DNA and BSA Binding, Molecular Docking Interactions and ADMET Properties of New PEPPSI-Type Palladium Complexes
Abstract
1. Introduction
2. Results and Discussion
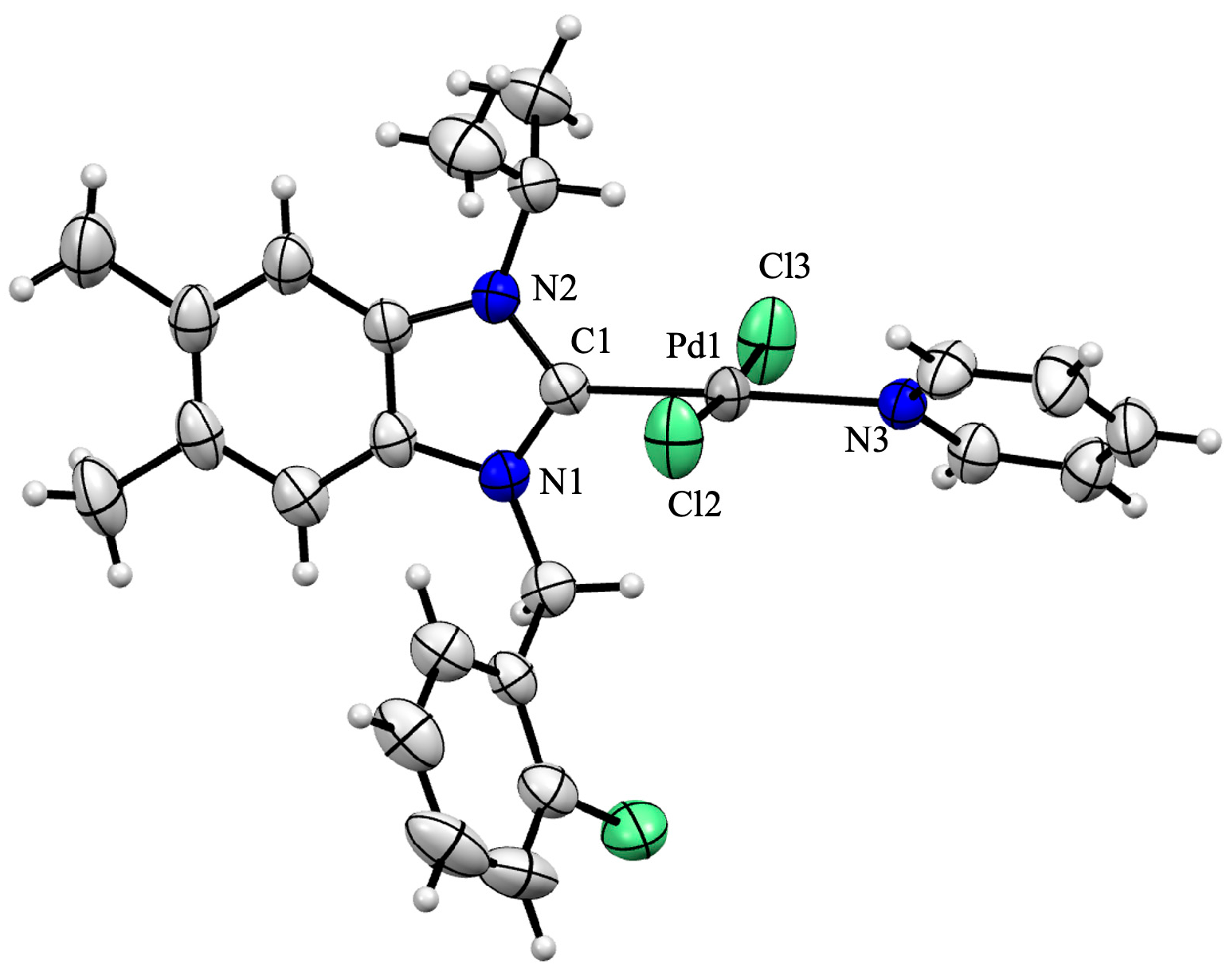
2.1. Synthesis and Structural Analysis of PEPPSI-Type Palladium Complexes
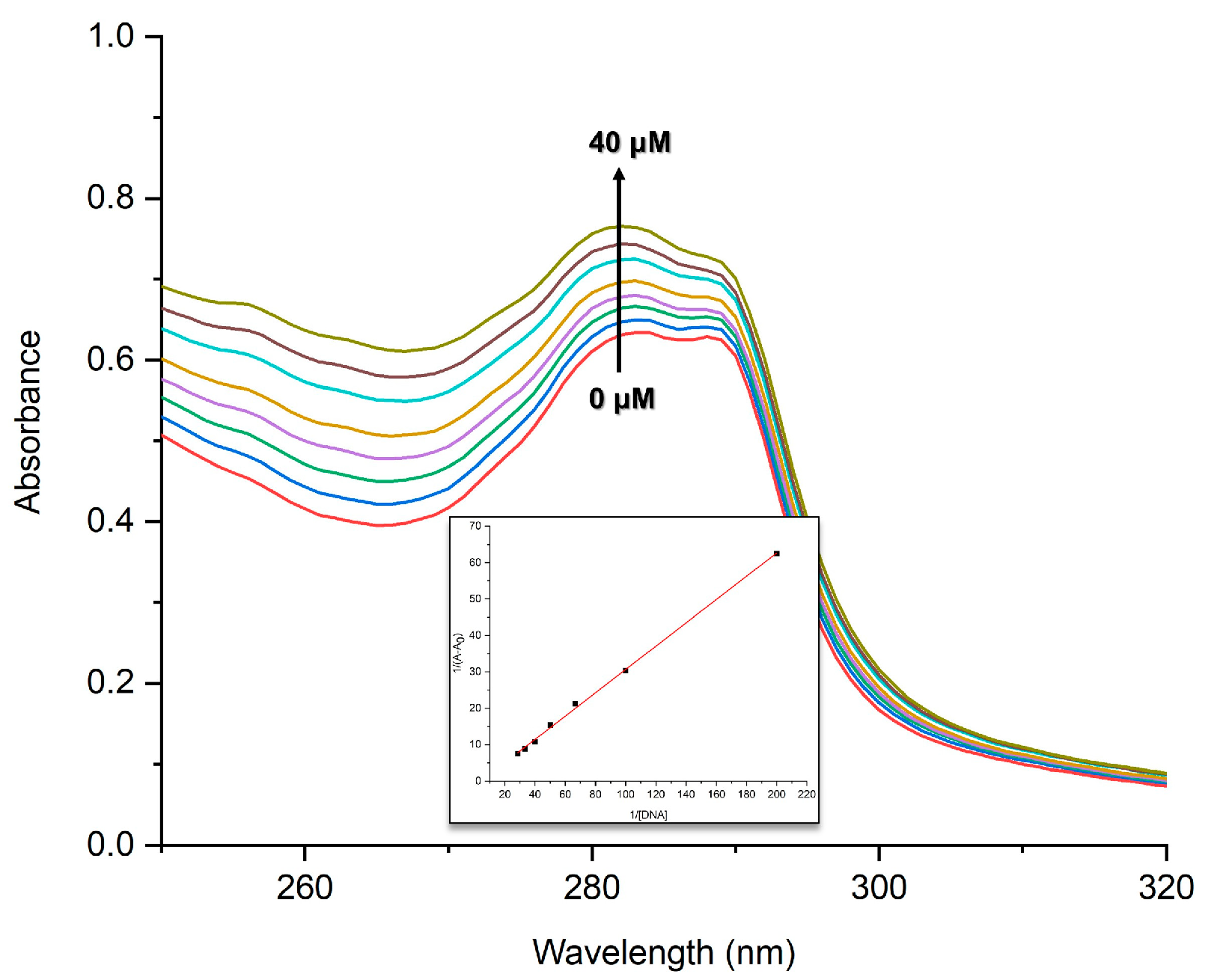
2.2. DNA-Binding Analysis
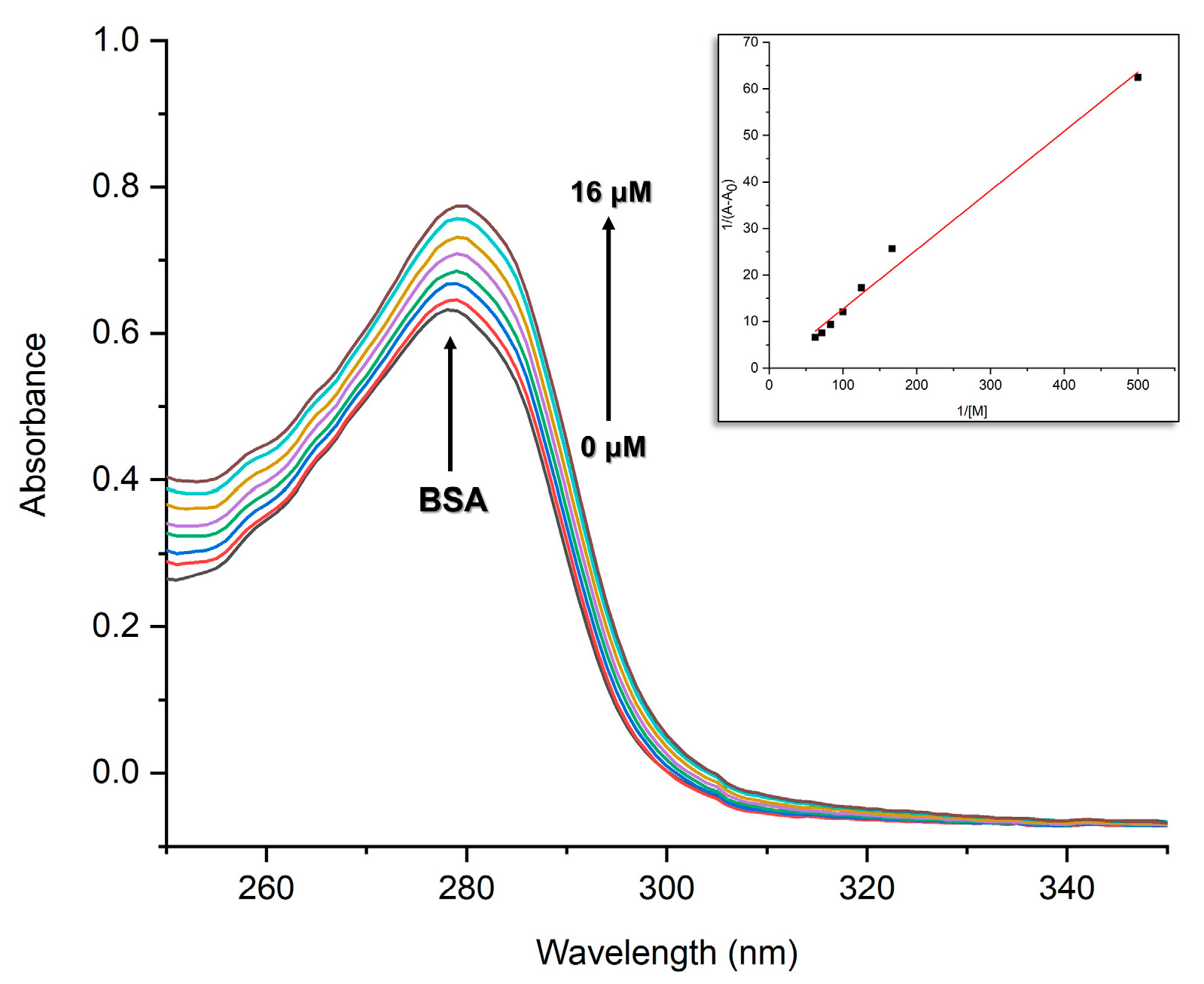
2.3. BSA-Binding Analysis
2.4. ADME Analysis
3. Materials and Methods
3.1. General
3.2. General Procedure for the Preparation of Benzimidazolium Salts
3.3. General Procedure for the Preparation of PEPPSI-Type Complexes
3.4. X-Ray Crystal Structure Analysis
| CCDC depository | 2,504,152 | chemical formula | C24H26Cl3N3Pd | |
| color/shape | yellow/block | formula weight (g∙mol−1) | 569.23 | |
| crystal system | monoclinic | space group | C2/c | |
| unit cell parameters | a (Å) | 25.0789 (11) | volume (Å3) | 5351.3 (4) |
| b (Å) | 13.4807 (6) | Z | 8 | |
| c (Å) | 15.9890 (8) | D (g cm−3) | 1.413 | |
| α (°) | 90 | μ (mm−1) | 1.008 | |
| β (°) | 98.130 (2) | Tmin, Tmax | 0.6281/0.7454 | |
| γ (°) | 90 | F (000) | 2304 | |
| crystal size (mm) | 0.203 × 0.104 × 0.1 | index ranges | −31 ≤ h ≤ 31 | |
| θ range for data collection (°) | 2.998 ≤ θ ≤ 26.610 | −17 ≤ k ≤ 17 | ||
| reflections collected | 37690 | −20 ≤ l ≤ 20 | ||
| data/restraints/parameters | 5680/0/285 | goodness-of-fit on F2 | 1.010 | |
| final R indices (I > 2.0 σ(I)) | R1 = 0.0427 | R indices (all data) | R1 = 0.0813 | |
| wR2 = 0.0783 | wR2 = 0.0961 | |||
| Δρmax, Δρmin (e Å−3) | 0.949, −0.684 | |||
3.5. Stability Test
3.6. DNA Binding Analysis
3.7. BSA Binding Analysis
3.8. Molecular Docking Method
3.9. ADME Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, S.P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014. [Google Scholar]
- Díez-González, S. N-Heterocyclic Carbenes: From Laboratory to Curiosities to Efficient Synthetic Tools, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2017. [Google Scholar]
- Wanzlick, H.-W.; Schönherr, H.-J. Direct synthesis of a mercury salt-carbene complex. Angew. Chem. Int. Ed. 1968, 7, 141–142. [Google Scholar] [CrossRef]
- Öfele, K. 1,3-Dimethyl-4-imidazolinyliden-(2)-pentacarbonylchrom ein neuer Übergangsmetall-carben-komplex. J. Organomet. Chem. 1968, 12, 42–43. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Köcher, C. N-Heterocyclic carbenes. Angew. Chem. Int. Ed. 1997, 36, 2162–2187. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef]
- Naz, N.; Saqib, S.; Ashraf, R.; Majeed, M.I.; Iqbal, M.A. Synthesis of new organoselenium compounds: Characterization and biological studies. Maced. J. Chem. Chem. Eng. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic carbene complexes of copper, nickel, and cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef]
- Gök, Y.; Akkoc, S.; Çelikal, Ö.Ö.; Özdemir, İ.; Günal, S. In vitro antimicrobial studies of naphthalen-1-ylmethyl substituted silver N-heterocyclic carbene complexes. Arab. J. Chem. 2019, 12, 2513–2518. [Google Scholar] [CrossRef]
- Bian, M.; Fan, R.; Zhao, S.; Liu, W. Targeting the thioredoxin system as a strategy for cancer therapy: Miniperspective. J. Med. Chem. 2019, 62, 7309–7321. [Google Scholar]
- Choo, K.B.; Mah, W.L.; Lee, S.M.; Lee, W.L.; Cheow, Y.L. Palladium complexes of bidentate pyridine N-heterocyclic carbenes: Optical resolution, antimicrobial and cytotoxicity studies. Appl. Organomet. Chem. 2018, 32, e4377. [Google Scholar]
- Catalano, A.; Mariconda, A.; Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Longo, P. Biological activities of ruthenium NHC complexes: An update. Antibiotics 2023, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Esarev, I.V.; Wu, C.; Kirsanova, A.A.; Türck, S.; Lippmann, P.; Jones, P.G.; Babak, M.V.; Ott, I. Silver N-heterocyclic biscarbene complexes: Potent inhibitors of thioredoxin reductase with anticancer activity in vitro and in vivo. Chem. Asian J. 2025, 20, e202401672. [Google Scholar] [CrossRef] [PubMed]
- Touj, N.; Chakchouk-Mtibaa, A.; Mansour, L.; Harrath, A.H.; Al-Tamimi, J.; Mellouli, L.; Özdemir, İ.; Yasar, S.; Hamdi, N. Synthesis, spectroscopic properties and biological activity of new Cu(I) N-Heterocyclic carbene complexes. J. Mol. Struct. 2019, 1181, 209–219. [Google Scholar] [CrossRef]
- Estrada-Ortiz, N.; Guarra, F.; de Graaf, I.A.M.; Marchetti, L.; de Jager, M.H.; Groothuis, G.M.M.; Gabbiani, C.; Casini, A. Anticancer gold N-heterocyclic carbene complexes: A comparative in vitro and ex vivo study. ChemMedChem 2017, 12, 1429–1435. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily prepared air-and moisture-stable Pd-NHC (NHC = N-heterocyclic carbene) complexes: A reliable, user-friendly, highly active palladium precatalyst for the Suzuki-Miyaura reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Travers, A.; Muskhelishvili, G. DNA structure and function. FEBS J. 2015, 282, 2279–2295. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Gupta, S.P.; Masand, N.; Balasubramanian, K. Experimental and computational models to understand protein-ligand, metal-ligand and metal-DNA interactions pertinent to targeted cancer and other therapies. Eur. J. Med. Chem. Rep. 2024, 10, 100133. [Google Scholar] [CrossRef]
- Jevtovic, V.; Golubović, L.; Alshammari, B.; Alshammari, M.R.; Rajeh, S.Y.; Alreshidi, M.A.; Alshammari, O.A.O.; Rakić, A.; Dimić, D. Crystal structure, theoretical analysis, and protein/DNA binding activity of iron (III) complex containing differently protonated pyridoxal–S-methyl-isothiosemicarbazone ligands. Int. J. Mol. Sci. 2024, 25, 7058. [Google Scholar] [CrossRef]
- Palchaudhuri, R.; Hergenrother, P.J. DNA as a target for anticancer compounds: Methods to determine the mode of binding and the mechanism of action. Curr. Opin. Biotechnol. 2007, 18, 497–503. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Liu, H.; Wang, R.; Chen, J. Studies on the interaction between antibiotics and DNA. Talanta 2007, 73, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Panicker, R.R.; Sivaramakrishna, A. Studies on synthesis and influence of sterically driven Ni(II)-terpyridine (NNN) complexes on BSA/DNA binding and anticancer activity. J. Inorg. Biochem. 2024, 257, 112553. [Google Scholar] [CrossRef]
- Sen, S.; Chowdhury, N.; Kim, T.-W.; Paul, M.; Debnath, D.; Jeon, S.; Bagchi, A.; Im, J.; Biswas, G. Anticancer, antibacterial, antioxidant, and DNA-binding study of metal-phenalenyl complexes. Bioinorg. Chem. Appl. 2022, 2022, 8453159. [Google Scholar] [CrossRef]
- Önbaş, S.C.; Serdaroğlu, G.; Şahin, N.; Üstün, E.; Özdemir, İ. Synthesis, characterization, computational evaluation, CO-releasing properties, and molecular docking interactions of new [Mn(CO)3(bpy)L] PF6- type molecules. ACS Omega 2025, 10, 30798–30814. [Google Scholar] [CrossRef] [PubMed]
- Saygıdeğer Demir, B.; İnce, S.; Yilmaz, M.K.; Sezan, A.; Derinöz, E.; Taskin-Tok, T.; Saygideger, Y. DNA binding and anticancer properties of new Pd(II)-phosphorus Schiff base metal complexes. Pharmaceutics 2022, 14, 2409. [Google Scholar] [CrossRef]
- Watanabe, K.; Seki, N. Biology and development of DNA-targeted drugs, focusing on synthetic lethality, DNA repair, and epigenetic modifications for cancer: A review. Int. J. Mol. Sci. 2024, 25, 752. [Google Scholar] [CrossRef]
- Sleep, D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 793–812. [Google Scholar]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar]
- Üstün, E.; Şahin, N. Insight into the effect of Ca2+, Mg2+ and Zn2+ on serum albumin interaction of benzimidazole-type new isopropyl substituted N-heterocyclic carbene molecules. J. Photochem. Photobiol. A Chem. 2024, 447, 115282. [Google Scholar] [CrossRef]
- Krishnan, D.; Sheela, A. A review on DNA/BSA binding and cytotoxic properties of multinuclear Schiff’s base complexes. Results Chem. 2023, 5, 100732. [Google Scholar] [CrossRef]
- Tarai, S.K.; Pan, A.; Biswas, P.; Bhaduri, R.; Mandal, S.; Paul, A.; Baitalik, S.; Bhattacharjee, A.; Moi, S.C. Anticancer behavior of pyrrolidine-based palladium(II) complexes and biophysical approach on their DNA, BSA binding activity, molecular docking, and DFT study. Langmuir 2023, 39, 10947–10964. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, A.S.D.; Dehghanian, E.; Mansouri-Torshizi, H.; Feizi-Dehnayebi, M. Computational and experimental examinations of new antitumor palladium(II) complex: CT-DNA-/BSA-binding, in silico prediction, DFT perspective, docking, molecular dynamics simulation and ONIOM. J. Biomol. Struct. Dyn. 2024, 42, 5447–5469. [Google Scholar] [CrossRef] [PubMed]
- Mvelase, S.T.; Benson, S.O.; Omondi, R.O.; Kumah, R.T.; Fatokun, A.A.; Ojwach, S.O. Structural, DNA/BSA binding interactions and cytotoxicity studies of carboxamide (pyridyl)pyrazine palladium (II) complexes. J. Mol. Struct. 2025, 1322, 140267. [Google Scholar]
- Ulu, Ö.D.; Serin, S.; Özdemir, İ. NHC-based Pd–PEPPSI complexes: Synthesis, characterization, DFT studies and catalytic activity in Suzuki-Miyaura cross coupling. J. Indian Chem. Soc. 2025, 102, 101689. [Google Scholar] [CrossRef]
- Atakol, A.; Yiğit, B.; Akdan, H.; Evren, E.; Celepci, D.B.; Yiğit, M.; Aygün, M.; Özdemir, İ. PEPPSI-type N-heterocyclic carbene palladium(II) complexes as catalysts in the direct C5 arylation of furan and thiophene. Inorg. Chimica Acta 2025, 580, 122612. [Google Scholar] [CrossRef]
- Yamada, T.; Tominari, Y.; Tanaka, S.; Mizuno, M. Infrared spectroscopy of ionic liquids consisting of imidazolium cations with different alkyl chain lengths and various halogen or molecular anions with and without a small amount of water. J. Phys. Chem. B 2017, 121, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Şahin, N.; Özdemir, İ.; Sémeril, D. Palladium-catalyzed cross-coupling reaction via C-H activation of furanyl and thiofuranyl substrates. Inorganics 2024, 12, 175. [Google Scholar] [CrossRef]
- Şahin, N.; Zengin, S.; Özdemir, İ.; Sémeril, D. C-H activation of furanyl and thiofuranyl substrates catalyzed by trans-dichloro [1-cinnamyl-3-arylmethyl-benzimidazol-2-yliden]pyridine palladium(II) complexes. Polyhedron 2024, 261, 117144. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Düşünceli, S.D.; Kaloğlu, M.; Şahin, O.; Üstün, E. Synthesis, characterization, crystal structure, and BSA-binding properties with the presence of Ca2+, Mg2+, and Zn2+ ions by N(7)-substituted theophyllines. ChemistrySelect 2025, 10, e01358. [Google Scholar] [CrossRef]
- Hu, W.; Deng, S.; Huang, J.; Lu, Y.; Le, X.; Zheng, W. Intercalative interaction of asymmetric copper(II) complex with DNA: Experimental, molecular docking, molecular dynamics and TDDFT studies. J. Inorg. Biochem. 2013, 127, 90–98. [Google Scholar] [CrossRef]
- Sorasaenee, K.; Fu, P.K.-L.; Angeles-Boza, A.M.; Dunbar, K.R.; Turro, C. Inhibition of transcription in vitro by anticancer active dirhodium(II) complexes. Inorg. Chem. 2003, 42, 1267–1271. [Google Scholar] [CrossRef]
- Raza, M.K.; Gautam, S.; Garai, A.; Mitra, K.; Kondaiah, P.; Chakravarty, A.R. Monofunctional BODIPY-appended imidazoplatin for cellular imaging and mitochondria-targeted photocytotoxicity. Inorg. Chem. 2017, 56, 11019–11029. [Google Scholar] [CrossRef]
- Lavanya, M.; Haribabu, J.; Ramaiah, K.; Yadav, C.S.; Chitumalla, R.K.; Jang, J.; Karvembu, R.; Reddy, A.V.; Jagadeesh, M. 2′-Thiophenecarboxaldehyde derived thiosemicarbazone metal complexes of copper(II), palladium(II) and zinc(II) ions: Synthesis, spectroscopic characterization, anticancer activity and DNA binding studies. Inorg. Chim. Acta 2021, 524, 120440. [Google Scholar] [CrossRef]
- Ali, M.S.; Muthukumaran, J.; Jain, M.; Santos-Silva, T.; Al-Lohedan, H.A.; Al-Shuail, N.S. Molecular interactions of cefoperazone with bovine serum albumin: Extensive experimental and computational investigations. J. Mol. Liq. 2021, 337, 116354. [Google Scholar] [CrossRef]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Şahin, N. Palladium-N-heterocyclic carbene complexes: Synthesis, characterization and catalytic application of C-H activation for carboxaldehyde derivatives. ChemistrySelect 2024, 9, e202403032. [Google Scholar] [CrossRef]
- heldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Struct. Chem. 2015, 71, 3–8. [Google Scholar]
- Jimenez, J.; Chakraborty, I.; Del Cid, A.M.; Mascharak, P.K. Five-and six-coordinated silver(I) complexes derived from 2,6-(pyridyl)iminodiadamantanes: Sustained release of bioactive silver toward bacterial eradication. Inorg. Chem. 2017, 56, 4784–4787. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Struct. Biol. 2012, D68, 1278–1289. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Serdaroğlu, G.; Uludag, N.; Üstün, E. Efficient synthesis of chromeno [2,3-b]pyridine derivatives using Zn(OTf)2 as a catalyst: DFT computations, molecular docking and ADME studies. J. Mol. Liq. 2023, 375, 121364. [Google Scholar] [CrossRef]
- Hkiri, S.; Coşkun, K.A.; Üstün, E.; Samarat, A.; Tutar, Y.; Şahin, N.; Sémeril, D. Silver(I) complexes based on oxadiazole-functionalized α-aminophosphonate: Synthesis, structural study, and biological activities. Molecules 2022, 27, 8131. [Google Scholar] [CrossRef] [PubMed]
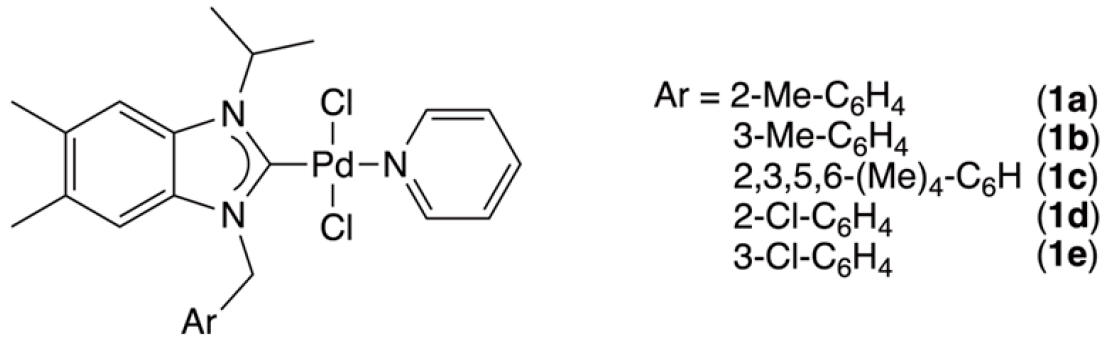

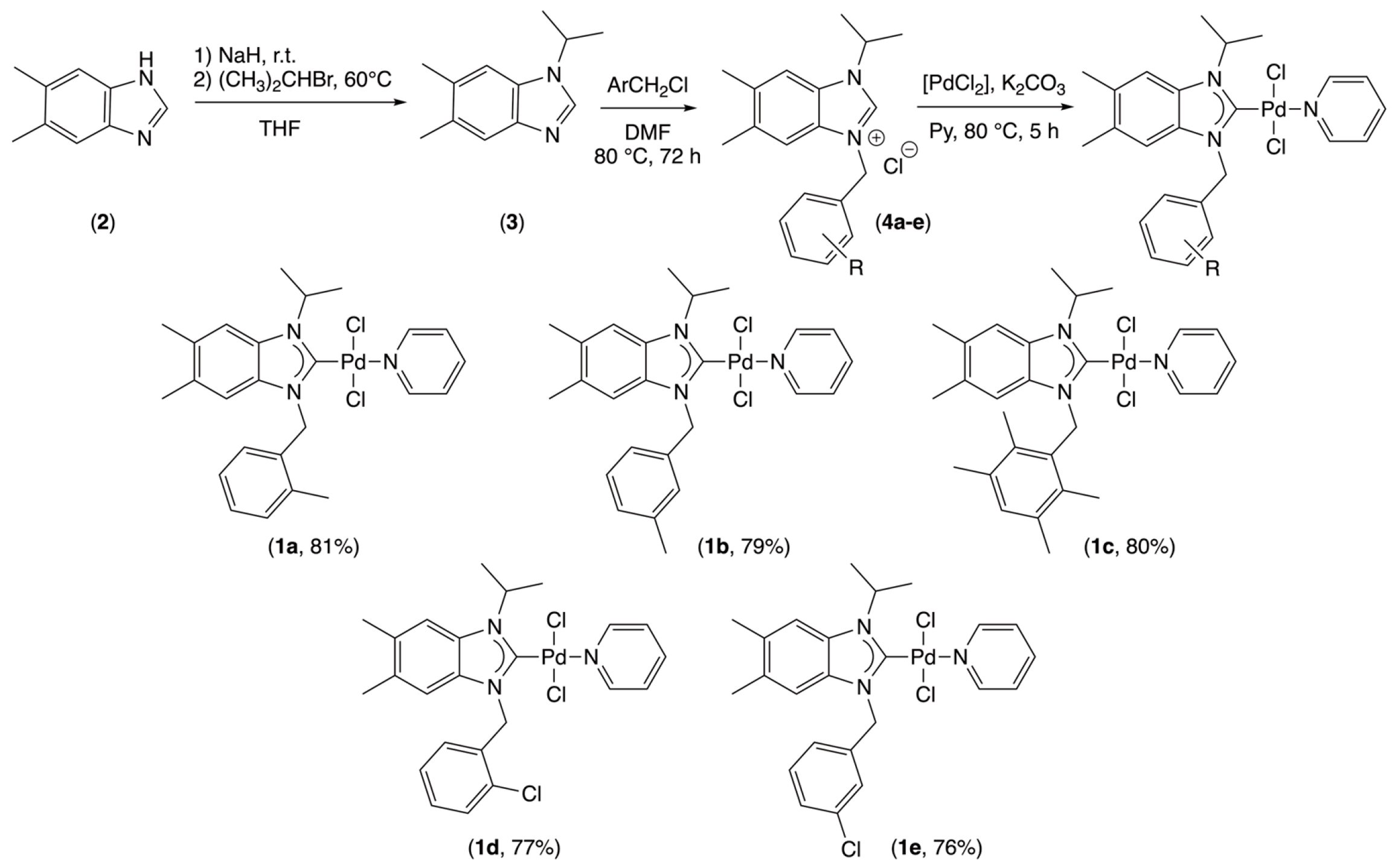
| Arylmethyl | Benzimidazolium Salts | Palladium Complexes | ||||||
|---|---|---|---|---|---|---|---|---|
| FT-IR ν(CN) (cm−1) | 1H NMR NCHN (ppm) | 13C NMR NCHN (ppm) | FT-IR ν(CN) (cm−1) | 13C NMR NC(Pd)N (ppm) | ||||
| 2-Methylbenzyl | 4a | [24] | 1557 | 11.69 | 141.80 | 1a | 1447 | 161.04 |
| 3-Methylbenzyl | 4b | 1554 | 11.87 | 141.43 | 1b | 1447 | 160.68 | |
| 2,3,5,6-tetramethylbenzyl | 4c | 1552 | 11.20 | 141.14 | 1c | 1444 | 160.27 | |
| 2-Chlorobenzyl | 4d | [24] | 1557 | 11.78 | 141.50 | 1d | 1441 | 161.30 |
| 3-Chlorobenzyl | 4e | 1557 | 11.86 | 141.31 | 1e | 1444 | 161.16 | |
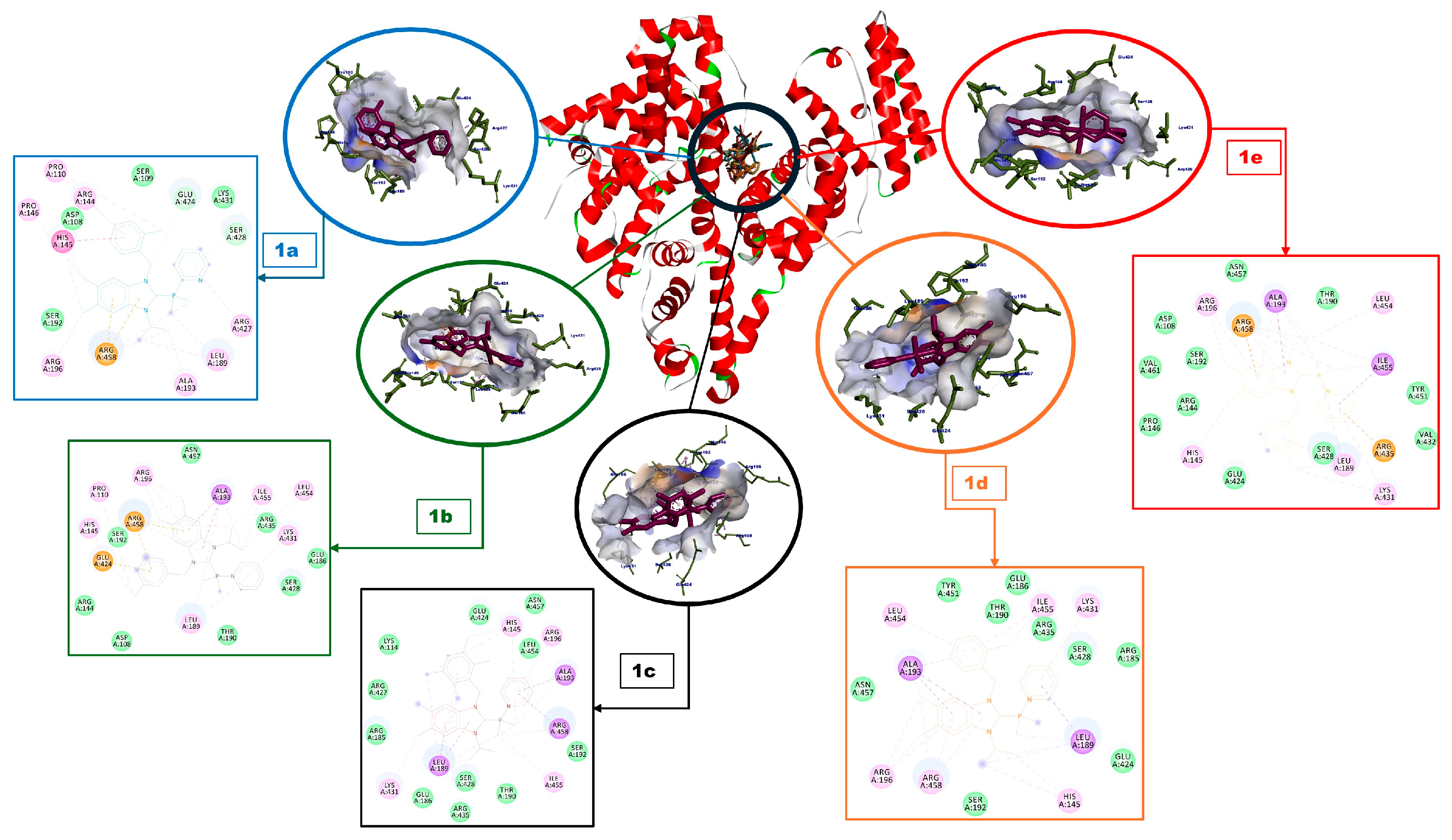
| Complex | BA (kcal/mol) | Amino Acids Residue |
|---|---|---|
| 1a | −7.79 | Glu424, Ser428 (H-bonds), His145, Arg458 (π-interactions), Pro110, Arg144, Pro146, Leu189, Ala193, Arg196, Arg457 (alkylic interactions), Asp108, Ser109, Ser191, Lys431 (van der Waals interactions) |
| 1b | −7.79 | Ala193, Glu424, Arg458 (π-interactions), Pro110, His145, Leu189, Arg196, Lys431, Leu454, Ile455 (alkylic interactions), Asp108, Arg144, Glu186, Thr190, Ser192, Ser428, Arg435, Asn457 (van der Waals interactions) |
| 1c | −7.67 | Leu189, Ala193, Arg458 (π-interactions), His145, Arg196, Lys431, Ile455 (alkylic interactions), Lys114, Arg185, Glu186, Thr190, Ser192, Glu424, Arg427, Ser428, Srg435, Leu454, Asn457 (van der Waals interactions) |
| 1d | −8.02 | Leu189, Ala193 (π-interactions), His145, Arg196, Lys431, Leu454, Ile455, Arg458 (alkylic interactions), Arg185, Glu186, Thr190, Ser192, Glu424, Ser428, Arg435, Tyr451, Asn457 (van der Waals interactions) |
| 1e | −7.97 | Ala193, Arg435, Ile455, Arg458 (π-interactions), His145, Leu189, Arg196, Lys431, Leu454 (alkylic interactions), Asp108, Arg144, Pro146, Thr190, Glu424, Ser428, Val432, Tyr451, Asn457, Val461 (van der Waals interactions) |
| Complex | MW (g/mol) | MlogP [54] | TPSA (Å2) | BBB | CYP3A4 | CYP2C9 | Pgp | Skin Permeation |
|---|---|---|---|---|---|---|---|---|
| 1a | 558.8 | 5.08 | 9.72 | Yes | No | Yes | Yes | −3.51 |
| 1b | 558.8 | 5.08 | 9.72 | Yes | No | Yes | Yes | −3.65 |
| 1c | 590.9 | 5.67 | 9.72 | Yes | No | Yes | Yes | −3.10 |
| 1d | 569.2 | 5.08 | 9.72 | Yes | No | Yes | Yes | −4.00 |
| 1e | 569.2 | 5.08 | 9.72 | Yes | No | Yes | Yes | −4.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Üstün, E.; Şahin, N.; Sémeril, D. DNA and BSA Binding, Molecular Docking Interactions and ADMET Properties of New PEPPSI-Type Palladium Complexes. Inorganics 2025, 13, 391. https://doi.org/10.3390/inorganics13120391
Üstün E, Şahin N, Sémeril D. DNA and BSA Binding, Molecular Docking Interactions and ADMET Properties of New PEPPSI-Type Palladium Complexes. Inorganics. 2025; 13(12):391. https://doi.org/10.3390/inorganics13120391
Chicago/Turabian StyleÜstün, Elvan, Neslihan Şahin, and David Sémeril. 2025. "DNA and BSA Binding, Molecular Docking Interactions and ADMET Properties of New PEPPSI-Type Palladium Complexes" Inorganics 13, no. 12: 391. https://doi.org/10.3390/inorganics13120391
APA StyleÜstün, E., Şahin, N., & Sémeril, D. (2025). DNA and BSA Binding, Molecular Docking Interactions and ADMET Properties of New PEPPSI-Type Palladium Complexes. Inorganics, 13(12), 391. https://doi.org/10.3390/inorganics13120391








