Electrochemical Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxaldehyde Using Mn(III)–Schiff Base Catalysts
Abstract
1. Introduction
2. Results
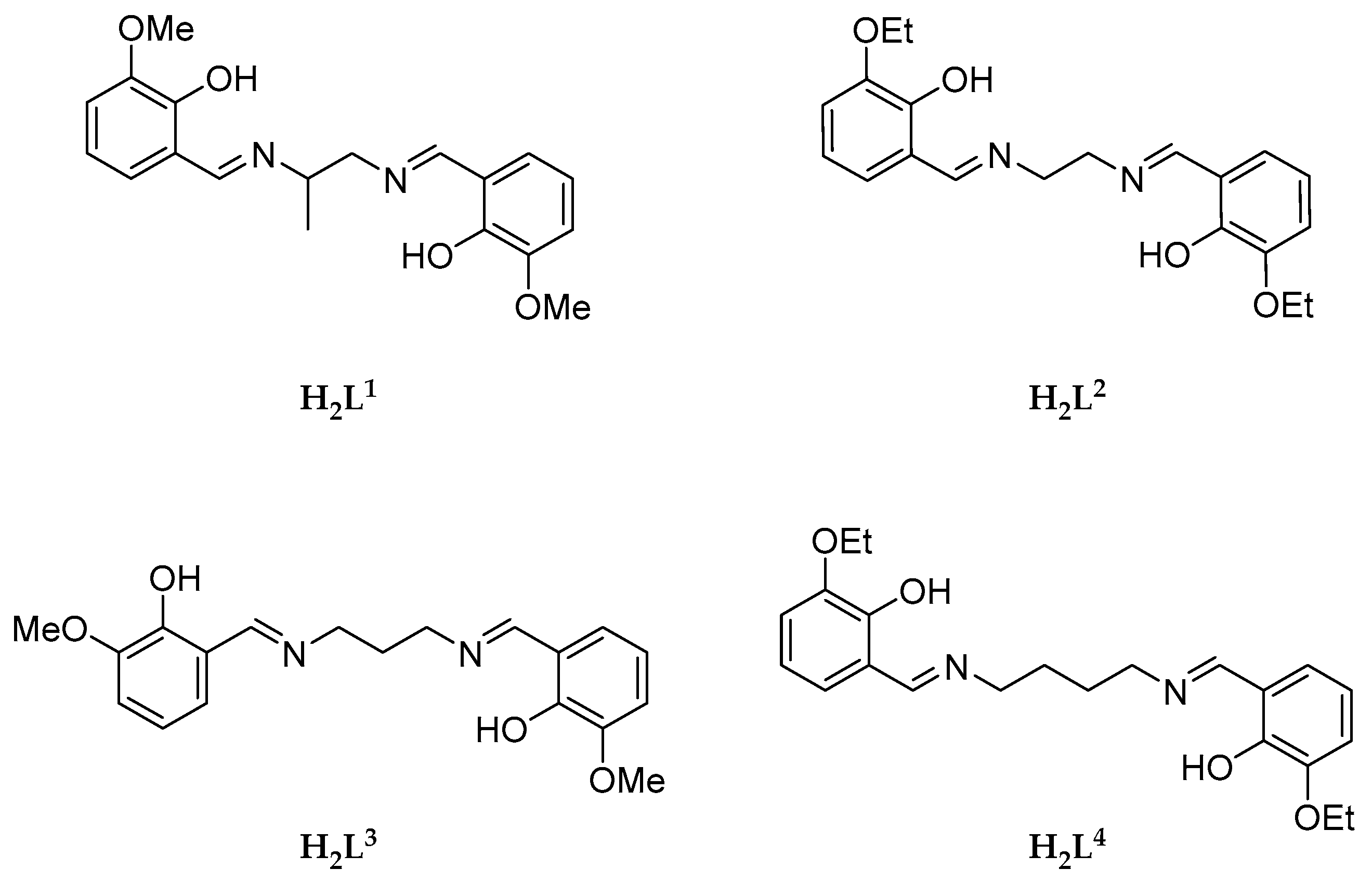
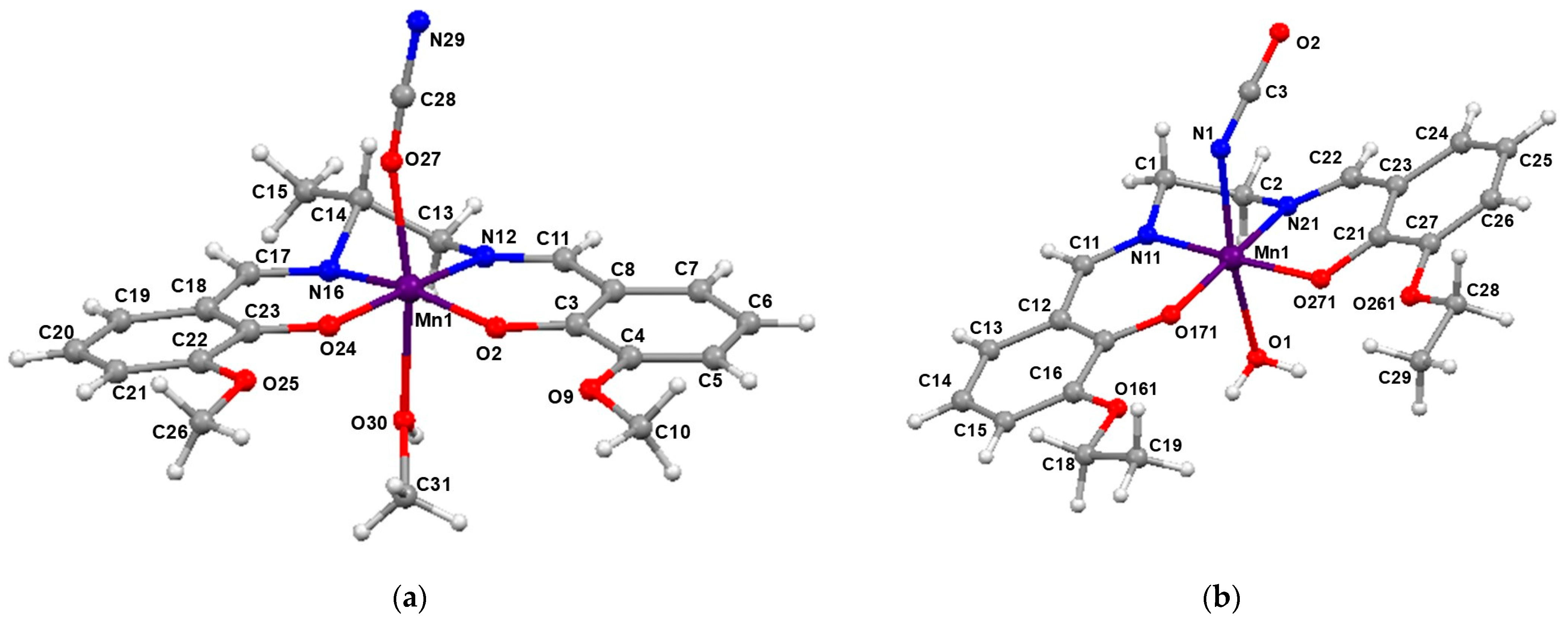
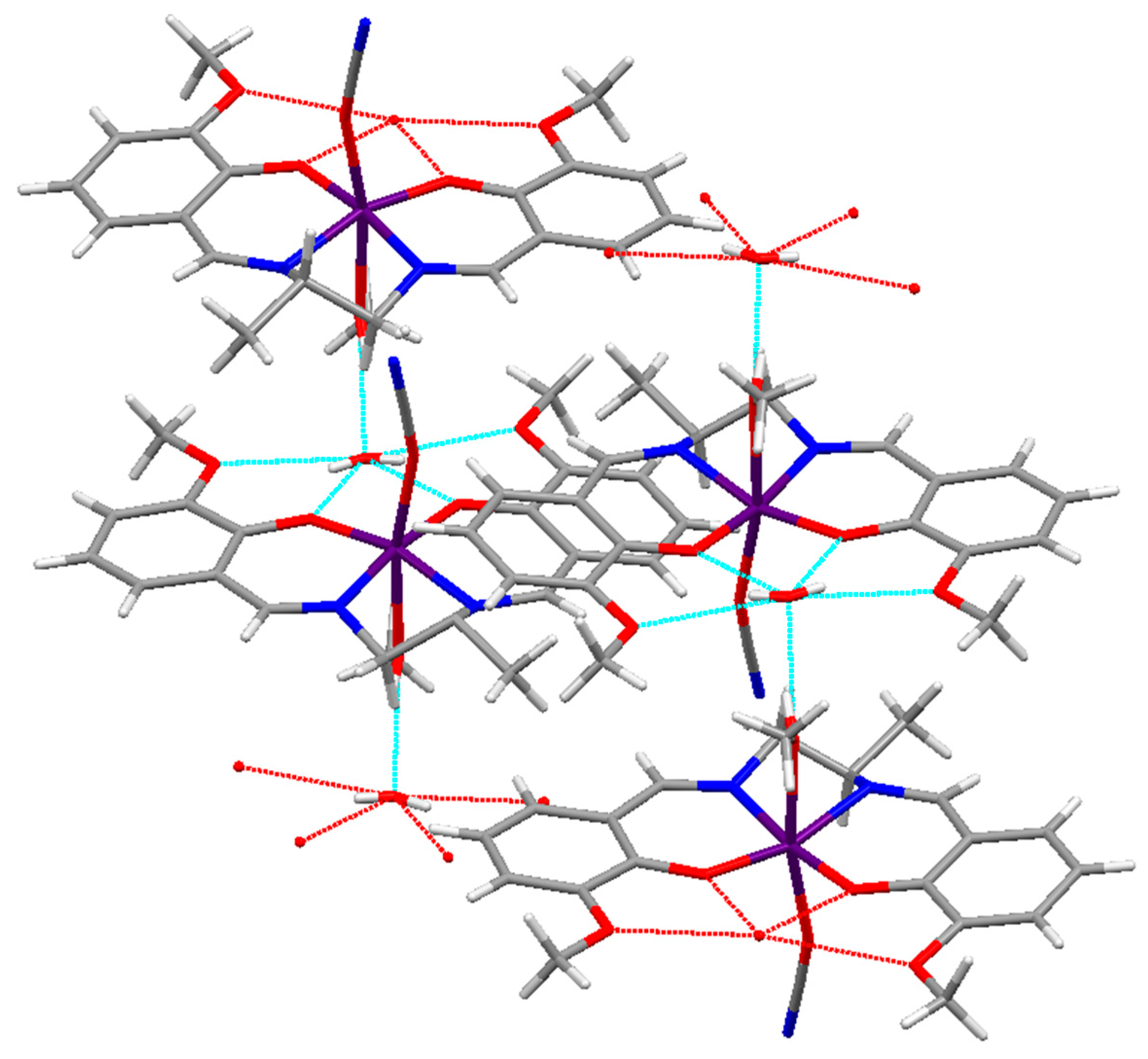
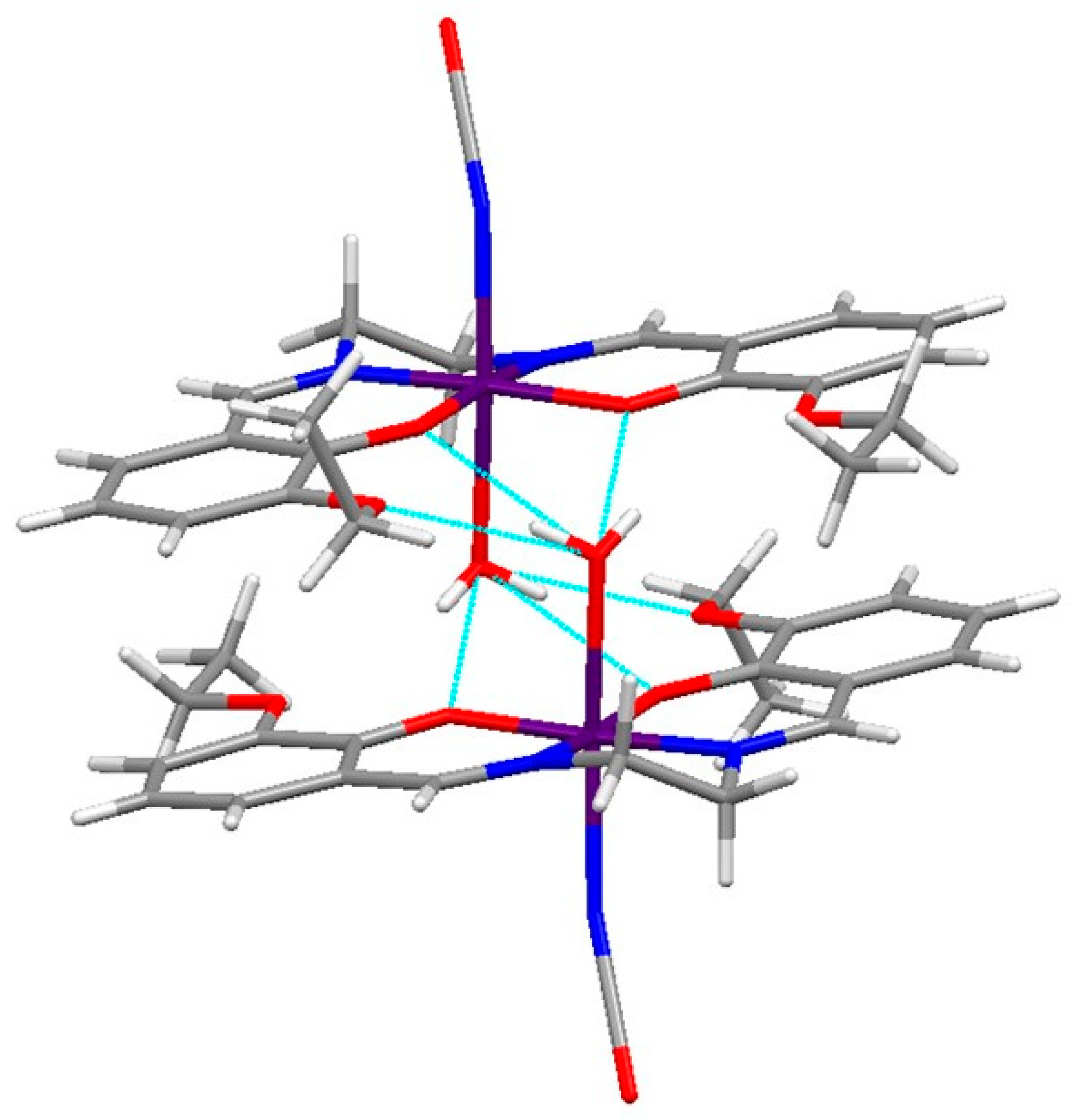
2.1. Characterization of the Manganese Complexes
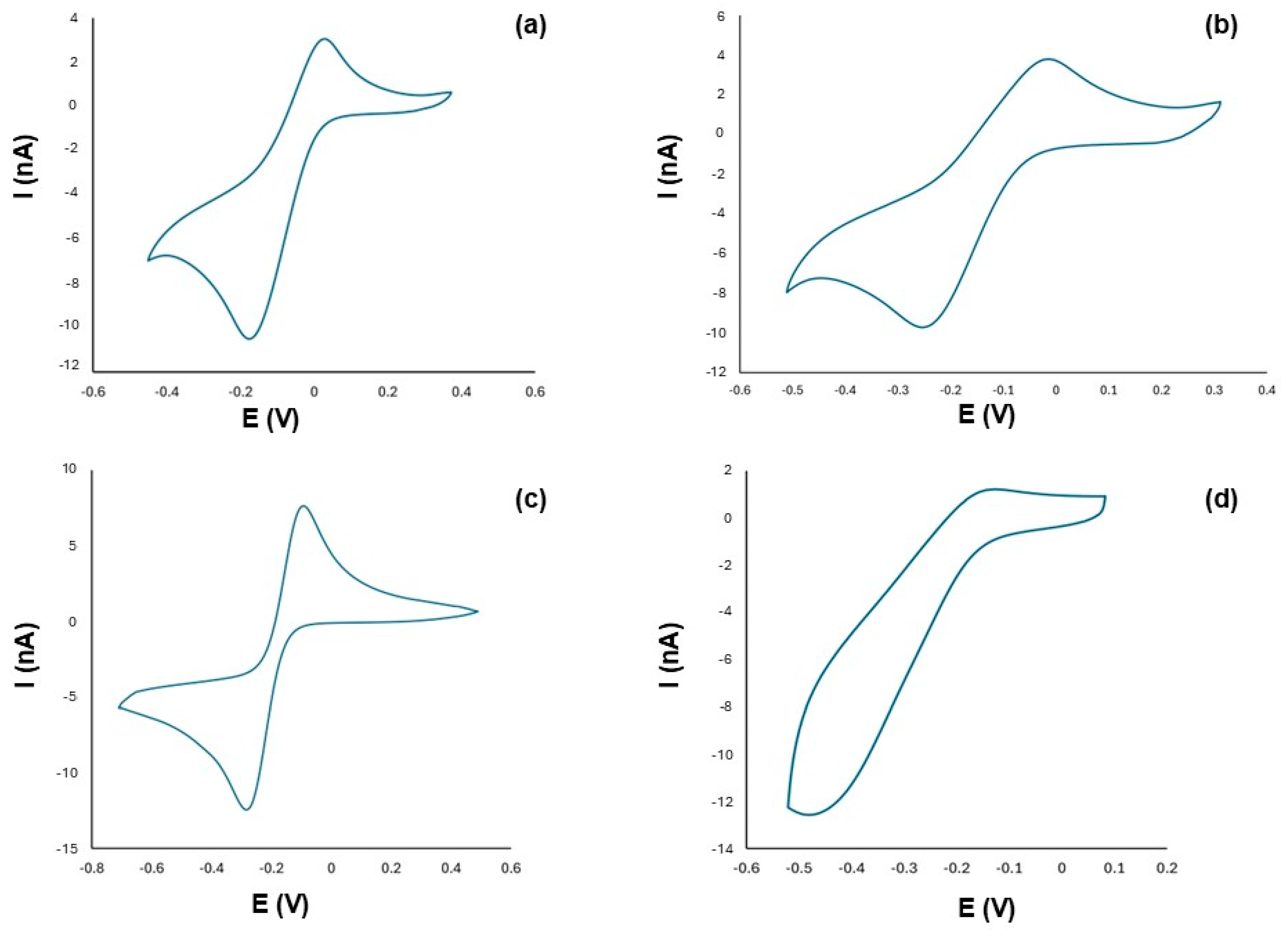
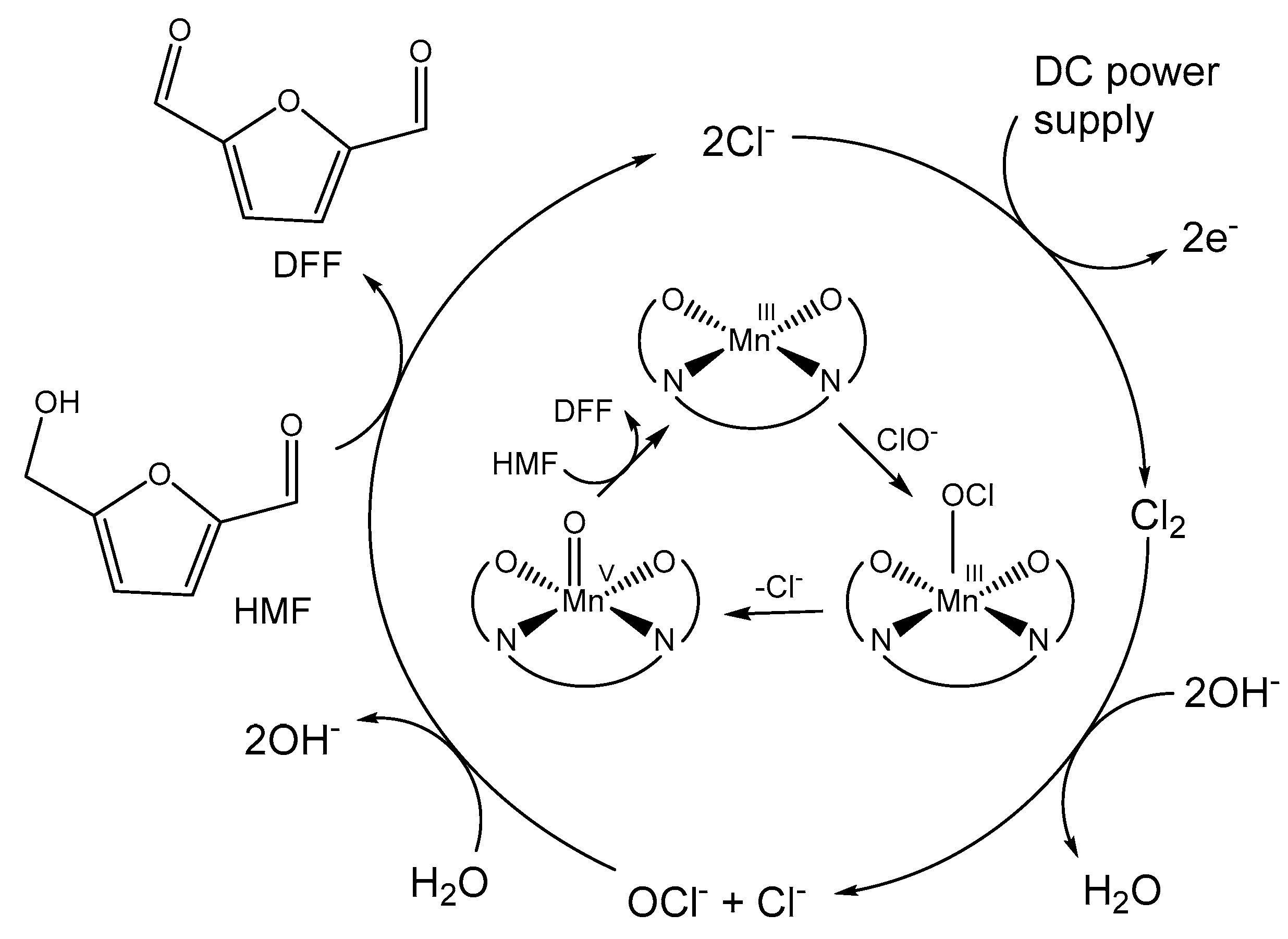
2.2. Electrochemical and Catalytic Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Complexes
4.3. Peroxidase-Like Function of the Complexes
4.4. Electrochemical Oxidations of 5-Hydroxymethylfurfural
4.5. Crystallographic Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sousa, A.F.; Silvestre, A.J.D. Plastics from renewable sources as green and sustainable alternatives. Curr. Op. Green. Sustain. Chem. 2022, 33, 100557. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Nikolaidis, G.N.; Efstathiadou, V.L.; Kapnisti, M.; Papageorgiou, G.Z. Blending as a process for controlling the properties of poly (ethylene 2,5-furandicarboxylate) (PEF): Fully biobased PEF/PBF blends. Polymer 2023, 266, 125615. [Google Scholar] [CrossRef]
- Xanthopoulou, E.; Zamboulis, A.; Terzopoulou, Z.; Bikiaris, D.N.; Kourtidou, D.; Tarani, E.; Chrissafis, K.; Papageorgiou, G.Z. Towards novel lignin-based aromatic polyesters: In-depth study of the thermal degradation and crystallization of poly (propylene vanillate). Thermochim. Acta 2022, 709, 179145. [Google Scholar] [CrossRef]
- Kammoun, M.; Margellou, A.; Toteva, V.B.; Aladjadjiyan, A.; Sousa, A.F.; Luis, S.V.; Garcia-Verdugo, E.; Triantafyllidis, K.S.; Richel, A. The key role of pretreatment for the one-step and multi-step conversions of European lignocellulosic materials into furan compounds. RSC Adv. 2023, 13, 21587. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Montes, M.L.; Soccio, M.; Siracusa, V.; Gazzano, M.; Lotti, N.; Cyras, V.P.; Manfredi, L.B. Chitin Nanocomposite Based on Plasticized Poly (lactic acid)/Poly (3-hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials. Polymers 2022, 14, 3177. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Tsetsou, I.; Ioannidis, R.O.; Nikolaidis, G.N.; Exarhopoulos, S.; Kasmi, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. A New Era in Engineering Plastics: Compatibility and Perspectives of Sustainable Alipharomatic Poly (ethylene terephthalate)/Poly (ethylene 2,5-furandicarboxylate) Blends. Polymers 2021, 13, 1070. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.F.; Patrício, R.; Terzopoulou, Z.; Bikiaris, D.N.; Stern, T.; Wenger, J.; Loos, K.; Lotti, N.; Siracusa, V.; Szymczyk, A.; et al. Recommendations for replacing PET on packaging, fiber, and film materials with biobased counterparts. Green Chem. 2021, 23, 8795. [Google Scholar] [CrossRef]
- Wu, X.; Galkin, M.V.; Stern, T.; Sun, Z.; Barta, K. Fully lignocellulose-based PET analogues for the circular economy. Nat. Commun. 2022, 13, 3376. [Google Scholar] [CrossRef]
- Kayishaer, A.; Annatelli, M.; Hansom, C.M.; Mouterde, L.M.M.; Peru, A.A.M.; Aricò, F.; Allais, F.; Fadlallah, S. Green Synthesis of UV-Reactive Polycarbonates from Levoglucosenone and 5-Hydroxymethyl Furfural. Macromol. Rapid Commun. 2024, 45, 2300483. [Google Scholar] [CrossRef]
- Trapasso, G.; Annatelli, M.; Dalla Torre, D.; Aricò, F. Synthesis of 2,5-furandicarboxylic acid dimethyl ester from galactaric acid via dimethyl carbonate chemistry. Green Chem. 2022, 24, 2766. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Lotti, N. New Random Aromatic/Aliphatic Copolymers of 2,5-Furandicarboxylic and Camphoric Acids with Tunable Mechanical Properties and Exceptional Gas Barrier Capability for Sustainable Mono-Layered Food Packaging. Molecules 2023, 28, 4056. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Dussin, A.; Xanthopoulou, E.; Bikiaris, D.N.; Botta, L.; Fiore, V.; Pegoretti, A. Compatibilization of Polylactide/Poly (ethylene 2,5-furanoate) (PLA/PEF) Blends for Sustainable and Bioderived Packaging. Molecules 2022, 27, 6371. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558. [Google Scholar] [CrossRef]
- Bianchi, E.; Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Salatelli, E.; Lotti, N. Biobased and Compostable Multiblock Copolymer of Poly (l-lactic acid) Containing 2,5-Furandicarboxylic Acid for Sustainable Food Packaging: The Role of Parent Homopolymers in the Composting Kinetics and Mechanism. Biomacromolecules 2023, 24, 2356. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Tuning the Properties of Furandicarboxylic Acid-Based Polyesters with Copolymerization: A Review. Polymers 2020, 12, 1209. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Klonos, P.A.; Kluge, M.; Zamboulis, A.; Terzopoulou, Z.; Kourtidou, D.; Magaziotis, A.; Chrissafis, K.; Kyritsis, A.; Bikiaris, D.N.; et al. Unlocking the potential of furan-based poly (ester amide)s: An investigation of crystallization, molecular dynamics and degradation kinetics of novel poly (ester amide)s based on renewable poly(propylene furanoate). Polym. Chem. 2021, 12, 5518. [Google Scholar] [CrossRef]
- Bouyahya, C.; Patrício, R.; Paço, A.; Lima, M.S.; Fonseca, A.C.; Rocha-Santos, T.; Majdoub, M.; Silvestre, A.J.D.; Sousa, A.F. Isosorbide and 2,5-Furandicarboxylic Acid Based (Co)Polyesters: Synthesis, Characterization, and Environmental Degradation. Polymers 2022, 14, 3868. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Colmenares, J.C.; Tsiplakides, D.; Triantafyllidis, K.S. Nanoengineered Electrodes for Biomass-Derived 5-Hydroxymethylfurfural Electrocatalytic Oxidation to 2,5-Furandicarboxylic Acid. ACS Sustain. Chem. Eng. 2021, 9, 1970. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Nikolaidis, G.N.; Ionannidis, R.O.; Rinis, K.; Papageorgiou, D.G.; Klonos, P.A.; Achilias, D.S.; Kapnisti, M.; Terzopoulou, Z.; Bikiaris, D.N. A Step Forward in Thermoplastic Polyesters: Understanding the Crystallization and Melting of Biobased Poly (ethylene 2,5-furandicarboxylate) (PEF). ACS Sustain. Chem. Eng. 2022, 10, 7050. [Google Scholar] [CrossRef]
- Demet, A.E.; Gimello, O.; Arletti, R.; Tanchoux, N.; Sougrati, M.T.; Stievano, L.; Quignard, F.; Centi, G.; Perathoner, S.; Di Renzo, F. 5-Hydroxymethylfurfural Oxidation to 2,5-Furandicarboxylic Acid on Noble Metal-Free Nanocrystalline Mixed Oxide Catalysts. Catalysts 2022, 12, 814. [Google Scholar] [CrossRef]
- Jung, S.; Kim, K.S.; Park, G.H.; Cha, H.G.; Jeong, H.; Kang, M.J. Strategies on utilizing biomass derived 5-hydroxymethylfurfural by catalytic reactions: Pathways and mechanisms. Mat. Today Sustain. 2025, 29, 101058. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, L.; Yu, X.; Zhang, S.; Chen, G. Co-Immobilization of Tri-Enzymes for the Conversion of Hydroxymethylfurfural to 2,5-Diformylfuran. Molecules 2019, 24, 3648. [Google Scholar] [CrossRef] [PubMed]
- Le, D.D.; Nguyen, T.H.; Phan, H.; Tran, P.H. A highly efficient, green, and straightforward approach for 2,5-diformyl-furan synthesis from carbohydrates using carbonized sugarcane bagasse and KBr. Appl. Catal. A Gen. 2023, 662, 119265. [Google Scholar] [CrossRef]
- Tjallins, G.; Boverio, A.; Jager, A.W.; Kaya, S.G.; Mattevi, A.; Fraaije, M.W. Efficient Oxidation of 5-Hydroxymethylfurfural Using a Flavoprotein Oxidase from the Honeybee Apis mellifera. ChemBioChem 2023, 24, e202300588. [Google Scholar] [CrossRef]
- Pal, P.; Saravanamurugan, S. Heterostructured manganese catalysts for the selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. ChemCatChem 2020, 12, 2324. [Google Scholar] [CrossRef]
- Lai, J.H.; Zhou, S.L.; Cheng, F.; Guo, D.W.; Liu, X.X.; Xu, Q.; Yin, D.L. Efficient and Selective Oxidation of 5-Hydroxymethylfurfural into 2,5-Diformylfuran Catalyzed by Magnetic Vanadium-Based Catalysts with Air as Oxidant. Catal. Lett. 2020, 150, 1301. [Google Scholar] [CrossRef]
- Raut, A.B.; Shende, V.S.; Bhanage, B.M. The one-step transformation of fructose to 2,5-diformylfuran over Ru metal supported on montmorillonite. New J. Chem. 2020, 44, 13659. [Google Scholar] [CrossRef]
- Mahendran, S.; Srinivasan, W.; Karthikeyan, G.; Pachamuthu, M.P. Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over niobium incorporated MCM-41 catalyst. Mol. Catal. 2021, 510, 111682. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, B.J.; Wang, S.P.; Li, M.C.; Li, C.M.; Shen, Z.L. Selective oxidation of biomass-based 5-hydroxymethylfurfural to 2,5-diformylfuran catalyzed by multicomponent molybdenum-based catalyst. J. Chem. Tech. Biotech. 2022, 97, 2487. [Google Scholar] [CrossRef]
- Ding, S.P.; Gabriel, J.B.; Peppel, T.; Haida, S.; Rabeah, J.; Steinfeldt, N.; Strunk, J. Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran with ZnIn2S4 2D nanosheets and atmospheric O2 under visible light. Sustain. Energy Fuels 2023, 7, 4396. [Google Scholar] [CrossRef]
- Pang, Y.J.; Chen, N.; Zhao, Z.Z.; Zhang, L.; Broekman, J.O.P.; Wei, J.N.; Li, X.J.; Lin, L.; Huang, H. A unique air-assisted DMSO oxidation pathway for the highly efficient synthesis of 2,5-diformylfuran from 5-hydroxymethylfurfural/fructose. Green Chem. 2023, 25, 9680. [Google Scholar] [CrossRef]
- Xu, X.W.; Shi, J.H.; Sun, Q.; Cao, Q.; She, Y.B.; Li, M.C.; Shen, Z.L. High-efficiency synthesis of 2,5-diformylfuran from 5-hydroxymethylfurfural using iron-bismuth-molybdenum oxides. Mol. Catal. 2025, 570, 114681. [Google Scholar] [CrossRef]
- Qu, D.; He, S.; Chen, L.; Ye, Y.; Ge, Q.; Cong, H.; Jiang, N.; Ha, Y. Paired electrocatalysis in 5-hydroxymethylfurfural valorization. Front. Chem. 2022, 10, 1055865. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Jiang, N.; Sun, Y. A General Strategy for Decoupled Hydrogen Production from Water Splitting by Integrating Oxidative Biomass Valorization. J. Am. Chem. Soc. 2016, 138, 13639. [Google Scholar] [CrossRef]
- Cha, H.G.; Choi, K.-S. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nat. Chem. 2015, 7, 328. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, X.; Yang, Y.; Liu, Y.; Liu, Y.; Zhao, L.; Wang, P.; Zhang, Y.; Lin, Y.; Wei, Y. Enhancing the Electrocatalytic Oxidation of 5-Hydroxymethylfurfural Through Cascade Structure Tuning for Highly Stable Biomass Upgrading. Nano-Micro Lett. 2024, 16, 275. [Google Scholar] [CrossRef]
- Yang, Z.; Hui, J.; Fan, W.; Liu, P.; Zhang, C.; Dong, S.; Yang, Z. Selenate-based heterojunction with cobalt–nickel paired site for electrocatalytic oxidation of 5-hydroxymethylfurfural coupling water splitting to produce hydrogen. J. Energy Chem. 2025, 101, 156. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, Z.; Wang, C. Construction of an Sc-NiFe-LDH electrocatalyst for highly efficient electrooxidation of 5-hydroxymethylfurfural at industrial current density. Nanoscale 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, F.; Wang, G.; Li, T.-T.; Liu, Z.; Wang, Y. Low-Crystalline Cobalt Iron Oxide-Supported Single Ru Atoms and Ru Clusters for 2,5-Hydroxymethylfurfural Electro-Oxidation Coupled with Hydrogen Evolution. ACS Sustain. Chem. Eng. 2024, 12, 11767. [Google Scholar] [CrossRef]
- Rouco, L.; González-Noya, A.M.; Pedrido, R.; Maneiro, M. Pursuing the Elixir of Life: In vivo antioxidative effects of manganosalen complexes. Antioxidants 2020, 9, 727. [Google Scholar] [CrossRef]
- Liu, W.; Groves, J.T. Manganese porphyrins catalyze selective C−H bond halogenations. J. Am. Chem Soc. 2010, 132, 12847. [Google Scholar] [CrossRef]
- Palopoli, C.; Duhayon, C.; Tuchagues, J.P.; Signorella, S. Synthesis, characterization, and reactivity studies of a water-soluble bis (alkoxo)(carboxylato)-bridged diMn III complex modeling the active site in catalase. Dalton Trans. 2014, 43, 17145. [Google Scholar] [CrossRef] [PubMed]
- González-Riopedre, G.; Fernández-García, M.I.; Gómez-Fórneas, E.; Maneiro, M. Biomimetic catalysts for oxidation of veratryl alcohol, a lignin model compound. Catalysts 2013, 3, 232. [Google Scholar] [CrossRef]
- Rouco, L.; Fernández-García, M.I.; González-Noya, A.M.; González-Riopedre, G.; Tyryshkin, A.M.; Maneiro, M. Electrochemical Conversion of the Lignin Model Veratryl Alcohol to Veratryl Aldehyde Using Manganese (III)-Schiff Base Homogeneous Catalysts. Appl. Sci. 2019, 9, 3430. [Google Scholar] [CrossRef]
- Rouco, L.; Liberato, A.; Fernández-Trujillo, M.J.; Máñez, A.; Basallote, M.G.; Alvariño, R.; Alfonso, A.; Botana, L.M.; Maneiro, M. Salen-manganese complexes for controlling ROS damage: Neuroprotective effects, antioxidant activity and kinetic studies. J. Inorg. Biochem. 2020, 203, 110918. [Google Scholar] [CrossRef]
- Rouco, L.; Alvariño, R.; Alfonso, A.; Fernández-Fariña, S.; González-Noya, A.M.; Martínez-Calvo, M.; Pedrido, R.; Rodríguez-Silva, L.; Maneiro, M. Understanding the Factors That Influence the Antioxidant Activity of Manganosalen Complexes with Neuroprotective Effects. Antioxidants 2024, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Atanasov, M.; Bandemehr, J.; Neese, F.; Pietzonka, C.; Kraus, F.; Karttunen, A.J.; Seibald, M.; Heymann, G.; Huppertz, H. Coexistence of two different distorted octahedral [MnF6]3- sites in K3[MnF6]: Manifestation in spectroscopy and magnetism. Chem. Eur. J. 2021, 27, 9801. [Google Scholar] [CrossRef]
- Zhu, L.N.; Jin, Y.W.; Li, X.Z.; Wang, J.; Kong, D.M.; Mi, H.F.; Liao, D.Z.; Shen, H.X. Synthesis, structure and DNA cleavage activity of two 4,4′-dimethyl-2,2′-bipyridyl manganese (II) complexes. Inorg. Chim. Acta 2008, 361, 29. [Google Scholar] [CrossRef]
- Paul, P.; Bhowmik, K.R.N.; Roy, S.; Deb, D.; Das, N.; Bhattacharjee, M.; Purkayastha, R.N.D.; Male, L.; Mckee, V.; Pallepogu, R.; et al. Synthesis, structural features, antibacterial behaviour and theoretical investigation of two new manganese (III) Schiff base complexes. Polyhedron 2018, 151, 407. [Google Scholar] [CrossRef]
- Palopoli, C.; Gómez, G.; Foi, A.; Doctorovich, F.; Mallet-Ladeira, S.; Hureau, C.; Signorella, S. Dimerization, redox properties and antioxidant activity of two manganese (III) complexes of difluoro- and dichloro-substituted Schiff-base ligands. J. Inorg. Biochem. 2017, 167, 49. [Google Scholar] [CrossRef]
- Ortabay, S.; Karakurt, T.; Kaya, B.; Sahin, O.; Ülküseven, B. Manganese (III) complexes with a tetradentate thiosemicarbazone. Structural characterization, electrochemistry, antioxidant capability, molecular docking and dynamics simulation on the potential inhibitory activity of cyclin-dependent kinase 2. Polyhedron 2024, 261, 117128. [Google Scholar] [CrossRef]
- Talebi, A.; Salehi, M.; Jesus, A.J.L.; Kubicki, M.; Fausto, R.; Golbedaghi, R. A New Azide-Bridged Polymeric Manganese (III) Schiff Base Complex with an Allylamine-Derived Ligand: Structural Characterization and Activity Spectra. Inorganics 2024, 12, 234. [Google Scholar] [CrossRef]
- Childs, R.E.; Bardsley, W.G. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 1975, 145, 93. [Google Scholar] [CrossRef]
- Liu, S.W.; Xu, N.H.; Tan, C.Y.; Fang, W.; Tan, Y.; Jiang, Y.Y. A sensitive colorimetric aptasensor based on trivalent peroxidase-mimic DNAzyme and magnetic nanoparticles. Anal. Chim. Acta 2018, 1018, 86. [Google Scholar] [CrossRef]
- Liberato, A.; Fernández-Trujillo, M.J.; Máñez, A.; Maneiro, M.; Rodríguez-Silva, L.; Basallote, M.G. Pitfalls in the ABTS Peroxidase Activity Test: Interference of Photochemical. Inorg. Chem. 2018, 57, 14471. [Google Scholar] [CrossRef]
- Mukimin, A.; Wijaya, K.; Kuncaka, A. Oxidation of remazol brilliant blue r (RB. 19) with in situ electro-generated active chlorine using Ti/PbO2 electrode. Sep. Purif. Technol. 2012, 95, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Concepcion, J.J.; Song, N.; Meyer, T.J. Chloride-assisted catalytic water oxidation. Chem. Commun. 2014, 50, 8053. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N.; Daniele, S.; de Faveri, M.D. Electrochemical oxidation of dyeing baths bearing disperse dyes. Indus. Eng. Chem. Res. 2000, 39, 3241. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Green, D.; McMillan, E. Efficient oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran using Mn(III)-salen catalysts. Catal. Commun. 2008, 9, 286. [Google Scholar] [CrossRef]
- Chellmami, A.; Harikengaram, S. Mechanism of oxidation of aryl methyl sulfoxides with sodium hypochlorite catalyzed by (salen)MnIII complexes. J. Mol. Catal. A. Chem. 2006, 247, 260. [Google Scholar] [CrossRef]
- Procner, M.; Orzel, Ł.; Stochel, G.; van Eldik, R. A Kinetic Study on the Efficient Formation of High-Valent Mn(TPPS)-oxo Complexes by Various Oxidants. Catalysts 2020, 10, 610. [Google Scholar] [CrossRef]
- Mahía, J.; Maestro, M.A.; Vázquez, M.; Bermejo, M.R.; González, A.M.; Maneiro, M. N,N’-Bis (2-tosylaminobenzylidene)-1,2-ethanediamine. Acta Cryst. 2000, 56, 492. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-97 (shelxs 97 and shelxl 97), Programs for Crystal Structure Analyses; University of Göttingen: Göttingen, Germany, 1998. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Scaling and Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. MERCURY CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466. [Google Scholar] [CrossRef]
| Compound | HMF to DFF a | Peroxidase Activity b | E1/2 (mV) c | ΔE (mV) d |
|---|---|---|---|---|
| 1 | 78 ± 4 | 65 ± 4 | −74 | 202 |
| 2 | 70 ± 3 | 49 ± 3 | −128 | 237 |
| 3 | 10 ± 1 | 3 ± 0.5 | −183 | 193 |
| 4 | 11 ± 1 | 2 ± 0.5 | −300 | 347 |
| 1a | 2 | |
|---|---|---|
| Empirical formula | C21H24MnN3O6·H2O | C21H24MnN3O6 |
| Formula weight | 487.39 | 469.37 |
| Temperature [K] | 100(2) | 100(2) |
| Wavelength [Å] | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P 21/c | P-1 |
| a [Å] | 12.2376(4) | 12.5606(5) |
| b [Å] | 14.3006(6) | 13.3548(5) |
| c [Å] | 13.6772(5) | 13.7492(5) |
| α [°] | 90 | 88.901(2) |
| β [°] | 116.362(2) | 86.201(2) |
| γ [°] | 90 | 63.478(2) |
| Volume [Å3] | 2144.66(14) | 2058.96(14) |
| Z | 4 | 4 |
| Density (calculated) [g cm−3] | 1.509 | 1.514 |
| Absorption coefficient [mm−1] | 0.665 | 0.686 |
| Theta range for data collection [°] | 1.86 to 26.47 | 1.48 to 26.42 |
| Reflections collected | 21229 | 50020 |
| Independent reflections | 4414 | 8413 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0582; wR2 = 0.1403 | R1 = 0.0319; wR2 = 0.068 |
| R indices (all data) | R1 = 0.127; wR2 = 0.1721 | R1 = 0.0456; wR2 = 0.0735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Sisto, U.; Fernández-Fariña, S.; Fernández-García, M.I.; González-Noya, A.M.; Velo-Heleno, I.; Maneiro, M. Electrochemical Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxaldehyde Using Mn(III)–Schiff Base Catalysts. Inorganics 2025, 13, 30. https://doi.org/10.3390/inorganics13020030
Barreiro-Sisto U, Fernández-Fariña S, Fernández-García MI, González-Noya AM, Velo-Heleno I, Maneiro M. Electrochemical Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxaldehyde Using Mn(III)–Schiff Base Catalysts. Inorganics. 2025; 13(2):30. https://doi.org/10.3390/inorganics13020030
Chicago/Turabian StyleBarreiro-Sisto, Uxía, Sandra Fernández-Fariña, María Isabel Fernández-García, Ana M. González-Noya, Isabel Velo-Heleno, and Marcelino Maneiro. 2025. "Electrochemical Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxaldehyde Using Mn(III)–Schiff Base Catalysts" Inorganics 13, no. 2: 30. https://doi.org/10.3390/inorganics13020030
APA StyleBarreiro-Sisto, U., Fernández-Fariña, S., Fernández-García, M. I., González-Noya, A. M., Velo-Heleno, I., & Maneiro, M. (2025). Electrochemical Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxaldehyde Using Mn(III)–Schiff Base Catalysts. Inorganics, 13(2), 30. https://doi.org/10.3390/inorganics13020030









