Novel Trimethoprim-Based Metal Complexes and Nanoparticle Functionalization: Synthesis, Structural Analysis, and Anticancer Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. HD Description
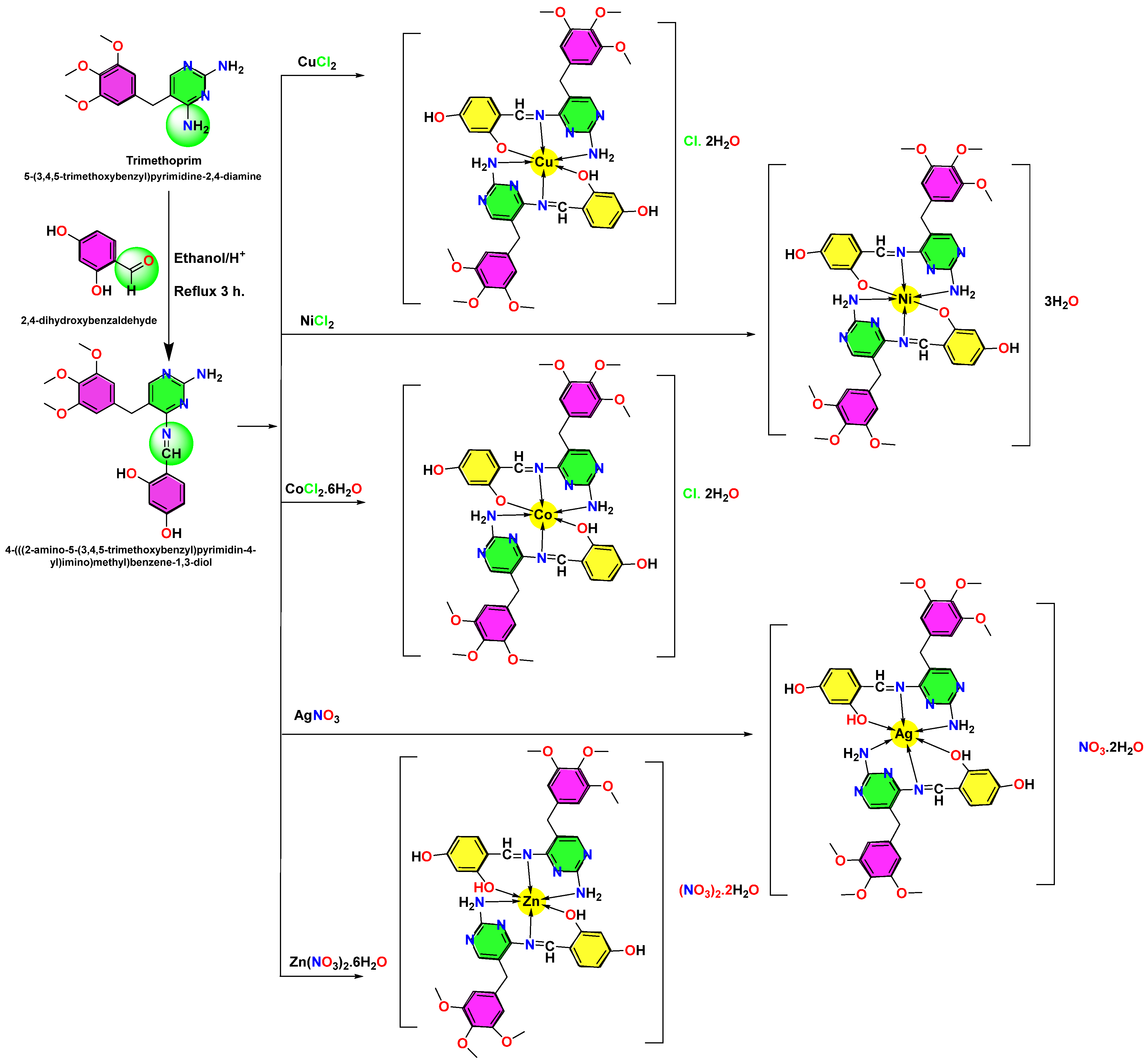
2.2. Complex Description
2.2.1. FTIR Spectra
2.2.2. 1H NMR
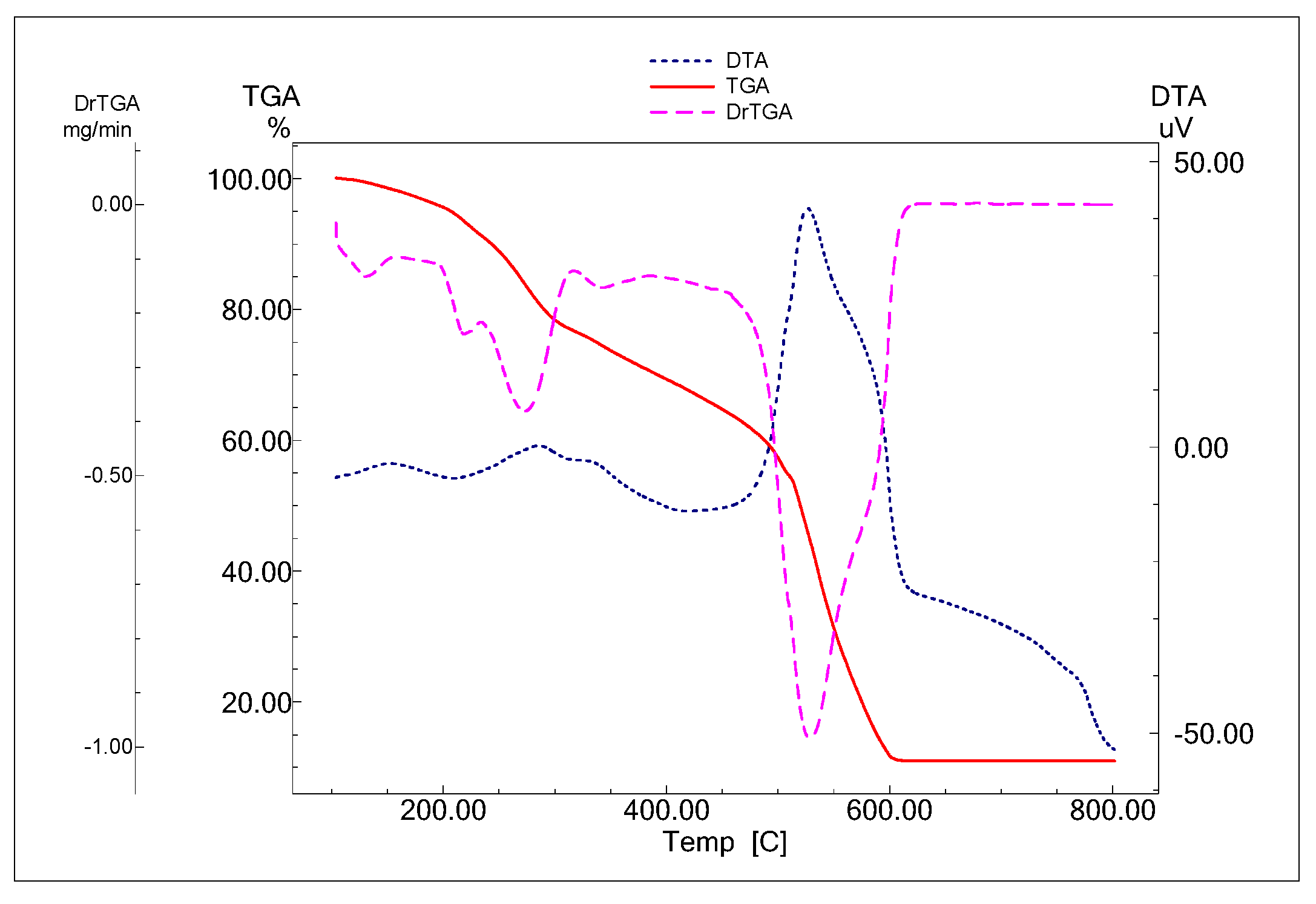
2.2.3. Thermal Analysis
2.2.4. Magnetic Moment and UV-Visible Spectra
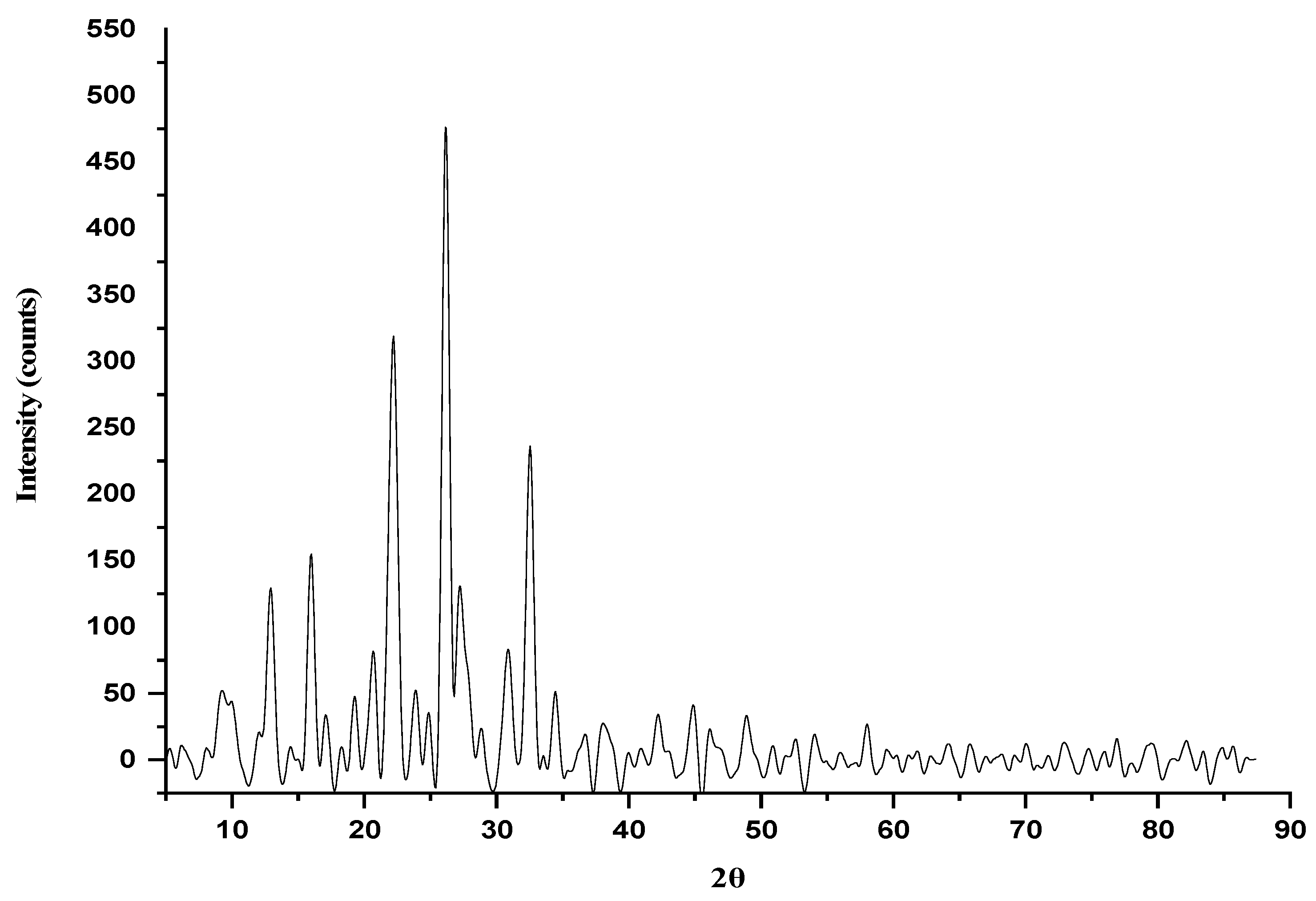
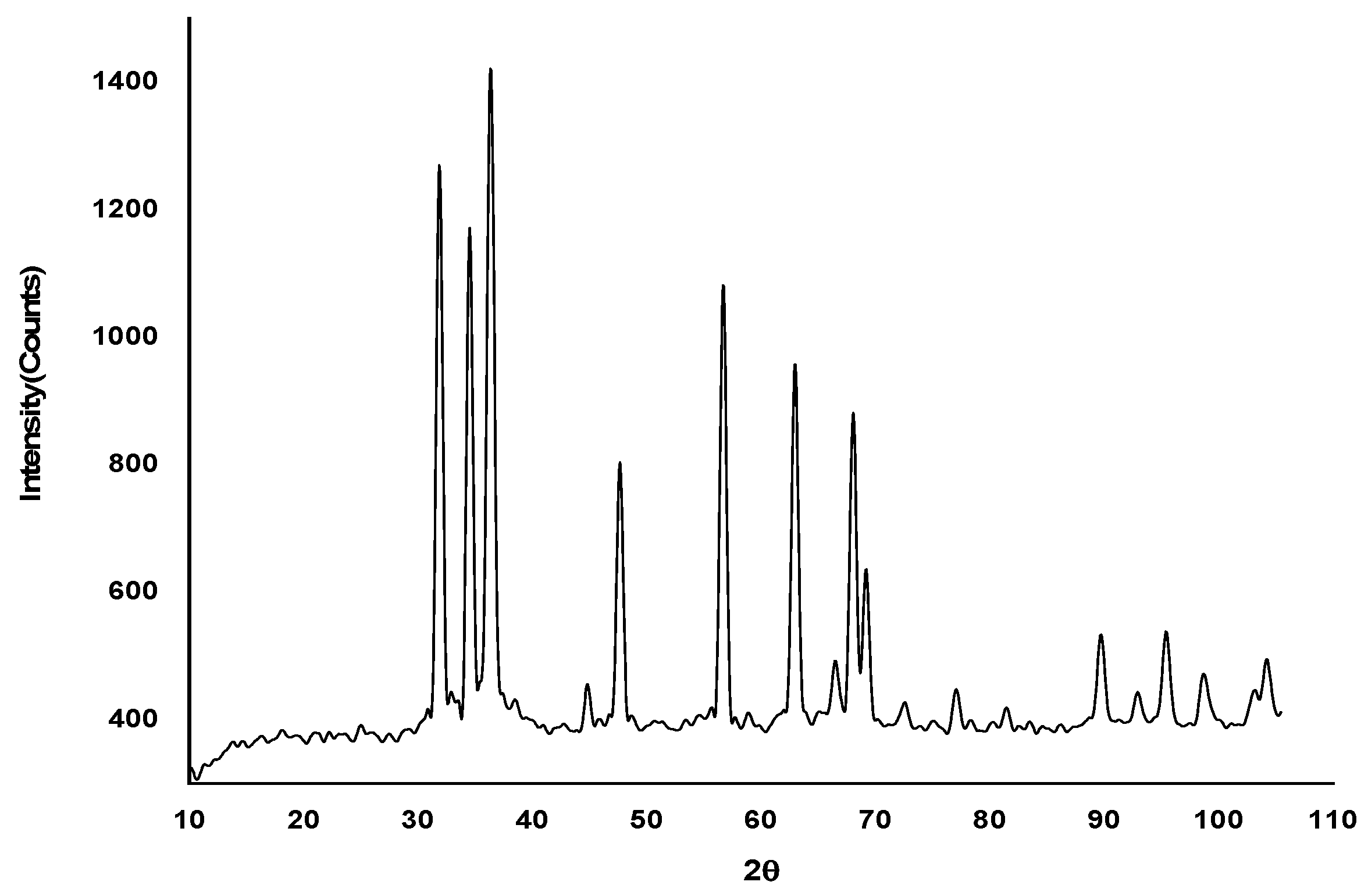
2.2.5. X-Ray Diffraction Studies (XRD)
2.3. Characterization of the Nanoparticles
2.3.1. UV–Vis Spectra for ZnONPs
2.3.2. UV–Vis Spectral for GNPs
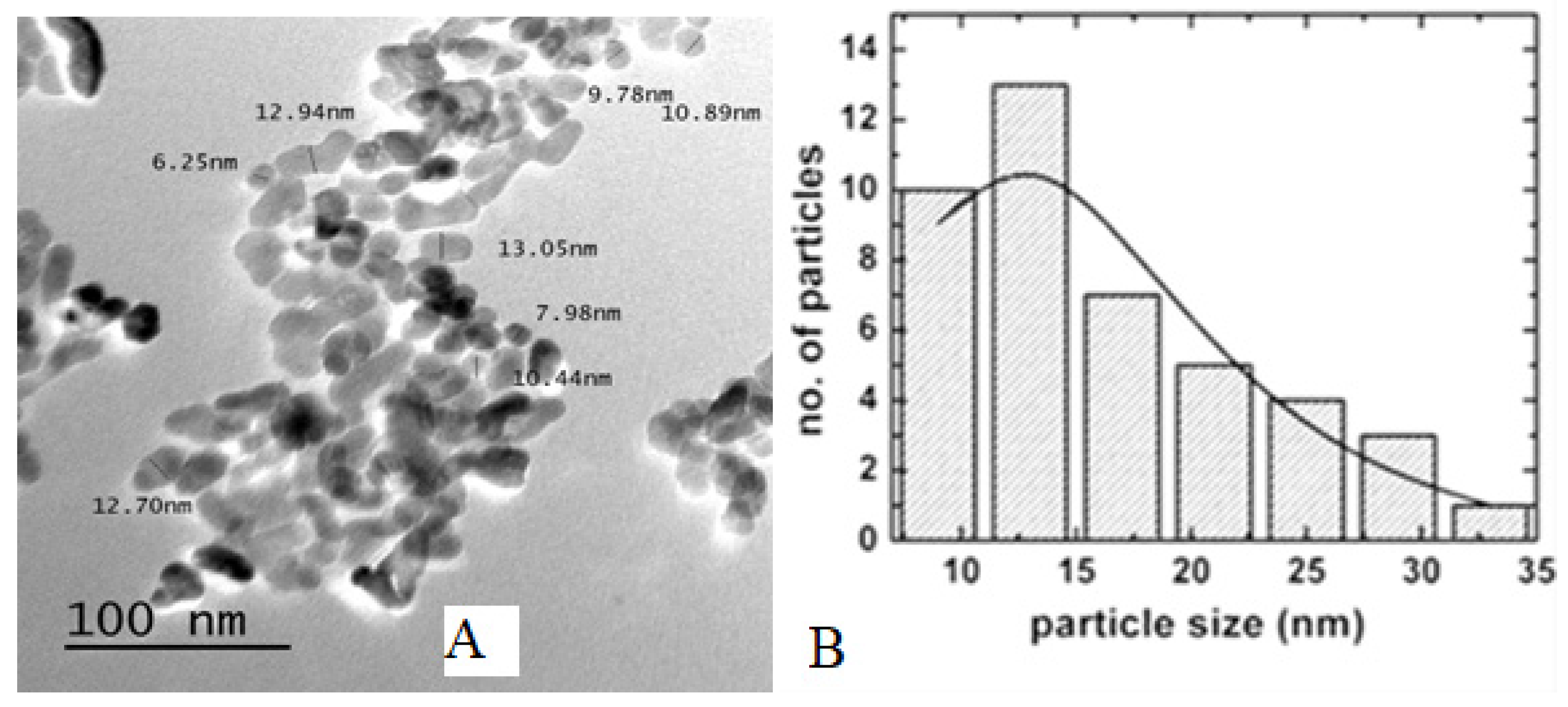
2.3.3. TEM
2.3.4. X-Ray Diffraction Studies (XRD)
2.3.5. FTIR
2.4. Computational Study
2.4.1. Molecular Mechanical Method (MM2)
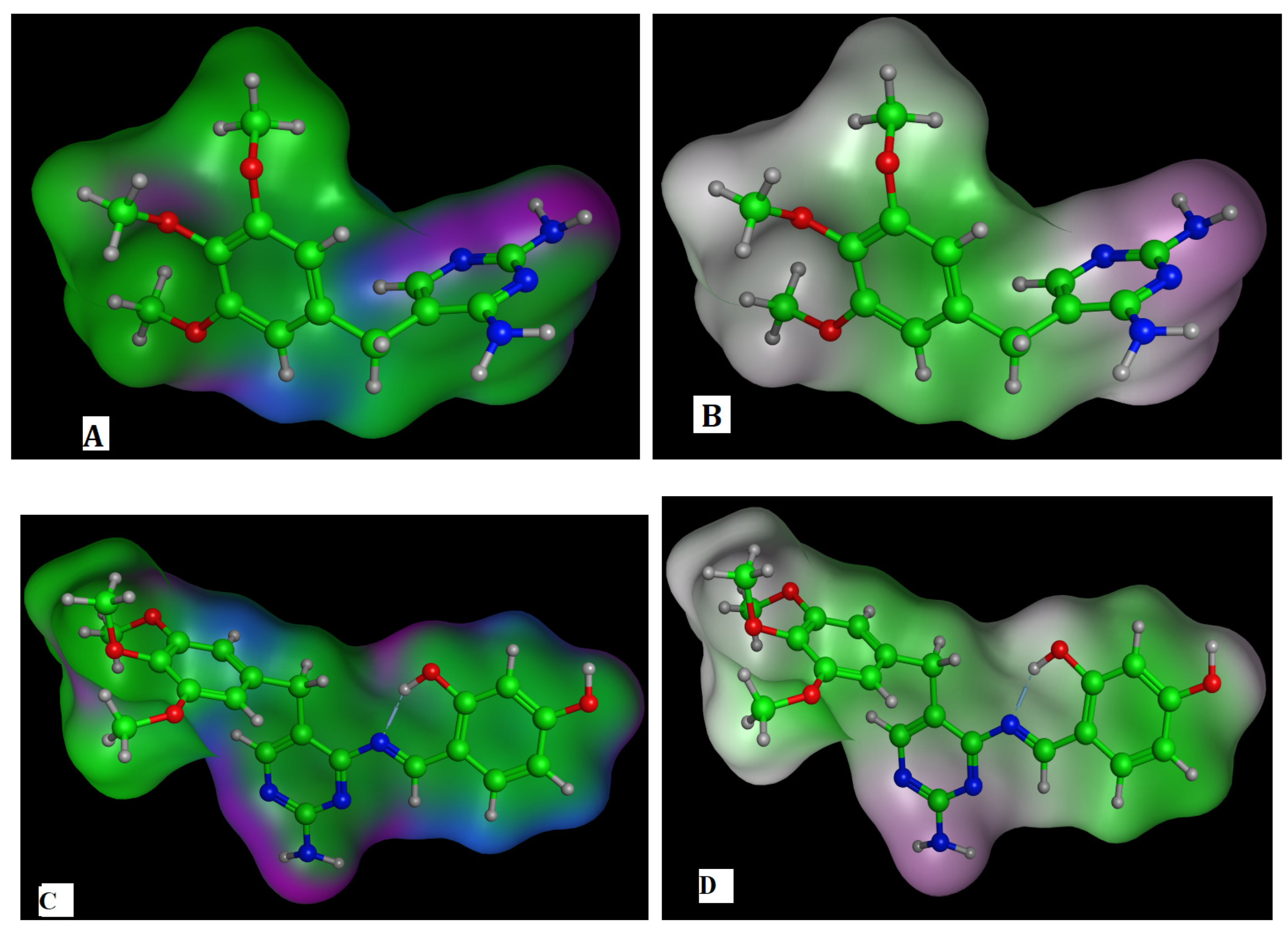
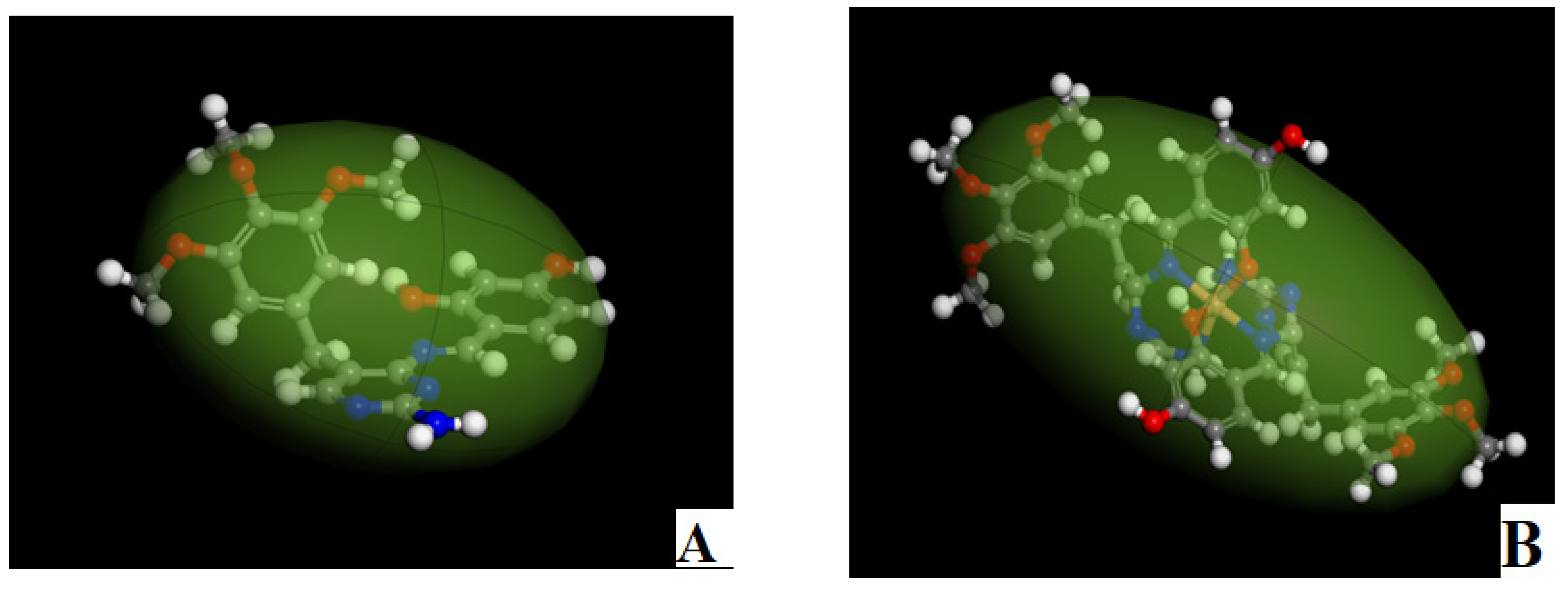
2.4.2. The Surface Properties of HD
2.4.3. The DFT for TMP and HD
- Changes between ground states outline local, non-local, and global hardness/softness functions.
- Soft-soft and hard-hard interactions are favored, with interactions progressing toward maximum hardness under chemical potential.
- These principles aid in understanding molecular behavior and response to different chemicals [52].
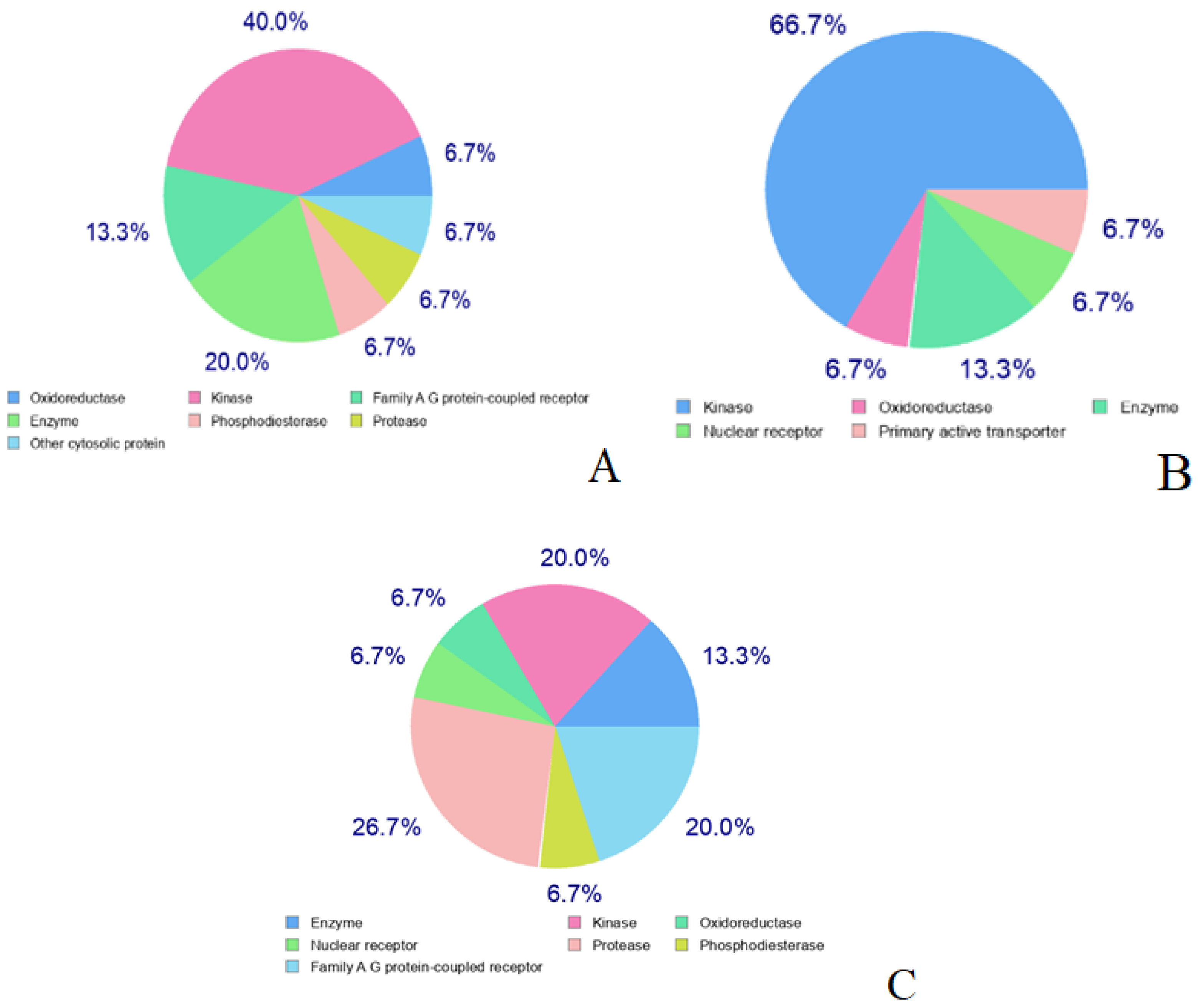
2.4.4. Target-Ligand Interaction Prediction
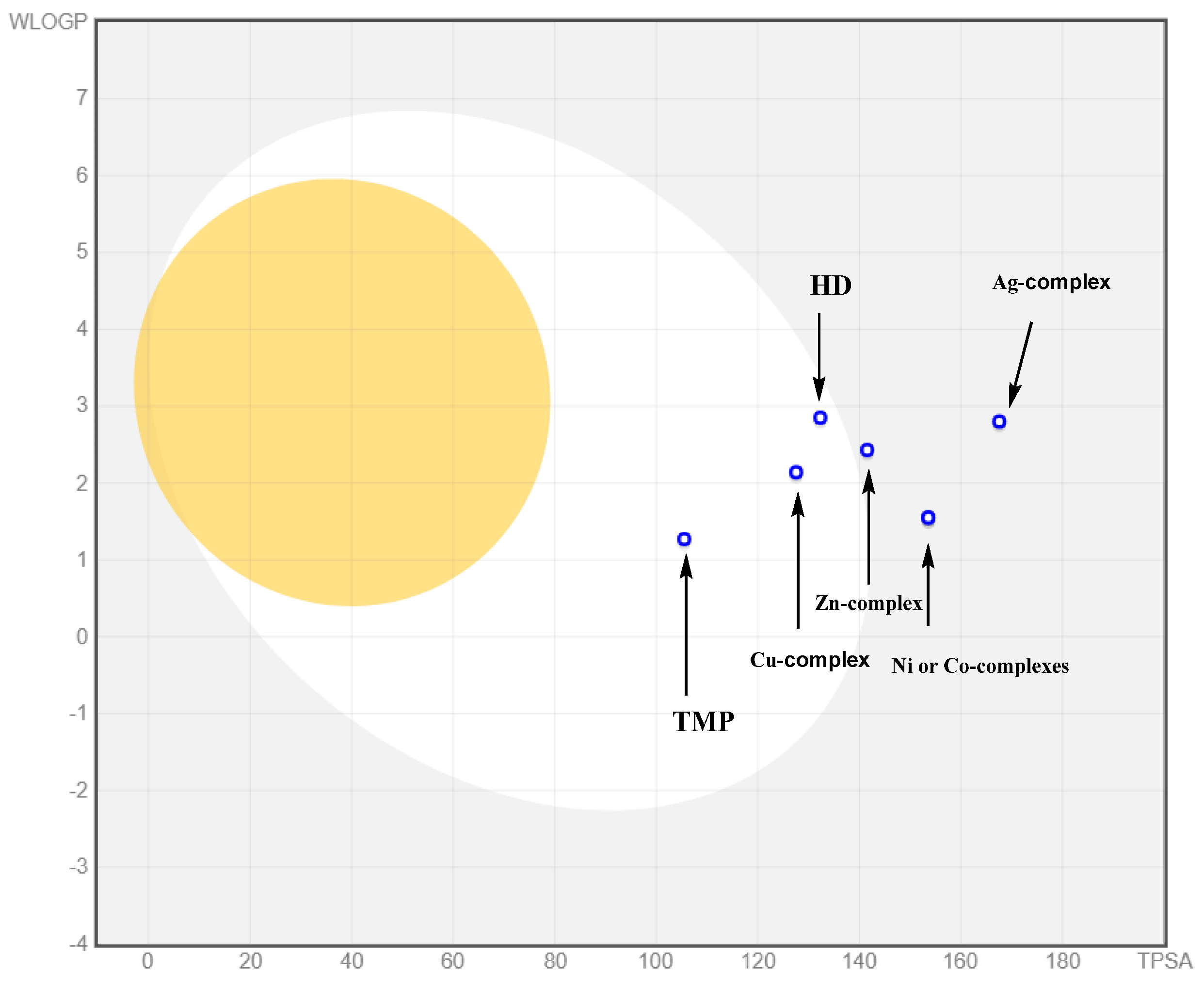
2.4.5. Biological Availability Estimation
2.4.6. Pharmacokinetic Properties
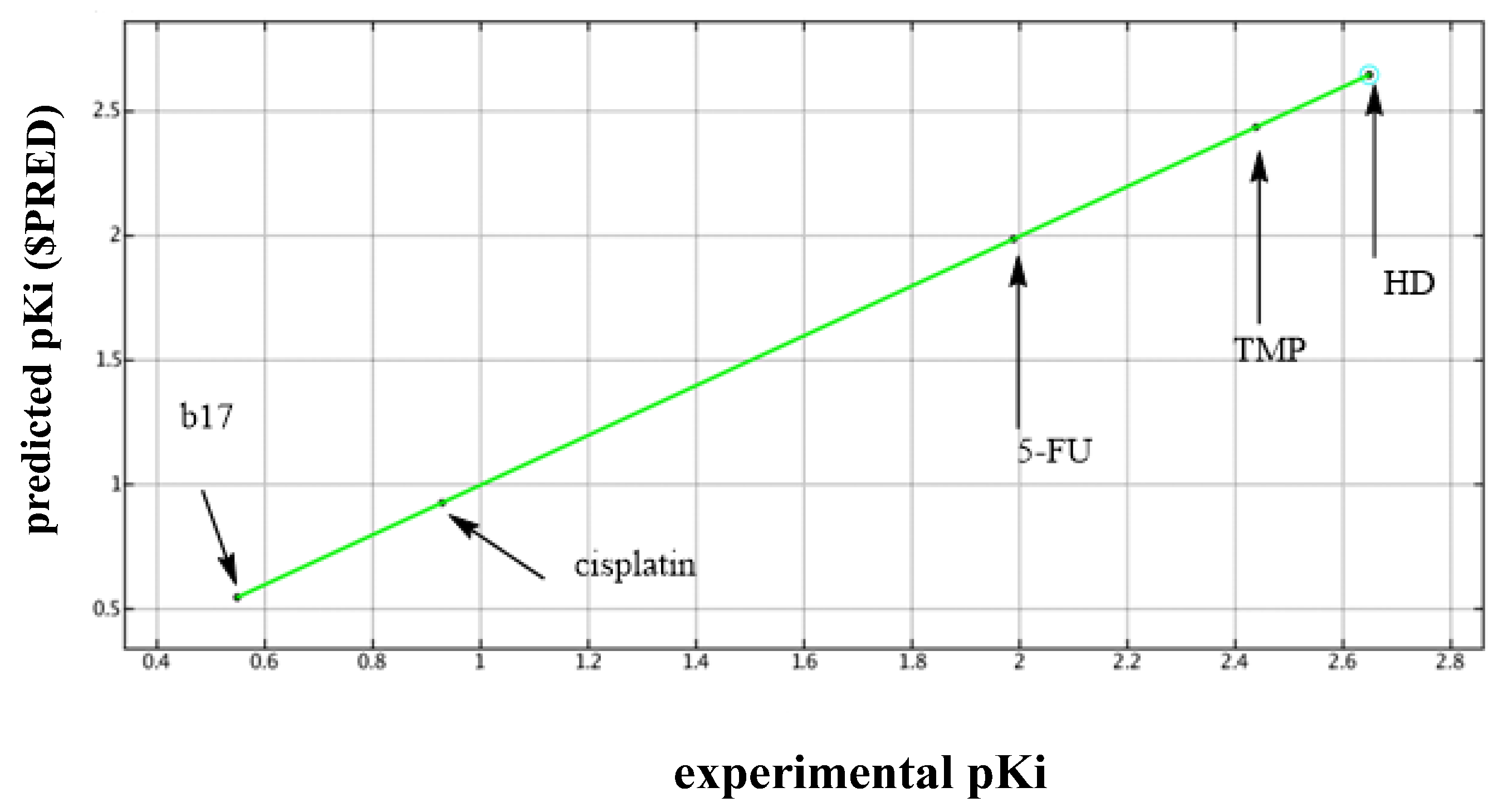
2.4.7. Quantitative Structure-Activity Relationship (QSAR)
D-QSAR Model
Principal Component Analysis (PCA)
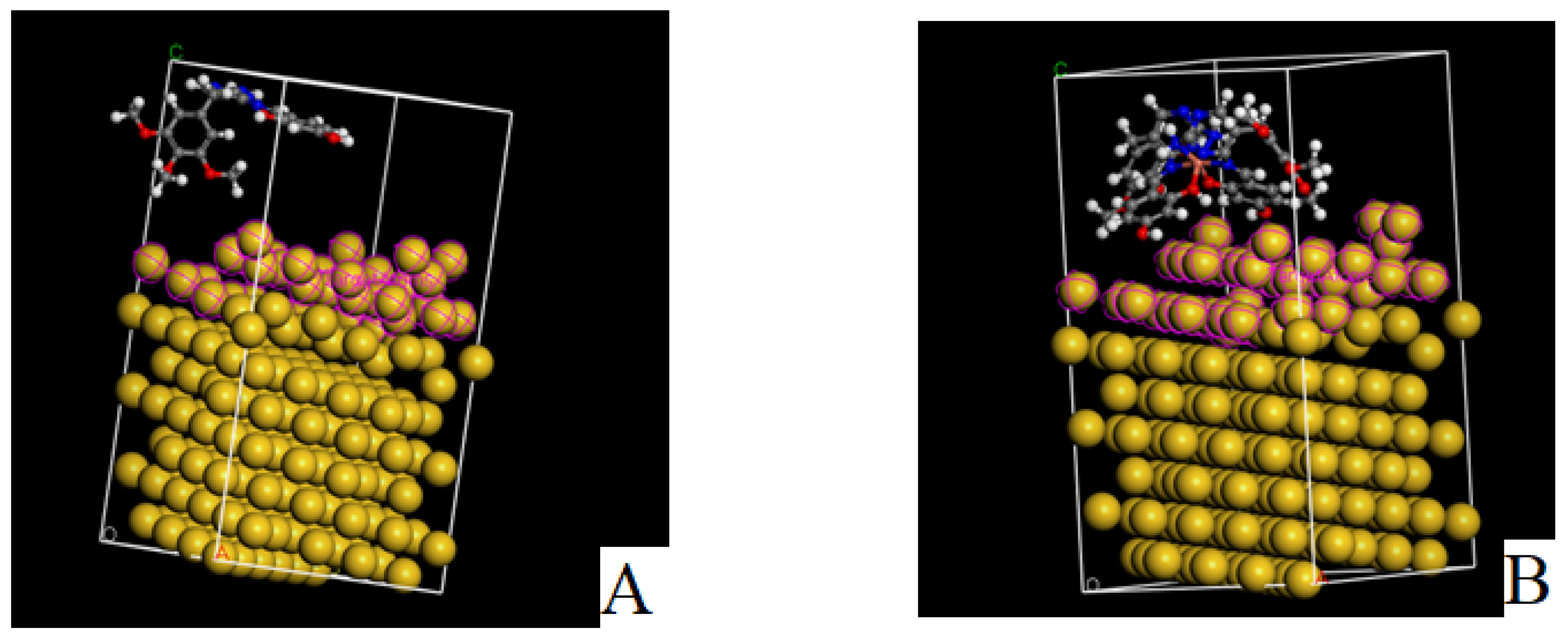
2.4.8. Prediction of Mechanical Properties of Nanoparticles
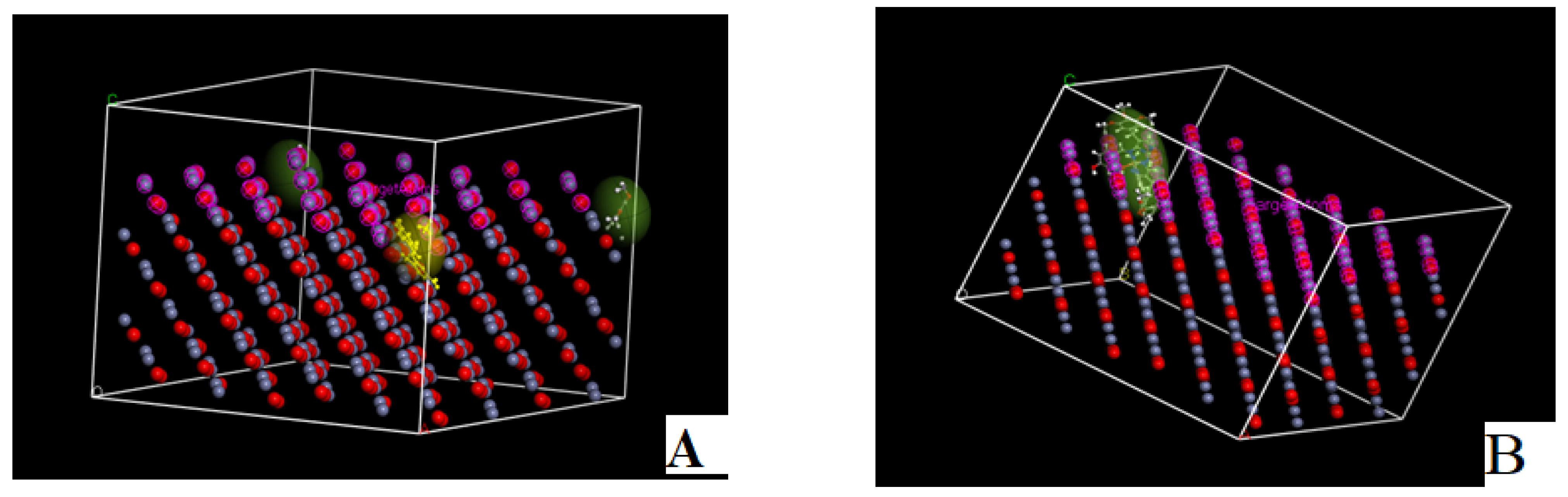
2.4.9. Molecular Docking
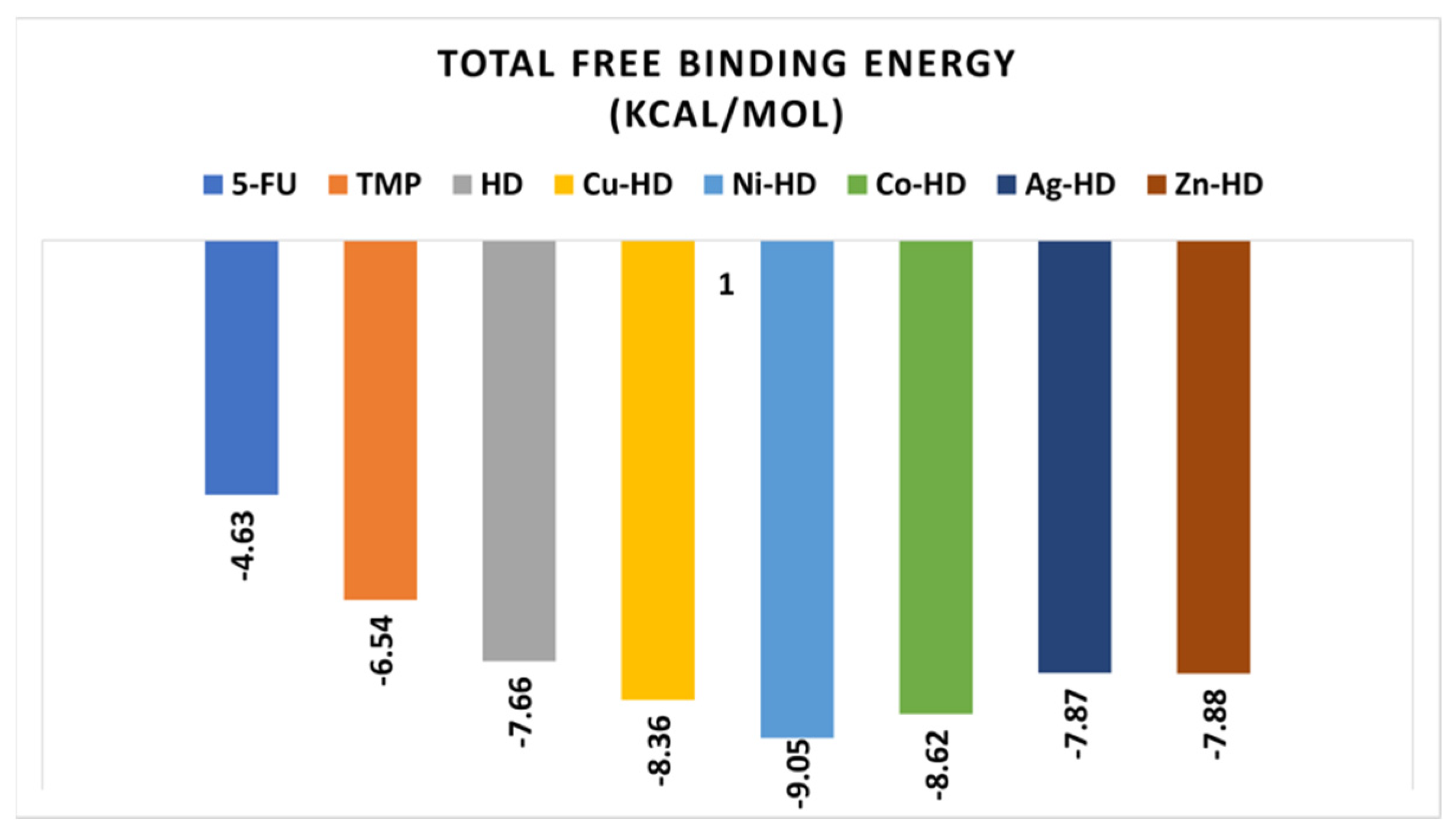
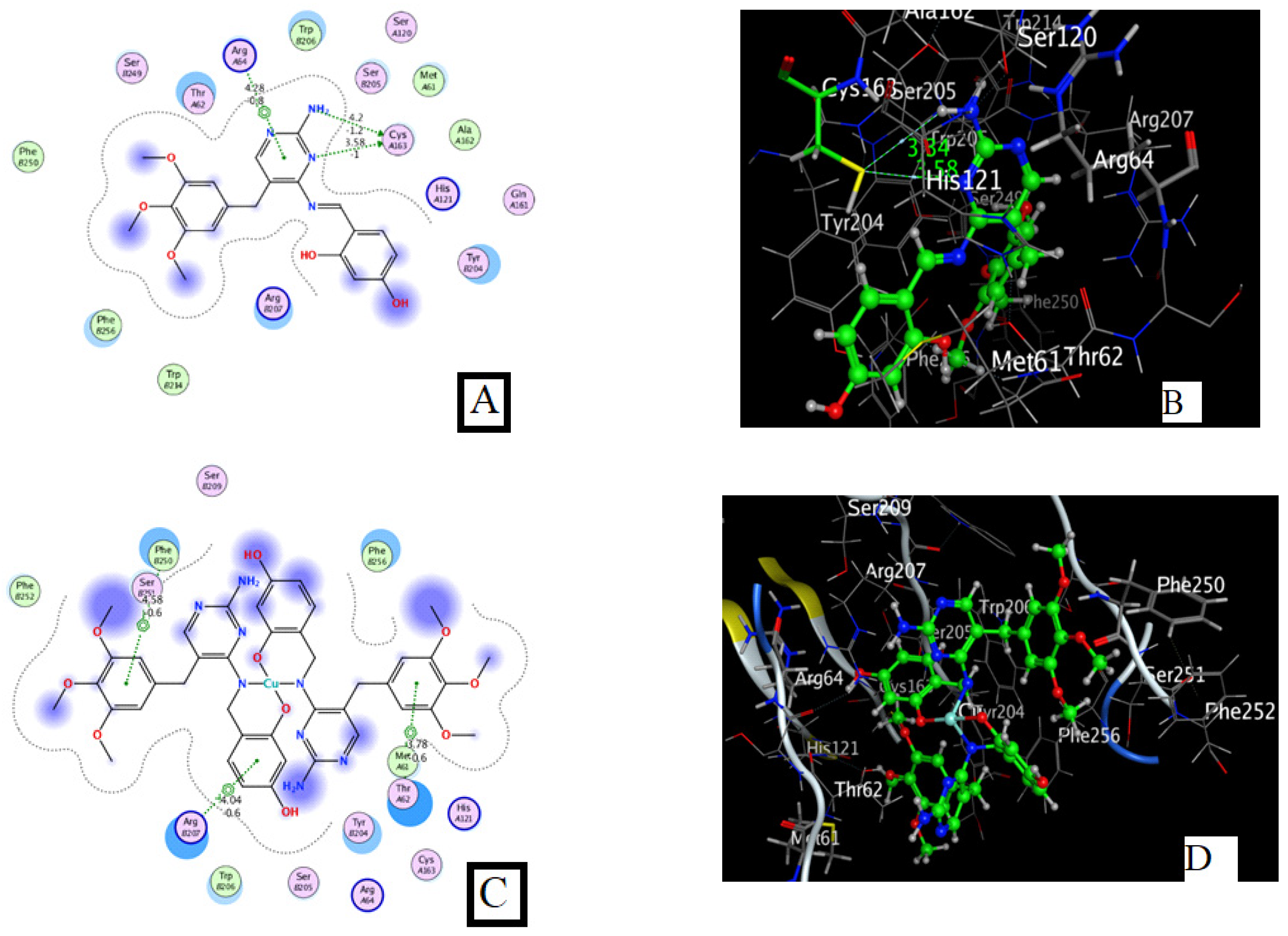
Docking of 5-Fluorouracil and Trimethoprim with Caspase-3 (PDB Code: 3GJQ)
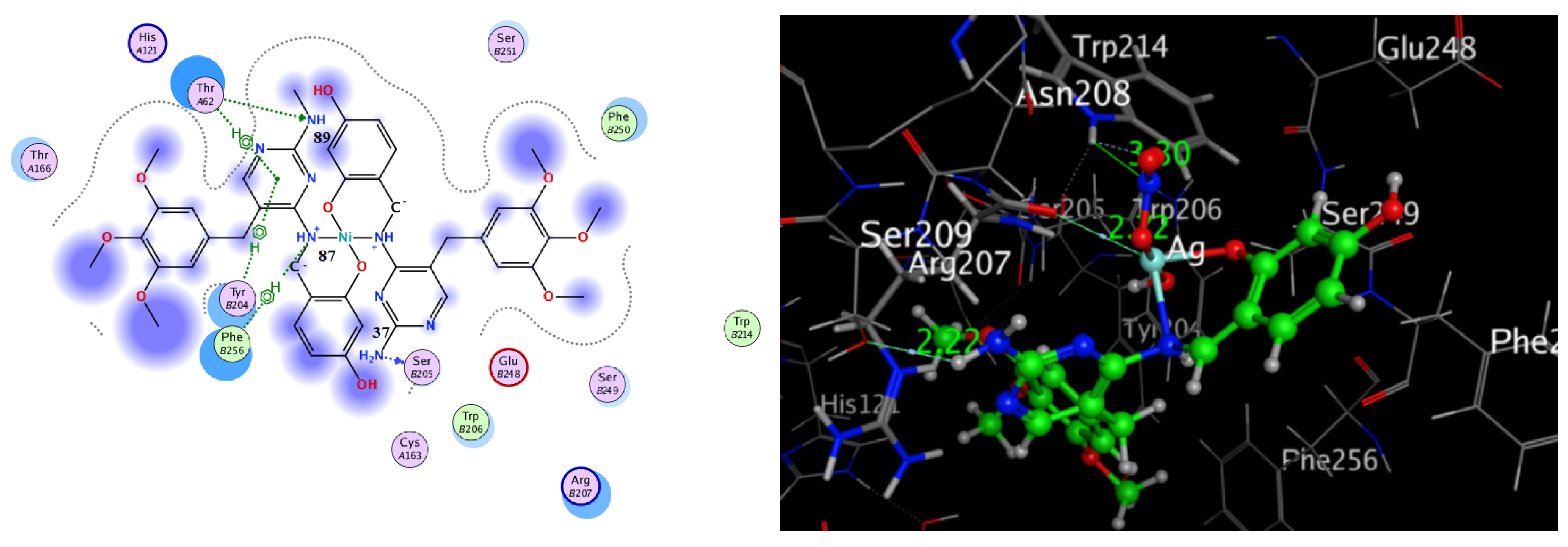
Docking of HD and Its Complexes with Caspase-3 (PDB Code: 3GJQ)
2.5. In Vitro Antitumor Activity
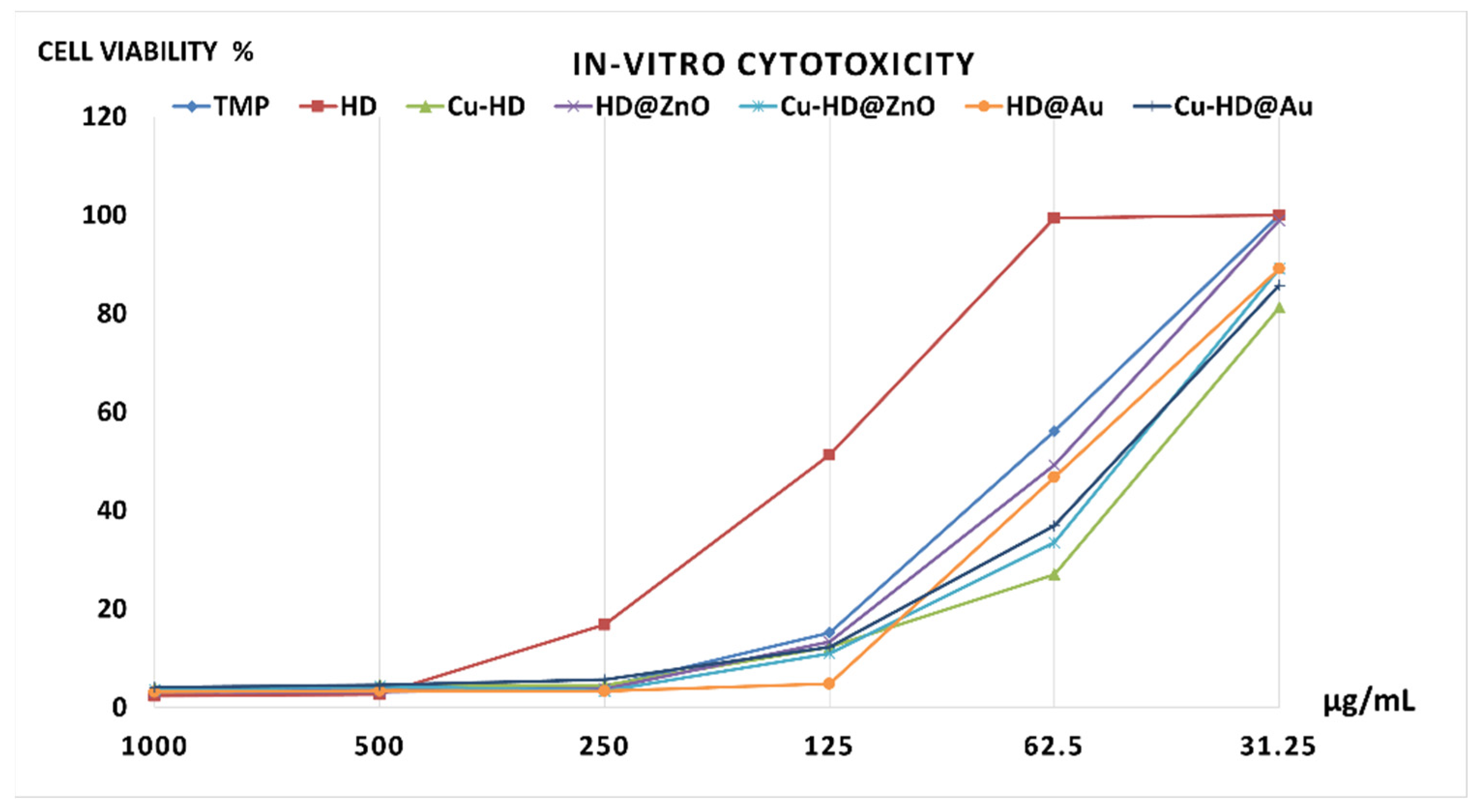
2.5.1. In Vitro Anticancer Activity by MTT Assay
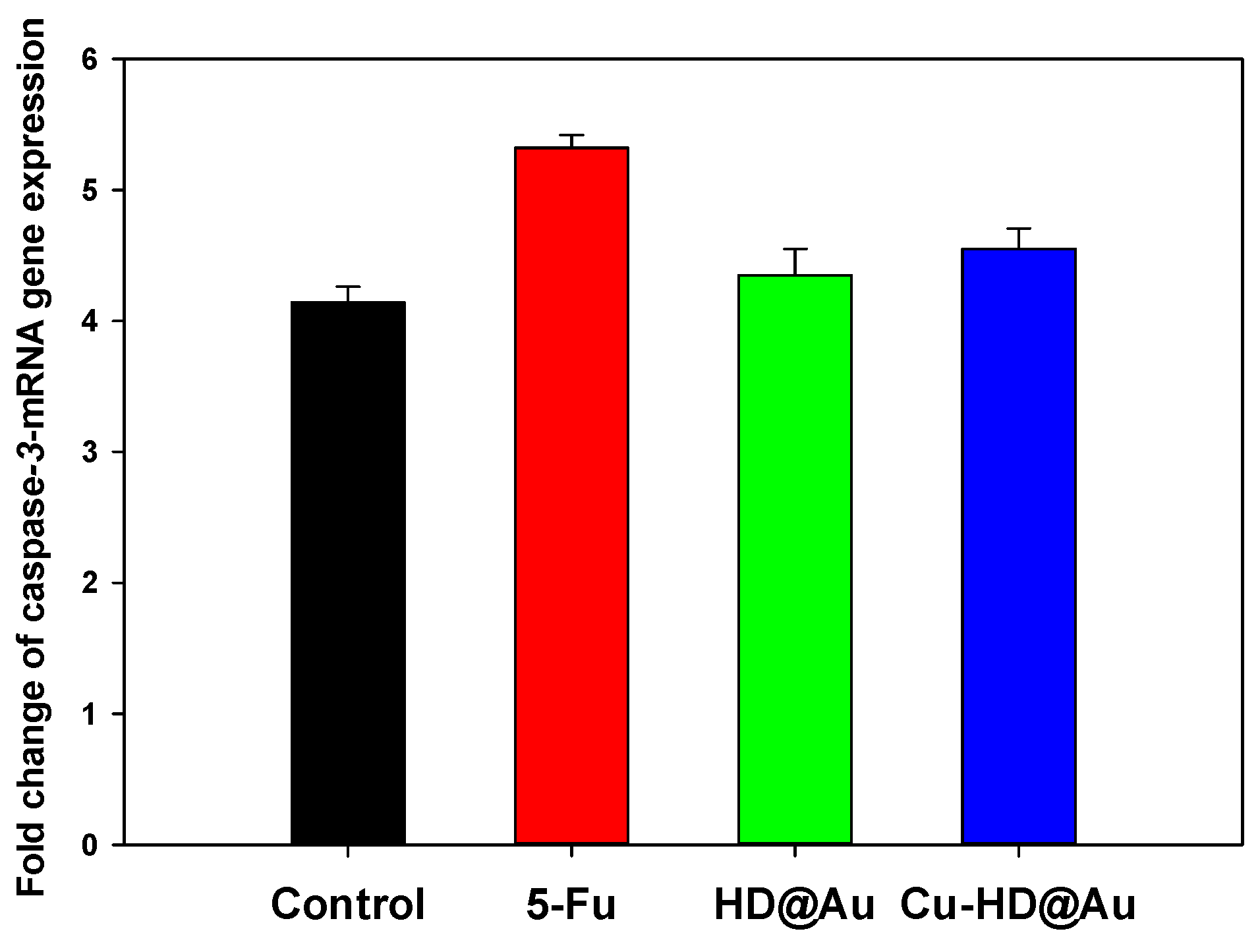
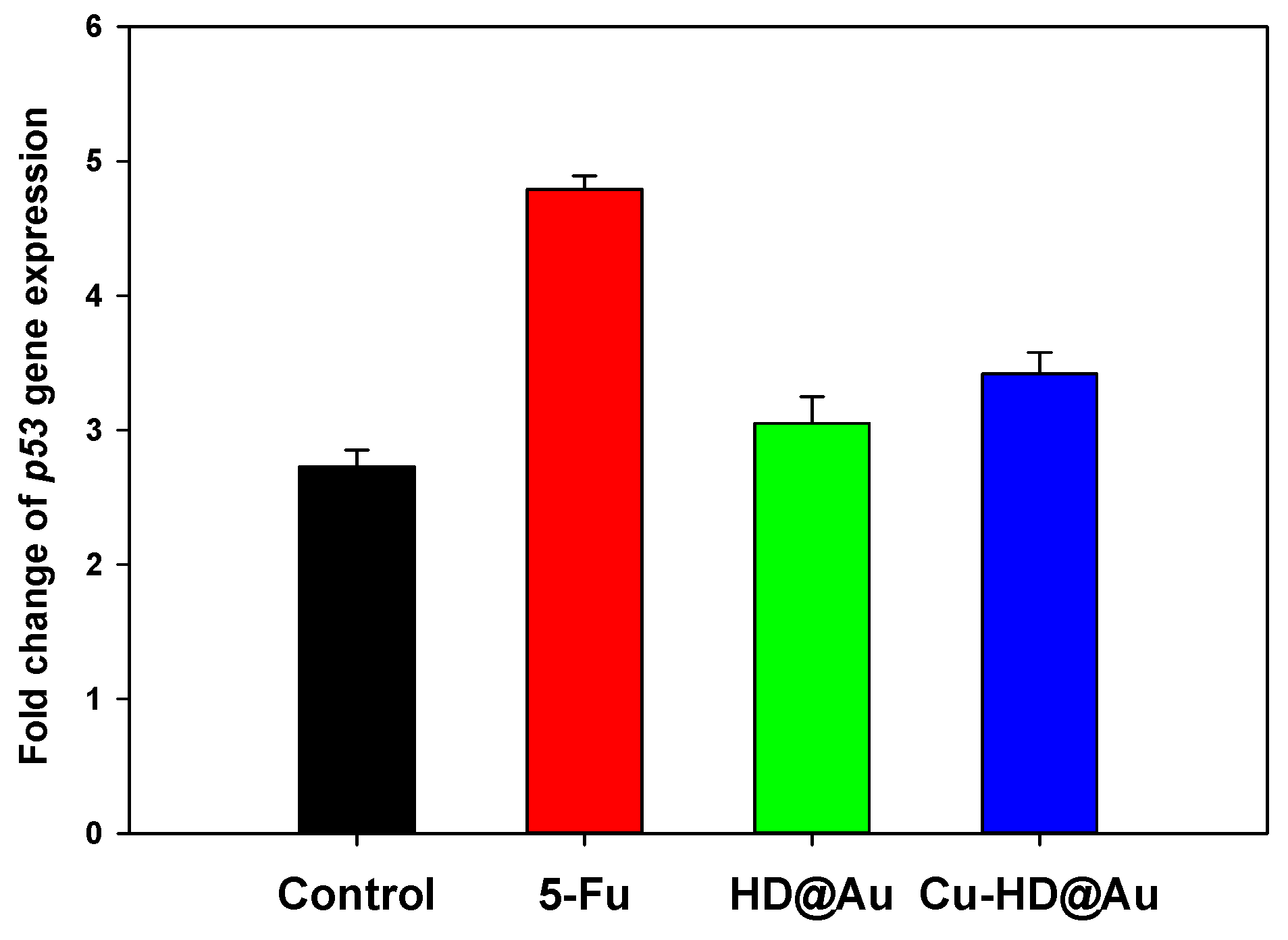
2.5.2. Quantitative Real-Time PCR
3. Experimental
3.1. Chemicals and Instrumentation
3.2. Synthesis
3.2.1. The HD Synthesis
3.2.2. The Complex Synthesis
3.2.3. ZnO Nanoparticles
3.2.4. The Au Nanoparticles
3.2.5. Functionalization @ZnO or @Au Nanoparticles
3.3. In Silico Studies
3.4. Antitumor Activity Evaluation
3.5. Quantitative Real-Time PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ali, E.-S.; Abeer, O.; Sama, A.-A.; Omar, A.-S. Anti-Cancer and Anti-Microbial Activity Studies of Some Complexes of Trimethoprim. J. Cancer Res. Updates 2013, 2, 14–20. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. Int. J. Pharm. Med. Chem. 2007, 340, 117–126. [Google Scholar] [CrossRef]
- Abbas, A.M.; Aboelmagd, A.; Kishk, S.M.; Nasrallah, H.H.; Boyd, W.C.; Kalil, H.; Orabi, A.S. A Novel Ibuprofen Derivative and Its Complexes: Physicochemical Characterization, DFT Modeling, Docking, In Vitro Anti-Inflammatory Studies, and DNA Interaction. Molecules 2022, 27, 7540. [Google Scholar] [CrossRef]
- Abbas, A.M.; Fisal, S.R.; Orabi, A.S. Novel β-lactam antibiotic derivative and its complexes: DFT, frontier energy levels, DNA interaction, docking, physicochemical and antimicrobial properties. J. Mol. Struct. 2020, 1218, 128487. [Google Scholar] [CrossRef]
- Abbas, A.M.; Fisal, S.R.; Orabi, A.S. Enhancement of the biochemical activity of some market antibiotics by chemical modification: Synthesis, characterization, and biochemical evaluation. J. Chin. Chem. Soc. 2021, 68, 131–149. [Google Scholar] [CrossRef]
- Abbas, A.M.; Fisal, S.R.; Radwan, A.; Makhlouf, M.; Orabi, A.S. Novel action for ampicillin derivative and its complexes: Physicochemical, thermal analysis, DNA interaction, docking with FabH protein, in silico, and in vitro studies. J. Mol. Liq. 2022, 351, 118333. [Google Scholar] [CrossRef]
- Scozzafava, A.; Menabuoni, L.; Mincione, F.; Mincione, G.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis of sulfonamides incorporating dtpa tails and of their zinc complexes with powerful topical antiglaucoma properties. Bioorg. Med. Chem. Lett. 2001, 11, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Enabling the chemistry of life. Nature 2001, 409, 226–231. [Google Scholar] [CrossRef]
- Bertini, I.; Gray, H.B.; Lippard, S.J.; Valentine, J.S. Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Mahmoud, M.A.; Sallam, S.A.; Abdelrahman, R.B.; Abbas, A.M. Studies of the molecular docking, antibacterial, antifungal, and DNA interaction of mixed-ligand Ni (II) complexes with gabapentin or pregabalin and diimine coligands. Inorg. Chim. Acta 2024, 564, 121959. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Idemudia, O.G. Synthesis, Characterization, and Antibacterial Studies of Pd(II) and Pt(II) Complexes of Some Diaminopyrimidine Derivatives. Bioinorg. Chem. Appl. 2013, 2013, 549549. [Google Scholar] [CrossRef]
- Demirezen, N.; Tarınç, D.; Polat, D.; Çeşme, M.; Gölcü, A.; Tümer, M. Synthesis of trimethoprim metal complexes: Spectral, electrochemical, thermal, DNA-binding and surface morphology studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 94, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Habib, U. DFT studies of temperature effect on coordination chemistry of Cu(II)-trimethoprim complexes. J. Coord. Chem. 2018, 71, 1102–1113. [Google Scholar] [CrossRef]
- Sekhon, B.S.; Randhawa, H.S.; Sahai, H.K. On the coordinating behaviour of trimethoprim towards some metal ions. Synth. React. Inorg. Met.-Org. Chem. 1999, 29, 309–321. [Google Scholar]
- Albedair, L.A. Iron(III), gold(III), platinum(IV) and palladium(II) trimethoprim drug complexes: Synthesis, spectroscopic, morphological and anticancer assessments. Rev. Roum. Chim. 2021, 65, 1145–1152. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W. Comparison of chemical and biological properties of metal nanoparticles (Au, Ag), with metal oxide nanoparticles (ZnO-NPs) and their applications. Adv. J. Chem. Sect. A 2020, 3, 111–236. [Google Scholar]
- Achilonu, C.C.; Kumar, P.; Swart, H.C.; Roos, W.D.; Marais, G.J. Zinc Oxide: Gold Nanoparticles (ZnO: Au NPs) Exhibited Antifungal Efficacy Against Aspergillus niger and Aspergillus candidus. BioNanoScience 2024, 14, 799–813. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold nanoparticles as potent anticancer agent: Green synthesis, characterization, and in vitro study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar]
- ElShaer, A.; Hanson, P.; Worthington, T.; Lambert, P.; Mohammed, A.R. Preparation and Characterization of Amino Acids-Based Trimethoprim Salts. Pharmaceutics 2012, 4, 179–196. [Google Scholar] [CrossRef]
- Florey, K.; Brittain, H.G.; Prankerd, R.J. Analytical Profiles of Drug Substances; Brittain, H.G., Ed.; Academic Press Inc.: Cambridge, MA, USA, 1993. [Google Scholar]
- Ungurean, A.; Leopold, N.; David, L.; Chiş, V. Vibrational spectroscopic and DFT study of trimethoprim. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 52–58. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Krishnan, A.R.; Saleem, H.; Thanikachalam, V.; Manikandan, G.; Erdogdu, Y. FT-IR, FT-Raman, NMR spectral analysis and theoretical NBO, HOMO–LUMO analysis of bis(4-amino-5-mercapto-1,2,4-triazol-3-yl)ethane by ab initio HF and DFT methods. J. Mol. Struct. 2010, 981, 59–70. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 386. [Google Scholar]
- Puviarasan, N.; Arjunan, V.; Mohan, S. FT-IR and FT-Raman Studies on 3-Aminophthalhydrazide and N-Aminophthalimide. Turk. J. Chem. 2002, 26, 4. [Google Scholar]
- Wang, L.; Cao, C.; Cao, C. Substituent effects on the stretching vibration of C═N in multi-substituted benzylideneanilines. J. Phys. Org. Chem. 2019, 32, e3969. [Google Scholar] [CrossRef]
- Manius, G.J. Trimethoprim. In Analytical Profiles of Drug Substances; Elsevier: Amsterdam, The Netherlands, 1978; Volume 7, pp. 445–475. [Google Scholar]
- Montazerozohori, M.; Musavi, S.A.; Masoudiasl, A.; Naghiha, A.; Dusek, M.; Kucerakova, M. Synthesis, spectral, crystal structure, thermal behavior, antimicrobial and DNA cleavage potential of two octahedral cadmium complexes: A supramolecular structure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Anacona, J.R. Synthesis and characterization of metal complexes with ampicillin. J. Coord. Chem. 1998, 44, 173–182. [Google Scholar] [CrossRef]
- El-Gamel, N.E.A. Metal chelates of ampicillin versus amoxicillin: Synthesis, structural investigation, and biological studies. J. Coord. Chem. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Abbas, A.; Salem, H.; Orabi, A. Synthesis, characterization, in vitro and in silico assessment of novel diclofenac derivative metal complexes. J. Mol. Struct. 2024, 1318, 139191. [Google Scholar] [CrossRef]
- Barszcz, B. Coordination properties of didentate N,O heterocyclic alcohols and aldehydes towards Cu(II), Co(II), Zn(II) and Cd(II) ions in the solid state and aqueous solution. Coord. Chem. Rev. 2005, 249, 2259–2276. [Google Scholar] [CrossRef]
- Yoshimura, T.; Miyake, C.; Imoto, S. Ultraviolet and Infrared Spectra of Cupferron and Neocupferron. Bull. Chem. Soc. Jpn. 1972, 45, 1424–1430. [Google Scholar] [CrossRef]
- Tong, M.M.; Brewer, D.G. Nature of the Coordination Bond in Metal Complexes of Substituted Pyridine Derivatives. VI. Electronic Spectra of Some Complexes of Copper(II). Can. J. Chem. 1971, 49, 102–104. [Google Scholar] [CrossRef]
- Dholakiya, P.P.; Patel, M.N. Preparation, magnetic, spectral, and biocidal studies of some transition metal complexes with 3, 5-dibromosalicylideneaniline and neutral bidentate ligands. Synth. React. Inorg. Met.-Org. Chem. 2002, 32, 819–829. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Abdulreazak, H.; Abood, H. Synthesis, Characterization, Theoretical Crystal Structure, and Antibacterial Activities of Some Transition Metal Complexes of the Thiosemicarbazone (Z)-2-(pyrrolidin-2-ylidene)hydrazinecarbothioamide. Bioinorg. Chem. Appl. 2011, 2011, 483101. [Google Scholar] [CrossRef]
- Altomare, A.; Camalli, M.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R. EXPO2009: Structure solution by powder data in direct and reciprocal space. J. Appl. Crystallogr. 2009, 42, 1197–1202. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Singh, G.; Babele, P.K.; Kumar, A.; Srivastava, A.; Sinha, R.P.; Tyagi, M.B. Synthesis of ZnO nanoparticles using the cell extract of the cyanobacterium, Anabaena strain L31 and its conjugation with UV-B absorbing compound shinorine. J. Photochem. Photobiol. B Biol. 2014, 138, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Yamaguchi, I. Optical Absorption Study of the Surface Plasmon Resonance in Gold Nanoparticles Immobilized onto a Gold Substrate by Self-Assembly Technique. J. Phys. Chem. B 2003, 107, 10321–10324. [Google Scholar] [CrossRef]
- Henglein, A. Physicochemical properties of small metal particles in solution: “microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 1993, 97, 5457–5471. [Google Scholar] [CrossRef]
- Hasan, M.; Bethell, D.; Brust, M. The fate of sulfur-bound hydrogen on formation of self-assembled thiol monolayers on gold: 1H NMR spectroscopic evidence from solutions of gold clusters. J. Am. Chem. Soc. 2002, 124, 1132–1133. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Soontaranon, S.; Hemvichian, K.; Lertsarawut, P.; Laksee, S.; Picha, R. Study on particle size and size distribution of gold nanoparticles by TEM and SAXS. Radiat. Phys. Chem. 2022, 191, 109842. [Google Scholar] [CrossRef]
- Mayoral, Á.; Agúndez, J.; Pascual-Valderrama, I.M.; Pérez-Pariente, J. Generation of gold nanoparticles according to procedures described in the eighteenth century. Gold Bull. 2014, 47, 161–165. [Google Scholar] [CrossRef]
- Chandrappa, K.G.; Venkatesha, T.V.; Vathsala, K.; Shivakumara, C. A hybrid electrochemical–thermal method for the preparation of large ZnO nanoparticles. J. Nanopart. Res. 2010, 12, 2667–2678. [Google Scholar] [CrossRef]
- Mitra, P.; Mondal, S. Structural and Morphological Characterization of ZnO thin Films Synthesized by SILAR. Prog. Theor. Appl. Phys. 2013, 1, 17–31. [Google Scholar]
- Hisaindee, S.; Al-Kaabi, L.; Ajeb, S.; Torky, Y.; Iratni, R.; Saleh, N.i.; AbuQamar, S.F. Antipathogenic effects of structurally-related Schiff base derivatives: Structure–activity relationship. Arab. J. Chem. 2015, 8, 828–836. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, M.; Agrawal, S.; Chandra, S.; Ojha, H.; Pathak, M. Synthesis, characterization of 1,2,4-triazole Schiff base derived 3D-metal complexes: Induces cytotoxicity in HepG2, MCF-7 cell line, BSA binding fluorescence and DFT study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 246–257. [Google Scholar] [CrossRef]
- Rauf, A.; Shah, A.; Munawar, K.S.; Khan, A.A.; Abbasi, R.; Yameen, M.A.; Khan, A.M.; Khan, A.R.; Qureshi, I.Z.; Kraatz, H.-B.; et al. Synthesis, spectroscopic characterization, DFT optimization and biological activities of Schiff bases and their metal (II) complexes. J. Mol. Struct. 2017, 1145, 132–140. [Google Scholar] [CrossRef]
- Gázquez, J.L. Hardness and softness in density functional theory. In Chemical Hardness; Sen, K.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 27–43. [Google Scholar]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of Octanol−Water Partition Coefficients by Guiding an Additive Model with Knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.A.d. Pharmacokinetic study with computational tools in the medicinal chemistry course. Braz. J. Pharm. Sci. 2011, 47, 797–805. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Frimayanti, N. 2D, 3D-QSAR, and pharmacophore studies on thiazolidine-4-carboxylic acid derivatives as neuraminidase inhibitors in H3N2 influenza virus. Med. Chem. Res. 2014, 23, 1447–1453. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Vyas, R.; Karthikeyan, M.; Vyas, R. Open-source tools, techniques, and data in chemoinformatics. In Practical Chemoinformatics; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–92. [Google Scholar]
- Ferrara, P.; Apostolakis, J.; Caflisch, A. Evaluation of a fast implicit solvent model for molecular dynamics simulations. Proteins Struct. Funct. Bioinform. 2002, 46, 24–33. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-P.; Sun, G.-P.; Shen, Y.-X.; Wei, W.; Peng, W.-R.; Wang, H. Antiproliferation and apoptosis induction of paeonol in HepG2 cells. World J. Gastroenterol. WJG 2007, 13, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Guo, P.; Pi, C.; He, Y.; Yang, H.; Hou, Y.; Feng, X.; Jiang, Q.; Wei, Y.; Zhao, L. Synergistic Effects of Curcumin and 5-Fluorouracil on the Hepatocellular Carcinoma In vivo and vitro through regulating the expression of COX-2 and NF-κB. J. Cancer 2020, 11, 3955–3964. [Google Scholar] [CrossRef]
- Xu, W.; Pan, Y.; Wang, H.; Li, H.; Peng, Q.; Wei, D.; Chen, C.; Zheng, J. Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line. Molecules 2017, 22, 467. [Google Scholar] [CrossRef]
- Lange, J.H.; Reinders, J.-H.; Tolboom, J.T.; Glennon, J.C.; Coolen, H.K.; Kruse, C.G. Principal component analysis differentiates the receptor binding profiles of three antipsychotic drug candidates from current antipsychotic drugs. J. Med. Chem. 2007, 50, 5103–5108. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, W.; Xin, F.; Li, Y. “Materials Studio” Simulation Study of the Adsorption and Polymerization Mechanism of Sodium Silicate on Active Silica Surface at Different Temperatures. Int. J. Metalcast. 2021, 15, 1091–1098. [Google Scholar] [CrossRef]
- Tran, N.V.; Tieu, A.K.; Zhu, H.; Ta, H.T.T.; Ta, T.D.; Le, H.M. First-Principles Study of the Adsorption and Depolymerization Mechanisms of Sodium Silicate on Iron Surfaces at High Temperature. J. Phys. Chem. C 2018, 122, 20827–20840. [Google Scholar] [CrossRef]
- Liu, H.; Song, L. Materials Studio simulation for the adsorption properties of CO2 molecules at the surface of sodium silicate and potassium silicate solution under different pressure conditions. Int. J. Met. 2022, 16, 242–251. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef]
- Li, L.; Larsen, A.H.; Romero, N.A.; Morozov, V.A.; Glinsvad, C.; Abild-Pedersen, F.; Greeley, J.; Jacobsen, K.W.; Nørskov, J.K. Investigation of Catalytic Finite-Size-Effects of Platinum Metal Clusters. J. Phys. Chem. Lett. 2013, 4, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Teobaldi, G.; Zerbetto, F. Adsorption of organic molecules on gold electrodes. J. Phys. Chem. C 2007, 111, 13879–13885. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. PDB—5IKT: The Structure of Tolfenamic Acid Bound to Human Cyclooxygenase-2. Available online: https://www.rcsb.org/structure/5IKT (accessed on 21 April 2025).
- Abbas, A.M.; Nasrallah, H.H.; Aboelmagd, A.; Kishk, S.M.; Boyd, W.C.; Kalil, H.; Orabi, A.S. Design, Synthesis, Anti-Inflammatory Activity, DFT Modeling and Docking Study of New Ibuprofen Derivatives. Int. J. Mol. Sci. 2024, 25, 3558. [Google Scholar] [CrossRef]
- Alnakib, N.; Abdel-Hamid, N.; El-Senduny, F.; EL-Gharieb, M. Assessment of Alpha-fetoprotein levels and the effect of 5-Fluorouracil on Cytotoxicity of HepG2 cell line. Alfarama J. Basic Appl. Sci. 2022, 3, 210–219. [Google Scholar] [CrossRef]
- Wu, X.-X.; Kakehi, Y.; Mizutani, Y.; Lu, J.; Terachi, T.; Ogawa, O. Activation of caspase-3 in renal cell carcinoma cells by anthracyclines or 5-fluorouracil. Int. J. Oncol. 2001, 19, 19–24. [Google Scholar] [CrossRef]
- Orabi, A.S.; Abou El-Nour, K.M.; Youssef, M.F.; Salem, H.A. Novel and highly effective composites of silver and zinc oxide nanoparticles with some transition metal complexes against different microorganisms. Arab. J. Chem. 2020, 13, 2628–2648. [Google Scholar] [CrossRef]
- Sangari, N.U. Synthesis and characterization of nano sized ZnO using conventional and microwave heating methods. Int. J. ChemTech Res. 2014, 7, 181. [Google Scholar]
- Tabrizi, A.; Ayhan, F.; Ayhan, H. Gold Nanoparticle Synthesis and Characterisation. Hacet. J. Biol. Chem. 2009, 37, 217–226. [Google Scholar]
- El-Nour, K.M.A.; Salam, E.T.A.; Soliman, H.M.; Orabi, A. Gold nanoparticles as a direct and rapid sensor for sensitive analytical detection of biogenic amines. Nanoscale Res. Lett. 2017, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Shehata, M.R.; Atlam, F.M.; Abdel-Mawgoud, A.A.H. Some new Ag (I), VO (II) and Pd (II) chelates incorporating tridentate imine ligand: Design, synthesis, structure elucidation, density functional theory calculations for DNA interaction, antimicrobial and anticancer activities and molecular docking studies. Appl. Organomet. Chem. 2019, 33, e4699. [Google Scholar] [CrossRef]
- Slater, T.F.; Sawyer, B.; Sträuli, U. Studies on succinate-tetrazolium reductase systems: III. Points of coupling of four different tetrazolium salts III. Points of coupling of four different tetrazolium salts. Biochim. Biophys. Acta 1963, 77, 383–393. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, g.; Broekhoven, M.G.; Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Alazzouni, A.S.; Dkhil, M.A.; Gadelmawla, M.H.; Gabri, M.S.; Farag, A.H.; Hassan, B.N. Ferulic acid as anticarcinogenic agent against 1,2-dimethylhydrazine induced colon cancer in rats. J. King Saud Univ.-Sci. 2021, 33, 101354. [Google Scholar] [CrossRef]
- Valiyari, S.; Jahanban-Esfahlan, R.; Shahneh, F.Z.; Yaripour, S.; Baradaran, B.; Delazar, A. Cytotoxic and apoptotic activity of Scrophularia oxysepala in MCF-7 human breast cancer cells. Toxicol. Environ. Chem. 2013, 95, 1208–1220. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]







| Compound | Molecular Weight | Color | Melting Point (°C) | Ω * | CHNM% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C% | H% | N% | M% ** | |||||||
| Calc. Found | Calc. Found | Calc. Found | A | B | Calc. | |||||
| HD (C21H22N4O5) | 410.44 | Brown | 182 | --- | 61.46 61.42 | 5.40 5.59 | 13.65 13.58 | --- | --- | --- |
| [Cu(HD)2]Cl.2H2O C42H47CuN8O12Cl | 954.88 | Dirty green | >250 | 65 | 52.83 52.23 | 4.96 4.85 | 11.74 11.77 | 6.69 | 7.02 | 6.81 |
| [Ni(HD)2]3H2O C42H49N8NiO13 | 932.59 | Apple green | >250 | 10 | 54.09 54.11 | 5.30 5.25 | 12.02 12.11 | 6.89 | 7.86 | 6.30 |
| [Co(HD)2]Cl.2H2O C42H47ClCoN8O12 | 950.27 | Brick brown | >250 | 68 | 53.09 53.15 | 4.99 5.05 | 11.79 11.85 | 6.69 | 7.59 | 6.20 |
| [Ag(HD)2]NO3.2H2O C42H48 AgN9O14 | 1026.76 | Brick brown | >250 | 60 | 49.13 49.55 | 4.71 4.62 | 12.28 12.35 | 10.25 | 10.95 | 10.50 |
| [Zn(HD)2](NO3)2.2H2O C42H47N10O18Zn | 1045.27 | Yellow-orange | >250 | 130 | 48.26 48.35 | 4.53 4.56 | 13.40 13.45 | 6.86 | 6.78 | 6.24 |
| Property | Model Name | TMP | HD | Cu-HD | Ni-HD | Co-HD | Ag-HD | Zn-HD |
|---|---|---|---|---|---|---|---|---|
| Absorption | Water Solubility (log mol/L) | −2.721 | −4.009 | −2.971 | −2.971 | −2.791 | −2.915 | −2.97 |
| Caco2 permeability (log Papp in 10−6 cm/s) | 0.649 | 0.188 | 0.085 | 0.085 | 0.085 | 0.027 | 0.085 | |
| Intestinal absorption (%) | 76.824 | 78.705 | 70.234 | 70.332 | 70.283 | 64.077 | 69.826 | |
| Skin Permeability (Log Kp) | −2.857 | −2.734 | −2.735 | −2.735 | −2.735 | −2.735 | −2.735 | |
| P-Glycoprotein substrate | Yes | Yes | No | No | No | Yes | No | |
| P-Glycoprotein I inhibitor | No | No | Yes | Yes | Yes | Yes | Yes | |
| P-Glycoprotein II inhibitor | No | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.M.; Nasrallah, H.H.; Aboelmagd, A.; Boyd, W.C.; Kalil, H.; Orabi, A.S. Novel Trimethoprim-Based Metal Complexes and Nanoparticle Functionalization: Synthesis, Structural Analysis, and Anticancer Properties. Inorganics 2025, 13, 144. https://doi.org/10.3390/inorganics13050144
Abbas AM, Nasrallah HH, Aboelmagd A, Boyd WC, Kalil H, Orabi AS. Novel Trimethoprim-Based Metal Complexes and Nanoparticle Functionalization: Synthesis, Structural Analysis, and Anticancer Properties. Inorganics. 2025; 13(5):144. https://doi.org/10.3390/inorganics13050144
Chicago/Turabian StyleAbbas, Abbas M., Hossam H. Nasrallah, A. Aboelmagd, W. Christopher Boyd, Haitham Kalil, and Adel S. Orabi. 2025. "Novel Trimethoprim-Based Metal Complexes and Nanoparticle Functionalization: Synthesis, Structural Analysis, and Anticancer Properties" Inorganics 13, no. 5: 144. https://doi.org/10.3390/inorganics13050144
APA StyleAbbas, A. M., Nasrallah, H. H., Aboelmagd, A., Boyd, W. C., Kalil, H., & Orabi, A. S. (2025). Novel Trimethoprim-Based Metal Complexes and Nanoparticle Functionalization: Synthesis, Structural Analysis, and Anticancer Properties. Inorganics, 13(5), 144. https://doi.org/10.3390/inorganics13050144










