Unsymmetrical Bis(thiosemicarbazone) Ligands and Their Nickel(II) Complexes: Synthesis, Characterization and Photocatalytic Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Infrared Spectroscopy
2.2. UV-Vis Spectroscopy
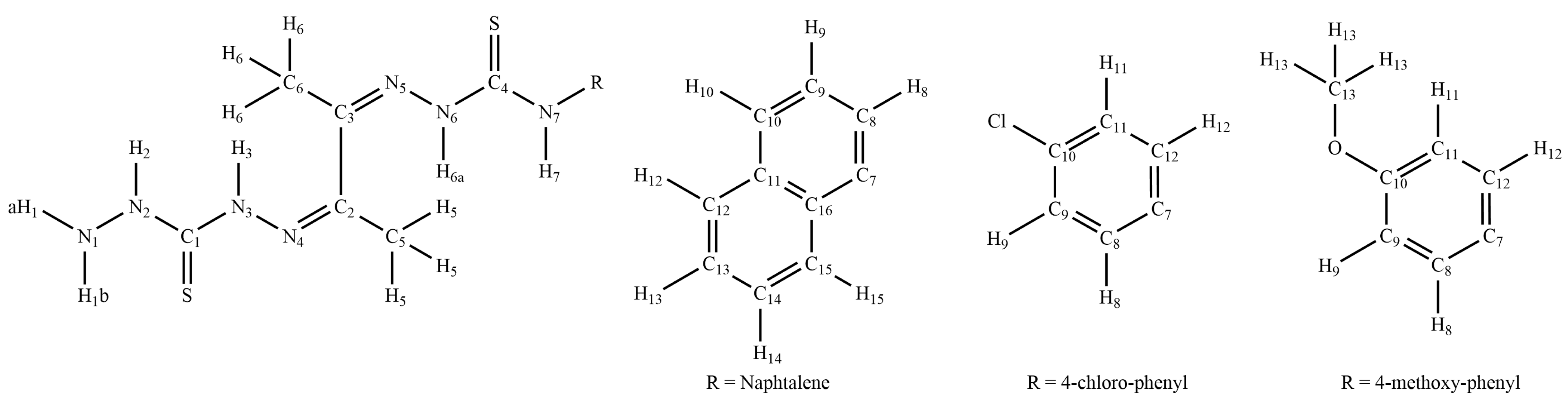
2.3. NMR Spectroscopy
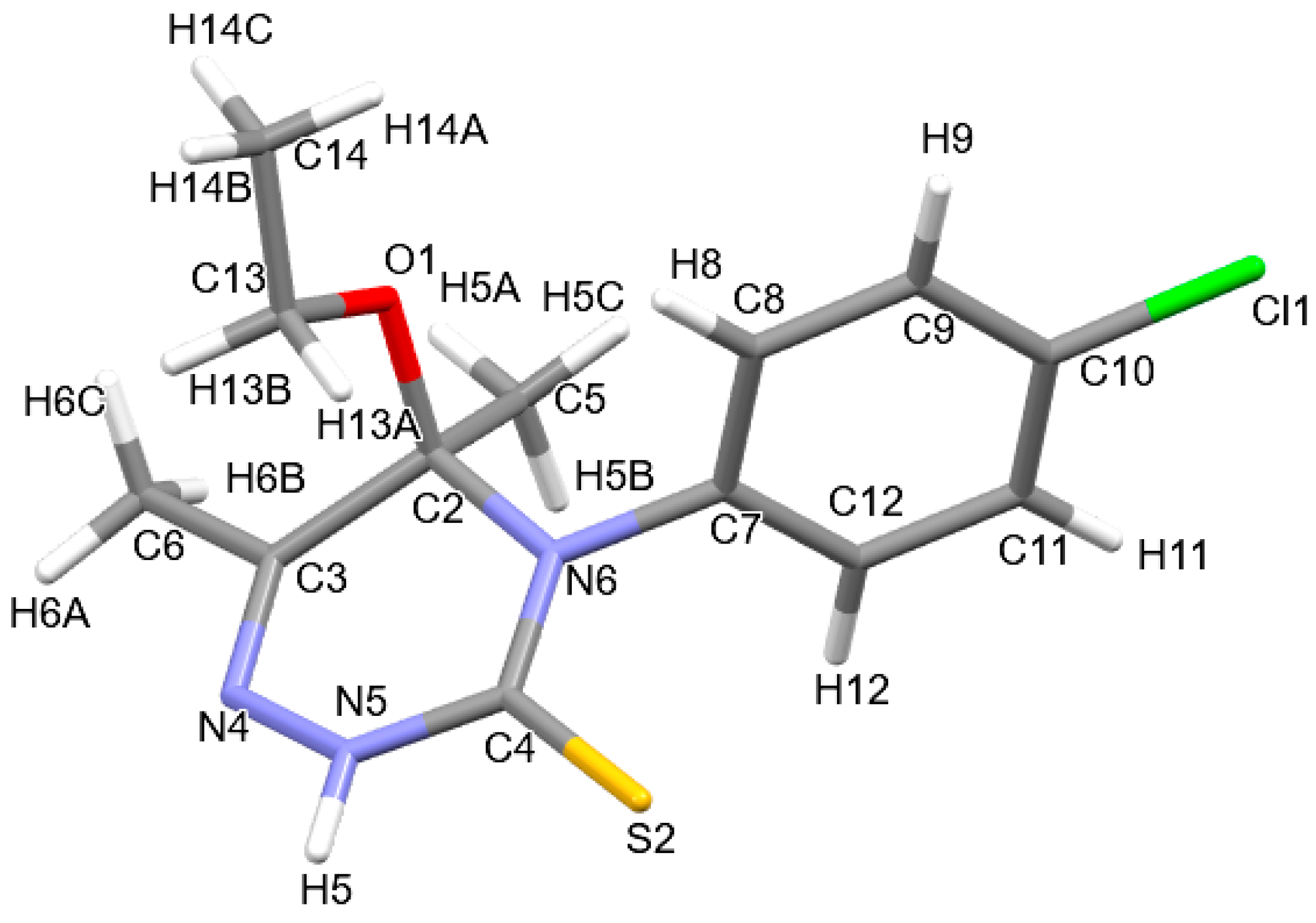
2.4. Crystal Structures
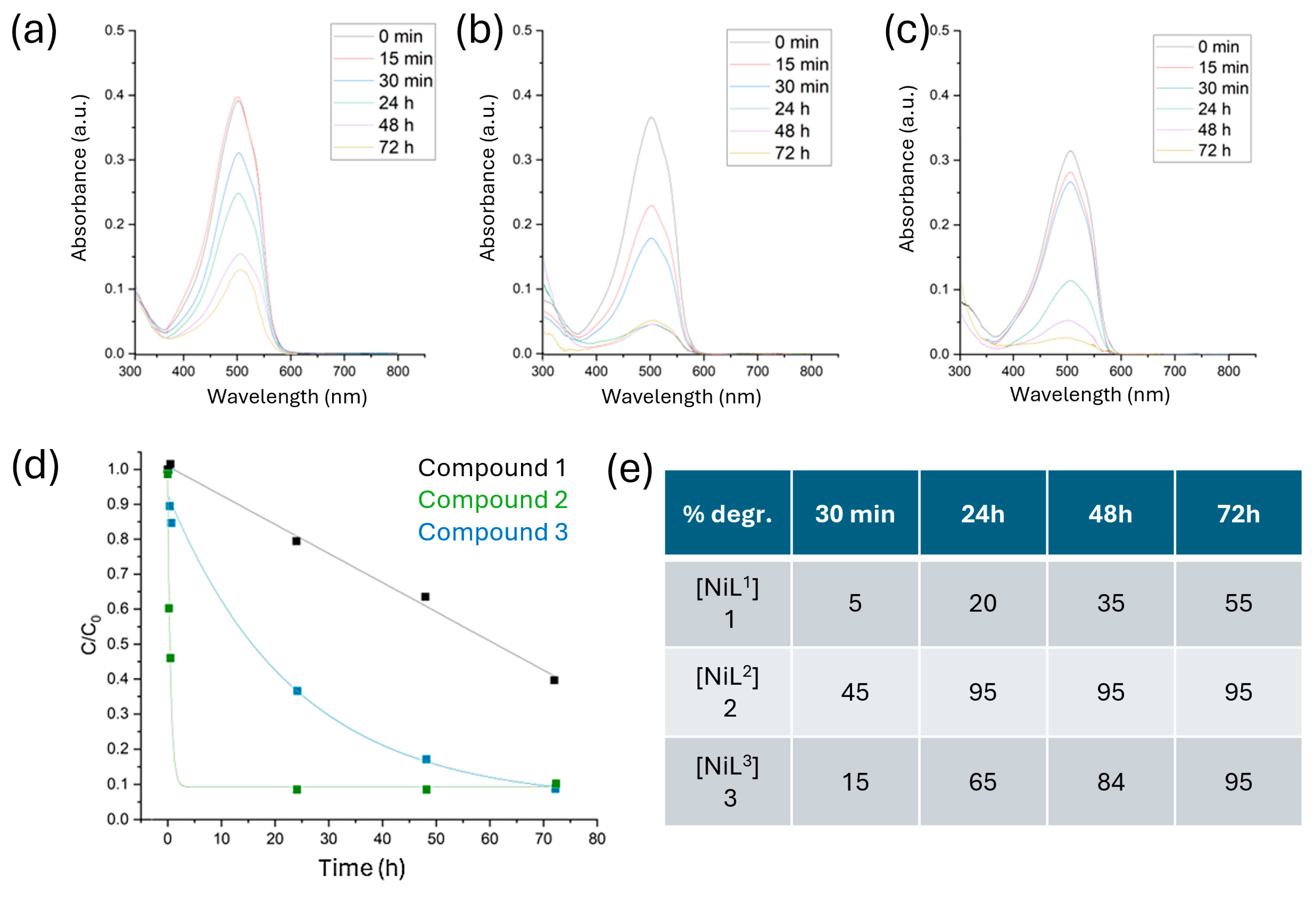
2.5. Photocatalytic Degradation of MO
2.6. Theoretical Calculations
2.7. Photocatalytic Water Splitting
3. Experimental Section
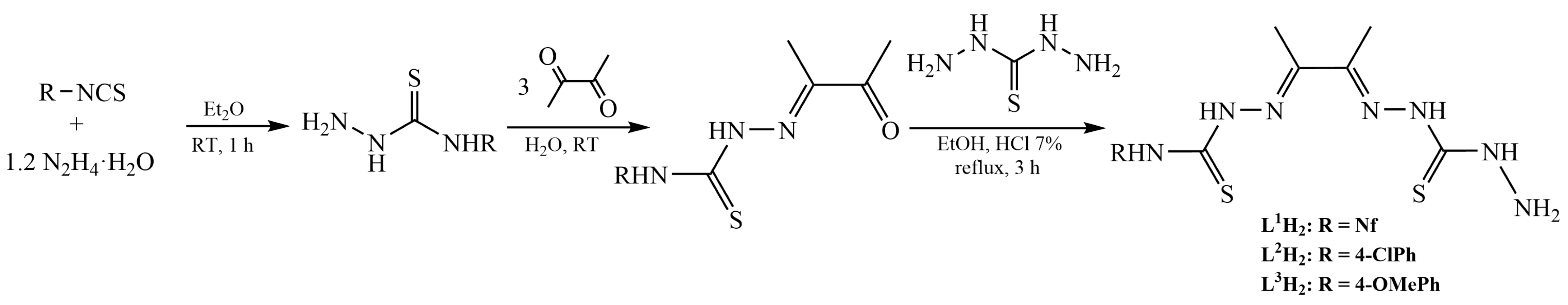
3.1. Synthesis of the Organic Compounds
3.2. Synthesis of the Coordination Compounds
3.3. Computational Studies
3.4. Testing of the Photocatalytic Activity for the Degradation of Methyl Orange
3.5. Testing of the Photocatalytic Activity for Water Splitting
3.6. Crystallographic Data and Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Drinking-Water, (n.d.). Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 18 December 2024).
- Akika, F.Z.; Rouibah, K.; Benamira, M.; Avramova, I. Elaboration of the new heterostructure of NiAl2O4/kaolin and its photocatalytic activity towards methyl green under solar light irradiation. Inorg. Chem. Commun. 2023, 154, 110878. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 714, 137222. [Google Scholar] [CrossRef]
- Matsuyama, K.; Kawahara, Y.; Shoji, A.; Kato, T.; Okuyama, T. Supercritical CO2-assisted formation of metal-organic framework-loaded porous polystyrene membranes for dye removal. J. Appl. Polym. Sci. 2023, e54347. [Google Scholar] [CrossRef]
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: A review of recent developments. RSC Adv. 2023, 13, 19335. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipouya, S.; Mousavi, S.A.; Shokrgozar, A.; Mousavi, D.V. Improving dye removal and antifouling performance of polysulfone nanofiltration membranes by incorporation of UiO-66 metal-organic framework. J. Environ. Chem. Eng. 2022, 10, 107535. [Google Scholar] [CrossRef]
- Barragán, B.E.; Costa, C.; Márquez, M.C. Biodegradation of azo dyes by bacteria inoculated on solid media. Dyes Pigment. 2007, 75, 73. [Google Scholar] [CrossRef]
- Au, V.K.-M.; Kwan, S.Y.; Lai, M.N.; Low, K.-H. Dual-functional mesoporous copper(II) metal-organic frameworks for the remediation of organic dyes. Chem. Eur. J. 2021, 27, 9174. [Google Scholar] [CrossRef] [PubMed]
- Rizala, M.Y.; Saleha, R.; Taufik, A. Characterization and photocatalytic activity of Ag/Mn3O4/graphene composites under visible light irradiation for organic dyes degradation. J. Environ. Chem. Eng. 2020, 8, 103610. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Passino, R.; Agostiano, A. UV-induced photocatalytic degradation of azo dyes by organic-capped ZnO nanocrystals immobilized onto substrates. Appl. Catal. B-Environ. 2005, 55, 81. [Google Scholar] [CrossRef]
- Carvalho, S.S.F.; Rodrigues, A.C.C.; Lima, J.F.; Carvalho, N.M.F. Photocatalytic degradation of dyes by mononuclear copper(II) complexes from bis-(2-pyridylmethyl)amine NNN-derivative ligands. Inorg. Chim. Acta 2020, 512, 119924. [Google Scholar] [CrossRef]
- Jennifer, S.J.; Jana, A.K. Influence of pyrazine/piperazine based guest molecules in the crystal structures of uranyl thiophene dicarboxylate coordination polymers: Structural diversities and photocatalytic activities for the degradation of organic dye. Cryst. Growth Des. 2017, 17, 5318. [Google Scholar] [CrossRef]
- Ghosh, K.; Harms, K.; Franconetti, A.; Frontera, A.; Chattopadhyay, S. A triple alkoxo bridged dinuclear cobalt(III) complex mimicking phosphatase and showing ability to degrade organic dye contaminants by photocatalysis. J. Organomet. Chem. 2019, 883, 52. [Google Scholar] [CrossRef]
- Bajaj, K.; Buchanan, R.M.; Grapperhaus, C.A. Antifungal activity of thiosemicarbazones, bis(thiosemicarbazones), and their metal complexes. J. Inorg. Biochem. 2021, 225, 111620. [Google Scholar] [CrossRef] [PubMed]
- Miglioli, F.; De Franco, M.; Bartoli, J.; Scaccaglia, M.; Pelosi, G.; Marzano, C.; Rogolino, D.; Gandin, V.; Carcelli, M. Anticancer activity of new water-soluble sulfonated thiosemicarbazone copper(II) complexes targeting disulfide isomerase. Eur. J. Inorg. Chem. 2024, 276, 116697. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Galván-Hidalgo, J.M.; Pérez-Cuéllar, G.; Huerta-Landa, K.A.; González-Hernández, A.; Gómez-García, O.; Andrade-Pavón, D.; Ramírez-Apan, T.; Hernández, K.D.R.; Hernández, S.; et al. New Organotin (IV) compounds derived from dehydroacetic acid and thiosemicarbazides: Synthesis, rational design, cytotoxic evaluation, and molecular docking simulation. Bioinorg. Chem. Appl. 2023, 2023, 901843. [Google Scholar] [CrossRef] [PubMed]
- Parrilha, G.L.; Santos, R.G.D.; Beraldo, H. Applications of radiocomplexes with thiosemicarbazones and bis(thiosemicarbazones) in diagnostic and therapeutic nuclear medicine. Coord. Chem. Rev. 2022, 458, 214418. [Google Scholar] [CrossRef]
- Murugan, K.; Vijayapritha, S.; Viswanathamurthi, P.; Saravanan, K.; Vijayan, P.; Ojwach, S.O. Ru (II) complexes containing (2-(pyren-1-ylmethylene) hydrazinyl) benzothiazole: Synthesis, solid-state structure, computational study and catalysis in N-alkylation reactions. Inorg. Chim. Acta 2020, 512, 119864. [Google Scholar] [CrossRef]
- Ravindran, A.; Sindhuja, D.; Bhuvanesh, N.; Karvembu, R. Synthesis of 1, 2-Disubstituted Benzimidazoles via Acceptorless Dehydrogenative Coupling Using Ru (II)-Arene Catalysts Containing Ferrocene Thiosemicarbazone. Eur. J. Inorg. Chem. 2022, 2022, e202200181. [Google Scholar] [CrossRef]
- Haddad, A.Z.; Cronin, S.P.; Mashuta, M.S.; Buchanan, R.M.; Grapperhaus, C.A. Metal-assisted ligand-centered electrocatalytic hydrogen evolution upon reduction of a bis(thiosemicarbazonato)Cu(II) complex. Inorg. Chem. 2017, 56, 11254. [Google Scholar] [CrossRef] [PubMed]
- Straistari, T.; Hardré, R.; Fize, J.; Shova, S.; Giorgi, M.; Réglier, M.; Artero, V.; Orio, M. Hydrogen evolution reactions catalyzed by a bis(thiosemicarbazone) cobalt complex: An experimental and theoretical study. Chem. Eur. J. 2018, 24, 8779. [Google Scholar] [CrossRef]
- Barrozo, A.; Orio, M. Unraveling the catalytic mechanisms of H2 production with thiosemicarbazone nickel complexes. RSC Adv. 2021, 11, 5232. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Tapias, B.; Halder, S.; Mendiola, M.A.; Roy, N.; Sahu, N.; Sinha, C.; Jana, K.; López-Torres, E. New tin(IV) and organotin(IV) complexes with a hybrid thiosemicarbazone/hydrazone ligand: synthesis, crystal structure and antiproliferative activity. Bioinorg. Chem. Appl. 2024, 2024, 1018375. [Google Scholar] [CrossRef]
- Huedo, C.; Zani, F.; Mendiola, M.A.; Pradhan, S.; Sinha, C.; López-Torres, E. Synthesis, antimicrobial activity and molecular docking of di- and triorganotin(IV) derivatives with thiosemicarbazide derivatives. Appl. Organomet. Chem. 2019, 33, e4700. [Google Scholar] [CrossRef]
- Papadakis, M.; Mehrez, J.; Wehrung, I.; Delmotte, L.; Giorgi, M.; Hardré, R.; Orio, M. Stereochemical tailoring of nickel-based electrocatalysts for hydrogen evolution reaction. ChemCatChem 2024, 16, e202400426. [Google Scholar] [CrossRef]
- Phipps, C.A.; Hofsommer, D.T.; Toda, M.J.; Nkurunziza, F.; Shah, B.; Spurgeon, J.M.; Kozlowski, P.M.; Buchanan, R.M.; Grapperhaus, C.A. Ligand-centered hydrogen evolution with Ni(II) and Pd(II)DMTH. Inorg. Chem. 2022, 61, 9792. [Google Scholar] [CrossRef]
- Barrozo, A.; Orio, M. From ligand- to metal-centered reactivity: Metal substitution effect in thiosemicarbazone-based complexes for H2 production. ChemPhysChem 2022, 23, e202200056. [Google Scholar] [CrossRef]
- Burón, R.; Jiménez-Gómez, D.; Calatayud, D.G.; Iglesias-Juez, A.; Fresno, F.; Mendiola, M.A.; López-Torres, E. Synthesis, characterization, and photocatalytic activity for water remediation and hydrogen evolution of Zn(II) and Ni(II) bis(thiosemicarbazone) complexes. Appl. Organomet. Chem. 2024, 38, e7408. [Google Scholar] [CrossRef]
- Bilyj, J.K.; Riley, M.J.; Bernhardt, P.V. Isomerism and reactivity of nickel(II) acetylacetonate bis(thiosemicarbazone) complexes. Dalton Trans. 2018, 47, 2018. [Google Scholar] [CrossRef]
- González-García, C.; García-Pascual, C.; Burón, R.; Calatayud, D.G.; Perles, J.; Mendiola, M.A.; López-Torres, E. Structural variety, fluorescence and photocatalytic activity of dissymmetric thiosemicarbazone complexes. Polyhedron 2022, 223, 115945. [Google Scholar] [CrossRef]
- Abrosimov, R.; Moosmann, B. The HOMO-LUMO Gap as Discriminator of Biotic from Abiotic Chemistries. Life 2024, 14, 1330. [Google Scholar] [CrossRef] [PubMed]
- Shittu, F.B.; Iqbal, A.; Ahmad, M.N.; Yusop, M.R.; Ibrahim, M.N.M.; Sabare, S.; Wilson, L.D.; Yantom, D.H.Y. Insight into the photodegradation mechanism of bisphenol-A by oxygen doped mesoporous carbon nitride under visible light irradiation and DFT calculations. RSC Adv. 2022, 12, 10409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-X.; Xie, Z.-L.; Zhan, S.-Z. A photocatalytic system with a bis (thiosemicarbazonato)-nickel over CdS nanorods for hydrogen evolution from water under visible light. Inorg. Chem. Commun. 2019, 102, 5. [Google Scholar] [CrossRef]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E.; et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
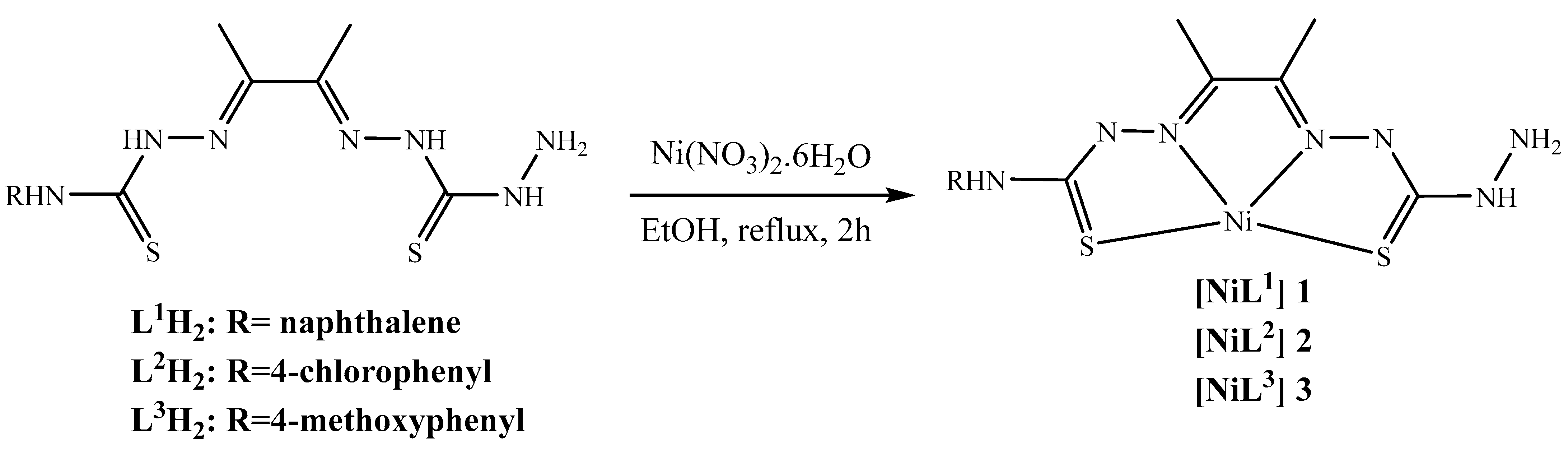
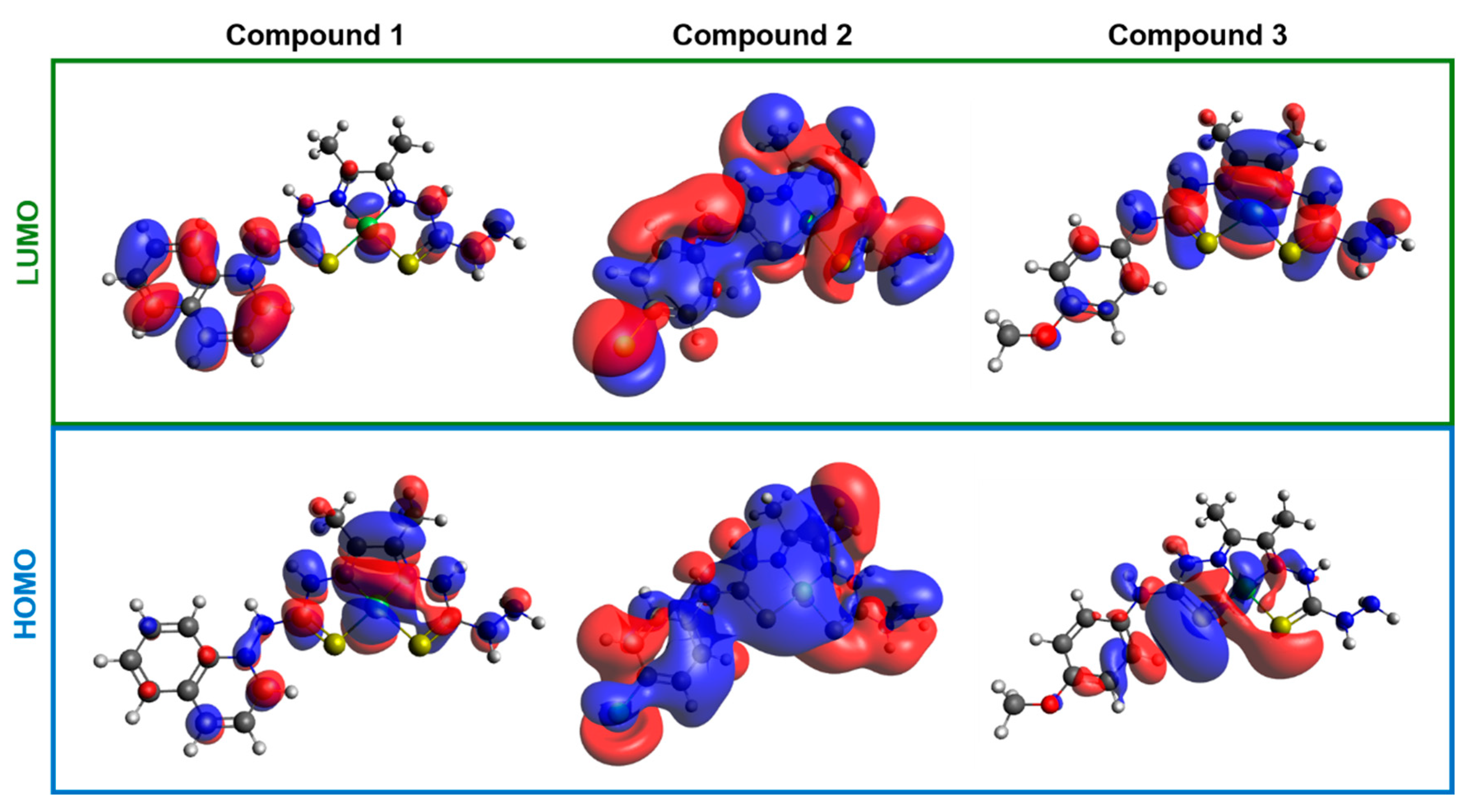
| Complex | EHOMO | MO Composition | ELUMO | MO Composition | H–L Gap |
|---|---|---|---|---|---|
| 1 | −9.061 | 75% ligand 25% Ni | −3.354 | 95% ligand 5% Ni | 5.707 |
| 2 | −3.924 | 50% ligand 50% Ni | −2.432 | 50% ligand 50% Ni | 1.492 |
| 3 | −3.237 | 85% ligand 15% Ni | −2.265 | 50% ligand 50% Ni | 0.972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burón, R.; Calatayud, D.G.; Mendiola, M.A.; López-Torres, E. Unsymmetrical Bis(thiosemicarbazone) Ligands and Their Nickel(II) Complexes: Synthesis, Characterization and Photocatalytic Activity. Inorganics 2025, 13, 40. https://doi.org/10.3390/inorganics13020040
Burón R, Calatayud DG, Mendiola MA, López-Torres E. Unsymmetrical Bis(thiosemicarbazone) Ligands and Their Nickel(II) Complexes: Synthesis, Characterization and Photocatalytic Activity. Inorganics. 2025; 13(2):40. https://doi.org/10.3390/inorganics13020040
Chicago/Turabian StyleBurón, Rodrigo, David G. Calatayud, M. A. Mendiola, and Elena López-Torres. 2025. "Unsymmetrical Bis(thiosemicarbazone) Ligands and Their Nickel(II) Complexes: Synthesis, Characterization and Photocatalytic Activity" Inorganics 13, no. 2: 40. https://doi.org/10.3390/inorganics13020040
APA StyleBurón, R., Calatayud, D. G., Mendiola, M. A., & López-Torres, E. (2025). Unsymmetrical Bis(thiosemicarbazone) Ligands and Their Nickel(II) Complexes: Synthesis, Characterization and Photocatalytic Activity. Inorganics, 13(2), 40. https://doi.org/10.3390/inorganics13020040










