Research Progress on Graphene Oxide (GO)/Chitosan (CS) Multifunctional Nanocomposites for Drug Delivery
Abstract
1. Introduction
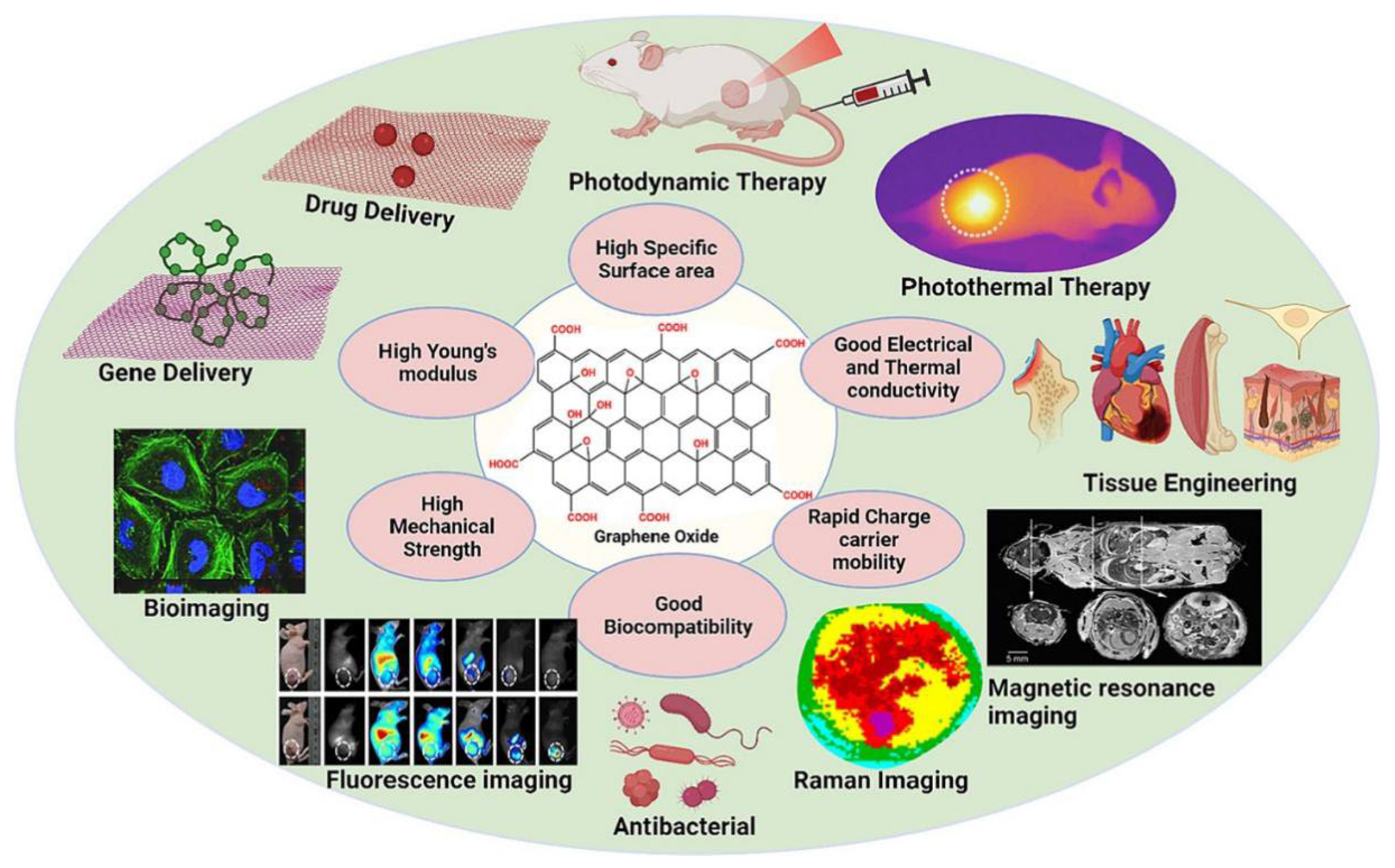
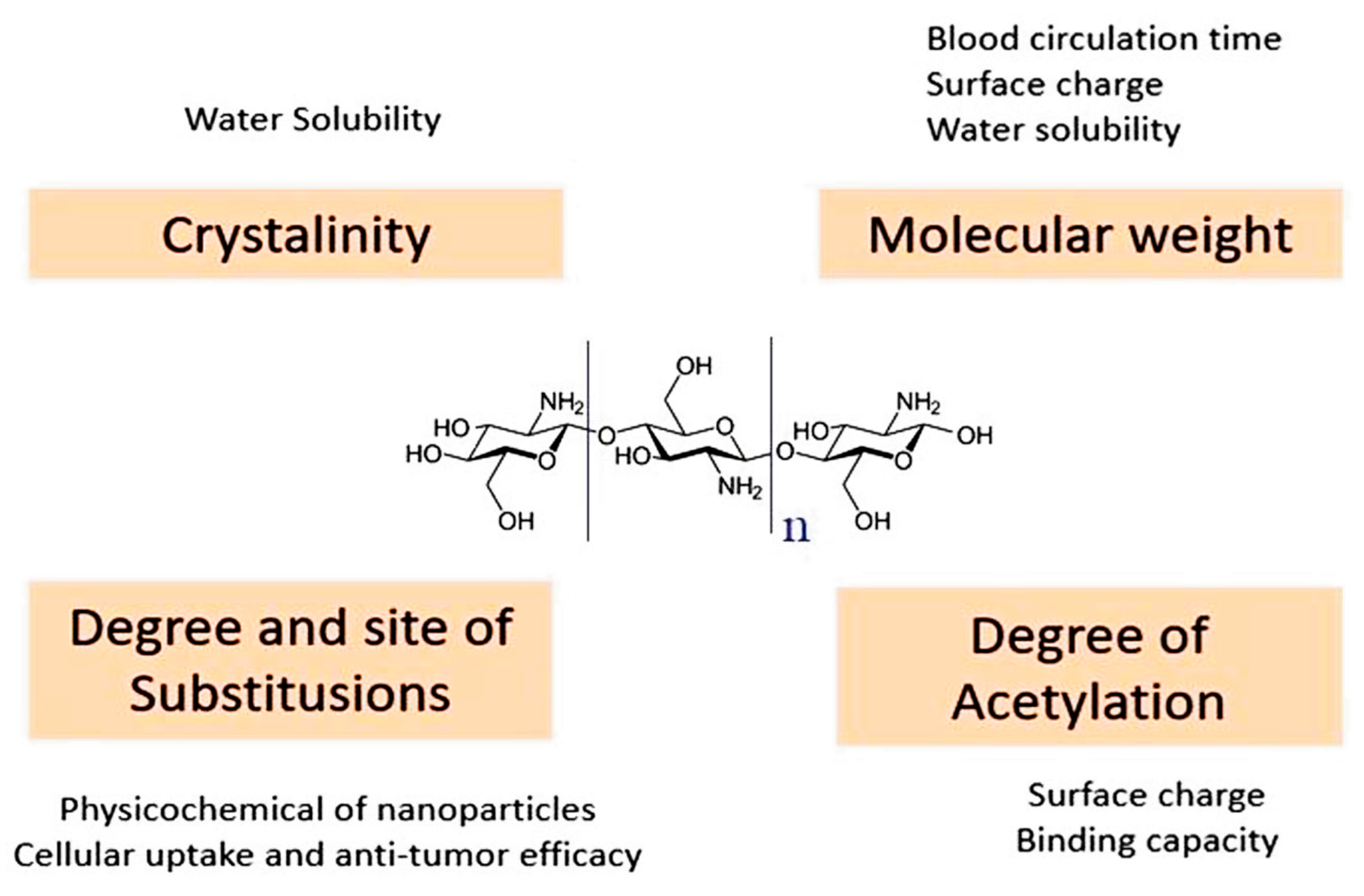
2. Properties of GO and CS
3. Types of DDSs Based on GO/CS
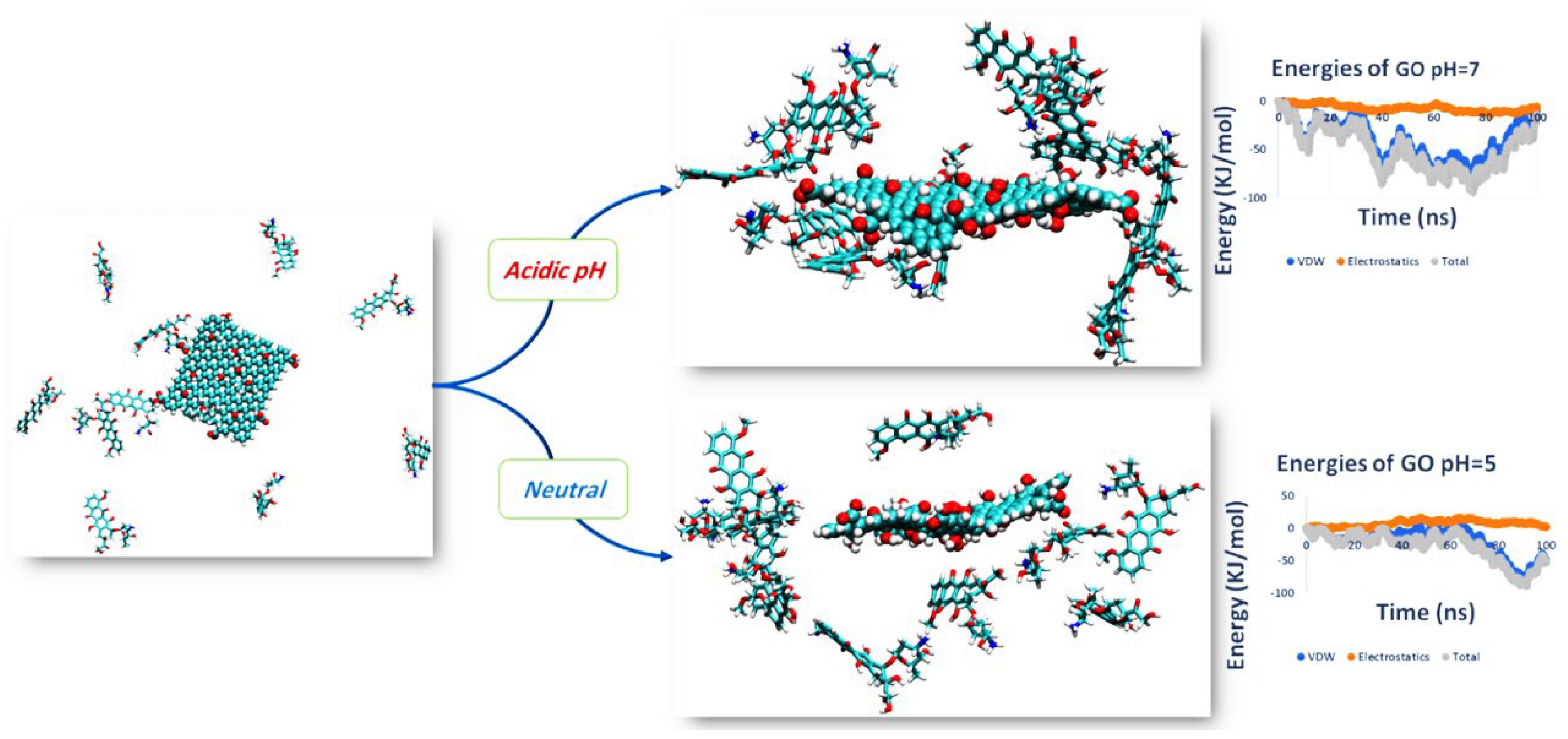
3.1. PH Sensitive Release Type DDS
3.2. Redox Responsive Type DDS
3.3. Thermal Sensitive Type DDS
3.4. Magnetic Targeting Type DDS
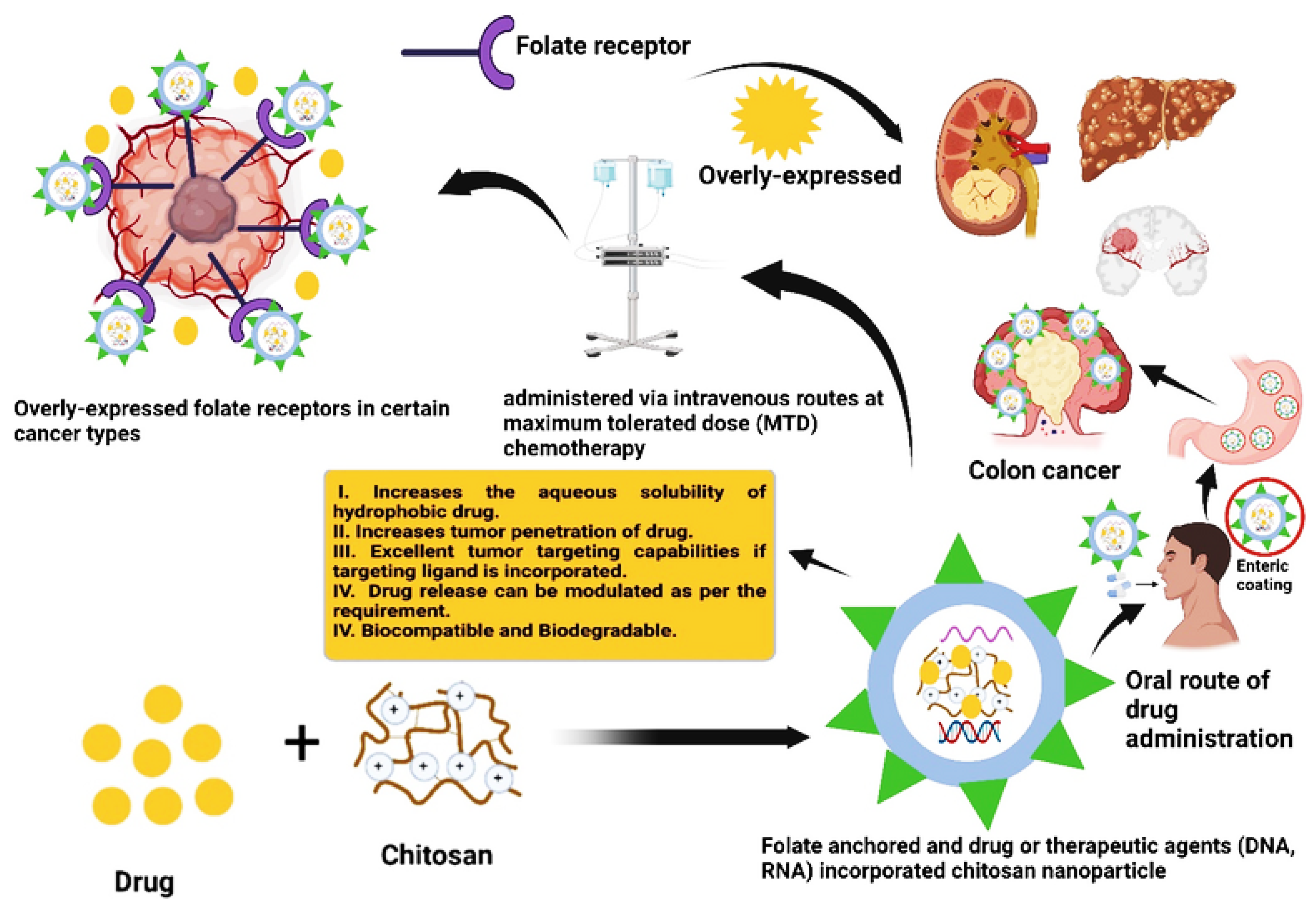
3.5. Folic Acid Targeted Type DDS
3.6. Photosensitive Type DDS
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306. [Google Scholar] [PubMed]
- Pan, Q.; Lv, Y.; Williams, G.R.; Tao, L.; Yang, H.; Li, H.; Zhu, L. Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohydr. Polym. 2016, 151, 812–820. [Google Scholar] [PubMed]
- Gooneh-Farahani, S.; Naimi-Jamal, M.R.; Naghib, S.M. Stimuli-responsive graphene-incorporated multifunctional chitosan for drug delivery applications: A review. Expert Opin. Drug Deliv. 2019, 16, 79–99. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar]
- Anirudhan, T.; Sekhar, V.C.; Athira, V. Graphene oxide based functionalized chitosan polyelectrolyte nanocomposite for targeted and pH responsive drug delivery. Int. J. Biol. Macromol. 2020, 150, 468–479. [Google Scholar]
- Wang, C.; Zhang, Z.; Chen, B.; Gu, L.; Li, Y.; Yu, S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 2018, 516, 332–341. [Google Scholar]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar]
- Xu, C.; Hu, Y.; Yang, Z. Influence of Oxidation Temperature on the Structure of Graphene Oxide. J. Liaocheng Univ. Nat. Sci. Ed. 2024, 37, 57–63. [Google Scholar] [CrossRef]
- Liu, J.; Dong, J.; Zhang, T.; Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 2018, 286, 64–73. [Google Scholar]
- Jafari, Z.; Rad, A.S.; Baharfar, R.; Asghari, S.; Esfahani, M.R. Synthesis and application of chitosan/tripolyphosphate/graphene oxide hydrogel as a new drug delivery system for Sumatriptan Succinate. J. Mol. Liq. 2020, 315, 113835. [Google Scholar]
- Hosseini, S.M.; Mazinani, S.; Abdouss, M.; Kalhor, H.; Kalantari, K.; Amiri, I.S.; Ramezani, Z. Designing chitosan nanoparticles embedded into graphene oxide as a drug delivery system. Polym. Bull. 2022, 79, 541–554. [Google Scholar]
- Deb, A.; Vimala, R. Natural and synthetic polymer for graphene oxide mediated anticancer drug delivery—A comparative study. Int. J. Biol. Macromol. 2018, 107, 2320–2333. [Google Scholar] [PubMed]
- Liu, B.; Che, C.; Liu, J.; Si, M.; Gong, Z.; Li, Y.; Zhang, J.; Yang, G. Fabrication and antitumor mechanism of a nanoparticle drug delivery system: Graphene oxide/chitosan oligosaccharide/γ-polyglutamic acid composites for anticancer drug delivery. ChemistrySelect 2019, 4, 12491–12502. [Google Scholar]
- Shan, H.; Xiao, J.; Duan, G.; Jiang, Y.; Lu, Q. Preparation and Property Study of Chitosan ZIF-8 Aerogel Composites. J. Liaocheng Univ. Nat. Sci. Ed. 2023, 36, 43–52. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Rajaei, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M.; Rahdar, A.; Díez-Pascual, A.M. Chitosan/agarose/graphene oxide nanohydrogel as drug delivery system of 5-fluorouracil in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104307. [Google Scholar]
- Figueroa, T.; Aguayo, C.; Fernández, K. Design and characterization of chitosan-graphene oxide nanocomposites for the delivery of proanthocyanidins. Int. J. Nanomed. 2020, 15, 1229–1238. [Google Scholar]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green synthesized chitosan/chitosan nanoforms/nanocomposites for drug delivery applications. Polymers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Biomedical applications of chitosan-graphene oxide nanocomposites. iScience 2022, 25, 103629. [Google Scholar]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Sharifi-Rad, J.; Mendoza-Muñoz, N.; González-Torres, M.; Urbán-Morlán, Z.; Florán, B.; Cortes, H.; Leyva-Gómez, G. Chitosan-decorated nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101896. [Google Scholar]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Mao, S.; Sun, W.; Wang, Y.; Kissel, T. Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur. J. Pharm. Biopharm. 2008, 70, 874–881. [Google Scholar] [CrossRef]
- Das, P.N.; Raj, K.G. Chitosan coated graphene oxide incorporated sodium alginate hydrogel beads for the controlled release of amoxicillin. Int. J. Biol. Macromol. 2024, 254, 127837. [Google Scholar]
- Kesharwani, P.; Halwai, K.; Jha, S.K.; Al Mughram, M.H.; Almujri, S.S.; Almalki, W.H.; Sahebkar, A. Folate-engineered chitosan nanoparticles: Next-generation anticancer nanocarriers. Mol. Cancer 2024, 23, 244. [Google Scholar]
- Abbasian, M.; Roudi, M.-M.; Mahmoodzadeh, F.; Eskandani, M.; Jaymand, M. Chitosan-grafted-poly (methacrylic acid)/graphene oxide nanocomposite as a pH-responsive de novo cancer chemotherapy nanosystem. Int. J. Biol. Macromol. 2018, 118, 1871–1879. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Yaqoob, Z.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Haider, S.; Busra, F.M. Chitosan/poly vinyl alcohol/graphene oxide based pH-responsive composite hydrogel films: Drug release, anti-microbial and cell viability studies. Polymers 2021, 13, 3124. [Google Scholar] [CrossRef]
- Marapureddy, S.G.; Thareja, P. Synergistic effect of chemical crosslinking and addition of graphene-oxide in Chitosan—Hydrogels, films, and drug delivery. Mater. Today Commun. 2022, 31, 103430. [Google Scholar]
- Tsai, C.-C.; Young, T.-H.; Chen, G.-S.; Cheng, N.-C. Developing a glyoxal-crosslinked chitosan/gelatin hydrogel for sustained release of human platelet lysate to promote tissue regeneration. Int. J. Mol. Sci. 2021, 22, 6451. [Google Scholar] [CrossRef]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef] [PubMed]
- Majidi, H.J.; Babaei, A.; Bafrani, Z.A.; Shahrampour, D.; Zabihi, E.; Jafari, S.M. Investigating the best strategy to diminish the toxicity and enhance the antibacterial activity of graphene oxide by chitosan addition. Carbohydr. Polym. 2019, 225, 115220. [Google Scholar]
- Karthika, V.; AlSalhi, M.S.; Devanesan, S.; Gopinath, K.; Arumugam, A.; Govindarajan, M. Chitosan overlaid Fe3O4/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci. Rep. 2020, 10, 18912. [Google Scholar]
- Eltahir, S.; Jagal, J.; Abdelkareem, M.A.; Ghoneim, M.M.; Rawas-Qalaji, M.M.; Greish, K.; Haider, M. Thermosensitive injectable graphene oxide/chitosan-based nanocomposite hydrogels for controlling the in vivo release of bupivacaine hydrochloride. Int. J. Pharm. 2022, 621, 121786. [Google Scholar]
- Gooneh-Farahani, S.; Naghib, S.M.; Naimi-Jamal, M.R.; Seyfoori, A. A pH-sensitive nanocarrier based on BSA-stabilized graphene-chitosan nanocomposite for sustained and prolonged release of anticancer agents. Sci. Rep. 2021, 11, 17404. [Google Scholar]
- Zhao, X.; Wei, Z.; Zhao, Z.; Miao, Y.; Qiu, Y.; Yang, W.; Jia, X.; Liu, Z.; Hou, H. Design and development of graphene oxide nanoparticle/chitosan hybrids showing pH-sensitive surface charge-reversible ability for efficient intracellular doxorubicin delivery. ACS Appl. Mater. Interfaces 2018, 10, 6608–6617. [Google Scholar]
- Sheng, Y.; Dai, W.; Gao, J.; Li, H.; Tan, W.; Wang, J.; Deng, L.; Kong, Y. pH-sensitive drug delivery based on chitosan wrapped graphene quantum dots with enhanced fluorescent stability. Mater. Sci. Eng. C 2020, 112, 110888. [Google Scholar]
- Rajabzadeh-Khosroshahi, M.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Rasekh, B. Chitosan/agarose/graphitic carbon nitride nanocomposite as an efficient pH-sensitive drug delivery system for anticancer curcumin releasing. J. Drug Deliv. Sci. Technol. 2022, 74, 103443. [Google Scholar]
- Le, Q.H.; Neila, F.; Smida, K.; Li, Z.; Abdelmalek, Z.; Tlili, I. pH-responsive anticancer drug delivery systems: Insights into the enhanced adsorption and release of DOX drugs using graphene oxide as a nanocarrier. Eng. Anal. Bound. Elem. 2023, 157, 157–165. [Google Scholar]
- Cui, X.; Dong, L.; Zhong, S.; Shi, C.; Sun, Y.; Chen, P. Sonochemical fabrication of folic acid functionalized multistimuli-responsive magnetic graphene oxide-based nanocapsules for targeted drug delivery. Chem. Eng. J. 2017, 326, 839–848. [Google Scholar]
- Guo, Q.; Cao, H.; Li, X.; Liu, S. Thermosensitive hydrogel drug delivery system containing doxorubicin loaded CS–GO nanocarriers for controlled release drug in situ. Mater. Technol. 2015, 30, 294–300. [Google Scholar]
- Fong, Y.T.; Chen, C.-H.; Chen, J.-P. Intratumoral delivery of doxorubicin on folate-conjugated graphene oxide by in-situ forming thermo-sensitive hydrogel for breast cancer therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, J.; Wang, T.; Gao, X.; Wan, Q.; Pei, X. Preparation and characterization of chitosan/β-glycerophosphate thermal-sensitive hydrogel reinforced by graphene oxide. Front. Chem. 2018, 6, 565. [Google Scholar]
- Wang, H.; Sun, D.; Zhao, N.; Yang, X.; Shi, Y.; Li, J.; Su, Z.; Wei, G. Thermo-sensitive graphene oxide–polymer nanoparticle hybrids: Synthesis, characterization, biocompatibility and drug delivery. J. Mater. Chem. B 2014, 2, 1362–1370. [Google Scholar]
- Kazemi, S.; Pourmadadi, M.; Yazdian, F.; Ghadami, A. The synthesis and characterization of targeted delivery curcumin using chitosan-magnetite-reduced graphene oxide as nano-carrier. Int. J. Biol. Macromol. 2021, 186, 554–562. [Google Scholar]
- Abdel-Bary, A.S.; Tolan, D.A.; Nassar, M.Y.; Taketsugu, T.; El-Nahas, A.M. Chitosan, magnetite, silicon dioxide, and graphene oxide nanocomposites: Synthesis, characterization, efficiency as cisplatin drug delivery, and DFT calculations. Int. J. Biol. Macromol. 2020, 154, 621–633. [Google Scholar]
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids Surf. B Biointerfaces 2019, 176, 462–470. [Google Scholar]
- Ashuri, A.; Miralinaghi, M.; Moniri, E. Evaluation of folic acid-conjugated chitosan grafted Fe3O4/graphene oxide as a pH-and magnetic field-responsive system for adsorption and controlled release of gemcitabine. Korean J. Chem. Eng. 2022, 39, 1880–1890. [Google Scholar]
- Jun, S.W.; Manivasagan, P.; Kwon, J.; Mondal, S.; Ly, C.D.; Lee, J.; Kang, Y.-H.; Kim, C.-S.; Oh, J. Folic acid–conjugated chitosan-functionalized graphene oxide for highly efficient photoacoustic imaging-guided tumor-targeted photothermal therapy. Int. J. Biol. Macromol. 2020, 155, 961–971. [Google Scholar]
- Sontakke, A.D.; Gupta, P.; Banerjee, S.K.; Purkait, M.K. Chitosan-grafted folic acid decorated one-dimensional GONS: A biocompatible drug cargo for targeted co-delivery of anticancer agents. Int. J. Biol. Macromol. 2024, 271, 132621. [Google Scholar]
- Dinçer, C.A.; Getiren, B.; Gökalp, C.; Ciplak, Z.; Karakeçili, A.; Yildiz, N. An anticancer drug loading and release study to ternary GO-Fe3O4-PPy and Fe3O4@PPy-NGQDs nanocomposites for photothermal chemotherapy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127791. [Google Scholar]
- Ramezani Farani, M.; Khadiv-Parsi, P.; Riazi, G.H.; Shafiee Ardestani, M.; Saligheh Rad, H. PEGylation of graphene/iron oxide nanocomposite: Assessment of release of doxorubicin, magnetically targeted drug delivery and photothermal therapy. Appl. Nanosci. 2020, 10, 1205–1217. [Google Scholar]
- Barrera, C.C.; Groot, H.; Vargas, W.L.; Narváez, D.M. Efficacy and molecular effects of a reduced graphene Oxide/Fe3O4 nanocomposite in photothermal therapy against cancer. Int. J. Nanomed. 2020, 15, 6421–6432. [Google Scholar]
- Jhang, J.-W.; Chou, Y.-H.; Wang, T.-H.; Hsieh, M.-H.; Chiang, W.-H. One-pot green reduction and surface decoration of graphene oxide nanosheets with PEGylated chitosan for application in cancer photothermal therapy. J. Taiwan Inst. Chem. Eng. 2022, 134, 104359. [Google Scholar]
- Chen, L.; Hong, W.; Duan, S.; Li, Y.; Wang, J.; Zhu, J. Graphene quantum dots mediated magnetic chitosan drug delivery nanosystems for targeting synergistic photothermal-chemotherapy of hepatocellular carcinoma. Cancer Biol. Ther. 2022, 23, 281–293. [Google Scholar]
- Su, Z.; Sun, D.; Zhang, L.; He, M.; Jiang, Y.; Millar, B.; Douglas, P.; Mariotti, D.; Maguire, P.; Sun, D. Chitosan/silver nanoparticle/graphene oxide nanocomposites with multi-drug release, antimicrobial, and photothermal conversion functions. Materials 2021, 14, 2351. [Google Scholar] [CrossRef]
- Li, S.; Xiao, L.; Deng, H.; Shi, X.; Cao, Q. Remote controlled drug release from multi-functional Fe3O4/GO/Chitosan microspheres fabricated by an electrospray method. Colloids Surf. B Biointerfaces 2017, 151, 354–362. [Google Scholar]
- Sheng, Y.; Cao, C.; Liang, Z.; Yin, Z.-Z.; Gao, J.; Cai, W.; Kong, Y. Construction of a dual-drug delivery system based on oxidized alginate and carboxymethyl chitosan for chemo-photothermal synergistic therapy of osteosarcoma. Eur. Polym. J. 2022, 174, 111331. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Ma, L.; Shi, Q.; Li, J.; Lv, Y.; Song, C. Research Progress on Graphene Oxide (GO)/Chitosan (CS) Multifunctional Nanocomposites for Drug Delivery. Inorganics 2025, 13, 98. https://doi.org/10.3390/inorganics13040098
Hu Y, Ma L, Shi Q, Li J, Lv Y, Song C. Research Progress on Graphene Oxide (GO)/Chitosan (CS) Multifunctional Nanocomposites for Drug Delivery. Inorganics. 2025; 13(4):98. https://doi.org/10.3390/inorganics13040098
Chicago/Turabian StyleHu, Yanqiu, Lei Ma, Qi Shi, Jinghang Li, Yuguang Lv, and Chaoyu Song. 2025. "Research Progress on Graphene Oxide (GO)/Chitosan (CS) Multifunctional Nanocomposites for Drug Delivery" Inorganics 13, no. 4: 98. https://doi.org/10.3390/inorganics13040098
APA StyleHu, Y., Ma, L., Shi, Q., Li, J., Lv, Y., & Song, C. (2025). Research Progress on Graphene Oxide (GO)/Chitosan (CS) Multifunctional Nanocomposites for Drug Delivery. Inorganics, 13(4), 98. https://doi.org/10.3390/inorganics13040098





