1. Introduction
The continuous development of the medical field has driven the demand for enhanced biomedical materials with superior functionality, safety, and patient comfort. Currently, ceramics, medical stainless steel (316L SS), and titanium alloys (e.g., Ti6Al4V, TC4) dominate the medical implant market due to their mechanical strength, corrosion resistance, and biocompatibility. However, these materials have notable limitations that restrict their application in modern medical practices. For instance, 316L SS is prone to corrosion in physiological environments, leading to the release of Ni ions, which can cause inflammatory and allergic reactions such as nickel allergy [
1]. Additionally, its wear resistance and antimicrobial properties are insufficient for advanced medical applications. Although TC4 titanium alloy offers better corrosion resistance and biocompatibility, its inert surface often necessitates additional activation treatments to improve cell adhesion and reduce bacterial colonization [
2,
3].
One of the most critical challenges of using traditional materials is the high risk of device-associated infections primarily caused by bacterial adhesion and biofilm formation on implant surfaces [
4]. Such infections contribute to prolonged patient recovery, high morbidity, and increased healthcare costs. The global rise of drug-resistant and multidrug-resistant bacteria has further amplified this issue, necessitating the development of novel antimicrobial biomaterials [
5,
6,
7]. Conventional disinfection and sterilization techniques often fail to eradicate biofilms, prompting the exploration of advanced materials capable of intrinsic antimicrobial activity.
Recent advancements in materials science have highlighted the potential of bulk metallic glasses (BMGs) as next-generation biomaterials. BMGs are unique metallic alloys with amorphous structures characterized by the absence of grains, grain boundaries, and dislocations. This structure endows BMGs with exceptional mechanical properties, superior corrosion resistance, and improved biocompatibility as compared with traditional crystalline metals [
8,
9]. Thus, BMGs have a good prospect for biomedical use and potentially as biomedical materials, especially as surgical instruments such as gastrointestinal endoscopes, razor blades, and other medical devices such as artificial implants [
8,
10]. In particular, Fe-based BMGs have emerged as promising candidates for biomedical applications due to their cost-effectiveness and compatibility with magnetic resonance imaging (MRI), outperforming alternatives like 316L SS [
11,
12]. However, despite their outstanding corrosion resistance and mechanical strength, the antimicrobial properties of Fe-based BMGs remain underexplored.
Among the various strategies for imparting antimicrobial properties, the incorporation of Ag into biomaterials has gained significant attention. Ag is widely recognized as a potent antimicrobial agent due to its ability to disrupt bacterial membranes and metabolic pathways through the generation of reactive oxygen species (ROS) [
13]. Unlike antibiotics, Ag remains effective against multidrug-resistant bacteria, making it an ideal additive for biomedical applications [
14,
15,
16,
17,
18,
19]. Incorporating Ag into Fe-based BMGs could potentially enhance their antimicrobial performance while retaining the intrinsic advantages of BMGs, such as high strength and biocompatibility.
This study seeks to address the gap in the development of antimicrobial Fe-based BMGs by examining the effects of Ag addition on their structural, thermal, mechanical, and electrochemical properties, as well as their biocompatibility and antimicrobial efficacy. Previous work by Li et al. demonstrated the development of Fe
55Cr
20Mo
5P
13C
7 BMG as a novel biomedical implant material, showcasing excellent corrosion resistance and biocompatibility [
3]. In this work, Ag is incorporated into this Fe-based BMG to further enhance its antibacterial properties. The antimicrobial effectiveness of these Ag-containing Fe-based BMGs is evaluated using both Gram-negative bacteria (
E. coli) and Gram-positive bacteria (
S. aureus), pathogens commonly implicated in healthcare-associated infections. Comparative studies with 316L SS and TC4 alloys are also conducted to benchmark the performance of the developed materials.
This work not only demonstrates the enhanced antimicrobial properties of Fe-based BMGs through Ag addition but also highlights their potential as cost-effective, biocompatible materials for biomedical implants. By addressing the dual challenges of bacterial infection and material biocompatibility, this study contributes to advancing the field of medical materials and presents Fe-based BMGs as promising candidates for next-generation implantable devices.
2. Results and Discussion
2.1. Structural, Thermal, and Mechanical Analyses
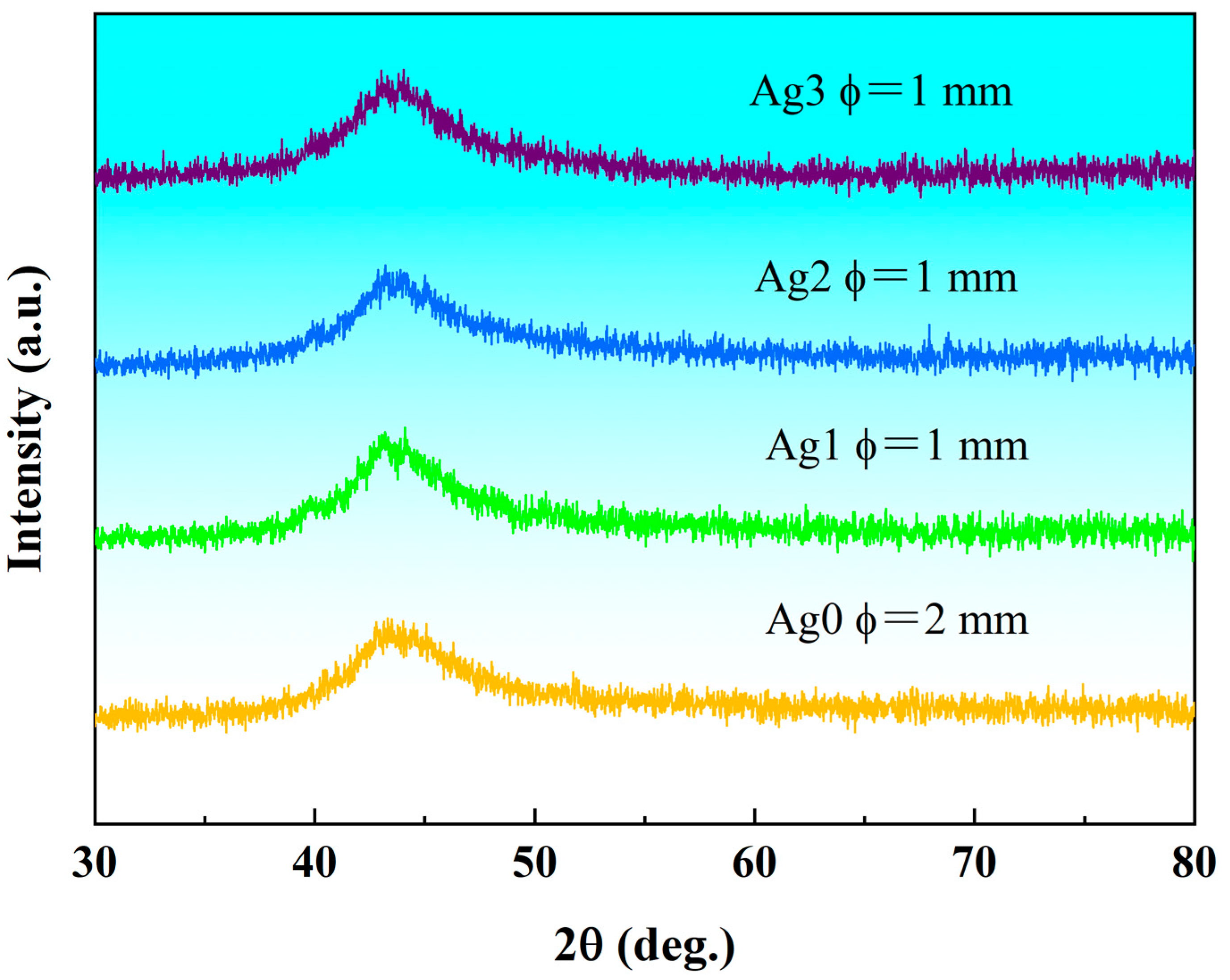
The X-Ray diffraction (XRD) patterns of the as-cast Fe
55-xCr
20Mo
5P
13C
7Ag
x (x = 0, 1, 2, 3 at.%) samples, shown in
Figure 1, reveal the critical diameter for fully amorphous formation (
Dmax). All samples exhibit a broad diffuse peak near the diffraction angle 2θ = 45°, with no discernible sharp-crystallization peaks. This confirms that the Fe-based bulk metallic glasses (BMGs) prepared in this study possess a fully amorphous structure, free from any crystalline phases. Notably, the absence of toxic or rare earth elements further underscores the suitability of these alloys for biomedical applications. For simplicity in the subsequent discussion, the Fe
55Cr
20Mo
5P
13C
7Ag
0, Fe
54Cr
20Mo
5P
13C
7Ag
1, Fe
53Cr
20Mo
5P
13C
7Ag
2, and Fe
52Cr
20Mo
5P
13C
7Ag
3 BMGs will be referred to Ag0, Ag1, Ag2, and Ag3, respectively.
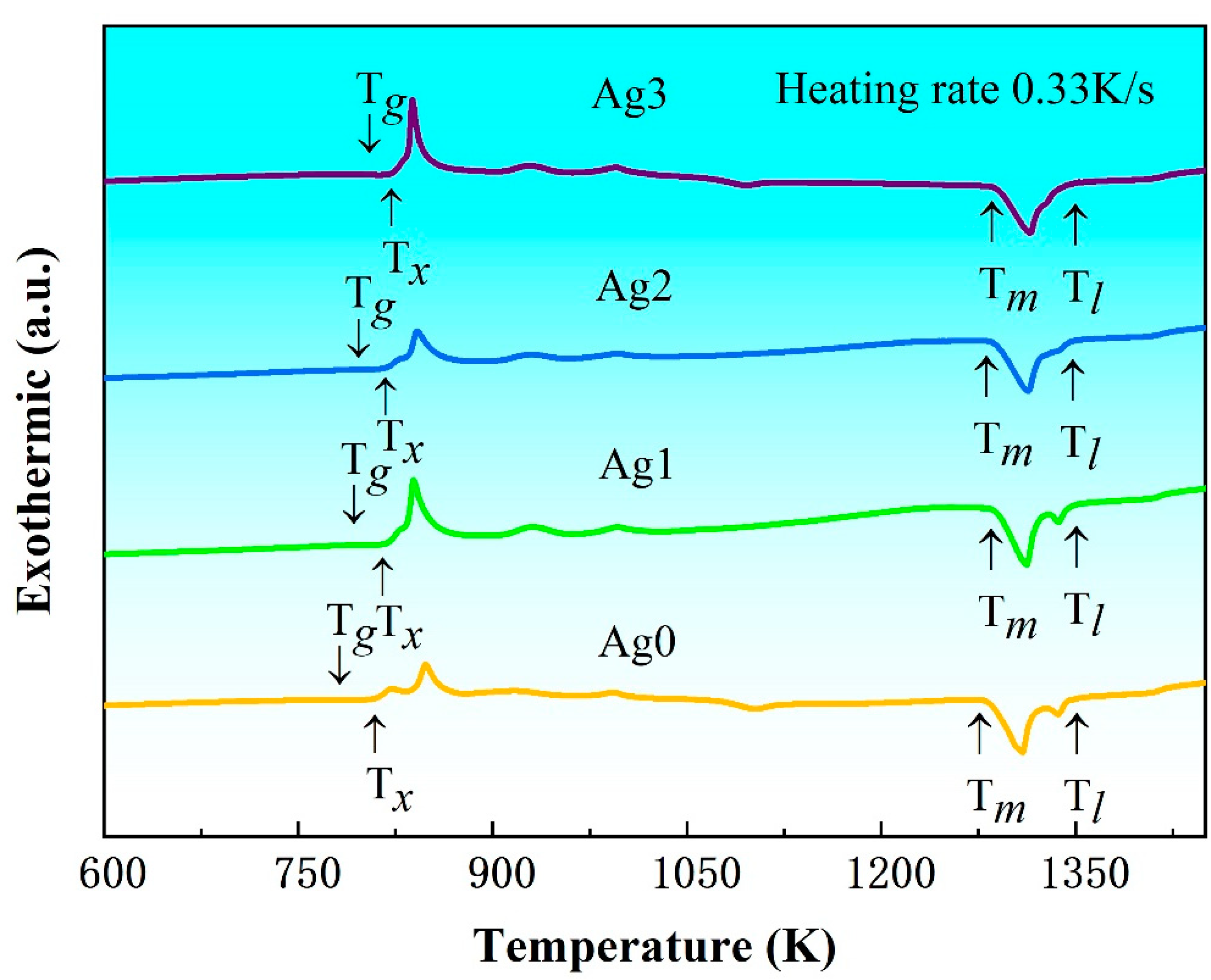
Figure 2 presents the DSC curves of the as-cast Fe-based BMG samples at a heating rate of 0.33 K/s. All samples exhibit a pronounced glass transition, followed by a supercooled liquid-phase region and a multi-step crystallization process [
3]. These thermal features are indicative of their amorphous natures and provide a basis for determining essential thermodynamic parameters, such as glass transition temperature (
Tg), onset crystallization temperature (
Tx), melting temperature (
Tm), and liquidus temperature (
Tl), which are summarized in
Table 1. The analysis reveals that both
Tg and
Tx increase with the addition of Ag content in the Fe-based BMGs.
To investigate the effect of Ag content on the glass-forming ability (GFA) of the Fe-based alloys, the three most commonly used GFA criteria, i.e., the reduced glass transition temperature
Trg (=
Tg/
Tl), the γ parameter (=
Tx/(
Tg +
Tl)) and the supercooled liquid-phase region Δ
Tx (=
Tx −
Tg), were calculated and are listed in
Table 1. These results show that the
Trg and γ values remain relatively consistent at approximately 0.58 and 0.38, respectively, across all samples. However, Δ
Tx decreases as Ag content increases, indicating a reduction in GFA with higher Ag content. This trend aligns with the experimentally determined results of
Dmax. The observed reduction in GFA can be attributed to the enthalpy of mixing between the Ag and Fe atoms, which is calculated as +28 kJ/mol [
20]. This positive enthalpy value indicates a repulsive interaction between Ag and Fe atoms. Such interactions promote the precipitation of the α-Fe phase, thereby disrupting the amorphous structure and significantly reducing the GFA of Fe-based BMGs. This behavior is analogous to the effect observed with the addition of Cu in Fe-based amorphous alloys [
21], where similarly repulsive atomic interactions favor the nanocrystallization of α-Fe and hinder glass formation.
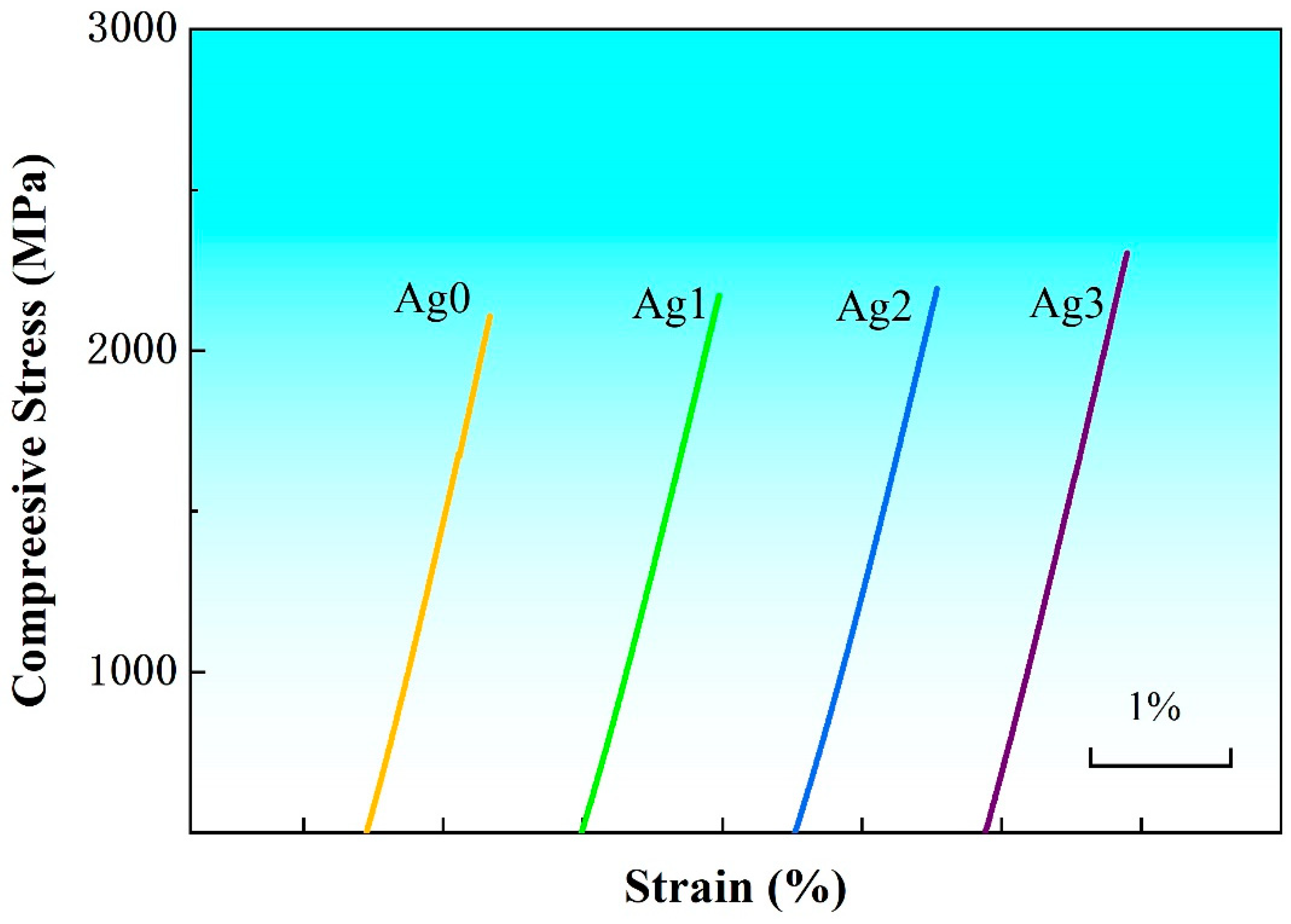
Figure 3 shows the compression curves of Fe-based BMGs at room temperature, and the test samples are all 1 mm in diameter and 2 mm in length. All the samples exhibit brittle fracture after linear elastic deformation. The Fe-based BMGs exhibit ultrahigh compression strength (
σf) exceeding 2 GPa, which gradually increases with the increase in Ag content and reaches the largest value of 2.34 GPa for the Ag3. It is well known that the strength of amorphous alloys is proportional to their
Tg [
22]. It can be seen from
Table 1 that the variation in
σf of the Fe-based BMGs is consistent with their
Tg values, showing a positive correlation.
2.2. Electrochemical Measurement
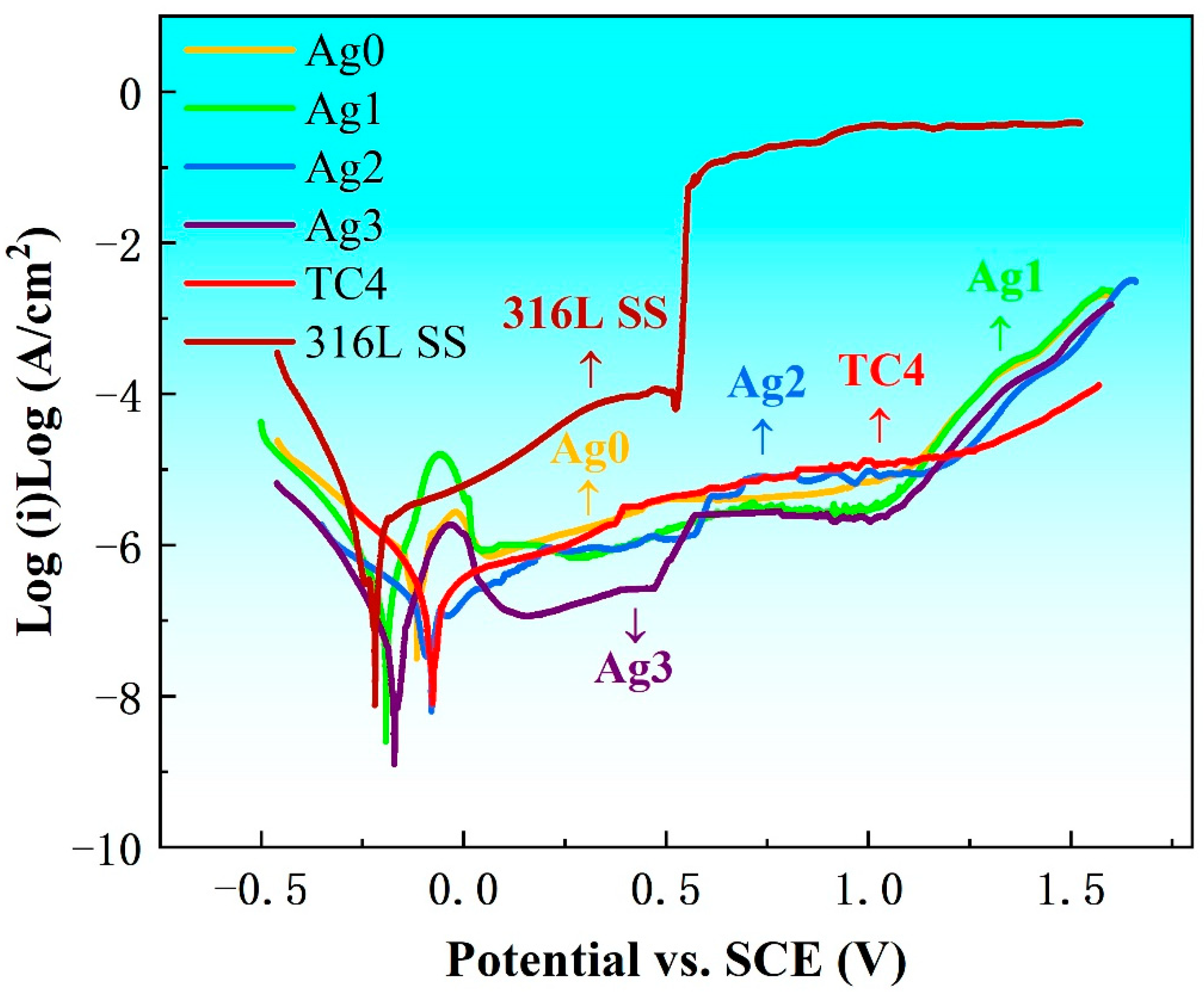
Figure 4 presents the potentiodynamic polarization curves of Fe-based BMGs, 316L SS, and TC4 in Hank’s solution at 37 °C. These tests, conducted in a thermostatic water bath under an atmospheric environment, provide valuable insights into the electrochemical corrosion parameters, including self-corrosion potential (
Ecorr), self-corrosion current density (
Icorr), pitting potential (
Epit), and corrosion rate (
CR), as summarized in
Table 2. The
Icorr values serve as indicators of the corrosion resistance of the materials, while the
Epit value and the width of the passivation potential region (∆
E =
Epit −
Ecorr) represent the stability of the passive film. The 316L SS shows the highest
Icorr and the poorest passivation behavior, reflecting its inferior corrosion resistance. In contrast, TC4 demonstrates good corrosion resistance, with
Icorr values comparable to Ag2 and Ag3. However, TC4 falls short in passivation stability as compared with Ag3. Among the Fe-based BMGs, the incorporation of Ag significantly improves their corrosion performance. As the Ag content increases,
Icorr decreases, and the corrosion resistance improves. Ag3 displays the lowest
Icorr and the most stable passivation behavior, indicating superior corrosion resistance. Its wide passivation potential range and minimal
Icorr highlight its effectiveness in resisting corrosive environments. These findings underscore the potential of Ag3 for biomedical applications, as it outperforms both 316L SS and TC4 in corrosion resistance and passivation stability.
2.3. Metal Ion Release
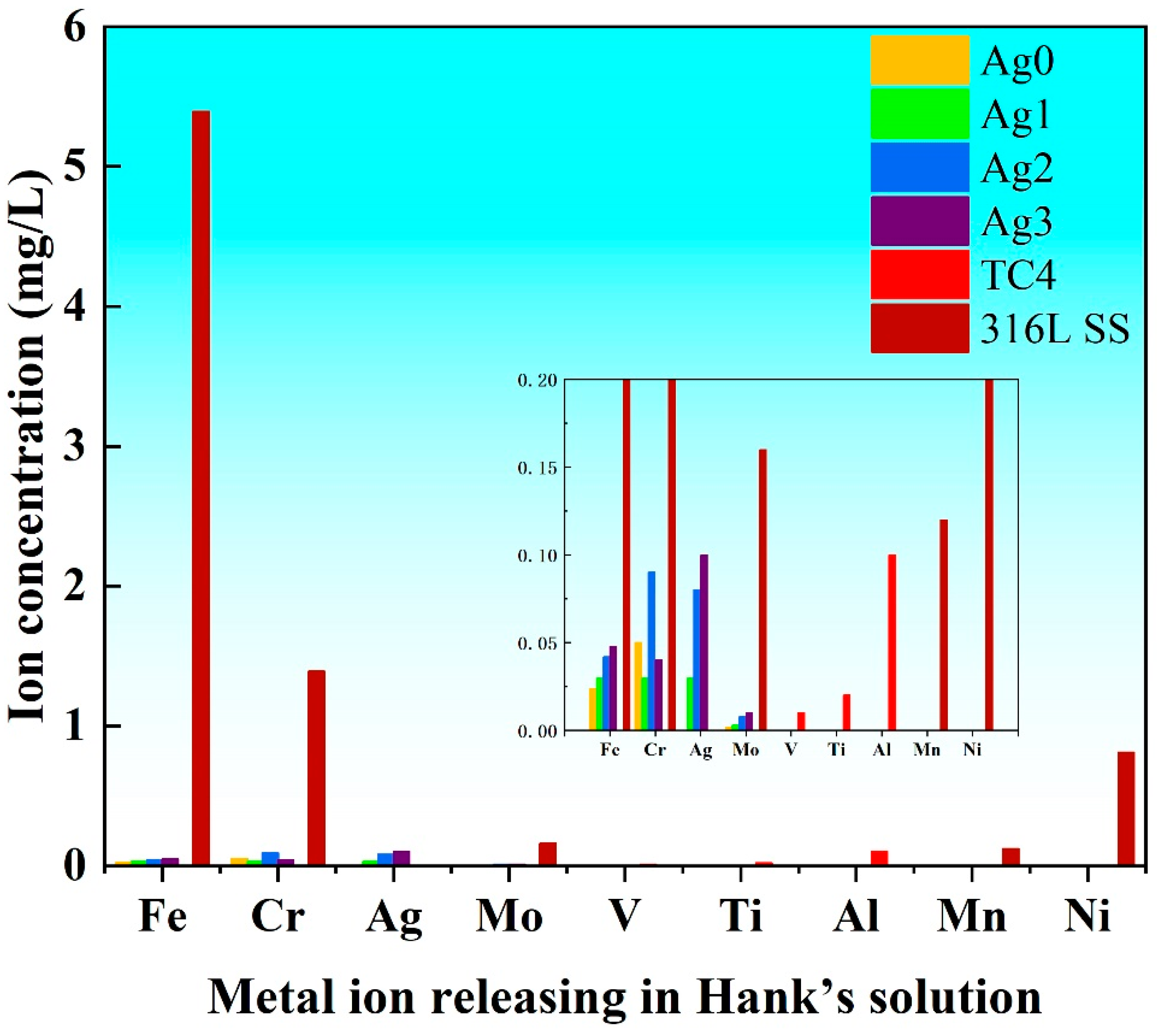
Figure 5 illustrates the ion concentrations released into Hank’s solution from the Fe-based BMGs, 316L SS, and TC4 after potentiodynamic polarization. The results reveal that 316L SS releases significantly higher concentrations of Fe, Cr, Mo, Mn, and Ni ions as compared with the Fe-based BMGs. This elevated ion release is attributed to the inferior corrosion resistance of 316L SS, which leads to a greater dissolution of metal ions in physiological environments. Notably, the release of Ni ions is particularly concerning, as Ni can interfere with enzymatic activity and trigger inflammatory and allergic responses [
23]. Consequently, the high levels of Ni released from 316L SS significantly compromises its biocompatibility. In contrast, both TC4 and Fe-based BMGs demonstrate minimal ion release, a direct result of their superior corrosion resistance. Among the Fe-based BMGs, those with higher Ag contents exhibit a slightly increased release of Ag ions, reflecting the incorporation of Ag as an antimicrobial agent. Nevertheless, the overall ion release from Fe-based BMGs remains exceptionally low, effectively minimizing the risk of harmful ions leaching into the body. These findings highlight the potential of the Fe-based BMGs as safe and reliable materials for biomedical implants, offering a combination of excellent corrosion resistance and minimal ion release, which are critical for maintaining biocompatibility and preventing adverse reactions in clinical applications.
2.4. Biocompatibility
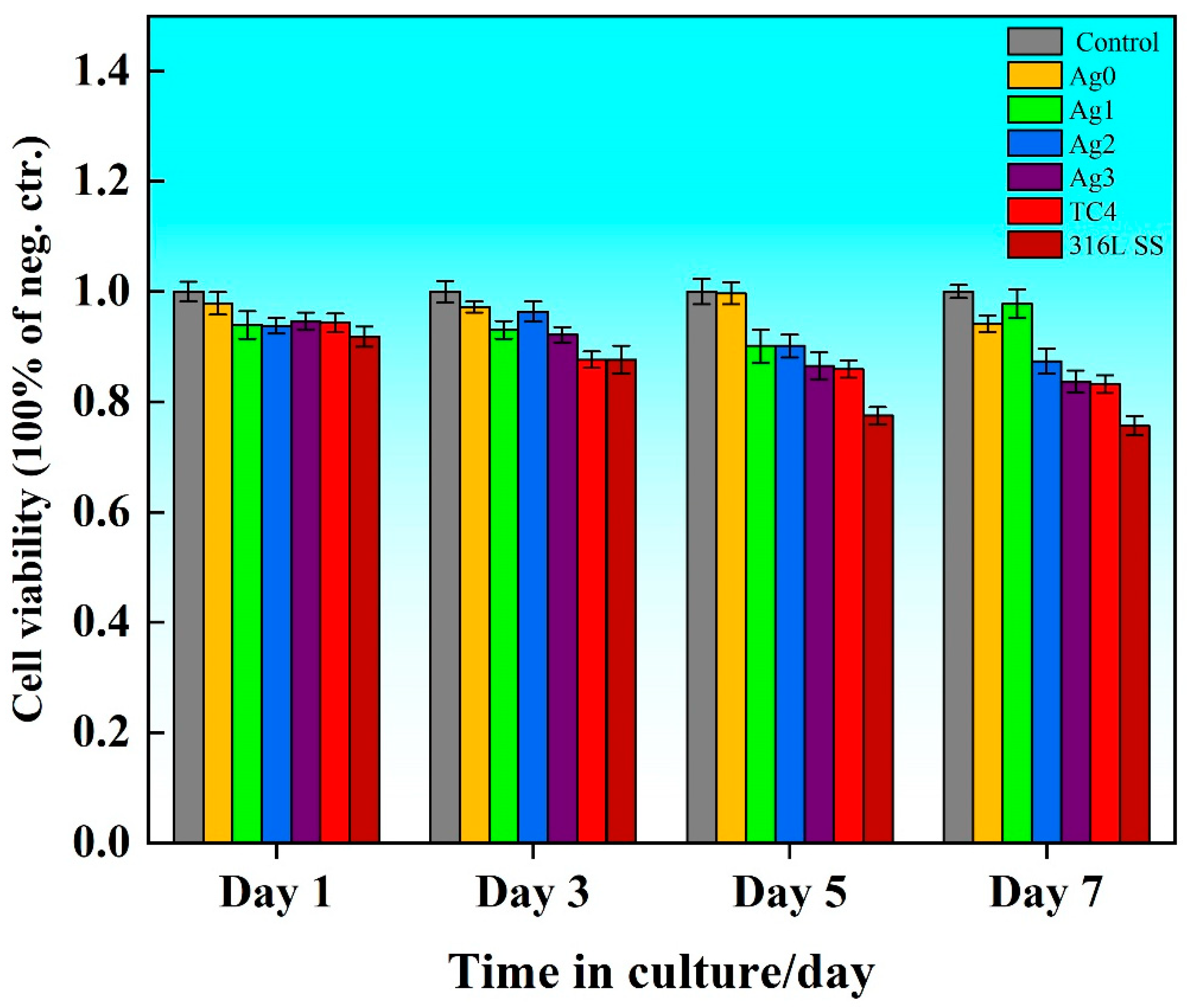
Figure 6 illustrates the cell viability ratio (
Rcv) of the Fe-based BMGs, 316L SS, and TC4 extracts over 1, 3, 5, and 7 days of incubation. The survival rate for all experimental groups ranged from 75% to 99%, corresponding to a toxicity grade of 0 or I, which aligns with the national standards for toxicity testing of medical and hygienic materials [
24]. Among the tested materials, 316L SS consistently exhibited the lowest cell viability as compared to the control, attributed to the release of cytotoxic ions such as Ni. In contrast, the cell viability of the Fe-based BMG was 8% and 1% higher than that of 316L SS and TC4, respectively, although a slight decrease was observed with the increasing Ag content. Compared with Ag0, the cell viability of Ag3 decreased by 10%.
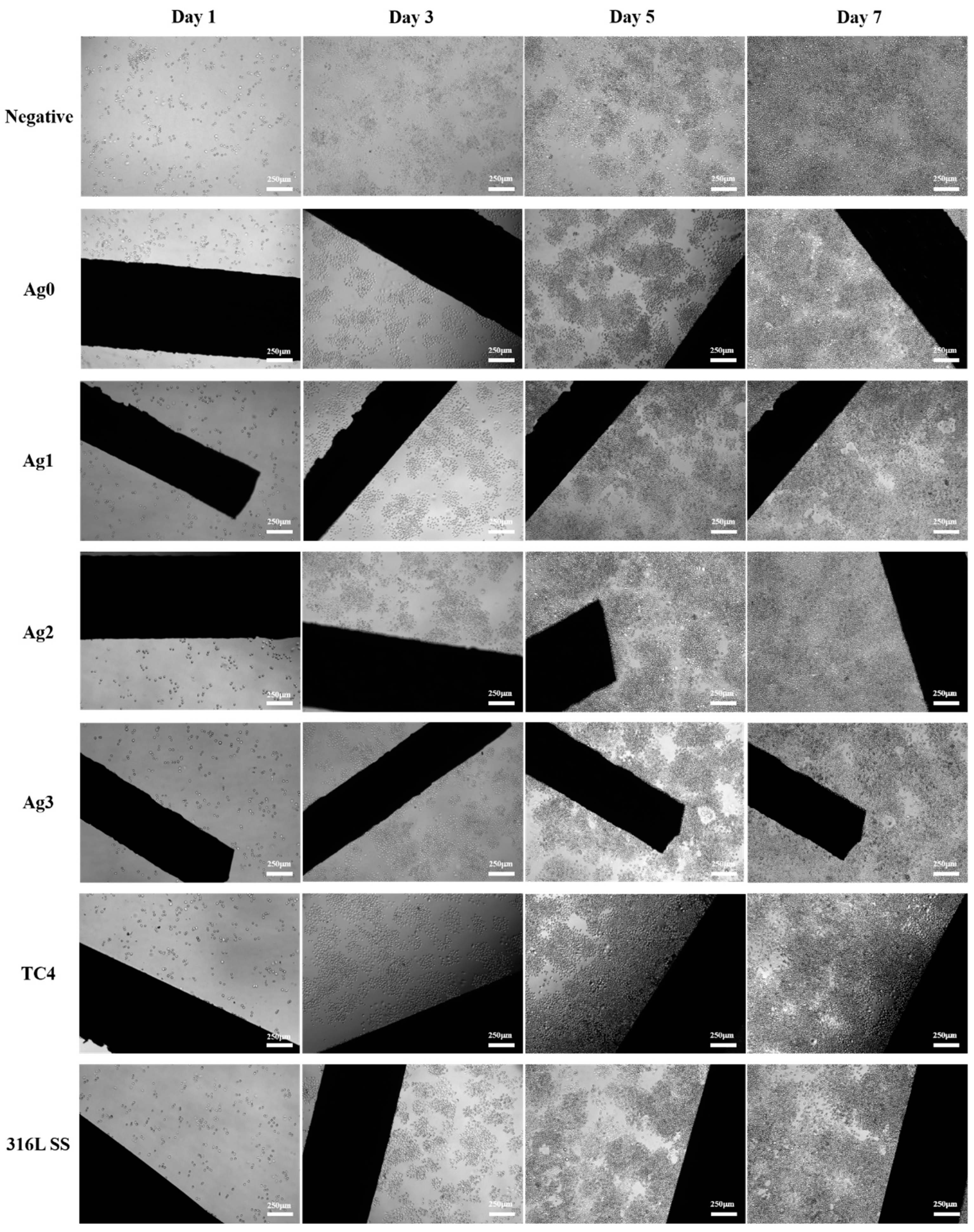
Figure 7 presents the adherent morphology of L929 cells under an inverted fluorescence microscope after 1, 3, 5, and 7 days of culture. By day 7, all samples showed a substantial increase in cell density, with cells spreading and extending in a shuttle-like pattern at low density. The results reveal no significant differences between the control and experimental groups, indicating that none of the tested materials had adverse effects on cell growth.
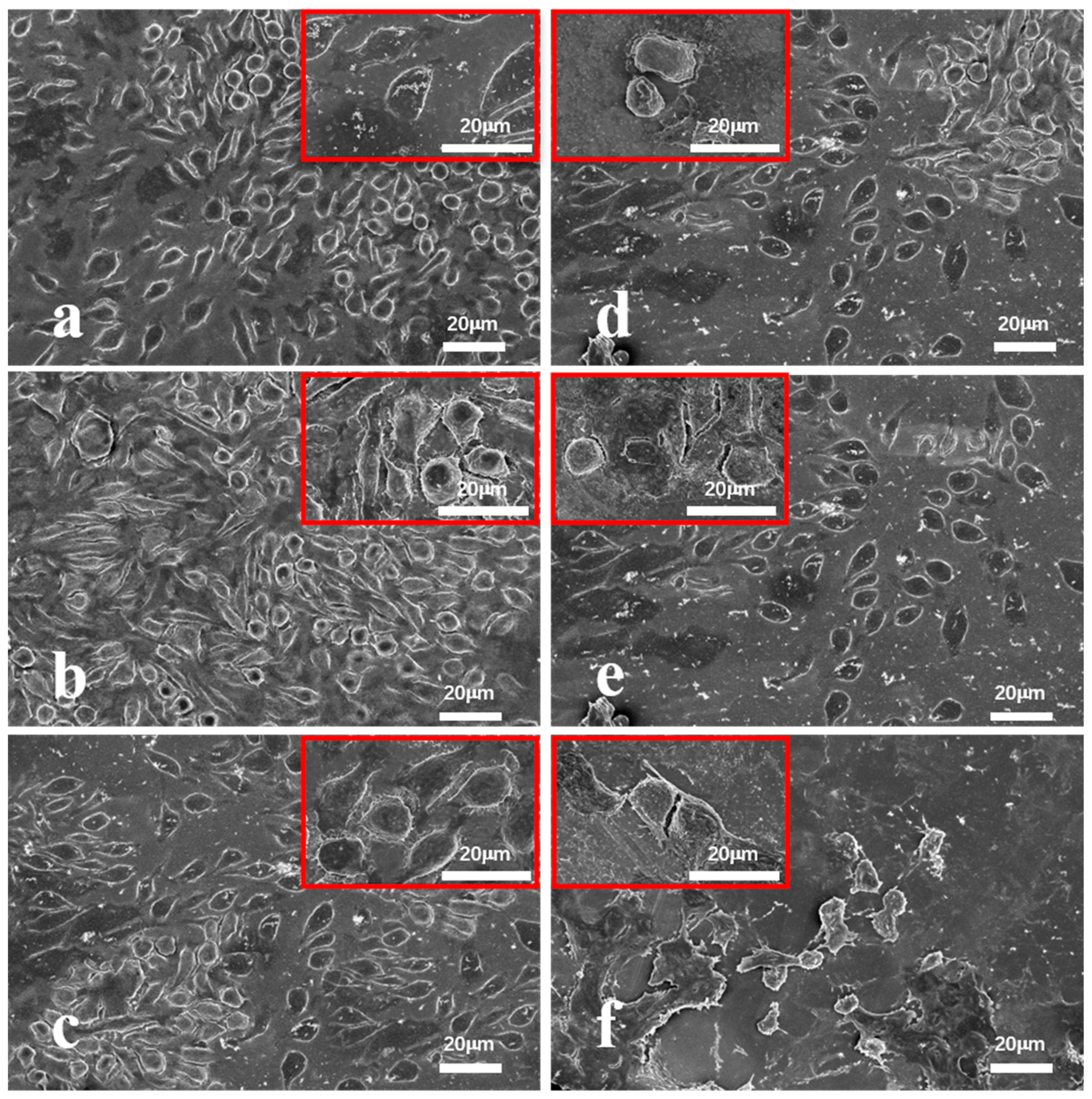
To further examine cell attachment and morphology,
Figure 8 provides SEM images of L929 cells adhering to the surfaces of the Fe-based BMGs, TC4, and 316L SS after 7 days of culture. The surface of the Fe-based BMGs was nearly completely covered with cells, reflecting their superior biocompatibility. In contrast, the surfaces of TC4 and 316L SS showed fewer adherent cells. The reduced cell attachment on TC4 is attributed to its biological inertness, which requires surface activation to improve cell adhesion. Meanwhile, 316L SS significantly reduced cell viability and attachment due to the release of toxic ions, such as Ni and Cr. SEM observations also showed intact cell morphology on all Fe-based BMG surfaces, indicating healthy cell growth without signs of rupture.
These findings collectively highlight the superior biocompatibility of the Fe-based BMGs as compared with TC4 and 316L SS, demonstrated by higher cell attachment, better cell morphology, and stronger cell activity across all tests.
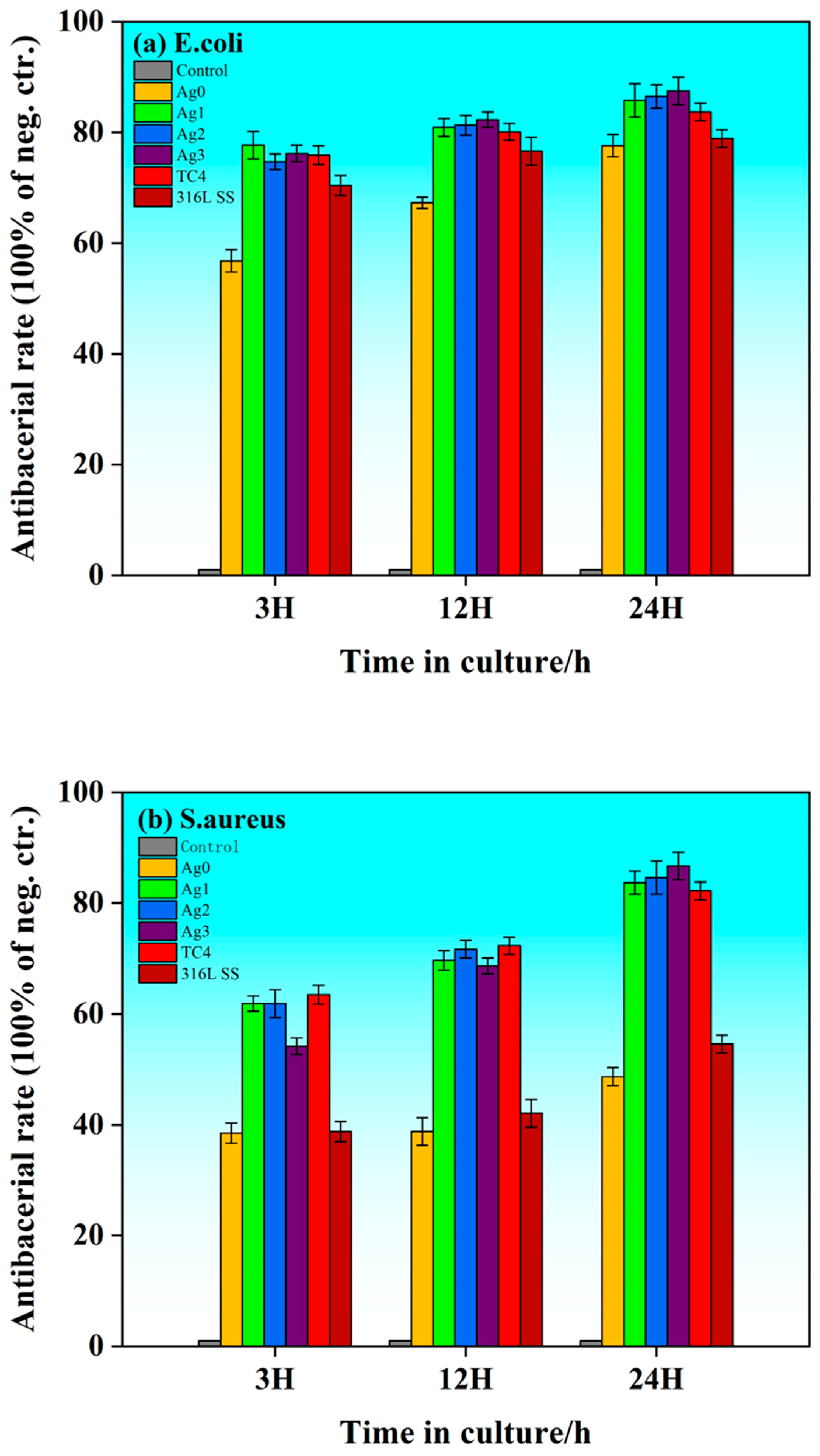
2.5. Antibacterial Activity
Figure 9 illustrates the bacterial inhibition rates of the Fe-based BMGs, 316L SS, and TC4 against
E. coli and
S. aureus at 3, 12, and 24 h of incubation. All six materials exhibit antibacterial properties to varying degrees. The Fe-based BMGs reduce bacterial attachment due to their amorphous microstructure, which limits the growth and proliferation of bacteria. Similarly, TC4 impedes bacterial attachment, owing to its inert surface. Meanwhile, 316L SS exhibits antibacterial performance partly due to the release of ions such as Cr, Fe, Mo, and Ni. However, its effectiveness remains inferior to the Ag-containing Fe-based BMGs.
The addition of Ag significantly enhances the antimicrobial performance of the Fe-based BMGs. At the early stage (3 h), the bacterial inhibition rate is similar across the samples, except for the Ag0, likely due to insufficient release of Ag ions. Over time (12–24 h), the antimicrobial performance of the Ag-containing Fe-based BMGs improves markedly, surpassing both TC4 and 316L SS. This enhanced performance can be attributed to the release of Ag+ ions, which trigger the reactive oxygen species (ROS) mechanism. ROS, including superoxide radicals, hydrogen peroxide, and hydroxyl radicals, disrupt bacterial membranes and induce oxidative damage, ultimately leading to cell death. Among the samples, the Ag3 demonstrates the highest antibacterial effect, achieving an inhibition rate of 87.5% against E. coli. Similarly, for S. aureus, the Ag3 exhibits an antimicrobial rate of 86.7%, significantly outperforming 316L SS (54.6%) and the Ag0 (48.7%).
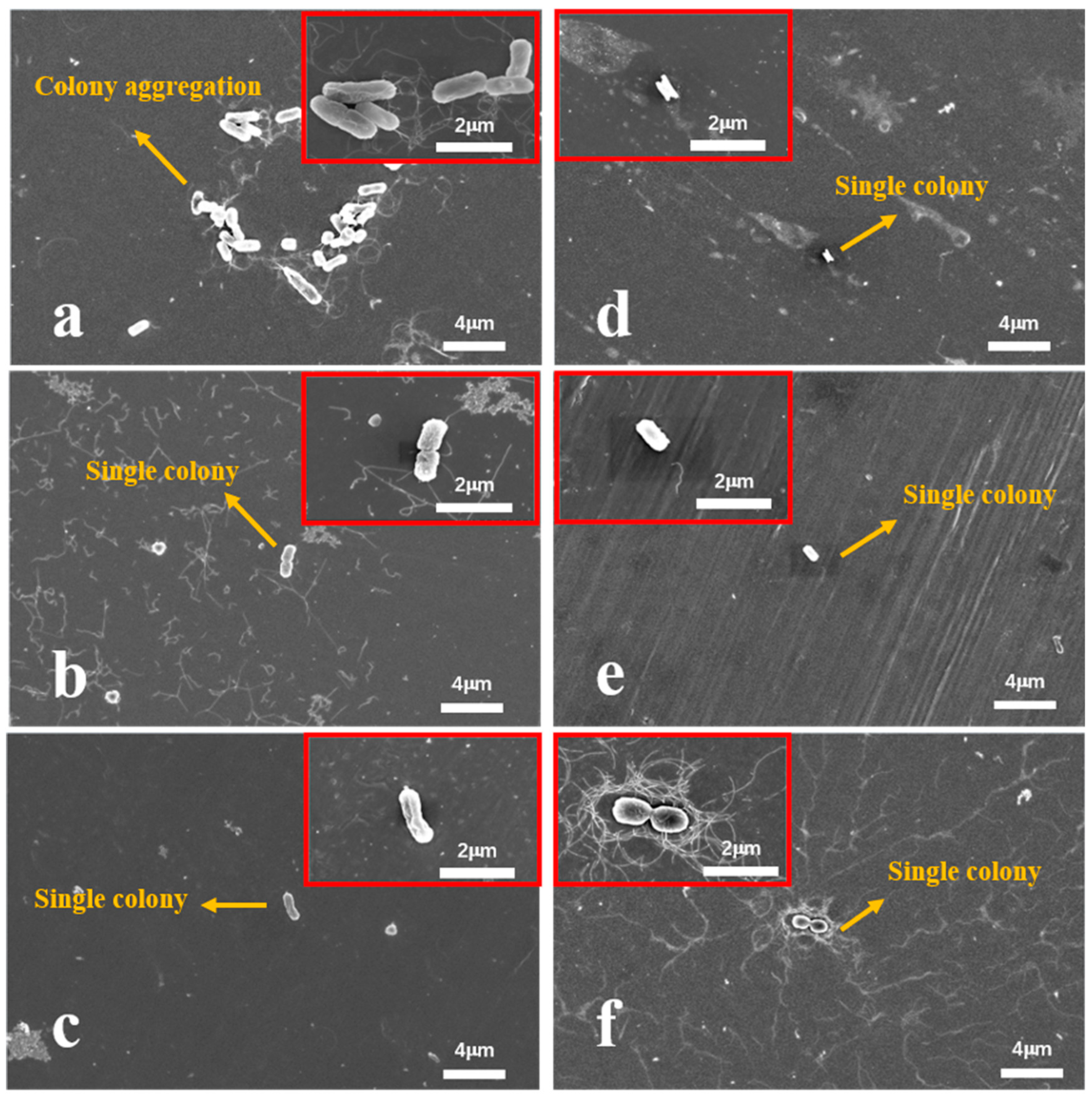
To further analyze the antibacterial behavior, bacterial adhesion was observed using FESEM after 24 h of incubation.
Figure 10 shows minimal
E. coli adhesion on the surfaces of the Ag-containing Fe-based BMGs, with only a few bacterial colonies observed on the Ag3. In contrast, the Ag0 exhibits higher bacterial aggregation, reflecting its weaker antimicrobial performance.
Figure 11 highlights similar trends for
S. aureus. The Ag0 and 316L SS show substantial bacterial adhesion, with dense colonies covering their surfaces. Interestingly, the diameter of
S. aureus colonies on the Ag0 is relatively small (0.2 μm), indicating limited bacterial growth. However, as the Ag content increases, bacterial adhesion is significantly reduced. The surface of the Ag3 shows only a few isolated bacterial colonies, demonstrating its superior antimicrobial effectiveness.
3. Materials and Methods
3.1. Material Preparation
Fe55−xCr20Mo5P13C7Agx (x = 0, 1, 2, 3 at.%) master alloy ingots were synthesized through induction melting of high-purity raw materials, including Fe (99.9 wt.%), Cr (99.9 wt.%), Mo (99.9 wt.%), graphite (99.9 wt.%), Ag (99.9 wt.%), and Fe3P (99.5 wt.%), under a high-purity argon atmosphere after a vacuum of approximately 10−5 Pa. The resulting alloy ingots were subsequently processed into glassy alloy rods of varying diameters using a copper-mold suction casting technique under the protection of pure argon gas.
3.2. Material Characterization
The amorphous structure of the as-cast alloy samples was verified using X-Ray diffraction (XRD) analysis performed on a Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with Cu Kα radiation (operating at 30 kV and 30 mA) at room temperature. The thermal behavior of the samples was analyzed via differential scanning calorimetry (DSC, NETZSCH, Görlitz, Germany) under an argon atmosphere, with a heating rate of 0.33 K/s.
To evaluate the mechanical properties, compression testing was conducted. The test samples, each with a diameter of 1 mm, were precisely cut to lengths of 2.5 mm. The ends of these samples were meticulously polished and smoothened using 2000-grit sandpaper to achieve a final length of 2 mm. Compression tests were performed at room temperature using an electronic universal testing machine (ETM105D, Wance, Shenzhen, China). To ensure data reliability, each test was repeated five times for each alloy composition.
3.3. Electrochemical Measurements
Electrochemical testing was conducted to evaluate the corrosion resistance of the Fe-based BMGs, 316L SS, and TC4, all prepared as 1 mm diameter bars. The experiments were performed using a Zahner Zennium X (Zahner, Neuss, Germany) electrochemical workstation in a three-electrode cell configuration. The setup included a platinum electrode as the auxiliary electrode, a saturated calomel electrode as the reference, and the test sample as the working electrode. The temperature was maintained at 37 °C using a water bath, with Hank’s solution (simulated body fluids, pH 7.4, supplied by China Beijing Biotech Ltd., Beijing, China) serving as the electrolyte.
Before testing, all samples were thoroughly polished with progressively finer grit sandpaper (600, 800, 1200, 2000, 3000, 4000, and 6500), followed by cleaning with alcohol via brief ultrasonication and air drying to ensure clean, smooth surfaces. The samples were immersed in the electrolyte for 30 min to establish a stable open-circuit potential before electrochemical polarization. The polarization curves were recorded at a scan rate of 1 mV/s over a potential range of −0.5 V to 1.6 V. The electrochemical corrosion parameters, including corrosion potential (Ecorr), corrosion current density (Icorr), pitting potential (Epit), and corrosion rate (CR), were derived from the kinetic potential polarization curves to comprehensively assess the corrosion resistance of the tested materials.
3.4. ICP Test
The concentration of ions released into Hank’s solution following kinetic potential polarization was quantified using full-spectrum, direct-reading inductively coupled plasma atomic-emission spectrometry (ICP-AES) with a PerkinElmer Optima 8000 spectrometer (PerkinElmer, Waltham, MA, USA). Each experimental group was measured in triplicate, and the results were averaged to ensure accuracy and reliability.
3.5. Cytocompatibility Evaluation
3.5.1. Cell Culture
Mouse embryonic fibroblasts (L929 cell line, Servicebio) were maintained in a basal medium consisting of Minimum Essential Medium (MEM, Servicebio) supplemented with 10% fetal bovine serum (FBS, Servicebio) and 1% penicillin–streptomycin antibiotics (Servicebio).The mouse embryonic fibroblasts (L929 cell line) were obtained from Servicebio (Wuhan, China)(Cat#: STCC20025P). The NCTC clone 929 [L cell, L-929, derived from C3H/An L species] cell line is a clone of the L cell line created in March of 1948. The L cell line is among the first established continuous cell lines, with L-929 being one of the early clonal derivatives. The parent L cell line was developed from the subcutaneous loose connective and adipose tissue of a 100-day-old male C3H/An mouse. At passage 95, the L cell line was employed to isolate single cells by capillary cloning to generate the L-929 clone. The cells were cultured in a humidified incubator at 37 °C with 5% CO2 to ensure optimal growth conditions. After initial incubation, the cells were washed with phosphate-buffered saline (PBS, Servicebio) and replenished with fresh medium. Following approximately 48 h of incubation, the cells were allowed to proliferate until they reached confluence at the bottom of the culture flask.
To harvest the cells, 0.25% trypsin solution was used to enzymatically detach them from the culture surface. The reaction was terminated by adding a growth medium comprising 44 mL MEM, 5 mL FBS, and 1 mL penicillin–streptomycin solution. The cells were subsequently counted using the plate counting method, and the cell concentration was adjusted to 10,000 cells per well for downstream experiments.
3.5.2. Cell Counting Kit-8 (CCK-8) Test
To ensure consistent and controlled experimental conditions, the samples used in this study, including the Fe-based BMGs, 316L SS, and TC4, were prepared as 3 mm × 1 mm sheets and polished to a mirror finish. The samples were thoroughly cleaned using an ultrasonic machine with anhydrous ethanol and deionized water for 10 min, followed by drying and sterilization in an autoclave to guarantee sterility. Three replicates of each sample were prepared and handled in an ultra-clean biological laboratory to maintain contamination-free conditions.
The prepared samples were inoculated into 24-well plates along with a blank group (no cells) and a control group (cells without material). Mouse embryonic fibroblast cells (5000 cells/well) were seeded into the 24-well plates and incubated in a humidified incubator at 37 °C with 5% CO2. Cell viability was assessed at four time points: 1, 3, 5, and 7 days. At each time point, 90 μL of cell suspension from each well was transferred to a 96-well plate, and 10 μL of CCK-8 reagent was added. The mixture was homogenized and incubated at 37 °C for 2 h. Following incubation, spectrophotometric absorbance measurements were taken at 450 nm using an enzyme-linked microplate reader.
For each experiment, three replicates (sample and control) were analyzed to ensure data reliability. The relative cell viability (Rcv) of each sample was calculated using the following formula: Rcv = (cell activity in experimental extract)/(cell activity in negative control). Calculate the standard deviation of the data from multiple experimental results using the standard deviation formula, and then use the standard deviation to represent the error. In the graph, set the length of the error bars to the standard deviation.
3.5.3. Cell Adhesion
Cell growth and adhesion were systematically evaluated at intervals of 1, 3, 5, and 7 days during the CCK-8 experiments. Observations and photographic documentation were performed using inverted fluorescence microscopy (Leica DMi8, Wetzlar, Germany). Following the 7-day incubation period, the samples co-cultured with cells were carefully removed and rinsed three times with phosphate-buffered saline (PBS) to eliminate any non-adherent cells. Subsequently, the adherent cells on the sample surfaces were fixed with 3% glutaraldehyde at room temperature for 1.5 h, protected from light to prevent degradation.
To prepare the samples for further analysis, they were sequentially dehydrated using ethanol solutions of increasing concentrations (30%, 50%, 70%, 95%, and 100%), with each step lasting 15 min at room temperature. Once dehydrated, the samples were dried in a vacuum atmosphere to preserve their structures. The final prepared samples were examined using an ultra-high-resolution cold field emission scanning electron microscope (FESEM, Hitachi, Tokyo, Japan) to investigate the surface morphology and growth patterns of the adhering cells.
3.6. Antibacterial Evaluation
3.6.1. Bacterial Culture
Healthcare-associated infections are frequently caused by pathogenic microorganisms, with E. coli and S. aureus being among the most prevalent culprits. To investigate the antimicrobial properties of the developed materials, this study employed E. coli (ATCC) and S. aureus (ATCC) as representative experimental bacteria, encompassing both Gram-negative and Gram-positive strains.
Bacterial cultures were grown on Luria–Bertani (LB) agar medium plates (Servicebio). The LB solid medium was prepared by dissolving the dry powder in purified water, followed by sterilization in an autoclave at 121 °C for 30 min. After cooling the sterilized medium to 50–60 °C, it was poured into sterile Petri dishes under ultra-clean conditions and allowed to solidify. Bacteria were extracted from frozen stock cultures and inoculated onto the prepared LB plates under aseptic conditions. The inoculated plates were incubated at 37 °C for 24 h in a constant-temperature incubator, facilitating the formation of distinct bacterial colonies.
On the subsequent day, fresh broth cultures were prepared to ensure viable bacterial populations for the experiments. This was achieved by diluting bacterial suspensions in fresh LB broth to a final concentration of 1 × 10⁵ CFU/mL, corresponding to a spectrophotometric optical density (OD) of 0.001 at 600 nm. This standardized preparation ensured reproducibility and reliability in evaluating the antimicrobial efficacy of the tested materials.
3.6.2. Antibacterial Activity Evaluation
To control the effective area, the samples (Fe-based BMGs, 316L SS, and TC4) were prepared as 3 mm × 1 mm sheets and polished to a mirror finish. All samples were cleaned with anhydrous ethanol and deionized water in an ultrasonic cleaner for 10 min, dried, and sterilized in an autoclave to ensure sterility. The sterilized samples were submerged in 24-well plates containing 1 mL of E. coli or S. aureus LB medium with a bacterial concentration of 1 × 10⁵ CFU/mL. Blank groups (no bacteria) and control groups (bacteria without material) were also included. The plates were incubated statically at 37 °C for 24 h. For each experiment, three replicates (sample and control) were analyzed to ensure data reliability. The following formula was employed: Calculate the standard deviation of the data from multiple experimental results using the standard deviation formula, and then use the standard deviation to represent the error. In the graph, set the length of the error bars to the standard deviation.
Bacterial inhibition was monitored spectrophotometrically at 600 nm, with measurements taken after 3 h (early stage), 12 h (intermediate stage), and 24 h (late stage). The inhibition rate was calculated using the following formula:
After 24 h, the samples were gently rinsed three times with PBS, and surface-adhering bacteria were fixed with 3% glutaraldehyde at room temperature in the dark for 1.5 h. The samples were sequentially dehydrated with ethanol solutions (30%, 50%, 70%, 95%, and 100%) for 15 min each at room temperature and dried under vacuum. Bacterial adhesion and growth were subsequently analyzed using an ultra-high-resolution cold field emission scanning electron microscope (FESEM, Hitachi, Tokyo, Japan).
4. Conclusions
In this study, Fe55-xCr20Mo5P13C7Agx (x = 0, 1, 2, 3 at.%, denoted as Ag0, Ag1, Ag2, and Ag3, respectively) bulk metallic glasses (BMGs) were prepared to investigate the effects of Ag addition on their antimicrobial and biomedical properties. The key conclusions are as follows:
- (1)
The glass-forming ability (GFA) of the Fe-based BMGs decreased with increasing Ag content, as indicated by a reduction in the critical diameter for glass formation from 2.0 mm for Ag0 to 1.0 mm for Ag3. This decline was attributed to the positive enthalpy of the mixing between Ag and Fe, which promotes crystalline phase precipitation.
- (2)
The Fe-based BMGs exhibited superior corrosion resistance as compared with 316L SS and TC4 in Hank’s solution at 37 °C. The Ag3 demonstrated the lowest corrosion current density and the most stable passivation behavior, making it highly resistant to localized corrosion.
- (3)
The Fe-based BMGs showed excellent biocompatibility, outperforming 316L SS and TC4 in terms of cell viability and adhesion. SEM analysis confirmed superior cell attachment and morphology on the Fe-based BMG surfaces, and minimal harmful ion release ensured their safety as implant materials.
- (4)
The addition of Ag significantly enhanced the antimicrobial properties of the Fe-based BMGs. Ag3 achieved the highest bacterial inhibition rates, with 87.5% against E. coli and 86.7% against S. aureus, surpassing both 316L SS and TC4. This was attributed to the Ag+ ions triggering the production of reactive oxygen species (ROS), which disrupt bacterial membranes and induce cell death.
- (5)
Combining exceptional mechanical properties, corrosion resistance, biocompatibility, and antimicrobial performance, the present Ag-containing Fe-based BMGs, particularly Ag3, emerge as promising candidates for next-generation biomedical implants. These materials provide a cost-effective, high-performance alternative to conventional implants, addressing critical challenges such as bacterial infections and implant failures.
Additionally, there are several promising research directions worth further exploration in this study, which are summarized as follows:
- (1)
As a potential orthopedic implant material, its osteogenic ability can be further assessed by culturing osteoblasts and testing their cellular activity in the samples.
- (2)
Biofilms play a crucial role in antibacterial research. Therefore, future studies could focus on dynamic biofilm research and design multifunctional antibacterial biofilms on the material surface.
- (3)
This study lacks in vivo experimental validation, which limits a comprehensive understanding of the material’s performance in a real biological environment. Future research should strengthen in vivo experiments to further validate the clinical relevance of the in vitro results, providing a more solid foundation for the application of Fe-based amorphous alloys in the biomedical field.