Abstract
Chemical–structural characteristics of three differently synthesized research-benchmark Mn-Na-W-Ox/SiO2 catalysts for the Oxidative Coupling of Methane (OCM) were systematically studied in this research. XRD, EDX, ICP-OES, and SEM/FIB-SEM techniques, as well as Carrier Gas Hot Extraction (CGHE) and high-temperature XRD analyses, were performed to explain the functional features of the studied catalysts, in particular, the features affecting the quantity and quality of the interactions of oxygen and methane with the catalyst surface and with other molecular and radical species. These enable tracking the potential for the oxygen activation and dynamic transformation of the solid-state chemistry on the surface and sub-surface of these Mn-Na-W-Ox/SiO2 catalysts. These catalysts were synthesized, respectively, via the sol–gel synthesis method (Cat1) and the incipient wetness impregnation of the non-structured silica support (Cat2) and structured SBA-15 silica support (Cat3), under different sets of temperatures and gas compositions. The catalysts with the homogenous distribution of active components, namely Cat1 and Cat3, showed similar trends in terms of their dynamic interaction with oxygen species. They also showed higher levels of crystallinity of the active materials and higher catalytic selectivity towards ethane and ethylene. An explanation is given as to how the structural characteristics of the catalysts on the nanometer–micrometer scale contribute to these. The gained knowledge will be crucial in the selection and treatment of the support and developing a proper synthesis approach for the ultimate goal of designing a selective OCM catalyst.
1. Introduction
The catalytic Oxidative Coupling of Methane—OCM—to produce ethylene, the smallest olefin molecule and the largest-scale hydrocarbon produced worldwide, has been the subject of almost a half-century of research [1,2,3,4]. The main potential for this process is the abundance of feedstock methane, as the main component of natural gas and shale gas [5,6]; on a smaller scale, it is available in the form of flare gas [7], as the gas streams resulting from processing the biomasses [8], and even as the side product of industrial processes such as ethane and naphtha crackers [2,4]. Despite these, OCM has not yet transitioned from developing technology [9,10] to an established mainstream technology for commercial ethylene production. This primarily requires a more stable-selective catalyst as well as an improved controllable operation in the reactor section [11,12,13]. These are the known challenges for this net-exothermic set of gas and catalytic reactions, through which methane is activated with oxygen to either form ethane and ethylene, or undesirably end up in the form of carbon oxides, in particular when operating at high-temperatures [14,15]. Reaction sets R-1 to R-5 represent the main involved catalytic and gas-phase reactions, with their reactant stoichiometry and thermal requirements shaping the selectivity of this gas-catalytic system. These include methane coupling, methane partial and complete oxidation, ethylene deep-oxidation, as well as the thermal and oxi-dehydrogenation of ethane. An extended list of reactions with explanations can be found elsewhere [16].
| Surface and Gas Reactions [−802.64 kJ·mol−1] | (R-1) |
| Surface and Gas Reactions [−176.5 kJ·mol−1] | (R-2) |
| Surface and Gas Reactions [−104.89 kJ·mol−1] | (R-3) |
| Surface and Gas Reactions [−1323 kJ·mol−1] | (R-4) |
| Gas Reaction [136.96 kJ·mol−1] | (R-5) |
A comprehensive understanding of the interactions of the reactive molecules and radicals with the catalyst and the involved physical–chemical phenomena could enable further specifying the contributions of the catalyst characteristics in shaping its selectivity [4]. Based on such an understanding, the desired chemical–structural characteristics for a stable-selective catalyst and the potential to establish them could be identified and secured. This should be accomplished through a panoptic study that considers the specifications of the reaction mechanism on the catalyst scale as well as the operating conditions on the reactor scale. On one hand, the reaction mechanism analysis helps explain how nanometer- to micrometer-scale phenomena influence catalytic performance, considering the chemical and structural properties of the catalyst. These properties are determined by the synthesis method used, the type and quantity of active components, and the type and structural characteristics of the support material. On the other hand, phenomenological analysis of the direct and indirect impacts of the operating parameters, such as temperature and concentration of the reactive species, should also be part of such a study. Having considered these, this research aims to particularly deepen the phenomenological-understanding of the interactions of the reactive species with the OCM catalysts, representing different sets of chemical–structural characteristics being established using different types of silica-support and synthesis methods. This includes an investigation of the characteristics, functionality, and performance of three Mn-Na-W-Ox/SiO2 catalysts, synthesized via the sol–gel method (Cat1) through the simultaneous pre-hydrolysis of the sources of silicate along with the metal nitrates, and via the incipient wetness impregnation of non-structured silica (Cat2) and structured SBA-15 silica (Cat3) supports, respectively. They represent three distinct sets of structural characteristics for this promising benchmark catalyst [17,18,19,20,21,22,23,24,25,26]. In this research, special attention is devoted to analyzing the dynamic transformations of these three catalysts under different sets of operating conditions. In doing so, in addition to characterizing these catalysts using ex situ XRD, EDX, and SEM/FIB-SEM techniques, their material characteristics were also monitored during Carrier Gas Hot Extraction–CGHE–and high-temperature XRD measurements. This enabled analyzing the dynamic transformation of the phases of the involved material under different sets of operating temperatures and gas compositions. The proper analysis of the resulting data could facilitate understanding and possibly overcome the challenging task of correlating the results of the characterization techniques with the actual characteristics of the catalysts at reaction temperatures.
2. Results and Discussion
2.1. Comparative Analysis of the Characteristics of the Three Synthesized Catalysts
In the sol–gel synthesis of the Mn-Na-W-Ox/SiO2 catalysts, by adding all metal precursors within one solution, all metal cations are distributed homogeneously to establish the mean closest distance from each other across the catalytic structure, as statistically expected. This is a distinguished feature of the sol–gel synthesis method compared to the incipient wetness impregnation method, where the precursors are added sequentially, shaping separate layers with relatively heterogeneously distributed concentrations of the active materials. From a structural point of view, mesopores are formed all across the structure using the sol–gel method as tungsten- and sodium-containing precursors are added simultaneously during the synthesis procedure. Hereby, the well-distributed sodium content over the entire catalyst body will result in the sintering of the silica structure under catalyst calcination conditions, which ultimately shrinks the silica matrix, resulting in a denser structure [20]. Moreover, the purchased non-structured (Davisil) and structured silica (SBA-15) used in Cat2 and Cat3 have different densities. Therefore, these three catalysts (Cat1, Cat2, Cat3) have different densities. The bulk density of the sol–gel catalyst (1.22 g∙cm−3) is almost/nearly more than twice that of the impregnated non-structured silica support catalyst (density of 0.56 g∙cm−3) and almost five times that of the SBA-15 supported catalyst (density of 0.25 g∙cm−3). Moreover, during the drying process of the sol–gel catalyst, the shrinkage of the catalyst structure also occurs. This step strongly affects the structure and the characteristics (e.g., porosity, pore size distribution and surface area) of this catalyst.
On the other hand, using the ICP-OES technique, the actual compositions of the synthesized catalysts were determined. The measured ICP data for the three investigated catalysts are shown in Table 1.

Table 1.
The ICP data for the three investigated 1.9%Mn-0.8%Na-3.1%W/SiO2 catalysts.
As seen in Table 1, the mass fractions of the active components in the sol–gel bulk catalyst (Cat1) are close to the targeted composition, i.e., 1.9%Mn-0.8%Na-3.1%W. The observed deviation between the targeted and established compositions of the wet-impregnated catalysts (Cat2 and Cat3) is within the usual variance of the impregnated quantities and is due to the fact that in any impregnation procedure, a portion of the active impregnating component will end up not impregnating the support and become wasted.
After checking the overall composition, the structural characteristics of the samples were also characterized based on Brunauer–Emmett–Teller—BET—theory and Barrett–Joyner–Halenda—BJH—analysis, measured via N2 adsorption–desorption at a cryogenic temperature of 77 K using the QuadraSorb SI (Quantachrome Instruments, Boynton Beach, FL, USA). Table 2 summarizes the main structural characteristics of all catalysts and their silica support materials in comparison.

Table 2.
Main characteristics of the investigated sample materials (catalysts Cat1, Cat2, Cat3, and the supports).
The reported data in Table 2 include the average pore size and volume of the micropores and mesopores in each sample as well as the specific surface area, which all are less than 6 m2 g−1. Having considered the original surface areas of the supports, it can be concluded that the measured relatively low surface areas of the catalyst samples is primarily caused by the applied high calcination temperature (i.e., θ ≥ 800 °C). The SBA-15 support in Cat3 with a high surface area, particularly the high surface area of its micropores, provides better accessibility to the metal precursors while being impregnated. Scanning the surface and sub-surface of Cat3 and Cat1 by FIB SEM-EDX indicated a relatively homogenous distribution of the active components across Cat1, established using the sol–gel synthesis method.
Both catalysts, Cat1 and Cat3, were characterized to have finer distributions of the active components and relatively better catalytic selectivity than Cat2 as shown in Supplementary Material and previous publications [20,27,28]. Further typical SEM images and EDX analyses of these three catalysts are available in the Supplementary Material.
2.2. Catalytic Activity and Functionality Analyses
2.2.1. Qualitative/Quantitative Measures for Catalytic Functionality and Activity Analyses
The mechanistic aspects and the detailed analysis of the interaction of the molecular and radical reactants species with the Mn-Na-W-Ox/SiO2 catalyst, accounting the involved reactions and the impacts of the OCM reactor conditions, have been investigated as the key information required for efficiently exploiting this catalyst in a proper reactor feeding policy [14,29,30,31,32,33].
The rate and contribution of the involved reactions under different sets of operation conditions will determine the main OCM catalyst/reactor performance indicators as defined by (a) the portion of the whole consumed methane, which appears in the form of each specific product [Selectivity, S], (b) the percentage of the inlet methane appearing in the form of each specific product [Yield, Y], and (c) the portion of the inlet methane converted to the desired and undesired products [methane conversion, X].
Depending on the targeted range of the reaction temperature and the established local concentration of the reactants, the relative contributions of the surface catalytic reactions and the gas-phase reactions on shaping the OCM reactor performance will be different [4]. For instance, the gas-phase reactions will be intensified at higher temperatures, whereas the contribution of the catalyst in determining the selectivity will be reduced [12,14,29]. However, it should be mentioned that the reaction temperature representing the bulk temperature of the macro-scale reactor as well as the micro-scale spot temperature over the catalyst surface, both play important roles in determining the ultimate selectivity and yield of the gaseous products exiting the reactor [4,34]. The comparative intensity of the gas-phase reactions and the surface reactions and their dependency on the operating temperature have been extensively investigated. However, the interactive contributions of the catalyst’s and reactor’s characteristics in that context have not yet been analyzed sufficiently.
2.2.2. Catalyst Scale: The Local Reaction Intensity Established Due to Catalyst Characteristics
The nanometer- to micrometer-scale phenomena (catalyst scale) are usually explained with the view on the reaction mechanism [4]. In doing so, it is assumed that the reaction performance indicators (e.g., conversion and selectivity) reflect the balance of the activation rates of oxygen and methane molecules, followed by the chain of parallel and consecutive radical and molecular transformations. In fact, not only the rate of reactions but also their intensity per area or volume of the catalytic bed will affect the ultimately observed performance indicators. A comprehensive thermal-reaction analysis should consider these along with the reactor-scale thermal-dimensional characteristics to track the impacts of the variation in the controllable parameters (e.g., set reactor temperature, feed composition) on the local thermal-reaction conditions and the involved phenomena. Nevertheless, a catalytically dominated/facilitated relatively low-temperature OCM operation is preferred and should be targeted, mainly for securing a selective, safe, and robust operation [4,15,35]. In any case, the local spot temperature and the thermal impact in the sub-micrometer scale of the catalyst surface on the selective catalytic conversion can be explained through its contributions on the intensity and the rate of generation and consumption of species over the catalyst’s surface. This has been explained by tracking the interactive impacts of the local spot temperature and gas composition at a given cross-section of the reactor [4].
The key inferences to be made related to the characteristics and functionality of the catalyst are reviewed here. The coupling reaction is emboldened when a well-distributed right intensity of the radical generation is established over the catalyst surface. The radicals and molecular species, which are present in disproportion to the capacity of the selective catalytic conversion of the catalyst, undergo undesired oxidation by the interaction with oxygen species (i.e., lattice oxygen and adsorbed oxygen) [33]. In fact, the type and quantities of the active catalytic species (e.g., manganese) and other material characteristics of the catalyst (e.g., electrical conductivity or its oxygen extraction potential as explained in Section 2.3.1) will determine the capacity of a catalyst for a selective conversion under given reaction environment. On the other side, the energy-excited species can engage each other even far from the catalyst surface and become involved in the predominantly unselective gas-phase reactions. Having reviewed all these, it can be concluded that a balanced rate of methane activation on the catalyst surface by the surface-adsorbed oxygen and sub-surface oxygen supply is desired to secure a selective catalytic conversion [4,34]. In this manner, tuning the factors affecting the local intensity of the reactions, including the activation reactions and sequential desired coupling, and understanding the oxidation reactions, are crucial in tailoring the selective catalytic performance of the catalyst. These phenomena are interconnected through a dynamic mechanism and are closely related to the dispersion and the quantity of the involved active phases across the catalyst body. These material phases, particularly on the sub-surface (i.e., tens of atomic layers) and over the surface of the catalyst, can be correlated with the rate of the generation and the selectivity of the conversion of the methyl radicals. Therefore, the rate and the conversion of methyl radicals were studied in this research using two in situ characterization techniques, namely, high-temperature oxygen extraction (as explained in Section 2.3.1) and high-temperature XRD measurements under reaction temperatures (as explained in Section 2.3.2).
2.2.3. Reactor Scale: Bulk Reaction Intensity Dynamically Affected by Operation Parameters
The phenomena in the reactor scale should be also taken into analysis while reviewing the observed performance of catalysts tested in a reactor. In this context, the bulk reaction intensity and thermal performance should be particularly analyzed. For instance, the catalytic performance of all three catalysts (Cat1, Cat2, Cat3) has been tested using standard bench-scale fixed-bed reactors, through co-feeding as well as oxygen-dosing reactor operations under comparable sets of conditions, showing a superior selective performance of Cat1 and Cat3 in comparison to Cat2 [20,27,28]. Also, by applying a low methane-to-oxygen ratio of 2.5, Cat1 and Cat2 showed 72% and 57% C2 selectivity, respectively, for a comparable level of methane conversion in the range of 25–26% (see Supplementary Material). However, it should be highlighted that Cat1 can maintain such a high C2 selectivity and C2 yield as long as a sufficiently high reaction temperature and intensity could be maintained. The observed catalytic performance of Cat1 and Cat2 under oxygen dosing in a membrane reactor also demonstrated a relatively more sensitive behavior of Cat1 towards the variation in parameters such as methane-to-oxygen ratios and inert gas dilution, which strongly affect the reaction temperature [20]. On the other side, by applying an average methane-to-oxygen ratio of 4, Cat3 and Cat2 showed 73% and 66% C2 selectivity, respectively, for a comparable level of methane conversion in the range of 14–15% [27,28]. Since the thermal and reaction performances of the OCM catalytic system are strongly intertwined, the thermal requirements of each of these catalysts to reach a comparable range of methane conversion are different. This, along with the details of such comparison reflecting the specifications of the catalytic testing, as well as their typical performance, are available in Supplementary Material. Extra data revealing their typical trends of catalytic performance, demonstrating the relatively higher selective performance of Cat1 and Cat3 compared to Cat2, along with the details of typical experimentation, the experimental setup, and the conditions, can be found in earlier reports [20,27,28,29,31].
After analyzing the impacts of the local reaction intensity in the catalyst scale (i.e., through reaction mechanism study) [4], as well as on the reactor performance (i.e., through thermal-reaction engineering), it is important to track how the variation in tunable design parameters (e.g., furnace set temperature or the feed composition) can affect the concentration and temperature profiles on the bulk reaction intensity along the reactor. In doing so, their impacts on the reaction intensity on the reactor scale should be first well-understood. This also includes analyzing the interactive impacts of the gas composition and reaction temperature. In this context, it should be highlighted that the rate of reactions, particularly the coupling reaction, can be correlated with the local temperature and the gas composition at each cross-section and local spot of the reactor. Not being able to directly monitor and track these along the reactor, the set temperature and composition (indicated by the reactants’ ratio and the gas dilution) of the feed stream could be used for such a correlation [14]. In any case, the dynamic trajectories of the concentration and temperature along the reactor should be carefully analyzed. The dynamic change in the thermal operation represented by the temporal and local temperature profiles and their impact on the catalytic reaction performance hints at the crucial role of thermal effects and the non-isothermality of this reaction system on its selective catalytic performance [2,4,12,29,33]. Studying the recorded transition behavior on the reactor scale provides valuable understanding of the operation of OCM reactors in order to optimize the design of the reactor and select the optimal operating conditions. Table 3 represents a typical dynamic change in an OCM reactor performance in correlation with the variation in temperature and concentration profiles along the reactor. Three sets of recorded reactor performance indicators (i.e., C2 selectivity, C2 and ethylene yields) in three time-steps of 3 min, 15 min, and 28 min, respectively, are shown in Table 3, representing three states of such a typical transition behavior. The established temperature profiles, represented in this table by the recorded deviated temperature from the steady state ∆T (base state), drive the reactor performance in each of these states. Such a dynamic behavior reflects the contributions of the phenomena, both in the catalyst and reactor scales.

Table 3.
Typical transient performance behavior of the OCM reactor using Cat2.
As seen in Table 3, when the interaction of the reaction atmosphere and the catalyst has resulted in a +110 K temperature deviation (state-1) from the base state (state 3: returning to the targeted reaction temperature 800 °C), the C2 and ethylene selectivity have been significantly improved due to the ascending temperature trajectory. The observed temperature deviation in the form of a sharp ascending temperature trajectory can be initiated by a disturbance or by varying an operating parameter such as a sudden increase in the feed flow rate, in particular its inlet oxygen content. When the time passes by and the thermal shock is damped down to +31 K, methane conversion and C2 selectivity are reduced too. Ultimately, when the impact of the thermal shock is completely over, half an hour later, the C2 and ethylene selectivity are further reduced to their original values. Such a dynamic trajectory can be utilized for improving the thermal-reaction performance on an OCM reactor. In an attempt to bridge the catalyst-scale and reactor-scale performances, while considering their correspondingly involved phenomena, the performed analysis should also consider the interaction mechanism of the gaseous species with the catalyst surface [4]. This is particularly interesting while heating the catalytic system under an ascending ramp temperature and monitoring the dynamic behavior. Different rates of catalytic surface reactions, primarily methane radical generation and their consequent secondary reactions towards C2 via coupling reactions or to carbon oxides, can be established and explained by interaction of the reactor-scale and catalyst-scale phenomena. For instance, the applied ramping temperature will affect the rate of radical generation, preferably by establishing a well-distributed moderate intensity of methane activation via balanced sub-surface and surface oxygen species [4]. These could lead to an improved reactor performance when combined with establishing the right bulk temperature and concentration profiles of reactive species in the reactor scale. Tracking the catalyst characteristics, in terms of its ability to interact with the reactive species and the observed dynamic transformation of its solid-state chemistry while changing the operating conditions, could be an important part of such an analysis, as discussed in Section 2. This is needed to be studied to understand the requirements for tailoring the right activation rate of oxygen and methane molecules on the surface of the catalyst, as well as the distribution of the reaction intensity along and across the catalytic bed, to secure an overall selective catalytic performance. Such a study enables tracking the impacts of catalyst characteristics as well as the operating parameters such as temperature and concentration profiles along the reactor on the overall reactor performance.
2.3. Results of Catalysts Characterization
After analyzing the involved reaction mechanism and thermal-reaction engineering aspects determining the OCM catalytic performance, as reviewed in Section 2.2, the characteristics of the catalysts confirming these are discussed in this section (Section 2.3). In that context, the catalyst characteristics such as the homogeneity of the distributed active components, and in particular the physical–chemical properties affecting their interaction with the oxygen species, are studied. All three Mn-Na-W-Ox/SiO2 catalysts (Cat1, Cat2, Cat3) were characterized in the targeted range of the reaction temperature using the Carrier Gas Hot Extraction (CGHE) technique and high-temperature XRD measurements. The specifications of these experiments along with their results are provided in this section. The promise is that studying the phase transformation under varying reaction conditions could provide valuable information when the results are analyzed along with the fundamental features of the involved materials such as their potential for oxygen release. In this manner, we tried to connect the results of the CGHE measurements with the capacity of the catalytic material in terms of lattice-oxygen release, correlating the observed trends with the identified phases such as different oxidation states of the tungsten and manganese using HT-XRD under the reaction temperature. Previously reported characterization data could also support the implemented characterization strategy in this research [11,20,21,22,23].
2.3.1. Carrier Gas Hot Extraction—CGHE
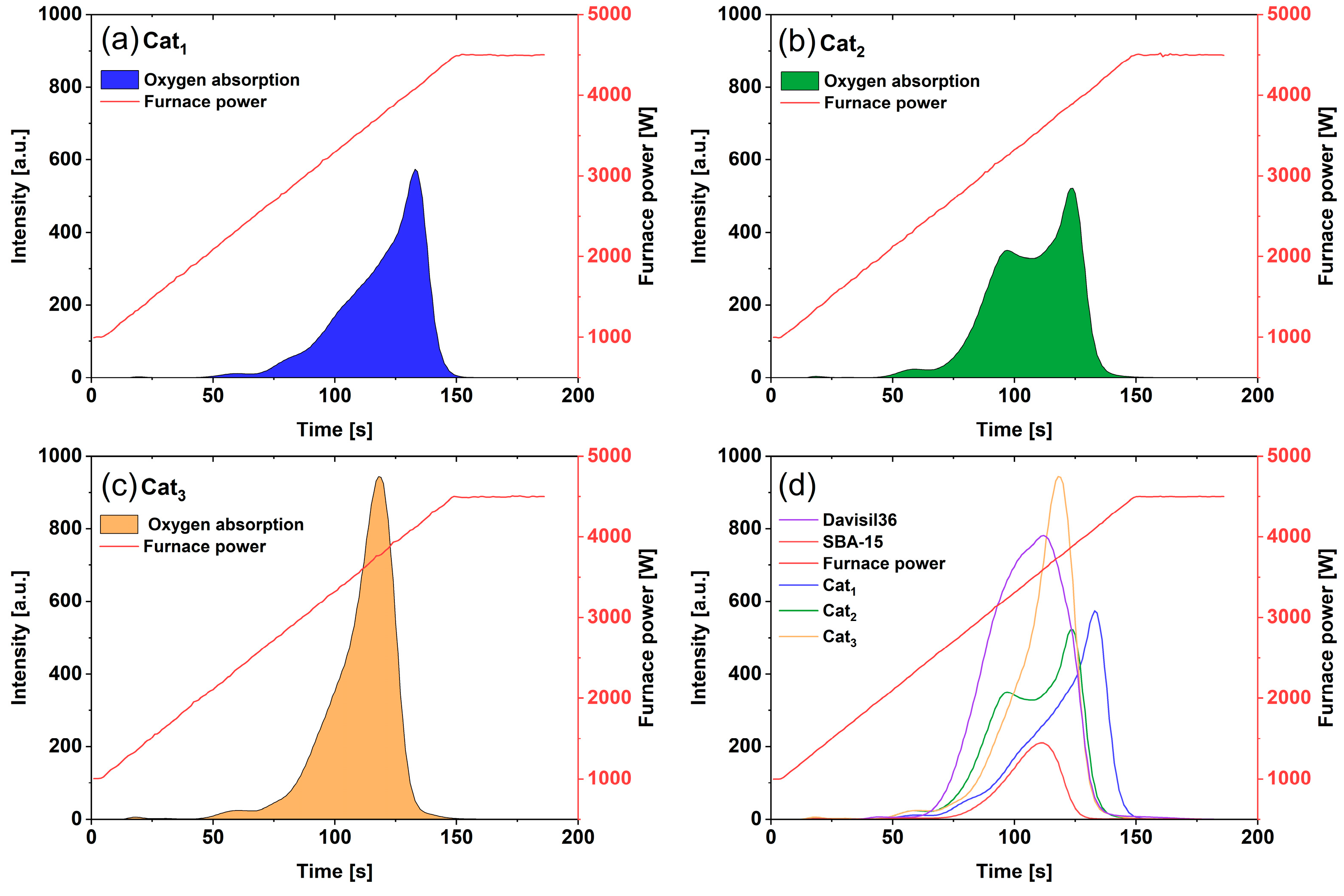
The CGHE technique was used in this research to characterize the behavior of the catalytic samples in the form of the releasing trend of their extractable oxygen content under an ascending temperature. Monitoring the trend of oxygen release in this way enables measuring the potential of the catalysts to interact with the gaseous species, particularly with regard to the activation and transfer of oxygen species. In this manner, while ramping up the temperature, the source of carbon erected around the catalyst sample reacts with the oxygen gradually released from the sample. Depending on the oxygen-bonding strength within the sample’s material structure, the released oxygen trend is measured quantitatively by tracking the concentration of the formed CO and CO2 using IR spectroscopy (LECO Corporation, St Joseph, MI, USA). The recorded peaks represent the released oxygen while the temperature rises over time as an indication of the strength of the oxygen binding within the material before being released. The capability of oxygen release from all three catalysts by applying the standard procedure under ramp temperature was measured. The results were represented in the form of the peaks, as shown in Figure 1, demonstrating the release trends of extractable oxygen from these samples.

Figure 1.
Recorded trends of extracted oxygen from the catalysts and support samples via the CGHE technique: (a): Cat1; (b): Cat2; (c): Cat3; and (d): comparative visualization of trends for Cat1, Cat2, Cat3, and the supports for Cat2 (Davisil36) and Cat3 (SBA-15).
Figure 1 shows the behavior of the catalysts and the silica supports, respectively, in terms of releasing oxygen. This can affect the rate of methyl radicals’ generation under the OCM reaction atmosphere and thereby the rate of the competing undesired deep oxidation and desired coupling reactions [4,22,33]. A moderate rate of lattice-oxygen transfer to the catalyst surface, thereby establishing a moderate methyl radical generation, has been explained to favor selective catalytic conversion [4]. As a result, a rapid and extensive oxygen release could indicate the potential for a sudden increase in the methyl radical generation under reaction conditions. This can hamper the selectivity, as the excess generated methyl radicals will go far away from the surface and contribute to the gas phase unselective reactions [4]. Also in this regard, the sol–gel catalyst (Cat1) and the structured SBA-15 (Cat3) supported catalyst show similar oxygen-releasing behavior with similar gradual and relatively milder potential to be engaged with methane molecules and generate methyl radicals on the surface. This can be partially associated with the structural characteristics and relatively more homogenous distribution of the active catalytic elements in these catalysts in comparison to Cat2, which has shown an early release of oxygen followed by another distinct sharp oxygen release step. The early release of oxygen in impregnated catalysts (Cat2 and Cat3), for instance at 100 s of time, compared to the bulk catalyst (Cat1), is worth noticing. This could be explained by the fact that the active components in the Cat1 catalyst have, on average, less access to the catalyst surface due to their distribution all across this bulk catalyst. This also partially explains the relatively lower activity of this catalyst compared to Cat2 for instance, particularly at lower reactor temperatures. On the other hand, the amorphous silica support in Cat2 has shown a comparably higher oxygen-release potential than the structured support of Cat3 (SBA-15). These suggest a relatively less-controlled rate of sub-surface oxygen-transfer/release and, thereby, a less-controlled activation of reactants and generation of methyl radicals on the surface of Cat2 in comparison to Cat1 and Cat3, which is partially responsible for its relatively less selective catalytic performance. As explained in Section 2.2.2, a gradual, mild, controlled, and distributed potential for oxygen release is the key for securing the desired activity and selectivity in the catalyst scale. Securing homogeneously distributed catalytic materials across the catalyst structure, as is the case for Cat1, as well as accordingly setting the operating temperature and feed composition, could have resulted in a high selective catalytic performance, as has been demonstrated [20]. However, it should be highlighted that Cat1 can maintain such high C2 selectivity and yield as long as a sufficiently high reaction temperature and intensity could be maintained. Such relatively more sensitive behavior of Cat1 towards the variation in parameters compared to Cat2 has been also demonstrated [20].
The distinguished features of the catalyst associated with the fast or slowly releasing oxygen is in line with the previously published understanding of the OCM catalytic mechanism [4]. This mechanism considers the competitive contributions of adsorbed oxygen and lattice-oxygen species on the catalyst surface, perceived to be responsible for the desired and undesired reactions, which ultimately determine the catalyst selectivity under various sets of operating conditions [4]. Moreover, as seen in Figure 1, by comparing the observed trends of Cat2 and Cat3 and their respective support materials Davisil636 and SBA-15, the presence of active catalytic components (Mn, W, and Na) in the support of these catalysts has resulted in a shift in the trends of oxygen extraction. This reflects the distinguished contributions of the catalytically active materials, identifiable with the operando XRD measurements of the involved material phases, on the extractable oxygen either directly from them or through their interaction with the silica support.
The final aspect to be discussed in Figure 1 is the observed similar trends of the oxygen extraction of Cat1 and Cat3, synthesized via the sol–gel and wet-impregnation methods, which could be correlated with their distinguished selective catalytic performance. Not only the trends, but also the relatively lower quantity of the initial extracted oxygen in these catalysts, particularly Cat1 compared to Cat2, could be highlighted. On the other side, it has been observed that a relatively higher temperature for Cat1, containing a relatively lower concentration of the active catalytic components in the material layers close to its catalyst surface, is needed to secure its highest selective performance [20]. Therefore, it could be concluded that mostly the active catalytic components in the material layers close to the catalyst surface are involved in the oxygen interaction with the surface and affect the surface catalytic activation reaction mechanism. The role and potential of the adsorbed oxygen species and the lattice oxygen on the catalyst surface and the resulting activation of the molecular reactants there, can be measured by other characterization techniques, such as transient responses analysis [33,36]. Further investigations are needed to elaborate the particular role of the fine distribution of the active components and their interaction with the support material on the oxygen release behavior of the catalytic system for the undesired carbon oxides formation and desired coupling reactions on the surface.
Having reviewed these, it should be mentioned that the applied heating procedure in the CGHE method can be qualitatively correlated with the rising temperature in an OCM catalytic bed. In that regard, the observed pattern of the oxygen extraction can support the explanation of the recorded dynamic performance of the catalytic reactor reported in Section 3.3 (Table 3). The ascending temperature trajectory in both cases enables securing the flux of oxygen release on the catalyst surface, which facilitates the coupling reactions and improves the selectivity. In particular, this is an important aspect in determining the oxygen releasing rate and capacity of the catalyst to be properly addressed for the design and operation of a chemical-looping OCM reactor system.
2.3.2. High-Temperature XRD Measurements
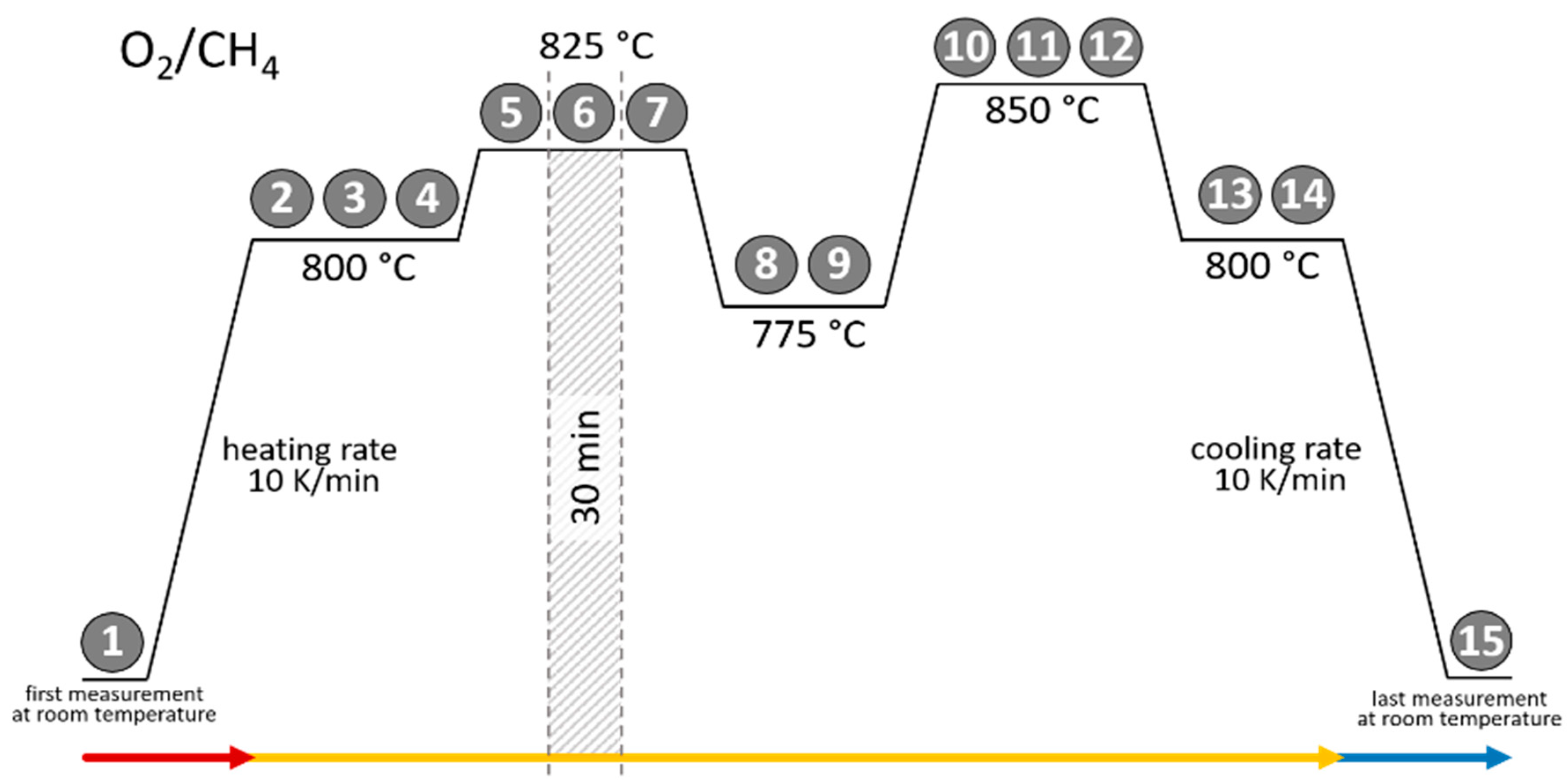
All three catalysts (Cat1, Cat2, Cat3) were analyzed using in situ high-temperature XRD measurements. First of all, the material composition of all three fresh catalysts was determined by comparing the observed diffraction patterns with the database PDF5+ showing various oxidic compounds containing the main elements (Na, Mn, W, (Si)) as well as the silica support (Supplementary Material). This information can be used to track the dynamic of change in crystal structures, formation of new compounds, loss of compounds, and/or melting/recrystallization at different temperatures and reaction atmospheres in terms of the methane-to-oxygen ratio and dilution, as well as gas hourly space velocities. Figure 2 shows the applied temperature profile during the high-temperature XRD measurements to track the phase transformations of the sample catalysts under ramping ascending/descending temperatures. In any attempt to correlate the structural characteristics of OCM catalysts with its reactivity/selectivity, it is essential to study the compounds present under the reaction temperature and preferably under the reaction atmosphere.

Figure 2.
Temperature trajectory of the in situ XRD measurements for all three catalysts under a varying reaction atmosphere (each number represents a single 30 min XRD measurement). Arrows represent the time periods (red arrow: initial heating; yellow arrow: varying conditions; blue arrow: final cool-down).
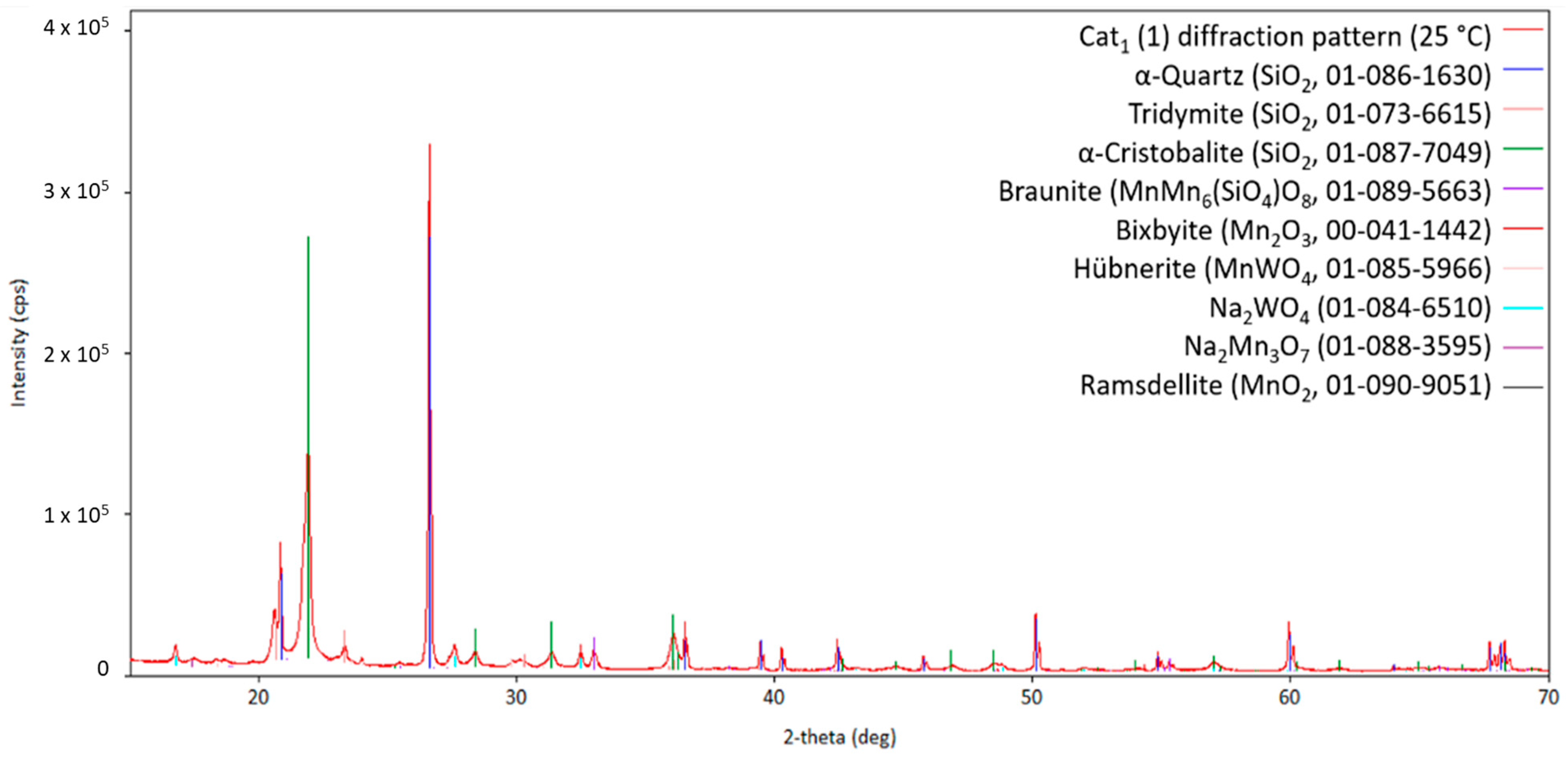
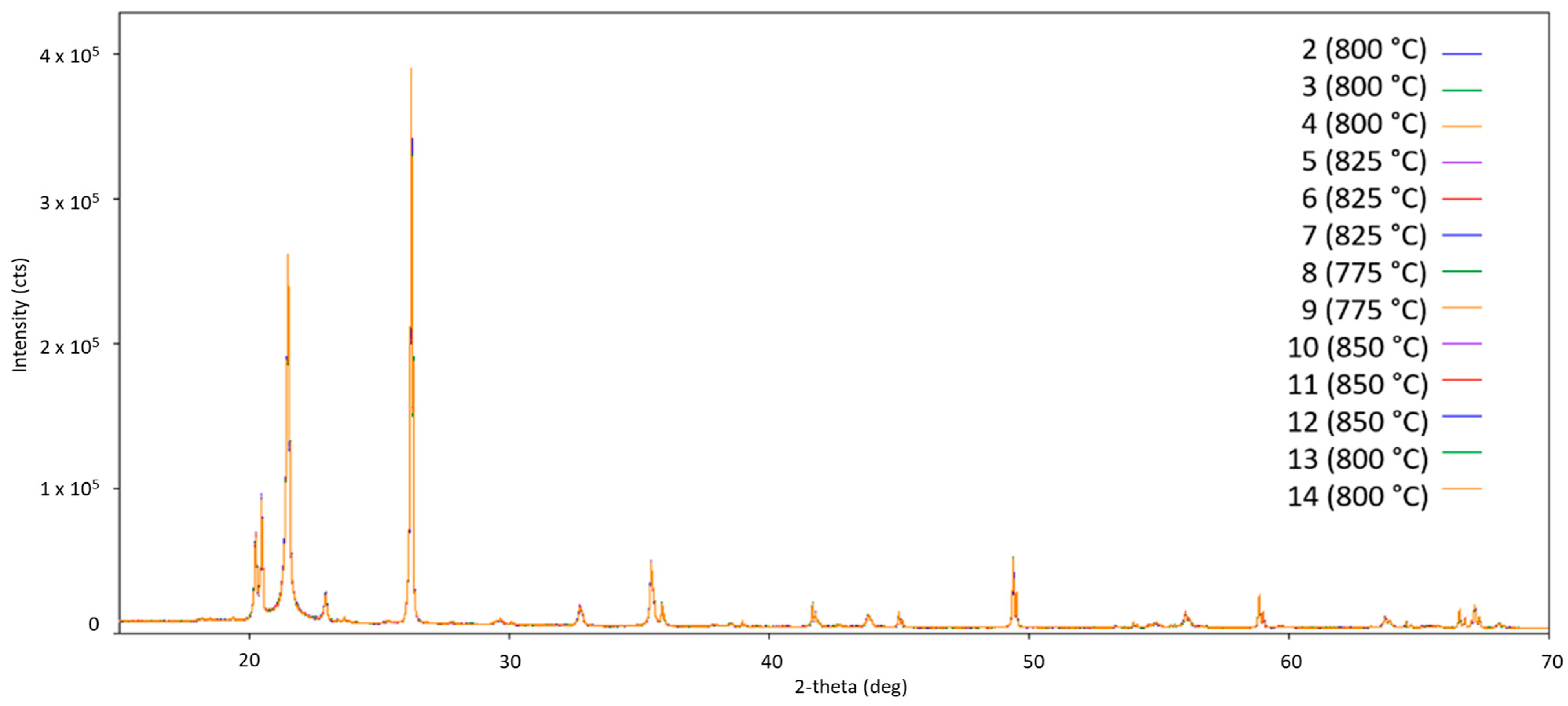
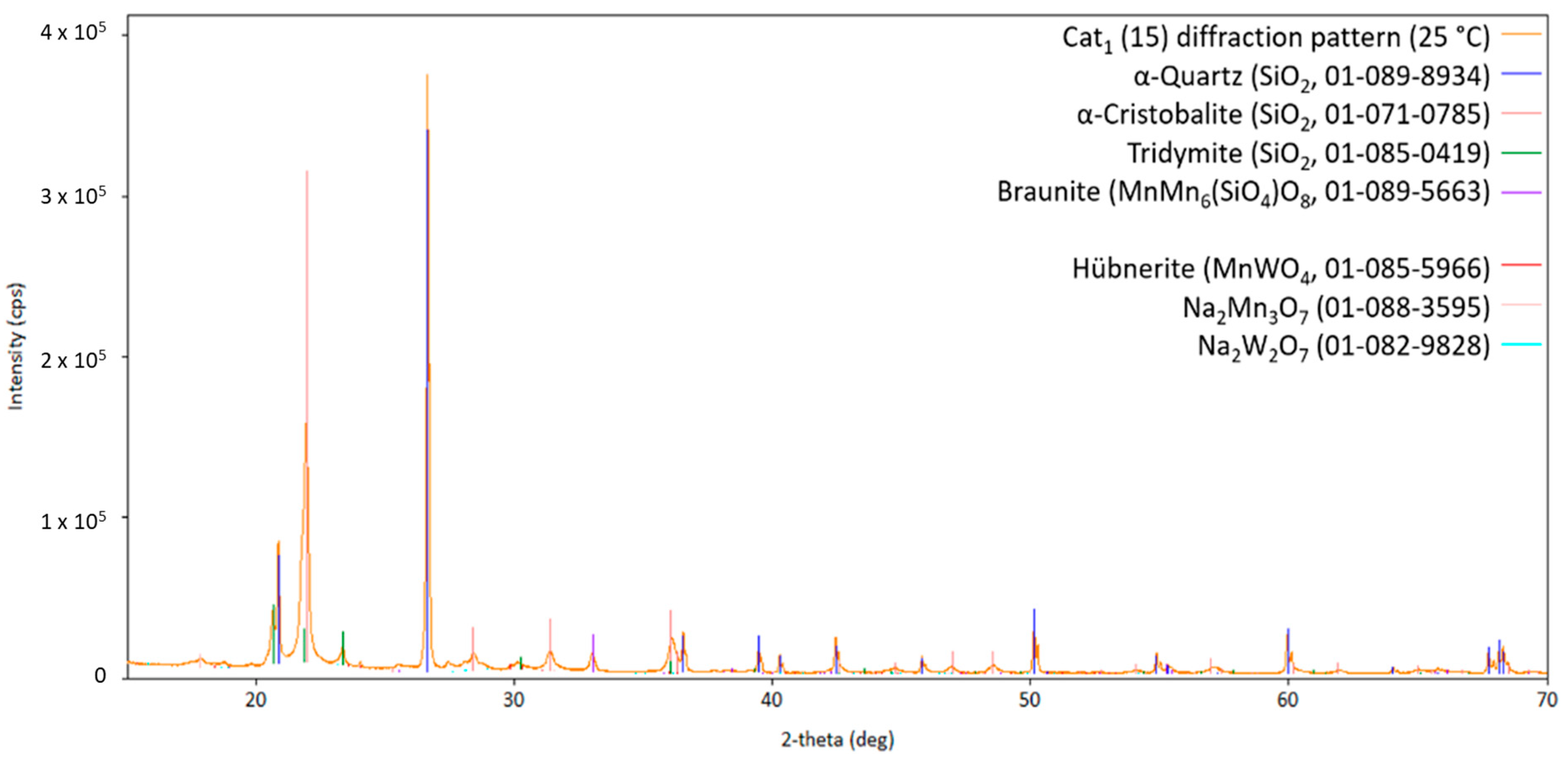
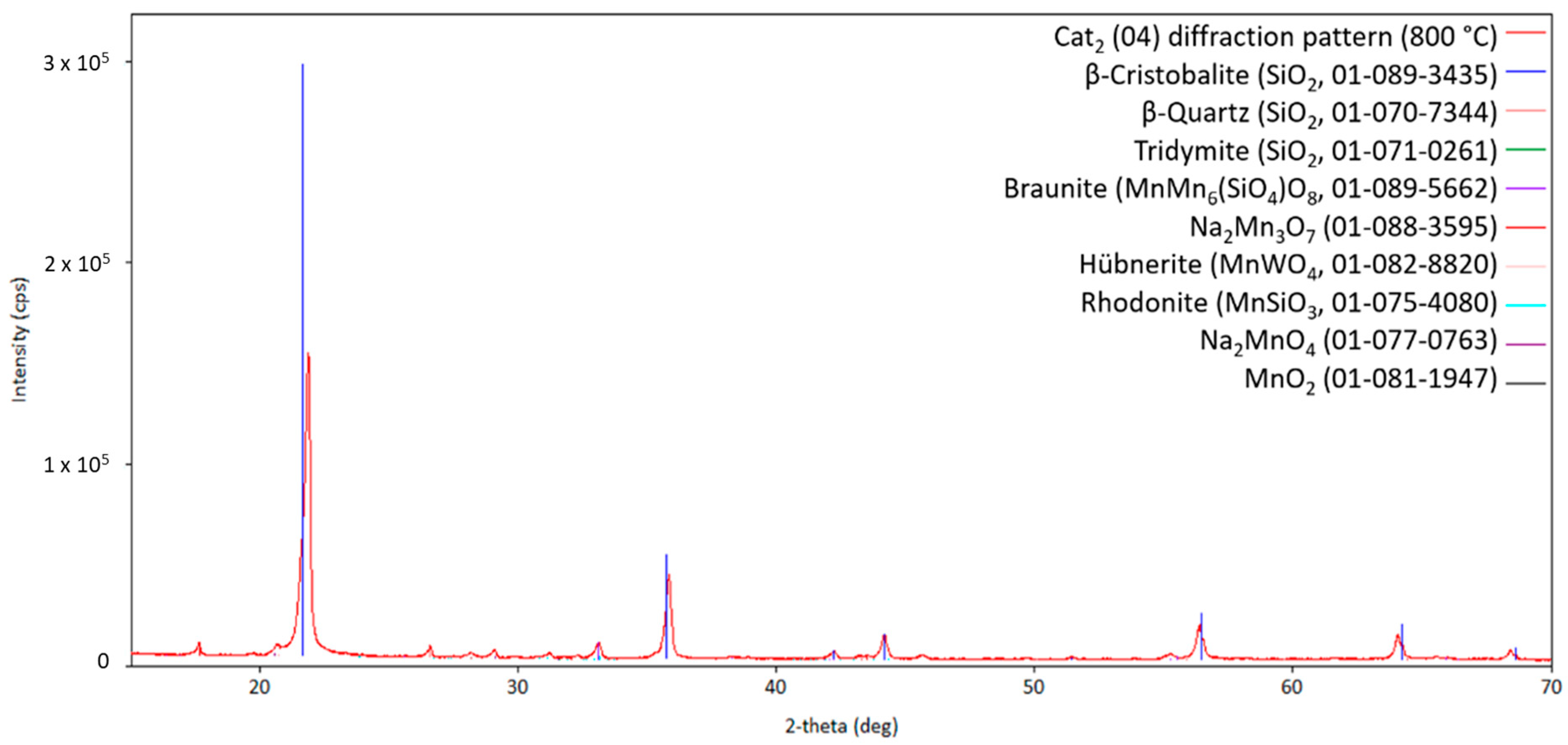
Starting the measurement from room temperature, Figure 3 shows the obtained diffraction pattern for the fresh Cat1 at measurement point 1. Figure 4 shows the diffraction patterns at higher temperatures (measurements points 2–14) for comparison. The comparison of the diffraction patterns in Figure 3 and Figure 4 shows that no significant change could be observed in the sample once the transformation of the fresh catalyst to the catalytic active one has been completed (almost identical diffraction patterns, Figure 4). Moreover, the initially observed Na2WO4 (PDF card no. 01-084-6510) is no longer present at higher temperatures (Figure 4), indicating either decomposition, reaction, or melting in the observed temperature range. This also holds for the other identified sodium-containing ternary oxide Na2Mn3O7 (PDF card no. 01-084-6510). Similar observations have been reported in the literature [22].

Figure 3.
Diffraction pattern of Cat1 initially measured at 25 °C (measurement point 1 indicated in Figure 2) with the respective theoretical reflection positions of various compounds identified by the database PDF5+ (in brackets, the sum formula and card no. are stated; CuKα1,2 radiation).

Figure 4.
Comparison of all obtained diffraction patterns for Cat1 at higher temperatures under oxygen/methane gas atmosphere (number represents the corresponding measurement point in Figure 2; in brackets, the temperature is given; CuKα1,2 radiation).
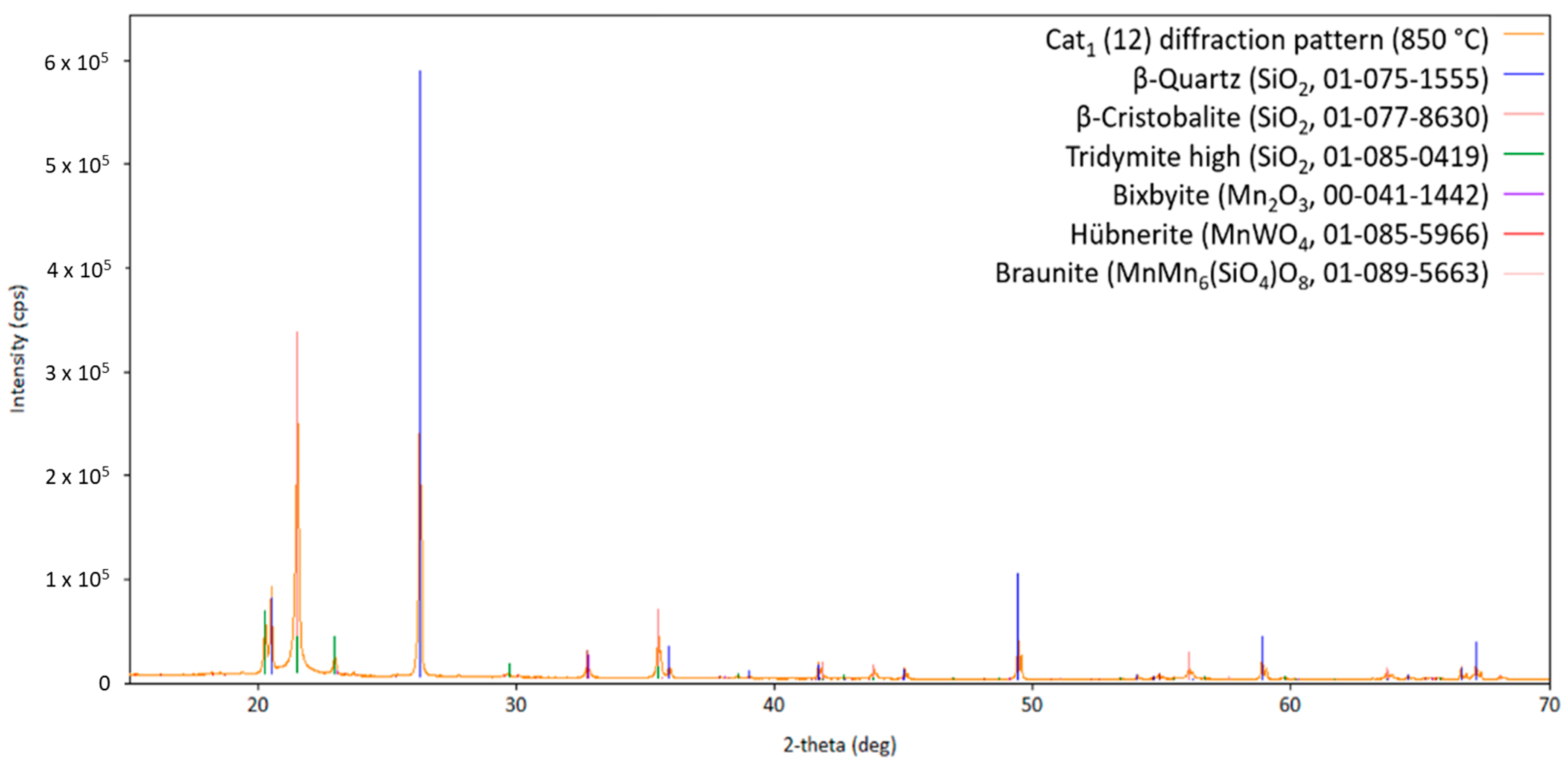
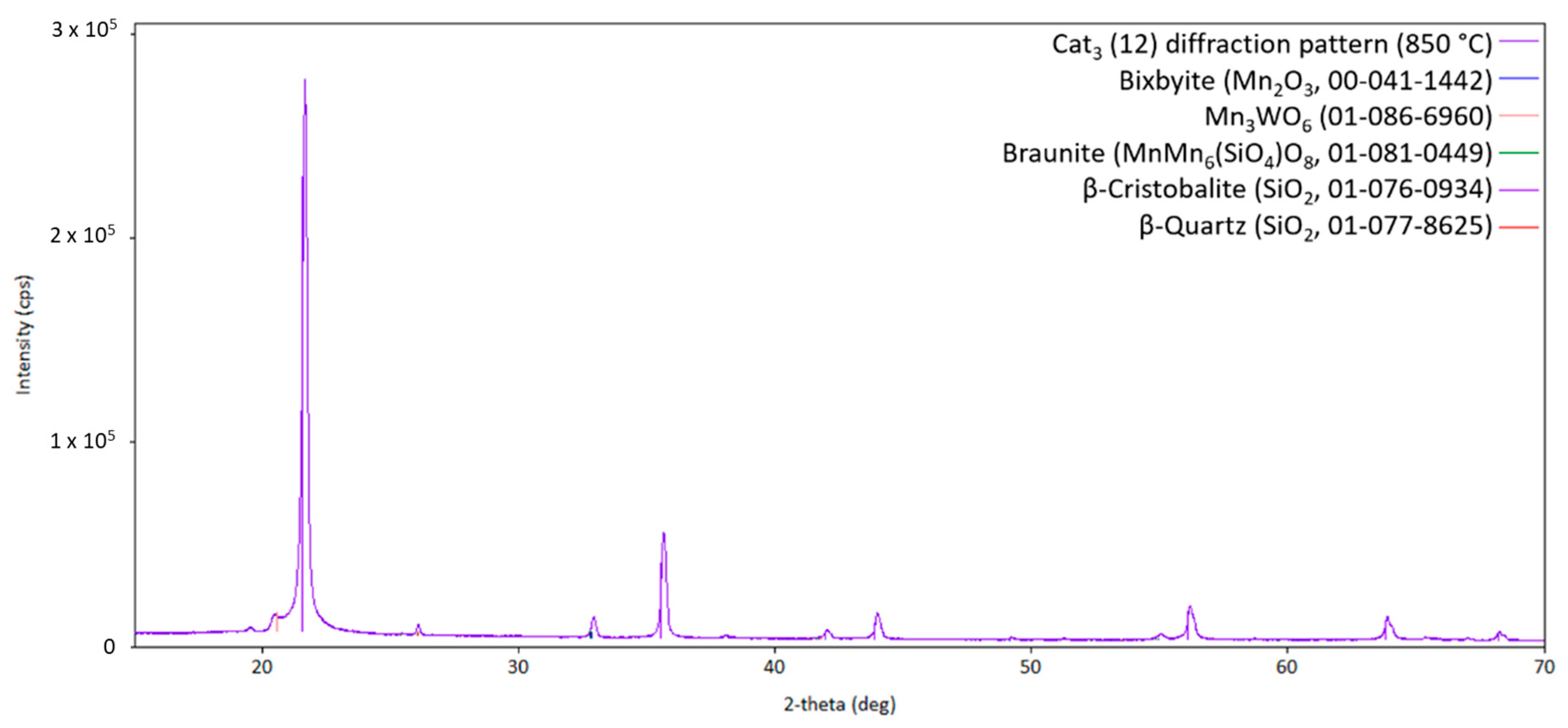
One has to keep in mind that identifying several minority phases from a diffraction pattern at higher temperatures is challenging due to a lack of crystallographic information in this temperature region, overlapping of reflections from different phases, low intensity of the reflections of the catalytic active phases caused by the small loading on the silica support, preferred orientation of the silica support, or due to different crystal structures with a high crystallographic relationship. Nevertheless, Figure 5 shows the diffraction pattern of three high-temperature silica variations (quartz, cristobalite, and tridymite; silica support), as well as Bixbyite, Hübnerite, and Braunite. Interestingly, no sodium-containing compound can be observed in Cat1. In order to check if sodium has been lost during the heat treatment or just the melting and recrystallization of sodium-containing compound(s) has occurred, the diffraction pattern at room temperature after the heat treatment has been analyzed for clarification, as shown in Figure 6.

Figure 5.
Diffraction pattern of Cat1 at 850 °C (measurement point 12 shown in Figure 2) with the respective theoretical reflection positions of various compounds identified by the database PDF5+ (in brackets, the sum formula and card no. are stated; CuKα1,2 radiation).

Figure 6.
Diffraction pattern of Cat1 at 25 °C (cooled down; measurement point 15 in Figure 2) with the respective theoretical reflection positions of various compounds identified by the database PDF5+ (in brackets, the sum formula and card no. are stated; CuKα1,2 radiation).
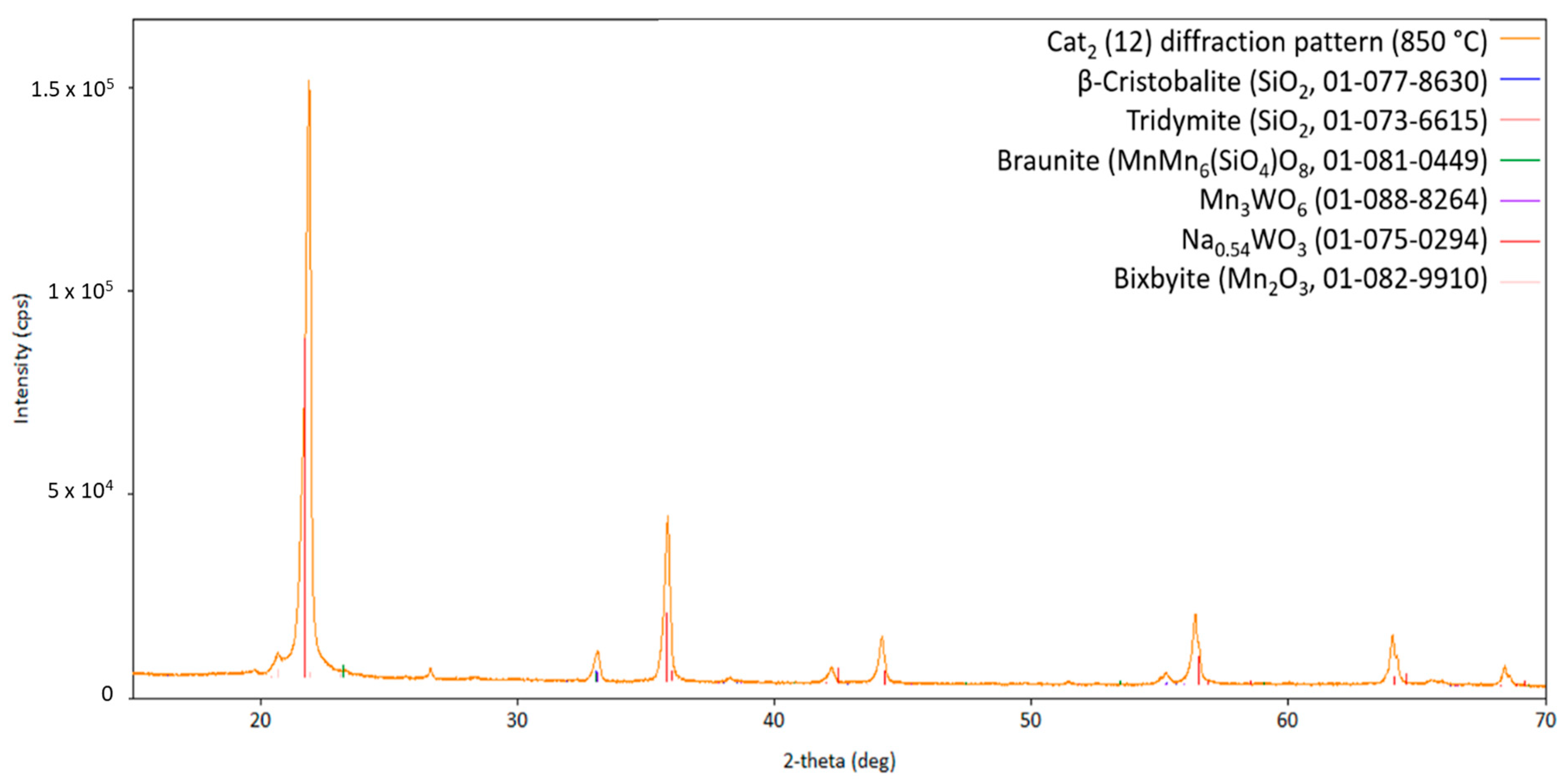
Obviously, the silica support, Braunite, and Hübnerite are stable under the applied conditions, since no decomposition of these phases can be observed over the whole temperature range from heating up to cooling down. The initially observed manganese oxides, Mn2O3 and MnO2, are no longer present, most probably due to their high-temperature reaction with the sodium tungstate forming Na2Mn3O7 and MnWO4 and also the reaction with the silica support forming more MnMn6(SiO4)O8. Moreover, in comparison to the presented high-temperature XRD patterns, two different sodium-containing phases, namely Na2Mn3O7 and Na2W2O7, can be observed after cooling down, strengthening the assumption that the sodium-containing phases are in a molten state under reaction conditions and recrystallize during cool-down.
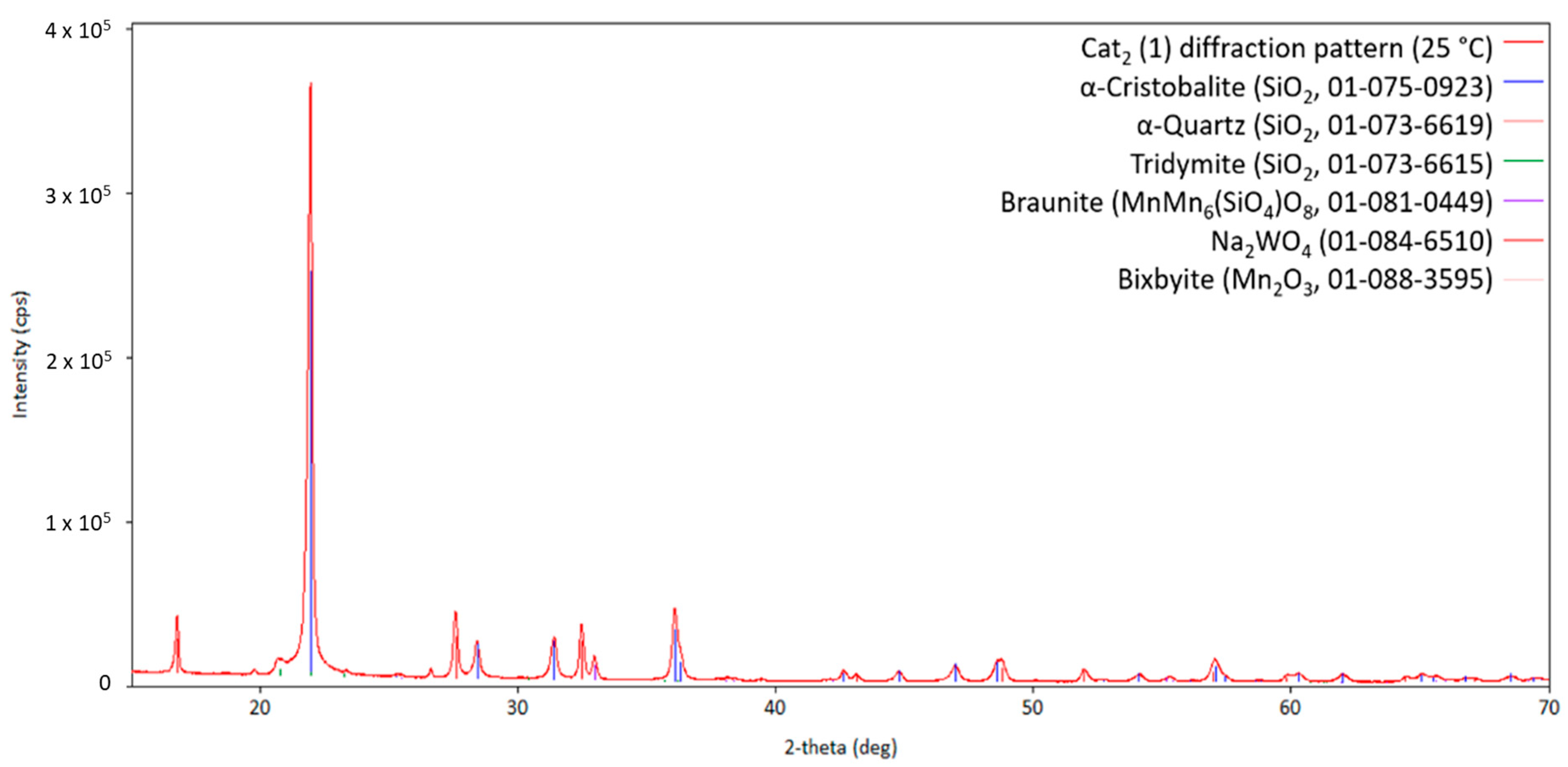
The diffraction pattern of the fresh Cat2 is presented in Figure 7. In comparison to Cat1, the main silica phase of Cat2 crystallizes in the cristobalite structure and less variants are formed during preparation of the fresh catalyst, namely, Braunite, Bixbyite, and disodium tungstate Na2WO4. Considering the observed patterns of Cat1, it is assumed that the initially observed Na2WO4 phase will vanish at high temperatures in favor of forming molten Na2Mn3O7 and Na2W2O7, which are recrystallizing when cooling down. Figure 8 shows an overview of the XRD patterns obtained at higher temperatures.

Figure 7.
Diffraction pattern of Cat2 initially at 25 °C (measurement point 1 shown in Figure 2) with the respective theoretical reflection positions of various compounds identified by the database PDF5+ (in brackets, the sum formula and card no. are stated; CuKα1,2 radiation).

Figure 8.
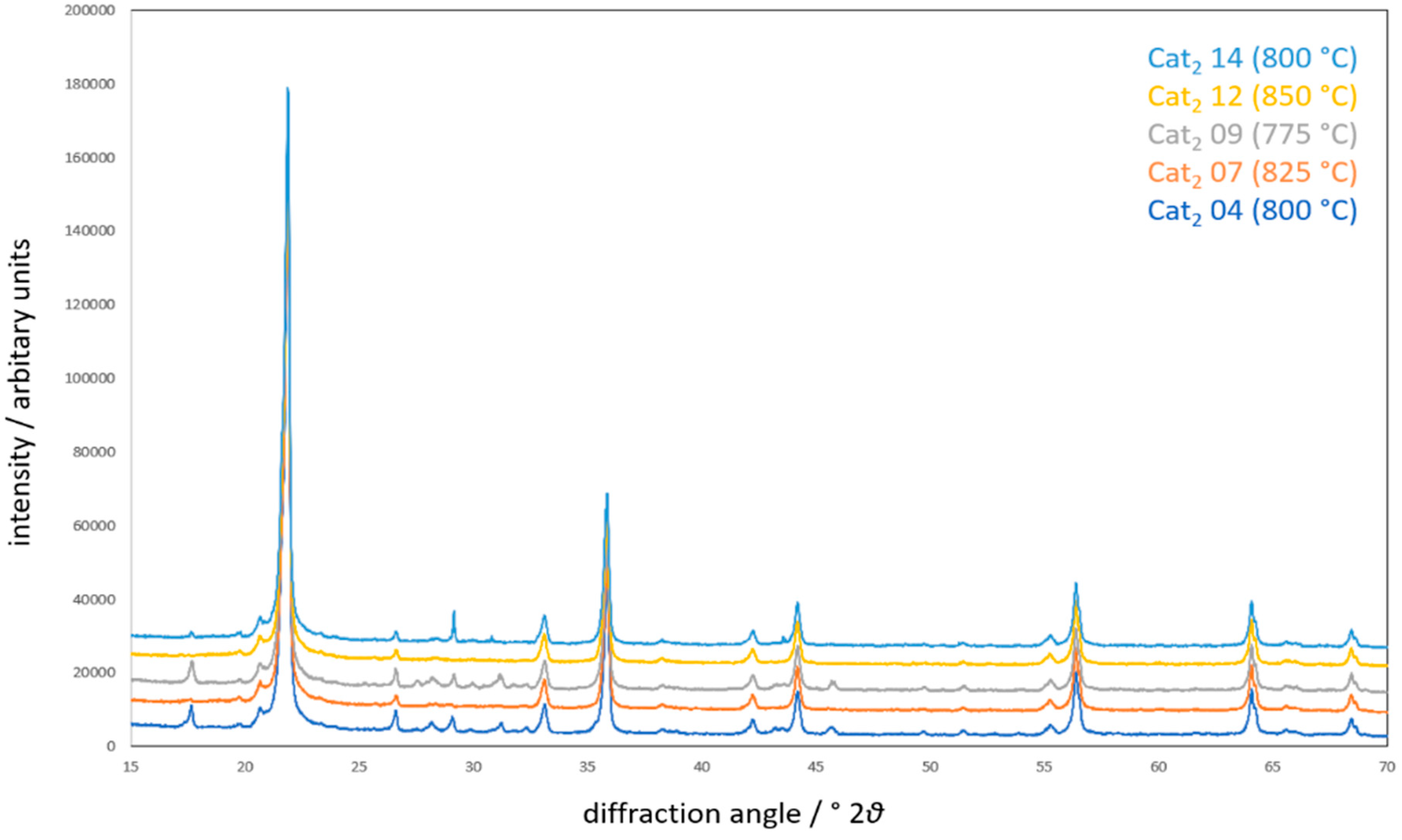
Diffraction patterns of Cat2 at different temperatures (measurement points 4, 7, 9, 12, and 14 in reference to Figure 2; CuKα1,2 radiation).
In Figure 8, it can be clearly seen that Na2Mn3O7 is being formed up to 800 °C and will melt/recrystallize at around 825 °C. Details of all phases in all diffraction patterns are presented in the Supplementary Material. Interestingly, various compounds are additionally formed during the heat treatment, most probably in contact with the gaseous atmosphere containing methane and oxygen, changing the oxygen partial pressure of the system compared to the reaction conditions during the catalyst preparation. The specific compounds additionally formed under catalytic conditions are presented in Figure 9 and Figure 10. Clearly, the catalytic active elements will partially react with the silica support to form oxidic compounds with different manganese to tungsten ratios. These formations indicate a very dynamic state of the silica support catalyst, hereby enabling the activation of methane and its further oxidation. However, from the composition of the cooled down catalyst, it can be concluded that a loss of sodium occurs during the heat treatment, as indicated by the transformation of Na2Mn3O7 to Na2Mn8O16.

Figure 9.
Diffraction pattern of Cat2 at 800 °C (measurement point 4 in Figure 2; CuKα1,2 radiation) with the respective theoretical reflection positions of the stated compounds.

Figure 10.
Diffraction pattern of Cat2 at 850 °C (measurement point 12 in Figure 2; CuKα1,2 radiation) with the respective theoretical reflection positions of different compounds.
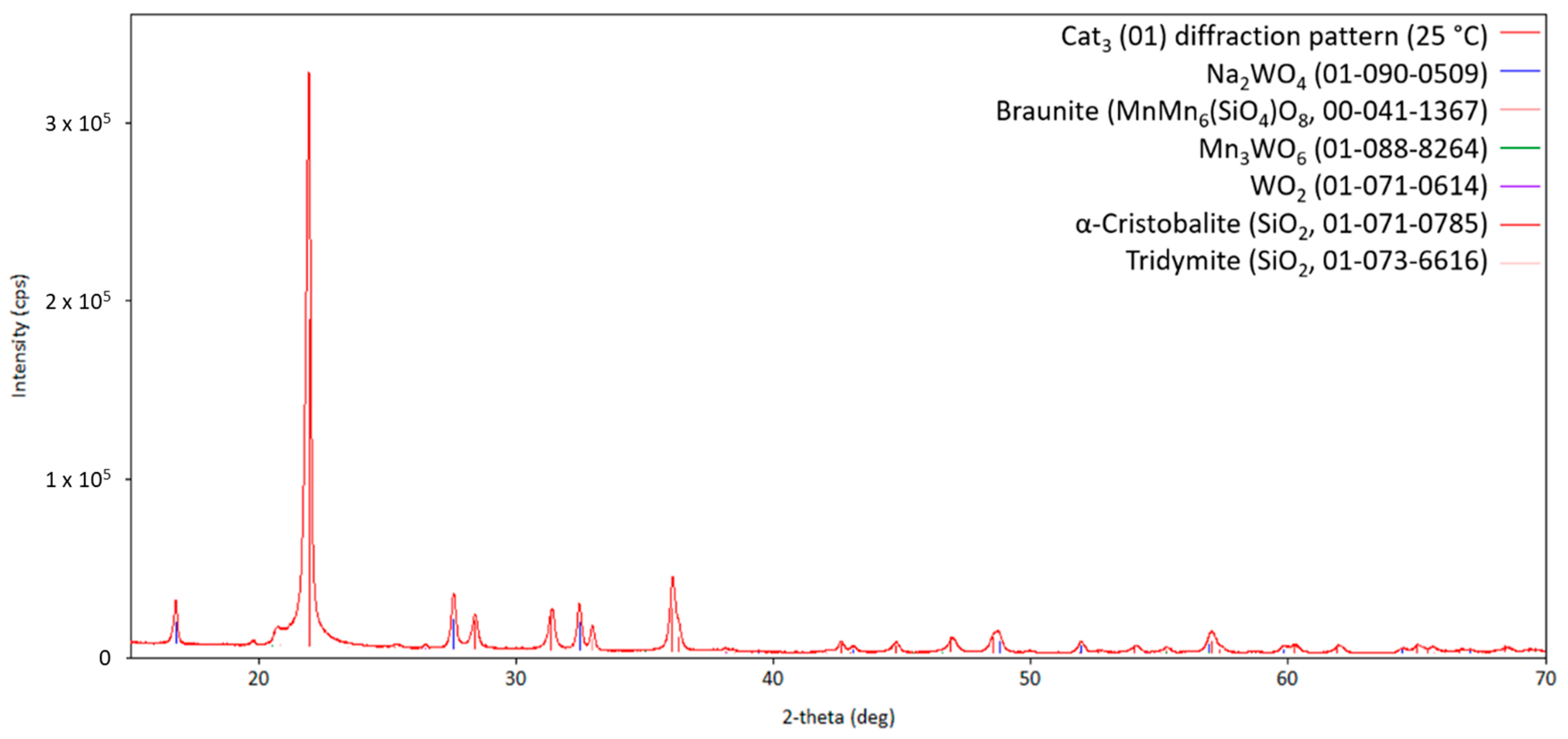
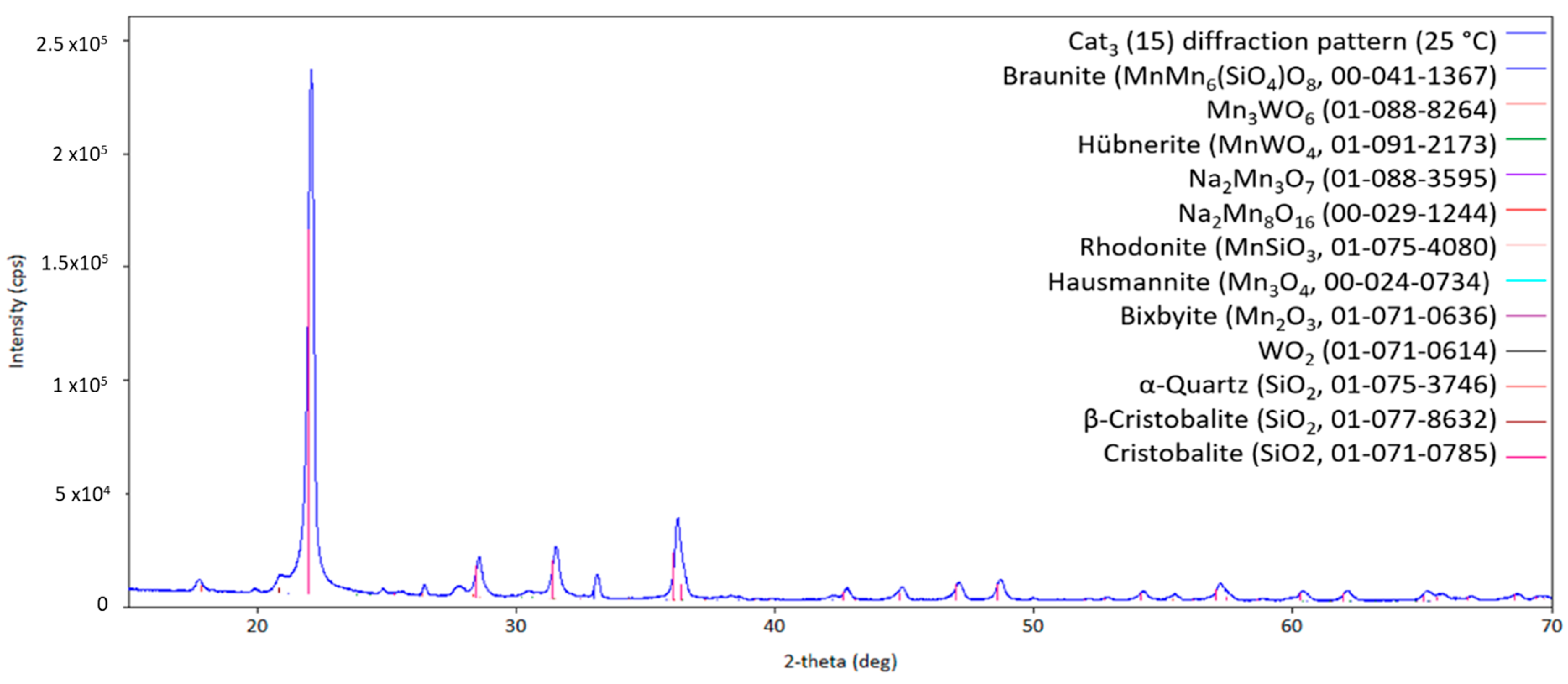
Finally, the SBA-15-supported catalyst Cat3 has been investigated by high-temperature X-ray diffraction. The diffraction pattern of the fresh catalyst Cat3 is presented in Figure 11.

Figure 11.
Diffraction pattern of the initial Cat3 at 25 °C (measurement point 1 in Figure 2; CuKα1,2 radiation) with the respective theoretical reflection positions of different compounds.
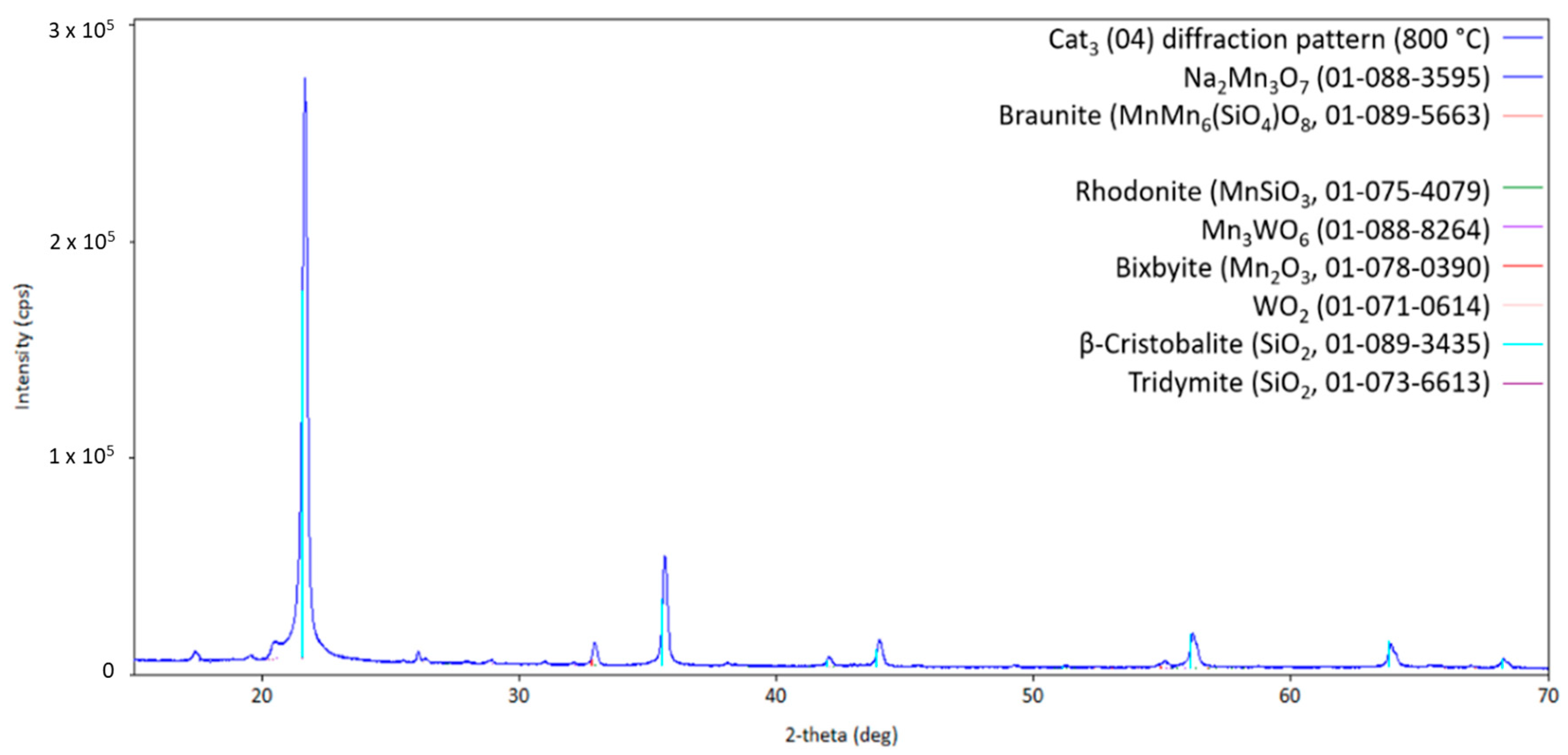
Here, the silica main phase crystallizes in the cristobalite structure in a way that is comparable to Cat2. Braunite and Na2WO4 are also formed compared to the other two catalysts (Cat1 and Cat2). However, WO2 and Mn3WO6 are also present in the freshly made catalyst Cat3. As shown in Figure 12, at higher temperatures, Na2Mn3O7 can be observed, indicating the reaction of Na2WO4 with the manganese-containing compounds. During the heat treatment under the atmosphere with a given CH4/O2 ratio, the oxidation state of manganese will vary between +2 and +4. The observed change in oxidation states also holds for tungsten-containing compounds, hereby changing from +4 to +6 and vice versa. At temperatures higher than 800 °C, the sodium-containing compound Na2Mn3O7 will melt, as can be inferred from the pattern shown in Figure 13, and recrystallizes when cooling down, as shown in Figure 14.

Figure 12.
Diffraction pattern of Cat3 at 800 °C (measurement point 4 in Figure 2; CuKα1,2 radiation) with the respective theoretical reflection positions of different compounds.

Figure 13.
Diffraction pattern of Cat3 at 850 °C (measurement point 12 in Figure 2; CuKα1,2 radiation) with the respective theoretical reflection positions of different compounds.

Figure 14.
Diffraction pattern of Cat3 after cooling down at 25 °C (measurement point 15 in Figure 2; CuKα1,2 radiation) with the respective theoretical reflection positions of different compounds.
In contrast to the two other catalysts, Rhodanite MnSiO3 is also formed during the heat treatment of Cat3, indicating a more reactive silica support when using SBA-15. Obviously, Rhodanite will also melt and recrystallize around 800 °C. The formation of some of the compounds during the heat treatment, primarily as a result of changing the oxygen partial pressure, could be considered an indication for the stability of oxides at a certain temperature range. The formation of Na2Mn8O16 can also indicate a loss of sodium under catalytic conditions (decreased sodium to manganese ratio).
To summarize, the observed transformation of the low-temperature silica phases to their high-temperature phases are comparable with the well-known trends in the literature. Moreover, the meta-stable silica phases (e.g., α-cristobalite) are also stabilized at room temperature after heat treatment, most probably due to the presence of the catalytic active materials containing sodium and/or manganese. Additionally, the silica support will form stoichiometric compounds with manganese. Hereby, MnMn6(SiO4)O8 can be observed in all three catalysts to be stable under the applied conditions. Obviously, the silica support of Cat3 (initially SBA-15) seems to be more reactive, also forming Rhodanite MnSiO3 during the heat treatment. Furthermore, the manganese and tungsten will form various oxidic compounds, either with sodium (e.g., Na2Mn3O7), with each other (e.g., Mn3WO6), or with pure binary oxides (e.g., WO2 or Mn3O4) with different oxidation states as function of temperature and oxygen partial pressure. Tracking the appearance of these phases can be used to identify their potential contributions to the selective methane conversion in the investigated range of operating conditions [18,21]. In particular, the role of tungsten-containing oxides with tungsten in its oxidation state +4 and +6, respectively, and manganese-containing oxides with manganese in its oxidation state +2, +3, and +4, respectively, in connection with the rate of oxygen lattice transport, have been investigated under different oxidizing and reducing atmospheres [17,18,21,37,38,39,40].
Furthermore, Na2WO4 being present in all fresh catalysts will most likely form Na2Mn3O7 with the manganese-containing oxides under the applied reaction conditions. Na2Mn3O7 will then melt around 800 °C and recrystallize when cooling down. However, this has not been recorded, for instance in the reported phase transformation recorded through the operando XRD analysis of the amorphous silica catalyst (Cat2) [11]. However, the molten phase of Na2Mn3O7 can contribute to bridging (e.g., via facilitating the electron or lattice oxygen transfer) the active components (e.g., manganese and tungsten) with each other and the support. For instance, this can result in the formation of the MnWO4 phase at lower temperatures.
Additionally, different oxidic manganese phases, present in different oxidation states, have been detected in the applied temperature range, reflecting the impact of the change in the oxygen partial pressure. For instance, the Mn2O3 phase has been detected in a wide temperature range in this study, as well as in the operando CT-XRD analysis, indicating that the manganese phases even deep into the catalyst bulk body are also reduced and contribute to lattice oxygen transfer [11]. Some of the manganese-containing phases (e.g., MnWO4), including the ones established with the silica support (e.g., MnMn6SiO12), have also been identified as potential contributors to the activation mechanism being involved in the oxygen transfer mechanism and oxygen storage capacity [11,22]. Therefore, the dynamic of the possible transformation of these phases as a function of the oxygen partial pressure can be particularly informative for understanding the catalytic reaction mechanism. Under a higher oxygen partial pressure (particularly at lower temperatures), mixed metal oxides usually appear with higher oxidation numbers of one cation (e.g., MnWO4 or Na2Mn3O7). However, this does not hold for all compounds present, most probably due to redox reactions between manganese and tungsten. This reduces their capability to be further oxidized and to contribute to a dynamic generation–transfer–release of lattice oxygen and thereby to activate methane over the catalyst surface with the rate and mechanism resulting in the formation of carbon oxides. In contrast, under a reductive atmosphere (particularly at higher temperatures), the catalyst becomes more active as more active metal centers (e.g., manganese and tungsten) can selectively engage with the molecular oxygen and methane [41]. For the oxygen-lean atmosphere representing oxygen dosing while using a high methane-to-oxygen ratio, a typical and reversible trend of the transformation of metal-oxide phases towards the ones with lower oxidative states, for instance Mn2O3 and MnMn6SiO12-Phases to Mn2WO4, have been reported [11,22]. In the temperature range and oxygen-to-methane range, at which the first Mn-W-O phase starts to be formed, metal oxides preferably contribute to activating methane and selectivity, converting the radical species over the catalyst. In detail, the Mn-W-O phases (Mn3WO6 and MnWO4, respectively) contribute to tuning the dynamic of oxygen storage and utilizing the oxygen vacancies across the Na-Mn-W-O/SiO2 catalysts, in particular in the incipient wetness-impregnated catalysts. The ultimate goal is to characterize the required phases involved in establishing a controlled rate of lattice oxygen transfer and oxygen activation on the catalyst surface to secure the desired dynamic of methane activation and methyl radical generation [4]. This is in contrast with the sudden uncontrolled spotted release of oxygen, which can result in very low or very high activation rates. On the one hand, such a sudden release of oxygen could be expected in a catalyst, over which the metal oxides have been inhomogeneously distributed, for instance by forming clusters of metal oxides. On the other hand, the rates of lattice-oxygen transfer and surface catalytic activation can be affected differently in a different range of operating conditions. For instance, sodium-containing phases could differently contribute to the lattice oxygen and regulating the activation rate as they transform between the solid and molten states in a different range of reaction temperatures.
Having considered the procedures and the observed patterns of the high-temperature XRD and the CGHE technique, the interaction of the gaseous species with the catalyst with its internal lattice-oxygen dynamic, both affected by the reaction temperature, will influence the quantity and quality of the products formed.
3. Materials and Methods: Synthesis and Characterization of Mn-Na-W/SiO2 Catalysts
Because of its distinguished catalytic behavior, indicated by a relatively stable operation and demonstrated high C2 (C2H4 + C2H6) selectivity and yield, Mn-Na-W-Ox/SiO2 catalysts have been frequently investigated since 1992 [24]. For instance, the characteristics and the performance of the Mn-Na-W-Ox/SiO2 catalysts synthesized via various preparation methods [20,26,27,28] and the impacts of the involved microscopic and macroscopic phenomena [11,12,20,21,22,23,27,28,29,42,43,44] have been extensively investigated. However, for an OCM catalyst in general, more systematic research is needed before reaching a general understanding concerning the roles and contributions of the support and the catalytic-active components as well as their interactions. For the Mn-Na-W-Ox/SiO2 catalyst in particular, the interactive promoting effects of the metal components, for instance sodium on the functionality of manganese and tungsten, while interacting with the silica support, have been thoroughly studied [20,25,27,44].
In fact, the attempts to improve the performance of OCM catalysts, in general, have usually relied on narrow-viewed analyses guided by scattered characterization data, some of which do not even reflect the special dynamic nature of such high-temperature catalytic systems. Nevertheless, such analyses and data can become specifically enlightening when they are re-evaluated in the context of studying the interactive effects of the involved parameters. Having this in mind, the characterization strategy in this research is categorized and orchestrated to the following:
- (1)
- characterize each synthesized Mn-Na-W-Ox/SiO2 catalyst and study structural characteristics, in particular, for the known properties of a selective catalyst. In this paper, the details of the synthesis and an overview of the ex situ characterization as well as the catalytic performance of the three synthesized Mn-Na-W-Ox/SiO2 catalysts (Cat1, Cat2, Cat3) are reported, along with a review of the current understanding of the general functionality of the Mn-Na-W-Ox/SiO2 catalyst.
- (2)
- analyze and explain the dynamic activation mechanism and catalytic behavior of the Mn-Na-W-Ox/SiO2 catalysts under reaction conditions. In this context, the solid-state chemistry of the transformation of metal-oxide phases, in particular, the manganese and tungsten oxides during the reaction, are analyzed and correlated with the selective and stable performance of the catalyst. The results were presented and discussed in Section 2, with a focus on studying the interactions of oxygen species with and within the catalytic structures under varying operating conditions, namely temperature and gas composition.
3.1. General Review of the Involved Material Characteristics for Mn-Na-W-Ox/SiO2 Catalyst
Manganese-containing compounds have been one of the most promising promoters, not only for OCM catalysts, but also for other catalytic oxidative reactions. It is therefore not surprising that manganese is part of the composition of one of the well-performing OCM catalysts, namely, the Mn-Na-W-Ox catalyst. In fact, the combination of manganese, tungsten, and sodium has been the most commonly used mixed metal-oxide OCM catalyst. On the other side, silica, as a support, provides a great level of flexibility to establish the desired structural characteristics of the catalyst. The functionalities of the promoters and the characteristics of silica are stimulated by the high-temperature range of the OCM reaction.
The structural analysis of the OCM catalyst, with a focus on the role of the nanometer–micrometer scale properties of the support along with the possible shrinkage of the catalyst structure due to the interaction of the cations with the silica and the impact of the calcination temperature, have been thoroughly carried out for OCM and other catalytic applications [11,20,37,44,45,46,47,48].
Particularly, in analyzing the interaction between the silica and the cations in the Mn-Na-W-Ox/SiO2 catalyst, the transformation of silica to cristobalite and its contribution to the catalytic performance have been extensively studied [18,20,27,28,42,43,44,47,48]. More specifically, the contribution of tungsten species to the catalyst functionality along with the facilitating effect of sodium in the transformation of amorphous silica into crystalline cristobalite and tridymite have been discussed in detail [47,48]. Parallel to the chemical aspects of such a material transformation, the transformation of the physical properties of the material should also be taken into account in order to track all contributions shaping the catalytic performance.
XRD analysis is usually performed to check the presence of the most important phases after synthesizing and calcinating an OCM catalyst. However, the capability of the XRD results for further improving the current understanding of the catalyst functionality, particularly regarding the contributions of the support and promoters, is limited and subject to uncertainty. This is mainly due to the appearance of molten phases and the dynamic nature of phase transformations and their associated phenomena at such high operation temperatures [22,23,38,39]. For instance, Na2WO4, which has been identified as an important phase to be correlated with the selective performance of this catalyst, has a melting temperature lower than the usual targeted temperature range of the OCM reaction; therefore, its crystalline phase and potential impact on the catalytic performance cannot be easily detected and studied [11]. Nevertheless, having considered the amorphous or molten features of this catalyst [38,39,47,49], the appearance of some detectable phases (e.g., tridymite) in the high-temperature range has been identified to indirectly indicate the formation of molten Na2WO4.
3.2. Catalyst Synthesis
In terms of catalyst compositions, 1.9wt.%Mn-5wt.%Na2WO4/SiO2 has been previously investigated as one of the most frequently studied OCM catalyst compositions [18,20,24,25,26]. The impact of varying the preparation route of this catalyst on the observed catalytic performances has also been previously studied. In this research, the sol–gel method and impregnation method of two different silica supports were employed to represent this catalyst with the same composition but with different structural characteristics.
3.2.1. Sol–Gel Catalyst Characteristics
The details of the sol–gel synthesis of the catalyst 1.9%Mn-0.8%Na-3.1%W/SiO2 (Cat1), its characteristics, and typical performance, representing the selective bulk OCM catalyst, have been provided elsewhere [20]. The synthesis started with the mixing of 32.3 g of tetraethyl orthosilicate (TEOS Si(OC2H5)4, Sigma-Aldrich, Taufkirchen, Germany: CAS-No. 78-10-4) as the source of silica, with the sources of metal precursors with ethanol (min. 99.5% Purity, Sigma-Aldrich: CAS-No. 64-17-5) in a vessel. Then, a solution of water and nitric acid (70%, Sigma-Aldrich: CAS-No. 7697-37-2) was added dropwise to TEOS and ethanol mixture while it was vigorously stirred. Then, the mixture was stirred for 3 h at room temperature. In the next step, in order to obtain 10 g of the 1.9%Mn-5.0%Na2WO4/SiO2 catalyst, calculated amounts of anhydrous sodium acetate (0.28 g, Sigma-Aldrich: CAS-No. 127-09-3), Manganese (II) acetate tetrahydrate (0.85 g, Riedel-de Haen, Charlotte, NC, USA: CAS-No. 6156-78-1) and ammonium meta-tungstate hydrate (0.42 g, Sigma-Aldrich: CAS-No. 12333-11-8) were mixed in a separate vessel containing ethanol and water with molar ratios of ethanol/H2O = 4.4/6.4 (in reference to each mole of TEOS in the pre-hydrolysis). Nitric acid was continuously added to control the pH value of the solution in the range of 2–3. After mixing, this solution was added dropwise to the pre-hydrolyzed silica solution and the mixture was vigorously stirred for 3 h. Pre-hydrolyzing the TEOS enables us to obtain a structure over which the cations are well-dispersed. This gel was then allowed to dry at 90 °C for 2 h. Afterwards, the catalyst was calcined at 850 °C for eight hours in air with a temperature ramp of 10 °C·min−1 and subsequently crushed and sieved to reach the required particle size in the 35–60 mesh range. It was ensured that the resulting powder size was similar to the other two catalysts before being placed and tested in the packed-bed reactor.
3.2.2. Impregnating Non-Structured and Mesoporous Structured Silica-Supports
The wetness-impregnation method has often been utilized for preparing Mn-Na-W-Ox/SiO2 catalysts. The non-structured and structured silica supports, namely the purchased Silica Gel (Davisil Grade-636, Sigma-Aldrich, min. 99% Purity, CAS-No. 112926-00-8) and SBA-15 (ACS Material LLC, min. 99% Purity, Pasadena, CA, USA: CAS-No. 7631-86-9), have specific surface areas of 480 m2/g and 630 m2/g, respectively. These silica supports were sequentially impregnated by the solution containing the metal precursors. The SBA-15-impregnated catalyst (Cat3) was chosen, as it has shown a distinguishably selective performance [27,28] along with the reference non-structured silica-supported Mn-Na-W-Ox/SiO2 catalyst (Cat2), representing different structural characteristics.
The silica supports were impregnated by an aqueous solution containing manganese (II) nitrate tetrahydrate (Mn(NO3)2 ∙ 4 H2O, Sigma-Aldrich: CAS-No. 20694-39-7). After being dried under air at a temperature of 130 °C for at least 5 h, the granules were impregnated with a second aqueous solution containing sodium tungstate dihydrate (Na2WO4 ∙ 2 H2O, Merck, Darmstadt, Germany: CAS-No. 10213-10-2). The samples were then dried for 8 h at 130 °C and annealed at 800 °C for 8 h in air. Details of the applied wetness-impregnation method and the resulting characteristics can be found elsewhere [20,27,28].
3.3. Characterization Techniques
The Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) technique was applied to ascertain the bulk composition of the three catalysts using the Horiba Ultima 2 ICP optical emission spectrometer.
The structural characteristics of the samples were characterized based on Brunauer–Emmett–Teller (BET) theory and Barrett–Joyner–Halenda (BJH) analysis, measured via N2 adsorption–desorption at a cryogenic temperature of 77 K using the QuadraSorb SI (Boynton Beach, FL, USA). The BET surface area, micropores, mesopores, and pore size were evaluated using Quadrawin software Version 5.04 (Quantachrome Instruments, Boynton Beach, FL, USA).
Carrier Gas Hot Extraction (CGHE) measurements were conducted using an ONH836 Elemental Analyzer (LECO Corporation, St Joseph, MI, USA). Helium was used as the inert carrier gas. For the oxygen analyses, a temperature ramp was applied by gradually increasing the furnace power from 160 W to 4500 W over 150 s, followed by a holding period of 30 s at 4500 W. The room temperature X-ray diffraction patterns of the samples were measured using a Philips/PANalytical PW 1830 XRD (Malvern Panalytical GmbH, Kassel, Germany). The XRD measurements at high temperatures were conducted by using a Rigaku SmartLab 3 kW system (Rigaku SE, Neu-Isenburg, Germany) operating in Bragg–Brentano geometry (θ/θ-setup) with nickel-filtered Cu-Kα1,2 radiation (40 kV/40 mA) detected by the 1D-silicon strip detector D/teX Ultra 250. The measurements were carried out in the reaction chamber ReactorX using a black quartz sample holder in the 2θ range of 15–70° 2θ, with a step size of 0.010° 2θ and a scan rate of 2° 2θ/min. The reactor was heated up in steps, as indicated later in the text, with a heating/cooling rate of 10 ± 0.1 K·min−1. A total of 50 mL·min−1 of synthetic air (20.5% O2 in N2, AlphaGaz 1, AirLiquide GmbH, Düsseldorf, Germany) and 20 mL·min−1 of methane (CH4, N55, AirLiquide GmbH, Germany) were used as the catalytic gas atmosphere. The software package SmartLab II Guidance (Rigaku SE, Germany) has been used for data collection, whereas the software package PDXL 2 (Rigaku SE, Germany) in combination with the crystallographic database PDF-5+ 2024 (ICDD, USA) have been used for data analysis.
4. Conclusions
Three different types of Na-Mn-W-Ox/SiO2 catalysts were synthesized by the sol–gel method as well as the wetness-impregnation method using non-structured silica gel and SBA-15, respectively. They were characterized by the Carrier Gas Hot Extraction CGHE technique with respect to their capacity regarding oxygen release and sub-surface lattice-oxygen transfer. Furthermore, the as-prepared fresh catalysts were characterized by in situ high-temperature X-ray diffraction under catalytic OCM conditions.
The main focus was on the investigation of the material and structural properties of the catalysts and their influence on the OCM catalytic properties. In particular, the positive impact of the fine distribution of the active catalytic material over the catalyst structure, as confirmed by SEM imaging, was studied. Such a fine distribution was established by implementing the sol–gel method for synthesizing Cat1 or by using a mesoporous support such as SBA-15, which enables the better distribution of precursors during the wetness impregnation of Cat3. The relatively homogenous distribution of active catalytic materials across Cat1 and Cat3 were demonstrated using FIB-SEM EDX analysis. In any case, it was argued that the fine-distributed catalytic materials will homogenize the rate and distribution of oxygen activation, lattice-oxygen transfer, and ultimately, methyl radical generation over the catalyst’s surface and their selective conversion towards ethane and ethylene. At the same time, the homogenous distribution of the active sites provides a relatively more homogeneous distribution of the generated reaction heat and reduces the chance of a local hot-spot formation, thereby leading to the better distribution of the adsorbed/desorbed species on the surface, which also contribute to the improvement of the selectivity. On the other side, the transformations of the involved material phases, such as the molten Na2Mn3O7 phase, were studied using the high-temperature XRD technique in connection with their potential in releasing oxygen and accordingly affecting the selective catalytic conversion of the species. The observed selective conversion of catalysts under high methane-to-oxygen ratios was correlated with the moderate potential of metal oxides with low oxidation states in contributing to oxygen activation and lattice-oxygen transfer, and thereby moderate rates of methyl radical generation over the catalyst surface. The obtained knowledge paves the way to tailor the material and structural characteristics of the OCM catalyst to establish a controlled rate of oxygen activation and a desired dynamic of methane activation and methyl radical generation on the catalyst surface. Thereby, selective performance can be secured for the co-feed, chemical-looping, and oxygen-dosing operations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13040106/s1, Figure S1: SEM images of the studied catalysts (Top-left: Cat1; Top-right: Cat2; Bottom: Cat3); Figure S2: Detailed results of SEM-EDX analysis of the studied catalysts (Bottom: Cat1; Middle: Cat2; Top: Cat3); Figure S3: Typical water-fall HT-XRD pattern graph representing the main phases involved at different temperatures according to the sample numbers shown in Figure 2; Figure S4: Initial diffraction pattern of Cat2 at 25 °C (measurement no. 1; CuKα1,2 radiation); Figure S5: Diffraction pattern of Cat2 at 800 °C (measurement no. 4; CuKα1,2 radiation); Figure S6: Diffraction pattern of Cat2 at 825 °C (measurement no. 7; CuKα1,2 radiation); Figure S7: Diffraction pattern of Cat2 at 775 °C (measurement no. 9; CuKα1,2 radiation); Figure S8: Diffraction pattern of Cat2 at 850 °C (measurement no. 12; CuKα1,2 radiation); Figure S9: Diffraction pattern of Cat2 at 800 °C (measurement no. 14; CuKα1,2 radiation); Figure S10: Diffraction pattern of Cat2 cooled down at 25 °C (measurement no. 15; CuKα1,2 radiation); Figure S11: Diffraction pattern of Cat3 initial at 25 °C (measurement no. 1; CuKα1,2 radiation); Figure S12: Diffraction pattern of Cat3 at 800 °C (measurement no. 4; CuKα1,2 radiation); Figure S13: Diffraction pattern of Cat3 at 825 °C (measurement no. 7; CuKα1,2 radiation); Figure S14: Diffraction pattern of Cat3 at 775 °C (measurement no. 9; CuKα1,2 radiation); Figure S15: Diffraction pattern of Cat3 at 850 °C (measurement no. 12; CuKα1,2 radiation); Figure S16: Diffraction pattern of Cat3 at 800 °C (measurement no. 14; CuKα1,2 radiation); Figure S17: Diffraction pattern of Cat3 at 25 °C (measurement no. 15; CuKα1,2 radiation); Table S1: (Top): Performance indicators of Cat2 and Cat3 under comparable moderate range of methane conversion (CH4/O2 ratio: 4); (Middle): Performance indicators of Cat1 and Cat2 under comparable moderate range of methane conversion (CH4/O2 ratio: 2.5); (Bottom): Comparing the performance indicators of Cat1 and Cat2 in the conditions, where C2+ yield and C2+ selectivity of respectively greater than 20% and 50% have been recorded.
Author Contributions
H.R.G.: Conceptualization; methodology; investigation; visualization; formal analysis; validation; supervision, project administration; writing—original draft and editing. S.B.: Methodology; investigation; visualization; formal analysis; validation; (all for XRD measurements and analysis); writing—original draft and editing. R.K.-E.: BET and CGHE measurements; writing—review. A.T.S.: writing—review. O.G.: Conceptualization; methodology; investigation; visualization; formal analysis; validation; supervision, project administration; writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Deutsche Forschungsgemeinschaft DFG for the Rigaku SmartLab 3kW within their major instrument program (Großgeräteförderung INST 131/734-1 FUGG) is gratefully acknowledged.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors also acknowledge Holger Kropf for conducting the FIB-SEM characterization at Helmholtz-Zentrum Berlin (HZB) and Milijana Batinic for catalyst synthesis and ICP-OES measurement.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Cat1 | Sol–gel made Mn-Na-W-Ox/SiO2 catalyst |
| Cat2 | Wetness-Impregnated Mn-Na-W-Ox/SiO2 catalyst over non-structured silica |
| Cat3 | Wetness-Impregnated Mn-Na-W-Ox/SiO2 catalyst over structured SBA-15 |
| CGHE | Carrier Gas Hot Extraction |
| C2 | C2H4+C2H6 |
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| FIB-SEM | Focused Ion Beam Scanning Electron Microscope |
| HT-XRD | High-Temperature X-Ray Diffraction |
| ICP | Inductively Coupled Plasma |
| OCM | Oxidative Coupling of Methane |
References
- Keller, G.; Bhasin, M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar] [CrossRef]
- Mazheika, A.; Geske, M.; Müller, M.; Schunk, S.A.; Rosowski, F.; Kraehnert, R. Data-driven Design of Catalytic Materials in Methane Oxidation Based on a Site Isolation Concept. ACS Catal. 2024, 14, 12297–12309. [Google Scholar] [CrossRef]
- Zavyalova, U.; Holena, M.; Schlög, R.; Baerns, M. Statistical analysis of past catalytic data on oxidative methane coupling for new insight into the composition of high-performance catalysts. ChemCatChem 2011, 3, 1935–1947. [Google Scholar] [CrossRef]
- Godini, H.R.; Bhasin, M.M. Oxidative Coupling of Methane: A Review Study on the Catalytic Performance. Molecules 2024, 29, 4649. [Google Scholar] [CrossRef] [PubMed]
- Galadima, A.; Muraza, O. Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: A review. J. Ind. Eng. Chem. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Natural Gas and the Environment—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/energyexplained/natural-gas/natural-gas-and-the-environment.php (accessed on 12 March 2024).
- World Bank Global Flaring Data. 2022. Available online: www.worldbank.org/en/programs/gasflaringreduction/global-flaring-data (accessed on 1 February 2025).
- Teixeira Penteado, A.; Lovato, G.; Pérez Ortiz, A.; Esche, E.; Domingues Rodrigues, J.A.; Godini, H.R.; Orjuela, A.; Gušča, J.; Repke, J.U. Economic Potential of Bio-Ethylene Production via Oxidative Coupling of Methane in Biogas from Anaerobic Digestion of Industrial Effluents. Processes 2021, 9, 1613. [Google Scholar] [CrossRef]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances future prospect in catalysts for oxidativecoupling of methane to ethylene A review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]
- Ortiz-Bravo, C.A.; Chagas, C.A.; Toniolo, F.S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies. J. Nat. Gas Sci. Eng. 2021, 96, 104254. [Google Scholar] [CrossRef]
- Matras, D.; Vamvakeros, A.; Jacques, S.D.; Grosjean, N.; Rollins, B.; Poulston, S.; Stenning, G.B.; Godini, H.R.; Drnec, J.; Cernik, R.J.; et al. Effect of thermal treatment on the stability of Na-Mn-W/SiO2 Catalyst for the Oxidative Coupling of Methane. Faraday Discuss. 2021, 229, 176–196. [Google Scholar] [CrossRef]
- Godini, H.R.; Fleischer, V.; Görke, O.; Jaso, S.; Schomäcker, R.; Wozny, G. Thermal-reaction analysis of Oxidative Coupling of Methane. Chem. Ing. Tech. 2014, 86, 1906–1915. [Google Scholar] [CrossRef]
- Weinberger, S.; Lakhapatri, S.; Radaelli, G.; Cizeron, J.; Pellizzari, R.; McCormick, J.; Sheridan, D.; Reid, C.; Freer, E.; Edwards, J.D.; et al. Reactors and Systems for Oxidative Coupling of Methane. U.S. Patent 10927056 B2, 22 February 2021. [Google Scholar]
- Kim, M.; Repke, J.U.; Schomäcker, R.; Khodadadi, A.A.; Wozny, G.; Görke, O.; Godini, H.R. Recognition of Oxidative Coupling of Methane Reactor Performance Patterns. Chem. Eng. Technol. 2022, 45, 694–708. [Google Scholar]
- Cantrell, R.D.; Ghenciu, A.; Campbell, K.D.; Minahan, D.M.A.; Bhasin, M.M.; Westwood, A.D.; Nielsen, K.A. Catalysts for the Oxidative Dehydrogenation of Hydrocarbons. U.S. Patent 6576803 B2, 10 June 2003. [Google Scholar]
- Stansch, Y.; Mleczko, L.; Baerns, M. Comprehensive Kinetics of Oxidative Coupling of Methane over the La2O3/CaO Catalyst. Ind. Eng. Chem. Res. 1997, 36, 2568–2579. [Google Scholar]
- Wang, D.J.; Rosynek, M.P.; Lunsford, J.H. Oxidative Coupling of Methane over Oxide Supported Sodium Manganese Catalysis. J. Catal. 1995, 155, 390–402. [Google Scholar] [CrossRef]
- Ji, S.; Xiao, T.; Li, S.; Chou, L.; Zhang, B.; Xu, C.; Hou, R.; York, A.P.E.; Green, M.L.H. Surface WO4 tetrahedron: The essence of the oxidative coupling of methane over M-W-Mn/SiO2 catalysts. J. Catal. 2003, 220, 47–56. [Google Scholar] [CrossRef]
- Malekzadeh, A. Structural features of Na2WO4–MOx/SiO2 catalysts in oxidative coupling of methane reaction. Catal. Commun. 2008, 9, 960–965. [Google Scholar]
- Godini, H.R.; Gili, A.; Görke, O.; Arndt, S.; Simon, U.; Thomas, A.; Schomäcker, R.; Wozny, G. Sol-gel method for synthesis of Mn-Na2WO4/SiO2 catalyst for methane oxidative coupling. Catal. Today 2014, 236, 12–22. [Google Scholar]
- Sadjadi, S.; Jašo, S.; Godini, H.R.; Arndt, S.; Wollgarten, M.; Blume, R.; Görke, O.; Schomäcker, R.; Wozny, G.; Simon, U. Feasibility study of the Mn–Na2WO4/SiO2 catalytic system for the oxidative coupling of methane in a fluidized-bed reactor. Catal. Sci. Technol. 2015, 5, 942–952. [Google Scholar]
- Werny, M.J.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Fluctuating Storage of the Active Phase in a Mn-Na2WO4/SiO2 Catalyst for the Oxidative Coupling of Methane. Angew. Chem. Int. Ed. 2020, 59, 14921–14926. [Google Scholar] [CrossRef]
- Ortiz-Bravo, C.A.; Figueroa, S.J.A.; Portela, R.; Chagas, C.A.; Bañares, M.A.; Souza Toniolo, F. Elucidating the structure of the W and Mn sites on the Mn-Na2WO4/SiO2 catalyst for the oxidative coupling of methane (OCM) at real reaction temperatures. J. Catal. 2022, 408, 423–435. [Google Scholar] [CrossRef]
- Fang, X.; Li, S.; Lin, J.; Gu, J.; Yan, J.D. Preparation and characterization of catalyst for oxidative coupling of methane. J. Mol. Catal. 1992, 8, 255–262. (In Chinese) [Google Scholar]
- Gu, S.; Kang, J.; Lee, T.; Shim, J.; Choi, J.W.; Suh, D.J.; Lee, H.; Yoo, C.; Baik, H.; Choi, J.; et al. Na2WO4/Mn supported on all-silica delaminated zeolite for the optimal oxidative coupling of methane via the effective stabilization of tetrahedral WO4: Elucidating effects of support precursors with different crystal structures, Al-addition, and morphologies. Chem. Eng. J. 2023, 457, 141057. [Google Scholar]
- Wang, J.; Chou, L.; Zhang, B.; Song, H.; Zhao, J.; Yang, J.; Li, S. Comparative study on oxidation of methane to ethane and ethylene over Na2WO4–Mn/SiO2 catalysts prepared by different methods. J. Mol. Catal. A Chem. 2006, 245, 272–277. [Google Scholar] [CrossRef]
- Yildiz, M.; Aksu, Y.; Simon, U.; Otremba, T.; Kailasam, K.; Göbel, C.; Girgsdies, F.; Görke, O.; Rosowski, F.; Thomas, A.; et al. Silica material variation for the MnxOy-Na2WO4/SiO2. Appl. Catal. A Gen. 2016, 525, 168–179. [Google Scholar] [CrossRef]
- Yildiz, M. Influences of Support Material Variation on Structure and Catalytic Performance of MnxOy-Na2WO4/SiO2 Catalyst for the Oxidative Coupling of Methane. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, 2014. [Google Scholar]
- Kim, M.; Arndt, S.; Yildiz, M.; Schomäcker, R.; Görke, O.; Repke, J.U.; Wozny, G.; Godini, H.R. Reaction Engineering of Oxidative Coupling of Methane: Experimental Observations and Analysis of the Impacts of Operating Parameters. Chem. Eng. Res. Des. 2021, 172, 84–98. [Google Scholar] [CrossRef]
- Takanabe, K.; Iglesia, E. Mechanistic Aspects and Reaction Pathways for Oxidative Coupling of Methane on Mn/Na2WO4/SiO2 Catalysts. J. Phys. Chem. C 2009, 113, 10131–10145. [Google Scholar] [CrossRef]
- Godini, H.R.; Gili, A.; Gorke, O.; Simon, U.; Hou, K.; Wozny, G. Performance analysis of a porous packed-bed membrane reactor for Oxidative Coupling of Methane: Structural and operational characteristics. Energy Fuels 2014, 28, 877–890. [Google Scholar] [CrossRef]
- Machin, I.; Pereira, P.; de Gouveia, V.; Rosa, F. Modeling of Catalytic Oxidative Coupling of Methane. ACS Prepr. Symp. 1992, 37, 173–178. [Google Scholar]
- AZanina, V.A.; Kondratenko, H.; Lund, J.; Li, J.; Chen, Y.; Li, G.; Jiang, E.V. Kondratenko, The Role of Adsorbed and Lattice Oxygen Species in Product Formation in the Oxidative Coupling of Methane over M2WO4/SiO2 (M = Na, K, Rb, Cs). ACS Catal. 2022, 12, 15361–15372. [Google Scholar] [CrossRef]
- Baerns, M.; Kondratenko, E.V. Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2008. [Google Scholar]
- Wu, T.; Wei, Y.; Xiong, J.; Yang, Y.; Wang, Z.; Han, D.; Zhao, Z.; Liu, J. Ca and Sr co-doping induced oxygen vacancies in 3DOM La2−xSrxCe2−yCayO7−δ catalysts for boosting low-temperature oxidative coupling of methane. J. Energy Chem. 2024, 91, 331–344. [Google Scholar] [CrossRef]
- Zanina, A.; Kondratenko, V.A.; Makhmutov, D.; Lund, H.; Li, J.; Chen, J.; Li, Y.; Jiang, G.; Kondratenko, E.V. Elucidating the Role of Oxygen Species in Oxidative Coupling of Methane over Supported MnOx-Na2WO4-containing Catalysts. ChemCatChem 2023, 16, e202300885. [Google Scholar] [CrossRef]
- Ji, S.F.; Xiao, T.C.; Li, S.B.; Xu, C.Z.; Hou, R.L.; Coleman, K.S.; Green, M.L. The relationship between the structure and the performance of Na-W-Mn/SiO2 catalysts for the oxidative coupling of methane. Appl. Catal. A Gen. 2002, 225, 271–284. [Google Scholar]
- Kou, Y.; Zhang, B.; Niu, J.Z.; Li, S.B.; Wang, H.L.; Tanaka, T.; Yoshida, S. Amorphous Features of Working Catalysts: XAFS and XPS Characterization of Mn/Na2WO4/SiO2 as Used for the Oxidative Coupling of Methane. J. Catal. 1998, 173, 399–408. [Google Scholar]
- Nipan, G.D. Phase States of Na/W/Mn/SiO2 Composites at Temperatures of Catalytic Oxidative Coupling of Methane. Inorg. Mater. 2014, 50, 1012–1017. [Google Scholar] [CrossRef]
- Dedov, A.G.; Nipan, G.D.; Loktev, A.S.; Tyunyaev, A.A.; Ketsko, V.A.; Parkhomenko, K.V.; Moiseev, I.I. Oxidative coupling of methane: Influence of the phase composition of silica-based catalyst. Appl. Catal. A Gen. 2011, 406, 1–12. [Google Scholar]
- YGordienko, T.; Usmanov, V.; Bychkov, V.; Lomonosov, Z.; Fattakhova, Y.; Tulenin, D.; Shashkin, M. Sinev, Oxygen availability and catalytic performance of NaWMn/SiO2 mixed oxide and its components in oxidative coupling of methane. Catal. Today 2016, 278, 127–134. [Google Scholar]
- Pak, S.; Lunsford, J.H. Thermal effects during the oxidative coupling of methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO catalysts. Appl. Catal. A Gen. 1998, 168, 131–137. [Google Scholar]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn–Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is Really Known? Appl. Catal. A Gen. 2012, 425, 53–61. [Google Scholar]
- Tiyatha, W.; Chukeaw, T.; Sringam, S.; Witoon, T.; Chareonpanich, M.; Rupprechter, G.; Seubsai, A. Oxidative coupling of methane-comparisons of MnTiO3-Na2WO4 and MnOx-TiO2-Na2WO4 catalysts on different silica supports. Sci. Rep. 2022, 12, 2595. [Google Scholar] [CrossRef]
- Dey, S.; Kumar, V.P. The performance of highly active manganese oxide catalysts for ambient conditions carbon monoxide oxidation. Curr. Res. Green Sustain. Chem. 2020, 3, 100012. [Google Scholar] [CrossRef]
- Dardouri, R.; Gannouni, A.; Zina, M.S. Structural and Oxidative Properties of Manganese Incorporated Mesostructure Silica for Methane Oxidation. Adv. Mater. Sci. Eng. 2019, 2019, 6024876. [Google Scholar] [CrossRef]
- Palermo, A.; Holgado, J.; Lee, A.; Tikhov, M.; Lambert, R. Critical influence of the amorphous silica-to-cristobalite phase transition on the performance of Mn/Na2WO4/SiO2 catalysts for the oxidative coupling of methane. J. Catal. 1998, 177, 259–266. [Google Scholar]
- Yu, Y.; Movick, W.J.; Obata, K.; Yoshida, S.; Takanabe, K. Mn-Na2WO4/SiO2-tridymite with improved stability and selectivity for high-pressure oxidative coupling of methane. Chem. Eng. J. 2024, 500, 156938. [Google Scholar]
- Takanabe, K.; Khan, A.M.; Tang, Y.; Nguyen, L.; Ziani, A.; Jacobs, B.W.; Elbaz, A.M.; Sarathy, S.M.; Tao, F. Integrated In Situ Characterization of a Molten Salt Catalyst Surface: Evidence of Sodium Peroxide and Hydroxyl Radical Formation. Angew. Chem. Int. Ed. 2017, 56, 10403. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).