Abstract
Coordination-driven Cu(I) complexes constitute an interesting class of materials with rich opto-electronic properties and diverse applications. Various homo- and heteroleptic Cu(I) complexes have been reported in the literature. In continuation with our quest for new materials, we report herein two novel coordination-driven self-assembled Cu(I) complexes: the homoleptic (1) and the heteroleptic (2) complexes based on the 6,6′-bis(phenylethynyl)-2,2′-bipyridine (L1) and 2,9-dimethyl-1,10-phenanthroline (dmph) ligands. L1 was prepared by a Pd(II)-catalyzed Sonogashira cross-coupling reaction between phenylactylene and 6,6′-dibromo-2,2′-bipyridine. Homo- and heteroleptic Cu(I) complexes were obtained by the self-assembly of L1 and dmph ligands. Complexes (1) and (2) were obtained in high yields, and are soluble in common organic solvents and stable at room temperature over a long period of time. The optical (absorption and emission) properties of both complexes were evaluated. The optical properties in solution are a function of the ligands and varied for the complexes. Complex (2) was also characterized by single-crystal X-ray diffraction and the intermolecular interaction was studied using Hirschfeld surface analysis. In the solid state, complex (2) exhibited four-coordinate distorted tetrahedral geometry around Cu(I). Density functional theory (B3LYP/6-311++G(d,p) was utilised to determine various molecular descriptors.
1. Introduction
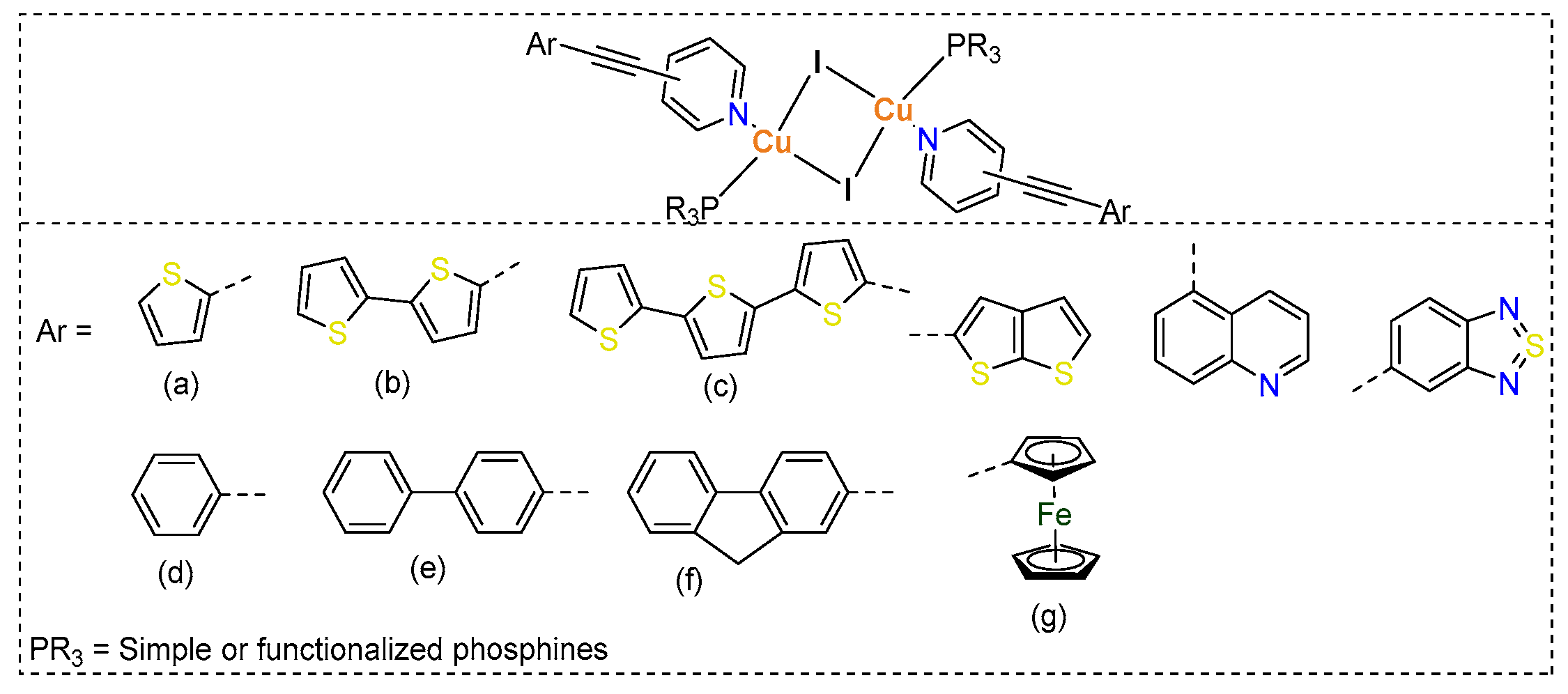
In the ongoing quest for innovative materials with remarkable opto-electronic properties and applications, researchers are investigating a diverse array of small to large molecular systems. Transition metals-based coordination compounds remain at the forefront among the emerging new functional materials due to their diverse photophysical properties and applications [1]. Over the last four decades, there has been a significant surge in the development of d10 ion-based complexes, particularly Cu(I) complexes [2,3]. Unlike traditional organic molecules, Cu(I) complexes can be easily prepared with a diverse range of ligands, allowing for easy manipulation of their structural, photophysical, and electrochemical features [4,5]. To date, a wide variety of Cu(I)-based dimers, tetramers, oligomers, and polymers are known [3,6], with applications in opto-electronics, catalysis, and biologicals [7,8,9]. The physicochemical and photophysical properties of Cu(I) complexes can be fine-tuned and regulated by the ligands and co-ligands used [9]. The most commonly used ligands come from N-donor ligands, which impart high stability to the complexes. Numerous studies have shown that by carefully fine-tuning the coordinated ligands in various complexes, researchers can create a wide array of compounds that exhibit distinct and precisely controlled photophysical properties. These tailored characteristics can lead to innovative applications in different fields. For instance, by adjusting the structure and electronic properties of the ligands, it is possible to enhance light absorption, emission, and overall stability of these complexes, thereby expanding their utility in advanced technologies [9,10,11]. We, in the past, studied various dimeric and tetrameric Cu(I) complexes bearing ethynylpyridine ligands and triarylphosphines as co-ligands (Chart 1). Our studies highlighted that the Cu(I) complexes containing pyridine ligands attached to 4-ethynyl ferrocene display fascinating electrochemical properties [12,13]. In these complexes, the ferrocene units serve as electron reservoirs or sinks, which significantly influence their emission properties in a detrimental manner. Taking this observation into consideration, we shifted our attention to a thorough examination of the structural and photophysical properties of Cu(I) complexes linked to both carbocyclic and heterocyclic ethynylpyridine derivatives [14,15]. We demonstrated linker-dependent optical features in these complexes. We also showed that dimeric complexes incorporating heteroarylethynyl groups are not only highly emissive but also demonstrate reasonable light to electricity conversion efficiency.
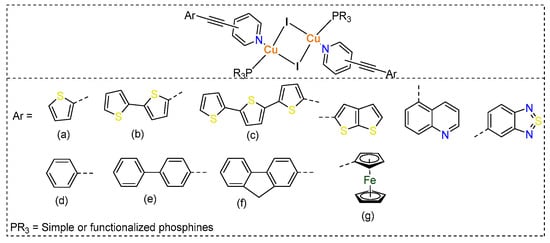
Chart 1.
Chemical structures of some neutral Cu(I) complexes prepared by self-assembly procedure using arylethynyl based N-donor ligands (a–g).
Herein, we report two novel coordination-driven self-assembled Cu(I) complexes: the homoleptic (1) and the heteroleptic (2) complexes bearing 6,6′-bis(phenylethynyl)-2,2′-bipyridine and 2,9-dimethyl-1,10-phenanthroline. The absorption and emission properties of both complexes were evaluated, and DFT calculations were performed to understand the influence of a coordination sphere on their optical properties and the nature of their excited states. We discuss the X-ray single-crystal structure of the heteroleptic complex in detail. Finally, we investigated the non-covalent interactions within the complex using Hirschfeld surface analysis.
2. Results
2.1. Chemistry
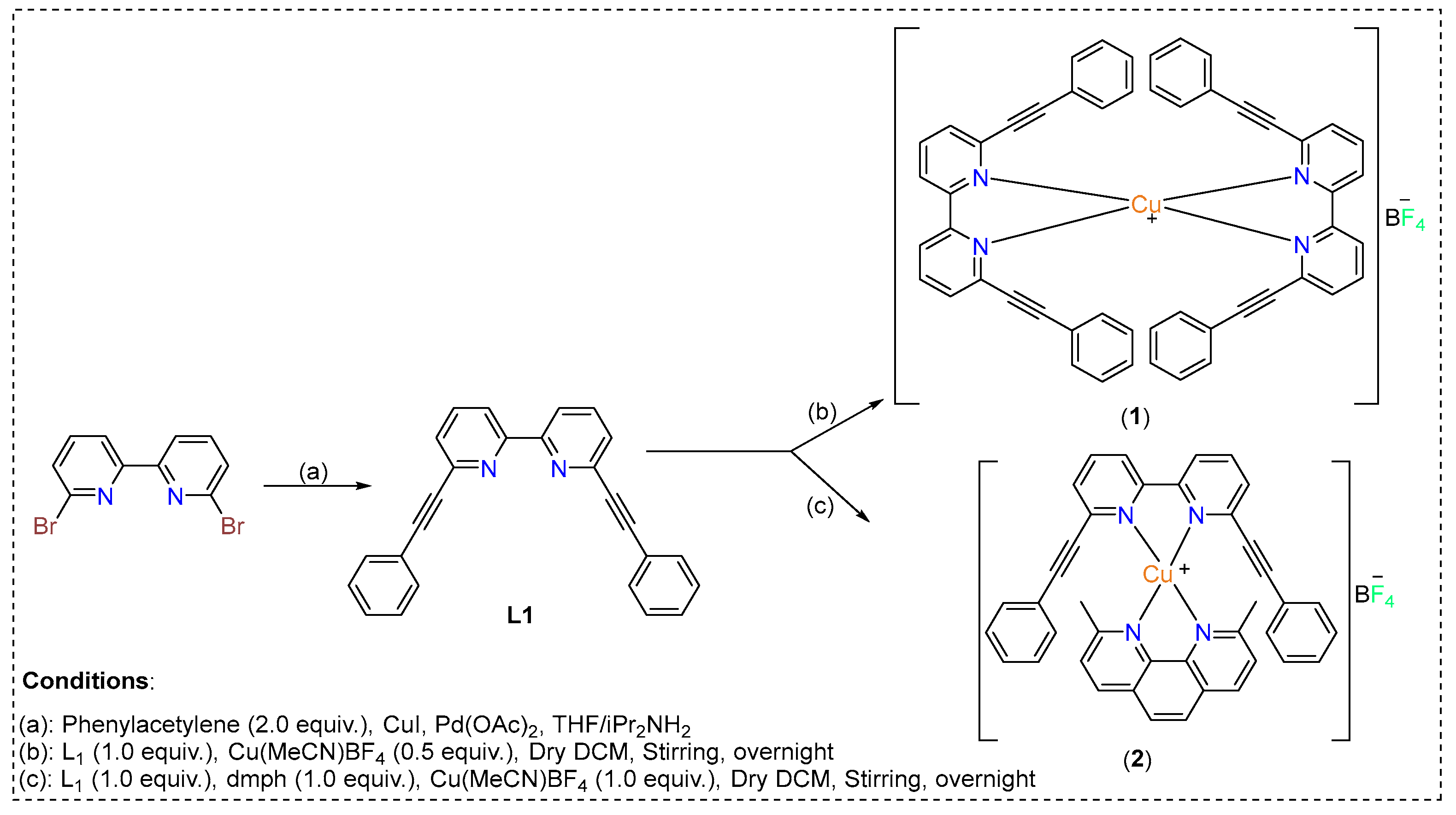
The synthetic protocol of the ligands and complexes are depicted in Scheme 1. The ligand 6,6′-bis(phenylethynyl)-2,2′-bipyridine (L1) was prepared by a Pd(II)/Cu(I)-catalyzed Sonogashira cross-coupling reaction between 6,6′-dibromo 2,2′-bipyridine and phenylacetylene [16]. The chemical structure of the ligand was confirmed using 1H/13C-NMR (Supporting Information). Homo- and heteroerotic Cu(I) complexes were obtained by self-assembly of the 6,6′-bis(phenylethynyl)-2,2′-bipyridine (L1) and 2,9-dimethyl-1,10-phenanthroline (dmph) ligands.
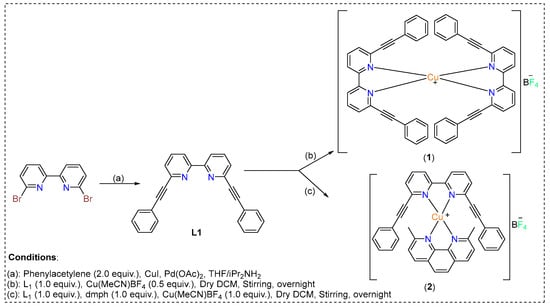
Scheme 1.
Synthetic scheme for obtaining homoleptic (1) and heteroleptic (2) Cu(I) complexes based on 6,6′-bis(phenylethynyl)-2,2′-bipyridine.
Formulation of the synthesized ligands and the Cu(I) complexes were established by elemental analysis, FT-IR, 1H-NMR, 13C-NMR, and mass spectrometry. The IR spectrum of L1 shows shifts in the bands on the formation of complexes 1 and 2 (Figures S2 and S3) attributed to increased conjugation. The observed ν(C≡C) stretching frequencies of the acetylide-functionalized arylpyridine is 2207 cm−1. The copper complexes 1 and 2 displayed a ν(C≡C) stretching frequency of 2218 and 2221 cm−1, respectively. In the 1H-NMR spectrum of L1 (Figure S4), the characteristic protons of the pyridyl ring resonated at high chemical shifts in the range of 8.49–8.47 ppm. The chemical shifts of other phenylic protons resonated at 7.84 to 7.39 ppm, supporting the formation of L1. The C=N and -C≡C- carbons were identified by the presence of high chemical shifts in the 13C spectrum at 155 ppm and 120.83 ppm (Figure S5). The same techniques are applied to confirm the formulation of complex (1). The characterization of the complex has been provided in the experimental section. The Cu(I) complex (2) was characterized by 1H-NMR and 13C-NMR (Figures S6–S9) In 1H-NMR, the significant protons of the complex appeared at high chemical shifts due to resonating downfield in the range of 8.60–8.17 ppm and 7.92–7.58 ppm due to pyridyl and phenanthroline protons, respectively. The chemical shift of protons of the phenyl ring was found to be at 7.32–6.03 ppm. In 13C-NMR, the chemical shifts of the -C=N and -C≡C- carbons were found at 157.87, 151.86, and 122.66 pm, showing the formation of the complex. Finally, the molecular structure of the complex was confirmed by X-ray single-crystal structure analysis.
2.2. X-Ray Single-Crystal Structure
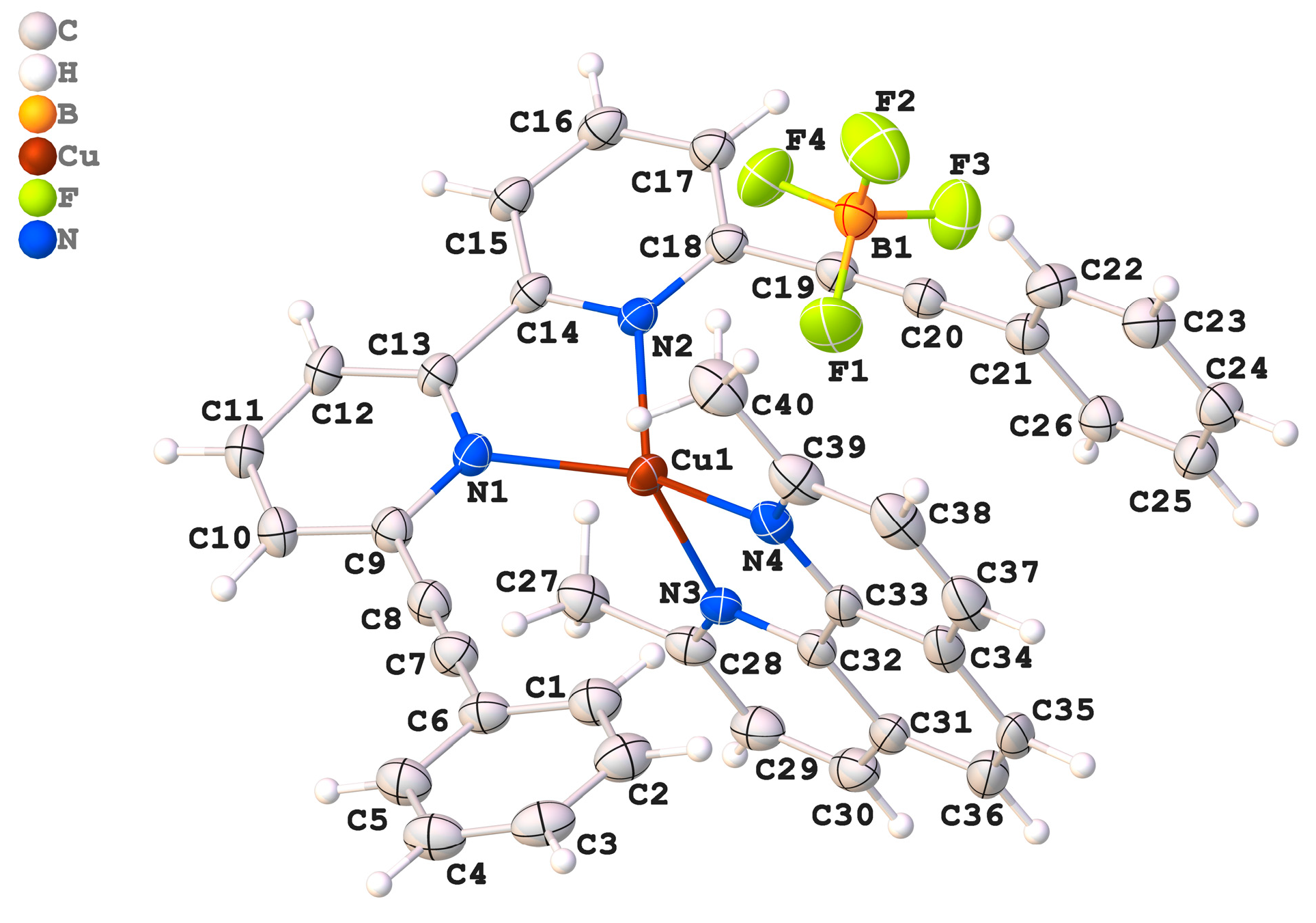
The compound Cu[(C30H20N2)(C14H14N2)]BF4, referred to as (2).BF4, crystallized within the centrosymmetric triclinic space group P-1 (Table 1). This indicates that the crystal structure possesses an inversion center, reflecting a symmetrical arrangement of its components (Figure 1). This compound forms a 1:1 complex of Cu(I) that is intricately built using two distinct ligands. The first ligand, 2-(2-phenylethynyl)-6-(6-(2-phenylethynyl)pyridin-2-yl)pyridine, is a conjugated bidentate ligand stemming from the presence of phenylethynyl substituents attached to a bipyridine core. The second ligand, 2,9-dimethyl-1,10-phenanthroline (dmph), is a planar, aromatic bidentate ligand that coordinates effectively with the metal center via its nitrogen atoms. The coordination geometry around the Cu(I) center is tetrahedral.

Table 1.
Crystal data and structure refinement for (2).BF4.
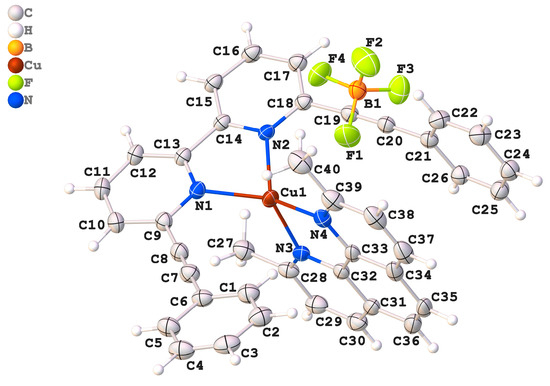
Figure 1.
An ORTEP drawing of compound (2).BF4, with the atom-numbering scheme. Displacement ellipsoids for non-H atoms are drawn at the 50% probability level. H atoms are shown as spheres of arbitrary radii.
The bond lengths around the Cu- atom are important for evaluating the stability and reliability of the coordination environment. The measured values are Cu1–N1 = 2.083(3) Å, Cu1–N2 = 1.989(2) Å, Cu1–N3 = 2.015(2) Å, and Cu1–N4 = 2.055(3) Å. When compared to previously reported analogs, Cu1–N1 = 2.0419(12)–1.988(2) Å, Cu1–N2 = 2.100(2)–2.0849 Å, Cu1–N3 = 1.988(2)–1.9760(11) Å, and Cu1–N4 = 2.100(2)–1.9760(11) Å [17,18], these values fall well within the typical range for Cu N coordination and confirm the integrity and stability of the coordination environment. The N2–C14 bond is 1.3399(5) Å, which closely matches the bond lengths observed in similar structures, reported as 1.3350(2), 1.327(3), and 1.3379(18) Å [17,18]. The bond angles surrounding the central copper atom (Cu1) provide crucial insights into its geometric arrangement. In the studied complex, the angle defined by the nitrogen atoms, N1–Cu1–N2, is 82.7(1)°, which is slightly higher than reported Cu complexes [17,18]. However, the bond angle is notably smaller than the ideal bond angle of a tetrahedron (109.5°), suggesting the presence of steric hindrances or electronic distortions. In the counter ion, the B-F bond lengths vary between 1.346(5) Å and 1.360(5) Å. This value is found to be close to the theoretically calculated values (1.3602 Å to 1.3801 Å). We observe that the tetrafluroborate anion has a slightly distorted tetrahedral geometry, and the distortion is attributed to the packing effect.
The structural data obtained by SCXRD were further complemented by density functional theory (DFT) calculations (B3LYP/6-311++G(d,p)) [19]. In the DFT-optimized structure, there were no imaginary frequencies present, indicating that the calculated structure possesses a favorable and energetically stable configuration. To assess the reliability of the computational methods utilized, we further compared selected crystal structure parameters, specifically bond lengths and angles, with the theoretically derived optimization data for heteroleptic complex (2) (Figure S1). Remarkably, the correlation coefficients were found to be R2 = 0.9828 for bond lengths and R2 = 0.9993 for bond angles. These values indicate an exceptionally strong agreement with the experimental data.
2.3. Hirshfeld Surface (HS) Investigation
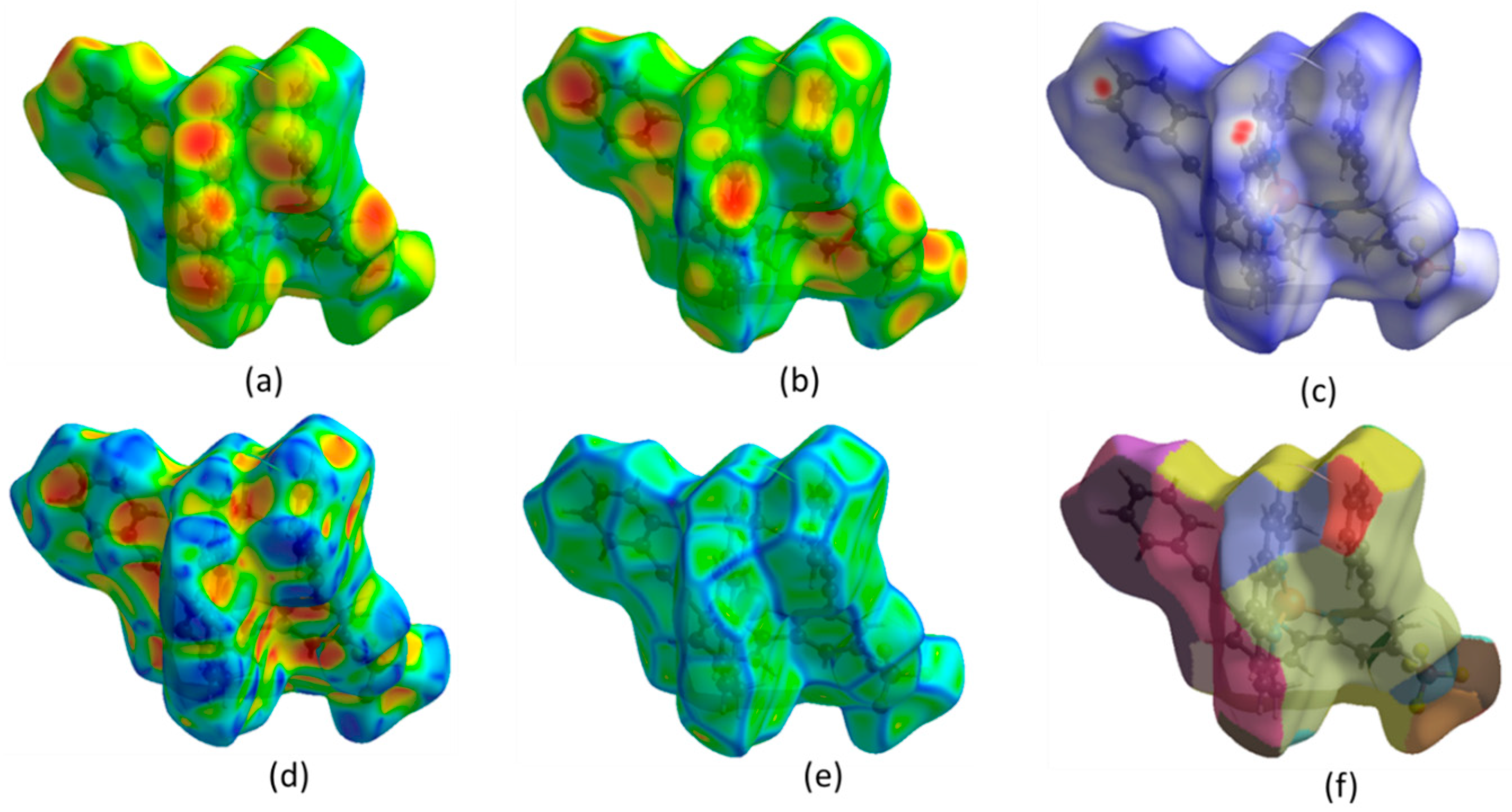
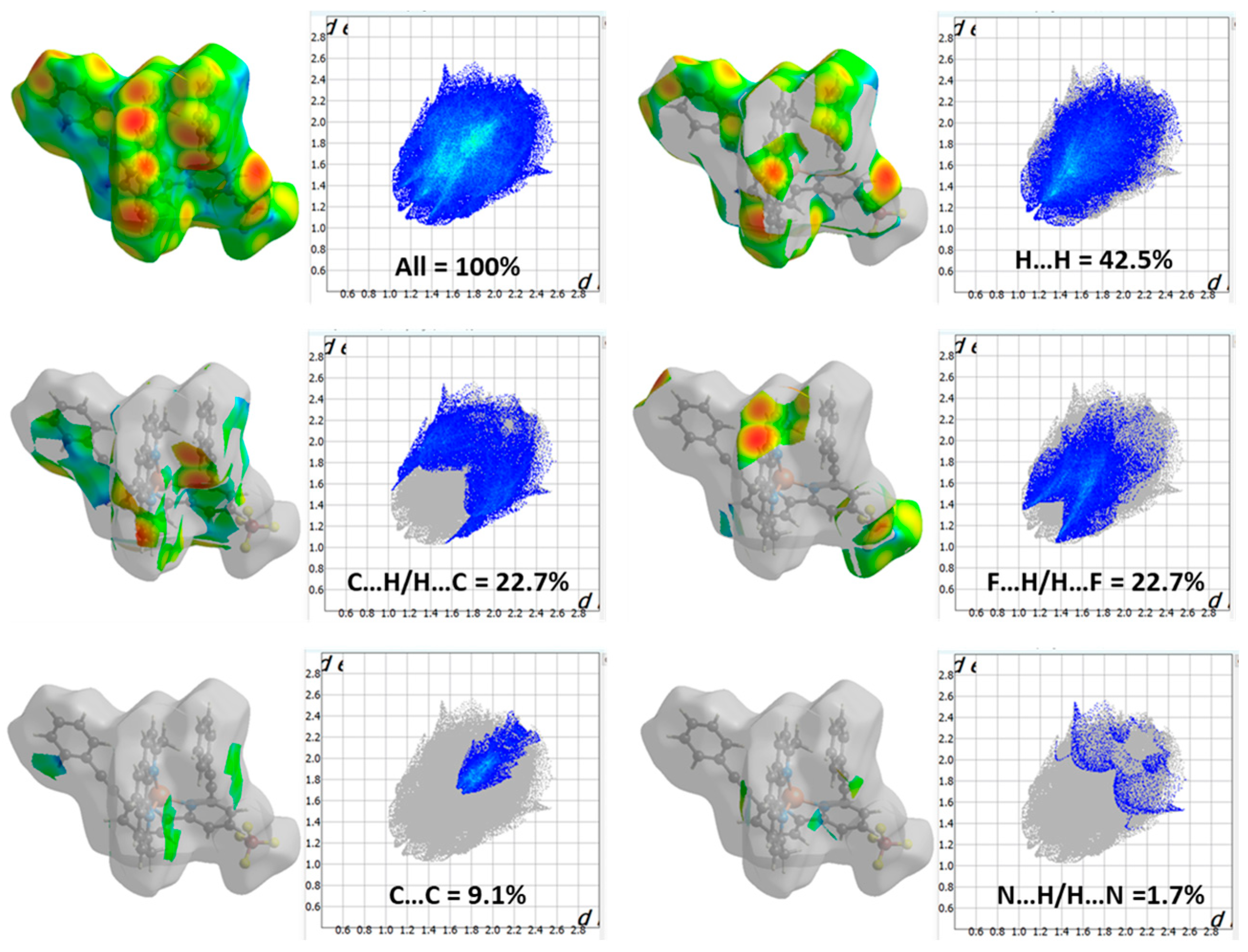
The Hirshfeld surface (HS) analysis provides a comprehensive examination of intermolecular interactions and their quantitative contributions to the arrangement of crystals. In HS analysis, the concept of normalized contact distance is crucial for understanding molecular interactions. Using Crystal Explorer (v3.1) [20,21,22], the HS and the corresponding two-dimensional fingerprint plots were generated (Figure 2). Intermolecular contacts in a crystal structure can be visualized using a color-coded system based on van der Waals separations. Red regions indicate strong interactions (short distances), white represents balanced interactions, and blue signifies weak interactions (longer distances). Two-dimensional fingerprint plot maps di (distance inside the molecular surface) and de (distance outside) (Figure 2a,b) were created, providing a quantitative analysis of molecular interactions. The dnorm surface highlights contacts: red (shorter-than-van der Waals), white (equal), and blue (longer) (Figure 2c). The shape index reflects molecular complementarity (Figure 2d), while curvedness identifies flat versus highly curved regions (Figure 2e). The fragment patch (Figure 2f) isolates specific molecular fragments for interaction analysis. These visualizations aid in understanding molecular packing, hydrogen bonding, and π-π stacking interactions, which are crucial for structural characterization.
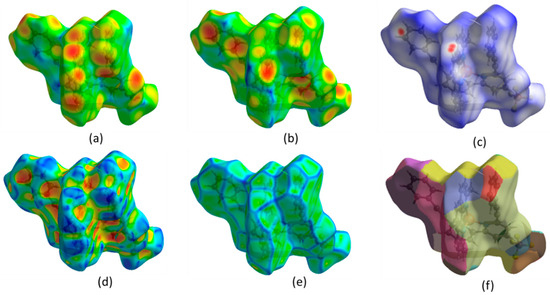
Figure 2.
(a) Hirshfeld surfaces (HS) mapped onto di (distance inside), (b) de (distance outside), (c) dnorm (normalized contact distance), (d) shape index, (e) curvedness, (f) fragment patch.
The 2D fingerprint map (Figure 3) quantitatively represents these interactions, highlighting their relative contributions to the overall Hirshfeld surface. The dominant interaction observed is H…H (42.5%), which reflects van der Waals contacts and close packing consequences, playing a vital role in the stabilization of the molecular arrangement. The effective existence of C…H contacts (22.7%) suggests contributions from non-covalent interactions such as C-H•••π interactions or weak hydrogen bonding, which can influence molecular organization. Similarly, F…H interactions (22.7%) indicate the involvement of fluorine in intermolecular contacts, potentially contributing to weak hydrogen bonding or dipole-induced interactions. The presence of C…C interactions (9.1%) suggests the occurrence of π•••π stacking, which is common in conjugated systems and may contribute to the structural stabilization through aromatic interactions. N…H interactions (1.7%) likely correspond to hydrogen bonding, where nitrogen acts as a hydrogen bond acceptor, albeit with a minor contribution. The relatively low percentages of N…C (0.8%) and F…C (0.5%) indicate minimal direct nitrogen-to-carbon and fluorine-to-carbon contacts, possibly due to steric constraints or unfavorable orientations. These outcomes reveal that van der Waals interactions and hydrophobic forces, especially reflected in the dominance of H…H and C…H interactions, significantly influence the molecular packing. Further, the prominence of F…H contacts emphasizes the role of fluorine in modulating intermolecular forces. Rather than merely affirming the stability of the complex, this investigation furnishes a deeper understanding of the specific non-covalent interactions (NCIs) governing the crystal packing, offering valuable insights into the molecular arrangement in the solid state [23,24].
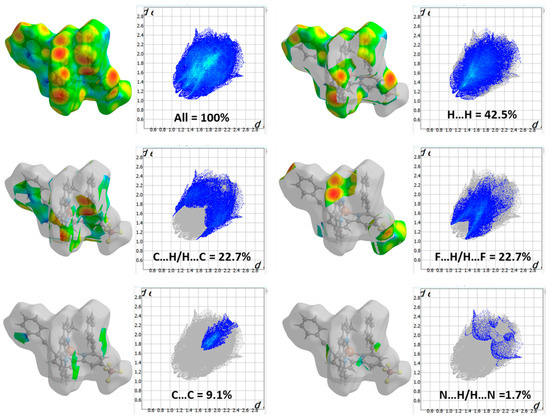
Figure 3.
The 2D fingerprint map shows the interatomic interactions contributing to the Hirshfeld surface of heteroleptic complex (2).
2.4. Photophysical Studies
2.4.1. Absorption Studies
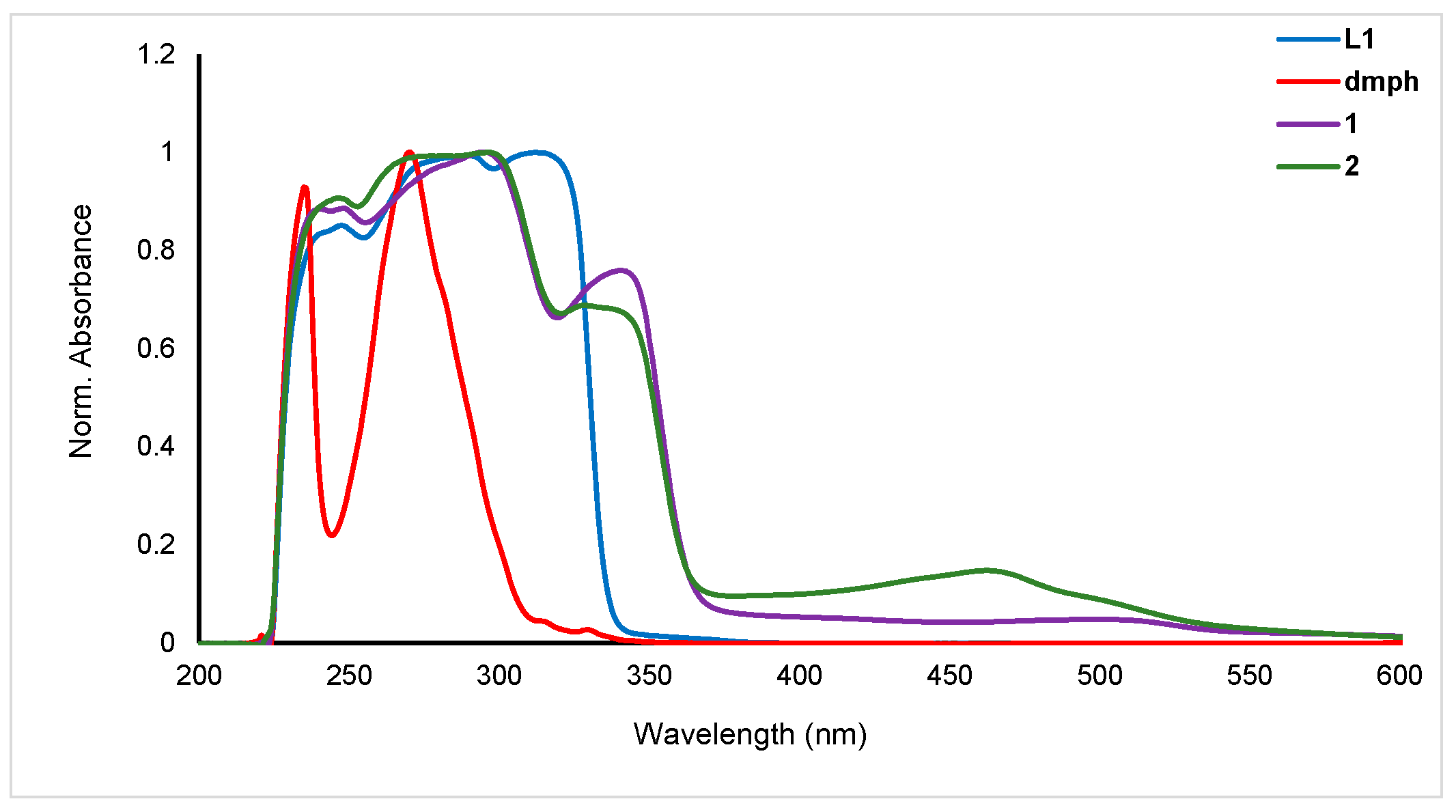
The electronic spectra of the ligands (L1 and dmph) and the homoleptic (1) and heteroleptic (2) complexes were recorded in dichloromethane (DCM) at room temperature (Figure 4). L1 exhibited peaks in the UV region at 240 (2.26 × 103 M−1 cm−1), 309 (2.71 × 103 M−1 cm−1), and 320 nm (2.67 × 103 M−1 cm−1) whereas dmph exhibited two strong peaks at 240 (2.4 × 103 M−1 cm−1) and 267 nm (2.55 × 103 M−1 cm−1). The homoleptic complex (1) showed red-shifted peaks at 267 (2.47 × 103 M−1 cm−1), 293 (2.79 × 103 M−1 cm−1), and 337 nm (0.03 × 103 M−1 cm−1) while the heteroleptic complex (2) showed peaks due to both ligands at 238 (2.54 × 103 M−1 cm−1), 300.0 (2.87 × 103 M−1 cm−1), 337 (1.99 × 103 M−1 cm−1), and 461 nm (0.43 × 103 M−1 cm−1). The absorption peaks in the UV spectrum (λmax < 400 nm) are due to the π→π* transition attributed to the arylethynylpyridine group. In the UV range, the main features come from the N,N ligands, while the bands seen in the visible spectrum, down to about 500 nm, are caused by metal-to-ligand charge transfer (MLCT) transitions between the metal cation and the phenanthroline units [25]. The dual absorption behavior highlights the significance of both the ligands and the metal center in the optical properties of these complexes. Compared to the neutral pyridine ligands-based complexes, these complexes showed red-shifted absorption maxima. These values are blue-shifted compared to earlier heteroleptic Cu(I) complexes. For example, Gardner and coworkers [26] reported a series of heteroleptic Cu(I) diimine complexes for DSSC application. The reported complexes when absorbed on TiO2 exhibited absorption in the visible region (451–531 nm). However, the values are comparable to the heteroleptic complexes such as [Cu(dmph)(PPh3)2]BF4 (λmax = 365 nm in MeOH) [27], [Cu(dmph) (DPEphos)]BF4 (λmax = 383 nm in DCM) [28], and others [29].
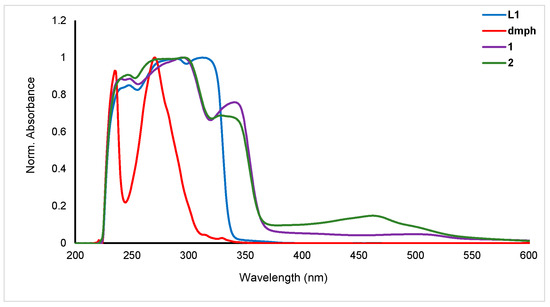
Figure 4.
Electronic spectra of the ligand (L1 and dmph), homoleptic (1) and heteroleptic (2) complexes.
2.4.2. Photoluminescence Studies
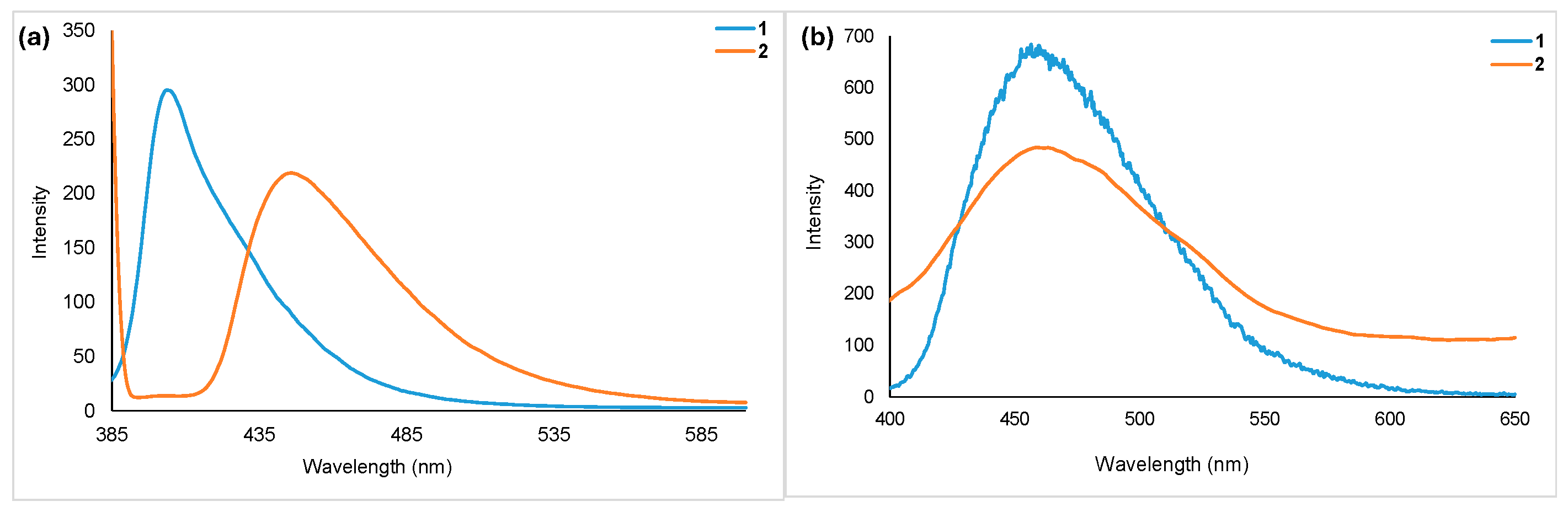
Emission spectra of homoleptic (1) and heteroleptic (2) complexes collected in DCM and in the solid state at room temperature are shown in Figure 5. As observed in the absorption spectra, here the heteroleptic complex showed red-shifted emission (λem = 446 nm) compared to the homoleptic complex (λem = 406 nm). In the solid state, both complexes emitted at red-shifted wavelengths (λem = 458 for 1 and 462 for 2). The quantum yields measured relative to coumarin 460 are 0.56 (2) and 0.91 (1).
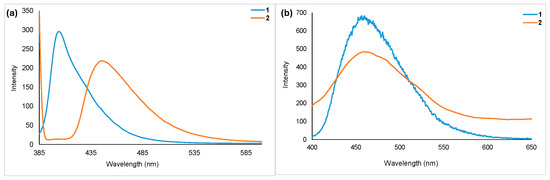
Figure 5.
Room temperature PL spectra of homoleptic (1) and heteroleptic (2) complexes. Data collected in (a) DCM (c = 1 × 10−4 M, λexc = 370), and (b) in solid state (λexc = 370).
2.5. Computational Studies
2.5.1. Frontier Molecular Orbital (FMO) Analysis
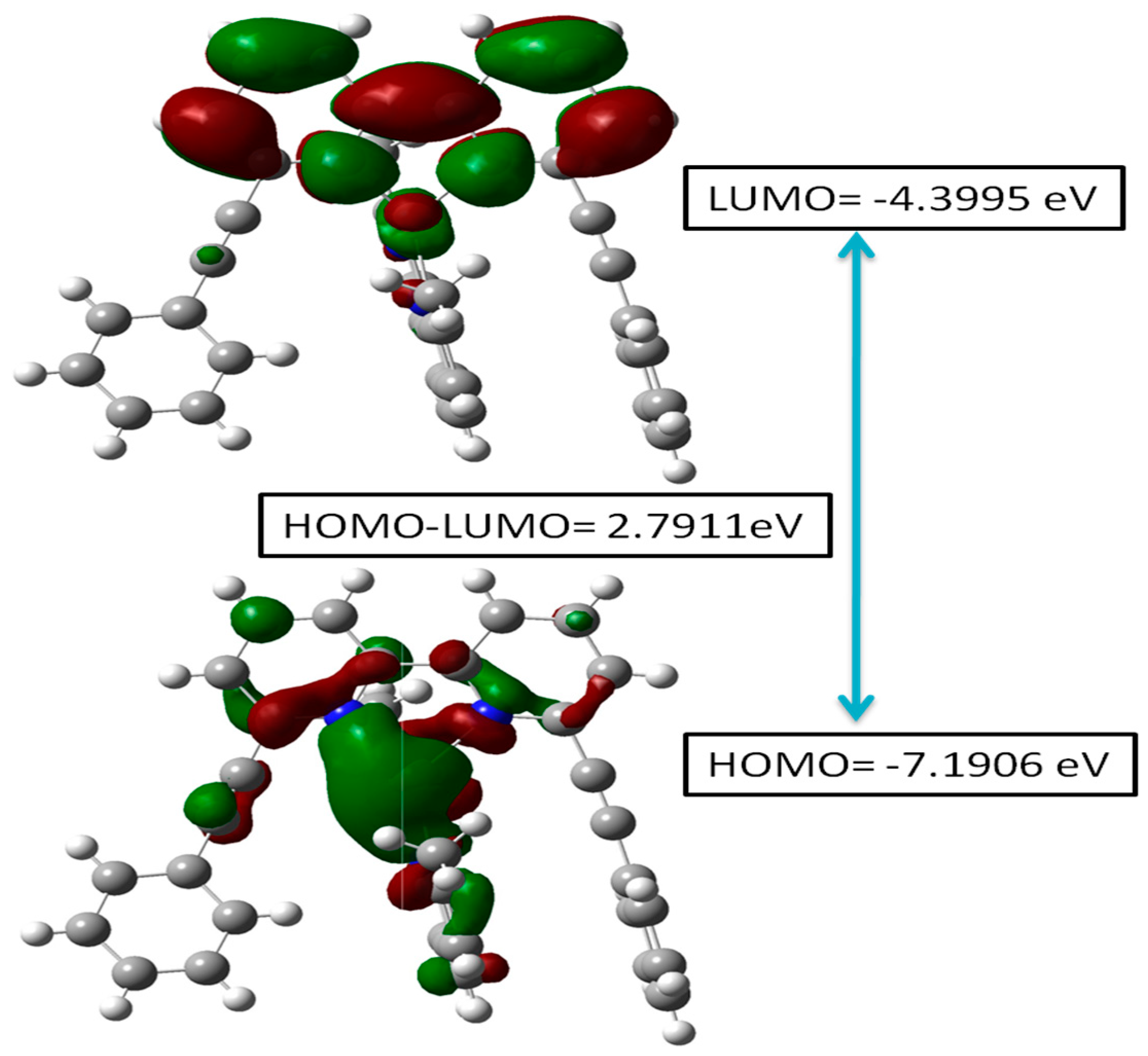
The energy band gap (EHOMO-LUMO/Eg) and frontier molecular orbital (FMO) analysis provide key insights into the electronic effects as well as the chemical reactivity of the heteroleptic complex (2) [30,31]. As Figure 6 depicts, the HOMO is at −7.1906 eV, while the LUMO is at −4.399 eV, resulting in an energy gap of 2.7911 eV. The low LUMO energy enables electron acceptance, assembling it into an efficient electron-accepting site, whereas the higher HOMO energy enables electron donation. A smaller energy gap enhances electron excitation, impacting the molecule’s optical properties [32] and potential applications of the complex.
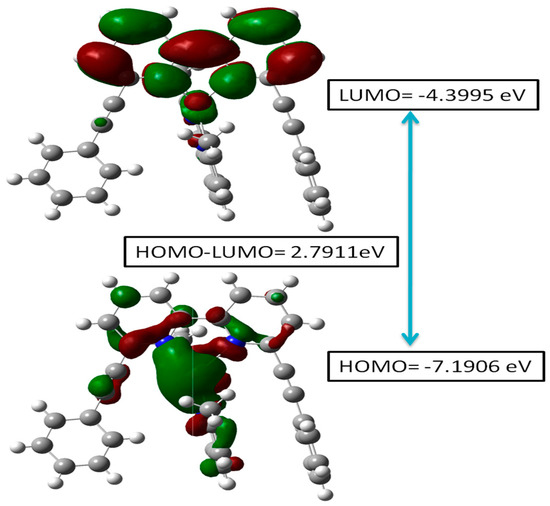
Figure 6.
HOMO-LUMO energy diagram for the heteroleptic complex (2).
2.5.2. Molecular Electrostatic Potential (MEP) Study
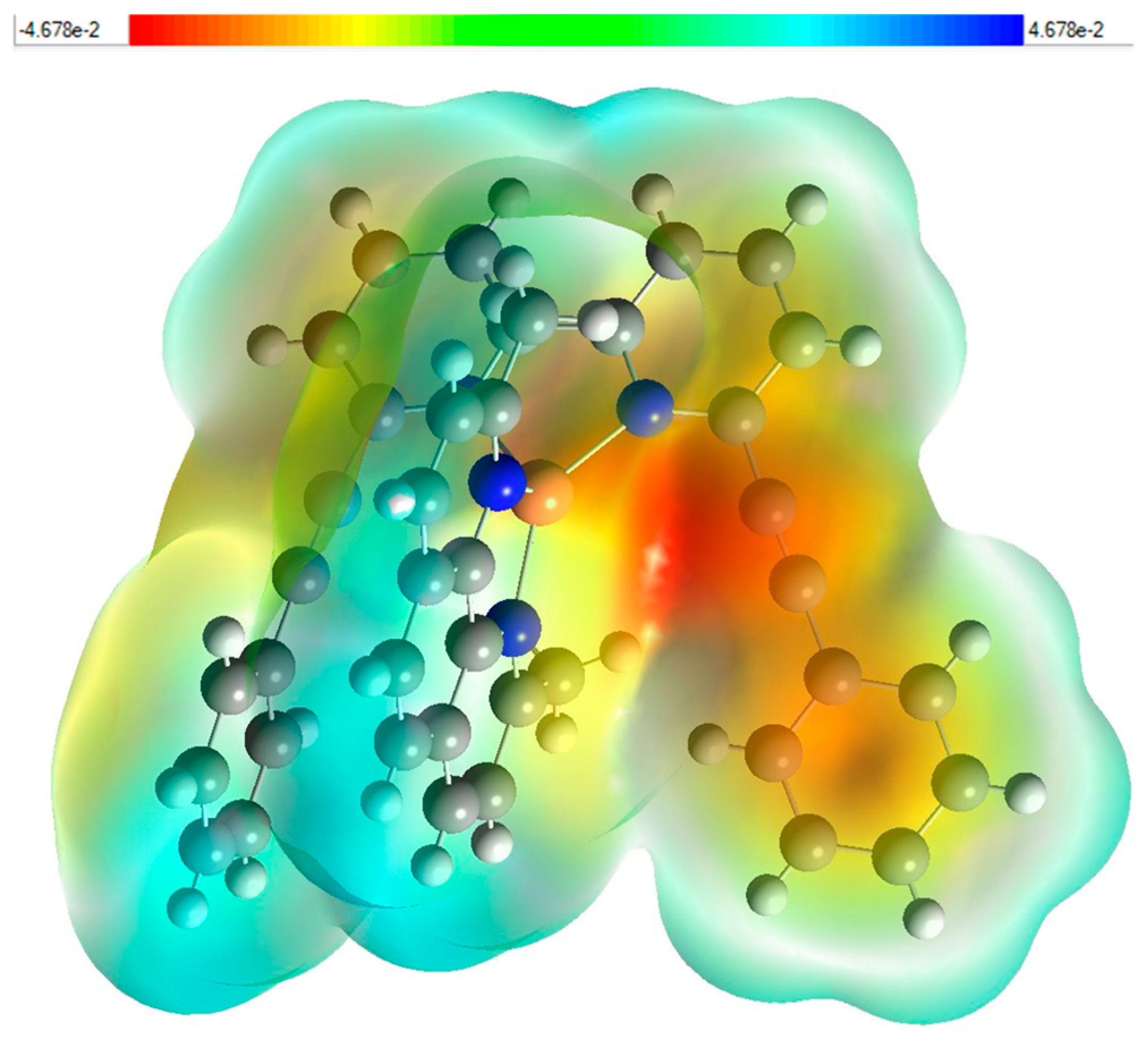
MEP was calculated by DFT using B3LYP/6-311++G(d,p) basis set. Figure 7 illustrates the three-dimensional charge distribution of molecular complex (2), providing a detailed color-coded representation of the electrostatic potential across the molecule. The corresponding color scale ranges from −4.678 × 102 to 4.678 × 102. In this visualization, the regions colored in red, orange, or yellow denote areas of high electronegativity. Conversely, the blue areas highlight regions of electropositivity, while the white areas signify neutral regions that exhibit no significant charge. Notably, the yellow regions of the MEP are prominently distributed over the phenyl ring of 2-(2-phenylethynyl)-6-(6-(2-phenylethynyl)pyridin-2-yl)pyridine, extending to the phenylethynyl bond and partially overlapping with the pyridine group. This suggests a strong presence of negative charge in these areas, which may play a significant role in the molecule’s reactivity. Additionally, the blue regions are closely associated with the 2,9-dimethyl-1,10-phenanthroline group, highlighting areas of positive charge that may attract nucleophiles in chemical reactions. This detailed mapping of the electrostatic landscape provides valuable insights into the molecule’s chemical behavior and potential interactions.
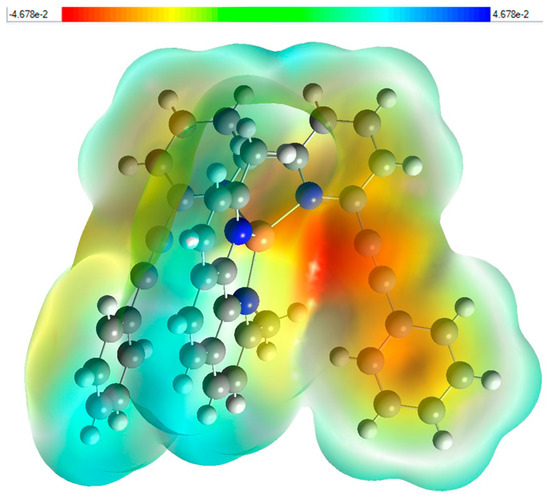
Figure 7.
Molecular electrostatic potential (MEP) study (e) fragment Patch (f) for (2).
3. Materials and Methods
General Procedure
All reactions were conducted under dry argon using the Schlenk line technique. Chemicals obtained from Sigma Aldrich (St. Louis, MO, USA) and TCI Chemicals (Tokyo, Japan) were used as received unless stated otherwise. NMR spectra in CDCl3 were recorded on a Bruker Advance III HD 700 MHz spectrometer (Billerica, MA, USA) with a 5 mm TCI H/C/N cryoprobe, referenced to solvent resonances. IR spectra were recorded via ATR on diamond using a Cary 630 FT-IR spectrometer (Agilent Technologies, Santa Clara, CA, USA). UV–vis spectra were obtained with an Agilent Cary 5000 spectrophotometer (Santa Clara, CA, USA) in a 1 cm quartz cuvette. Emission spectra were recorded on a PerkinElmer LS 55 fluorescence spectrometer (Waltham, MA, USA). Mass spectra were taken with a Kratos MS 890 spectrometer (Manchester, UK) using EI and ESI techniques. Column chromatography used 230−400 mesh silica gel (Merck, Darmstadt, Germany). Microanalyses were performed at the Department of Chemistry, Sultan Qaboos University (Muscat, Oman).
Synthesis of acetylide-functionalized bipyridyl ligand(L1):
6,6-Dibromo-2,2′-bipyridine (1 mmol, 0.31 g) was dissolved in dry THF-di-isopropylamine (1:1) in a 3-necked round-bottomed flask under argon atmosphere. Phenyl acetylene (2.25 mmol, 0.23 g, 0.25 mL) was added dropwise from a pressure equalizing dropping funnel. The reaction mixture was stirred at 80 °C and progress of the reaction was monitored by TLC. After the completion of the reaction, the crude product was obtained by removing the solvent mixture under reduced pressure. The crude product was purified by silica column chromatography using hexane-dichloromethane (1:1) as the eluant. Removal of the solvents under vacuo gave a creamy solid in 81% yield. M.p. = 156 °C; IR (ATR): 2207 (-C≡C). 1H-NMR (700 MHz, CDCl3): δ 8.49–8.47 (d, 2H, J = 7.0 Hz, H-3,3′), 7.84–7.82 (t, 2H, J = 7.77, 15.54 Hz, H-4,4′), 7.65–7.64 (q, HPh), 7.58–7.57 (dd, 2H, J = 6.5 Hz, H-5,5′), 7.39–7.38 (m, HPh). 13C-NMR (176 MHz, CDCl3): δ 155.95, 142.81, 137.11, 132.10, 128.99, 128.39, 127.66, 122.33, 120.83 (C≡C). Exact mass = 356.13 amu for C26H16N2; observed mass (m/z): 357.05 amu [M + 1]+. CHN cal. C, 87.62; H, 4.52; N, 7.86%; found: C, 87.69; H, 4.48; N, 7.82%.
Synthesis of Cu (I) complexes
Complex (1) and complex (2) were prepared by the reported method. L1 (1 mmol, 0.36 g) was dissolved in dry DCM (10 mL) in a round-bottomed flask under argon atmosphere. Cu(MeCN)BF4 salt (1 mmol, 0.19 g) dissolved in acetonitrile (10 mL) was added to the L1 solution. For complex (2), an equivalent mole of dmph dissolved in DCM was added to the reaction mixture. After the completion of the reaction, the crude product was obtained by filtration and purified by crystallization using diethyl ether, dichloromethane, and MeOH. Fine crystals of Cu(I) complex were obtained.
Complex (1):
Yellow solid; yield = 58%, m.p. = 220 °C; IR (ATR): 2218 (-C≡C), 1454 (C=N). 1H-NMR (700 MHz, CDCl3): δ 8.49–8.47 (d, 4H, J = 7.91 Hz, Hbpy, 3′), 7.96–7.95 (d, 4H, J = 7.77, Hz, Hbpy-4,4′), 7.88–7.86 (t, J = 7.77, 15.75 Hz, HPh) 7.63–7.62 (d, J = 7.49 Hz, HPh), 7.39 (m, HPh), 7.32–7.30 (t, J = 7.49, 7.56 Hz, HPh), 7.17–7.15 (t, J = 7.77, 7.77 Hz, HPh), 6.74–6.72 (d, J = 7.00, Hbpy). 13C-NMR (176 MHz, CDCl3): δ 151.53, 141.40, 137.52, 131.16, 129.72, 128.66, 128.40, 121.20, 120.62, 90.99, 87,18. Exact mass = 862.20 amu for C52H32BCuF4N4; observed mass (m/z): 863.15 amu [M + 1]+. CHN cal. C, 72.35; H, 3.74; N, 6.49%; found: C, 72.42; H, 3.68; N, 6.39%.
Complex (2):
Light yellow solid; yield= 45%; IR (ATR): 2221 (-C≡C),1453 (C=N). 1H-NMR (700 MHz, CDCl3): (δ) (ppm): 8.60 (d, 2H, J = 7.0 Hz, Pyridine), 8.44 (d, 1H, J = 7.7 Hz, Pyridine), 8.17 (t, 2H, J = 7.0 Hz, Pyridine), 7.92 (d, 1H, J = 7.0 Hz, pyridine), 7.82 (t, 1H, J = 14.0 Hz, Phenanthroline), 7.72 (d, 1H = 7.7 Hz, Phenanthroline), 7.67 (t, 2H, J = 7.7 Hz, phenanthroline), 7.58–7.54 (m, 2H, Phenanthroline), 7.32 (d, 1H, J = 7.0 Hz, Ar-H), 7.25 (t, 1H, J = 7.0 Hz, Ar-H), 7.10 (t, 1H, J = 7.0 Hz, Ar-H), 7.02 (t, 1H, J = 7.0 Hz), 6.75 (t, 2H, J = 7.0 Hz, Ar-H), 6.65 (t, 2H, J = 7.0 Hz, Ar-H), 6.03 (t, 2H, J = 7.0 Hz, Ar-H), 2.45 (s, 6H, CH3): 13C-NMR (176 MHz, CDCl3): (δ): 157.87 (-C=N-) pyridine, 151.86 (-C=N-, Phenanthroline), 143.24, 141.50, 138.76, 137.73, 136.58, 134.37, 132.24, 131.29, 130.67, 129.87, 128.99, 128.03, 127.33, 126.26, 125.57 (Aromatics), 122.66 (Alkyne), 121.44 (Alkyne), 29.80 (CH3): Exact mass = 714.16 amu for C40H28BCuF4N4; observed mass (m/z): 715.09 amu [M + 1]+. CHN cal. C, 67.19; H, 3.95; N, 7.84%, found: C, 85.71, H, 4.23, N, 7.59%.
X-ray Crystallography
Three-dimensional X-ray diffraction data were collected on a Bruker Kappa Apex CCD diffractometer at low temperature by the ϕ-ω scan method. Reflections were measured from a hemisphere of data collected from frames, each of them covering 0.3° in ω. A total of 59758 for 6 reflections measured were corrected for Lorentz and polarization effects and for absorption by multi-scan methods based on symmetry-equivalent and repeated reflections. Of the total, 4744 independent reflections exceeded the significance level (|F|/σ|F|) > 4.0. After data collection, an multi-scan absorption correction (SADABS) was applied [33], and the structure was solved by direct methods and refined by full matrix least-squares on F2 data using the SHELX suite of programs [34]. Hydrogen atoms were located in a difference Fourier map and were left to refine freely, except for C(20), which were included in calculation position and refined in the riding mode. Refinements were performed with allowance for the thermal anisotropy of all non-hydrogen atoms. A final difference Fourier map showed no residual density outside: 0.403 and −0.265 e.Å−3·A weighting scheme w = 1/[σ2(Fo2) + (0.052700 P)2 + 0.584300P] for 6, where P = (|Fo|2 + 2|Fc|2)/3 was used in the latter stages of refinement.
4. Conclusions
Two new diimine copper(I) complexes, homoleptic complex (1) and heteroleptic complex (2), have been synthesized by the coordination-driven self-assembly of L1 and dmph ligands. The complexes have been characterized by elemental analysis and multi-spectroscopic techniques. The solid-state structure of complex (2) was confirmed by single-crystal X-ray diffraction analysis. Complex (2) has a distorted tetrahedral coordination geometry around Cu(I), influenced by the steric and electronic properties of the L1 and dmph ligands. The structural data obtained by SCXRD were complemented by computational calculations. Hirshfeld surface analysis revealed the presence of several dominant NCIs, notably, H…H and C…H interactions, stabilizing complex (2) in the solid state and F…H contact, emphasizing the role of fluorine-bearing counter anions in modulating intermolecular forces in complex (2). Optical absorption spectroscopy revealed dual absorption behavior, implying significant role of the ligands and the metal center on the optical properties of the complexes. Computational studies provided an energy gap of 2.7911 eV for complex (2). A small energy gap makes complex (2) a potential candidate for opto-electronic applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13040104/s1. Figure S1: Correlation diagram for bond distance (a) and angles (b) in the heteroleptic complex (2), Figure S2: FT-IR of complex 1, Figure S3: FT-IR of complex 2, Figure S4: 1H-NMR of L1, Figure S5: 13C-NMR of L1, Figure S6: 1H-NMR of complex 1, Figure S7: 13C-NMR of complex 1, Figure S8: 1H-NMR complex 2, Figure S9: 13C-NMR of complex 2
Author Contributions
Conceptualization, R.A.A.-B.; methodology, R.A.A.-B. and M.M.; software, M.S.H.F.; validation, R.A.A.-B. and M.S.K. formal analysis, I.J.A.-B.; investigation, I.J.A.-B. and M.M.; resources, M.S.K.; data curation, I.J.A.-B., M.M., and M.S.H.F.; writing—original draft preparation, I.J.A.-B., M.M., and M.S.H.F.; writing—review and editing, R.A.A.-B. and M.S.K.; visualization, R.A.A.-B. and M.S.K.; supervision, R.A.A.-B. and M.S.K.; funding acquisition, R.A.A.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the Ministry of Higher Education, Research, and Innovation (MoHERI) of the Sultanate of Oman under the Block Funding Program, Projects No [BFP/RGP/EI/22/229] and [BFP/GRG/EI/22/089] and His Majesty’s Trust Fund for Strategic Research (Grant No. SR/SCI/CHEM/2021/01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
R.A.B. acknowledges the Ministry of Higher Education, Research, and Innovation (MoHERI) of the Sultanate of Oman [Project No. BFP/RGP/EI/22/229] and [Project No. BFP/GRG/EI/22/089]. M.S.K. acknowledges His Majesty’s Trust Fund for Strategic Research (Grant No. SR/SCI/CHEM/2021/01).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATR | Attenuated Total Reflectance |
| 13C-NMR | Carbon-13 Nuclear Magnetic Resonance Spectroscopy |
| DCM | Dichloromethane |
| DFT | Density functional theory |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| FMOs | Frontier molecular orbital |
| 1H-NMR | Proton Nuclear Magnetic Resonance Spectroscopy |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| TLC | Thin-layer chromatography |
References
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) hybrid inorganic–organic materials with intriguing stimuli responsive and optoelectronic properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
- Peng, R.; Li, M.; Li, D. Copper(I) halides: A versatile family in coordination chemistry and crystal engineering. Coord. Chem. Rev. 2010, 254, 1–18. [Google Scholar] [CrossRef]
- Ilmi, R.; Juma Al-busaidi, I.; Haque, A.; Khan, M.S. Recent progress in coordination chemistry, photo-physical properties, and applications of pyridine-based Cu(I) complexes. J. Coord. Chem. 2018, 71, 3045–3076. [Google Scholar]
- Leitl, M.J.; Zink, D.M.; Schinabeck, A.; Baumann, T.; Volz, D.; Yersin, H. Copper(I) Complexes for Thermally Activated Delayed Fluorescence: From Photophysical to Device Properties. Top. Curr. Chem. 2016, 374, 25. [Google Scholar] [CrossRef]
- Bizzarri, C.; Flechon, C.; Fenwick, O.; Cacialli, F.; Polo, F.; Galvez-Lopez, M.D.; Yang, C.H.; Scintilla, S.; Sun, Y.H.; Frohlich, R.; et al. Luminescent Neutral Cu(I) Complexes: Synthesis, Characterization and Application in Solution-Processed OLED. Ecs J. Solid State Sci. Technol. 2016, 5, R83–R90. [Google Scholar] [CrossRef]
- Safko, J.P.; Kuperstock, J.E.; McCullough, S.M.; Noviello, A.M.; Li, X.; Killarney, J.P.; Murphy, C.; Patterson, H.H.; Bayse, C.A.; Pike, R.D. Network formation and photoluminescence in copper (I) halide complexes with substituted piperazine ligands. Dalton Trans. 2012, 41, 11663–11674. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent copper (I) complexes with halogenido-bridged dimeric core. Coord. Chem. Rev. 2016, 306, 636–651. [Google Scholar]
- Liu, Y.; Yiu, S.-C.; Ho, C.-L.; Wong, W.-Y. Recent advances in copper complexes for electrical/light energy conversion. Coord. Chem. Rev. 2018, 375, 514–557. [Google Scholar] [CrossRef]
- Beaudelot, J.; Oger, S.; Perusko, S.; Phan, T.-A.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive copper complexes: Properties and applications. Chem. Rev. 2022, 122, 16365–16609. [Google Scholar] [CrossRef]
- Hilmey, D.G.; Paquette, L.A. Exo-and endo-receptors in one. A novel class of supramolecular structures housing transition-metal-binding bi-and terpyridine units alongside lithium ion-selective trispirotetrahydrofuranyl components. J. Org. Chem. 2004, 69, 3262–3270. [Google Scholar] [CrossRef]
- Miller, M.T.; Gantzel, P.K.; Karpishin, T.B. Effects of sterics and electronic delocalization on the photophysical, structural, and electrochemical properties of 2, 9-disubstituted 1, 10-phenanthroline copper (I) complexes. Inorg. Chem. 1999, 38, 3414–3422. [Google Scholar]
- Shah, H.H.; Al-Balushi, R.A.; Al-Suti, M.K.; Khan, M.S.; Woodall, C.H.; Sudlow, A.L.; Raithby, P.R.; Kociok-Kohn, G.; Molloy, K.C.; Marken, F. New multi-ferrocenyl- and multi-ferricenyl- materials via coordination-driven self-assembly and via charge-driven electro-crystallization. Inorg. Chem. 2013, 52, 12012–12022. [Google Scholar] [CrossRef]
- Shah, H.H.; Al-Balushi, R.A.; Al-Suti, M.K.; Khan, M.S.; Marken, F.; Sudlow, A.L.; Kociok-Kohn, G.; Woodall, C.H.; Raithby, P.R.; Molloy, K.C. New di-ferrocenyl-ethynylpyridinyl triphenylphosphine copper halide complexes and related di-ferricenyl electro-crystallized materials. Dalton Trans. 2014, 43, 9497–9507. [Google Scholar]
- Jayapal, M.; Haque, A.; Al-Busaidi, I.J.; Al-Rasbi, N.; Al-Suti, M.K.; Khan, M.S.; Al-Balushi, R.; Islam, S.M.; Xin, C.; Wu, W.; et al. Dicopper(I) Complexes Incorporating Acetylide-Functionalized Pyridinyl-Based Ligands: Synthesis, Structural, and Photovoltaic Studies. Inorg. Chem. 2018, 57, 12113–12124. [Google Scholar] [CrossRef]
- Haque, A.; Al Balushi, R.A.; Al-Busaidi, I.J.; Ilmi, R.; Al Rasbi, N.; Jayapal, M.; Khan, M.S.; Raithby, P.R. Synthesis, optical spectroscopy, structural, and DFT studies on dimeric iodo-bridged copper(I) complexes. J. Organomet. Chem. 2019, 892, 75–82. [Google Scholar]
- Hissler, M.; Harriman, A.; Khatyr, A.; Ziessel, R. Intramolecular triplet energy transfer in pyrene–metal polypyridine dyads: A strategy for extending the triplet lifetime of the metal complex. Chem.–A Eur. J. 1999, 5, 3366–3381. [Google Scholar]
- Nisbet, M.L.; Hiralal, E.; Poeppelmeier, K.R. Crystal structures of three copper (II)-2,2′-bipyridine (bpy) compounds, [Cu(bpy)2(H2O)][SiF6]·4H2O,[Cu(bpy)2(TaF6)2] and [Cu(bpy)3][TaF6]2 and a related coordination polymer, [Cu(bpy)(H2O)2SnF6]n. Struct. Rep. 2021, 77, 158–164. [Google Scholar]
- Nisbet, M.L.; Poeppelmeier, K.R. Crystal structures of [Cu(phen)(H2O)3(MF6)]·H2O (M = Ti, Zr, Hf) and [Cu(phen)(H2O)2F]2[HfF6]·H2O. Struct. Rep. 2021, 77, 165–170. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009; Volume 121, pp. 150–166. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer (v17); The University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar]
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Struct. Rep. 2019, 75, 308–318. [Google Scholar]
- Leoni, E.; Mohanraj, J.; Holler, M.; Mohankumar, M.; Nierengarten, I.; Monti, F.; Sournia-Saquet, A.; Delavaux-Nicot, B.; Nierengarten, J.-F.; Armaroli, N. Heteroleptic copper (I) complexes prepared from phenanthroline and bis-phosphine ligands: Rationalization of the photophysical and electrochemical properties. Inorg. Chem. 2018, 57, 15537–15549. [Google Scholar]
- Franchi, D.; Leandri, V.; Pizzichetti, A.R.P.; Xu, B.; Hao, Y.; Zhang, W.; Sloboda, T.; Svanstrom, S.; Cappel, U.B.; Kloo, L. Effect of the ancillary ligand on the performance of Heteroleptic Cu (I) diimine complexes as dyes in dye-sensitized solar cells. ACS Appl. Energy Mater. 2022, 5, 1460–1470. [Google Scholar]
- Rader, R.A.; McMillin, D.R.; Buckner, M.T.; Matthews, T.G.; Casadonte, D.J.; Lengel, R.K.; Whittaker, S.B.; Darmon, L.M.; Lytle, F.E. Photostudies of 2,2′-bipyridine bis (triphenylphosphine) copper (1+), 1,10-phenanthroline bis (triphenylphosphine) copper (1+), and 2,9-dimethyl-1,10-phenanthroline bis (triphenylphosphine) copper (1+) in solution and in rigid, low-temperature glasses. Simultaneous multiple emissions from intraligand and charge-transfer states. J. Am. Chem. Soc. 1981, 103, 5906–5912. [Google Scholar]
- Kuang, S.-M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and structural characterization of Cu (I) and Ni (II) complexes that contain the Bis [2-(diphenylphosphino) phenyl] ether ligand. Novel emission properties for the Cu (I) species. Inorg. Chem. 2002, 41, 3313–3322. [Google Scholar]
- Mani, G.; Subramaniyan, V. Homoleptic and heteroleptic copper (I) complexes bearing diimine-diphosphine ligands. In Copper (I) Chemistry of Phosphines, Functionalized Phosphines and Phosphorus Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–258. [Google Scholar]
- Khalid, M.; Ali, A.; Jawaria, R.; Asghar, M.A.; Asim, S.; Khan, M.U.; Hussain, R.; ur Rehman, M.F.; Ennis, C.J.; Akram, M.S. First principles study of electronic and nonlinear optical properties of A–D–π–A and D–A–D–π–A configured compounds containing novel quinoline–carbazole derivatives. RSC Adv. 2020, 10, 22273–22283. [Google Scholar]
- Khalid, M.; Shafiq, I.; Mahmood, K.; Hussain, R.; Ur Rehman, M.F.; Assiri, M.A.; Imran, M.; Akram, M.S. Effect of different end-capped donor moieties on non-fullerenes based non-covalently fused-ring derivatives for achieving high-performance NLO properties. Sci. Rep. 2023, 13, 1395. [Google Scholar]
- Gong, L.; Ma, C.; Liu, T.; Lv, J.; Xun, X. Theoretical study on functionalized acrylonitrile compounds with a large second-order nonlinear optical response. New J. Chem. 2020, 44, 19623–19629. [Google Scholar]
- Sheldrick, G.M. SADABS; Version 2.03; University of Göttingen: Göttingen, Germany, 2002. [Google Scholar]
- Sheldrick, G.M. SHELXL-2016/6: Program for Crystal Structure Determination; University of Göttingen: Göttingen, Germany, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).