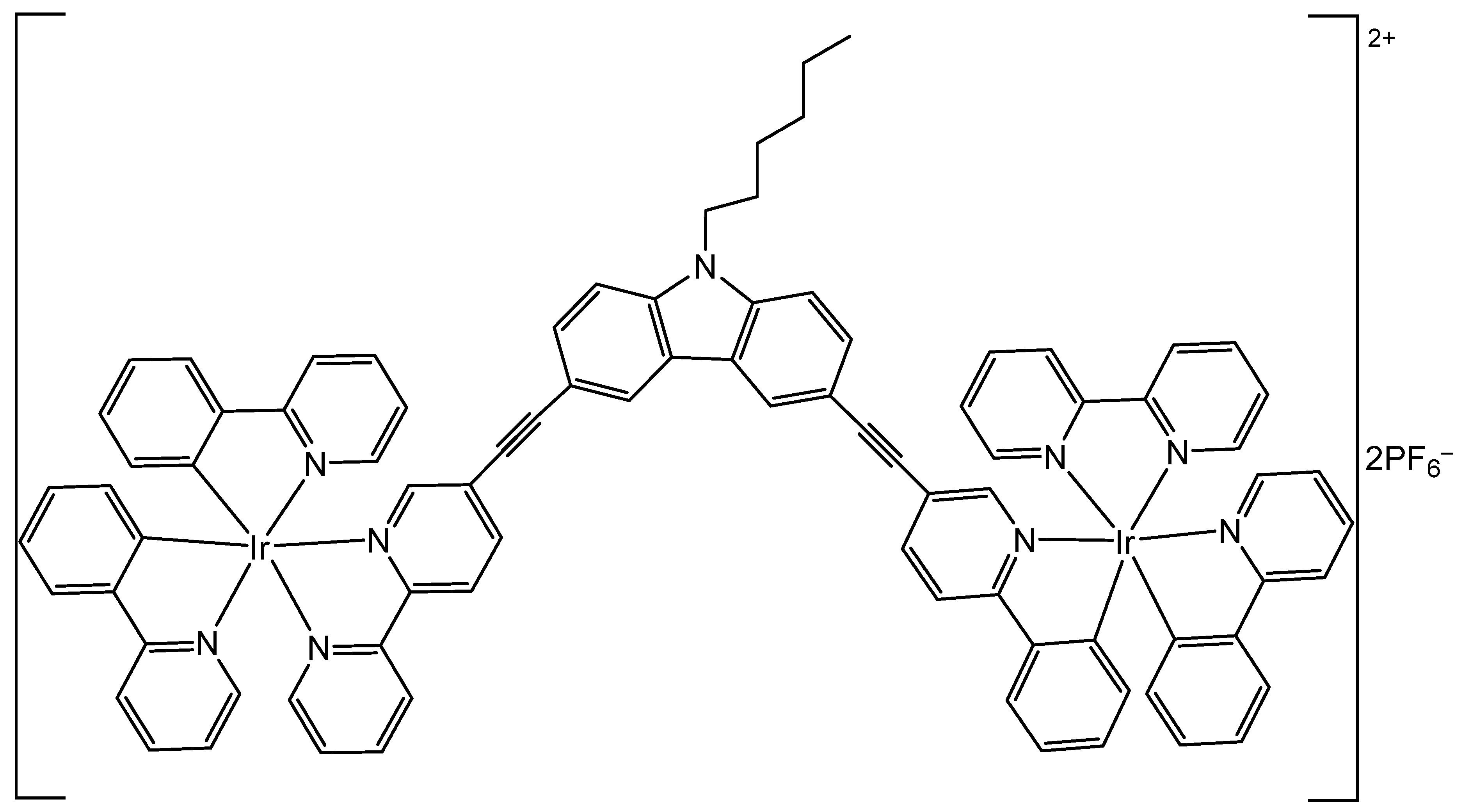
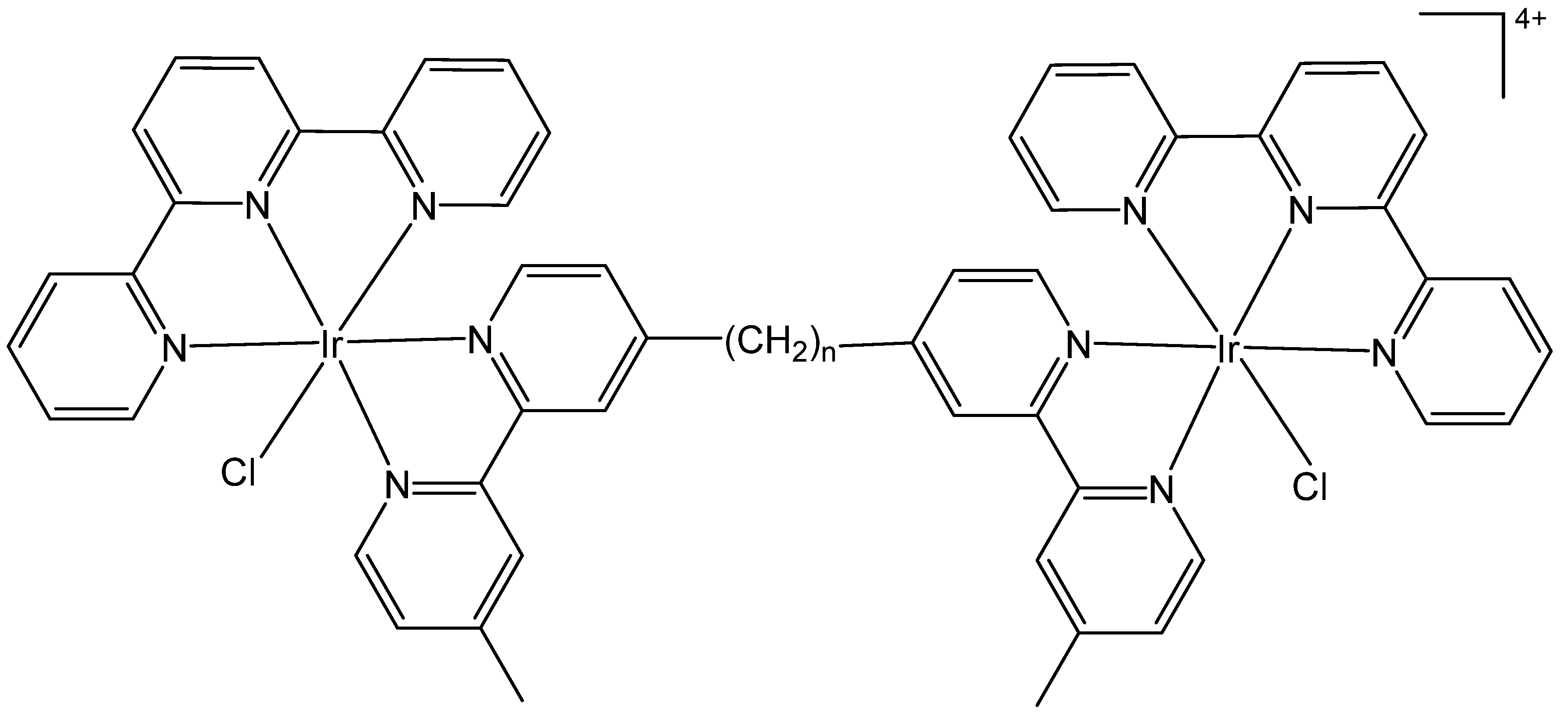
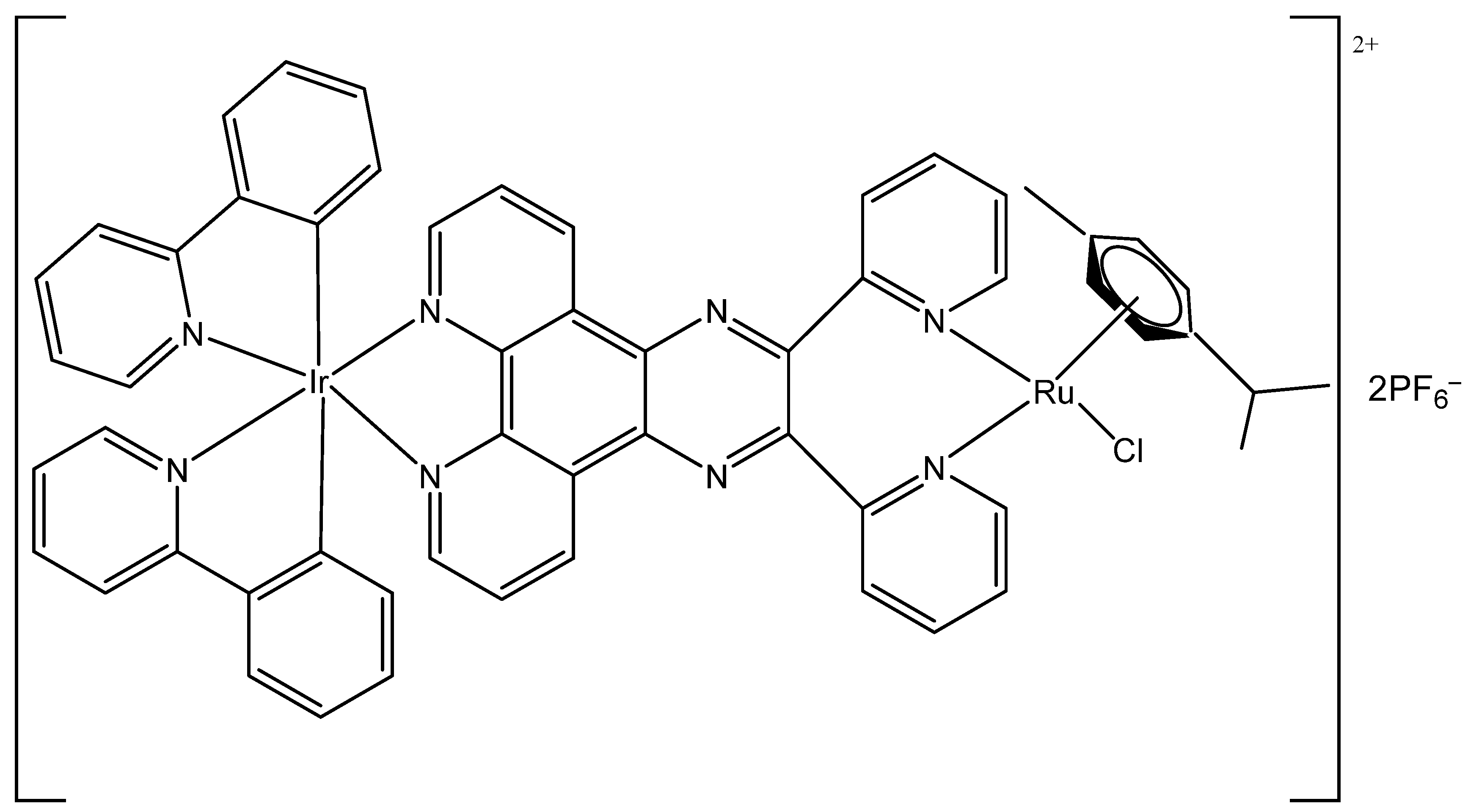
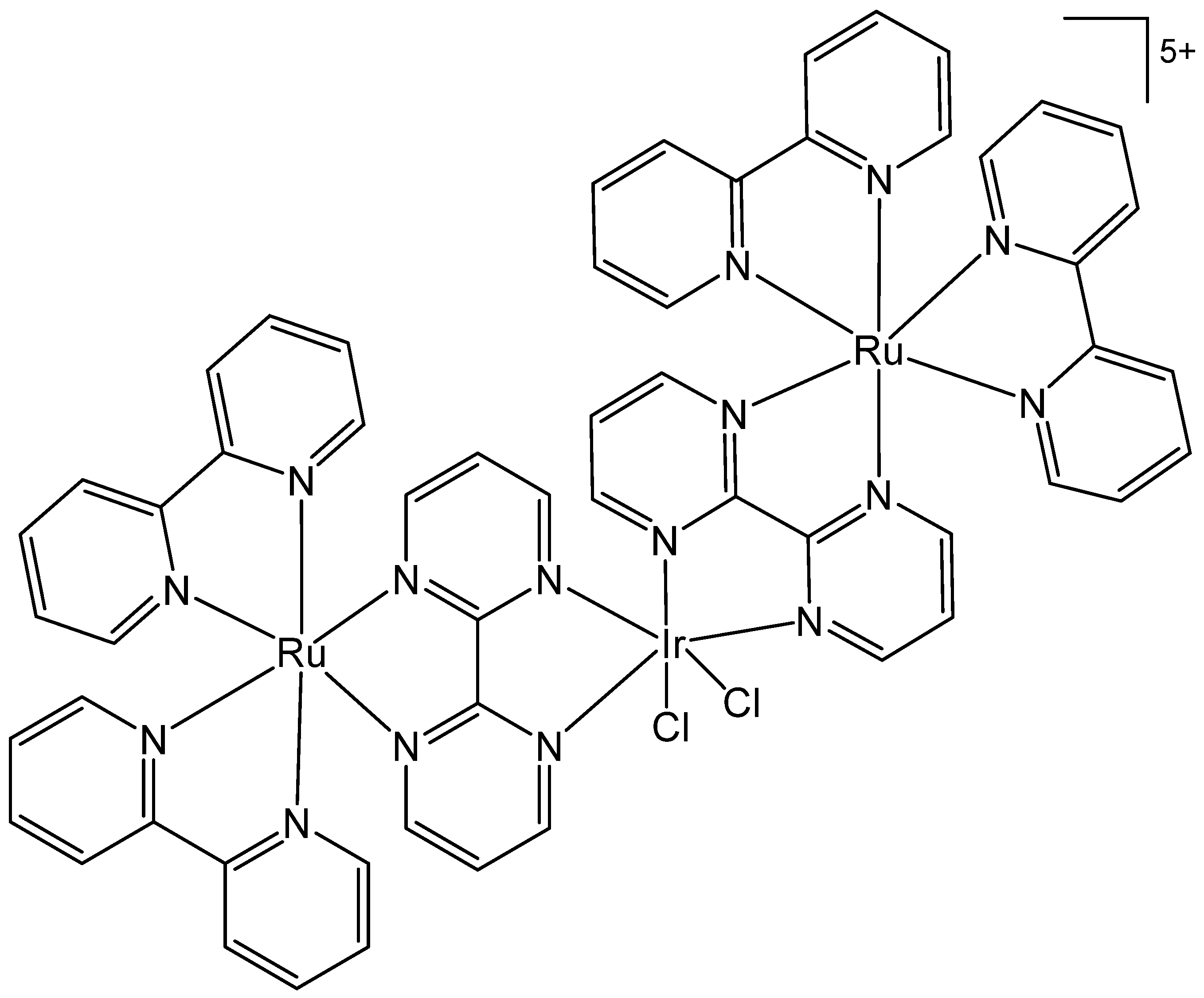
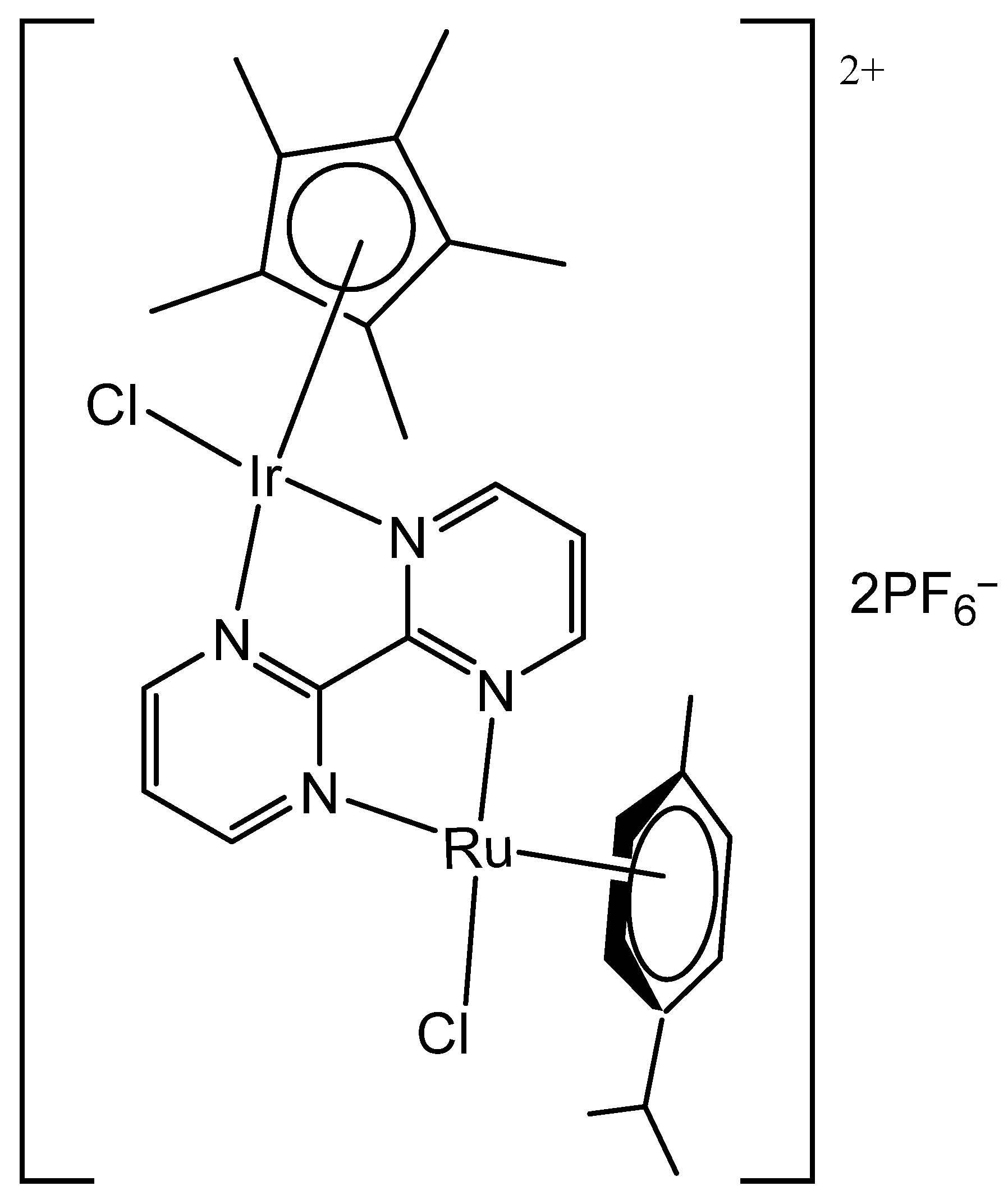
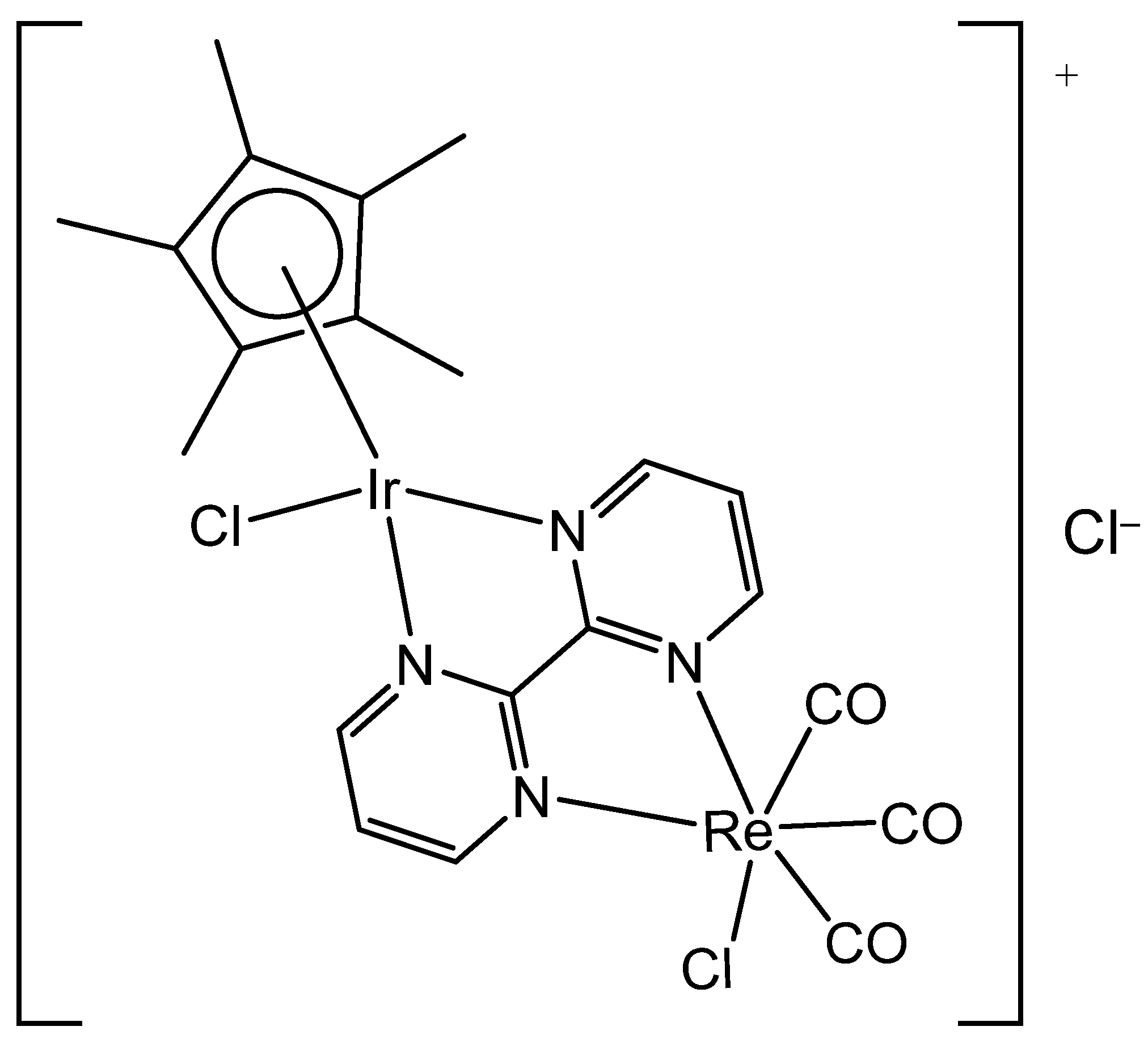
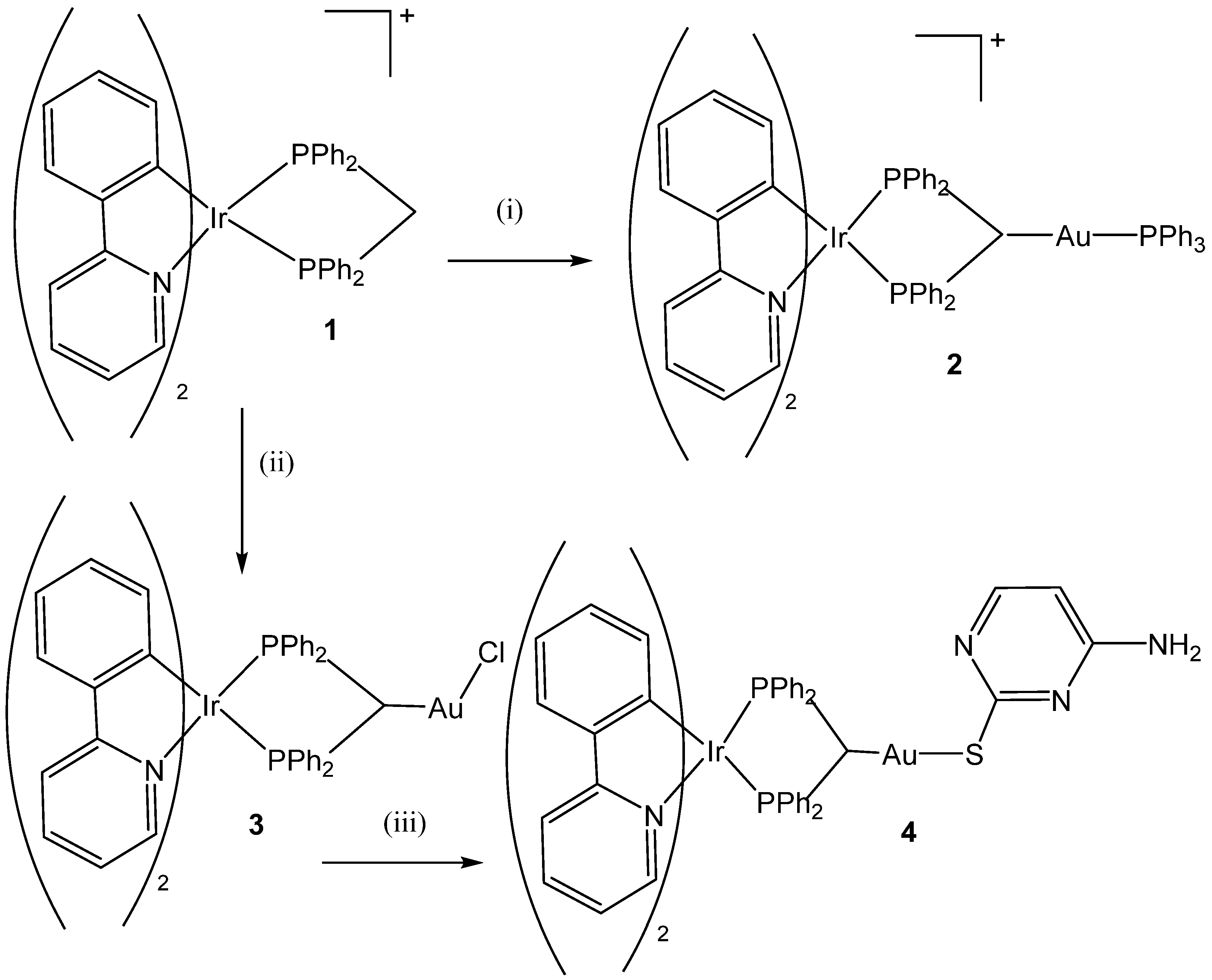
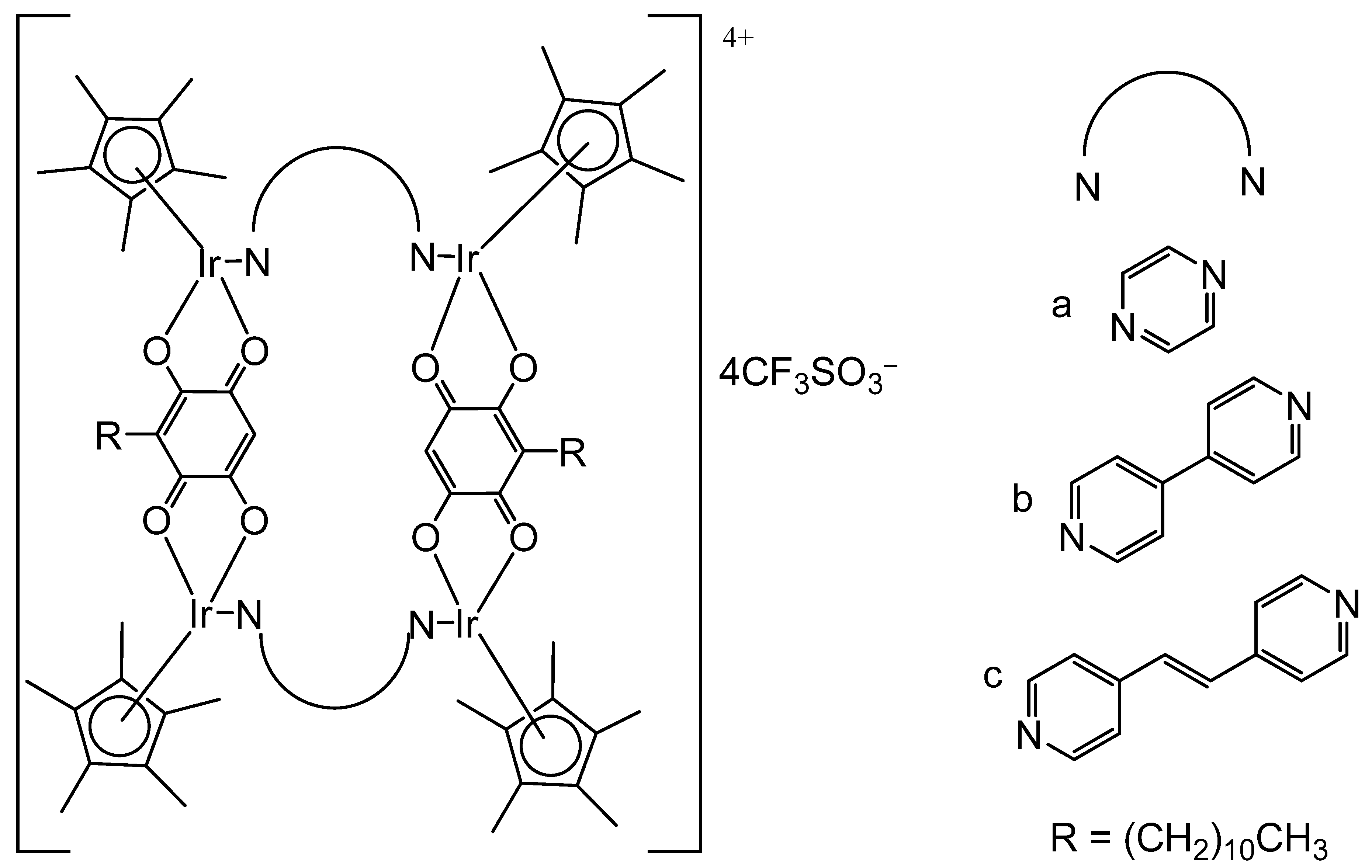
3. Heteronuclear Iridium(III) Complexes
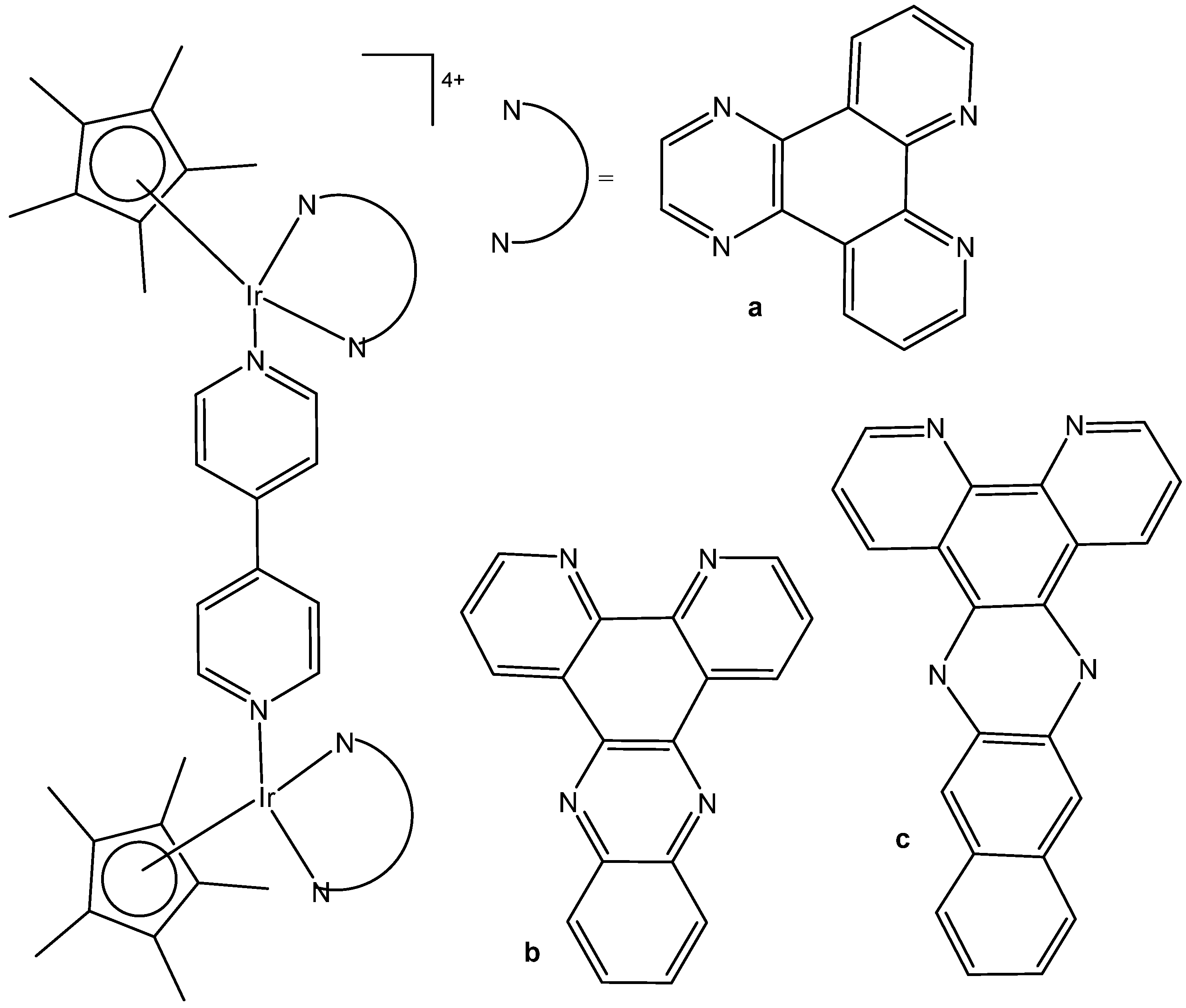
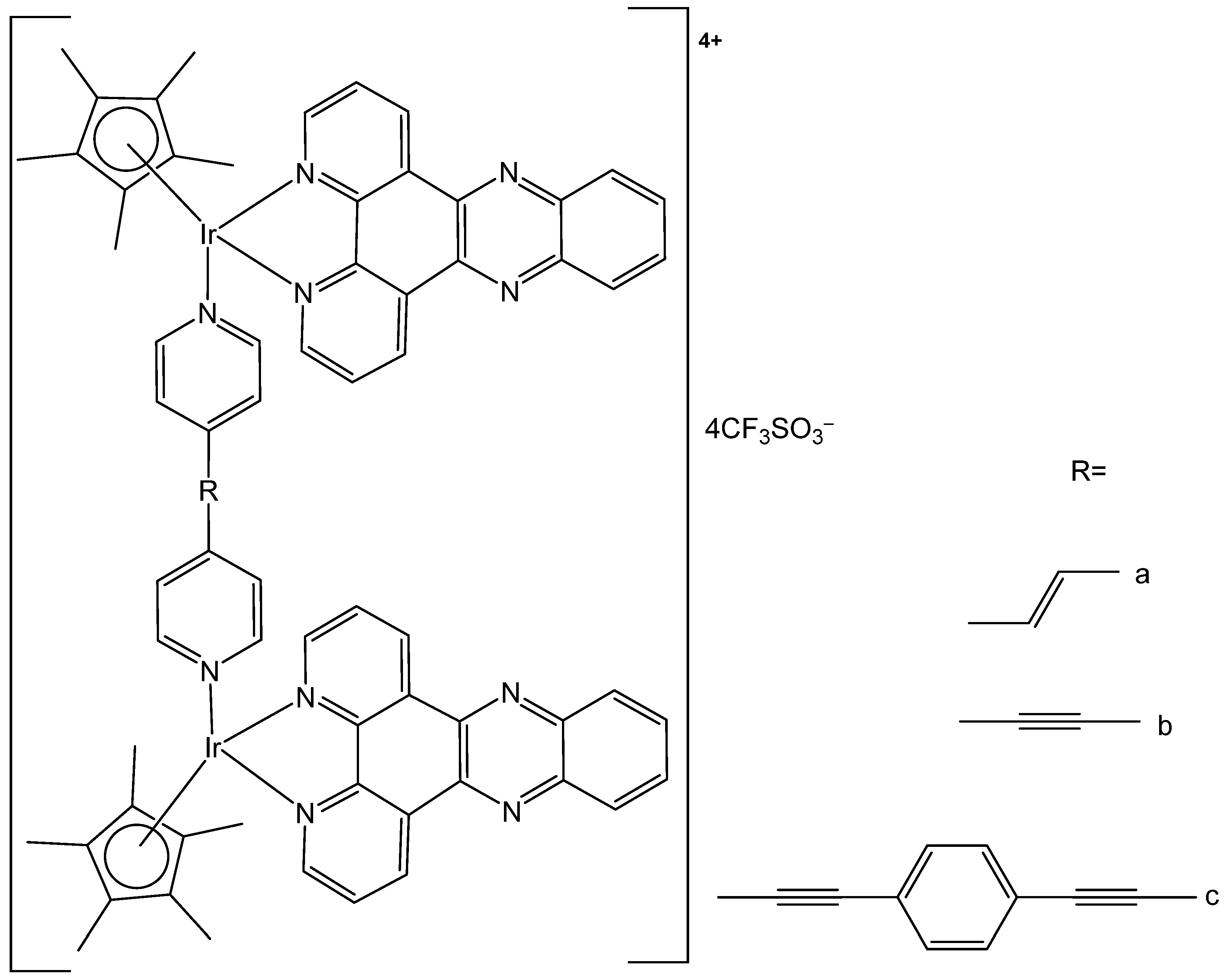
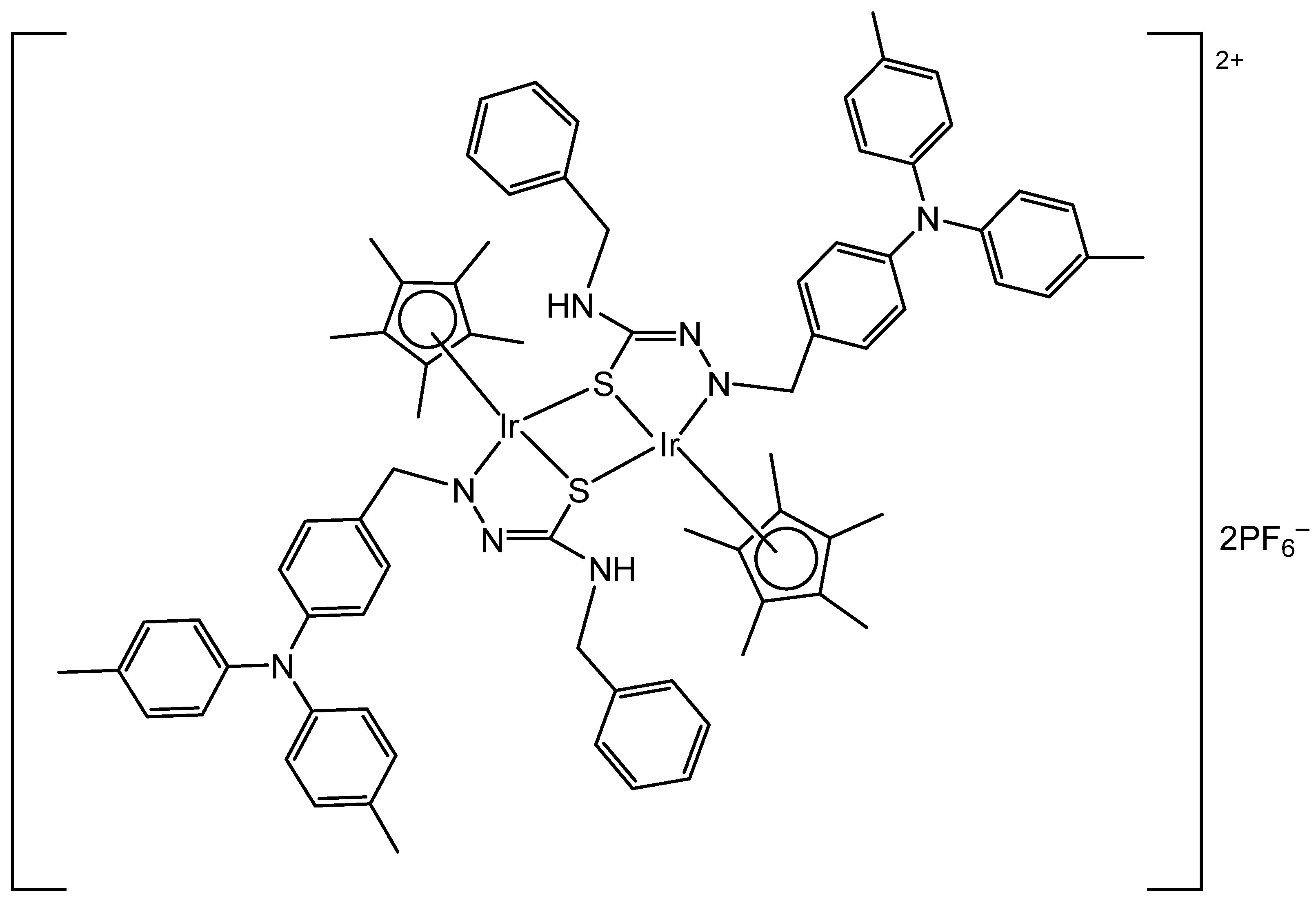
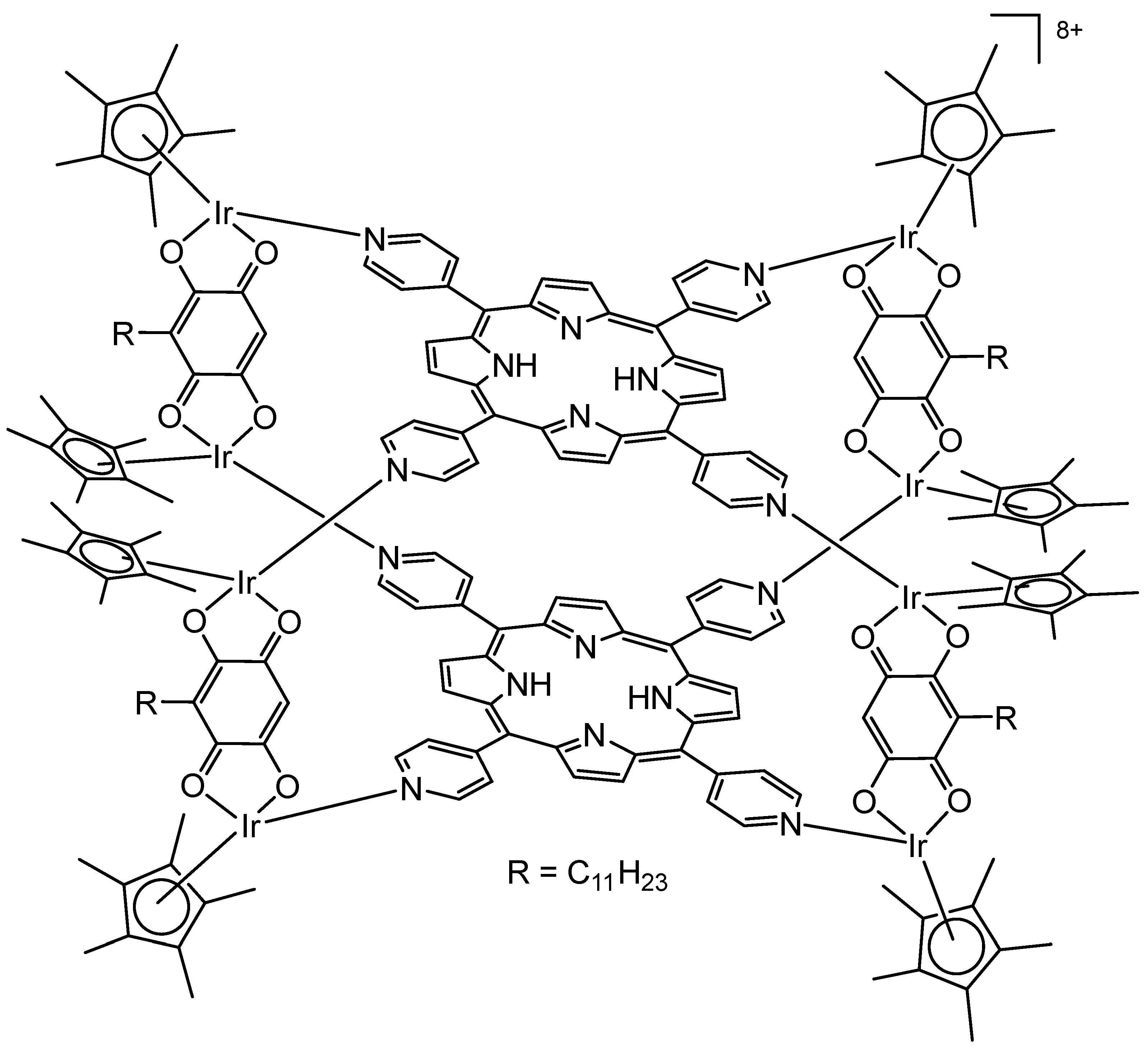
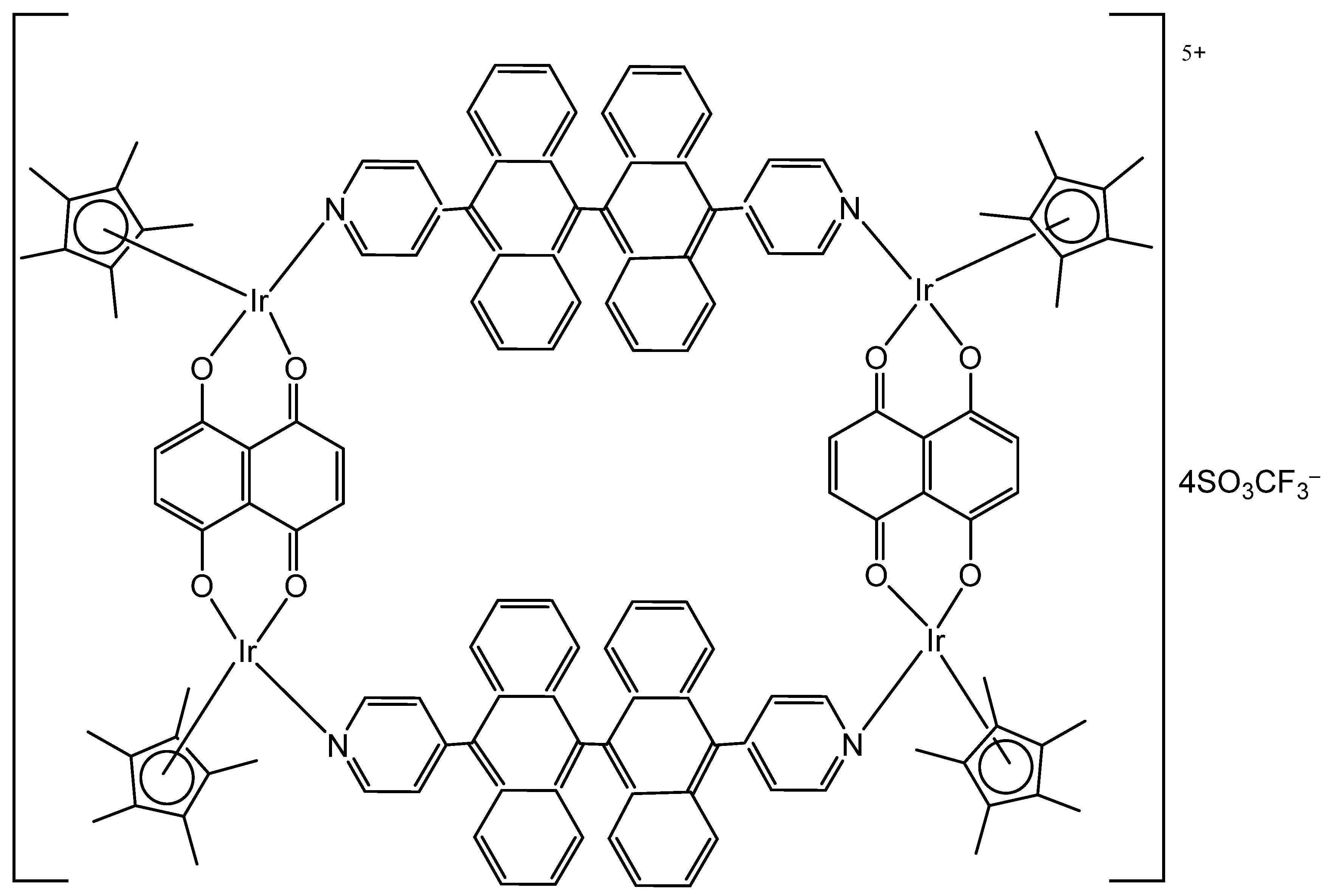
4. Metallacages
| Compound | IC50 | Cell Lines | Ref. |
|---|---|---|---|
| [Ir2(m-pzpy)(η5-Cp*)2Cl3]PF6 | >100 μM | HT-29 | [65] |
| [{(η5-Cp*)Ir(dppz)}2(μ-pyz)]4+ | 3.5 μM | MCF-7 | [66] |
| 1.8 μM | HT-29 | ||
| [{(η5-C5Me5)Ir(dppz)}2(μ-4,4′-bpy)]4+ | 3.1 μM | MCF-7 | |
| 3.8 μM | HT-29 | ||
| [{(η5-Cp*)Ir(dppz)}2(μ-dpee)]4+ | 0.61 μM | MCF-7 | [67] |
| [{(η5-Cp*)Ir(dppz)}2(μ-dpey)]4+ | 0.49 μM | MCF-7 | |
| [{(η5-Cp*)Ir(dppz)}2(μ-dpeb)]4+ | 2.2 μM | MCF-7 | |
| [Ir2(μ-L*)(η5-Cp*)2Cl2](PF6)2 | 3.1 μM | A2780 | [69] |
| 6.0 μM | MCF-7 | ||
| [Ir2(μ-L**)(η5-Cp*)2Cl2](PF6)2 | 12.6 μM | HepG2 | [70] |
| 16.5 μM | A549 | ||
| [{(ppy)2Ir}(µ-phpy){Ru(p-cym)Cl}](PF6)2 | 0.92 µM | MCF7 | [83] |
| [Ir(η5-Cp*)Cl(μ-bpm)Ru(η6-p-cym)Cl](PF6)2 | 1.9 μM | MDA-MB-468 | [90] |
| 6.2 μM | Caco-2 | ||
| [Ir(η5-Cp*)Cl(μ-bpm)ReCl(CO)3]Cl | 24.1 μM | MDA-MB-468 | [91] |
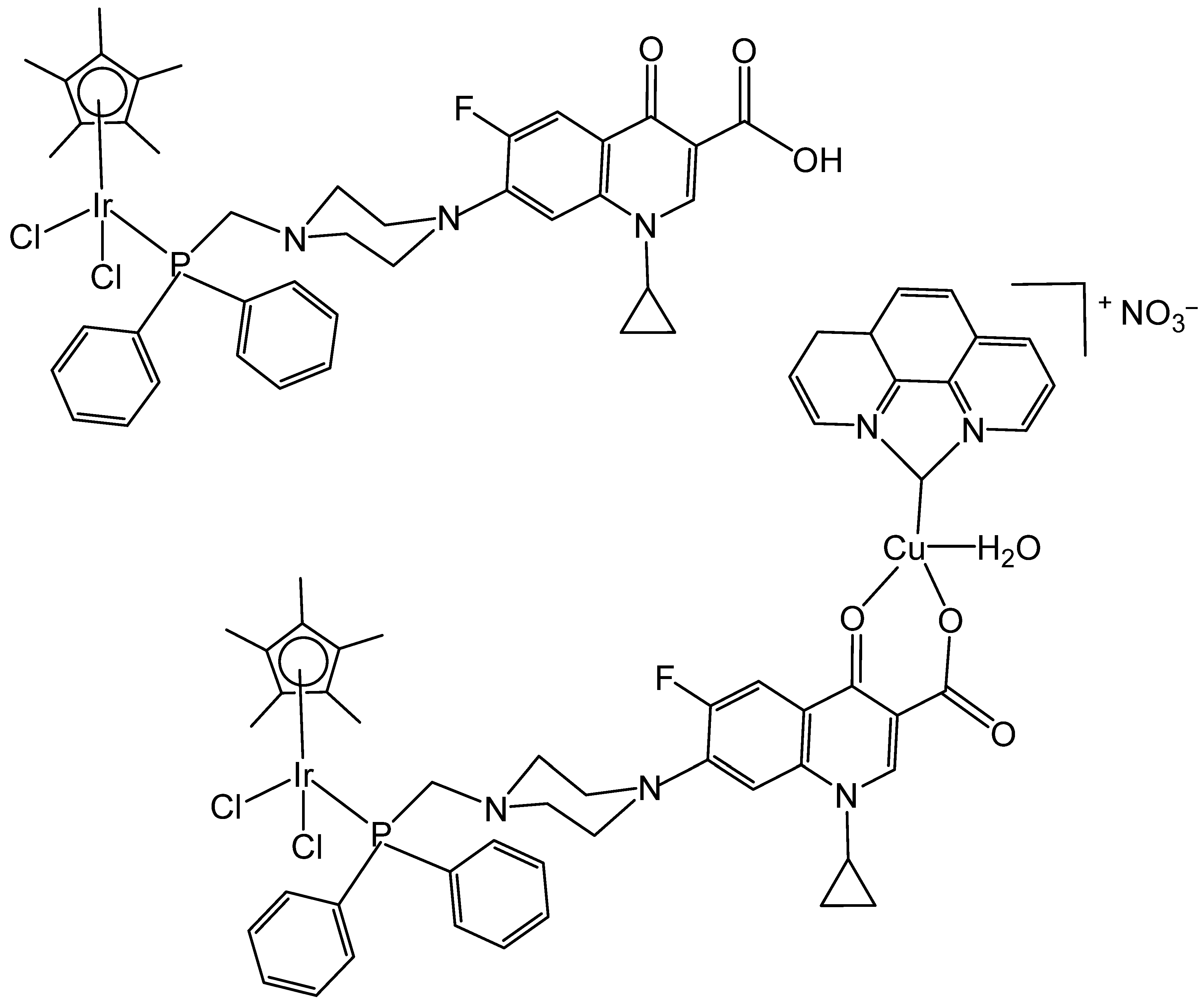
| [Ir(η5-Cp*)Cl2(μ-pcfx)Cu(phen)(H2O)]NO3 | 1.3 pM | DU145 | [94] |
| 35.5 nM | A549 | ||
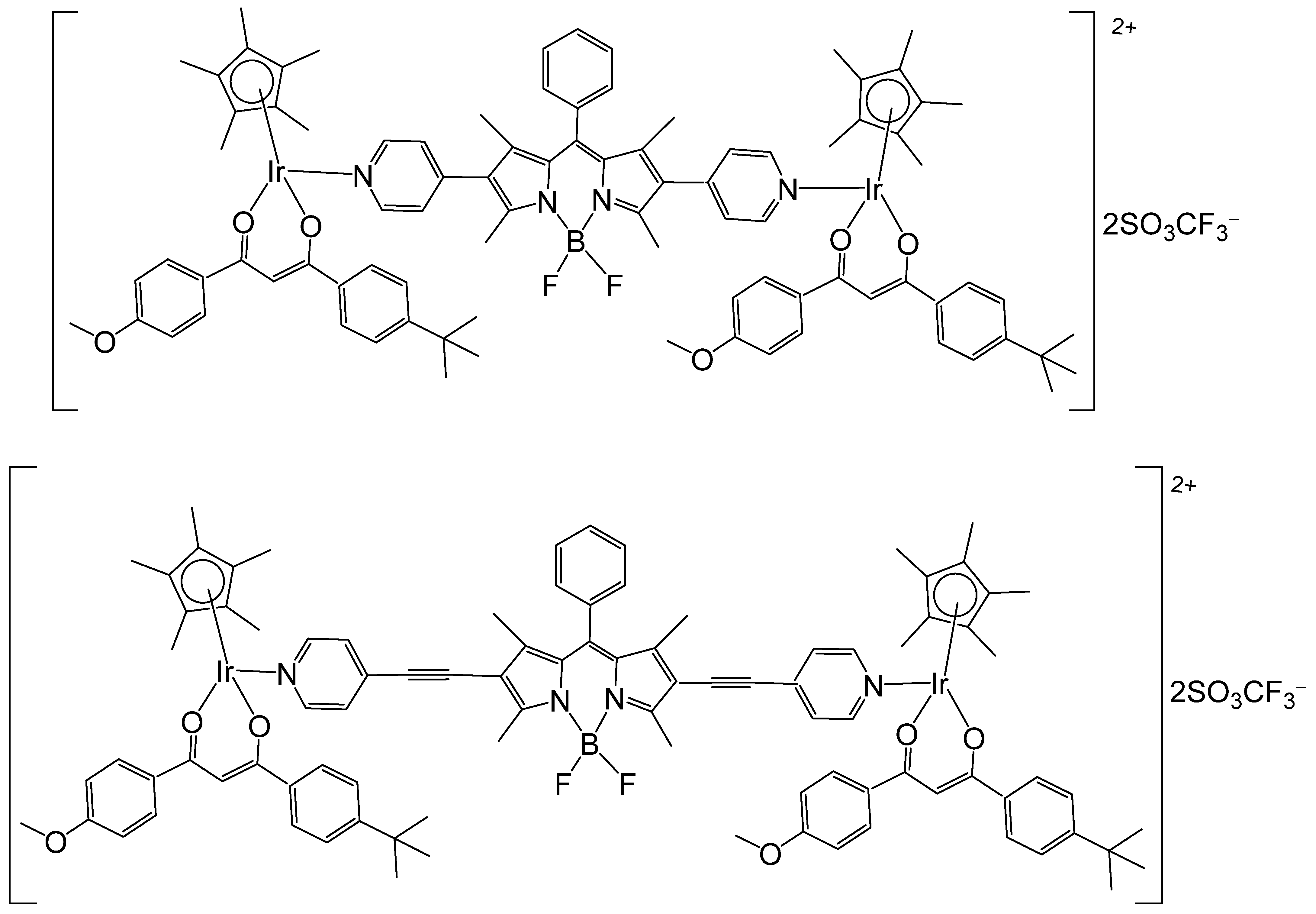
| [Ir4(μ-emb)2(μ-dpee)2(η5-Cp*)4](CF3SO3)4 | 0.6 μM | HeLa | [106] |
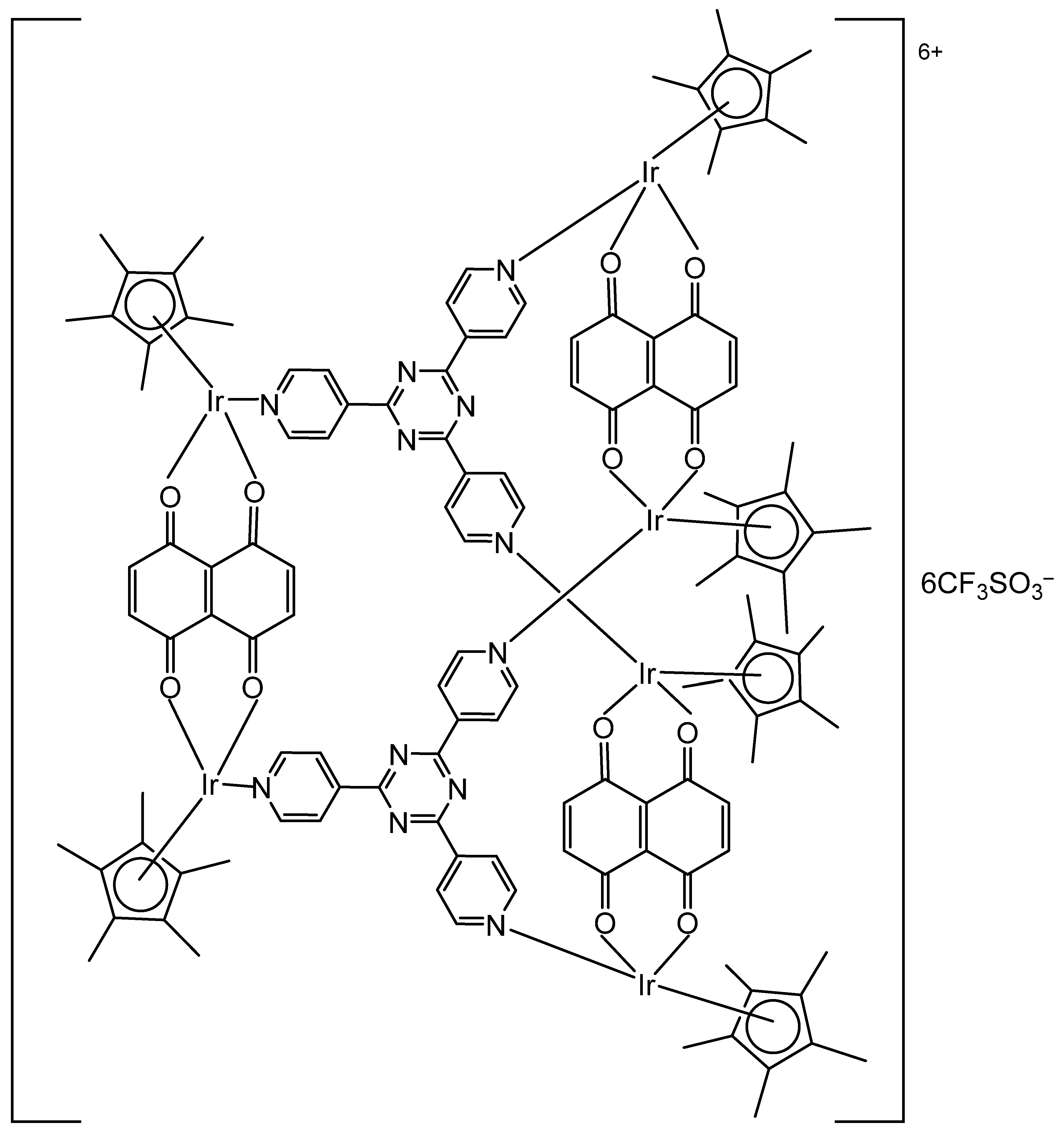
| [(Cp*Ir)6(tpt)2(dhnq)3]6+ | 1 μM | HT-29 | [108] |
5. Conclusions and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Sudhindra, P.; Roy, N.; Paira, P. Advances in novel iridium (III) based complexes for anticancer applications: A review. Inorg. Chim. Acta 2020, 513, 119925. [Google Scholar] [CrossRef]
- Kostova, I. Cytotoxic Organometallic Iridium (III) Complexes. Molecules 2025, 30, 801. [Google Scholar] [CrossRef] [PubMed]
- Pracharova, J.; Vigueras, G.; Novohradsky, V.; Cutillas, N.; Janiak, C.; Kostrhunova, H. Exploring the effect of polypyridyl ligands on the anticancer activity of phosphorescent iridium(III) complexes: From proteosynthesis inhibitors to photodynamic therapy agents. Chemistry 2018, 24, 4607–4619. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Lord, R.; Wilson, R.; Phillips, R.; Sridharan, V.; McGowan, P. Synthesis of iridium and ruthenium complexes with (N, N), (N, O) and (O, O) coordinating bidentate ligands as potential anti-cancer agents. Dalton Trans. 2012, 41, 13800–13802. [Google Scholar] [CrossRef]
- Cao, R.; Jia, J.; Ma, X.; Zhou, M.; Fei, H. Membrane localized iridium(iii) complex induces endoplasmic reticulum stress and mitochondriamediated apoptosis in human cancer cells. J. Med. Chem. 2013, 56, 3636–3644. [Google Scholar] [CrossRef]
- Cao, J.J.; Tan, C.P.; Chen, M.H.; Wu, N.; Yao, D.Y.; Liu, X.G. Targeting cancer cell metabolism with mitochondria-immobilized phosphorescent cyclometalated iridium(iii) complexes. Chem. Sci. 2017, 8, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.-P.; Meng, T.; Tan, M.-X.; Liu, Y.-C.; Luo, X.-J.; Zou, B.-Q. Synthesis and in vitro biological evaluation of three 4′-(4-methoxyphenyl)-2, 2′:6′, 2″-terpyridine iridium(iii) complexes as new telomerase inhibitors. Eur. J. Med. Chem. 2018, 143, 1387–1395. [Google Scholar] [CrossRef]
- Hao, L.; Li, Z.-W.; Zhang, D.-Y.; He, L.; Liu, W.; Yang, J. Monitoring mitochondrial viscosity with anticancer phosphorescent ir(III) complexes via twophoton lifetime imaging. Chem. Sci. 2019, 10, 1285–1293. [Google Scholar] [CrossRef]
- Redrado, M.; Minana, M.; Coogan, M.P.; Concepcion Gimeno, M.; Fernandez-Moreira, V. Tunable emissive ir(iii) benzimidazole-quinoline hybrids as promising theranostic lead compounds. Chemmedchem 2022, 2022, e202200244. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Lu, X.-R.; Li, M.-F.; Ji, L.-N.; Mao, Z.-W. Cyclometalated iridium(III) n-heterocyclic carbene complexes as potential mitochondrial anticancer and photodynamic agents. Dalton Trans. 2017, 46, 11363–11371. [Google Scholar] [CrossRef]
- Monti, F.; Kessler, F.; Delgado, M.; Frey, J.; Bazzanini, F.; Accorsi, G. Charged bis-cyclometalated iridium(III) complexes with carbene-based ancillary ligands. Inorg. Chem. 2013, 52, 10292–10305. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.-R.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Coumarin-appended phosphorescent cyclometalated iridium(iii) complexes as mitochondria-targeted theranostic anticancer agents. Dalton Trans. 2016, 45, 13042–13051. [Google Scholar] [CrossRef]
- Hearn, J.M.; Romero-Canelón, I.; Qamar, B.; Liu, Z.; Hands-Portman, I.; Sadler, P.J. Organometallic iridium (III) anticancer complexes with new mechanisms of action: NCI-60 screening, mitochondrial targeting, and apoptosis. ACS Chem. Biol. 2013, 8, 1335–1343. [Google Scholar] [CrossRef]
- Liu, Z.; Romero-Canelón, I.; Qamar, B.; Hearn, J.M.; Habtemariam, A.; Barry, N.P.; Sadler, P.J. The potent oxidant anticancer activity of organoiridium catalysts. Angew. Chem. 2014, 126, 4022–4027. [Google Scholar] [CrossRef]
- Liu, Z.; Sadler, P.J. Formation of glutathione sulfenate and sulfinate complexes by an organoiridium (III) anticancer complex. Inorg. Chem. Front. 2014, 1, 668–672. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Banerjee, S.; Hughes, G.M.; Bridgewater, H.E.; Song, J.I.; Breeze, B.G.; Sadler, P.J. Ligand-centred redox activation of inert organoiridium anticancer catalysts. Chem. Sci. 2020, 11, 5466–5480. [Google Scholar] [CrossRef]
- Starha, P.; Habtemariam, A.; Romero-Canelon, I.; Clarkson, G.J.; Sadler, P.J. Hydrosulfide adducts of organo-iridium anticancer complexes. Inorg. Chem. 2016, 55, 2324–2331. [Google Scholar] [CrossRef]
- Prathima, T.S.; Choudhury, B.; Ahmad, M.G.; Chanda, K.; Balamurali, M.M. Recent developments on other platinum metal complexes as target-specific anticancer therapeutics. Coord. Chem. Rev. 2023, 490, 215231. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Shao, M.; Zhang, P.; Song, S.; Yang, G. Cyclometalated iridium(iii) dithioformic acid complexes as mitochondriatargeted imaging and anticancer agents. J. Inorg. Biochem. 2022, 233, 111855. [Google Scholar] [CrossRef]
- Xiong, K.; Zhou, Y.; Lin, X.; Kou, J.; Lin, M.; Guan, R. Cyclometalated iridium(III) complexes as mitochondria-targeting photosensitizers against cisplatin-resistant cells. Photochem. Photobiol. 2022, 98, 85–91. [Google Scholar] [CrossRef]
- Wang, F.-X.; Chen, M.-H.; Hu, X.-Y.; Ye, R.-R.; Tan, C.-P.; Ji, L.-N. Ester-modified cyclometalated iridium(iii) complexes as mitochondria-targeting anticancer agents. Sci. Rep. 2016, 6, 38954. [Google Scholar] [CrossRef]
- Wang, L.; Guan, R.; Xie, L.; Liao, X.; Xiong, K.; Rees, T.W. An er-targeting iridium(III) complex that induces immunogenic cell death in non-small-cell lung cancer. Angew. Chem. Int. Ed. Engl. 2021, 60, 4657–4665. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H.; Banerjee, S.; Clarkson, G.J.; Ge, C.; Imberti, C. Nucleus-targeted organoiridium–albumin conjugate for photodynamic cancer therapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liang, C.; Sadhukhan, T.; Banerjee, S.; Fan, Z.; Li, T. Invitro and in-vivo photocatalytic cancer therapy with biocompatible iridium(iii) photocatalysts. Angew. Chem. Int. Ed. Engl. 2021, 60, 9474–9479. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Wang, M.M.; Huang, Y.; Qian, Y.; Xue, X. The cyclometalated iridium (III) complex based on 9-anthracenecarboxylic acid as a lysosomal-targeted anticancer agent. J. Inorg. Biochem. 2022, 235, 111913. [Google Scholar] [CrossRef]
- Acuña, M.I.; Rubio, A.R.; Martínez-Alonso, M.; Busto, N.; Rodríguez, A.M.; Davila-Ferreira, N.; Domínguez, F. Targets, mechanisms and cytotoxicity of half-sandwich Ir(III) complexes are modulated by structural modifications on the benzazole ancillary ligand. Cancers 2023, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Rong, Y.; Sadhukhan, T.; Liang, S.; Li, W.; Yuan, Z. Singlecell quantification of a highly biocompatible dinuclear iridium(III) complex for photocatalytic cancer therapy. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202098. [Google Scholar] [CrossRef]
- Ouyang, C.; Chen, L.; Rees, T.W.; Chen, Y.; Liu, J.; Ji, L. A mitochondriatargeting hetero-binuclear Ir(III)–Pt (II) complex induces necrosis in cisplatin-resistant tumor cells. Chem. Commun. 2018, 54, 6268–6271. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, R.; Liao, X.; Ouyang, C.; Rees, T.W.; Liu, J. A mitochondria-targeting dinuclear ir–ru complex as a synergistic photoactivated chemotherapy and photodynamic therapy agent against cisplatin-resistant tumour cells. Chem. Commun. 2019, 55, 12547–12550. [Google Scholar] [CrossRef]
- Thomas, S.; Balónová, B.; Cinatl, J.; Wass, M.; Serpell, C.; Id, O. Thiourea and guanidine compounds and their iridium complexes in drug-resistant cancer cell lines: Structure-activity relationships and direct luminescent imaging. Chemmedchem 2022, 15, 349–353. [Google Scholar] [CrossRef]
- Xiong, K.; Chen, Y.; Ouyang, C.; Guan, R.-L.; Ji, L.-N.; Chao, H. Cyclometalated iridium(iii) complexes as mitochondria-targeted anticancer agents. Biochimie 2016, 125, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, C.-P.; Zhang, W.; He, L.; Ji, L.-N.; Mao, Z.-W. Phosphorescent iridium(iii)-bis-n-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents. Biomaterials 2015, 39, 95–104. [Google Scholar] [CrossRef]
- Ouyang, M.; Zeng, L.; Huang, H.; Jin, C.; Liu, J.; Chen, Y. Fluorinated cyclometalated iridium(iii) complexes as mitochondria-targeted theranostic anticancer agents. Dalton Trans. 2017, 46, 6734–6744. [Google Scholar] [CrossRef]
- Song, X.-D.; Kong, X.; He, S.-F.; Chen, J.-X.; Sun, J.; Chen, B.-B. Cyclometalated iridium(iii)-guanidinium complexes as mitochondria-targeted anticancer agents. Eur. J. Med. Chem. 2017, 138, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wan, D.; Wang, Y.-J.; Yi, Q.-Y.; Guo, B.-H.; Liu, Y.-J. An iridium (III) complex as potent anticancer agent induces apoptosis and autophagy in b16 cells through inhibition of the akt/mtor pathway. Eur. J. Med. Chem. 2018, 145, 302–314. [Google Scholar] [CrossRef]
- Yi, Q.-Y.; Wan, D.; Tang, B.; Wang, Y.-J.; Zhang, W.-Y.; Du, F. Synthesis, characterization and anticancer activity in vitro and in vivo evaluation of an iridium (III) polypyridyl complex. Eur. J. Med. Chem. 2018, 145, 338–349. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, K.-N.; Zheng, Y.; Cao, J.-J.; Zhang, M.-F.; Tan, C.-P. Cyclometalated iridium(iii) complexes induce mitochondria-derived paraptotic cell death and inhibit tumor growth in vivo. Dalton Trans. 2018, 47, 6942–6953. [Google Scholar] [CrossRef]
- Millett, A.J.; Habtemariam, A.; Romero-Canelon, I.; Clarkson, G.J.; Sadler, P.J. Contrasting anticancer activity of half-sandwich iridium(III) complexes bearing functionally diverse 2-phenylpyridine ligands. Organometallics 2015, 34, 2683–2694. [Google Scholar] [CrossRef]
- Křikavová, R.; Romanovová, M.; Jendželovská, Z.; Majerník, M.; Masaryk, L.; Zoufalý, P.; Nemec, I. Impact of the central atom and halido ligand on the structure, antiproliferative activity and selectivity of half-sandwich Ru(II) and Ir(III) complexes with a 1,3,4-thiadiazole-based ligand. Dalton Trans. 2023, 52, 12717–12732. [Google Scholar] [CrossRef]
- Novohradsky, V.; Zerzankova, L.; Stepankova, J.; Kisova, A.; Kostrhunova, H.; Liu, Z.; Brabec, V. A dual-targeting, apoptosis-inducing organometallic half-sandwich iridium anticancer complex. Metallomics 2014, 6, 1491–1501. [Google Scholar] [CrossRef]
- Guo, L.; Li, P.; Li, J.; Gong, Y.; Li, X.; Wen, T.; Liu, Z. Potent half-sandwich 16-/18-electron iridium (III) and ruthenium(II) anticancer complexes with readily available amine–imine ligands. Inorg. Chem. 2023, 62, 21379–21395. [Google Scholar] [CrossRef]
- Mou, Z.D.; Deng, N.; Zhang, F.; Zhang, J.; Cen, J.; Zhang, X. Halfsandwich Schiff-base Ir(III) complexes as anticancer agents. Eur. J. Med. Chem. 2017, 138, 72–82. [Google Scholar] [CrossRef]
- Li, J.; Guo, L.; Tian, Z.; Tian, M.; Zhang, S.; Xu, K. Novel halfsandwich iridium(III) imino-pyridyl complexes showing remarkable in vitro anticancer activity. Dalton Trans. 2017, 46, 15520–15534. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, M.; Jiang, M.; Yang, F.; Zhang, Z. Current status of iridium-based complexes against lung cancer. Front. Pharmacol. 2022, 13, 1025544. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P. Anticancer iridium (III) cyclopentadienyl complexes. Inorg. Chem. Front. 2025, 12, 897–954. [Google Scholar] [CrossRef]
- Ma, L.; Li, L.; Zhu, G. Platinum-containing heterometallic complexes in cancer therapy: Advances and perspectives. Inorg. Chem. Front. 2022, 9, 2424–2453. [Google Scholar] [CrossRef]
- Notaro, A.; Gasser, G. Monomeric and dimeric coordinatively saturated and substitutionally inert Ru (II) polypyridyl complexes as anticancer drug candidates. Chem. Soc. Rev. 2017, 46, 7317–7337. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.L.; White, T.A.; Winkel, B.S.; Brewer, K.J. Redox, spectroscopic, and photophysical properties of Ru−Pt mixed-metal complexes incorporating 4, 7-diphenyl-1, 10-phenanthroline as efficient DNA binding and photocleaving agents. Inorg. Chem. 2011, 50, 463–470. [Google Scholar] [CrossRef]
- López-Hernández, J.E.; Nayeem, N.; Cerón-Carrasco, J.P.; Ahad, A.; Hafeez, A.; León, I.E.; Contel, M. Platinum(IV)–Gold(I) Agents with Promising Anticancer Activity: Selected Studies in 2D and 3D Triple-Negative Breast Cancer Models. Chem. Eur. J. 2023, 29, e202302045. [Google Scholar] [CrossRef]
- Wenzel, M.; Bigaeva, E.; Richard, P.; Le Gendre, P.; Picquet, M.; Casini, A.; Bodio, E. New heteronuclear gold(I)-platinum(II) complexes with cytotoxic properties: Are two metals better than one? J. Inorg. Biochem. 2014, 141, 10–16. [Google Scholar] [CrossRef]
- Pete, S.; Roy, N.; Kar, B.; Paira, P. Construction of homo and heteronuclear Ru (II), Ir (III) and Re (I) complexes for target specific cancer therapy. Coord. Chem. Rev. 2022, 460, 214462. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathy, S.K.; Paul, D.; Laha, P.; Santra, M.K.; Patra, S. Asymmetrically Coordinated Heterodimetallic Ir–Ru System: Synthesis, Computational, and Anticancer Aspects. Inorg. Chem. 2023, 62, 7003–7013. [Google Scholar] [CrossRef] [PubMed]
- Serratrice, M.; Maiore, L.; Zucca, A.; Stoccoro, S.; Landini, I.; Mini, E.; Massai, L.; Ferraro, G.; Merlino, A.; Messori, L. Cytotoxic properties of a new organometallic platinum(ii) complex and its gold(i) heterobimetallic derivatives. Dalton Trans. 2016, 45, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Bellam, R.; Jaganyi, D.; Robinson, R.S. Heterodinuclear Ru–Pt Complexes Bridged with 2,3-Bis(pyridyl)pyrazinyl Ligands: Studies on Kinetics, Deoxyribonucleic Acid/Bovine Serum Albumin Binding and Cleavage, In Vitro Cytotoxicity, and In Vivo Toxicity on Zebrafish Embryo Activities. ACS Omega 2022, 7, 26226–26245. [Google Scholar] [CrossRef] [PubMed]
- Bellam, R.; Jaganyi, D.; Mambanda, A.; Robinson, R. Role of a 2,3-bis(pyridyl)pyrazinyl chelate bridging ligand in the reactivity of Ru(ii)–Pt(ii) dinuclear complexes on the substitution of chlorides by thiourea nucleophiles—A kinetic study. New J. Chem. 2018, 42, 12557–12569. [Google Scholar] [CrossRef]
- Askari, B.; Rudbari, H.A.; Micale, N.; Schirmeister, T.; Maugeri, A.; Navarra, M. Anticancer study of heterobimetallic platinum(II)-ruthenium(II) and platinum(II)-rhodium(III) complexes with bridging dithiooxamide ligand. J. Organomet. Chem. 2019, 900, 120918. [Google Scholar] [CrossRef]
- Ćoćić, D.; Jovanović-Stević, S.; Jelić, R.; Matić, S.; Popović, S.; Djurdjević, P.; Baskić, D.; Petrović, B. Homo- and hetero-dinuclear Pt(ii)/Pd(ii) complexes: Studies of hydrolysis, nucleophilic substitution reactions, DNA/BSA interactions, DFT calculations, molecular docking and cytotoxic activity. Dalton Trans. 2020, 49, 14411–14431. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, R.; Liao, X.; Ouyang, C.; Liu, J.; Ji, L.; Chao, H. Mitochondrial DNA targeting and impairment by a dinuclear Ir–Pt complex that overcomes cisplatin resistance. Inorg. Chem. Front. 2020, 7, 1864–1871. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Sáez, J.; Rodriguez-Yoldi, M.J.; Cerrada, E. Heteronuclear complexes with promising anticancer activity against colon cancer. Biomedicines 2024, 12, 1763. [Google Scholar] [CrossRef]
- Štarha, P. Multinuclear biologically active Ru, Rh, Os and Ir arene complexes. Coord. Chem. Rev. 2021, 431, 213690. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enz. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Redrado, M.; Fernández-Moreira, V.; Gimeno, M.C. Theranostics Through the Synergistic Cooperation of Heterometallic Complexes. ChemMedChem 2021, 16, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Muhamedi, A.; Batsanov, A.S. Phosphorescent mono- and diiridium(III) complexes cyclometalated by fluorenyl- or phenyl-pyridino ligands with bulky substituents, as prospective OLED dopants. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 392–399. [Google Scholar] [CrossRef]
- De Palo, A.; Draca, D.; Murrali, M.G.; Zacchini, S.; Pampaloni, G.; Mijatovic, S.; Marchetti, F. A comparative analysis of the in vitro anticancer activity of iridium (III){η5-C5Me4R} complexes with variable R groups. Intern. J. Mol. Sci. 2021, 22, 7422. [Google Scholar] [CrossRef]
- Lapasam, A.; Pinder, E.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, structure and bonding modes of pyrazine based ligands of Cp* Rh and Cp* Ir complexes: The study of in-vitro cytotoxicity against human cell lines. J. Organomet. Chem. 2019, 899, 120887. [Google Scholar] [CrossRef]
- Nazif, M.A.; Bangert, J.A.; Ott, I.; Gust, R.; Stoll, R.; Sheldrick, W.S. Dinuclear organoiridium (III) mono-and bis-intercalators with rigid bridging ligands: Synthesis, cytotoxicity and DNA binding. J. Inorg. Biochem. 2009, 103, 1405–1414. [Google Scholar] [CrossRef]
- Nazif, M.A.; Rubbiani, R.; Alborzinia, H.; Kitanovic, I.; Wölfl, S.; Ott, I.; Sheldrick, W.S. Cytotoxicity and cellular impact of dinuclear organoiridium DNA intercalators and nucleases with long rigid bridging ligands. Dalton Trans. 2012, 41, 5587–5598. [Google Scholar] [CrossRef]
- Parveen, S.; Hanif, M.; Leung, E.; Tong, K.; Yang, A.; Astin, J.; De Zoysa, G.H.; Steel, T.R.; Goodman, D.; Movassaghi, S.; et al. Anticancer organorhodium and -iridium complexes with low toxicity in vivo but high potency in vitro: DNA damage, reactive oxygen species formation, and haemolytic activity. Chem. Commun. 2019, 55, 12016–12019. [Google Scholar] [CrossRef]
- Štarha, P.; Hošek, J.; Trávníček, Z.; Dvořák, Z. Cytotoxic dimeric half-sandwich Ru(II), Os(II) and Ir(III) complexes containing the 4,4′-biphenyl-based bridging ligands. Appl. Organomet. Chem. 2020, 34, e5785. [Google Scholar] [CrossRef]
- Masaryk, L.; Koczurkiewicz-Adamczyk, P.; Milde, D.; Nemec, I.; Sloczynska, K.; Pękala, E.; Štarha, P. Dinuclear half-sandwich Ir(III) complexes containing 4,4′-methylenedianiline-based ligands: Synthesis, characterization, cytotoxicity. J. Organomet. Chem. 2021, 938, 121748. [Google Scholar] [CrossRef]
- Steel, T.R.; Tong, K.K.; Söhnel, T.; Jamieson, S.M.; Wright, L.J.; Crowley, J.D.; Hartinger, C.G. Homodinuclear organometallics of ditopic N,N-chelates: Synthesis, reactivity and in vitro anticancer activity. Inorg. Chim. Acta 2021, 518, 120220. [Google Scholar] [CrossRef]
- Kavukcu, S.B.; Ensarioğlu, H.K.; Karabıyık, H.; Vatansever, H.S.; Türkmen, H. Cell death mechanism of organometallic ruthenium(II) and iridium(III) arene complexes on HepG2 and Vero cells. ACS Omega 2023, 8, 37549–37563. [Google Scholar] [CrossRef]
- Shao, M.; Yao, M.; Liu, X.; Gao, C.; Liu, W.; Guo, J.; Liu, Z. In vitro and in vivo of triphenylamineappended fluorescent half-sandwich iridium(III) thiosemicarbazones antitumor complexes. Inorg. Chem. 2021, 60, 17063–17073. [Google Scholar] [CrossRef]
- Sava, G.; Zorzet, S.; Perissin, L.; Mestroni, G.; Zassinovich, G.; Bontempi, A. Coordination metal complexes of Rh (I), Ir (I) and Ru (II): Recent advances on antimetastatic activity on solid mouse tumors. Inorg. Chim. Acta 1987, 137, 69–71. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakaguchi, S. A novel catalysis of [{IrCl (Cod)} 2] Complex in Organic syntheses. Bull. Chem. Soc. Jpn. 2004, 77, 909–920. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; McCarthy, W.; Conway-Kenny, R.; Twamley, B.; Zhao, J.; Draper, S.M. Novel ruthenium and iridium complexes of N-substituted carbazole as triplet photosensitisers. Chem. Commun. 2018, 54, 1073–1076. [Google Scholar] [CrossRef]
- Pandrala, M.; Sundaraneedi, M.K.; Ammit, A.J.; Woodward, C.E.; Wallace, L.; Keene, F.R.; Collins, J.G. Differential anticancer activities of the geometric isomers of dinuclear iridium (III) complexes. Eur. J. Inorg. Chem. 2015, 34, 5694–5701. [Google Scholar] [CrossRef]
- Liu, B.; Monro, S.; Lystrom, L.; Cameron, C.G.; Colón, K.; Yin, H.; Kilina, S.; McFarland, S.A.; Sun, W. Photophysical and Photobiological Properties of Dinuclear Iridium(III) Bis-Tridentate Complexes. Inorg. Chem. 2018, 57, 9859–9872. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Saunders, G.C.; Raymond, O.; Ma, X.; Henderson, W. Pyrrole thioamide complexes of organo-rhodium(III), -iridium(III) and -ruthenium(II). Inorg. Chim. Acta 2020, 506, 119545. [Google Scholar] [CrossRef]
- Giorgi, E.; Binacchi, F.; Marotta, C.; Cirri, D.; Gabbiani, C.; Pratesi, A. Highlights of New Strategies to Increase the Efficacy of Transition Metal Complexes for Cancer Treatments. Molecules 2023, 28, 273. [Google Scholar] [CrossRef]
- Shi, H.; Carter, O.W.; Ponte, F.; Imberti, C.; Gomez-Gonzalez, M.A.; Cacho-Nerin, F.; Sadler, P.J. A Photodynamic and Photochemotherapeutic Platinum-Iridium Charge-Transfer Conjugate for Anticancer Therapy. Ang. Chem. Intern. Ed. 2024, 63, e202400476. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, X.; Wang, W.; Shi, Z.; Zhang, Y.; Tian, Q.; Duan, C. Biomedical applications of multinuclear Pt (II)/Ru (II)/Ir (III) metallo-supramolecular assemblies for intensive cancer therapy. Coord. Chem. Rev. 2023, 495, 215366. [Google Scholar] [CrossRef]
- Tripathy, S.K.; De, U.; Dehury, N.; Pal, S.; Kim, H.S.; Patra, S. Dinuclear [{(p-cym) RuCl}2(μ-phpy)](PF6)2 and heterodinuclear [(ppy)2Ir(μ-phpy)Ru(p-cym)Cl](PF6)2 complexes: Synthesis, structure and anticancer activity. Dalt. Trans. 2014, 43, 14546–14549. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, P.Y.; Wang, Y.T.; Yang, G.F.; Zhang, A.; Miao, Z.H. An update on poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors: Opportunities and challenges in cancer therapy. J. Med. Chem. 2016, 59, 9575–9598. [Google Scholar] [CrossRef]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Nallas, G.N.A.; Jones, S.W.; Brewer, K.J. Bipyrimidine-bridged mixed-metal trimetallic complexes of ruthenium (II) with rhodium (III) or iridium (III), {[(bpy)2Ru (bpm)]2MCl2}5+. Inorg. Chem. 1996, 35, 6974–6980. [Google Scholar] [CrossRef]
- Kar, B.; Roy, N.; Pete, S.; Moharana, P.; Paira, P. Ruthenium and iridium based mononuclear and multinuclear complexes: A Breakthrough of Next-Generation anticancer metallopharmaceuticals. Inorg. Chim. Acta 2020, 512, 119858. [Google Scholar] [CrossRef]
- Bridgewater, J.S.; Vogler, L.M.; Molnar, S.M.; Brewer, K.J. Tuning the spectroscopic and electrochemical properties of polypyridyl bridged mixed-metal trimetallic ruthenium (II), iridium (III) complexes: A spectroelectrochemical study. Inorg. Chim. Acta 1993, 208, 179–188. [Google Scholar] [CrossRef]
- Molnar, S.M.; Jensen, G.E.; Vogler, L.M.; Jones, S.W.; Laverman, L.; Bridgewater, J.S.; Brewer, K.J. Photochemical properties of mixed-metal supramolecular complexes. J. Photochem. Photobiol. A Chem. 1994, 80, 315–322. [Google Scholar] [CrossRef]
- Roy, N.; Sen, U.; Moharana, P.; Babu, L.T.; Kar, B.; Vardhan, S.; Paira, P. 2,2′- Bipyrimidine-based luminescent Ru(II)/Ir(III)–arene monometallic and homo- and hetero-bimetallic complexes for therapy against MDA-MB-468 and Caco-2 cells. Dalton Trans. 2021, 50, 11725–11729. [Google Scholar] [CrossRef]
- Roy, N.; Shanavas, S.; Kar, B.; Thilak Babu, L.; Das, U.; Vardhan, S.; Paira, P. G2/M-Phase-inhibitory mitochondrial-depolarizing Re(I)/Ru(II)/Ir(III)-2,2′-bipyrimidine-based heterobimetallic luminescent complexes: An assessment of in vitro antiproliferative activity and bioimaging for targeted therapy toward human TNBC cells. ACS Omega 2023, 8, 12283–12297. [Google Scholar] [CrossRef]
- Redrado, M.; Benedi, A.; Marzo, I.; García-Otín, A.L.; Fernández-Moreira, V.; Concepción Gimeno, M. Multifunctional Heterometallic IrIII−AuI Probes as Promising Anticancer and Antiangiogenic Agents. Chem. Eur. J. 2021, 27, 9885–9897. [Google Scholar] [CrossRef] [PubMed]
- Luengo, A.; Marzo, I.; Reback, M.; Daubit, I.M.; Fernández-Moreira, V.; Metzler-Nolte, N.; Gimeno, M.C. Luminescent bimetallic IrIII/AuI peptide bioconjugates as potential theranostic agents. Chem. Eur. J. 2020, 26, 12158–12167. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Koziel, S.; Pucelik, B.; Barzowska, A.; Siczek, M.; Malik, M.; Wojtala, D.; Niorettini, A.; Kyziol, A.; Sebastian, V.; et al. Liposomal binuclear Ir(III)-Cu(II) coordination compounds with phosphino-fluoroquinolone conjugates for human prostate carcinoma treatment. Inorg. Chem. 2022, 61, 19261–19273. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, S.; Komarnicka, U.K.; Ziółkowska, A.; SkórskaStania, A.; Pucelik, B.; Płotek, M.; Sebastian, V.; Bieńko, A.; Stochel, G.; Kyzioł, A. Anticancer potency of novel organometallic Ir(III) complexes with phosphine derivatives of fluoroquinolones encapsulated in polymeric micelles. Inorg. Chem. Front. 2020, 7, 3386–3401. [Google Scholar] [CrossRef]
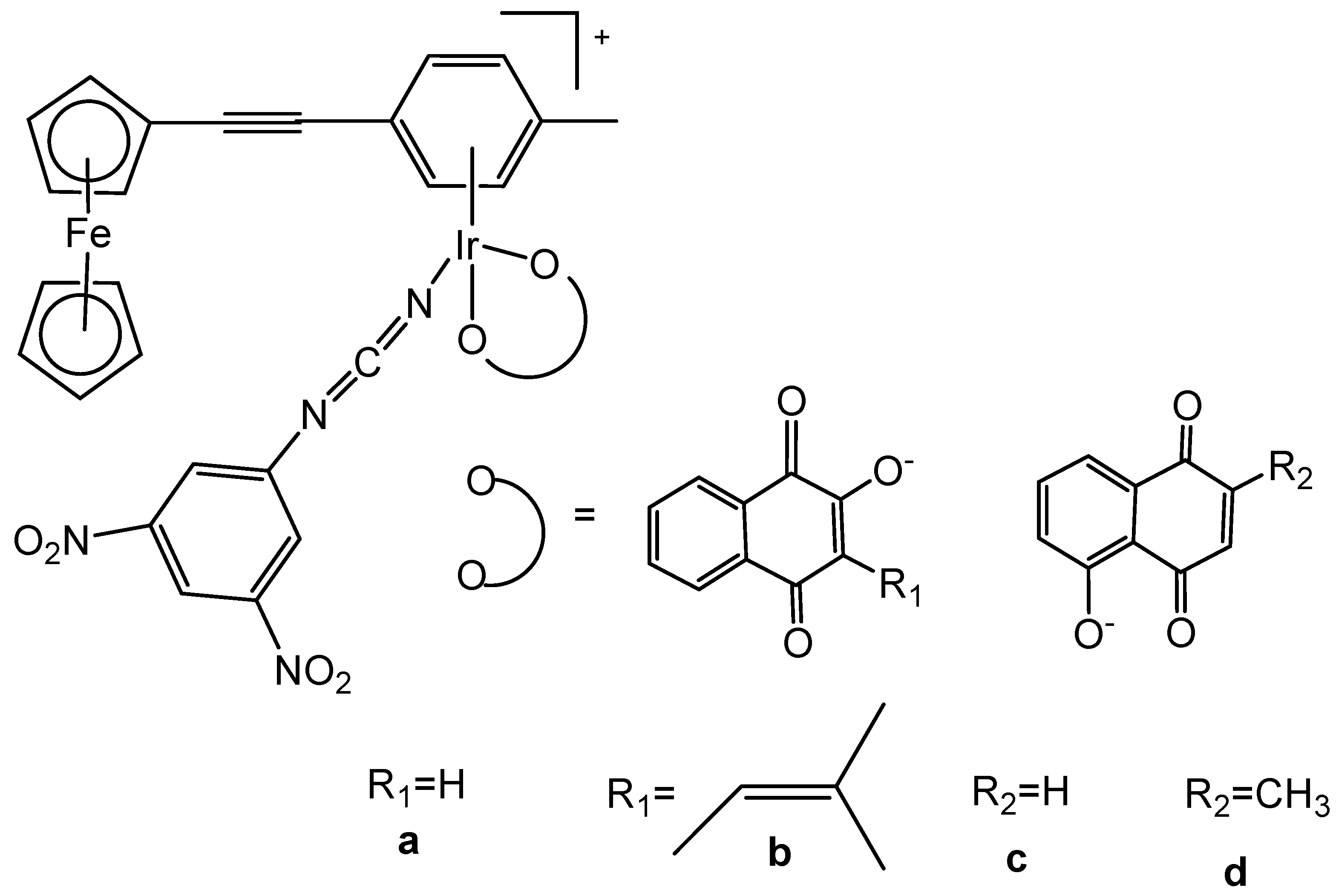
- Tabrizi, L.; Chiniforoshan, H. Designing new iridium (III) arene complexes of naphthoquinone derivatives as anticancer agents: A structure–activity relationship study. Dalton Trans. 2017, 46, 2339–2349. [Google Scholar] [CrossRef]
- Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
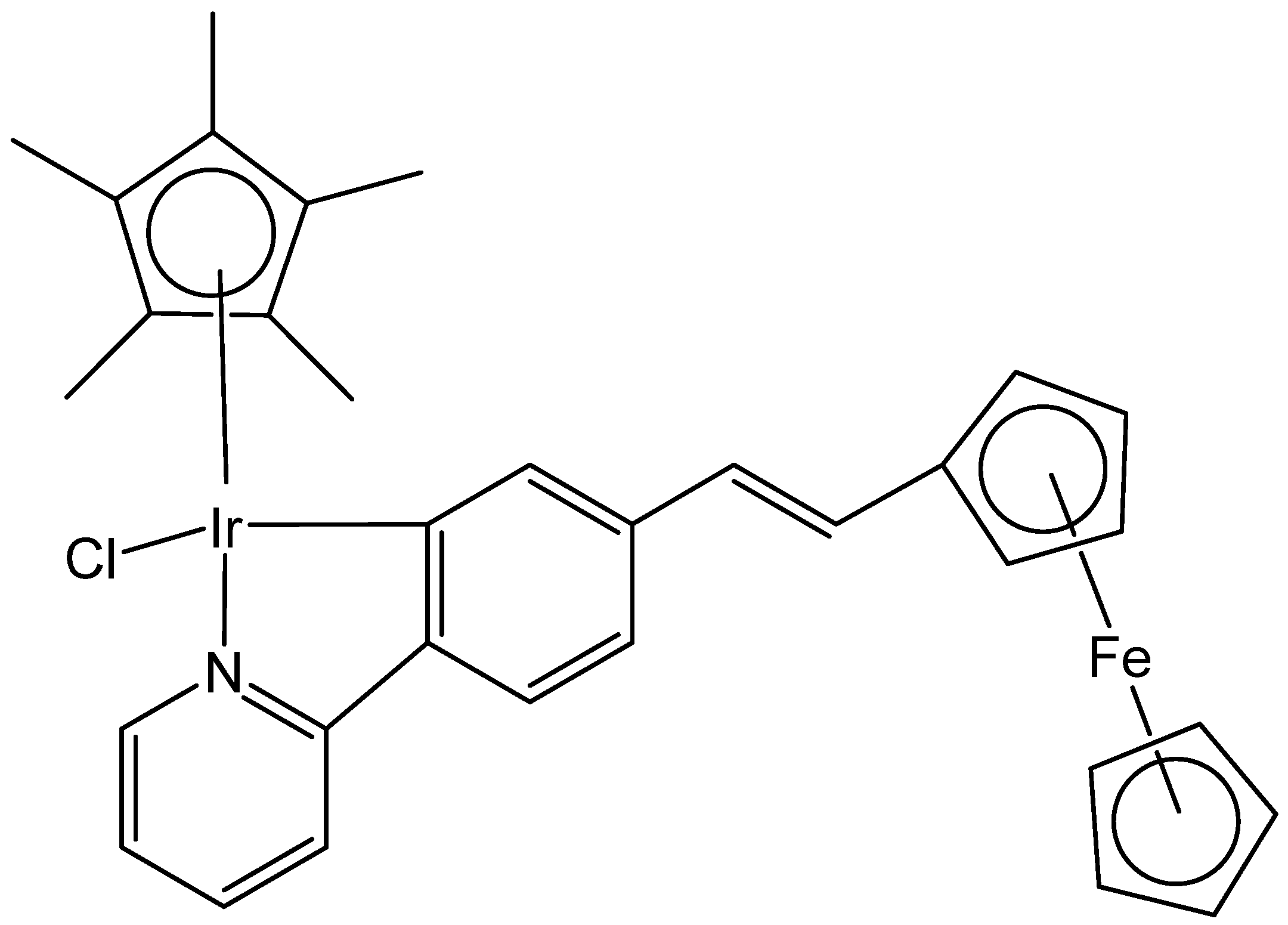
- Ge, X.; Chen, S.; Liu, X.; Wang, Q.; Gao, L.; Zhao, C.; Liu, Z. Ferrocene-appended iridium (III) complexes: Configuration regulation, anticancer application, and mechanism research. Inorg. Chem. 2019, 58, 14175–14184. [Google Scholar] [CrossRef]
- Palao, E.; Sola-Llano, R.; Tabero, A.; Manzano, H.; Agarrabeitia, A.R.; Villanueva, A.; Ortiz, M.J. AcetylacetonateBODIPY-biscyclometalated iridium(III) complexes: Effective strategy towards smarter fluorescent photosensitizer agents. Chemistry 2017, 23, 10139–10147. [Google Scholar] [CrossRef]
- Gupta, G.; Cherukommu, S.; Srinivas, G.; Lee, S.W.; Mun, S.H.; Jung, J.; Lee, C.Y. BODIPY-based Ru (II) and Ir (III) organometallic complexes of avobenzone, a sunscreen material: Potent anticancer agents. J. Inorg. Biochem. 2018, 189, 17–29. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Petrini, A.; Pettinari, C.; Lupidi, G.; Smolenski, P.; Dyson, P.J. From sunscreen to anticancer agent: Ruthenium (II) arene avobenzone complexes display potent anticancer activity. Organometallics 2016, 35, 3734–3742. [Google Scholar] [CrossRef]
- Gupta, G.; Das, A.; Ghate, N.B.; Kim, T.; Ryu, J.Y.; Lee, J.; Lee, C.Y. Novel BODIPY-based Ru (II) and Ir (III) metalla-rectangles: Cellular localization of compounds and their antiproliferative activities. Chem. Commun. 2016, 52, 4274–4277. [Google Scholar] [CrossRef]
- Gupta, G.; Das, A.; Park, K.C.; Tron, A.; Kim, H.; Mun, J. Self-assembled novel BODIPY-based palladium supramolecules and their cellular localization. Inorg. Chem. 2017, 56, 4615–4621. [Google Scholar] [CrossRef]
- Gupta, G.; Mahesh Kumar, J.; Garci, A.; Rangaraj, N.; Nagesh, N.; Therrien, B. Anticancer Activity of Half-Sandwich RhIII and IrIII Metalla-Prisms Containing Lipophilic Side Chains. ChemPlusChem 2014, 79, 610–618. [Google Scholar] [CrossRef]
- Gupta, G.; Murray, B.S.; Dyson, P.J.; Therrien, B. Synthesis, molecular structure and cytotoxicity of molecular materials based on water soluble half-sandwich Rh (III) and Ir (III) tetranuclear metalla-cycles. Materials 2013, 6, 5352–5366. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Kumar, J.M.; Garci, A.; Nagesh, N.; Therrien, B. Exploiting natural products to build metallaassemblies: The anticancer activity of embelin-derived Rh (III) and Ir(III) metalla-rectangles. Molecules 2014, 19, 6031–6046. [Google Scholar] [CrossRef]
- Nikolovska-Coleska, Z.; Xu, L.; Hu, Z.; Tomita, Y.; Li, P.; Roller, P.P.; Wang, S. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J. Med. Chem. 2004, 47, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Denoyelle-Di-Muro, E.; Mbakidi, J.P.; Leroy-Lhez, S.; Sol, V.; Therrien, B. Delivery of porphin to cancer cells by organometallic Rh (III) and Ir (III) metalla-cages. J. Organomet. Chem. 2015, 787, 44–50. [Google Scholar] [CrossRef]
- Martir, D.R.; Zysman-Colman, E. Photoactive supramolecular cages incorporating Ru (II) and Ir (III) metal complexes. Chem. Commun. 2019, 55, 139–158. [Google Scholar] [CrossRef]
- Gupta, G.; Oggu, G.S.; Nagesh, N.; Bokara, K.K.; Therrien, B. Anticancer activity of large metalla-assemblies built from half-sandwich complexes. CrystEngComm. 2016, 18, 4952–4957. [Google Scholar] [CrossRef]
- Gupta, G.; Patel, P.; Lee, J.; Kaushik, N.; Choi, E.H.; Kaushik, N.K.; Lee, C.Y. Bi-anthracene based ruthenium and iridium metalla-rectangles: Coordination driven self-assembly and anti-cancer activities. Inorg. Chem. Commun. 2025, 174, 113916. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; He, C.; Zhang, R.; Duan, C. Multicomponent self-assembly of a pentanuclear Ir–Zn heterometal–organic polyhedron for carbon dioxide fixation and sulfite sequestration. Chem. Commun. 2016, 52, 5104–5107. [Google Scholar] [CrossRef] [PubMed]
- Oldknow, S.; Martir, D.R.; Pritchard, V.E.; Blitz, M.A.; Fishwick, C.W.; Zysman-Colman, E.; Hardie, M.J. Structure-switching M3L2 Ir (III) coordination cages with photo-isomerising azo-aromatic linkers. Chem. Sci. 2018, 9, 8150–8159. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Lu, Y.; Guo, J.; Zhu, C.; He, H.; Su, C. An iridium (III)-palladium (II) metal-organic cage for efficient mitochondria-targeted photodynamic therapy. Chin. Chem. Lett. 2020, 31, 1183–1187. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yu, H.J.; Wang, Y.P.; Li, C.J.; Wang, X.F.; Ye, C.G.; Su, C.Y. A photoactive Ir–Pd bimetallic cage with high singlet oxygen yield for efficient one/two-photon activated photodynamic therapy. Mater. Chem. Front. 2022, 6, 948–955. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostova, I. Homo- and Hetero-Multinuclear Iridium(III) Complexes with Cytotoxic Activity. Inorganics 2025, 13, 156. https://doi.org/10.3390/inorganics13050156
Kostova I. Homo- and Hetero-Multinuclear Iridium(III) Complexes with Cytotoxic Activity. Inorganics. 2025; 13(5):156. https://doi.org/10.3390/inorganics13050156
Chicago/Turabian StyleKostova, Irena. 2025. "Homo- and Hetero-Multinuclear Iridium(III) Complexes with Cytotoxic Activity" Inorganics 13, no. 5: 156. https://doi.org/10.3390/inorganics13050156
APA StyleKostova, I. (2025). Homo- and Hetero-Multinuclear Iridium(III) Complexes with Cytotoxic Activity. Inorganics, 13(5), 156. https://doi.org/10.3390/inorganics13050156








